Abstract
OBJECTIVE:
Coronavirus disease 2019 is expected to have a widespread and significant impact on pulmonary tuberculosis services. This study aims to investigate the effect of comprehensive short-term, outpatient pulmonary rehabilitation program among treated pulmonary tuberculosis patients during the coronavirus disease 2019 pandemic.
MATERIAL AND METHODS:
Forty-five pulmonary tuberculosis patients who completed their tuberculosis treatment were randomly allocated to the outpatient pulmonary rehabilitation group and the other 45 were allocated to the control group for 4 weeks. The pulmonary rehabilitation program comprised supervised endurance and resistance training, breathing techniques, self-management strategies, and patient education. The outcome measures evaluated in both groups were functional capacity assessed by a 6-minute walk test, health-related quality of life (short-form 36 questionnaire), pulmonary function (forced expiratory volume in 1 second/forced vital capacity, forced vital capacity, and forced expiratory volume in 1 second), and dyspnea by modified Medical Research Council dyspnea scale. All measurements were performed at enrolment and after completion of 4 weeks protocol in both the groups.
RESULTS:
There was a significant improvement in 6-minute walk distance in meters (P = .001) and percentage predicted (P = .014) in the pulmonary rehabilitation group compared to the control group. All domains of the short-form 36 questionnaire showed significant improvement post-pulmonary rehabilitation (P < 0.05), and modified Medical Research Council dyspnea scale also significantly improved in the pulmonary rehabilitation group (P < .001). However, no significant differences were observed in any of the pulmonary function measures (P > .05) between the groups after 4 weeks.
CONCLUSION:
Short-term pulmonary rehabilitation program in pulmonary tuberculosis demonstrated improvement in functional capacity, quality of life, and dyspnea. However, it failed to register changes in pulmonary function. The study results provide motivation to consider the implementation of a short-term pulmonary rehabilitation program after pulmonary tuberculosis treatment to reduce the impairment the patient may suffer even after microbiological cure.
Keywords: COVID-19 pandemic, exercise tolerance, lung function impairment, pulmonary rehabilitation, pulmonary tuberculosis, quality of life
MAIN POINTS
The burden of pulmonary tuberculosis (PTB) during coronavirus disease 2019 (COVID-19) has negatively influenced PTB management strategies.
The long-term sequelae of PTB patients results in deteriorated functional capacity, pulmonary measures, and excessive breathlessness, ultimately affecting the overall health-related quality of life (HRQoL). This results in enhanced symptomatic burden that cannot be recovered solely by pharmacological management.
Pulmonary rehabilitation (PR) strategies have the potential to break the vicious cycle. It has proven beneficial impact on various chronic respiratory conditions. However, the efficacy of short-term PR program on patients with PTB still remains inconclusive.
The findings of the present study can wave paths for clinicians to optimally deliver short-term PR in post-PTB patients to maximize gains in functional capacity, HRQoL, and dyspnea. However, the efficacy of PR on pulmonary measures in these patients is still debatable.
INTRODUCTION
By distorting the healthcare system at several levels, the coronavirus disease 2019 (COVID-19) pandemic has impacted pulmonary tuberculosis (PTB) control strategies and the occurrence of catastrophic expenses. The prioritization has also resulted in a drastic shift in healthcare personnel’s role toward COVID-19-related services which has led to a reduction in the number of consultation services and the delivery of PTB-related programs at both hospitals and community levels. Due to the allocation of numerous PTB-related resources to COVID-19 programs, there is also a reduction in the availability of healthcare services for efficient PTB management strategies.1 Between 2020 and 2025, the globe might see an additional 6.3 million cases of PTB, and 1.4 million PTB deaths are suspected.2 Social distancing measures established in several countries to restrict COVID-19 transmission had an immediate and substantial impact on the delivery of pulmonary rehabilitation (PR) programs.3
In most impacted nations, PR programs included a minimum of 6-7 weeks duration (24 supervised sessions) followed by 3 months of maintenance phase.4,5 Pulmonary rehabilitation setups were discontinued to protect vulnerable population from contracting the virus. However, the dire need for PR cannot be neglected; therefore, formal discussions were conducted among healthcare professionals on how to continue delivering PR services in the midst of the crisis.6
Short-term PR programs can be considered as an efficient and effective treatment strategy at the time of the crisis where delivering long-term PR can be challenging. Short-term PR programs have shown immediate benefits in patients with various chronic respiratory conditions.7 However, there have been no published evaluations on the efficacy of short-term PR during the COVID-19 pandemic on post-PTB pharmacologically treated patients. Therefore, the aim of the present study is to evaluate the effect of short-term PR on functional capacity, pulmonary function, health-related quality of life (HRQoL), and dyspnea during the COVID-19 pandemic on post-PTB-treated patients.
MATERIAL AND METHODS
The present randomized control trial was conducted after obtaining ethical clearance from the Institutional Ethics Committee of Sharda University, Greater Noida, India, with ethical approval number SU/SMS&R/76-A/2022/01. The study was designed according to the Consolidated Standard of Reporting Trials (CONSORT) guidelines designed for reporting Randomized control trials. Potential participants were educated about the protocol based on eligibility criteria, signed informed consent was acquired from all participants, and research methods were followed based on the Declaration of Helsinki, 1964.
Inclusion criteria for both groups were patients with PTB 18 years of age and older, who exhibit shortness of breath on exertion, who have limitations in the activities of daily living, and who have a documented history of smear-positive PTB with pharmacological treatment completed more than 6 months prior to the study enrolment.8 Subjects who have not participated in any physical activity or rehabilitation program during the previous 6 months were included. Subjects with a previous history of other respiratory diseases such as chronic obstructive pulmonary disease, asthma, and interstitial lung disease as well as cardiovascular diseases such as myocardial infarction, angina, and congestive heart failure were excluded. Patients over the age of 80 and those with orthopedic or cognitive impairments who would be unable to participate in the rehabilitation program on a regular basis were also excluded from the present study.
Sample Size Calculation
Software G. Power 3.1.9.2 (Franz F, Universitat Kiel, Kiel, Germany) was used to determine the sample from a previous study conducted by Singh et al8 using data of changes in 6-minute walk distance (6MWD). A total of 45 subjects were considered necessary in each group, and the power (1 – beta) was 0.80 with an effect size of 0.384.
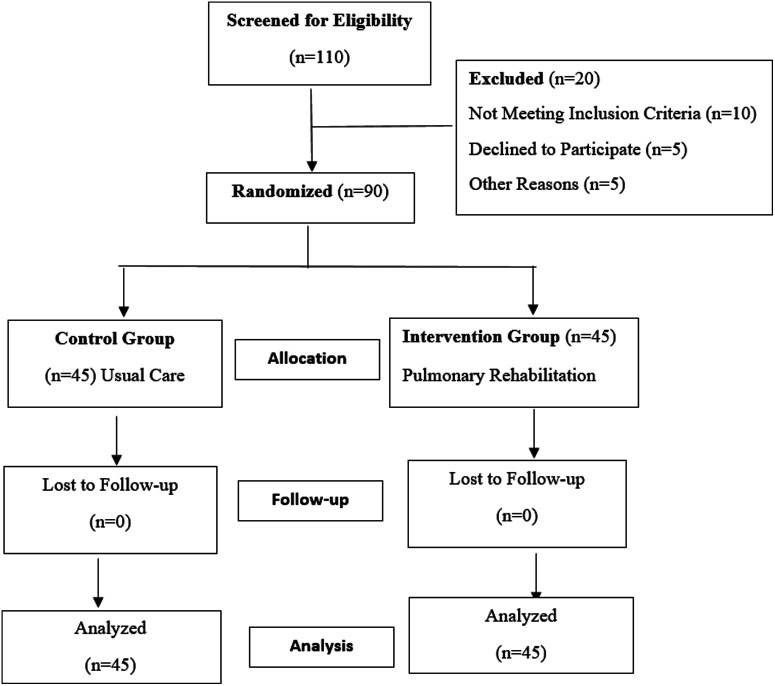
The respiratory outpatient service helped in enrolling 90 stable PTB patients who had completed their pharmacological treatment. Patients who met the inclusion criteria were allocated randomly to either the rehabilitation or control group using sealed envelopes created and jumbled by an impartial individual unaffiliated with the study procedure before trial was initiated. The allocator opened the envelope sequentially, only after the participant’s name was written on it. Patients who were allocated to the rehabilitation group had to complete a minimum of 16 sessions of an outpatient PR program over the course of 4 weeks (4 sessions per week). The complete exercise prescription for this PR program was carried out under the guidance of a licensed physiotherapist at the hospital while adhering to COVID-19-appropriate behavior because it was an institution-based program.
Pulmonary rehabilitation sessions were conducted in the morning hours to allow participants to travel back home in time before the evening rush hour, and the standard operating procedure for data collection was modified to ensure 2-m distancing between the study staff and study participant. Study participants underwent COVID-19 testing before starting PR and as needed during the hospital-based sessions. Before accessing the PR room, all participants and staff were required to undergo temperature measurement using a hand-held non-contact thermometer and wash their hands with soap or alcohol-based hand sanitizer. The maximum number of participants participating in the PR session was reduced from 12 to 8 to ensure social distancing between participants. All study staff were required to wear N95 masks at all times and underwent COVID-19 training with an emphasis on infection prevention and control, and the study participants were screened for signs and symptoms of the disease.
Assessment
A week before baseline testing, all patients were made aware of the research procedures and potential hazards. Each patient got a primary health examination prior to initial testing. The baseline testing was conducted over 2 days. On day 1, participants were requested to complete the short form-36 questionnaire (SF-36) which is generic HRQoL questionnaire, following baseline dyspnea assessment which was conducted through modified Medical Research Council (mMRC) dyspnea scale. On day 2, the participants underwent a pulmonary function test, as recommended by the American Thoracic Society and the European Respiratory Society (ATS/ERS) guidelines. Measurements were made of the forced vital capacity (FVC), forced expiratory volume (FEV1) in 1 second, and FEV1/FVC ratio. According to ATS/ERS recommendations, blood pressure, heart rate, oxygen saturation (SpO2), and dyspnea were measured before and after the 6-minute walk test (6MWT). Each patient was instructed to walk at their own pace covering the largest distance possible in 6 minutes. The participant’s total distance covered was calculated in meters and percentage. Enright9 equation was used to estimate the 6MWD predicted for both males and females.
Following baseline testing, participants were randomly allocated to the PR or control group. Participant assigned to the control group were asked to continue their normal routine strategies for 4 weeks. After completion of the 4 weeks, outcome measures were reassessed for both the groups.
Pulmonary Rehabilitation Intervention
The outpatient PR sessions were individually tailored to each patient’s performance during the initial assessment which comprised static circuit training to increase peripheral muscle strength and general mobility with breathing exercises, supervised endurance and resistance training, stretching of upper and lower extremity muscles, self-management, and patient education. Breathing exercises are applied in each session and last for 30 minutes.10
The endurance exercise program was applied in the form of ground walking. The walking prescription mostly represents the activities of daily living. It is functional, simple, inexpensive, and readily applicable. The walk speed or intensity of the training was calculated based on the distanced covered during 6MWT, as suggested by Chandrasekaran and Reddy.11
Maximum distance that may be walked in 20-minute walk distance (20MWD) = actual 6MWD × 3.33 m
Initial training distance to be walked in 20 minute for first 1 week = 80/100 × 20MWD m
For the second week, distance to be walked in 20 minutes = 85/100 × 20MWD m
For the third week, distance to be walked in 20 minutes = 90/100 × 20MWD m
For the fourth week, the subjects performed walking at the actual 6MWD covered in the initial testing.
This way the subjects began from 80% of their walking distance and progressively increased to 100% of the walk intensity derived from actual 6MWT conducted over a period of 4 weeks. Walking speed was gradually increased as the patients gained confidence and correlated with an intensity of 11-13 points on the Borg scale for the initial 2 weeks and progressively increased up to 15 on the Borg scale for the final 2 weeks, taking care that SpO2 did not drop below 90%. Walking distance was 20 minutes twice a day for 4 weeks based on the above prescription. The training was done under the supervision of a physical therapist on 4 weekdays. On the weekend, the patients were instructed to perform walking as prescribed during supervised sessions.
Resistance training was performed 3 times per week at 50%-70% of 1 repetition maximum intensity (1-RM).4 After determining the 1-RM for each exercise, the load at 50%-70% of 1-RM was approximated. Quadriceps, hamstrings, hip flexors, abductors, and extensors were all worked out. The deltoid, triceps, and biceps muscles were also exercised by the upper extremity strength training. Three sets of 8-10 repetitions of each exercise were performed with 2-3 minutes of rest interval between the sets. Resistance training intensity was progressively increased: 50% of 1-RM for the first week, 60% of 1-RM for the middle 2 weeks, and 70% of 1-RM for the fourth week.12
Stretching exercises targeting specific muscle groups were recommended to ensure good posture and proper body mechanics and to minimize the incidence of joint and muscle injury resulting in improved respiratory mechanics.13 Five minutes of flexibility training was conducted at the beginning and end of each exercise session. One set of 4-5 stretching exercises was performed for 15-30 seconds (seated single-leg hamstring stretch, standing quadriceps stretch, chest stretch, overhead reach stretch, and wall cat stretch).14
The researcher gave the intervention group a systematic program consisting of self-management of symptoms, involving relaxation techniques to minimize dyspnea, cessation of smoking, and nutritional suggestions based on the nutritionist’s personalized recommendations.
The control group on the other hand continued with their usual activities and, if necessary, medical care as prescribed by a qualified practitioner.
Statistical Analysis
Statistical Package for Social Sciences (SPSS) version 21.0 (IBM Corp.; Armonk, NY, USA) was used for the data analysis. Results were reported in mean and SD for continuous data, whereas discrete (categorical) data were presented as number (n) and percentage (%). The normal distribution of data was tested by the Shapiro–Wilk test, skewness, and histogram. Variables that depicted non-normal distribution were log-transformed.
The outcome variables between groups were compared at baseline and 4 weeks later using an independent t-test. To compute the difference between PR versus control group following 4 weeks, standardized mean difference and 95% CI were estimated. Statistical significance was set at P ≤ .05.
RESULTS
As demonstrated in Table 1, there were no appreciable variations in baseline demographic, clinical, or outcome characteristics between the PR and the control group. The 2 groups were well-matched in terms of age, weight, and height in comparison to the control group. After 4 weeks, the PR group demonstrated a significant improvement in 6MWD in meters (P = .001) and percentage predicted (P = .014). The PR group showed a significant improvement in all SF-36 domains. Modified Medical Research Council (dyspnea scale) scores in the PR group also significantly increased (P = .001). However, as shown in Table 2, there were no differences between the groups after 4 weeks in the pulmonary function measurements: FEV1 (L) (P = .142), FVC (L) (P = .115), and FEV1/FVC (P = .497).
Table 1.
Baseline Clinical and Demographic Features of the Subject (n = 90)
| Variables | PR Group (n = 45), Mean ± SD | Control Group (n = 45), Mean ± SD | P |
|---|---|---|---|
| Age, years | 35.20 ± 11.69 | 35.04 ± 7.74 | .95 |
| 9 × height, cm | 152.25 ± 5.46 | 152.32 ± 5.37 | .94 |
| Weight, kg | 54.93 ± 10.2 | 53.72 ± 11.8 | .54 |
| 6MWT, m | 286.85 ± 44.64 | 352.37 ± 36.17 | .17 |
| 6MWD, % | 64.2 ± 13.6 | 69.4 ± 12.7 | .30 |
| 6MWD, RHR (bpm) | 81.95 ± 9.36 | 76.3 ± 11.4 | .48 |
| 6MWD, HRmax (bpm) | 104.6 ± 16.88 | 105.45 ± 19.91 | .38 |
| PFT | |||
| FEV1, L | 1.29 ± 0.44 | 1.25 ± 0.46 | .61 |
| FVC, L | 1.64 ± 0.47 | 1.57 ± 0.51 | .46 |
| FEV1/FVC | 100.37 ± 16.0 | 101.45 ± 15.91 | .71 |
| mMRC | 3.38 ± 0.49 | 3.60 ± 0.49 | .93 |
| SF-36 | |||
| Physical functioning | 42.4 ± 7.3 | 47.7 ± 8.1 | .42 |
| Role physical | 49.6 ± 13.6 | 51.2 ± 9.5 | .43 |
| Bodily pain | 43.3 ± 7.3 | 42.7 ± 8.3 | .67 |
| General health | 50.2 ± 7.1 | 51.2 ± 6.9 | .42 |
| Vitality | 62.5 ± 8.0 | 61.9 ± 7.1 | .70 |
| Social functioning | 56.5 ± 12.3 | 56.5 ± 12.3 | .98 |
| Role emotional | 76.3 ± 11.4 | 63.3 ± 14.1 | .07 |
| Mental health | 65.1 ± 8.3 | 62.9 ± 10.5 | .20 |
All values expressed in mean ± SD.
6MWT, 6-minute walk test; 6MWD, 6-minute walk distance; RHR, Resting heart rate; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; mMRC, modified Medical Research Council (dyspnea scale); PFT, pulmonary function testing; PR, pulmonary rehabilitation; SF-36, 36-item short-form survey.
Table 2.
Four-Week Outcome Variable Standardized Mean Differences Between Groups
| Outcome Variables |
PR Group (n = 45) | Control Group (n = 45) |
t-Value |
PR Group vs. Control Group Standardized Mean Difference Random (95% CI) |
P
|
||
|---|---|---|---|---|---|---|---|
| Baseline | Four weeks | Baseline | Four weeks | ||||
| 6MWT, m | 286.85 ± 44.64 | 484.15 ± 48.01 | 313.0 ± 48.17 | 321.32 ± 43.46 | 13.03 | 1.44 (0.60-2.29) | .001* |
| 6MWD, % predicted | 64.2 ± 13.6 | 83.2 ± 11.4 | 69.4 ± 12.7 | 71.1 ± 12.6 | 11.3 | 0.98 (0.19-1.77) | .014* |
| 6MWD, RHR (bpm) | 81.95 ± 9.36 | 73.60 ± 9.82 | 76.3 ± 11.4 | 88.9 ± 6.3 | 14.9 | –9.2(69.00-78.20) | <.001 |
| 6MWD, HRmax (bpm) | 104.6 ± 16.88 | 107.6 ± 18.50 | 105.45 ± 19.91 | 108.2 ± 19.30 | 18.7 | –17.36 (98.94-116.3) | .381 |
| FEV1, L | 1.29 ± 0.44 | 1.44 ± 0.64 | 1.25 ± 0.46 | 1.34 ± 0.58 | 5.59 | 0.51(–0.25 to 1.26) | .142 |
| FVC, L | 1.64 ± 0.47 | 1.64 ± 0.47 | 1.57 ± 0.51 | 1.57 ± 0.51 | 6.03 | 0.18 (–0.56 to 0.92) | .115 |
| FEV1/FVC | 100.37 ± 16.0 | 100.37 ± 16.0 | 101.45 ± 15.91 | 101.45 ± 15.9 | 2.43 | 0.02 (–0.72 to 0.76) | .497 |
| mMRC | 3.38 ± 0.49 | 0.02 ± 0.12 | 3.60 ± 0.49 | 0.50 ± 0.65 | 17.96 | 1.49 (–2.34 to –0.64) | .005* |
| Physical functioning | 42.4 ± 7.3 | 88.9 ± 6.3 | 47.7 ± 8.1 | 50.8 ± 8.3 | 23.8 | 1.22 (–2.03 to –0.40) | .003* |
| Role physical | 49.6 ± 13.6 | 79.3 ± 10.0 | 51.2 ± 9.5 | 54.3 ± 9.8 | 13.6 | 1.22 (–2.03 to –0.40) | .003* |
| Bodily pain | 43.3 ± 7.3 | 87.8 ± 6.9 | 42.7 ± 8.3 | 46.0 ± 9.1 | 26.5 | 1.22 (–2.03 to –0.40) | .003* |
| General health | 50.2 ± 7.1 | 89.7 ± 7.2 | 51.2 ± 6.9 | 54.6 ± 7.1 | 18.4 | 1.15 (–1.96 to –0.34) | .007* |
| Vitality | 62.5 ± 8.0 | 88.6 ± 6.8 | 61.9 ± 7.1 | 64.0 ± 8.0 | 19.8 | 1.06 (–1.039 to –1.082) | <.001* |
| Social functioning | 56.5 ± 12.3 | 89.4 ± 5.7 | 56.5 ± 12.3 | 59.1 ± 11.9 | 15.77 | 1.019 (1.011 to –1.028) | <.001* |
| Role emotional | 76.3 ± 11.4 | 88.7 ± 5.1 | 63.3 ± 14.1 | 65.8 ± 13.8 | 14.53 | 1.052 (1.016-1.091) | .005* |
| Mental health | 65.1 ± 8.3 | 88.4 ± 5.1 | 62.9 ± 10.5 | 65.8 ± 10.9 | 17.93 | 1.49 (–2.34 to –0.64) | .005* |
The mean and SD of the values are shown. Percent in FVC denotes a percentage change in FVC.
*Significant difference between groups after 6 weeks.
6MWT, 6-minute walk test; 6MWD, 6-minute walk distance; RHR, Resting heart rate; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; mMRC, modified Medical Research Council (dyspnea scale); PFT, pulmonary function testing; PR, pulmonary rehabilitation.
DISCUSSION
In the current study, the PR group had a substantial improvement in 6MWD by 198 m compared to controls; these findings are supported by previous trials conducted in PTB patients.12,15 Adults with post-PTB develop skeletal muscle dysfunction accountable to physical inactivity and systemic inflammation which is often compounded by impaired nutrition. Such patients enter a vicious cycle, which results in declining body mass index, progressive morbidity, and increased mortality.16 Individuals affected by chronic respiratory conditions tend to avoid exercise and become increasingly deconditioned and developed exercise intolerance during exercise testing and training. Pulmonary rehabilitation results in breaking this vicious cycle and improves the exercise tolerance of the patient.17 We may speculate that improvement in functional capacity might result from decreased ventilatory demand and switching toward aerobic metabolism as suggested by the depressed lactate levels,13 which ultimately results in improved muscle performance and subsequent alleviation of muscle fatigue, thereby resulting in enhanced exercise tolerance.13
We observed significant improvements in HRQoL assessed by the SF-36, a reliable and validated generic HRQoL questionnaire appropriate for assessing patients with PTB. These improvements in HRQoL may be more significant than physiological measurements.18 According to a study by Rossi et al19, symptoms of mental health deterioration are present at quite high rates since the pandemic, which eventually resulted in a low quality of life. However, the current study found that by the end of the PR program, the patient’s mental health improved significantly, as assessed by the SF-36 questionnaire. The findings of the current study are consistent with previous research that found that PTB patients’ HRQoL significantly improved following the completion of PR sessions on both clinical and statistical level.17 Exercise-related biological processes included improved hypothalamic–pituitary–adrenal axis modulation, higher endogenous opioid release, and lower systemic inflammation, all of which are speculated to enhance mental and emotional well-being following PR and thereby improving HRQoL among PTB patients.20
We found no improvement in spirometric measures in PTB post-PR. The finding of the present study is in agreement with Mbatchou Ngahane et al21 who demonstrated a connection between pulmonary function deterioration and the interval between the onset of disease symptoms and the diagnosis of tuberculosis. Moreover, cumulative age-related FEV1 decline predominantly in young adults will cause even more significant impairment in pulmonary function later.22 The reason for no significant improvement in pulmonary function in the current study is a longer delay in diagnosis and treatment due to the COVID-19 pandemic, which has eventually been linked to a decline in pulmonary function outcomes.
The current study has a few limitations. First, it would be fascinating to look into the effects of a long-term PR in individuals who have been pharmacologically cured of PTB. Second, other more relevant outcome variables such as chest x-rays, sputum examination, arterial blood gas analysis, and inflammatory markers like eosinophil might provide a comprehensive picture of this population's physiological responses to PR. Despite these limitations, the study has potential strengths; the lack of dropouts from the current trial suggests that participants adhered to the treatment protocol. This suggests that short-term PR may be an effective strategy to improve outcome measures in PTB patients with an increased adherence rate. As the protocol was implemented during the time of COVID-19, COVID-19 appropriate measures have been taken for all of the patients.
CONCLUSION
The results of the study indicate that a short-term PR program enhances PTB patient’s functional capacity, HRQoL, and dyspnea scores. However, short-term PR was insufficient to register changes in pulmonary function in these PTB patients.
Figure 1.
Study flowchart.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Sharda University (Approval No: SU/SMS&R/76-A/2022/01).
Informed Consent: Patients who volunteered to participate in the trial provided written informed consent.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.O.; Design – M.J.,; Supervision – A.P.; Materials - M.O., I.A.; Data Collection and/ or Processing – I.A.; Analysis and/or Interpretation – I.A.; Literature Review – A.M., M.O., M.J.; Writing – M.O., A.M.; Critical Review – M.J., A.M.
Acknowledgments: The authors would like to thank the undergraduate students, interns, and the other staff of the Physiotherapy Department for their assistance in data collection and the supervision of the rehabilitation sessions.
Declaration of Interests: The authors have no conflicts of interest to declare.
Funding: This study received no funding.
References
- 1. Giuntoli M, Bonicoli E, Bugelli G, Valesini M, Manca M, Scaglione M. Lessons learnt from COVID 19: an Italian multicentric epidemiological study of orthopaedic and trauma services. J Clin Orthop Trauma. 2020;11(4):721 727. 10.1016/j.jcot.2020.05.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zumla A, Marais BJ, McHugh TD.et al. COVID-19 and tuberculosis—threats and opportunities. Int J Tuberc Lung Dis. 2020;24(8):757 760. 10.5588/ijtld.20.0387) [DOI] [PubMed] [Google Scholar]
- 3. Houchen-Wolloff L, Steiner MC. Pulmonary rehabilitation at a time of social distancing: prime time for telerehabilitation. Thorax. 2020;75(6):446 447. 10.1136/thoraxjnl-2020-214788) [DOI] [PubMed] [Google Scholar]
- 4. Ries AL. Pulmonary rehabilitation: summary of an evidence-based guideline. Respir Care. 2008;53(9):1203 1207. [PubMed] [Google Scholar]
- 5. Horton EJ, Mitchell KE, Johnson-Warrington V.et al. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax. 2018;73(1):29 36. 10.1136/thoraxjnl-2016-208506) [DOI] [PubMed] [Google Scholar]
- 6. Orooj M, Moiz JA, Mujaddadi A, Ali MS, Talwar D. Effect of pulmonary rehabilitation in patients with asthma COPD overlap syndrome: a randomized control trial. Oman Med J. 2020;35(3):e136. 10.5001/omj.2020.54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zaki N, Alashwal H, Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: a systematic review. Diabetes Metab Syndr. 2020;14(5):1133 1142. 10.1016/j.dsx.2020.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh SK, Naaraayan A, Acharya P, Menon B, Bansal V, Jesmajian S. Pulmonary rehabilitation in patients with chronic lung impairment from pulmonary tuberculosis. Cureus. 2018;10(11):e3664. 10.7759/cureus.3664) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Enright PL. The six-minute walk test. Respir Care. 2003;48(8):783 785. [PubMed] [Google Scholar]
- 10. Dechman G, Wilson CR. Evidence underlying breathing retraining in people with stable chronic obstructive pulmonary disease. Phys Ther. 2004;84(12):1189 1197. 10.1093/ptj/84.12.1189) [DOI] [PubMed] [Google Scholar]
- 11. Chandrasekaran B, Reddy KC. Six-minute walk test as a guide for walking prescription for patients with chronic obstructive pulmonary diseases. Indian J Respir Care. 2018;7(2):73. 10.4103/ijrc.ijrc_19_17) [DOI] [Google Scholar]
- 12. Jones R, Kirenga BJ, Katagira W.et al. A pre–post-intervention study of pulmonary rehabilitation for adults with post-tuberculosis lung disease in Uganda. Int J Chronic Obstruct Pulm Dis. 2017;12:3533-3539. 10.2147/COPD.S146659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida N, Yoshiyama T, Asai E, Komatsu Y, Sugiyama Y, Mineta Y. Exercise training for the improvement of exercise performance of patients with pulmonary tuberculosis sequelae. Intern Med. 2006;45(6):399 403. 10.2169/internalmedicine.45.1505) [DOI] [PubMed] [Google Scholar]
- 14. Muñoz-Torrico M, Rendon A, Centis R.et al. Is there a rationale for pulmonary rehabilitation following successful chemotherapy for tuberculosis? J Bras Pneumol. 2016;42(5):374 385. 10.1590/S1806-37562016000000226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Grass D, Manie S, Amosun SL. Effectiveness of a home-based pulmonary rehabilitation program in pulmonary function and health-related quality of life for patients with pulmonary tuberculosis: a pilot study. Afr Health Sci. 2014;14(4):866 872. 10.4314/ahs.v14i4.14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siafakas NM, Vermeire P, Pride NB.et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society task force. Eur Respir J. 1995;8(8):1398 1420. 10.1183/09031936.95.08081398) [DOI] [PubMed] [Google Scholar]
- 17. Decramer ML, Hanania NA, Lötvall JO, Yawn BP. The safety of long-acting β2-agonists in the treatment of stable chronic obstructive pulmonary disease. Int J Chronic Obstruct Pulm Dis. 2013;8:53-64. 10.2147/COPD.S39018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown J, Capocci S, Smith C, Morris S, Abubakar I, Lipman M. Health status and quality of life in tuberculosis. Int J Infect Dis. 2015;32:68 75. 10.1016/j.ijid.2014.12.045) [DOI] [PubMed] [Google Scholar]
- 19. Rossi R, Socci V, Talevi D.et al. COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. Front Psychiatry. 2020;11:790. 10.3389/fpsyt.2020.00790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tselebis A, Bratis D, Pachi A.et al. A pulmonary rehabilitation program reduces levels of anxiety and depression inCOPD patients. Multidiscip Respir Med. 2013;8(1):1 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mbatchou Ngahane BH, Nouyep J, Nganda Motto M.et al. Post-tuberculous lung function impairment in a tuberculosis reference clinic in Cameroon. Respir Med. 2016;114:67 71. 10.1016/j.rmed.2016.03.007) [DOI] [PubMed] [Google Scholar]
- 22. Maguire GP, Anstey NM, Ardian M.et al. Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. Int J Tuberc Lung Dis. 2009;13(12):1500 1506. [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a