Key Points
Question
Does treatment with niraparib prolong progression-free survival in patients with newly diagnosed advanced ovarian cancer?
Finding
In the PRIME phase 3 randomized clinical trial of 384 patients with newly diagnosed advanced ovarian cancer after receiving first-line platinum-based chemotherapy, including those who underwent R0 resection at primary debulking surgery, treatment with niraparib with an individualized starting dose significantly extended progression-free survival vs placebo and reduced the risk of disease progression or death by 55%.
Meaning
The results of this randomized clinical trial support niraparib monotherapy as a standard of care after first-line platinum-based chemotherapy in a broad patient population with advanced ovarian cancer.
Abstract
Importance
The efficacy of niraparib maintenance therapy with an individualized starting dose (ISD) warrants further investigation in a broad population with newly diagnosed advanced ovarian cancer (aOC), including patients without postoperative residual disease.
Objective
To evaluate the efficacy and safety of niraparib with an ISD in a broad population with newly diagnosed aOC (R0 resection permitted).
Design, Setting, and Participants
This multicenter, randomized, double-blind, placebo-controlled, phase 3 study was conducted in China and enrolled 384 patients with newly diagnosed aOC who received primary or interval debulking surgery and responded to treatment with first-line platinum-based chemotherapy. By data cutoff (September 30, 2021), median follow-up for progression-free survival (PFS) was 27.5 (IQR, 24.7-30.4) months.
Interventions
Patients were randomized 2:1 to receive niraparib or placebo with ISD (200 mg/d for those with a body weight of <77 kg and/or platelet count of <150 ×103/μL [to convert to ×109/μL, multiply by 1] at baseline; 300 mg/d otherwise) stratified by germline BRCA variant status, tumor homologous recombination deficiency status, neoadjuvant chemotherapy, and response to first-line platinum-based chemotherapy.
Main Outcomes and Measurements
The primary end point was blinded, independent central review–assessed PFS in the intention-to-treat population.
Results
A total of 384 patients were randomized (255 niraparib [66.4%]; median [range] age, 53 [32-77] years; 129 placebo [33.6%]; median [range] age, 54 [33-77] years), and 375 (247 niraparib [65.9%], 128 placebo [34.1%]) received treatment at a dose of 200 mg per day. Median PFS with niraparib vs placebo was 24.8 vs 8.3 months (hazard ratio [HR], 0.45; 95% CI, 0.34-0.60; P < .001) in the intention-to-treat population; not reached vs 10.8 months (HR, 0.40; 95% CI, 0.23-0.68) and 19.3 vs 8.3 months (HR, 0.48; 95% CI, 0.34-0.67) in patients with and without germline BRCA variants, respectively; not reached vs 11.0 months (HR, 0.48; 95% CI, 0.34-0.68) and 16.6 vs 5.5 months (HR, 0.41; 95% CI, 0.22-0.75) in homologous recombination deficient and proficient patients, respectively; and 24.8 vs 8.3 months (HR, 0.44; 95% CI, 0.32-0.61) and 16.5 vs 8.3 months (HR, 0.27; 95% CI, 0.10-0.72) in those with optimal and suboptimal debulking, respectively. Similar proportions of niraparib-treated and placebo-treated patients (6.7% vs 5.4%) discontinued treatment due to treatment-emergent adverse events.
Conclusion and Relevance
This randomized clinical trial found that niraparib maintenance therapy prolonged PFS in patients with newly diagnosed aOC regardless of postoperative residual disease or biomarker status. The ISD was effective and safe in the first-line maintenance setting.
Trial Registration
ClinicalTrials.gov Identifier: NCT03709316
This randomized clinical trial examines the efficacy and safety of treatment with niraparib with an individualized starting dose in Chinese patients with newly diagnosed advanced ovarian cancer.
Introduction
Ovarian cancer is the leading cause of death among gynecologic cancers.1 At diagnosis, approximately 75% of patients with ovarian cancer present with advanced disease.2,3 The standard treatment for newly diagnosed advanced ovarian cancer is surgical cytoreduction and platinum-based chemotherapy. Although 59% to 81% of patients respond to treatment with first-line chemotherapy, around 75% of these patients relapse within a median time of 18 to 24 months without maintenance therapy.4
Bevacizumab that was added to chemotherapy and then used alone as first-line maintenance therapy provided limited improvement in progression-free survival (PFS) vs chemotherapy alone5 and extended overall survival (OS) only in patients with stage IV disease.6 Polyadenosine diphosphate–ribose polymerase (PARP) inhibitors, such as olaparib and niraparib, can substantially extend PFS in the first-line maintenance setting and transformed the maintenance treatment landscape. Olaparib improved PFS vs placebo among a subset of patients with BRCA-variant tumors.7 Olaparib plus bevacizumab extended PFS vs bevacizumab alone, but only in patients with homologous recombination deficiency (HRD).8
The phase 3 PRIMA study demonstrated that niraparib, a potent, highly selective PARP inhibitor, significantly prolonged PFS vs placebo in the first-line maintenance setting regardless of HRD status.9 However, PRIMA mainly enrolled patients at a very high risk of disease progression and excluded patients with stage III disease who had undergone R0 resection at primary debulking surgery and used an individualized starting dose (ISD) based on baseline body weight and platelet count, a strategy to improve the safety profile of niraparib,10 in around 35% of patients only.9,11 Thus, the efficacy and safety of niraparib with ISD still need further prospective assessment. The objective of this PRIME randomized clinical trial was to evaluate the efficacy and safety of niraparib with the prospective adoption of ISD as maintenance therapy in a broad patient population with newly diagnosed advanced ovarian cancer who responded to treatment with first-line platinum-based chemotherapy, regardless of postoperative residual disease status or biomarker status.
Methods
Study Design and Participants
The PRIME study was a randomized, double-blind, placebo-controlled, phase 3 randomized clinical trial conducted at 29 hospitals in China (Supplement 1, Supplement 2, and eTable 1 in Supplement 3). The trial was conducted in accordance with the Declaration of Helsinki, the Good Clinical Practice, and all relevant local laws and regulations. The study was approved by the ethics committees of all participating centers. All patients provided written informed consent. This report followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Eligible patients were 18 years or older and had a new diagnosis of histologically confirmed, high-grade (dominantly) serous or endometrioid epithelial ovarian cancer, fallopian tube carcinoma, or primary peritoneal carcinoma (collectively defined as ovarian cancer) that was classified as stage III or IV according to the International Federation of Gynecology and Obstetrics criteria. Patients who received primary or interval debulking surgery were eligible regardless of cytoreductive surgery outcome (optimal, residual tumor ≤1 cm; or suboptimal, residual tumor >1 cm). Patients received 6 to 9 cycles of platinum-based chemotherapy and had a complete response or partial response as assessed by investigators. The full eligibility criteria are provided in the eMethods in Supplement 3.
Randomization and Masking
Patients were centrally randomized 2:1 to receive niraparib or placebo (both provided by the study sponsor) through an interactive web-response system, as stratified by germline BRCA variant status (yes or no), tumor HRD status (positive or negative including unknown) on the HRD assay (BGI Genomics), receipt of neoadjuvant chemotherapy (yes or no), and clinical response to treatment with first-line platinum-based chemotherapy (complete or partial). The randomization algorithm was developed by statisticians who were not involved in the study. Before randomization, peripheral blood samples and tumor samples underwent central testing for germline BRCA variant status (gBRCA testing assay; BGI Genomics) and tumor HRD status (HRD testing assay), respectively. The details of tumor HRD testing are provided in the eMethods in Supplement 3.
Patients with germline BRCA variants were randomized immediately, but those without were randomized after their tumor HRD status was identified. Study participants, investigators, central assessors, and the study sponsor were masked to the treatment assignment, and masking was achieved using a placebo with an identical appearance to niraparib.
Procedures
Patients with a body weight of fewer than 77 kg and/or a platelet count of less than 150 × 103/μL (to convert to × 109/μL, multiply by 1) at baseline received niraparib, 200 mg (2 100-mg capsules), or matched placebo orally once daily (QD).10 Other patients received niraparib, 300 mg (3 100-mg capsules), or matched placebo orally QD. Patients were treated in 28-day cycles for 36 months or until disease progression, death, intolerable toxic effects, withdrawal, or loss to follow-up, whichever occurred first.
Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 4.03. Criteria for treatment interruptions and dose reductions are provided in eTables 2 and 3 and eFigures 1 to 3 in Supplement 3. Without any treatment interruption or dose reduction during the first 2 cycles, the daily dose for patients receiving 200 mg could be increased to 300 mg at the investigators’ discretion. Patients with treatment interruptions for more than 28 consecutive days discontinued participation in the study. The complete discontinuation criteria are provided in the eMethods in Supplement 3.
Tumor assessment was conducted using computed tomography or magnetic resonance imaging once every 12 weeks until disease progression as assessed by blinded independent central review (BICR; provided by Parexel) according to the Response Evaluation Criteria in Solid Tumors, version 1.1.12 For patients who discontinued treatment, tumor assessment was continued as planned until disease progression or initiation of subsequent anticancer therapy.
End Points
The primary end point was BICR-assessed PFS in the intention-to-treat (ITT) population. Progression-free survival was defined as the duration from randomization to disease progression or death of any cause, whichever occurred earlier. A sensitivity analysis was conducted to assess the agreement between BICR-assessed and investigator-assessed PFS.
The secondary end points included OS (duration from randomization to death of any cause) and time to first subsequent anticancer therapy (TFST; duration from randomization to the initiation of first subsequent anticancer therapy or death) in the ITT population, as well as PFS and OS in the HRD subgroup, comprising patients with germline BRCA variants, positive tumor HRD status, or both.
Statistical Analysis
It was estimated that 246 PFS events would provide a power of 90% to detect a significant difference in PFS between the niraparib and placebo groups at a 2-sided significance level of .05, assuming a 0.65 hazard ratio (HR) for disease progression or death (niraparib vs placebo) in the ITT population with a median PFS (mPFS) of 10.8 months with niraparib vs 7.0 months with placebo. A sample size of 381 patients (254 niraparib [66.7%]; 127 placebo [33.3%]) was determined as appropriate. The trial was planned to include 20% or more patients with germline BRCA variants. The primary efficacy analysis was conducted on the occurrence of 246 PFS events or on September 30, 2021, whichever came earlier.
A hierarchical testing method was used to test PFS and TFST sequentially in the ITT population. Progression-free survival was analyzed with a stratified log-rank test using the randomization stratification factors. A stratified Cox proportional hazards model was used to calculate the HR (niraparib vs placebo) and its 95% CI, in which the baseline hazard function was allowed to vary across randomization strata and treatment group was the only covariate. The proportional hazards assumption was verified graphically using log-log (event times) vs log (time) plots. The PFS function was estimated using the Kaplan-Meier method. All secondary efficacy outcomes were analyzed using similar methods to the primary efficacy analysis. Given the many homologous recombination indeterminate patients, post hoc subgroup analyses were conducted separately for them and homologous recombination–proficient (HRp) patients, who constituted 1 subgroup in the statistical analysis plan (SAP; Supplement 2). Similarly, a post hoc subgroup analysis was performed for patients with suboptimal debulking, who were combined with those with missing surgical outcome as 1 subgroup in the SAP. All other reported subgroup analyses were prespecified in the SAP. No multiple testing adjustment was conducted for subgroup analyses.
An efficacy analysis was conducted in the ITT population, which comprised all randomized patients. A safety analysis was conducted in the safety population, which comprised all patients who received 1 dose or more of the study drug. All statistical analyses were performed with the SAS software, version 9.4 or later (SAS Institute). The BICR-assessed PFS is reported unless otherwise stated.
Results
Study Population
Between June 29, 2018, and November 11, 2019, 384 patients were enrolled and randomized (255 niraparib [66.4%], 129 placebo [33.6%]) (Figure 1). All the randomized patients received 1 dose or more of the study drug; thus they were included for the efficacy and safety analyses. The starting daily dose was 200 mg in 375 patients (97.7%; 247 niraparib [65.9%], 128 placebo [34.1%]) and 300 mg in 9 patients (2.3%; 8 niraparib, 1 placebo). At the data cutoff (September 30, 2021), 131 patients (34.1%; 102 niraparib [77.9%], 29 placebo [22.1%]) were still receiving the study treatment.
Figure 1. Patient Flow Diagram.
Patient demographic and baseline characteristics were well balanced between the niraparib and placebo groups (Table). Of the 384 randomized patients, the median age was 54 years (range, 32-77 years), 108 (28.1%) had stage IV disease, 180 (46.9%) received neoadjuvant chemotherapy, 50 (13.0%) achieved suboptimal debulking, and 69 (18.0%) had a partial response to platinum-based chemotherapy. A total of 125 patients (32.6%) carried germline BRCA variants. The homologous recombination status was deficient in 257 patients (66.9%), proficient in 85 (22.1%), and indeterminate in 42 (10.9%).
Table. Baseline Characteristics.
| Characteristics | Niraparib (n = 255) | Placebo (n = 129) |
|---|---|---|
| Age, median (range), y | 53 (32-77) | 54 (33-77) |
| Weight, median (range), kg | 59.0 (39.5-100.0) | 57.0 (37.0-97.0) |
| ECOG score, No. (%) | ||
| 0 | 98 (38.4) | 52 (40.3) |
| 1 | 157 (61.6) | 77 (59.7) |
| Cytoreductive surgery outcome, No. (%)a | ||
| Optimal debulking (R0/R1) | 193 (75.7) | 105 (81.4) |
| Suboptimal debulking (R2) | 36 (14.1) | 14 (10.9) |
| Missing | 26 (10.2) | 10 (7.8) |
| FIGO stage, No. (%) | ||
| III | 182 (71.4) | 94 (72.9) |
| A | 8 (3.1) | 5 (3.9) |
| B | 14 (5.5) | 8 (6.2) |
| C | 154 (60.4) | 80 (62.0) |
| Not specified | 6 (2.4) | 1 (0.8) |
| IV | 73 (28.6) | 35 (27.1) |
| Primary tumor location, No. (%) | ||
| Ovary | 229 (89.8) | 117 (90.7) |
| Fallopian tube | 19 (7.5) | 9 (7.0) |
| Peritoneum | 7 (2.7) | 3 (2.3) |
| Histologic subtype | ||
| Serous ovarian cancer | 253 (99.2) | 128 (99.2) |
| Endometrioid carcinoma | 2 (0.8) | 0 |
| Other | 0 | 1 (0.8) |
| Neoadjuvant chemotherapy, No. (%) | ||
| Yes | 121 (47.5) | 59 (45.7) |
| No | 134 (52.5) | 70 (54.3) |
| Clinical response after platinum-based chemotherapy, No. (%) | ||
| Complete response | 212 (83.1) | 103 (79.8) |
| Partial response | 43 (16.9) | 26 (20.2) |
| Cancer antigen 125 level, No. (%) | ||
| ≤35 U/mL | 252 (98.8) | 128 (99.2) |
| >35 U/mL | 3 (1.2) | 1 (0.8) |
| Germline BRCA variant status, No. (%)b | ||
| Germline BRCA variant | 85 (33.3) | 40 (31.0) |
| Nongermline BRCA variant | 170 (66.7) | 89 (69.0) |
| Homologous recombination status, No. (%)c | ||
| Deficient | 170 (66.7) | 87 (67.4) |
| Proficient | 60 (23.5) | 25 (19.4) |
| Indeterminate | 25 (9.8) | 17 (13.2) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; HRD, homologous recombination deficiency.
SI conversion factor: To convert cancer antigen 125 to kU/L, multiply by 1.
Optimal debulking was defined as the largest residual tumor nodule measuring 1 cm or less; suboptimal debulking was defined as the largest residual tumor nodule measuring more than 1 cm.
Germline BRCA variants included pathogenic and suspected pathogenic ones; the others were classified as nongermline BRCA variants.
The homologous recombination status was deficient if a patient had germline BRCA variants and/or the tumor HRD status was positive on the HRD assay, proficient if a patient had no germline BRCA variants and the tumor HRD status was negative on the HRD assay, and indeterminate if a patient had no germline BRCA mutations and the tumor HRD status was unknown on the HRD assay.
Efficacy
By the data cutoff, median follow-up for PFS was 27.5 months, with 27.5 months (95% CI, 25.3-27.6) in the niraparib group and 27.6 months (95% CI, 24.9-30.3) in the placebo group. A total of 209 patients (54.4%; 123 niraparib [58.9%], 86 placebo [41.4%]) had disease progression or died (Figure 2A). The median study drug exposure (range) was 19.3 (0.1-38.5) months with niraparib and 10.2 (0.4-38.2) months with placebo. The compliance rate (percentage of administered capsules to planned capsules) was at least 80% in 238 (93.3%) of niraparib-treated patients and 127 (98.4%) of placebo-treated patients. The median relative dose intensity (percentage of administered doses to initially planned doses; range) was 100.0% (range, 26.0%-147.0%) with niraparib and 100.0% (range, 36.0%-147.0%) with placebo.
Figure 2. Progression-Free Survival and Overall Survival in the Intention-to-Treat Population.
NE indicates not estimable; NR, not reached.
In the ITT population, mPFS was 24.8 months (95% CI, 19.2 to not estimable [NE]) with niraparib vs 8.3 months (95% CI, 7.3-11.1) with placebo (HR, 0.45; 95% CI, 0.34-0.60; P < .001) (Figure 2A). The results of PFS assessment by investigators were comparable with those by BICR (eFigure 4 and eTable 4 in Supplement 3).
In the HRD subgroup, mPFS was not reached (95% CI, 22.3 to NE) vs 11.0 months (95% CI, 8.3-13.8) with niraparib vs placebo (HR, 0.48; 95% CI, 0.34-0.68) (eFigure 5 in Supplement 3). In the ITT population, median TFST was 29.2 months (95% CI, 22.4 to NE) with niraparib and 11.9 months (95% CI, 8.8-14.8) with placebo (HR, 0.45; 95% CI, 0.34-0.59; P < .001) (eFigure 6 in Supplement 3). Data for OS were not mature in the ITT population (Figure 2B). By the data cutoff, 65 patients (16.9%; 37 niraparib [56.9%], 28 placebo [43.1%]) had died (HR, 0.63; 95% CI, 0.38-1.03), and the estimated OS rate at 24 months was 87.3% for niraparib vs 82.7% for placebo. Data for OS for the HRD subgroup are provided in eTable 5 in Supplement 3.
Exploratory subgroup analyses (Figure 3) consistently showed that niraparib improved PFS vs placebo. Niraparib significantly prolonged mPFS vs placebo in patients with germline BRCA variants (not reached vs 10.8 months; HR, 0.40; 95% CI, 0.23-0.68) and those without (19.3 vs 8.3 months; HR, 0.48; 95% CI, 0.34-0.67) (eFigure 7 in Supplement 3). In HRp and homologous recombination–indeterminate patients (eFigure 8 in Supplement 3), mPFS with niraparib vs placebo was 16.6 vs 5.5 months (HR, 0.41; 95% CI, 0.22-0.75) and 13.8 vs 5.4 months (HR, 0.36; 95% CI, 0.15-0.86), respectively. Niraparib significantly extended mPFS vs placebo in those with optimal debulking (24.8 vs 8.3 months; HR, 0.44; 95% CI, 0.32-0.61) and those with suboptimal debulking (16.5 vs 8.3 months; HR, 0.27; 95% CI, 0.10-0.72).
Figure 3. Exploratory Subgroup Analyses of Progression-Free Survival.
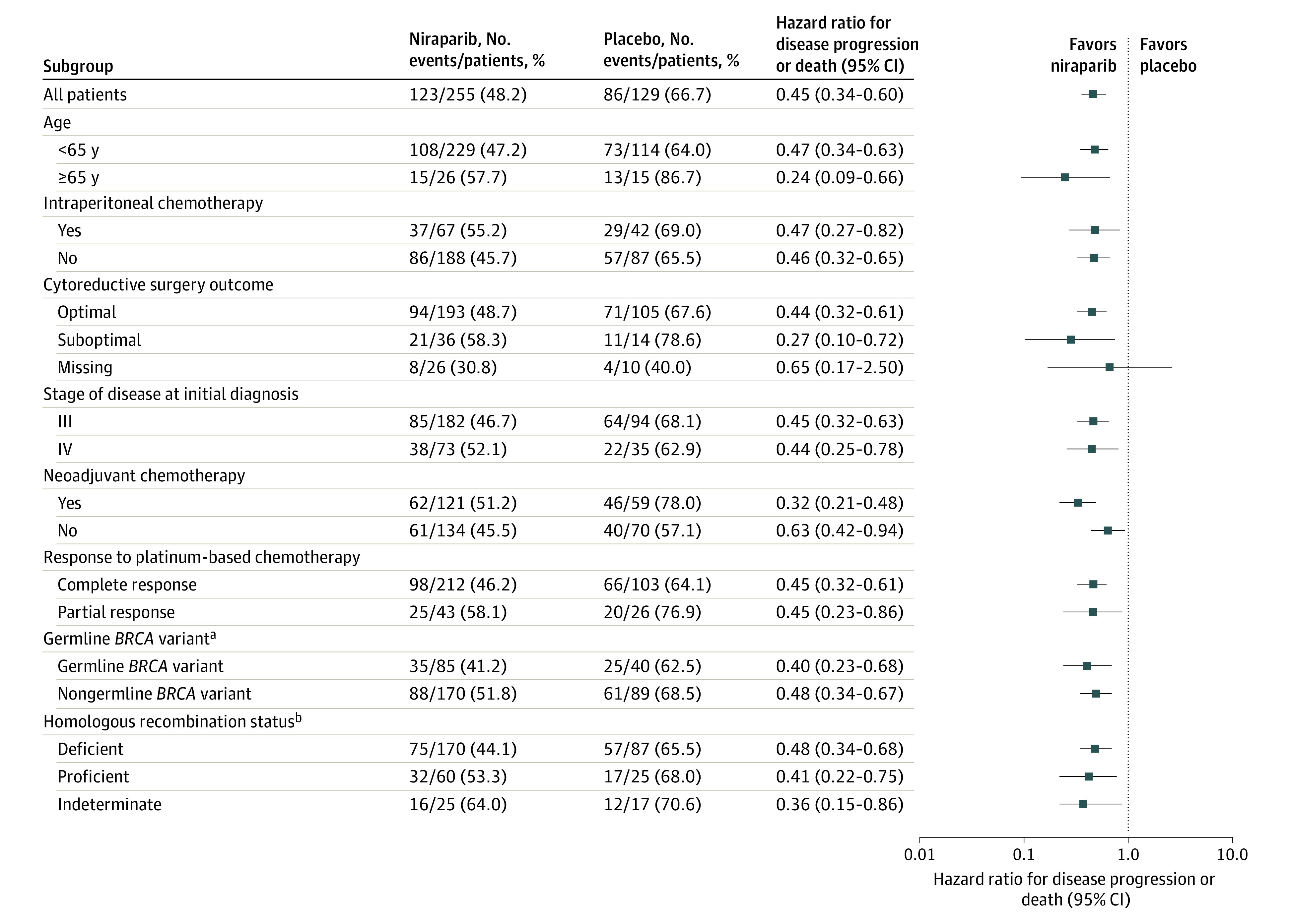
aThe germline BRCA variants include pathogenic and suspected pathogenic ones; the others were classified as nongermline BRCA variants.
bThe homologous recombination status was deficient if a patient had germline BRCA variants and/or the tumor homologous recombination deficiency (HRD) status was positive on the HRD assay (BGI Genomics), proficient if a patient had no germline BRCA variants and the tumor HRD status was negative on the HRD assay, and indeterminate if a patient had no germline BRCA variants and the tumor HRD status was unknown on the HRD assay.
Safety
Treatment-emergent adverse events (TEAEs) are presented in eTables 6 and 7 in Supplement 3. Grade 3 or higher TEAEs were reported in 139 niraparib-treated patients (54.5%) and 23 placebo-treated patients (17.8%). In the niraparib group, the most common grade 3 or higher TEAEs included anemia (18.0%), neutrophil count decreased (17.3%), platelet count decreased (14.1%), and white blood cell count decreased (6.7%). Serious TEAEs occurred in 48 niraparib-treated patients (18.8%) and 11 placebo-treated patients (8.5%). The niraparib group had 1 case each of acute myeloid leukemia and myelodysplastic syndrome, and the patient with acute myeloid leukemia died of this TEAE.
Dose reductions due to TEAEs occurred in 103 (40.4%) and 8 (6.2%) niraparib-treated and placebo-treated patients, respectively. The most common TEAEs leading to dose reductions in the niraparib group were hematologic TEAEs, including platelet count decreased (24.3%), anemia (10.2%), neutrophil count decreased (9.8%), and white blood cell count decreased (3.1%) (eTable 8 in Supplement 3). Most TEAEs were well-controlled, which led to low treatment discontinuation rates in both groups (niraparib [6.7%] vs placebo [5.4%]).
Discussion
The PRIME randomized clinical trial prospectively demonstrated that as maintenance therapy, niraparib with ISD significantly prolonged PFS vs placebo in the ITT population with newly diagnosed advanced ovarian cancer who responded to treatment with first-line platinum-based chemotherapy, irrespective of postoperative residual disease status or biomarker status. It also showed an improved tolerability profile, without new safety signals identified.
PRIME included a broader, more representative patient population compared with PRIMA through the inclusion of patients with stage III disease who achieved R0 resection at primary debulking surgery. As a result, more patients in PRIME had favorable clinical characteristics, such as stage III disease (71.9% vs 64.9%), no neoadjuvant chemotherapy (53.1% vs 33.3%), and a complete response to platinum-based chemotherapy (82.0% vs 69.4%).9 An exploratory analysis demonstrated that PFS was significantly extended by niraparib vs placebo, regardless of those clinical characteristics (Figure 3).
As the RADAR analysis of the NOVA study identified platelet count and body weight at baseline as risk factors for increased incidence of grade 3 or higher thrombocytopenia in niraparib-treated patients,10 PRIMA changed from a fixed starting dose at 300 mg, QD to an ISD based on body weight and platelet count at baseline through protocol amendment. As a result, 35% of patients in PRIMA used an ISD.9,11 In contrast, all patients in PRIME received an ISD. Notably, comparable proportions of placebo-treated patients from PRIME and PRIMA experienced grade 3 or higher TEAEs (17.8% vs 18.9%) and treatment discontinuation due to TEAEs (5.4% vs 2.5%), but grade 3 or higher TEAEs (54.5% vs 70.5%) and treatment discontinuation due to TEAEs (6.7% vs 12.0%) were reported much less frequently in niraparib-treated patients from PRIME than in their counterparts from PRIMA.9 These results indicate that ISD can lead to better safety and tolerability profiles of niraparib than a fixed starting dose at 300 mg QD and further support the use of ISD in clinical practice.
The treatment outcome with niraparib appeared to be better in PRIME than in PRIMA, as demonstrated by the much longer mPFS with niraparib (24.8 vs 13.8 months).9 This better treatment outcome may be attributed to the previously mentioned more favorable clinical characteristics, improved tolerability because of the prospective adoption of ISD (which could lead to better adherence and efficacy), and the higher proportion of patients with BRCA variants, who were more responsive to treatment with niraparib than those without.13,14 Patients with germline BRCA variants comprised 33.3% of niraparib-treated patients in PRIME, which was already higher than the proportion of those with BRCA-variant tumors among niraparib-treated patients from PRIMA (31.2%),9 and another 4.0% to 6.4% of niraparib-treated patients in PRIME were expected to carry only somatic BRCA variants.15,16,17
Niraparib in the current study afforded PFS benefits vs placebo regardless of biomarker status (Figure 3), which was consistent with the findings from PRIMA.9 Focusing on patients with BRCA variants, the treatment effect of niraparib among those with germline BRCA variants from PRIME was similar to those of niraparib in PRIMA, rucaparib in ATHENA-MONO among patients with BRCA-variant tumors,9,18 and olaparib among Chinese patients with BRCA variants from SOLO1 (HR, 0.40 vs 0.40, 0.40, and 0.46, respectively).19
At the time of study initiation, no commercial HRD assay was approved in China; the laboratory-developed, unvalidated BGI HRD assay was used in this study. The HRD assay and myChoice test (Myriad Genetics), which was used in PRIMA, considered BRCA variants and evaluated genomic instability based on loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions, but they are not interchangeable due to differences in HRD scoring algorithms and the means to determine the HRD-defining threshold.9,20,21 Therefore, it warrants caution to compare homologous recombination-related results across studies because they used different methods to determine homologous recombination status. Nevertheless, in homologous recombination deficient patients identified in this study, niraparib conferred a similar extent of PFS benefits vs placebo as niraparib in PRIMA and rucaparib in ATHENA-MONO (FoundationOne CDx assay for HRD testing) in respective homologous recombination–deficient populations (HR, 0.48 vs 0.43 and 0.47).9,18 In HRp patients, who are typically deemed to be less responsive to treatment with PARP inhibitors, niraparib appeared to reduce the risk of disease progression or death vs placebo by a larger extent in PRIME than PRIMA (HR, 0.41 vs 0.68), which could be partly attributed to the previously described increased adherence due to improved tolerability.
Limitations
This study had several limitations. First, the laboratory-developed, unvalidated HRD assay was used to determine tumor HRD status. Homologous recombination status determined in this study in PRIME still demonstrated a prognostic value, given the much shorter mPFS among HRp vs homologous recombination–deficient patients (5.5 vs 11.0 months) from the placebo group. Second, R0 and R1 resections were recorded as optimal debulking, which made subgroup analysis for patients with R0 resection impossible. Third, the trial evaluated only efficacy and safety outcomes and did not assess the effect of the study treatment on quality of life.
Conclusions
This randomized clinical trial found that niraparib maintenance therapy significantly reduced the risk of disease progression or death vs placebo in a broad patient population with newly diagnosed advanced ovarian cancer after a response to treatment with first-line platinum-based chemotherapy, irrespective of postoperative residual disease status or biomarker status. An ISD of niraparib based on body weight and platelet count at baseline was effective and safe in the first-line maintenance setting.
Trial protocol
Statistical analysis plan
eTable 1. List of study sites and principal investigators
eMethods.
eTable 2. Dose modification schemes for niraparib
eTable 3. Dose modifications for non-hematologic toxicities
eFigure 1. Management and dose modification for thrombocytopenia
eFigure 2. Management and dose modification for neutropenia
eFigure 3. Management and dose modification for anemia
eFigure 4. Investigator-assessed progression-free survival in the intention-to-treat population
eFigure 5. Progression-free survival assessed by blinded independent central review in the HRD subgroup
eFigure 6. Time to first subsequent anti-cancer therapy in the intention-to-treat population
eFigure 7. Progression-free survival assessed by blinded independent central review in patients with (A) and without (B) germline BRCA mutations
eFigure 8. Progression-free survival assessed by blinded independent central review in homologous recombination proficient (A) and indeterminate (B) patients
eTable 4. Agreement between investigators and blinded independent central review in disease progression assessment
eTable 5. Overall survival
eTable 6. Summary of treatment-emergent adverse events (TEAEs)
eTable 7. Treatment-emergent adverse events occurring in ≥10% of patients in either study group
eTable 8. Treatment-emergent adverse events leading to dose reductions
Data sharing statement
References
- 1.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284-296. doi: 10.3322/caac.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240-1253. doi: 10.1016/S0140-6736(18)32552-2 [DOI] [PubMed] [Google Scholar]
- 3.Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204(6):466-478. doi: 10.1016/j.ajog.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 4.Gadducci A, Cosio S. Randomized clinical trials and real world prospective observational studies on bevacizumab, PARP inhibitors, and immune checkpoint inhibitors in the first-line treatment of advanced ovarian carcinoma: a critical review. Anticancer Res. 2021;41(10):4673-4685. doi: 10.21873/anticanres.15281 [DOI] [PubMed] [Google Scholar]
- 5.Burger RA, Brady MF, Bookman MA, et al. ; Gynecologic Oncology Group . Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473-2483. doi: 10.1056/NEJMoa1104390 [DOI] [PubMed] [Google Scholar]
- 6.Tewari KS, Burger RA, Enserro D, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37(26):2317-2328. doi: 10.1200/JCO.19.01009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495-2505. doi: 10.1056/NEJMoa1810858 [DOI] [PubMed] [Google Scholar]
- 8.Ray-Coquard I, Pautier P, Pignata S, et al. ; PAOLA-1 Investigators . Olaparib plus bevacizumab as first-Line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416-2428. doi: 10.1056/NEJMoa1911361 [DOI] [PubMed] [Google Scholar]
- 9.González-Martín A, Pothuri B, Vergote I, et al. ; PRIMA/ENGOT-OV26/GOG-3012 Investigators . Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391-2402. doi: 10.1056/NEJMoa1910962 [DOI] [PubMed] [Google Scholar]
- 10.Berek JS, Matulonis UA, Peen U, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29(8):1784-1792. doi: 10.1093/annonc/mdy181 [DOI] [PubMed] [Google Scholar]
- 11.Mirza MR, Gonzalez Martin A, Graybill W, et al. Evaluation of an individualized starting-dose of niraparib in the PRIMA/ENGOT-OV26/GOG-3012 study. J Clin Oncol. 2020;38(15)(suppl):6050. doi: 10.1200/JCO.2020.38.15_suppl.6050 [DOI] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 13.Mirza MR, Monk BJ, Herrstedt J, et al. ; ENGOT-OV16/NOVA Investigators . Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164. doi: 10.1056/NEJMoa1611310 [DOI] [PubMed] [Google Scholar]
- 14.Wu XH, Zhu JQ, Yin RT, et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2021;32(4):512-521. doi: 10.1016/j.annonc.2020.12.018 [DOI] [PubMed] [Google Scholar]
- 15.You Y, Li L, Lu J, et al. Germline and somatic BRCA1/2 mutations in 172 Chinese women with epithelial ovarian cancer. Front Oncol. 2020;10:295. doi: 10.3389/fonc.2020.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, Yang J, Li L, Cao D, Yu M, Shen K; BGI Group . Germline and somatic mutations in homologous recombination genes among Chinese ovarian cancer patients detected using next-generation sequencing. J Gynecol Oncol. 2017;28(4):e39. doi: 10.3802/jgo.2017.28.e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Shao D, Li L, et al. Germline and somatic mutations of multi-gene panel in Chinese patients with epithelial ovarian cancer: a prospective cohort study. J Ovarian Res. 2019;12(1):80. doi: 10.1186/s13048-019-0560-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40(34):3952-3964. doi: 10.1200/JCO.22.01003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Zhu J, Yin R, et al. Olaparib maintenance therapy in patients with newly diagnosed advanced ovarian cancer and a BRCA1 and/or BRCA2 mutation: SOLO1 China cohort. Gynecol Oncol. 2021;160(1):175-181. doi: 10.1016/j.ygyno.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 20.Telli ML, Timms KM, Reid J, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22(15):3764-3773. doi: 10.1158/1078-0432.CCR-15-2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, Shao M, Meng P, et al. GSA: an independent development algorithm for calling copy number and detecting homologous recombination deficiency (HRD) from target capture sequencing. BMC Bioinformatics. 2021;22(1):562. doi: 10.1186/s12859-021-04487-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eTable 1. List of study sites and principal investigators
eMethods.
eTable 2. Dose modification schemes for niraparib
eTable 3. Dose modifications for non-hematologic toxicities
eFigure 1. Management and dose modification for thrombocytopenia
eFigure 2. Management and dose modification for neutropenia
eFigure 3. Management and dose modification for anemia
eFigure 4. Investigator-assessed progression-free survival in the intention-to-treat population
eFigure 5. Progression-free survival assessed by blinded independent central review in the HRD subgroup
eFigure 6. Time to first subsequent anti-cancer therapy in the intention-to-treat population
eFigure 7. Progression-free survival assessed by blinded independent central review in patients with (A) and without (B) germline BRCA mutations
eFigure 8. Progression-free survival assessed by blinded independent central review in homologous recombination proficient (A) and indeterminate (B) patients
eTable 4. Agreement between investigators and blinded independent central review in disease progression assessment
eTable 5. Overall survival
eTable 6. Summary of treatment-emergent adverse events (TEAEs)
eTable 7. Treatment-emergent adverse events occurring in ≥10% of patients in either study group
eTable 8. Treatment-emergent adverse events leading to dose reductions
Data sharing statement




