Abstract
Paenibacillus polymyxa is a plant growth–promoting rhizobacteria (PGPR) that has significant biocontrol properties. Wheat sheath blight caused by Rhizoctonia cerealis is a significant soil–borne disease of wheat that causes significant losses in wheat production, and the biological control against the disease has received extensive attention. P. polymyxa ZYPP18 was identified using morphological and molecular characterization. An antagonistic activity experiment verified that ZYPP18 inhibits the growth of R. cerealis on artificial growth media. A detached leaf assay verified that ZYPP18 inhibits the expansion of wheat sheath blight on the detached leaf. ZYPP18 has been found to possess plant growth–promoting properties, as well as the ability to solubilize phosphate and generate indole–3–acetic acid. Results from hydroponic experiments showed that wheat seedlings treated with ZYPP18 grew faster. Additionally, pot experiments and field experiments demonstrated that ZYPP18 effectively controls the occurrence of wheat sheath blight. ZYPP18 reduced the incidence of wheat sheath blight in wheat seedlings by 37.37% and 37.90%, respectively. The control effect of ZYPP18 on wheat sheath blight was 56.30% and 65.57%, respectively. These findings provide evidence that P. polymyxa ZYPP18 is an effective biological factor that can control disease and promote plant growth.
Keywords: Paenibacillus polymyxa, plant growth promotion, antagonism, wheat sheath blight
1. Introduction
Plant growth–promoting rhizobacteria (PGPRs) have received increasing attention for their plant disease control ability and environmentally friendly potential [1,2]. PGPRs were verified to benefit plant root growth and produce antimicrobial substances, making them essential in the context of biocontrol [3]. PGPRs enhance plant growth through multiple direct or indirect mechanisms, including gibberellin, auxin, and indole acetic acid auxin synthesis, biological nitrogen fixation and phosphate dissolution [4,5], organic acid production and reduction of Ethylene content, and increased root density and length through 1–aminocyclopropane 1–carboxylate deaminase (ACC) production [6,7]. Numerous plant growth–promoting rhizobacteria (PGPR) are known to biosynthesize and exude defense–related metabolites that serve to trigger systemic resistance (ISR) within the host plant. In addition, PGPRs can suppress pathogen growth through multiple modes of action, including direct competition for nutrients and niches, enzyme lysis, antibiosis, and signal interference [8,9,10].
Wheat is one of the three major crops and is the main food staple [11]. Wheat sheath blight caused by Rhizoctonia cerealis is a significant soil–borne disease of wheat, which infects wheat roots and causes stems to shrink [12]. The predominant method of controlling soil–borne diseases, such as wheat sheath blight, typically involves the application of chemical fungicides, which may result in environmental pollution and the emergence of fungal resistance [13]. Biological control involves using antagonistic microbes and metabolites to control the disease, demonstrating excellent effects and impressive prospects [14].
The genus Paenibacillus is Gram–positive and sporulating. With over 150 species identified, this genus holds paramount importance in agriculture as PGPRs [15,16]. Paenibacillus polymyxa is one of the most critical species out of all of these species [17].
P. polymyxa has been reported to enhance plant growth and disease resistance through the biosynthesis and secretion of plant growth hormones, such as dissolved inorganic phosphates and indole–3–acetic acid (IAA) [15,18,19,20,21,22,23]. Many of the strains of P. polymyxa can directly inhibit the plant pathogen, thereby protecting the plant from pathogen infection, mainly due to the production of antibiotic compounds such as polymyxins, hydrolytic proteases with glycosyl groups, fusaricidin polypeptide antibiotics, and beta–glucans [24,25,26,27]. The valuable properties exhibited by P. polymyxa strains have garnered significant interest in their potential application for biofertilization and biocontrol development [28].
In this study, a novel strain of P. polymyxa was isolated and subjected to biocontrol assays. Results indicated that the strain exhibited significant plant growth–promoting effects on wheat and demonstrated potent biocontrol activity against wheat sheath blight disease. Collectively, these findings suggest that the identified P. polymyxa strain holds significant promise as a viable biocontrol agent in agricultural practices.
2. Results
2.1. Bacterium Identification
One isolate with a distinct inhibitory effect on R. cerealis was picked out for further study. The isolate formed light pale yellowish colonies with a thick central part surrounded by a glistening visible part on the LA plate (Figure 1A). The isolate was a Gram–positive and spore–forming bacterium and exhibited small rod–shaped structures typical of the genus Paenibacillus (Figure 1B,C). The isolate showed alignment as ZYPP18. The 16S rRNA, gyrA, and rpoB genes were cloned and sequenced. The partial sequences of the three genes were compared to the sequences using the BLAST search program in NCBI’s GenBank, which showed high correlations with the genes of the species belonging to Paenibacillus spp. Three phylogenetic trees were constructed using MEGA 11 with the partial sequences of 16S rRNA, gyrA, and rpoB of ZYPP18 and other typical strains of Paenibacillus spp. (Figure 1D–F).
Figure 1.
Bacterium identification of P. polymyxa ZYPP18. (A) Colony morphology of ZYPP18 on LA cultured for 3 day; (B) Gram stain; (C) Spore character; (D–F) Phylogenetic tree based on the 16S rRNA gene, gyrA gene, and rpoB gene. (D) 16S rRNA gene; (F) gyrA gene; (E) rpoB gene.
2.2. Antagonistic Activity
2.2.1. Inhibition Effect on R. cerealis
P. polymyxa ZYPP18 had strong antagonistic activity against R. cerealis, with an inhibition rate of 92.68% (Figure 2). The fermentation filtrate of ZYPP18 also inhibited the mycelial growth of R. cerealis (Figure 3). When treated with the fermentation filtrate of ZYPP18 cultured for 3 d, 4 d, and 5 d, at 1/2 concentration, the inhibition rates were 45.73%, 46.45%, and 48.90%, respectively (Figure 3A,B).
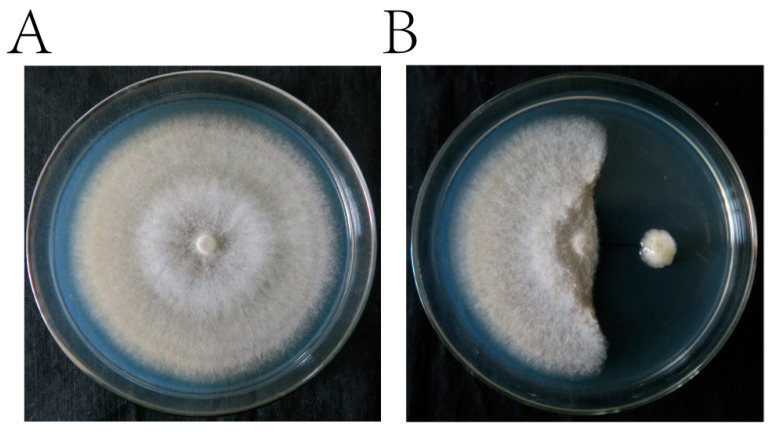
Figure 2.
Antagonistic activity of P. polymyxa ZYPP18 against R. cerealis. (A) R. cerealis cultured on PDA for 7 d; (B) R. cerealis and ZYPP18 were co–cultured on PDA for 7 d.
Figure 3.
The inhibition effect of the fermentation filtrate of P. polymyxa ZYPP18 on the growth of R. cerealis. (A) the growth of R. cerealis on the PDA plates (CK) and the plates of PDA mixed with different ratios of the fermentation filtrate of ZYPP18 cultured in LB for 3 d, 4 d, and 5 d; (B) inhibition rate. Bar diagrams represent the mean of three replicates. Error bars indicate the standard deviation (SD) of the three replicates. Different lowercase letters mean the fermentation filtrate of inhibition rate cultured for 3 d, 4 d, and 5 d there were significant differences (p < 0.05).
2.2.2. Disease Inhibition Effect on Detached Leaves
The experiment was conducted by inoculating R. cerealis on detached wheat seedling leaves sprayed with either sterile water or P. polymyxa ZYPP18. The control group treated with sterile water showed a complete susceptibility to R. cerealis, as evidenced by lesion expansion with an average length of 2.50 cm after 5 days of inoculation. In contrast, the group treated with P. polymyxa ZYPP18 exhibited mild to no infection by R. cerealis, with an average lesion length of only 0.075 cm after the same duration. This clearly indicates the remarkable inhibitory effect of P. polymyxa ZYPP18 against wheat sheath blight disease, as depicted in Figure 4.
Figure 4.
Inhibition effect of P. polymyxa ZYPP18 on wheat sheath blight expansion on detached leaves. (A) the disease expansion 5 d after the inoculation of R. cerealis on the detached leaves sprayed with sterile water (control) or a bacterial suspension of P. polymyxa ZYPP18 (ZYPP18); (B) the lesion length of wheat sheath blight on the detached wheat leaves. The line graph indicates the mean of the four replicates. Error bars indicate the standard deviation (SD) of the four replicates.
2.2.3. Detection of Genes Related to Antibacterial Substance Synthesis
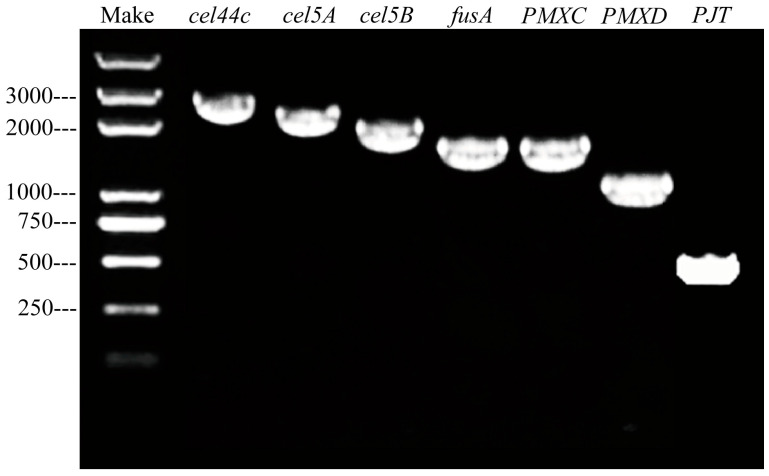
The partial fragmentation genes encoding the antibiotics polymyxin C (PMXC), polymyxin D (PMXD), Fusaricidins (fusA), β–glucanase (PJT), hydrolysis of proteases CEL44C–MAN26A (cel44C), and Cellulase A (cel5A) and Cellulase B (cel5B) were detected by PCR amplification (Figure 5). It indicates that B. polymyxa ZYPP18 possesses the genes related to antibacterial substance synthesis, implying the function of antibacterial substances in the biological effect.
Figure 5.
Detection of the genes related to antibacterial substance synthesis in P. polymyxa ZYPP18.
2.3. Plant Growth Promotion
2.3.1. Growth–Promoting Effect on Wheat Seedlings
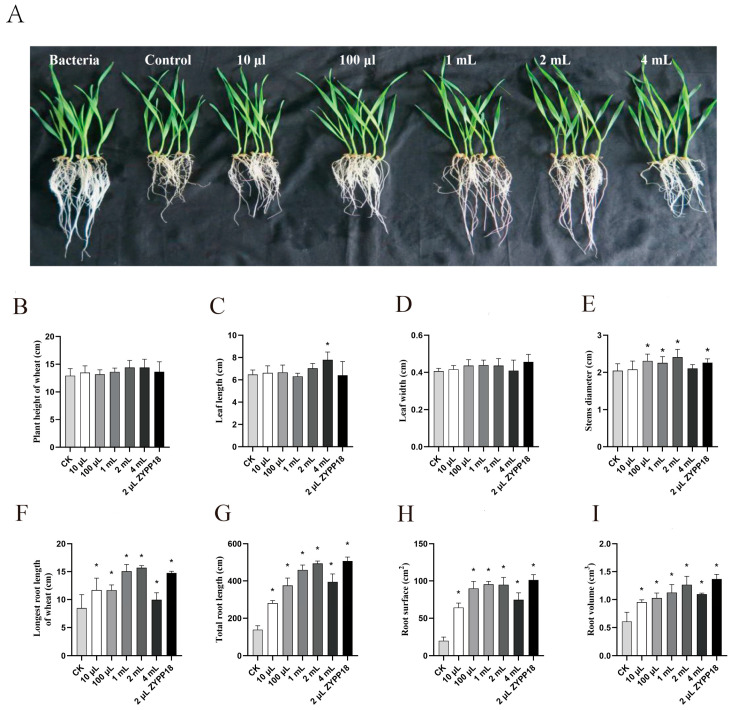
Wheat treated with a fermentation filtrate and bacteria of P. polymyxa ZYPP18 exhibited significant growth–promoting effects (Figure 6). ZYPP18 had discernible impacts on multiple phenotypic traits related to wheat seedling growth, including plant height, stem diameter, leaf length, and leaf width (Figure 6B–E). Our findings indicate that ZYPP18 did not elicit noticeable changes in wheat plant height. However, treatment with 4 mL of fermentation filtrate led to a significant increase in wheat leaf length, while no significant effects were observed in leaf width and the remaining parameters. Notably, treatments with 100 μL, 1 mL, 2 mL of fermentation filtrate, and 2 μL of bacterial suspension successfully promoted wheat stem diameter growth. The fermentation filtrate treatment and bacteria of ZYPP18 benefited the growth of the wheat root (Figure 6F–I). All the treatments with different volumes of fermentation filtrate and bacteria of ZYPP18 significantly promoted the growth of root length, root surface area, and root volume. As the volume of fermentation filtrate was increased, it was observed that the promoting effect on wheat roots also increased. Notably, a relatively weaker promoting effect was observed upon treatment with 4 mL of fermentation filtrate. It is speculated that the deleterious impact of fermentation filtrate on root growth may become more pronounced at higher volumes exceeding 4 mL. The findings of our study underscore the capacity of ZYPP18 to promote the growth of wheat, with a particular emphasis on root development.
Figure 6.
The growth–promoting effect of B. polymyxa ZYPP18 on wheat seedlings. (A) wheat seedlings grown in the Hoagland nutritional fluid with ZYPP18 or different contents of fermentation filtrate of ZYPP18; (B–I) the growth–promoting effect when treated with ZYPP18 or different contents of fermentation filtrate. (B) Longest root length of wheat, (C) Leaf length; (D) Leaf width; (E) Stems diameter; (F) Longest root length of wheat; (G) Total root length; (H) Root surface; (I) Root volume. Bar diagrams represent the mean of five replicates. Error bars indicate the standard deviation (SD) of the five replicates. Bar diagrams marked with “*” indicate significant differences between the treatment and CK (p < 0.05).
2.3.2. Phosphorus Solubilization Activity
As shown in Table 1, B. polymyxa ZYPP18 can dissolve phosphate, which could dissolve organic phosphorus or inorganic phosphorus. The ratio of the transparent circle to colony diameter of ZYPP18 (D/d) was 1.4 in the organic phosphorus medium and 1.6 in the inorganic medium (Table 1).
Table 1.
Phosphorus solubilization activity of B. polymyxa ZYPP18.
| Phosphorus | Colony Diameter of ZYPP18 (d) (mm) | Transparent Circle (D) (mm) | Transparent Circle/ZYPP18 Diameter (D/d) |
|---|---|---|---|
| Organic phosphorus | 8.3 ± 0.6 | 12.0 ± 0.0 | 1.4 ± 0.1 |
| Inorganic phosphorus | 13.3 ± 1.5 | 20.7 ± 0.6 | 1.6 ± 0.1 |
The enzyme activity results provide evidence of the phosphate–releasing ability of B. polymyxa ZYPP18 (Table 2). Phosphatase activity, including acid phosphatase, alkaline phosphatase, and neutral phosphatase, was detected in the cultures of ZYPP18 cultured for 3 d and 5 d.
Table 2.
Acid–base neutral phosphatase activity of B. polymyxa ZYPP18.
| Phosphatase | Phosphatase Activity of ZYPP18 Cultured for 3 d (mg/mL−1, 37 °C, 24 h) | Phosphatase Activity of ZYPP18 Cultured for 5 d (mg/mL−1, 37 °C, 24 h) |
|---|---|---|
| Acid phosphatase | 3.014 ± 0.004 | 1.305 ± 0.002 |
| Alkaline phosphatase | 13.846 ± 0.007 | 15.972 ± 0.002 |
| Neutral phosphatase | 0.473 ± 0.001 | 0.461 ± 0.003 |
Explanatory note: the data denote the mean ± SD (n = 3).
2.3.3. IAA Production
For L–Ser–inducing IAA production, the IAA contents tested were 3.66 mg/mL in the R2A medium with 200 mg/L L–tryptophan, 3.05 mg/mL in the LB medium with 200 mg/L L–tryptophan, and 2.74 mg/mL in LB (Figure 7A). The gene that encodes IAA in ZYPP18 was detected by PCR amplification, and a band of approximately 1800 bp was detected (Figure 7B). Therefore, B. polymyxa ZYPP18 possesses the IAA synthesis gene and can produce IAA.
Figure 7.
IAA detection in B. polymyxa ZYPP18. (A) Determination of the IAA content; (B) IAA–encoding gene detection. Bar diagrams represent the mean of three replicates. Error bars indicate the standard deviation (SD) of the three replicates. Bars diagrams marked with “*” indicate significant differences between different culture mediums (p < 0.05).
2.4. Control Effect of ZYPP18 on Wheat Sheath Blight in Pot and Field Experiments
The pot experiment showed that the disease incidence of wheat seedlings treated with R. cerealis was 36.90%, while it was 23.00% when treated with R. cerealis and ZYPP18 (Figure 8A). Inoculation with ZYPP18 reduced the incidence of wheat sheath blight in wheat seedlings by 37.37%. The disease index of wheat seedlings treated only with R. cerealis was determined to be 40.11, whereas the application of ZYPP18 in conjunction with R. cerealis significantly decreased the disease index to 17.53 (Figure 8B). The observed control efficacy of ZYPP18 against wheat sheath blight was estimated to be 56.30% (Table 3).
Figure 8.
Disease incidence and disease index of wheat sheath blight in pot and field experiments. (A) Disease incidence in pot experiment; (B) Disease index in pot experiment; (C) Disease incidence in field experiment; (D) Disease index in field experiment. Bar diagrams represent the mean of three replicates. Error bars indicate the standard deviation (SD) of the three replicates. Bars diagrams marked with “*” indicate significant differences between treatments and CK or between treatments (p < 0.05).
Table 3.
Control effect of B. polymyxa ZYPP18 on wheat sheath blight.
| Experiments | Control Effect (%) |
|---|---|
| Pot experiments | 56.30 ± 0.55 |
| Field experiments | 65.57 ± 0.22 |
Explanatory note: the data denote the mean ± SD (n = 3).
The field experiment showed that the disease incidence of wheat seedlings treated with R. cerealis was 61.69%, while it was 38.31% when treated with R. cerealis and ZYPP18 (Figure 8C). Inoculation with ZYPP18 reduced the incidence of wheat sheath blight in wheat seedlings by 37.90%. The disease index of wheat seedlings treated with R. cerealis was 59.75, while it was 20.57 when treated with R. cerealis and ZYPP18 (Figure 8D). The control effect of ZYPP18 on wheat sheath blight was 65.57% (Table 3).
3. Discussion
Biological control represents an efficacious method for managing plant diseases, which leverages the capabilities of beneficial microorganisms and biological metabolites to suppress plant pathogens, improve plant immunity, and modify the plant growth environment [29,30,31]. In this study, P. polymyxa ZYPP18 was verified as being such a microorganism, possessing the dual capacity of producing antibiotic substances and promoting plant growth.
P. polymyxa is widely isolated from the rhizosphere soils of many plants, including crops such as wheat [32] and rice [33]; vegetables such as tomatoes [34], beans [35], peppers, and cucumber beans [36]; and other plants such as maize [37], sunflower [38], and Dendrobium [39]. B. polymyxa has been demonstrated to possess the ability to suppress numerous plant diseases [40]. For example, P. polymyxa HX–140 inhibits the growth of the pathogen of cucumber wilt disease [41]. In addition, P. polymyxa Nl4 effectively controlled pear Valsa canker caused by Valsa pyri [42], while P. polymyxa Y–1 controlled rice bacterial disease [43]. To date, there have been no studies reporting on the utilization of P. polymyxa strains for the purpose of biological control of wheat sheath blight. In this study, ZYPP18, a biocontrol bacterium isolated and screened from the rhizosphere soil of healthy tobacco, had an obvious inhibitory effect on wheat sheath wilt through plant tests, including an in vitro leaf experiment, a pot experiment, and a field experiment. The field control effect of ZYPP18 on wheat sheath blight was 65.57%. Therefore, it can be exploited as an environmentally biological agent or microbial–friendlier alternative to chemical fertilizers.
P. polymyxa is the main microbial population for the biological control of plant diseases, owing to its notable features such as high stress resistance, production of a variety of lipopeptide antibiotics, and broad bacteriostatic spectrum [23,24,25,27]. We revealed that P. polymyxa ZYPP18 had strong antagonistic activity against R. cerealis with an inhibition rate of 92.68%. The fermentation filtrate of ZYPP18 also inhibited the mycelial growth of R. cerealis. Several genes related to antibacterial substance synthesis were detected in ZYPP18, including the genes encoding the antibiotics Polymyxin, Fusaricidins, β–glucanase, hydrolysis of proteases CEL44C–MAN26A, and cellulase. This indicates that P. polymyxa ZYPP18 has multiple disease–resistant mechanisms.
IAA plays a crucial role in the establishment of a stable seedling root system by promoting the development of secondary roots. Conversely, bacteria with the function of solubilizing phosphorus secrete organic acids and some metal ions to accrete and convert the insoluble phosphorus in the soil into soluble phosphorus, which can be absorbed by plants and promotes plant growth. Notably, genome analysis has revealed that the majority of P. Polymyxa strains harbor genes involved in gluconic acid synthesis and that encode glucose–1–dehydrogenase and gluconate dehydrogenase, which aid in the production of gluconate–dissolved phosphorus [28]. Weselowski [28] reported that P. polymyxa CR1 could dissolve inorganic phosphates. Moreover, P. polymyxa harbors a gene responsible for the synthesis of IAA precursors and which encodes aminotrans–ferase L–tryptophan for oxidative deamination to indole–3–pyruvate (IPA), a crucial intermediate for IAA biosynthesis [44,45]. In this regard, ZYPP18 exhibited proficient IAA production and decarboxylation of phosphorus. Notably, the promoting effect of the ZYPP18 fermentation filtrate on wheat growth was positively correlated with the concentration of filtrate. However, excess filtrate concentrations compromised wheat growth, possibly due to high IAA concentration–induced growth inhibition. Thus, an optimal filtrate concentration is pivotal for obtaining the maximum promotive potential of ZYPP18 on wheat growth.
4. Materials and Methods
4.1. Isolation and Identification of the Strain
Soil samples were collected from a tobacco field in Zhucheng City, Shandong Province, China. Dilution plate techniques were employed to isolate the bacteria. Soil samples were serially diluted with ten–fold dilutions up to 10−5 in sterile water. A total of 100 μL of soil diluent were plated on LA (10 g tryptone, 5 g yeast powder, 10 g NaCl, 15 g agar, and 1000 mL deionized water) plates and cultured at 28 °C. The preferred isolates were picked out and cultured on LA plates to observe their morphological characteristics.
The bacteria cultured in LB were collected, and genomic DNA was extracted using an OMEGA bacterial DNA kit for amplified 16S rRNA. The 16S rRNA, gyrA gene, and rpoB gene fragments were PCR amplified using universal bacterial primers. The primers for the 16S rRNA gene were 27f (5′–AGAGTTTGATCCTGGCTCAG–3′) and 1492r (5′–CGGTGTGTACAAGGCCC–3′) [46]; the primer pair for gyrA1 was gyrA–F (5′–CAGTCAGGAAATGCGTACGTCCTT–3′) and gyrA–R (5′–CAAGGTAATGCTCCAGGCATTGCT–3′); and the primer pair for rpoB was rpoB_2292f–F (5′–GACGTGGGATGGCTACAACT–3′) and rpoB_3354r (5′–ATTGTCGCCTTTAACGATGG–3′) [47]. The 16S rRNA, gyrA gene, and rpoB gene sequences obtained were compared to the sequences using the BLAST search program in NCBI’s GenBank (https://www.ncbi.nlm.nih.gov/) (accessed on 5 January 2022). The phylogenetic tree was constructed using MEGA 11, and Maximum Likelihood was used [48,49].
4.2. In Vitro Antagonism Test against R. cerealis
4.2.1. Antimicrobial Activity of Antagonistic Bacteria
The strain of R. cerealis was isolated from the samples of wheat sheath blight and kept in the Key Laboratory of Agricultural Microbiology, Shandong Agricultural University. A 5 mm dish of R. cerealis was placed onto the center of a PDA plate, and then 2 μL of bacterial suspension (≈1 × 108 CFU/mL) were dropped onto the same petri dish 2 cm away from the dish of R. cerealis, with the one without drops of bacterial suspension used as a control. Treatments and controls were replicated three times. The plates were incubated at 28 °C. When the colony of the control was just full–grown on the PDA plate, then the fungal colony radius was measured. The inhibition rate was calculated as follows. Inhibition rate (%) = (radius of control fungal colony − radius of treated fungal colony)/radius of control fungal colony × 100%.
4.2.2. Antimicrobial Activity of Fermentation Filtrate
The antagonistic bacteria were cultured in 150 mL LB media at 28 °C and 150 rpm for 3, 4, and 5 days. The fermentation filtrate was obtained by filtration with a 0.22 μm sterile membrane. The PDA (200 g potato, 15 g glucose, 18 g agar, and 1000 mL water) and 2 × PDA (200 g potato, 15 g glucose, 18 g agar, and 500 mL water) were prepared. The fermentation filtrate was added to the 2 × PDA media with a volume ratio of 1:1, 1:3, 1:5, and 1:9, and replenished to the same volume with sterile water. A 5 mm dish of R. cerealis was placed onto the center of a PDA plate with different concentrations of fermentation filtrate and cultured at 28 °C. The fungal dish incubated onto the PDA medium without the fermentation filtrate was treated as a control. Treatments and controls were replicated three times. The diameter of the fungal colony was measured when the colony of the control was just full–grown on the PDA plate, and the inhibition rate was calculated using the following formula. Inhibition rate (%) = (diameter of control fungal colony − diameter of treated fungal colony)/diameter of control fungal colony × 100%.
4.2.3. Detached Leaves Experiment
The detached leaves of wheat seedlings were used to determine the effect of the bacteria on wheat sheath blight by measuring the disease expansion on the leaf surface. A 5 cm length of leaf from the second wheat leaf of the seedling was cut and sterilized with alcohol. The bacteria were grown in LB at 28 °C at 180 r/min for 24 h, and the bacterial suspension was collected. The pellet was resuspended in sterile water and adjusted to 1 × 108 CFU/mL. The bacterial suspension was sprayed on the detached leaf, then a 5 mm fungal dish of R. cerealis was inoculated on the center of the leaf surface. Next, the leaves were incubated in Petri dishes at 25 °C, and the disease lesion was measured daily. The detached leaves sprayed with sterile water were treated as a control. Treatments and controls were replicated four times.
4.2.4. Detection of the Genes Related to Antimicrobial Substance Synthesis
The bacteria cultured in LB were collected, and genomic DNA was extracted using an OMEGA bacterial DNA extraction kit according to the manufacturer’s instructions. The primers for detection of the genes related to antibacterial substance production, including Polymyxin C, Polymyxin D, Fusaricidins, and hydrolysis of proteases, β–glucanase, and cellulases, were designed using Primer 5.0 [50]. All the primers are listed in Table 4. PCR was performed using the thermal cycle to detect the genes associated with antimicrobial substance synthesis.
Table 4.
Primers used for the detection of the genes related to antimicrobial substance synthesis.
| Antibiotic | Primer | Sequence 5′–3′ |
|---|---|---|
| Polymyxin C | PMXC–F | TACACGGTCTTTGTGGTCATC |
| PMXC–R | GCGTACAGTCCCTTCATCTTC | |
| Polymyxin D | PMXD–F | CTCGCAGGTTTACTTCGTTT |
| PMXD–R | CCAATGCTGGGATTCGTTAT | |
| Fusaricidins | fusA–F | CAAGGATTCGACCGTAGGTG |
| fusA–R | GTAGGGATTATGGCTGACCG | |
| Hydrolysis of proteases | cel44C–F | TTTGGTTACCGCATGGGGTG |
| cel44C–R | TTTCGGACGGAGAGGAGAGTGT | |
| β–Glucanase | PJT–F | TACTAATTGCTCGTATATTTTACCCA |
| PJT–R | TTGCGAATGTGTTCTGGGAACC | |
| Cellulase A | cel5A–F | CTGCTCAACCTGGTCAACG |
| cel5A–R | GCTCAAGGGCATTAGTTCTC | |
| Cellulase B | cel5B–F | CTTGCTGTTGGCATTGAGC |
| cel5B–R | CCTTTGCGAATCCATCTTTC |
4.3. Growth–Promoting Properties Test
4.3.1. Plant Growth–Promoting Experiments
A plant growth–promoting experiment was performed with a hydroponic experiment. The wheat seeds were sterilized for 10 min with 1% NaClO, washed three times with sterile water, and incubated at 25 °C for germination. When the seeds germinated and grew to 1 cm high, the seedlings were transplanted into pots with 500 mL Hoagland nutrient solution (945 mL Ca(NO3)2·4H20, 607 mL KNO3, 115 mL NH4HPO4, 493 mL MgSO4·7H2O, 2.5 mL Fe–citrate, and 5 mL micro–elements). Next, 10 μL, 100 μL, 1 mL, 2 mL, and 4 mL of fermentation filtrate and 2 μL of bacteria were inoculated in the nutrient solution, respectively. Ten wheat seedlings were planted in each pot, and three pots were treated as treatments and incubated in a light incubator with a 14 h light/10 h dark cycle at 25 °C. The seedlings’ height, total root length, leaf length, and leaf width were measured using a ruler. The total root length, root volume, and root surface area were measured using a root scanner (WinRHIZO, AgriPheno, Shanghai, China).
4.3.2. Phosphate Solubilization Assay
For detection of the phosphorus solubilization activity, the bacteria were cultured on the Pikovskaya (PVK) inorganic phosphorus medium and the Mongina medium for 7 d at 28 °C.
The PVK and Mongina mediums were prepared as follows: PVK medium, 10 g glucose, 0.5 g (NH4)2SO4, 0.2 g NaCl, 0.2 g KCl, 0.03 g MgSO4·7H2O, 0.03 g MnSO4, 0.003 g FeSO4, 0.5 g yeast extract, 10 g agar, 1000 mL distilled water, and pH = 7.0; Ca3(PO4)2 was sieved, sterilized separately, and then mixed with the medium; Mongina medium, 10 g glucose, 0.5 g (NH4)2SO4, 0.3 g MgSO4·7H2O, 0.3 g NaCl, 0.3 g KCl, 0.36 g FeSO4·7H2O, 0.03 g MnSO4·H2O, and 5 g CaCO3, 18 g agar.
The bacteria were cultured in the Mongina medium for 3 d and 5 d at 28 °C, 150 r/min. Determination of bacterial phosphatase activity was measured spectrophotometrically by the disodium phenyl phosphate method of Li [51].
The bacteria were incubated in the acidic acetic acid–sodium acetate buffer with pH 4.6, pH 7.0, and pH 9 for 24 h; then the OD value was measured at OD570. The activity was calculated by calculating the amount of phenol production in 1 mL of bacterial solution after 24 h. All assays were carried out in triplicate.
4.3.3. IAA Production Assay
The bacteria were cultured in R2A and LB with 200 mg/L L–tryptophan at 28 °C for 3 days, and then IAA quantification was carried out using the Salkowski method [52]. The bacteria cultured in LB IAA were quantified as well. The non–inoculated mediums were conducted at 28 °C for 3 days and used as negative controls. The bacteria were centrifuged at 10,000 r/min, and then the supernatant was mixed with the Salkowski reagent. The optical density was measured at 530 nm. The experiment was repeated thrice. A standard curve was generated with the optical densities for 0, 5, 10, 20, 40, and 60 mg/L of indole acetic acid.
4.3.4. IAA Gene Detection
The bacteria were cultured in LB, and the genomic DNA was extracted as mentioned above. The gene encoding IAA was detected by PCR amplification using primers of primer1 (GGGAATTCTTACTCGTCCCCCATCAGC) and primer2 (CTCGGATCCCCAATGAGTGCACAAATTCC) [21].
4.4. In Vivo Plant Experiment
4.4.1. Pot Experiment
Sterilized wheat seeds were inoculated with 5 fungal disks of R. cerealis and cultured at 28 °C for 20 d to obtain the inoculant of the wheat seeds. The wheat seeds were sterilized for 10 min with 1% NaClO, washed three times with sterile water, and incubated at 25 °C for germination. When the seeds germinated and grew to 1 cm high, the seedlings were transplanted into the pots. Twenty wheat seedlings were planted in each pot, and three pots were treated as treatments and incubated at 25 °C; 20 days after, 100 mL sterile water with 500 μL bacteria (about 1 × 108 CFU/mL) were inoculated into the soil in the pot. The wheat seed inoculants were inoculated near the wheat roots. No–bacterial treatment and inoculating with inactivated wheat seed inoculant served as controls. Twenty days after, the disease incidence and disease index were investigated.
The disease incidence of wheat was investigated according to the method of Youssef [53]. Disease incidence = (number of diseased plants/total number of investigated plants) × 100%; Disease index = Σ (number of diseased plants at all levels × representative of all levels)/(total number of plants under investigation × the highest level of representative value) × 100; Control effect = (control disease index − treatment of disease index)/control disease index × 100%.
4.4.2. Field Experiment
The field plot employed in the experiment was 1.5 m wide and 5 m long. Wheat seeds were sowed in soil with a 4–5 cm depth and six rows were planted. Each treatment was replicated three times. Two days after sowing, 1mL bacteria were inoculated into the soil (about 1 × 108 CFU/mL) per row. Twenty days after, 10 g of wheat seed inoculant were inoculated in each row. No–bacterial treatment and inoculating with inactivated wheat seed inoculant served as controls. After 20 d of inoculation with the wheat seed inoculant, the diseases were surveyed as per the methods in the pot experiment.
4.5. Statistical Analysis
GraphPad Prism 8 software was used for data collation and graphing. One–way ANOVA (p < 0.05) was performed using IBM SPSS Statistics 20 software, and Duncan’s and Dunnett’s T3 methods were used for multiple comparisons. Marking of significant differences.
Author Contributions
Conceptualization, Z.L.; Data curation, C.P.; Formal analysis, S.M. and W.W.; Validation, X.L. and Y.M.; Writing—review & editing, C.L. and S.F. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Natural Science Foundation of China, grant number 31770685, 31270687 and Shandong Agricultural Science and Technology Fund Project, grant number 2019LY003.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rai S., Omar A.F., Rehan M., Al–Turki A., Sagar A., Ilyas N., Sayyed R.Z., Hasanuzzaman M. Crop microbiome: Their role and advances in molecular and omic techniques for the sustenance of agriculture. Planta. 2022;257:27. doi: 10.1007/s00425-022-04052-5. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya P.N., Jha D.K. Plant growth–promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 3.Bhat M.A., Mishra A.K., Jan S., Bhat M.A., Kamal M.A., Rahman S., Shah A.A., Jan A.T. Plant Growth Promoting Rhizobacteria in Plant Health: A Perspective Study of the Underground Interaction. Plants. 2023;12:629. doi: 10.3390/plants12030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Andrade L.A., Santos C.H.B., Frezarin E.T., Sales L.R., Rigobelo E.C. Plant Growth–Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms. 2023;11:1088. doi: 10.3390/microorganisms11041088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez H., Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999;17:319–339. doi: 10.1016/S0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 6.Spaepen S., Vanderleyden J., Remans R. Indole–3–acetic acid in microbial and microorganism–plant signaling. Fems Microbiol. Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 7.Glick B.R., Cheng Z., Czarny J., Duan J. Promotion of plant growth by ACC deaminase–producing soil bacteria. New Perspect. Approaches Plant Growth–Promot. Rhizobacteria Res. 2007;119:329–339. doi: 10.1007/s10658-007-9162-4. [DOI] [Google Scholar]
- 8.Compant S., Duffy B., Nowak J., Clément C., Barka E.A. Use of plant growth–promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005;71:4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lugtenberg B., Kamilova F. Plant–growth–promoting rhizobacteria. Annu. Rev. Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- 10.Beneduzi A., Ambrosini A., Passaglia L.M.P. Plant growth–promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012;35:1044–1051. doi: 10.1590/S1415-47572012000600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hlaváčová M., Klem K., Rapantová B., Novotná K., Urban O., Hlavinka P., Trnka M. Interactive effects of high temperature and drought stress during stem elongation, anthesis and early grain filling on the yield formation and photosynthesis of winter wheat. Field Crops Res. 2018;221:182–195. doi: 10.1016/j.fcr.2018.02.022. [DOI] [Google Scholar]
- 12.Hamada M.S., Yin Y., Chen H., Ma Z. The escalating threat of Rhizoctonia cerealis, the causal agent of sharp eyespot in wheat. Pest Manag. Sci. 2011;67:1411–1419. doi: 10.1002/ps.2236. [DOI] [PubMed] [Google Scholar]
- 13.McMillan V.E., Canning G., Moughan J., White R.P., Gutteridge R.J., Hammond–Kosack K.E. Exploring the resilience of wheat crops grown in short rotations through minimising the build–up of an important soil–borne fungal pathogen. Sci. Rep. 2018;8:9550. doi: 10.1038/s41598-018-25511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He D.C., He M.H., Amalin D.M., Liu W., Alvindia D.G., Zhan J. Biological control of plant diseases: An evolutionary and eco–economic consideration. Pathogens. 2021;10:1311. doi: 10.3390/pathogens10101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand R., Grayston S., Chanway C. N–2–fixation and seedling growth promotion of lodgepole pine by endophytic Paenibacillus polymyxa. Microb. Ecol. 2013;66:369–374. doi: 10.1007/s00248-013-0196-1. [DOI] [PubMed] [Google Scholar]
- 16.Grady E.N., MacDonald J., Liu L., Richman A., Yuan Z.C. Current knowledge and perspectives of Paenibacillus: A Review. Microb. Cell Factories. 2016;15:203. doi: 10.1186/s12934-016-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langendries S., Goormachtig S. Paenibacillus Polymyxa, a jack of all trades. Environ. Microbiol. 2021;23:5659–5669. doi: 10.1111/1462-2920.15450. [DOI] [PubMed] [Google Scholar]
- 18.Lee B., Farag M.A., Park H.B., Kloepper J.W., Lee S.H., Ryu C.M. Induced resistance by a long–chain bacterial volatile: Elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS ONE. 2012;7:e48744. doi: 10.1371/journal.pone.0048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaheen M., Li J., Ross A.C., Vederas J.C., Jensen S.E. Paenibacillus polymyxa PKB1 produces variants of polymyxin B–Type antibiotics. Chem. Biol. 2011;18:1640–1648. doi: 10.1016/j.chembiol.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Kim J.F., Jeong H., Park S.Y., Kim S.B., Park Y.K., Choi S.K., Ryu C.M., Hur C.G., Ghim S.Y., Oh T.K., et al. Genome sequence of the polymyxin–producing plant–probiotic rhizobacterium Paenibacillus polymyxa E681. J. Bacteriol. 2010;192:6103–6104. doi: 10.1128/JB.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eastman A.W., Heinrichs D.E., Yuan Z.C. Comparative and genetic analysis of the four sequenced Paenibacillus polymyxa genomes reveals a diverse metabolism and conservation of genes relevant to plant–growth promotion and competitiveness. BMC Genom. 2014;15:851. doi: 10.1186/1471-2164-15-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H., Wang J., Sun H., Han X., Peng Y., Liu J., Liu K., Ding Y., Wang C., Du B. Transcriptome profilesreveal the growth–promoting mechanisms of Paenibacillus Polymyxa YC0136 on tobacco (Nicotiana Tabacum L.) Front. Microbiol. 2020;11:584174. doi: 10.3389/fmicb.2020.584174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogbe A.A., Gupta S., Stirk W.A., Finnie J.F., Van Staden J. Growth–Promoting Characteristics of Fungal and Bacterial Endophytes Isolated from a Drought–Tolerant Mint Species Endostemon obtusifolius (E. Mey. ex Benth.) NE. Br. Plants. 2023;12:638. doi: 10.3390/plants12030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Z., Kisla D., Zhang L., Yuan C., Green–Church K.B., Yousef A.E. Isolation and identification of a Paenibacillus polymyxa strain that coproduces a novel lantibiotic and polymyxin. Appl. Environ. Microbiol. 2007;73:168–178. doi: 10.1128/AEM.02023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raza W., Yang X., Wu H., Wang Y., Xu Y., Shen Q. Isolation and characterisation of fusaricidin–type compound–producing strain of Paenibacillus polymyxa SQR–21 active against Fusarium oxysporum f.sp. nevium. Eur. J. Plant Pathol. 2009;125:471–483. doi: 10.1007/s10658-009-9496-1. [DOI] [Google Scholar]
- 26.Cho K.M., Hong S.Y., Lee S.M., Kim Y.H., Kahng G.G., Kim H., Yun H.D. A cel44C–man26A gene of endophytic Paenibacillus polymyxa GS01 has multi–glycosyl hydrolases in two catalytic domains. Appl. Microbiol. Biotechnol. 2006;73:618–630. doi: 10.1007/s00253-006-0523-2. [DOI] [PubMed] [Google Scholar]
- 27.Martin N.I., Hu H., Moake M.M., Churey J.J., Whittal R., Worobo R.W., Vederas J.C. Isolation, structural characterization, and properties of mattacin (polymyxin M), a cyclic peptide antibiotic produced by Paenibacillus kobensis M. J. Biol. Chem. 2003;278:13124–13132. doi: 10.1074/jbc.M212364200. [DOI] [PubMed] [Google Scholar]
- 28.Weselowski B., Nathoo N., Eastman A.W., MacDonald J., Yuan Z.C. Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production. BMC Microbiol. 2016;16:244. doi: 10.1186/s12866-016-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Souza Vandenberghe L.P., Garcia L.M.B., Rodrigues C., Camara M.C., de Melo Pereira G.V., de Oliveira J., Soccol C.R. Potential applications of plant probiotic microorganisms in agriculture and forestry. AIMS Microbiol. 2017;3:629–648. doi: 10.3934/microbiol.2017.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burketova L., Trda L., Ott P.G., Valentova O. Bio–based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015;33:994–1004. doi: 10.1016/j.biotechadv.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Li J., Zhao Q., Wuriyanghan H., Yang C. Biocontrol Bacteria Strains Y4 and Y8 Alleviate Tobacco Bacterial Wilt Disease by Altering Their Rhizosphere Soil Bacteria Community. Rhizosphere. 2021;19:100390. doi: 10.1016/j.rhisph.2021.100390. [DOI] [Google Scholar]
- 32.Mohite B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013;13:638–649. doi: 10.4067/S0718-95162013005000051. [DOI] [Google Scholar]
- 33.Bal H.B., Das S., Dangar T.K., Adhya T.K. ACC deaminase and IAA producing growth promoting bacteria from the rhizosphere soil of tropical rice plants. J. Basic Microbiol. 2013;53:972–984. doi: 10.1002/jobm.201200445. [DOI] [PubMed] [Google Scholar]
- 34.Khan Z., Son S.H., Akhtar J., Gautam N.K., Kim Y.H. Plant growth–promoting rhizobacterium (Paenibacillus polymyxa) induced systemic resistance in tomato (Lycopersicon esculentum) against root–knot nematode (Meloidogyne incognita) Indian J. Agric. Sci. 2012;82:603–607. [Google Scholar]
- 35.Mohamed I., Eid K.E., Abbas M.H.H., Salem A.A., Ahmed N., Ali M., Shah G.M., Fang C. Use of plant growth promoting Rhizobacteria (PGPR) and mycorrhizae to improve the growth and nutrient utilization of common bean in a soil infected with white rot fungi. Ecotoxicol. Environ. Saf. 2019;171:539–548. doi: 10.1016/j.ecoenv.2018.12.100. [DOI] [PubMed] [Google Scholar]
- 36.Han H.S., Supanjani, Lee K.D. Effect of co–inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ. 2006;52:130–136. doi: 10.17221/3356-PSE. [DOI] [Google Scholar]
- 37.Chen B., Han H., Hou J., Bao F., Tan H., Lou X., Wang G., Zhao F. Control of Maize Sheath Blight and Elicit Induced Systemic Resistance Using Paenibacillus polymyxa Strain SF05. Microorganisms. 2022;10:1318. doi: 10.3390/microorganisms10071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan N., Zandi P., Ali S., Mehmood A., Adnan S.M. Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Front. Microbiol. 2018;9:2507. doi: 10.3389/fmicb.2018.02507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi W., Chen C., Gan X. Active metabolites from the endophyte Paenibacillus polymyxa Y–1 of Dendrobium nobile for the control of rice bacterial diseases. Front. Chem. 2022;10:879724. doi: 10.3389/fchem.2022.879724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daud N.S., Rosli M.A., Azam Z.M., Othman N.Z., Sarmidi M.R. Paenibacillus polymyxa bioactive compounds for agricultural and biotechnological applications. Biocatal. Agric. Biotechnol. 2019;18:101092. doi: 10.1016/j.bcab.2019.101092. [DOI] [Google Scholar]
- 41.Zhai Y., Zhu J.X., Tan T.M., Xu J.P., Shen A.R., Yang X.B., Li J.L., Zeng L.B., Wei L. Isolation and characterization of antagonistic Paenibacillus polymyxa HX–140 and its biocontrol potential against Fusarium wilt of cucumber seedlings. BMC Microbiol. 2021;21:75. doi: 10.1186/s12866-021-02131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan H., Yuan M., Shi B., Wang Z., Huang T., Qin G., Hou H., Wang L., Tu H. Biocontrol activity and action mechanism of Paenibacillus polymyxa strain Nl4 against pear Valsa canker caused by Valsa pyri. Front. Microbiol. 2022;13:950742. doi: 10.3389/fmicb.2022.950742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi W., Chen C., Gan X. Polymyxin B1 and E2 from Paenibacillus polymyxa Y–1 for controlling rice bacterial disease. Front. Cell. Infect. Microbiol. 2022;28:866357. doi: 10.3389/fcimb.2022.866357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phi Q.T., Park Y.M., Ryu C.M., Park S.H., Ghim S.Y. Functional identification and expression of indole–3–pyruvate decarboxylase from Paenibacillus polymyxa E681. J. Microbiol. Biotechnol. 2008;18:1235–1244. [PubMed] [Google Scholar]
- 45.Sun H., Zhang J., Liu W., E W., Wang X., Li H., Cui Y., Zhao D., Liu K., Du B., et al. Identification and combinatorial engineering of indole–3–acetic acid synthetic pathways in Paenibacillus polymyxa. Biotechnol. Biofuels Bioprod. 2022;15:81. doi: 10.1186/s13068-022-02181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank J.A., Reich C.I., Sharma S., Weisbaum J.S., Wilson B.A., Olsen G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008;74:2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rooney A.P., Price N.P., Ehrhardt C., Swezey J.L., Bannan J.D. Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. Int. J. Syst. Evol. Microbiol. 2009;59:2429–2436. doi: 10.1099/ijs.0.009126-0. [DOI] [PubMed] [Google Scholar]
- 48.Tamura K., Stecher G., Kumar S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen X.X., Li Y., Hittinger C.T., Chen X.X., Rokas A. An investigation of irreproducibility in maximum likelihood phylogenetic inference. Nat. Commun. 2020;11:6096. doi: 10.1038/s41467-020-20005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lalitha S. Biotech Software and Internet Report. Volume 1. Carnegie Institution for Science; Washington, DC, USA: 2000. Primer Premier 5; pp. 270–272. [DOI] [Google Scholar]
- 51.Li F.D., Yu Z.N., He S.J. Experimental Techniques in Agricultural Microbiology. Chinese Agricultural Press; Beijing, China: 1996. pp. 137–139. [Google Scholar]
- 52.Reasoner D.J., Geldreich E.E. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L., Guo N., Feng Y., Duan M., Li C. Effect of Piriformospora indica–induced systemic resistance and basal immunity against Rhizoctonia cerealis and Fusarium graminearum in wheat. Front. Plant Sci. 2022;13:836940. doi: 10.3389/fpls.2022.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.