Abstract
Background
Chronic kidney disease (CKD) and atrial fibrillation (AF) are increasing in prevalence globally and share common risk factors.
Our aim was to characterise real-world evidence on direct oral anticoagulant (DOAC) prescribing for people with AF and CKD, in terms of adherence, persistence and renal dose titration.
Methods
PubMed, EMBASE and CINAHL were searched from inception to June 2022. Our search terms included a combination of Medical Subject Headings (MeSH) terms and keywords including ‘atrial fibrillation’, ‘chronic kidney disease’, ‘adherence’, ‘persistence’, ‘direct oral anticoagulants’ and ‘dosing’. Data extraction and quality assessment were undertaken by two reviewers independently. Meta-analyses for pooled estimates were performed using DerSimonian and Laird random-effects models. Age, sex, diabetes, hypertension and heart failure were chosen as variables of interest.
Results
From 19 studies, a total of 252 117 patients were included with CKD and AF. Meta-analysis was only possible in seven studies with 128 406 patients, five on DOAC dose titration and two on adherence. There were insufficient studies on persistence. Our meta-analysis of dosing showed that 68% of patients with CKD and AF had correct dosing. There was no evidence to show any association between correct DOAC dosing and variables of interest. Overall, 67% of patients were DOAC adherent.
Conclusion
Adherence and correct dosing of DOACs were suboptimal compared with other medications in the pooled studies with respect to CKD and AF. Thus, further research is required as the lack of generalisation of findings is a rate-limiting factor for improved DOAC management in AF and CKD.
PROSPERO registration number
CRD;42022344491.
Keywords: atrial fibrillation, medication adherence, meta-analysis, systematic reviews as topic, drug monitoring
WHAT IS ALREADY KNOWN ON THIS TOPIC
Guidelines recommend using direct-acting oral anticoagulants (DOACs) for atrial fibrillation (AF) as first-line anticoagulants in those without moderate to severe mitral stenosis or prosthetic valves. DOAC dosage is adjusted in renal impairment. Patients with AF and chronic kidney disease (CKD) have a higher risk of stroke, cardiovascular morbidity and all-cause mortality compared with patients who have either condition alone. DOACs reduce these risks if managed correctly. The improvement in outcomes with DOACs in patients with AF is dependent on adherence to the correct dose. Hence, the aim of this research is to explore these fundamental factors and to evaluate associations between them and their determinants.
WHAT THIS STUDY ADDS
We found that there is a prescribing gap in appropriate dose reduction of DOACs in patients who have AF and CKD and that adherence to DOAC therapy is poor in this group. Little was found about DOAC persistence in this group of patients or the determinants of DOAC correct prescribing, adherence and persistence in CKD and AF.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Further research is needed in primary care, since these clinicians are routinely involved in and responsible for DOAC management, including correct DOAC dosing, adherence, and persistence.
Introduction
Direct oral anticoagulants (DOACs) changed the landscape of atrial fibrillation (AF) treatment since their introduction in 2010. DOAC prescribing has significantly increased while vitamin K antagonist (VKA) prescribing has declined in the USA and Europe, including the UK.1 Clinical trials have shown that DOACs are at least non-inferior to VKA for the prevention of stroke and systemic embolism, and all have consistently superior safety profiles, particularly with reduced risk of intracranial bleeding.2–5 Therefore, among patients with AF who are suitable for DOAC treatment, the current UK NICE guidance, European Society of Cardiology guidelines and joint American cardiac societies guidelines all advocate DOACs over VKAs as a preferable anticoagulation approach.5–7
There are more than 800 million people on the globe who suffer from chronic kidney disease (CKD), affecting over 10% of the population.8 AF is the the most common arrhythmia worldwide.9 The global prevalence of AF was estimated to be 59.7 million worldwide with nearly five million new cases occurring every year.9 The prevalence of each condition increases with age, and patients with both conditions have a higher risk of stroke, cardiovascular morbidity and all-cause mortality compared with those with either AF or CKD alone.8 10 11
DOACs do not require therapeutic monitoring of anticoagulant effect, unlike VKAs.11 12 However, this does not obviate the need for regular clinical review and dose titration in CKD.13 14 One may speculate that, in comparison to people receiving VKAs, a reduced frequency of interaction with healthcare professionals might lead to reduced treatment adherence in people on DOACs. Thus, we undertook a comprehensive systematic review and meta-analysis of DOAC monitoring, adherence and persistence in CKD.15
Methods
Study design
This study was designed in accordance with the Meta-analysis Of Observational Studies in Epidemiology.16 The protocol was peer-reviewed and registered (CRD42022344491) with the International Prospective Register of Systematic Reviews, PROSPERO.
Eligibility criteria
We included studies that assessed DOAC correct dosing, adherence and persistence among patients with AF and CKD with a participant age of 18 years and over. All studies with participants under the age of 18 years and any patient that did not have CKD and AF were excluded. Studies reporting participants on DOACs for reasons other than AF, such as deep venous thrombosis, pulmonary embolism, moderate or severe mitral stenosis, and mechanical valves were excluded.
Dialysis patients and those with CKD stage 5 were excluded from the systematic review as current guidelines state that DOACs should not be routinely prescribed to patients with a creatinine clearance <15 mL/min.6 7 16
We excluded case reports/series, systematic literature reviews and conference posters. Studies that were not published in English language, or that used subjective adherence measures, such as the Morisky Medication Adherence Scale, or medication event monitoring systems were also excluded.17
Search strategy
We searched the PubMed, EMBASE and CINAHL databases from 30 June 2008 to 30 June 2022 (online supplemental table 1) with snowballing effect. We did not apply any language restrictions during our initial search, and our search terms included a combination of Medical Subject Headings (MeSH) terms and keywords including ‘atrial fibrillation’, ‘chronic kidney disease’, ‘adherence’, ‘persistence’, ‘direct oral anticoagulants (DOAC)’.
openhrt-2023-002340supp001.pdf (72.4KB, pdf)
Data extraction and screening
Information extracted included study design, population characteristics, sample size, definition and measure for the outcome measure. Multiple reports from the same studies were grouped during the data collection process and any duplicates were removed. In addition, we contacted authors of studies to request additional data that were not reported in the manuscript.
We conducted a two-stage screening process to improve the quality of the final dataset. In the first stage, two reviewers independently mapped abstracts against the inclusion criteria. Studies were retained if they met all criteria. Rejection of studies was on agreement between both reviewers. Any instances of disagreement were retained for full-text screening. In the second stage of screening, the full-text articles of each study retained from stage 1 were independently reviewed by both reviewers. Studies were retained or rejected according to consensus between the two reviewers as shown in the PRISMA diagram below. Any disagreements between the reviewers were resolved by discussion. We additionally searched the references of included studies to identify any further articles for screening and analysis.
Outcomes assessed
We extracted three primary variables: (1) fidelity of prescribing to recommended doses in CKD, (2) adherence to prescribed dose and (3) persistence of treatment. We assessed the fidelity of prescribing against manufacturers’ recommendations on dose titration in CKD (table 1).
Table 1.
Dosing criteria according to renal guidelines
| Dosing criteria for AF | Edoxaban | Apixaban | Dabigatran | Rivaroxaban |
| Normal dosing regime | 60 mg once per day | 5 mg two times per day | 150 mg two times per day | 20 mg once per day |
| Reduced dosing regime | 30 mg once per day | 2.5 mg two times per day | 110 mg two times per day | 15 mg once per day |
| Renal criteria for dose reduction | CrCl 15–50 mL/min | CrCl 15–30 mL/min or ≥2 of: Age>80 years, body weight ≤60 kg, creatinine ≥133 umol/L | CrCl 30–50 mL/min | CrCl 15–50 mL/min |
| Contraindication (renal) | CrCl ≤15 mL/min | CrCl ≤15 mL/min | CrCl ≤30 mL/min | CrCl ≤15 mL/min |
CrCI, creatinine clearance.
We assessed adherence using the proportion of days covered (PDC) over the year following the index date, according to the following formula, with good adherence defined as PDC>80%, in line with other studies.18 19
*Whichever is shorter.
We assessed persistence as the proportion of patients without any gaps longer than 90 days between prescriptions in the year following the index date.20 21
Study quality assessment
All studies were observational studies. As such, it was important to assess the risk of bias, reliability of the analysis and validity of the evaluation conducted. The Newcastle-Ottawa Scale (NOS) was used to conduct this assessment.22 NOS allows 9 points of risk bias associated with the study group, comparability within the groups based on outcomes as well as exposure and outcomes. A risk of bias table has been made available as online supplemental table 2. Studies were ranked according to low risk (7–9 stars), high risk (4–6 stars) or very high risk (1–3 stars) of bias.23
openhrt-2023-002340supp002.pdf (48.5KB, pdf)
Statistical analysis plan
A systematic synthesis was performed along with a meta-analysis. For the statistical synthesis, we grouped two or more studies that shared comparable outcome data (eg, ORs) and pooled these data in meta-analysis. Pairwise meta-analysis was performed using random effects model24 to account for variation in how the outcome measure was assessed. We reported the pooled estimates of effect with 95% CI.
Heterogeneity among the studies was determined using the Q-test; a p value <0.1 implies heterogeneity between the studies. A tau-squared value and I2 statistic with 95% CI were also reported, where an I2 value of <30%, 30%–59%, 60%–90% and more than 90% inferred low, moderate, substantial and considerable heterogeneity, respectively. To assess the risk of publication bias, we used funnel plots and performed Egger’s test to determine asymmetry. Data synthesis was performed in RStudio software, V.1.3.959, using the package metafor.25–27
Results
Study selection
A total of 837 studies were explored initially where 732 were retained following removal of duplicates. Following title and abstract review, 538 articles were excluded and 194 articles underwent full-text review. Studies were limited to those in the English language, and those published after 2008, the year DOACs were first licensed. We also excluded systematic reviews, clinical trials, conference abstracts, letters and case reports. Following this, 147 were excluded, leaving 19 eligible studies28–47 for systematic literature review. After assessing studies that used the same data sources, and therefore, with overlapping populations, we included seven studies28–34 in the meta-analysis, of which two dealt with medication adherence33 34 and five28–32 dealt with fidelity of prescribing to recommended doses (figure 1).
Figure 1.
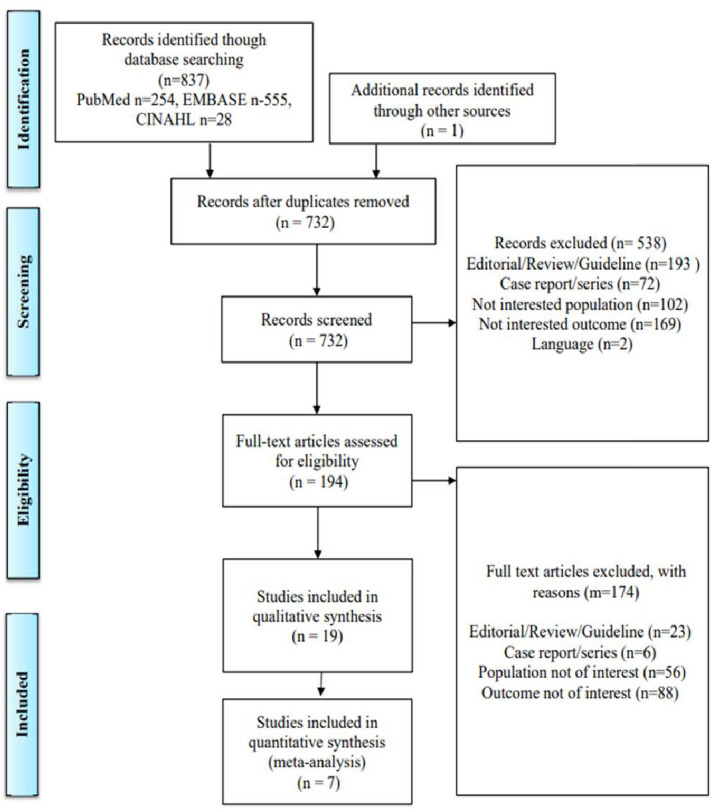
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram of studies identified by searches, screened and included in final analysis.
Study characteristics
Of the 19 studies,28–46 all were observational (table 2).
Table 2.
Studies in qualitative analysis
| Study name | Renal function (CrCl, eGFR or not specified) | Poss CKD specific/CKD criteria | Study type | Country | Length of study |
DOAC and if comparator | Total sample size /CKD sample size |
Outcome CKD DOAC dosing /adherence/persistence |
Primary care/community/hospital |
| 1. Briasoulis et al32 | CKD dose for 15–30 mL/min | AF specific/not CKD specific | Retrospective cohort | USA | 2010–2016 5 years |
Dabigatran rivaroxaban | Total 14 863 9221 CKD |
Correct dosing 52.74% |
Primary care |
| 2. Leef et al28 | CKD defined by eGFR into CKD 3–5 | AF/not CKD specific | Retrospective cohort | USA | 2003–2015 | Dabigatran rivaroxaban | 230 762 1312 CKD | Correct dosing 83.23% | Hospital and primary care |
| 3. Ting et al29 | CrCl<50 mL/min | CKD-specific -moderate/severe renal impairment including haemodialysis patients | Retrospective cohort | USA | June 2015–Dec 2018 Median follow-up 20 months |
Dabigatran edoxaban rivaroxaban apixaban | 207 CKD patients total | Correct dosing 70.5% | Hospital |
| 4. Erdogan et al31 | CKD defined as eGFR<50 | AF/not CKD specific | Cohort (25.2% retrospective/remaining prospectively) | Turkey | April 2017–May 2018 | Rivaroxaban apixaban dabigatran | 302 overall 105 CKD |
Correct dosing 60.95% |
Hospital outpatients |
| 5. Jackson et al30 | CrCl 30—50 mL/min | CKD specific -Moderate renal impairment CrCl 30–50 mL/min |
Retrospective cohort | USA | 20 Feb 2013 and 12 July 2016 2-year follow-up |
Rivaroxaban apixaban dabigatran edoxaban | 1134 CKD patients | 65.5% correct DOAC dosing | Hospital outpatients |
| 6. Banerjee et al33 | CrCl/eGFR not discussed but assumed CKD 3–5. | Not CKD Specific | Retrospective cohort | UK | 2011–2016 5 years |
Dabigatran edoxaban apixaban rivaroxaban | 36 652, of which 18 731 had CKD | Adherence (A)—overall 70.4%. Also, persistence 67.7% | Primary care |
| 7. Shore et al34 | CKD not defined | Not CKD Specific | Retrospective cohort | USA | October 2010–September 2012 | Dabigatran | 5372 of which 652 had CKD | 71.4% adherence | Primary care |
| 8. Choi et al44 | CKD not defined. CrCl not defined |
Not CKD Specific | Retrospective cohort | Korea | 1 July 2015–31 December 2016 15 months follow-up |
Dabigatran, rivaroxaban, apixaban | 56 504 of which 2566 had CKD | Mention that patients with CKD were underdosed. No statistical analysis given | Hospital and primary care |
| 9. Xing et al46 | CKD not defined. No CrCl defined |
Not CKD specific | Retrospective cohort | Denmark | 2011–2017 | Dabigatran, rivaroxaban, apixaban | 24 489 of which 892 had CKD | CKD dosing. Dose reduction seen in CKD. No analysis |
Hospital and primary care |
| 10. Akao et al38 | Dosing acc to CrCl 15 >100 mL/min | Not CKD specific | Retrospective cohort | Japan | Jan 2012–Jan 2019 | Dabigatran, rivaroxaban, apixaban and edoxaban | 32,713, of which 6325 CKD | Appropriate dosing/warfarin therapy associated with CKD/no analysis in CKD | Hospital |
| 11. Beshir et al36 | CKD not defined | Not CKD specific | Retrospective cohort | Malaysia | 2010–2015 | Dabigatran | Total 192 of which 9 patients CKD | Persistence | Hospital |
| 12. Guo et al45 | CKD classified. Used eGFR than CrCl |
Not CKD specific | Retrospective cohort | China | October 2014–Dec 2018 | Rivaroxaban dabigatran | 5742 of which 698 CKD | DOAC dosing. Incorrect dosing associated with CKD. Not enough analysis | Hospital |
| 13. Pagès et al43 | Definitions of CKD as stage 3,4,5 | Not CKD Specific |
Retrospective cohort | France | Sept 2016–August 2017 | Dabigatran, rivaroxaban and apixaban grouped together. | 405 of which 111 | Adherence | Hospital |
| 14. Dhamane et al35 | eGFR/CrCl not discussed | Not CKD specific | Retrospective cohort | USA | Jan 2012–June 2019 | Dabigatran, rivaroxaban, apixaban. Edoxaban excluded | 362 823 of which 58 804 | Persistence | Hospital and primary care |
| 15. Charlton et al40 | No CrCl given | Not CKD specific | Retrospective cohort | Spain | Jan 2009–Dec 2015 | Apixaban dabigatran rivaroxaban | 12 257 of which 1790 CKD | Adherence. Not enough data for CKD |
Primary care |
| 16. Piccini et al39 | eGFR rate<60 | Not CKD specific | Retrospective cohort | USA | Jan 2013– September 2017 |
Apixaban rivaroxaban dabigatran edoxaban | 33 235 of which 10 707 CKD | Correct dosing. Not enough data for CKD |
Hospital |
| 17. Sato et al37 | CrCl <50 mL/min | Not CKD Specific |
Retrospective cohort | Japan | Sept 2011–Jan 2016 | Dabigatran, rivaroxaban, apixaban and edoxaban | 2272 CKD 1460 |
Correct dosing. Not enough data for CKD |
Hospital |
| 18. Yeo et al42 | No CrCl given | Not CKD specific | Retrospective cohort | Singapore | 2010–2014 | No specific DOAC | 2299, of which 1139 CKD | Adherence. Grouped with clopidogrel and other anticoagulants |
Hospital |
| 19. Maura et al41 | No CrCl | Not CKD Specific | Retrospective observational | France | 2012–2013 | Rivaroxaban/dabigatran | 22 267 571 CKD |
Adherence. CKD looked at; no specific data given |
Hospital outpatients |
AF, atrial fibrillation; CKD, chronic kidney disease; CrCl, creatinine clearance; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate.
The studies included a total of 661 855 patients with AF taking DOACs of which 19.3% (n=128 406) had CKD. Ten studies28–32 37 38 44–46 reported monitoring, six33 34 39–43 reported adherence and three33 35 36 reported persistence. Information required for meta-analysis was only found in seven studies with 252 117 patients, five on correct dosing28–32 and two on adherence.33 34 Within these studies, only 3.5% (9800) of the patients had CKD. Four of the studies were from USA,28–30 32 34 one from Asia31 and one from Europe.33 The earliest data was collected from 2010, and the latest from 2019, with the publications ranging from 2014 to 2021. Five studies were from primary or community care alone,32–34 40 four were from a combination of primary and secondary care,28 35 44 46 and the remainder were from hospital outpatients. There were only two studies which were CKD-specific29 30 but these were relatively small, with 207 and 1134 patients only; the data from others was a subanalysis of AF studies with DOACs not specific to CKD. Most compared the findings to VKA.
Study quality
The quality of the studies was generally good. The mean quality score of the studies using the Newcastle-Ottawa Quality Assessment Scale was 7.2 (median=7). All studies scored 7 to 9 for the quality assessment and, hence, had a low risk of bias (online supplemental table 2). However, the majority of the studies did not provide details relating to the loss of individuals during the follow-up period.
Correct dosing
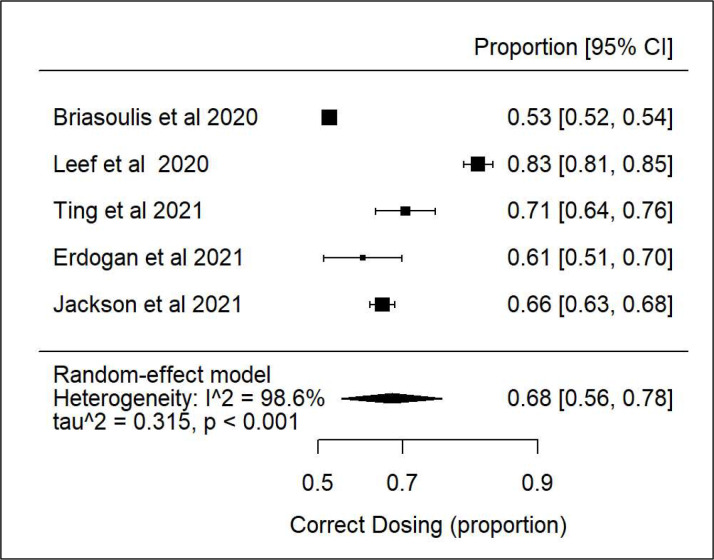
Our meta-analysis on correct dosing of the five studies28–32 showed that there was high heterogeneity and that 68% of patients with CKD and AF had correct dosing (figure 2).
Figure 2.
Forest plot: correct direct oral anticoagulant dosing according to creatinine clearance.
Sub-analysis
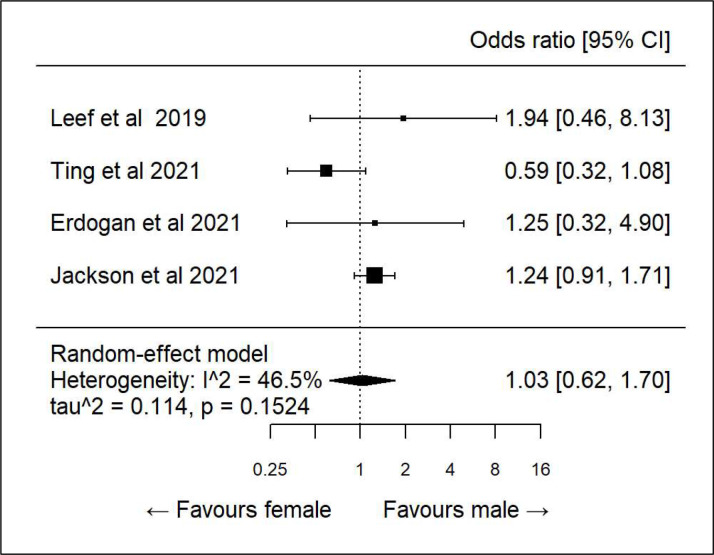
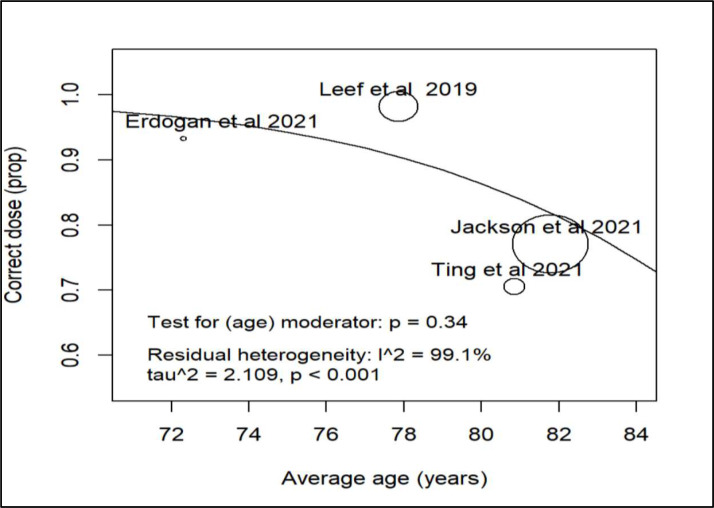
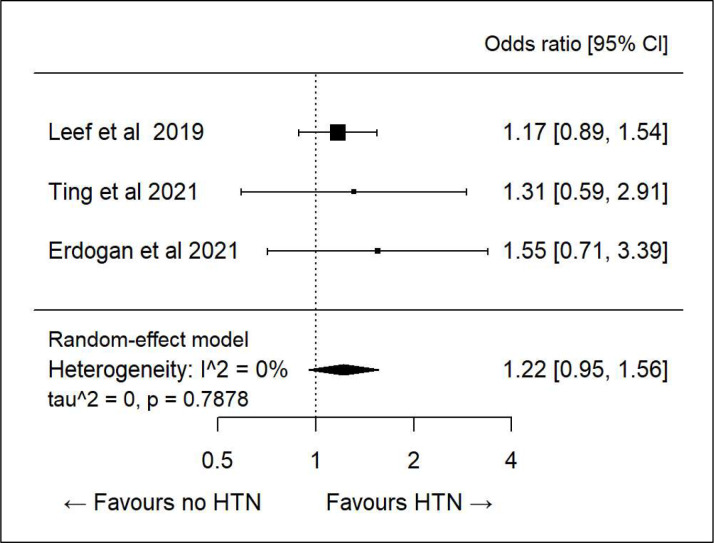
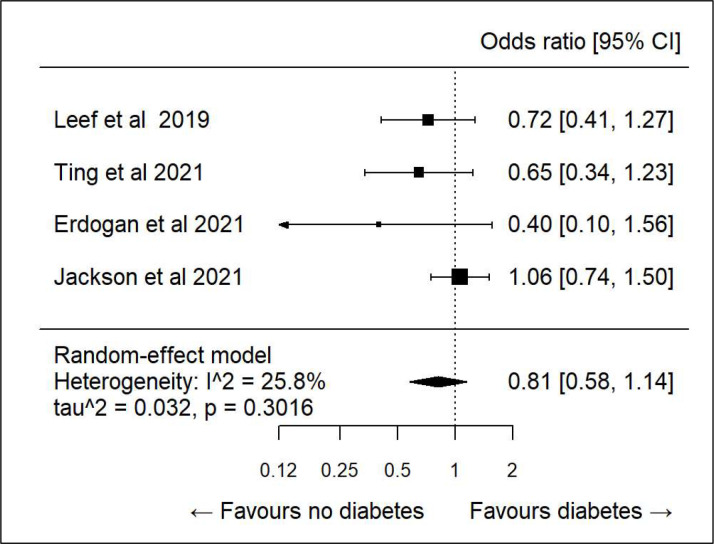
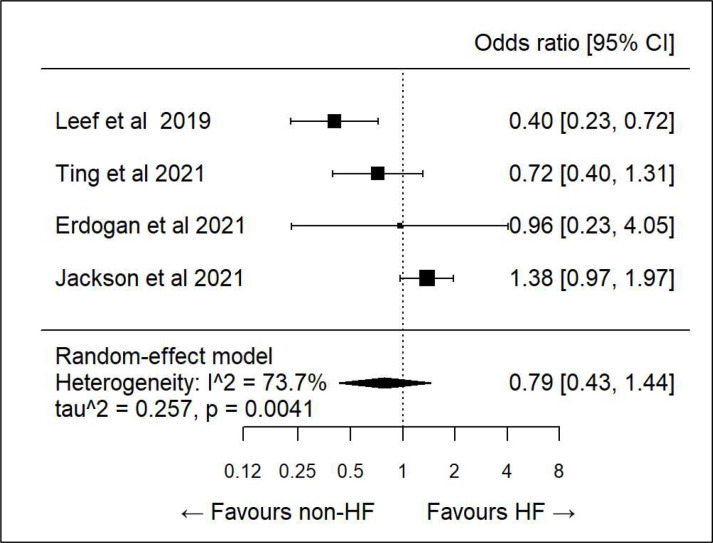
We did further subanalysis on various demographics to see if there was any association between specific determinants and correct dosing. There was only sufficient data to evaluate age, sex, diabetes, hypertension and heart failure. All showed high heterogeneity and hence any association was inconclusive (figures 3–7).
Figure 3.
Subanalysis correct direct oral anticoagulant dosing and sex.
Figure 4.
Subanalysis correct direct oral anticoagulant dosing and age.
Figure 5.
Subanalysis: correct direct oral anticoagulant dosing and hypertension (HTN).
Figure 6.
Subanalysis: correct direct oral anticoagulant dosing and diabetes.
Figure 7.
Subanalysis: correct dosing and heart failure (HF).
Adherence
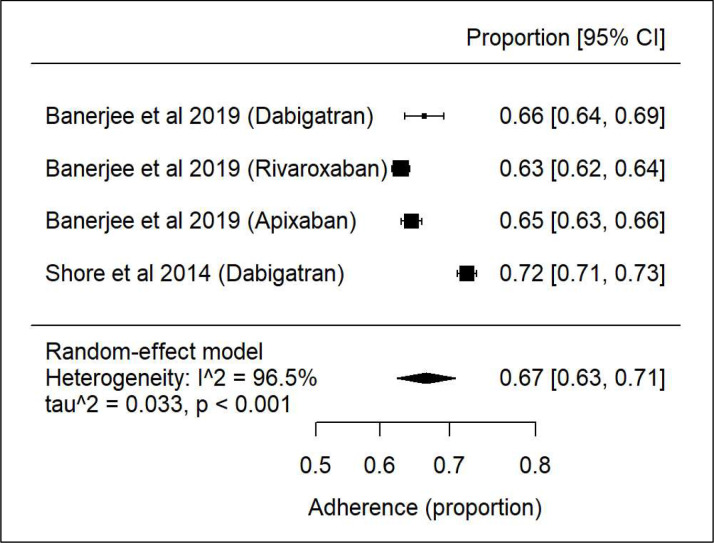
Again, there was high heterogeneity33 34 (figure 8) with 67% of patients’ adherent to DOACs. Subanalysis of the two papers33 34 with reference to adherence was not possible as there were not sufficient data.
Figure 8.
Forest plot showing adherence of direct oral anticoagulants in chronic kidney disease and atrial fibrillation.
Persistence
Only three papers.33 35 36 looked at DOAC persistence in CKD and AF. However, as CKD was not part of their original primary outcome this was not evaluated, and their definitions of persistence differed.
It was difficult to make any combined analysis. Banerjee et al33 found that 67.7% of patients persisted on anticoagulants for a full year after the index prescription, corresponding to 61.8% of patients on dabigatran, 74.7% on rivaroxaban and 81.6% apixaban compared with 63.6% of VKA patients. However, sample size was not reported for each specific DOAC, but only for the overall CKD cohort. Beshir et al36 had a small sample size of 195 and overall persistence in AF was 86.5% at 1 year and 83.4% at 2 years. This was relatively better than VKA (warfarin) which was 83.4%. However, only 9 AF patients had CKD and out of this 55.5% (n=5) had good persistence.
In Dhamane et al,35 66 826 patients had CKD. Of these, 55.6% were on apixaban, 21.0% were on rivaroxaban and 3.9% were on dabigatran. CKD was associated with non-persistence with HR 1.02 but no other details on sample sizes were found in the study.
Publication bias
The funnel plot for correct dosing showed some asymmetry on visual inspection (online supplemental figure 1). However, Egger’s test for a regression intercept found a p value of 0.9042 which implies a low probability of publication bias. As the number of studies in the meta-analysis was small, no sensitivity analysis was done.
openhrt-2023-002340supp003.pdf (125KB, pdf)
Discussion
This systematic review found that seven papers were suitable for meta-analysis with most addressing correct DOAC dosing and the remainder focusing on DOAC adherence. There were no papers to compare persistence. Despite an abundance of publications on the use of DOACs in AF, there is a lack of data currently available in the context of AF and CKD.
Correct DOAC dosing
Our analysis shows that 68% of patients with correct DOAC dosing is in line with other studies for overall correct DOAC dosing in AF.37 48 This has increased since the DOACs was initially introduced. Most systematic literature reviews in this area have focused on risk of bleeding and stroke38–40 on the assumption that real-world DOAC dosing mirrors the adherence and persistence typical of clinical trials. This is the first systematic literature review and meta-analysis which is specific to DOAC monitoring in CKD and AF. It was not possible to do subanalysis of the various dosing structures of standard and low doses of each DOAC as the studies did not divide it according to CKD groups 3–4 which covers the overarching grouping of CKD for which DOACs are licensed; the lower doses will generally be in those with CKD; some may be in those without CKD if they are older or have low body weights as in the case of apixaban according to guidelines. There were too few studies available to assess correct dosing by individual drug.
Adherence
The DOAC adherence was suboptimal but similar to other studies with AF overall.33 41 49 However, this is much lower than other chronic disease medications.50 Very few studies had adherence at CKD level and hence limited data is available. However, the findings of Shore et al34 showed good adherence to dabigatran.34 This was done very early on in 2014 when DOACs had recently been introduced, and hence patients may have been seen more often which increased adherence. In addition, the numbers of patients involved on dabigatran with CKD are much less due to stringent guidelines with respect to creatinine clearance. Eighty per cent to 85% of dabigatran is excreted by the kidneys via glomerular filtration, and hence it can only be used in moderate renal impairment unlike the factor Xa inhibitors (apixaban, rivaroxaban and edoxaban). There were no adequate studies on adherence in CKD and AF as we only had two studies to show a link between non-adherence and worsening kidney disease.
Adherence with two times per day dosing regimens is assumed to be lower than with once per day regimens in real-world settings and in patients with a high pill burden. Conversely, two times per day dosing is expected to deliver a more stable anticoagulant effect over the course of 24 hours. A meta-analysis of the four key efficacy RCTs of DOAC in AF revealed that two times per day dosing provided a better benefit–risk equation than once per day dosing. The economic impact of non-adherence is well documented with significantly higher annual adjusted per-patient medical costs (inpatient and outpatient).43
We found no associations between adherence and any determinants. Although it has been shown in other studies that increasing comorbidity (by CHA2DS2VASc) was associated with decreased likelihood of non-adherence.33 41 Age ≥75 years, diabetes, female gender and anaemia were also associated with reduced risk of non-adherence, while hypertension and vascular disease were associated with increased risk. Adherence was non-linearly associated with time since the introduction of DOACs, increasing for approximately 2 years (to early 2013) before starting to decrease, returning to its original level by early 2015 and then dropping below its original level.
Persistence
Beshir et al36 and Banerjee et al33 differed on the documentation of what persistence was with the former deeming individuals to be persistent if no gaps of >90 days appear in the prescription history in the year following the index date while the latter felt it was >60 days following the index date. Thus, only qualitative analysis was possible. The limited findings in both studies were consistent with those of other studies in AF overall that showed persistence in DOACs to be better than VKAs.30 32
Dhamane et al35 also deemed patients persistent with no gaps >60 days and said non-persistence was affected by CKD with HR 1.02 but no further details were given.35
Further analysis at drug level was not possible overall for comparison but Banerjee et al33 reported a higher risk of non-persistence among dabigatran users than rivaroxaban and warfarin users. The two times per day dosing of dabigatran was thought to be a possible explanation for these observations. However, patients receiving apixaban, which also has a two times per day dosing regimen, had a lower risk of non-persistence than those receiving rivaroxaban and warfarin. This suggests that factors other than the dosing regimen play a major role in the lower persistence associated with dabigatran.
No studies have shown the determinants of persistence in CKD and AF. However, in just AF, heart failure, vascular disease, CKD, prior bleeding and alcohol misuse were associated with increased risk of non-persistence, while hypertension and age >65 years were associated with reduced risk.33 41 Although the persistence rate of DOACs was higher than VKA, suboptimal persistence with DOAC therapy remains a great concern for patients with AF and CKD.
Limitations and strengths
Our study has several strengths. There is a high level of congruence between our findings and those reported in the existing literature. This is a timely systematic review that synthesises the evidence on extent of poor adherence to oral anticoagulants, its determinants, and clinical and economic outcomes, among patients with AF and CKD. We focused on mainly observational studies to evaluate the evidence on patients’ real-world medication-taking behaviour. We considered all oral anticoagulants, including the newer drugs (apixaban, rivaroxaban, dabigatran and edoxaban), and aimed to generate pooled adherence at the individual drug level.
Our study also had some limitations. First and foremost, there were very few studies specifically on combined CKD, AF and DOAC. Second, there was heterogeneity of the included studies, possibly due to variations in definitions of adherence, small numbers, as well as follow-up durations. We tried to conduct subgroup analyses to pool the same definitions, however, residual heterogeneity persisted. Third, there was no universal tool available to assess the risk of bias for systematic reviews of observational studies. We used the Newcastle-Ottawa Scale, a commonly used tool, however, due to the similar methodology for our included studies; this tool did not differentiate between study quality and rated all included studies at the level of good quality. Finally, the included studies used scripts prescribed than dispensed, which does not necessarily mean they were taken. It is therefore possible that adherence and persistence rates are even lower than from the papers.
Conclusion
Adherence and correct dosing to DOACs were suboptimal in the pooled studies with respect to CKD and AF. Future research is needed as the lack of statistically significant sample sizes prevents the generalisability of findings. This is a rate-limiting factor for improved clinical and patient-reported outcomes. Insufficient data was available on persistence in CKD and AF to make any conclusions. As the ageing population increases with the identification of AF and CKD, there is greater importance of clinician awareness to DOAC adherence, persistence and appropriate dosing and its association to sex, age, ethnicity, presence or absence of comorbidities and CHA₂DS₂-VASc scores.
Footnotes
Contributors: SE, RAK, GYHL, BCTF, SdL: conceptualisation, methodology and writing-original draft preparation. RAK, GD: supervision, investigation and writing-original draft preparation. SE and RAK performed the searches and extracted data from the eligible studies. SE and RAK assessed the quality of the studies, and SE conducted the statistical analysis with critical appraisal from GD and RAK. GD and RAK contributed equally to the draft. SE led the drafting of the manuscript, with review and contributions from RAK, GD, BCTF, GYHL and SdL. All authors contributed to the article and approved the submitted version. SE is responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data used in this study has been shown as supplementary material.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Vora P, Morgan Stewart H, Russell B, et al. Time trends and treatment pathways in prescribing individual oral anticoagulants in patients with nonvalvular atrial fibrillation: an observational study of more than three million patients from Europe and the United States. Int J Clin Pract 2022;2022:6707985. 10.1155/2022/6707985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 5.NICE . Atrial fibrillation: diagnosis and management. 2021. Available: https://www.nice.org.uk/guidance/ng196
- 6.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American college of cardiology/American heart Association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of Thoracic Surgeons. Circulation 2019;140:e125–51. 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 2022;12:7–11. 10.1016/j.kisu.2021.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLOS ONE 2016;11:e0158765. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ocak G, Khairoun M, Khairoun O, et al. Chronic kidney disease and atrial fibrillation: a dangerous combination. PLOS ONE 2022;17:e0266046. 10.1371/journal.pone.0266046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen N-N, Zhang C, Wang N, et al. Effectiveness and safety of under or over-dosing of direct oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of 148909 patients from 10 real-world studies. Front Pharmacol 2021;12:645479. 10.3389/fphar.2021.645479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connors JM. Testing and monitoring direct oral anticoagulants. Blood 2018;132:2009–15. 10.1182/blood-2018-04-791541 [DOI] [PubMed] [Google Scholar]
- 14.Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in Anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2017;70:2621–32. 10.1016/j.jacc.2017.09.1087 [DOI] [PubMed] [Google Scholar]
- 15.Rhee T-M, Lee S-R, Choi E-K, et al. Efficacy and safety of oral anticoagulants for atrial fibrillation patients with chronic kidney disease: a systematic review and meta-analysis. Front Cardiovasc Med 2022;9:885548. 10.3389/fcvm.2022.885548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 17.Aursulesei V, Costache II. Anticoagulation in chronic kidney disease: from guidelines to clinical practice. Clin Cardiol 2019;42:774–82. 10.1002/clc.23196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raebel MA, Schmittdiel J, Karter AJ, et al. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care 2013;51:S11–21. 10.1097/MLR.0b013e31829b1d2a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes CA, Deshpande S, Sorio-Vilela F, et al. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin 2018;34:1613–25. 10.1080/03007995.2018.1477747 [DOI] [PubMed] [Google Scholar]
- 20.Cataldo N, Pegoraro V, Ripellino C, et al. Non-persistence risk and health care resource utilization of Italian patients with non-valvular atrial fibrillation. Recenti Prog Med 2018;109:113–21. 10.1701/2865.28904 [DOI] [PubMed] [Google Scholar]
- 21.Ribic C, Crowther M. Thrombosis and anticoagulation in the setting of renal or liver disease. Hematology American Society of Hematology Education Program 2016;2016:188–95. 10.1182/asheducation-2016.1.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA, O’Connell D, Peterson J, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of Nonrandomised studies in meta-analyses. 2010. Available: http://www.ohri.ca/programs/ clinical_ epidemiology/oxford.asp
- 23.Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers to authors’ assessments. BMC Med Res Methodol 2014;14:45. 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 25.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 27.RStudio Team . RStudio: integrated development environment for R. PBC. Boston, MA: RStudio, 2020. Available: http://www.rstudio.com [Google Scholar]
- 28.Leef GC, Perino AC, Askari M, et al. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation: insights from the veterans health administration. J Pharm Pract 2020;33:647–53. 10.1177/0897190019828270 [DOI] [PubMed] [Google Scholar]
- 29.Ting C, Rhoten M, Dempsey J, et al. Evaluation of direct oral anticoagulant prescribing in patients with moderate to severe renal impairment. Clin Appl Thromb Hemost 2021;27:1076029620987900. 10.1177/1076029620987900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson LR, Schrader P, Thomas L, et al. Dosing of direct oral anticoagulants in patients with moderate chronic kidney disease in US clinical practice: results from the outcomes Registry for better informed treatment of AF (ORBIT-AF II). Am J Cardiovasc Drugs 2021;21:553–61. 10.1007/s40256-021-00473-x [DOI] [PubMed] [Google Scholar]
- 31.Erdogan T, Erdogan O, Ozturk S, et al. Non-vitamin K antagonist oral anticoagulant use at doses inappropriately lower than recommended in outpatient older adults: a real-life data. Eur Geriatr Med 2021;12:809–16. 10.1007/s41999-021-00452-0 [DOI] [PubMed] [Google Scholar]
- 32.Briasoulis A, Gao Y, Inampudi C, et al. Characteristics and outcomes in patients with atrial fibrillation receiving direct oral anticoagulants in off-label doses. BMC Cardiovasc Disord 2020;20:42. 10.1186/s12872-020-01340-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee A, Benedetto V, Gichuru P, et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: a population-based study. Heart 2020;106:119–26. 10.1136/heartjnl-2019-315307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the veterans health administration. Am Heart J 2014;167:810–7. 10.1016/j.ahj.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhamane AD, Hernandez I, Di Fusco M, et al. Non-persistence to oral anticoagulation treatment in patients with non-valvular atrial fibrillation in the USA. Am J Cardiovasc Drugs 2022;22:333–43. 10.1007/s40256-021-00501-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beshir SA, Chee K-H, Lo Y-L. Factors associated with abrupt discontinuation of dabigatran therapy in patients with atrial fibrillation in Malaysia. Int J Clin Pharm 2016;38:1182–90. 10.1007/s11096-016-0350-1 [DOI] [PubMed] [Google Scholar]
- 37.Sato T, Aizawa Y, Kitazawa H, et al. The characteristics and clinical outcomes of direct oral anticoagulantsin patients with atrial fibrillation and chronic kidney disease: from the database of a single-center registry. J Atr Fibrillation 2020;13:2308. 10.4022/jafib.2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akao M, Shimizu W, Atarashi H, et al. Oral anticoagulant use in elderly Japanese patients with non-valvular atrial fibrillation― subanalysis of the ANAFIE Registry. Circ Rep 2020;2:552–9. 10.1253/circrep.CR-20-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccini JP, Xu H, Cox M, et al. Adherence to guideline-directed stroke prevention therapy for atrial fibrillation is achievable. Circulation 2019;139:1497–506. 10.1161/CIRCULATIONAHA.118.035909 [DOI] [PubMed] [Google Scholar]
- 40.Charlton A, Vidal X, Sabaté M, et al. Factors associated with primary nonadherence to newly initiated direct oral anticoagulants in patients with Nonvalvular atrial fibrillation. J Manag Care Spec Pharm 2021;27:1210–20. 10.18553/jmcp.2021.27.9.1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maura G, Pariente A, Alla F, et al. Adherence with direct oral anticoagulants in nonvalvular atrial fibrillation new users and associated factors: a French nationwide cohort study. Pharmacoepidemiol Drug Saf 2017;26:1367–77. 10.1002/pds.4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeo S-H, Toh M, Lee SH, et al. Impact of medication nonadherence on stroke recurrence and mortality in patients after first-ever ischemic stroke: insights from registry data in Singapore. Pharmacoepidemiol Drug Saf 2020;29:538–49. 10.1002/pds.4981 [DOI] [PubMed] [Google Scholar]
- 43.Pagès A, Sabatier R, Sallerin B. Factors associated with the choice of oral anticoagulant class in the older patients: an observational study. J Cardiovasc Pharmacol Ther 2020;25:332–7. 10.1177/1074248420917811 [DOI] [PubMed] [Google Scholar]
- 44.Choi Y-J, Uhm J-S, Kim T-H, et al. Differences in anticoagulation strategy and outcome in atrial fibrillation patients with chronic kidney disease: a CODE-AF registry study. Int J Arrhythm 2020;21. 10.1186/s42444-020-0011-2 [DOI] [Google Scholar]
- 45.Guo Y, Kotalczyk A, Imberti JF, et al. Poor adherence to guideline-directed anticoagulation in elderly Chinese patients with atrial fibrillation: a report from the optimal Thromboprophylaxis in elderly Chinese patients with atrial fibrillation (ChiOTEAF) registry. Eur Heart J Qual Care Clin Outcomes 2023;9:169–76. 10.1093/ehjqcco/qcab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xing LY, Barcella CA, Sindet-Pedersen C, et al. Dose reduction of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: a Danish nationwide cohort study. Thromb Res 2019;178:101–9. 10.1016/j.thromres.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 47.Cho MS, Yun JE, Park JJ, et al. Outcomes after use of standard- and low-dose non-vitamin K oral anticoagulants in Asian patients with atrial fibrillation. Stroke 2018:STROKEAHA118023093. 10.1161/STROKEAHA.118.023093 [DOI] [PubMed] [Google Scholar]
- 48.Joy M, Williams J, Emanuel S, et al. Trends in direct oral anticoagulant (DOAC) prescribing in English primary care (2014–2019). Heart 2023;109:195–201. 10.1136/heartjnl-2022-321377 [DOI] [PubMed] [Google Scholar]
- 49.Ozaki AF, Choi AS, Le QT, et al. Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2020;13:e005969. 10.1161/CIRCOUTCOMES.119.005969 [DOI] [PubMed] [Google Scholar]
- 50.Lemstra M, Nwankwo C, Bird Y, et al. Primary nonadherence to chronic disease medications: a meta-analysis. Patient Prefer Adherence 2018;12:721–31. 10.2147/PPA.S161151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002340supp001.pdf (72.4KB, pdf)
openhrt-2023-002340supp002.pdf (48.5KB, pdf)
openhrt-2023-002340supp003.pdf (125KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data used in this study has been shown as supplementary material.