Key Points
Question
Does the all-oral metronomic chemotherapy regimen vinorelbine plus cyclophosphamide plus capecitabine (VEX) provide a better clinical outcome and longer disease control compared with weekly paclitaxel for patients with estrogen receptor (ER)–positive, ERBB2 [formerly HER2/neu])-negative advanced breast cancer requiring chemotherapy?
Findings
In this phase 2 randomized clinical trial of 140 patients, time to treatment failure among patients receiving VEX was significantly improved, with a median of 8.3 months vs 5.7 months among patients receiving paclitaxel, as was progression-free survival, to a median of 11.1 months with VEX vs 6.9 months with paclitaxel.
Meaning
These results suggest that metronomic VEX may be one of the chemotherapy options for patients with ER+/ERBB2− advanced breast cancer.
Abstract
Importance
In spite of the effectiveness of endocrine therapy plus cyclin-dependent kinase (CDK) 4/6 inhibitors as the first-line treatment for estrogen receptor (ER)-positive, erb-b2 receptor tyrosine kinase 2 (ERBB2 [formerly HER2/neu])-negative (ER+/ERBB2−) metastatic breast cancer (MBC), patients eventually develop resistance, and eventually most will receive chemotherapy. The METEORA-II trial compared a metronomic all-oral treatment with intravenous (IV) chemotherapy.
Objective
To compare the efficacy of the oral vinorelbine plus cyclophosphamide plus capecitabine (VEX) regimen vs weekly IV paclitaxel among patients with ER+/ERBB2− MBC who are candidates for chemotherapy.
Design, Setting, and Participants
This phase 2 randomized clinical trial including 140 women 18 years and older (randomized 1:1) with ER+/ERBB2− MBC was carried out from September 13, 2017, to January 14, 2021 at 15 centers in Italy. Eligible patients could have received 1 prior line of chemotherapy for MBC and/or 2 lines of endocrine therapy (including CDK4/6 inhibitors).
Interventions
In 4-week cycles, patients received either metronomic oral VEX or weekly IV paclitaxel.
Main Outcomes and Measures
The primary end point was investigator-assessed time to treatment failure (TTF) defined as the interval between the date of randomization to the end of treatment (because of disease progression or lack of tolerability or because further trial treatment was declined). Secondary end points included progression-free survival (PFS), overall survival (OS), and disease control rate (complete or partial response or stable disease lasting for at least 24 weeks).
Results
In total, 133 patients received either VEX (n = 70) or paclitaxel (n = 63) in 4-weekly cycles. The median age was 61 (range, 30-80) years. The VEX treatment significantly prolonged TTF vs paclitaxel (hazard ratio [HR], 0.61; 95% CI, 0.42-0.88; P = .008), median TTF was 8.3 (95% CI, 5.6-11.1) months for VEX vs 5.7 (95% CI, 4.1-6.1) months for paclitaxel, and the 12-month TTF was 34.3% for VEX vs 8.6% for paclitaxel. The median PFS was 11.1 (95% CI, 8.3-13.8) months vs 6.9 (95% CI, 5.4-10.1) months favoring VEX (HR, 0.67; 95% CI, 0.46-0.96, P = .03). The 12-month PFS was 43.5% for VEX vs 21.9% for paclitaxel. No difference in OS was found. The TF event for 55.6% of patients was progression of disease; for 23% it was AEs. More patients assigned to VEX had at least 1 grade 3 or 4 targeted adverse event (VEX, 42.9%; 95% CI, 31.1%-55.3% vs paclitaxel, 28.6%; 95% CI, 17.9%-41.3%), but essentially no alopecia.
Conclusion and Relevance
This randomized clinical trial found significantly prolonged TTF and PFS for oral VEX but no improvement in OS compared with intravenous paclitaxel, despite increased but still manageable toxic effects. The VEX regimen may provide more prolonged disease control than weekly paclitaxel for ER+/ERBB2− MBC.
Trial Registration
ClinicalTrials.gov Identifier: NCT02954055
This randomized clinical trial compares the efficacy, in terms of clinical outcomes and disease control rate, of an all-oral metronomic vinorelbine plus cyclophosphamide plus capecitabine regimen with weekly paclitaxel among patients with estrogen receptor–positive, ERBB2-negative metastatic breast cancer.
Introduction
Estrogen receptor (ER)–positive, ERBB2 (formerly HER2/neu)–negative (ER+/ERBB2−) metastatic breast cancer (MBC) remains incurable in most cases.1 Despite the efficacy of endocrine therapies (ETs) alone and in combination with cyclin-dependent kinase (CDK) 4/6 inhibitors,2 most responding patients ultimately develop resistance and most will receive chemotherapy.3 A metronomic oral chemotherapy regimen comprising vinorelbine plus cyclophosphamide plus capecitabine (VEX) showed promise in a single-institution phase 2 trial,4 reporting median times to disease progression of 25.1 and 11.2 months for untreated and pretreated patients with MBC, respectively. The METEORA-II trial aimed to investigate whether metronomic VEX was superior to standard weekly paclitaxel in patients with ER+/ERBB2− MBC requiring chemotherapy.
Methods
Study Design
The METEORA-II trial was a phase 2 randomized, open-label study of metronomic oral VEX vs intravenous (IV) weekly paclitaxel given in 4-week cycles (the study protocol is provided in Supplement 1). The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients
Eligible patients with ER+/ERBB2− MBC who were treated with at most 1 prior line of chemotherapy, were randomized in a 1:1 ratio to receive oral VEX or weekly IV paclitaxel until disease progression (determined through imaging every 12 weeks) or lack of tolerability or until further trial treatment was declined (eMethods in Supplement 2); a maximum 3-week delay of trial treatment administration was allowed.
All patients provided written informed consent. The study was approved by the local ethics and/or institutional review boards for all participating sites (eAppendix 1 in Supplement 2) and the Italian health authority and was conducted according to principles of the Declaration of Helsinki and ICH Guidelines for Good Clinical Practice.
End Points
The primary end point was investigator-assessed time to treatment failure (TTF), defined as the interval between the date of randomization to the end-of-treatment date. The statistical design required 123 TF events (2-sided α = .05; 80% power). The secondary end points were progression-free survival (PFS), defined as time from randomization until disease progression according to the RECIST 1.1 criteria5 or death; rate of disease control (complete or partial response or stable disease lasting for at least 24 weeks); and overall survival (OS), defined as time from randomization to death from any cause. The distributions were compared using log-rank tests, hazard ratios (HRs) estimated using Cox proportional hazards regression models, and median and 12-month TTF estimated by the Kaplan-Meier method (eMethods in Supplement 2).
Analyses were performed with SAS statistical software, version 9.4 (SAS Institute Inc), and R, version 4.1.1 for Windows (R Foundation for Statistical Computing). A 2-sided P = .05 indicated statistical significance.
Results
Patients and Treatment
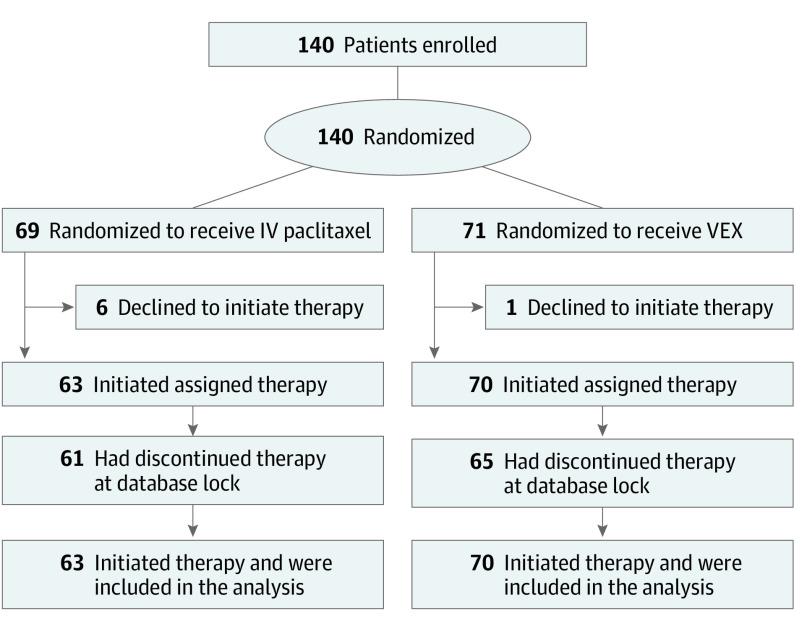
The METEORA-II trial enrolled 140 female patients in 15 centers in Italy between September 13, 2017, and January 14, 2021. A total of 133 patients (median [range] age, 61 [30-80] years) started treatment (VEX [n = 70]; paclitaxel [n = 63]) and comprised the analysis populations (Figure 1). The median time from MBC diagnosis to randomization was 11.2 (95% CI, 2.2-25.6) months; (eTable 1 in Supplement 2). In total, 21 patients (15.8%) had received chemotherapy for MBC before enrollment; 75 patients (56.4%) had received only prior ET for MBC, usually with a CDK4/6 inhibitor (35 patients [46.6%]).
Figure 1. METEORA-II Trial CONSORT Flow Diagram.
IV indicates intravenous; VEX, vinorelbine plus cyclophosphamide plus capecitabine.
Primary Outcome
Of the 133 patients, 128 had a TF event. The median TTF for VEX was 8.3 (95% CI, 5.6-11.1) vs 5.7 (95% CI, 4.1-6.1) months for paclitaxel (HR, 0.61; 95% CI, 0.42-0.88; P = .008; Figure 2A), and the 12-month freedom from treatment failure rate for VEX was 34.3% vs 8.6% for paclitaxel.
Figure 2. Time-to-treatment Failure (TTF) in the METEORA-II Trial.
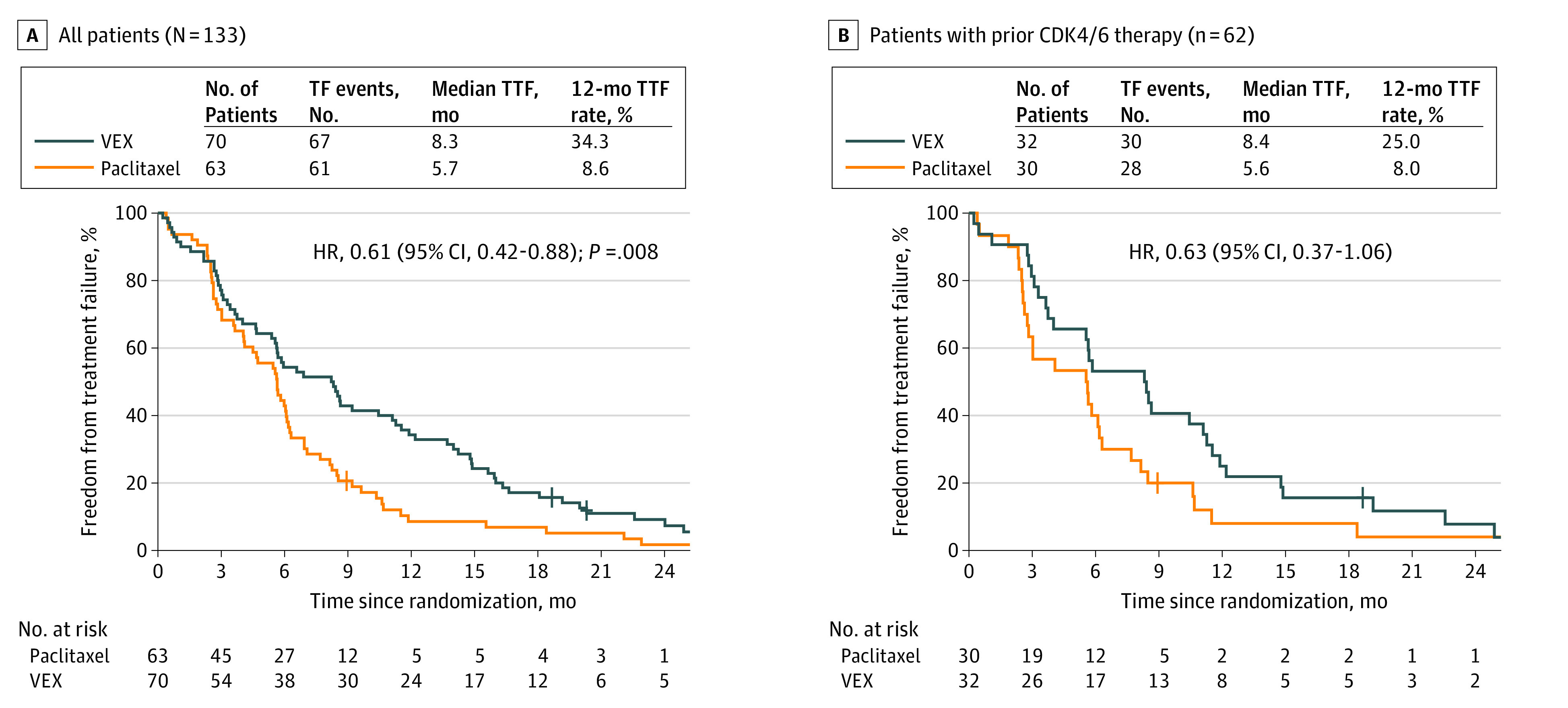
The end-of-treatment date for defining TTF was the date the last paclitaxel intravenous dose was administered plus 7 days or the date when at least 1 of the 3 oral medications (vinorelbine plus cyclophosphamide plus capecitabine) was taken for the last time. A maximum 3-week delay of administration of any study medication was allowed. VEX indicates vinorelbine plus cyclophosphamide plus capecitabine.
The treatment effects were similar across the subgroups defined by prior therapy for MBC (eTable 2 in Supplement 2). Patients previously treated with CDK4/6 inhibitors had a median TTF of 8.4 (95% CI, 3.8-11.3) vs 5.6 (95% CI, 2.8-6.3) months for the VEX vs paclitaxel groups (HR, 0.63; 95% CI, 0.37-1.06); the 12-month freedom from treatment failure rate for VEX was 25.0% vs 8.0% for paclitaxel (Figure 2B).
Secondary End Points
The median PFS was 11.1 (8.3-13.8) months for VEX vs 6.9 (5.4-10.1) months for paclitaxel, with 118 patients having a PFS event reported (HR, 0.67; 95% CI, 0.46-0.96, P = .03; eFigure, A in Supplement 2). The 12-month PFS was 43.5% (VEX) vs 21.9% (paclitaxel). The disease control rate was 68.6% (48 patients; 95% CI, 56.4-79.1) in the VEX group vs 55.6% (35 patients; 95% CI, 42.5-68.1) in the paclitaxel group. Overall survival did not differ between the groups; 61 patients (45.9%) died, with a median OS of 29.5 (95% CI, 19.4-undefined) months for VEX vs 33.7 (95% CI, 20.0-undefined) months for paclitaxel (HR, 0.98; 95% CI, 0.59-1.63; P = .90; eFigure, B in Supplement 2).
Treatment and Safety
In total, 1203 cycles (466 paclitaxel; 737 VEX) were administered (eTables 3-8 in Supplement 2). For 48 patients (36.1%; 95% CI, 27.9%-44.9%), at least 1 targeted grade 3 or 4 AE was reported (Table). The frequency of targeted grade 3 or 4 AEs was higher in the VEX group (42.9%; 95% CI, 31.1%-55.3%) than in the paclitaxel group (28.6%; 95% CI, 17.9%-41.3%). In total, 61 patients (45.9%; 95% CI, 37.2%-54.7%) had a grade 3 to 5 targeted or other AE reported (eTable 9 in Supplement 2). Patients in the paclitaxel group had greater paresthesia and a higher incidence of alopecia, compared with essentially no alopecia in the VEX group.
Table. Targeted Adverse Events Reported Until the Time-to-Treatment Failure Event, According to Treatment Assignmenta.
| Adverse event | Maximum AE grade, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients receiving paclitaxel (n = 63) | Patients receiving VEX (n = 70) | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Anemia | 20 (31.7) | 21 (33.3) | 2 (3.2) | NR | 19 (27.1) | 6 (8.6) | 4 (5.7) | NR |
| Alopecia | 2 (3.2) | 19 (30.2) | NR | NR | NR | 2 (2.9) | NR | NR |
| Palmar-plantar erythrodysesthesia syndrome | 3 (4.8) | 2 (3.2) | NR | NR | 4 (5.7) | 1 (1.4) | 4 (5.7) | NR |
| Anaphylaxis | NR | NR | 1 (1.6) | NR | NR | NR | NR | NR |
| Allergic reaction | 2 (3.2) | 4 (6.3) | 1 (1.6) | NR | 1 (1.4) | 1 (1.4) | NR | NR |
| Anorexia | NR | 1 (1.6) | NR | NR | 1 (1.4) | 4 (5.7) | 1 (1.4) | NR |
| Diarrhea | 8 (12.7) | 4 (6.3) | NR | NR | 15 (21.4) | 7 (10.0) | 1 (1.4) | NR |
| Constipation | 9 (14.3) | 4 (6.3) | NR | NR | 4 (5.7) | 2 (2.9) | NR | NR |
| Mucositis oral | 5 (7.9) | 2 (3.2) | NR | NR | 4 (5.7) | 1 (1.4) | NR | NR |
| Nausea | 12 (19.0) | 5 (7.9) | NR | NR | 19 (27.1) | 9 (12.9) | 2 (2.9) | NR |
| Vomiting | 3 (4.8) | 6 (9.5) | NR | NR | 7 (10.0) | 1 (1.4) | 1 (1.4) | NR |
| Peripheral sensory neuropathy | 16 (25.4) | 14 (22.2) | 5 (7.9) | NR | 4 (5.7) | NR | NR | NR |
| Infection | 5 (7.9) | 14 (22.2) | 1 (1.6) | NR | 2 (2.9) | 9 (12.9) | 2 (2.9) | NR |
| Arthralgia and/or myalgia | 13 (20.6) | 7 (11.1) | 1 (1.6) | NR | 13 (18.6) | 4 (5.7) | NR | NR |
| Injection site reaction | NR | 1 (1.6) | NR | NR | NR | NR | NR | NR |
| Fatigue | 17 (27.0) | 17 (27.0) | 1 (1.6) | NR | 17 (24.3) | 12 (17.1) | 5 (7.1) | NR |
| Acute coronary syndrome | NR | 1 (1.6) | NR | NR | NR | NR | NR | NR |
| Supraventricular tachycardia | 1 (1.6) | 1 (1.6) | 1 (1.6) | NR | 2 (2.9) | 1 (1.4) | 1 (1.4) | NR |
| Ventricular arrhythmia | 1 (1.6) | NR | NR | NR | NR | NR | NR | NR |
| Optic nerve disorder | 1 (1.6) | NR | NR | NR | 1 (1.4) | NR | NR | NR |
| Thrombocytopenia | 1 (1.6) | NR | NR | NR | 8 (11.4) | NR | NR | NR |
| Neutrophil count decreased | 2 (3.2) | 10 (15.9) | 8 (12.7) | 1 (1.6) | 2 (2.9) | 7 (10.0) | 11 (15.7) | 9 (12.9) |
| Aspartate aminotransferase increased | 16 (25.4) | 3 (4.8) | NR | NR | 12 (17.1) | 1 (1.4) | 3 (4.3) | NR |
| Any targetedb AE | 4 (6.3) | 40 (63.5) | 17 (27.0) | 1 (1.6) | 14 (20.0) | 21 (30.0) | 21 (30.0) | 9 (12.9) |
| Other grade 3 or 4 AE | NR | NR | 7 (11.1) | 1 (1.6) | NR | NR | 18 (25.7) | 5 (7.1) |
Abbreviations: AE, adverse event; NR, none reported.
Data on AEs were collected without regard to trial medication, and AEs were assessed according to the terminology and grading of severity used the Common Terminology Criteria for Adverse Events dictionary, version 4. NR indicates that no AEs of that grade were reported. There were 3 deaths during study treatment, 1 in the paclitaxel group and 2 in the VEX group (see eAppendix 2 in Supplement 2).
Two targeted AEs, heart failure and sinus bradycardia, were not reported for any patients and are not included here.
Three deaths during the study treatment and 1 death not clearly attributable to progression of breast cancer were reported, 3 in the VEX group and 1 in the paclitaxel group (eAppendix 2 in Supplement 2).
Discussion
The METEORA-II randomized clinical trial met its primary objective, demonstrating that in patients with ER+/ERBB2− MBC, the oral chemotherapy combination with metronomic VEX provided a clinical benefit in terms of TTF that was superior to the standard treatment with IV weekly paclitaxel. In the overall patient population, the median TTF was 8.3 months for VEX vs 5.7 months for paclitaxel. At 12 months, more than one-third vs fewer than 10% of patients, respectively, were still receiving therapy.
These results were consistent in the subgroup pretreated with CDK4/6 inhibitors, reflecting the current standard of first-line chemotherapy administered after ET and CDK4/6 inhibitor failure.6 The results for PFS and disease control were consistent with those for TTF.
Previous studies7,8 on first-line IV chemotherapy compared with oral chemotherapy showed similar outcomes; as these trials date back more than a decade, they had heterogeneous populations, and most women8 had luminal disease. Across several randomized phase 2 or 3 studies,9 the median PFS ranged from 6.0 to 7.9 months with first-line single-agent capecitabine in the ERBB2− population. Accordingly, the PFS and TTF outcomes obtained with VEX are promising and reassuring, although we cannot rule out that the improvement observed with VEX was due to a combination of the 3 drugs, which may have delayed the onset of resistant disease.
Unexpectedly, grade 3 or 4 targeted AEs were more frequently reported in the VEX group; however, grade 4 AEs were either rare or not found except for the neutrophil count. In contrast, patients in the paclitaxel group, as expected, had greater paresthesia and a higher incidence of alopecia, compared with minimal alopecia in the VEX group. Hematologic toxic effects were known and frequently reported in a previous study4 with metronomic chemotherapy, although they were mainly grade 1 or 2 and of short duration, while asthenia was less frequently reported10 than in the METEORA study.
Metronomic VEX’s safety profile in METEORA-II was similar to that of other metronomic regimens in MBC and still manageable.11,12 Another important aspect is the cost of treatment. Inequities are being created because of the rising expense of cancer treatment.13 The metronomic VEX schedule combines 3 oral drugs whose monthly overall costs are limited, allowing a less expensive but still effective medicine for both high-income and low- and middle-income countries.
Limitations
This is a randomized open-label trial comparing IV with oral treatment has the potential for bias, including in the documentation of disease progression. METEORA-II chose to emphasize a more clinically relevant end point of TTF that encompasses all the reasons patients discontinue therapy. Nevertheless, the PFS and TTF results are consistent. Seven patients, including 6 who were assigned to paclitaxel, withdrew before treatment initiation, which is methodologically undesirable; interestingly, declining the IV or desiring the oral regimen were the stated reasons. Furthermore, due to a lack of financial support, the study protocol did not include a quality-of-life assessment.
Conclusions
The METEORA-II randomized clinical trial demonstrated the benefit of a metronomic oral regimen over an IV chemotherapy regimen in patients with ER+/ERBB2− MBC but no improvement in OS. When deciding on chemotherapy, oral VEX offers a longer disease control than weekly paclitaxel, despite the increased but still manageable toxic effects. Additionally, oral VEX is a home-based treatment that requires fewer hospital visits and does not cause hair loss.
Trial Protocol
eAppendix 1
eMethods
eTable 1. Characteristics of the 133-Patient METEORA-II Efficacy Analysis Population
eFigure 1. METEORA-II Progression-Free Survival and Overall Survival
eTable 2. Subgroup Analyses
eTable 3. Numbers of Cycles
eTable 4. Reasons for TTF Event
eTable 5. Paclitaxel Dosing
eTable 6. Paclitaxel Treatment Modifications
eTable 7. VEX Agent Stopping
eTable 8. VEX Treatment Modifications
eTable 9. Patients Experiencing ≥1 Targeted AE
eAppendix 2
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 (suppl 7). [DOI] [PubMed] [Google Scholar]
- 3.Munzone E, Pagan E, Bagnardi V, et al. Systematic review and meta-analysis of post-progression outcomes in ER+/HER2- metastatic breast cancer after CDK4/6 inhibitors within randomized clinical trials. ESMO Open. 2021;6(6):100332. doi: 10.1016/j.esmoop.2021.100332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montagna E, Palazzo A, Maisonneuve P, et al. Safety and efficacy study of metronomic vinorelbine, cyclophosphamide plus capecitabine in metastatic breast cancer: a phase II trial. Cancer Lett. 2017;400:276-281. doi: 10.1016/j.canlet.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 6.Martin JM, Handorf EA, Montero AJ, Goldstein LJ. Systemic therapies following progression on first-line CDK4/6-inhibitor treatment: analysis of real-world data. Oncologist. 2022;27(6):441-446. doi: 10.1093/oncolo/oyac075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talbot DC, Moiseyenko V, Van Belle S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer. 2002;86(9):1367-1372. doi: 10.1038/sj.bjc.6600261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stockler MR, Harvey VJ, Francis PA, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29(34):4498-4504. doi: 10.1200/JCO.2010.33.9101 [DOI] [PubMed] [Google Scholar]
- 9.O’Shaughnessy JA, Kaufmann M, Siedentopf F, et al. Capecitabine monotherapy: review of studies in first-line HER-2-negative metastatic breast cancer. Oncologist. 2012;17(4):476-484. doi: 10.1634/theoncologist.2011-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cazzaniga ME, Cortesi L, Ferzi A, et al. ; VICTOR Study Group . Metronomic chemotherapy with oral vinorelbine (mVNR) and capecitabine (mCAPE) in advanced HER2-negative breast cancer patients: is it a way to optimize disease control? Final results of the VICTOR-2 study. Breast Cancer Res Treat. 2016;160(3):501-509. doi: 10.1007/s10549-016-4009-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cazzaniga ME, Capici S, Cordani N, et al. Metronomic chemotherapy for metastatic breast cancer treatment: clinical and preclinical data between lights and shadows. J Clin Med. 2022;11(16):4710. doi: 10.3390/jcm11164710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nature Reviews Clinical Oncology. 2010;7(8):455-465. [DOI] [PubMed] [Google Scholar]
- 13.Prager GW, Braga S, Bystricky B, et al. Global cancer control: responding to the growing burden, rising costs and inequalities in access. ESMO Open. 2018;3(2):e000285. doi: 10.1136/esmoopen-2017-000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1
eMethods
eTable 1. Characteristics of the 133-Patient METEORA-II Efficacy Analysis Population
eFigure 1. METEORA-II Progression-Free Survival and Overall Survival
eTable 2. Subgroup Analyses
eTable 3. Numbers of Cycles
eTable 4. Reasons for TTF Event
eTable 5. Paclitaxel Dosing
eTable 6. Paclitaxel Treatment Modifications
eTable 7. VEX Agent Stopping
eTable 8. VEX Treatment Modifications
eTable 9. Patients Experiencing ≥1 Targeted AE
eAppendix 2
Nonauthor Collaborators
Data Sharing Statement