Abstract
Enterovirus 71 (EV71) infection is one of the main causes of severe hand, foot and mouth disease (HFMD), which is usually accompanied by a marked inflammatory response. The excessive inflammatory response has been implicated to serve an important role in EV71-caused HFMD. Pyroptosis is a type of inflammatory programmed cell death. Therefore, a novel treatment strategy against EV71 infection could aim to alleviate the inflammatory response through inhibition of EV71-induced pyroptosis. The present study revealed that metformin had this therapeutic potential. A cell model of EV71 infection was established, cell viability was measured by CCK8 assay, cell damage was measured by LDH release kit, and the dead and dying cells were excluded by propidium iodide staining. The intracellular levels of DEP domain-containing mTOR interacting protein (DEPTOR) and pyroptosis-associated molecules were measured by western blot analysis, the NLRP3 expression was assessed by immunofluorescence labeling, and virus titers in cell culture supernatants were determined by a cell culture infectious dose 50 assay. The results demonstrated that EV71 infection could induce pyroptosis in a time- and dose-dependent manner, and metformin could inhibit EV71-induced pyroptosis. The mechanism of metformin inhibiting EV71-induced pyroptosis was explored next. Subsequent experiments indicated that metformin could increase the levels of DEPTOR, which were decreased by EV71. Finally, overexpression of DEPTOR in cells could reduce EV71-induced pyroptosis. Overall, the present study demonstrated that metformin could exert a novel pharmacodynamic anti-pyroptosis effect in the treatment of EV71 infection by upregulating DEPTOR expression.
Keywords: enterovirus 71, pyroptosis, metformin, DEP domain-containing mTOR-interacting protein
Introduction
Enterovirus 71 (EV71) belongs to the small ribonucleovirus family of the enterovirus genus, and is a non-envelope single-stranded RNA virus (1). Patients who are <6 years old with EV71 infection may suffer from brain stem encephalitis, neurogenic pulmonary edema, cardiac arrest and other fatal clinical symptoms, including death (2,3).
Previous studies have demonstrated that viral infections, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (4), influenza virus (5), hepatitis B virus (6) and West Nile virus (7), can induce pyroptosis, and our previous study has also indicated that EV71 infection could induce pyroptosis (8). Pyroptosis is a type of inflammatory programmed cell death. NLR family, pyrin domain-containing 3 (NLRP3) activation triggers NLRP3-inflammasome assembly, which results in pyroptosis initiation when viral infections occur. Caspase-1 is a downstream molecule of the inflammasome and activated caspase-1 initiates pyroptosis by cleaving gasdermin D (GSDMD). Activated caspase-1 also cleaves Pro-IL-1β into mature IL-1β. When pyroptosis occurs, the cleaved GSDMD forms a pore channel on the cell surface, which causes membrane rupture, cell death and release of the inflammatory cytokines IL-1β and IL-18. Therefore, IL-1β, which can induce the inflammatory reaction, is a sensitive marker of the activation of GSDMD (9-14).
It has been demonstrated that excessive inflammatory activation is one of the main causes of severe hand, foot and mouth disease (HFMD) (2,3). We hypothesized that the suppression of virus-induced cytotoxicity and pyroptosis with drugs can be a novel therapeutic strategy for EV71 infection. At present, certain researchers have tried to explore potential inhibitors to reduce the formation of GSDMD pore channels; for example, combination of dimethyl fumarate and cysteine residues limits the oligomerization of GSDMD in cells (15). In addition, the inhibition of diabetes-induced pyroptosis by inhibiting the thioredoxin interacting protein (TXNIP)/NLRP3 signaling pathway has been observed (16). In another study, paeoniflorin has been used to inhibit the activation of caspase-11, thereby aiming to inhibit the activation of the inflammatory caspase cascade to suppress inflammation (17).
Metformin was originally extracted from goat beans to treat diabetes (18). It has been demonstrated that metformin reduces liver glucose generation via the AMP-activated protein kinase (AMPK) signaling pathway (19). A previous study has revealed that metformin inhibits the production of IL-1β by macrophages (20), and that it interferes with the AMPK signaling pathway to suppress inflammation (21). Metformin has also been revealed to inhibit NLRP3(22). Another study has demonstrated that metformin inhibits cardiac muscle cell pyroptosis by inhibiting the TXNIP-NLRP3-GSDMD signaling pathway (23).
A recent study has indicated that metformin treatment can lower the mortality rate of diabetic patients infected with SARS-CoV-2(24). At present, to the best of our knowledge, there is no report on the effect of metformin treatment against EV71 infection or the effect of metformin on EV71-induced pyroptosis.
Previous studies suggest that metformin can increase DEP domain-containing mTOR-interacting protein (DEPTOR) levels in hepatoma cells, ureteral epithelial cells and rats with unilateral ureteral obstruction (25,26). DEPTOR is a component of the mTOR complex (mTORC)1 and mTORC2 and binds to mTOR to suppress its kinase activity (27). A study has demonstrated that DEPTOR serves an important role in immune regulation and that DEPTOR is higher in T cells before inflammatory activation (28). Zhai et al (29) revealed that DEPTOR deficiency in T cells is associated with NLRP3-activated IL-1β release. Another study has revealed that decreased DEPTOR levels induce the release of a large number of chemokines in endothelial cells, such as chemokine (C-X-Cmotif) ligand 9 (CXCL9), CXCL10, CXCL11, CX3CL1, chemokine (C-C motif) ligand 5 (CCL5) and CCL20(30). It has been revealed that DEPTOR is also regulated by the nuclear receptors of steroids, which can increase the levels of DEPTOR mRNA in cells (31). An androgen receptor repressor, prochloramide, is used in clinical treatment to control SARS-CoV-2 infection (32). It has been demonstrated that DEPTOR is regulated by the androgen receptor, which serves a negative role in the regulation of DEPTOR mRNA levels (33). Therefore the present study investigated whether EV71 could induce a change in the DEPTOR protein levels and what consequences this had on EV71-induced pyroptosis. In addition, in terms of inhibiting EV71-induced pyroptosis, the present study aimed to find a commercial drug that may be used in the treatment of EV71 infection and investigate its anti-pyroptosis mechanism.
Materials and methods
Reagents and antibodies
DMEM (cat. no. PYG0073), penicillin and streptomycin (cat. no. PYG0016), trypsin, BCA protein detection kit (cat. no. AR0146), ECL western blot detection kit (cat. no. AR1172), SDS-PAGE loading buffer (5X) (cat. no. AR1112-10), horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L) (cat. no. BA1054) and GSDMD antibody (cat. no. A02842) were purchased from Boster Biological Technology. Cell Counting Kit (CCK-8) (cat. no. 40203ES76) was purchased from Yeasen Biotechnology (Shanghai) Co., Ltd.. Bovine serum albumin (cat. no. abs9157) was purchased from Absin Bioscience Inc., Fetal bovine serum (cat. no. 04-001-1A) were purchased from Biological Industries, metformin (cat. no. IM0140) were obtained from Beijing Solarbio Science & Technology Co., Ltd. DAPI (cat. no. C1002), Lipofectamine 8000 (cat. no. C0533-0.5ml), propidium iodide (PI; cat. no. ST511) and Alexa Fluor 488 goat anti-rabbit IgG antibody (cat. no. A0423) were purchased from Beyotime Biotechnology Company. The caspase-1 antibody (cat. no. A0964) and caspase 1 p20 (cat. no. A23429) were purchased from ABclonal Biotech Co., Ltd. The lactate dehydrogenase (LDH) detection kit (cat. no. WLA072a) and Pro-IL-1β (cat. no. WL02257) and NLRP3 (cat. no. WL02635) antibodies were purchased from Wanleibio Co., Ltd. The GAPDH (cat. no. BS65656) antibody was obtained from Bioworld Technology, Inc. The β-actin (cat. no. AB0061) antibody was purchased from Shanghai Abways Biotechnology Co., Ltd. The DEPTOR/DEPDC6 (D9F5) antibody (cat. no. #11816) was purchased from Cell Signaling Technology, Inc. pcDNA 3.1 and pcDNA 3.1-DEPTOR were purchased from Shanghai GenePharma Co., Ltd.
Cells and virus
GES-1 human normal gastric epithelial cells were purchased from Boster Biological Technology and RD human rhabdomyosarcoma cells were purchased from the National Collection of Authenticated Cell Cutures, and cultured in DMEM containing 10% fetal bovine serum and 1% penicillin and streptomycin in an incubator with 5% CO2 at 37˚C. EV71 was obtained from Professor Zhendong Zhao (Institute of Pathogen Biology, Chinese Academy of Medical Sciences & Peking Union Medical College). GES-1 cells were infected with EV71 at various multiplicity of infections (MOIs) of 1, 2, 3, 4 or 5 for different times (12, 18 or 24 h) at 37˚C, and cells cultured in full culture medium without EV71 were used as the mock group (mock infection).
Cell culture infectious dose 50 (CCID 50) assay
RD cells (1x104/well) were seeded into 96-well plates and cultured in an incubator at 37˚C with 5% CO2 for 12 h. Subsequently, RD cells were infected with EV71 that was serially diluted from 1x101 to 1x1011 times, and cells in a total of eight wells per 96-well plate were infected with EV71 at the same dilution and cultured at 37˚C. The 96-well plates were observed once per day for 5 days. The wells with ~50% cytopathic effect of RD cells were observed under a microscope and recorded, and the CCID 50 results were calculated using the Reed-Münch method (34).
Measurement of LDH release
GES-1 cells were infected with EV71 at various MOIs of 1, 2, 3, 4 or 5 or infected with EV71 at MOI 5 and treated with metformin (1, 3 or 5 µM) for 24 h and then the cell supernatant was collected at 400 x g for 5 min at 4˚C. The LDH release of the cell supernatant was measured using an LDH detection kit. LDH activity (%) was calculated as follows: (LDHtreatment-LDHcontrol)/(LDHmax-LDHcontrol) x100.
Western blotting
GES-1 cells from different groups were collected in 1.5-ml centrifuge tubes and lysed with 60 µl RIPA lysis buffer (Beyotime Institute of Biotechnology). The total protein concentration was measured using a BCA kit. A total of 30 µg total extracted protein from each group was separated by 12% SDS-PAGE and then transferred to a nitrocellulose (NC) membrane. The NC membrane was blocked with 5% skimmed milk for 2 h at room temperature and then incubated with NLRP3 (1:1,000), GSDMD (1:1,000), caspase-1 (1:1,000), caspase-1 P20 (1:1,000), Pro-IL-1β (1:5,000), DEPTOR (1:1,000), GAPDH (1:5,000) and β-actin (1:5,000) antibodies overnight at 4˚C. Subsequently, the NC membrane was washed three times with TBST (0.1% Tween) and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:5,000) at room temperature for 2 h. Finally, immunoblots were incubated with an ECL chromogenic kit and observed using a ChemiDoc™ MP Imaging System (Bio-Rad Laboratories, Inc.). ImageJ software (version 6.0; National Institutes of Health) was used for densitometry.
Cell Counting Kit-8 (CCK-8) assay
GES-1 cells seeded onto 96-well plates were infected with EV71 at MOI 1, 2, 3, 4, or 5 or infected with EV71 at MOI 5 and treated with metformin (1, 3 or 5 µM) for 24 h at 37˚C with 5% CO2. At 2 h before the end of the culture, 20 µl CCK-8 reagent was added to the 96-well plates. Subsequently, the absorption of each sample was detected at 450 nm using a microplate reader (BioTek Instruments, Inc.). The cell viability was calculated using the following formula: Cell viability (%)=(Absorbancetreatment-Absorbanceblank)/(Absorbancecontrol-Absorbanceblank) x100%.
Immunofluorescence staining
GES-1 cells were seeded into 24-well plates (1x105 cells/well). When the EV71 infected-GES-1 cell density reached 60-70%, GES-1 cells were fixed with 4% paraformaldehyde at 4˚C for 20 min. Subsequently, the cells were permeated with 0.5% Triton X-100 at room temperature for 15 min and blocked with 5% bovine serum albumin at room temperature for 1 h. Then the cells were incubated with the NLRP3 antibody (1:200) at 4˚C overnight, followed by incubation with Alexa Fluor 488 goat anti-rabbit IgG (1:200) at room temperature for 1 h, and then protected from light and stained with 1 µg/ml DAPI solution in PBS for 5 min at room temperature. The cells were again protected from light and washed three times with PBS after each staining step. All images were observed under an inverted fluorescence microscope (x200 magnification2; Nikon Corporation).
PI staining
GES-1 cells in the logarithmic phase were seeded into 24-well plates (1.2x105/well) for 12 h and then treated with EV71, metformin (5 µM) or EV71 and metformin (5 µM) for 24 h at 37˚C. When the density of the treated cells reached ~80%, GES-1 cells were stained with 4 µM PI for 30 min at 37˚C. The images were captured using an inverted fluorescence microscope (magnification, x200; Nikon Corporation).
DNA transfection
GES-1 cells were plated into 6-well plates (50x104/well) for 12 h. Subsequently, 2.5 µg pcDNA 3.1 (negative control with empty vector) or pcDNA 3.1-DEPTOR and 5 µl Lipofectamine 8000 were added to 125 µl fresh DMEM (without bovine serum and antibiotics) and gently mixed. Then, the mixture was added to the 6-well plate. Cells were cultured at 37˚C, and subsequent experiments were carried out 48 h later.
Statistical analysis
GraphPad Prism 8 software (GraphPad Software; Dotmatics) was used for statistical tests. All experiments were independently repeated three times and data are presented as the mean ± SD. Multiple groups were analyzed through one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
EV71 infection causes cell damage and reduces cell viability
GES-1 cells were treated with EV71 at the different MOIs (0, 1, 2, 3, 4 and 5) for 24 h. The cell viability of each group was detected using a CCK-8 assay (Fig. 1A). Subsequently, GES-1 cells were treated with EV71 at a MOI of 1, 3 and 5 for 24 h, and the cell damage level was measured using an LDH release assay (Fig. 1B). The results demonstrated that EV71 decreased cell viability and increased cell damage in a dose-dependent manner.
Figure 1.
Shift in cell viability and cell damage caused by enterovirus 71. (A) Cell viability was detected using a Cell Counting Kit-8 assay. (B) Concentration of LDH in the culture supernatant as detected by an LDH release assay. **P<0.01 compared with the mock group. LDH, lactate dehydrogenase; MOI, multiplicity of infection.
EV71 infection induces pyroptosis
The present study further examined whether pyroptosis is involved in EV71-induced cell damage. GES-1 cells were infected with EV71 at different MOIs (0, 1, 2, 3, 4 and 5) for 24 h. The protein levels of NLRP3, GSDMD, caspase-1 and Pro-IL-1β in cells were detected by western blotting. The results demonstrated that EV71 increased the levels of NLRP3, and decreased the levels of GSDMD, caspase-1 and Pro-IL-1β in a dose-dependent manner (Fig. 2A-C). Subsequently, GES-1 cells were infected with EV71 at a MOI of 5 for 12, 18 and 24 h. Compared to those of the mock group, the protein levels of GSDMD, and Pro-IL-1β were reduced in a time-independent manner, and caspase-1 was decreased significantly at 24 h (Fig. 2D and E). GES-1 cells were infected with EV71 at a MOI of 5 for 24 h, and the NLRP3 levels were examined using immunofluorescence staining. The results indicated that intracellular NLRP3 was upregulated and showed intracellular aggregation (Fig. 2F). These suggested that EV71 infection could induce cell pyroptosis.
Figure 2.
EV71 induces cell pyroptosis. Western blotting was performed to detect the levels of (A) NLRP3 and caspase-1, (B) GSDMD and (C) Pro-IL-1β, with β-actin or GAPDH as an internal reference. Western blot analysis of the levels of (D) caspase-1 and GSDMD and (E) Pro-IL-1β, with β-actin or GAPDH as internal references. (F) NLRP3 staining in GES-1 cells was detected using an immunofluorescence assay (magnification, x200). *P<0.05 and **P<0.01 compared with the mock group. EV71, enterovirus 71; GSDMD, gasdermin D; NLRP3, NLR family, pyrin domain-containing 3; MOI, multiplicity of infection; hpi, hours post inoculation.
Metformin reverses EV71-induced cytotoxicity and cell viability inhibition
The present study evaluated whether metformin was able to affect the viability of EV71-infected cells and reverse EV71-induced cytotoxicity. Shifts in cell viability and cell damage were detected using CCK-8 and LDH release assays. The cells were treated with EV71 (MOI=5) alone or combined with metformin at different concentrations (1, 3 and 5 µM) for 24 h. The cell viability was increased and cell damage was inhibited by 5 µM metformin treatment in EV71-infected cells compared with cells infected with EV71 only (Fig. 3A and B). Next, The GES-1 cells were stained with PI (red fluorescence), which was used to stain the dead and dying cells. EV71-treated cells exhibited cell injury and the number of GES-1 cells were decreased compared with that in the uninfected group, while treatment with metformin reversed the EV71-induced cell damage (Fig. 3C).
Figure 3.
Effect of metformin on the cell viability inhibition and cell damage in EV71-infected GES-1 cells. (A) Cell viability was measured using a Cell Counting Kit-8 assay. (B) Cell damage was assessed using an LDH release assay. (C) Cells were stained using PI (red fluorescence) and observed under a fluorescence microscope (magnification, x200). *P<0.05 and **P<0.01 compared with the mock group; #P<0.05 and ##P<0.01 compared with the EV71-infected group (without metformin treatment). EV71, enterovirus 71; MOI, multiplicity of infection; LDH, lactate dehydrogenase; PI, propidium iodide.
Metformin inhibits EV71-induced pyroptosis in GES-1 cells
In order to define whether metformin against EV71 induced cell pyroptosis, GES-1 cells were treated with EV71 and 2 µM metformin. Metformin upregulated Pro-IL-1β and downregulated caspase-1 P20 in EV71-infected cells compared with the EV71 infected group (P<0.01; Fig. 4A and B). EV71-infected GES-1 cells were treated with different concentrations (0, 1, 2, 3, 4 and 5 µM) of metformin for 24 h. The results revealed that NLRP3 and caspase-1 P20 were downregulated, and GSDMD, caspase-1 and Pro-IL-1β were upregulated (P<0.01; Fig. 4C-F) compared with the EV71 group. This indicated that metformin exerted substantial inhibitory effects on EV71-induced pyroptosis.
Figure 4.
Effect of metformin on EV71-induced cell pyroptosis. (A) Western blot analysis was performed to investigate the levels of caspase-1 P20. GAPDH was used as an internal reference. (C) Western blot analysis was performed to investigate the levels of caspase-1 and caspase-1 P20. GAPDH was used as an internal reference. (B, E and F) Western blot analysis was performed to determine Pro-IL-1β protein expression in GES-1 cells. β-actin was used as an internal reference. (D) Protein levels of NLRP3 and GSDMD in GES-1 cells were examined by western blotting. GAPDH was used as an internal reference. **P<0.01 compared with the mock group. ##P<0.01 compared with the EV71-infected group (without metformin treatment). EV71, enterovirus 71; GSDMD, gasdermin D; NLRP3, NLR family, pyrin domain-containing 3; MET, metformin MOI, multiplicity of infection.
Metformin upregulates DEPTOR protein expression in GES-1 cells
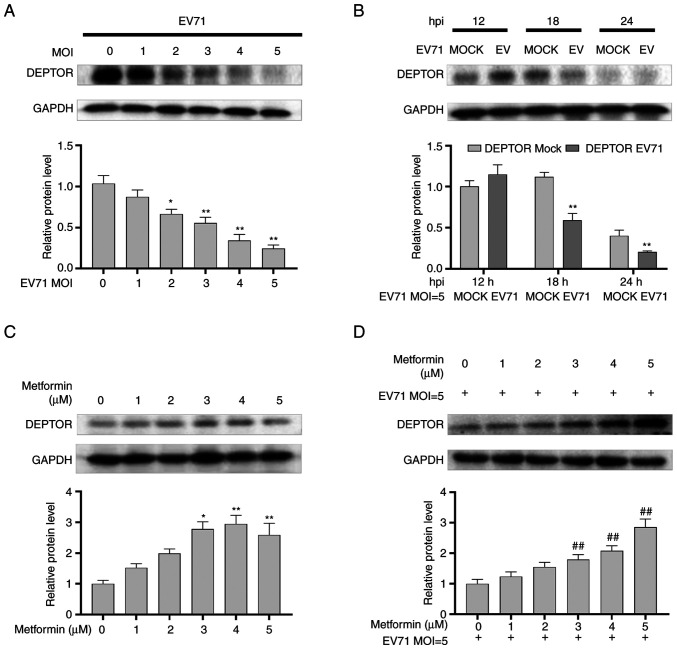
GES-1 cells were infected with EV71 at various MOIs (0, 1, 2, 3, 4 and 5) for 24 h, and the results indicated that the protein levels of DEPTOR were decreased in a dose-dependent manner in EV71-infected GES-1 cells (P<0.01; Fig. 5A). Subsequently, GES-1 cells were infected with EV71 (MOI=5) at varying times (12, 18 and 24 h), and western blot analysis revealed the degradation of DEPTOR in a time-dependent manner compared with the mock group at the same time (P<0.01; Fig. 5B). Next, GES-1 cells were incubated with varying concentrations (0, 1, 2, 3, 4 and 5 µM) of metformin for 24 h, and the results indicated that metformin increased DEPTOR levels in a dose-dependent manner (P<0.01; Fig. 5C). Finally, DEPTOR protein levels were upregulated in EV71-infected and metformin-treated (0, 1, 2, 3, 4 and 5 µM) GES-1 cells compared with EV71-infected alone GES-1 cells (P<0.01; Fig. 5D).
Figure 5.
Shift in the levels of DEPTOR caused by EV71 and metformin in GES-1 cells. (A) Western blotting was performed to detect the changes in the protein levels of DEPTOR in GES-1 cells infected with different MOI for EV71. GAPDH was used an internal reference. (B) Western blotting was performed to detect the changes in the protein levels of DEPTOR in GES-1 cells infected with EV71 for different times. GAPDH was used an internal reference. (C) Western blot analysis of DEPTOR protein expression in GES-1 cells treated with different concentrations of metformin for 24 h. GAPDH was used as an internal reference. (D) Western blot analysis of the changes in DEPTOR protein expression in EV71-infected GES-1 cells treated with different concentrations (0, 1, 2, 3, 4 and 5 µM) of metformin. GAPDH was used as an internal reference. *P<0.05 and **P<0.01 compared with the mock group or without metformin treatment group; ##P<0.01 compared with the EV71-infected group (without metformin treatment). DEPTOR, DEP domain-containing mTOR interacting protein; EV71, enterovirus 71; MOI, multiplicity of infection; hpi, hours post inoculation.
The results demonstrated that metformin could reverse the downregulating effect of EV71 on DEPTOR. The alterations of DEPTOR were accompanied by changes in pyroptosis that were regulated by EV71 or metformin, as aforementioned. These findings suggested a potential association between pyroptosis and DEPTOR expression.
Overexpression of DEPTOR inhibits EV71-induced pyroptosis
EV71 infection induced pyroptosis and the degradation of DEPTOR, which were reversed by metformin treatment. Next, the present study aimed to further investigate the association between pyroptosis and DEPTOR. pcDNA 3.1 and pcDNA 3.1-DEPTOR were transfected into GES-1 cells, which were then infected with EV71. The changes of pyroptosis-related proteins were observed. The results showed that after EV71 infection, compared with that of the empty vector expression group, the level of GSDMD and DEPTOR was increased (P<0.01; Fig. 6A), caspase-1 level was increased (P<0.01; Fig. 6B), caspase-1 P20 level was decreased (P<0.05; Fig. 6B), the expression of NLRP3 was reduced (P<0.01; Fig. 6C) and the level of Pro-IL-1β was increased (P<0.01; Fig. 6D) in the DEPTOR overexpression group. The results demonstrated that DEPTOR overexpression led to inhibition of pyroptosis and indicated that metformin inhibited EV71-induced pyroptosis via upregulating DEPTOR.
Figure 6.
Possible involvement of DEPTOR in the regulatory effect of metformin on pyroptosis. Western blot analysis of the changes in the levels of (A) GSDMD, (B) caspase-1 and caspase-1 P20, (C) NLRP3 and (D) Pro-IL-1β in GES-1 cells overexpressing DEPTOR. β-actin or GAPDH was used as a loading control. #P<0.05; ##P<0.01 compared with the NC with EV71-infected group. DEPTOR, DEP domain-containing mTOR-interacting protein; EV71, enterovirus 71; GSDMD, gasdermin D; NLRP3, NLR family, pyrin domain-containing 3; MOI, multiplicity of infection; NC, negative control (empty vector).
Discussion
EV71 infection causes HFMD in infants and children, particularly severe infections with pulmonary edema and neurological complications, which pose a serious threat to the life and health of children. There is no specific drug to control EV71 infection at present, thus it is important to investigate anti-infection mechanisms and find effective antiviral drugs (35). Viral infection induces pyroptosis and causes an inflammation response. We hypothesized that targeting the virus-induced pyroptosis and its initiated inflammation can be an innovative strategy for antiviral treatment. In the present study, the inhibitory effect of metformin on EV71-induced pyroptosis and the upregulating effect of metformin on DEPTOR levels were revealed. It was demonstrated that DEPTOR was involved in the inhibitory process of EV71-induced pyroptosis, which provided a reference for discovering effective anti-EV71 infection drugs.
The present study first confirmed that EV71 infection increased cell damage and decreased cell viability, and that 5 µM metformin treatment reversed this cytotoxicity. This finding suggests that metformin may play a protective role in EV71 infections. EV71 infected GES-1 cells exhibited pyroptosis, accompanied by caspase-1 activation, GSDMD increase and IL-1β maturation. This finding is consistent with the work of Yogarajah et al (36) showing that EV71 induced host cell pyroptosis. Other studies have demonstrated that EV71 3C protease inhibits the activation of GSDMD (36-38). It is possible that the viral protease prolongs the proliferation stage of the virus in cells by inhibiting pyroptosis in the early stage of infection (39). However, a large number of aggregating progeny viruses eventually lead to the occurrence of pyroptosis in the later stage of infection, which causes the release of mature viruses and inflammatory cytokines, increases infection and pathogenesis, and causes further serious pathological damage to the body. This hypothesis is supported by the marked intracellular elevation of viral structure protein VP1 and the pyroptosis-related proteins NLRP3, GSDMD, Caspase-1 and Pro IL-1β 24 h after infection (40). The present study subsequently tried to find a marketed drug that could suppress pyroptosis to shorten its clinical trial period. It was revealed that metformin could inhibit EV71-induced cell damage and pyroptosis in a dose-dependent manner. This finding demonstrated the anti-pyroptosis effect of metformin in a novel model, which further supplemented the pharmacological effects of metformin.
The anti-pyroptosis mechanism of metformin was investigated further, and it was revealed that metformin could regulate DEPTOR. DEPTOR, as a metabolic pathway inhibitor protein, is upregulated by corticosteroids (31). It was explored whether the levels of DEPTOR changed in the EV71-infection model and whether metformin could regulate DEPTOR. The results demonstrated that EV71 infection reduced, while metformin increased the cellular levels of DEPTOR which is consistent with the findings of Obara et al (25). In the present cell model of EV71 infection, metformin reversed EV71-induced DEPTOR reduction in a dose-dependent manner, suggesting that metformin may exert its effect by upregulating the expression levels of DEPTOR.
The present study subsequently aimed to determine whether DEPTOR is involved in the regulation of virus-induced pyroptosis. The effects of DEPTOR overexpression on NLRP3, Pro-IL-1β, caspase-1, caspase-1 P20 and GSDMD were detected. The results demonstrated that DEPTOR overexpression could suppress EV71-induced pyroptosis. Zhai et al (29) indicated that the reduced level of DEPTOR can activate NLRP3 and mature IL-1β in macrophages. DEPTOR knockdown experiments were conducted in metformin-treated cells in the present study with an aim to observe whether the effect of metformin on pyroptosis can be inhibited. However, knockdown experiments require prolonged cell cultivation that make the expression level of DEPTOR in cells too low to detect. This is a limitation of the present study and an aim in future research to improve upon. The present study demonstrated that metformin could inhibit EV71-induced pyroptosis by upregulating DEPTOR. However, the mechanism via which metformin upregulates DEPTOR and how DEPTOR regulates pyroptosis should be investigated in the future.
In future studies on this topic, the first consideration should be to further investigate the association between DEPTOR and the pyroptosis pathway. Peterson et al (41) revealed that overexpression of DEPTOR activates the mTORC2/AKT signaling pathway, leading to an increase in phosphorylation at the Ser-473 site of AKT. Zhao et al (42) revealed that activation of AKT inhibits NLRP3 degradation, thereby suppressing NLRP3 oligomerization and activation. Considering the relationship between AKT and mTORC2, it may be hypothesized that DEPTOR may affect AKT activity through mTORC2 to regulate NLRP3 oligomerization and inhibit pyroptosis.
In conclusion, the findings of the present study suggested that EV71 infection induced GES-1 cell pyroptosis, while metformin suppressed EV71-induced pyroptosis, alleviated cytotoxicity and the inflammatory response. The implicated mechanism may involve the upregulation of DEPTOR by metformin.
Acknowledgements
The authors would like to thank Professor Zhendong Zhao (Institute of Pathogen Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China) for his gift of cell lines and virus.
Funding Statement
Funding: The present study was supported by the National Natural Science Foundation of China (grant no. 81301426), the Provincial Natural Science Foundation of Shanxi (grant no. 201901D111329), the Mega Research and Development Projects of Lüliang (grant no. 2020SHFZ38), the Program of Fenyang College, Shanxi Medical University (grant no. 2020B01) and the Key Laboratory Platform Construction Projects of Lüliang (grant no. 2020ZDSYS17).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XiaZ and CS conceived and designed the experiments. CS, XiaZ, JH, LC and XinZ performed the experiments. CS, JD, QH analyzed the data. XiaZ and CS wrote the manuscript. XiaZ and JH revised the manuscript. All authors have read and approved the final manuscript. XiaZ and CS confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Huang SW, Kiang D, Smith DJ, Wang JR. Evolution of re-emergent virus and its impact on enterovirus 71 epidemics. Exp Biol Med (Maywood) 2011;236:899–908. doi: 10.1258/ebm.2010.010233. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Hu L, Xu F, Liu C, Li J. A case report of a teenager with severe hand, foot, and mouth disease with brainstem encephalitis caused by enterovirus 71. BMC Pediatr. 2019;19(59) doi: 10.1186/s12887-019-1428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing J, Wang K, Wei H, Wei D. Pathologic and molecular studies of enterovirus 71 infection in a fatal case from a recent epidemic in China. Medicine (Baltimore) 2018;97(e13447) doi: 10.1097/MD.0000000000013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X, Liu Y, Huang Z, Xu W, Hu W, Yi L, Liu Z, Chan H, Zeng J, Liu X, et al. SARS-CoV-2 non-structural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1. Cell Death Differ. 2022;29:1240–1254. doi: 10.1038/s41418-021-00916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie WH, Ding J, Xie XX, Yang XH, Wu XF, Chen ZX, Guo QL, Gao WY, Wang XZ, Li D. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm Res. 2020;69:683–696. doi: 10.1007/s00011-020-01351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, Sodhi K, Treuting PM, Busch MP, Norris PJ, Gale M Jr. IL-1β signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012;8(e1003039) doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Hao J, Sun C, Du J, Han Q, Li Q. Total astragalosides decrease apoptosis and pyroptosis by inhibiting enterovirus 71 replication in gastric epithelial cells. Exp Ther Med. 2022;23(237) doi: 10.3892/etm.2022.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 10.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 11.Kesavardhana S, Malireddi RKS, Kanneganti T. Caspases in cell death, inflammation, and gasdermin-induced pyroptosis. Annu Rev Immunol. 2020;38:567–595. doi: 10.1146/annurev-immunol-073119-095439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 13.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion: Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, Wilson R, Jiang Z, Khalighinejad F, Muneeruddin K, et al. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369:1633–1637. doi: 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An X, Zhang Y, Cao Y, Chen J, Qin H, Yang L. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 2020;12(1516) doi: 10.3390/nu12051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian DD, Wang M, Liu A, Gao MR, Qiu C, Yu W, Wang WJ, Zhang K, Yang L, Jia YY, et al. Antidepressant effect of paeoniflorin is through inhibiting pyroptosis CASP-11/GSDMD pathway. Mol Neurobiol. 2021;58:761–776. doi: 10.1007/s12035-020-02144-5. [DOI] [PubMed] [Google Scholar]
- 18.Bailey CJ. Metformin: Historical overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 19.Agius L, Ford BE, Chachra SS. The metformin mechanism on gluconeogenesis and AMPK activation: The metabolite perspective. Int J Mol Sci. 2020;21(3240) doi: 10.3390/ijms21093240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qing L, Fu J, Wu P, Zhou Z, Yu F, Tang J. Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway. Am J Transl Res. 2019;11:655–668. [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Li X, Zhang W, He J, Xu B, Lei B, Wang Z, Cates C, Rousselle T, Li J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism. 2018;83:256–270. doi: 10.1016/j.metabol.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan J, Ding D, Feng G, Yang Y, Zhou Y, Ma L, Guo H, Lu Z, Jin Q. Metformin reduces chondrocyte pyroptosis in an osteoarthritis mouse model by inhibiting NLRP3 inflammasome activation. Exp Ther Med. 2022;23(222) doi: 10.3892/etm.2022.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia Y, Cui R, Wang C, Feng Y, Li Z, Tong Y, Qu K, Liu C, Zhang J. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biol. 2020;32(101534) doi: 10.1016/j.redox.2020.101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuel SM, Varghese E, Büsselberg D. Therapeutic potential of metformin in COVID-19: Reasoning for its protective role. Trends Microbiol. 2021;29:894–907. doi: 10.1016/j.tim.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obara A, Fujita Y, Abudukadier A, Fukushima T, Oguri Y, Ogura M, Harashima S, Hosokawa M, Inagaki N. DEPTOR-related mTOR suppression is involved in metformin's anti-cancer action in human liver cancer cells. Biochem Biophys Res Commun. 2015;460:1047–1052. doi: 10.1016/j.bbrc.2015.03.148. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Wang Y, Li Y, Lu L, Peng Y, Zhang S, Xia A. Metformin attenuates renal interstitial fibrosis through upregulation of Deptor in unilateral ureteral obstruction in rats. Exp Ther Med. 2020;20(1) doi: 10.3892/etm.2020.9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caron A, Briscoe DM, Richard D, Laplante M. DEPTOR at the nexus of cancer, metabolism, and immunity. Physiol Rev. 2018;98:1765–1803. doi: 10.1152/physrev.00064.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wedel J, Bruneau S, Liu K, Kong SW, Sage PT, Sabatini DM, Laplante M, Briscoe DM. DEPTOR modulates activation responses in CD4+ T cells and enhances immunoregulation following transplantation. Am J Transplant. 2018;19:77–88. doi: 10.1111/ajt.14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai Y, Lin P, Feng Z, Lu H, Han Q, Chen J, Zhang Y, He Q, Nan G, Luo X, et al. TNFAIP3-DEPTOR complex regulates inflammasome secretion through autophagy in ankylosing spondylitis monocytes. Autophagy. 2018;14:1629–1643. doi: 10.1080/15548627.2018.1458804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruneau S, Nakayama H, Woda CB, Flynn EA, Briscoe DM. DEPTOR regulates vascular endothelial cell activation and proinflammatory and angiogenic responses. Blood. 2013;122:1833–1842. doi: 10.1182/blood-2013-03-488486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laplante M, Horvat S, Festuccia WT, Birsoy K, Prevorsek Z, Efeyan A, Sabatini DM. DEPTOR cell-autonomously promotes adipogenesis, and its expression is associated with obesity. Cell Metab. 2012;16:202–212. doi: 10.1016/j.cmet.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cadegiani FA, McCoy J, Gustavo Wambier C, Vaño-Galván S, Shapiro J, Tosti A, Zimerman RA, Goren A. Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: Results from a randomized, double-blinded, placebo-controlled trial. Cureus. 2021;13(e13492) doi: 10.7759/cureus.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanno Y, Zhao S, Yamashita N, Yanai K, Nemoto K, Inouye Y. Androgen receptor functions as a negative transcriptional regulator of DEPTOR, mTOR inhibitor. J Toxicol Sci. 2015;40:753–758. doi: 10.2131/jts.40.753. [DOI] [PubMed] [Google Scholar]
- 34.Reed LJ, Muench H. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 35.Nayak G, Bhuyan SK, Bhuyan R, Sahu A, Kar D, Kuanar A. Global emergence of Enterovirus 71: A systematic review. Beni Suef Univ J Basic Appl Sci. 2022;11(78) doi: 10.1186/s43088-022-00258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yogarajah T, Ong KC, Perera D, Wong KT. AIM2 inflammasome-mediated pyroptosis in enterovirus A71-infected neuronal cells restricts viral replication. Sci Rep. 2017;7(5845) doi: 10.1038/s41598-017-05589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai J, Chen X, Liu Q, Zhou X, Long JE. Characteristics of enterovirus 71-induced cell death and genome scanning to identify viral genes involved in virus-induced cell apoptosis. Virus Res. 2019;265:104–114. doi: 10.1016/j.virusres.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Lei X, Xiao X, Yang C, Lu W, Huang Z, Leng Q, Jin Q, He B, Meng G, Wang J. Reciprocal regulation between enterovirus 71 and the NLRP3 inflammasome. Cell Rep. 2015;12:42–48. doi: 10.1016/j.celrep.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Ning X, Jiang Z. Caspases control antiviral innate immunity. Cell Mol Immunol. 2017;14:736–747. doi: 10.1038/cmi.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang SM, Lei HY, Liu CC. Cytokine immunopathogenesis of enterovirus 71 brain stem encephalitis. Clin Dev Immunol. 2012;2012(876241) doi: 10.1155/2012/876241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao W, Shi CS, Harrison K, Hwang IY, Nabar NR, Wang M, Kehrl JH. AKT regulates NLRP3 inflammasome activation by phosphorylating NLRP3 serine 5. J Immunol. 2020;205:2255–2264. doi: 10.4049/jimmunol.2000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.