Abstract
Background
Successful implementation of practice guidelines has been challenging in the treatment of acute coronary syndrome (ACS), leaving room for improvement. A nationwide registry can provide more information than that recorded in the National Health Insurance Research Database (NHIRD).
Methods
We conducted a prospective, nationwide, multi-center ACS full spectrum registry involving 3600 patients admitted to hospitals within 24 hours of the onset of myocardial infarction with ST-segment elevation or ACS without ST-segment elevation. In total, 41 sites including medical centers and regional hospitals were selected across Taiwan. The data for each patient are collected at 3 time points for the main study: during hospitalization, 6 months, and 12 months after the discharge. The milestone for first patient in was reached on January 7, 2022, and complete enrollment is expected before October 2023. The primary aims of the main study are to determine the degree of guideline-directed medical therapies and to identify prognostic predictors associated with 1-year composite outcomes, including death, myocardial infarction, stroke, and unplanned coronary revascularization in ACS patients. Thereafter, the patient data will be analyzed every 3 to 5 years for up to 20 years after discharge using the NHIRD in the extended study.
Conclusions
We hypothesized that a greater increase in the implementation of guideline-directed medical therapies can be observed. The results of the current study will add new and important information regarding a broad spectrum of ACS to drive further investigations.
Keywords: Acute coronary syndrome, Guideline-directed medical therapies, Outcomes, Registry
Abbreviations
ACS, Acute coronary syndrome
ACS-FS, Taiwan Acute Coronary Syndrome Full Spectrum Registry
CABG, Coronary artery bypass grafting
DCF, Data collection form
ECG, Electrocardiography
GRACE, Global Registry of Acute Coronary Events
ICF, Informed consent form
IRB, Institutional Review Board
MI, Myocardial Infarction
NHIRD, National Health Insurance Research Database
NSTE-ACS, Non-ST-segment elevation acute coronary syndrome
PCI, Percutaneous coronary intervention
STEMI, ST-segment elevation myocardial infarction
T-ACCORD, Taiwan Acute Coronary Syndrome Descriptive
T-FORMOSA, TSOC-Fully Organized Registry for the Management Of Symptomatic ACS Study
TIMI, Thrombolysis In Myocardial Infarction
TSOC, Taiwan Society of Cardiology
TSOC ACS-DM, Acute Coronary Syndrome-Diabetes Mellitus Registry of the Taiwan Society of Cardiology
INTRODUCTION
Heart disease, and mainly coronary artery disease, remains the second leading cause of death in Taiwan.1 Acute coronary syndrome (ACS) is the most serious coronary artery disease and represents a heterogeneous spectrum of conditions. International2-5 and local6,7 clinical practice guidelines have been developed to provide physicians with an evidence-based approach to daily patient care. However, data from international registries and observational studies in Taiwan have demonstrated that many patients do not receive best evidence-based practice as mandated by clinical practice guidelines.8-12 Therefore, successful implementation of practice guidelines has been challenging in the treatment of ACS, leaving significant room for improvement.8
The National Health Insurance Research Database (NHIRD) is a useful tool to understand the status of ACS in Taiwan.13 However, disadvantages of the NHIRD include a lack of diagnostic and treatment strategies, details of procedures and complications, lifestyle information and laboratory data, and the impact of contemporary interdisciplinary healthcare. Furthermore, disease coding bias and errors are still major concerns. These disadvantages can mostly be overcome by conducting registry studies. The Taiwan Society of Cardiology (TSOC) has conducted four ACS registries,9-12 and the data have been analyzed and published previously in at least 23 original articles with respect to various aspects of ACS.9-12,14-32 The time of enrollment, different disease spectrums, number of participating hospitals and patients in these four registries plus the current study are summarized in Table 1.
Table 1. Summary of 5 ACS registries conducted by TSOC.
| Registry | T-ACCORD10 | ACS-FS11 | ACS STENT12 | TSOC ACS-DM13 | T-FORMOSA |
| Time of enrolment | April 2004-December 2006 | October 2008-January 2011 | April 2012-December 2015 | July 2013-December 2015 | January 2022-October 2023 |
| Disease spectrums | NSTE-ACS | ACS | ACS with stenting | ACS with DM | ACS |
| Participating hospital (n) | 27 | 39 | 24 | 27 | 41 |
| Patient (n) | 1331 | 3183 | 2357 | 1534 | Estimated 3600 |
| STEMI [n (%)] | 0 | 1665 (52.3) | 1294 (54.9) | 455 (29.7) | NA |
ACS, acute coronary syndrome; ACS-FS, Taiwan Acute Coronary Syndrome Full Spectrum Registry; DM, diabetes mellitus; NA, not available; NSTE-ACS, non-ST-segment elevation ACS; T-ACCORD, Taiwan Acute Coronary Syndrome Descriptive Registry; T-FORMOSA, TSOC-Fully Organized Registry for the Management Of Symptomatic ACS Study; TSOC ACS-DM, Acute Coronary Syndrome-Diabetes Mellitus Registry of the Taiwan Society of Cardiology.
Many ACS registries have been conducted in Western33,34 and Asia-Pacific countries.35,36 The results from different countries may be used for comparisons between countries, however generalizability of the results in different populations and countries is still questionable. Furthermore, previous large-scale randomized controlled trials have usually included a small number of Asian patients.37 Therefore, it is arguable whether the results of such trials can be generalized to Asian patients.38-40 Furthermore, different management patterns and different outcomes of a single disease across different ethnicities and regions are usually present. For example, the efficacy and safety of ticagrelor seems to be different between Taiwan41,42 and other East Asian countries.38,39 Therefore, large-scale nationwide registries covering the entire spectrum of ACS to assess current practice and outcomes in a ‘real-world’ situation within a specific time interval to fill this knowledge gap are needed in Taiwan.
To address these issues, the TSOC initiated a new large-scale, nationwide, multi-center, prospective registry, the TSOC-Fully Organized Registry for the Management Of Symptomatic ACS Study (T-FORMOSA Study), to determine the degree of guideline-directed medical therapies and to identify prognostic predictors associated with 1-year composite outcomes, with the long-term outcomes clarified using the NHIRD of Taiwan. We hypothesized that the 1-year outcomes of patients with ACS would be improved in the current era compared to those in the era of the previous ACS-FS registry, owing to a greater increase in the implementation of guideline-directed medical therapies.
MATERIALS AND METHODS
Study design
The current study was originally designed as a prospective, nationwide, and multi-center registry. The study protocol was initially approved by the TSOC and the first initiating site, the Institutional Review Board (IRB) of National Cheng Kung University Hospital (identifier: B-ER-110-450). The approved version was reviewed and approved by all other participating sites thereafter. All study participants are asked to sign an informed consent form (ICF), and this study follows the regulations of the ethics committees of National Cheng Kung University Hospital and the other participating sites. The investigators and study nurses fully explain the study protocol and collect signed informed consent forms from potential trial participants or authorized surrogates.
Eligible participants
Inclusion criteria
This study is ongoing and consecutively enrolling ACS patients (target number: 3600) (age ≥ 20 years) admitted within 24 hours or transferred from a non-trial site if they are at the non-trial site for less than 12 hours of symptom onset. ACS is defined as patients with unstable angina, myocardial infarction (MI) without ST-segment elevation or MI with ST-segment elevation (STEMI). Unstable angina and non-STEMI are further categorized as non-ST-segment elevation ACS (NSTE-ACS).
Exclusion criteria
Patients are excluded if they meet at least one of the following situations before screening: 1) ACS accompanied by or precipitated by significant co-morbidity e.g. motor vehicle accidents, trauma, severe gastrointestinal bleeding, peri-operative or peri-procedural MI; 2) having been enrolled in the current study already with recurrent ACS; 3) participating in an investigational drug study.
Selection of participating sites and investigators, and enrollment of participants
At least three full-time cardiologists were required at a single qualified participating site, which was required to have an independent IRB (or to collaborate with an outside IRB with regular monitoring) and could provide 24-hour primary percutaneous coronary intervention (PCI) service. A qualified participating site was either a medical center or regional hospital covering a sufficient geographic area to allow for a representative sample. Cardiologists taking care of ACS patients at the qualified participating sites were selected as the participating physicians. The geographic distribution and number of participating sites in each city or county in Taiwan are shown in Figure 1. The participating hospitals and the names of the principal investigators are listed in Supplementary Table 1.
Figure 1.
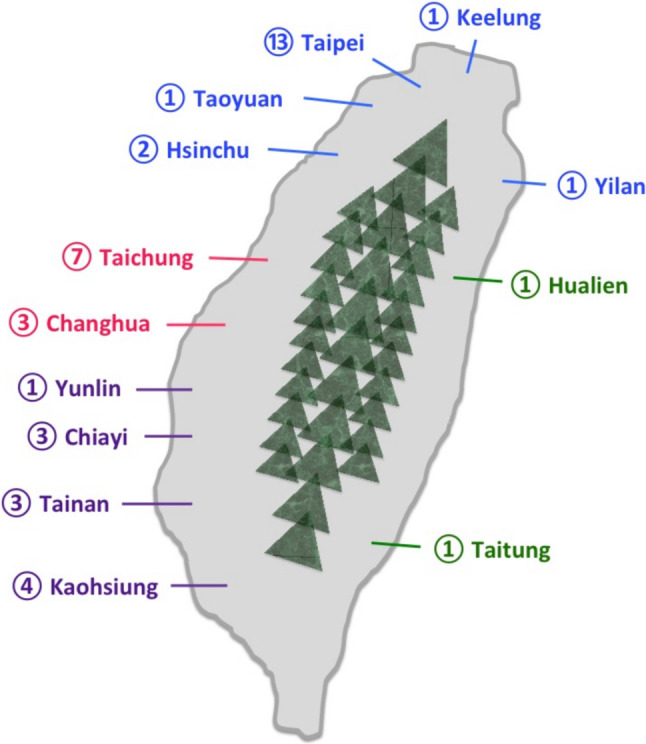
The geographic distribution and number of participating sites in each city or county in Taiwan. The numbers in circles denote how many participating sites there are in each city or county, and different colors indicate in which part of Taiwan the participating sites are located.
Each participating site was requested to enroll 50-200 patients during the recruitment period of the study. The participating physicians should enroll consecutive patients who meet the study criteria confirmed by the participating physicians, thereby limiting bias of subject selection as far as possible.
Duration, visits, and data collection
This is a non-interventional registry. The patients are treated according to the physician’s discretion, local and international guidelines, evidence-based strategies, and local labeling.
Main study data are recorded prospectively and entered via data collection forms (DCFs) at the baseline visit (V1) and subsequent visits (V2 at 6 months and V3 at 12 months after discharge) until 1 year of follow-up. Data are collected from medical records and obtained during hospitalization, and/or on clinical visits (or by telephone if the patients do not make contact for more than 10 days of the scheduled visit date). One year after discharge, the data will be followed as the extended study, using the NHIRD every 3-5 years up to a total of 20 years.
The duration of the main part of the study is approximately 3 years with an estimated 12- to 18-month recruitment period in total and 12-month follow-up for each participant. The milestone for the first site first patient in was achieved on January 7, 2022, and the last participant will be expected to be enrolled before October 2023. The timeline is illustrated in Table 2. Each patient will be followed up for approximately 20 years after discharge or until death or withdrawal of consent by the patient or participating physician.
Table 2. Timeline of the T-FORMOSA study.
| Time | Task |
| November to December 2021 | First IRB approval and site correspondence training |
| January 3 2022 | First site first patient in |
| 45200 | Last patient in |
| 45413 | Interim report of the baseline characteristics |
| October to December 2024 | Data locked |
| 45778 | Reporting the main results |
| Every 3-5 years up to 2045 | Following up long-term outcomes using NHIRD |
IRB, Institutional Review Board; NHIRD, national health insurance research database; T-FORMOSA, TSOC-Fully Organized Registry for the Management Of Symptomatic ACS Study.
The collected items are summarized as follows.
V1: The following data about the patient during hospitalization are recorded:
• Inclusion and exclusion criteria.
• Demographics (date of birth, sex, and ethnicity).
• Height and weight, and waist circumference, if available.
• Medical history (transient ischemic attack/stroke; coronary artery disease; MI; PCI; coronary artery bypass grafting; positive stress test; peripheral arterial disease; atrial fibrillation; heart failure; malignancy; chronic obstructive pulmonary disease; chronic renal disease; obstructive sleep apnea; gout, etc).
• Cardiovascular risk factors (previous cardiovascular history; smoking status including e-cigarettes; hypertension; hyperlipidemia; diabetes mellitus; hyperuricemia; family history).
• Date and time of symptom onset, and whether they were transferred by ambulance or from another hospital.
• Date and time of hospital presentation; out-of-hospital or in-hospital cardiac arrest.
• Presenting symptoms, vital signs, arterial oxygen saturation, routine or indicated oxygen supplement or morphine treatment, door-to-electrocardiography (ECG) time, diagnostic ECG, door-to-enzyme time, cardiac-specific enzyme levels, and initial complications.
• Killip class (I to IV), or Thrombolysis In Myocardial Infarction (TIMI) risk score/Global Registry of Acute Coronary Events (GRACE) score.
• HAS-BLED bleeding score, high bleeding risk.
• Serum lipid profile, hemoglobin A1C, uric acid, natriuretic peptide level, creatinine, white cell count and differential count, etc. (if performed by the hospital).
• In-hospital therapies: interventions (COVID-19 screening immediately before the primary PCI or not; cardiac catheterization; coronary artery stenosis; culprit vessel(s) and flow pattern; PCI; door-to-wire time and door-to-balloon time; stent types; vascular access; thrombus aspiration strategy; total ischemic time if available; non-culprit lesion intervention; image- or physiology-guided therapy; coronary artery bypass grafting; intra-aortic balloon pumping; mechanical life support; others); other procedures (echocardiography; modalities to evaluate left ventricular ejection fraction; in-hospital healthcare).
• In-hospital medications: drug treatments (Thrombolytics – streptokinase, alteplase, reteplase, tenecteplase; Anticoagulants – warfarin, unfractionated heparin, low molecular weight heparin, fondaparinux, non-vitamin K oral anticoagulants; Antiplatelets – glycoprotein IIb/IIIa, aspirin, clopidogrel, ticagrelor, prasugrel, ticlopidine, others; Other Medications – angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, sacubitril/valsartan, calcium channel antagonists, beta-blockers, digoxin, diuretics, nitrates, nicorandil, anti-arrhythmic drugs, statins, proprotein convertase subtilisin/kexin type 9 inhibitors, ezetimibe, other lipid-lowering agents, spironolactone or eplerenone, oral or injected sugar-lowering agents, ranolazine, varenicline, colchicine, ivabradine, others).
• In-hospital medications are collected from the time of admission to in-hospital therapies (long-term use; within 24 hours before or after admission to hospital; after 24 hours in hospital; at discharge; not prescribed).
• In-hospital events (death; MI; unplanned revascularization, new-onset congestive heart failure/pulmonary edema; cardiogenic shock; acute renal failure; stroke – hemorrhagic and non-hemorrhagic; stent thrombosis; TIMI major/minor bleeding; sustained ventricular tachycardia or ventricular fibrillation; new-onset atrial fibrillation).
• Date of discharge.
• Primary discharge diagnosis.
At 6 and 12 months (± 10 days visit) (V2 and V3):
• Outcome (death; MI; stroke; unplanned revascularization; planned revascularization; stent thrombosis; TIMI major/minor bleeding; rehospitalization; heart failure hospitalization or worsening heart failure).
• Healthcare (smoking abstinence; return to rehabilitation department, complete cardiac rehabilitation program; implementation of telehealth monitoring); serum levels of hemoglobin A1C, lipid profile; high potency statin use; echocardiography performed; HAS-BLED bleeding score; high bleeding risk.
• Medications (warfarin; non-vitamin K anticoagulants; aspirin; clopidogrel; ticagrelor; prasugrel; angiotensin-converting enzyme inhibitors; angiotensin receptor blockers; sacubitril/valsartan; calcium channel antagonists; beta-blockers; digoxin; diuretics; nitrates; nicorandil; anti-arrhythmic drugs; statins; proprotein convertase subtilisin/kexin type 9 inhibitors; ezetimibe; other lipid-lowering agents; spironolactone or eplerenone; oral or injected sugar-lowering agents; ranolazine; varenicline; colchicine; ivabradine, others).
Data obtained every 3-5 years (linked to the NHIRD) after discharge:
• Outcome (death; MI; stroke; revascularization; rehospitalization; major bleeding).
• Medications (warfarin; non-vitamin K oral anticoagulants; aspirin; clopidogrel; ticagrelor; prasugrel; angiotensin-converting enzyme inhibitors; angiotensin receptor blockers; sacubitril/valsartan; calcium channel antagonists; beta-blockers; digoxin; diuretics; nitrates; nicorandil; anti-arrhythmic drugs; statins; proprotein convertase subtilisin/kexin type 9 inhibitors; ezetimibe; other lipid-lowering agents; spironolactone or eplerenone; oral or injected sugar-lowering agents; ranolazine; varenicline; colchicine; ivabradine, others).
Logistic aspects
The DCFs are provided and the electronic data collection form (DCF) platform was established by the TSOC T-FORMOSA Study Steering Committee. Information collected in the DCFs are entered into the electronic DCF platform by the physician or designated representative. The representatives authorized by the participating physicians to make entries in the DCFs provide their names, positions, and contact information to the TSOC T-FORMOSA Study Steering Committee. The DCFs are completed as soon as possible after information is collected, preferably on the same day when the patient is due for treatment or an examination. The completed DCFs must be reviewed and signed on the electronic DCF platform by the physician.
Management of data
Data validation
The TSOC T-FORMOSA Study Steering Committee or the clinical research associate authorized by the TSOC may generate additional requests or queries to which the investigator is obliged to respond by confirming or after reconfirmation to revise the data questioned. The requests or queries with their responses are archived in the document held by the TSOC T-FORMOSA Study Steering Committee. Clinical endpoints will be adjudicated by the Clinical Endpoint Adjudication Taskforce.
Monitoring visits for data quality control
Site monitoring visits are performed by a study site monitor, who is a qualified and independent member of staff working for the TSOC. After a site has enrolled > 50 subjects, an Interim Monitoring Visit will be made based on the monitoring plan. Furthermore, a Remote Monitoring Visit will be made every 3 to 6 months, depending on the recruitment status. Auditing may be conducted by the IRB of each site annually as planned. All ICFs should be checked for completeness, and DCFs should be verified for the existence and eligibility of each patient. Complete source data verification is performed on at least 5% of the DCFs at 40% of the sites (Table 3).
Table 3. Requirement for site data quality control.
| DATA checked | Percentage of sampling | Details |
| Patient initials and date of birth | 100% of DCFs | The presence of source documents with medical records corresponding to this patient will be checked. |
| ICF | 100% of DCFs | The completeness of ICF signed by the patient or authorized designated surrogate will be checked. |
| Inclusion and exclusion criteria | 100% of DCFs | The eligibility of all patients will be checked. |
| All other data | At least 5% of DCFs in 40% of the sites | It means 1 out of 20 DCFs with corresponding source documents and medical records will be 100% checked in 2 out of 5 sites. The top recruiting sites take priority to monitor. |
| In sites with less than 20 enrolled patients, at least 1 DCF will be entirely cross-checked with source documents and medical records. |
DCFs, data collection forms; ICF, informed consent form.
The results of the monitoring visits are reported to the Steering Committee. If specific issues are identified at some sites, the percentage of sampling for quality control at the site of concern or at all sites must be appropriately increased, and corrective actions must be taken.
The Steering Committee encourages the principal investigators and site correspondents to reduce the number of participants who are "lost to follow-up" as best as they can and provide incentives to the principal investigators and site correspondents for complete follow-up.
Power estimation and sample size calculation
Due to the descriptive character of this study, there was no requirement for sample size calculation. The study sample size has therefore been chosen arbitrarily.
There are about 50 new ACS cases per 100,000 people per year in Taiwan.6,44 Based on the known background incidence rate of 0.0025, a sample of 2395 patients achieves 80% power to detect an additional incidence rate of 0.003 with a precision of 0.2% and 95% confidence interval. Considering a dropout rate of 20%, 3000 subjects is required, and 3600 was chosen to compare with the previous TSOC ACS-FS and ACS-DM registries.
Primary aims of the main study
The primary aims of the main study are to determine the degree of implementation of guideline-directed medical therapies, including dual anti-platelet treatment, lipid-lowering therapy, an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, and a beta-blocker, and to identify prognostic predictors associated with 1-year composite outcomes, including death, MI, stroke, and unplanned coronary revascularization.
Statistical analysis
Analysis population
Statistical analysis will be based on all patients enrolled in the registry. All data recorded will be analyzed in an explorative manner. The results will be presented in tables and figures.
Statistical methods
Parameters will be summarized using mean, median, standard deviation and interquartile range where appropriate for continuous data, and count or percentage for categorical data. Survival and "event-free survival" will be analyzed by Cox proportional hazards modeling to test the impact of clinical and demographic covariates, as well as variations in patient management. Where possible, propensity score matching will be undertaken to adjust for non-randomized comparisons. All statistical analyses will be performed with an alpha level of < 0.05 on two-sided testing considered as statistically significant. Analyses will be conducted as time to first event without double counting of events within analyses involving composite endpoints. Patients who are "lost to follow-up" will be censored at the time of last contact, with their vital status deemed as being alive and "event-free" at that time.
Planned analyses will include, but not be limited to:
• Description of the baseline clinical and demographic characteristics of patients presenting with intermediate or high-risk ACS in the Taiwanese context.
• Prevalence of risk factors.
• Description of the management strategies administered, including use of invasive management, and the use of clinical guideline-advocated therapies.
• Identification of high-risk groups where an increased risk of late reinfarction or death is observed.
• Evaluation of the relationship between the implementation of guideline-directed medical therapies/healthcare or risk predictors and late clinical outcomes.
• Validation of predictive models such as the TIMI and GRACE risk scores.
• Comparisons of the implementation of guideline-directed medical therapies and clinical outcomes between this and previous TSOC ACS registries.
DISCUSSION
The current study provides the best opportunity to compare adherence to the best evidence-based approach and clinical outcomes after ACS in Taiwan between the contemporary and previous eras. New features of the current registry study will include: 1) evaluation of the impact of management strategies such as routine oxygen supply, thrombus aspiration during primary PCI, non-culprit lesion intervention, image- or physiology-guided intervention, vascular access, single or dual anti-platelet treatment, dual or triple anti-thrombotic treatment; 2) evaluation of the implementation and prognostic impact of interdisciplinary healthcare; 3) evaluation of high-intensity statin use and low-density lipoprotein cholesterol goal attainment rate in the contemporary era; 4) evaluation of the prognostic impact of a full spectrum of medications, including contemporary cardiovascular- and reno-protective drugs such as sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists; 5) evaluation of the efficacy and safety of different designs of coronary stents; 6) evaluation, for the first time, of the long-term cardiovascular outcomes of ACS.
A recent study based on data from the NHIRD on the practice pattern of ACS in Taiwan from 2008 to 2015 showed that PCI and secondary preventive medications, including dual antiplatelet therapy, angiotensin converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers and statins, were less prescribed for NSTEMI compared with STEMI.9 Although the use of statins increased year by year in STEMI patients,9 about one quarter of NSTEMI patients were still not receiving statin therapy before discharge in 2015. Data from two nationwide ACS registries (2008-2010 and 2012-2015) conducted by the TSOC also showed that the prescription rate of secondary preventive medications for ACS was still relatively low compared with Western data, especially for patients with NSTE-ACS.11 However, owing to the introduction and adoption of the Disease-Specific Care Certification Program advocated by the Joint Commission of Taiwan, we may optimistically expect a significant improvement in adherence to guideline-directed medical therapies and clinical outcomes after the index ACS.44
The long-term outcomes and prognostic predictors of ACS may change with the innovation and development of evidence-based diagnosis and management strategies, and the adoption of interdisciplinary healthcare. For example, the in-hospital mortality rate of STEMI stratified by Killip classification has dramatically improved45 compared to decades ago.46 Some surrogate biomarkers have become the most important predictors of cardiovascular events and have been shown to have an even greater prognostic value than other traditional risk factors.47 The current study will further demonstrate the long-term outcomes and prognostic predictors of ACS in Taiwan in the contemporary era.
CONCLUSIONS
In conclusion, the results of the current study will provide new and important information regarding a broad spectrum of ACS in the contemporary era in order to drive both researchers and clinicians to explore and solve potential unmet clinical issues.
DECLARATION OF CONFLICT OF INTEREST
Ting-Hsing Chao have been on the speaker bureau for AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, Pfizer, Sanofi, Tanabe, Orient EuroPharma, and TSH biopharm.
Tzung-Dau Wang has been on the speaker bureau for AstraZeneca, Daiichi-Sankyo, Medtronic, Novartis, Pfizer, and Omron.
All other authors declare no potential conflict of interest in relation to this work.
SUPPLEMENTARY MATERIAL
Supplementary Table 1. Participating hospitals and the names of principal investigators.
| Number | Location | Participating hospital | Principal investigator |
| 1 | Keelung City | Chang Gang Memorial Hospital, Keelung | Chun-Tai Mao |
| 2 | Yilan County | Lo-Hsu Medical Foundation Lotung Poh-Ai Hospital | Yu-Cheng Hsu |
| 3 | Taipei City | Tri-Service General Hospital, National Defense Medical Center | Cheng-Chung Cheng |
| 4 | Taipei City | Taipei Veterans General Hospital | Tse-Min Lu |
| 5 | Taipei City | National Taiwan University Hospital | Yen-Hung Lin |
| 6 | Taipei City | Shin Kong Wu Ho-Su Memorial Hospital | Kou-Gi Shyu |
| 7 | Taipei City | Cathay General Hospital | Chi-Hung Huang |
| 8 | Taipei City | Mackay Memorial Hospital, Taipei | Cheng-Ting Tsai |
| 9 | New Taipei City | Mackay Memorial Hospital, Tamsui | Cheng-Ting Tsai |
| 10 | Taipei City | Wan Fang Hospital, Taipei Medical University | Jen-Hung Huang |
| 11 | Taipei City | Taipei Medical University Hospital | Chun-Yao Huang |
| 12 | Taipei City | Cheng Hsin General Hospital | Wei-Hsian Yin |
| 13 | New Taipei City | Far Eastern Memorial Hospital | Yen-Wen Wu |
| 14 | New Taipei City | Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation | Shih-Jung Jang |
| 15 | New Taipei City | Shuang Ho Hospital | Kuan-Rau Chiou |
| 16 | Taoyuan City | Chang Gung Memorial Hospital, Linkou | Chi-Jen Chang |
| 17 | Hsinchu City | National Taiwan University Hospital Hsin-Chu Branch | Chao-Lun Lai |
| 18 | Hsinchu City | Mackay Memorial Hospital, Hsinchu | Po-Jung Yuan |
| 19 | Taichung City | Taichung Veterans General Hospital | Tsun-Jui Liu |
| 20 | Taichung City | China Medical University Hospital | Chiung-Ray Lu |
| 21 | Taichung City | Tungs’ Taichung MetroHarbor Hospital | Bao-Tzung Wu |
| 22 | Taichung City | Kuang Tien General Hospital, Shalu | Shih-Chung Huang |
| 23 | Taichung City | Kuang Tien General Hospital, Dajia | Chih-Ping Hsia |
| 24 | Taichung City | Chung Shan Medical University Hospital | Chin-Feng Tsai |
| 25 | Changhua County | Changhua Christian Hospital | Bing-Jung Yang |
| 26 | Changhua County | Show Chwan Memorial Hospital | Ho-Pang Yang |
| 27 | Changhua County | Chang Bing Show Chwan Memorial Hospital | Chih-Peng Hsu |
| 28 | Yunlin County | National Taiwan University Hospital Yunlin Branch | Fu-Chun Chiu |
| 29 | Chiayi City | Ditmanson Medical Foundation Chia-Yi Christian Hospital | Han-Lin Tsai |
| 30 | Chiayi County | Chang Gung Memorial Hospital, Chiayi | Jung-Jung Chang |
| 31 | Chiayi County | Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation | Tin-Kwang Lin |
| 32 | Tainan City | Chi Mei Medical Center | Chon-Seng Hong |
| 33 | Tainan City | National Cheng Kung University Hospital | Ting-Hsing Chao |
| 34 | Kaohsiung City | Kaohsiung Veterans General Hospital | Feng-Yu Kuo |
| 35 | Kaohsiung City | Chang Gung Memorial Hospital, Kaohsiung | Shu-Kai Hsueh |
| 36 | Kaohsiung City | Kaohsiung Medical University Hospital | Tsung-Hsien Lin |
| 37 | Kaohsiung City | E-Da Hospital | Chao-Ping Wang |
| 38 | Hualien County | Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation | Ji-Hong Wang |
| 39 | Taitung County | Taitung Mackay Memorial Hospital | Kuang-Te Wang |
| 40 | Tainan City | Tainan Municipal Hospital | Liang-Miin Tsai |
| 41 | Taichung City | Feng Yuan Hospital of the Ministry of Health and Welfare | Chen-Rong Tsao |
Acknowledgments
This research was funded with grants from the National Science Council, Executive Yuan, Taiwan (MOST 111-2314-B-006-019-MY3), Pfizer Limited, and Daiichi Sankyo Taiwan Limited. We sincerely thank to the grant support from the National Science Council, Pfizer Limited, and Daiichi Sankyo Taiwan Limited, and assistance from the T-FORMOSA principal investigators, site correspondences, and TSOC Clinical Research Associate Ms. Yu-Hua Chao. The T-FORMOSA study principal investigators include (in alphabetical order):
Chi-Jen Chang, Jung-Jung Chang, Ting-Hsing Chao, Cheng-Chung Cheng, Kuan-Rau Chiou, Fu-Chun Chiu, Chon-Seng Hong, Chih-Ping Hsia, Chih-Peng Hsu, Yu-Cheng Hsu, Shu-Kai Hsueh, Chi-Hung Huang, Chun-Yao Huang, Jen-Hung Huang, Shih-Chung Huang, Shih-Jung Jang, Feng-Yu Kuo, Chao-Lun Lai, Tin-Kwang Lin, Tsung-Hsien Lin, Yen-Hung Lin, Tsun-Jui Liu, Chiung-Ray Lu, Tse-Min Lu, Chun-Tai Mao, Kou-Gi Shyu, Cheng-Ting Tsai, Chin-Feng Tsai, Han-Lin Tsai, Liang-Miin Tsai, Chen-Rong Tsao, Chao-Ping Wang, Kuang-Te Wang, Ji-Hong Wang, Bao-Tzung Wu, Yen-Wen Wu, Bing-Jung Yang, Ho-Pang Yang, Wei-Hsian Yin, Po-Jung Yuan.
REFERENCES
- 1.Taiwan Department of Health and Welfare. Cause of death Statistics. https://www.mohw.gov.tw/np-128-2.html [Google Scholar]
- 2.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67:1235–1250. doi: 10.1016/j.jacc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 5.Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 6.Li YH, Wang YC, Wang YC, et al. 2018 guidelines of the Taiwan Society of Cardiology, Taiwan Society of Emergency Medicine and Taiwan Society of Cardiovascular Interventions for the management of non ST-segment elevation acute coronary syndrome. J Formos Med Assoc. 2018;117:766–790. doi: 10.1016/j.jfma.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Li YH, Lee CH, Huang WC, et al. 2020 focused update of the 2012 guidelines of the Taiwan Society of Cardiology for the management of ST-segment elevation myocardial infarction. Acta Cardiol Sin. 2020;36:285–307. doi: 10.6515/ACS.202007_36(4).20200619A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer T, Gitt AK, Jünger C, et al. Guideline-recommended secondary prevention drug therapy after acute myocardial infarction: predictors and outcomes of nonadherence. Eur J Cardiovasc Prev Rehabil. 2010;17:576–581. doi: 10.1097/HJR.0b013e328338e5da. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CI, Chen CP, Kuan PL, et al. The causes and outcomes of inadequate implementation of existing guidelines for antiplatelet treatment in patients with acute coronary syndrome: the experience from Taiwan Acute Coronary Syndrome Descriptive Registry (T-ACCORD Registry). Clin Cardiol. 2010;33:E40–E48. doi: 10.1002/clc.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shyu KG, Wu CJ, Mar GY, et al. Clinical characteristics, management and in-hospital outcomes of patients with acute coronary syndrome – observations from the Taiwan ACS full spectrum registry. Acta Cardiol Sin. 2011;27:135–144. [Google Scholar]
- 11.Li YH, Chiu YW, Cheng JJ, et al. Changing practice pattern of acute coronary syndromes in Taiwan from 2008 to 2015. Acta Cardiol Sin. 2019;35:1–10. doi: 10.6515/ACS.201901_35(1).20180716B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen KC, Yen DH, Chen CD, et al. In-hospital implementation of evidence-based medications is associated with improved survival in diabetic patients with acute coronary syndrome – data from TSOC ACS-DM registry. Acta Cardiol Sin. 2018;34:211–223. doi: 10.6515/ACS.201805_34(3).20180207B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CH, Fang CC, Tsai LM, et al. Patterns of acute myocardial infarction in Taiwan from 2009 to 2015. Am J Cardiol. 2018;122:1996–2004. doi: 10.1016/j.amjcard.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 14.Chu CY, Lin TH, Lai WT. The management and prognostic factors of acute coronary syndrome: evidence from the Taiwan acute coronary syndrome full spectrum registry. Acta Cardiol Sin. 2017;33:329–338. doi: 10.6515/ACS20161205A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei CC, Shyu KG, Cheng JJ, et al. The relation between the timing of percutaneous coronary intervention and outcomes in patients with acute coronary syndrome with routine invasive strategy – data from Taiwan acute coronary syndrome full spectrum data registry. Acta Cardiol Sin. 2016;32:39–48. doi: 10.6515/ACS20150722A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei CC, Shyu KG, Cheng JJ, et al. Diabetes and adverse cardiovascular outcomes in patients with acute coronary syndrome – data from Taiwan’s acute coronary syndrome full spectrum data registry. Acta Cardiol Sin. 2016;32:31–38. doi: 10.6515/ACS20150322A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou LP, Zhao P, Kao C, et al. Women were noninferior to men in cardiovascular outcomes among patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention from Taiwan acute coronary syndrome full-spectrum registry. Medicine (Baltimore) 2018;97:e12998. doi: 10.1097/MD.0000000000012998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin TH, Lai WT, Kuo CT, et al. Additive effect of in-hospital TIMI bleeding and chronic kidney disease on 1-year cardiovascular events in patients with acute coronary syndrome: data from Taiwan acute coronary syndrome full spectrum registry. Heart Vessels. 2015;30:441–450. doi: 10.1007/s00380-014-0504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua SK, Lo HM, Chiu CZ, et al. Use of CHADS2 and CHA2DS2-VASc scores to predict subsequent myocardial infarction, stroke, and death in patients with acute coronary syndrome: data from Taiwan acute coronary syndrome full spectrum registry. PLoS One. 2014;9:e111167. doi: 10.1371/journal.pone.0111167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang FT, Shyu KG, Wu CJ, et al. Predictors of 1-year outcomes in the Taiwan acute coronary syndrome full spectrum registry. J Formos Med Assoc. 2014;113:794–802. doi: 10.1016/j.jfma.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Lin TH, Hsin HT, Wang CL, et al. Impact of impaired glomerular filtration rate and revascularization strategy on one-year cardiovascular events in acute coronary syndrome: data from Taiwan acute coronary syndrome full spectrum registry. BMC Nephrol. 2014;15:66. doi: 10.1186/1471-2369-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei CC, Lee SH. Predictors of mortality in elderly patients with non-ST elevation acute coronary syndrome – data from Taiwan acute coronary syndrome full spectrum registry. Acta Cardiol Sin. 2017;33:377–383. doi: 10.6515/ACS20170126A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai CC, Chang KC, Liao PC, et al. Effects of door-to-balloon times on outcomes in Taiwanese patients receiving primary percutaneous coronary intervention: a report of Taiwan acute coronary syndrome full spectrum registry. Acta Cardiol Sin. 2015;31:215–225. doi: 10.6515/ACS20140721E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin TH, Lai WT, Hsin HT, et al. Effects of clopidogrel on mortality, cardiovascular and bleeding outcomes in patients with chronic kidney disease - data from Taiwan acute coronary syndrome full spectrum registry. PLoS One. 2013;8:e71917. doi: 10.1371/journal.pone.0071917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chua SK, Lo HM, Chiu CZ, et al. Prognostic impact of renal dysfunction in patients with acute coronary syndrome-role beyond the CHA2 DS2-VASc score: data from Taiwan acute coronary syndrome full spectrum registry. Nephrology (Carlton) 2016;21:583–591. doi: 10.1111/nep.12653. [DOI] [PubMed] [Google Scholar]
- 26.Huang CH, Lin HC, Tsai CD, et al. The interaction effects of meteorological factors and air pollution on the development of acute coronary syndrome. Sci Rep. 2017;7:44004. doi: 10.1038/srep44004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai CC, Lin TH, Yip HK, et al. One-year cardiovascular outcomes of drug-eluting stent versus bare-metal stent implanted in diabetic patients with acute coronary syndrome. J Chin Med Assoc. 2016;79:239–247. doi: 10.1016/j.jcma.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Li YH, Chiu YW, Cheng JJ, et al. Duration of clopidogrel-based dual antiplatelet therapy and clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention - a real-world observation in Taiwan from 2012 to 2015. Circ J. 2019;83:1317–1323. doi: 10.1253/circj.CJ-18-1283. [DOI] [PubMed] [Google Scholar]
- 29.Chen CW, Hsieh YC, Hsieh MH, et al. Predictive power of in-hospital and long-term mortality of the GRACE, TIMI, revised CADILLAC and PAMI score in NSTEMI patients with diabetes – data from TSOC ACS-DM registry. Acta Cardiol Sin. 2020;36:595–602. doi: 10.6515/ACS.202011_36(6).20200326A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang CA, Hsieh YC, Huang CY, et al. Comparison between ticagrelor versus clopidogrel in long term outcomes of Taiwanese diabetic subjects with acute coronary syndrome undergoing successful revascularization: from TSOC ACS-DM registry. Medicine (Baltimore) 2020;99:e19969. doi: 10.1097/MD.0000000000019969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jong CB, Chen KY, Hsieh MY, et al. Metformin was associated with lower all-cause mortality in type 2 diabetes with acute coronary syndrome: a nationwide registry with propensity score-matched analysis. Int J Cardiol. 2019;291:152–157. doi: 10.1016/j.ijcard.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Shyu KG. Improvement of outcomes in acute coronary syndrome (ACS) by getting with the guidelines: from Taiwan ACS-full spectrum registry to Taiwan ACS-DM registry. J Taiwan Cardiovasc Interv. 2019;8:8–15. [Google Scholar]
- 33.Peterson ED, Roe MT, Chen AY, et al. The NCDR ACTION registry-GWTG: transforming contemporary acute myocardial infarction clinical care. Heart. 2010;96:1798–1802. doi: 10.1136/hrt.2010.200261. [DOI] [PubMed] [Google Scholar]
- 34.Wallentin L, Gale CP, Maggioni A, et al. EuroHeart: European unified registries on heart care evaluation and randomized trials. Eur Heart J. 2019;40:2745–2749. doi: 10.1093/eurheartj/ehz599. [DOI] [PubMed] [Google Scholar]
- 35.Lee KH, Jeong MH, Ahn YK, et al. Gender differences of success rate of percutaneous coronary intervention and short term cardiac events in Korea acute myocardial infarction registry. Int J Cardiol. 2008;130:227–234. doi: 10.1016/j.ijcard.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 36.Honda S, Nishihira K, Kojima S, et al. Rationale, design, and baseline characteristics of the prospective Japan acute myocardial infarction registry (JAMIR). Cardiovasc Drugs Ther. 2019;33:97–103. doi: 10.1007/s10557-018-6839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 38.Goto S, Huang CH, Park SJ, et al. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J. 2015;79:2452–2460. doi: 10.1253/circj.CJ-15-0112. [DOI] [PubMed] [Google Scholar]
- 39.Park DW, Kwon O, Jang JS, et al. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. 2019;140:1865–1877. doi: 10.1161/CIRCULATIONAHA.119.041766. [DOI] [PubMed] [Google Scholar]
- 40.Kuo FY, Lee CH, Lan WR, et al. Effect of CYP2C19 status on platelet reactivity in Taiwanese acute coronary syndrome patients switching to prasugrel from clopidogrel: switch study. J Formos Med Assoc. 2022;121:1786–1797. doi: 10.1016/j.jfma.2022.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Chen IC, Lee CH, Fang CC, et al. Efficacy and safety of ticagrelor versus clopidogrel in acute coronary syndrome in Taiwan: a multicenter retrospective pilot study. J Chin Med Assoc. 2016;79:521–530. doi: 10.1016/j.jcma.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Lee CH, Cheng CL, Kao Yang YH, et al. Cardiovascular and bleeding risks in acute myocardial infarction newly treated with ticagrelor vs. clopidogrel in Taiwan. Circ J. 2018;82:747–756. doi: 10.1253/circj.CJ-17-0632. [DOI] [PubMed] [Google Scholar]
- 43.Yin WH, Lu TH, Chen KC, et al. The temporal trends of incidence, treatment, and in-hospital mortality of acute myocardial infarction over 15 years in a Taiwanese population. Int J Cardiol. 2016;209:103–113. doi: 10.1016/j.ijcard.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Liao HH, Wang PC, Yeh EH, et al. Impact of disease-specific care certification on clinical outcome and healthcare performance of myocardial infarction in Taiwan. J Chin Med Assoc. 2020;83:156–163. doi: 10.1097/JCMA.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 45.Lee PT, Chao TH, Huang YL, et al. Analysis of the clinical characteristics, management, and causes of death in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention from 2005 to 2014. Int Heart J. 2016;57:541–546. doi: 10.1536/ihj.15-454. [DOI] [PubMed] [Google Scholar]
- 46.Killip T, 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 47.Batra G, Lindbäck J, Becker RC, et al. Biomarker-based prediction of recurrent ischemic events in patients with acute coronary syndromes. J Am Coll Cardiol. 2022;80:1735–1747. doi: 10.1016/j.jacc.2022.08.767. [DOI] [PubMed] [Google Scholar]


