Abstract
Herbal medicine has been widely applied for a range of diseases in China since antiquity. Cassia obtusifolia L. and Cassia tora L. are plants whose seeds have high reported medicinal values and have been documented to function as a laxative, to lower lipid level and to lower blood pressure. The main active ingredient in Cassia seeds is aurantio-obtusin (AO), which is an anthraquinone monomer compound. Currently, AO is listed in China as a quality control index component of Cassia seeds. In clinical practice in China, AO is typically used to treat obesity, diabetes and its complications, non-alcoholic fatty liver disease and allergic reactions. In addition, AO has been reported to confer insecticidal activities and antimalarial effects. Previous studies have even suggested that AO is a potential therapeutic candidate for a variety of diseases with research value. Therefore, the present review summarizes and discuss the existing literature on AO to provide a review of its pharmacological activity and mechanism of action, with the aim of providing a basis for its development and utilization in a clinical setting.
Keywords: aurantio-obtusin, traditional Chinese medicine, pharmacological activity, mechanism of action
1. Introduction
Cassia seeds are the dried mature seeds of Cassia obtusifolia L. or Cassia tora L., which belongs to the Leguminosae family (1). The seeds are widely used in China, Japan and Korea for improving visual acuity, and having laxative, antioxidant, neuroprotective and anti-bacterial effects, in addition to lowering the blood pressure (2-5). Cassia seeds contain anthraquinones, naphthopyrrolidones and fatty acids, with anthraquinone being the main active ingredient. Anthraquinones have a planar and rigid anthracene ring in the 9th and 10th positions, with two ketone groups, so that this structure allows the absorption of light at specific wavelengths (6) (Fig. 1), which also have reported anticancer, antitumor, antioxidant and antimalarial biological activities (7-10). The main anthraquinones in Cassia seeds include aurantio-obtusin (AO), chrysophanol, emodin and rhein (11).
Figure 1.

Chemical structure of anthraquinone.
As a lipophilic anthraquinone compound extracted from Cassia seeds, AO is the main bioactive component of Cassia seeds and is currently listed as a quality control index component of Cassia seeds in the Pharmacopoeia of the People's Republic of China (5). The Pharmacopoeia of the People's Republic of China stipulates that Cassia seeds should contain not less than 0.080% of AO on a dried basis. AO is also known as 1,3,7-trihydroxy-2,8-dimethoxy-6-methyl-9,10-anthracenedione (Fig. 2) and has a variety of documented pharmacological effects, including anti-hyperlipidemic (12,13), and anti-inflammatory effects (14,15) This renders AO to be a potential candidate for the treatment of various diseases. However, to the best of our knowledge, there are no systematic reviews on this topic at present. Therefore, the present paper reviewed and discussed the relevant literature on AO and its pharmacological activity.
Figure 2.

Chemical structure of aurantio-obtusin.
2. Pharmacological activity
Treatment of obesity
Obesity is a condition in which adipose tissues accumulates excessively in the body to an extent that it exerts detrimental effects on health (16). It is characterized by weight gain, which is caused by excessive fat accumulation due to excessive daily food intake and insufficient calorific expenditure (17). Obesity increases the risk of coronary artery disease (18), hypertension (19), type 2 diabetes (20), asthma (21), cancer (17), venous thromboembolism (22), periodontal disease (23) and Coronavirus disease 2019 (Covid-19) (24,25).
For the treatment of obesity, reducing daily intake whilst increasing daily calorific expenditure and increasing the metabolic rate in the body can confer a significant effects. In a previous study on the effects of AO on obesity, hepatic lipid metabolism and insulin sensitivity using high-fat diet-induced obese mice, AO was found to significantly reduce body weight and inhibit lipid accumulation in the liver and the white adipose tissue (WAT) (26). The mechanism of action was found to be mainly due to AO increasing peroxisome proliferator-activated receptor (PPAR)-α mRNA expression and decreasing PPAR-γ mRNA expression in the liver. PPAR-α expression can inhibit triglyceride synthesis and promote fatty acid oxidation (27) in another study, while decreasing PPAR-γ expression can reduce the differentiation of preadipocytes into adipocytes to decrease fatty acid storage (28). This suggests to a certain extent the inhibitory effects AO can exert against obesity.
Treatment of diabetic complications
Diabetes is a condition in which the combination of genetic and environmental factors contributes to either absolute or relative insulin deficiency and reduced insulin sensitivity in target tissue cells (29). This results in metabolic disorders in the body, which are characterized by hyperglycemia (29). Diabetic complications caused by the prolonged exposure to hyperglycemic conditions are the main causes of organ dysfunction and even mortality in patients with diabetes (30). Diabetic complications can affect almost all organs of the body, including the nervous system, heart, kidney, eyes and blood vessels (29), which can be classified as macroangiopathy and microangiopathy. Macroangiopathy includes cardiovascular and heart disease, whereas microangiopathy includes diabetic nephropathy, cataract and retinopathy (31,32). Diabetes has also been reported to predispose patients to the more severe forms of Covid-19, which increases the risk of poorer prognosis (33). Advanced glycation end products (AGEs) and aldose reductase (AR) are two important contributing components to the complications of diabetes (34). Therefore, AR inhibitors have been proposed to be a viable option for the treatment of diabetes mellitus. In the AGE formation and rat lens aldose reductase (RLAR) inhibition assay (35), AO showed no inhibitory activity on AGE formation, but showed significant inhibitory activity against RLAR with an IC50 value of 13.6 µM, suggesting that AO can exert inhibitory effects against AR. In addition, it was found in another study that AO can activate the insulin signaling pathway to increase sensitivity to insulin whilst also improving obesity (26). Therefore, AO is a potential candidate for the treatment of diabetic complications and associated diseases.
Reduction of non-alcoholic fatty liver disease (NAFLD)
NAFLD is defined as a disease caused by excessive hepatic adipose accumulation associated with insulin resistance (IR) (36). It is a general term used for a range of diseases with histological hepatic alterations, including simple hepatic steatosis, non-alcoholic hepatitis characterized by hepatocellular damage with inflammation and varying degrees of fibrosis, cirrhosis and hepatocellular carcinoma (37,38). IR is therefore a key factor in the pathogenesis of NAFLD. It can, on the one hand, lead to lipolysis in adipose tissue, thus providing free fatty acids to the liver, and on the other hand, it can promote de novo synthesis, leading to further accumulation of fatty acids in the liver (39). However, the specific mechanism driving the pathogenesis of NAFLD remains unclear, where the main strategy of treatment is to target the IR and intrahepatic lipid accumulation (40). Under conditions of high ester and high glucose conditions, AO has been found to improve IR by downregulating the mRNA expression of genes associated with lipid metabolism such as PPAR-γ and FAS, whilst suppressing the mRNA expression of inflammatory cytokines such as IL-6, IL-1β, MCP-1 and TNF-α in WAT (26). In addition, in a mouse model of NAFLD induced by high-sugar and high-fat conditions and in oleic and palmitic acid-treated mouse primary hepatocytes, AO was found to significantly promote autophagic flow and activate the transcription factor EB (41). This inhibited ab initio lipid synthesis and suppressed lipid accumulation to improve hepatic steatosis (41). Altogether, this provides a pharmacological treatment avenue for NAFLD and related complications.
Antiallergic effect
Allergy is an acquisitive hypersensitivity by the immune system to harmless environment substances (42). This spectrum includes allergic rhinitis, allergic asthma, food allergies and atopic dermatitis (also known as eczema) (43,44). Immunoglobulin E (IgE) is one of the key drivers of allergic responses (45). Although it is the least abundant antibody in the human serum, it can induce an effective inflammatory immune response in various tissues and organs, whilst also serving as a Th2 biomarker involved in the regulation of Th2 inflammatory responses (46,47). Previous studies on the effects of AO on IgE-mediated allergic responses and lipopolysaccharide (LPS)-induced RAW264.7 cells have found that AO can inhibit the expression of TNF-α and IL-4 mRNA whilst also suppressing the expression of prostaglandin E2 and cyclooxygenase-2 (48,49).
Aryl hydrocarbon receptors (AhRs) is a ligand-activated transcription factor and is present in important signaling pathways in the mammalian immune system (50). In addition, they can regulate the differentiation of monocytes into dendritic cells and that of T cells into regulatory T cells and Th17 cells (51). AhRs also serves an important role in anticancer effects, energy metabolism, immunity and drug metabolism (50,52,53), such that it has been shown that activation of AhRs decreases the immune response; AO exhibits significant AhRs activity and it may be a significant natural AhR agonist (54). It has also been found that the natural plant extract mixture AF-343, obtained from Cassia tora L., Ulmus pumila L. and Taraxacum officinale, is potentially a natural candidate for the prevention and treatment of mast cell-induced allergic diseases, such as allergic inflammation (55,56). Since the natural active compounds of AF-343 also include AO, this suggests the possible benefits of using AO for the treatment of allergy-related disorders.
Treatment of asthma and chronic obstructive pulmonary disease
In 2019, chronic obstructive pulmonary disease (COPD) and asthma were respiratory diseases with high morbidity and mortality rates in China, the United States and other regions (57,58). Airway smooth muscle contraction is one of the causes of both of the aforementioned diseases, rendering bronchodilators to be an effective drug for their treatment (59). In a previous study on the effects of Cassia seeds on airway smooth muscle contraction (60), the ethanolic extract of Cassia seeds have been found to inhibit the contraction of airway smooth muscle by inhibiting voltage-dependent L-type-mediated Ca2+ influx. Further studies have demonstrated that the main component of the ethanol extract of Cassia seeds that can induce the relaxation of airway smooth muscle is AO. Therefore, AO may serve as a viable therapeutic agent for the treatment of asthma and COPD.
Other effects
Mosquitoes are vectors of a number of diseases, such as malaria, dengue fever, dengue shock syndrome and yellow fever (61,62). Therefore, controlling their population can control these aforementioned infectious diseases (63). Generally, control is done at their larval stages because they are more accessible compared with adults, where they are more concentrated and less likely to change their habitat (64). AO has been shown to be effective for controlling the larvae of Anopheles gambiae, with a median lethal dose of 1 mg/ml (65,66). In addition, besides killing this species of mosquitoes, AO can also protect against cowpea weevil beetle infestation, which can be applied as a protective agent for stored cowpea seeds and other crops (67). Furthermore, a previous review of plant-based insecticides from 2000 to 2018 showed that AO can be used as a potential larvicide (68).
In addition, AO showed >15% inhibition on the senescence-associated secretory phenotype (SASP) (69). SASP is a bioactive secretion produced during senescence of cells which can mediate non-cell-autonomous effects of senescence (70,71). Therefore, inhibition of SASP formation may have a role in slowing down cellular senescence. It has also been shown that AO can significantly reduce total serum cholesterol levels, triglyceride and low-density lipoprotein levels in hyperlipidemic rats (12). In Xuezhiling tablets, a Chinese patented medicine used for the treatment of hyperlipidemia, tests have identified AO to be one of the active ingredients, which is associated with the anti-hyperlipidemic effect of Xuezhiling (13). This suggests AO to be a potential drug for the treatment of hyperlipidemia. Bate-site amyloid precursor protein cleaving enzyme-1 (BACE1) inhibitors can significantly reduce the concentration of cerebrospinal fluid amyloid plaques of β-protein and are promising drugs for the treatment of Alzheimer's disease (72). It has been found that AO has a strong inhibitory effect on BACE1 with IC50 values of 50.9-190 µg/ml (73), which suggests that AO has some application in the development of drugs for the prevention and treatment of Alzheimer's disease.
3. Mechanism of action
Actions on vasopressin receptors
Vasopressin (AVP), a nonapeptide, is mainly synthesized in the hypothalamic supraoptic nucleus, paraventricular nucleus and the supraoptic nucleus (74). It can also be produced in other areas of the brain and organs, such as the medial amygdala, the nucleus of the terminal bed and adrenal chromophores (74,75). AVP can only be found in mammals and is involved in the regulation of blood pressure, water and salt balance, social behavior (such as learning and cognition) and regulation of emotion (such as anxiety, fear and depression) (76,77). AVP acts through three different vasopressin receptors (78): V1a, V1b and V2 receptors, all of which belong to different isoforms of G protein-coupled receptors (79). A previous study has demonstrated that V1a is the most abundant and widely distributed vasopressin receptor (80). V1a-knockout mice show a significant reduction in anxious behavior but also severely impaired social cognition performance (81,82). V1a is mainly distributed in the brain and is involved in regulation of emotional and adaptive behaviors, pain, circadian rhythm cortisol synthesis and secretion (82).
In a study that evaluated the functional effects of anthraquinones from Cassia seeds on various G protein-coupled receptors (83), it was previously found that only AO exhibits specific V1a receptor antagonism, with an IC50 value of 67.70±2.41 µM. In further experiments in a C57Bl/6 mouse model of transient cerebral ischemia/reperfusion injury, therapy with AO (10 mg/kg, p.o.) significantly decreased the severity of injury in the cortex regions, medial cornu ammonis 1 and dorsal medial cornu ammonis 1, which indicated a neuroprotective effect of AO. These results emphasize the possible antagonistic effect of AO on the V1a receptor. At present, there have been reports proposing the use of V1a receptor antagonists for the treatment of Raynaud's syndrome, dysmenorrhea, preterm labor, reduction of cell proliferation and bone metastasis growth in desmoplastic refractory prostate cancer in vivo (84-86), which could be used as a potential therapeutic in multiple disease types.
Action on the PI3K/Akt/endothelial nitric oxide synthase (eNOS) signaling pathway in endothelial cells
PI3K/Akt/eNOS is an important regulatory signaling pathway in endothelial cells, and previous studies have also found that activation of the PI3K/Akt/eNOS signaling pathway can not only promote angiogenesis (87,88), improve renal microcirculation (89) and protect endothelial cells from injury (90), but can also suppress diabetes-induced atrial remodeling and atrial fibrillation (91), and can improve cardiac function in rats with myocardial infarction (92). Thus, these data suggest that the PI3K/Akt/eNOS signaling pathway plays an important role in regulating vascular activity. In a study investigating the effects of AO on isolated mesenteric arteries and its mechanism of action, AO was previously found to have an important role in activating the PI3K/Akt/eNOS signaling pathway by phosphorylating Ser473 to activate Akt. This enhanced eNOS activation by phosphorylating Ser1177 and Thr495 to stimulate nitric oxide (NO) production in endothelial cells (93). Therefore, these observations suggest that AO can serve as a potential vasodilator.
Thrombin inhibition
AO is a potent thrombin inhibitor (94) that can readily inhibit this enzyme, with a Ki value of 10.30 µM, which was shown by previous kinetic studies. Docking simulations showed that AO can bind both the catalytic cavity and two anion-binding exosites (ABE) 1 and ABE2. Specifically, the hydroxyl group at the C-7 site and the methoxy group at the C-8 site were found to produce a critical interaction with human thrombin by forming hydrogen bonds (94). Furthermore, it has been previously shown that anthraquinones isolated from Cassia seeds have a thrombin-inhibiting function against thrombin-mediated Z-GGRAMC acetic acid (a thrombin-specific fluorescent substrate for the detection of thrombin production in PRP and platelet-deficient plasma) hydrolysis (95), where further docking experiments revealed that AO has an improved inhibitory effect (94,96). Therefore, AO could potentially be the lead compound for the exploration of novel thrombin inhibitors. They can be used to inhibit thrombus formation and/or vascular embolism, which can reduce the incidence of myocardial infarction, acute ischemic stroke, venous thromboembolism and pulmonary embolism (97-99). AO can be a potential therapeutic alternative for the treatment and prevention of thrombotic diseases.
Actions on the NF-κB signaling pathway
NF-κB has an instrumental role in immune homeostasis and chronic inflammation (100-102). AO has been previously found to exert anti-inflammatory effects by interrupting the activation of MAPK and NF-κB signaling, in addition to suppressing IL-6 generation in the IL-1β-treated lung epithelial A549 cells (14). In the mouse airway inflammation model of LPS-induced acute lung injury, AO exerted an inhibitory effect on the inflammatory response (14). Additionally, AO treatment was able to ameliorate acute lung injury by inactivating the MAPK and NF-κB signaling pathways (15). By studying the effect of AO on the LPS-induced inflammatory response in the mouse macrophage RAW264.7 model, it was also demonstrated that AO can prevent inflammation through inhibition of NF-κB activation (49).
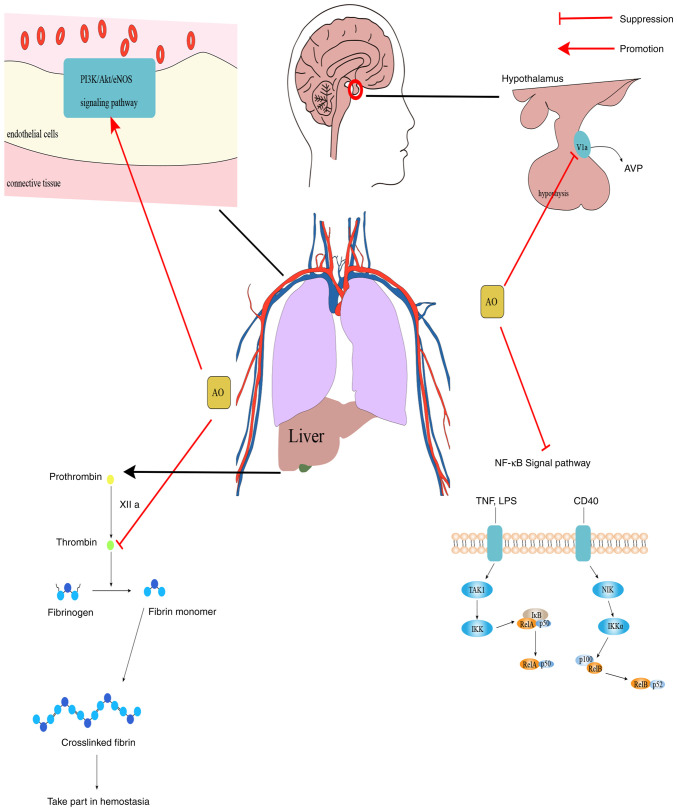
In summary, AO can exert varying degrees of inhibitory effects on vasopressin and thrombin signaling, whilst also conferring agonistic effects on the PI3K/Akt/eNOS and NF-κB signaling pathways (Fig. 3). These can potentially be exploited for the development of therapeutic agents for the corresponding diseases.
Figure 3.
Mechanism diagram of AO. PI3K/Akt/eNOS is a signaling pathway that mainly operates in endothelial cells and serves an important regulatory role in dilating blood vessels and protecting endothelial cells. NF-κB signaling serves a key role in regulating the immune response to infection. AO can activate PI3K/Akt/eNOS and plays an important role in vasodilation. AO can also inhibit NF-κB, thrombin and inhibit V1a, which prevent inflammation, are involved in brain emotion regulation and glycogen decomposition, this can be exploited as a potential therapeutic intervention for the prevention and treatment of thrombotic diseases and neurological diseases. AO, aurantio-obtusin; eNOS, endothelial nitric oxide synthase; V1a, vasopressin receptor; NIK, NF-κB-inducing kinase; TAK1, transforming growth factor-β-activated kinase 1; AVP, vasopressin; LPS, lipopolysaccharide.
4. Biosafety
Anthraquinones generally have some hepatic and renal toxicity, where AO is of no exception (103,104). Whilst examining the effects of oral administration of different doses of AO on hepatotoxicity in rats, it was previously found that medium (40 mg/kg) and high doses (200 mg/kg) of AO can cause liver damage (5). Furthermore, in a study in which Cassia seed aqueous extract was administered orally to rats at doses of 4.37, 15.75 and 47.30 g/kg for 28 days, histopathological changes in the livers of male rats (47.30 g/kg group) and female rats (15.75 and 47.30 g/kg groups) were found; it was demonstrated that the aqueous extract can induce hepatotoxicity in rats, where AO was one of the components that caused the hepatotoxicity (105). AO induces hepatotoxicity by activating the nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain-containing 3 inflammatory vesicle signaling pathway (106), which also causes nephrotoxicity and colorectal melanosis (107). In addition to this, AO may increase the toxicity of certain drugs. AO has been observed to significantly increase the toxicity of irinotecan compared with glucoaurantio-obtusin (108). Therefore, the biosafety of AO should be considered when developing it for the treatment of various diseases in the body.
5. Conclusions
In summary, as one of the main active components of Cassia seeds, AO has certain pharmacological activities and medicinal values that can be explored as a potential drug for various human diseases. However, when considering AO for potential drug development, it cannot be ignored that AO can exert certain hepatic and renal toxicity.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the Project of Science and Technology Department of Sichuan Province (grant no. 2021YJ0445), Foundation of Affiliated Hospital of Southwest Medical University (grant no. 21072) and Innovation and Entrepreneurship Training Program for Students of Southwest Medical University (grant no. 202210632176).
Availability of data and materials
Not applicable.
Authors' contributions
ZW and YL conceived the study. YL and XS designed the study, drafted, reviewed and edited the manuscript, and produced all the figures. XH, YX and TL wrote the manuscript. YL, XS and TL analyzed the relevant literature. All authors read and approved the final manuscript. Data sharing is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lee GY, Cho BO, Shin JY, Jang SI, Cho IS, Kim HY, Park JS, Cho CW, Kang JS, Kim JH, Kim YH. Tyrosinase inhibitory components from the seeds of Cassia tora. Arch Pharm Res. 2018;41:490–496. doi: 10.1007/s12272-018-1032-4. [DOI] [PubMed] [Google Scholar]
- 2.Luo H, Wu H, Wang L, Xiao S, Lu Y, Liu C, Yu X, Zhang X, Wang Z, Tang L. Hepatoprotective effects of Cassiae Semen on mice with non-alcoholic fatty liver disease based on gut microbiota. Commun Biol. 2021;4(1357) doi: 10.1038/s42003-021-02883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YJ, Lee S, Jin J, Woo H, Choi YK, Park KG. Cassiaside C Inhibits M1 polarization of macrophages by downregulating glycolysis. Int J Mol Sci. 2022;23(1696) doi: 10.3390/ijms23031696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang B, Xie L, Peng S, Sun K, Jin J, Zhen Y, Qin K, Cai B. Nine components pharmacokinetic study of rat plasma after oral administration raw and prepared Semen Cassiae in normal and acute liver injury rats. J Sep Sci. 2019;42:2341–2350. doi: 10.1002/jssc.201900007. [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Li J, Tang X, Wang Y, Ma Z, Gao Y. Metabolomics of aurantio-obtusin-induced hepatotoxicity in rats for discovery of potential biomarkers. Molecules. 2019;24(3452) doi: 10.3390/molecules24193452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mugas ML, Calvo G, Marioni J, Céspedes M, Martinez F, Vanzulli S, Sáenz D, Di Venosa G, Nuñez Montoya S, Casas A. Photosensitization of a subcutaneous tumour by the natural anthraquinone parietin and blue light. Sci Rep. 2021;11(23820) doi: 10.1038/s41598-021-03339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou Y, Cao Z, Wang J, Chen X, Chen YQ, Li Y, Liu J, Zhao Y, Wang A, He B. A Series of Novel HDAC inhibitors with anthraquinone as a cap group. Chem Pharm Bull (Tokyo) 2020;68:613–617. doi: 10.1248/cpb.c20-00206. [DOI] [PubMed] [Google Scholar]
- 8.Watroly MN, Sekar M, Fuloria S, Gan SH, Jeyabalan S, Wu YS, Subramaniyan V, Sathasivam KV, Ravi S, Mat Rani NNI, et al. Chemistry, biosynthesis, physicochemical and biological properties of rubiadin: A promising natural anthraquinone for new drug discovery and development. Drug Des Devel Ther. 2021;15:4527–4549. doi: 10.2147/DDDT.S338548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Guo F, Guan Y, Chen T, Ma K, Zhang L, Wang Z, Su Q, Feng L, Liu Y, Zhou Y. Novel anthraquinone compounds inhibit colon cancer cell proliferation via the reactive oxygen Species/JNK Pathway. Molecules. 2020;25(1672) doi: 10.3390/molecules25071672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szymańska M, Majerz I. Effect of substitution of hydrogen atoms in the molecules of anthrone and anthraquinone. Molecules. 2021;26(502) doi: 10.3390/molecules26020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panigrahi GK, Verma N, Singh N, Asthana S, Gupta SK, Tripathi A, Das M. Interaction of anthraquinones of Cassia occidentalis seeds with DNA and Glutathione. Toxicol Rep. 2018;5:164–172. doi: 10.1016/j.toxrep.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang N, Dong N, Pang L, Xu H, Ji H. Quantitative determination and pharmacokinetic study of aurantio-obtusin in rat plasma by liquid chromatography-mass spectrometry. J Chromatogr Sci. 2014;52:1059–1064. doi: 10.1093/chromsci/bmt159. [DOI] [PubMed] [Google Scholar]
- 13.Nie C, Zhang F, Ma X, Guo R, Zhou S, Zhao L, Xu H, Xiao X, Wang Z. Determination of quality markers of Xuezhiling tablet for hyperlipidemia treatment. Phytomedicine. 2018;44:231–238. doi: 10.1016/j.phymed.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Kwon KS, Lee JH, So KS, Park BK, Lim H, Choi JS, Kim HP. Aurantio-obtusin, an anthraquinone from cassiae semen, ameliorates lung inflammatory responses. Phytother Res. 2018;32:1537–1545. doi: 10.1002/ptr.6082. [DOI] [PubMed] [Google Scholar]
- 15.He YQ, Zhou CC, Yu LY, Wang L, Deng JL, Tao YL, Zhang F, Chen WS. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol Res. 2021;163(105224) doi: 10.1016/j.phrs.2020.105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137(111315) doi: 10.1016/j.biopha.2021.111315. [DOI] [PubMed] [Google Scholar]
- 17.Munafò A, Frara S, Perico N, Di Mauro R, Cortinovis M, Burgaletto C, Cantarella G, Remuzzi G, Giustina A, Bernardini R. In search of an ideal drug for safer treatment of obesity: The false promise of pseudoephedrine. Rev Endocr Metab Disord. 2021;22:1013–1025. doi: 10.1007/s11154-021-09658-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stadler JT, Marsche G. Obesity-Related changes in high-density lipoprotein metabolism and function. Int J Mol Sci. 2020;21(8985) doi: 10.3390/ijms21238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mongioì LM, La Vignera S, Cannarella R, Cimino L, Compagnone M, Condorelli RA, Calogero AE. The role of resveratrol administration in human obesity. Int J Mol Sci. 2021;22(4362) doi: 10.3390/ijms22094362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayoral LP, Andrade GM, Mayoral EP, Huerta TH, Canseco SP, Rodal Canales FJ, Cabrera-Fuentes HA, Cruz MM, Pérez Santiago AD, Alpuche JJ, et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J Med Res. 2020;151:11–21. doi: 10.4103/ijmr.IJMR_1768_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuder MM, Nyenhuis SM. Optimizing lifestyle interventions in adult patients with comorbid asthma and obesity. Ther Adv Respir Dis. 2020;14(1753466620906323) doi: 10.1177/1753466620906323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purdy JC, Shatzel JJ. The hematologic consequences of obesity. Eur J Haematol. 2021;106:306–319. doi: 10.1111/ejh.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganesan SM, Vazana S, Stuhr S. Waistline to the gumline: Relationship between obesity and periodontal disease-biological and management considerations. Periodontol 2000. 2021;87:299–314. doi: 10.1111/prd.12390. [DOI] [PubMed] [Google Scholar]
- 24.Landecho MF, Marin-Oto M, Recalde-Zamacona B, Bilbao I, Frühbeck G. Obesity as an adipose tissue dysfunction disease and a risk factor for infections-Covid-19 as a case study. Eur J Intern Med. 2021;91:3–9. doi: 10.1016/j.ejim.2021.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gammone MA, D'Orazio N. COVID-19 and Obesity: Overlapping of two pandemics. Obes Facts. 2021;14:579–585. doi: 10.1159/000518386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo CY, Liao WT, Qiu RJ, Zhou DS, Ni WJ, Yu CP, Zeng Y. Aurantio-obtusin improves obesity and insulin resistance induced by high-fat diet in obese mice. Phytother Res. 2021;35:346–360. doi: 10.1002/ptr.6805. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita S, Masuda D, Matsuzawa Y. Pemafibrate, a new selective PPARα modulator: Drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr Atheroscler Rep. 2020;22(5) doi: 10.1007/s11883-020-0823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciavarella C, Motta I, Valente S, Pasquinelli G. Pharmacological (or Synthetic) and nutritional agonists of PPAR-γ as candidates for cytokine storm modulation in COVID-19 disease. Molecules. 2020;25(2076) doi: 10.3390/molecules25092076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prabhakar PK. Pathophysiology of diabetic secondary complication and their management. Curr Diabetes Rev. 2021;17:395–396. doi: 10.2174/157339981704210326092455. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Shen S, Cui Z, Nie H, Han D, Yan H. Screening and isolating major aldose reductase inhibitors from the seeds of evening primrose (Oenothera biennis) Molecules. 2019;24(2709) doi: 10.3390/molecules24152709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Julius A, Renuka RR, Hopper W, Babu Raghu P, Rajendran S, Srinivasan S, Dharmalingam K, Alanazi AM, Arokiyaraj S, Prasath S. Inhibition of aldose reductase by novel phytocompounds: A heuristic approach to treating diabetic retinopathy. Evid Based Complement Alternat Med. 2022;2022(9624118) doi: 10.1155/2022/9624118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thakur S, Gupta SK, Ali V, Singh P, Verma M. Aldose Reductase: A cause and a potential target for the treatment of diabetic complications. Arch Pharm Res. 2021;44:655–667. doi: 10.1007/s12272-021-01343-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19) Diabetes Metab Res Rev. 2021;37(e3377) doi: 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodda D, Rama Rao A, Veeresham C. In vitro and in vivo evaluation of pterostilbene for the management of diabetic complications. J Ayurveda Integr Med. 2020;11:369–375. doi: 10.1016/j.jaim.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang DS, Lee GY, Kim YS, Lee YM, Kim CS, Yoo JL, Kim JS. Anthraquinones from the seeds of Cassia tora with inhibitory activity on protein glycation and aldose reductase. Biol Pharm Bull. 2007;30:2207–2210. doi: 10.1248/bpb.30.2207. [DOI] [PubMed] [Google Scholar]
- 36.Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Implications for liver transplantation. Transplantation. 2019;103:22–27. doi: 10.1097/TP.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 37.Arrese M, Arab JP, Barrera F, Kaufmann B, Valenti L, Feldstein AE. Insights into nonalcoholic fatty-liver disease heterogeneity. Semin Liver Dis. 2021;41:421–434. doi: 10.1055/s-0041-1730927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. American Family Physician: Nonalcoholic Fatty Liver Disease. https://www.aafp.org/pubs/afp/issues/2020/1115/p603-s1.html. Accessed April 2, 2023. [Google Scholar]
- 39.Makri E, Goulas A, Polyzos SA. Epidemiology, pathogenesis, diagnosis and emerging treatment of nonalcoholic fatty liver disease. Arch Med Res. 2021;52:25–37. doi: 10.1016/j.arcmed.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou F, Ding M, Gu Y, Fan G, Liu C, Li Y, Sun R, Wu J, Li J, Xue X, et al. Aurantio-Obtusin attenuates non-alcoholic fatty liver disease through AMPK-Mediated autophagy and fatty acid oxidation pathways. Front Pharmacol. 2021;12(826628) doi: 10.3389/fphar.2021.826628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu T, Dong Y, Yang C, Zhao M, He Q. Pathogenesis of children's allergic diseases: Refocusing the role of the gut microbiota. Front Physiol. 2021;12(749544) doi: 10.3389/fphys.2021.749544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Y, Chen CL, Yu D, Liu Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy. 2021;76:456–470. doi: 10.1111/all.14639. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen SMT, Rupprecht CP, Haque A, Pattanaik D, Yusin J, Krishnaswamy G. Mechanisms governing anaphylaxis: Inflammatory cells, mediators, endothelial gap junctions and beyond. Int J Mol Sci. 2021;22(7785) doi: 10.3390/ijms22157785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu-De Z, Bei-Bei G, Xi-Juan W, Hai-Bo L, Li-Li Z, Feng-Xia L. Serum IgE Predicts difference of population and allergens in allergic diseases: Data from Weifang City, China. Mediators Inflamm. 2021;2021(6627087) doi: 10.1155/2021/6627087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zellweger F, Eggel A. IgE-associated allergic disorders: Recent advances in etiology, diagnosis, and treatment. Allergy. 2016;71:1652–1661. doi: 10.1111/all.13059. [DOI] [PubMed] [Google Scholar]
- 48.Kim M, Lim SJ, Lee HJ, Nho CW. Cassia tora seed extract and its active compound aurantio-obtusin inhibit allergic responses in IgE-Mediated mast cells and anaphylactic models. J Agric Food Chem. 2015;63:9037–9046. doi: 10.1021/acs.jafc.5b03836. [DOI] [PubMed] [Google Scholar]
- 49.Hou J, Gu Y, Zhao S, Huo M, Wang S, Zhang Y, Qiao Y, Li X. Anti-Inflammatory effects of aurantio-obtusin from seed of cassia obtusifolia L. through Modulation of the NF-κB pathway. Molecules. 2018;23(3093) doi: 10.3390/molecules23123093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song JY, Casanova-Nakayama A, Möller AM, Kitamura SI, Nakayama K, Segner H. Aryl hydrocarbon receptor signaling is functional in immune cells of rainbow trout (Oncorhynchus mykiss) Int J Mol Sci. 2020;21(6323) doi: 10.3390/ijms21176323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Disner GR, Lopes-Ferreira M, Lima C. Where the Aryl hydrocarbon receptor meets the microRNAs: Literature review of the last 10 years. Front Mol Biosci. 2021;8(725044) doi: 10.3389/fmolb.2021.725044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamashita N, Kanno Y, Yoshikawa M, Ozawa M, Sanada N, Nemoto K, Kizu R. Polycyclic aromatic hydrocarbons induce CYP3A5 gene expression via aryl hydrocarbon receptor in HepG2 cells. J Toxicol Sci. 2021;46:25–29. doi: 10.2131/jts.46.25. [DOI] [PubMed] [Google Scholar]
- 53.Vogel CFA, Van Winkle LS, Esser C, Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors-Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34(101530) doi: 10.1016/j.redox.2020.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amakura Y, Yoshimura M, Takaoka M, Toda H, Tsutsumi T, Matsuda R, Teshima R, Nakamura M, Handa H, Yoshida T. Characterization of natural aryl hydrocarbon receptor agonists from cassia seed and rosemary. Molecules. 2014;19:4956–4966. doi: 10.3390/molecules19044956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu H, Bin NR, Sugita S. Diverse exocytic pathways for mast cell mediators. Biochem Soc Trans. 2018;46:235–247. doi: 10.1042/BST20170450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee EK, Song J, Seo Y, Koh EM, Kim SH, Jung KJ. Inhibitory Effects of AF-343, a Mixture of Cassia tora L., Ulmus pumila L., and Taraxacum officinale, on Compound 48/80-Mediated Allergic Responses in RBL-2H3 Cells. Molecules. 2020;25(2434) doi: 10.3390/molecules25102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. WHO: Global health estimates:leading causes of death. Cause specific mortality 2000-2019. WHO, Genova, Switzerland, 2019. [Google Scholar]
- 58.Dumitrache MD, Jieanu AS, Scheau C, Badarau IA, Popescu GDA, Caruntu A, Costache DO, Costache RS, Constantin C, Neagu M, Caruntu C. Comparative effects of capsaicin in chronic obstructive pulmonary disease and asthma (Review) Exp Ther Med. 2021;22(917) doi: 10.3892/etm.2021.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen YY, Yu MF, Zhao XX, Shen J, Peng YB, Zhao P, Xue L, Chen W, Ma LQ, Qin G, et al. Paracetamol inhibits Ca2+ permeant ion channels and Ca(2+) sensitization resulting in relaxation of precontracted airway smooth muscle. J Pharmacol Sci. 2020;142:60–68. doi: 10.1016/j.jphs.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 60.She YS, Ma LQ, Liu BB, Zhang WJ, Qiu JY, Chen YY, Li MY, Xue L, Luo X, Wang Q, et al. Semen cassiae extract inhibits contraction of airway smooth muscle. Front Pharmacol. 2018;9(1389) doi: 10.3389/fphar.2018.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duong V, Lambrechts L, Paul RE, Ly S, Lay RS, Long KC, Huy R, Tarantola A, Scott TW, Sakuntabhai A, Buchy P. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci USA. 2015;112:14688–14693. doi: 10.1073/pnas.1508114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H, Halverson S, Ezinwa N. Mosquito-Borne Diseases. Prim Care. 2018;45:393–407. doi: 10.1016/j.pop.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Gan SJ, Leong YQ, Bin Barhanuddin MFH, Wong ST, Wong SF, Mak JW, Ahmad RB. Dengue fever and insecticide resistance in Aedes mosquitoes in Southeast Asia: A review. Parasit Vectors. 2021;14(315) doi: 10.1186/s13071-021-04785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fillinger U, Lindsay SW. Larval source management for malaria control in Africa: Myths and reality. Malar J. 2011;10(353) doi: 10.1186/1475-2875-10-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raman Ibrahim NBB, Puchooa D, Govinden-Soulange J, Facknath S. Cassia species: a potential source of biopesticides. Journal of Plant Diseases and Protection. 2021;128:339–351. [Google Scholar]
- 66.Mbatchou VC, Tchouassi DP, Dickson RA, Annan K, Mensah AY, Amponsah IK, Jacob JW, Cheseto X, Habtemariam S, Torto B. Mosquito larvicidal activity of Cassia tora seed extract and its key anthraquinones aurantio-obtusin and obtusin. Parasit Vectors. 2017;10(562) doi: 10.1186/s13071-017-2512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mbatchou VC, Dickson RA, Amponsah IK, Mensah AY, Habtemariam S. Protection effect of the anthraquinones, cassiatorin and aurantio-obtusin from seeds of Senna tora against cowpea weevil attack. Asian Pac J Trop Biomed. 2018;8:98–105. [Google Scholar]
- 68.Piplani M, Bhagwat DP, Singhvi G, Sankaranarayanan M, Balana-Fouce R, Vats T, Chander S. Plant-based larvicidal agents: An overview from 2000 to 2018. Exp Parasitol. 2019;199:92–103. doi: 10.1016/j.exppara.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Lim H, Park BK, Shin SY, Kwon YS, Kim HP. Methyl caffeate and some plant constituents inhibit age-related inflammation: Effects on senescence-associated secretory phenotype (SASP) formation. Arch Pharm Res. 2017;40:524–535. doi: 10.1007/s12272-017-0909-y. [DOI] [PubMed] [Google Scholar]
- 70.Birch J, Gil J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020;34:1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohtani N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): Can it be controlled by senolysis? Inflamm Regen. 2022;42(11) doi: 10.1186/s41232-022-00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheltens P, De trooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung HA, Ali MY, Jung HJ, Jeong HO, Chung HY, Choi JS. Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against β-secretase and cholinesterases. J Ethnopharmacol. 2016;191:152–160. doi: 10.1016/j.jep.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 74.Török B, Fazekas CL, Szabó A, Zelena D. Epigenetic modulation of vasopressin expression in health and disease. Int J Mol Sci. 2021;22(9415) doi: 10.3390/ijms22179415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe J, Takayanagi Y, Yoshida M, Hattori T, Saito M, Kohno K, Kobayashi E, Onaka T. Conditional ablation of vasopressin-synthesizing neurons in transgenic rats. J Neuroendocrinol. 2021;33(e13057) doi: 10.1111/jne.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glavaš M, Gitlin-Domagalska A, Dębowski D, Ptaszyńska N, Łęgowska A, Rolka K. Vasopressin and its analogues: From natural hormones to multitasking peptides. Int J Mol Sci. 2022;23(3068) doi: 10.3390/ijms23063068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Atanu FO, Batiha GE. Arginine vasopressin and pathophysiology of COVID-19: An innovative perspective. Biomed Pharmacother. 2021;143(112193) doi: 10.1016/j.biopha.2021.112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mondritzki T, Mai TA, Vogel J, Pook E, Wasnaire P, Schmeck C, Hüser J, Dinh W, Truebel H, Kolkhof P. Cardiac output improvement by pecavaptan: A novel dual-acting vasopressin V1a/V2 receptor antagonist in experimental heart failure. Eur J Heart Fail. 2021;23:743–750. doi: 10.1002/ejhf.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szczepanska-Sadowska E, Wsol A, Cudnoch-Jedrzejewska A, Żera T. Complementary role of oxytocin and vasopressin in cardiovascular regulation. Int J Mol Sci. 2021;22(11465) doi: 10.3390/ijms222111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Che K, Muttenthaler M, Kurzbach D. Conformational selection of vasopressin upon V1a receptor binding. Comput Struct Biotechnol J. 2021;19:5826–5833. doi: 10.1016/j.csbj.2021.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sparapani S, Millet-Boureima C, Oliver J, Mu K, Hadavi P, Kalostian T, Ali N, Avelar CM, Bardies M, Barrow B, et al. The biology of vasopressin. Biomedicines. 2021;9(89) doi: 10.3390/biomedicines9010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lago TR, Brownstein MJ, Page E, Beydler E, Manbeck A, Beale A, Roberts C, Balderston N, Damiano E, Pineles SL, et al. The novel vasopressin receptor (V1aR) antagonist SRX246 reduces anxiety in an experimental model in humans: A randomized proof-of-concept study. Psychopharmacology (Berl) 2021;238:2393–2403. doi: 10.1007/s00213-021-05861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paudel P, Kim DH, Jeon J, Park SE, Seong SH, Jung HA, Choi JS. Neuroprotective effect of aurantio-obtusin, a putative vasopressin V(1A) receptor antagonist, on transient forebrain ischemia mice model. Int J Mol Sci. 2021;22(3335) doi: 10.3390/ijms22073335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lemmens-Gruber R, Kamyar M. Vasopressin antagonists. Cell Mol Life Sci. 2006;63:1766–1779. doi: 10.1007/s00018-006-6054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lemmens-Gruber R, Kamyar M. Pharmacology and clinical relevance of vasopressin antagonists. Internist (Berl) 2008;49:629–630. doi: 10.1007/s00108-008-2017-z. 628. 632-4. [DOI] [PubMed] [Google Scholar]
- 86.Ripoll GV, Pifano M, Garona J, Alonso DF. Commentary: Arginine vasopressin receptor 1a is a therapeutic target for castration-resistant prostate cancer. Front Oncol. 2020;9(1490) doi: 10.3389/fonc.2019.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L, Yan C, Xie X, Lin Z, Panayi AC, et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology. 2021;19(150) doi: 10.1186/s12951-021-00894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S, Wang Y, Wang Z, Chen L, Zuo B, Liu C, Sun D. Enhanced renoprotective effect of GDNF-modified adipose-derived mesenchymal stem cells on renal interstitial fibrosis. Stem Cell Res Ther. 2021;12(27) doi: 10.1186/s13287-020-02049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C, Sun L, Zhang W, Tang Y, Li X, Jing R, Liu T. Limb ischemic preconditioning ameliorates renal microcirculation through activation of PI3K/Akt/eNOS signaling pathway after acute kidney injury. Eur J Med Res. 2020;25(10) doi: 10.1186/s40001-020-00407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J, Huang Y, Hu X, Bian X, Nian S. Gastrodin prevents homocysteine-induced human umbilical vein endothelial cells injury via PI3K/Akt/eNOS and Nrf2/ARE pathway. J Cell Mol Med. 2021;25:345–357. doi: 10.1111/jcmm.16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xue X, Ling X, Xi W, Wang P, Sun J, Yang Q, Xiao J. Exogenous hydrogen sulfide reduces atrial remodeling and atrial fibrillation induced by diabetes mellitus via activation of the PI3K/Akt/eNOS pathway. Mol Med Rep. 2020;22:1759–1766. doi: 10.3892/mmr.2020.11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song W, Liang Q, Cai M, Tian Z. HIF-1α-induced up-regulation of microRNA-126 contributes to the effectiveness of exercise training on myocardial angiogenesis in myocardial infarction rats. J Cell Mol Med. 2020;24:12970–12979. doi: 10.1111/jcmm.15892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li S, Li Q, Lv X, Liao L, Yang W, Li S, Lu P, Zhu D. Aurantio-obtusin relaxes systemic arteries through endothelial PI3K/AKT/eNOS-dependent signaling pathway in rats. J Pharmacol Sci. 2015;128:108–115. doi: 10.1016/j.jphs.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 94.Yu X, Wei LH, Zhang JK, Chen TR, Jin Q, Wang YN, Zhang SJ, Dou TY, Cao YF, Guo WZ, et al. Anthraquinones from Cassiae semen as thrombin inhibitors: In vitro and in silico studies. Phytochemistry. 2019;165(112025) doi: 10.1016/j.phytochem.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 95.Tarandovskiy ID, Artemenko EO, Panteleev MA, Sinauridze EI, Ataullakhanov FI. Antiplatelet agents can promote two-peaked thrombin generation in platelet rich plasma: Mechanism and possible applications. PLoS One. 2013;8(e55688) doi: 10.1371/journal.pone.0055688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varghese R, George Priya Doss C, Kumar RS, Almansour AI, Arumugam N, Efferth T, Ramamoorthy S. Cardioprotective effects of phytopigments via multiple signaling pathways. Phytomedicine. 2022;95(153859) doi: 10.1016/j.phymed.2021.153859. [DOI] [PubMed] [Google Scholar]
- 97.Alkarithi G, Duval C, Shi Y, Macrae FL, Ariëns RAS. Thrombus structural composition in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2021;41:2370–2383. doi: 10.1161/ATVBAHA.120.315754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu H, Wang Y, Zhang Y, Xu F, Chen J, Duan L, Zhang T, Wang J, Zhang F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020;32(101500) doi: 10.1016/j.redox.2020.101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao Y, Xie R, Yodsanit N, Ye M, Wang Y, Wang B, Guo LW, Kent KC, Gong S. Hydrogen peroxide-responsive platelet membrane-coated nanoparticles for thrombus therapy. Biomater Sci. 2021;9:2696–2708. doi: 10.1039/d0bm02125c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williams LM, Gilmore TD. Looking Down on NF-κB. Mol Cell Biol. 2020;40:e00104–20. doi: 10.1128/MCB.00104-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harrold AP, Cleary MM, Bharathy N, Lathara M, Berlow NE, Foreman NK, Donson AM, Amani V, Zuercher WJ, Keller C. In vitro benchmarking of NF-κB inhibitors. Eur J Pharmacol. 2020;873(172981) doi: 10.1016/j.ejphar.2020.172981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Son M, Wang AG, Tu HL, Metzig MO, Patel P, Husain K, Lin J, Murugan A, Hoffmann A, Tay S. NF-κB responds to absolute differences in cytokine concentrations. Sci Signal. 2021;14(eaaz4382) doi: 10.1126/scisignal.aaz4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang B, Xie Y, Guo M, Rosner MH, Yang H, Ronco C. Nephrotoxicity and Chinese Herbal Medicine. Clin J Am Soc Nephrol. 2018;13:1605–1611. doi: 10.2215/CJN.11571017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Y, Mapa MST, Sprando RL. Liver toxicity of anthraquinones: A combined in vitro cytotoxicity and in silico reverse dosimetry evaluation. Food Chem Toxicol. 2020;140(111313) doi: 10.1016/j.fct.2020.111313. [DOI] [PubMed] [Google Scholar]
- 105.Yang J, Zhu A, Xiao S, Zhang T, Wang L, Wang Q, Han L. Anthraquinones in the aqueous extract of Cassiae semen cause liver injury in rats through lipid metabolism disorder. Phytomedicine. 2019;64(153059) doi: 10.1016/j.phymed.2019.153059. [DOI] [PubMed] [Google Scholar]
- 106.Hu M, Lin L, Liu J, Zhong Y, Liang B, Huang Y, Li Z, Lin X, Wang B, Zhang B, et al. Aurantio-obtusin induces hepatotoxicity through activation of NLRP3 inflammasome signaling. Toxicol Lett. 2022;354:1–13. doi: 10.1016/j.toxlet.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 107.Wang J, Zhao Y, Xiao X, Li H, Zhao H, Zhang P, Jin C. Assessment of the renal protection and hepatotoxicity of rhubarb extract in rats. J Ethnopharmacol. 2009;124:18–25. doi: 10.1016/j.jep.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 108.Yu J, Han JC, Gao YJ. Biotransformation of glucoaurantio-obtusin towards aurantio-obtusin increases the toxicity of irinotecan through increased inhibition towards SN-38 glucuronidation. Phytother Res. 2014;28:1577–1580. doi: 10.1002/ptr.5162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.