Abstract
Background
Recurrent gliomas are therapeutically challenging diseases with few treatment options available. One area of potential therapeutic vulnerability is the presence of targetable oncogenic fusion proteins.
Methods
To better understand the clinical benefit of routinely testing for fusion proteins in adult glioma patients, we performed a retrospective review of 647 adult patients with glioma who underwent surgical resection at our center between August 2017 and May 2021 and whose tumors were analyzed with an in-house fusion transcript panel.
Results
Fifty-two patients (8%) were found to harbor a potentially targetable fusion with 11 (21%) of these patients receiving treatment with a fusion-targeted inhibitor. The targetable genes found to be involved in a fusion included FGFR3, MET, EGFR, NTRK1, NTRK2, BRAF, ROS1, and PIK3CA.
Conclusions
This analysis demonstrates that routine clinical testing for gene fusions identifies a diverse repertoire of potential therapeutic targets in adult patients with glioma and can offer rational therapeutic options for patients with recurrent disease.
Keywords: adult gliomas, fusion proteins, targeted therapies
Recurrence of adult glioma following radiation and alkylating chemotherapy represents a significant therapeutic challenge. Re-operation, re-irradiation, and/or retreatment with chemotherapy agents such as temozolomide and lomustine may provide benefits for select patients with progressive disease, but survival outcomes remain poor.1–4 Robust clinical development programs to identify new agents and targets, including immune, cellular, and targeted therapies, have lagged with no new regulatory approvals since bevacizumab in 2009. Novel effective treatments for recurrent glioma are desperately needed.
In addition to their importance for making integrated brain tumor diagnoses, oncogenic fusions represent unique therapeutic vulnerabilities in a subset of patients with glioma.5,6 Fusion proteins are drivers of malignant growth that often function through the inhibition of tumor suppressor genes or activation of oncogenes promoting aberrant cellular behavior.7 Testing for such fusions is routinely performed in other malignancies, and fusion inhibitors are mainstays of the therapeutic arsenal in lung cancer, bladder cancer, and sarcomas.8–10 The recent pace of development of fusion inhibitors is remarkable with multiple new drugs approved by the US Food and Drug Administration (FDA) over the past several years.10–13 Adult gliomas can also harbor fusion proteins, and there is growing clinical experience using FDA-approved or off-label fusion inhibitors as effective therapies for these patients.14–19
The University of Pennsylvania Health System has been routinely evaluating surgically resected brain tumors with an RNA-based fusion transcript panel (FTP) for the detection of fusion transcripts and oncogenic isoforms since 2017. The use of transcript panels has identified interesting and clinically beneficial therapeutic targets at a notable rate. To better characterize the fusions detected by this panel and its clinical utility, we undertook a retrospective analysis of our electronic medical record system (EMR), identifying all patients for whom a resected glioma was analyzed with the FTP since its inception. Here we report the results of the FTP testing in a large cohort of glioma patients and highlight the role the assay has played in guiding clinical management. These results add significantly to the best of our knowledge of the frequency and diversity of fusions with potential clinical relevance in patients with glioma and underscore the need for dedicated clinical trials of therapies targeted against the most common glioma-associated fusions.
Methods
Fusions were identified using a customized Archer FusionPlex panel for detection of gene fusions using next-generation sequencing (ArcherDx, Boulder, CO). This sequencing strategy utilizes gene-specific primers for regions of critical genes associated with oncogenic arrangements paired with random primers, allowing for detection of novel fusion partners. Specimens were received either as formalin-fixed, paraffin-embedded (FFPE) tissue or fresh tissue samples preserved in PreservCyt (Hologic, Marlborough, MA). Total nucleic acid was extracted from submitted specimens, and primarily RNA-derived reads were analyzed for fusion detection. This assay was performed at the Center for Personalized Diagnostics at the Hospital of the University of Pennsylvania according to standard operating procedures, the initial validation of which has been previously described.20 Samples were tested on version 1.0 of this panel until July 9, 2018. Subsequent samples were tested on version 2.0 of this panel which included an expanded list of targeted genes and a decreased minimum required nucleic acid input (from 100 ng to 10 ng). Version 1.0 could detect previously described or novel fusions targeting critical rearrangements involving ALK, BRAF, EGFR (including the non-fusion aberrant isoform EGFRvIII), EML4, ERG, ESR1, FGFR1, FGFR2, FGFR3, MET (including MET exon 14 skippings), NRG1, NTRK1, NTRK2, NTRK3, RET, ROS1, TERT, and TMPRSS2. Version 2.0 expanded upon this list to detect fusions in additional genes, with the full list of gene targets in Table 1.
Table 1.
Fifty-Six Genes Assessed in the Penn Fusion Transcript Panel
| Fusion Transcript Panel | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| AKT1 | ALK | AXL | BCOR | BRAF | CALCA | CAMTA1 | CCNB3 | CCND1 | CIC |
| EGFR | EML4 | EPC1 | ERBB2 | ERG | ESR1 | EWSR1 | FGFR1 | FGFR2 | FGFR3 |
| FOXO1 | FUS | GLI1 | HMGA2 | JAZF1 | KRT20 | KRT7 | MEAF6 | MET | MKL2 |
| NCOA2 | NRG1 | NTRK1 | NTRK2 | NTRK3 | PDGFB | PIK3CA | PLAG2 | PMS2 | PPARG |
| PTH | RAF1 | RET | ROS1 | SLC5A5 | SS18 | STAT6 | TAF15 | TCF12 | TERT |
| TFE3 | TFG | THADA | TMPRSS2 | USP6 | YWHAE | ||||
This study was approved by an independent institutional review board at the Hospital of the University of Pennsylvania (HUP IRB 827290). Electronic Health Records were reviewed to identify all patients for whom a FTP was ordered on resected brain tissue of any underlying histology (Ab.D.). The results of these panels as well as additional molecular information and key demographic and clinical variables were collated (Ab.D.). All charts and surgical and molecular pathology reports were manually reviewed to confirm the diagnosis of glioma, as well as the specific type, and to confirm the reported results of the FTP panel (S.K. and M.P.N.). Four authors (S.K., M.P.N., Ar.D., and S.B.) reviewed the charts of the patients in whom a potentially targetable fusion was identified to evaluate the clinical implications of the fusion testing. A complete list of the tumors with fusions detected is provided in Supplementary Table 1.
Statistical analyses were performed using Stata software, version 16 (StataCorp). Overall survival (OS) was defined as the time from initial surgical resection until death from any cause. The Kaplan–Meier method was used to estimate median OS. Log-rank tests were used to assess crude differences in survival according to the following categorizations, respectively: (1) at least one fusion detected versus no fusion detected, (2) at least one targetable fusion detected versus no fusion or only non-targetable fusion(s) detected, (3) at least one targetable fusion detected versus at least one non-targetable fusion detected, and (4) more than one fusion detected versus only one fusion detected.
Results
Over the study period of August 2017–May 2021, a total of 801 unique patients were identified to have had at least one FTP performed on tissue resected during brain surgery. Several of these patients had multiple FTPs performed as they underwent multiple resections through their treatment course. Of the 801 patients identified, 7 patients did not have a formal surgical pathology report for our review and thus histology of the resected lesion could not be confirmed. These patients were excluded from analysis. An additional 128 patients were excluded based on histology of the resected lesion demonstrating a metastatic lesion to the central nervous system from a non-glial malignancy or neuro-epithelial neoplasm other than a glioma. A total of 666 patients (83%) were found to have a resected glial neoplasm. Among these 666 patients, the FTP was unable to be performed on resected tissue of 19 patients due to insufficient total nucleic acid quality and/or quantity. Our final cohort consisted of 647 patients.
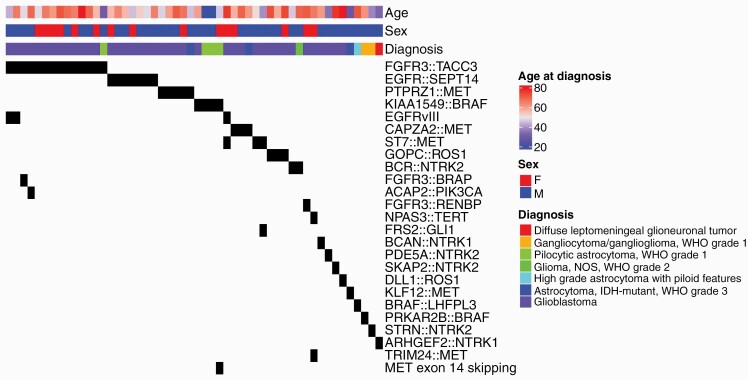
The final histopathologic diagnoses using 2021 WHO criteria for the resulting 647 patients in our cohort are presented in Table 2. The method of retrospectively classifying tumors by 2021 WHO criteria is described (Supplementary Note). Of the evaluated 647 patients, 156 patients (24%) were identified to harbor a fusion and/or EGFRvIII, which is also detected by the FTP. Fifty-two patients were identified to harbor a potentially targetable fusion (other than EGFRvIII), representing 8% of the cohort. The targetable genes found to be involved in a fusion included FGFR3, MET, EGFR, NTRK1, NTRK2, BRAF, ROS1, and PIK3CA, as listed in Table 3. These targetable fusions were identified in 9% of patients with IDH-wild type (WT) astrocytomas, 4% of patients with IDH-mutant astrocytomas, and no patients with oligodendrogliomas. The histologic diagnoses, age, gender, and specific fusion proteins identified in the 52 patients harboring potentially targetable fusions are presented as an Oncoprint analysis in Figure 1.
Table 2.
Targetable Fusions by 2021 WHO Integrated Diagnosis
| WHO 2021 Integrated Diagnosis | WHO Grade | Number of Patients | Fusion Identified | Targetable Fusion (Not Including EGFRvIII) |
|---|---|---|---|---|
| GBM, IDH WT | 4 | 468 | 141 | 40 |
| Astrocytoma, IDH mutant | 4 | 29 | 1 | 1 |
| Astrocytoma, IDH mutant | 3 | 32 | 3 | 2 |
| Astrocytoma, IDH mutant | 2 | 17 | 0 | 0 |
| Oligodendroglioma, IDH mutant, 1p19 codeleted | 3 | 22 | 0 | 0 |
| Oligodendroglioma, IDH mutant, 1p19 codeleted | 2 | 29 | 0 | 0 |
| Oligoastrocytoma, IDH-mutant | 2 | 1 | 0 | 0 |
| Astrocytoma, NF1 | 2 | 2 | 0 | 0 |
| DMG | 4 | 7 | 0 | 0 |
| HGAP | NA | 1 | 1 | 1 |
| Glioma, NOS | 2 | 4 | 1 | 1 |
| Spinal glioma, NOS | 3–4 | 3 | 1 | 0 |
| Low-grade glioma, NEC | 1–2 | 2 | 0 | 0 |
| PXA | 3–4 | 1 | 0 | 0 |
| PXA | 2 | 1 | 0 | 0 |
| Pilocytic astrocytoma | 1 | 11 | 4 | 4 |
| Gangliocytoma/ Ganglioglioma | 1 | 5 | 2 | 2 |
| DNET | 1 | 3 | 0 | 0 |
| Glioneuronal tumor, BRAF V600E mutated | 1–2 | 1 | 0 | 0 |
| Glioneuronal tumor | 1 | 1 | 0 | 0 |
| DLGNT | 3 | 1 | 1 | 1 |
| Low-grade neuro-epithelial neoplasm | 1–2 | 1 | 0 | 0 |
| PLNTY | 1 | 1 | 0 | 0 |
| Ependymal neoplasm | NA | 3 | 0 | 0 |
| Malignant spindle and round cell neoplasm | NA | 1 | 1 | 0 |
| Total | 647 | 156 | 52 |
Table 3.
Targetable Fusions Identified
| Targetable Gene Partner | Variant | Total Number of Fusions |
|---|---|---|
| FGFR3 |
FGFR3::TACC3 (n = 4) FGFR3::BRAP (n = 1) FGFR3::RENBP (n = 1) |
16 |
| MET |
PTPRZ1::MET (n = 5) CAPZA2::MET (n = 3) ST7::MET (n = 3) KLF12::MET (n = 1) MET exon 14 skip (n = 1) TRIM24::MET (n = 1) |
14 |
| EGFR | EGFR::SEPT14 (n = 7) | 7 |
| BRAF |
KIAA1549::BRAF (n = 4)
BRAF::LHFPL3 (n = 1) PRKAR2B::BRAF (n = 1) |
6 |
| NTRK2 |
BCR::NTRK2 (n = 2)
STRN::NTRK2 (n = 1) PDE5A::NTRK2 (n = 1) SKAP2::NTRK2 (n = 1) |
5 |
| NTRK1 |
ARHGEF2::NTRK1 (n = 1)
BCAN::NTRK1 (n = 1) |
2 |
| ROS 1 |
GOPC::ROS1 (n = 3)
DLL1::ROS1 (n = 1) |
4 |
| PIK3CA | ACAP2::PIK3CA (n = 1) | 1 |
| Total | 55 |
Figure 1.
Oncoprint analysis of patients identified to have a targetable fusion protein. Tumors are grouped from most frequently to least frequently seen fusion, with age, sex, and diagnosis indicated at the top.
Four patients in the cohort were found to harbor 2 fusion proteins on a single FTP assessment, as presented in the first 4 rows of Table 4. Three of the tumors with 2 fusions were GBMs; one was a pilocytic astrocytoma. The pilocytic astrocytoma and one GBM each had 2 different targetable fusions, and the other 2 patients with glioblastoma harbored MET fusions with a non-targetable second fusion. The 46-year-old patient who had been diagnosed with a pilocytic astrocytoma 42 years previously had a reresection; the recurrent tumor was found to simultaneously harbor both a KIAA1549::BRAF fusion and a MET exon 14 skipping fusion. The patient passed 3 months later.
Table 4.
Patients With Multiple Concurrent Fusions and Patients With Multiple FTPs Performed
| Diagnosis | Fusions on First Sequencing | Fusions on Second Sequencing | Interval Treatment |
Vital Status | Survival (Months) |
|---|---|---|---|---|---|
| Pilocytic astrocytoma |
KIAA1549::BRAF; MET exon 14 skipping |
n/a | Radiation, resections, and multiple chemotherapy agents | deceased | 506 |
| Glioblastoma, MGMT promoter methylation detected |
NPAS3::TERT;
TRIM24:MET |
n/a | Chemoradiation | deceased | 4 |
| Glioblastoma, MGMT promoter methylation not detected |
ACAP2::PIK3CA;
FGFR3:TACC3 |
n/a | Chemoradiation, Avastin | deceased | 13 |
| Glioblastoma, MGMT promoter methylation not detected |
ST7::MET;
FRS2:GLI1 |
n/a | Chemoradiation | deceased | 18 |
| Glioblastoma, MGMT promoter methylation not detected | FGFR3::BRAP | FGFR3::TACC3 | chemoradiation, lenvatinib (4 months), cyberknife, pembrolizumab (6 months), and cyberknife, bevacizumab (2 months) | deceased | 20 |
| Recurrent/residual glioblastoma | FGFR3::TACC3 | FGFR3::TACC3 | chemoradiation, second resection with carmustine implant, bevacizumab (16 months), third resection, re-irradiation, pembrolizumab (4 months), and fourth resection, lenvatinib (7 months) | deceased | 41 |
| Glioblastoma | ST7::MET | None detected | Chemoradiation, second resection | deceased | 3 |
Additionally, 3 patients in the cohort were sequenced on sequential surgical resections, which revealed rare consistency between the 2 assays over time. Among these patients, only 1 patient’s tumor showed the same fusion (FGFR3::TACC3) on both studies. The other 2 patients showed temporal heterogeneity. A 49-year-old patient with an IDH-WT glioblastoma was found to have an FGFR3::BRAP fusion on initial resection. In this case, a repeat resection performed for progressive disease approximately 20 months following the first resection identified an FGFR3::TACC3 fusion; the FGFR3::BRAP fusion was not detected in the recurrence specimen. A 61-year-old male with an IDH-WT glioblastoma was found to harbor an ST7::MET fusion on initial resection, while a second resection performed months later showed no detectable fusions. Table 4 presents survival and therapies received between FTP assessments. Although statistical conclusions cannot be drawn from the small number of patients, survival times appear to be within the range typically seen for glioblastoma.
Review of the electronic medical record for the cohort indicated that 11 of the 52 patients harboring a targetable fusion received treatment with a fusion inhibitor (21%). Nine of the eleven patients had a diagnosis of GBM, but with an unusually young age range. Four of these nine patients were aged 46–48 years, with 2 other patients aged 54 and 57 years. The median survival for the 9 GBM patients was 21 months. Targeted agents tended to be received late in the course of disease, and included TAS-120, lenvatinib, larotrectinib, trametinib, crizotinib, selitrectinib, and osimertinib. Three patients with glioblastoma each received 2 sequential fusion-targeted agents as a result of disease progression following initiation of the first drug. The patients who received a fusion inhibitor had received treatment with an average of 1.9 prior systemic therapies (range = 1–3) and 5 patients (45%) received bevacizumab either prior to or concurrently with the fusion inhibitor (Table 5).
Table 5.
Patients Treated With Fusion Inhibitors
| Diagnosis | Fusion | Age at Diagnosis (years) | Therapy | Number of Prior Systemic Therapies | Prior Systemic Therapies | Systemic Therapies Following Fusion Inhibitor | Clinical/Imaging Response | Survival (months) |
|---|---|---|---|---|---|---|---|---|
| Glioblastoma, IDH-wild type, WHO grade 4 | FGFR3::TACC3 | 46 | Lenvatinib | 3 | Temozolomide, Bevacizumab, Pembrolizumab | Received lenvatinib for final 7 months, during slow clinical progression in context of stable imaging. | 41 | |
| Glioblastoma, IDH-wild type, WHO grade 4 | FGFR3::BRAP; FGFR3::TACC3 | 48 | TAS-120/ Lenvatinib | 1 | Temozolomide | FGFR3 fusions both seen in right frontal tumor, which was stable on treatment (chemoradiation and TAS-120). New left parietal lesion developed, no fusions detected. Lenvatinib initiated. Left-sided disease continued to worsen, right remained stable. | 20 | |
| Glioblastoma, IDH-wild type, WHO grade 4 | FGFR3::TACC3 | 66 | Lenvatinib | 2 | Temozolomide, CCNU | Bevacizumab | Responded well to Lenvatinib for 5 months, then tumor progression, and was subsequently treated with bevacizumab and tumor-treating fields for final 14 months | 32 |
| Glioblastoma, IDH-wild type, WHO grade 4 | FGFR3::TACC3 | 82 | TAS-120/ Lenvatinib | 1 | Temozolomide | Bevacizumab | Imaging and clinical improvement with TAS-120 initially, but withdrawn due to side effects. Rapid progression, and started on lenvatinib shortly before passing | 8 |
| Glioblastoma, IDH-wild type, WHO grade 4 | EGFR::SEPT14 | 46 | Osimertinib | 3 | Temozolomide, CCNU, Bevacizumab | Pembrolizumab | Progression of multifocal tumor on osimertinib (given for last 6 months of course); tissue tested to reveal fusion was parietal, area of progression was frontal and deep | 17 |
| Glioblastoma, IDH-wild type, WHO grade 4 | EGFR::SEPT14 | 54 | Osimertinib | 1 | Temozolomide | Anti - PD1, Anti-GITR, Bevacizumab, CCNU, Carboplatin | Began to progress on osimertinib after 2.5 months of treatment, passed 7 months later | 33 |
| Glioblastoma, IDH-wild type, WHO grade 4 | BCR::NTRK2 | 46 | Larotrectinib/ Selitrectinib | 3 | Temozolomide, CCNU, Bevacizumab | Initial progression on chemoradiation, then a year on Larotrectinib with no progression, then progressed, and was started on compassionate use selitrectinib, but continued decline | 21 | |
| Glioblastoma, IDH-wild type, WHO grade 4 | SKAP2::NTRK2 | 67 | Larotrectinib late 2021 for 1 month | 1 | Temozolomide | Initiated larotrectinib upon eventual radiographic progression after 31 months of stability; imaging continued to progress, so switched to different therapy after 1 month of lartrectinib | alive 45 months | |
| Glioblastoma, IDH-wild type, WHO grade 4 | CAPZA2::MET | 57 | Crizotinib | 3 | Temozolomide, Bevacizumab, CCNU | Was only able to procure a 2-week supply of crizotinib; subsequently rapid progression, clinically and on imaging. | 20 | |
| High-grade glioma with piloid features, IDH-wild type | BRAF::LHFPL3 | 72 | Trametinib | 1 | Temozolomide | Pembrolizumab | One month after initiation of trametinib, imaging was stable, but slow progression was seen after a couple of months, particularly outside radiation field. | 13 |
| Diffuse leptomeningeal glioneuronal tumor, IDH-wild type | ARHGEF2::NTRK1 | 8 (32) | Larotrectinib | 2 | Temozolomide, Bevacizumab | Originally posterior fossa, 24 years later recurred as a left temporal lobe mass with broad dural attachment and cerebellar mass. Initiated larotrectinib in setting of continued clinical decline and transitioned to hospice care. | 312 |
The most common fusion gene partner identified in the cohort was FGFR3 with 15 patients harboring FGFR3 gene fusions. Three distinct FGFR3 fusions were identified: FGFR3::TACC3, FGFR3::BRAP, and FGFR3::RENBP. Four patients in the cohort with glioblastoma were treated with FGFR3-targeting agents including TAS-120 (futibatinib), a direct FGFR inhibitor, and lenvatinib, a multikinase inhibitor with activity against FGFR. One patient with glioblastoma received lenvatinib 24 mg daily for 7 months and discontinued the drug in the setting of clinical decline and intolerance. The patient passed away shortly thereafter. Another patient with glioblastoma initiated lenvatinib 24 mg daily after prior treatment with TAS-120 and continued treatment for 3 months, at which point MRI demonstrated disease progression. The patient received additional therapies and passed away approximately 8 months later. # #3A third patient with glioblastoma received lenvatinib 24 mg daily for 5 months at which point the drug was stopped for progression. The patient passed 14 months later. #4A fourth patient with glioblastoma initiated lenvatinib 24 mg daily after prior treatment with TAS-120. The patient had clinical decline and was transitioned to hospice the following month, and passed later that month.
Fourteen patients in the cohort were found to harbor a MET fusion. Six different fusions were identified including PTRPRZ1::MET, CAPZA2::MET, ST7::MET, KLF12::MET, MET exon 14 skip, and TRIM24::MET. One patient in the cohort with a glioblastoma harboring a CAPZA2::MET fusion was treated with crizotinib 250 mg twice daily for a brief time; MRI showed progression, and the patient transitioned to hospice and passed a couple of months later.
Seven patients in the cohort were identified to harbor EGFR::SEPT14 fusions. Two of these patients, both with glioblastoma, received osimertinib to target EGFR. One patient received osimertinib 80 mg daily for 6 months at which point MRI demonstrated further progression and the drug was discontinued. The patient was transitioned to hospice and passed soon thereafter. A second patient with glioblastoma initiated treatment with osimertinib 80 mg daily, with follow-up MRI 10 weeks after osimertinib demonstrated progression prompting discontinuation. The patient was enrolled in a clinical trial and passed 7 months later.
Six patients in the cohort were identified to harbor a BRAF fusion. Three different fusions were identified including KIAA1549::BRAF, BRAF::LHFPL3, and PRKAR2B::BRAF. One patient with a high-grade glioma with piloid features harboring a BRAF::LHFPL3 fusion was treated with trametinib. The patient has been treated with trametinib 1 mg daily for approximately 3 months, at which point surveillance MRI demonstrated disease progression. The patient passed a few months later.
Seven patients in the cohort harbored a fusion with NTRK: 5 patients with NTRK2 and 2 patients with NTRK1. Six unique fusions were identified including BCR::NTRK2, STRN::NTRK2, PDE5A::NTRK2, SKAP2::NTRK2, ARHGEF2::NTRK1, and BCAN::NTRK1. One patient with glioblastoma harboring a BCR::NTRK2 fusion was treated with larotrectinib 100 mg twice daily for a year, at which point disease progression was demonstrated. The patient has been initiated on selitrectinib 100 mg twice daily for 3 months, with disease progression and clinical decline. The patient passed 2 months later. A second patient with glioblastoma harboring SKAP2::NTRK2 initiated larotrectinib 100 mg twice daily upon disease progression 2 and a half years into his course. However, his disease continued to progress through a month of therapy, and he was subsequently initiated on a clinical trial and follow-up is ongoing. A patient with a Diffuse Leptomeningeal Glioneuronal Tumor (DLGNT) harboring ARHGEF2::NTRK1 has been treated with larotrectinib 100 mg twice daily for 2 months, but had continued disease progression. The patient was transitioned to hospice and passed 5 months later.
Four patients in the cohort were identified to harbor fusions with ROS1. Two unique fusions were identified: GOPC::ROS1 and DLL1::ROS1. No patients with ROS1 fusions received targeted therapy. Additionally, one patient in the cohort harbored an ACAP2::PIK3CA fusion and did not receive a targeted fusion inhibitor.
Survival analysis did not show a significant difference in overall survival (OS) between patients with glioblastoma harboring a fusion compared to glioblastoma patients with no fusions, nor between patients with glioblastoma harboring a targetable fusion compared to glioblastoma patients with either no fusion or a non-targetable fusion. Median OS for patients with glioblastoma identified to have at least one fusion was 17.2 months (95% CI, 11.7–20.1 months) v. 15.4 months among patients with glioblastoma who did not harbor a fusion (95% 14.4–17.0 months) (log-rank P = .9). Additionally, median OS for patients with glioblastoma harboring a targetable fusion was 17.2 months (95% CI, 10.9–20.4 months) versus 15.4 months (95% CI 14.4–17.0 months) for patients with either no fusion or a non-targetable fusion (log-rank P = .82).
Discussion
This study presents our institutional experience routinely testing gliomas for oncogenic fusions. We identified 52 patients with gliomas harboring genetic fusions involving 8 different partner genes that could be targeted with either off-label use of targeted agents approved for other cancers, fusion inhibitors indicated for all cancers harboring a given genetic lesion, or as part of clinical trials evaluating novel fusion inhibitors. Taken together, these results demonstrate that fusion transcripts are identified at a notable rate in patients with glioma, the fusion repertoire is diverse and unique, and there can be significant clinical relevance to performing fusion testing in this population.
To the best of our knowledge, our study represents the largest reported cohort of adult glioma patients evaluated with a FTP and is the only study to describe the clinical implications of such testing. Woo et al. reported on next-generation sequencing of 356 diffuse gliomas identifying 53 cases of glioblastoma harboring an oncogenic gene fusion.21 Na et al. reported the results of testing 135 diffuse gliomas with a 55-gene RNA panel for fusions, identifying fusions in approximately 10% of cases.22 Ferguson et al. reported on the use of an ArcherDx FusionPlex Assay to evaluate 390 gliomas and found 36 to harbor a potentially targetable fusion.23 Similarly, Subramanian et al. tested 404 gliomas using an ArcherDx FusionPlex Assay, identifying 39 to harbor a potentially targetable fusion.24 Our work with an additional and larger cohort yields results consistent with the prior studies, and expands on previous studies to present aggregate and patient-level information on how fusion identification impacts clinical management. In so doing, our results support the performance of fusion testing in gliomas.
The fusions identified in our cohort consist of both well-described genetic aberrations with known oncogenic properties as well as changes that have not been previously reported in the literature. Of the FGFR3 fusions identified, both the FGFR3::TACC3 and FGFR3::BRAP fusions have been previously reported, while the FGFR3::RENBP fusion has not been reported to date.23–25 Several agents have received approval from the FDA for the treatment of cancers with FGFR fusions including pemigatinib, infigratinib, and erdafitinib.26,27 Within gliomas, targeting of tumors with FGFR fusions has been described using futibatinib, infigratinib, and investigational agent JNJ-42756493.25,28,29
Amongst the MET fusions identified, PTPRZ1::MET, CAPZA2::MET, and ST7::MET have been previously described in gliomas and a TRIM24::MET fusion has been previously described in a neonatal brain tumor; conversely, the KLF12::MET fusion has not been previously reported.23,30,31 Additionally, MET exon 14 skippings which were identified in a patient with pilocytic astrocytoma have too been reported in gliomas.32,33 Multiple targeted therapies are currently in use for patients with malignancies harboring certain MET aberrations, including crizotinib, capmatinib, and tepotinib.11 Among glioma patients, Hu et al. demonstrated efficacy and safety of PLB-1001, a MET kinase inhibitor, in a small number of patients with histologic grade 4 astrocytomas with MET exon 14 skipping or PTPRZ1::MET fusions that had progressed from lower grade astrocytomas.33
EGFR::SEPT14 was the only EGFR fusion identified in our cohort. SETP14 has been reported to be the most common fusion partner with EGFR in glioblastoma, commonly joining the first 24 exons of EGFR with exon 10 of SEPT14 and putatively leading to constitutive activation.34 Functional studies with EGFR::SEPT14 fusion-positive glioma cells have demonstrated increased growth as well as sensitivity to EGFR inhibition.34 Multiple EGFR-targeted therapies have been evaluated amongst glioma patients though no specific targeting of EGFR::SEPT14 has been reported.35
Of the 3 unique fusions involving BRAF identified in our cohort, BRAF::LHFPL3 has not been reported in the medical literature, and it is unclear how the fusion might contribute to pathogenesis as it is not predicted to contain the kinase domain of BRAF. Based on the reported exonic breakpoints, this novel fusion would not be predicted to maintain the same reading frame from the first to the second partner gene. Conversely, PRKAR2B::BRAF has been reported rarely in ganglioglioma and KIAA1549::BRAF is well described in pilocytic astrocytomas.36BRAF fusions often lead to loss of the N-terminal autoinhibitory region of BRAF leading to activation of signaling.37 Several targeted agents have received FDA approval for the treatment of tumors with BRAF point mutations; however, no agents have thus far received approval for BRAF fusions. Among glioma patients, BRAF fusion targeting with selumetinib and trametinib has shown some efficacy in pediatric low-grade glioma patients harboring KIAA1549::BRAF fusions.38,39
The NTRK1 fusions identified in our cohort, BCAN::NTRK1 and ARHGEF2::NTRK1, have both been previously reported in glioma.34,40 Of NTRK2 fusions identified, only BCR::NTRK2 has previously been reported in glioma.41 Of note, the novel PDE5A::NTRK2 fusion was noted to not maintain the same reading frame from the first to the second partner gene within the captured read lengths. Two targeted agents, larotrectinib and entrectinib, have received FDA approval for treatment of certain solid tumors with an NTRK gene fusion, and both have shown some signs of efficacy amongst glioma patients.42–44
The GOPC::ROS1 fusion identified in the cohort has previously been described in gliomas, whereas the DLL1:ROS1 fusion has not been previously described in the medical literature.45 Multiple agents have received FDA approval for the treatment of certain non-small cell lung cancer patients with rearrangement of ROS1.12 Among glioma patients, successful targeting of ROS1 fusions in pediatric and young adult patients was detailed in the STARTRK-NG Trial evaluating entrectinib.46
The ACAP2::PIK3CA fusion identified has not been reported previously in glioma. PIK3CA targeting is commonly employed in the treatment of advanced breast cancer with the combination of alpelisib, an alpha-specific PI3K inhibitor, and fulvestrant, an anti-hormonal agent, and additional PI3K-targeted agents are being evaluated in clinical trials.47 There are no reports of glioma patients receiving targeted treatment for PIK3CA fusions.
As the discussion above outlines, a diverse array of oncogenic fusion proteins was found within glioma patients including both previously described fusions as well as others not previously reported in the literature. Several of these fusions have demonstrated targetability in prior studies in glioma patients, and the identification of these fusions informed treatment decisions for patients in our cohort. In addition to highlighting fusion diversity, our work also confirms the findings that gene fusions are more commonly found in IDH-WT tumors as opposed to IDH-mutant tumors and that FGFR3 is the most common targetable gene partner in fusions identified in glioma patients.
In our cohort, 9% (40/468) of glioblastomas were found to harbor a fusion protein as compared to 5% (3/61) of IDH-mutant histologically high-grade astrocytomas. Similar rates were found in the analyses by Subramanian and Ferguson.23,24 Subramanian et al. found that among histologic WHO Grade 4 astrocytomas in their cohort, 6.7% of IDH-mutated tumors (n = 1/15) had a potentially targetable fusion as compared to 12.6% of IDH-WT tumors (n = 22/175). Ferguson et al. similarly found fusions were more frequent in IDH-WT tumors in their cohort (12%, n = 31/262) as compared to IDH-mutant tumors (4%; n = 4/109). This potential difference in frequency between IDH-mutant and IDH-WT disease is of high clinical relevance. Targeting mutant IDH is an attractive therapeutic option for patients with recurrent disease with either direct IDH inhibitors or other targeted agents including PARP inhibitors.48 Recurrent IDH-WT lesions are often more difficult to treat given that no therapies demonstrate an overall survival benefit. Targeted fusion inhibitors may represent meaningful therapeutic options in this subpopulation of patients whose tumors harbor an oncogenic fusion.
Our work also confirms the finding that FGFR3 fusions are found in adult gliomas at a particularly notable rate. We identified 16 FGFR3 fusions representing 29% of the targetable fusions found. FGFR3 fusions were similarly the most commonly found targetable fusions in the works by Subramanian and Ferguson.23,24 The concordance of these findings across studies highlights the importance of clinical development efforts for brain-penetrant FGFR inhibitors given the frequency of this aberration. Encouragingly, clinical trials are underway evaluating infigratinib, pemigatinib, and erdafitinib in adult and pediatric gliomas (NCT05222165, NCT05267106, NCT04424966, and NCT03210714).
Finally, our results also identify the important role fusion-targeted therapies can play in the sequence of therapies glioma patients receive. Bevacizumab, an anti-VEGF antibody, is routinely used in the care of patients with recurrent glioma as it can prolong progression-free survival, decrease local inflammation, and help reduce steroid requirement.49 However, the use of bevacizumab may prohibit future clinical trial eligibility for these patients, limiting therapeutic options. A recent analysis of clinical trials for glioblastoma identified that roughly one-third of clinical trials do not allow for prior use of bevacizumab within their eligibility criteria.50 Our data demonstrate that 45% of the patients in the cohort who received a fusion inhibitor did so either following or concurrently with bevacizumab. This highlights the role fusion inhibitors can play for patients following bevacizumab initiation when trial eligibility may be additionally limited.
Our study seeks to accurately report the fusion repertoire of this cohort, but it must be noted that interpretation and reporting of such fusion results can be nuanced. Most gene fusions identified in this cohort resulted in what is commonly thought of as a fusion gene; however, several gene rearrangements within the cohort, including cases with PTPRZ1::MET, ST7::MET, CAPZA2::MET, and ACAP2::PIK3CA, were noted to join often only the first exon of one gene to the entire coding length of another gene. While these rearrangements are considered as fusion genes, the oncogenic event in these cases may involve overexpression of the full-length partner gene. Additionally, one fusion identified in this cohort (KLF12::MET) is noted to include only the non-coding first exon of KLF12 which strongly suggests this mechanism (ie, overexpression of MET through promoter swapping). Additional points of complexity that often arise in evaluation of gene fusions and, though not noted for each fusion individually within the discussion, were occasionally observed within this cohort include: Identification of multiple fusion transcripts, which may be due to splicing heterogeneity or overall complexity due to overexpression; identification of atypical transcripts that include intronic sequences which likely still represent RNA-derived reads but could be DNA-derived; identification of fusion transcripts which do not appear to maintain the same reading frame from the first to second gene partner within the captured read length; and identification of fusions with unusual breakpoints. In this study, all fusions which were deemed to be clinically reportable at the time of testing were considered as eligible for inclusion.
Furthermore, 4 identified fusions in the cohort (3 FGFR3::TACC3 fusions and one EGFR::SEPT14 fusion) did not fully meet strict reporting criteria but were clinically reported as indeterminate at the discretion of the laboratory. These fusions were included for analysis as they were determined to be clinically meaningful. Finally, as gene-specific primers were used to specifically target commonly rearranged exons of the target genes, rare rearrangements or those with unusual breakpoints may be missed.
Though our study is notable in several ways as described above, it is limited in its current potential clinical impact as fusion inhibitors have not yet demonstrated significant survival benefits to patients with primary central nervous system malignancies. Despite this, we hope that routine identification of these fusions helps spur additional clinical development programs to create new generation of CNS penetrant fusion inhibitors.
Conclusion
Our institutional experience suggests that routine fusion testing of adult gliomas identifies genetic aberrations with potential therapeutic relevance at a clinically meaningful rate. Such testing may open therapeutic opportunities specifically for patients with glioblastoma, as these patients have severely limited treatment options in the recurrent setting. Prospective clinical trials are ultimately needed to establish the efficacy of targeted therapies for patients with glioma and specific oncogenic fusion proteins. In the meantime, increased testing for fusion proteins in the neuro-oncology clinic will add to the field’s experience with targeting these fusions in glioma and enhance the feasibility of conducting prospective clinical trials in these rare patient subgroups.
Supplementary Material
Contributor Information
Shawn Kothari, Division of Hematology/Oncology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Anna C Dusenbery, Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Abigail Doucette, Division of Hematology/Oncology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Daniel Y Zhang, Biochemistry and Molecular Biophysics Graduate Group, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Dominique Ballinger, Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Arati Desai, Electronic Phenotyping Core, Abramson Cancer Center, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Jennifer J D Morrissette, Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Stephen J Bagley, Division of Hematology/Oncology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
MacLean P Nasrallah, Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Funding
Funding from National Institute of Health Grant: 5T32CA009615-32 enabled S.K. to complete this study.
Conflict of interest statement
Subsequent to working on the project, ACD was employed by Strata Oncology. The remaining authors declare no conflicts of interest related to this work.
Authorship statement
MPN, SK, and SB conceived the study. AbD performed data extraction to identify the study cohort. SK, MPN, ArD, DYZ, DB and SB performed retrospective review of surgical pathology reports, fusion transcript analyses, and patient charts. ACD performed literature review, editing, and provided subject matter expertise. JJDM reviewed the data, provided subject matter expertise and additional case review. All authors contributed to the writing of the manuscript.
References
- 1. Ostrom QT, Cioffi G, Waite K, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;24(suppl 5):1–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perry JR, Rizek P, Cashman R, Morrison M, Morrison T.. Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: The “rescue” approach. Cancer. 2008;113(8):2152–2157. [DOI] [PubMed] [Google Scholar]
- 3. Perry JR, Belanger K, Mason W, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051–2057. [DOI] [PubMed] [Google Scholar]
- 4. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 5. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO Classification of Tumours Editorial Board. Central nervous system tumours [Internet]. Lyon (France): International Agency for Research on Cancer; 2021. [cited 2022 09 22]. (WHO classification of tumours series, 5th ed.; vol. 6). Available from: https://tumourclassification.iarc.who.int/chapters/45. [Google Scholar]
- 7. Schram AM, Chang MT, Jonsson P, Drilon A.. Fusions in solid tumours: Diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol. 2017;14(12):735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Majeed U, Manochakian R, Zhao Y, Lou Y.. Targeted therapy in advanced non-small cell lung cancer: Current advances and future trends. J Hematol Oncol. 2021;14(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pollack SM, Ingham M, Spraker MB, Schwartz G.. Emerging targeted and immune-based therapies in sarcoma. J Clin Oncol. 2018;36(2):125–135. [DOI] [PubMed] [Google Scholar]
- 10. Tran L, Xiao JF, Agarwal N, Duex JE, Theodorescu D.. Advances in bladder cancer biology and therapy. Nat Rev Cancer. 2021;21(2):104–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo R, Luo J, Chang J, et al. MET-dependent solid tumours — Molecular diagnosis and targeted therapy. Nat Rev Clin Oncol. 2020;17(9):569–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drilon A, Jenkins C, Iyer S, et al. ROS1-dependent cancers — Biology, diagnostics and therapeutics. Nat Rev Clin Oncol. 2021;18(1):35–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cocco E, Scaltriti M, Drilon A.. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu T, Wang H, Huang X, et al. Gene fusion in malignant glioma: An emerging target for next-generation personalized treatment. Transl Oncol. 2018;11(3):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Rhun E, Preusser M, Roth P, et al. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019;80:101896. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Liang D, Chen J, et al. Targeted therapy with anlotinib for a patient with an oncogenic FGFR3-TACC3 fusion and recurrent glioblastoma. Oncologist. 2021;26(3):173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ziegler D, Wong M, Mayoh C, et al. Potent clinical and radiological response to larotrectinib in TRK fusion-driven high-grade glioma. Br J Cancer. 2018;119(6):693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. You G, Fan X, Hu H, Jiang T, Chen CC.. Fusion genes altered in adult malignant gliomas. Front Neurol. 2021;12:715206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grogan PT, Deming D, Helgager J, et al. Entrectinib demonstrates prolonged efficacy in an adult case of radiation-refractory NTRK fusion glioblastoma. Neurooncology Adv. 2022;4(1):vdac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sussman RT, Oran A, Paolillo C, et al. Validation of a next-generation sequencing assay targeting RNA for the multiplexed detection of fusion transcripts and oncogenic isoforms. Arch Pathol Lab Med. 2020;144(1):90–98. [DOI] [PubMed] [Google Scholar]
- 21. Woo HY, Na K, Yoo J, et al. Glioblastomas harboring gene fusions detected by next-generation sequencing. Brain Tumor Pathol. 2020;37(4):136–144. [DOI] [PubMed] [Google Scholar]
- 22. Na K, Kim H, Shim HS, et al. Targeted next-generation sequencing panel (TruSight Tumor 170) in diffuse glioma: A single institutional experience of 135 cases. J Neurooncol. 2019;142(3):445–454. [DOI] [PubMed] [Google Scholar]
- 23. Ferguson SD, Zhou S, Huse J, et al. Targetable gene fusions associate with the IDH wild-type astrocytic lineage in adult gliomas. J Neuropathol Exp Neurol. 2018;77(6):437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Subramaniam DS, Xiu J, Mehta S, et al. RNA-Seq analysis of glioma tumors to reveal targetable gene fusions. J Clin Oncol. 2017;35(15):2019–2019. [Google Scholar]
- 25. Di Stefano A, Fucci A, Frattini V, et al. Detection, characterization, and inhibition of FGFR–TACC fusions in IDH wild-type glioma. Clin Cancer Res. 2015;21(14):3307–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krook MA, Reeser J, Erns G, et al. Fibroblast growth factor receptors in cancer: Genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br J Cancer. 2021;124(5):880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Javle M, Roychowdhury S, Kelley RK, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6(10):803–815. [DOI] [PubMed] [Google Scholar]
- 28. Bahleda R, Meric-Bernstam F, Goyal L, et al. Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1–4 inhibitor in patients with advanced solid tumors. Ann Oncol. 2020;31(10):1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lassman A, Sanchez JM, Cloughesy T, et al. Infigratinib in patients with recurrent gliomas with fibroblast growth factor receptor (FGFR) alterations: A multicenter phase II study. Clin Cancer Res. 2021;28(11):2270–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bao Z, Chen H, Yang M, et al. RNA-seq of 272 gliomas revealed a novel, recurrent PTPRZ1-MET fusion transcript in secondary glioblastomas. Genome Res. 2014;24(11):1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hiemenz MC, Skrypek MM, Cotter J, Biegel J.. Novel TRIM24 - MET fusion in a neonatal brain tumor. JCO Precs Oncol.. 2019;3:1–6. [DOI] [PubMed] [Google Scholar]
- 32. Frampton GM, Ali S, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5(8):850–859. [DOI] [PubMed] [Google Scholar]
- 33. Hu H, Mu Q, Boa Z, et al. Mutational landscape of secondary glioblastoma guides MET-targeted trial in brain tumor. Cell. 2018;175(6):1665–1678.e18. [DOI] [PubMed] [Google Scholar]
- 34. Frattini V, Trifonov V, Chan J, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45(10):1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. An Z, Aksoy O, Zheng T, Fan Q, Weiss W.. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene. 2018;37(12):1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oon M, Low S, Kuick C, et al. An unusual ganglioglioma with pseudopapillary features and PRKAR2B-BRAF fusion. J Neuropathol Exp Neurol. 2021;80(10):1000–1003. [DOI] [PubMed] [Google Scholar]
- 37. Ross J, Wang K, Chmielecki J, et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer. 2016;138(4):881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fangusaro J, Onar-Thomas A, Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: A multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Selt F, Van Tilburg C, Bison B, et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neurooncol. 2020;149(3):499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kurozumi K, Nakano Y, Ishida J, et al. High-grade glioneuronal tumor with an ARHGEF2–NTRK1 fusion gene. Brain Tumor Pathol. 2019;36(3):121–128. [DOI] [PubMed] [Google Scholar]
- 41. Jones K, Bossler A, Bellizzi A, Snow A.. BCR-NTRK2 fusion in a low-grade glioma with distinctive morphology and unexpected aggressive behavior. Cold Spring Harb Mol Case Stud. 2019;5(2):a003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doz F, Van Tilburg C, Geoerer B, et al. Efficacy and safety of larotrectinib in TRK fusion-positive primary central nervous system tumors. Neuro Oncol. 2022;24(6):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keddy C, Neff T, Huan J, et al. Mechanisms of targeted therapy resistance in a pediatric glioma driven by ETV6-NTRK3 fusion. Cold Spring Harbor Mol Case Stud. 2021;7(5):a006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mayr L, Guntner A, Madlener S, et al. Cerebrospinal fluid penetration and combination therapy of entrectinib for disseminated ROS1/NTRK-fusion positive pediatric high-grade glioma. J Pers Med. 2020;10(4):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sievers P, Stichel D, Sill M, et al. GOPC:ROS1 and other ROS1 fusions represent a rare but recurrent drug target in a variety of glioma types. Acta Neuropathol. 2021;142(6):1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Desai AV, Ronbinson G, Guavain K, et al. Entrectinib in children and young adults with solid or primary CNS tumors harboring NTRK, ROS1 or ALK aberrations (STARTRK-NG). Neuro Oncol. 2022;24(10):1776–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arafeh R, Samuels Y.. PIK3CA in cancer: The past 30 years. Semin Cancer Biol. 2019;59:36–49. [DOI] [PubMed] [Google Scholar]
- 48. Han S, Liu Y, Cai S, et al. IDH mutation in glioma: Molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 50. Bagley SJ, Kothari S, Rahman R, et al. Glioblastoma clinical trials: Current landscape and opportunities for improvement. Clin Cancer Res. 2022;28(4):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.