Abstract
A heartbeat generates tiny mechanical vibrations, mainly due to the opening and closing of heart valves. These vibrations can be recorded by accelerometers and gyroscopes applied on a subject’s chest. In particular, the local 3D linear accelerations and 3D angular velocities of the chest wall are referred to as seismocardiograms (SCG) and gyrocardiograms (GCG), respectively. These signals usually exhibit a low signal-to-noise ratio, as well as non-negligible amplitude and morphological changes due to changes in posture and the sensors’ location, respiratory activity, as well as other sources of intra-subject and inter-subject variability. These factors make heartbeat detection a complex task; therefore, a reference electrocardiogram (ECG) lead is usually acquired in SCG and GCG studies to ensure correct localization of heartbeats. Recently, a template matching technique based on cross correlation has proven to be particularly effective in recognizing individual heartbeats in SCG signals. This study aims to verify the performance of this technique when applied on GCG signals. Tests were conducted on a public database consisting of SCG, GCG, and ECG signals recorded synchronously on 100 patients with valvular heart diseases. The results show that the template matching technique identified heartbeats in GCG signals with a sensitivity and positive predictive value (PPV) of 87% and 92%, respectively. Regression, correlation, and Bland–Altman analyses carried out on inter-beat intervals obtained from GCG and ECG (assumed as reference) reported a slope of 0.995, an intercept of 4.06 ms (R2 > 0.99), a Pearson’s correlation coefficient of 0.9993, and limits of agreement of about ±13 ms with a negligible bias. A comparison with the results of a previous study obtained on SCG signals from the same database revealed that GCG enabled effective cardiac monitoring in significantly more patients than SCG (95 vs. 77). This result suggests that GCG could ensure more robust and reliable cardiac monitoring in patients with heart diseases with respect to SCG.
Keywords: gyrocardiography, seismocardiography, heartbeat detection, template matching, heart rate, mechanocardiography
1. Introduction
Heart rate monitoring plays a key role in the assessment of cardiovascular function. Continuous, long-term cardiac monitoring has long been recognized as a critical task to improve the diagnosis and treatment of cardiac diseases [1,2,3,4]. To date, electrocardiography (ECG) is widely recognized as the gold standard for heart rate measurement via recording the myocardial electrical activity. However, ECG examinations can only be performed by skilled clinical professionals using approved medical devices. In addition, various practical drawbacks, such as detachments/slipping of electrodes, drying of electrolytic gel, and susceptibility to electromagnetic interferences, make electrocardiography not appealing for continuous, long-term monitoring, particularly in non-clinical settings [5,6,7,8,9,10,11]. Heart rate monitors based on plethysmographic techniques are available as alternatives to ECG, as well as in the form of wearable devices (e.g., smartwatches and fitbands). However, their strong sensitivity to motion artifacts usually limits their application to continuous, long-term monitoring for clinical purposes [11,12,13,14,15,16,17,18]. Cardio-mechanical monitoring techniques represent a further alternative for non-invasive assessment of cardiac function. Many of these techniques record the mechanical activity of the heart from a subject’s chest, essentially replicating the ancient practice of palpation [11,19]. From the end of the 19th century, various techniques have been proposed to record the tiny vibrations induced on the human body by the mechanical activity of the cardiovascular system [20]. Indeed, specific events of the cardiac cycle could be monitored by analyzing the peaks and valleys of cardio-mechanical signals. However, the bulky instrumentation and complexity of signal interpretation, as well as the considerable inter-subject variability, led most of these techniques to fall into disuse in favor of the emerging ultrasound imaging, which offered the unprecedented opportunity to visually inspect the anatomy and physiology of the cardiovascular system. Indeed, ultrasound imaging and Doppler techniques are currently reference clinical examinations for assessing the mechanical properties of the myocardium, which cannot be evaluated from ECG. However, ultrasound techniques also require highly qualified specialists, and are not suitable for continuous, long-term cardiac monitoring.
In the last two decades, the manufacturing of small, lightweight, inexpensive sensors based on micro-electromechanical system (MEMS) technologies has rejuvenated the interest in Ballistocardiography (BCG), which records the mechanical vibrations of the whole body due to blood ejection towards the feet [21,22,23,24,25,26], as well as Seismocardiography (SCG), which measures the infrasonic three-dimensional accelerations of the precordium induced by heart contractions [26,27,28,29,30,31,32,33]. In addition, the availability of modern electronic stethoscopes fostered the use of phonocardiography (PCG) to record heart sounds [34,35,36,37,38,39].
Recently, novel, non-invasive, cardio-mechanical monitoring techniques have been introduced, namely Forcecardiography (FCG) [40,41,42,43,44,45,46] and Gyrocardiography (GCG) [47,48,49,50], and even the combination of SCG with BCG [51] and GCG [52,53] has been investigated. FCG captures precordial vibrations using broadband force sensors, which are able to monitor respiration, infrasonic cardiac vibrations, and heart sounds, all simultaneously from a single site on the chest. On the other hand, GCG makes use of tri-axial gyroscopes to measure the infrasonic angular velocities of the thorax induced by heart contraction. Indeed, up to 60% of cardiac vibrational energy has been reported to be contained in the GCG signal [49]. Furthermore, peaks and valleys of GCG signals also have a clear correlation with important cardiac cycle events, thus allowing for the estimation of cardiac time intervals of diagnostic relevance [49,50]. Generally, the angular velocities around the latero–lateral axis (GCGx) and cranio–caudal axis (GCGy) are analyzed. During the cardiac cycle, the twisting and untwisting motion of the heart, which results from the contraction and relaxation of the helically oriented myocardial fibers, causes the chest wall to move accordingly. The specific arrangement of myofibrils, which form two oppositely oriented eight-shaped configurations, is responsible for the torsional motion of the heart. Indeed, during systole, the apex of the heart rotates counterclockwise, while the base rotates clockwise; on the contrary, during diastole, the opposite occurs [54,55,56]. This movement is mainly captured by the y component of the GCG signal [49].
Beat-by-beat heart rate monitoring from GCG signals is feasible. Specifically, precise identification of the temporal locations of all heartbeats and accurate measurement of inter-beat intervals are demanded to accomplish this task. Generally, heartbeats are localized by taking advantage of the a priori knowledge of R-peaks on a simultaneous ECG recording [49,57,58,59]. However, GCG-based heartbeat detectors that remove the need for concurrent ECG signals have also been proposed. In this regard, various approaches are available, such as short-term autocorrelation [60,61]; envelope extraction through wavelet [62] or Hilbert [63,64,65] transform, even combined with adaptative thresholding [66], clustering [67] or autoregressive models [68]; calculation of the kinetic energy waveform followed by Sparse Fast Fourier Transform [69]; and the autocorrelated differential algorithm [51,70]. A great part of these methods is based on rather complex algorithms and has only been tested on GCG signals recorded from subjects without any history of cardiovascular diseases. On the contrary, the method presented in [67], which combines GCG envelope extraction and unsupervised k-means clustering, was also assessed on a limited cohort of 12 patients with coronary artery disease. Moreover, GCG signals from 40 patients with atrial fibrillation, 21 patients with coronary artery disease, and 21 patients with myocardial ischemia were considered in [61], but any information is reported on the performance of heartbeat detection and the accuracy of inter-beat interval measurements. Heartbeats have also been recognized by combining GCG and SCG recordings [51,65,71]. As an example, independent component analysis was first employed in [65] for data fusion; then, an envelope-based heartbeat detector was tested on 19 patients with coronary artery disease. Furthermore, in [69], heartbeats were located on the kinetic energy waveform, which was obtained as the ensemble averaging of SCG and GCG signals. Deep learning methods for biosignal analysis [72,73,74] have also been proposed for ECG-free heartbeat detection in SCG [75] and BCG [76] signals, but not in GCG signals.
This study aims to demonstrate the suitability of a template matching approach for ECG-free heartbeat detection in GCG signals. The algorithm proposed here is based on the well-established normalized cross-correlation (NCC) function [77,78,79] to assess the morphological similarity between a selected template and signal chunks. This allows for capturing the heartbeat pattern in the signal without any a priori assumptions about the signal waveform. The performance of the proposed approach was extensively evaluated on SCG signals of 100 patients with one or more valvular heart diseases (VHDs) [80], made available in a public database [81]. To the best of our knowledge, for the first time in the literature, a template matching algorithm has been shown to provide high precision in heartbeat detection, as well as high accuracy in inter-beat intervals estimation, on a large cohort of pathological subjects [80]. As the database considered also contained simultaneous GCG recordings, the feasibility of this method on GCG signals from the same 100 VHD patients was evaluated in this work. To this end, the temporal locations of heartbeats were identified from both GCG signals and reference ECG recordings, and inter-beat interval measurements were compared via statistical analyses. The results showed that even on GCG signals the template matching offers a simple, yet effective and robust way for continuous heart rate monitoring.
2. Materials and Methods
2.1. Dataset
GCG and ECG signals of 100 VHD patients (59 males and 41 females, age 68 ± 14 years) from a public database [81] were considered in this study. The subjects suffered from one or more VHDs at a moderate or severe stage. In particular, 23 subjects were affected by mitral valve stenosis, 51 by mitral valve regurgitation, 39 by aortic valve stenosis, 28 by aortic valve regurgitation, 40 by tricuspid valve regurgitation, and 59 subjects suffered from more than one VHD (more detailed information on the patients is available in [81] and at the database repository). GCG and ECG signals were recorded on subjects’ chest by means of a tri-axial MEMS gyroscope and an ECG front-end, respectively, integrated in the Shimmer 3 ECG module (Shimmer Sensing, Dublin, Ireland). Signals were acquired simultaneously at a sampling rate of 256 Hz (patient IDs #CP-01 to #CP-70 and #UP-01 to #UP-21) or 512 Hz (patients IDs #UP-22 to #UP-30), from subjects lying supine and breathing at a natural pace. Only GCGy signals and ECG leads II were considered for the analysis. Recordings corresponding to patient ID #UP-28 were discarded due to the lack of a simultaneous ECG signal. In addition, four recordings (patient IDs #CP-24, #CP-31, #CP-55, and #UP-22) were excluded from the analysis because of poor GCG signal quality. Therefore, signals from a total of 95 subjects were analyzed in this study.
2.2. Signals Pre-Processing
Matlab® R2018b (The MathWorks, Inc., 1 Apple Hill Drive, Natick, MA 01760, USA) was used for all signal processing operations. First of all, a linear interpolation was carried out via the Matlab® function interp1 to oversample the signals up to 1 kHz, in order to improve their temporal resolution. Afterwards, the ECG signal was filtered in the 0.5–40 Hz frequency band using a fourth order zero-lag Butterworth band-pass filter; then, the 50 Hz powerline interference and its higher harmonics were filtered out by means of a comb notch filter; finally, the temporal locations of R-peaks were identified by the well-kwown Pan and Tompkins algorithm [82], implemented in the BioSigKit Matlab® toolbox [83]. On the other hand, the GCG signal was processed via a fourth order zero-lag Butterworth filter with 7–30 Hz pass band. Figure 1 shows an example of pre-processed ECG and GCG signals from patient #CP-63.
Figure 1.
An example of pre-processed ECG (blue line) and GCG (green line) signals from patient #CP-63. Red points mark R peak locations.
2.3. Template Matching
A template matching algorithm, previously proposed in a SCG [80] study, was implemented for heartbeat localization in GCG signals. As already reported in [80], the template matching first requires selecting a heartbeat template within the GCG signal, then calculating the NCC function between the selected template and GCG signal chunks, and finally identifying the temporal locations of the NCC peaks.
2.3.1. Template Selection
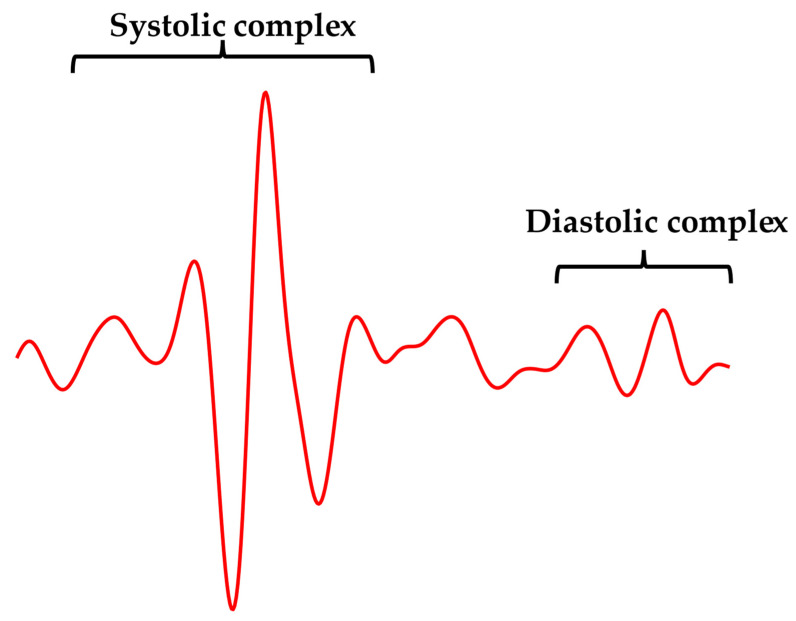
First, a template was identified in each GCG signal in order to capture the typical heartbeat waveform, which was regarded as a morphological reference. As illustrated in Figure 2, the template was selected according to the following criterion: “the template should start two or three waves before the highest local maximum in the systolic complex, where the amplitude of the waves is significantly lower than systolic peak magnitude, and should end after the last wave in the diastolic complex”. All GCG signals included in the dataset met this criterion. Hence, a template comprising both the systolic and diastolic complexes of the selected cardiac cycle was chosen for all of the GCG signals analyzed. Figure 3 shows an example of template selection for subject #CP-63.
Figure 2.
An example of the recommended template waveform extracted from subject #CP-63.
Figure 3.
An example of an ECG signal (blue line), GCG signal (green line), and selected template (red line) from subject #CP-63. The template corresponds to that highlighted in Figure 2. Red points mark R-peak locations.
2.3.2. Heartbeats Localization on Normalized Cross-Correlation Function
The NCC function was considered as a similarity measure between the template and signal chunks to find those that most resemble the template, as in [80]. Local maxima in the NCC function corresponded to signal chunks with highest local similarity to the selected template, so they marked the temporal locations of heartbeats. Therefore, once a template was selected, the NCC function was computed between the template and GCG signal chunks, after local mean removal. Then, the local maxima were identified in the NCC function obtained via the Matlab® function findpeaks, by setting a minimum peak prominence of 0.5 and a minimum peak distance of 500 ms for all of the GCG signals included in the dataset. Figure 4 shows some examples of heartbeat detection in the NCC function for patients #CP-63 and #CP-08.
Figure 4.
Some excerpts of ECG (blue line), GCG (green line), and NCC (orange line) signals from subjects: (a) #CP-08 and (b) #CP-63. Red and black points mark the temporal locations of R peaks and NCC peaks, respectively.
2.3.3. Inter-Beat Intervals Estimation
Once NCC peak detection was completed, wrong and missed heartbeat detections among all NCC peaks were annotated with the support of reference ECG signals, as reported in [80]. Multiple NCC peaks within one cardiac cycle were considered as false positives (FPs); single NCC peaks within one cardiac cycle occurring at temporal locations that were unlikely to correspond to matched heartbeat templates were considered as detection errors (DEs); cardiac cycles with no NCC peaks were considered as false negatives (FNs). DEs essentially marked cardiac cycles where only one FP was found, and no true heartbeat was detected, so they contributed both to the number of FPs and FNs. Finally, inter-beat intervals were estimated from ECG and GCG signals as temporal differences between consecutive R-peaks and NCC-peaks, respectively, as depicted in Figure 5. Inter-beat intervals corrupted by FNs and DEs were obviously removed from the analysis.
Figure 5.
An example of inter-beat interval estimation from subject #CP-63 on ECG (blue line) and NCC (orange line) signals. For the ECG signal, the inter-beat interval was estimated as the temporal difference between two consecutive R-peaks (grey double arrow). For the NCC signal, the inter-beat interval measurement was obtained as the temporal difference between two consecutive NCC peaks (electric blue double arrow).
2.4. Statistical Analyses
Sensitivity and positive predictive value (PPV) were considered as performance evaluation metrics of heartbeat detection. These metrics are defined by the following mathematical expressions:
| (1) |
| (2) |
where TP, FN, FP, and DE indicate the number of true positives, false negatives, false positives, and detection errors, respectively.
Furthermore, regression, correlation, and Bland–Altman [84,85] analyses were carried out via the Matlab® function bland–altman-and-correlation-plot [86], to compare the inter-beat interval measurements obtained from GCG signals with those provided by the reference ECG signals. In addition, the results obtained from the analysis of GCG signals were compared with those reported in the previous work on SCG signals [80], to assess the performance of the proposed template matching algorithm in the estimation of inter-beat intervals from the GCG and SCG signals acquired on the same VHD patients.
3. Results
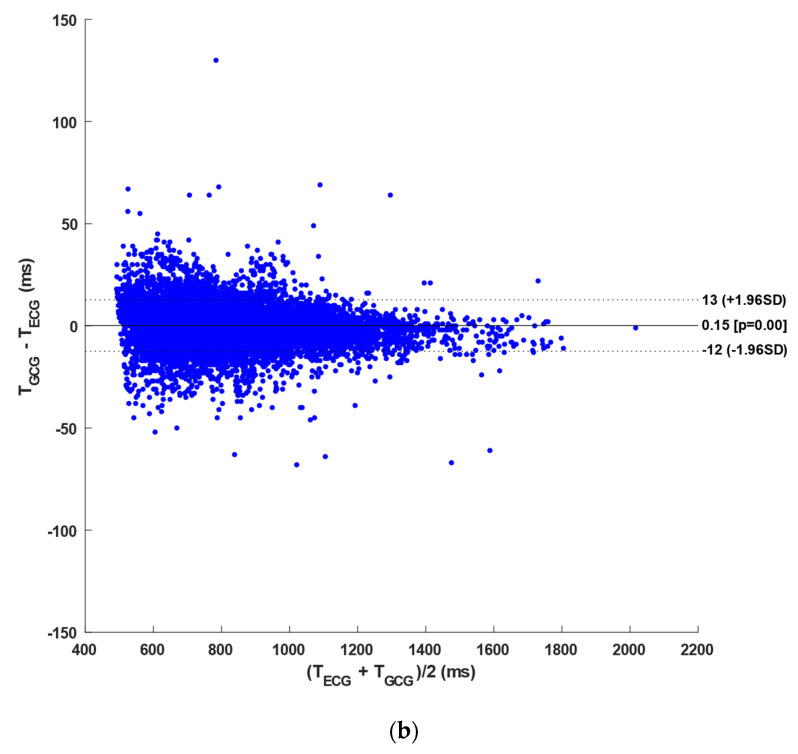
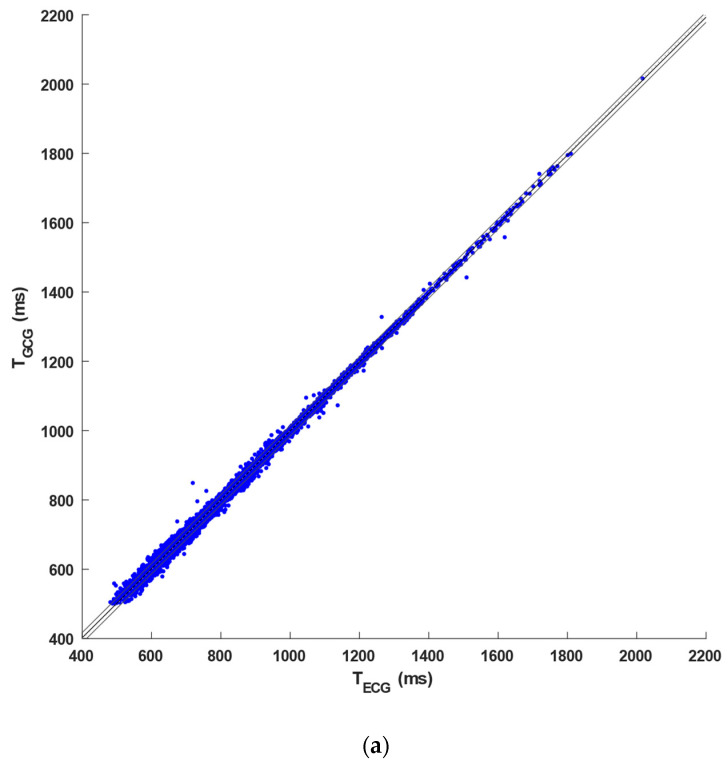
The number of ECG heartbeats (R-peaks) and GCG heartbeats (NCC-peaks) identified for each subject, together with the number of FPs, FNs, and DEs, are outlined in Table A1 in Appendix A. This table also reports the number of inter-beat intervals compared via the statistical analyses. An overall number of 40,527 and 38,565 heartbeats were identified in the ECG and GCG signals, respectively. In particular, 35,383 TPs, 526 FPs, 2486 FNs, and 2656 DEs were detected in the GCG signals. Hence, heartbeat detection in GCG signals achieved a sensitivity of 87% and a PPV of 92%. Furthermore, an overall number of 31,831 inter-beat intervals were compared via regression, correlation, and Bland–Altman analyses. The regression and correlation analysis reported a slope of 0.995, an intercept of 4.06 ms (R2 > 0.99), and a Pearson’s correlation coefficient r of 0.9993 (p < 0.001), as illustrated in Figure 6a. Moreover, limits of agreement (LoA) of [−12.85, 13.15] ms, with a 95% confidence interval (95% CI) of [−12.97, 13.27] ms, and a negligible bias of 0.15 ms (p < 0.001), with a 95% CI of [0.08, 0.22] ms, were obtained from the Bland–Altman analysis (see Figure 6b). The results of statistical analyses are also summarized in Table 1.
Figure 6.
Statistical analyses on inter-beat intervals obtained from GCG signals of the 95 VHD patients included in the dataset: (a) results of the regression and correlation analysis and (b) results of the Bland–Altman analysis.
Table 1.
Results of statistical analyses on the GCG signals of the 95 VHD patients included in the dataset: 95% CIbias is the 95% confidence interval of the bias and 95% CILoA is the 95% confidence interval of the limits of agreement.
| Sample size | N° of subjects | 95 |
| N° of compared inter-beat intervals | 31,831 | |
| Performance of heartbeat detection | Sensitivity (%) | 87 |
| PPV (%) | 92 | |
| Results of regression analysis | Slope | 0.995 |
| Intercept (ms) | 4.06 | |
| R2 | 0.9985 | |
| Results of correlation analysis | r | 0.9993 (p < 0.001) |
| Results of Bland-Altman analysis | Bias (ms) | 0.15 (p < 0.001) |
| 95% CIbias (ms) | [0.08, 0.22] | |
| LoA (ms) | [−12.85, 13.15] | |
| 95% CILoA (ms) | [−12.97, 13.27] |
To compare the performance of heartbeat detection in GCG and SCG signals from the same database [81], Table A2 in Appendix A reports, for each of the 100 VHD patients, the number of heartbeats identified in the ECG signals; the number of TPs, FPs, and FNs detected in the GCG recordings analyzed in this study; and the number of TPs, FPs, and FNs recognized in the SCG recordings obtained in a previous study [80]. In particular, the table also highlights which patients were excluded from the analyses of GCG and/or SCG signals due to very poor signal quality (see dashes in the table). In addition, a comparison of the performances achieved in the inter-beat interval estimation from GCG and SCG recordings against the reference ECG was carried out. To this aim, 76 patients were considered, for which both GCG and SCG analysis was feasible, and then regression, correlation, and Bland–Altman analyses of inter-beat intervals were performed. In detail, a total of 41,320 heartbeats were detected in the ECG signals, while 35,383 TPs, 3182 FPs, and 5142 FNs were identified in GCG recordings, and 29,583 TPs, 2976 FPs, and 3678 FNs were detected in the SCG recordings. Hence, the GCG signals scored a sensitivity of 89% and a PPV of 93%, while SCG scored a sensitivity of 90% and a PPV of 91.5%. Moreover, a slope of 0.995, an intercept of 4.51 ms (R2 > 0.99), and a Pearson’s correlation coefficient r of 0.9993 (p < 0.001) were obtained for GCG signals, while a slope of 0.995, an intercept of 3.92 ms (R2 > 0.99), and a Pearson’s correlation coefficient r of 0.9993 (p < 0.001) were found for the SCG signals (see Figure 7a). As shown in Figure 7b, the Bland–Altman analyses reported a negligible bias of 0.16 ms (p < 0.001), with a 95% CI of [0.09, 0.23] ms, and LoA of [−11.84, 12.16] ms, with a 95% CI of [−11.97, 12.29] ms, for the GCG signals, as well as a non-significant bias (p = 0.09), with 95% CI of [0.01, 0.17] ms, and LoA of [−12.91, 13.09] ms, with 95% CI of [−13.05, 13.23] ms, for the SCG signals. The results of these statistical analyses are also outlined in Table 2 (see [80] for further details on the results obtained for SCG signals).
Figure 7.
Statistical analyses on inter-beat intervals obtained from GCG signals of the 76 VHD patients: (a) results of the regression and correlation analysis and (b) results of the Bland–Altman analysis.
Table 2.
Results of statistical analyses performed on GCG and SCG signals from the same 76 VHD patients: 95% CIbias is the 95% confidence interval of the bias and 95% CILoA is the 95% confidence interval of the limits of agreement.
| GCG | SCG | ||
|---|---|---|---|
| Sample size | N° of subjects | 76 | 76 |
| N° of compared inter-beat intervals | 26,308 | 26,913 | |
| Performance of heartbeat detection | Sensitivity (%) | 89 | 90 |
| PPV (%) | 93 | 91.5 | |
| Results of regression analysis | Slope | 0.995 | 0.995 |
| Intercept (ms) | 4.51 | 3.92 | |
| R2 | 0.9987 | 0.9986 | |
| Results of correlation analysis | r | 0.9993 (p < 0.001) |
0.9993 (p < 0.001) |
| Results of Bland-Altman analysis | Bias (ms) | 0.16 (p < 0.001) |
0.09 (p = 0.09) |
| 95% CIbias (ms) | [0.09, 0.23] | [0.01, 0.17] | |
| LoA (ms) | [−11.84, 12.16] | [−12.91, 13.09] | |
| 95% CILoA (ms) | [−11.97, 12.29] | [−13.05, 13.23] | |
4. Discussion
The proposed method was able to recognize heartbeats in GCG signals of 95 out of 99 patients (96%) with a sensitivity and PPV of 87% and 92%, respectively, also providing accurate measurements of inter-beat intervals compared with ECG (LoA of about ± 13 ms). It is worth noting that, when applied on SCG, the templatematching method ensured accurate heartbeat detection in 77 out of 99 patients (78%) with similar values of sensitivity and PPV, as well as of LoA for inter-beat interval estimation. In both SCG and GCG, the inability to ensure an acceptable performance in heartbeat detection was due to an extremely poor signal quality.
The measurement errors in inter-beat intervals obtained by SCG and GCG compared with ECG have various contributions. First of all, assuming the ideal capability of locating the timing of the aortic valve opening with maximum accuracy in SCG and GCG signals (AO peaks), it must be considered that the time delay between ECG R peaks and AO peaks, which is a component of the pre-ejection period, changes over time. Therefore, if the R–AO time delays of two consecutive heartbeats are different, then the related AO–AO interval will differ from the R–R interval, thus leading to a measurement error. Moving forward to less ideal conditions, it is important to consider that motion artifacts and noise may corrupt the heartbeat morphology in SCG and GCG signals, thus potentially leading to imprecise localization of the AO peaks, and eventually to errors in inter-beat intervals measurements. Finally, it must be considered that in the proposed approach the heartbeat markers are the peaks of NCC between the selected template and the signals, and not the actual AO peaks. This is important as the time delay between the NCC peaks and the actual AO peaks may change over time, because the heartbeat morphology is not always exactly the same of the template, so the local maximum of NCC may not always occur at a fixed time distance from the related actual AO peak. Again, different AO–NCC-peaks time delays in two consecutive heartbeats would lead to differences between NCC peak–NCC peak intervals and AO–AO intervals, and eventually in additional measurement errors with respect to R–R intervals. Anyway, the impact of these errors on the parameters of clinical relevance, such as the mean heart rate and HRV indices, has yet to be evaluated, and will be the subject of a future study.
This is the first study that assessed the performance of an algorithm for ECG-free heartbeat detection in GCG signals on such a large cohort of subjects. In addition, it is also the first time that the heart monitoring performances of SCG and GCG have been thoroughly compared for such a large cohort of pathological subjects. Indeed, the results suggest that, for patients affected by valvular heart diseases, the heartbeat morphology is more stable in GCG than in SCG signals. This result has important implications in the development of devices and methods for continuous, long-term patient surveillance based on cardio-mechanical monitoring techniques. In fact, the superior performances achieved by GCG suggest that its use could ensure a more reliable and robust monitoring of actual patients. However, considering that electronic microchips of inertial measurement units incorporating both accelerometers and gyroscopes are widely available nowadays, it might be advantageous to apply sensor fusion approaches to design heartbeat detectors that jointly use SCG and GCG signals, in order to leverage their strengths and compensate for their weaknesses. Undoubtedly, the availability of a quantitative index of signal quality could help to dynamically identify the most reliable signal, thus possibly improving the performance of heartbeat detection.
Overall, the proposed templatematching method achieves very high performance on both SCG and GCG signals, which makes it effective for ECG-free cardiac monitoring. The modest computational burden of the proposed method also makes it very efficient and suitable for implementation on resource-constrained computing platforms, possibly also in real time. To date, not many ECG-free methods have been proposed in the literature. The few proposed methods are based on rather complex procedures, or on computationally intensive transformations, or even on machine learning and deep learning techniques, which require very high-performing processors. However, such artificial intelligence methodologies can potentially provide valuable support for the classification and interpretation of signals to offer not only information on heart rhythm, but also on other cardiovascular functions and pathologies.
The results obtained in this study cannot be directly compared with those of previous studies for different reasons. First, the performance of different algorithms must be compared on the same data for consistency, but none of the methods proposed in the literature has been assessed on the same database. Furthermore, other methods have been evaluated on much smaller cohorts of subjects. Data from these cohorts were likely to be characterized by a lower inter-subject variability, which has long been recognized as a factor affecting the accuracy of biosignal analysis. In addition, these cohorts mainly consisted of healthy subjects, sometimes with the presence of a small number of pathological subjects. It is well known that cardio-mechanical signals from pathological subjects exhibit atypical waveforms, with higher morphological variability and difficulty in annotation and interpretation compared with healthy subjects [87], thus making the heartbeat detection task far more complex. Therefore, the methods proposed in the literature have been tested on signals with undoubtedly higher quality and with lower inter-subject variability than those considered in this study, so it is more likely that the results of other methods have been overestimated.
Obviously, the need for manual selection of the heartbeat template is the main limitation for the use of the template-matching method by unskilled users who are unable to follow the suggested guidelines. Therefore, an automated template selection algorithm could be beneficial to improve the reproducibility of the proposed technique.
5. Conclusions
This study presents an extensive performance analysis of an ECG-free heartbeat detection method in Gyrocardiography signals. The method is based on a template matching approach and was tested on GCG signals from a public database of 100 patients with valvular heart diseases. The algorithm has previously been tested on Seismocardiography signals from the same database.
This study provides, for the first time, an extensive comparison of heart rate monitoring performances of SCG and GCG on a large cohort of pathological subjects. The morphology of heartbeats turned out to be more stable in GCG than in SCG signals, thus providing a superior performance in heartbeat detection. According to these results, GCG could ensure a more reliable and robust monitoring of actual patients. However, these findings should be confirmed also on patients affected by other cardiac diseases.
Overall, the proposed method achieves very high performance for both SCG and GCG signals, and presents a modest computational burden compared with other methods proposed in the literature. These aspects make the template matching algorithm effective and efficient for ECG-free cardiac monitoring. It is also suitable for real-time implementation on resource-constrained computing platforms, which is appealing to enable the continuous, long-term monitoring of heart rate.
Appendix A
Table A1.
Number of heartbeats detected in the ECG and GCG signals, along with the number of FP, FN, and DE identified in GCG signals and the number of compared inter-beat intervals.
| Patient ID # |
ECG Heartbeats (R-Peaks) |
GCG Heartbeats (NCC-Peaks) |
FP | FN | DE | Compared Inter-Beat Intervals |
|---|---|---|---|---|---|---|
| CP-01 | 448 | 448 | 0 | 0 | 2 | 443 |
| CP-02 | 651 | 633 | 3 | 21 | 3 | 609 |
| CP-03 | 481 | 474 | 2 | 9 | 9 | 451 |
| CP-04 | 661 | 629 | 2 | 34 | 16 | 562 |
| CP-05 | 509 | 502 | 2 | 9 | 6 | 484 |
| CP-06 | 294 | 286 | 2 | 10 | 7 | 264 |
| CP-07 | 451 | 450 | 3 | 4 | 5 | 435 |
| CP-08 | 544 | 544 | 0 | 0 | 4 | 536 |
| CP-09 | 364 | 320 | 2 | 46 | 57 | 190 |
| CP-10 | 506 | 485 | 19 | 40 | 41 | 347 |
| CP-11 | 656 | 627 | 3 | 32 | 57 | 500 |
| CP-12 | 423 | 425 | 9 | 7 | 3 | 404 |
| CP-13 | 837 | 829 | 0 | 8 | 1 | 819 |
| CP-14 | 472 | 429 | 6 | 48 | 27 | 319 |
| CP-15 | 630 | 618 | 0 | 12 | 16 | 590 |
| CP-16 | 355 | 354 | 1 | 2 | 4 | 342 |
| CP-17 | 362 | 366 | 13 | 9 | 42 | 272 |
| CP-18 | 601 | 599 | 1 | 3 | 1 | 593 |
| CP-19 | 484 | 473 | 8 | 19 | 32 | 404 |
| CP-20 | 391 | 393 | 3 | 1 | 0 | 388 |
| CP-21 | 247 | 247 | 0 | 0 | 2 | 242 |
| CP-22 | 610 | 534 | 5 | 81 | 71 | 368 |
| CP-23 | 235 | 235 | 0 | 0 | 0 | 234 |
| CP-25 | 602 | 494 | 10 | 118 | 147 | 166 |
| CP-26 | 389 | 379 | 2 | 12 | 6 | 352 |
| CP-27 | 130 | 130 | 1 | 1 | 0 | 127 |
| CP-28 | 238 | 239 | 1 | 0 | 2 | 233 |
| CP-29 | 290 | 192 | 0 | 98 | 20 | 91 |
| CP-30 | 523 | 512 | 0 | 11 | 3 | 500 |
| CP-32 | 527 | 493 | 15 | 49 | 91 | 298 |
| CP-33 | 449 | 487 | 40 | 2 | 10 | 424 |
| CP-34 | 462 | 455 | 2 | 9 | 5 | 436 |
| CP-35 | 484 | 483 | 13 | 14 | 125 | 269 |
| CP-36 | 386 | 409 | 26 | 3 | 197 | 73 |
| CP-37 | 342 | 343 | 3 | 2 | 3 | 331 |
| CP-38 | 406 | 339 | 23 | 90 | 53 | 161 |
| CP-39 | 518 | 515 | 0 | 3 | 39 | 448 |
| CP-40 | 509 | 413 | 0 | 96 | 19 | 328 |
| CP-41 | 349 | 349 | 0 | 0 | 1 | 346 |
| CP-42 | 346 | 343 | 6 | 9 | 18 | 296 |
| CP-43 | 460 | 408 | 15 | 67 | 138 | 159 |
| CP-44 | 321 | 321 | 0 | 0 | 4 | 312 |
| CP-45 | 357 | 359 | 18 | 16 | 58 | 232 |
| CP-46 | 499 | 489 | 3 | 13 | 21 | 441 |
| CP-47 | 537 | 457 | 11 | 91 | 38 | 319 |
| CP-48 | 637 | 588 | 33 | 82 | 121 | 313 |
| CP-49 | 451 | 425 | 13 | 39 | 27 | 325 |
| CP-50 | 781 | 679 | 0 | 102 | 30 | 547 |
| CP-51 | 621 | 621 | 0 | 0 | 52 | 543 |
| CP-52 | 728 | 494 | 0 | 234 | 54 | 212 |
| CP-53 | 562 | 497 | 0 | 65 | 49 | 377 |
| CP-54 | 397 | 366 | 1 | 32 | 36 | 275 |
| CP-56 | 742 | 629 | 0 | 113 | 6 | 511 |
| CP-57 | 507 | 507 | 1 | 1 | 3 | 499 |
| CP-58 | 525 | 525 | 1 | 1 | 1 | 521 |
| CP-59 | 405 | 405 | 1 | 1 | 1 | 400 |
| CP-60 | 512 | 503 | 0 | 9 | 10 | 484 |
| CP-61 | 397 | 397 | 0 | 0 | 2 | 393 |
| CP-62 | 572 | 500 | 1 | 73 | 5 | 424 |
| CP-63 | 610 | 609 | 0 | 1 | 0 | 607 |
| CP-64 | 382 | 396 | 14 | 0 | 4 | 373 |
| CP-65 | 369 | 369 | 0 | 0 | 5 | 361 |
| CP-66 | 468 | 468 | 1 | 1 | 0 | 465 |
| CP-67 | 539 | 514 | 10 | 35 | 25 | 429 |
| CP-68 | 327 | 348 | 23 | 2 | 1 | 320 |
| CP-69 | 587 | 585 | 0 | 2 | 2 | 578 |
| CP-70 | 422 | 429 | 19 | 12 | 44 | 324 |
| UP-01 | 239 | 232 | 22 | 29 | 58 | 111 |
| UP-02 | 286 | 268 | 5 | 23 | 66 | 134 |
| UP-03 | 264 | 218 | 1 | 47 | 34 | 135 |
| UP-04 | 258 | 247 | 7 | 18 | 23 | 183 |
| UP-05 | 462 | 412 | 2 | 52 | 122 | 178 |
| UP-06 | 458 | 398 | 0 | 60 | 16 | 325 |
| UP-07 | 376 | 372 | 1 | 5 | 3 | 359 |
| UP-08 | 257 | 257 | 0 | 0 | 2 | 252 |
| UP-09 | 286 | 285 | 2 | 3 | 7 | 267 |
| UP-10 | 165 | 161 | 1 | 5 | 12 | 131 |
| UP-11 | 417 | 386 | 25 | 56 | 10 | 297 |
| UP-12 | 339 | 336 | 2 | 5 | 25 | 283 |
| UP-13 | 106 | 104 | 0 | 2 | 7 | 89 |
| UP-14 | 340 | 341 | 1 | 0 | 9 | 321 |
| UP-15 | 214 | 215 | 1 | 0 | 0 | 213 |
| UP-16 | 221 | 220 | 4 | 4 | 36 | 150 |
| UP-17 | 613 | 544 | 1 | 70 | 62 | 421 |
| UP-18 | 350 | 351 | 1 | 0 | 3 | 343 |
| UP-19 | 116 | 116 | 11 | 11 | 45 | 26 |
| UP-20 | 617 | 494 | 3 | 126 | 55 | 329 |
| UP-21 | 305 | 308 | 3 | 0 | 2 | 300 |
| UP-23 | 565 | 570 | 5 | 0 | 3 | 558 |
| UP-24 | 349 | 335 | 0 | 14 | 11 | 308 |
| UP-25 | 244 | 222 | 12 | 34 | 44 | 131 |
| UP-26 | 160 | 158 | 0 | 2 | 1 | 154 |
| UP-27 | 269 | 276 | 22 | 15 | 74 | 131 |
| UP-29 | 146 | 146 | 1 | 1 | 5 | 133 |
| UP-30 | 228 | 230 | 2 | 0 | 39 | 150 |
| Total | 40,527 | 38,565 | 526 | 2486 | 2656 | 31,831 |
Table A2.
Comparison of SCG and GCG results on the whole database. The table reports the number of heartbeats detected in the ECG signals, together with the number of TP, FP, and FN detected in both SCG and GCG recordings (the number of DE was added to those of FP and FN). Black dashes indicate the SCG and/or GCG recordings discarded from the analysis due to very poor signal quality. Recordings of patient #UP-28 were not analyzed because ECG was unavailable (NA: not available).
| Patient ID # |
ECG Heartbeats (R-Peaks) |
SCG | GCG | ||||
|---|---|---|---|---|---|---|---|
| TP | FP | FN | TP | FP | FN | ||
| CP-01 | 448 | 448 | 0 | 0 | 446 | 2 | 2 |
| CP-02 | 651 | 606 | 37 | 45 | 627 | 6 | 24 |
| CP-03 | 481 | ― | ― | ― | 463 | 11 | 18 |
| CP-04 | 661 | 607 | 47 | 54 | 611 | 18 | 50 |
| CP-05 | 509 | 496 | 11 | 13 | 494 | 8 | 15 |
| CP-06 | 294 | ― | ― | ― | 277 | 9 | 17 |
| CP-07 | 451 | 445 | 8 | 6 | 442 | 8 | 9 |
| CP-08 | 544 | 520 | 18 | 24 | 540 | 4 | 4 |
| CP-09 | 364 | 222 | 120 | 142 | 261 | 59 | 103 |
| CP-10 | 506 | 329 | 176 | 177 | 425 | 60 | 81 |
| CP-11 | 656 | 580 | 82 | 76 | 567 | 60 | 89 |
| CP-12 | 423 | 406 | 27 | 17 | 413 | 12 | 10 |
| CP-13 | 837 | 824 | 5 | 12 | 828 | 1 | 9 |
| CP-14 | 472 | 278 | 199 | 194 | 396 | 33 | 75 |
| CP-15 | 630 | 620 | 3 | 10 | 602 | 16 | 28 |
| CP-16 | 355 | 332 | 28 | 23 | 349 | 5 | 6 |
| CP-17 | 362 | ― | ― | ― | 311 | 55 | 51 |
| CP-18 | 601 | ― | ― | ― | 597 | 2 | 4 |
| CP-19 | 484 | 445 | 48 | 39 | 433 | 40 | 51 |
| CP-20 | 391 | 390 | 1 | 1 | 390 | 3 | 1 |
| CP-21 | 247 | 244 | 3 | 3 | 245 | 2 | 2 |
| CP-22 | 610 | 528 | 67 | 82 | 458 | 76 | 152 |
| CP-23 | 235 | 197 | 28 | 38 | 235 | 0 | 0 |
| CP-24 | (1036) | ― | ― | ― | ― | ― | ― |
| CP-25 | 602 | ― | ― | ― | 337 | 157 | 265 |
| CP-26 | 389 | 371 | 10 | 18 | 371 | 8 | 18 |
| CP-27 | 130 | 130 | 0 | 0 | 129 | 1 | 1 |
| CP-28 | 238 | 232 | 5 | 6 | 236 | 3 | 2 |
| CP-29 | 290 | ― | ― | ― | 172 | 20 | 118 |
| CP-30 | 523 | 508 | 2 | 15 | 509 | 3 | 14 |
| CP-31 | (948) | ― | ― | ― | ― | ― | ― |
| CP-32 | 527 | 353 | 133 | 174 | 387 | 106 | 140 |
| CP-33 | 449 | 432 | 24 | 17 | 437 | 50 | 12 |
| CP-34 | 462 | 452 | 8 | 10 | 448 | 7 | 14 |
| CP-35 | 484 | ― | ― | ― | 345 | 138 | 139 |
| CP-36 | 386 | 268 | 109 | 118 | 186 | 223 | 200 |
| CP-37 | 342 | 190 | 145 | 152 | 337 | 6 | 5 |
| CP-38 | 406 | 356 | 64 | 50 | 263 | 76 | 143 |
| CP-39 | 518 | 510 | 8 | 8 | 476 | 39 | 42 |
| CP-40 | 509 | 246 | 259 | 263 | 394 | 19 | 115 |
| CP-41 | 349 | 341 | 3 | 8 | 348 | 1 | 1 |
| CP-42 | 346 | 272 | 68 | 74 | 319 | 24 | 27 |
| CP-43 | 460 | 346 | 132 | 114 | 255 | 153 | 205 |
| CP-44 | 321 | 318 | 2 | 3 | 317 | 4 | 4 |
| CP-45 | 357 | 326 | 17 | 31 | 283 | 76 | 74 |
| CP-46 | 499 | ― | ― | ― | 465 | 24 | 34 |
| CP-47 | 537 | 505 | 17 | 32 | 408 | 49 | 129 |
| CP-48 | 637 | 560 | 66 | 77 | 434 | 154 | 203 |
| CP-49 | 451 | 399 | 58 | 52 | 385 | 40 | 66 |
| CP-50 | 781 | ― | ― | ― | 649 | 30 | 132 |
| CP-51 | 621 | ― | ― | ― | 569 | 52 | 52 |
| CP-52 | 728 | 494 | 38 | 234 | 440 | 54 | 288 |
| CP-53 | 562 | 561 | 0 | 1 | 448 | 49 | 114 |
| CP-54 | 397 | ― | ― | ― | 329 | 37 | 68 |
| CP-55 | 793 | 339 | 263 | 454 | ― | ― | ― |
| CP-56 | 742 | 533 | 160 | 206 | 623 | 6 | 119 |
| CP-57 | 507 | 501 | 5 | 6 | 503 | 4 | 4 |
| CP-58 | 525 | 523 | 1 | 2 | 523 | 2 | 2 |
| CP-59 | 405 | 405 | 1 | 0 | 403 | 2 | 2 |
| CP-60 | 512 | 502 | 1 | 10 | 493 | 10 | 19 |
| CP-61 | 397 | 396 | 7 | 1 | 395 | 2 | 2 |
| CP-62 | 572 | ― | ― | ― | 494 | 6 | 78 |
| CP-63 | 610 | 608 | 0 | 2 | 609 | 0 | 1 |
| CP-64 | 382 | 381 | 10 | 1 | 378 | 18 | 4 |
| CP-65 | 369 | 365 | 1 | 4 | 364 | 5 | 5 |
| CP-66 | 468 | 467 | 1 | 1 | 467 | 1 | 1 |
| CP-67 | 539 | ― | ― | ― | 479 | 35 | 60 |
| CP-68 | 327 | 275 | 55 | 52 | 324 | 24 | 3 |
| CP-69 | 587 | 585 | 0 | 2 | 583 | 2 | 4 |
| CP-70 | 422 | 376 | 36 | 46 | 366 | 63 | 56 |
| UP-01 | 239 | 190 | 51 | 49 | 152 | 80 | 87 |
| UP-02 | 286 | ― | ― | ― | 197 | 71 | 89 |
| UP-03 | 264 | ― | ― | ― | 183 | 35 | 81 |
| UP-04 | 258 | 244 | 7 | 14 | 217 | 30 | 41 |
| UP-05 | 462 | ― | ― | ― | 288 | 124 | 174 |
| UP-06 | 458 | 371 | 29 | 87 | 382 | 16 | 76 |
| UP-07 | 376 | 370 | 3 | 6 | 368 | 4 | 8 |
| UP-08 | 257 | 255 | 1 | 2 | 255 | 2 | 2 |
| UP-09 | 286 | 278 | 0 | 8 | 276 | 9 | 10 |
| UP-10 | 165 | 151 | 6 | 14 | 148 | 13 | 17 |
| UP-11 | 417 | 343 | 71 | 74 | 351 | 35 | 66 |
| UP-12 | 339 | 260 | 87 | 79 | 309 | 27 | 30 |
| UP-13 | 106 | 92 | 1 | 14 | 97 | 7 | 9 |
| UP-14 | 340 | 333 | 5 | 7 | 331 | 10 | 9 |
| UP-15 | 214 | 189 | 0 | 25 | 214 | 1 | 0 |
| UP-16 | 221 | 195 | 19 | 26 | 180 | 40 | 40 |
| UP-17 | 613 | 596 | 7 | 17 | 481 | 63 | 132 |
| UP-18 | 350 | 348 | 1 | 2 | 347 | 4 | 3 |
| UP-19 | 116 | ― | ― | ― | 60 | 56 | 56 |
| UP-20 | 617 | 613 | 2 | 4 | 436 | 58 | 181 |
| UP-21 | 305 | 303 | 2 | 2 | 303 | 5 | 2 |
| UP-22 | (700) | ― | ― | ― | ― | ― | ― |
| UP-23 | 565 | 565 | 2 | 0 | 562 | 8 | 3 |
| UP-24 | 349 | 329 | 11 | 20 | 324 | 11 | 25 |
| UP-25 | 244 | ― | ― | ― | 166 | 56 | 78 |
| UP-26 | 160 | ― | ― | ― | 157 | 1 | 3 |
| UP-27 | 269 | 246 | 37 | 23 | 180 | 96 | 89 |
| UP-28 | NA | NA | NA | NA | NA | NA | NA |
| UP-29 | 146 | 141 | 1 | 5 | 140 | 6 | 6 |
| UP-30 | 228 | 228 | 16 | 0 | 189 | 41 | 39 |
| Total | 41,320 | 29,583 | 2976 | 3678 | 35,383 | 3182 | 5142 |
Author Contributions
Conceptualization, J.C. and E.A.; methodology, S.P., J.C. and E.A.; software, S.P.; formal analysis, S.P. and E.A.; investigation, S.P., J.C., D.E., P.B. and E.A.; data curation, S.P.; writing—original draft preparation, S.P., J.C. and E.A.; writing—review and editing, S.P., J.C., D.E., P.B. and E.A.; visualization, S.P.; supervision, P.B. and E.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the relevant research data will be made available upon request after the publication of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hieronymi U., Kramme R. Cardiovascular Monitoring. In: Kramme R., Hoffmann K.P., Pozos R.S., editors. Springer Handbook of Medical Technology. Springer; Berlin/Heidelberg, Germany: 2011. pp. 955–970. [DOI] [Google Scholar]
- 2.Perret-Guillaume C., Joly L., Benetos A. Heart Rate as a Risk Factor for Cardiovascular Disease. Prog. Cardiovasc. Dis. 2009;52:6–10. doi: 10.1016/j.pcad.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Polley C., Jayarathna T., Gunawardana U., Naik G., Hamilton T., Andreozzi E., Bifulco P., Esposito D., Centracchio J., Gargiulo G. Wearable Bluetooth Triage Healthcare Monitoring System. Sensors. 2021;21:7586. doi: 10.3390/s21227586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galli A., Montree R.J.H., Que S., Peri E., Vullings R. An Overview of the Sensors for Heart Rate Monitoring Used in Extramural Applications. Sensors. 2022;22:4035. doi: 10.3390/s22114035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berbari E.J. Principles of electrocardiography. In: Bronzino J.D., editor. Biomedical Engineering Handbook. CRC Press; Boca Raton, FL, USA: 1999. pp. 231–240. [Google Scholar]
- 6.Nazeran H. Electrocardiography, Computers in. In: Webster J.G., editor. Encyclopedia of Medical Devices and Instrumentation. 2nd ed. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2006. pp. 34–51. [Google Scholar]
- 7.Clark J.W.J. The Origin of Biopotentials. In: Webster J.G., editor. Medical Instrumentation: Application and Design. 4th ed. John Wiley & Sons; Hoboken, NJ, USA: 2010. pp. 147–158. [Google Scholar]
- 8.Vieau S., Iaizzo P.A. Basic ECG Theory, 12-Lead Recordings and Their Interpretation. In: Iaizzo P.A., editor. Handbook of Cardiac Anatomy, Physiology and Devices. 3rd ed. Springer; Cham, Switzerland: 2015. pp. 321–334. [Google Scholar]
- 9.Sattar Y., Chhabra L. Electrocardiogram. StatPearls Publishing; Treasure Island, FL, USA: 2023. [(accessed on 7 April 2023)]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549803/ [Google Scholar]
- 10.Luz E.J., Schwartz W.R., Cámara-Chávez G., Menotti D. ECG-based heartbeat classification for arrhythmia detection: A survey. Comput. Methods Programs Biomed. 2016;127:144–164. doi: 10.1016/j.cmpb.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Landreani F., Caiani E.G. Smartphone accelerometers for the detection of heart rate. Expert Rev. Med. Devices. 2017;14:935–948. doi: 10.1080/17434440.2017.1407647. [DOI] [PubMed] [Google Scholar]
- 12.De Pinho Ferreira N., Gehin C., Massot B. A Review of Methods for Non-Invasive Heart Rate Measurement on Wrist. IRBM. 2021;42:4–18. doi: 10.1016/j.irbm.2020.04.001. [DOI] [Google Scholar]
- 13.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007;28:R1–R39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 14.Pereira T., Tran N., Gadhoumi K., Pelter M.M., Do D.H., Lee R.J., Colorado R., Meisel K., Hu X. Photoplethysmography based atrial fibrillation detection: A review. NPJ Digit. Med. 2020;3:3. doi: 10.1038/s41746-019-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswas D., Simões-Capela N., Van Hoof C., Van Helleputte N. Heart Rate Estimation From Wrist-Worn Photoplethysmography: A Review. IEEE Sens. J. 2019;19:6560–6570. doi: 10.1109/JSEN.2019.2914166. [DOI] [Google Scholar]
- 16.Andreozzi E., Sabbadini R., Centracchio J., Bifulco P., Irace A., Breglio G., Riccio M. Multimodal Finger Pulse Wave Sensing: Comparison of Forcecardiography and Photoplethysmography Sensors. Sensors. 2022;22:7566. doi: 10.3390/s22197566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine J., Branan K.L., Rodriguez A.J., Boonya-ananta T., Ajmal, Ramella-Roman J.C., McShane M.J., Coté G.L. Sources of Inaccuracy in Photoplethysmography for Continuous Cardiovascular Monitoring. Biosensors. 2021;11:126. doi: 10.3390/bios11040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail S., Akram U., Siddiqi I. Heart rate tracking in photoplethysmography signals affected by motion artifacts: A review. EURASIP J. Adv. Signal. Process. 2021;5:5. doi: 10.1186/s13634-020-00714-2. [DOI] [Google Scholar]
- 19.Hajar R. The Pulse in Ancient Medicine Part 1. Heart Views. 2018;19:36–43. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_23_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luisada A.A., Singhal A., Portaluppi F. Assessment of Left Ventricular Function by Noninvasive Methods. Adv. Cardiol. 1985;32:111–141. doi: 10.1159/000410758. [DOI] [PubMed] [Google Scholar]
- 21.Gordon J.W. On certain molar movements of the human body produced by the circulation of blood. J. Anat. Physiol. 1877;11:533–536. [PMC free article] [PubMed] [Google Scholar]
- 22.Burger H.C., Noordergraaf A. Physical basis of ballistocardiography. III. Am. Heart J. 1956;51:179–185. doi: 10.1016/0002-8703(56)90079-5. [DOI] [PubMed] [Google Scholar]
- 23.Starr I. The relation of the ballistocardiogram to cardiac function. Am. J. Cardiol. 1958;2:737–747. doi: 10.1016/0002-9149(58)90271-6. [DOI] [PubMed] [Google Scholar]
- 24.Knoop A.A. NASA Contractor Report—NASA CR. National Aeronautics and Space Administration; Washington, DC, USA: 1965. Experimental investigations on ultra-low frequency displacement ballistocardiography: NASA TT F-269; pp. 1–107. [PubMed] [Google Scholar]
- 25.Sadek I., Biswas J., Abdulrazak B. Ballistocardiogram signal processing: A review. Health Inf. Sci. Syst. 2019;7:10. doi: 10.1007/s13755-019-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inan O.T. Recent advances in cardiovascular monitoring using ballistocardiography; Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); San Diego, CA, USA. 28 August–1 September 2012; pp. 5038–5041. [DOI] [PubMed] [Google Scholar]
- 27.Zanetti J., Salerno D. Seismocardiography: A new technique for recording cardiac vibrations: Concept, method, and initial observations. J. Cardiovasc. Technol. 1990;9:2. [Google Scholar]
- 28.Inan O.T., Migeotte P.F., Park K.S., Etemadi M., Tavakolian K., Casanella R., Zanetti J., Tank J., Funtova I., Prisk G.K., et al. Ballistocardiography and Seismocardiography: A Review of Recent Advances. IEEE J. Biomed. Health Inform. 2015;19:1414–1427. doi: 10.1109/JBHI.2014.2361732. [DOI] [PubMed] [Google Scholar]
- 29.Taebi A., Solar B.E., Bomar A.J., Sandler R.H., Mansy H.A. Recent Advances in Seismocardiography. Vibration. 2019;2:64–86. doi: 10.3390/vibration2010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanetti J.M., Tavakolian K. Seismocardiography: Past, present and future; Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Osaka, Japan. 3–7 July 2013; pp. 7004–7007. [DOI] [PubMed] [Google Scholar]
- 31.Crow R.S., Hannan P., Jacobs D., Hedquist L., Salerno D.M. Relationship between Seismocardiogram and Echocardiogram for Events in the Cardiac Cycle. Am. J. Noninvas. Cardiol. 1994;8:39–46. doi: 10.1159/000470156. [DOI] [Google Scholar]
- 32.Khosrow-Khavar F., Tavakolian K., Blaber A., Menon C. Automatic and Robust Delineation of the Fiducial Points of the Seismocardiogram Signal for Noninvasive Estimation of Cardiac Time Intervals. IEEE Trans. Biomed. Eng. 2017;64:1701–1710. doi: 10.1109/TBME.2016.2616382. [DOI] [PubMed] [Google Scholar]
- 33.Di Rienzo M., Vaini E., Castiglioni P., Merati G., Meriggi P., Parati G., Faini A., Rizzo F. Wearable seismocardiography: Towards a beat-by-beat assessment of cardiac mechanics in ambulant subjects. Auton. Neurosci. 2013;178:50–59. doi: 10.1016/j.autneu.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Rappaport M.B., Sprague H.B. The graphic registration of the normal heart sounds. Am. Heart J. 1942;23:591–623. doi: 10.1016/S0002-8703(42)90541-6. [DOI] [Google Scholar]
- 35.Dimond E.G., Benchimol A. Phonocardiography. Calif. Med. 1961;94:139–146. [PMC free article] [PubMed] [Google Scholar]
- 36.Ismail S., Siddiqi I., Akram U. Localization and classification of heart beats in phonocardiography signals—A comprehensive review. EURASIP J. Adv. Signal. Process. 2018;2018:26. doi: 10.1186/s13634-018-0545-9. [DOI] [Google Scholar]
- 37.Giordano N., Knaflitz M. A Novel Method for Measuring the Timing of Heart Sound Components through Digital Phonocardiography. Sensors. 2019;19:1868. doi: 10.3390/s19081868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenstein J. Phonocardiography; its application to clinical medicine. S. Afr. Med. J. 1955;29:123–134. [PubMed] [Google Scholar]
- 39.Vermarien H. Phonocardiography. In: Webster J.G., editor. Encyclopedia of Medical Devices and Instrumentation. 2nd ed. John Wiley & Sons; Hoboken, NJ, USA: 2006. pp. 278–289. [Google Scholar]
- 40.Andreozzi E., Fratini A., Esposito D., Naik G., Polley C., Gargiulo G.D., Bifulco P. Forcecardiography: A Novel Technique to Measure Heart Mechanical Vibrations onto the Chest Wall. Sensors. 2020;20:3885. doi: 10.3390/s20143885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreozzi E., Gargiulo G.D., Esposito D., Bifulco P. A Novel Broadband Forcecardiography Sensor for Simultaneous Monitoring of Respiration, Infrasonic Cardiac Vibrations and Heart Sounds. Front. Physiol. 2021;12:725716. doi: 10.3389/fphys.2021.725716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andreozzi E., Centracchio J., Punzo V., Esposito D., Polley C., Gargiulo G.D., Bifulco P. Respiration Monitoring via Forcecardiography Sensors. Sensors. 2021;21:3996. doi: 10.3390/s21123996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andreozzi E., Centracchio J., Esposito D., Bifulco P. A Comparison of Heart Pulsations Provided by Forcecardiography and Double Integration of Seismocardiogram. Bioengineering. 2022;9:167. doi: 10.3390/bioengineering9040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centracchio J., Andreozzi E., Esposito D., Gargiulo G.D. Respiratory-Induced Amplitude Modulation of Forcecardiography Signals. Bioengineering. 2022;9:444. doi: 10.3390/bioengineering9090444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centracchio J., Andreozzi E., Esposito D., Gargiulo G.D., Bifulco P. Detection of Aortic Valve Opening and Estimation of Pre-Ejection Period in Forcecardiography Recordings. Bioengineering. 2022;9:89. doi: 10.3390/bioengineering9030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centracchio J., Esposito D., Gargiulo G.D., Andreozzi E. Changes in Forcecardiography Heartbeat Morphology Induced by Cardio-Respiratory Interactions. Sensors. 2022;22:9339. doi: 10.3390/s22239339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jafari Tadi M., Lehtonen E., Pankaala M., Saraste A., Vasankari T., Teras M., Koivisto T. Gyrocardiography: A new non-invasive approach in the study of mechanical motions of the heart. Concept, method and initial observations; Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Orlando, FL, USA. 16–20 August 2016; pp. 2034–2037. [DOI] [PubMed] [Google Scholar]
- 48.Jafari Tadi M., Lehtonen E., Saraste A., Tuominen J., Koskinen J., Teräs M., Airaksinen J., Pänkäälä M., Koivisto T. Gyrocardiography: A New Non-invasive Monitoring Method for the Assessment of Cardiac Mechanics and the Estimation of Hemodynamic Variables. Sci. Rep. 2017;7:6823. doi: 10.1038/s41598-017-07248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sieciński S., Kostka P.S., Tkacz E.J. Gyrocardiography: A Review of the Definition, History, Waveform Description and Applications. Sensors. 2020;20:6675. doi: 10.3390/s20226675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dehkordi P., Tavakolian K., Tadi M.J., Zakeri V., Khosrow-Khavar F. Investigating the estimation of cardiac time intervals using gyrocardiography. Physiol. Meas. 2020;41:055004. doi: 10.1088/1361-6579/ab87b2. [DOI] [PubMed] [Google Scholar]
- 51.D’Mello Y., Skoric J., Xu S., Roche P.J.R., Lortie M., Gagnon S., Plant D.V. Real-Time Cardiac Beat Detection and Heart Rate Monitoring from Combined Seismocardiography and Gyrocardiography. Sensors. 2019;19:3472. doi: 10.3390/s19163472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hossein A., Mirica D.C., Rabineau J., Del Rio J.I.J., Morra S., Gorlier D., Nonclercq A., van de Borne P., Migeotte P.F. Accurate Detection of Dobutamine-induced Haemodynamic Changes by Kino-Cardiography: A Randomised Double-Blind Placebo-Controlled Validation Study. Sci. Rep. 2019;9:10479. doi: 10.1038/s41598-019-46823-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hossein A., Rabineau J., Gorlier D., Del Rio J.I.J., van de Borne P., Migeotte P.F., Nonclercq A. Kinocardiography Derived from Ballistocardiography and Seismocardiography Shows High Repeatability in Healthy Subjects. Sensors. 2021;21:815. doi: 10.3390/s21030815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tendulkar A.P., Harken A.H. Mechanics of the normal heart. J. Card Surg. 2006;21:615–620. doi: 10.1111/j.1540-8191.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- 55.Buckberg G., Hoffman J.I., Mahajan A., Saleh S., Coghlan C. Cardiac mechanics revisited: The relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–2587. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 56.Bloechlinger S., Grander W., Bryner J., Dünser M.W. Left ventricular rotation: A neglected aspect of the cardiac cycle. Intensive Care Med. 2011;37:156–163. doi: 10.1007/s00134-010-2053-8. [DOI] [PubMed] [Google Scholar]
- 57.Yang C., Tavassolian N. A feasibility study on a low-cost, smartphone-based solution of pulse transit time measurement using cardio-mechanical signals; Proceedings of the IEEE Healthcare Innovations and Point of Care Technologies (HI-POCT); Bethesda, MD, USA. 6–8 November 2017; pp. 93–96. [DOI] [Google Scholar]
- 58.Sieciński S., Kostka P.S., Tkacz E.J. Heart Rate Variability Analysis on Electrocardiograms, Seismocardiograms and Gyrocardiograms on Healthy Volunteers. Sensors. 2020;20:4522. doi: 10.3390/s20164522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siecinski S., Kostka P.S., Tkacz E.J. Time Domain And Frequency Domain Heart Rate Variability Analysis on Gyrocardiograms; Proceedings of the Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); Montreal, QC, Canada. 20–24 July 2020; pp. 2630–2633. [DOI] [PubMed] [Google Scholar]
- 60.Lahdenoja O., Hurnanen T., Iftikhar Z., Nieminen S., Knuutila T., Saraste A., Kiviniemi T., Vasankari T., Airaksinen J., Pänkäälä M., et al. Atrial Fibrillation Detection via Accelerometer and Gyroscope of a Smartphone. IEEE J. Biomed. Health Inform. 2018;22:108–118. doi: 10.1109/JBHI.2017.2688473. [DOI] [PubMed] [Google Scholar]
- 61.Iftikhar Z., Lahdenoja O., Jafari Tadi M., Hurnanen T., Vasankari T., Kiviniemi T., Airaksinen J., Koivisto T., Pänkäälä M. Multiclass Classifier based Cardiovascular Condition Detection Using Smartphone Mechanocardiography. Sci. Rep. 2018;8:9344. doi: 10.1038/s41598-018-27683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hurnanen T., Kaisti M., Jafari Tadi M., Vähä-Heikkilä M., Nieminen S., Iftikhar Z., Paukkunen M., Pänkäälä M., Koivisto T. Heartbeat Detection Using Multidimensional Cardiac Motion Signals and Dynamic Balancing; Proceedings of the European Medical and Biological Engineering Confernce (EMBEC) & Nordic-Baltic Conference on Biomedical Engineering and Medical Physics (NBC); Tampere, Finland. 11–15 June 2017; [DOI] [Google Scholar]
- 63.Jafari Tadi M., Lehtonen E., Teuho J., Saraste A., Pänkäälä M., Teräs M., Koivisto T. A miniaturized MEMS motion processing system for nuclear medicine imaging applications; Proceedings of the 2016 Computing in Cardiology Conference (CinC); Vancouver, BC, Canada. 11–14 September 2016. [Google Scholar]
- 64.Jafari Tadi M., Teuho J., Lehtonen E., Saraste A., Pänkäälä M., Koivisto T., Teräs M. A novel dual gating approach using joint inertial sensors: Implications for cardiac PET imaging. Phys. Med. Biol. 2017;62:8080–8101. doi: 10.1088/1361-6560/aa8b09. [DOI] [PubMed] [Google Scholar]
- 65.Jafari Tadi M., Lehtonen E., Teuho J., Koskinen J., Schultz J., Siekkinen R., Koivisto T., Pänkäälä M., Teräs M., Klén R. A Computational Framework for Data Fusion in MEMS-Based Cardiac and Respiratory Gating. Sensors. 2019;19:4137. doi: 10.3390/s19194137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hernandez J.E., Cretu E. Simple Heart Rate Monitoring System with a MEMS Gyroscope for Sleep Studies; Proceedings of the Annual Information Technology, Electronics and Mobile Communication Conference (IEMCON); Vancouver, BC, Canada. 1–3 November 2018; pp. 61–67. [DOI] [Google Scholar]
- 67.Kaisti M., Jafari Tadi M., Lahdenoja O., Hurnanen T., Saraste A., Pänkäälä M., Koivisto T. Stand-Alone Heartbeat Detection in Multidimensional Mechanocardiograms. IEEE Sens. J. 2019;19:234–242. doi: 10.1109/JSEN.2018.2874706. [DOI] [Google Scholar]
- 68.Jia W., Li Y., Bai Y., Mao Z.-H., Sun M., Zhao Q. Estimation of heart rate from a chest-worn inertial measurement unit; Proceedings of the 2015 International Symposium on Bioelectronics and Bioinformatics (ISBB); Beijing, China. 14–17 October 2015; pp. 148–151. [DOI] [Google Scholar]
- 69.Lee H., Lee H., Whang M. An Enhanced Method to Estimate Heart Rate from Seismocardiography via Ensemble Averaging of Body Movements at Six Degrees of Freedom. Sensors. 2018;18:238. doi: 10.3390/s18010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aboulezz E., Skoric J., D’Mello Y., Hakim S., Clairmonte N., Lortie M., Plant D.V. Analyzing Heart Rate Estimation from Vibrational Cardiography with Different Orientations; Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Montreal, QC, Canada. 20–24 July 2020; pp. 2638–2641. [DOI] [PubMed] [Google Scholar]
- 71.Lahdenoja O., Humanen T., Jafari Tadi M., Pänkäälä M., Koivisto T. Heart rate variability estimation with joint accelerometer and gyroscope sensing; Proceedings of the 2016 Computing in Cardiology Conference (CinC); Vancouver, BC, Canada. 11–14 September 2016; pp. 717–720. [Google Scholar]
- 72.Hu Y., Wong Y., Wei W., Du Y., Kankanhalli M., Geng W. A novel attention-based hybrid CNN-RNN architecture for sEMG-based gesture recognition. PLoS ONE. 2018;13:e02060492018. doi: 10.1371/journal.pone.0206049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia Y., Wulan N., Wang K., Zhang H. Detecting atrial fibrillation by deep convolutional neural networks. Comput. Biol. Med. 2018;93:84–92. doi: 10.1016/j.compbiomed.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Jia Z., Ji J., Zhou X., Zhou Y. Hybrid spiking neural network for sleep electroencephalogram signals. Sci. China Inf. Sci. 2022;65:140403. doi: 10.1007/s11432-021-3380-1. [DOI] [Google Scholar]
- 75.Duraj K.M., Siecinski S., Doniec R.J., Piaseczna N.J., Kostka P.S., Tkacz E.J. Heartbeat detection in seismocardiograms with semantic segmentation; Proceedings of the 44th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Glasgow, UK. 11–15 July 2022; pp. 662–665. [DOI] [PubMed] [Google Scholar]
- 76.Zhang S., Zhang H., Lin Z., Huat Ng S. Automated and precise heartbeat detection in ballistocardiography signals using bidirectional LSTM. Franklin Open. 2022;1:30–38. doi: 10.1016/j.fraope.2022.05.001. [DOI] [Google Scholar]
- 77.Yoo J.C., Han T.H. Fast Normalized Cross-Correlation. Circuits Syst. Signal Process. 2009;28:819–843. doi: 10.1007/s00034-009-9130-7. [DOI] [Google Scholar]
- 78.Briechle K., Hanebeck U.D. Template matching using fast normalized cross correlation; Proceedings of the Society of Photo-Optical Instrumentation Engineers (SPIE) 4387, Optical Pattern Recognition XII; Orlando, FL, USA. 20 March 2001; [DOI] [Google Scholar]
- 79.Chen Y.H., Chen H.H., Chen T.C., Chen L.G. Robust heart rate measurement with phonocardiogram by on-line template extraction and matching; Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Boston, MA, USA. 30 August–3 September 2011; pp. 1957–1960. [DOI] [PubMed] [Google Scholar]
- 80.Centracchio J., Parlato S., Esposito D., Bifulco P., Andreozzi E. ECG-Free Heartbeat Detection in Seismocardiography Signals via Template Matching. Sensors. 2023;23:4684. doi: 10.3390/s23104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang C., Fan F., Aranoff N., Green P., Li Y., Liu C., Tavassolian N. An Open-Access Database for the Evaluation of Cardio-Mechanical Signals From Patients with Valvular Heart Diseases. Front. Physiol. 2021;12:750221. doi: 10.3389/fphys.2021.750221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan J., Tompkins W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985;32:230–236. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- 83.Sedghamiz H. BioSigKit: A Matlab Toolbox and Interface for Analysis of BioSignals. J. Open. Source Softw. 2018;3:671. doi: 10.21105/joss.00671. [DOI] [Google Scholar]
- 84.Altman D.G., Bland J.M. Measurement in medicine: The analysis of method comparison studies. Statistician. 1983;32:307–317. doi: 10.2307/2987937. [DOI] [Google Scholar]
- 85.Giavarina D. Understanding Bland Altman analysis. Biochem. Med. 2015;25:141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ran K. Bland-Altman and Correlation Plot, MATLAB Central File Exchange. 2020. [(accessed on 15 March 2023)]. Available online: https://www.mathworks.com/matlabcentral/fileexchange/45049-bland-altman-and-correlation-plot.
- 87.Işilay Zeybek Z.M., Racca V., Pezzano A., Tavanelli M., Di Rienzo M. Can Seismocardiogram Fiducial Points Be Used for the Routine Estimation of Cardiac Time Intervals in Cardiac Patients? Front. Physiol. 2022;13:825918. doi: 10.3389/fphys.2022.825918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the relevant research data will be made available upon request after the publication of the paper.