Abstract
Background
The Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) is a scoring system that is easy to use in outpatient clinics or at the bedside, and was developed to predict the survival of heart failure patients after hospitalization.
Objectives
This study aims to evaluate the relationship between the MAGGIC score and cardiorenal syndrome (CRS) in patients with acute decompensated heart failure with reduced ejection fraction (HFrEF).
Methods
This retrospective, single-center study, included 706 patients with New York Heart Association II-IV who were hospitalized and discharged for acute decompensated heart failure between 2016 and 2021. CRS type 1 was defined as acute worsening of cardiac function leading to renal dysfunction. Patients were divided into two groups: those with CRS and those without. The MAGGIC score of all patients was determined. The primary outcome was the occurrence of CRS.
Results
CRS developed in 132 patients. The MAGGIC score was higher in CRS (+) patients compared to CRS (-) patients (30.70 ± 8.09 vs. 23.96 ± 5.59, p < 0.001). After a multivariable analysis, MAGGIC score [odds ratio (OR): 3.92, p < 0.001], sodium (OR: 0.92, p = 0.003), N terminal pro B type natriuretic peptide (OR: 1.78, p = 0.009), hs troponin (OR: 1.28, p = 0.044), MRA (OR: 0.61, p = 0.019) and furosemide dose (OR: 1.03, p = 0.001) were found to be independent predictors of CRS development. The MAGGIC score was associated with CRS development (area under curve = 0.778).
Conclusions
The MAGGIC score may be associated with CRS in HFrEF patients.
Keywords: Cardiorenal syndrome, Heart failure, MAGGIC risk score
Abbreviations
ACE-I, Angiotensin-converting enzyme inhibitor
AKI, Acute kidney injury
ARB, Angiotensin-receptor blockers
BMI, Body mass index
CI, Confidence interval
CRP, C-reactive protein
CRS, Cardiorenal syndrome
DM, Diabetes mellitus
EF, Ejection fraction
HF, Heart failure
HFrEF, Heart failure with reduced ejection fraction
hs, High sensitive
HT, Hypertension
LVEF, Left ventricular ejection fraction
MAGGIC, Meta-Analysis Global Group in Chronic Heart Failure
MRA, Mineralocorticoid receptor antagonist
NT pro-BNP, N-terminal pro-brain natural peptide
NYHA, New York Heart Association
OR, Odds ratio
PASP, Pulmonary artery systolic pressure
SBP, Systolic blood pressure
SD, Standard deviation
INTRODUCTION
Heart failure (HF) is one of the leading health problems worldwide due to its increasing frequency and prevalence. Patients with HF often present with other organ dysfunction, and primary disease in either the heart or kidney often causes dysfunction in other organs.1 Cardiorenal syndrome (CRS) type 1 is defined as the progression of renal dysfunction secondary to acute decompensated heart failure.2 CRS is seen in one third of patients with acute decompensated heart failure and is associated with a poor prognosis.3,4
Predictive scoring models such as the Seattle Heart Failure Model, the Heart Failure Survival Score, and the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) score are widely used to predict mortality in HF patients and determine the best approach.5 The MAGGIC score is the most commonly used of these scoring systems, and it has been shown to be able to predict all-cause death in patients with both preserved and reduced left ventricular ejection fraction.6 As the development of CRS in patients with heart failure is associated with a poor prognosis, it is important to detect patients with the potential to develop CRS at an early stage.7 To the best of our knowledge, there are no studies on the predictive ability of the MAGGIC scoring system for the development of CRS in HF patients. Therefore, the aim of this study was to evaluate the relationship between the MAGGIC score and CRS in patients with acute decompensated heart failure with reduced ejection fraction (HFrEF).
METHODS
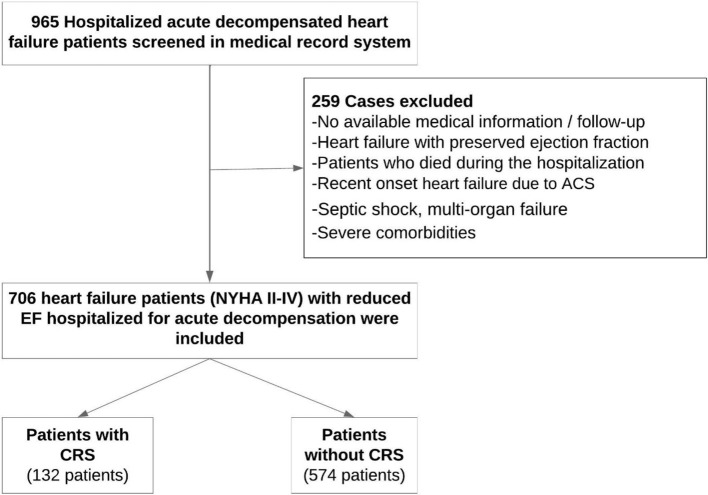
This retrospective, single-center study included 706 patients with New York Heart Association (NYHA) II-IV who were hospitalized and discharged for acute decompensated heart failure between 2016 and 2021. We enrolled patients with reduced left ventricular ejection fraction [left ventricular ejection fraction (LVEF) < 40%]. Patients with the following characteristic were excluded: < 18 years of age, presence of active malignant diseases, septic shock, multi-organ failure, severe comorbidities with the potential to impact the prognosis, pregnancy, implanted ventricular-assist devices, and recent onset heart failure due to acute coronary syndrome. We also excluded patients who died during the hospitalization due to worsening HF and those with missing data making calculation of the MAGGIC score impossible. The patients were divided into two groups: those with CRS and those without (Figure 1). The study was approved by the local Ethical Committee.
Figure 1.
Flowchart of the study. ACS, acute coronary syndrome; CRS, cardiorenal syndrome; EF, ejection fraction; NYHA, New York Heart Association.
Clinical variables, laboratory test panels, electrocardiography recordings, and echocardiography reports of the patients were obtained from medical records filed during hospitalization. Data regarding demographics (age, sex, and ethnicity), medical history (ischemic heart disease, diabetes mellitus, hypertension and atrial fibrillation), current medical treatment, and NYHA functional class were collected from patient files. The dose of furosemide was taken as the average of the intravenous dose administered for the first three days during hospitalization. The eGFR level of all patients was calculated according to the Cockcroft-Gault formula. Treatment of patients with acute decompensated heart failure was arranged according to current guidelines. MAGGIC risk scores of all participating patients were calculated according to baseline data from the initial hospitalization for acute decompensated heart failure.
Transthoracic echocardiography examinations were performed using a Philips Epiq 7 echocardiography device X5-1 transthoracic probe (Philips Epiq7; Philips Healthcare, Inc., Andover, MA, USA). The standard evaluation included M-mode, 2-dimensional, and Doppler studies according to the recommendations of the American Society of Echocardiography.8 LVEF was calculated using Simpson’s method by manually drawing the endocardial boundaries from apical four-chamber views from the diastole and end systole images on all sections from the apex to the basal.9
Definitions
HFrEF was defined as heart failure symptoms and signs along with a LVEF of less than 40%.10 The NYHA functional classification of heart failure was based on the symptoms of the patients and the amount of exertion they could manage without provocation of those symptoms. CRS is divided into 5 subgroups:11 1) acute CRS (Type 1): an acute worsening of cardiac function leading to renal dysfunction; 2) chronic CRS (Type 2): chronic abnormalities in cardiac function leading to renal dysfunction; 3) acute reno-cardiac syndrome (Type 3): acute worsening of renal function causing cardiac dysfunction; 4) chronic reno-cardiac syndrome (Type 4): chronic abnormalities in renal function leading to cardiac disease; 5) secondary CRS (Type 5): systemic conditions causing simultaneous dysfunction of the heart and kidneys. Only patients with CRS type 1 were included in this study. Acute kidney injury (AKI) was defined according to the KDIGO criteria as an increase in serum creatinine of 0.3 mg/dL within 48 h or an increase in serum creatinine by ≥ 50% within seven days.12 Based on whether or not AKI occurred during hospitalization, the patients were divided into CRS-1 and no-CRS-1 groups.
The MAGGIC score (www.heartfailurerisk.org, accessed on 11 January 2021) was calculated according to the final model by Pocock et al. and included the following 13 independent predictors of mortality: age, sex, EF, NYHA class, body mass index, serum creatinine, systolic blood pressure, time since HF diagnosis, diabetes, current smoking, chronic obstructive pulmonary disease, and current therapy with beta-blockers, ACE inhibitors and angiotensin receptor blockers.6
The primary outcome of this study was the occurrence of CRS. The MAGGIC score of all patients was determined, and during follow-up, the patients who developed CRS were identified.
Statistical analysis
All statistical tests were conducted using the Statistical Package for the Social Sciences 25.0 for Windows (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to analyze normality of the data. Continuous data are expressed as mean ± standard deviation (SD), and categorical data are expressed as percentages. A chi-square test was used to assess differences in categorical variables between groups. The Student’s t-test or Mann-Whitney U test was used to compare unpaired samples as needed. Univariate and multivariate logistic regression analyses were used to identify independent variables associated with CRS. After performing univariate analysis, statistically significant variables were selected into the multivariate logistic regression analysis with the stepwise method. The results of univariate and multivariate regression analyses were presented as odds ratio (OR) with 95% confidence interval (CI). Receiver operating characteristic (ROC) curves were obtained, and the optimal values with the greatest total sensitivity and specificity in the prediction of CRS were determined. The MAGGIC score, high sensitive (hs) troponin, N-terminal pro-brain natural peptide (NT pro-BNP) and furosemide dose parameters in the ROC curve analysis were included in the binary logistic regression analysis. A combined model, which was created with the obtained probability value and CRS development predictors (MAGGIC score, hs troponin, and furosemide dose), was analyzed using ROC curves. Significance was assumed at a 2-sided p < 0.05.
RESULTS
The clinical and demographic characteristics of the 706 patients with HFrEF included in the study are shown in Table 1. CRS developed in 132 patients (CRS+). Thirty-six of the 132 (26.5%) patients in the CRS (+) group were female, while 193 of 574 (33.6%) patients in the CRS (-) group were female, and there was no statistically significant difference (p = 0.11). Systolic blood pressure was lower in the CRS (+) group than in the CRS (-) group (p = 0.001). BMI (p = 0.01) and NYHA class (p < 0.001) were higher in the CRS (+) group than in the CRS (-) group. There were no significant differences between the groups in terms of the frequency of chronic diseases such as hypertension (HT), atrial fibrillation, and ischemic heart disease. However, diabetes mellitus (DM) was higher in the CRS (+) group than in the CRS (-) group (p = 0.008). When the groups were compared in terms of laboratory tests, the CRS (+) patients had lower serum hemoglobin, sodium and albumin levels, and higher creatinine and C-reactive protein (CRP) levels compared to the CRS (-) patients. There were no significant differences in serum potassium and glucose levels between the groups. There was no significant difference in serum NT pro-BNP level between the groups (p = 0.056). Hs troponin levels were higher in the patients who developed CRS than in those who did not (p < 0.001). While EF (p < 0.001) was lower in the CRS (+) patients compared to the CRS (-) patients, left atrial diameter (p = 0.01) and PASP (p = 0.001) were higher. The use of mineralocorticoid receptor antagonists (MRAs) (p = 0.001), dopamine (p < 0.001), and furosemide (p < 0.001) was higher in the CRS (+) patients compared to the CRS (-) patients. ACEi/ARB (p < 0.001) and statin (p < 0.001) use was lower in the CRS (+) patients compared to the CRS (-) patients. There were no significant differences between the groups in the use of beta-blockers, digoxin, and oral anticoagulants.
Table 1. Baseline characteristics and outcomes of the CRS (+) and CRS (-) patients in study group.
| Parameters | CRS (+) (N = 132) | CRS (-) (N = 574) | p value |
| Age (years) | 64.4 ± 11.1 | 63.9 ± 11.7 | 0.630 |
| Female, n (%) | 35 (26.5) | 193 (33.6) | 0.113 |
| Systolic blood pressure (mmHg) | 116.1 ± 22.2 | 123.2 ± 22.5 | 0.001 |
| BMI (kg/m2) | 29.2 ± 6.9 | 27.9 ± 4.9 | 0.010 |
| NYHA class | 3.2 ± 0.6 | 2.8 ± 0.6 | < 0.001 |
| Medical history, n (%) | |||
| Hypertension | 68 (51.5) | 342 (59.6) | 0.090 |
| Diabetes mellitus | 69 (52.5) | 227 (39.5) | 0.008 |
| Ischaemic heart disease | 83 (62.9) | 359 (62.5) | 0.940 |
| Atrial fibrillation | 30 (22.7) | 113 (19.7) | 0.431 |
| Laboratory findings | |||
| Creatinine (mg/dl) | 1.8 ± 1.3 | 1.2 ± 0.5 | < 0.001 |
| Hemoglobin (g/dl) | 11.4 ± 1.6 | 12.4 ± 2.3 | < 0.001 |
| Sodium (mmol/l) | 133.4 ± 5.6 | 137.1 ± 4.7 | < 0.001 |
| Potassium (mmol/l) | 4.4 ± 0.7 | 4.3 ± 0.6 | 0.100 |
| Glucose (mg/dl) | 129.8 ± 54.9 | 138.3 ± 67.4 | 0.170 |
| Albumin (g/dl) | 3.4 ± 0.5 | 3.5 ± 0.6 | 0.007 |
| CRP (mg/l) | 36.8 ± 51.7 | 26.8 ± 40.6 | 0.010 |
| NT pro-BNP (pg/ml) | 864 (244-1765) | 704 (183-1734) | 0.056 |
| hs troponin (ng/l) | 60 (12-200) | 12 (6-31) | < 0.001 |
| eGFR (ml/min) | 45.1 ± 25.9 | 65.8 ± 42.4 | < 0.001 |
| Echocardiographic findings | |||
| Ejection fraction (%) | 24.4 ± 6.4 | 27.8 ± 6.0 | < 0.001 |
| Left atrial diameter (mm) | 46.7 ± 7.9 | 44.8 ± 7.7 | 0.010 |
| PASP (mmHg) | 49.3 ± 13.3 | 43.8 ± 13.8 | 0.001 |
| Treatment | |||
| Beta-blocker, n (%) | 100 (75.8) | 450 (78.4) | 0.510 |
| ACE-I/ARB, n (%) | 70 (53) | 441 (76.8) | < 0.001 |
| MRA, n (%) | 58 (43.9) | 164 (28.6) | 0.001 |
| Digoxin, n (%) | 20 (15.2) | 124 (21.6) | 0.090 |
| Statin, n (%) | 52 (39.4) | 296 (51.6) | 0.015 |
| Dopamine, n (%) | 77 (58.3) | 87 (15.2) | < 0.001 |
| Anticoagulant, n (%) | 47 (35.6) | 180 (31.4) | 0.340 |
| Furosemide dose* (mg/day) | 298.9 ± 179.0 | 211.4 ± 126.5 | < 0.001 |
| Outcomes | |||
| Maggic score | 30.7 ± 8.0 | 23.9 ± 5.5 | < 0.001 |
| Length of stay (days) | 22.9 ± 15.8 | 14.9 ± 9.4 | < 0.001 |
* The average of the dose given intravenously for the first 3 days.
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blockers; BMI, body mass index; CRS, cardiorenal syndrome; CRP, C-reactive protein; hs troponin, high sensitive troponin; eGFR, estimated Glomerular filtration rate; MRA, mineralocorticoid receptor antagonist; NT pro-BNP, N-terminal pro-brain natural peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure.
The MAGGIC score was higher in the CRS (+) patients compared to the CRS (-) patients (30.7 ± 8.0 vs. 23.9 ± 5.5, p < 0.001). The length of hospital stay was longer in the CRS (+) patients compared to the CRS (-) patients (22.9 ± 15.8 vs. 14.9 ± 9.4, p < 0.001).
Logistic regression was carried out using univariate and multivariate analyses to predict the occurrence of CRS in the included HFrEF patients. MAGGIC score, hemoglobin, HT, sodium, CRP, albumin, PASPs, furosemide dose, NT pro-BNP, hs troponin, MRA, dopamine and statin use were evaluated in univariate analysis. The multivariate analysis included MAGGIC score, hemoglobin, sodium, CRP, albumin, PASPs, furosemide dose, NT pro-BNP, hs troponin, MRA, dopamine and statin use, which were statistically significant in the univariate analysis. MAGGIC score (OR: 3.92, p < 0.001), sodium (OR: 0.92, p = 0.003), NT pro-BNP (OR: 1.78, p = 0.009), hs troponin (OR: 1.28, p = 0.044), MRA use (OR: 0.61, p = 0.019), and furosemide dose (OR: 1.03, p = 0.001), were determined to be independent predictors of CRS development (Table 2).
Table 2. Univariate and multivariate logistic regression analyzes to identify independent predictors of CRS development in HFrEF patients.
| Variable | Univariate | Multivariate | ||||
| OR | 95% CI | p value | OR | 95% CI | p value | |
| MAGGIC score | 2.23 | 1.68-3.79 | < 0.001 | 3.92 | 1.98-7.84 | < 0.001 |
| Hemoglobin | 0.80 | 0.73-0.88 | < 0.001 | 0.96 | 0.83-1.11 | 0.633 |
| HT | 0.72 | 0.49-1.05 | 0.090 | |||
| Sodium | 0.87 | 0.84-0.90 | < 0.001 | 0.92 | 0.87-0.97 | 0.003 |
| CRP | 1.03 | 1.01-1.05 | 0.010 | 1.00 | 0.99-1.00 | 0.942 |
| Albumin | 0.66 | 0.49-0.89 | 0.008 | 0.55 | 0.34-0.99 | 0.019 |
| PASP (mmHg) | 1.02 | 1.01-1.04 | 0.001 | 1.01 | 0.99-1.04 | 0.107 |
| Furosemide dose | 1.03 | 1.02-1.05 | < 0.001 | 1.03 | 1.01-1.05 | 0.001 |
| Dopamine | 0.87 | 0.660-0.992 | < 0.001 | 0.90 | 0.79-1.87 | 0.088 |
| hs troponin | 1.32 | 1.05-1.78 | 0.032 | 1.28 | 1.04-1.56 | 0.044 |
| NT pro-BNP | 1.89 | 1.23-2.78 | 0.001 | 1.78 | 1.20-2.57 | 0.009 |
| MRA | 0.61 | 0.44-0.75 | 0.001 | 0.76 | 0.62-0.87 | 0.019 |
| Statin | 0.61 | 0.41-0.89 | 0.012 | 1.02 | 0.52-1.99 | 0.940 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blockers; CI, confidence interval; CRP, C-reactive protein; CRS, cardiorenal syndrome; DM, diabetes mellitus; HT, hypertension; hs troponin, high sensitive troponin; LVEF, left ventricular ejection fraction; MAGGIC, Meta-Analysis Global Group in Chronic Heart Failure; MRA, mineralocorticoid receptor antagonis; NT pro-BNP, N-terminal pro-brain natural peptide; NYHA, New York Heart Association; OR, odds ratio; PASP, pulmonary artery systolic pressure; SBP, systolic blood pressure.
The specificity and sensitivity of the MAGGIC score, hs troponin, NT pro-BNP and furosemide dose to predict the development of CRS were evaluated by ROC analysis. The area under the curve (AUC) for MAGGIC score was 0.778 (0.736-0.821, p < 0.001). The MAGGIC score was determined to have a cutoff value of 27.5 with 70% sensitivity and 73% specificity. In addition, the AUCs for hs troponin (cutoff 21.8 ng/ml, sensitivity 70%, specificity 70%), NT pro-BNP (cutoff 811 pg/ml, sensitivity 53%, specificity 53%), and furosemide dose (cutoff 230 mg, sensitivity 66%, specificity 60%) were 0.712 (0.661-0.763, p < 0.001), 0.550 (0.495-0.605, p = 0.076), and 0.648 (0.591-0.704, p < 0.001), respectively (Figure 2). ROC analysis was then performed to assess all the combined predictors (MAGGIC score, hstroponin, and furosemide dose) in a single model (Figure 3). The AUC of this model was 0.812 (0.760-844), with 73% sensitivity and 72% specificity.
Figure 2.

The ROC curves for Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) score, high sensitive (hs) troponin, N-terminal pro-brain natural peptide (NT pro-BNP) and furosemide dose.
Figure 3.

ROC analysis with a single model created with all of the combine data [Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) score, high sensitive (hs) troponin and furosemide dose].
DISCUSSION
The aim of this study was to evaluate the relationship between the MAGGIC score and CRS in patients with acute decompensated heart failure with reduced ejection fraction. The main results were as follows: i) the MAGGIC score was higher in the CRS (+) group compared to the CRS (-) group (30.70 ± 8.09 vs. 23.96 ± 5.59, p < 0.001); ii) the MAGGIC score (OR: 3.92, p < 0.001), sodium (OR: 0.92, p = 0.003), NT pro-BNP (OR: 1.78, p = 0.009), hs troponin (OR: 1.28, p = 0.044), MRA use (OR: 0.61, p = 0.019) and furosemide dose (OR: 1.03, p = 0.001) were independent predictors of CRS development; and iii) in ROC analysis, the MAGGIC score was determined to have a cutoff value of 27.5 with 70% sensitivity and 73% specificity.
CRS is a common clinical condition in patients with heart failure and it is associated with a poor prognosis. Many studies have revealed the negative impact of kidney disease on mortality in patients with heart failure. Kidney function measurements are a component of many heart failure risk classification scores.5-7 Several risk models have been developed, although only some are currently used in clinical practice. In a recent study, the MAGGIC risk score was found to have the highest accuracy in predicting mortality in general HF patients when compared to other prognostic risk scores.13 Although the MAGGIC score is mainly used as a prognostic predictor in patients with heart failure, it has been used for different purposes in many studies to evaluate prognosis after valve surgery, risk of death in patients with ICD, and hospitalization before ablation.14-17
In the past decade, several clinical risk scores for predicting the occurrence of AKI in patients with AHF or AMI, as well as in patients after cardiac surgery or coronary angiography, have been published.18 However, there is currently no standardized risk score.
In a study by Forman et al., 27% of 1004 hospitalized HF patients developed AKI. DM, creatinine ≥ 1.5 mg/dl and systolic BP > 160 mmHg were found to be independent predictors of AKI.19 Wang et al. developed a clinical risk score to predict the development of AKI in 1709 Chinese patients hospitalized for acute HF. This scoring system included parameters such as age (≥ 70 years), SBP < 90 mmHg, serum sodium < 130 mmol/L, NYHA class 4, ≥ 3 admissions for AHF, proteinuria, serum creatinine > 104 μmol/L, and IV furosemide dose > 80 mg/day.20 In 2015, Cheng et al. performed an external validation of Forman’s risk score using the same 1709 patients, and the results were similar.17 In 2011, in a study by Breidthardt et al. involving 657 patients admitted to the emergency department with acute HF, the Forman risk score was confirmed, and AKI developed in 136 (21%) patients. In their study, the presence of chronic kidney disease was found to be an independent predictor of AKI.7 Palazzuoli et al. examined the role of biomarkers in the development of CRS in 2014, and the prevalence of CRS was found to be high in patients with high natriuretic peptide and troponin levels.21 In a prospective study conducted in China by Zhou et al. in 2016, the data of 507 patients were examined and AKI occurred in 33% of them. The authors developed a clinical scoring system to identify patients at high risk of developing AKI. This risk model included five clinical factors (age, gender, CKD history, serum albumin level, and NT-proBNP) and two urinary biomarkers, urinary angiotensinogen and urinary NGAL, both of which were found to be predictors of AKI. The risk model obtained in the study showed high discriminatory power (optimism-corrected c statistic of 0.859).22 Their study was the first clinical scoring system to be derived and validated for the early prediction of AKI in patients with acute decompensated HF, including clinical risk factors and AKI biomarkers. In our study, the MAGGIC score was found to be an independent predictor of CRS development in HF patients (OR: 2.75, p < 0.001), and the MAGGIC score could predict the development of CRS with a cutoff value of 27.5 with 79% sensitivity and 70% specificity.
Many mechanisms have been proposed for the development of CRS in HF patients. Renal hypoperfusion develops due to the decrease in arterial filling pressure. High intra-abdominal pressures in patients with acute HF may contribute to renal dysfunction by causing renal compression and decreased perfusion. In addition, the renin-angiotensin aldosterone system is activated due to central nervous system stimulation in these patients. As a result of all these pathways, renal vasoconstriction and hypoperfusion occur. Depending on the decrease in renal blood flow, a decrease in glomerular filtration rate is observed. As a result, necrosis and apoptosis develop in renal cells. In addition, due to oxidative stress and inflammation occurring in HF patients, cytokine release, mitochondrial dysfunction, and endothelial dysfunction may occur, and AKI may develop directly.23-25 Likewise, studies have shown that the parameters that make up the MAGGIC score increase the risk of developing CRS.26-29 In the present study, we showed that the MAGGIC scoring system, whose prognostic importance is known in HF patients, could predict the development of CRS in this patient group.
Our study has some limitations. First, the study was retrospective and conducted at a single center. Due to the retrospective design, casual relationships are unknown. Second, biomarkers with proven prognostic importance such as troponin and BNP were not measured. Third, we lacked follow-up data such as post-discharge death and rehospitalization. Fourth, patients with HFrEF were included in the study but those with HFpEF were excluded.
CONCLUSIONS
In conclusion, the MAGGIC score may be associated with the development of CRS in HFrEF patients. Advanced HF complicated by CRS is difficult to manage, and irreversible renal failure limits the patient’s candidacy for advanced HF treatments such as transplant or left ventricular assist device therapy. Therefore, it is important to detect the development of CRS early in HF patients. Studies in which the MAGGIC score is combined with biomarkers proven to be associated with CRS are needed.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
Acknowledgments
None.
AUTHOR CONTRIBUTION
All authors contributed substantially to study conception and design, or acquisition of data, or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content and final approval of the version to be published.
FUNDING
None.
REFERENCES
- 1.Tomasoni D, Adamo M, Lombardi CM, Metra M. Highlights in heart failure. ESC Heart Fail. 2019;6:1105–1127. doi: 10.1002/ehf2.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronco C, McCullough P, Anker SD, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 4.Bader FM, Attallah N. Insights into cardiorenal interactions in acute decompensated heart failure. Curr Opin Cardiol. 2017;32:203–208. doi: 10.1097/HCO.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 5.Codina P, Lupón J, Borrellas A, et al. Head-to-head comparison of contemporary heart failure risk scores. Eur J Heart Fail. 2021;23:2035–2044. doi: 10.1002/ejhf.2352. [DOI] [PubMed] [Google Scholar]
- 6.Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: a risk score based on 39,372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 7.Breidthardt T, Socrates T, Noveanu M, et al. Effect and clinical prediction of worsening renal function in acute decompensated heart failure. Am J Cardiol. 2011;107:730–735. doi: 10.1016/j.amjcard.2010.10.056. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 9.Schiller NB, Acquatella H, Ports TA, et al. Left ventricular volume from paired biplane two-dimensional echocardiography. Circulation. 1979;60:547–555. doi: 10.1161/01.cir.60.3.547. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 11.House AA, Anand I, Bellomo R, et al. Definition and classification of Cardio-Renal Syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1416–1420. doi: 10.1093/ndt/gfq136. [DOI] [PubMed] [Google Scholar]
- 12.Ostermann M, Bellomo R, Burdmann EA, et al. Controversies in acute kidney injury: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020;98:294–309. doi: 10.1016/j.kint.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canepa M, Fonseca C, Chioncel O, et al. Performance of prognostic risk scores in chronic heart failure patients enrolled in the European Society of Cardiology Heart Failure Long-Term Registry. JACC: Heart Fail. 2018;6:452–462. doi: 10.1016/j.jchf.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Cirasa A, La Greca C, Pecora D, et al. Catheter ablation of atrial fibrillation in heart failure: clinical, prognostic, and echocardiographic outcome. J Interv Card Electrophysiol. 2021;60:221–229. doi: 10.1007/s10840-020-00727-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhuo DX, Bilchick KC, Shah KP, et al. MAGGIC, STS, and EuroSCORE II risk score comparison after aortic and mitral valve surgery. J Cardiothorac Vasc Anesth. 2021;35:1806–1812. doi: 10.1053/j.jvca.2020.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hioki H, Watanabe Y, Kozuma K, et al. The MAGGIC risk score predicts mortality in patients undergoing transcatheter aortic valve replacement: sub-analysis of the OCEAN-TAVI registry. Heart Vessels. 2019;34:1976–1983. doi: 10.1007/s00380-019-01443-9. [DOI] [PubMed] [Google Scholar]
- 17.Canepa M, Palmisano P, Dell’Era G, et al. Usefulness of the MAGGIC score in predicting the competing risk of non-sudden death in heart failure patients receiving an implantable cardioverter-defibrillator: a sub-analysis of the OBSERVO-ICD registry. J Clin Med. 2021;11:121. doi: 10.3390/jcm11010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng H, Chen YP. Clinical prediction scores for type 1 cardiorenal syndrome derived and validated in chinese cohorts. Cardiorenal Med. 2015;5:12–19. doi: 10.1159/000369479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Wang YN, Cheng H, Yue T, Chen YP. Derivation and validation of a prediction score for acute kidney injury in patients hospitalized with acute heart failure in a Chinese cohort. Nephrology. 2013;18:489–496. doi: 10.1111/nep.12092. [DOI] [PubMed] [Google Scholar]
- 21.Palazzuoli A, Masson S, Ronco C, Maisel A. Clinical relevance of biomarkers in heart failure and cardiorenal syndrome: the role of natriuretic peptides and troponin. Heart Fail Rev. 2014;19:267–284. doi: 10.1007/s10741-013-9391-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhou LZ, Yang XB, Guan Y, et al. Development and validation of a risk score for prediction of acute kidney injury in patients with acute decompensated heart failure: a prospective cohort study in China. J Am Heart Assoc. 2016;5:e004035. doi: 10.1161/JAHA.116.004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangaswami J, Bhalla V, Blair JE, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139:e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 24.Pliquett RU. Cardiorenal syndrome: an updated classification based on clinical hallmarks. J Clin Med. 2022;11:2896. doi: 10.3390/jcm11102896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437–1444. doi: 10.1093/eurheartj/ehv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costanzo MR. The cardiorenal syndrome in heart failure. Heart Fail Clin. 2020;16:81–97. doi: 10.1016/j.hfc.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Ananthram MG, Gottlieb SS. Renal dysfunction and heart failure with preserved ejection fraction. Heart Fail Clin. 2021;17:357–367. doi: 10.1016/j.hfc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5:543–551. doi: 10.1016/j.jchf.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Pérez JP, Bou XC. Cardiorenal syndrome. Nefrologia. 2008;28:29–32. [PubMed] [Google Scholar]