Abstract
Background
Paraspinal muscle fat infiltration is closely related to the occurrence and development of lumbar spine disorders and postoperative complications. This study aimed to explore the effects of age, sex, muscle, and level on paraspinal muscle fat infiltration among Chinese adults to identify the best single level of assessing whole-level paraspinal muscle fat infiltration and to define the standardized identification thresholds for paraspinal muscle fat infiltration by means of magnetic resonance imaging.
Methods
This was a single-center, cross-sectional study conducted on 336 asymptomatic Chinese volunteers aged 20 to 69 years recruited from Beijing and surrounding communities through designed advertisements from May 2022 to October 2022. The fat signal fraction of multifidus (FSFMF), erector spinae (FSFES), psoas major (FSFPM), and the sum of multifidus, erector spinae, and psoas major (FSFTotal) at lumbar levels L1–L5 were measured with magnetic resonance imaging. The Student t-test and Mann-Whitney test were performed, and Pearson correlations and intraclass correlation coefficients were determined. Subgroups were compared using analysis of variance followed by a post hoc Bonferroni test or Kruskal-Wallis test.
Results
FSFTotal (14.02%±4.71% vs. 10.34±4.08%; P<0.001), FSFMF (21.14%±6.77% vs. 16.21%±6.26%; P<0.001), and FSFES (15.97%±5.56% vs. 12.37%±4.80%; P<0.001) were higher in females than in males and increased with age and lumbar level, whereas FSFPM did not significantly differ by age (all P values >0.05) or sex (P=0.12) and showed a decreasing trend from L1 to L5. The FSFTotal at L4 showed both the strongest correlation (Pearson correlation coefficient =0.95; P<0.001) and agreement (intraclass correlation coefficient =0.92; P<0.001) with the whole-level FSFTotal. Pathological paraspinal muscle fat infiltration identification thresholds of FSFTotal, FSFMF, FSFES, and FSFPM were 10.0–33.9%, 19.2–47.4%, 16.2–43.6%, and 4.8%, respectively, in each age (range, 20–69 years) and sex group.
Conclusions
In asymptomatic Chinese adults, paraspinal muscle fat infiltration can be influenced by age, sex, muscle type, and location. The L4 level can serve as an optimal substitution in whole-level fat infiltration measurement. We present the first data concerning the identification thresholds of pathological paraspinal muscle fat infiltration, which will provide a valuable resource for researchers in the field.
Keywords: Paraspinal muscles, fat infiltration, fat signal fraction (FSF), magnetic resonance imaging (MRI), asymptomatic Chinese adults
Introduction
Paraspinal muscle is a vital body composition component and plays an important role in maintaining spinal stability, balance, and sagittal alignment (1-3). Typically, paraspinal muscle fat infiltration is a normal and slow process. However, excessive fat accumulation within muscles can initiate pathological changes, ultimately resulting in the loss of muscle strength and muscle mass (4). In recent years, the study of the associations between paraspinal muscle fat infiltration and spinal disease has attracted considerable attention. Paraspinal muscle fat infiltration has been shown to correlate with the occurrence and development of low back pain, lumbar disc herniation, lumbar spinal stenosis, and degenerative lumbar scoliosis (5-8). A more recent systematic review has demonstrated that paraspinal muscle fat infiltration is a predictor of postoperative complications, such as proximal junctional kyphosis, adjacent segment degeneration, and pedicle screw loosening (9). Fortunately, the evidence suggests that physical activity and regular exercise are effective approaches for slowing down or even reversing fat infiltration (10,11). However, although improving paraspinal muscle fat infiltration is able to attenuate the severity of lumbar spine disease and the rates of postoperative complication (12,13), the timing of the intervention is essential. In fact, functional exercise might be too late and have limited effectiveness for patients who have already developed lumbar spine symptoms or disease (14). Therefore, it is necessary to more deeply understand the characteristics of paraspinal muscle fat infiltration in asymptomatic populations to better enable the early identification and intervention of abnormal fat infiltration.
In recent years, numerous studies have revealed that fat infiltration may vary markedly by age, sex, muscles, and disc level (15-20). Moreover, L4 has also been found to be the best level for representing the pathological change of the whole lumbar region (21,22). However, in these studies, most or all study populations were White, with Asian populations being scarce. Even in the few studies restricted to Asian populations (23,24), Chinese populations were not evaluated or included. In our opinion, while some of the conclusions from these studies may seem logical or intuitive, additional validation with underlying solid data should be undertaken. Additionally, as a large and aging country, China has an extremely high number of patients with lumbar disease. Undoubtedly, understanding these characteristics would be valuable for planning and developing a more targeted intervention strategy. More importantly, some of the abovementioned studies suggested that their findings should not be extrapolated to other populations since ethnic differences exist related to paraspinal muscle fat infiltration (19-21). Unfortunately, we have not seen any work that has identified the characteristics of paraspinal muscle fat infiltration in the Chinese population or has confirmed whether the findings from other populations can be applied to the Chinese population.
It is also important to note that the accurate and correct identification of pathological paraspinal muscle fat infiltration is an essential prerequisite for timely intervention. Currently, pathological paraspinal muscle fat infiltration is generally identified based on the Goutallier classification (25). However, although the Goutallier classification is intuitive and has been proven to correlate with functional status and clinical outcomes (26), questions regarding its interobserver or intraobserver reliability remain unresolved (27). Furthermore, the subjectivity of the scorer and the lack of quantitative data not only limit the utility of this semiquantitative classification but obstruct communication and comparison between studies (27,28). By contrast, quantitative evaluation has higher reliability and is capable of overcoming such limitations via objective and continuous measures (29). Thus, defining the standardized identification thresholds for quantitative assessments of pathological paraspinal muscle fat infiltration is urgently needed. However, to our knowledge, no such attempts have been reported so far.
The objectives of the present study were to (I) clarify the effects of age, sex, muscle, and level on paraspinal muscle fat infiltration among Chinese adults; (II) identify the best single level to assess whole-level paraspinal muscle fat infiltration; and (III) define the standardized identification thresholds for paraspinal muscle fat infiltration by means of magnetic resonance imaging (MRI). We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1131/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of the Beijing Chaoyang Hospital (institutional review board ethics approval No. 2022-KE-509) and registered in the Chinese Clinical Trial Registry (registry No. ChiCTR2200059380). Study participation was voluntary, and written informed consent was acquired from all participants.
Participants
This was a cross-sectional, single-center study. Chinese volunteers were recruited from Beijing and surrounding communities through designed advertisements during the period from May 2022 to October 2022. The inclusion criteria were the following: (I) age between 20 and 69 years and (II) an Oswestry Disability Index (ODI) score <20% (30). The exclusion criteria were following: (I) contraindications for MRI or inability to tolerate the MRI procedure; (II) body mass index (BMI) ≤18.5 kg/m2 or BMI ≥30 kg/m2; (III) significant painful episodes related to the spine; (IV) a history of spinal or pelvic surgery; (V) a history of neurological, psychiatric, musculoskeletal, or severe systemic diseases; and (VI) usage of medications known to affect muscle structure or function. In particular, participants who had imaging evidence of lumbar degenerative changes but without symptoms (ODI score <20%) were also included in the study. Age, sex, height, and weight were recorded for every participant. The BMI and ODI of participants were also calculated and recorded.
Imaging acquisition
MRI can objectively, noninvasively, and accurately evaluate the fat infiltration of paraspinal muscle (31). In this study, MRI data acquisition of the lumbar spine was performed on a 3.0 T magnetic resonance scanner (MAGNETOM Verio; Siemens Healthineers, Erlangen, Germany). The scanning range was set from the superior L1 endplate to the superior S1 endplate. The MR imaging sequences and parameters are summarized in Table 1. All participants were scanned in the supine position and were instructed to close their eyes but stay awake, move as little as possible, and think of nothing in particular during the scans. All MRI scans were performed by one qualified and trained radiology technician. After the scan, the original images were saved in DICOM format in the picture archiving and communication system for further processing.
Table 1. Magnetic resonance imaging sequences and parameters.
| Parameters | Sequence | |
|---|---|---|
| T1-weighted | T2-weighted | |
| Sequence type | Turbo spin echo | Turbo spin echo |
| Repetition time (msec) | 400–600 | 3,000–3,100 |
| Echo time (msec) | 10–15 | 70–80 |
| Flip angle (°) | 90 | 150–160 |
| Echo train length | 29 | 26 |
| Slice thickness (mm) | 3 | 3 |
| Acquisition matrix | 256×224 | 512×256 |
| Field of view (mm) | 280×280 | 200×220 |
| Number of excitations | 1 | 4 |
Image analysis
All the images were displayed and analyzed using ImageJ software (version 1.40; US National Institutes of Health, Bethesda, MD, USA). The midsagittal T1-weighted imaging slices were used to position the lumbar level, and each lumbar level was defined as the region between the superior endplate of the upper vertebra and the superior endplate of the vertebra (or sacrum) immediately below, including the intervertebral disc. Among all axial T2-weighted imaging slices, every third slice was selected to quantify paraspinal muscle fat infiltration (17). The quantification procedure was split into four key steps (Figure 1). Briefly, according to the scale bar within the original image, the line selection tool was used to convert pixel units into millimeters. Additionally, to reduce the inhomogeneity artifacts, coil shading, and corruption by noise (32), the brightness and contrast of all images were normalized using ImageJ’s integrated contrast normalization function prior to segmentation (Figure 1A). We then manually outlined the fascial boundary of each region of interest (ROI), including the right and left multifidus (MF), erector spinae (ES), and psoas major (PM) (Figure 1B). For each ROI, the automatic threshold method and color segmentation technique were applied to separate fat and water signals (Figure 1C). In fact, manual thresholding relies on individuals for visually determining thresholds and can be affected by interoperator subjectivity (33). To avoid subjective selection of the threshold, an automatic threshold scheme, the Otsu thresholding method, was used to threshold images in this study (34). However, although the Otsu thresholding method is fully automated and has been shown capable of avoiding human error or inconsistency in segmenting an image, this method can lead to the loss of meaningful data (35). Therefore, when necessary, the thresholds were adjusted manually to ensure accuracy. Finally, the cross-sectional area (CSA) of the fat and water signals within each ROI was measured and recorded (Figure 1D). Two spinal surgeons with 5 years of clinical experience and 5 years of experience in reading musculoskeletal MRI assessed each image independently, and the average value was considered to be the final result. Fat signal fraction (FSF) was calculated using the following formula: FSF = [CSAfat/(CSAwater + CSAfat)] ×100% (36).
Figure 1.
Measurements of the paraspinal muscles and fat using ImageJ software. (A) Image contrast and brightness were normalized using ImageJ software. (B) The fascial boundary of each paraspinal muscle was manually outlined. (C) The automatic threshold method and color segmentation technique were applied to separate fat and water signals. (D) The cross-sectional area of the fat and water signals within each paraspinal muscle were measured and recorded. MF, multifidus; ES, erector spinae; PM, psoas major.
Sample size estimation
The sample size was estimated using PASS software (version 15.0; NCSS, Kaysville, UT, USA). We made the following assumptions: based on the standard deviation of the paraspinal muscle FSF obtained in a previous study (17), a 95% confidence level, and a 1% desired precision, the minimum sample size was estimated to be 293. Considering an expected withdrawal or dropout rate of approximately 20%, the target sample size was set to 366.
Statistical analysis
The normality of distributions was examined using the Kolmogorov-Smirnov test. Normally distributed data are presented as the mean ± standard deviation, whereas nonnormally distributed data are presented as the median and interquartile range. The differences between 2 groups were tested using an unpaired Student t-test or Mann-Whitney test depending on the outcome of the normality assessment. Multiple comparisons among subgroups were tested using one-way analysis of variance (ANOVA) or Kruskal-Wallis one-way ANOVA. If the ANOVA detected significant differences, the Bonferroni post hoc test or Dunn multiple comparisons post hoc test was performed. Pearson correlation coefficients (r) were used to explore the relationships between continuous variables. Intraclass correlation coefficients (ICCs) were used to assess the interobserver reliability of the FSF quantitative measures and the agreement between the whole-level FSF and the single-level FSF. For all correlation coefficients, less than 0.5 was considered poor, 0.5 to 0.9 moderate, and greater than 0.9 excellent. The single-level FSF that showed the best correlation and agreement with the whole-level FSF was determined to be the optimal level (37). As only increased FSF values are considered pathologic, an FSF ≥95th percentile was defined as the reference value for pathological fat infiltration (38). Statistical analysis was performed using SPSS software (version 20.0; IBM Corp., Armonk NY, USA). Statistical significance was set at P<0.05.
Results
Participant recruitment
Of the 1,673 individuals approached, 587 individuals agreed to be screened for study eligibility. Of these, 251 individuals were excluded, of whom 205 did not meet the inclusion criteria or met at least one of the exclusion criteria, 40 declined to participate, 5 were excluded due to poor imaging quality, and 1 was unable to tolerate MRI. In the final analysis, 336 participants were included (Figure 2).
Figure 2.
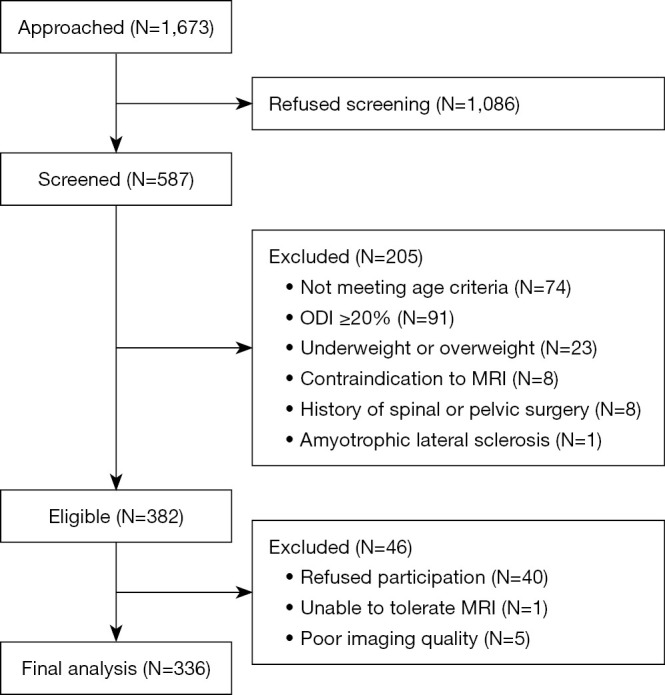
Flowchart of participant inclusion in the study. ODI, Oswestry Disability Index; MRI, magnetic resonance imaging.
Participant characteristics
The demographic characteristics of the participants and the specific number of participants within each age group are summarized in Tables 2,3, respectively. The analyzed population comprised 148 males (44.05%) and 188 females (55.95%), with a mean age of 42.56±12.76 years. Mean BMI was 21.80±1.88 kg/m2. All of the data sets were normally distributed except for the FSFPM data. The ICCs for FSFMF, FSFES, and FSFPM were 0.93, 0.91, and 0.95, respectively, indicating excellent interobserver reliability.
Table 2. Demographic characteristics and measurement outcomes of paraspinal muscles for the entire cohort and by sex.
| Variables | All (N=336) | Male (N=148) | Female (N=188) | P value |
|---|---|---|---|---|
| Age (years) | 42.56±12.76† | 43.91±13.40 | 41.50±12.19 | 0.22 |
| Height (m) | 1.66±0.10 | 1.73±0.08 | 1.61±0.07 | <0.001* |
| Body weight (kg) | 60.42±10.48 | 67.75±8.84 | 54.61±7.78 | <0.001* |
| BMI (kg/m2) | 21.80±1.88 | 22.69±1.40 | 21.10±1.94 | 0.06 |
| FSFMF (%) | 18.96±6.97 | 16.21±6.26 | 21.14±6.77 | <0.001* |
| FSFES (%) | 14.38±5.53 | 12.37±4.80 | 15.97±5.56 | <0.001* |
| FSFPM (%) | 1.40 (0.88–2.42)‡ | 1.22 (0.68–2.35) | 1.62 (0.95–2.57) | 0.12 |
| FSFTotal (%) | 12.39±4.79 | 10.34±4.08 | 14.02±4.71 | <0.001* |
†, mean ± standard deviation (all such values) for normally distributed data. ‡, median with interquartile range in parentheses (all such values) for nonnormally distributed data. *, statistical significance (P<0.05). P value indicates the male group versus the female group. BMI, body mass index; FSF, fat signal fraction; MF, multifidus; ES, erector spinae; PM, psoas major. FSFTotal indicates the fat signal fraction of the sum of the multifidus, erector spinae, and psoas major.
Table 3. Number of participants within each age group.
| Variables | Age range (years) | Total | ||||
|---|---|---|---|---|---|---|
| 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | ||
| Male, n | 16 | 62 | 23 | 20 | 27 | 148 |
| Female, n | 28 | 78 | 28 | 33 | 21 | 188 |
| Total, n | 44 | 140 | 51 | 53 | 48 | 336 |
Sex
Overall, there were significant differences between males and females in the FSFMF, FSFES, and FSF of the sum of MF, ES, and PM (FSFTotal) (all P values <0.001), whereas there were no significant differences in FSFPM between sexes (Table 2). The FSF values stratified by age and sex are presented in Table 4. Within each age group, males had a significantly lower FSFMF (except those aged 50–59 years), FSFES, and FSFTotal compared to females (all P values <0.05). In addition, although not statistically significant, a similar trend was observed in FSFPM.
Table 4. Measurement outcomes of paraspinal muscles stratified by age and sex.
| Variables | Sex | Age group (years) | P value‡ | ||||
|---|---|---|---|---|---|---|---|
| 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | |||
| FSFMF (%) | Both sexes | 15.81±5.81§ | 16.40±5.34 | 18.04±4.75 | 24.29±7.16 | 23.60±6.21 | <0.001* |
| Male | 12.41±2.25 | 13.46±4.48 | 15.33±5.12 | 21.64±6.92 | 21.28±6.30 | <0.001* | |
| Female | 17.75±6.38 | 18.73±4.84 | 20.02±3.40 | 25.94±7.00 | 27.20±4.20 | <0.001* | |
| P value† | 0.01* | <0.001* | 0.01* | 0.13 | 0.02* | ||
| FSFES (%) | Both sexes | 10.66±3.79 | 12.12±3.69 | 13.82±4.07 | 19.09±5.14 | 19.69±5.10 | <0.001* |
| Male | 8.29±1.86 | 9.91±2.59 | 11.74±4.08 | 16.52±3.26 | 17.70±4.80 | <0.001* | |
| Female | 12.02±3.99 | 13.88±3.50 | 15.35±3.43 | 20.70±5.52 | 22.77±4.07 | <0.001* | |
| P value† | 0.008* | <0.001* | 0.02* | 0.04* | 0.01* | ||
| FSFPM (%) | Both sexes | 0.92 (0.67–1.86)¶ | 1.24 (0.65–2.03) | 1.79 (1.19–2.54) | 2.31 (1.17–4.51) | 2.12 (1.24–3.34) | 0.07 |
| Male | 0.85 (0.46–3.27) | 0.92 (0.63–1.87) | 2.20 (1.07–5.16) | 1.81 (0.87–4.34) | 1.39 (0.68–2.28) | 0.03 | |
| Female | 0.94 (0.70–1.48) | 1.40 (0.79–2.43) | 1.39 (1.21–2.31) | 2.37 (1.47–5.23) | 2.79 (1.79–4.72) | 0.06 | |
| P value† | 0.67 | 0.06 | 0.59 | 0.20 | 0.08 | ||
| FSFTotal (%) | Both sexes | 9.49±3.46 | 10.43±3.31 | 11.89±3.17 | 16.64±4.61 | 16.14±4.34 | <0.001* |
| Male | 7.30±1.46 | 8.19±2.22 | 10.11±3.42 | 14.04±3.64 | 14.39±4.23 | <0.001* | |
| Female | 10.74±3.68 | 12.20±2.95 | 13.21±2.30 | 18.27±4.49 | 18.88±3.00 | <0.001* | |
| P value† | 0.006* | <0.001* | 0.01* | 0.02* | 0.006* | ||
†, males versus females performed by Student t-test or Mann-Whitney test. ‡, between age group comparisons performed by one-way ANOVA or Kruskal-Wallis one-way ANOVA. §, mean ± standard deviation (all such values) for normally distributed data. ¶, median with interquartile range in parentheses (all such values) for nonnormally distributed data. *, statistical significance (P<0.05). FSFTotal indicates the fat signal fraction of the sum of the multifidus, erector spinae, and psoas major. ANOVA, analysis of variance; FSF, fat signal fraction; MF, multifidus; ES, erector spinae; PM, psoas major.
Age
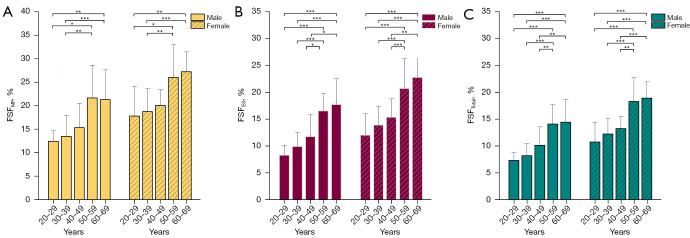
In both sexes, FSFMF, FSFES, and FSFTotal were significantly different among the age groups (all P values <0.001), whereas the differences in FSFPM across age groups were not significant (Table 4). Post hoc comparisons between age groups revealed significantly higher FSFMF, FSFES, and FSFTotal in the middle-aged and older participants compared to the younger participants (all P values <0.05) (Figure 3).
Figure 3.
Fat signal fraction of paraspinal muscles according to age and sex. (A) The left picture shows the fat signal fraction of the multifidus stratified by age and sex. (B) The middle picture shows the fat signal fraction of the erector spinae stratified by age and sex. (C) The right picture shows the fat signal fraction of the sum of the multifidus, psoas major, and erector spinae stratified by age and sex. *, Bonferroni adjusted P value <0.05; **, adjusted P value <0.01; ***, adjusted P value <0.001. FSF, fat signal fraction; MF, multifidus; ES, erector spinae. FSFTotal indicates the fat signal fraction of the sum of the multifidus, erector spinae, and psoas major. Bars and error bars indicate the means and standard deviations, respectively.
Lumbar level
FSFMF, FSFES, and FSFTotal showed an increasing trend from L1 to L5, whereas FSFPM showed the same trend but in the opposite direction. At each lumbar level, the FSFMF, FSFES (except at L4), and FSFTotal were significantly higher in females than in males (all P values <0.05). FSFPM was significantly different between males and females only at the L3 level (Figure 4).
Figure 4.

Fat signal fraction of paraspinal muscles at each lumbar spine level for males and females. The yellow, red, and green bars indicate the mean; the blue bars indicate the median; and all error bars indicate 95% confidence intervals. *, P value <0.05; **, P value <0.01; ***, P value <0.001. MF, multifidus; ES, erector spinae; PM, psoas major; total, the sum of multifidus, psoas major, and erector spinae.
Optimal level to predict whole-level FSFTotal
Pearson correlation coefficients between variables ranged between 0.72 and 0.95; On the other hand, the ICCs were between 0.29 and 0.92 (Table 5). All correlation coefficients were statistically significant (P<0.001). In all cases, the FSFTotal at L4 showed both the strongest correlation (r=0.95) and agreement (ICC=0.92) with the whole-level FSFTotal.
Table 5. Correlation coefficients between the FSFTotal for each single level and the whole level†.
| Level | L1 | L2 | L3 | L4 | L5 | L1–L5 |
|---|---|---|---|---|---|---|
| L1 | 0.90 | 0.84 | 0.78 | 0.72 | 0.89‡ | |
| L2 | 0.89 | 0.90 | 0.84 | 0.75 | 0.93 | |
| L3 | 0.73 | 0.84 | 0.90 | 0.76 | 0.94 | |
| L4 | 0.50 | 0.60 | 0.80 | 0.85 | 0.95¶ | |
| L5 | 0.29 | 0.34 | 0.46 | 0.70 | 0.91 | |
| L1–L5 | 0.65§ | 0.75 | 0.90 | 0.92¶ | 0.63 |
†, all correlation coefficients were statistically significant (P<0.001). ‡, the top right half shows the Pearson correlation coefficients. §, the lower left half shows the intraclass correlation coefficients. ¶, the highest correlation coefficient. FSFTotal indicates the fat signal fraction of the sum of the multifidus, erector spinae, and psoas major.
Reference values for paraspinal muscle fat infiltration
Based on the above results, we subsequently used the FSF values at the L4 level to simplify the evaluation of the whole-level FSF. Table 6 shows the proposed thresholds for identifying pathological paraspinal muscle fat infiltration were derived from the 95th percentiles for each age group and sex.
Table 6. The 95th percentiles for FSFTotal, FSFMF, FSFES, and FSFPM of the L4 level by age and sex.
| Variables | Sex | Age, years | ||||
|---|---|---|---|---|---|---|
| 20–29 | 30–39 | 49–49 | 50–59 | 60–69 | ||
| FSFTotal (%) | Male | 10.0 | 14.5 | 15.6 | 22.5 | 25.8 |
| Female | 14.5 | 18.8 | 20.5 | 25.4 | 33.9 | |
| FSFMF (%) | Male | 19.2 | 24.3 | 26.2 | 34.2 | 41.5 |
| Female | 21.5 | 27.9 | 32.3 | 36.3 | 47.4 | |
| FSFES (%) | Male | 16.2 | 20.7 | 21.6 | 31.9 | 36.3 |
| Female | 19.5 | 25.7 | 28.3 | 35.4 | 43.6 | |
| FSFPM (%) | Both sexes | 4.8† | ||||
†, for all age range (20–69 years). FSFTotal indicates the fat signal fraction of the sum of the multifidus, erector spinae, and psoas major. FSF, fat signal fraction; MF, multifidus; ES, erector spinae; PM, psoas major.
Discussion
Fat infiltration is an important indicator of paraspinal muscle degeneration as well as a key predictor of spinal disease and postoperative complications (5-9). The last decade has seen a dramatic increase in the interest and attention given to paraspinal muscle fat infiltration. However, fundamental questions about its influencing factors, measurement protocols, and identification criteria have been largely ignored to date. The present study is the first attempt to systematically describe these fundamental questions to uncover the characteristics of paraspinal muscle fat infiltration.
While muscle fat infiltration is thought to be a result of the aging process (15), it is not without controversy. An ultrasound-based study demonstrated no significant association between MF fat infiltration and age in healthy individuals (39). However, in another analysis of ultrasonography images for asymptomatic participants, only MF showed a correlation with age (40). Fortunately, our results are consistent with most studies demonstrating that fat infiltration is increasingly common with age among paraspinal muscles (21,23). Beyond this point, we observed that FSFTotal increased significantly in participants aged 40–59 years and represented almost half of the overall increases. In contrast, the increasing trend of muscle fat infiltration in the other age groups was relatively slow. Furthermore, these observations appeared to be an independent age effect, which was not explained by sex differences. Therefore, we speculate that the relationship between aging and muscle fat infiltration may evolve with age. Our results suggest that middle-aged adults are an important population for possible future preventive interventions.
Previous research has shown that there are sex differences in muscle fat infiltration (17). Muscle fat infiltration appears to be lower in healthy males compared to females (40). In this study, we observed similar results among the Chinese population and demonstrated that muscle fat infiltration is more common in females. Moreover, we also found that the sex differences appeared to become more striking in the 60–69 age group of (most likely postmenopausal) women. This finding not only supports the current notion that female gonadal hormones affect the development of muscle fat infiltration (41) but also implicates their role in protecting muscle. However, FSFTotal maintained significant sex differences even when we restricted our analysis to only men and premenopausal women. Thus, sex differences in muscle fat infiltration may arise from various mechanisms, a point that should be further explored in future research.
In a previous study, Lee et al. reported differences in fat infiltration between paraspinal muscles, with the highest in ES, followed by MF, and the lowest in PM (23). They attributed these differences to anatomical differences in muscles; that is, direction and length. However, in the present study, our data revealed that fat infiltration was lowest in the PM, while that in the MF was higher than that in the ES. At first glance, some of these results seem to be conflicting. Interestingly, although differences among the three paraspinal muscles do exist, we found that the FSF values of both MF and ES were approximately at the same level and were significantly higher than that of the PM, which is similar to the findings from previous studies (16,21,23). From the standpoint of muscle function, MF and ES act predominantly as extensor muscles in the lumbar region, whereas PM is mainly involved in lumbar flexion. On the one hand, these lines of evidence suggest that the lumbar extensors may be more susceptible to the effect of fat infiltration compared to lumbar flexors. On the other hand, our findings may provide an alternative explanation for the differences between paraspinal muscle fat infiltration, which is functional differences. Remarkably, whether the differences are anatomical or functional will necessarily involve the characteristics of the muscle itself. Thus, we favor the hypothesis that paraspinal muscle fat infiltration is muscle-dependent. Nevertheless, the use of additional measurements, such as electromyography or the finite element model, will be required to further validate this hypothesis.
Numerous reports in the literature have shown that the paraspinal muscle exhibits a level-dependent increase in fat infiltration from L1 to S1 and that the lower lumbar levels are more likely to have higher fat infiltration (17,18,21,23,42). It has been suggested that this phenomenon could be explained by the cantilever hypothesis, which views the spine as a kind of cantilever fixed to the sacropelvic complex (23). Since more force is loaded as it gets closer to the fixed side of a cantilever (43), they therefore believe that lower lumbar levels may bear higher stress. This hypothesis seems reasonable, considering the fact that excessive non-physiological muscle stress can cause intramuscular fat infiltration (44). However, our data showed that the fat infiltration of PM tended to occur at higher lumbar levels. Clearly, the cantilever hypothesis does not fully account for our findings. Interestingly, we do not think this hypothesis is entirely false. In our opinion, regarding paraspinal muscles as a cantilever fixed at the fixed end of the muscle may be a more rational hypothesis than the spine as a cantilever. From an anatomical point of view, the PM originates from the 12th thoracic vertebra, whereas the MF and ES are derived from the dorsal sacrum. Unlike those of the MF and ES, higher lumbar levels are closer to the fixed end of the PM, and this might have been the reason for the PM displaying the opposite fat infiltration pattern to that of the MF and ES. It can be seen that the extent of paraspinal muscle fat infiltration does not depend on the level of the lumbar spine but rather on the relative distance from the fixed end of the muscle, which we refer to as a distance-dependent of fat infiltration. Furthermore, it should be noted that because the paraspinal muscle has two or more fixed ends, fat infiltration may be more than just a gradual decreasing unidirectional change trend from the fixed end to its distal end. In fact, a previous study on thigh muscle found that proximal and distal fat infiltration of the quadriceps was significantly higher than central fat infiltration (45). Furthermore, another study on muscle fat infiltration in the cervical multifidus showed that the fat infiltration value tended to increase from cervical vertebral level C5 to C3 (46). Therefore, despite the lack of FSF data on paraspinal muscle in other spinal regions, we believe that fat infiltration is relatively high for the proximal and distal portions of each paraspinal muscle and gradually decreases toward the middle section. It will be interesting to see whether subsequent studies bear out this speculation.
Previous studies have suggested there to be ethnic differences in paraspinal muscle fat infiltration (20,21). The degree of paraspinal muscle fat infiltration among Asian individuals is more severe than among White individuals (20). Moreover, Asians also have a more rapid progression rate of paraspinal muscle fat infiltration even when compared to White individuals of the same age group, level, and muscle (21). Thus, the recognition of ethnic differences in paraspinal muscle fat infiltration might be of clinical importance, as this might help prevent or reduce the severity of paraspinal muscle fat infiltration among Asians. However, the reasons for the fat infiltration differences between ethnicities, particularly the pathophysiologic mechanism, remain poorly understood. It was previously reported that Asians have a higher percentage of body fat, greater visceral adiposity, and a stronger tendency toward the predominance of insulin resistance than do White individuals (47,48). In our view, these differences are worth considering in the future and may have the potential to identify the source of ethnic differences in paraspinal muscle fat infiltration.
Measuring the CSA of fat and water signal within each lumbar cross-sectional MRI slice is considered as the recommended method to assess whole-level FSFTotal (17,23,49). However, this is a cumbersome and downright inefficient process. In contrast, estimating the whole-level FSFTotal from single-lumbar-level cross-sectional MRI slices may be an attractive alternative measurement protocol (49). However, although several studies have previously demonstrated that L4 can represent overall muscle degeneration by using Pearson correlation analysis (17,22), we considered the real problem in the literature not to be a shortage of the most appropriate level but rather a lack of a suitable identification method. In statistics, the Pearson correlation coefficient only measures the strength of the linear dependence between two variables. A high correlation or association does not always imply excellent agreement (50). In fact, apart from the Pearson correlation coefficient, we also needed to gain insight into how similar the data are between the two datasets. Notably, in a recent study investigating total macroscopic fat infiltration in children from 1 to 18 years old, the agreement between datasets was well appreciated (51). As a result, we used a new approach that combines Pearson correlation coefficients with ICC in this study. Interestingly, L4 still remained the optimal level to represent the extent of whole-level fat infiltration. Although the results of our study are consistent with those reported previously, we believe that our results, accounting for both correlation and agreement, are more robust than are those from previous studies.
We consider that the present Goutallier classification has several drawbacks. First, the Goutallier classification is a visual grading system and depends on the experience of the assessor (27). Therefore, a degree of subjectivity is inevitably present (28). Second, this classification has the inherent disadvantages of qualitative measurements. The lack of quantitative data and a cutoff value often introduces measurement error, leading to difficulties in grading (29). Third, previous studies have suggested that several factors, including age, sex, and muscle, could greatly influence paraspinal muscle fat infiltration (17). As a result, this classification may be oversimplified since it is not adjusted for these factors, which may underestimate or overestimate the true severity of fat infiltration. Finally, this classification does not detail the specific disc level used to assess paraspinal muscle fat infiltration, resulting in the level selected in different studies being quite confusing (49).
Therefore, a definition of threshold and standardization of assessment methods is a critical issue for the identification of pathological paraspinal muscle fat infiltration. Since slight fat infiltration of the paraspinal muscle can also be observed in adults and even in adolescents (52), we used the 95th percentile threshold to define pathological paraspinal muscle fat infiltration. Specifically, only 5% of the asymptomatic Chinese adults were considered to have pathological fat infiltration. In fact, the 95th percentile is a commonly used criteria for many diseases, such as hypertension and obesity (38,53). It is able to strike a balance between false positives and false negatives and make study results easily comparable (54). In addition, the use of the 95th percentile is generally considered a reasonable choice, as it can effectively distinguish pathological conditions from normal variants while avoiding overdiagnosis (55). On the other hand, as our data showed that the FSF values could vary based on age, sex, and level, they needed to be standardized to ensure accuracy at thresholds. Based on these considerations, we defined the pathological paraspinal muscle fat infiltration as FSF values ≥95th percentile, stratified by age and sex at the L4 level. These new and standardized identification thresholds have important implications for researchers planning future studies and for clinicians devising better screening or individualized rehabilitative strategies for patients in this population. Furthermore, we observed that the PM-based identification threshold is a fixed value that does not appear to be moderated by either age or sex (Table 4). More specifically, direct measurements of the FSFPM at the L4 level may enhance the identification efficiency of pathological paraspinal muscle fat infiltration since there is no requirement to stratify patients on the basis of age or sex. Overall, the unique stable characteristics that PM exhibits perhaps make it a promising muscle for identifying pathological paraspinal muscle fat infiltration. However, in addition to stability, an ideal diagnostic marker should be reliable and reproducible (56). Thus, a larger cohort of patients should be used to assess the clinical potential of this candidate muscle in future research.
Some limitations in our study should be mentioned. First, the purpose of our study was not to provide a diagnostic tool for daily clinical practice but to present a detailed benchmark reference and an interesting starting point for subsequent studies. Consequently, despite its potential scientific value, our method is more complicated and time-consuming compared with the Goutallier classification. Future studies are needed to simplify our method, by perhaps developing an efficient deep learning-based segmentation system, to achieve the appropriate balance of clinical practice and research. Second, we only assessed the lumbar segments of asymptomatic Chinese adults aged 20–69 years. Indeed, there is some evidence suggesting that paraspinal muscle fat infiltration could be even higher in populations with spinal disease (57). Moreover, an ultrasound-based study revealed that fat infiltration might be influenced by the location of muscle, with different fat infiltration patterns existing among different muscle locations (40). In light of these reports, caution is needed when generalizing the study results with other populations, ages, or spinal segments. Third, the ODI scores were self-reported and thus may be subject to some degree of erroneous reporting and misclassification. Fourth, although we carefully adjusted for several important confounders, we cannot rule out that unmeasured and/or unknown confounders might have impacted results. Fifth, it should be noted that while we found the L4 level to be the optimal level to reflect the extent of whole-level fat infiltration, the FSF values at this level are not equivalent to those at the whole level. Additionally, the exact location of each MRI slice at the L4 level may not be anatomically identical across individuals. In the future, we will also have to increase our efforts to develop an accurate and reliable computational model for predicting whole-level fat infiltration. Finally, although our findings may provide a useful reference for future research in this field and highlight the potential for further investigation into the pathological mechanisms of paraspinal muscle fat infiltration, the definition of a threshold is rather inconsistent across studies. Moreover, the clinical applicability of the new identification thresholds remains to be decided. Therefore, additional longer-term follow-up studies on larger populations are necessary to determine whether the threshold defined in our study, the 95th percentile, is truly rational.
Conclusions
Paraspinal muscle fat infiltration can be affected by age, sex, muscle type, and location among asymptomatic adults aged 20–69 years in China. Middle-aged and older adults, females, extensor muscles, and the fixed ends of muscle tend to display a higher value of FSF. Our data suggest that L4 is the best compromise level to assess whole-level fat infiltration. This study provides the first data concerning identification thresholds of pathological paraspinal muscle fat infiltration. These findings could lay the groundwork for subsequent paraspinal muscle fat infiltration studies.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was funded by the Beijing Municipal Science and Technology Commission (Nos. Z191100007619036 and L192046) and the Key medical Disciplines/Schools of Shijingshan District.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of the Beijing Chaoyang Hospital (institutional review board ethics approval No. 2022-KE-509) and registered in the Chinese Clinical Trial Registry (registration No. ChiCTR2200059380). Study participation was voluntary, and written informed consent was acquired from all participants.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-1131/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-1131/coif). The authors have no conflicts of interest to declare.
References
- 1.Ignasiak D, Valenzuela W, Reyes M, et al. The effect of muscle ageing and sarcopenia on spinal segmental loads. Eur Spine J 2018;27:2650-9. 10.1007/s00586-018-5729-3 [DOI] [PubMed] [Google Scholar]
- 2.Jun HS, Kim JH, Ahn JH, et al. The Effect of Lumbar Spinal Muscle on Spinal Sagittal Alignment: Evaluating Muscle Quantity and Quality. Neurosurgery 2016;79:847-55. 10.1227/NEU.0000000000001269 [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Jia R, Li J, et al. Research Progress on the Mechanism of Lumbarmultifidus Injury and Degeneration. Oxid Med Cell Longev 2021;2021:6629037. 10.1155/2021/6629037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front Endocrinol (Lausanne) 2016;7:69. 10.3389/fendo.2016.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teichtahl AJ, Urquhart DM, Wang Y, et al. Fat infiltration of paraspinal muscles is associated with low back pain, disability, and structural abnormalities in community-based adults. Spine J 2015;15:1593-601. 10.1016/j.spinee.2015.03.039 [DOI] [PubMed] [Google Scholar]
- 6.Stevens S, Agten A, Timmermans A, et al. Unilateral changes of the multifidus in persons with lumbar disc herniation: a systematic review and meta-analysis. Spine J 2020;20:1573-85. 10.1016/j.spinee.2020.04.007 [DOI] [PubMed] [Google Scholar]
- 7.Chen YY, Pao JL, Liaw CK, et al. Image changes of paraspinal muscles and clinical correlations in patients with unilateral lumbar spinal stenosis. Eur Spine J 2014;23:999-1006. 10.1007/s00586-013-3148-z [DOI] [PubMed] [Google Scholar]
- 8.Shafaq N, Suzuki A, Matsumura A, et al. Asymmetric degeneration of paravertebral muscles in patients with degenerative lumbar scoliosis. Spine (Phila Pa 1976) 2012;37:1398-406. 10.1097/BRS.0b013e31824c767e [DOI] [PubMed] [Google Scholar]
- 9.Gengyu H, Jinyue D, Chunjie G, et al. The predictive value of preoperative paraspinal muscle morphometry on complications after lumbar surgery: a systematic review. Eur Spine J 2022;31:364-79. 10.1007/s00586-021-07052-3 [DOI] [PubMed] [Google Scholar]
- 10.Taaffe DR, Henwood TR, Nalls MA, et al. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology 2009;55:217-23. 10.1159/000182084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol (1985) 2008;105:1498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2005;60:882-7. 10.1093/gerona/60.7.882 [DOI] [PubMed] [Google Scholar]
- 13.Leng J, Han G, Zeng Y, et al. The Effect of Paraspinal Muscle Degeneration on Distal Pedicle Screw Loosening Following Corrective Surgery for Degenerative Lumbar Scoliosis. Spine (Phila Pa 1976) 2020;45:590-8. 10.1097/BRS.0000000000003336 [DOI] [PubMed] [Google Scholar]
- 14.Wesselink EO, Pool JJM, Mollema J, et al. Is fatty infiltration in paraspinal muscles reversible with exercise in people with low back pain? A systematic review. Eur Spine J 2023;32:787-96. 10.1007/s00586-022-07471-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahlqvist JR, Vissing CR, Hedermann G, et al. Fat Replacement of Paraspinal Muscles with Aging in Healthy Adults. Med Sci Sports Exerc 2017;49:595-601. 10.1249/MSS.0000000000001119 [DOI] [PubMed] [Google Scholar]
- 16.Crawford RJ, Volken T, Valentin S, et al. Rate of lumbar paravertebral muscle fat infiltration versus spinal degeneration in asymptomatic populations: an age-aggregated cross-sectional simulation study. Scoliosis Spinal Disord 2016;11:21. 10.1186/s13013-016-0080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford RJ, Filli L, Elliott JM, et al. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. AJNR Am J Neuroradiol 2016;37:742-8. 10.3174/ajnr.A4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry DB, Rodriguez-Soto AE, Englund EK, et al. Multiparametric MRI characterization of level dependent differences in lumbar muscle size, quality, and microstructure. JOR Spine 2020;3:e1079. 10.1002/jsp2.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khil EK, Choi JA, Hwang E, et al. Paraspinal back muscles in asymptomatic volunteers: quantitative and qualitative analysis using computed tomography (CT) and magnetic resonance imaging (MRI). BMC Musculoskelet Disord 2020;21:403. 10.1186/s12891-020-03432-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hida T, Eastlack RK, Kanemura T, et al. Effect of race, age, and gender on lumbar muscle volume and fat infiltration in the degenerative spine. J Orthop Sci 2021;26:69-74. 10.1016/j.jos.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 21.Crawford RJ, Elliott JM, Volken T. Change in fatty infiltration of lumbar multifidus, erector spinae, and psoas muscles in asymptomatic adults of Asian or Caucasian ethnicities. Eur Spine J 2017;26:3059-67. 10.1007/s00586-017-5212-6 [DOI] [PubMed] [Google Scholar]
- 22.Hebert JJ, Kjaer P, Fritz JM, et al. The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: a 9-year population-based prospective cohort study. Spine (Phila Pa 1976) 2014;39:1417-25. 10.1097/BRS.0000000000000424 [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Park SW, Kim YB, et al. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J 2017;17:81-7. 10.1016/j.spinee.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Hasebe Y, Yamamoto M, et al. Risk Factor Analysis for Fat Infiltration in the Lumbar Paraspinal Muscles in Patients With Lumbar Degenerative Diseases. Geriatr Orthop Surg Rehabil 2022;13:21514593211070688. 10.1177/21514593211070688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goutallier D, Postel JM, Bernageau J, et al. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994;(304):78-83. [PubMed] [Google Scholar]
- 26.Gladstone JN, Bishop JY, Lo IK, et al. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007;35:719-28. 10.1177/0363546506297539 [DOI] [PubMed] [Google Scholar]
- 27.Oh JH, Kim SH, Choi JA, et al. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res 2010;468:1558-64. 10.1007/s11999-009-0818-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somerson JS, Hsu JE, Gorbaty JD, et al. Classifications in Brief: Goutallier Classification of Fatty Infiltration of the Rotator Cuff Musculature. Clin Orthop Relat Res 2016;474:1328-32. 10.1007/s11999-015-4630-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandelli F, Nüesch C, Zhang Y, et al. Assessing Fatty Infiltration of Paraspinal Muscles in Patients With Lumbar Spinal Stenosis: Goutallier Classification and Quantitative MRI Measurements. Front Neurol 2021;12:656487. 10.3389/fneur.2021.656487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nonclercq O, Berquin A. Predicting chronicity in acute back pain: validation of a French translation of the Orebro Musculoskeletal Pain Screening Questionnaire. Ann Phys Rehabil Med 2012;55:263-78. 10.1016/j.rehab.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 31.Si F, Yuan S, Zang L, et al. Paraspinal Muscle Degeneration: A Potential Risk Factor for New Vertebral Compression Fractures After Percutaneous Kyphoplasty. Clin Interv Aging 2022;17:1237-48. 10.2147/CIA.S374857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fortin M, Omidyeganeh M, Battié MC, et al. Evaluation of an automated thresholding algorithm for the quantification of paraspinal muscle composition from MRI images. Biomed Eng Online 2017;16:61. 10.1186/s12938-017-0350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergouignan L, Chupin M, Czechowska Y, et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage 2009;45:29-37. 10.1016/j.neuroimage.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 34.Otsu N. A threshold selection method from gray-level histograms. IEEE Transactions on Systems, Man, and Cybernetics 1979;9:62-6. 10.1109/TSMC.1979.4310076 [DOI] [Google Scholar]
- 35.Luo TL, Eisenberg MC, Hayashi MAL, et al. A Sensitive Thresholding Method for Confocal Laser Scanning Microscope Image Stacks of Microbial Biofilms. Sci Rep 2018;8:13013. 10.1038/s41598-018-31012-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacher S, Hajdu SD, Maeder Y, et al. Differentiation between benign and malignant vertebral compression fractures using qualitative and quantitative analysis of a single fast spin echo T2-weighted Dixon sequence. Eur Radiol 2021;31:9418-27. 10.1007/s00330-021-07947-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schweitzer L, Geisler C, Pourhassan M, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr 2015;102:58-65. 10.3945/ajcn.115.111203 [DOI] [PubMed] [Google Scholar]
- 38.Dong Y, Ma J, Song Y, et al. National Blood Pressure Reference for Chinese Han Children and Adolescents Aged 7 to 17 Years. Hypertension 2017;70:897-906. 10.1161/HYPERTENSIONAHA.117.09983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masaki M, Ikezoe T, Fukumoto Y, et al. Association of sagittal spinal alignment with thickness and echo intensity of lumbar back muscles in middle-aged and elderly women. Arch Gerontol Geriatr 2015;61:197-201. 10.1016/j.archger.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 40.Yoshiko A, Kaji T, Sugiyama H, et al. Muscle quality characteristics of muscles in the thigh, upper arm and lower back in elderly men and women. Eur J Appl Physiol 2018;118:1385-95. 10.1007/s00421-018-3870-7 [DOI] [PubMed] [Google Scholar]
- 41.Link JC, Reue K. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu Rev Nutr 2017;37:225-45. 10.1146/annurev-nutr-071816-064827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urrutia J, Besa P, Lobos D, et al. Is a single-level measurement of paraspinal muscle fat infiltration and cross-sectional area representative of the entire lumbar spine? Skeletal Radiol 2018;47:939-45. 10.1007/s00256-018-2902-z [DOI] [PubMed] [Google Scholar]
- 43.Sander D, Tian Z, Kirschner J. Cantilever measurements of surface stress, surface reconstruction, film stress and magnetoelastic stress of monolayers. Sensors (Basel) 2008;8:4466-86. 10.3390/s8074466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhury S, Dines JS, Delos D, et al. Role of fatty infiltration in the pathophysiology and outcomes of rotator cuff tears. Arthritis Care Res (Hoboken) 2012;64:76-82. 10.1002/acr.20552 [DOI] [PubMed] [Google Scholar]
- 45.Greve T, Burian E, Zoffl A, et al. Regional variation of thigh muscle fat infiltration in patients with neuromuscular diseases compared to healthy controls. Quant Imaging Med Surg 2021;11:2610-21. 10.21037/qims-20-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbott R, Pedler A, Sterling M, et al. The geography of fatty infiltrates within the cervical multifidus and semispinalis cervicis in individuals with chronic whiplash-associated disorders. J Orthop Sports Phys Ther 2015;45:281-8. 10.2519/jospt.2015.5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kodama K, Tojjar D, Yamada S, et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care 2013;36:1789-96. 10.2337/dc12-1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim S, Choi SH, Park YJ, et al. Visceral fatness and insulin sensitivity in women with a previous history of gestational diabetes mellitus. Diabetes Care 2007;30:348-53. 10.2337/dc06-1405 [DOI] [PubMed] [Google Scholar]
- 49.Han G, Jiang Y, Zhang B, et al. Imaging Evaluation of Fat Infiltration in Paraspinal Muscles on MRI: A Systematic Review with a Focus on Methodology. Orthop Surg 2021;13:1141-8. 10.1111/os.12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. 10.1016/S0140-6736(86)90837-8 [DOI] [PubMed] [Google Scholar]
- 51.Marunowski K, Świętoń D, Bzyl W, et al. Reference values for MRI-derived psoas and paraspinal muscles and macroscopic fat infiltrations in paraspinal muscles in children. J Cachexia Sarcopenia Muscle 2022;13:2515-24. 10.1002/jcsm.13049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kjaer P, Bendix T, Sorensen JS, et al. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med 2007;5:2. 10.1186/1741-7015-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul IM, Savage JS, Anzman-Frasca S, et al. Effect of a Responsive Parenting Educational Intervention on Childhood Weight Outcomes at 3 Years of Age: The INSIGHT Randomized Clinical Trial. JAMA 2018;320:461-8. 10.1001/jama.2018.9432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phung D, Thai PK, Guo Y, et al. Ambient temperature and risk of cardiovascular hospitalization: An updated systematic review and meta-analysis. Sci Total Environ 2016;550:1084-102. 10.1016/j.scitotenv.2016.01.154 [DOI] [PubMed] [Google Scholar]
- 55.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137-47. 10.7326/0003-4819-139-2-200307150-00013 [DOI] [PubMed] [Google Scholar]
- 56.Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology 2008;70:1827-35. 10.1212/01.wnl.0000311445.21321.fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elliott J, Jull G, Noteboom JT, et al. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976) 2006;31:E847-55. 10.1097/01.brs.0000240841.07050.34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as