This randomized clinical trial investigates if low-dose atropine, 0.01%, eye drops delivered nightly over a 2-year period can slow the progression of myopia in children aged 5 to 12 years in the US.
Key Points
Question
Do low-dose atropine, 0.01%, eye drops delivered nightly over 2 years slow the progression of myopia in children aged 5 to 12 years in the US?
Finding
In this randomized clinical trial including 187 children, atropine, 0.01%, eye drops did not slow myopia progression or axial elongation.
Meaning
Results do not support the use of atropine, 0.01%, eye drops nightly to slow progression of myopia in US children; future studies of myopia control should test stronger concentrations of atropine or optical and environmental approaches to reduce myopia progression, which may reduce the risk of adult myopic macular degeneration and retinal detachment.
Abstract
Importance
Controlling myopia progression is of interest worldwide. Low-dose atropine eye drops have slowed progression in children in East Asia.
Objective
To compare atropine, 0.01%, eye drops with placebo for slowing myopia progression in US children.
Design, Setting, and Participants
This was a randomized placebo-controlled, double-masked, clinical trial conducted from June 2018 to September 2022. Children aged 5 to 12 years were recruited from 12 community- and institution-based practices in the US. Participating children had low to moderate bilateral myopia (−1.00 diopters [D] to −6.00 D spherical equivalent refractive error [SER]).
Intervention
Eligible children were randomly assigned 2:1 to 1 eye drop of atropine, 0.01%, nightly or 1 drop of placebo. Treatment was for 24 months followed by 6 months of observation.
Main Outcome and Measures
Automated cycloplegic refraction was performed by masked examiners. The primary outcome was change in SER (mean of both eyes) from baseline to 24 months (receiving treatment); other outcomes included change in SER from baseline to 30 months (not receiving treatment) and change in axial length at both time points. Differences were calculated as atropine minus placebo.
Results
A total of 187 children (mean [SD] age, 10.1 [1.8] years; age range, 5.1-12.9 years; 101 female [54%]; 34 Black [18%], 20 East Asian [11%], 30 Hispanic or Latino [16%], 11 multiracial [6%], 6 West/South Asian [3%], 86 White [46%]) were included in the study. A total of 125 children (67%) received atropine, 0.01%, and 62 children (33%) received placebo. Follow-up was completed at 24 months by 119 of 125 children (95%) in the atropine group and 58 of 62 children (94%) in the placebo group. At 30 months, follow-up was completed by 118 of 125 children (94%) in the atropine group and 57 of 62 children (92%) in the placebo group. At the 24-month primary outcome visit, the adjusted mean (95% CI) change in SER from baseline was −0.82 (−0.96 to −0.68) D and −0.80 (−0.98 to −0.62) D in the atropine and placebo groups, respectively (adjusted difference = −0.02 D; 95% CI, −0.19 to +0.15 D; P = .83). At 30 months (6 months not receiving treatment), the adjusted difference in mean SER change from baseline was −0.04 D (95% CI, −0.25 to +0.17 D). Adjusted mean (95% CI) changes in axial length from baseline to 24 months were 0.44 (0.39-0.50) mm and 0.45 (0.37-0.52) mm in the atropine and placebo groups, respectively (adjusted difference = −0.002 mm; 95% CI, −0.106 to 0.102 mm). Adjusted difference in mean axial elongation from baseline to 30 months was +0.009 mm (95% CI, −0.115 to 0.134 mm).
Conclusions and Relevance
In this randomized clinical trial of school-aged children in the US with low to moderate myopia, atropine, 0.01%, eye drops administered nightly when compared with placebo did not slow myopia progression or axial elongation. These results do not support use of atropine, 0.01%, eye drops to slow myopia progression or axial elongation in US children.
Trial Registration
ClinicalTrials.gov Identifier: NCT03334253
Introduction
Myopia is increasing in prevalence worldwide.1,2,3 By 2050, more than 44 million people in the US are predicted to have myopia.4 Myopia onset is typically between 7 and 16 years of age5 with refractive error and axial length increasing until early adulthood.6 Although blurred distance vision from myopia can be corrected with eyeglasses, contact lenses, and refractive surgery, these interventions do not affect axial elongation of the eye. Longer axial length is associated with increased risk of vision impairment from retinal detachment, myopic macular degeneration, and choroidal neovascularization.7,8 Given these potential complications, many treatments to control myopia are being prescribed including topical atropine,9,10,11,12,13,14,15,16,17,18,19,20,21 soft multifocal contact lenses,21,22,23,24 overnight orthokeratology,21,25,26,27,28 specialized spectacle lenses,27,28,29 increased outdoor activity,30,31,32 and chromatic interventions.33,34 Recently, low-dose atropine eye drops have also been used in Hong Kong to delay the onset of myopia in some children.35
Atropine eye drops (0.5%-1.0%) have been used for decades to slow myopia progression.11,12,14,15,16 Although these concentrations of atropine appeared effective, they are associated with photophobia and near blur, reducing their acceptance.12,14,18 In recent trials in Asia, lower-concentration atropine eye drops (0.01%-0.05%) have slowed the progression of childhood myopia by approximately 50%.17,18 In the Atropine Treatment of Myopia (ATOM) series of randomized clinical trials, concentrations of 1.0%, 0.5%, 0.1%, and 0.01% atropine12,18 slowed myopic progression over 2 years, with a concentration of 0.01% reducing progression by as much as 60% (compared with a historical placebo control) while having the fewest adverse effects18 and least myopic rebound after atropine cessation.9,10 These results provided the rationale for choosing atropine, 0.01%, when our randomized clinical trial was launched.
Although the number of children using low-dose atropine eye drops is unknown, ophthalmologists and optometrists are prescribing these eye drops for myopia control in the US and around the world. In 2017, the American Academy of Ophthalmology found sufficient level 1 evidence supporting the use of low-dose atropine to slow myopia progression.36 An international survey in 2018 noted that 345 of 493 pediatric ophthalmologists (70%) reported prescribing eye drops to slow myopia progression, with atropine, 0.01%, being a common concentration.37
To date, randomized clinical trials of low-dose atropine to slow myopia progression have been conducted primarily in East Asia,10,13,38,39,40,41 with no published results from large, randomized clinical trials in the US. Herein, we report the efficacy and safety of 1 drop of atropine, 0.01%, nightly compared with placebo for slowing myopia progression over 2 years in US children aged 5 to 12 years, with an assessment of myopia progression over 30 months (6 months after stopping treatment).
Methods
Trial Conduct and Oversight
The study was funded by the National Eye Institute of the National Institutes of Health and conducted according to the tenets of the Declaration of Helsinki by the Pediatric Eye Disease Investigator Group at 12 US sites. The protocol and Health Insurance Portability and Accountability Act–compliant informed consent forms were approved by the required institutional review boards. A parent or guardian gave written informed consent, and children gave written assent when required. Participants received a $50 stipend per completed visit. An investigational new drug application was approved by the US Food and Drug Administration for the conduct of the trial. An independent data and safety monitoring committee provided oversight. The protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively. The study results are presented in accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.42
Trial Design and Participants
The trial was conducted from June 2018 to September 2022. Eligible children were aged 5 to 12 years with myopia of −1.00 diopter (D) to −6.00 D spherical equivalent refractive error (SER), astigmatism of 1.50 D or less in both eyes, and anisometropia less than 1.00 D. SER at each study visit was the mean of 3 cycloplegic autorefractions per eye, obtained 30 minutes after 2 drops of cyclopentolate, 1%, were administered 4 minutes apart. No previous myopia control treatment was permitted. eTable 1 in Supplement 3 lists complete eligibility criteria. Axial length, flat corneal radius, anterior chamber depth, and lens thickness of each eye were measured. Race and ethnicity were investigator reported using National Institutes of Health–specified categories, with the exception that the Asian group was split into East Asian and West Asian, to define the cohort and to conduct preplanned subgroup analyses, with an a priori hypothesis that there may be a difference in treatment effect among racial and ethnic groups. The following race and ethnicity categories were included: Black, East Asian, Hispanic or Latino, a multiple race category, West Asian/South Asian, and White. Parental myopia was caregiver reported. Iris color (brown vs not brown) was determined subjectively by the investigator.
Eligible children completed a 2- to 4-week run-in phase of nightly artificial tears to assess their adherence to eye drops. Adherence was assessed from calendar logs, with 90% or greater use of artificial tears and greater than 75% eyeglass wear required for randomization; the intent was to enroll children more likely to continue treatment and wear their glasses and to minimize the potential influence of undercorrection on myopia progression.43,44
Children were randomly assigned 2:1 to 1 eye drop of atropine, 0.01%, or 1 eye drop of placebo nightly via the Pediatric Eye Disease Investigator Group website using a permuted block design stratified by iris color (brown vs not brown) and clinical site. Atropine and placebo eye drops were provided in identical, preservative-free, single-use ampules by Vyluma Inc. Atropine ampules contained atropine, 0.01%, in solution prepared for ocular use; placebo ampules contained only the solution. An independent laboratory confirmed the accuracy of the atropine concentrations in the study drug and placebo ampules from randomly selected samples of study drug and placebo at 2 time points. No instance of mislabeling was identified.
One eye drop was prescribed to be administered to each eye nightly for 24 months, followed by 6 months without eye drops. No other myopia control treatments could be used. Calendars were provided to record eye drop use and eyeglass wear. Unused ampules were collected and counted (eMethods in Supplement 3).
Follow-up visits were at 6, 12, 18, 24 (primary outcome), and 30 months from randomization. Because of the COVID-19 pandemic, virtual visits by telephone or video were allowed for the 6-, 12-, and 18-month visits (eMethods in Supplement 3). At follow-up visits, participants were queried about adherence to study eye drops and eyeglass wear and the occurrence of adverse events. Participants also completed the Eye Drop Symptom Questionnaire before study testing (eMethods in Supplement 3). Adherence to the schedule for study eye drops and eyeglass wear (percentage of waking hours) was deemed excellent (>75%), good (51%-75%), fair (26%-50%), or poor (≤25%) based on calendar review and parental report. Additional study procedures are detailed in the eMethods in Supplement 3.
Outcomes
The primary outcome was the change in cycloplegic SER (mean of 3 autorefractions for each eye and the mean of both eyes for each participant) from baseline to 24 months (while receiving treatment). The same autorefractor was used at each visit (eMethods in Supplement 3). A key secondary outcome was the change in SER from baseline to 30 months, 6 months after treatment had been discontinued. Other secondary outcomes included the following: change in axial length from baseline to 24 months and from baseline to 30 months, proportions of participants with myopia progression of 0.50 D or greater, 1.00 D or greater, and 2.00 D or greater from baseline to 12, 24, and 30 months, and mean binocular near point of accommodation at 6 months.
Statistical Analysis
A sample size of 186 participants provided 97% power to reject the null hypothesis of no difference in mean change in SER from baseline to 24 months under the following assumptions: (1) 2:1 randomization, (2) treatment group difference of 0.50 D, (3) SD of 0.80 D, (4) type I error of 5% (2-sided), and (5) up to 10% loss to follow-up.
Statistical analyses followed the intent-to-treat principle and included all randomized participants. The primary analysis was a comparison of mean change in SER from baseline to 24 months (while receiving treatment) between treatment groups using a longitudinal discrete-time mixed model adjusting for baseline SER, age, iris color (brown vs not brown), and race (East Asian vs non–East Asian participants). The mean change in SER from baseline to 24 months and from baseline to 30 months in each treatment group and the treatment group difference (atropine − placebo) with corresponding 95% CI were estimated using the maximum likelihood method.
Continuous secondary outcomes were analyzed using the same method as the primary analysis, and the proportions of the binary secondary outcomes were calculated within each treatment group and compared between the groups using the Barnard unconditional exact test. Treatment group differences for SER change from baseline to 24 and 30 months in prespecified subgroups were evaluated by adding an interaction term (treatment by time by subgroup) into the model used for the primary analysis.
The overall false discovery rate for secondary analyses was controlled at the 5% level and the 95% CIs were adjusted using the 2-stage step-up procedure.45 Additional statistical methods are outlined in the eMethods in Supplement 3. All P values are 2-tailed, and a P value < .05 was considered significant. Analyses were performed from March to December 2022 using SAS, version 9.4 (SAS Institute).
Results
Participants and Follow-Up
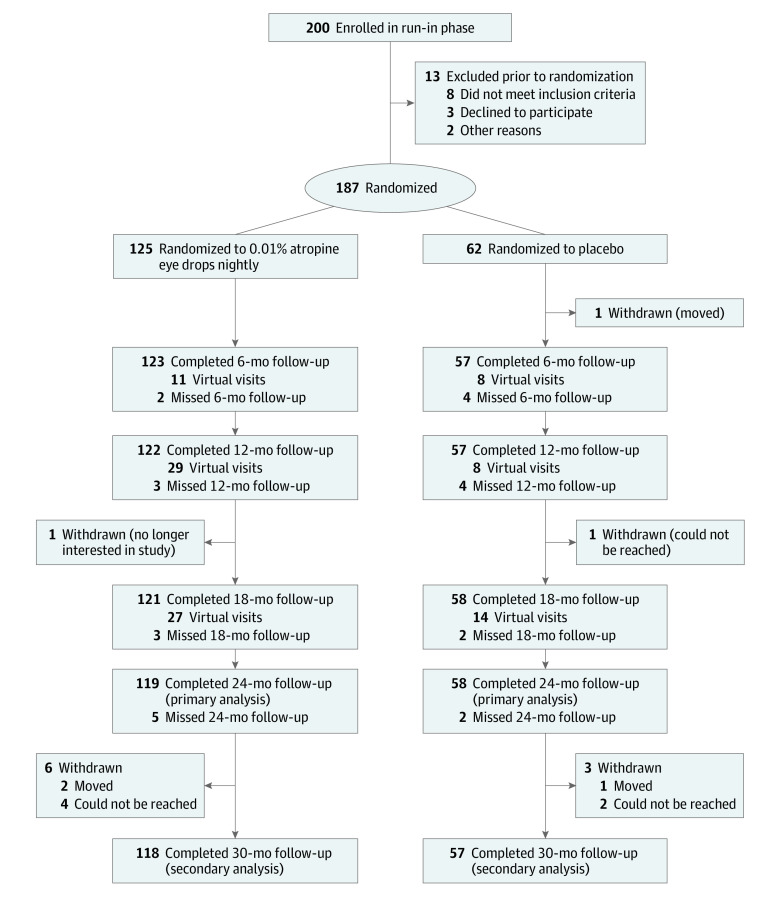
Two hundred participants were enrolled into the run-in phase between June 2018 and February 2020 (Figure 1); 13 were ineligible for randomization. Thus, 187 children were randomly assigned to treatment groups: 125 (67%) in the atropine group and 62 (33%) in the placebo group. Mean (SD) age was 10.1 (1.8) years (range, 5.1-12.9 years); 101 children (54%) were female, and 86 were male (46%). Investigators reported that participants were from the following race and ethnicity categories: 34 Black (18%), 20 East Asian (11%), 30 Hispanic or Latino (16%), 11 multiracial (6%), 6 West/South Asian (3%), and 86 White (46%). Mean (SD) SER at baseline was −2.83 (1.10) D, and mean (SD) axial length was 24.4 (0.8) mm. Baseline demographic and clinical characteristics were similar for both groups (Table 1).
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram.
Completed visits included both in-office and virtual visits at 6, 12, and 18 months; all visits at 24 months and 30 months were completed in office.
Table 1. Baseline Characteristics of Randomized Participants According to Treatment Groupa.
| Characteristic | Treatment group | |
|---|---|---|
| Atropine (n = 125) | Placebo (n = 62) | |
| Sex, No. (%) | ||
| Female | 65 (52) | 36 (58) |
| Male | 60 (48) | 26 (42) |
| Age, No. (%), y | ||
| 5-<7 | 6 (5) | 3 (5) |
| 7-<9 | 28 (22) | 13 (21) |
| 9-<11 | 50 (40) | 20 (32) |
| 11-<13 | 41 (33) | 26 (42) |
| Mean (SD) | 10.1 (1.8) | 10.1 (1.8) |
| Range | 5.1 to 12.9 | 5.1 to 12.9 |
| Race/ethnicity, No. (%) | ||
| Black | 20 (16) | 14 (23) |
| East Asian | 16 (13) | 4 (6) |
| Hispanic or Latino | 21 (17) | 9 (15) |
| Multiracial | 6 (5) | 5 (8) |
| West Asian/South Asian | 4 (3) | 2 (3) |
| White | 58 (46) | 28 (45) |
| Eye color, No. (%) | ||
| Brown | 82 (66) | 41 (66) |
| Not brown | 43 (34) | 21 (34) |
| No. of biological parents with myopia, No. (%) | ||
| 0 | 16 (13) | 11 (18) |
| 1 | 51 (41) | 24 (39) |
| 2 | 52 (42) | 20 (32) |
| Myopia history unknown for one or both parents | 6 (5) | 7 (11) |
| Current refractive correction (single-vision spectacles or contact lenses), No. (%) | ||
| Yes | 125 (100) | 61 (98) |
| No | 0 (0) | 1 (2) |
| Distance visual acuity in habitual refractive correction (mean of both eyes), No. (%), logMAR | ||
| <-0.2 | 0 (0) | 1 (2) |
| −0.2 to <−0.1 | 18 (14) | 5 (8) |
| −0.1 to <0.0 | 62 (50) | 35 (56) |
| 0.0 to <0.1 | 36 (29) | 16 (26) |
| 0.1 to <0.2 | 9 (7) | 5 (8) |
| Mean (SD) | −0.02 (0.08) | −0.03 (0.07) |
| Range | −0.20 to 0.16 | −0.21 to 0.16 |
| Mean right and left eye flat corneal radius, Db | ||
| Mean (SD) | 43.4 (1.4) | 43.6 (1.4) |
| Range | 40.1 to 47.0 | 39.2 to 49.7 |
| Mean right and left eye anterior chamber depth, mm | ||
| Mean (SD) | 3.8 (0.2) | 3.9 (0.2) |
| Range | 3.2 to 4.5 | 3.4 to 4.6 |
| Mean right and left eye lens thickness, mmc | ||
| Mean (SD) | 3.4 (0.2) | 3.3 (0.2) |
| Range | 3.0 to 3.9 | 3.0 to 3.5 |
Abbreviation: D, diopter.
All percentages are column percentages (out of total number of participants randomly assigned to the treatment group).
Flat corneal radius was not available for 1 participant in the placebo group.
Lens thickness was not available for some participants (n = 53 in the atropine group and n = 27 in the placebo group) because the biometer used did not provide a lens thickness reading due to measurement difficulty.
Follow-up was completed at 24 months by 119 of 125 children (95%) in the atropine group and 58 of 62 children (94%) in the placebo group. At 30 months, follow-up was completed by 118 of 125 children (94%) in the atropine group and 57 of 62 children (92%) in the placebo group (Figure 1). Adherence to eye drop treatment based on calendar logs was excellent (76% to 100% of the time) for at least 93% of the atropine group (399 of 430) across all visits and for at least 96% of the placebo group (193 of 201) across all visits. Ampule usage is depicted in eFigure 8 in Supplement 3. Three participants (2.4%) in the atropine group and 1 participant (1.6%) in the placebo group permanently stopped treatment. Six participants temporarily stopped treatment (1 [17%] atropine; 5 [83%] placebo). Two participants (1.6%) in the atropine group received additional myopia progression interventions (eTable 2 in Supplement 3).
Efficacy Outcomes
In the atropine and placebo groups, respectively, mean (SD) SER was −2.83 (1.17) D and −2.83 (0.97) D at baseline and −3.64 (1.46) D and −3.54 (1.08) D at 24 months. At the 24-month primary outcome visit (while receiving treatment), the adjusted mean (95% CI) change in SER from baseline was −0.82 (−0.96 to −0.68) D and −0.80 (−0.98 to −0.62) D in the atropine and placebo groups, respectively (adjusted difference atropine − placebo = −0.02D; 95% CI, −0.19 to 0.15 D; P = .83) (Table 2, Figure 2, and eFigures 1 and 2 in Supplement 3). The adjusted mean (95% CI) changes in axial length from baseline to 24 months were 0.44 (0.39-0.50) mm in the atropine group and 0.45 (0.37-0.52) mm in the placebo group (adjusted difference = −0.002 mm; 95% CI, −0.106 to 0.102 mm) (Table 2, Figure 2, and eFigure 4 in Supplement 3). Sensitivity analyses produced similar results (eResults in Supplement 3).
Table 2. Spherical Equivalent Refractive Error (SER) and Axial Length by Treatment Groupa,b.
| Variable | Baseline | 12-mo Visit | 24-mo Visit | 30-mo Visitc | ||||
|---|---|---|---|---|---|---|---|---|
| Atropine (n = 125) | Placebo (n = 62) | Atropine (n = 93) | Placebo (n = 49) | Atropine (n = 119) | Placebo (n = 58) | Atropine (n = 118) | Placebo (n = 57) | |
| Mean right and left eye SER, No. (%), D | ||||||||
| <−6.00 | 0 | 0 | 1 (1) | 1 (2) | 7 (6) | 1 (2) | 11 (9) | 3 (5) |
| −6.00 to <−5.00 | 5 (4) | 1 (2) | 9 (10) | 1 (2) | 11 (9) | 5 (9) | 10 (8) | 3 (5) |
| −5.00 to <−4.00 | 17 (14) | 5 (8) | 17 (18) | 9 (18) | 26 (22) | 14 (24) | 26 (22) | 15 (26) |
| −4.00 to <−3.00 | 27 (22) | 19 (31) | 18 (19) | 13 (27) | 31 (26) | 17 (29) | 35 (30) | 19 (33) |
| −3.00 to <−2.00 | 37 (30) | 22 (35) | 30 (32) | 18 (37) | 26 (22) | 17 (29) | 19 (16) | 13 (23) |
| −2.00 to <−1.00 | 39 (31) | 15 (24) | 18 (19) | 7 (14) | 17 (14) | 4 (7) | 16 (14) | 4 (7) |
| −1.00 to <0.00 | 0 | 0 | 0 | 0 | 1 (1) | 0 | 1 (1) | 0 |
| Mean (SD) | −2.83 (1.17) | −2.83 (0.97) | −3.22 (1.27) | −3.22 (1.05) | −3.64 (1.46) | −3.54 (1.08) | −3.81 (1.56) | −3.69 (1.16) |
| Median | −2.60 | −2.81 | −2.94 | −3.00 | −3.52 | −3.51 | −3.66 | −3.65 |
| Change of mean right and left eye SER from baseline (outcome − baseline), No. (%), D | ||||||||
| ≤−3.50 | NA | NA | 0 | 0 | 0 | 0 | 1 (1) | 0 |
| >−3.50 to −3.00 | 0 | 0 | 1 (1) | 0 | 2 (2) | 1 (2) | ||
| >−3.00 to −2.50 | 0 | 0 | 1 (1) | 0 | 5 (4) | 0 | ||
| >−2.50 to −2.00 | 0 | 0 | 5 (4) | 2 (3) | 5 (4) | 3 (5) | ||
| >−2.00 to −1.50 | 1 (1) | 1 (2) | 8 (7) | 7 (12) | 8 (7) | 6 (11) | ||
| >−1.50 to −1.00 | 5 (5) | 2 (4) | 19 (16) | 9 (16) | 22 (19) | 11 (19) | ||
| >−1.00 to −0.50 | 22 (24) | 17 (35) | 37 (31) | 18 (31) | 35 (30) | 20 (35) | ||
| >−0.50 to 0.00 | 57 (61) | 27 (55) | 46 (39) | 18 (31) | 36 (31) | 13 (23) | ||
| >0 | 8 (9) | 2 (4) | 2 (2) | 4 (7) | 4 (3) | 3 (5) | ||
| Mean (SD) | −0.39 (0.36) | −0.45 (0.35) | −0.78 (0.64) | −0.74 (0.60) | −0.94 (0.77) | −0.88 (0.71) | ||
| Median | −0.31 | −0.40 | −0.58 | −0.63 | −0.72 | −0.77 | ||
| Adjusted treatment group difference of mean change in SER from baseline at 24 and 30 mo (atropine – placebo) (95% CI), Dd | NA | NA | NA | NA | −0.02 (−0.19 to 0.15)e | −0.04 (−0.25 to 0.17)f | ||
| Participants with ≥0.5 D progression in myopia from baseline, No. (%) | NA | NA | 35 (38) | 20 (41) | 78 (66) | 37 (64) | 83 (70) | 41 (72) |
| Participants with ≥1 D progression in myopia from baseline, No. (%) | NA | NA | 6 (6) | 3 (6) | 34 (29) | 18 (31) | 44 (37) | 21 (37) |
| Participants with ≥2 D progression in myopia from baseline, No. (%) | NA | NA | 0 | 0 | 7 (6) | 2 (3) | 14 (12) | 4 (7) |
| Mean right and left eye axial length, No. (%), mmg | ||||||||
| 22.0 to <23.0 | 5 (4) | 3 (5) | 1 (1) | 1 (2) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| 23.0 to <24.0 | 33 (26) | 12 (19) | 23 (25) | 9 (18) | 18 (15) | 8 (14) | 18 (15) | 7 (12) |
| 24.0 to <25.0 | 51 (41) | 30 (48) | 36 (40) | 22 (45) | 50 (42) | 25 (43) | 44 (37) | 23 (40) |
| 25.0 to <26.0 | 33 (26) | 15 (24) | 26 (29) | 14 (29) | 37 (31) | 21 (36) | 40 (34) | 20 (35) |
| 26.0 to <27.0 | 3 (2) | 2 (3) | 5 (5) | 2 (4) | 12 (10) | 3 (5) | 15 (13) | 6 (11) |
| 27.0 to <28.0 | 0 | 0 | 0 | 1 (2) | 1 (1) | 1 (2) | 1 (1) | 1 (2) |
| Mean (SD) | 24.4 (0.8) | 24.4 (0.8) | 24.7 (0.8) | 24.7 (0.8) | 24.9 (0.9) | 24.9 (0.8) | 24.9 (0.9) | 24.9 (0.8) |
| Median | 24.4 | 24.3 | 24.6 | 24.6 | 24.8 | 24.7 | 24.9 | 24.8 |
| Change of mean right and left eye axial length from baseline (outcome – baseline), mmg | ||||||||
| <0 | NA | NA | 4 (4) | 1 (2) | 1 (1) | 0 | 0 | 0 |
| 0 to <0.25 | 54 (59) | 25 (51) | 41 (34) | 19 (33) | 30 (25) | 15 (26) | ||
| 0.25 to <0.50 | 28 (31) | 20 (41) | 39 (33) | 18 (31) | 37 (31) | 18 (32) | ||
| 0.50 to <0.75 | 4 (4) | 2 (4) | 22 (18) | 14 (24) | 31 (26) | 11 (19) | ||
| 0.75 to <1.00 | 1 (1) | 1 (2) | 8 (7) | 5 (9) | 6 (5) | 8 (14) | ||
| 1.00 to <1.25 | 0 | 0 | 7 (6) | 2 (3) | 10 (8) | 4 (7) | ||
| 1.25 to <1.50 | 0 | 0 | 0 | 0 | 1 (1) | 1 (2) | ||
| ≥1.50 | 0 | 0 | 1 (1) | 0 | 3 (3) | 0 (0) | ||
| Mean (SD) | 0.22 (0.17) | 0.25 (0.16) | 0.42 (0.29) | 0.41 (0.27) | 0.51 (0.35) | 0.49 (0.32) | ||
| Median | 0.20 | 0.23 | 0.38 | 0.36 | 0.44 | 0.45 | ||
| Adjusted treatment group difference of mean change in axial length from baseline at 24 and 30 mo (atropine – placebo) (95% CI), mmh,i | NA | NA | NA | NA | −0.002 (−0.106 to 0.102)j | 0.009 (−0.115 to 0.134)k | ||
Abbreviations: D, diopter; NA, not applicable.
All percentages in this table are column percentages (of total number of participants randomly assigned to the treatment group who completed the specified visit).
In-person follow-up at the 12-month visit was reduced due to the COVID-19 public health emergency. Virtual visits were conducted when semiannual visits could not be completed in person but excluded cycloplegic autorefraction. Study eye drops were delivered to all participants.
Study treatment was discontinued at the 24-month visit; 30-month visit was without treatment.
The adjusted treatment group difference of mean change in SER from baseline adjusted for baseline SER, age, iris color (brown vs nonbrown), and race (East Asian vs non–East Asian participants), to account for potential residual confounding.
The adjusted mean change in SER from baseline at 24 months was −0.82 D vs −0.80 D in the atropine and placebo groups, respectively.
The adjusted mean change in SER from baseline at 30 months was −0.97 D vs −0.93 D in the atropine and placebo groups, respectively.
The total number of participants in the atropine group with axial length measurement at 12 months was 91.
The adjusted treatment group difference of mean change in axial length from baseline adjusted for baseline axial length, age, iris color (brown vs nonbrown), and race (East Asian vs non–East Asian participants), to account for potential residual confounding.
The CIs were adjusted to control the overall false discovery rate for the multiple secondary outcomes at 5%.
The adjusted mean change in axial length from baseline at 24 months was 0.44 mm vs 0.45 mm in the atropine and placebo groups, respectively.
The adjusted mean change in axial length from baseline at 30 months was 0.53 mm vs 0.52 mm in the atropine and placebo groups, respectively.
Figure 2. Adjusted Change in Spherical Equivalent Refractive Error (SER) and Axial Length (and 95% CI) Between Baseline and Outcome Visits (Outcome − Baseline) (Mean of Right and Left Eyes).
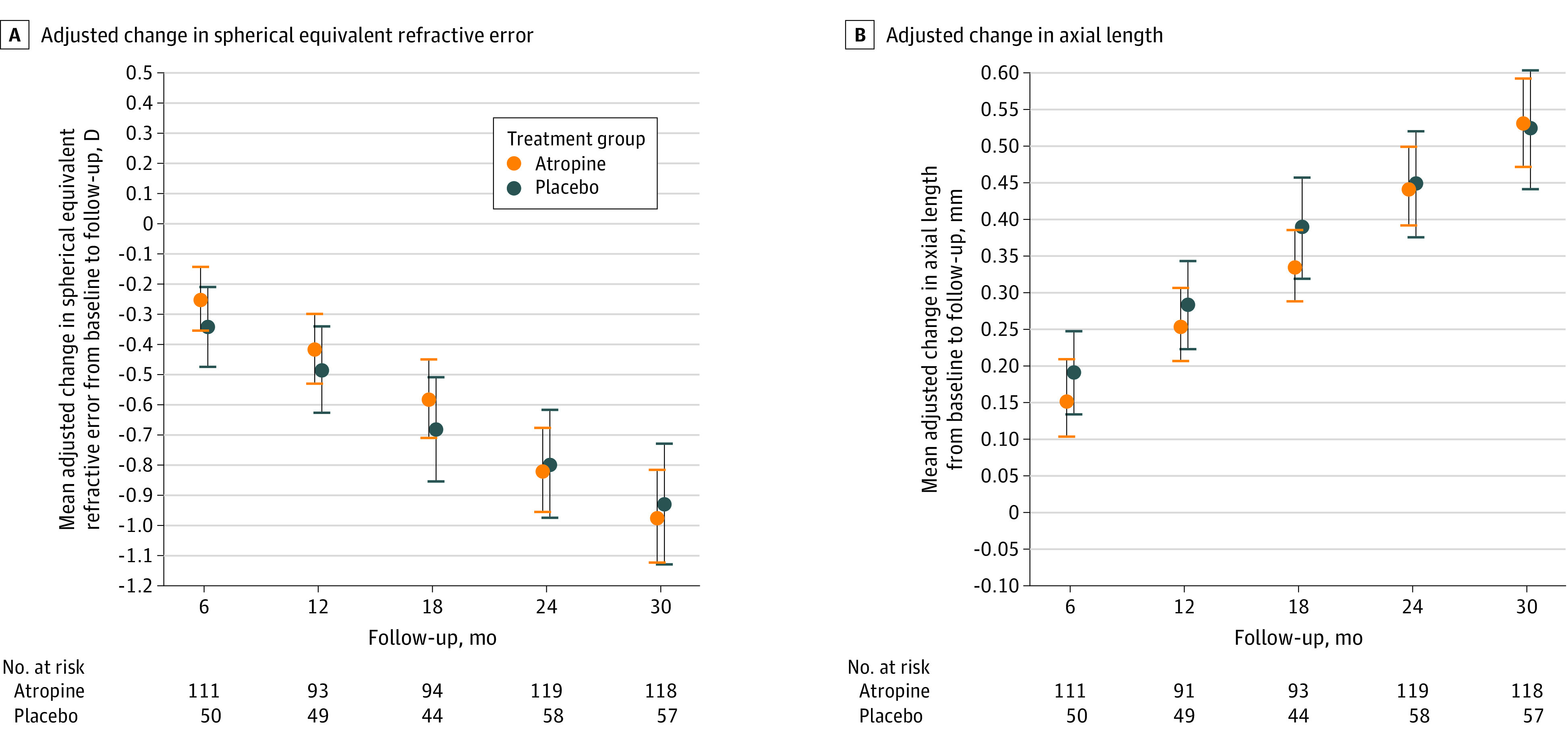
The change in SER (diopter [D]) from baseline adjusted for baseline SER, age, iris color (brown vs nonbrown), and race (East Asian vs non–East Asian participants). The adjusted treatment group difference of mean change in axial length from baseline adjusted for baseline axial length, age, iris color (brown vs nonbrown), and race (East Asian vs non–East Asian participants).
At the 30-month secondary outcome visit (6 months after stopping treatment), the mean (SD) change in SER from baseline was −0.94 (0.77) D and −0.88 (0.71) D in the atropine and placebo groups, respectively (adjusted difference = −0.04 D; 95% CI, −0.25 to 0.17 D) (eFigures 3 and 5 in Supplement 3). The mean (SD) change in axial length from baseline was 0.51 (0.35) mm in the atropine group and 0.49 (0.32) mm in the placebo group (adjusted difference = 0.009 mm; 95% CI, −0.115 to 0.134 mm). The absence of a benefit from atropine, 0.01%, at 24 and 30 months was consistent in subgroups based on age, sex, race and ethnicity, eye color, and baseline SER (eFigures 6 and 7 in Supplement 3).
Adverse Events
There were 2 serious systemic adverse events unrelated to study medication (hip surgery and COVID-19 hospitalization) (Table 3). Ocular adverse effects were reported at least once by 87% of participants (109 of 125) in the atropine group and 90% of participants (56 of 62) in the placebo group. Of these adverse events, 89% (398 of 445) were considered mild in the atropine group and 90% (198 of 220) were considered mild in the placebo group. Among the participants, common ocular adverse events were eye irritation at time of instillation (atropine, 72% [90 of 125]; placebo, 82% [51 of 62]), photophobia (atropine, 26% [32 of 125]; placebo, 27% [17 of 62]), and blurred vision (atropine, 14% [17 of 125]; placebo, 16% [10 of 62]).
Table 3. Safety Outcomes During the 30-Month Study Perioda.
| Adverse events | No. (%) | |
|---|---|---|
| Atropine (n = 125) | Placebo (n = 62) | |
| Serious ocular adverse events | ||
| Participants with a serious ocular adverse event | 0 | 0 |
| Any ocular adverse event | ||
| Participants with an ocular adverse event | 109 (87) | 56 (90) |
| Ocular adverse events (reported at eye level), No.b | 445 | 220 |
| Mild | 398 (89) | 198 (90) |
| Moderate | 43 (10) | 21 (10) |
| Severec | 4 (1) | 1 (<1) |
| Types and number of ocular adverse events | ||
| Eye irritation | 90 (72) | 51 (82) |
| Photophobia | 32 (26) | 17 (27) |
| Vision blurred | 17 (14) | 10 (16) |
| Allergic conjunctivitis | 10 (8) | 5 (8) |
| Ocular discomfort | 8 (6) | 5 (8) |
| Visual impairment | 6 (5) | 2 (3) |
| Blepharitis | 6 (5) | 1 (2) |
| Meibomian gland dysfunction | 3 (2) | 3 (5) |
| Eye pain | 5 (4) | 0 |
| Otherd | 30 (24) | 9 (15) |
| Serious systemic adverse eventse | ||
| Participants with serious systemic adverse event | 2 (2) | 0 |
| Serious systemic adverse events, No. | 2 | 0 |
| Any systemic adverse event | ||
| Participants with a systemic adverse event | 28 (22) | 18 (29) |
| Systemic adverse events, No. | 64 | 37 |
| Mild | 42 (66) | 28 (76) |
| Moderate | 21 (33) | 9 (24) |
| Severef | 1 (2) | 0 |
| Types and number of systemic adverse events | ||
| COVID-19 infection | 7 (6) | 3 (5) |
| Headache | 3 (2) | 6 (10) |
| Acne | 3 (2) | 1 (2) |
| Pharyngitis streptococcal | 3 (2) | 1 (2) |
| Seasonal allergy | 2 (2) | 1 (2) |
| Influenza | 1 (1) | 2 (3) |
| Pneumonia | 3 (2) | 0 (0) |
| Upper respiratory tract infection | 2 (2) | 1 (2) |
| Otherg | 18 (14) | 10 (16) |
Participants and caregivers were queried about adverse events since their prior visit as part of history taking before administering the Eye Drop Symptom Questionnaire and any testing procedures. The Medical Dictionary for Regulatory Activities preferred term was used to describe each event.
Ocular adverse events were reported at eye level (eg, irritation in both eyes was reported as 2 ocular adverse events).
Four ocular adverse events with severe intensity were reported by 2 participants in the atropine group: 1 reported burning in both eyes after study drug instillation and 1 reported pain in both eyes. One ocular adverse event with severe intensity that was reported in the placebo group was swelling and bruising in 1 eyelid due to injury not related to study drug.
Ocular adverse events that each occurred in less than 3% of participants.
Two participants in the atropine group reported a serious adverse event. One underwent hip surgery to treat a preexisting condition before study entry. Another patient was hospitalized owing to COVID-19 infection.
One participant in the atropine group reported a systemic adverse event with severe intensity (apnea); none of the participants in the placebo group reported a systemic adverse event with severe intensity.
Systemic adverse events that each occurred in less than 2% of the participants.
Eye Drop Symptom Questionnaire
Mean scores for items on the Eye Drop Symptom Questionnaire (scale 0-3; 0 = never and 3 = always) were similar in both treatment groups, indicating the treatment was well tolerated (eTable 3 in Supplement 3).
Discussion
We found that nightly low-dose atropine, 0.01%, eye drops did not slow myopia progression or axial elongation when compared with placebo over 24 months in a cohort of US children aged 5 to 12 years with spherical equivalent myopia of −1.00 to −6.00 D. These results do not support the nightly use of low-dose atropine, 0.01%, eye drops to slow myopia progression in US children. Given that this trial was double-masked and placebo-controlled, had excellent reported treatment adherence, minimal (5%) loss to follow-up at 24 months, and had sufficient statistical power, we have no reason to suspect that our results are due to bias or chance.
The lack of benefit from atropine, 0.01%, in our study differs from the results of 5 clinical trials in East Asian12,18,38,40 and South Asian41 populations with similar age and refractive error eligibility criteria. In the ATOM218 trial in Singapore (N = 400; mean age, 9.5 years; age range, 6-12 years), investigators reported a substantial difference in SER myopia progression (−0.49 D vs −1.20 D) but not axial elongation (0.41 mm vs 0.38 mm) over a 2-year period with atropine, 0.01%, compared with a historical placebo control from their earlier ATOM trial.12 The lack of a randomized placebo control group in the ATOM2 trial reduces the certainty that there was a treatment benefit on myopia progression. It is noteworthy that the ATOM2 trial did not find a difference in axial elongation over 2 years, similar to the outcome in our randomized clinical trial. In the randomized Low-Concentration Atropine for Myopia Progression (LAMP) trial of 3 atropine concentrations plus placebo conducted in Hong Kong (N = 438; mean age of approximately 8.5 years; range, 4-12 years),38 investigators found a reduction in myopia progression (−0.59 D vs −0.81 D; P < .004) with atropine, 0.01%, after 1 year of treatment compared with placebo and a reduction in axial length elongation (0.36 mm vs 0.41 mm; P = .18).38 The higher atropine concentrations (0.05%, 0.025%) were more effective than 0.01% in reducing myopia progression. Investigators in Japan conducted a 2-year trial of atropine, 0.01% (N = 168; mean age = 9.0 years; 94% follow-up) and found a reduction (mean difference = 0.22 D at 2 years; 95% CI, 0.09-0.35 D) in myopia progression.40 In a 1-year randomized clinical trial of atropine, 0.01%, in South Asian children from India (N = 100; mean age = 10.7 years; 92% follow-up), there was a reduction in mean SER progression41 (−0.16 D in the atropine group vs −0.35 D in placebo group; P = .02). A 1-year randomized clinical trial of atropine, 0.01%, in Beijing, China (N = 220; mean age = 9.6 years; age range, 6-12 years; 72% follow-up) also reported a reduction in myopia progression (mean difference = 0.26 D over 1 year; 95% CI, 0.12-0.41 D).39
In contrast, a 2-year randomized clinical trial conducted in Western Australia (WA-ATOM)13 with similar age and refractive error eligibility criteria to our study (N = 153; mean age = 11.5 years; age range, 6-16 years) found that myopia progression of −0.64 D with atropine, 0.01%, compared with −0.78 D with placebo was not statistically significant (adjusted mean difference = 0.14 D over 2 years; 95% CI, −0.03 to +0.29 D). The authors suggested that the greater loss to follow-up of placebo-group participants with faster-progressing myopia in their study may have contributed to not finding a treatment effect. In contrast to the studies in Asia,17,18,38,39,40 but similar to our study, 49% of children in the WA-ATOM trial were of European ancestry, and 18% were of East Asian ethnicity.
The absence of treatment benefit in our US-based study, in contrast with studies performed in East Asia and India, could be due to several factors. The lack of a 2-year contemporaneous placebo control and some loss to follow-up in the ATOM2 (11% over 2 years)18 and LAMP (20% over 1 year)17 studies could explain why our study results differ from theirs. However, the study populations differed in race and ethnicity, and there may be racial differences in response to atropine eye drops. We enrolled fewer Asian children, whose myopia progresses more quickly, and we included Black children, whose myopia progresses less quickly, compared with other races.46,47 The placebo participants in the ATOM trial progressed at −1.20 D over 2 years,12,18 whereas the rate of myopia progression in our placebo group was approximately −0.75 D over 2 years, similar to rates previously published for myopic US school children.46,48 This rate was also similar to the placebo progression rate of −0.78 D over 2 years in the WA-ATOM study.13 In addition, it is possible that our atropine, 0.01%, eye drop formulation was different than the 0.01% formulations used in other studies. Of note, at 2 different times, we independently confirmed the concentration of drug and placebo used in our trial.
It is possible that a different atropine concentration is needed for US children to experience a benefit. A dose-dependent effect has been noted with 0.025% and 0.05% concentrations performing better than 0.01% for 1-year myopic progression in the LAMP trial.17,38 Age was unlikely a factor as the mean age and age range of the children were similar across the studies.
This eye drop treatment was well tolerated aside from some irritation during eye drop instillation. In our study, approximately 1 in 4 children in both the atropine and placebo groups reported photophobia at least once but continued with treatment. In the atropine, 0.01%, group in the LAMP2 trial, 1% of children reported photophobia at 1 year,38 although 34% of children were prescribed photochromic lenses by 2 years.17
Limitations
Limitations of our study include a lack of an objective measure of eye drop use. Our study finding of good-to-excellent family-reported eye drop compliance and counts of returned eye drop ampules may overestimate usage. COVID-19 shutdowns, which occurred during the treatment phase of our study, led to some virtual visits without refractions at 6, 12, and 18 months. However, the children still received and continued to take their study eye drops. Further, 95% of participants completed their in-office primary outcome visits.
Conclusions
In conclusion, this multicenter, placebo-controlled, randomized clinical trial with high participant retention and treatment adherence found that atropine, 0.01%, eye drops did not slow myopia progression over 2 years of treatment in US children aged 5 to 12 years with low to moderate myopia. Future studies of pharmacologic myopia control in US children should consider increased atropine concentrations, new pharmaceuticals, objective measures of treatment adherence, alternative eye drop delivery systems and schedules, as well as evaluating the impact of environmental and genetic factors and optical interventions on myopia control treatment.
Trial Protocol
Statistical Analysis Plan
eMethods
eResults
eTable 1. Eligibility Criteria
eTable 2. Treatment Modifications During 24 Months of Follow-Up
eTable 3. Responses to Eye Drop Symptom Questionnaire at Each Study Visit
eFigure 1. Change in Spherical Equivalent Refractive Error (D) Between Baseline and Outcome Visits (Outcome − Baseline) (Mean of OD and OS)
eFigure 2. Change in Spherical Equivalent Refractive Error (D) Between Baseline and Outcome Visits (Outcome − Baseline) at 24 Months (Mean of OD and OS)
eFigure 3. Change in Spherical Equivalent Refractive Error (D) Between Baseline and Outcome Visits (Outcome − Baseline) at 30 Months (Mean of OD and OS)
eFigure 4. Change in Axial Length (mm) Between Baseline and Outcome Visits (Outcome − Baseline) (Mean of OD and OS)
eFigure 5. Myopia Progression at the Follow-up Visits
eFigure 6. Mean Change in Spherical Equivalent Refractive Error (SER) From Baseline at 24 Months in Subgroups
eFigure 7. Mean Change in Spherical Equivalent Refractive Error (SER) From Baseline at 30 Months in Subgroups
eFigure 8. Estimated Prescribed Medication Used Based on Return of Unused Ampules
Nonauthor Collaborators. The Pediatric Eye Disease Investigator Group
Data Sharing Statement
References
- 1.Williams KM, Bertelsen G, Cumberland P, et al. ; European Eye Epidemiology (E(3)) Consortium . Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology. 2015;122(7):1489-1497. doi: 10.1016/j.ophtha.2015.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitale S, Sperduto RD, Ferris FL III. Increased prevalence of myopia in the US between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009;127(12):1632-1639. doi: 10.1001/archophthalmol.2009.303 [DOI] [PubMed] [Google Scholar]
- 3.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042. doi: 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 4.National Eye Institute . Nearsightedness (myopia) tables. Accessed June 2, 2022. https://www.nei.nih.gov/learn-about-eye-health/eye-health-data-and-statistics/nearsightedness-myopia-data-and-statistics/nearsightedness-myopia-tables
- 5.Kleinstein RN, Sinnott LT, Jones-Jordan LA, Sims J, Zadnik K; Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study Group . New cases of myopia in children. Arch Ophthalmol. 2012;130(10):1274-1279. doi: 10.1001/archophthalmol.2012.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COMET Group . Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET). Invest Ophthalmol Vis Sci. 2013;54(13):7871-7884. doi: 10.1167/iovs.13-12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, Flitcroft DI. The risks and benefits of myopia control. Ophthalmology. 2021;128(11):1561-1579. doi: 10.1016/j.ophtha.2021.04.032 [DOI] [PubMed] [Google Scholar]
- 8.Bullimore MA, Brennan NA. Myopia control: why each diopter matters. Optom Vis Sci. 2019;96(6):463-465. doi: 10.1097/OPX.0000000000001367 [DOI] [PubMed] [Google Scholar]
- 9.Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157(2):451-457.e1. doi: 10.1016/j.ajo.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 10.Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123(2):391-399. doi: 10.1016/j.ophtha.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 11.Chou AC, Shih YF, Ho TC, Lin LL. The effectiveness of 0.5% atropine in controlling high myopia in children. J Ocul Pharmacol Ther. 1997;13(1):61-67. doi: 10.1089/jop.1997.13.61 [DOI] [PubMed] [Google Scholar]
- 12.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285-2291. doi: 10.1016/j.ophtha.2006.05.062 [DOI] [PubMed] [Google Scholar]
- 13.Lee SS, Lingham G, Blaszkowska M, et al. Low-concentration atropine eye drops for myopia control in a multi-racial cohort of Australian children: a randomised clinical trial. Clin Exp Ophthalmol. 2022;50(9):1001-1012. doi: 10.1111/ceo.14148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. J Ocul Pharmacol Ther. 1999;15(1):85-90. doi: 10.1089/jop.1999.15.85 [DOI] [PubMed] [Google Scholar]
- 15.Song YY, Wang H, Wang BS, Qi H, Rong ZX, Chen HZ. Atropine in ameliorating the progression of myopia in children with mild to moderate myopia: a meta-analysis of controlled clinical trials. J Ocul Pharmacol Ther. 2011;27(4):361-368. doi: 10.1089/jop.2011.0017 [DOI] [PubMed] [Google Scholar]
- 16.Syniuta LA, Isenberg SJ. Atropine and bifocals can slow the progression of myopia in children. Binocul Vis Strabismus Q. 2001;16(3):203-208. [PubMed] [Google Scholar]
- 17.Yam JC, Li FF, Zhang X, et al. Two-year clinical trial of the Low-Concentration Atropine for Myopia Progression (LAMP) study: phase 2 report. Ophthalmology. 2020;127(7):910-919. doi: 10.1016/j.ophtha.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 18.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-354. doi: 10.1016/j.ophtha.2011.07.031 [DOI] [PubMed] [Google Scholar]
- 19.Gong Q, Janowski M, Luo M, et al. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017;135(6):624-630. doi: 10.1001/jamaophthalmol.2017.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee LC, Hsieh MW, Chen YH, Chen PL, Chien KH. Characteristics of responders to atropine 0.01% as treatment in Asian myopic children. Sci Rep. 2022;12(1):7380. doi: 10.1038/s41598-022-10978-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walline JJ, Lindsley KB, Vedula SS, et al. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2020;1(1):CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019;96(8):556-567. doi: 10.1097/OPX.0000000000001410 [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Pomeda A, Pérez-Sánchez B, Valls I, Prieto-Garrido FL, Gutiérrez-Ortega R, Villa-Collar C. MiSight Assessment Study Spain (MASS): a 2-year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):1011-1021. doi: 10.1007/s00417-018-3906-z [DOI] [PubMed] [Google Scholar]
- 24.Walline JJ, Walker MK, Mutti DO, et al. ; BLINK Study Group . Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. JAMA. 2020;324(6):571-580. doi: 10.1001/jama.2020.10834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho P, Cheung SW. Retardation of Myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53(11):7077-7085. doi: 10.1167/iovs.12-10565 [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Xu F, Zhang T, et al. Orthokeratology to control myopia progression: a meta-analysis. PLoS One. 2015;10(4):e0124535. doi: 10.1371/journal.pone.0124535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rappon J, Chung C, Young G, et al. Control of myopia using diffusion optics spectacle lenses: 12-month results of a randomised controlled, efficacy and safety study (CYPRESS). Br J Ophthalmol. 2022;bjophthalmol-2021-321005. doi: 10.1136/bjo-2021-321005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao J, Huang Y, Li X, et al. Spectacle lenses with aspherical lenslets for myopia control vs single-vision spectacle lenses: a randomized clinical trial. JAMA Ophthalmol. 2022;140(5):472-478. doi: 10.1001/jamaophthalmol.2022.0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam CSY, Tang WC, Tse DY, et al. Defocus Inc Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol. 2020;104(3):363-368. doi: 10.1136/bjophthalmol-2018-313739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in china: a randomized clinical trial. JAMA. 2015;314(11):1142-1148. doi: 10.1001/jama.2015.10803 [DOI] [PubMed] [Google Scholar]
- 31.Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120(5):1080-1085. doi: 10.1016/j.ophtha.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 32.Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125(8):1239-1250. doi: 10.1016/j.ophtha.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 33.Khanal S, Norton TT, Gawne TJ. Amber light treatment produces hyperopia in tree shrews. Ophthalmic Physiol Opt. 2021;41(5):1076-1086. doi: 10.1111/opo.12853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang Y, Zhu Z, Tan X, et al. Effect of repeated low-level red-light therapy for myopia control in children: a multicenter randomized controlled trial. Ophthalmology. 2022;129(5):509-519. doi: 10.1016/j.ophtha.2021.11.023 [DOI] [PubMed] [Google Scholar]
- 35.Yam JC, Zhang XJ, Zhang Y, et al. Effect of low-concentration atropine eye drops vs placebo on myopia incidence in children: the LAMP2 randomized clinical trial. JAMA. 2023;329(6):472-481. doi: 10.1001/jama.2022.24162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pineles SL, Kraker RT, VanderVeen DK, et al. Atropine for the prevention of myopia progression in children: a report by the American Academy of Ophthalmology. Ophthalmology. 2017;124(12):1857-1866. doi: 10.1016/j.ophtha.2017.05.032 [DOI] [PubMed] [Google Scholar]
- 37.Zloto O, Wygnanski-Jaffe T, Farzavandi SK, Gomez-de-Liaño R, Sprunger DT, Mezer E. Current trends among pediatric ophthalmologists to decrease myopia progression-an international perspective. Graefes Arch Clin Exp Ophthalmol. 2018;256(12):2457-2466. doi: 10.1007/s00417-018-4078-6 [DOI] [PubMed] [Google Scholar]
- 38.Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113-124. doi: 10.1016/j.ophtha.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 39.Wei S, Li S-M, An W, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138(11):1178-1184. doi: 10.1001/jamaophthalmol.2020.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hieda O, Hiraoka T, Fujikado T, et al. ; ATOM-J. Study Group . Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol. 2021;65(3):315-325. doi: 10.1007/s10384-021-00822-y [DOI] [PubMed] [Google Scholar]
- 41.Saxena R, Dhiman R, Gupta V, et al. Atropine for the treatment of childhood myopia in India: multicentric randomized trial. Ophthalmology. 2021;128(9):1367-1369. doi: 10.1016/j.ophtha.2021.01.026 [DOI] [PubMed] [Google Scholar]
- 42.Eldridge SM, Chan CL, Campbell MJ, et al. ; PAFS consensus group . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung K, Mohidin N, O’Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002;42(22):2555-2559. doi: 10.1016/S0042-6989(02)00258-4 [DOI] [PubMed] [Google Scholar]
- 44.Adler D, Millodot M. The possible effect of undercorrection on myopic progression in children. Clin Exp Optom. 2006;89(5):315-321. doi: 10.1111/j.1444-0938.2006.00055.x [DOI] [PubMed] [Google Scholar]
- 45.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93(3):491-507. doi: 10.1093/biomet/93.3.491 [DOI] [Google Scholar]
- 46.Jones-Jordan LA, Sinnott LT, Chu RH, et al. ; CLEERE Study Group . Myopia progression as a function of sex, age, and ethnicity. Invest Ophthalmol Vis Sci. 2021;62(10):36. doi: 10.1167/iovs.62.10.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shamp W, Brennan NA, Bullimore MA, Cheng X, Maynes E. Influence of age and race on axial elongation in myopic children. Invest Ophthalmol Vis Sci. 2022;63(7):257. [DOI] [PubMed] [Google Scholar]
- 48.Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses vs single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44(4):1492-1500. doi: 10.1167/iovs.02-0816 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods
eResults
eTable 1. Eligibility Criteria
eTable 2. Treatment Modifications During 24 Months of Follow-Up
eTable 3. Responses to Eye Drop Symptom Questionnaire at Each Study Visit
eFigure 1. Change in Spherical Equivalent Refractive Error (D) Between Baseline and Outcome Visits (Outcome − Baseline) (Mean of OD and OS)
eFigure 2. Change in Spherical Equivalent Refractive Error (D) Between Baseline and Outcome Visits (Outcome − Baseline) at 24 Months (Mean of OD and OS)
eFigure 3. Change in Spherical Equivalent Refractive Error (D) Between Baseline and Outcome Visits (Outcome − Baseline) at 30 Months (Mean of OD and OS)
eFigure 4. Change in Axial Length (mm) Between Baseline and Outcome Visits (Outcome − Baseline) (Mean of OD and OS)
eFigure 5. Myopia Progression at the Follow-up Visits
eFigure 6. Mean Change in Spherical Equivalent Refractive Error (SER) From Baseline at 24 Months in Subgroups
eFigure 7. Mean Change in Spherical Equivalent Refractive Error (SER) From Baseline at 30 Months in Subgroups
eFigure 8. Estimated Prescribed Medication Used Based on Return of Unused Ampules
Nonauthor Collaborators. The Pediatric Eye Disease Investigator Group
Data Sharing Statement