Abstract
Objectives
To compare severity and clinical outcomes from Omicron as compared with the Delta variant and to compare outcomes between Omicron sublineages.
Methods
We searched the WHO COVID-19 Research database for studies that compared clinical outcomes for patients with Omicron variant and the Delta variant, and separately Omicron sublineages BA.1 and BA.2. A random-effects meta-analysis was used to pool estimates of relative risk (RR) between variants and sublineages. Heterogeneity between studies was assessed using the I2 index. Risk of bias was assessed using the tool developed by the Clinical Advances through Research and Information Translation team.
Results
Our search identified 1494 studies and 42 met the inclusion criteria. Eleven studies were published as preprints. Of the 42 studies, 29 adjusted for vaccination status; 12 had no adjustment; and for 1, the adjustment was unclear. Three of the included studies compared the sublineages of Omicron BA.1 versus BA.2. As compared with Delta, individuals infected with Omicron had 61% lower risk of death (RR 0.39, 95% CI 0.33 to 0.46) and 56% lower risk of hospitalisation (RR 0.44, 95% CI 0.34 to 0.56). Omicron was similarly associated with lower risk of intensive care unit (ICU) admission, oxygen therapy, and non-invasive and invasive ventilation. The pooled risk ratio for the outcome of hospitalisation when comparing sublineages BA.1 versus BA.2 was 0.55 (95% 0.23 to 1.30).
Discussion
Omicron variant was associated with lower risk of hospitalisation, ICU admission, oxygen therapy, ventilation and death as compared with Delta. There was no difference in the risk of hospitalisation between Omicron sublineages BA.1 and BA.2.
PROSPERO registration number
CRD42022310880.
Keywords: COVID-19, SARS-CoV-2, Critical Illness
WHAT IS ALREADY KNOWN ON THIS TOPIC
While Omicron, currently the dominant variant of SARS-CoV-2, is known to have increased transmissibility and infectivity, true clinical severity remains uncertain.
WHAT THIS STUDY ADDS
Our systematic review evaluates clinical severity and outcomes for individuals infected with Omicron compared with Delta as well as sublineages of Omicron (BA.1 and BA.2). Our results suggest that Omicron is associated with 61% lower risk of death and 56% lower risk of hospitalization. There were no differences in severity or clinical outcomes between Omicron sublineages.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
With waning immunity, changes in testing policies and procedures, cocirculation of numerous sublineages and variations in clinical management strategies, assessing severity for each new emerging variant is essential in order to optimise clinical care. Our findings thus provide important information for key stakeholders.
Background
Globally, over 6.9 million deaths from COVID-19 have been reported to the WHO as of 24 May 2023.1 In the 3.5 years since the first reports of SARS-CoV-2, the virus has continuously evolved, and multiple variants of concern have emerged.2 While the emergence of future variants is a known and expected phenomenon, not all mutations confer a fitness advantage to the virus. Mutations that enhance pathogenicity, infectivity, transmissibility and/or antigenicity may confer important survival advantages, leading to newer and potentially deadlier waves of the pandemic.3 In parallel, it is important to recognise the changing host response as the pandemic continues, resulting in increasing population immunity due to prior infection(s) and the increasing availability of vaccines, as well as effective treatment options.4–6
In late November 2021, researchers from South Africa reported the emergence of a newer variant (B.1.1.529) to WHO after observing a rapid increase in cases from Gauteng province with the unique finding of S-gene target failure on PCR testing.7 The WHO Technical Advisory Group on SARS-CoV-2 Virus Evolution recommended classification as variant of concern (VOC) based on its transmissibility and immune escape properties. WHO named this VOC Omicron on 26 November 2021.8
Omicron rapidly replaced Delta to become the dominant circulating variant globally.2 While reports from several countries9–11 suggested that patients infected with Omicron were experiencing symptoms that were less severe than Delta, much of this information was unadjusted for potential confounding factors such as prior immunity from infection or vaccination and availability of COVID-19-specific therapeutics. Using information available in the Global Clinical Platform for COVID-19, WHO published a report (number of patients included 34 442) suggesting lower severity with Omicron as compared with Delta.12 Similar to other published reports, the WHO analysis also suffered from challenges such as small patient numbers from a limited number of settings and limited information on vaccination status and prior infection.
Despite the relative lower severity, the intense circulation of Omicron has resulted in significant mortality, with 1 243 850 reported deaths in 2022 globally.1 For WHO member states, public health institutions and the public, understanding the clinical impact of emerging variants is vital to ensure an appropriate public health response, including adequate resource allocation and to update clinical management guidelines. In this context and given the public health implications, the WHO Steering Committee for COVID-19 Clinical Guidelines requested a comprehensive review of the literature to better understand the severity of Omicron compared with previously circulating VOCs. The aim of this review was to compare severity and clinical outcomes for individuals infected with the Omicron variant as compared with the Delta variant and additionally between BA.1 and BA.1 sublineages of Omicron.
Methods
Review questions, inclusion criteria and outcomes
The initial review question was focused on the severity of Omicron as compared with the Delta variant. However, during the literature appraisal process, the emergence of sublineages BA.1 and BA.2 of Omicron triggered the expansion of the review to include severity of these sublineages (prompting an updated literature search). Therefore, review questions of interest were (1) among patients infected with Omicron variant, what is the severity and clinical outcomes as compared with patients infected with the Delta variant? and (2) among patients infected with the BA.2 sublineage of Omicron, what is the severity and clinical outcomes as compared with BA.1 sublineage?
Eligible studies were those with primary data on humans of any age group focusing on clinical features and clinical outcomes among patients with Omicron variant and Delta variant. Outcomes of interest were admission to hospital, admission to intensive care unit (ICU), receipt of oxygen therapy (low flow and high flow), receipt of non-invasive ventilation, receipt of invasive ventilation, receipt of kidney replacement therapy or any other organ support, and death at longest follow-up as reported in each study. Editorials, commentaries, viewpoints, letters (correspondence) and abstracts with no full text available were excluded.
Search strategy
The search was developed and run by a Health Information Specialist (KK). The WHO COVID-19 Research database, a specialised, comprehensive COVID-19 resource, maintained by the WHO Library, was searched.13 This database includes peer reviewed publications from all major scientific databases (including Embase, MEDLINE and Web of Science), preprint articles and other grey literature sources.14 The initial search was run on 26 January 2022 with no date filters, and an update was run on 16 May 2022. No restrictions on language were applied. More details on the search strategy can be found in the online supplemental material 1.
bmjgh-2023-012328supp001.pdf (780.3KB, pdf)
Study selection
Titles/abstracts of all retrieved citations and full texts were reviewed independently and in duplicate by two authors (PR and BKTV). In case of disagreement, a third reviewer (NVM) was available. Consensus was achieved for inclusion of articles.
Data extraction
A standardised data extraction form was developed and piloted jointly by BKTV and PR prior to data extraction. Data were extracted on the number of patients for the different comparisons (Omicron vs Delta and BA.1 vs BA.2), on severity and clinical outcomes. In case of disagreement, a third reviewer (VM) was available. Consensus was achieved for data extraction.
Study quality assessment
Study quality was assessed using the risk of bias (RoB) in observational studies tool developed by the Clinical Advances through Research and Information Translation research team.15 The tool assesses RoB across eight domains (selection of exposed and non-exposed from the same population, confidence in the assessment of exposure, confidence that outcome was not present at the start of the study, whether the study adjusted for the key covariates of interest, confidence in the assessment of presence or absence of prognostic factors, confidence in the assessment of outcome, adequacy of follow-up and similarity of cointerventions). Quality was assessed independently and in duplicate by two authors (PR and BKTV). In addition, we also developed rules to arrive at an overall quality rating (see online supplemental figure 8).
Data analysis
All analyses were undertaken in R (R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org).
Categorical variables are reported as counts and percentages and continuous variables as mean and SD (or median and IQR). We summarise qualitatively and quantitatively the severity and outcomes for patients by variant and sublineage. Hospitalisation is reported as a proportion of the entire study cohort of patients with Omicron and Delta, whereas in-hospital outcomes such as ICU admission, receipt of oxygen therapy, etc, are reported as a proportion of the total number of patients hospitalised for each variant.
The restricted maximum likelihood method, the default method in the metafor package V.3.0 in R,16 was used for analysis. We performed a random-effects meta-analysis for each outcome by combining the effect estimates of studies where they were available. When studies did not report effect estimates, we calculated the relative risk (RR) from the numbers of participants in each comparison group. In cases where studies reported the HR or OR, we calculated the risk ratio using the methods suggested by Schor et al17 and Zhang and Yu,18 respectively. For all studies, we extracted and pooled the adjusted estimates where available.
Heterogeneity between studies was assessed using the Q statistic with a significance level of p<0.10, and the I2 index was used to assess the degree of heterogeneity between study estimates.19 RoB plots were created using a standard R package for visualising RoB assessments.20
A post hoc sensitivity analysis was undertaken by pooling the results for different outcomes by incorporating only those studies that adjusted for vaccination and only studies at low RoB. We specified a significance level of p<0.05 for overall effect estimate in the meta-analysis for the random-effects model.
Study registration and deviations from protocol
The review was registered prior to any data extraction and analysis on the International Prospective Register of Systematic Reviews (CRD42022310880).21
Our original review question did not include the Omicron sublineage comparison and was added after the widespread emergence of BA.2. The sensitivity analysis of studies that adjusted for vaccination and studies at low RoB was also not part of the original analysis plan.
We conducted and reported this review in accordance with Meta-Analyses of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guidelines.22 23 We used Covidence to conduct this systematic review and meta-analysis (Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia).
Patient and public involvement
Given the nature of this research, we did not involve patients or the public in the design or conduct or reporting.
Results
Our search identified 1494 records, and after excluding duplicates and records not relevant to the study question, 57 full-text reports were assessed for eligibility. The PRISMA flow diagram (online supplemental material 1) provides the detailed reasons for exclusions. From the 57 full-text reports, 43 were included in the review9–11 24–63 and analysis. One additional study60 was excluded as the data reported in the paper was unclear and the study authors did not respond to emails, leaving us with 42 studies. A total of 6 174 807 patients were included in this review.
Table 1 describes the characteristics of the included studies. Five of six WHO regions were represented (Africa, Americas, Eastern Mediterranean, Europe and South-East Asia), and most studies were from high-income or upper-middle-income settings: 12 from the USA,9 25 26 29 30 32 36 40 44 53 54 57 7 from the UK,27 31 37 42 46 50 59 7 from South Africa,33 51 52 55 56 58 61 5 from France24 28 34 38 62 and two from Norway.35 39 There was one study each from Denmark,47 Germany,41 India,45 Indonesia,49 Italy,63 Portugal,10Turkey46 and Qatar.43 Of the included studies, 11 were published as preprints.6–8 19 25 32 44 45 49 52 56 Sixteen studies reported on adult patients and 3 on children, and 21 studies included a combination of adults and children. Of the 42 studies, 29 adjusted for vaccination status either in the study design or in the analysis stage; 12 had no adjustment; and 1 study was unclear. Three of the included studies compared the sublineages of Omicron (BA.1 vs BA.2).61–63
Table 1.
Characteristics of included studies
| First author | Country | WHO region | World Bank income | Year of publication | Publication type | Method used to identify variants | Participants, total n | |
| Omicron versus Delta | ||||||||
| 1 | Abdullah et al55 | South Africa | African | Upper middle income | 2021 | Peer reviewed | Time period | 4428 |
| 2 | Adhikari et al40 | USA | Americas | High income | 2022 | Peer reviewed | WGS+time period | 1343 |
| 3 | Vieillard-Baron et al24 | France | European | High income | 2022 | Preprint | Mutations in spike protein of SARS-CoV-2 | 3761 |
| 4 | Auvigne et al28 | France | European | High income | 2022 | Peer reviewed | RT-qPCR with mutation screening multiplex | 184 364 |
| 5 | Bager et al47 | Denmark | European | High income | 2022 | Peer reviewed | RT-PCR using a specific Omicron marker | 188 980 |
| 6 | Bal et al38 | France | European | High income | 2022 | Preprint+peer review | WGS | 6223 |
| 7 | Birol Ilter et al46 | Turkey and UK | European | Upper middle income/high income | 2022 | Peer reviewed | Time period | 231 |
| 8 | Bouzid et al34 | France | European | High income | 2022 | Peer reviewed | WGS+SGTF+detection of mutation | 1716 |
| 9 | Butt et al43 | Qatar | Eastern Mediterraean | High income | 2022 | Peer reviewed | WGS+time period | 1970 |
| 10 | Ulloa et al11 | Canada | Americas | High income | 2021 | Preprint | WGS+SGTF | 25 802 |
| 11 | Christensen et al53 | USA | Americas | High income | 2022 | Preprint+peer review | WGS+SGTF | 20 196 |
| 12 | Fall et al36 | USA | Americas | High income | 2022 | Preprint+peer review | WGS | 2027 |
| 13 | Goga et al51 | South Africa | African | Upper middle income | 2021 | Preprint+peer review | Time period | 39 929 |
| 14 | Grint et al50 | UK | European | High income | 2022 | Preprint | SGTF | 330 380 |
| 15 | Gunadi et al49 | Indonesia | Southeast Asian | Lower middle income | 2022 | Preprint | WGS | 352 |
| 16 | Hussey et al58 | South Africa | African | Upper middle income | 2022 | Preprint+peer review | RdRp target delay to detect Omicron | 1636 |
| 17 | Jassat et al33 | South Africa | African | Upper middle income | 2022 | Preprint+peer review | Time period | 1 935 877 |
| 18 | Krutikov et al59 | UK | European | High income | 2022 | Peer reviewed | WGS+SGTF+time periods with a subanalysis of confirmed | 545 |
| 19 | Lauring et al29 | USA | Americas | High income | 2022 | Preprint+peer review | WGS+time period | 1338 |
| 20 | Leiner et al41 | Germany | European | High income | 2022 | Preprint+peer review | Time period | 3403 |
| 21 | Lewnard et al9 | USA | Americas | High income | 2022 | Preprint | SGTF | 69 579 |
| 22 | Davies et al56 | South Africa | African | Upper middle income | 2022 | Preprint+peer review | Time period | 9547 |
| 23 | Menni et al42 | UK | European | High income | 2022 | Peer reviewed | Time period | 9980 |
| 24 | Modes et al26 | USA | Americas | High income | 2022 | Peer reviewed | Time period | 1076 |
| 25 | Nyberg et al27 | UK | European | High income | 2022 | Peer reviewed | WGS | 1 516 702 |
| 26 | Paredes et al32 | USA | Americas | High income | 2022 | Preprint+peer review | WGS | 38 477 |
| 27 | Pascall et al37 | UK | European | High income | 2022 | Preprint | WGS | 3854 |
| 28 | Peralta-Santos et al10 | Portugal | European | High income | 2022 | Preprint | WGS+SGTF | 15 978 |
| 29 | Raju et al45 | India | Southeast Asian | Lower middle Income | 2022 | Peer reviewed | SGTF | 1809 |
| 30 | Robinson et al25 | USA | Americas | High income | 2022 | Preprint+peer review | WGS | 441 |
| 31 | Sheikh et al48 | Scotland | European | High income | 2022 | Peer reviewed | SGTF | 143 504 |
| 32 | Šmid et al60 | Czech Republic | European | High income | 2022 | Peer reviewed | WGS+time period | No author response, hence excluded |
| 33 | Stålcrantz et al35 | Norway | European | High income | 2022 | Peer reviewed | Unclear | 1075 |
| 34 | Wang et al30 | USA | Americas | High income | 2022 | Preprint | Time period | 294 214 |
| 35 | Wang et al54 | USA | Americas | High income | 2022 | Preprint | Time period | 28 080 |
| 36 | Wang et al57 | USA | Americas | High income | 2022 | Preprint | Time period | 14 396 |
| 37 | Ward et al31 | UK | European | High income | 2022 | Peer reviewed | SGTF | 1 035 149 |
| 38 | Whittaker et al39 | Norway | European | High income | 2022 | Preprint+peer review | WGS+SGTF | 125 269 |
| 39 | Wolter et al52 | South Africa | African | Upper middle income | 2022 | Peer reviewed | WGS+SGTF | 11 495 |
| 40 | Wrenn et al44 | USA | Americas | High income | 2022 | Peer reviewed | WGS | 752 |
| Total | 6 075 878 | |||||||
| BA.1 versus BA.2 | ||||||||
| 1 | Wolter et al61 | South Africa | African | Upper middle income | 2022 | Preprint | SGTF | 95 470 |
| 2 | Gautret et al62 | France | European | High income | 2022 | Peer reviewed | WGS | 3000 |
| 3 | Loconsole et al63 | Italy | European | High income | 2022 | Peer reviewed | WGS+SGTF | 459 |
| Total | 98 929 | |||||||
RdRP, RNA-dependent RNA P; RT-qPCR, quantitative reverse transcriptase PCR; SGTF, S-gene target failure; WGS, whole-genome sequencing.
For the Omicron versus Delta comparison, we were able to pool data from the studies for the following outcomes: death at longest follow-up, hospitalisation, ICU admission, receipt of oxygen therapy (low flow and high flow), and receipt of non-invasive and invasive ventilation.
Figure 1 is a forest plot of death at the longest follow-up comparing patients infected with the Omicron as compared with Delta. Overall, individuals hospitalised with Omicron had a 61% lower risk of dying (RR 0.39, 95% CI :0.33 to 0.46).
Figure 1.
Forest plot of death: Omicron versus Delta. *Death at longest follow-up: studies included in the review followed patients for different time periods, some until hospital discharge, some for 14 days, some for 30 days etc. RE, Random Effects.
Figure 2 is a forest plot of hospitalisation comparing patients infected with the Omicron variant as compared with the Delta variant. Overall, individuals infected with Omicron had a 56% lower (RR 0.44, 95% CI 0.34 to 0.56) risk of being admitted to the hospital.
Figure 2.

Forest plot comparing hospitalisation: Omicron versus Delta.
Similarly, patients infected with Omicron had a 54% lower risk (RR 0.46, 95% CI 0.37 to 0.57) of admission to ICU (online supplemental figure 1), 52% lower risk (RR 0.48, 95% CI 0.32 to 0.71) of receiving low-flow oxygen (online supplemental figure 2), 49% lower risk (RR 0.51, 95% CI 0.29 to 0.92) of receiving high-flow oxygen (online supplemental figure 3) and 59% lower risk (RR 0.41, 95% CI 0.29 to 0.57) of receiving invasive ventilation (online supplemental figure 4). There was no difference in the receipt of non-invasive ventilation between the variants (RR 0.90, 95% CI 0.69 to 1.19) (online supplemental figure 5).
For the BA.1 versus BA.2 comparison, we were able to pool studies only for the outcome of need for hospitalisation. Figure 3 is a forest plot comparing hospitalisation between the sublineages, and as can be seen, the risk of hospitalisation did not differ (RR 0.55, 95% CI 0.23 to 1.30).
Figure 3.

Forest plot comparing hospitalisation for Omicron sublineages.
Sensitivity analysis
Figures 4 and 5 show hospitalisation and death when pooling only studies that explicitly adjusted for vaccination. This analysis found similar results regarding the lower risk of hospitalisation and death following infection with Omicron compared with infection with Delta.
Figure 4.

Forest plot comparing hospitalisation in studies adjusting for vaccination: Omicron versus Delta.
Figure 5.
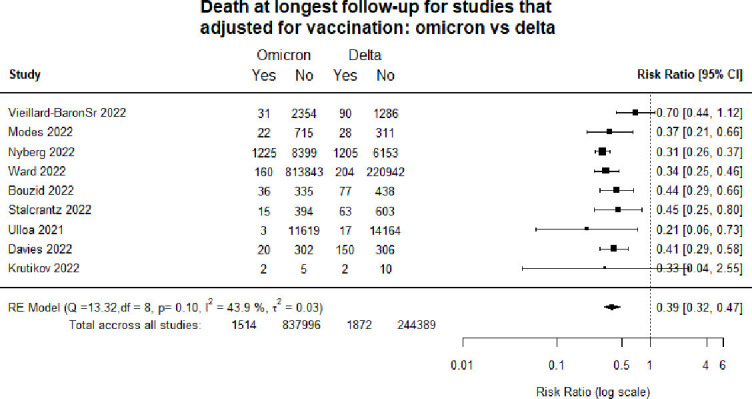
Forest plot comparing death in studies adjusting for vaccination: Omicron versus Delta.
Similarly, pooling only studies with a low RoB (online supplemental figure 6 and 7) found that the risk of hospitalisation and death was lower with infection with Omicron compared with infection with Delta.
Study quality
Online supplemental figure 8 provides a visual representation of the quality of the included studies. Nearly half of the studies were at high RoB.
Discussion
Our systematic review comparing outcomes for the Omicron variant as compared with Delta shows a lower risk of death and hospitalisation. There was also a lower risk of receipt of oxygen therapy, and invasive ventilation. Our sensitivity analysis pooling only studies that explicitly adjusted for vaccination and studies at low RoB also corroborates these findings. Comparing sublineages, the results show that the risk of hospitalisation did not differ between BA.1 versus BA.2.
These results broadly corroborate those of an earlier WHO report12 which suggested lower odds of developing severe or critical disease (OR 0.43, 95% CI 0.41 to 0.46) and lower hazards of in-hospital mortality (HR 0.62, 95% CI 0.59 to 0.65), but add more confidence and precision to the estimates as our study included over 300 times the number of patients and covers more countries than those included in the WHO report. Prior studies have demonstrated enhanced transmissibility with Omicron64 and the capacity for immune evasion.64 65 Considering these characteristics that confer a ‘fitness advantage’, we found that our data demonstrating lower clinical severity is reassuring.
There are various factors that may explain the lower observed severity and mortality of Omicron compared with Delta. Higher levels of both infection-derived and vaccine-derived immunity may confound the association, resulting in lower apparent severity of infection particularly as immunity from vaccines and infection protects against severity and hospitalisation more than it protects against infection.66 67 Although the analysis limited to studies adjusting for vaccination showed a reduced severity from Omicron, there was inadequate adjustment for immunity from prior infection, which is likely to partially lower the overall risk too. Also, our analysis showed no statistical difference in severity between BA.1 and BA.2, and other studies comparing multiple waves of Omicron have not observed substantial changes in severity for more recent variants (BA.4/BA.5 compared with BA.1/BA.2).68
In our review, we have considered severity and outcomes from two perspectives, hospitalisation among infected and in-hospital outcomes for those that needed admission.69 However, it must be noted that hospitalisation as a measure of severity is not very specific; it can often ‘be with COVID-19’ rather than ‘because of COVID-19’. Despite this limitation, we included hospitalisation as a key outcome as it represents a concrete measure of healthcare use and is also a measure of the burden on potentially overwhelmed healthcare systems.
As such, while higher population immunity from prior infection and vaccination during Omicron waves of 2022 and 2023 may explain some of the reduced severity observed, it is likely that the intrinsic severity of Omicron is lower than that of previous variants, potentially due to its tropism for upper airways and hence lower risk of lower respiratory tract infection, as suggested by in vitro studies.70 71
Assessing the impact of new and emerging variants on severity and clinical outcomes remains critical as the pandemic continues. With waning immunity, changes in testing, surveillance and reporting, cocirculation of multiple lineages and variability in clinical management, assessing severity for each new emerging variant is essential in order to optimise clinical care and reduce the impact of COVID-19. COVID-19 surveillance must continue to be strengthened globally and include linking epidemiological, laboratory and clinical data. It is also important to note that most studies in our review come from high-income settings. There is thus a need to understand outcomes from newer variants and sublineages from low-income and lower middle-income settings.
Strengths and limitations
This is the first systematic review comparing clinical severity and outcomes for individuals infected with Omicron as compared with Delta. Our results provide important information for healthcare providers, public health officials, ministries of health and other policy makers around the world. Our review followed rigorous methods: preregistration of the protocol, adherence to MOOSE and PRISMA guidelines with duplicate and independent screening, data extraction and quality assessment.
Our review also has some limitations. First, nearly half of the included studies were at high RoB. About a third of included studies did not adjust for vaccination status, and this could have impacted the results. However, this is mitigated somewhat by the consistency of our results across the several outcomes and results from our sensitivity analysis. In recent months, several newer sublineages of Omicron have emerged. While our search strategy included BA.4 and BA.5, there were no published studies on these variants at the time of our search. Thus, our review does not provide information on these sublineages of Omicron, including those that are driving a 2023 surge in China (BF.7).72 We were unable to compare outcomes across different age groups and also unable to perform a sex-disaggregated analysis. Additional limitations of the results of the review include the substantial heterogeneity between studies and the fact that a quarter of the studies were only published as preprints, thus potentially limiting the generalisability of the results.
Conclusion
The Omicron variant as compared with Delta was associated with a lower risk of death and hospitalisation. There was also a lower risk of receipt of oxygen therapy, non-invasive ventilation and invasive ventilation; however, disentangling the relative effect of immunity from variant-specific properties remains challenging. Comparing sublineages, this study shows that the risk of hospitalisation did not differ between BA.1 versus BA.2.
Acknowledgments
We gratefully acknowledge the methods advice received from Professor Gordon Guyatt (McMaster University, Hamilton, Canada), Dr Diane Heels-Ansdell (McMaster University, Hamilton, Canada), Dr Neill Adhikari (Sunnybrook Health Sciences Centre, University of Toronto, Canada) and Dr Ani Movsisyan (WHO, Geneva, Switzerland).
Footnotes
Handling editor: Soumyadeep Bhaumik
Twitter: @DrPryanka, @mvankerkhove, @diazjv, @bharathkumartv
PR and BKTV contributed equally.
Contributors: PR, BKTV, LA, OLP and JD conceptualised the study. PR, BKTV and KK developed the search strategy. PR and BKTV developed the data extraction forms and conducted the study screenings and data extraction. VM and PR led the data analysis and visualisations. BKTV and PR led manuscript writing with contributions from all coauthors. All coauthors reviewed and approved the manuscript. BKTV will act as the guarantor for this manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
As this was a secondary analysis of previously published deidentified data, ethics clearance was not required.
References
- 1.World Health Organization . Available: https://covid19.who.int/ [Accessed 27 Feb 2023].
- 2.GISAID . Available: https://gisaid.org/hcov19-variants/ [Accessed 27 Feb 2023].
- 3.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021;19:409–24. 10.1038/s41579-021-00573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobrovitz N, Ware H, Ma X, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the Omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis 2023;23:556–67. 10.1016/S1473-3099(22)00801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Our World in Data . Available: https://ourworldindata.org/covid-vaccinations [Accessed 27 May 2023].
- 6.World Health Organization . Available: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4 [Accessed 27 May 2023].
- 7.World Health Organization . Available: https://www.who.int/docs/default-source/coronaviruse/2021-12-23-global-technical-brief-and-priority-action-on-omicron.pdf?sfvrsn=d0e9fb6c_8 [Accessed 27 Feb 2023].
- 8.World Health Organization . Available: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern [Accessed 26 Dec 2022].
- 9.Lewnard JA, Hong VX, Patel MM, et al. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat Med 2022;28:1933–43. 10.1038/s41591-022-01887-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peralta-Santos A, Rodrigues EF, Moreno J, et al. Omicron (BA.1) SARS-CoV-2 variant is associated with reduced risk of hospitalization and length of stay compared with Delta (B.1.617.2. Infectious Diseases (except HIV/AIDS) 2022. 10.1101/2022.01.20.22269406 [DOI] [Google Scholar]
- 11.Ulloa AC, Buchan SA, Daneman N, et al. Early estimates of SARS-CoV-2 Omicron variant severity based on a matched cohort study, Ontario, Canada. Epidemiology 2022. 10.1101/2021.12.24.21268382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Available: https://www.who.int/publications/i/item/9789240051829 [Accessed 26 Dec 2022].
- 13.World Health Organization . Available: https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/ [Accessed 27 Feb 2023].
- 14.World Health Organization . Available: https://www.who.int/publications/m/item/quick-search-guide-who-covid-19-database [Accessed 27 Feb 2023].
- 15.Distiller SR . Available: https://www.distillersr.com/resources [Accessed 27 Feb 2023].
- 16.Viechtbauer W. Conducting meta-analyses in R with the Metafor package. J Stat Softw 2010;36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 17.Shor E, Roelfs D, Vang ZM. The "Hispanic mortality paradox" revisited: meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants' mortality. Soc Sci Med 2017;186:20–33. 10.1016/j.socscimed.2017.05.049 [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 19.Cochrane Training . Available: https://training.cochrane.org/handbook/current/chapter-10#section-10-10-2 [Accessed 27 Feb 2023].
- 20.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021;12:55–61. 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 21.NIHR . Available: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=310880 [Accessed 26 Dec 2022].
- 22.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021;18:e1003583. 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieillard-Baron A, Flicoteaux R, Salmona M. Epidemiological characteristics and severity of Omicron variant cases in the APHP critical care units. Intensive Care Med 2022. 10.1101/2022.01.25.22269839 [DOI] [Google Scholar]
- 25.Robinson ML, Morris CP, Betz JF, et al. Impact of severe acute respiratory syndrome coronavirus 2 variants on inpatient clinical outcome. Clin Infect Dis 2023;76:1539–49. 10.1093/cid/ciac957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modes ME, Directo MP, Melgar M, et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance - one hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb Mortal Wkly Rep 2022;71:217–23. 10.15585/mmwr.mm7106e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: a cohort study. Lancet 2022;399:1303–12. 10.1016/S0140-6736(22)00462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auvigne V, Vaux S, Strat YL, et al. Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021-January 2022: a retrospective, population-based, matched cohort study. EClinicalMedicine 2022;48:101455. 10.1016/j.eclinm.2022.101455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from Omicron, Delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ 2022;376:e069761. 10.1136/bmj-2021-069761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Berger NA, Kaelber DC, et al. COVID infection rates, clinical outcomes, and racial/ethnic and gender disparities before and after Omicron emerged in the US. MedRxiv 2022. 10.1101/2022.02.21.22271300 [DOI] [Google Scholar]
- 31.Ward IL, Bermingham C, Ayoubkhani D, et al. Risk of COVID-19 related deaths for SARS-CoV-2 Omicron (B.1.1.529) compared with Delta (B.1.617.2): retrospective cohort study. BMJ 2022;378:e070695. 10.1136/bmj-2022-070695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paredes MI, Lunn SM, Famulare M, et al. Associations between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and risk of coronavirus disease 2019 (COVID-19) hospitalization among confirmed cases in Washington state: a retrospective cohort study. Clin Infect Dis 2022;75:e536–44. 10.1093/cid/ciac279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jassat W, Abdool Karim SS, Mudara C, et al. Clinical severity of COVID-19 in patients admitted to hospital during the Omicron wave in South Africa: a retrospective observational study. Lancet Glob Health 2022;10:e961–9. 10.1016/S2214-109X(22)00114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouzid D, Visseaux B, Kassasseya C, et al. Comparison of patients infected with Delta versus Omicron COVID-19 variants presenting to Paris emergency departments: a retrospective cohort study. Ann Intern Med 2022;175:831–7. 10.7326/M22-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stålcrantz J, Kristoffersen AB, Bøås H, et al. Milder disease trajectory among COVID-19 patients hospitalised with the SARS-CoV-2 Omicron variant compared with the Delta variant in Norway. Scand J Public Health 2022;50:676–82. 10.1177/14034948221108548 [DOI] [PubMed] [Google Scholar]
- 36.Fall A, Eldesouki RE, Sachithanandham J, et al. The displacement of the SARS-CoV-2 variant Delta with Omicron: an investigation of hospital admissions and upper respiratory viral loads. EBioMedicine 2022;79:104008. 10.1016/j.ebiom.2022.104008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascall DJ, Vink E, Blacow R, et al. Inconsistent directions of change in case severity across successive SARS-CoV-2 variant waves suggests an unpredictable future. Epidemiology 2022. 10.1101/2022.03.24.22272915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bal A, Simon B, Destras G, et al. Detection and prevalence of SARS-CoV-2 coinfections during the Omicron variant circulation in France. Nat Commun 2022;13:6316. 10.1038/s41467-022-33910-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whittaker R, Greve-Isdahl M, Bøås H, et al. COVID-19 hospitalization among children <18 years by variant wave in Norway. Pediatrics 2022;150:e2022057564. 10.1542/peds.2022-057564 [DOI] [PubMed] [Google Scholar]
- 40.Adhikari EH, MacDonald L, SoRelle JA, et al. COVID-19 cases and disease severity in pregnancy and neonatal positivity associated with Delta (B.1.617.2) and Omicron (B.1.1.529) variant predominance. JAMA 2022;327:1500–2. 10.1001/jama.2022.4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leiner J, Pellissier V, Hohenstein S, et al. Characteristics and outcomes of COVID-19 patients during B.1.1.529 (Omicron) dominance compared to B.1.617.2 (Delta) in 89 German hospitals. BMC Infect Dis 2022;22:802. 10.1186/s12879-022-07781-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet 2022;399:1618–24. 10.1016/S0140-6736(22)00327-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butt AA, Dargham SR, Loka S, et al. COVID-19 disease severity in children infected with the Omicron variant. Clin Infect Dis 2022;75:e361–7. 10.1093/cid/ciac275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrenn JO, Pakala SB, Vestal G, et al. COVID-19 severity from Omicron and Delta SARS-CoV-2 variants. Influenza Other Respir Viruses 2022;16:832–6. 10.1111/irv.12982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raju MK, Thangaraj JWV, Selvavinayagam TS, et al. Clinical profile of patients infected with suspected SARS-CoV-2 Omicron variant of concern, Tamil Nadu, India, December 2021-January 2022. Indian J Med Res 2022;155:165–70. 10.4103/ijmr.ijmr_312_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birol Ilter P, Prasad S, Mutlu MA, et al. Maternal and perinatal outcomes of SARS-CoV-2 infection in unvaccinated pregnancies during Delta and Omicron waves. Ultrasound Obstet Gynecol 2022;60:96–102. 10.1002/uog.24916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bager P, Wohlfahrt J, Bhatt S, et al. Risk of Hospitalisation associated with infection with SARS-CoV-2 Omicron variant versus Delta variant in Denmark: an observational cohort study. Lancet Infect Dis 2022;22:967–76. 10.1016/S1473-3099(22)00154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheikh A, Kerr S, Woolhouse M, et al. Severity of Omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design. Lancet Infect Dis 2022;22:959–66. 10.1016/S1473-3099(22)00141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunadi MSH, Hakim MS, Wibawa H, et al. Comparative analysis of the outcomes of COVID-19 between patients infected with SARS-CoV-2 Omicron and Delta variants: a retrospective cohort study. Public and Global Health [Preprint]. 10.1101/2022.04.30.22274532 [DOI]
- 50.Grint DJ, Wing K, Gibbs HP, et al. Accident and emergency (AE) attendance in England following infection with SARS-CoV-2 Omicron or Delta. Infectious Diseases (except HIV/AIDS) 2023. 10.1101/2022.05.03.22274602 [DOI] [Google Scholar]
- 51.Goga A, Bekker L-G, Garrett N, et al. Breakthrough SARS-CoV-2 infections during periods of Delta and Omicron predominance, South Africa. Lancet 2022;400:269–71. 10.1016/S0140-6736(22)01190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: a data linkage study. Lancet 2022;399:437–46. 10.1016/S0140-6736(22)00017-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christensen PA, Olsen RJ, Long SW, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the Omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol 2022;192:642–52. 10.1016/j.ajpath.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Berger NA, Kaelber DC, et al. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. MedRxiv 2022. 10.1101/2021.12.30.21268495 [DOI] [Google Scholar]
- 55.Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global Omicron variant COVID-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis 2022;116:38–42. 10.1016/j.ijid.2021.12.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davies M-A, Kassanjee R, Rousseau P, et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa. Trop Med Int Health 2022;27:564–73. 10.1111/tmi.13752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Berger NA, Kaelber DC, et al. COVID infection severity in children under 5 years old before and after Omicron emergence in the US. MedRxiv 2022. 10.1101/2022.01.12.22269179 [DOI] [Google Scholar]
- 58.Hussey H, Davies M-A, Heekes A, et al. Assessing the clinical severity of the Omicron variant in the Western Cape Province, South Africa, using the diagnostic PCR proxy marker of RdRp target delay to distinguish between Omicron and Delta infections - a survival analysis. Int J Infect Dis 2022;118:150–4. 10.1016/j.ijid.2022.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krutikov M, Stirrup O, Nacer-Laidi H, et al. Outcomes of SARS-CoV-2 Omicron infection in residents of long-term care facilities in England (VIVALDI): a prospective, cohort study. Lancet Healthy Longev 2022;3:e347–55. 10.1016/S2666-7568(22)00093-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Šmíd M, Berec L, Přibylová L, et al. Protection by vaccines and previous infection against the Omicron variant of severe acute respiratory syndrome Coronavirus 2. J Infect Dis 2022;226:1385–90. 10.1093/infdis/jiac161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolter N, Jassat W, von Gottberg A, et al. Clinical severity of Omicron sub-lineage BA.2 compared to BA.1 in South Africa. Infectious Diseases (except HIV/AIDS) 2023. 10.1101/2022.02.17.22271030 [DOI] [Google Scholar]
- 62.Gautret P, Hoang VT, Jimeno MT, et al. The severity of the first 207 infections with the SARS-CoV-2 Omicron BA.2 variant, in Marseille, France, December 2021-February 2022. J Med Virol 2022;94:3494–7. 10.1002/jmv.27760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loconsole D, Centrone F, Sallustio A, et al. Characteristics of the first 284 patients infected with the SARS-CoV-2 Omicron BA.2 subvariant at a single center in the Apulia region of Italy, January-March 2022. Vaccines (Basel) 2022;10:674. 10.3390/vaccines10050674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohsin M, Mahmud S. Omicron SARS-CoV-2 variant of concern: a review on its transmissibility, immune evasion, reinfection, and severity. Medicine (Baltimore) 2022;101:e29165. 10.1097/MD.0000000000029165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyngse FP, Mortensen LH, Denwood MJ, et al. Household transmission of the SARS-CoV-2 Omicron variant in Denmark. Nat Commun 2022;13:5573. 10.1038/s41467-022-33328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ssentongo P, Ssentongo AE, Voleti N, et al. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis 2022;22:439. 10.1186/s12879-022-07418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toubasi AA, Al-Sayegh TN, Obaid YY, et al. Efficacy and safety of COVID-19 vaccines: a network meta-analysis. J Evid Based Med 2022;15:245–62. 10.1111/jebm.12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolter N, Jassat W, Walaza S, et al. Clinical severity of SARS-CoV-2 Omicron BA.4 and BA.5 lineages compared to BA.1 and Delta in South Africa. Nat Commun 2022;13:5860. 10.1038/s41467-022-33614-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sigal A, Milo R, Jassat W. Estimating disease severity of Omicron and Delta SARS-CoV-2 infections. Nat Rev Immunol 2022;22:267–9. 10.1038/s41577-022-00720-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hui KPY, Ng K-C, Ho JCW, et al. Replication of SARS-CoV-2 Omicron BA.2 variant in ex vivo cultures of the human upper and lower respiratory tract. EBioMedicine 2022;83:104232. 10.1016/j.ebiom.2022.104232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamers MM, Mykytyn AZ, Breugem TI, et al. SARS-CoV-2 Omicron efficiently Infects human airway, but not alveolar epithelium. Microbiology 2023. 10.1101/2022.01.19.476898 [DOI] [Google Scholar]
- 72.The Hindu . Available: https://www.thehindu.com/sci-tech/health/covid-19-omicron-subvariant-bf7-linked-to-china-spike-first-found-in-india-in-july/article66289443.ece [Accessed 27 Feb 2023].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2023-012328supp001.pdf (780.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.



