This cluster randomized trial evaluates the effect of the Friendship Bench intervention on antiretroviral therapy outcome and mental health symptoms among patients with HIV in rural Zimbabwe.
Key Points
Question
Does the Friendship Bench intervention improve antiretroviral therapy (ART) adherence, viral suppression, and mental health symptoms in people living with HIV in rural Zimbabwe?
Findings
In this cluster randomized trial of 516 participants, the intervention showed no significant effect on adherence to ART.
Meaning
The intervention did not affect adherence, possibly due to the absence of skill-based adherence training and the ceiling effect.
Abstract
Importance
Common mental disorders (CMD), which include depression and anxiety, are prevalent among people living with HIV and are associated with suboptimal antiretroviral therapy (ART) adherence.
Objective
To assess the effect of a lay health worker–led psychological intervention on ART adherence, virologic suppression, and mental health symptoms.
Design, Setting, and Participants
Open-label pragmatic cluster trial with 1:1 block randomization of 16 health facilities in rural Bikita, Zimbabwe. Recruitment occurred from October 2018 to December 2019, and participants were followed up for 12 months, ending in December 2020. Participants were adults aged 18 years and older, who spoke English or Shona, screened positive for CMD (Shona Symptoms Questionnaire [SSQ]-14 score ≥9), received first-line ART for 6 or more months, had no World Health Organization stage 4 disease, no psychosis, were not pregnant, and provided informed consent. Data were analyzed from March 2021 to February 2022.
Intervention
The Friendship Bench, consisting of 6 lay health worker–led weekly problem-solving therapy sessions and optional peer-led group support.
Main Outcomes and Measures
The primary outcome was mean adherence during 2 to 6 months of follow-up, and the secondary outcomes were mean adherence during 1 to 12 months of follow-up, change in SSQ-14 and Patient Health Questionnaire (PHQ-9) scores (3, 6, 9, and 12 months), and viral load suppression (6 and 12 months).
Results
A total of 516 participants were recruited (244 in Friendship Bench and 272 in enhanced standard care facilities); 438 (84.9%) were female and the mean (SD) age was 45.6 (10.9) years. Mean (SD) adherence between 2 to 6 months was 89.9% (18.4%) in the Friendship Bench group and 87.2% (20.1%) in the control group. The intervention had no statistically significant effect on adherence between 2 to 6 months (unadjusted mean difference, 1.93 percentage points; 95% CI, −1.20 to 5.06 percentage points; P = .23), between months 1 to 12 (mean difference 0.79 percentage points; 95% CI, −2.14 to 3.71 percentage points; P = .60), or viral suppression. Declines in SSQ-14 scores from baseline to 3 months (difference, −1.65; 95% CI, −3.07 to −0.24), 6 months (difference, −1.57; 95% CI, −2.98 to −0.15), and 9 months (difference, −1.63; 95% CI, −3.05 to −0.22) were greater in the Friendship Bench than the standard care group (P < .05). There were no differences in the decline in the SSQ-14 scores from baseline to 12 months and in declines in PHQ-9 scores from baseline to 3, 6, 9, and 12 months.
Conclusions and Relevance
In this randomized trial of HIV-positive participants with CMD, the Friendship Bench intervention had no effect on adherence and viral suppression, possibly due to the absence of skill-based adherence training and a ceiling effect.
Trial Registration
ClinicalTrials.gov Identifier: NCT03704805
Introduction
Zimbabwe carries a high HIV burden, with an estimated 1.2 million people living with HIV in 2020.1 Antiretroviral treatment (ART) improves the life expectancy of people living with HIV,2 but lifelong retention and high adherence levels are vital for long-term ART effectiveness.3,4 The prevalence of common mental disorders (CMD), specifically depression and anxiety, among people living with HIV is high.5,6,7 This can be attributed to the bidirectional relationship between mental health and HIV. Mental health disorders potentially increase the risk of HIV acquisition and, conversely, living with HIV often exacerbates mental health conditions.8 Mental health problems can create challenges in treating HIV. They are associated with suboptimal adherence,9,10,11 inadequate viral suppression,12,13,14,15,16 low retention,17 and premature mortality.16
Treating mental disorders in people with HIV is vital for improving mental health18,19,20,21 and HIV management,21,22,23,24,25 yet mental disorders often remain undiagnosed and untreated.26,27 The Friendship Bench (FB), a lay health worker–led psychological intervention, was developed to increase access to treatment for CMD in Zimbabwe.28 The FB intervention reduced CMD symptoms in a trial in urban Zimbabwe.28 Its effectiveness in the rural settings and improving ART adherence and virologic suppression is unknown. This cluster randomized trial assessed the effect of the FB intervention on mental health symptoms and ART outcomes among adults living with HIV in the rural district of Bikita, where 1 in 5 adults with HIV screened positive for CMD.7
Methods
Ethics Statement
Ethics committees of the Medical Research Council of Zimbabwe, the Research Council of Zimbabwe, and the Canton of Bern, Switzerland approved the study. Individuals provided written informed consent. This report follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Design
The study was a pragmatic cluster randomized open-label superiority trial in 16 health facilities in rural Zimbabwe (see Supplement 1 for study protocol and statistical analysis plan). Participants were followed up every 3 months for 1 year between October 5, 2018, and December 18, 2020.
Setting
Bikita is a rural district about 300 km south of Harare. From 18 facilities participating in the International Epidemiology Databases to Evaluate AIDS Southern Africa (IeDEA-SA),29 we selected 15, excluding 3 for low patient numbers, and adding a non-IeDEA-SA site to ensure a balanced distribution of intervention and control facilities.
ART followed national treatment guidelines, except for additional CD4 testing at baseline and additional viral load testing at baseline, 6, and 12 months.30 Until April 2019, first-line regimens contained efavirenz or nevirapine and 2 nucleoside/nucleotide reverse transcriptase inhibitors.30 Subsequently, individuals receiving ART with viral loads below 1000 copies/mL were switched to a dolutegravir-based regimen. Following national treatment guidelines, participants with a viral load of more than 1000 copies/mL received enhanced adherence counseling and an additional viral load test to confirm virologic failure.31 See eMethods in Supplement 2 for further details.
Randomization and Masking
We randomized 16 health facilities in a 1:1 ratio to intervention or control group. Randomization was stratified by health facility size (3 strata). All facilities were randomized at the same time using block randomization with a block size of 2 within each stratum. Treatment assignment was known to participants, clinicians, evaluators, and data analysts. Participant recruitment occurred after randomization. See eMethods in Supplement 2 for further details.
Participants
We assessed study eligibility of individuals waiting for ART clinic appointment. Adults aged 18 years or older from Bikita who spoke English or Shona, screened positive for CMD (Shona Symptoms Questionnaire [SSQ]-14 score ≥9), had received first-line ART for at least 6 months, had no World Health Organization (WHO) stage 4 disease, no psychotic symptoms, were nonpregnant, and provided informed consent were eligible (Figure 1).
Figure 1. Trial Profile.
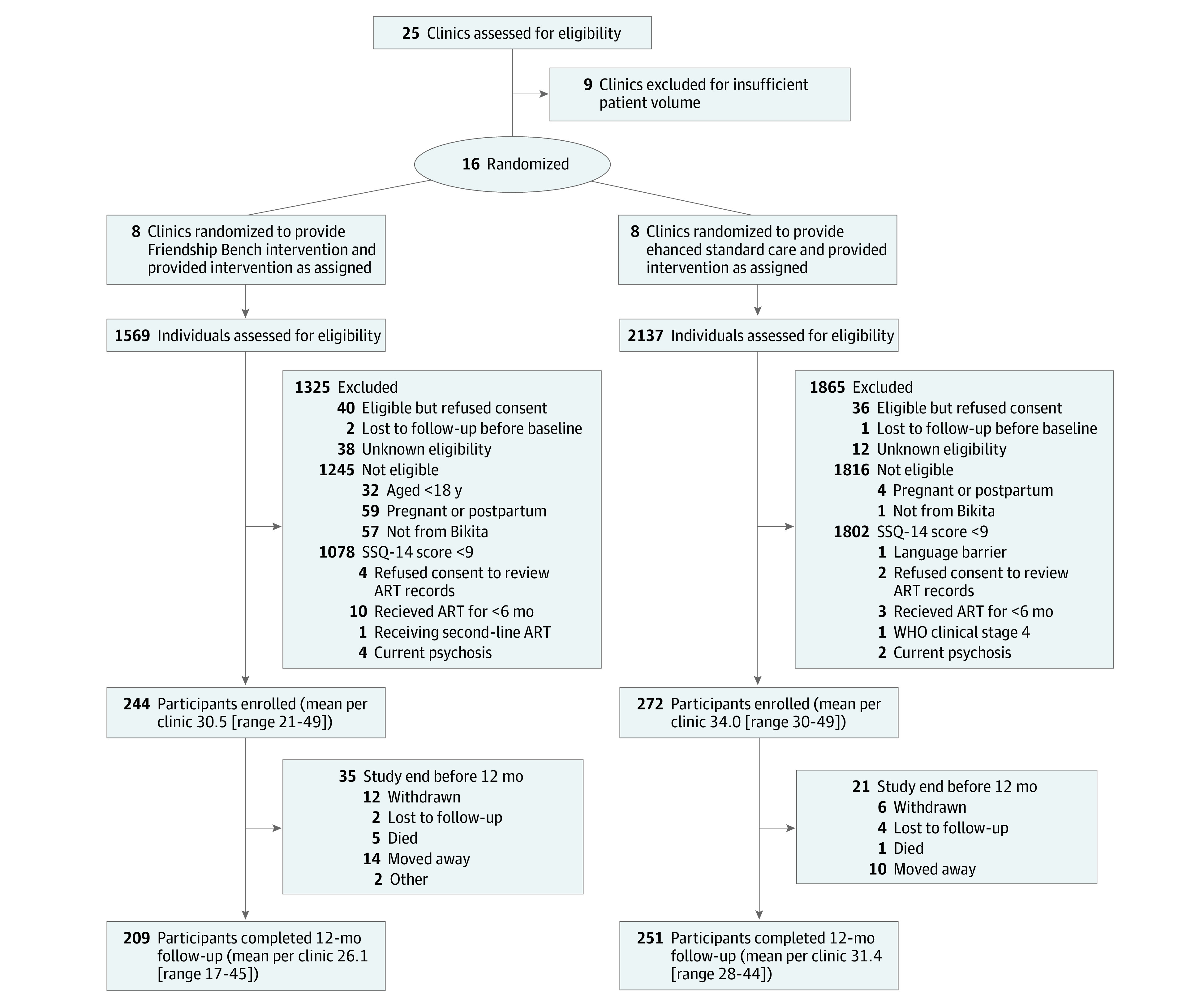
ART indicates antiretroviral therapy; SSQ-14, Shona Symptoms Questionnaire-14; WHO, World Health Organization.
Interventions
Participants in the intervention group were offered the FB intervention in addition to enhanced standard of care (SC). Participants in the control group received SC only.
The FB intervention consisted of 6 weekly individual counseling sessions and an optional peer-led group support. Trained lay health workers facilitated these sessions, following a structured approach to identify problems, including adherence issues, and to foster a positive attitude toward resolving them. Lay health workers were selected from the existing pool of village health workers, regardless of sex or HIV status. Two FB trainers trained lay health workers in the FB program’s manualized protocol. Clinic nurses, trained in FB supervision, regularly supervised lay health workers. The intervention was delivered on a park bench at the health facilities or in clinic consultation rooms, making it easily accessible for individuals.
After 4 sessions, participants were invited to join a peer-led group activity where they were trained in income-generating skills (eg, producing bags from recycled plastic). The support groups were initiated and facilitated by the lay health workers who provided the individual counseling sessions. These groups offered continuous support from lay health workers and peers. The FB intervention is described in detail elsewhere.28,32
SC consisted of nurse-led brief counseling, education regarding CMD, prescription of an antidepressant (fluoxetine), or referral to a psychiatric facility if indicated (see eMethods in Supplement 2). The nurses were trained in managing mental, neurological, and substance use disorders.33
Measures
We used the Medication Event Monitoring System (MEMS) electronic pill box (AARDEX) to measure adherence. We calculated monthly mean adherence as the percentage of days participants opened the box once or twice (depending on the regimen). We treated adherence of less than 10% as missing, assuming participants did not use their MEMS device. We assessed self-reported baseline adherence according to 30-day recall. eMethods in Supplement 2 provides further details.
Research assistants administered the SSQ-1434,35 and the Patient Health Questionnaire (PHQ-9)35,36 at baseline and the 3, 6, 9, and 12-month visits. The SSQ-14 is a locally developed brief screening tool to assess symptoms of depression and anxiety in the Shona language in Zimbabwe. The tool evaluates symptoms including overthinking, difficulties in concentration, irritability, stomachache, sleep disturbance, suicidal ideations, tearfulness, perceptual symptoms, and impaired functioning in the past week,35 with the score ranging from 0 (no symptoms) to 14 (all symptoms present).34 The tool was developed through ethnographic and qualitative research, which elicited idioms of distress related to mental disorders. With a cutoff score of 9 or more, the tool has demonstrated 88% sensitivity and 76% specificity for detecting depression or anxiety among people with HIV in urban Zimbabwe.35 The PHQ-9 is a screening tool for depression symptoms over the past 2 weeks. A score of 11 or more has 88% sensitivity and 71% specificity for detecting depression in people with HIV in urban Zimbabwe.35 At baseline, research assistants administered structured questionnaires on participants’ sociodemographic characteristics, perceived general health perception, ART knowledge, alcohol use (Alcohol Use Disorders Identification Test C [AUDIT-C]37), food insecurity (Household Food Insecurity Scale), and social support (Medical Outcomes Study Social Support Survey [MOS-SS]).
Outcomes
The primary outcome was mean adherence between 2 to 6 months of follow-up. Secondary outcomes included mean adherence between 1 to 12 months; change from baseline SSQ-14 and PHQ-9 score at 3, 6, 9, and 12 months; and change in viral suppression (viral load <1000 copies per mL) at months 6 and 12; and positive screening for CMD (SSQ-14 score ≥9) and depression (PHQ-9 score ≥11) at 3, 6, 9, and 12 months.
Sample Size
The study was powered to detect an absolute 10% difference in mean adherence24 between months 2 to 6, assuming a standard deviation of 20, an intracluster coefficient of 0.05, and a type I error of 5% (see Supplement 1). Sixteen clusters with 25 participants provide 92% power to detect such a difference. To allow for attrition, we set the target sample size at 480 participants (16 clusters with 30 participants).
Statistical Analysis
The primary analysis was per intention to treat. We first calculated participants’ monthly mean adherence scores and then analyzed these scores using linear mixed-effects models to assess the difference in mean adherence. Models included a random intercept and slope to account for the correlation within participants. A random intercept accounted for the clustering of individuals in health facilities, indicators defined treatment assignment, the month of analysis time (categorical), and interactions between the 2. Subsequently, we calculated the average difference in mean adherence between months 2 to 6 and 1 to 12 based on contrasts. We estimated odds ratios (ORs) for the difference in the proportion of participants with viral suppression at 6 and 12 months using logistic mixed-effect models. We conducted prespecified adjusted analyses of mean adherence and viral suppression, controlling for facility size, age, and sex. In post hoc sensitivity analyses, we adjusted for facility size, age, sex, self-reported baseline adherence, baseline SSQ-14 score, ART regimen, PHQ-9 score, WHO clinical stage, CD4 cell count, viral suppression, AUDIT-C score, MOS-SS score, and travel cost. We also assessed the difference in change from baseline in SSQ-14 and PHQ-9 scores using the same linear mixed-effects model as described previously but adjusted for the baseline score. We used the logistic mixed-effect models described previously to assess the difference in the proportion of participants with an SSQ-14 score of 9 or higher and a PHQ-9 score of 11 or higher at 3, 6, 9, and 12 months. We repeated analyses of primary and secondary outcomes using a per-protocol analysis, excluding participants who did not receive the allocated intervention and those with missing adherence data between months 2 to 6. Missing data were imputed using multiple imputation by chained equations.38 We used linear and logistic mixed effects models. All tests were 2-sided, and we considered a P value of less than .05 as statistically significant. Statistical analyses were completed in Stata, version 16 (StataCorp). eMethods in Supplement 2 provides further details. Data were analyzed from March 2021 to February 2022.
Results
Participants and Baseline Characteristics
In this study, 516 individuals (mean [SD] age 45.6 [10.9] years; 438 [84.9%] female) were included, with 244 participants in 8 clusters in the FB group and 272 participants in 8 clusters in the control group (Table 1). Most participants were married or cohabitating (265 participants [51.4%]) and had completed primary (229 participants [44.4%]) or secondary education (256 participants [49.6%]). The mean (SD) SSQ-14 score at baseline was 10.0 (1.1), and the mean (SD) PHQ-9 was 7.7 (3.5). The median (IQR) baseline CD4 cell count was 552 (401-721) cells/μL, few participants (29 participants [5.6%]) had a viral load of 1000 copies/mL or more, and most (405 participants [78.5%]) reported optimal adherence (ie, not having missed a single dose) in the 30 days before enrollment. Participants in the intervention group had a higher baseline mean PHQ-9 score, were more likely to be in WHO clinical stage 1, had lower MOS-SS and AUDIT-C scores, and had lower transportation costs than participants in the control group (Table 1).
Table 1. Sociodemographic and Clinical Characteristics of Participants at Baseline.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Friendship Bench (n = 244) | Standard of care (n = 272) | Total (N = 516) | |
| Age, mean (SD), y | 45.6 (11.3) | 45.5 (10.5) | 45.6 (10.9) |
| Sex | |||
| Female | 211 (86.5) | 227 (83.5) | 438 (84.9) |
| Male | 33 (13.5) | 45 (16.5) | 78 (15.1) |
| Marital status | |||
| Married/living together | 119 (48.8) | 146 (53.7) | 265 (51.4) |
| Widowed/divorced/separated | 111 (45.5) | 120 (44.1) | 231 (44.8) |
| Single | 13 (5.3) | 6 (2.2) | 19 (3.7) |
| Missing | 1 (0.4) | 0 | 1 (0.2) |
| Education | |||
| Primary | 114 (46.7) | 115 (42.3) | 229 (44.4) |
| Secondary or higher | 110 (45.1) | 146 (53.7) | 256 (49.6) |
| Missing | 20 (8.2) | 11 (4.0) | 31 (6.0) |
| SSQ-14 score, mean (SD)a | 10.1 (1.1) | 9.9 (1.1) | 10.0 (1.1) |
| PHQ-9 score, mean (SD)b | 8.3 (3.6) | 7.2 (3.2) | 7.7 (3.5) |
| Missing | 1 (0.4) | 0 | 1 (0.2) |
| Time on ART, median (IQR), y | 7.9 (5.0-9.7) | 6.6 (4.4-8.6) | 7.0 (4.8-9.1) |
| Missing | 9 (3.7) | 8 (2.9) | 17 (3.3) |
| WHO clinical stage | |||
| Stage 1 | 86 (35.2) | 56 (20.6) | 142 (27.5) |
| Stage 2 | 55 (22.5) | 97 (35.7) | 152 (29.5) |
| Stage 3 | 103 (42.2) | 119 (43.8) | 222 (43.0) |
| ART regimen | |||
| NNRTI-based | 237 (97.1) | 270 (99.3) | 507 (98.3) |
| Dolutegravir-based | 7 (2.9) | 2 (0.7) | 9 (1.7) |
| CD4 count in cells/μL, median (IQR) | 527 (368-697) | 572 (410-731) | 552 (401-721) |
| Missing | 69 (28.3) | 73 (26.8) | 151 (28.2) |
| Viral load | |||
| ≥1000 copies/mL | 9 (3.7) | 20 (7.4) | 29 (5.6) |
| <1000 copies/mL | 182 (74.6) | 200 (73.5) | 382 (74.0) |
| Missing | 53 (21.7) | 52 (19.1) | 105 (20.3) |
| Self-reported adherence, mean (SD) % | 98 (6) | 98 (4) | 98 (5) |
| Missing | 0 | 1 (0.4) | 1 (0.2) |
| Comprehensive ART knowledge | |||
| No | 41 (16.8) | 35 (12.9) | 76 (14.7) |
| Yes | 203 (83.2) | 237 (87.1) | 440 (85.3) |
| HFIS score, median (IQR) | 3 (0-8) | 3 (0-7) | 3 (0-7) |
| MOS-SS score, median (IQR) | 26 (18-35) | 32 (24-36) | 30 (20-36) |
| AUDIT-C screening outcome | |||
| Negative | 232 (95.1) | 251 (92.3) | 483 (93.6) |
| Positive | 8 (3.3) | 19 (7.0) | 27 (5.2) |
| Missing | 4 (1.6) | 2 (0.7) | 6 (1.2) |
| AUDIT-C score, mean (SD) | 0.3 (0.9) | 0.5 (1.4) | 0.4 (1.2) |
| Cost of transportation to facility (return), US $ | |||
| 0 | 126 (51.6) | 111 (40.8) | 237 (45.9) |
| <2 | 34 (13.9) | 14 (5.1) | 48 (9.3) |
| 2-5 | 52 (21.3) | 67 (24.6) | 119 (23.1) |
| >5 | 32 (13.1) | 80 (29.4) | 112 (21.7) |
Abbreviations: ART, antiretroviral therapy; AUDIT-C, Alcohol Use Disorders Identification Test-Concise Test; HFIS, Household Food Insecurity Access Scale; MOS-SS, Medical Outcomes Study Social Support; NNRTI, nonnucleoside reverse transcriptase inhibitors; PHQ, Patient Health Questionnaire; SSQ, Shona Symptoms Questionnaire; WHO, World Health Organization.
The SSQ-14 score ranges from 0 (no symptom) to 14 (all symptoms present).
The PHQ-9 score is ranging from 0 (no depressive symptoms) to 27 (severe depressive symptoms).
To recruit this sample, we assessed the eligibility of 3706 individuals between October 5, 2018, and December 19, 2019, and excluded 3190 participants (86.1%) (Figure 1). Of the 595 participants who met inclusion criteria, 79 (13.3%) refused to participate or were lost to follow-up before the baseline visit. The last 12-month follow-up visit was on December 18, 2020.
Outcomes
In unadjusted analyses, the primary outcome of mean (SD) ART adherence between months 2 and 6 was slightly higher in the FB group (89.9% [18.4]) than in the SC group (87.2% [20.1]) (mean difference, 1.93 percentage points; 95% CI, −1.20 to 5.06 percentage points; P = .23), but there was little difference in mean ART adherence after month 6 (Figure 2A, Table 2). The intracluster coefficient of the primary outcome was 0.006. Overall, between months 1 and 12, mean ART adherence was similar (mean difference, 0.79 percentage points; 95% CI, −2.14 to 3.71 percentage points; P = .60) (Table 2). The odds of virologic suppression at 6 months (OR, 2.20; 95% CI, 0.79 to 6.14; P = .13) and 12 months (OR, 1.60; 95% CI, 0.42 to 6.05; P = .49) were higher in the FB than in the SC group, but differences failed to reach conventional levels of statistical significance (Figure 2B and Table 2). Declines in SSQ-14 scores from baseline to 3 months (difference, −1.65; 95% CI, −3.07 to −0.24), 6 months (difference, −1.57; 95% CI, −2.98 to −0.15), and 9 months (difference, −1.63; 95% CI, −3.05 to −0.22) were more pronounced in the FB than the SC group (Figure 2C and Table 2). There were no differences in the decline in the SSQ-14 scores from baseline to 12 months (Figure 2C and Table 2) and in PHQ-9 scores (Figure 2D and Table 2). There was little evidence for a difference in the odds of screening positively for common mental disorders (SSQ-14 >9) or depression (PHQ-9 >11) at 3, 6, 9, or 12 months (Table 2). Results were similar in the prespecified adjusted (Table 2) and post hoc sensitivity analyses for the intention to treat and per-protocol populations (eTable 1 and eTable 2 in Supplement 2).
Figure 2. Adherence, Viral Load Suppression, Shona Symptoms Questionnaire (SSQ)-14 Score, and Patient Health Questionnaire (PHQ)-9 Score by Group.
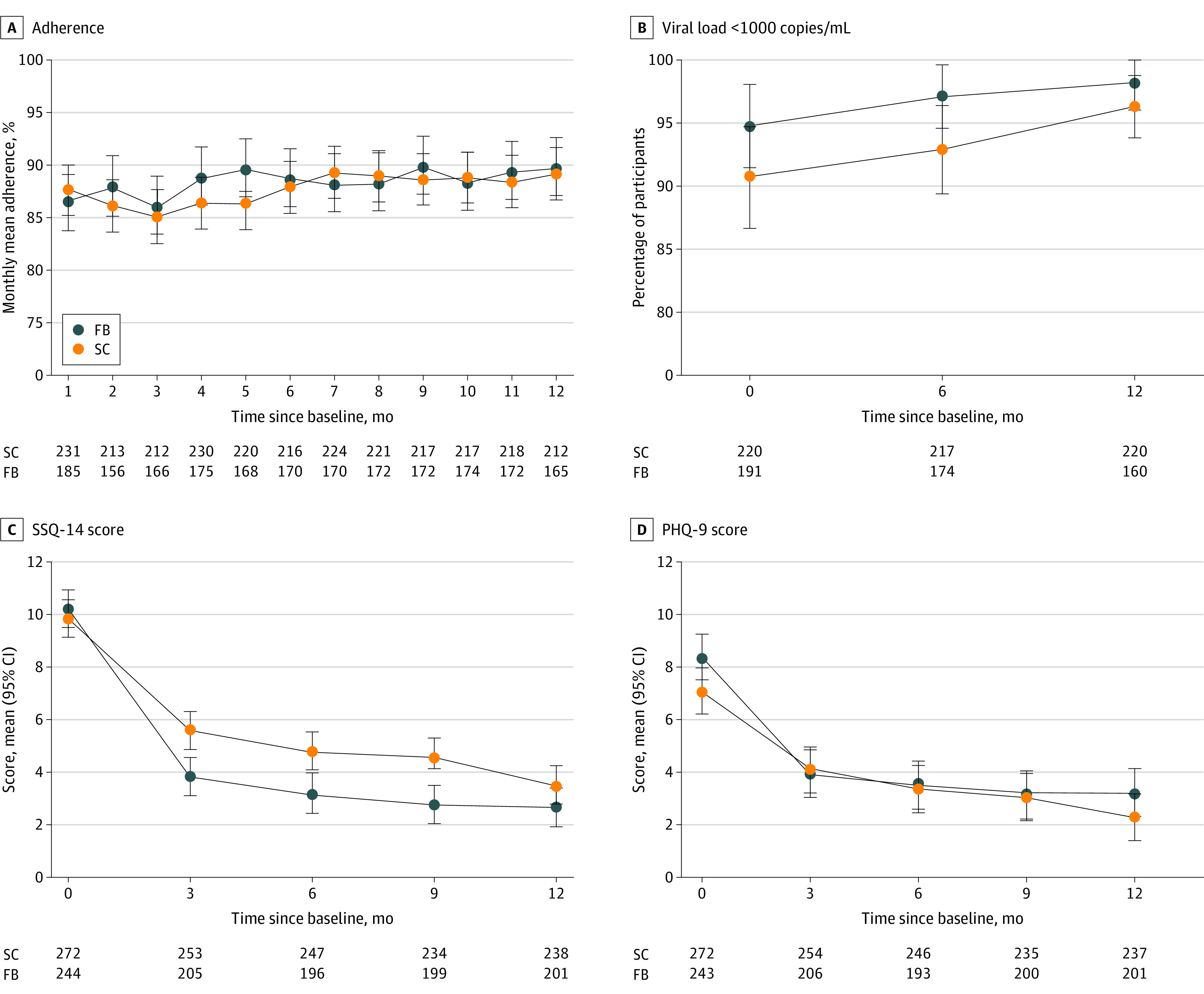
Figure shows mean monthly adherence scores (A), proportions of participants with viral load of less than 1000 copies/mL (B), mean SSQ-14 scores, and mean PHQ-9 scores. Error bars represent 95% CIs for means and proportions. FB indicates Friendship Bench; SC, standard care.
Table 2. Effect of the Friendship Bench Intervention on Adherence, Viral Load, and Mental Healtha.
| Outcome | Unadjusted analyses | Adjusted analysesb | ||
|---|---|---|---|---|
| Effect estimate | P value | Effect estimate | P value | |
| Adherence, mean difference (95% CI) | ||||
| Month 2-6 | 1.93 (−1.20 to 5.06) | .23 | 1.79 (−1.71 to 5.29) | .32 |
| Month 1-12 | 0.79 (−2.14 to 3.71) | .60 | 0.64 (−2.67 to 3.94) | .71 |
| Viral suppression, odds ratio (95% CI) | ||||
| Month 6 | 2.20 (0.79 to 6.14) | .13 | 2.26 (0.79 to 6.45) | .13 |
| Month 12 | 1.60 (0.42 to 6.05) | .49 | 1.75 (0.47 to 6.49) | .40 |
| SSQ-14 score, mean difference (95% CI) | ||||
| Month 3 | −1.65 (−3.07 to −0.24) | .02 | −1.65 (−3.13 to −0.16) | .03 |
| Month 6 | −1.57 (−2.98 to −0.15) | .03 | −1.56 (−3.05 to −0.07) | .04 |
| Month 9 | −1.63 (−3.05 to −0.22) | .02 | −1.63 (−3.11 to −0.15) | .03 |
| Month 12 | −0.78 (−2.19 to 0.63) | .28 | −0.77 (−2.25 to 0.71) | .31 |
| PHQ-9 score, mean difference (95% CI) | ||||
| Month 3 | −0.35 (−1.68 to 0.99) | .61 | −0.49 (−1.82 to 0.85) | .47 |
| Month 6 | 0.01 (−1.33 to 1.34) | .99 | −0.14 (−1.47 to 1.20) | .84 |
| Month 9 | −0.04 (−1.39 to 1.30) | .95 | −0.19 (−1.53 to 1.15) | .78 |
| Month 12 | 0.74 (−0.60 to 2.08) | .28 | 0.60 (−0.74 to 1.93) | .38 |
| SSQ-14 ≥9, odds ratio (95% CI) | ||||
| Month 3 | 0.38 (0.09 to 1.59) | .19 | 0.28 (0.07 to 1.12) | .07 |
| Month 6 | 0.41 (0.09 to 1.83) | .25 | 0.30 (0.07 to 1.30) | .11 |
| Month 9 | 0.34 (0.07 to 1.59) | .17 | 0.25 (0.05 to 1.13) | .07 |
| Month 12 | 1.54 (0.33 to 7.15) | .58 | 1.12 (0.25 to 5.01) | .88 |
| PHQ-9 ≥11, odds ratio (95% CI) | ||||
| Month 3 | 3.24 (0.60 to 17.46) | .17 | 2.16 (0.47 to 9.98) | .33 |
| Month 6 | 3.17 (0.47 to 21.37) | .24 | 2.10 (0.35 to 12.47) | .41 |
| Month 9 | 3.84 (0.49 to 30.30) | .20 | 2.55 (0.37 to 17.74) | .34 |
| Month 12 | 3.11 (0.47 to 20.49) | .24 | 2.06 (0.36 to 11.85) | .42 |
Abbreviations: PHQ, Patient Health Questionnaire; SSQ, Shona Symptoms Questionnaire.
All analyses were prespecified and accounted for clustering of individuals in health facilities.
Adjusted for facility size, age, and sex.
Retention
Retention in the FB and adherence interventions was high (Table 3). The median (IQR) number of sessions received among participants in the intervention group was 6 (6-6); 215 participants (88.1%) received all 6 sessions. In the intervention group, the proportion of participants who attended at least 1 group support session within a 3-month interval decreased from 165 (67.6%) to 125 (51.2%) from the first to the last interval. Few participants (6 [2.5%] in the FB group and 17 [6.3%] in the SC group) received antidepressants. Most participants with a viral load of 1000 copies/mL or more at the baseline visit (8 [72.7%] and 20 [76.9%] in FB and SC groups, respectively) attended all 3 adherence counseling sessions.
Table 3. Retention in Mental Health and Adherence Interventions.
| Intervention | Friendship bench (n = 244) | Standard of care (n = 272) | Total (n = 516) |
|---|---|---|---|
| Received allocated intervention | 223 (91.4) | 272 (100) | 495 (95.9) |
| Friendship Bench intervention | |||
| Individual counseling session 1 attended | 235 (96.3) | NA | 235 (45.5) |
| Individual counseling session 2 attended | 230 (94.3) | NA | 230 (44.6) |
| Individual counseling session 3 attended | 229 (93.9) | NA | 29 (44.4) |
| Individual counseling session 4 attended | 224 (91.8) | NA | 224 (43.4) |
| Individual counseling session 5 attended | 219 (89.8) | NA | 219 (42.4) |
| Individual counseling session 6 attended | 215 (88.1) | NA | 215 (41.7) |
| Group support attended | |||
| Between month 0 and 3 | 165 (67.6) | NA | 165 (32.0) |
| Between month 4 and 6 | 146 (59.8) | NA | 146 (28.3) |
| Between month 7 and 9 | 141 (57.8) | NA | 141 (27.3) |
| Between month 10 and 12 | 125 (51.2) | NA | 125 (24.2) |
| Standard of care | |||
| Nurse-led counseling attended | 242 (99.2) | 272 (100) | 514 (99.6) |
| Antidepressant prescribed | 6 (2.5) | 17 (6.3) | 23 (4.5) |
| Enhanced adherence counseling | |||
| VL ≥1000 copies/mL at baseline visit | |||
| Patients, No. | 11 | 26 | 37 |
| 1-2 sessions attended | 2 (18.2) | 5 (19.2) | 7 (18.9) |
| 3 sessions attended | 8 (72.7) | 20 (76.9) | 28 (75.7) |
| No session attended | 1 (9.1) | 0 | 1 (2.7) |
| Unknown | 0 | 1 (3.8) | 1 (2.7) |
| VL ≥1000 copies/mL 6-mo visit | |||
| Patients, No. | 6 | 17 | 23 |
| 1-2 sessions attended | 0 | 5 (29.4) | 5 (21.7) |
| 3 sessions attended | 5 (83.3) | 10 (58.8) | 15 (65.2) |
| No session attended | 1 (16.7) | 1 (5.9) | 2 (8.7) |
| Unknown | 0 | 1 (5.9) | 1 (4.3) |
| VL ≥1000 copies/mL at 12-mo visit | |||
| Patients, No. | 3 | 9 | 12 |
| 1-2 sessions attended | 0 | 2 (22.2) | 2 (16.7) |
| 3 sessions attended | 0 | 2 (22.2) | 2 (16.7) |
| Unknown | 3 (100) | 5 (55.6) | 8 (66.7) |
Abbreviations: NA, not applicable; VL, viral load.
Safety Outcomes
In total, 16 participants reported self-harm or attempted self-harm; 11 (4.5%) were in the FB group and 5 (1.8%) were in the SC group. In the FB group, 9 participants (3.7%) reported a history of self-harm before baseline, and 2 participants (0.8%) reported a new incident self-harm event after baseline. No new incident self-harm event after baseline was reported in the SC group. No psychiatric hospitalizations occurred. Five participants died in the FB group and 1 in the SC group. All deaths were unrelated to study procedures, and no deaths by suicide were recorded or suspected (eTable 3 in Supplement 2).
Discussion
In this cluster randomized trial, we examined the effect of the FB intervention on ART outcomes and mental health symptoms in adults with HIV in rural Zimbabwe. The intervention did not demonstrate a clear effect on ART adherence, viral suppression, and symptoms of depression (PHQ-9 scores) but had a beneficial effect on CMD symptoms (SSQ-14 scores). Characteristics of the study population at enrollment, including the high proportion of women, high baseline adherence, high baseline viral suppression rates, and relatively low mean PHQ-9 scores, may have contributed to the negative findings of this trial. The FB intervention was found to be a feasible and acceptable approach to address the mental health care needs of the rural study population. Implementing this psychological intervention led by lay health workers in rural health facilities was successful, with almost 90% of participants attending all 6 individual counseling sessions, reflecting their satisfaction and intervention appropriateness.
With the present study, 3 trials show that the FB intervention can reduce CMD symptoms (eFigure in Supplement 2). The first trial in urban Harare found that participants in the intervention group experienced a greater decrease in CMD and depression symptoms than participants in the enhanced standard care group.28 In contrast, our rural trial found a less pronounced decline in CMD symptoms and no effect on symptoms of depression. Differences could stem from urban vs rural settings, or the inclusion of a younger study population, or HIV-negative participants (42%) in the urban trial.28 Interestingly, decreases in the intervention group in CMD and depression symptoms were similar between the Harare trial and our study. The rural trial’s lower effectiveness can be attributed to differences in the rate of change within the control group. In the urban Harare trial,28 the 6-month scores remained high in controls, whereas in our rural trial, scores decreased both in the intervention and control group, suggesting that enhanced standard care may have been more effective in the rural setting. The third FB trial, involving Zimbabwean adolescents with HIV,39 showed improvements in CMD and depression symptoms compared with standard care, with relatively small differences in SSQ-14 and PHQ-9 scores of about 1 score point favoring the intervention.39
Two of the 3 trials examined virologic suppression (this study and the trial among adolescents39), and both failed to demonstrate any statistically significant effect (eFigure in Supplement 2). The present study also examined adherence and equally showed no robust intervention effect. A ceiling effect may have played a role in our study, as over 90% of participants had viral suppression and adherence was close to 90%. Given the well-established association between CMD and poor HIV outcomes,9,10,11,12,13,14,15,16 we expected lower baseline viral suppression and adherence rates. The large proportion of women in our study and the relatively older age may have contributed to these high rates. In a recent analysis of a South African cohort, we found considerably higher viral suppression and adherence rates in women than men and in older than in younger people.40 A ceiling effect is less likely for the trial among adolescents where more than one-third of participants had a viral load of 1000 copies/mL or more.39 Indeed, the nature of the FB intervention, which does not include skill-based adherence training, might be a more pervasive explanation for the lack of effect on viral suppression. Mental health interventions with integrated skill-based adherence training have been effective.41 For example, a trial of South African adults with depression showed that nurse-delivered cognitive-behavioral therapy improved depression scores, ART adherence, and viral suppression.25 Of note, a small pilot trial in Zimbabwe of a version of the FB intervention that incorporated skill-based training in adherence showed promising results regarding improved adherence and viral load suppression.42
Strengths and Limitations
Our study’s strengths include a large sample size, long follow-up, and pragmatic study design resembling clinical practice conditions in rural Zimbabwe. Further strengths of our study include using electronic pill bottles for adherence monitoring and using a locally developed tool to measure CMD symptoms.35
Our study has several limitations. First, the mental health screening tools used in this study have been validated in Harare but not in the rural study setting, where our study took place.35 Second, men were underrepresented in our study (and the Harare study28), and it remains unclear how well the FB intervention works for them. Third, we could not evaluate the contribution of individual counseling, support groups, and nonspecific attention to the outcomes of the FB intervention. Additionally, although we did not reimburse for individual counseling or group support sessions, the $3 transport reimbursement for 3 monthly study visits might have influenced FB intervention retention, even though it was first paid after participants completed individual counseling.
Conclusions
The FB intervention had no effect on adherence and viral suppression, possibly due to the absence of skill-based adherence training and ceiling effect. The intervention improved CMD symptoms, but the effect was smaller than previously shown in an urban setting. More work is needed to evaluate the effect of the approach on HIV outcomes in different populations, including young adults, men, and populations with more severe symptoms at higher risk of nonadherence and virologic failure. The further development of the FB intervention to incorporate adherence training may be a promising approach to reach those at high risk of poor HIV outcomes.
Trial Protocol and Statistical Analysis Plan
eMethods. Additional Information on Standard Care, Randomization, Adherence Measures, and Statistical Analyses
eTable 1. Effect of the Friendship Bench Intervention on Adherence, Viral Load, and Mental Health: Results From Prespecified and Post Hoc Sensitivity Intention-to-Treat Analyses
eTable 2. Effect of the Friendship Bench Intervention on Adherence, Viral Load, and Mental Health: Results From Prespecified and Post Hoc Sensitivity Per-Protocol Analyses
eTable 3. Number of Participants With Safety-Relevant Outcomes by Trial Group
eFigure. Forest Plot of Available Evidence of the Effect of the Friendship Bench Intervention on SSQ-14 Scores, PHQ-9 Scores, and Viral Suppression From 3 Trials
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Ministry of Health and Child Care (MOHCC) Zimbabwe . Zimbabwe population-based HIV impact assessment (ZIMPHIA 2020). 2020. Accessed December 9, 2021. https://phia.icap.columbia.edu/zimbabwe-2020-summary-sheet/
- 2.Johnson LF, Mossong J, Dorrington RE, et al. ; International Epidemiologic Databases to Evaluate AIDS Southern Africa Collaboration . Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10(4):e1001418. doi: 10.1371/journal.pmed.1001418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas AD, Zaniewski E, Anderegg N, et al. ; African regions of the International epidemiologic Databases to Evaluate AIDS (IeDEA) . Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc. 2018;21(2):e25084. doi: 10.1002/jia2.25084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford N, Darder M, Spelman T, Maclean E, Mills E, Boulle A. Early adherence to antiretroviral medication as a predictor of long-term HIV Virological suppression: five-year follow up of an observational cohort. PLoS One. 2010;5(5):e10460. doi: 10.1371/journal.pone.0010460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard C, Dabis F, de Rekeneire N. Prevalence and factors associated with depression in people living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0181960. doi: 10.1371/journal.pone.0181960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, O’Cleirigh CM. Anxiety symptoms and disorders among adults living with HIV and AIDS: a critical review and integrative synthesis of the empirical literature. Clin Psychol Rev. 2017;51:164-184. doi: 10.1016/j.cpr.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas AD, Kunzekwenyika C, Hossmann S, et al. ; IeDEA Southern Africa . Symptoms of common mental disorders and adherence to antiretroviral therapy among adults living with HIV in rural Zimbabwe: a cross-sectional study. BMJ Open. 2021;11(7):e049824. doi: 10.1136/bmjopen-2021-049824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet. 2007;370(9590):859-877. doi: 10.1016/S0140-6736(07)61238-0 [DOI] [PubMed] [Google Scholar]
- 9.Wykowski J, Kemp CG, Velloza J, Rao D, Drain PK. Associations between anxiety and adherence to antiretroviral medications in low- and middle-income countries: a systematic review and meta-analysis. AIDS Behav. 2019;23(8):2059-2071. doi: 10.1007/s10461-018-02390-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. 2014;11(3):291-307. doi: 10.1007/s11904-014-0220-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakimuli-Mpungu E, Bass JK, Alexandre P, et al. Depression, alcohol use and adherence to antiretroviral therapy in sub-Saharan Africa: a systematic review. AIDS Behav. 2012;16(8):2101-2118. doi: 10.1007/s10461-011-0087-8 [DOI] [PubMed] [Google Scholar]
- 12.Haas AD, Technau KG, Pahad S, et al. ; IeDEA Southern Africa Collaboration . Mental health, substance use and viral suppression in adolescents receiving ART at a paediatric HIV clinic in South Africa. J Int AIDS Soc. 2020;23(12):e25644. doi: 10.1002/jia2.25644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesko CR, Hutton HE, Fojo AT, Shen NM, Moore RD, Chander G. Depression and HIV viral nonsuppression among people engaged in HIV care in an urban clinic, 2014-2019. AIDS. 2021;35(12):2017-2024. doi: 10.1097/QAD.0000000000003005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenthal MA, Wanje G, Richardson BA, et al. A prospective study of depressive symptoms, condomless sex, and HIV viral load in HIV-positive female sex workers in Kenya. AIDS Behav. 2021;25(10):3047-3056. doi: 10.1007/s10461-021-03258-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regan M, Muhihi A, Nagu T, et al. Depression and viral suppression among adults living with HIV in Tanzania. AIDS Behav. 2021;25(10):3097-3105. doi: 10.1007/s10461-021-03187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas AD, Ruffieux Y, van den Heuvel LL, et al. ; IeDEA Southern Africa collaboration . Excess mortality associated with mental illness in people living with HIV in Cape Town, South Africa: a cohort study using linked electronic health records. Lancet Glob Health. 2020;8(10):e1326-e1334. doi: 10.1016/S2214-109X(20)30279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rooks-Peck CR, Adegbite AH, Wichser ME, et al. ; Prevention Research Synthesis Project . Mental health and retention in HIV care: a systematic review and meta-analysis. Health Psychol. 2018;37(6):574-585. doi: 10.1037/hea0000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heijden I, Abrahams N, Sinclair D. Psychosocial group interventions to improve psychological well-being in adults living with HIV. Cochrane Database Syst Rev. 2017;3(3):CD010806. doi: 10.1002/14651858.CD010806.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Luenen S, Garnefski N, Spinhoven P, Spaan P, Dusseldorp E, Kraaij V. The benefits of psychosocial interventions for mental health in people living with HIV: a systematic review and meta-analysis. AIDS Behav. 2018;22(1):9-42. doi: 10.1007/s10461-017-1757-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown LK, Kennard BD, Emslie GJ, et al. ; Adolescent Trials Network for HIVAIDS Interventions . Effective treatment of depressive disorders in medical clinics for adolescents and young adults living with HIV: a controlled trial. J Acquir Immune Defic Syndr. 2016;71(1):38-46. doi: 10.1097/QAI.0000000000000803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passchier RV, Abas MA, Ebuenyi ID, Pariante CM. Effectiveness of depression interventions for people living with HIV in Sub-Saharan Africa: a systematic review and meta-analysis of psychological and immunological outcomes. Brain Behav Immun. 2018;73:261-273. doi: 10.1016/j.bbi.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Zhao M, Chen S, et al. Effects of cognitive behavioral therapy on people living with HIV and depression: a systematic review and meta-analysis. Psychol Health Med. 2019;24(5):578-594. doi: 10.1080/13548506.2018.1549739 [DOI] [PubMed] [Google Scholar]
- 23.Sin NL, DiMatteo MR. Depression treatment enhances adherence to antiretroviral therapy: a meta-analysis. Ann Behav Med. 2014;47(3):259-269. doi: 10.1007/s12160-013-9559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safren SA, Bedoya CA, O’Cleirigh C, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV. 2016;3(11):e529-e538. doi: 10.1016/S2352-3018(16)30053-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safren SA, O’Cleirigh C, Andersen LS, et al. Treating depression and improving adherence in HIV care with task-shared cognitive behavioural therapy in Khayelitsha, South Africa: a randomized controlled trial. J Int AIDS Soc. 2021;24(10):e25823. doi: 10.1002/jia2.25823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruffieux Y, Efthimiou O, Van den Heuvel LL, et al. The treatment gap for mental disorders in adults enrolled in HIV treatment programmes in South Africa: a cohort study using linked electronic health records. Epidemiol Psychiatr Sci. 2021;30:e37. doi: 10.1017/S2045796021000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bull World Health Organ. 2004;82(11):858-866. [PMC free article] [PubMed] [Google Scholar]
- 28.Chibanda D, Weiss HA, Verhey R, et al. Effect of a primary care-based psychological intervention on symptoms of common mental disorders in Zimbabwe: a randomized clinical trial. JAMA. 2016;316(24):2618-2626. doi: 10.1001/jama.2016.19102 [DOI] [PubMed] [Google Scholar]
- 29.Chammartin F, Dao Ostinelli CH, Anastos K, et al. International epidemiology databases to evaluate AIDS (IeDEA) in sub-Saharan Africa, 2012-2019. BMJ Open. 2020;10(5):e035246. doi: 10.1136/bmjopen-2019-035246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Medicines and Therapeutics Policy Advisory Committee (NMTPAC) and The AIDS and TB Directorate; Ministry of Health and Child Care . Guidelines for Antiretroviral Therapy for the Prevention and Treatment. 2016. Accessed June 7, 2023. https://depts.washington.edu/edgh/zw/vl/project-resources/ZIM_ART_Guidelines_2016_-_review_final.pdf
- 31.AIDS and TB Programme Ministry of Health and Child Care Zimbabwe . Operational and service delivery manual for the prevention, care and treatment of HIV in Zimbabwe. 2017. Accessed June 7, 2023. https://differentiatedservicedelivery.org/wp-content/uploads/msf-zim-osdm-web-revised.pdf
- 32.Chibanda D, Bowers T, Verhey R, et al. The Friendship Bench programme: a cluster randomised controlled trial of a brief psychological intervention for common mental disorders delivered by lay health workers in Zimbabwe. Int J Ment Health Syst. 2015;9:21. doi: 10.1186/s13033-015-0013-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization . MhGAP intervention guide, version 2.0. 2016. Accessed June 7, 2023. https://www.who.int/publications/i/item/9789241549790
- 34.Patel V, Simunyu E, Gwanzura F, Lewis G, Mann A. The Shona Symptom Questionnaire: the development of an indigenous measure of common mental disorders in Harare. Acta Psychiatr Scand. 1997;95(6):469-475. doi: 10.1111/j.1600-0447.1997.tb10134.x [DOI] [PubMed] [Google Scholar]
- 35.Chibanda D, Verhey R, Gibson LJ, et al. Validation of screening tools for depression and anxiety disorders in a primary care population with high HIV prevalence in Zimbabwe. J Affect Disord. 2016;198:50-55. doi: 10.1016/j.jad.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. doi: 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 38.Rubin DBD. In: Rubin DB, ed. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons, Inc; 1987. [Google Scholar]
- 39.Simms V, Weiss HA, Chinoda S, et al. Peer-led counselling with problem discussion therapy for adolescents living with HIV in Zimbabwe: a cluster-randomised trial. PLoS Med. 2022;19(1):e1003887. doi: 10.1371/journal.pmed.1003887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas AD, Lienhard R, Didden C, et al. Mental health, ART adherence, and viral suppression among adolescents and adults living with HIV in South Africa: a cohort study. AIDS Behav. 2023;27:1849-1861. doi: 10.1007/s10461-022-03916-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safren SA, Otto MW, Worth JL. Life-steps: applying cognitive behavioral therapy to HIV medication adherence. Cogn Behav Pract. 1999;6(4):332-341. doi: 10.1016/S1077-7229(99)80052-2 [DOI] [Google Scholar]
- 42.Abas M, Nyamayaro P, Bere T, et al. Feasibility and acceptability of a task-shifted intervention to enhance adherence to HIV medication and improve depression in people living with HIV in Zimbabwe, a low income country in Sub-Saharan Africa. AIDS Behav. 2018;22:86-101. doi: 10.1007/s10461-016-1659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eMethods. Additional Information on Standard Care, Randomization, Adherence Measures, and Statistical Analyses
eTable 1. Effect of the Friendship Bench Intervention on Adherence, Viral Load, and Mental Health: Results From Prespecified and Post Hoc Sensitivity Intention-to-Treat Analyses
eTable 2. Effect of the Friendship Bench Intervention on Adherence, Viral Load, and Mental Health: Results From Prespecified and Post Hoc Sensitivity Per-Protocol Analyses
eTable 3. Number of Participants With Safety-Relevant Outcomes by Trial Group
eFigure. Forest Plot of Available Evidence of the Effect of the Friendship Bench Intervention on SSQ-14 Scores, PHQ-9 Scores, and Viral Suppression From 3 Trials
Nonauthor Collaborators
Data Sharing Statement


