Abstract
Objectives:
Umbilical artery (UA) Doppler indices are surrogate measures of placental function, most commonly used to assess fetal wellbeing in pregnancies with fetal growth restriction. Fetuses with trisomy 21 (t21) are reported to have elevated UA Doppler indices, but reference percentiles are currently lacking for this population. We hypothesized that gestational age-specific values of UA Doppler indices in pregnancies complicated by t21 will be elevated compared to established percentiles based on euploid pregnancies. We aimed to assess UA Doppler indices longitudinally in fetuses with t21 in order to demonstrate Doppler patterns across gestation in this population, compare them with euploid fetuses, and investigate their association with pregnancy outcomes.
Methods:
We conducted a retrospective cohort study of singleton pregnancies with confirmed fetal t21 who underwent UA Doppler surveillance antenatally from January 2012 to August 2019. UA Doppler indices, including systolic/diastolic (S/D) ratio, pulsatility index (PI), and resistance index (RI) were extracted from ultrasound reports or directly from ultrasound images. UA S/D, PI, and RI percentiles by gestational week were created from available observations from our cohort via a data-driven approach using a generalized additive model. A secondary analysis was run to statistically compare t21 values to established percentiles based on observations from a historical population of euploid fetuses.
Results:
UA Doppler measurements from 86 t21 fetuses and 130 euploid fetuses were included in our analysis. Median [IQR] maternal age in t21 pregnancies and euploid pregnancies were 35 years [29–38] and 30 years [27–33], respectively. As in euploid fetuses, we found a negative association between Doppler indices and gestational age in the t21 fetuses. Maternal tobacco use, obesity, or chronic hypertension had no significant effect on UA Doppler indices. As hypothesized, values for UA S/D ratio, PI, and RI at the 2.5th, 5th, 10th, 25th, 50th, 75th, 90th, 95th, and 97.5th percentiles by gestational week were significantly higher in t21 fetuses compared to euploid fetuses (p<.001). Overall, 55.8% (48/86) of the t21 fetuses demonstrated at least one Doppler value above the 95th percentile for gestational age based on euploid reference standard. At birth, eight (9.3%) of the t21 fetuses were small for gestational age. When these pregnancies were removed from analysis, UA Doppler indices remained significantly higher than established percentiles at each week of gestation (p<.001). Only three pregnancies ended in fetal demise in the t21 population, two of which had persistently elevated Dopplers above the 95th percentile per established reference percentiles.
Conclusions:
At each week of gestation, UA Doppler indices in t21 fetuses were significantly higher than established percentiles from a euploid population. Reference intervals based on euploid fetuses may therefore not be appropriate for antenatal surveillance of fetuses with t21. Prospective studies are needed to investigate the role and impact of serial UA Doppler velocimetry in the surveillance of pregnancies complicated by fetal t21.
Keywords: Doppler index, umbilical artery, trisomy 21, aneuploidy
Introduction:
Umbilical artery (UA) Doppler velocimetry is a noninvasive tool used to assess the resistance of blood flow through the umbilical artery and serves as a proxy for placental vascular resistance. Several indices are used in clinical practice, including UA pulsatility index (PI), resistance index (RI) and systolic/diastolic (S/D) ratio. Surveillance of fetal wellbeing by UA Doppler indices in high-risk pregnancies has been shown to reduce fetal morbidity and mortality [1]. Physicians rely on accurate reference ranges to assess the risk of perinatal death and morbidity and the need for closer surveillance or delivery.
Current commonly utilized longitudinal reference percentiles for UA Doppler indices were developed from a prospective study of uncomplicated, euploid pregnancies [2]. In the U.S. the incidence of trisomy 21 (t21) is about 1 in 700 live births [3]. Some studies have shown that pregnancies complicated by t21 have atypical placental development and elevated UA Doppler indices, but current literature lacks gestational age-specific reference percentiles for this population [4–8]. Furthermore, the impact of elevated UA Doppler indices on clinical outcome of pregnancies with t21 fetus is not fully explored. We hypothesize that gestational age-specific values of UA Doppler indices in pregnancies complicated by t21 will be elevated compared to established percentiles based on euploid pregnancies. We aim to assess UA Doppler indices longitudinally in fetuses with t21 to establish percentile values by gestational age, compare them with euploid fetuses, and investigate their association with pregnancy outcomes.
Methods:
Study Population
This was a retrospective cohort study of t21 pregnancies, approved by the Vanderbilt University Medical Center Institutional Review Board. Inclusion criteria were singleton pregnancies with prenatal suspicion for, and cytogenetic confirmation of, t21 seen in the division of Maternal Fetal Medicine (MFM) from January 1, 2012 to August 31, 2019. Exclusion criteria were: multiple gestation, major placental abnormalities detected by ultrasound, or lack of UA Doppler data recorded in the electronic medical record (EMR).
Clinical Data Collection
For all pregnant women referred to our Fetal Center with cytogenetically confirmed or suspected fetal t21, UA Doppler ultrasound was performed during serial ultrasound examinations by certified obstetric sonographers and read by MFM physicians at five different Vanderbilt University Medical Center sites in Tennessee. According to department policy, these women were scheduled for serial growth and Doppler assessments every 4 weeks in the 2nd and 3rd trimesters, with increased fetal surveillance frequency starting at 32–34 weeks to include at least weekly biophysical profile and UA Doppler assessment. The UA Doppler velocity waveforms were obtained according to established guidelines [9]. The peak systolic, end-diastolic, and time-averaged maximum velocities were determined, and S/D ratio, RI, and PI were automatically calculated by the software of the ultrasound machine. In our practice, an average of 3 waveforms is routinely obtained when reporting Doppler values. The ultrasound images with measurements were stored and values were reported. These values were extracted from ultrasound reports or directly from stored ultrasound images during the data collection process. Observations that had absent or intermittently absent end-diastolic flow were not used for analysis, as these were considered overtly abnormal regardless of systolic velocity.
Additional patient demographics, past medical and obstetric history, information about the course and outcome of pregnancy, including any complications, and delivery data were obtained from the EMR. Sex-specific growth charts for neonates with t21 were used to calculate birth weight percentiles [10]. Study data were collected and managed using Research Electronic Data Capture (REDCap), a secure web-based application designed to support data capture for research studies [11].
We then obtained a complete set of anonymized data from the principal investigator (and co-author of this current manuscript) of a 2005 study of 130 euploid fetuses (513 observations) to compare Doppler indices with our t21 population. The detailed methodology used for Doppler ultrasonography and for prospective data collection and evaluation in that study has been previously published [2].
Statistical Analysis
UA S/D ratio percentiles by gestational week were created for S/D ratio, RI, and PI using available observations in the t21 and euploid populations via generalized additive models for location, scale, and shape (GAMLSS) [12]. GAMLSS is a type of semi-parametric regression model. It requires a parametric distribution for Doppler indices and uses non-parametric smoothing functions to model the location, scale, and shape parameters of Doppler indices as a function of gestational age in weeks. Doppler index values were used to create descriptive reference curves with corresponding fitted curves across specific percentiles using GAMLSS R package [13]. Both sets of percentiles were then plotted on curve-fitted percentile charts. For each Doppler index, the curves across gestational weeks 19 to 40 for the t21 and euploid populations were statistically compared using a Wilcoxon Signed Rank Test. Multivariable linear regression was used to assess the association between gestational age and Doppler indices with the adjustment of covariates such as maternal hypertension, tobacco use during pregnancy, obesity, chronic hypertension, and fetal cardiac anomalies. The association between covariates and Doppler values at the 95th percentile was determined by using quantile regression [14].
Results:
We identified 101 singleton pregnancies with trisomy 21 between January 2012 and August 2019. Diagnosis of t21 was confirmed antenatally by chorionic villus sampling or amniocentesis or postnatally by peripheral blood chromosome analysis and/or clinical evaluation. The majority of t21 diagnoses were due to nondisjunction with no evidence of mosaicism. One twin pregnancy was excluded, and 10 singleton pregnancies were excluded due to insufficient or absent UA Doppler information. Three fetuses diagnosed with hydrops fetalis were excluded. One fetus with a single umbilical artery was excluded because studies have shown that two-vessel cords have decreased resistance to flow compared to three-vessel cords [15,16]. The remaining 86 pregnancies with at least one UA Doppler measurement were used to construct gestational age-based percentiles. The median [IQR] numbers of observations per fetus across gestation were 3[2–6], 3 [2–6], and 2 [0.75–4] for S/D, RI, and PI, respectively from the t21 population. Data from 130 euploid pregnancies from the study by Acharya et al. were used for comparative analysis, and included, on average, 4 assessments per subject, timed at 4 week intervals, between 19–42 weeks gestation[2].
Demographic and maternal data for our t21 population and the 130 euploid pregnancies from the study by Acharya et al. were compared (Supplemental Table 1). S/D ratio, RI, and PI were negatively associated with gestational age. Maternal tobacco use during pregnancy, obesity, and chronic hypertension did not have a significant effect on UA Doppler indices according to ANOVA analysis. Therefore, we did not exclude the women with these comorbidities from data analysis.
Of fetuses with t21, 44.2% were female, while 49.2% were female in the euploid population. Information on the mode of delivery, birthweight, placental weight, APGAR scores, and NICU admissions of the liveborn infants was collected (Supplemental Table 2). Twelve t21 pregnancies were delivered at outside hospitals, resulting in missing neonatal data. Placentas were not consistently sent to pathology in the t21 population, resulting in several missing data points. One live birth was missing placental weight in the euploid population.
UA S/D ratio percentiles by gestational week were created from 377 observations for all 86 t21 pregnancies using a generalized additive model. In the t21 population, less patients had PI Doppler values recorded than other indices, and those who did had them recorded at fewer instances. RI percentiles by gestational week were created from 370 observations from 85 fetuses and PI percentiles were created from 246 observations from 65 fetuses. Similarly, S/D ratio, RI, and PI percentiles were created from 507 observations in the euploid population. Using a Wilcoxon Signed Rank Test, we found that UA Doppler indices across all percentiles by gestational week were significantly higher in t21 pregnancies compared to euploid pregnancies (p<.001). Overall, 55.8% (48/86) of the t21 fetuses demonstrated at least one Doppler value above the 95th percentile for gestational age during prenatal ultrasound surveillance.
Structural cardiac anomalies were common in our t21 population. There were 53 cases of septal defects, including atrial septal defects, ventricular septal defects, and atrioventricular septal defects (AVSD). Forty-five (52.3%) t21 fetuses had what we considered to be major structural cardiac anomalies, including isolated AVSD (28), coarctation of the aorta (5), tetralogy of Fallot (ToF) (4), ToF with an AVSD (2), double outlet right ventricle with pulmonary stenosis (1), hypoplastic left heart (1), and Ebstein anomaly (1). The presence of a major cardiac anomaly did not have a statistically significant effect on Doppler indices when evaluated by multivariate linear regression (p=.68). Duodenal atresia, another common anomaly in t21, occurred in 14 (16.3%) pregnancies and was associated with higher Doppler values, though this was not statistically significant across all Doppler indices. Other gastrointestinal anomalies noted in our t21 population included one fetus each with tracheo-esophageal fistula, esophageal atresia, and omphalocele. Two fetuses had a Dandy Walker malformation or variant, and one had hydrocephaly.
Of the 86 pregnancies included, one was electively terminated at 30 4/7 weeks and three resulted in a fetal demise at 32 3/7, 31 3/7, and 38 2/7 weeks of gestation. Two of the three did have persistently elevated Dopplers (>95th percentile) as well as duodenal atresia and congenital heart disease (VSD and AVSD), but neither were growth restricted. Two of the mothers had a history of prior spontaneous abortions. A placental pathology report was available for one pregnancy, which showed no abnormalities except a placenta that was large for gestational age. In the euploid population there was one fetal demise at 42 weeks of gestation.
Nineteen fetuses in the t21 population had documented growth restriction (estimated fetal weight <10th percentile) during at least one prenatal growth ultrasound, of which 17 had at least one UA Doppler value that was ≥ 95th percentile according to current established percentiles [2]. Five of the pregnancies with FGR had absent or reversed UA end-diastolic flow during at least one Doppler ultrasound examination.
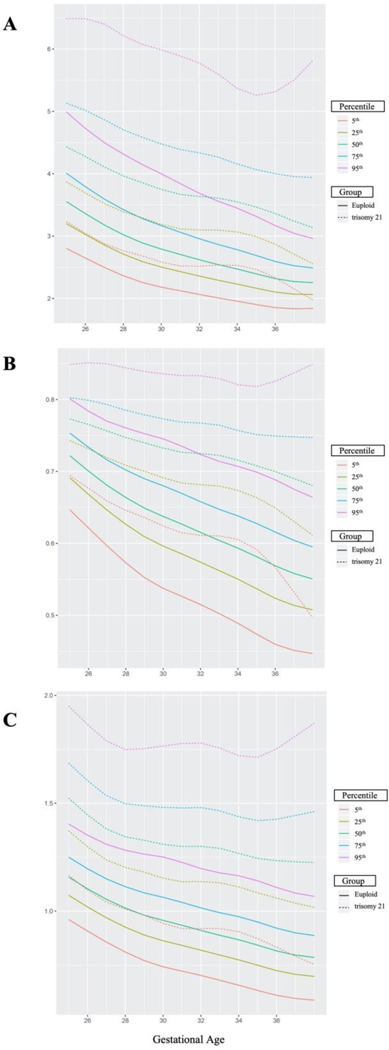
At birth, eight (9.3%) of the t21 fetuses were SGA, meaning their birth weight was <10th percentile for gestational age, while only two (1.5%) were SGA in the euploid population. Fetuses with t21 that were found to be SGA at birth were then removed from the generalized additive model to produce Doppler percentiles by gestational age because research has shown that SGA babies tend to have elevated UA Doppler indices [17–19]. Furthermore, SGA pregnancies had persistently elevated Doppler values in our study, with all being well above the 97.5th percentile according to current established percentiles [2]. UA S/D ratio percentiles by gestational week were created from 341 observations for non-SGA t21 pregnancies (Table 1) and 507 observations for non-SGA euploid pregnancies (Supplemental Table 3). RI percentiles by gestational week were created from 334 observations and PI percentiles were created from 229 observations for non-SGA t21 pregnancies and 507 observations each for non-SGA euploid pregnancies (Supplemental Tables 4–7). The number of individual Doppler assessments per gestational age, for each cohort, were recorded (Supplemental Table 8.) Curve-fitted percentile charts for UA Doppler S/D ratio, RI, and PI in the t21 and euploid populations were created. Figure 1 displays overlaid curve-fitted percentiles for the t21 and euploid populations. Once again, we found that UA Doppler indices across all gestational weeks were significantly higher in t21 pregnancies compared to euploid pregnancies (p<.001).
Table 1:
Reference values for serial measurements of the umbilical artery systolic/diastolic ratio in normally grown trisomy 21 fetuses. Created from 341 observations.
| Percentile | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gestation (wk) | 2.5th | 5th | 10th | 25th | 50th | 75th | 90th | 95th | 97.5th |
| 19 | 3.11 | 3.27 | 3.47 | 3.83 | 4.30 | 4.85 | 5.45 | 5.86 | 6.27 |
| 20 | 2.98 | 3.15 | 3.36 | 3.75 | 4.27 | 4.89 | 5.57 | 6.05 | 6.53 |
| 21 | 2.86 | 3.04 | 3.26 | 3.69 | 4.24 | 4.93 | 5.70 | 6.26 | 6.82 |
| 22 | 2.78 | 2.96 | 3.20 | 3.64 | 4.24 | 4.98 | 5.84 | 6.46 | 7.09 |
| 23 | 2.73 | 2.92 | 3.16 | 3.62 | 4.24 | 5.02 | 5.93 | 6.60 | 7.28 |
| 24 | 2.72 | 2.91 | 3.15 | 3.61 | 4.24 | 5.03 | 5.96 | 6.64 | 7.34 |
| 25 | 2.70 | 2.89 | 3.13 | 3.59 | 4.22 | 5.01 | 5.93 | 6.61 | 7.30 |
| 26 | 2.68 | 2.86 | 3.10 | 3.55 | 4.17 | 4.95 | 5.85 | 6.52 | 7.21 |
| 27 | 2.63 | 2.81 | 3.04 | 3.49 | 4.09 | 4.85 | 5.73 | 6.38 | 7.05 |
| 28 | 2.58 | 2.76 | 2.98 | 3.41 | 4.00 | 4.73 | 5.58 | 6.21 | 6.85 |
| 29 | 2.52 | 2.69 | 2.91 | 3.33 | 3.90 | 4.62 | 5.45 | 6.06 | 6.68 |
| 30 | 2.45 | 2.62 | 2.84 | 3.25 | 3.80 | 4.51 | 5.32 | 5.92 | 6.54 |
| 31 | 2.40 | 2.57 | 2.78 | 3.18 | 3.72 | 4.41 | 5.21 | 5.79 | 6.39 |
| 32 | 2.37 | 2.53 | 2.74 | 3.13 | 3.66 | 4.33 | 5.10 | 5.67 | 6.25 |
| 33 | 2.35 | 2.51 | 2.71 | 3.09 | 3.61 | 4.25 | 4.99 | 5.53 | 6.09 |
| 34 | 2.32 | 2.48 | 2.67 | 3.04 | 3.54 | 4.17 | 4.88 | 5.40 | 5.93 |
| 35 | 2.26 | 2.41 | 2.60 | 2.97 | 3.47 | 4.09 | 4.81 | 5.33 | 5.86 |
| 36 | 2.16 | 2.31 | 2.50 | 2.88 | 3.38 | 4.03 | 4.78 | 5.34 | 5.91 |
| 37 | 2.04 | 2.19 | 2.39 | 2.77 | 3.30 | 3.98 | 4.79 | 5.41 | 6.05 |
| 38 | 1.92 | 2.08 | 2.28 | 2.67 | 3.22 | 3.95 | 4.83 | 5.51 | 6.23 |
| 39 | 1.82 | 1.97 | 2.17 | 2.57 | 3.14 | 3.91 | 4.87 | 5.63 | 6.44 |
| 40 | 1.71 | 1.87 | 2.07 | 2.48 | 3.07 | 3.88 | 4.92 | 5.76 | 6.68 |
Figure 1:
Umbilical artery S/D ratio (A), resistance index (B), and pulsatility ratio (C) curve-fitted percentiles for non-SGA t21 and euploid pregnancies.
Discussion:
We found that values by gestational age for three commonly used UA Doppler indices were significantly higher in t21 compared to euploid pregnancies, even when SGA pregnancies were excluded. Prior studies have shown that pregnancies complicated by t21 have elevated Doppler indices, but these were limited by cross-sectional design, small population size, or inclusion of only first trimester measurements [4–7]. Studies in the second and third trimesters have reported high incidences of abnormal PI in t21, especially in the third trimester [7,20].
Strengths of our study include a larger sample size compared to previous studies and a longitudinal design allowing assessment of serial changes in UA Doppler indices throughout the second half of pregnancy. We found that UA Doppler indices are significantly higher in t21 pregnancies throughout the second half of pregnancies compared to euploid pregnancies, but this finding was not associated with higher rates of fetal demise in our population (3/86, 3.5%) than the 10% risk that has been previously described in the literature [21]. We therefore created percentile charts from our values, using a generalized additive model for location, scale, and shape, in order to demonstrate how UA Doppler indices from our t21 population differed from the euploid historical cohort.
UA Doppler indices are believed to reflect placental vascular resistance, and higher values of UA Doppler indices may be associated with increased placental vascular resistance in pregnancies with t21 fetuses. Prior reports have shown dysregulation in placental development and villous abnormalities in t21 placentas, which may explain our findings [22–27]. Histological differences described have included increased vascular abnormalities, inflammation, villous hypoplasia, and intervillous fibrin deposition [22–25]. Regression analysis found an overall negative relationship between mean Doppler indices and advancing gestational age, which is consistent with the established physiologic principle that placental resistance decreases throughout pregnancy [28–31].
Previous studies have found that the prevalence of SGA newborns in pregnancies complicated by t21 is higher than the general population at around 25–36% [32,33]. However, we found that only 9.3% of our t21 fetuses were SGA, and Dopplers were elevated compared to euploid pregnancies even when these pregnancies were removed. This suggests that higher placental vascular resistance, as indicated by higher values of UA Doppler indices, did not impair the growth of t21 fetuses. Prior studies have reported normal placental weights in t21, while others have found increased placental weights [34–36]. After correcting for neonatal birthweight, we found that the median birthweight-to-placental weight ratio, which is considered to be a proxy for placental efficiency [37], was higher in the euploid population when compared to the t21 population. Additional studies correlating UA Doppler indices with biochemical markers of placental function and placental histopathology in pregnancies with t21 fetuses are warranted to elucidate the effect of this aneuploidy on placental function, efficiency, and fetal growth.
Our study is not without limitations. Among our population of t21fetuses, 52.3% had major heart defects. Prior studies have found that neonates with t21 have a 33–48% chance of having any congenital heart defect [38–40]. These defects did not have a significant effect on Doppler indices, although the data were not stratified based on the type of cardiac anomaly. Studies to date are limited in number and size but have not found significant diagnostic value in UA Doppler indices in fetuses with complex congenital heart disease, beyond extremes of absent or reversed end-diastolic flow [41,42]. Additionally, no significant relationship has been found between UA Doppler indices and fetal cardiac function in experimental studies on sheep fetuses [43]. Duodenal atresia is also a common abnormality found in pregnancies complicated by t21 [44,45], which was present in 16.3% of our t21 population. This anomaly was associated with higher Doppler values, although this was not statistically significant across all Doppler indices. Interestingly, two of the three fetal demises occurred in fetuses with duodenal atresia, which raises the question as to whether this structural anomaly impacts circulatory function in the fetus and may be an independent risk factor for fetal compromise and death. Further studies are needed to investigate the effects of gastrointestinal tract obstruction on UA Doppler values. The high prevalence of anatomical malformations in our population of t21 fetuses can be explained by the higher frequency of complicated t21 pregnancies being referred to our tertiary care institution.
Importantly, when comparing the t21and euploid fetuses, we must acknowledge the group differences with higher maternal age and BMI in t21 pregnancies, as well as a different distribution in maternal race. This reflects differences in populations between the southeastern United States and Norway. The euploid population was prospectively selected, allowing for exclusion of mothers who had pre-existing conditions such as hypertension, diabetes, and maternal smoking. We chose not to exclude women with such comorbidities to avoid compromising our sample size. However, when statistically adjusting for tobacco use, hypertension, obesity, and additional factors such as fetal cardiac disease, we found that these covariates did not have a significant impact on UA Doppler indices. Due to limitations of retrospective data collection, we were unable to assess for the various additional maternal, fetal, and pregnancy factors that may affect Doppler velocimetry; however, our population is diverse and overall representative of the general U.S. maternal population, where 24.8% of mothers are obese [46], 7.2% smoke during pregnancy [47], and the rate of maternal chronic hypertension grows by 6% annually [48].
We also acknowledge the inherent limitations of retrospective studies, including the potential for selection bias and inconsistencies in data collection and reporting in the EMR. The S/D ratio, is the most commonly used UA Doppler index in the United States. This was also the most consistently collected UA Doppler variable during the study period, which resulted in fewer RI and PI observations. Observations were not evenly distributed across the range of gestational weeks, so the reference percentile <24 weeks and >38 weeks may be less reliable. Limited observations at 19 – 24 and 38 – 40 weeks can be explained by second trimester referrals to MFM and 40% of our patient population delivering preterm.
Conclusion:
At each week of gestation, UA Doppler indices in t21 fetuses were significantly higher than the established percentiles from a euploid population but were not associated with high risk of abnormal fetal growth or fetal demise. As such, higher values of Doppler indices in t21 fetuses may not indicate placental-fetal compromise, and reference intervals based on euploid fetuses are not necessarily appropriate for antenatal surveillance of fetuses with t21. Prospective studies are needed to investigate the role and impact of serial UA Doppler indices in the surveillance of pregnancies complicated by fetal t21.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the obstetric sonographers in the Division of Maternal Fetal Medicine at the Vanderbilt University Medical Center for their help with this study.
Footnotes
The authors report no conflict of interest.
Contributor Information
Karampreet Kaur, Vanderbilt University School of Medicine, Vanderbilt Surgical Outcomes Center for Kids, Nashville, TN, United States.
Ganesh Acharya, Department of Clinical Medicine, UiT-The Arctic University of Norway, Tromsø, Norway; Division of Obstetrics and Gynecology, Department of Clinical Science, Intervention and Technology Karolinska Institute, Stockholm, Sweden.
Heidi Chen, Vanderbilt University Medical Center Dept. of Biostatistics, Vanderbilt Surgical Outcomes Center for Kids, Nashville, TN, United States.
Chevis N. Shannon, Vanderbilt University Medical Center Dept. of Neurosurgery, Vanderbilt Surgical Outcomes Center for Kids, Nashville, TN, United States.
Brittany E. Lipscomb, Vanderbilt Surgical Outcomes Center for Kids, Nashville, TN, United States.
Randa Newman, Vanderbilt University Medical Center Dept. of Obstetrics and Gynecology, Division of Materna, Fetal Medicine, Nashville, TN, United States.
Lisa C. Zuckerwise, Vanderbilt University Medical Center Dept. of Obstetrics and Gynecology, Division of Maternal, Fetal Medicine Nashville, TN, United States; Vanderbilt Surgical Outcomes Center for Kids, Nashville, TN, United States.
References
- 1.Alfirevic Z, Neilson JP. Doppler ultrasonography in high-risk pregnancies: systematic review with meta-analysis. Am J Obstet Gynecol. 1995; 172: 1379–1387. [DOI] [PubMed] [Google Scholar]
- 2.Acharya G, Wilsgaard T, Berntsen GK, et al. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol. 2005; 192: 937–944. [DOI] [PubMed] [Google Scholar]
- 3.Mai CT, Isenburg JL, Canfield MA, et al. National Birth Defects Prevention N. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. 2019; 111: 1420–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez Crespo JM, Comas C, Ojuel H, et al. Fortuny A. Umbilical artery pulsatility index in early pregnancies with chromosome anomalies. Br J Obstet Gynaecol. 1996; 103: 330–334. [DOI] [PubMed] [Google Scholar]
- 5.Martinez JM, Borrell A, Antolin E, et al. Combining nuchal translucency with umbilical Doppler velocimetry for detecting fetal trisomies in the first trimester of pregnancy. Br J Obstet Gynaecol. 1997; 104: 11–14. [DOI] [PubMed] [Google Scholar]
- 6.Corry E, Mone F, Segurado R, et al. Placental disease and abnormal umbilical artery Doppler waveforms in trisomy 21 pregnancy: A case-control study. Placenta. 2016; 47: 24–28. [DOI] [PubMed] [Google Scholar]
- 7.Flöck A, Remig I, Müller A, et al. Conflicting umbilical artery Doppler findings in fetuses with trisomy 21. Arch Gynecol Obstet. 2015; 292: 613–617. [DOI] [PubMed] [Google Scholar]
- 8.Adams AD, Guedj F, Bianchi DW. Placental development and function in trisomy 21 and mouse models of Down syndrome: Clues for studying mechanisms underlying atypical development. Placenta. 2019; 89: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhide A, Acharya G, Bilardo CM, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol 2013; 41: 233–239. [DOI] [PubMed] [Google Scholar]
- 10.Zemel BS, Pipan M, Stallings VA, et al. Growth Charts for Children With Down Syndrome in the United States. Pediatrics. 2015; 136: e1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale and shape. Journal of the Royal Statistical Society: Series C (Applied Statistics) 2005; 54: 507–554. [Google Scholar]
- 13.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Australia, 2019. [Google Scholar]
- 14.Wei Y, Pere A, Koenker R, et al. Quantile regression methods for reference growth charts. Stat Med 2006; 25: 1369–1382. [DOI] [PubMed] [Google Scholar]
- 15.Goldkrand JW, Lentz SU, Turner AD, et al. Doppler velocimetry in the fetus with a single umbilical artery. J Reprod Med 1999; 44: 346–350. [PubMed] [Google Scholar]
- 16.Goldkrand JW, Pettigrew C, Lentz SU, et al. Volumetric umbilical artery blood flow: comparison of the normal versus the single umbilical artery cord. J Matern Fetal Med. 2001; 10: 116–121. [DOI] [PubMed] [Google Scholar]
- 17.Figueras F, Eixarch E, Gratacos E, et al. Predictiveness of antenatal umbilical artery Doppler for adverse pregnancy outcome in small-for-gestational-age babies according to customised birthweight centiles: population-based study. BJOG. 2008; 115: 590–594. [DOI] [PubMed] [Google Scholar]
- 18.McCowan LM, Erskine LA, Ritchie K. Umbilical artery Doppler blood flow studies in the preterm, small for gestational age fetus. Am J Obstet Gynecol. 1987; 156: 655–659. [DOI] [PubMed] [Google Scholar]
- 19.Baschat AA, Weiner CP. Umbilical artery doppler screening for detection of the small fetus in need of antepartum surveillance. Am J Obstet Gynecol. 2000; 182: 154–158. [DOI] [PubMed] [Google Scholar]
- 20.Bustos JC, Herrera A, Sepulveda W. Umbilical artery pulsatility index and half-peak systolic velocity deceleration time in fetuses with trisomy 21. J Matern Fetal Neonatal Med. 2019. DOI: 10.1080/14767058.2019.1575357. 1–7. [DOI] [PubMed] [Google Scholar]
- 21.Won RH, Currier RJ, Lorey F, et al. The timing of demise in fetuses with trisomy 21 and trisomy 18. Prenat Diagn. 2005; 25: 608–611. [DOI] [PubMed] [Google Scholar]
- 22.Frendo JL, Vidaud M, Guibourdenche J, et al. Defect of villous cytotrophoblast differentiation into syncytiotrophoblast in Down’s syndrome. J Clin Endocrinol Metab. 2000; 85: 3700–3707. [DOI] [PubMed] [Google Scholar]
- 23.Amiel A, Fejgin MD, Liberman M, et al. Senescence in amniocytes and placentas from trisomy 21 pregnancies. J Matern Fetal Neonatal Med. 2013; 26: 1086–1089. [DOI] [PubMed] [Google Scholar]
- 24.Pidoux G, Guibourdenche J, Frendo JL, et al. Impact of trisomy 21 on human trophoblast behaviour and hormonal function. Placenta 2004; 25 Suppl A: S79–84. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi F, Jacques SM, Johnson MP, et al. Trisomy 21 placentas: histopathological and immunohistochemical findings using proliferating cell nuclear antigen. Fetal Diagn Ther. 1997; 12: 210–215. [DOI] [PubMed] [Google Scholar]
- 26.Wright A, Zhou Y, Weier JF, et al. Trisomy 21 is associated with variable defects in cytotrophoblast differentiation along the invasive pathway. Am J Med Genet A. 2004; 130A: 354–364. [DOI] [PubMed] [Google Scholar]
- 27.Massin N, Frendo JL, Guibourdenche J, et al. Defect of syncytiotrophoblast formation and human chorionic gonadotropin expression in Down’s syndrome. Placenta. 2001; 22 Suppl A: S93–97. [DOI] [PubMed] [Google Scholar]
- 28.Gudmundsson S, Marsal K. Umbilical artery and uteroplacental blood flow velocity waveforms in normal pregnancy--a cross-sectional study. Acta Obstet Gynecol Scand. 1988; 67: 347–354. [PubMed] [Google Scholar]
- 29.Ferrazzi E, Gementi P, Bellotti M, et al. Doppler velocimetry: critical analysis of umbilical, cerebral and aortic reference values. Eur J Obstet Gynecol Reprod Biol. 1991; 38: 189–196. [DOI] [PubMed] [Google Scholar]
- 30.Kurmanavicius J, Florio I, Wisser J, et al. Reference resistance indices of the umbilical, fetal middle cerebral and uterine arteries at 24–42 weeks of gestation. Ultrasound Obstet Gynecol. 1997; 10: 112–120. [DOI] [PubMed] [Google Scholar]
- 31.Ertan AK, Hendrik HJ, Tanriverdi HA, et al. Fetomaternal Doppler sonography nomograms. Clin Exp Obstet Gynecol. 2003; 30: 211–216. [PubMed] [Google Scholar]
- 32.Clementi M, Calzolari E, Turolla L, et al. Neonatal growth patterns in a population of consecutively born Down syndrome children. Am J Med Genet Suppl. 1990; 7: 71–74. [DOI] [PubMed] [Google Scholar]
- 33.Weijerman ME, van Furth AM, Vonk Noordegraaf A, et al. Prevalence, neonatal characteristics, and first-year mortality of Down syndrome: a national study. J Pediatr. 2008; 152: 15–19. [DOI] [PubMed] [Google Scholar]
- 34.Kouvalainen K, Osterlund K. Placental weights in Down’s syndrome. Ann Med Exp Biol Fenn. 1967; 45: 320–322. [PubMed] [Google Scholar]
- 35.Shepard TH, FitzSimmons JM, Fantel AG, et al. Placental weights of normal and aneuploid early human fetuses. Pediatr Pathol. 1989; 9: 425–431. [DOI] [PubMed] [Google Scholar]
- 36.FitzSimmons J, Droste S, Shepard TH, et al. Growth failure in second-trimester fetuses with trisomy 21. Teratology. 1990; 42: 337–345. [DOI] [PubMed] [Google Scholar]
- 37.Hayward CE, Lean S, Sibley CP, et al. Placental Adaptation: What Can We Learn from Birthweight:Placental Weight Ratio? Front Physiol. 2016; 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freeman SB, Taft LF, Dooley KJ, et al. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998; 80: 213–217. [PubMed] [Google Scholar]
- 39.Khoury MJ, Erickson JD. Improved ascertainment of cardiovascular malformations in infants with Down’s syndrome, Atlanta, 1968 through 1989. Implications for the interpretation of increasing rates of cardiovascular malformations in surveillance systems. Am J Epidemiol. 1992; 136: 1457–1464. [DOI] [PubMed] [Google Scholar]
- 40.Wells GL, Barker SE, Finley SC, et al. Congenital heart disease in infants with Down’s syndrome. South Med J. 1994; 87: 724–727. [DOI] [PubMed] [Google Scholar]
- 41.Meise C, Germer U, Gembruch U. Arterial Doppler ultrasound in 115 second- and third-trimester fetuses with congenital heart disease. Ultrasound Obstet Gynecol. 2001; 17: 398–402. [DOI] [PubMed] [Google Scholar]
- 42.Al-Gazali W, Chapman MG, Chita SK, et al. Doppler assessment of umbilical artery blood flow for the prediction of outcome in fetal cardiac abnormality. Br J Obstet Gynaecol. 1987; 94: 742–745. [DOI] [PubMed] [Google Scholar]
- 43.Acharya G, Erkinaro T, Makikallio K, et al. Relationships among Doppler-derived umbilical artery absolute velocities, cardiac function, and placental volume blood flow and resistance in fetal sheep. Am J Physiol Heart Circ Physiol. 2004; 286: H1266–1272. [DOI] [PubMed] [Google Scholar]
- 44.Freeman SB, Torfs CP, Romitti PA, et al. Congenital gastrointestinal defects in Down syndrome: a report from the Atlanta and National Down Syndrome Projects. Clin Genet. 2009; 75: 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchin PJ, Levy JS, Schullinger JN. Down’s syndrome and the gastrointestinal tract. JClin Gastroenterol. 1986; 8: 111–114. [DOI] [PubMed] [Google Scholar]
- 46.Branum AM, Kirmeyer SE, Gregory EC. Prepregnancy Body Mass Index by Maternal Characteristics and State: Data From the Birth Certificate, 2014. Natl Vital Stat Rep. 2016; 65: 1–11. [PubMed] [Google Scholar]
- 47.Drake P, Driscoll AK, Mathews TJ. Cigarette Smoking During Pregnancy: United States, 2016. NCHS Data Brief. 2018. 1–8. [PubMed] [Google Scholar]
- 48.Ananth CV, Duzyj CM, Yadava S, et al. Changes in the Prevalence of Chronic Hypertension in Pregnancy, United States, 1970 to 2010. Hypertension. 2019; 74: 1089–1095 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.