Abstract

The utility of carbazole in photo-, electro-, and medicinal applications has ensured its widespread use also as the backbone in tridentate pincer ligands. In this review, the aim is to identify and illustrate the key features of the LNL-carbazolide binding to transition metal centers (with L = flanking donor moieties, e.g., C, N, P, and O-groups) in a systematic bottom-up progression to illustrate the marked benefits attainable from (i) the rigid aromatic carbazole scaffold (modulable in both the 1,8- and 3,6-positions), (ii) the significant electronic effect of central carbazole-amido binding to a metal, and the tunable sterics and electronics of both the (iii) flanking donor L-moieties and (iv) the wingtip R-groups on the L-donors, with their corresponding influence on metal coordination geometry, d-electron configuration, and resultant reactivity. Systematic implementation of the ligand design strategies not in isolation, but in a combinatorial approach, is showcased to demonstrate the potential for functional molecules that are not only modulable but also adaptable for wide-ranging applications (e.g., stereoselective (photo)catalysis, challenging small molecule activation, SET and redox applications, and even applications in chemotherapeutics) as an indication of future research efforts anticipated to stem from this versatile pincer assembly, not only for the transition metals but also for s-, p-, and f-block elements.
1. Introduction
1.1. Carbazole: the Core of the Privileged Pincer
The unique properties of the nitrogen-containing tricyclic 9H-carbazole prompted its rapid development in various disciplines such as photo-,1−5 electro-,6,7 and medicinal chemistry.1,3,8 The synthesis of the carbazole backbone itself has been extensively documented,3 and the low cost of the precursor carbazole renders elaborate backbone modification economically viable.3,9−22
The rigid and stable planar heterocycle boasts with proficient electron donating ability, charge transfer functionality, and excellent biocompatibility.1,7,23,24 Its efficient hole transporting capability7,25,26 has equated to carbazoles’ success in the fields of photo-1−5,11,27,28 and electrochemistry,6,10,29−31 also in donor–acceptor systems crucial toward preparation of organic light emitting diodes (OLEDs)23,31−35 with photoswitching ability.36 The smart electro- and photoactive application of carbazole extends further, with successful utilization in polymers and semiconducting polymers,5,15,24,37−43 electrochemiluminescence,44 and tailor-made photo(redox) catalysis.45−47 Carbazole-based organic compounds have also featured prominently in the field of medicinal chemistry spanning application as anticancer,8,48−54 antifungal,50,55,56 antioxidant,57,58 antiviral,59 anti-inflammatory,58,60 and antibacterial agents.50,55,61,62
This review is based on the distinctive use of the 9H-carbazole as the central moiety in the motif of a pincer ligand design, that can exploit the versatility of carbazole applications as listed above, while introducing the inherent advantages of pincer ligands coordinated to a relevant metal center.
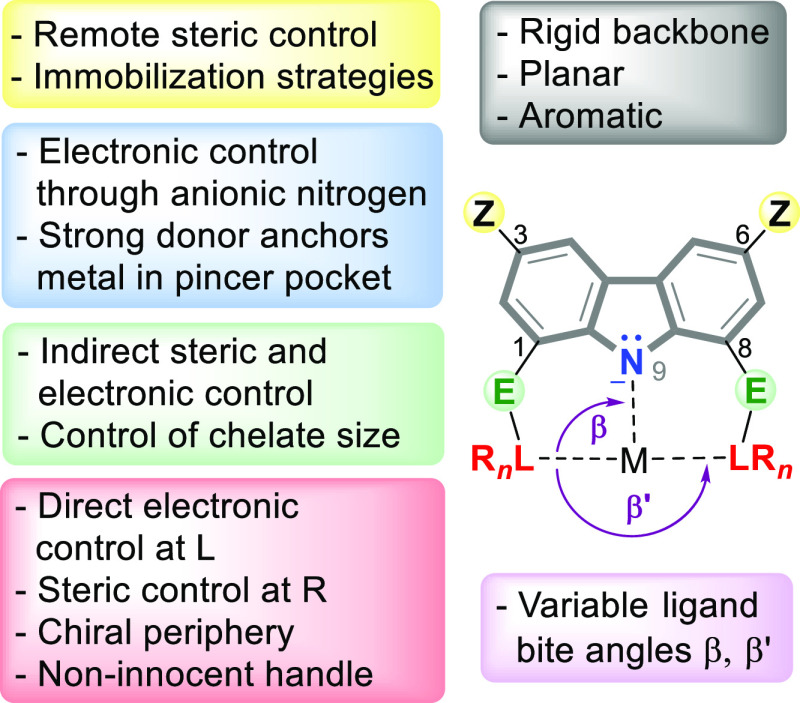
1.2. Inherent Benefits of the Carbazole-Based Pincer Ligand
The privileged pincer ligand platform has indulged a plethora of elements, reactivities, and applications.63−72 It has been widely celebrated for the stabilization it imparts to the chelated entity, and more recently, ligand noninnocence accessible through tailored ligand design.73−82 This includes redox noninnocence83−88 and ligand-metal-mediated processes,89−98 which have witnessed an exponential surge in interest due to the reactivity accessible through this multipronged approach to bond activation. Control over 5- or 6-membered chelation at position E (as exemplified for the carbazole-based pincer ligand shown in Figure 1) extends the range of reactivity available with a pincer ligand in hand, while introduction of chirality at R could lead to stereoselective processes.99−107 Immobilization strategies are another possibility, usually by modifying the backbone of the ligand at position Z (see Figure 1 per illustration), leading to an immobilized catalyst retaining its selectivity and reactivity while being recycled several times.108−112 These attributes, among others, have rendered pincer ligands an attractive platform from which to prepare tailored complexes.
Figure 1.
General representation of LNL-carbazolide ligand under review.
Assembly of carbazole in a pincer allows for a tailored ligand that harnesses the unique properties of carbazole constituting the backbone of the tridentate ligand. Subsequent coordination to a metal or main group element endows unique reactivity to the coordinated species, capitalizing on a pincer environment complemented by the use of a carbazole scaffold. Ligand fine-tuning is further realized through facile modification of control points Z, E, L, and R (Figure 1). These attributes have led the way during the design and synthesis of LNL-carbazolide coordinated complexes, with the isolated species finding use in a myriad of applications, including small molecule activation, stoichiometric transformation, catalysis, and photoluminescence, which are detailed throughout this review. One of the cornerstones of the tridentate LNL-carbazolide is its inherent stabilizing properties as illustrated throughout this review, conferring adequate stabilization to reactive and even elusive species leading to its subsequent isolation. In this respect, the rigid, aromatic carbazole scaffold (modulable in both the 1,8- and 3,6-positions) provides enhanced stability as a result of the connection of the flanking (E)L-donor groups to aromatic sp2-carbon atoms on the 1,8-positions of carbazole, yet wide variation in the bite angles of (E)L–M–N and L–M–L (β and β′, respectively, Figure 1) can be achieved as shown throughout this text.
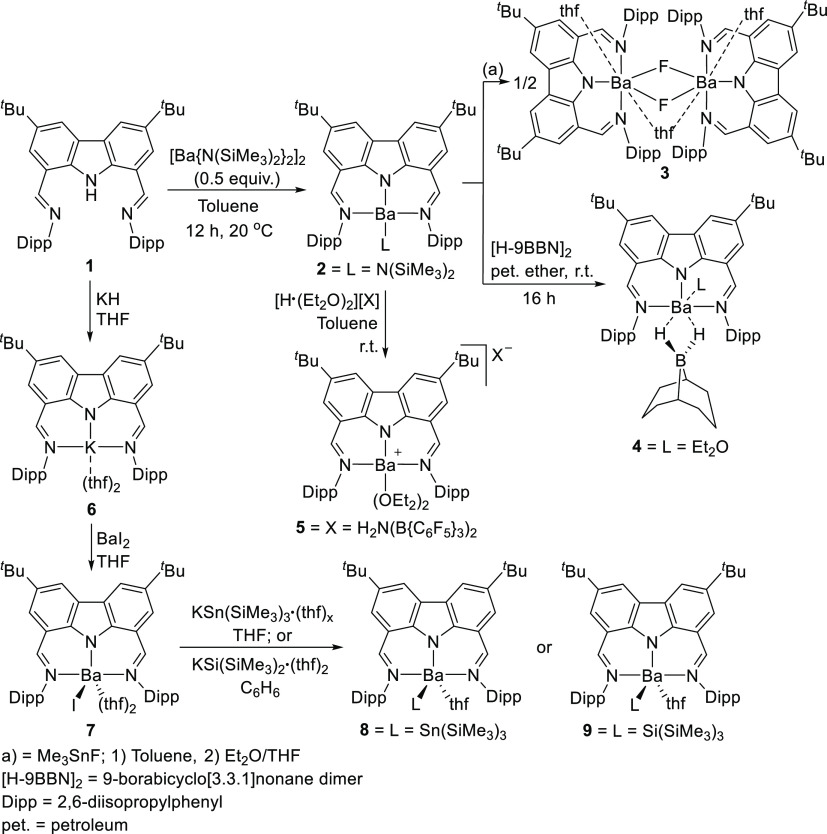
An example of the stabilizing properties of the carbazolide pincer was recently expressed through the synthesis and isolation of molecular barium fluoride and barium stannylide complexes (3 and 8, respectively, Scheme 1); a class of compounds previously inaccessible due to inefficient stabilization.113 Both thermally stable group 1 metal complexes (Li, Na, and K)114 and 3d-transition metal complexes (e.g., Co as a hydrophosphination catalyst precursor)115 containing the bis(imine)carbazole pincer ligand 1 have been previously reported. When employed to tame the group 2 metals, the resulting barium complex 2 displays catalytic performance comparable to the best catalyst in the benchmark catalytic hydrophosphination of styrene with HPPh2.113 The use of bis(imine)carbazole 1 inhibits ligand redistribution via the Schlenk equilibrium, a decomposition pathway plaguing the oxophilic and ionic alkaline earth metal complexes, allowing for the isolation of molecular barium complexes 3–5 and 7–9 (Scheme 1).116−119 Ligand scrambling of 2 was inhibited, even at 80 °C. In fact, only in the presence of excess ligand and at a reaction temperature of 80 °C did the homoleptic complex form. A range of Ba113,120,121 and analogous group 2 (Mg, Ca, and Sr)120 complexes were reported, with further reactivity studies on the Ca120 and Ba ( Scheme 1 and Scheme 38, vide infra)113,120,121 complexes providing insight into this scarcely reported class of compounds (see section 3.3.1 below).116−119
Scheme 1. Bis(imine)carbazolide Sufficiently Stabilizing Reactive Barium Complexes.
Scheme 38. NNN-Carbazolide Barium Complexes with Imine Donor Groups.
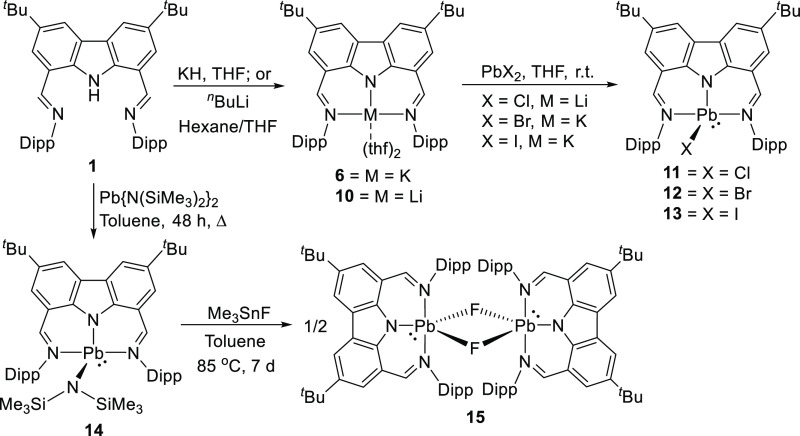
Yet another hallmark of pincer ligands, including the carbazolide pincer, is its ability to solubilize complexes otherwise insoluble in most solvents, as was the case for a recently reported class of lead(II) complexes.122 The chemistry of molecular lead is overshadowed by reports of metallic lead formation due to the decomposition of its organometallic complexes, in addition to its poor solubility generally forming insoluble precipitates.123−132 However, the carbazolide sufficiently stabilizes various lead(II) halide complexes (11–13, Scheme 2), while it was reported that these complexes are well soluble in solvents of low polarity, such as aromatic hydrocarbon and ether solvents.122 A rare example of a molecular lead(II) fluoride 15 was isolated by reacting 14 with the fluorinating reagent Me3SnF for 7 days at 85 °C (Scheme 2).
Scheme 2. Toward Stable and Soluble Molecular Lead(II) Halides and a Dimeric Lead(II) Fluoride.
These examples of the inherent benefits to be gained from the use of the carbazolide scaffold, and indeed the majority of the reports reviewed here, demonstrate the coordination of the LNL-carbazolide pincer ligand in the expected meridional geometry as per the definition of pincer ligands,133 as a result of the planar, rigid carbazole backbone. However, the scaffold is sufficiently flexible to allow for facial coordination if enforced by the coordination environment, as demonstrated by singular examples with hindered rotation of the donor L and wingtip R groups of the LNL-carbazolide pincer, with the use of coligands such as pentamethylcyclopentadienyl (see section 4.3.2),134 or in trigonal bipyramidal molecular geometries (section 2.1),135 leading inevitably to significant changes in the bite angles of the donating ligand sites.
1.3. Scope of the Review
This contribution aims to highlight the modality and functionality of the LNL-pincer ligand featuring carbazole as the backbone motif, with pincer complex formation through coordination of the carbazole’s anionic nitrogen and the two flanking donor groups. Using relevant examples of complex formation with the tridentate ligand, we will use a “bottom-up” perspective to delineate the key features of the coordinated LNL-carbazolide in the following order: (1) the amido nitrogen and its effects on the metal; (2) variations to the flanking donor groups EL (with L = C, N, P, or O-donor ligands) and their influence on the metal, in addition to the size of the chelate controlled by E; and (3) the facile modification of the R wingtip groups to incorporate steric bulk, chirality, or even a noninnocent moiety. The constructed carbazole-based scaffold is evaluated in the broader context of related pincer metal complexes where relevant and showcased in selected examples portraying the summative effects of the different building blocks toward various processes. Only LNL-pincer ligands featuring a carbazole backbone as the central N-donor ligand will be considered. Furthermore, only complexes in which the LNL-carbazolide ligand coordinates to the metal at all three coordinating sites will be scrutinized (i.e., carbazolide complexes featuring mono- or bidentate coordination of the carbazole scaffold will not be discussed in this review).
2. Electronic Consequences of the Carbazole Backbone
2.1. Electronic Effects of the Carbazolide-Nitrogen
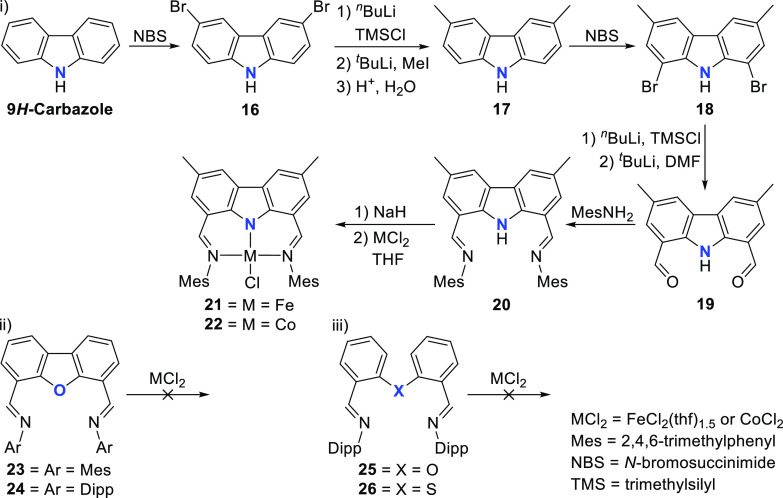
The report of Gibson et al. in 2003 already demonstrated the requirement for the donor amide of the carbazolide tridentate ligand toward pincer complex formation.136 The authors prepared the bis(imino)carbazole pincer ligand precursor 20, in addition to the analogue ligand featuring an oxygen (23 and 24) instead of the amido donor moiety (i and ii, Scheme 3). The dibrominated carbazole 18, accessed through bromination of the 3,6-dimethylcarbazole 17 with N-bromosuccinimide (NBS), was subjected to formylation via quenching of the 1,8-dilithiated intermediate with dimethylformamide (DMF), leading to 19. A Schiff-base condensation between the dialdehyde 19 and 2,4,6-trimethylaniline (MesNH2) yielded the precursor 20. Deprotonation of 20 with NaH followed by in situ coordination of the carbazolide to either FeCl2(thf)1.5 or CoCl2 yielded the complexes 21 and 22, respectively (i, Scheme 3). Contrasting this result, the attempted coordination of ligands 23–26 with either FeCl2(thf)1.5 or CoCl2 did not result in the formation of the targeted complexes, and only the starting material could be isolated (ii and iii, Scheme 3). For 20, the stronger donor amide in the five-membered pyrrolic heterocycle can be credited as one of the major contributing factors toward pincer complex formation compared to the softer neutral sulfur and oxygen (23–26) containing analogues, by securing the metal in the pincer pocket with strong amide coordination.136 It is worth mentioning that dibenzofuran-based pincer ligands featuring oxazoline donor groups instead of imines as in the analogues 23 and 24,137 or phosphines,138 did lead to successful pincer complexes with other metals such as nickel(II) prior to this report.
Scheme 3. Synthesis of (i) Bis(imine)carbazole Ligand and Reactions of Pincer Ligands (i–iii) with Fe or Co.
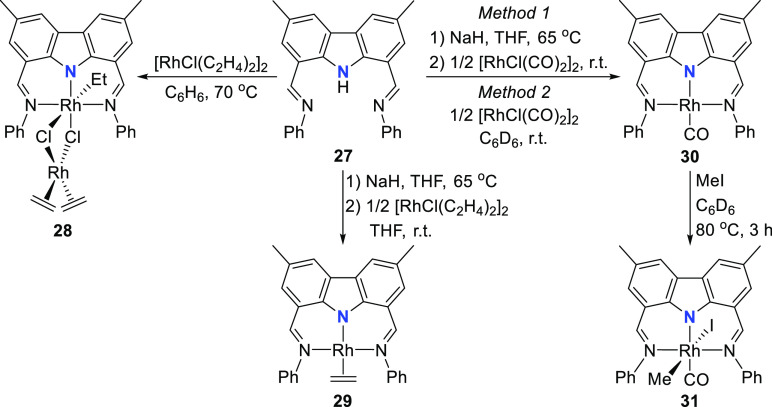
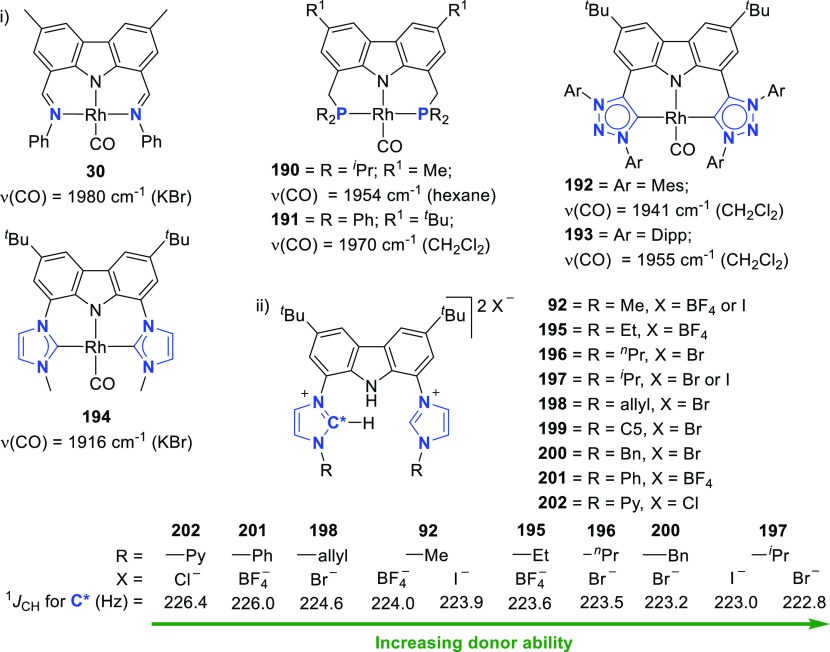
The anionic nitrogen donor of the carbazole-based NNN-pincer ligand was implicated in the fast oxidative addition of MeI across a rhodium(I) pincer coordinated metal center.139 Gibson, Haynes, and co-workers reported complex 30 to oxidatively add MeI across the metal 50 000 times faster compared to the carbonylation catalyst [RhI2(CO)2]− (Scheme 4). The authors disclosed the deprotonation of 27 with sodium hydride followed by in situ coordination of [RhCl(C2H4)2]2 and [RhCl(CO)2]2, leading to the isolation of the NNN-pincer coordinated rhodium(I) complexes 29 and 30, respectively. The rhodium carbonyl complex 30 could also be prepared through the direct metalation of ligand 27 with the rhodium precursor [RhCl(CO)2]2. Interestingly, reacting 27 with one equivalent of the dimeric precursor [RhCl(C2H4)2]2 yielded a dinuclear mixed valent complex 28 (Scheme 4). The dinuclear complex 28 features an NNN-pincer coordinated octahedral RhIII metal, bridged to a square planar RhI metal via two chlorido coligands. The carbonyl stretching frequency of 30 was measured to be 1980 cm–1,139 at a significantly higher energy than the rhodium(I) BIMCA (3,6-di-tert-butyl-1,8-bis(imidazol-2-ylidene-1-yl)carbazolide) complex 194 with a ν(CO) band at 1916 cm–1 (vide infra, Figure 5).140 Reacting 30 with excess MeI in C6D6 at room temperature resulted in slow formation of the octahedral rhodium complex 31, but complete conversion of 30 to 31 was noted after 3 h at 80 °C.139 The carbonyl stretching frequency shifted considerably from 1980 cm–1 for 30 to 2083 cm–1 for 31, an observation consistent with weaker back-donation going from square-planar RhI to octahedral RhIII complexes. Not only was the rate of oxidative addition faster than observed for the commercial carbonylation catalyst,141 but also it was reported to be faster than rhodium complexes coordinated by neutral donor ligands such as 2,2′-bipyridine,142 1,2-bis(diphenylphosphino)ethane,143,144 or PEt3.145
Scheme 4. Nucleophilic NNN-Carbazolide Accelerating MeI Oxidative Addition.
Figure 5.
Increasing ligand donor strength though modification of the flanking donor groups.
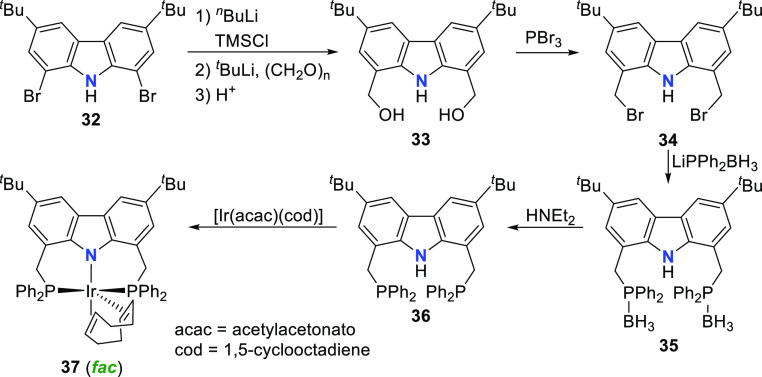
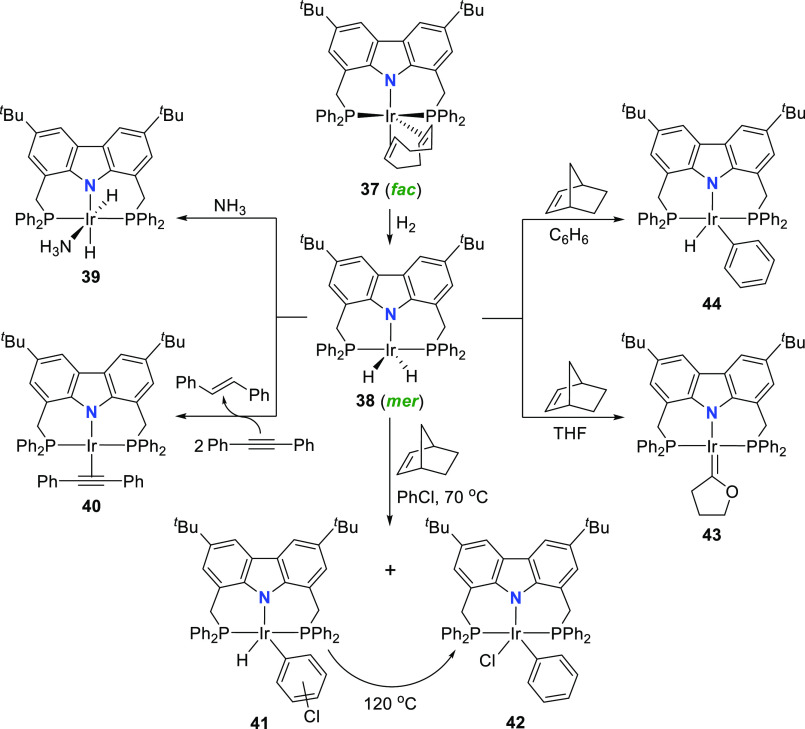
Activity in the benchmark catalytic transfer dehydrogenation reaction between cyclooctane (COA) and tert-butylethylene (TBE) at high temperatures (200 °C) to form cyclooctene (COE) and tert-butylethane (TBA) was communicated in the seminal report of a dihydrido iridium complex with a PCP-ligand.146 Subsequent DFT calculations showed that the thermodynamic favorability of oxidative addition of nonpolar substrates like H2 or RH to the fragment XML2 (M = Ir, Rh) increases as the σ-donating ability of coordinating group X decreases.147,148 These results prompted the interest of both the groups of Gade135 and Goldman and Brookhart149 to prepare PNP-pincer ligands featuring the more rigid carbazole backbone for complexation to iridium, compared to the diphenylamide pincer ligand studied by Ozerov et al.150−153 The use of hybrid ligands containing both soft phosphorus and hard amido donor atoms, a 6-membered chelate ring-size and flexibility introduced by using methylene spacers between the carbazole and the phosphines, were additionally rationalized for coordination to transition metals with large atomic radii by Gade et al.135 The ligand 36 was prepared from the starting material 1,8-dibromo-3,6-di-tert-butyl-9H-carbazole 32 (Scheme 5).154N-protection by a TMS group was followed by bromine substitution with hydroxymethylene and subsequent reaction with p-formaldehyde to yield 33. The carbazole-diol was then treated with PBr3 to yield the key intermediate 34. A borane-protected ligand 35 was prepared by reaction of 34 with the lithium-diphenylphosphine-BH3 adduct, which delivered the protioligand 36 after deprotection. The ligand precursor 36 was treated with [Ir(acac)(cod)] (acac = acetylacetonato, cod = 1,5-cyclooctadiene) (Scheme 5) to give the corresponding complex 37.135 Unlike the previously reported d8-metal complexes bearing this ligand in a meridional coordination mode,154 the molecular structure of 37 revealed a facial coordination of the distorted carbazole backbone for the trigonal bipyramidal coordination geometry.135 The strongest σ-donors occupy the axial positions, as demonstrated by the shorter axial diene bond (C=C bond length 1.408(7) Å) compared to the equatorial diene bond (C=C bond length 1.443(7) Å) of the cod ligand.155
Scheme 5. Synthesis of a PNP-Carbazole Ligand and Coordination to IrI.
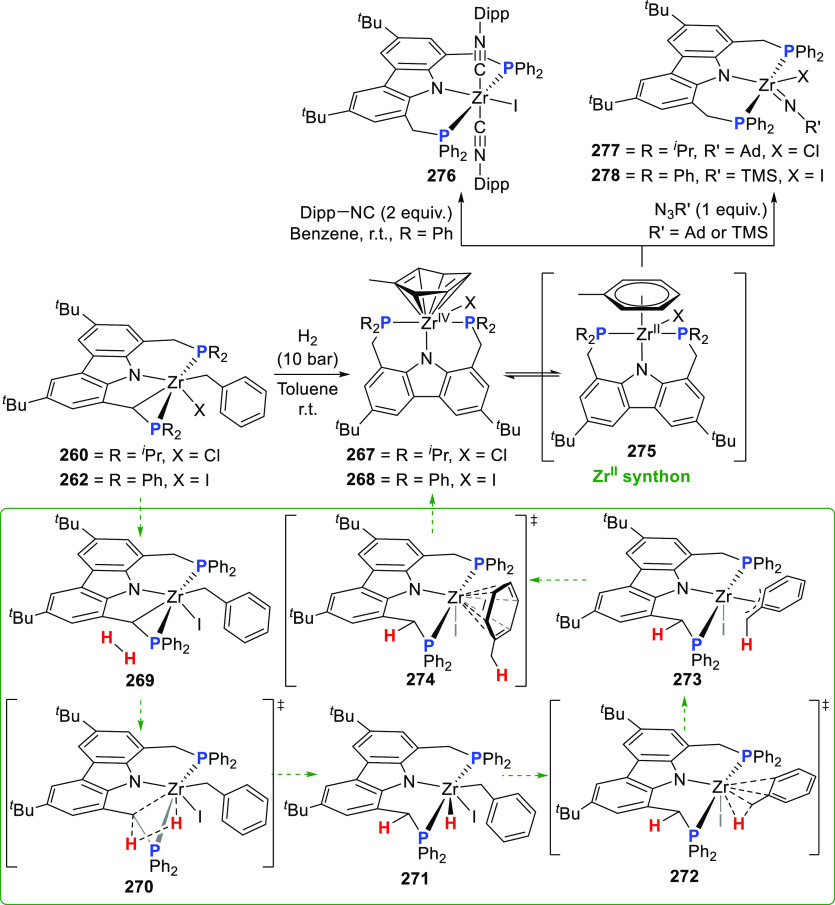
The iridium dihydrido complex 38 was accessed by reacting 37 with hydrogen at 10 bar (Scheme 6),135 while avoiding salt formation by using triethylborohydride sources.146,156,157 The trigonal bipyramidal geometry of the dihydrido iridium(III) complex 38 displays the PNP-pincer ligand coordinated in the more typical “pincer”-like mer-fashion.135 Although reaction of 38 with a solution of ammonia in THF resulted in the formation of the ammine complex 39 with no sign of any N–H activation, bond cleavage of both C–H and C–Cl bonds proceeded facilely (Scheme 6). Initial reactivity studies revealed that stoichiometric reaction of 38 with diphenylacetylene hydrogenates the alkyne selectively to trans-stilbene, with isolation of the corresponding alkyne complex 40 (Scheme 6). If the reaction is carried out under catalytic conditions (excess H2 and alkyne), complete hydrogenation of diphenylacetylene is observed. Similarly, reaction of norbornene with 38 led to the hydrogenation of norbornene and the formation of a reactive species that subsequently reacts with the reaction solvent employed. In the case of benzene as solvent, the C–H activated phenyl hydrido complex 44 is obtained, while the use of chlorobenzene as solvent indicates the formation of three different C–H activated products 41 and the C–Cl oxidative addition product 42. Complex 42 is thermodynamically favored, as shown by its selective formation following heating at 120 °C (Scheme 6). Finally, double C–H activation is achieved with the use of tetrahydrofuran as solvent, and Fischer carbene complex 43 is isolated.135
Scheme 6. Bond Activation Reactivity of a Dihydrido PNP-IrIII Complex.
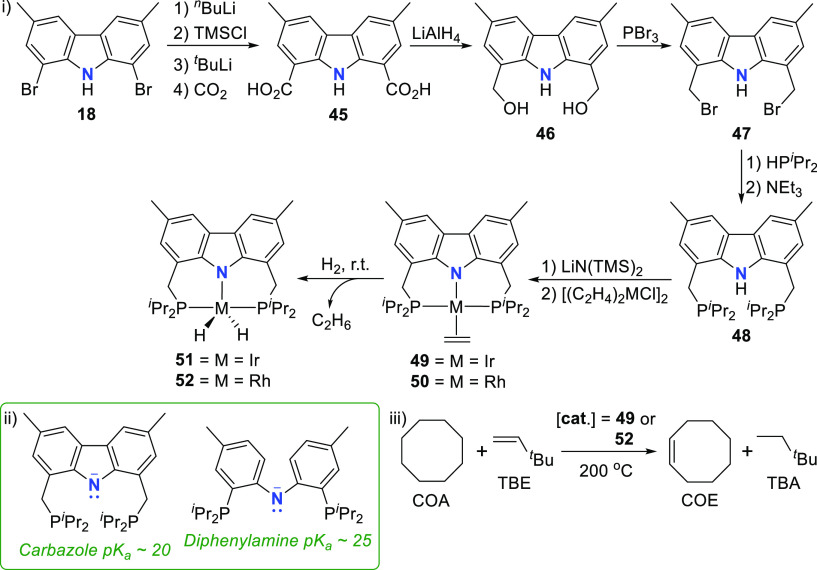
The closely related protioligand 48 with the carbazole backbone methylated in the 3,6-positions, and the phosphine donor moieties containing isopropyl substituents instead of the phenyl groups reported by Gade et al.,135 was prepared in a modified sequence, whereafter stepwise lithiation and coordination with the ethylene metal dimers (iridium(I), rhodium(I)) resulted in the formation of the corresponding olefin complexes 49 and 50 of the group 9 metals (i, Scheme 7).149,158 Treatment of the olefin complexes with hydrogen atmosphere at room temperature readily displaces the hydrogenated ethane, and the corresponding dihydrido metal complexes 51 (iridium(III))149 and 52 (rhodium(III))158 are formed. On the basis of the pKa values of the neutral ligands, shown in Scheme 7, the central nitrogen in 48 was expected to be a weaker σ-donor than the corresponding nitrogen in the diphenylamine-PNP ligand previously employed by Ozerov et al.150−153 in the benchmark transfer dehydrogenation reaction of COA with TBE. The more weakly σ-donating group at the central position of the pincer ligand was anticipated to favor the thermodynamics of C–H and/or H–H addition to 14-electron iridium-pincer fragments implicated in the well-known mechanism of the reaction (iii, Scheme 7).159 The use of 49 as the catalyst in this transformation, however, proved ineffective, with experimental and computational investigations indicating that hydrogenation of TBE is the rate-limiting step. Although TBE does insert into an Ir–H bond of 51, reductive elimination from the resulting IrIII alkyl hydride is thermodynamically very unfavorable and the +3 oxidation state is maintained.149 This result contrasts with prior studies of alkane transfer hydrogenation employing catalysts with PCP-pincer ligands where the iridium(III) alkyl hydride or dihydride is not thermodynamically favored over the 14-electron iridium(I).159 On the other hand, it was found that the RhIII state was not sufficiently accessible to allow an effective catalytic cycle based on the RhI/RhIII couple for the PCP-ligand.160 On the basis of these reports as well as their results with PNP-iridium,149 the groups of Goldman and Brookhart investigated the use of the rhodium analogue 50 as catalyst for alkane transfer dehydrogenation.158 As the thermodynamics are biased more toward the +1 oxidation state for rhodium than iridium,161 it was anticipated that the relatively high stability of the RhIII analogue would not preclude transfer hydrogenation. In support of the hypothesis, complex 52 was found to be an active catalyst for the dehydrogenation of COA with TBE, achieving TOFs (TOF = turnover frequency) of up to 10 min–1, as the first example of a rhodium-based catalyst that does not require light or hydrogen atmosphere for this transformation.158
Scheme 7. Synthesis of (i) Iridium(I) and Rhodium(I) Olefin Complexes with a PNP-Carbazolide Pincer Ligand and (ii) Comparative pKa Values for the Bis(diisopropylphosphino)carbazole and Corresponding Bis(diisopropylphosphino) Diphenylamine Ligands Employed in (iii) the Transfer Hydrogenation of COA with TBE to Form COE and TBA.
2.2. Metalloradical Reactivity Enabled by the Carbazolide
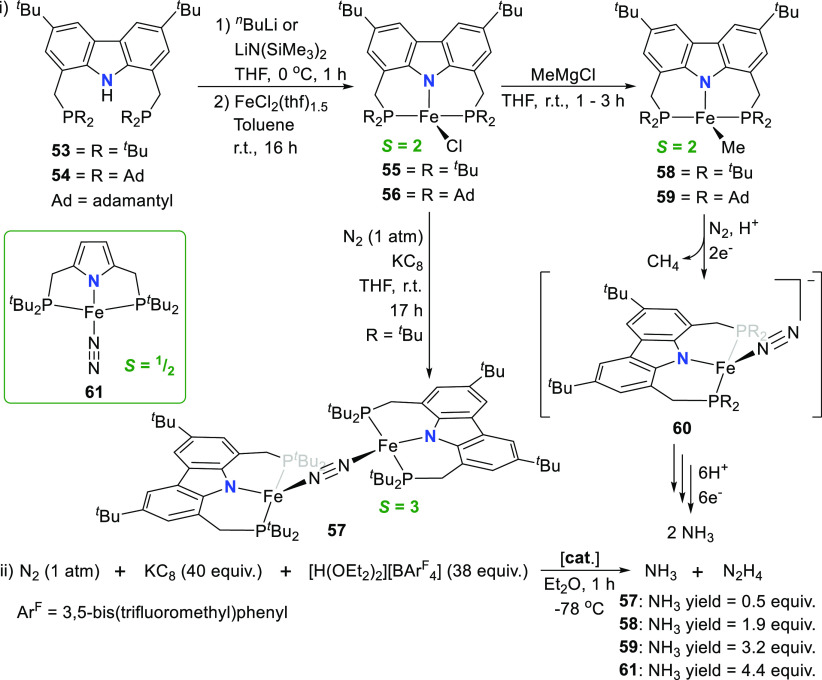
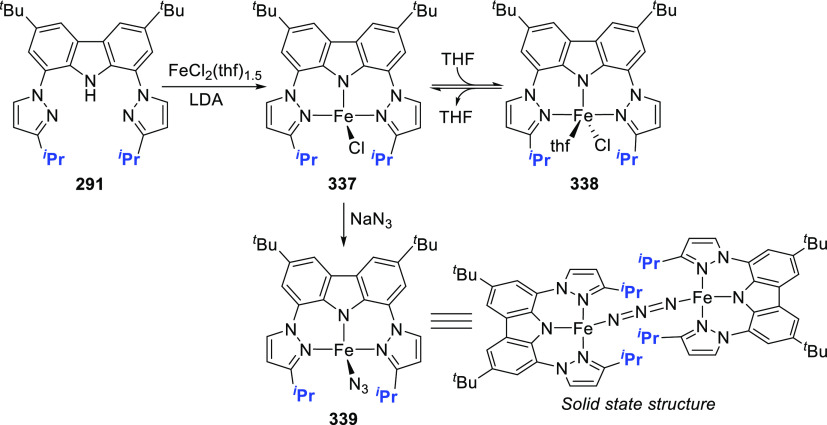
The group of Nishibayashi reported the synthesis of iron complexes coordinated by a monoanionic PNP-carbazolide pincer ligand, as catalysts for the fixation of nitrogen.162 The protioligands 53 and 54 with bulky tert-butyl or adamantyl (Ad) substituents on the phosphines, respectively (i, Scheme 8), were prepared following a modified procedure,154 followed by salt metathesis reaction of the in situ generated lithium complexes to yield the FeII–Cl complexes 55 and 56. The complexes exhibit distorted tetrahedral geometries around the iron atoms (τ4 = 0.79 for R = tBu and τ4 = 0.80 for R = Ad, where τ4 = 0.00 for perfect square planar geometry and τ4 = 1.00 for tetrahedral geometry),163 and the solution magnetic moments determined were consistent with a high spin S = 2 electronic configuration.162 Reduction of 56 with KC8 under nitrogen atmosphere failed to deliver identifiable products for the adamantyl-substituted complex, while a dinitrogen-bridged diiron complex 57 (S = 3) was isolated from the corresponding reduction of the tBu-substituted complex 55 (i, Scheme 8). Alternatively, reaction of 55 or 56 with MeMgCl afforded the corresponding methyl-complexes 58 and 59 in both cases. As for 55 and 56, a high spin tetrahedral geometry around the iron(II) ion was found, in sharp contrast to the low-spin pyrrole-based pincer FeI complex analogues with geometry indices τ4 of 0.11–0.13, e.g., 61 (Scheme 8) previously reported by the same group.164 The size of the chelate ring (vide infra, section 3.1) rather than the nature of the N-donor, was attributed as the cause of the geometry distortion. In the case of the rigid pyrrole-based PNP-ligand, square planar complex geometry is favored to prevent the formation of a dinuclear structure as found for 57. The activity of the iron complexes 57–59 and 61 in the catalytic reduction of dinitrogen to ammonia and hydrazine was probed using KC8 as reductant and [H(OEt2)2]BArF4 (ArF = 3,5-bis(trifluoromethyl)phenyl) as proton source under atmospheric nitrogen pressure (ii, Scheme 8). The highest yield of 4.4 equiv of ammonia and 0.2 equiv of hydrazine, based on the iron atom of the catalyst, was obtained for 61. However, a catalyst deactivation pathway for 61 was confirmed to occur via pyrrole-backbone protonation,164 rationalizing the use of the central carbazole moiety as a replacement central donor in the pincer scaffold.162 The strategy showed limited success as the pronounced influence of the molecular structure on the catalytic activity led to significantly lower yields obtained in the nitrogen reduction (ammonia yield < 0.5 equiv for 57, 1.9 equiv for 58, and 3.2 equiv for 59 with R = Ad) compared to the ammonia yield (4.4 equiv) for 61,164 although the possible formation of the corresponding anionic mononuclear iron(0) dinitrogen complexes 60 under catalytic reaction conditions (Scheme 8) was proposed.162
Scheme 8. Synthesis of (i) High-Spin PNP-Pincer Complexes of Iron for (ii) Catalytic Reduction of Dinitrogen.
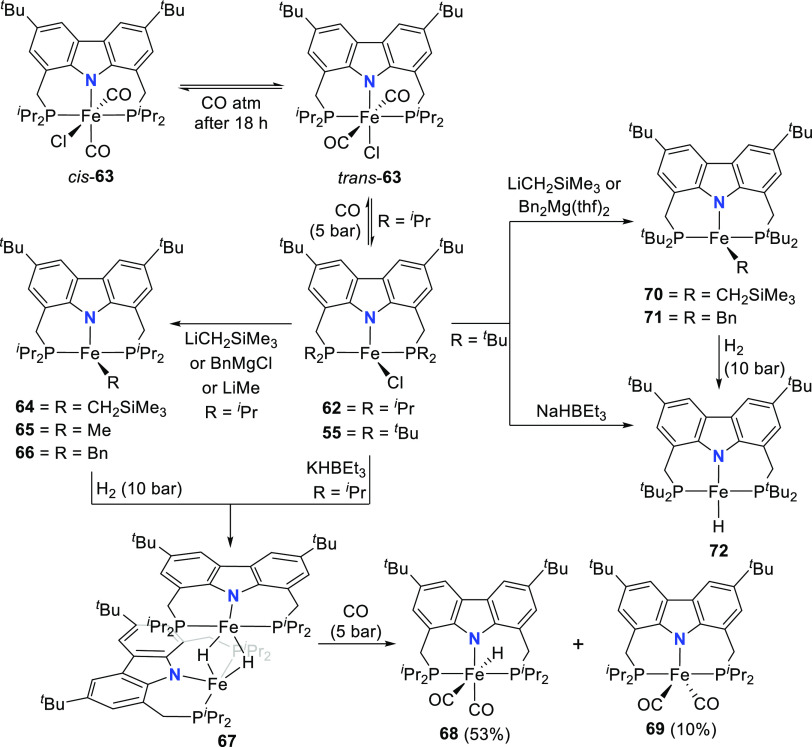
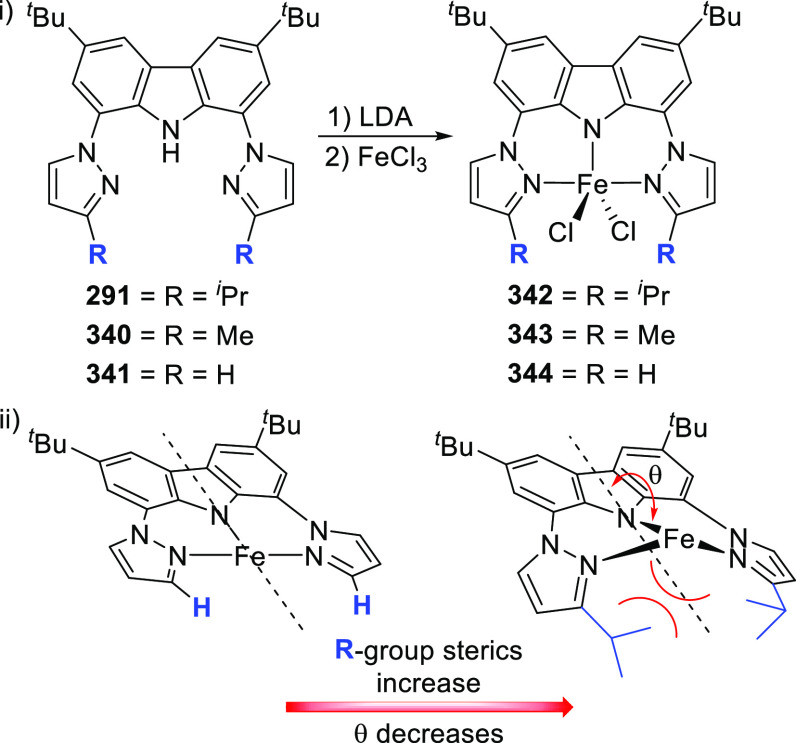
The related work of Gade et al. demonstrates that the reactivity of the iron complexes is governed also by the steric effects of the wingtip groups (phosphine substituents),165−167 (vide infra, section 4.1) as well as very prominently by the N(carbazolide)–Fe interaction, which leads directly to metalloradical reactivity.168,169 Their interest centered on the isolation and characterization of a paramagnetic, high-spin iron hydrido complex as the key intermediate in the catalytic cycle of iron-catalyzed olefin hydrogenation,170−172 in contrast to the known intermediate spin FeII-hydrido complex congener of 61 reported by Nishibayashi et al.164 Protioligands 312 (vide infra, Scheme 46)173 and 53,162 containing diisopropyl- or tert-butyl-substituted phosphine donor moieties, respectively, were employed as ligand precursors for the synthesis of the corresponding FeII–Cl complexes 62(167) and 55(166) (Scheme 9). The complexes 62 and 55 feature distorted tetrahedral geometry (e.g., for 62 a geometry index of τ4 = 0.77163 is observed where the acute P–N–P bite angle of 84.03(6)° is enforced by the rigidity of the carbazole backbone),167 similar to 56(162) with high spin state of S = 2 determined also in this case. Reaction of 62 with a strong field ligand (CO) results in a change of spin, and the diamagnetic dicarbonyl complexes trans-63 and cis-63 are in equilibrium (Scheme 9).167 Alkylation of 62 yields again distorted tetrahedral complexes 64–66 (Scheme 9) with solution magnetic moments measured that are consistent with a high-spin state. If chloride substitution is effected by reaction of diisopropyl-substituted 62 with KHBEt3, a complex displaying reduced magnetic susceptibility with significant antiferromagnetic coupling is observed to form, suggesting that the dimeric structure of the isolated complex 67 is maintained in solution. 67 is a dinuclear hydrido complex, also accessible from the hydrogenation of the alkylated complexes 64–66 (Scheme 9), with a highly distorted square-pyramidal geometry around each of the iron centers bridged by two H-atoms. The steric demand of the isopropyl-phosphino substituents results in a twist of the two molecular fragments to give a torsion angle of N(carbazolide)–Fe–Fe–N(carbazolide) of 88.81(12)° with respect to each other. Conversely, a similar reaction sequence for the bulkier tert-butyl-substituted 55 yielded a monomeric, square planar FeII-hydrido complex 72 with intermediate spin (Scheme 9),166 confirmed by the geometry index τ4 = 0.15.163 Treatment of 67 with excess CO (g) yielded a mixture of diamagnetic complex 68 and the iron(I) complex 69 as a minor byproduct.167 Attempts to prepare 69 by an independent route via reduction of 62 proved unsuccessful.
Scheme 46. Synthesis and Reactivity of High-Spin Cobalt(II) Chlorido, Low-Spin Cobalt(II) Hydrido and High-Spin Cobalt(I) Complexes Supported by PNP-Ligands.
Scheme 9. Synthesis and Reactivity of High-Spin PNP-Pincer Hydrido Complexes of Iron Containing a Carbazole-Based Ligand.
The alkyl-analogues of 64–66, high-spin complexes 70 and 71, were obtained using similar alkylating agents and analogously to 64–66 feature distorted tetrahedral geometry (Scheme 9).166 Hydrogenation of 70–71 also leads to the formation of FeII-hydrido 72 as a first example of a metal hydride with a paramagnetic ground state for which the hydrido ligand is directly detectable via solution 1H NMR spectroscopy. Extensive DFT calculations were employed for full assignment of the paramagnetic complexes, with unprecedented shifts of the recorded hydride resonances.166−169,174
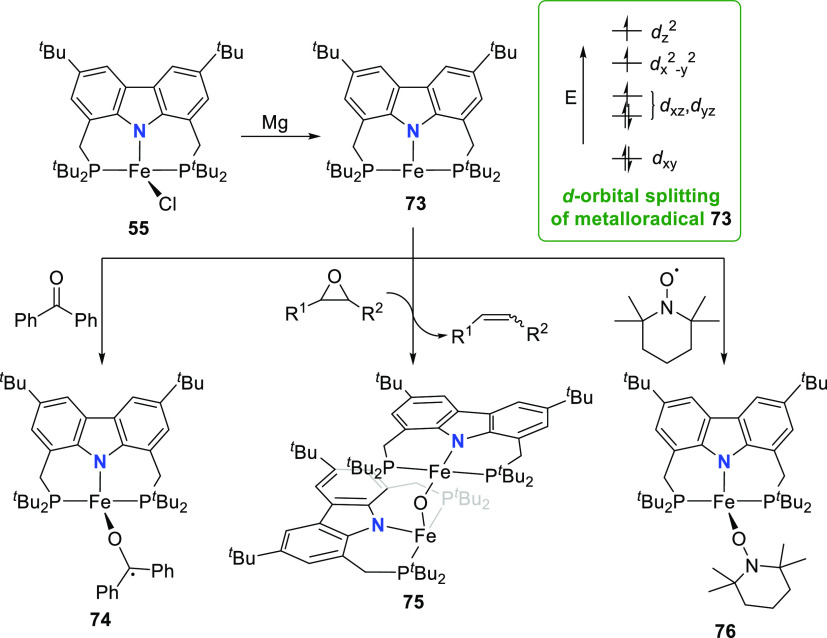
Remarkably, 55 could be reduced to a “naked” T-shaped FeI complex 73 stabilized by the PNP-pincer ligand with tBu-substituted phosphine donors (Scheme 10).168 Treatment of the precursor FeII-chlorido 55 with excess magnesium powder in the absence of a nitrogen atmosphere results in the formation of paramagnetic 73. The complex is revealed to have a high-spin electronic structure, confirmed by computed spin densities. The majority of unpaired spin is localized around the vacant coordination site to inform a metalloradical character of the iron center with assigned oxidation state of +1. This corroborates the observed chemical inertness of 73 with σ-donors such as THF and NEt3 and resembles the electronic “remote basicity” effected by the antibonding nature of the metal-carbazolide nitrogen in the Kohn-Sham HOMO calculated for T-shaped Au-CNC-carbazolide complexes (vide infra, section 2.3). The HOMO has dz2 character and is singly occupied (see Scheme 10),169 and its Fe–N antibonding character is further enhanced by the unpaired electron spins in the antibonding dx2–y2 and nonbonding dxz/dyz orbitals so that the half-filled orbital is effectively blocked for ligand binding.175 In addition, the calculated LUMO of 73 is oriented orthogonally to the FeNP2-plane and effectively shielded by the tert-butyl groups to further explain the observed reluctance toward adduct formation at the vacant coordination site.168
Scheme 10. Synthesis and Metalloradical Reactivity of a T-Shaped FeI Complex.
The metalloradical character of FeI complex 73 in conjunction with its resistance to Lewis acidic reactivity prompted the investigation of its single electron redox chemistry (Scheme 10). Reaction of benzophenone with 73 gives the thermodynamically stable iron benzophenone ketyl radical complex 74 with an expected (distorted) tetrahedral arrangement around the high-spin iron(II) center.168 However, end-on coordination of the ketyl ligand is unprecedented in iron chemistry.176 DFT analysis, EPR spectroscopy, and solution magnetic moments measured were consistent with a high-spin iron(II) d6 metal with an antiferromagnetically coupled ketyl radical.168 The stability of the alkoxide-FeII bond led to the assumption that ring-opening reaction with a strained cyclic ether would overcome the inertness of 73 observed with other oxygen-donor ligands. Testing of this hypothesis by addition of various epoxides to 73 generated the rare example of an oxido-bridged diferrous complex 75 and the secondary reaction product mixtures of trans- and cis-alkenes (Scheme 10). Such single-electron transfer (SET) to a ligand from T-shaped 73 was further demonstrated by the reaction with the stable radical 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO) to give the high-spin FeII alkoxide complex 76 (Scheme 10)169 with elongated O–N bond (1.423(3) Å), compared to free TEMPO (1.296(5) Å).177
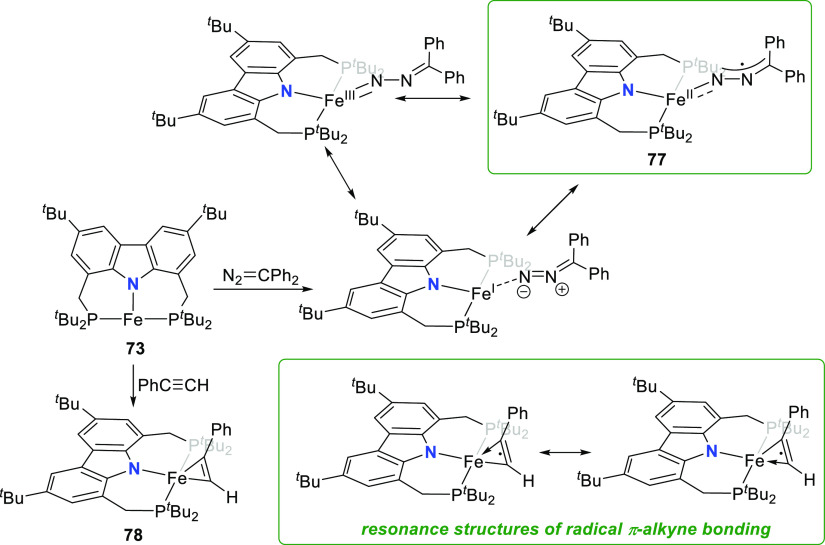
Extension of the SET reactivity was done by reaction of 73 with phenylacetylene (Scheme 11). Side-on coordination of the alkyne in the resulting complex 78 was confirmed in the molecular structure, with the steric demand of the phenyl substituent leading to an elongation of the Fe–P bond length on the side of the molecule to which the phenyl substituent is oriented (2.5662(6) Å) compared to the Fe–P bond distance of 2.3593(5) Å on the opposite side.169 A quartet ground state with three unpaired electrons was determined from the effective magnetic moment measured. This could indicate either a high-spin FeI or an intermediate-spin FeII with the unpaired spin density primarily located on the iron, if the π-alkyne was coordinated as a closed shell unit (either neutral or dianionic, respectively). Computed spin density distribution however showed significant localization of the spin density on the π-alkyne of 78 to indicate delocalization from the metal into the C≡C-π- and π*-orbitals, as shown in the representative resonance structures drawn in Scheme 11.
Scheme 11. Charge Transfer Reactivity Mediated by a T-Shaped FeI–PNP Complex.
The charge transfer reactivity of 73 was further illustrated by using diphenyl diazomethane as an electron acceptor.169 The complex 77 that forms contains a diazoalkane ligand coordinated end-on, with an Fe–diazomethane-N bond length indicative of a bond order exceeding one (1.765(2) Å), in an amido/imido-type coordination. Moreover, the bent angle of the coordinated diazoalkane CNN unit contrasts with the expectation of a linear CNN unit for a neutral bound ligand. Unlike the decoupled organic radical species found to bind in 74,168 the DFT modeled spin density of 77 is in accordance with an S = 2 iron(II) antiferromagnetically coupled with the diazoalkanyl radical.169 Based on the computed spin density and the tetrahedral coordination sphere of 77, the resonance form of a diazomethane ligand bonded in an amido-fashion to a high-spin iron(II), rather than the resonance structures depicting a neutral donor bound to FeI or a formal iron(III) center, is believed to be a more appropriate description of the bonding in 77 (Scheme 11).
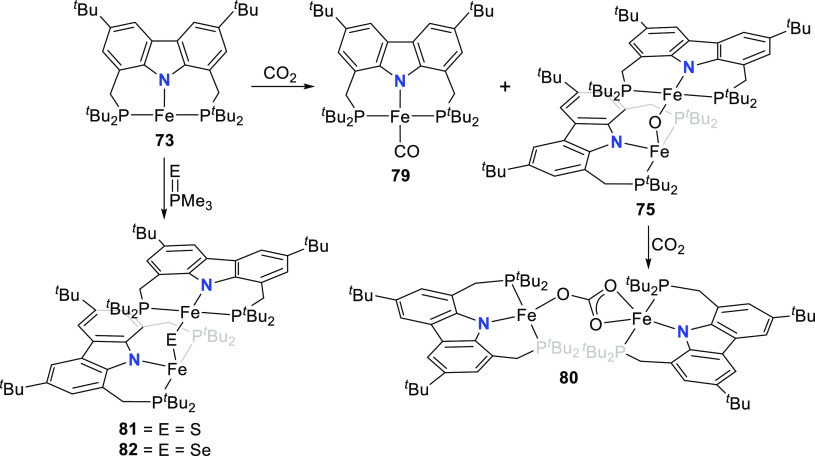
The ability of 73 to act as an oxygen atom abstractor was demonstrated by its reaction with epoxides to release the corresponding alkene and oxygen-bridged diiron(II) complex 75 (Scheme 10).168 The same oxophilicity resulted in formation of a reaction mixture containing both square planar monocarbonyl complex 79 and dinuclear 75, following reaction of 73 with carbon dioxide (Scheme 12).169 Continued reactivity of 75 with CO2 leads to a further CO2-to-CO transformation resulting in complex 80, a dinuclear μ-carbonato iron complex. The μ–κ:2κ1 coordination mode of the carbonato ligand yields a complex with one molecular fragment with a distorted tetrahedral geometry (τ4 = 0.79) and the other with distorted square pyramidal geometry (τ5 = 0.25) around the iron centers. Given the propensity of 73 to act as a chalcogen abstractor, the viability of similar reactivity with the heavier chalcogens (S and Se) was investigated by reaction of half an equivalent of trimethylphosphine sulfide or selenide, respectively, with 73 (Scheme 12).169 The corresponding dinuclear bridged sulfido 81 and selenido complexes 82 were isolated, with the pincer complex units oriented at approximate right angles to each other. Strong antiferromagnetic coupling between the iron centers was confirmed by solid-state magnetometry to result in linearly increasing molar susceptibility above 50 K.
Scheme 12. (i) Deoxygenation of Carbon Dioxide and (ii) Chalcogen Abstraction Mediated by a T-Shaped FeI–PNP Complex.
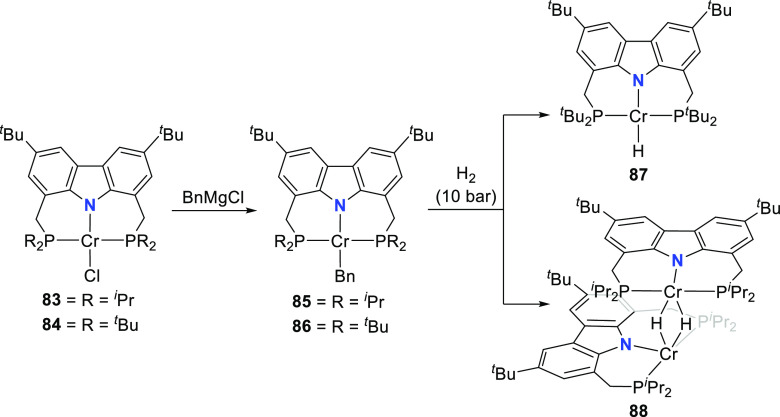
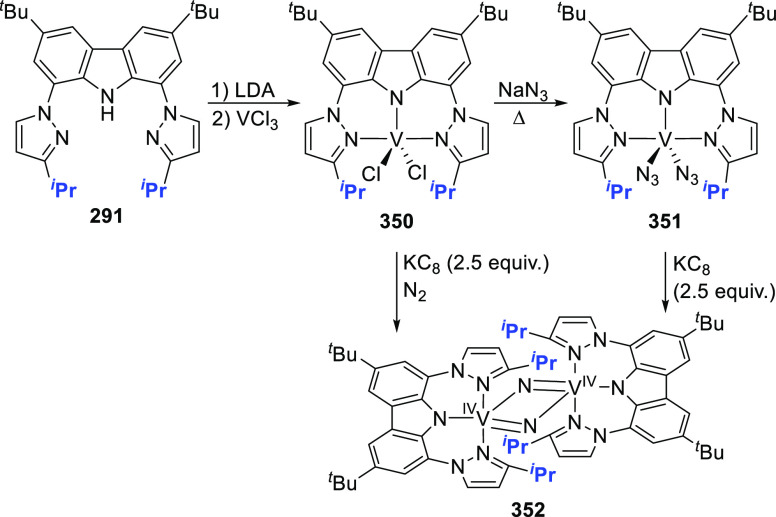
The versatile reactivity of T-shape complex 73 obtained from reduction of square planar, mononuclear iron(II)-hydrido 72 (Scheme 9–10) preceded investigation of the mid-first row transition metal chromium.178 As for its group 8 analogue, very few examples of low-valent CrII-hydrido complexes have been structurally characterized,179−184 due to the challenge of stabilizing the open-shell hydride complexes. A similar synthetic methodology was followed to prepare chromium(II) chlorido complexes 83 and 84 (Scheme 13), from the isopropyl- and tert-butyl-substituted protioligands 312 and 53, respectively.178 Both complexes were found to adopt high-spin ground states with four unpaired electrons in solution and displayed distorted square planar geometries, with the greater steric demand of the tBu-wingtips resulting in a geometry index τ4 = 0.27 for 84 compared to τ4 = 0.13 for 83. Treatment of 83 and 84 with benzyl magnesium chloride yields the corresponding alkylated complexes 85 and 86 (Scheme 13), again displaying square planar geometry in contrast to the analogous iron(II) complexes 64–66 and 70–71 with tetrahedral coordination spheres.167 In this instance, however, the bulkier pincer scaffold of 86 yields a more planar molecular arrangement than observed for 85.178 The wingtip-effect (vide infra, section 4) is prominent in the follow-up hydrogenation of the alkyl complexes 85 and 86, where the discrimination between a dinuclear hydrido complex 88 with two hydrido ligands bridging the two CrII ions and a mononuclear, square planar PNP-chromium(II) hydrido complex 87 (Scheme 13) is possible as a steric consequence of the wingtip groups. Both the di- and mononuclear chromium(II) hydrido complexes demonstrated insertion reactivity toward unsaturated C=O and C=N bonds, as anticipated for hydrido intermediates in catalytic reductions of unsaturated substrates.
Scheme 13. Synthesis of Di- and Mononuclear PNP-Pincer Complexes of Chromium(II) Hydride.
2.3. Access to Nucleophilic T-Shaped d10 Transition Metals
Carbazolide coordination enforcing a T-shaped geometry has also been reported for the group 10185 and 11186d10 transition metals, with the amido moiety fostering a nucleophilic metal that exhibits alternative reactivity profiles. Kunz and co-workers demonstrated this for the group 10 metals,185 accessing nucleophilic complexes with the introduction of their BIMCA ligand consisting of a carbazole backbone substituted by two NHC (N-heterocyclic carbene) moieties on the 1,8-positions.140 The strong σ-donor properties of NHCs, stabilizing metal centers in both low and high oxidation states, are known to provide for complexes with unique reactivity and/or high catalytic activity.71,187−189 Modulation of the electronic consequence at the metal center by manipulation of the L-donor groups (Figure 1) of the LNL-carbazole pincer is discussed in more detail in section 3.
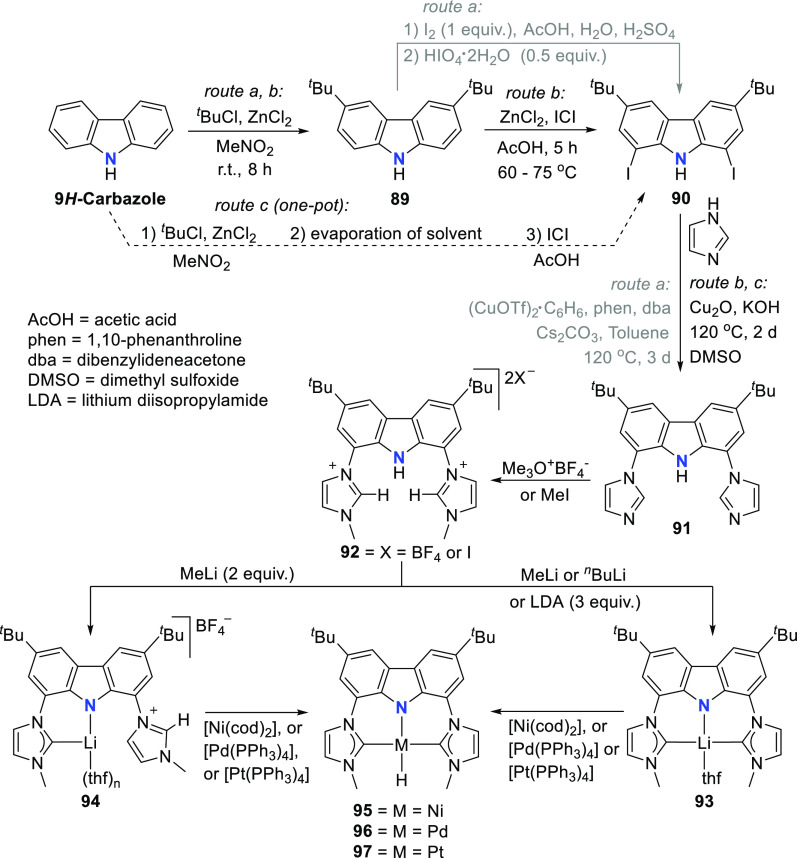
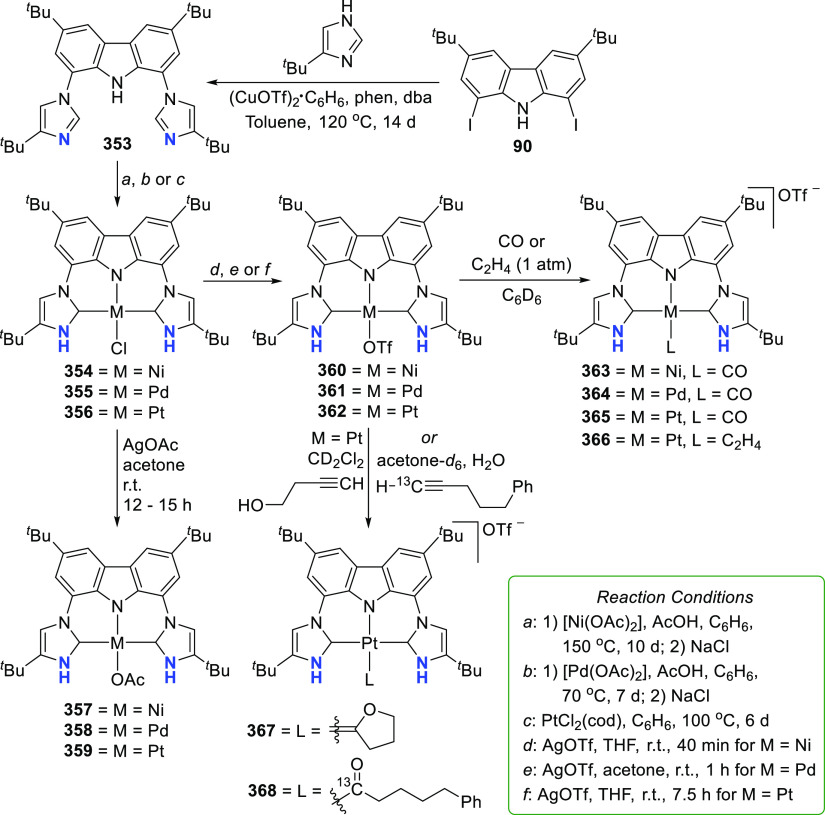
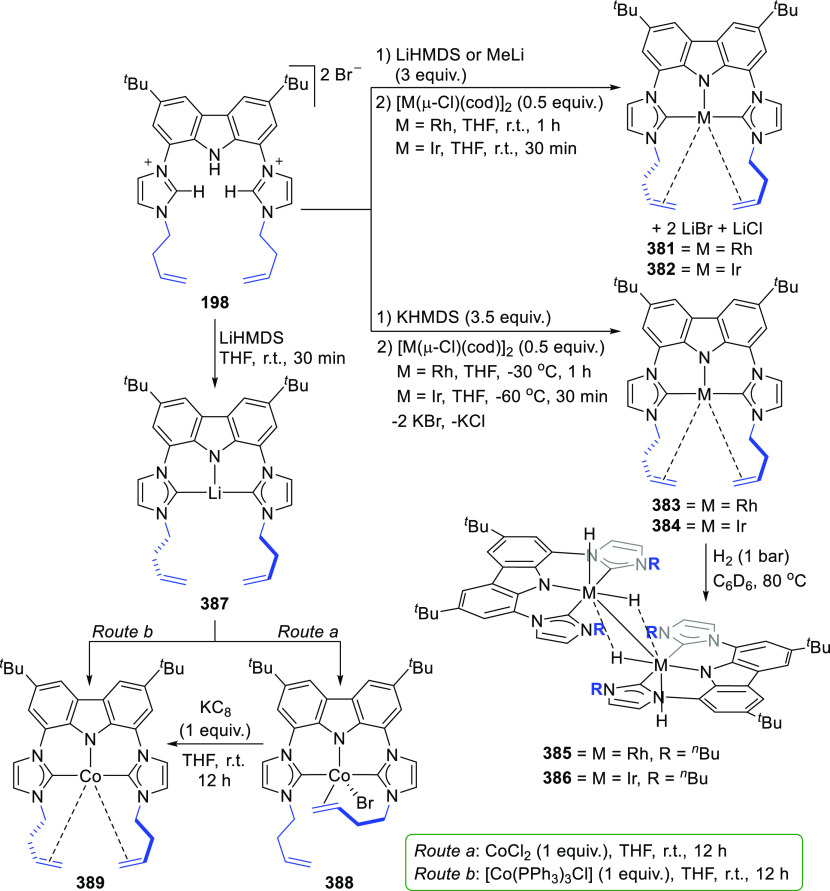
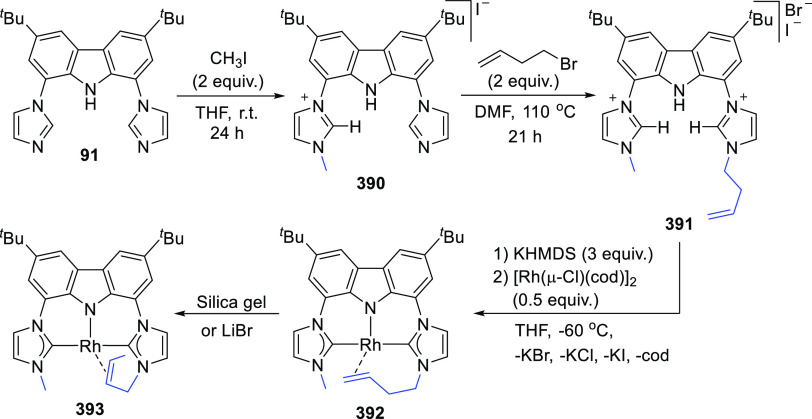
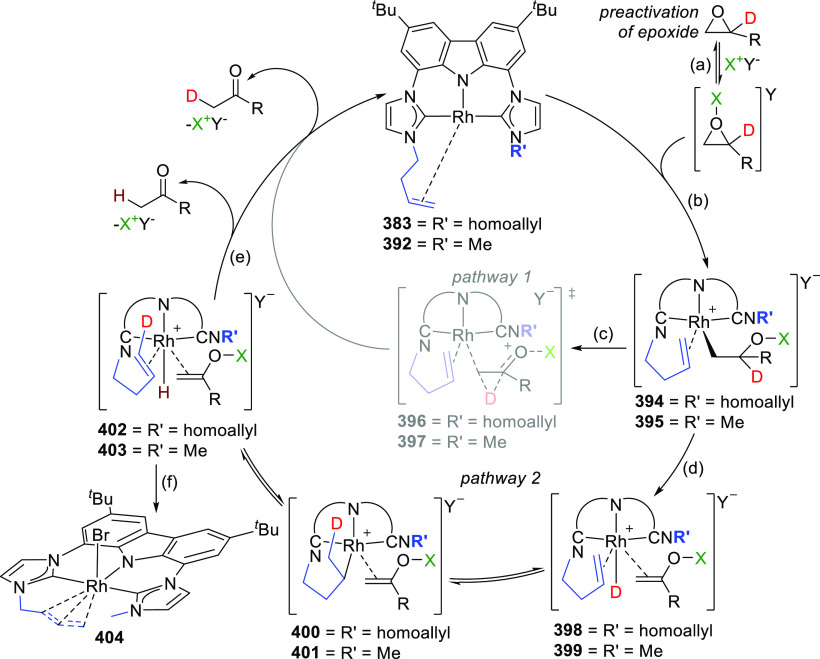
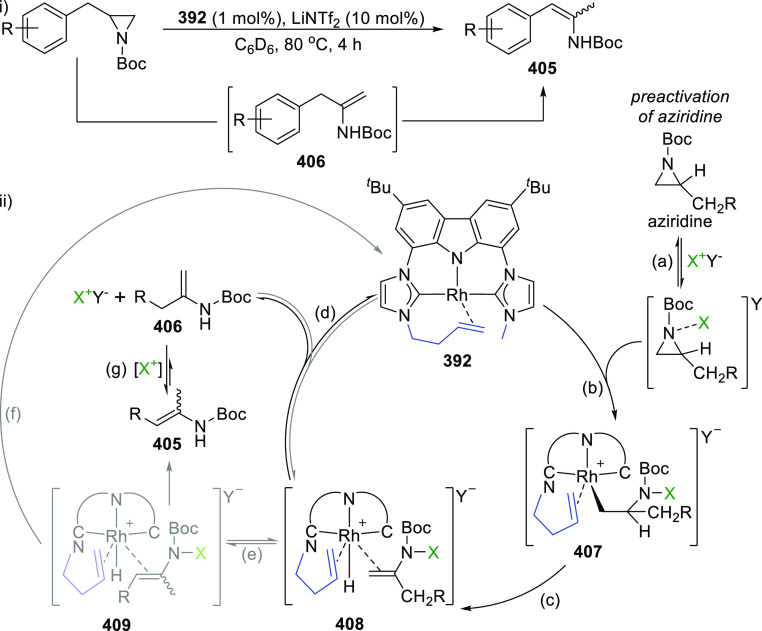
For the preparation of BIMCA ligand precursor, bis(imidazolium) salt 92, a 4-step synthesis was first reported (route a, Scheme 14) starting from 9H-carbazole, the 3,6-positions of which was first alkylated followed by iodination of the 1,8-positions.140 The resulting 3,6-di-tert-butyl-1,8-diiodocarbazole 90 was coupled with imidazole in a copper-catalyzed Ullman reaction to give a bis(imidazolyl)-substituted carbazole 91, which was further treated with methyl iodide or Meerwein’s salt (Me3O+BF4–) to give the protioligand, imidazolium salt 92. Optimization of the synthesis (route b and c, Scheme 14) afforded shorter reaction times and better yields, while employing more affordable, readily available and air-stable starting materials.190
Scheme 14. Synthesis of the BIMCA (3,6-di-tert-butyl-1,8-bis(imidazol-2-ylidene-1-yl)carbazolide) Ligand and Coordination to Group 10 Transition Metals.
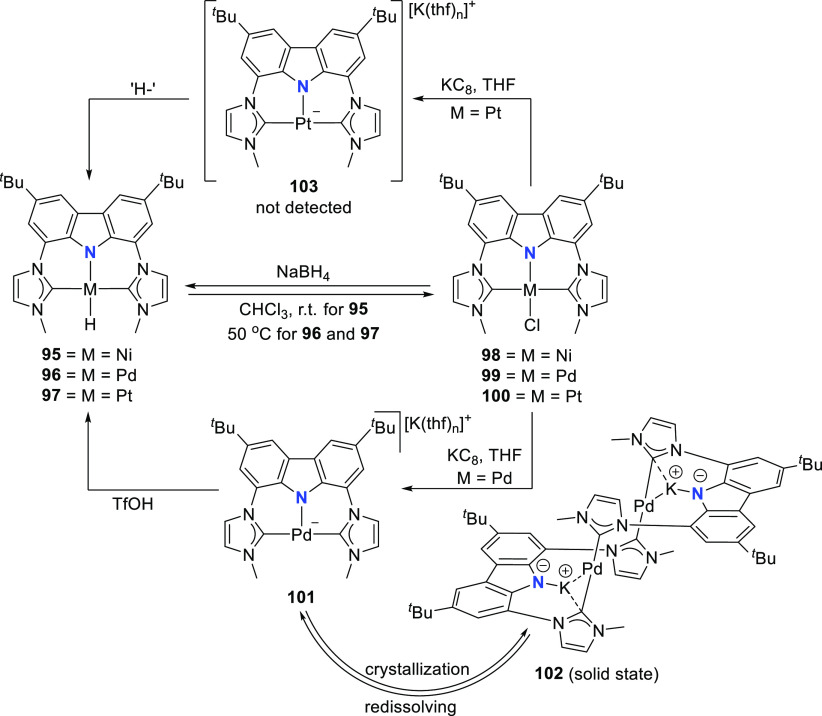
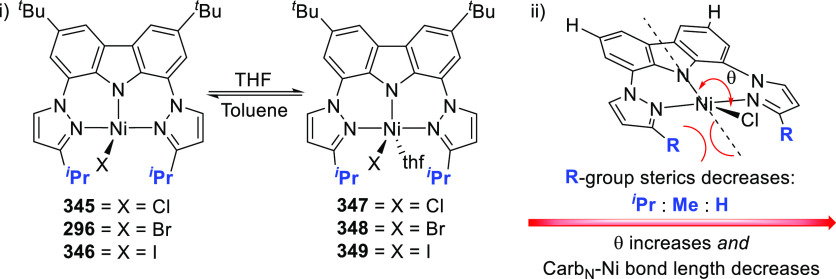
It was envisioned that the anionic BIMCA ligand coordinated with zerovalent group 10 metals (Ni, Pd, Pt) could provide access to very electron-rich and reactive anionic group 10 metal complexes.185 Transmetalation of in situ generated 93 with a Ni0, Pd0, or Pt0 precursor was attempted (Scheme 14), but the targeted anionic M0 complexes were not formed in the reaction. Surprisingly the MII-hydrido complexes 95–97 formed instead (Scheme 14). NMR and IR spectroscopic studies suggested that the complexes are monomeric. Further confirmation of this was provided by a single crystal X-ray structure determination of the Pd and Pt complexes 96 and 97, respectively. The complexes have isomorphic structures in which the BIMCA ligand is meridionally coordinated to the metal center, the square planar coordination of which is completed by the hydrido ligand trans to the carbazolide-nitrogen. The origin of the hydrido ligand in 95–97 was not clear, but several options were considered as the proton source, including the BIMCA ligand itself,185 in contrast to the analogous group 10 metal (Ni, Pt, Pd) hydrido complexes formed unambiguously from the N–H oxidative addition of the PNP-carbazole precursor 312 (vide infra, section 4.1) to M0 precursors (M = Ni, Pt, Pd).191 The formation of the hydrido complexes was explained by the partial deprotonation of the bis(imidazolium) salt 92, leading to a monodeprotonated lithiated species 94 (Scheme 14).185 As this intermediate reacts further with the M0 precursor, an anionic M0 complex is formed, where the BIMCA ligand is κ2-coordinated to the metal via the C atom of the NHC moiety and the N atom of anionic carbazole, resulting in a very basic metal complex. This facilitates the deprotonation of the uncoordinated imidazolium unit in the next step, resulting in simultaneous formation of the MII-hydrido complex with the formation of the carbene moiety. With this conclusion, the synthesis of the MII-hydrido complexes 95–97 was optimized, reducing the amount of base used to deprotonate 92, to two molar equivalents, to yield a mixture of fully deprotonated and partially deprotonated 93 and 94, respectively. Upon further reaction with the M0 precursors, the MII-hydrido complexes were isolated with improved yields (Scheme 14).
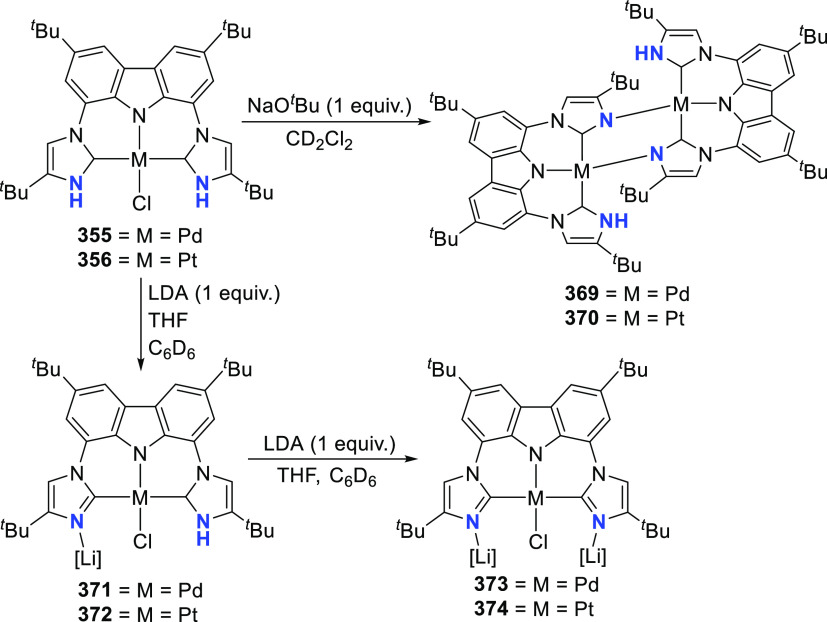
Interconversion of the MII hydrido complexes 95–97 and the corresponding stable chlorido complexes 98–100 was demonstrated (Scheme 15).185 Reduction of 100 with NaBH4 led to the formation of the hydrido complex 97, whereas reduction of 99 with KC8 results in the formation of a dimeric Pd0 biscarbene complex 102. The pincer-type coordination of the ligand collapses, and both BIMCA ligands are coordinated through their NHC moieties to one palladium(0) center while the anionic carbazolide is coordinated to potassium. In solution, the dimer 102 was observed to dissociate into the monomer 101. On the basis of NMR DOSY experiments, it was concluded that dimer dissociation and subsequent rearrangement results in the formation of a monomeric anionic pincer palladium(0) complex 101 (Scheme 15). DFT calculations suggested formation of a solvent-separated contact-ion pair in solution. When the complex was protonated with trifluoromethanesulfonic acid (TfOH), the formation of the PdII-hydrido complex 96 was observed (Scheme 15). The authors concluded that the anionic BIMCA M0 complexes are intermediates in the oxidative addition of 93 to the respective MII-hydrido complexes 95–97. In contrast to palladium, for which the reactive Pd0-intermediate was observed both in the solid state (102) and in solution (101), the reactive platinum(0) intermediate 103 (Scheme 15) was not observed. It is probably formed in situ and is very basic, which allows the abstraction of a proton from the ligand of another molecule. This metal-basicity was inferred from theoretical considerations, where the antibonding HOMO of all the calculated zerovalent [M(BIMCA)]− fragments are aligned along the N–M bonding axis, and the occupation of this orbital (trans to M–N) leads to elongated M–N bonds for the hydrido complexes 95–97, compared to their calculated metal-chlorido analogues.
Scheme 15. Syntheses of Group 10 Metal(II) Hydrido and Chlorido Complexes of BIMCA Ligand and Anionic M(0) Complexes Formed As a Result of the Reduction of Chlorido Complexes.
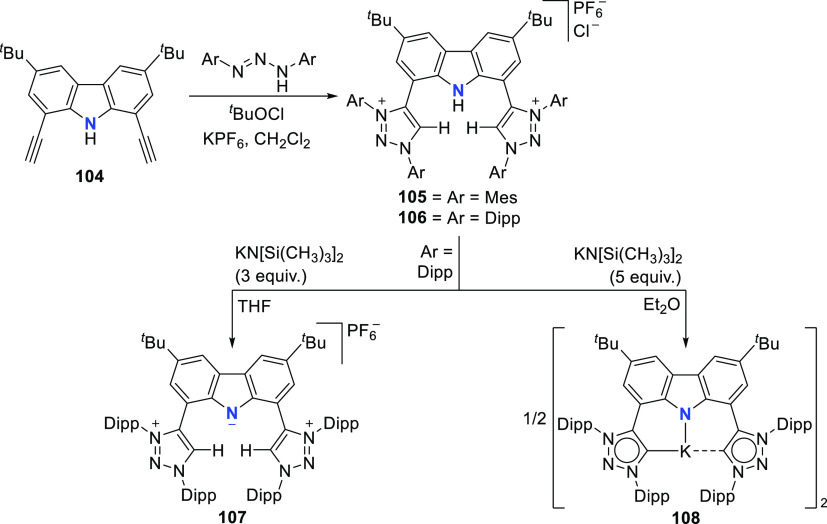
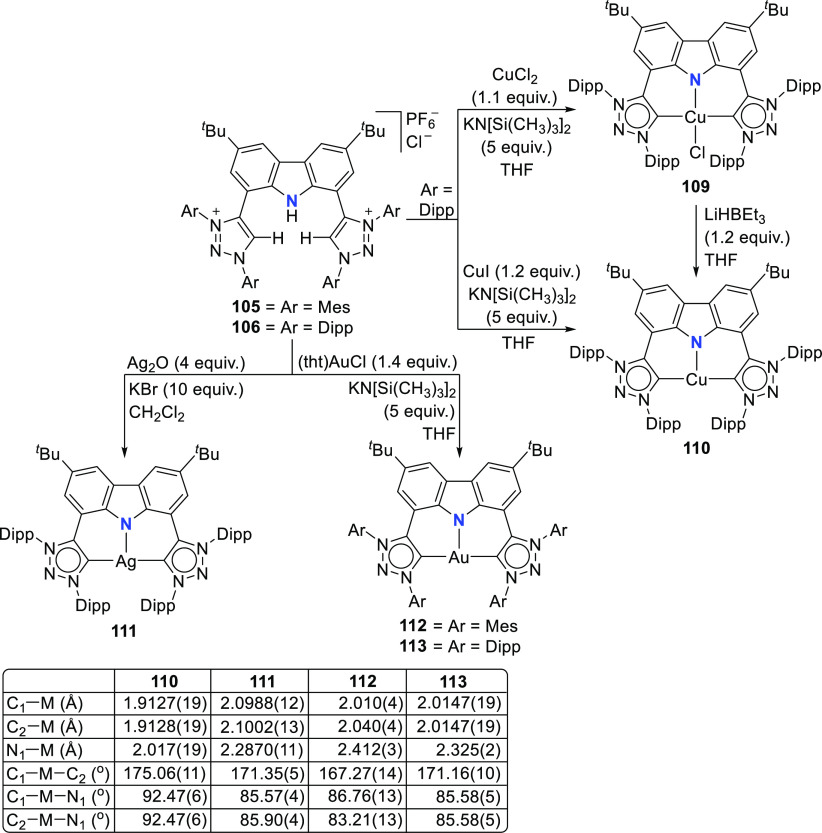
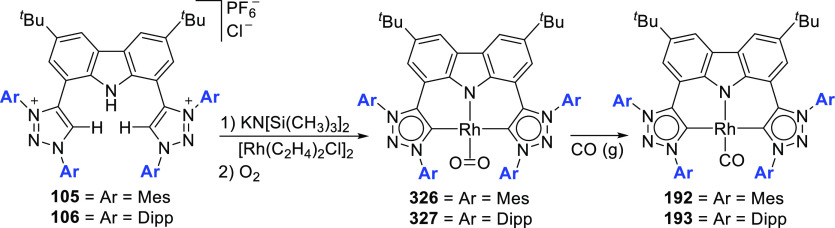
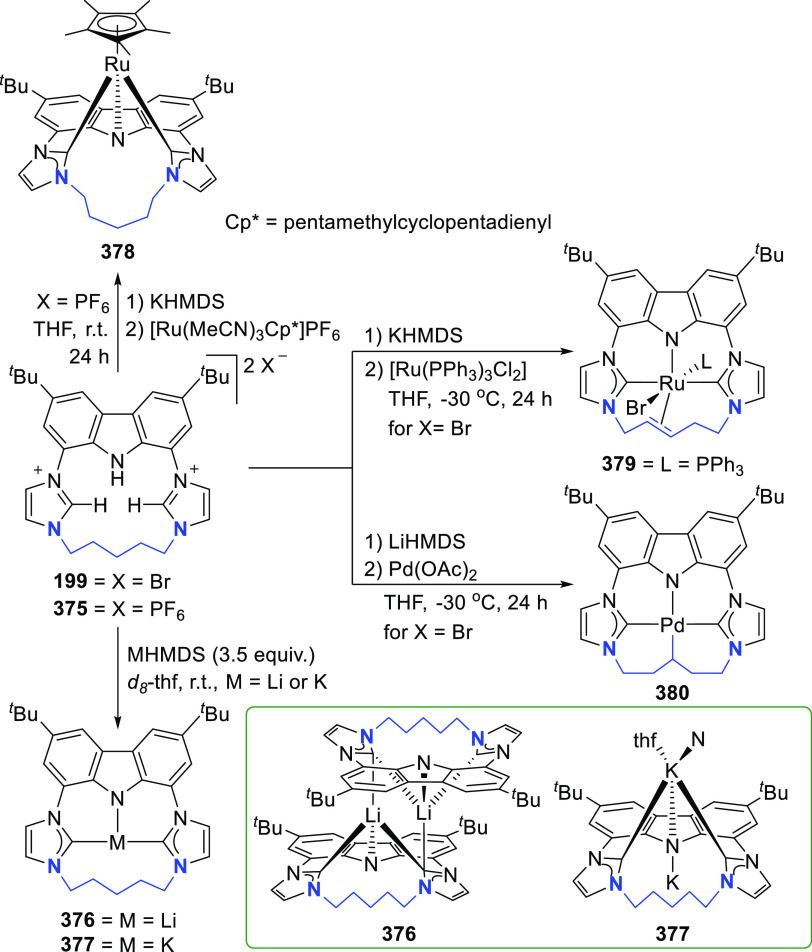
The BIMCA ligand was also coordinated to the coinage metals Cu and Au.192 In the case of copper, depending on the copper source used, either a paramagnetic neutral copper(II) chlorido complex or a dinuclear cationic copper(II) complex is formed. The corresponding CuI complex was not reported. However, if the identity of the flanking carbon-donor carbenes is changed, in addition to the wingtip steric bulk, access to a stable T-shaped CuI complex is granted.193 The monoanionic bis(triazolylidene)carbazolide analogue to the BIMCA ligand introduces mesoionic carbenes (MICs) as the C-donor ligands, with their proven strong σ-donating ability.194,195 The dicationic bis(triazolium)carbazole precursors 105(193) and 106(196) are obtained from the 1,3-dipolar cycloaddition reaction between the 1,3-diaryl-2-azoniaallene salt and 1,8-diethynylcarbazole 104 (Scheme 16). Treatment of the protioligand 106 with 3 equiv of potassium hexamethyldisilazane (KHMDS) results in a monodeprotonated cationic salt 107.193 However, increasing the amount of base to 5 equiv circumvents the equilibrium between the base and its conjugated acid amine to yield a fully deprotonated compound that can be isolated as the potassium salt 108 (Scheme 16), which is stable both in solution and in the solid state under anhydrous conditions.
Scheme 16. Synthesis of Dicationic Bis(triazolium)carbazole Ligand Salts.
Reaction of precursor 106 with an excess of KHMDS and copper(II) chloride produces the paramagnetic copper(II) complex 109 (Scheme 17).193 Treatment of 109 with superhydride (LiHBEt3) results in the reduction of CuII to CuI to yield the copper(I) complex 110, which is also obtained from the reaction of 106 with CuI in the presence of KHMDS (Scheme 17). The range of uncommon T-shaped d10 coinage metal complexes could be expanded to also include AgI (111)197 and AuI (112 and 113)186 as a result of the fine-tuned ligand anchoring the metal in the pincer pocket. More importantly, in the case of gold, “remote” basicity similar to the calculated [M(BIMCA)]− fragments185 was demonstrated, rendering the gold(I) site nucleophilic, a feature arising from both the unusual, strained T-shaped geometry combined with the strong electron-donating nature of the ligand.186 T-shaped AuI complexes 112 and 113(186) were prepared similarly to the CuI complex 110(193) by a reaction of in situ triply deprotonated protioligands 105 or 106 and the metal precursor (Scheme 17). On the other hand, the AgI complex 111(197) was obtained by the direct metalation of the ligand precursor with an excess of Ag2O in the presence of KBr and in the absence of light (Scheme 17). The T-shaped geometry of the metal center in 110–113 was confirmed by single crystal structure determinations.186,193,197 In the structural analysis of the complexes, deviation from the ideal T-shape geometry is a result of the metal atom displacement above the plane defined by the ligand. This distortion is most pronounced for 112, with 2,4,6-trimethylphenyl (mesityl = Mes) wingtips.186
Scheme 17. Syntheses of Coinage Metal Complexes Coordinated by the Bis(triazolylidene)carbazolide Ligand.
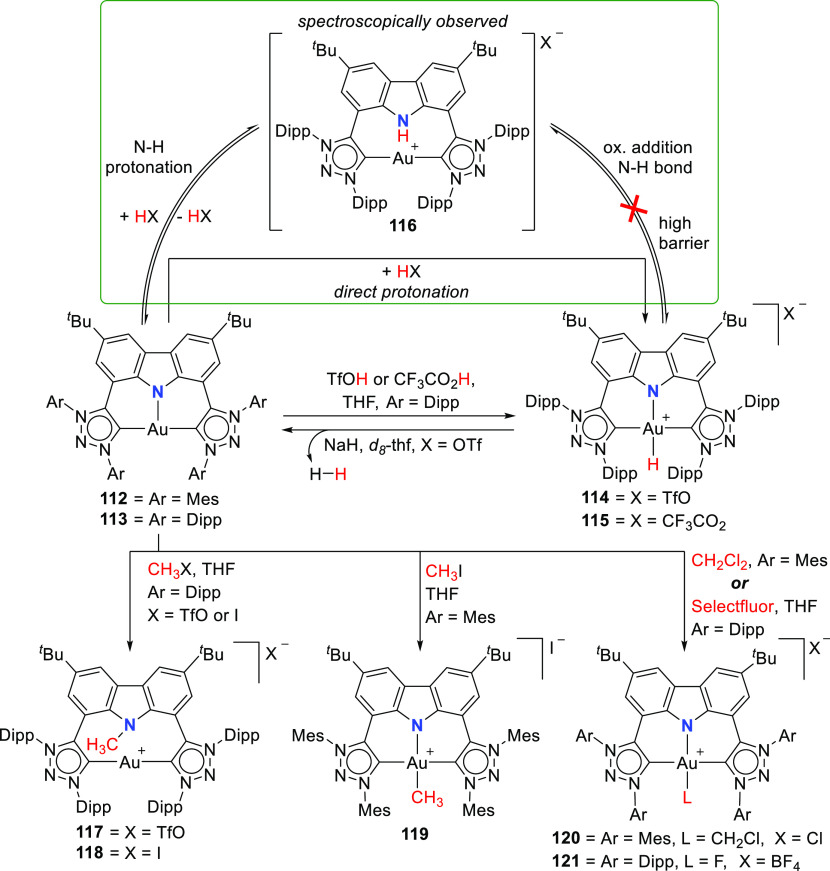
In the treatment of the gold(I) complexes 112 and 113 with acids, e.g., TfOH, trifluoroacetic, or acetic acid, the unprecedented formation of cationic gold(III) hydride complexes 114 and 115 (Scheme 18), with near ideal square planar geometry, was observed.186114 has a protic rather than hydridic character and does not react with acids. The converse reaction with NaH base leads to the reformation of 113 with simultaneous release of H2 gas after 3 days at room temperature (Scheme 18). Mechanistic considerations for the formation of 114 involved two possible pathways, either by direct protonation of the gold(I) center in 113 or alternatively by two steps involving the protonation of the amido nitrogen of the carbazolide, followed by oxidative addition of the N–H bond across the gold(I) center (Scheme 18). At first, the latter option seemed more promising and was supported by the NMR spectroscopic observation of the cationic gold(I) complex 116 with protonated amido N–H which was obtained from the stoichiometric reaction between 113 and TfOH. 116 was found to convert completely into AuIII complex 114 over an extended period of time. However, DFT calculations suggested that the oxidative addition of the N–H bond is energetically highly unfavorable, leaving the possibility of protonation occurring at both the nitrogen and the gold center as the more likely scenario. Further support for this was provided by the visualization of the HOMO of 113 showing the antibonding interaction between carbazolide-nitrogen and gold(I), resulting in a significant polarization of the corresponding occupied d-orbital at gold. This results in a remote metal-basicity which renders the gold(I) center nucleophilic and thus reactive toward electrophiles. This also explained the equilibrium between the cationic AuI complex 116 and the neutral AuI complex 113 leading to the formation of the observed gold(III) hydride complex 114.186 Subsequent quantum theoretical studies suggested that the high proton affinity at the gold(I) center of the T-shaped complexes is due to relativistic effects.198 As a result, the electron density at the gold center increases to the extent that it is similar to, and competes with, the electron-rich amido nitrogen, thus supporting the experimental observations.
Scheme 18. Oxidation of AuI Complexes to Square Planar Cationic AuIII Complexes.
Further electrophilic oxidation of the gold(I) complexes 112 and 113 was observed when the investigations were extended to electrophilic alkylation reagents.186 In addition, it was found that the steric factors of the ligand affect whether the electrophilic attack targets the nucleophilic gold(I) center or the amido nitrogen of the ligand. When 113 with sterically demanding 2,6-diisopropylphenyl (Dipp) wingtip groups was treated with methyl triflate or methyl iodide, alkylation of the amido nitrogen was observed resulting in the formation of cationic linear AuI complexes 117 or 118, respectively. In contrast, the reaction of methyl iodide with 112 bearing mesityl wingtips led to the alkylation of the gold(I) followed by oxidation of the metal to give the cationic AuIII-Me complex 119. The formation of the cationic AuIII-CH2Cl complex 120 was observed in the reaction of 112 with dichloromethane, whereas no reaction was observed with 113. The reaction of 113 with Selectfluor afforded 121, representing the first stable well-defined cationic gold(III) fluoride complex.197 The different reactivity presented by the Mes 112 and the Dipp analogue 113 may also partly be due to the different geometries of the gold(I) centers of the complexes, of which the reactive metal center in the mesityl analogue 112 is more distorted from the T-shape. This observation is in accordance with facile oxidative addition to AuI made possible by chelate-assisted ligand reactivity reported by Bourissou,199,200 or the use of reactive partners such as a strained biphenylene,201,202 where increased distortion raises the energy of the complex and preorganizes it for oxidative addition.
2.4. Extending the Reactivity of the Carbazolide-Nitrogen Lone Pair
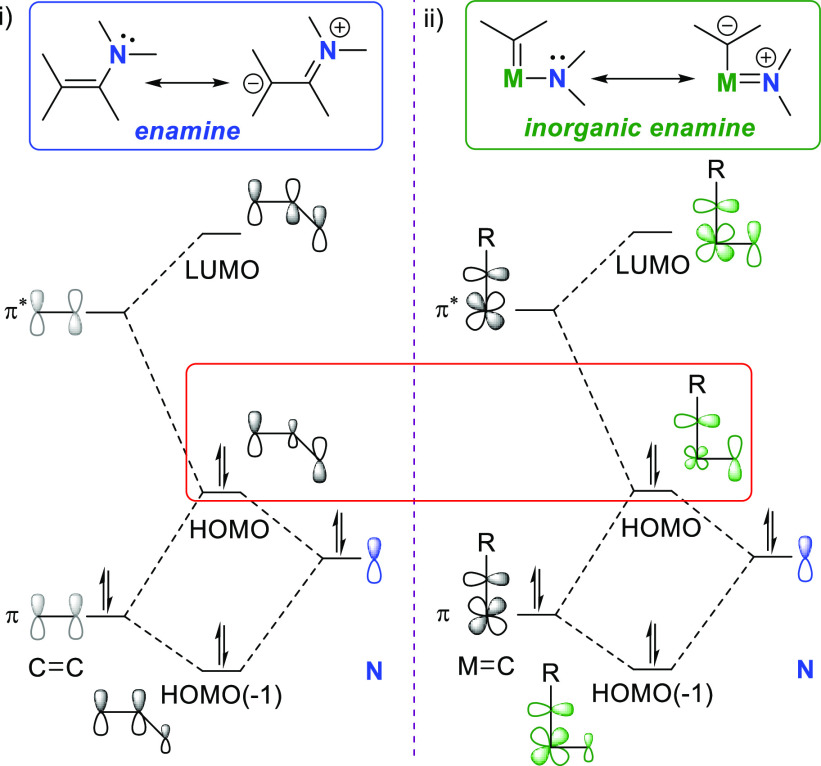
A pronounced electronic consequence of the carbazole-amido as central coordinating moiety of carbazole-based pincer ligands, is the so-called inorganic enamine effect203,204 observed in the bonding analysis of a trianionic ONO3– pincer alkylidyne complex of tungsten.205 The inorganic enamine effect is the term used to describe the elevated nucleophilicity of metal–carbon multiple bonds by constraining a nitrogen atom lone pair to be collinear with a metal-carbon multiple bond to allow for orbital overlap of the bonding and antibonding combination of the N lone pair with the M–C π-bond. Figure 2 depicts the relationship between organic enamines (i, Figure 2)206 and inorganic enamines. The inorganic enamine interaction has as consequence the destabilization of the HOMO with π* character, while the electron density from the amido lone pair is delocalized onto the α-carbon of the metal-carbon multiple bond (ii, Figure 2) to form highly nucleophilic metal alkylidenes/alkylidynes.205
Figure 2.
Resonance contributions and truncated qualitative orbital diagram of the bonding analogy between (i) enamines and (ii) amidoalkylidenes as analogous inorganic enamines.203,204
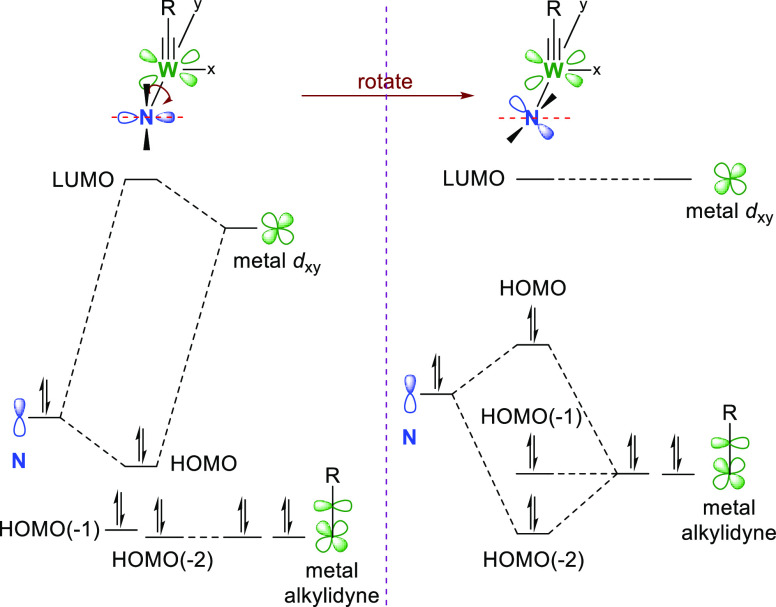
In their previous work, Veige et al. described alkylidyne tungsten complexes coordinated to a tridentate ONO-pincer ligand featuring a central amido flanked by two alkoxo-functionalized phenylene moieties.203,204 It was shown that the flanking biaryl moieties adjacent to the central amido rotate to create an inherent twist across the pincer backbone. Because the angle of the nitrogen atom p orbital approach and its energy match with the M–C π-bond naturally influences the magnitude of the orbital overlap, their target was to constrain the amido lone pair to be perfectly collinear with the metal-carbon multiple bonds to maximize orbital alignment for inorganic enamine orbital interaction (Figure 3). To accomplish this goal, a ligand precursor 122 representing a scaffold with the two aryl moieties of the previously reported ONO-pincer ligand was prepared from dibromo-precursor 18 (Scheme 19).205
Figure 3.
Orbital overlap for amido p-orbital aligned with dxy (left) and amido p-orbital rotated out of alignment, with corresponding truncated molecular orbital diagrams of preferential overlap of freely rotating N atom lone pair with unoccupied dxy orbital (left), and constrained N atom forced to overlap with one of the W≡C π-bonds (right).203,204
Scheme 19. Synthesis of Alkylidene, Alkylidyne, and Oxo-alkyl Complexes of Tungsten Coordinated to a Trianionic ONO-Carbazolide Pincer Ligand.
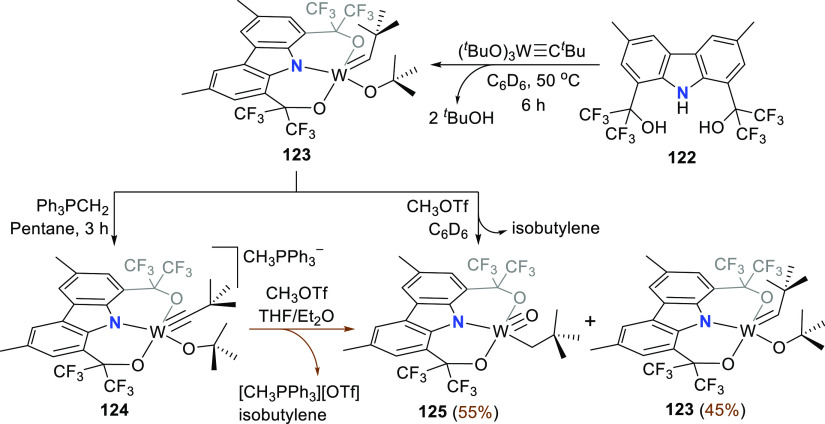
Metalation of 122 with (tBuO)3W≡CtBu yielded the alkylidene complex 123 with near-perfect square-pyramidal geometry (τ5207 = 0.059) (Scheme 19). The ONO3– trianionic pincer and the tert-butoxide ligand reside on the basal plane, with the planar carbazole forcing the N-aryl rings in a coplanar arrangement, while the alkylidene fragment occupies the axial position as determined from the molecular structure.205 The trigonal plane of the nitrogen atom is oriented perpendicular to the W=C bond axis, as designed. Treatment of 123 with Ph3PCH2 deprotonates the alkylidene and precipitates the anionic alkylidyne complex 124. Treatment of 124 with MeOTf did not result in the expected formation of a neutral metal alkylidyne derivative. Instead, complex 123 is regenerated alongside the oxo-alkyl complex 125 (Scheme 19). Formation of 123 was ascribed to the presence of adventitious protons, while formation of 125 from 123 was confirmed in an independent reaction with MeOTf and expulsion of isobutylene.
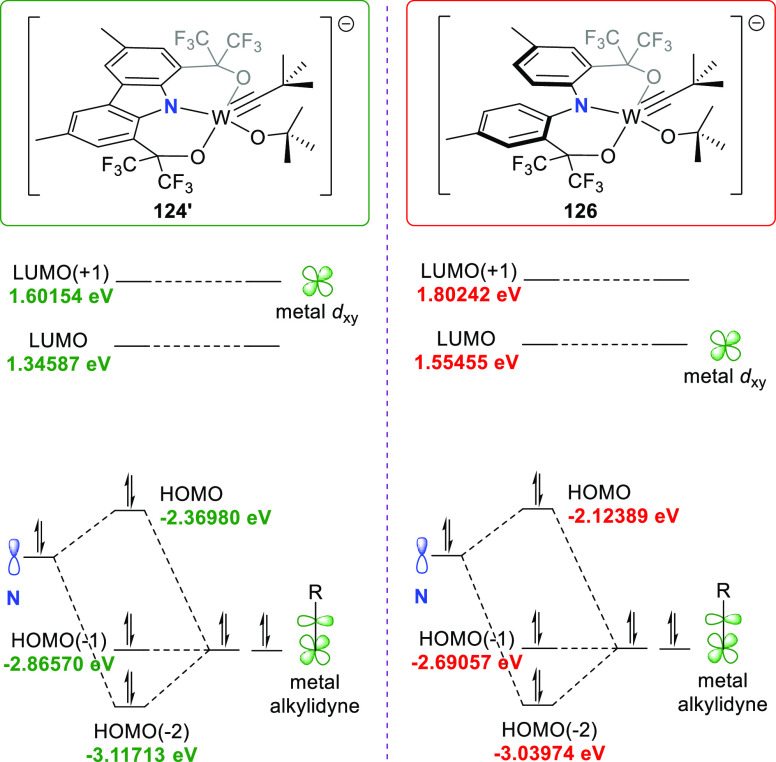
DFT calculations were performed to examine the electronic structure of the rigid anion of 124 (Figure 4, left) and to compare its orbital overlap with the previously reported, flexible analogue 126 (Figure 4, right).208 From the computed structures 124′ and 126, it was evident that the nitrogen atom lone pair is nearly collinear with the alkylidyne π-orbitals in both complexes to generate an inorganic enamine between the HOMO(−2) and the HOMO with an overlap of similar magnitude (Figure 3 and Figure 4, right).205 The rigid ligand was expected to have a significantly stronger overlap; however, several factors other than the p orbital orientation influences the magnitude of the overlap. As an example, a distinct difference was noted in the magnitude of the electron density on the nitrogen atom within the molecular orbital that overlap with the π-W–C bond for 124′ and 126. In 124′, the electron density is delocalized over the carbazole backbone, while it is largely N-centered in the twisted ligand of 126 with a more basic amido moiety. This can be further supported with the pKa values (Scheme 7). Thus, despite the prominent structural differences between 124 and 126 and achieving the restriction of the p orbital on the nitrogen atom for collinearity with the W–C π-bond and inorganic enamine reactivity, these factors alone are not sufficient to accomplish increased orbital overlap and influencing their relative energies. More generally, though, is the observation throughout this review that increased delocalization into the carbazole backbone ensures increased aromaticity across the backbone over the metal carbon bond, which certainly becomes important during redox and photocatalyzed processes, vide infra section 5.
Figure 4.
Truncated molecular orbital diagrams exhibiting inorganic enamine bonding combinations for the anions 124′ and 126.205,208
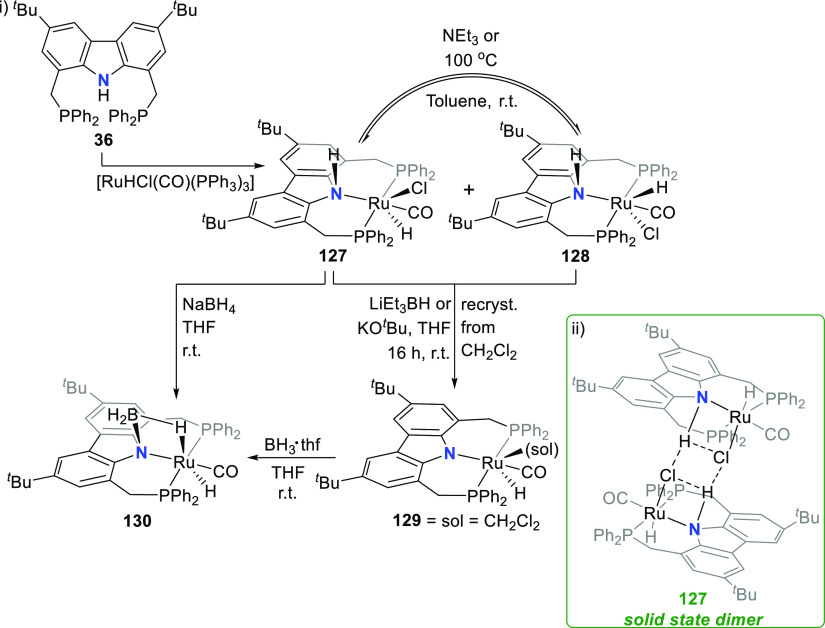
Another example implicating the carbazolide-nitrogen’s lone pair in directing complex reactivity is found in the observed metal-ligand cooperativity209 of a PNP-pincer complex of ruthenium.210 B–H activation was reported from the reaction of the borane-thf adduct with either a neutral protonated or an anionic PNP-pincer complex of ruthenium. The protonated complex 127 was prepared from the reaction of protioligand 36 with [RuHCl(CO)(PPh3)3] to form the two stereoisomeric hydrido complexes 127 and 128 (i, Scheme 20). The 1,2-dehydrochlorination reaction products contain both a metal-bound hydrido ligand as well as a protic NH moiety at the carbazole backbone of the pincer. The molecular structure of the isolated stereoisomer 127 displays an intermolecular hydrogen-bonding interaction between the chloride ligand and the carbazole-NH to form a dimeric structural arrangement around the central H2Cl2 cycle (ii, Scheme 20). If 127 is heated at 100 °C or treated with triethylamine, conversion to the stereoisomer 128 is observed, but 128 reverts to the steady-state equilibrium favoring 127 at room temperature (i, Scheme 20). Both 127 and 128 react with a strong base (KOtBu or LiEt3BH) to yield deprotonated 129 with an anionic carbazolide scaffold with significantly shorter Ru–N bond length (2.301 Å for 127 compared to 2.173 Å for 129), with weak solvent coordination completing the octahedral coordination sphere around the ruthenium metal. Reaction of BH3·thf with 129 at room temperature leads to the 1,2-addition of the BH3 moiety to the Ru–N functionality to form a RuNBH cycle in 130 (i, Scheme 20).210 The borane-bridged 130 can also be formed by reaction of 127 with sodium borohydride in solvent THF. Structural analysis of the X-ray diffraction data shows the ruthenium-bound H-atoms in the axial positions of the trigonal bipyramidal coordination geometry. The Ru–H bond that forms part of the RuNBH cycle is elongated (1.80 Å) compared to the other Ru–H (1.58 Å), and while the Ru···B distance (2.458(2) Å) is longer than the sum of the covalent radii (2.09 Å), it is shorter than those of 1η-B–H σ-type complexes.211 The authors surmised that the Ru···B distance reflects the geometric demands of the coordination mode of the BH3 unit to the Ru–N function in the complex rather than additional attractive interactions between the ruthenium and boron centers,210 with a B–N single bond (1.58(2) Å) instead of a B=N bond with π-character as expected for interaction with a quaternary nitrogen.212
Scheme 20. Synthesis of Hydrido Ruthenium(II) PNP-Pincer Complexes and Cooperative Reactivity with Borane.
3. Flanking Donor Effects
The effects of the donor moieties flanking the central carbazole moiety can be differentiated into effects governed primarily by the tethering E-group (connecting donor L and 1,8-carbazole positions, Figure 1) in section 3.1, the identity of the ligating L-group (in this review, only neutral C-, N-, and P-donors are reported, with singular examples of anionic O-donors) in section 3.2, or a combined effect of the EL-donors in section 3.3 leading to metal-ligand cooperativity.
3.1. Controlling the Size of the Lanthanide Chelate
When considering the effect of E in the L(E)N(E)L-carbazolide scaffold (Figure 1), the presence or absence of an E-moiety would dictate the formation of 5- or 6-membered metallacycles of the three “pincing” moieties. In turn, 5- versus 6-membered chelation could prevent or allow for intramolecular C–H activation of the E aliphatic tethers linking the 1,8-positions of carbazole with donor ligand moieties L, respectively. The 5-membered chelation leads to “open” chelate (less acute ligand bite angle), decreasing the propensity to undergo cyclometalation. On the other hand, a 6-membered chelate positions wingtip substituents in close proximity and correct geometry to undergo cyclometalative reactions (noted for carbazole and pyrrole backbones; see section 3.3 below).213 In this context, carbazole-based pincers provide an excellent alternative to the cyclopentadienyl ligands and their derivatives that are often employed in lanthanide organometallic chemistry and catalysis. Despite the frequent application of cyclopentadienyl ligands in this area, their steric and electronic modulation are limited, especially when likened against tridentate pincer ligands. Furthermore, multidentate coordination can impart additional stabilization to lanthanides against decomposition and ligand redistribution reactions, a drawback that has hindered the progress made with regard to lanthanide organometallics, especially when compared against the transition metals. Carbazole pincers address both concerns, with tridentate coordination increasing the stability of the complex in addition to facile electronic and steric fine-tuning leading to refined complex/catalyst.
3.1.1. 5-Membered Chelation
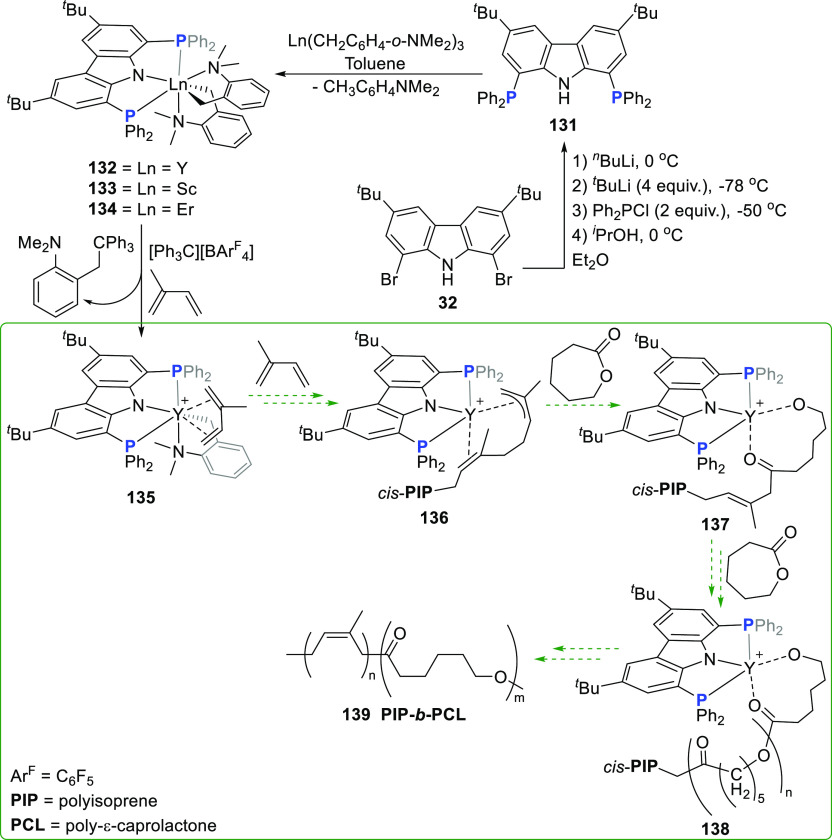
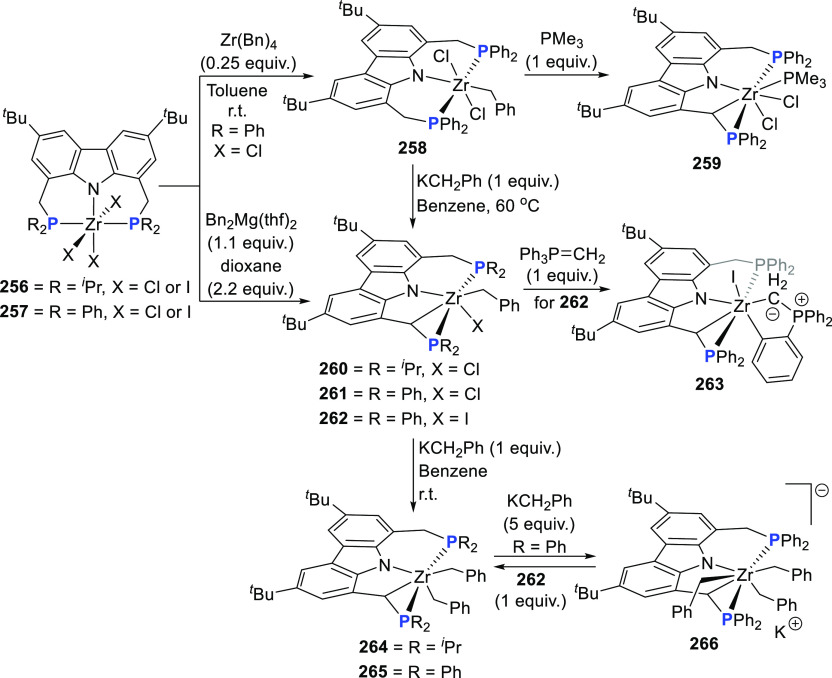
A carbazole pincer ligand containing phosphino-donor groups directly bonded to the carbazole 1,8-positions was employed by the group of Cui in the synthesis of well-defined rare earth metal pincer complexes containing 5-membered chelated metallacycles.214 The dibrominated carbazole precursor 32 was sequentially lithiated, whereafter metathesis with PPh2Cl yielded the protioligand precursor 131 (Scheme 21). The combination of the large, soft phosphorus donor and hard, anchoring carbazole-amido donor was anticipated to stabilize the dialkyl rare earth metal complexes, while the 5-membered chelation was specifically employed to preclude unpredictable C–H activation of the ligand backbone. Simultaneously, the tridentate coordination would circumvent the usual challenges of dimerization and ligand scrambling for the rare earth metals, even in a complex with relatively low steric crowding. Deprotonation and complexation of 131 were achieved by reaction with the rare earth metal tris(o-aminobenzyl) derivatives to yield the yttrium(III) (132), scandium(III) (133), and erbium(III) (134) complexes (Scheme 21). A striking structural feature observed in the crystal structure of seven-coordinate 132 is the large ligand bite angle [bond angle P–Y–P = 118.06(3)°] compared to the bite angles of 6-membered chelating PNP-carbazolide metal complexes (259, 263, and 266, vide infra, Scheme 40) with coordination number seven, where P–M–P bond angles can range between 89.9–101.6°, albeit that these contrasting examples feature an early transition metal with PN(C)P coordination mode of the carbazole-based pincer.173 NMR spectroscopic studies, as well as the solid state structure obtained for 132, confirmed the absence of solvent coordination, while both aminobenzyl ligands were found to coordinate as chelating bidentate ligands to yield the solvent-free seven coordinate monomer with the YIII deviating slightly from the planar pincer geometry as a result of the steric congestion of the diphenylphoshpine groups.214 In contrast, a related PNP-pincer dialkyl yttrium complex derived from flexible bis(diphenylphosphino) amido ligand displays thf coordination, with ligand P–M–P bite angles of 102.7–106.6°.215
Scheme 21. Synthesis of 5-Membered Chelating PNP-Pincer Lanthanide Complexes for Polymerization of Dienes.
Scheme 40. Reactivity of Cyclometalated Zr Complexes.
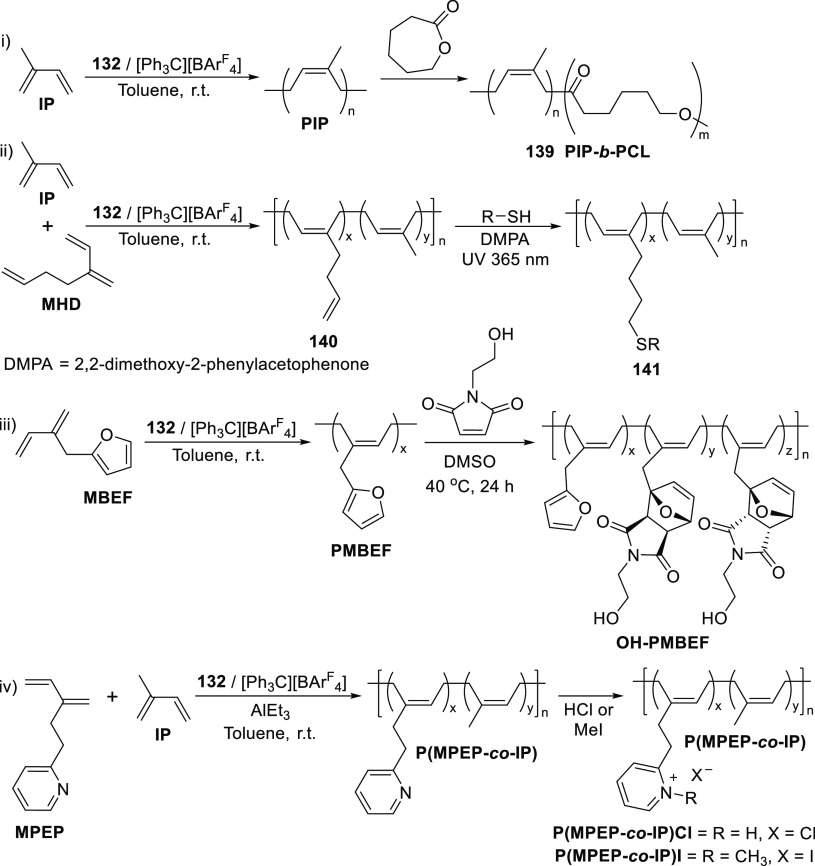
Addressing the need for new catalyst systems beyond that of the Ziegler–Natta type for the polymerization of 1,3-conjugated dienes requires catalysts that exhibit a living mode as well as high cis-1,4-selectivity to control the rheological and mechanical properties of these important rubbers.216,217 Cui et al. demonstrated that complexes 132 and 133 are highly active (complete conversion within 10 min) toward isoprene (IP) polymerization with borate activation instead of activation with a trialkylaluminum activator, whereas the erbium catalyst 134 was slightly less active (30 min conversion time).214 Not only was excellent cis-1,4-regularity obtained (>99%), but regularity was maintained at polymerization temperatures up to 80 °C. More remarkably, a living mode was exhibited for the 132 or 134 and [Ph3C][BArF4] (ArF = C6F5) catalyst systems, with molecular weights of the polyisoprene (PIP) increasing linearly with monomer to initiator ratio increase. Encouraged by these results, block copolymerization of IP and ε-caprolactone (ε-CL) was carried out to yield a 100% conversion to the PIP-b-PCL block copolymer 139 with designable molecular weight (i, Scheme 22). Abstraction of the alkyl moiety o-CH2C6H4NMe2 with a trityl cation [Ph3C][BArF4] is required to yield the cationic monoalkyl complex 135 with release of the coupling product Ph3CCH2C6H4NMe2-o (Scheme 21). NMR studies confirmed cis-η4 coordination of the IP monomer to the cationic 135, inserted into the Y–CH2 bond, and propagated to cationic yttrium polyisoprene (PIP) 136 as the active species. 136 initiates the polymerization of CL on the carbonyl carbon via cleavage of an acyl-oxygen bond (137), leading to the formation of copolymer 139 via 138. Notably, although the yttrium precursor Y(CH2C6H4-o-NMe2)3 is active in the polymerization, it is nonselective and not living, and the selectivity and activity in the dual catalysis of diene polymerization and ring-opening of polar ε-CL are therefore attributed to the introduction of the PNP-carbazolide ligand.
Scheme 22. Cis-1,4-Selective Living Diene Polymerization and Subsequent Post-Modification.
Expanding the catalytic polymerization repertoire of 132 to include (co)polymerization of 3-methylenehepta-1,6-diene (MHD) demonstrated that the high stereotacticity (cis-1,4-selectivity up to 98.5%) is maintained in the homopolymer PMHD and the copolymer 140 obtained with pendant vinyl groups ranging from 10–90% (ii, Scheme 22).218 Moreover, postmodification of the vinyl groups in every chain unit could be quantitatively effected. Conversion of the vinyl groups into a variety of thiol functionalities via a light-mediated thiol-ene reaction with photoinitiator DMPA (2,2-dimethoxy-2-phenylacetophenone) yields functionalized polybutadiene materials 141 with enhanced hydrophilicity. The copolymerization of polar and nonpolar monomers with subsequent functionalization of the polymers allows for modification or improvement of material properties. In this regard, living, cis-1,4-selective polymerization of 2-(2-methylidenebut-3-enyl)furan (MBEF) could be similarly achieved upon borate activation (iii, Scheme 22), without the need to mask the polar furan groups in the formation of PMBEF.219 Functional rubber materials are accessible after Diels–Alder addition of the furan groups in PMBEF with 1-(2-hydroxyethyl)-1H-pyrrole-2,5-dione to yield hydroxyl-functionalized polymers OH-PMBEF with 75% conversion containing a mixture of both endo and exo diastereomers. In contrast, copolymerization of IP with polar 2-(3-methylidenepent-4-en-1-yl)pyridine (MPEP) does require equimolar addition of triethylaluminum to form an adduct with the pyridine prior to polymerization (iv, Scheme 22).220 Nevertheless, copolymerization of MPEP and IP proceeds to yield a copolymer P(MPEP-co-IP) containing up to 17 mol % of the polar MPEP, compared to the commonly reported 10 mol % incorporation for other catalyst systems.221 Complete quaternization of the pendant pyridine nitrogen groups can be effected by treatment with HCl at room temperature to yield P(MPEP-co-IP)Cl while alkylation with methyl iodide yielded P(MPEP-co-IP)I.220 A self-standing and elastic film could be prepared from P(MPEP-co-IP)I as a result of cationic pyridine aggregation to form pseudo cross-link joints.
3.1.2. 6-Membered Chelation
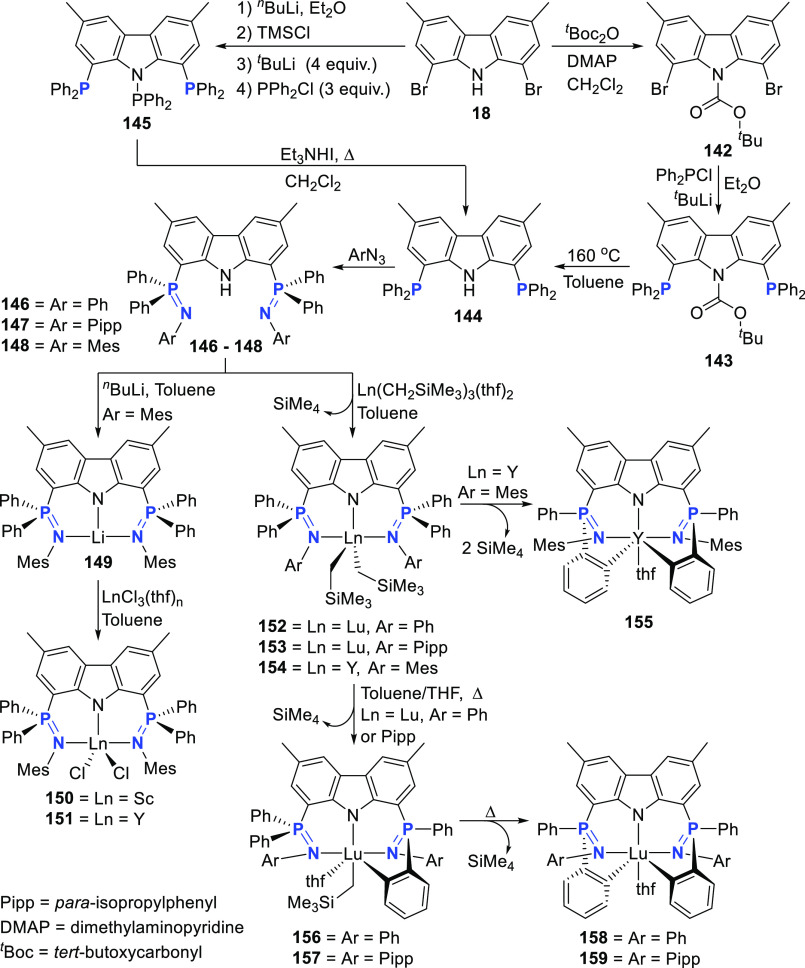
Cyclometalation reactivity of the E-linkers between carbazole and L-donors in the pincer ligand is not always well-controlled, but the reactivity can be harnessed for lanthanides or excluded by changing the nature of the E-linkers. The group of Hayes explored the chemistry of 6-membered chelated organolanthanides coordinated by a tridentate carbazolide.222 The authors reported the synthesis of a novel bis(phosphinimine)carbazole ligand, attributed to have appreciable electron-donating character, with easy substitution of the groups at both the phosphorus and nitrogen atoms, further increasing the handle on catalyst fine-tuning. Accordingly, the 1,8-diphosphine 144 was accessed from the dibromo 142 through phosphine substitution followed by deprotection of the carbazole-nitrogen (Scheme 23). The bis(phosphinimine) ligands 146 and 147 could be isolated from 144 and the corresponding azide under standard Staudinger reaction conditions. Alternatively, protioligand 148 was accessed from 144, in turn obtained from the deprotection of 145.223 An alkane elimination reaction between Lu(CH2SiMe3)3(THF)2 and protioligands 146 and 147 yielded the bis(phosphinimine)carbazolide lutetium complexes 152 and 153, respectively (Scheme 23).222 The complexes could only be characterized by solution NMR spectroscopic techniques below 0 °C, as they were determined to be thermally unstable preventing their isolation. Above 0 °C, metal-ligand reactivity results in thermal decomposition with two consecutive intramolecular phenyl C–H activation and metalation reactions, yielding the cyclometalated Lu complexes 158 and 159. A σ-bond metathesis was reported as the reaction pathway through which the phenyl ortho C–H bond reacts. Similarly, yttrium complexes could also be prepared from the bis(phosphinimine)carbazolide featuring increased steric bulk at the donor wingtip sites.223 Yttrium complex 155 was isolated after cyclometalation of 154 coordinated by ligand 148. Yttrium and scandium dichloro-complexes 150 and 151, respectively, could also be synthesized, determined to be stable even when heated up to 140 °C. The stability was attributed to the increased steric bulk (Scheme 23).
Scheme 23. Synthesis and Reactivity of Pincer Coordinated Lanthanide Complexes.
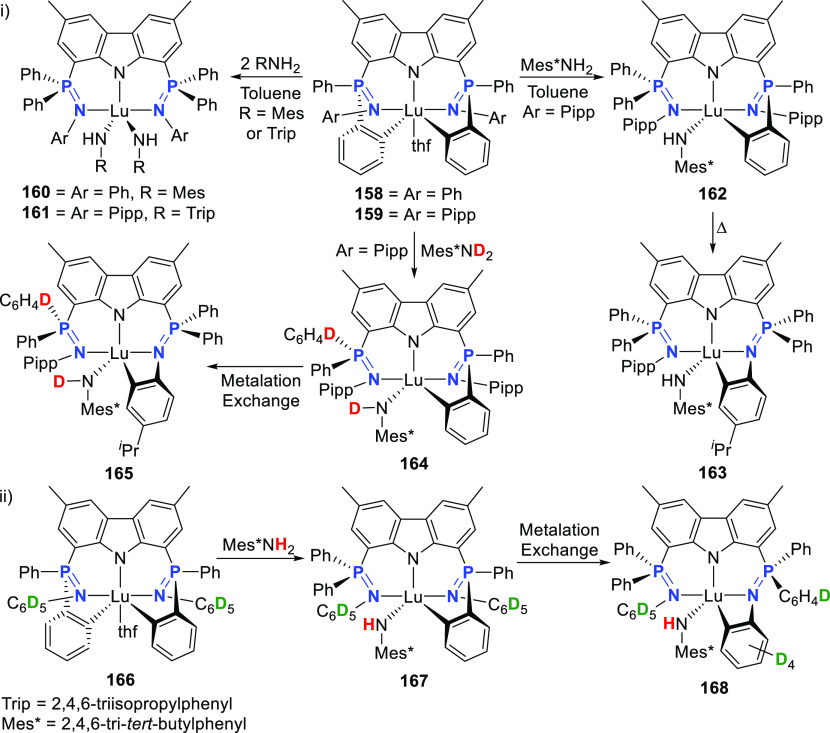
The reactivity of 158 and 159 (Scheme 23) was further investigated, exploiting the basic character of the phenyl carbon–metal bond.224 Specifically, 158 was reacted with two equivalents of 2,4,6-trimethylaniline (MesNH2), which yielded the corresponding bis(anilide) 160 (i, Scheme 24). Ligand-assisted reactivity facilitates N–H bond activation across the metal-phenyl bond, followed by metallacycle ring-opening and metal-anilide bond formation. Bis(anilide) formation was reported even when reacting 158 with only one equivalent of MesNH2. Similar reactivity was also noted when reacting the more sterically encumbered 159 with 2,4,6-triisopropylaniline (TripNH2), isolating the corresponding bis(anilide) 161 (i, Scheme 24). In contrast to the repeated double N–H bond activations observed for 158 and 159 (i, Scheme 24), mono(anilide) formation could be enforced when reacting the six coordinated lutetium with an aniline that has even greater steric bulk compared to MesNH2 or TripNH2. Thus, when reacting 159 with 2,4,6-tri-tert-butylaniline (Mes*NH2) in toluene, mono(anilide) 162 formed. Again, complex 162 could not be isolated but was characterized via NMR spectroscopy. The corresponding bis(anilide) was not formed, even in the presence of excess aniline or at a reaction temperature of 100 °C maintained for 24 h. Interestingly, the mono(anilide) 162 was found to be susceptible towards a thermally induced intramolecular rearrangement reaction, leading to 163.
Scheme 24. Metallacycle Ring Opening and ortho-Metalation Leading to Lutetium Anilide Formation.
Deuteration experiments allowed insight into the reaction mechanism toward 163 (i, Scheme 24).224 Reacting 159 with Mes*ND2 yielded 165 featuring a deuterium at the anilide nitrogen. This suggested initial N–D activation with metallacycle ring-opening leading to the observed 164, followed by direct metalation exchange between the two aryl substituents at the phosphinimine donor arm, namely the exchange of the phenyl group for the 4-isopropylphenyl substituent, resulting in 165. A second deuterium labeled experiment corroborated the results, with metallacycle ring-opening followed by direct metalation exchange of the aryl rings. Hence, reacting the deuterium-labeled 166 with Mes*NH2 yielded 168 via 167, with the proton still bound to the anilide nitrogen (ii, Scheme 24). Deuterium exchange between the two aryl substituents involved in the direct metalation exchange reaction was confirmed, further supporting the proposed reaction mechanism.
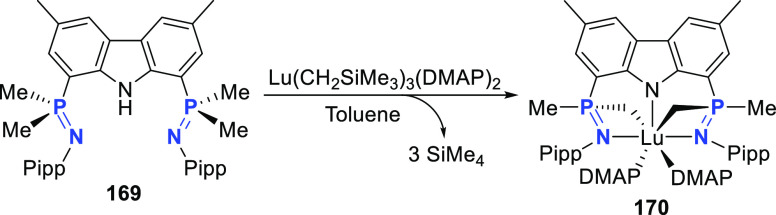
Decreasing the steric bulk at the P-site of the donor arm to methyl substituents also leads to C–H activation and cyclometalation.225 Ligand 169, with decreased steric bulk at the E-linker groups, was reacted with Lu(CH2SiMe3)3(thf)2 similar to previous routes toward the organolanthanides. However, complex isolation was not possible and neither was spectroscopic characterization as a result of extreme thermal instability. Stabilization of the targeted complex was brought about by substituting the lutetium precursor’s thf coligands with 4-dimethylaminopyridine (DMAP). As a result, coordination of ligand 169 to Lu(CH2SiMe3)3(DMAP)2 yielded the doubly cyclometalated Lu complex 170, with two methyl C–H activation and cyclometalation reactions leading to formation of the distorted pentagonal bipyramidal complex (Scheme 25). The complex was determined to be unreactive to anilines.
Scheme 25. Decreasing the Steric Bulk at the P-Site of the Donor Groups.
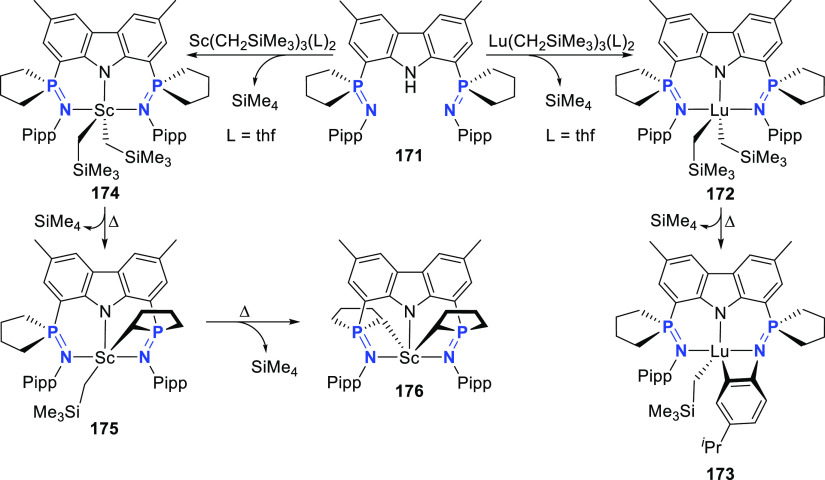
At the same instance of decreasing steric bulk at the E-site of the P-donor group (169, Scheme 25), Hayes et al. also introduced a cyclic phospholane at the phosphorus moieties.225 The corresponding bis(phospholane)carbazole ligand 171 was prepared and coordinated to lutetium and scandium (Scheme 26). Reacting ligand 171 with Lu(CH2SiMe3)3(thf)2 yielded 172 through an alkane elimination reaction. Complex 172 could only be characterized in situ with NMR spectroscopic methods, due to thermal instability leading to cyclometalation and formation of 173, followed by complex decomposition (Scheme 26). Noteworthy is the different result obtained upon treatment of ligand 171 with Sc(CH2SiMe3)3(thf)2, which yielded 176 through two consecutive cyclometalation reactions at the phospholane’s α-position, as determined by NMR analysis.
Scheme 26. Coordination of Bis(phospholane)carbazolide to Lutetium and Scandium.
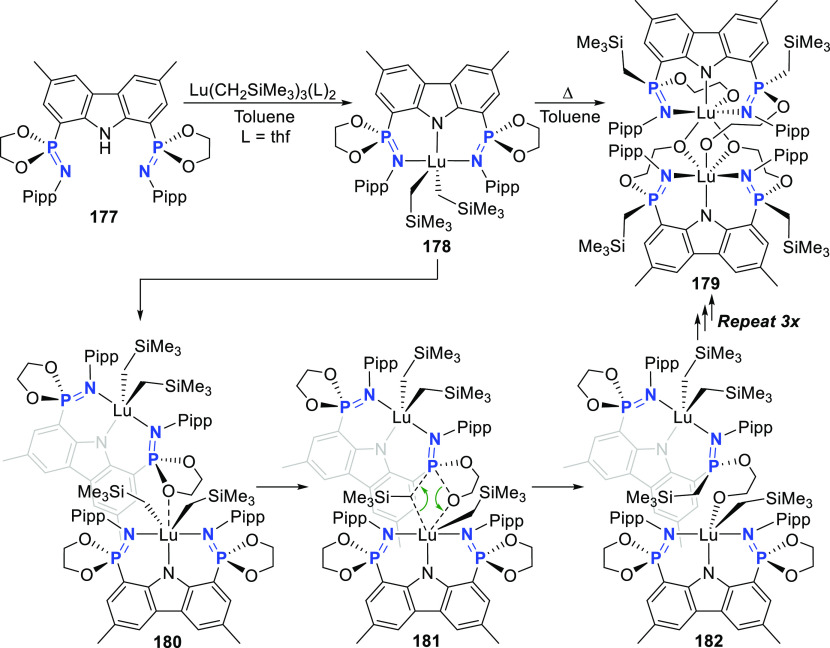
A variation of the phospholane donor group was also reported by Hayes and co-workers, introducing an oxygen at the phospholane’s α-position, which markedly changed the reactivity outcome.226 An alkane elimination reaction led to formation of the lutetium complex 178 from ligand 177 (Scheme 27), similar to what was described above. Again, it was disclosed that the bis(alkyl) complex 178 reacted further at higher temperatures. Unlike the phospholane 172, 178 reacts via a ring-opening insertion reaction of the dioxaphospholane donor group to yield the asymmetric dinuclear tetraalkoxide complex 179 (Scheme 27). A proposed mechanism is depicted in Scheme 27. The oxygen of a dioxaphospholane of 178 coordinates to a second lutetium metal center at 180, with concomitant ring-opening insertion with a four-centered transition state as the intermediary species (181, Scheme 27). From the four-centered transition state ensues the cleavage of the oxygen–phosphorus bond and formation of a new oxygen–lutetium bond at 182. The process repeats several times, ultimately leading to 179.
Scheme 27. Asymmetric Dinuclear Tetraalkoxide Lutetium Complex via a Cascade Ring-Opening Insertion Reaction.
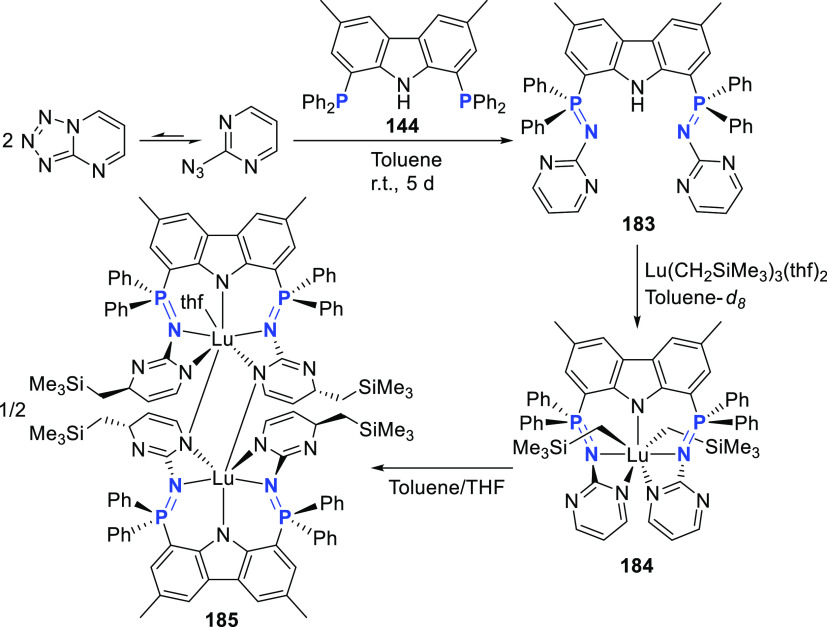
Varying the substituents at L (see Figure 1) from a bulky moiety to a pyrimidine functionality, changed the intramolecular reactivity of the formed lanthanide complex.227 It was reasoned that the lack of wingtip ortho C–H bonds, in addition to the potential denticity of the ligand being increased by introduction of more nitrogens in the ligand, would hamper the cyclometalative pathway encountered for previously reported bis(phosphinimine)carbazole ligands, which could lead to alternative reactivity. Ligand 183, prepared from 144 and 2-azidopyrimidine under Staudinger reaction conditions, was coordinated to Lu(CH2SiMe3)3(thf)2 yielding seven-coordinate complex 184 which was characterized in situ by NMR spectroscopy (Scheme 28). Complex 184 demonstrated increased thermal stability, with a half-life of more than 5 h compared to other bis(phosphinimine)carbazolide coordinated lutetium dialkyl species with half-lives of less than one hour. The increased thermal stability was ascribed to the coordination of two additional nitrogens from the pyrimidine wingtip groups. Furthermore, 184 does not undergo intramolecular cyclometalation as described above. After several hours in solution, 184 converted to the dinuclear 185, a reaction accelerated at elevated temperatures. Interestingly, alkyl migration gave rise to pyrimidine dearomatization, with the ensuing complex featuring an anionic nitrogen coordinating to one metal, while the same nitrogen coordinates as a neutral Lewis base to the second metal, evidenced by crystal structure analysis of 185.227 The authors reasoned that a 1,3-alkyl migration in 184 with subsequent isomerization, followed by a second 1,3-alkyl migration and isomerization, and finally dimerization, leads to 185 (Scheme 28).
Scheme 28. Alkyl Lutetium Leading to Dearomatization and Complex Dimerization.
Substitution of the phosphinimine donor functionalities with pyrazole groups primed the carbazole-based NNN-pincer to stabilize lanthanide complexes against thermal degradation.213,228 As such, an Ullmann-type amination of dibromocarbazole 18 with the corresponding substituted pyrazole yielded ligand 186 featuring a methyl or isopropyl wingtip substituent further reacted with lutetium in an alkane elimination reaction leading to 187 and 188 (Scheme 29). It was reported that heating a solution of either 187 and 188 in C6D6 to 75 °C for 12 h did not lead to any complex decomposition. Complex 188 was further subjected to hydrogenolysis in an effort to prepare a lutetium hydride complex, which was successfully isolated and crystallized. The crystal structure evidenced the trimetallic complex 189 with five hydride ligands bridging the three metals. Of further note was the NNC-coordination mode of one of the NNN-pincer ligands to a Lu metal. Monoanionic carbazolide coordination was described for two of the Lu metals, with NNN-pincer coordination as expected. The third metal is coordinated by a dianionic carbazole ligand, a result of pyrazole C–H bond activation. It was theorized that intramolecular C–H bond activation occurred via metalation of the carbon of the pyrazolyl donor moiety with concomitant H2 elimination.228 This could further suggest hemilabile decoordination of the donor group with subsequent deprotonation of the pyrazolyl C–H by the hydride ligand. Alternatively, it was reasoned that metalation could have occurred prior to H2 formation by elimination of tetramethylsilane from 188, a scenario less likely due to the complex’s high thermal stability. Hydrogenolysis of the sterically less encumbered complex 187 only resulted in decomposition, highlighting the requirement for steric shielding at the wingtip position. The presentation clearly demonstrates the donor influence on the complex, allowing for a more thermally robust complex that is not inert, therefore mediating the bond activation process.
Scheme 29. Lutetium Alkyl and Hydride Complexes Stabilized by a Bis(pyrazolyl)carbazolide.
3.2. Increased Metal Nucleophilicity Imparted by Electron-Donating Flanking Groups
One of the most obvious benefits of the LNL-carbazole scaffold is the ease with which the identity of the flanking donor groups L can be varied for direct modulation of the electronic consequence at the metal center. This is illustrated by the wide range of carbonyl stretching frequencies (1916–1980 cm–1) reported for square planar rhodium(I) carbonyl complexes 30, 190–194 coordinated to the LNL-carbazolide (i, Figure 5) and follows the expected trend with νCO decreasing when L is varied from N(imine) donors (30, vide supra section 2.1, Scheme 4)139 to phosphines (190,158191,154vide infra, section 4.4, Scheme 63) to C(carbene) donors (192–194, vide infra, Schemes 30 and 48).140,196,229 On the one end of the spectrum (i, Figure 5), less basic donor groups can function as hemilabile ligands, rendering the ligand coordination noninnocent as described for the s- and p-block metal complexes of the bis(imine)carbazolide ligand (as coordinated in 30 in Figure 5) in section 3.3.1.120,230 On the other hand, the carbazole-N scaffold already provides for a strong donor platform (section 2), which can be maximized with the use of strongly basic donor groups such as NHCs, increasing the propensity of the electron rich metals to favor classic two electron processes, e.g., oxidative addition as described in sections 2.1 and below. For the rhodium carbonyl complexes reported with L = C(carbene), the BIMCA coordinated 194 exhibits the lowest CO wavenumber,140 demonstrating that it is the strongest donor ligand even compared to the triazolylidene analogues 192 and 193.196 This result is surprising, as the mesoionic triazolylidenes are generally reported to be stronger donors compared to NHCs.194,195 One explanation for this contradiction could be the wingtip R-groups, as they also provide for a remote handle on the electronic properties at the metal center. This is evident comparing the difference in carbonyl ligand vibrations even within the same class of donors, i.e., 192 (1941 cm–1) and 193 (1955 cm–1) (Figure 5).196 Extrapolating to compare 192/193 with 194, it means that the presence of four aryl groups instead of small aliphatic substituents on the flanking heterocycles can markedly alter the electronic consequence at RhI.
Scheme 63. Synthesis and Coordination Chemistry of Achiral and Chiral PNP-Carbazolide Pincer Ligands.
Scheme 30. Synthesis and Reactivity of Rhodium and Iridium Complexes of BIMCA.
Scheme 48. Synthesis of Rhodium(I) Complexes of Bis(triazolylidene)carbazolide.
The overall fine-tunability of the flanking donors by remote wingtip groups is illustrated clearly by inspection of the imidazolium C–H 1JCH coupling constants of the library of available BIMCA ligand precursors (92, 195–202, ii, Figure 5).140,190 The effects of the N-wingtip groups on the donor properties of the BIMCA ligand are discussed in more detail in section 4.3.3. A strong electron-withdrawing N-substituent increases the p-character of the N–C–H σ-bond in imidazolium, which in turn increases the s-character of the C–H bond and a larger C–H coupling constant. On the basis of the observed coupling constants, it was concluded that 197 with a bulky N-alkyl substituent (iPr) is the strongest donor, while N-aryl-substituted 201 and N-heteroaryl-substituted 202 were identified as the weakest.
Kunz et al. accessed rhodium carbonyl complexes 194 and 204 of the BIMCA ligands (92 and 195) by transmetalation of in situ generated lithium complexes 93 and 203, respectively, with [Rh(CO)2Cl]2 (Scheme 30).140,231 Analogous iridium complexes 208 and 210 were obtained by a similar method (Scheme 30).232 The most prominent structural feature in the crystal structures of the rhodium complex 194 and iridium complexes 208 and 210 is the strong distortion of the CO ligand out of the square planar geometry around the metal center.140,232 The possibility of increased lability of the ligand due to carbonyl distortion was ruled out in 13CO exchange experiments performed on rhodium complex 194 (Scheme 30). No exchange was observed despite overpressure of 13CO gas, irradiation, or prolonged heating, in accordance with the strong π-back-donation inferred from IR spectroscopy.140
The nucleophilicity of the RhI center in 194 as a result of the electron-donating nature of the BIMCA ligand was demonstrated by the formal oxidative addition of methyl iodide, whereby the observed immediate reaction led to the formation of 205 (Scheme 30).140 The X-ray crystal structure of octahedral 205 exhibits the iodine and methyl group at the axial positions trans to each other, typical of oxidative addition by the SN2 mechanism. The carbonyl group was shown to be more labile in 205 than in 194 based on 13CO exchange experiments, where carbonyl exchange was observed at room temperature within a short time (Scheme 30).
The rhodium and iridium complexes (194, 204, 208, and 210) also showed reactivity toward allyl halides.231,232 The complexes reacted with allyl chloride and benzyl halides to the respective rhodium(III) and iridium(III) allyl complexes (211 and 212, M = Rh; 213 and 214, M = Ir) and benzyl complexes (215 and 216, M = Rh; 217, M = Ir) (Scheme 31). In the case of the rhodium complexes, it was reported that the reaction with benzyl chloride is much slower, attributed to the reaction with benzyl halides proceeding via an SN2 mechanism, while the reaction with allyl halides followed an SN2′ mechanism.231 Both the allyl and benzyl substituents in 211–214, 216, and 217, respectively, are η1-coordinated to the metal.231,232 In addition, 194 and 208 were reacted with linear and branched methallyl chlorides (Scheme 31). Reaction with 3-chloro-1-butene yielded η1-allyl complexes 218 and 219 with an internal double bond (E/Z ratio = 5:1, reported for 218) via an SN2′ reaction. The reaction with 1-chloro-2-butene also resulted in η1-allyl complexes 218 and 219, instead of the expected 220 and 221 with a terminal double bond, the formation of which was not observed at all.
Scheme 31. Reactivity of Group 9 Carbonyl Complexes of BIMCA with Allyl Halides.
In the case of rhodium complex 218, one possibility considered was that the observed isomerization yielding the complex proceeds via a reactive η3-allyl intermediate as observed in palladium-catalyzed allylic alkylation.233−236 However, the possibility of this pathway was ruled out as unlikely, as the formation of the η3-isomer was not observed even under irradiation or at elevated temperatures.231 Two other pathways were found more likely, either by a direct SN2 mechanism or by a 2-fold SN2′ reaction via 220 (i, Scheme 32). The possibility of an SN2 mechanism was justified by the observation that the reaction proceeds more slowly with 1-chloro-2-butane than with 3-chloro-1-butene. However, as the reaction with 1-chloro-2-butene is faster than that with benzyl chloride, which typically proceeds by a SN2 mechanism and should be three times faster than the reaction with allyl chloride, the more likely pathway was considered to be a 2-fold SN2′ reaction. First an SN2′ reaction of 194 with 1-chloro-2-butene yields 220in situ, which then proceeds via an SN2′ mechanism with the highly nucleophilic Rh complex 194 to form the thermodynamically more favorable complex 218 with an internal double bond. A crossover experiment was performed in which 194 was reacted with 212 (ii, Scheme 32). Monitoring the experiment by 1H NMR spectroscopy indicated that two-thirds of the η1-allyl ligand was transferred from 212 to 194, which resulted in the formation of 211 and 204. After 5 h, the ratio of the components in the reaction mixture had not changed, indicating that 211 and 204 are thermodynamically more stable compared to 194 and 212. This was also observed in the control experiment where 211 was reacted with 204. DFT calculations supported the experimental observation of higher stability of 211 and 204. On the basis of the above, it was concluded that in rhodium-catalyzed allylic alkylation the η1-allyl to η1-allyl isomerization can proceed without the formation of an η3-allyl intermediate.231 If the intermediate has a terminal allyl double bond, the reaction can proceed via the intermolecular route following the SN2′ metal transfer reaction.
Scheme 32. (i) Possible Pathways for the Formation of the Rhodium Complex 218 in the Reaction with 1-Chloro-2-butene and (ii) Crossover Experiment between 194 and 212.
Expansion of the reactivity profile of the BIMCA complexes to catalytic epoxide deoxygenation was probed.237 Using carbon monoxide as a direct deoxygenation agent, 194 was found to catalyze the conversion of propylene oxide into propylene with Lewis acid LiNTf2 as the cocatalyst, although the complex proved to be unstable at the elevated temperature required for the reaction. However, the iridium(I) analogue 208 showed greater stability and considerably higher activity, which even exceeded the activity of commercially available rhodium, iridium, cobalt, and iron complexes under the same reaction conditions. 208 catalyzed the full conversion of propylene oxide to propylene under optimized reaction conditions (5 mol % catalyst loading along with 30 mol % of LiNTf2 cocatalyst under 10 bar of CO in benzene-d6 at 80 °C) (i, Scheme 33). The catalyzed deoxygenation of terminal alkyl epoxides (without significant isomerization to internal olefins) and aryl epoxides (especially electron-poor ones) proceeded efficiently, while side reactions increased with electron-rich aryl epoxides. Of particular note is the retention or inversion of configuration observed for 1,2-disubstituted epoxides upon deoxygenation. 1,2-Dialkyl epoxides retain the configuration as was exemplified by 2-butene oxide, the cis-isomer of which converts almost exclusively to cis-2-butene while trans-2-butene oxide is converted to trans-2-butene (ii and iii, Scheme 33). In contrast, inversion of configuration was observed with doubly ester-functionalized epoxides. This was illustrated by the deoxygenation of diethyl-2,3-epoxy succinate, the cis-isomer of which reacts to diethyl fumarate (trans) and the trans-isomer to diethyl maleate (cis) (iv and v, Scheme 33). The (stereo) selectivity of deoxygenation was explained by two different substrate-dependent epoxide activation mechanisms, either by oxidative addition with alkyl epoxides leading to retention of configuration or by SN2 mechanisms in the case of ethyl carboxylates, resulting in inversion of the configuration.
Scheme 33. Catalytic Deoxygenation (in Solvent C6D6, 80 °C, 24 h) of (i) Terminal Epoxides, (ii) cis-2-Butene Oxide, (iii) trans-2-Butene Oxide, (iv) cis-Diethyl-2,3-epoxy Succinate, and (v) trans-Diethyl-2,3-epoxy Succinate with Carbon Monoxide.
Further studies provided information on the mechanism of the catalytic reaction (Scheme 34).237 The 16-electron IrI complex 208 was identified as the catalytically active species, as it did not form an 18-electron CO-coordinated complex when exposed to 10 bar of CO. As the outcome of the catalytic reaction was found to be substrate-dependent, the intermediate steps of the catalytic cycle were therefore considered for both terminal alkyl epoxides and internal 1,2-di(alkyl or ester) substituted epoxides. In addition, the investigations were carried out both in the presence of a Lewis acid and without to clarify the role of the cocatalyst in the reaction. Activation of the epoxide requires the presence of a Lewis acid cocatalyst for coordination to epoxide oxygen.
Scheme 34. Proposed Mechanism for the Deoxygenation of Epoxides with CO Catalyzed by 208.
In the case of the terminal epoxide, propylene oxide, it was observed that the activation also occurs without a Lewis acid, albeit slowly.237 In a reaction without Lewis acid, the formation and accumulation of intermediate 224 (i) (Scheme 34) with 2-irida-γ-lactone moiety was observed, the identity of which was verified by NMR and IR spectroscopy. When Lewis acid was present in the reaction, which was monitored by NMR spectroscopically, rapid formation of 224 (i) was observed. As the concentration of 224 decreases, product propene and active catalyst 208 is observed to form in the solution in addition to a new compound, identified as the Lewis acid adduct 225 (i) (Scheme 34). On the basis of these findings, it was concluded that the Lewis acid cocatalyst is not only necessary for preactivation of the epoxide but also mandatory for CO2 elimination (step e) (Scheme 34).
The retention of configuration observed for deoxygenation of 1,2-dialkyl substituted epoxides and the inversion observed for the ester-functionalized epoxides supported the conclusion that the activation of internal epoxides occurs by two different mechanisms (Scheme 34).237 With 1,2-dialkyl substituted epoxides, the opening of the epoxide ring (step b) most likely proceeds via oxidative addition under C–O bond cleavage to form the intermediate 222 where the configuration is preserved. An alternative SN2 mechanism would lead to inversion of configuration in the possible intermediate 223, which is the more likely mechanism for the activation of ester-functionalized epoxides. In the case of 1,2-dialkyl substituted epoxides, the formation of intermediates 224 (ii) or 224 (iii) was not observed, indicating that the oxidative addition is the rate-determining step of the reaction. In a reaction involving ester-functionalized epoxide substrates without the presence of Lewis acid cocatalyst, the intermediates 224 (iv) (from trans-substrate) and 224 (v) (from cis-substrate) were detected by NMR spectroscopy. The single crystal X-ray structures of the intermediates 224 (iv) and 224 (v) confirmed the inversion of the configuration and the formation of the 2-irida-γ-lactone moiety. The mechanism (Scheme 34) thus derived for the 208 catalyzed deoxygenation of epoxides with CO, includes preactivation of the epoxide as a result of Lewis acid coordination (step a), followed by epoxide activation (step b) where the epoxide ring opens, either by oxidative addition (step b) allowing the retention of configuration or by the SN2 mechanism (step b′), leading to inversion of configuration. This is followed by CO-induced migration of the alkoxide from Ir to a CO ligand to form the intermediate 224 (step c) which forms a Lewis acid adduct 225 (step d), with subsequent decarboxylation to reconstitute the active catalyst 208 with product release (step e).
Lee and co-workers employed modified BIMCA complexes of nickel(II) as switchable catalysts for cycloaddition or copolymerization of epoxides and carbon dioxide, demonstrating that not only the electron donating ability of the ligand but also the N-substituent wingtips in the donors adjacent to the NHC influence the catalytic performance (see section 4).238 The bis(imidazolium)carbazole precursors 92, 226, and 200 were reacted with excess triethylamine and Ni(OAc)2·4H2O (OAc = acetate) to yield the air- and moisture-stable nickel(II) acetate complexes 227–229 (i, Scheme 35),238 which are analogous to the nickel(II) complex 357 reported by Grotjahn (vide infra, section 4.3.1).239 The 1H NMR spectra of the complexes display resonances for the methyl protons of the acetate group at δ 1.68, 1.91, and 2.09 ppm for 229, 228, and 227, respectively.238 The observed trend of increasingly downfield chemical shifts indicates the influence of the wingtip groups on the electronic environment.
Scheme 35. (i) Synthesis of Nickel(II) Complexes of Bis(imidazolylidene)carbazolide, and (ii) Coupling of Carbon Dioxide with Cyclohexane to Cyclohexane Carbonate Catalyzed by 228 and Poly(cyclohexane Carbonate) Catalyzed by 229.
All complexes 227–229 were active in the cycloaddition of cyclohexene oxide (CHO) and CO2 to produce cyclohexene carbonate (CHC) without the presence of a cocatalyst.238 However, 228 outperformed the other two in its catalytic activity, showing activity with a TOF of 41 h–1 and quantitative conversion of CHO (99%) with almost full cis-CHC selectivity (>99%) under optimized conditions of 0.1 mol % catalyst loading and CO2 pressure of 500 psi at 170 °C (ii, Scheme 35). The results showed that the coupling activity of the complexes decreases by the order of 228 > 227 ∼ 229. This is presumably due to the electronic effect of the wingtip groups, whereby the more electron-donating nBu wingtip groups in 228 induce more nucleophilicity toward the acetate group for epoxide ring opening, which in turn leads to the observed higher catalytic activity of 228 in CHO/CO2 cycloaddition. However, further studies showed that as the catalyst loading is increased and the reaction temperature is decreased, complexes 229 and 227 catalyze the copolymerization of CHO and CO2 to poly(cyclohexene carbonate) (PCHC) instead of cycloaddition. A higher CHO conversion was achieved with 227, while a better copolymer selectivity was associated with the presence of 229. Under optimized conditions (0.25 mol % catalyst loading, CO2 pressure of 500 psi at 110 °C), 229 catalyzed the CHO/CO2 copolymerization into a narrowly dispersed poly(cyclohexene carbonate) with >99% carbonate-linkage content with moderate copolymerization selectivity (68% copolymer) (ii, Scheme 35).
Hohloch et al. also reported the coupling of carbon dioxide and epoxides mediated by a bis(carbene)carbazolide nickel(II) complex, but their approach involved mesoionic carbenes, namely N-fused triazolylidenes, as carbazolide flanking groups.240 The change in the L-donor group from NHC to the stronger σ-donor MIC194,195 was anticipated to provide access to a more catalytically active complex. Ligand synthesis involved a copper-catalyzed alkyne azide cycloaddition (CuAAC) under standard conditions between 1,8-diazido-3,6-di-tert-butylcarbazole 230 and a chloro-alkyne, 5-chloro-1-pentyne, or 6-chloro-1-hexyne, yielding the corresponding chloroalkyl-triazoles 231(240) and 232,241 which were treated with an excess of potassium iodide to give the bis(N-fused triazolium)carbazole salts 233(240) and 234,241 respectively (i, Scheme 36). Reaction of the ligand salts 233 and 234 with Ni(OAc)2·4H2O in the presence of excess triethylamine afforded the respective nickel(II) acetate complexes 235 and 236 (i, Scheme 36).240 In 235 and 236, the wingtip groups of the ligands differ only in one methylene group, but it was found to significantly affect the solubility of the complexes. Complex 236 was soluble in several coordinating, aromatic, and halogenated solvents, while 235 proved to be sparingly soluble in halogenated and coordinating solvents and insoluble in organic solvents.
Scheme 36. Synthesis of (i) Bis(triazolylidene)carbazolide Ligand Precursors and Their Nickel(II) Complexes and (ii) Cyclization of Epoxides and CO2 to Cyclic Carbonates Catalyzed by 235 and 236.
The catalytic activity of 235 and 236 was tested in the copolymerization of carbon dioxide and epoxides under the same conditions as Lee had reported for the nickel(II) complexes 227–229,238 except for a lower carbon dioxide pressure of 290 psi.240 However, 235 and 236 did not show significant activity in the coupling of carbon dioxide and cyclohexene oxide to polycarbonate. Instead, the replacement of NHC with MIC flanking donors led to an inversion of selectivity rather than the expected increase in catalytic potential, and the cyclization of carbon dioxides and epoxides to cyclic carbonates was observed instead of polycarbonate formation. The complexes 235 and 236 catalyzed the transformation of several epoxides into cyclic carbonates under pressurized carbon dioxide with a catalyst loading of 0.1 mol % at 130 °C (ii, Scheme 36). The general trend confirms the positive effect of the presence of cocatalyst bis(triphenylphosphine)iminium chloride ([PPN]Cl) on the activity and/or selectivity of the catalytic system. In addition, 236 showed higher catalytic activity than 235 which was concluded to be related to its better solubility.
3.3. Introducing a Noninnocent Handle at the EL-Group
3.3.1. Ligand-Assisted Reactivity for s- and p-Block Metal Complexes
The stabilizing attributes imparted by the tridentate carbazole-based ligand were utilized toward the preparation of the first example of a pincer coordinated gallylene, with hemilabile coordination evidenced during subsequent reactivity studies on 238.230 Ligand 1 was treated with KH, followed by GaCl3 in THF leading to the corresponding dichloride 237 (Scheme 37). Reacting 237 with two equivalents of KC8 yielded 238 which features a lone pair of electrons at the Ga metal, calculated to have a high s-orbital character. Reacting 238 with selenium powder led to dinuclear complex formation, with one of the imine donor moieties at each ligand decoordinating en route to 239. No reaction occurred between 238 and Cr(CO)6. However, complete conversion of 238 to 240 was noted when subjecting a reaction mixture of 238 and Cr(CO)6 to light irradiation with a 250 W UV lamp. Again, hemilabile imine decoordination occurred toward 240, with the lone pair of electrons on Ga coordinating the Cr(CO)5 metal. Ligand 1 was also coordinated to Al, leading to 241 that was further reacted with elemental iodine furnishing the aluminum diiodide 242 (Scheme 37). Reduction of 242 with [Mg(MesNacnac)]2 yielded the dinuclear AlIII243, presumed to have formed through a one electron reduction of 242, leading to an imino-based radical that undergoes dimerization and C–C bond formation. Crystal structure analysis confirmed the changes in bond lengths going from the imine in 242 to the amide of 243. The reverse reaction of oxidation to yield the imine 242 was not reported and would be interesting to determine if such reactivity is feasible, which could potentially lead to a redox switchable catalyst with the redox reactivity facilitated across the imine donor wingtip.
Scheme 37. NNN-Carbazole Pincer with Imine Donor Groups Exhibiting Hemilabile Coordination.
Imine reduction was also disclosed for the barium-coordinated bis(imine)carbazolide 244.120 The reduction reactivity was accompanied by imine hydrosilylation during attempts toward a barium hydride complex. Accordingly, reacting 244 with PhSiH3 resulted in the dinuclear barium complex 246 (Scheme 38). The authors hypothesized the formation of dinuclear 246 through imine reduction by a putative barium hydride 245, yielding the amide while imine hydrosilylation resulted in reductive decoordination of the second ligand’s imine moieties (Scheme 38). The order of reduction reactivity was not stated.
3.3.2. Ligand-Assisted Reactivity for Early Transition Metal Complexes
The PNP-ligand precursor 36 (vide supra, Scheme 5) previously reported by Gade et al.154 could be modified by introducing isopropyl phosphine substituents as an alternative to the phenyl phosphorus substituents in the protioligand 312 (see Scheme 46).173 As for the aforementioned PNP-Ln catalysts 132–134 (Scheme 21), the combination of the hard amido and soft phosphorus donor functionalities served as rationale for the choice of ligands for the group 4 metals, in anticipated stabilization of both electron-rich and electron-poor metal atoms. The trihalegenido PNP-pincer complexes 247 and 248 (M = TiIV), 249 and 250 (M = HfIV), (Scheme 39) and 256 and 257 (M = ZrIV) (Scheme 40) were prepared following ligand precursor deprotonation and metalation with the appropriate tetrachloride metal precursor to yield the trichlorido complexes, whereafter ligand exchange with trimethylsilyl iodide resulted in the corresponding triiodido complexes. Titanium complexes 247 and 248 were found to be suitable precursors for the alkylidene complexes containing Ti=C bonds, 251 and 252, respectively, obtained from the reaction with dibenzylmagnesium tetrahydrofuran with elimination of toluene. The analogous reaction with the hafnium complexes 249 and 250, however, did not yield related alkylidene complexes. Instead, the cyclometalated monoalkyl complexes 253 and 254 were isolated (Scheme 39). Similarly, the zirconium trihalogenido complexes 256 and 257 (Scheme 40), yielded analogous complexes 260–262, with the single crystal X-ray structure obtained for 262 exhibiting an η3-coordination mode of the benzyl ligand and confirming the tetradentate nature of the carbazolide ligand. This different reactivity of 249, 250, 256, and 257 compared to the Ti complexes 247 and 248 could be ascribed to the longer metal-ligand bonds and the resulting differences in the orientation of the phosphino-methylene units within the coordination sphere. The presence of the α-CH2 units adjacent to the phosphine donors dominates the alkylation reactivity patterns of these group 4 metal complexes to kinetically favor cyclometalation over α-hydrogen abstraction.173 The intramolecular C–H activation of the phosphine methylene could not be controlled; however, it could be suppressed in a “comproportionation” reaction with tetrabenzyl zirconium(IV) to yield the thermally unstable 258 (Scheme 40). In this case, the crystal structure indicated η2-bonding of the benzyl, and although the P–Zr–P bond angle (157.59(4)°/154.66(4)°) of 258 deviates significantly from linearity as a structural consequence of the tilted arrangement adopted by the carbazole backbone, no C–H activation and cyclometalation were observed. 258 could be deprotonated by benzyl potassium at elevated temperatures to proceed to the cyclometalated 261 (Scheme 40). Alternatively, donor-induced hydrogen atom abstraction and cyclometalation were facilitated by the addition of 1 mol equiv of trimethyl phosphine to 258. One of the ligand methylene groups was deprotonated to yield cyclometalated 259 with concurrent loss of toluene.
Scheme 39. Synthesis and C–H Activation Reactivity of Cyclometalated PNP-Pincer Complexes of Group 4 Metals.
The remaining coordination site occupied by the halogenido ligands in 253, 254, and 260–262 was readily benzylated by treatment with benzyl potassium to afford the dibenzyl cyclometalated complexes 255 (M = Hf, Scheme 39) and 264 and 265 (M = Zr, Scheme 40), respectively.173 In this instance, one benzyl ligand was found to be coordinated in a η3-fashion, while the second displayed η2-coordination for the zirconium complexes 264 and 265 compared to the η1,η3-bonding exhibited by the dibenzyl hafnium complex 255 (Scheme 39). Further reaction with excess benzyl potassium results in the formation of the anionic tribenzyl zirconate 266, which could be comproportioned with 262 to regenerate 265 (Scheme 40). An ortho-metalated methylene triphenylphosphorane complex 263 was produced by reaction of 262 with methylene triphenylphosphorane. The five-membered metallacycle containing the ylide carbon atom is slightly puckered, as shown in the crystal structure of this complex, and confirms sp3 hybridization at the ylide carbon.
The suitability of cyclometalated zirconium complexes 260 and 262 as candidates to serve as zirconium(II) synthons was considered due to the presence of a PNP-pincer ligand that not only stabilizes a variety of coordination geometries but also potentially has redox states lower than + IV.242 This is essential for the development of stoichiometric and catalytic group 4 metal chemistry that does not merely involve redox-neutral transformations.243−245 Toward this effort, 260 and 262 were treated with molecular hydrogen, leading to the hydrogenolysis of the cyclometalated Zr–C bond to yield zirconium η6-arene complexes 267 and 268 (Scheme 41).242 Formally, the puckered arene is formulated as an arene-1,4-diido species, rendering these compounds as ZrIV species. The folding of the arene ligand was attributed to the metal-to-ligand δ back-bonding of the zirconium dxy-orbital into the LUMO of the arene, confirmed by visualization of the HOMO of 267 with DFT calculations. For a zirconium(II) metal, the complex HOMO would have been a metal-localized nonbonding orbital. However, of the two resonance forms displayed for (267 and 268)/275 in Scheme 41, the zircona-norbornadiene formulation (267 and 268) appears to adequately represent the ground state metal valency (+IV) and metal-ligand bonding interaction. However, it also means that the displacement of the neutral arene by other ligating moieties should provide access to ZrII complexes, validating 275 as ZrII synthon stabilized by the PNP-pincer.
Scheme 41. Mechanism of Hydrogenolytic Formation of η6-Arene Zr PNP-Pincer Complexes and Their Reactivity As Zirconium(II) Synthons.
The hydrogenolysis mechanism of 260/262 was postulated to proceed through a labile hydrido zirconium intermediate too short-lived to be detected directly, with consequent reductive elimination leading to the η6-arene complexes 267/268.242 Deuteration studies confirmed an intramolecular reaction sequence after the initial hydrogenolysis of the Zr–C bond in the cyclometalated pincer ligand, but the individual reaction steps of the initial hydrogenolysis remained ambiguous. Either hydrogenolysis at the benzyl-C–Zr gives rise to a η6-toluene hydrido intermediate in which the PNP ligand remains cyclometalated, followed by reductive elimination, or hydrogenolysis by σ-bond metathesis occurs first at the cyclometalated ligand to yield a hydrido-benzyl intermediate. DFT modeling of the two alternative reaction pathways for 268 formation was performed, and the most plausible reaction pathway is presented in Scheme 41 (bottom). Approach of a dihydrogen molecule to yield intermediate 269 is followed by intramolecular heterolytic splitting of dihydrogen to protonate the methyne group of the ligand in 270 to yield the zirconium benzyl hydrido intermediate 271, significantly more stable than the alternative η1-toluene hydrido intermediate computed. Hereafter, the terminal hydride in 271 attacks the methylene group of the benzyl ligand via 272 and 273 to form the zirconium(II) η6-toluene transition state 274 through C–H coupling with subsequent rearrangement to the ground state structure of 268.
Vindication of the supposed role of 268/275 as the ZrII synthon was obtained by the reaction with 2,6-diisopropylphenyl isocyanide (Scheme 41).242 Clean conversion to the mononuclear 276 was observed, while the computed HOMO was interpreted as consisting primarily of a Zr-centered d-orbital lone pair engaged in back-bonding interactions into the C≡N π* bonds to validate its description as a zirconium(II) species. The further demonstration of 267/268 as zirconium(II) precursors 275 was performed by reaction of 267 with 1-azidoadamantane, and 268 with trimethylsilylazide, to yield the imido complexes 277 and 278, respectively, with the release of toluene and evolution of nitrogen (Scheme 41). The azides act as two-electron oxidants where the “masked” ZrII reagents 275 are oxidized to the corresponding ZrIV species 277 and 278 during the Zr=N bond formation.
Addition of pyridine to 267 (Scheme 42) also displaces the toluene ligand, resulting in complex 279 with a coordinated 2,2′-bipyridine (bpy) ligand,242 through a combination of C–H activation and C–C coupling and molecular hydrogen release, as a unique reactivity reported for group 4 metals.246−248 The structural data obtained from the X-ray structure of 279 indicated distinctly different Cpy–Cpy and C–N bond lengths, corroborated by spectroscopic studies to identify the bonding of the bipyridine as a dianionic, diamido-type ligand (bpy2–), resulting from the reductive coupling of pyridine facilitated by a transient ZrII species. The scope of this transformation and the cooperative reactivity of the PNP ligand in the mechanism of the transformation were investigated by including substituted pyridines as substrates.249 For pyridines methylated in the meta-position, unselective conversion to a variety of unidentifiable products was observed, while 4-picoline substrate yielded exclusively the analogous 280 complex with coordinated bpy2– and concurrent loss of hydrogen (Scheme 42). Conversely, when the ortho-methylated pyridine was employed as a substrate, resultant complex 287 displayed a cyclometalated pincer backbone from one of the methylene bridges neighboring a phosphine donor (Scheme 42). Extending the range of 4-substituted pyridine substrates led to the emergence of a general reactivity trend: pyridine substrates with reduced electron density in the aromatic ring did not undergo reductive coupling, while the reaction with an electron-rich pyridine yielded the coupled heterocycle product along with significant amounts of side products. Only substrates with similar electronic properties to the unsubstituted pyridine, e.g., alkyl and sp2 substituted pyridines, gave the desired bipyridyl ligand. If, however, a substituent with a further increase in electron donating character was employed, such as N,N-dimethylamine in DMAP, selective conversion into the cyclometalated pyridyl complex 288 was observed (Scheme 42). As for 287, ortho C–H activation of the pyridine leads to the formation of an η2-pyridyl, although an additional substrate (DMAP) molecule is coordinated to the metal in 288. A chemically noninnocent role of the ligand backbone was deemed to be a possible pathway toward the reductive coupling of the pyridines, by way of an initial C–H activation step forming an (η2-pyridyl) zirconium(IV) hydride species. Repeating the reaction with pyridine-d5 did not lead to the expected incorporation of deuterium into the ligand backbone, whereby a cooperative cyclometalation step involving the PNP ligand backbone could be excluded from this reaction sequence.249 Thus, isolated complexes 287 and 288 are the result of a “dead-end” competitive reaction pathway rather than a reaction step en route to bpy2– formation.
Scheme 42. Dehydrogenative Coupling of Pyridines Mediated by PNP-ZrII Synthon.
When isoquinoline was reacted with 267, a C–C coupling reaction was indeed observed to occur, but without the loss of the two hydrogen atoms at the bridge carbons of product 289 (Scheme 42), with the solid state structures displaying an anti-disposition of the H atoms.249 To confirm that C–H activation of the pyridine via a ZrIV and η2-pyridyl intermediate was not the most likely reaction pathway, DFT calculations for both the aforementioned route, as well as a route incorporating C–C bond formation as the first step, were carried out. The elevated activation barrier for the former route focused the study on an initial C–C coupling step of the two pyridines with either syn- or anti-orientation of the hydrogen atoms, relative to the formed bipyridine plane in the intermediate. The results indicated a syn C–C coupling sequence, as illustrated in Scheme 42 (bottom). The almost thermoneutral substitution of toluene by pyridine and concomitant formation of the low-valent ZrII species 281 is followed by coordination of the second pyridine to form 282. C–C bond formation leading to the ZrIV species 283 is exergonic via 282 with singlet spin state. Subsequent isomerization to 284, to position the C–H bond close to the zirconium, is endergonic but leads to facile C–H bond cleavage to form 285. The second C–H bond cleavage is achieved through 286 in a strongly exergonic σ-bond metathesis to yield product 279. The competitive dead-end pathway yielding 288 and 289, however, were computed to show lower energy transition states (Scheme 42), compared to the analogous transition state for the unsubstituted pyridine substrate (excluded also by the deuteration experiments), demonstrating a clear preference for the pathway leading to the cyclometalated η2-pyridyl complexes. This result is in agreement with the experimental observations, where further reaction with another equivalent of substituted pyridine is precluded by the subsequent high lying transition states.
3.3.3. Ligand Cooperativity for Late Transition Metal Complexes
The group of Lee focused their efforts on the bis(pyrazole)carbazole class of ligands,250 noting the advantage of incorporating the pyrazole donor group as a hemilabile site modulating complex reactivity.251−253 Accordingly, the synthesis of a carbazole coordinated iron bis(trimethylsilyl)amide complex was reported.250 The authors introduced steric bulk at the pincer’s donor wingtip sites R (Figure 1), which increased steric strain between the ligand and the bis(trimethylsilyl)amide coligand. The result is an air- and moisture-sensitive bidentate complex with an uncoordinated pyrazole donor group (292, Scheme 43), which leads to increased complex reactivity as was reported for the catalytic hydrosilylation reaction. In fact, 1 mol % of 292 catalyzed the hydrosilylation of various aryl and/or alkyl carbonyls with phenylsilane at ambient temperature in less than 60 min, with excellent conversion and TOFs reported. Attempted isolation of the reactive catalytic intermediate, postulated to be an iron hydride, proved nontrivial. The authors turned to calculations which validated the possible formation of three- and four-coordinate iron hydrido intermediates, with the four-coordinate iron hydride 293 lower in energy compared to the three-coordinate intermediate. Thus, “rollover” of the pyrazole donor moiety with subsequent coordination forming the pinced complex results in increased stability of the complex intermediate. Presumably then, its subsequent decoordination in the next catalytic cycle again increases the reactivity of the complex toward hydrosilylation.
Scheme 43. Synthesis and Reactivity of Hemilabile Bis(pyrazole)carbazolide Iron Complexes.
In addition to the isolation of 292, a trigonal bipyramidal complex 295 with tridentate carbazolide coordination was also reported, obtained due to exposure of 292 to an ambient environment (Scheme 43).250 The two OSiMe3 groups originate from the hydrolysis of N(SiMe3)2 and do not crowd the coordination environment around the metal, allowing for tridentate coordination. Heating a solution of 292 yielded the dinuclear complex 294 with dianionic tridentate coordination, in addition to bridging interactions between the ligand and the second metal. The dinuclear complex could also be prepared by heating a solution of ligand 290 and Fe[N(SiMe3)2]2.
The NNN-carbazolide coordinated nickel bromide 296 could be prepared through the deprotonation of ligand 291 followed by in situ metalation with NiBr2. 296 was treated with NaN3, resulting in isolation of the targeted nickel azido complex 297 (Scheme 44).254 Irradiation of 297 yielded an orange-colored product in high yields. Evidence for unique reactivity was initially noted during an NMR spectroscopic investigation, which indicated a C1 molecular symmetry for the product, suggesting modification of the carbazole supporting ligand. This was confirmed with a crystal structure of 299, which revealed an unprecedented double C–H activation. The first ligand-assisted reaction engages the C–H of the isopropyl wingtip moiety and the formed nickel nitridyl intermediate 298, leading to isopropyl C–N bond formation. The second C–H activation step involves hemilabile pyrazole decoordination and rollover of the donor moiety at 303 leading to 304 (Scheme 44). Calculations suggest an agostic interaction to proceed after pyrazole rollover, followed by a [2σ + 2π] transition state with ensuing formation of the nickel amine complex 299. A thermal route toward 299 was also probed, which was proposed to proceed via a NiIII imido intermediate. Reduction of 296 with 1.5 equiv of KC8 leads to formation of the paramagnetic T-shape NiI300 with an S = 1/2 ground state. Both color change and gas evolution were noted when reacting 300 with TMSN3 and isolating 299 as the major product of the reaction (Scheme 44). Additional computational investigations supported the replacement of nickel for iron or cobalt, calculated to follow a similar reactivity profile as determined for the nickel, albeit with different energy profiles.255 Calculations further suggested that multiple photoexcitation events are required to drive the reaction forward, from photolysis of the azide to the rollover C–H activation step, especially in the case of Fe and Co.
Scheme 44. Double Intramolecular C–H Activation Leading to 299 via a Nickel-Nitridyl Intermediate.
Changing the donor wingtips from the bulky Dipp groups to Mes substituents introduced another noninnocent handle on the bis(imino)carbazole NNN-pincer ligand.256 The handle was leveraged during the preparation of the cyclometalated platinum complex 309, isolated after reacting 308 with 5 mol % 310 or with triethylamine which induced mesityl wingtip cyclometalation (Scheme 45). The cyclometalated 309 could also be obtained by subjecting 311 to vacuum conditions at elevated temperature or placing it over molecular sieves. Complex 311 was in turn isolated by reacting 310 with water (Scheme 45). The reactivity of the bis(imino)carbazolide showcases a hemilabile as well as redox noninnocent combinatorial effect, in addition to exhibiting cyclometalative reactivity through ligand donor wingtip group fine-tuning.
Scheme 45. Wingtip C–H Activation Leading to Cyclometalative Reactivity.
4. Wingtip Effects
4.1. Wingtip Sterics Controlling Reactivity at the Metal
The significant effect of the wingtip steric demand was demonstrated by the minor modifications of the PNP-carbazolide pincer wingtip groups.257 The achiral PNP-protioligands 312(173) and 53,162 containing isopropyl or tert-butyl substituents, respectively, on the donor phosphorus atoms were deprotonated and reacted with [CoCl2(thf)1.1] to yield the d7-high spin cobalt(II) chloride complexes 314 and 313 (Scheme 46).257 Both cobalt(II) complexes display distorted tetrahedral coordination, but with a notable disparity between the N(carbazolide)–Co–Cl bond angles: in the case of 313, a decrease in the bond angle from 122.6° for 314 to 117.1° for 313 is observed due to the increased steric demand of the tBu groups compared to the iPr groups, resulting in the chlorido ligand seemingly “pushed” toward the carbazolide-nitrogen. This steric effect results in different reactivity of the cobalt(II) chloride complexes toward treatment with the hydride transfer reagent NaHBEt3. In the case of 314, facile chloride substitution to yield the low-spin d7 cobalt(II)-hydride complex 315 takes place (Scheme 46). Few examples of isolated pincer-supported CoII–H complexes are known; the rarity is ascribed to the propensity of CoII hydride complexes to undergo one-electron reductions.258−260 On the other hand, reaction of 313 with NaHBEt3 leads to the reduction of the cobalt(II) to yield a coordinatively unsaturated high-spin CoI complex 317 with T-shape geometry conferred by the tridentate PNP pincer ligand (Scheme 46),257 only the second example of such a three-coordinate cobalt(I) complex.261 Presumably, reduction proceeds via the cobalt(II)-hydrido intermediate 316, whereafter reductive elimination of molecular hydrogen results in the formation of 317. Alternatively, direct reduction of 313 with reducing agent Na/Hg also yields 317 (Scheme 46).257
The reactivity of high-spin CoI complex 317 was investigated by reaction with carbon monoxide, hydrogen, as well as tert-butyl chloride and phenyl disulfide (Scheme 46).257 Treatment of 317 with CO yielded the diamagnetic low spin d8 complex 318, while exposure of 317 to a hydrogen atmosphere (10 bar) leads to the formation of the diamagnetic cobalt(III) dihydrido complex 321. Under the given reaction conditions, an equilibrium between 317 and 321 was observed. Consequently, isolation of 321 was not possible, and deuteration studies were conducted with deuterium hydride gas to confirm dihydride formation and not dihydrogen coordination. Isotope scrambling was observed to occur, by way of bond scission processes of the dihydrogen isotopomers after an oxidative addition step with rapid H/D exchange. DFT calculations were employed to differentiate between the two possible reaction sequences for H/D exchange. Participation of the carbazole-amide moiety in hydrogen activation leading to a protonated amide was excluded by the highly endergonic H2 extrusion reaction step. Instead, a direct exchange pathway was found likely by proceeding through intermediate 322 after additional molecular H2 coordination (Scheme 46). H atom scrambling (323) and consequent σ-bond metathesis between coordinated dihydrogen and one hydride ligand leads to formation of 322′ with a dihydrogen coordinated trans to the amide donor. From 322′, the reaction sequence proceeds with the extrusion of the coordinated H2 to form the “mixed” dihydride 321′. Besides the two-electron process of oxidative addition leading to 321, one-electron reductions were confirmed using the substrates favoring radical-type transformations, both reaction with tBuCl and phenyl disulfide resulted in one-electron oxidations to yield 319 and 320, respectively (Scheme 46).
The potential activity of CoIII dihydride 321 and the CoII hydride 315 in the catalytic hydrogenation of alkenes was screened with substrate norbornene under dihydrogen pressure (10 bar) at room temperature.257315 mediated the transformation readily at a catalyst loading of 2 mol % in under 5 min, but a slow transformation to norbornane was only observed for 321 at 60 °C (i, Scheme 47). Further investigation into the activity of 315 by substrate variation proved that although aryl-substituted terminal alkenes were readily hydrogenated, disubstituted alkenes were only converted over longer time periods or at elevated temperatures. This result points to a slow insertion step of the alkene into the stable Co–H bond of 315. This mechanistic assumption was probed with stoichiometric transformations (ii, Scheme 47). 314 was treated with a solution of phenethylmagnesium chloride, directly forming 315 to verify a fast equilibrium between insertion of the alkene and predominant β-H elimination of the styrene to reform 315. In a second elementary reaction, the σ-bond metathesis of H2 with the Co–C bond was demonstrated by the reaction of the benzyl complex 324 with H2 at elevated pressure. Rapid cleavage of the Co–C bond yields 315, to confirm that slow insertion of the alkene is the limiting step. The insertion of the disubstituted alkenes is therefore less likely to proceed as the steric demand of the alkenes increases.
Scheme 47. (i) Catalytic Alkene Hydrogenation and (ii) Stoichiometric Transformations to Model the Elementary Reaction Steps of the Alkene Hydrogenation Mechanism.
The wingtip steric effects were also pronounced with rhodium complexes of the bis(triazolylidene)carbazolide ligand employed in catalytic dimerization and hydrothiolation of alkynes.196 The rhodium complexes 326 and 327 were obtained by treatment of the ligand salts 105 and 106, respectively, with excess KHMDS in the presence of metal precursor [Rh(C2H4)2Cl]2 followed by exposure to oxygen (Scheme 48). The crystal structure of 327 further confirmed the molecular structure of the complex, showing a square planar geometry around the rhodium(I) center with molecular oxygen coordinated in a side-on fashion. Treatment of 326 and 327 with carbon monoxide resulted in the formation of the respective carbonyl complexes 192 and 193, useful as probes for the electron-donating ability of the CNC-ligands, as discussed in section 3.2.
The catalytic activity of complexes 326 and 327 was investigated in the homodimerization and hydrothiolation of alkynes using 1-hexyne as a model substrate.196 In dimerization, 327 with bulkier Dipp wingtip groups proved to be inactive. In contrast, the Mes analogue 326 showed high catalytic activity with excellent selectivity as only the gem-enyne isomer is formed in the reaction, with complete conversion in less than an hour with a catalyst loading of 1 mol % at 80 °C (i, Scheme 49). Further experiments with a range of substrates showed 326 to display high functional group tolerance while maintaining the high selectivity. In addition, the complex is stable in atmospheric conditions and does not need a cocatalyst to function. In the hydrothiolation of alkynes, for which the reaction conditions were optimized using 1-hexyne that was hydrothiolated with thiophenol, both complexes 326 and 327 showed catalytic activity at 1 mol % catalyst loading at 80 °C, yielding α-vinyl sulfide with over 90% selectivity (ii, Scheme 49). 326 showed excellent selectivity toward α-vinyl sulfide isomers comparable to the best-known catalysts in the hydrothiolation of aliphatic alkynes with aliphatic thiols. On the other hand, in hydrothiolation involving aryl-substituted substrates, 326 displayed a decrease in catalytic performance.
Scheme 49. (i) Homodimerization of Terminal Alkynes, (ii) Terminal Alkyne Hydrothiolation, (iii) Bis-Hydrothiolation in a Sequential One-Pot Reaction of Dithiol with Various Alkynes, and (iv) Sequential Alkyne Dimerization and Hydrothiolation.
326 also showed high catalytic activity and selectivity in the sequential bis-hydrothiolation of two different alkynes to nonsymmetrical bis-α-α′-vinyl sulfides (iii, Scheme 49).196 In the reaction catalyzed by 326, the alkyne was first reacted with a dithiol to afford mono-α-vinyl sulfide, followed by the addition of a different alkyne yielding nonsymmetrical bis-α-α’-vinyl sulfides with high selectivity. For terminal alkynes, the formation of bis-α-vinyl sulfides was observed, while for internal alkynes, the reaction yielded β-E-vinyl sulfides. Remarkably, 326 showed catalytic activity even in a one-pot sequential dimerization and hydrothiolation tandem reaction, in which the alkyne dimerization of dimethylaminopropyne was followed by the addition of 1-hexanethiol leading to syn-addition of the thiol across the internal alkyne affording the formation of 1,3- and 1,4-gem-ene-β-E-vinyl sulfides as the main products (iv, Scheme 49).
4.2. Electronic Consequences of Wingtip Sterics
A fine balance is drawn for steric bulk at the wingtip sites, with increasing steric bulk leading to complexes with higher reactivity that can lead to decomposition. Conversely, small wingtip substituents might not provide sufficient steric bulk for stabilizing the complex which could also result to decomposition or unwanted complex formation. This was illustrated by investigating various iron complexes, of which 332 and 333 were reported to be stable even under atmospheric conditions (Scheme 50).262 However, FeIII complexes 330 and 331, featuring increased steric bulk at the imine donor wingtip positions, were found to decompose in air and also exhibited diminished stability in solution while under an inert atmosphere. The tendency of the complexes to decompose was speculated to be a result of the bulky mesitylimino wingtip substituents. The sterically demanding ligand can result to unfavorable interactions between the wingtip substituents and the chloride ligands, possibly resulting to imine decoordination which decreases the stability of the complex. The analogue of 330, the FeII complex 21 (Scheme 3), was isolated and reported to be stable. Ligand 27, with a lower steric demand when compared against the mesityl containing pincer ligands, imparted sufficient stabilization to the iron center allowing for subsequent reactivity studies, further advancing the scope of iron complexes accessible. Reaction of 332 with MeLi lead to the formation and isolation of 334. Two consecutive chloride abstractions, first with AgSbF6 followed by Ag(acac) resulted to 335 and 336, respectively (Scheme 50).
Scheme 50. FeIII Complex Stability and Reactivity Effected by Wingtip Steric Bulk.
Lee et al. furthered their investigation into the bis(pyrazole)carbazolide iron class of compounds (see section 3.3.3), in this case isolating complexes with varying steric bulk at the wingtip positions.263 For the FeII complexes, evidence of the influence that the wingtip sterics exhorts on the complex was noted when instead of the ligand 290 the less sterically hindered ligand 291 was employed, allowing for the formation of iron complexes 337–339 in which the metal center is coordinated by all three pincer nitrogen donor atoms. The coordination sphere is completed by chlorido, thf and chloride, or azido coligands (337, 338, and 339, respectively, Scheme 51), in contrast to 292 (Scheme 43) where the bulky bis(trimethylsilyl)amide coligand accompanies bidentate coordination of the carbazolide ligand.264 Slight modulation of steric bulk on the wingtip positions (e.g., changing from tert-butyl to isopropyl substituents) can thus similarly control complex reactivity by variation of coordination number, where tridentate coordination increases complex stability at the expense of ligand hemilability.
Scheme 51. Bis(pyrazole)carbazolide Coordinated FeII Complexes.
Bis(pyrazole)carbazolide iron(III) complexes 342–344 were prepared (i, Scheme 52)263 following a similar procedure as for FeII (Scheme 52)264 and NiII complexes (Scheme 44).265 Crystal structure and electrochemical analysis, in addition to theoretical calculations, EPR, Mössbauer, and electronic absorption spectroscopy, again evidenced the influence that the wingtip steric bulk exhorts on the reactivity of the synthesized complexes.263 Increasing the steric bulk from H to Me to iPr at the wingtip position leads to an out-of-plane distortion of iron from the plane of the carbazole backbone (ii, Scheme 52). The donor pyrazole groups also have to deviate from planarity in order to accommodate iron’s out-of-plane movement. On the basis of the results obtained, the authors suggested that the out-of-plane deviation decreases the extent of orbital overlap between the carbazole’s nitrogen p-orbital and the metal’s d-orbital. This could decrease electron donation from the ligand to the metal, reducing the ligand’s overall electronic effect on the complex as manifested in the redox potential of the complexes investigated. The steric bulk at the wingtip position also influences complex formation, as the authors had to prepare 342 at low concentrations to reduce the possible formation of byproducts, one of which was speculated to be an iron complex coordinated by two carbazolide pincers.
Scheme 52. Wingtip Sterics Dictating Iron’s Reactivity.
The series of NiII complexes coordinated by the bis(pyrazole)carbazolide pincer disclosed by Lee and co-workers was expanded (vide supra, section 3.3.3).265 The inclusion of bulky isopropyl wingtip substituents at the pyrazole donor groups was rationalized by their effort to access nickel(II) complexes with a higher reactivity compared against the overall kinetically inert square planar nickel analogues. The authors noted the tendency of the wingtip steric bulk of the bis(pyrazole)carbazolide to point toward the fourth coordination site. This ligand design strategy compounds the influence of the wingtip steric bulk on the metal, allowing for increased steric pressure at the metal center and forcing the metal to adopt a geometry higher in energy due to an alteration of the molecular orbital’s energy and electronic population. Thus, complexes 345, 296, and 346 isolated after treatment of 291 with LDA followed by the corresponding nickel halide precursor exhibited a seesaw geometry around the four-coordinate metal center and not a square planar coordination environment (i, Scheme 53). Dissolving the complexes in THF resulted in its coordination and formation of the corresponding five-coordinated complexes 347–349. The thf ligand could be removed under reduced pressure followed by dissolving the solid in a noncoordinating solvent. All complexes were determined to be paramagnetic. Theoretical investigation confirmed the paramagnetic nature of the complexes, describing two SOMOs, the dx2–y2 and dz2 orbitals, energetically separated from three low-lying filled d-orbitals. Calculations further supported the experimentally determined electronic spectra, describing the lowest energy transition to be ligand-to-metal charge transfer (LMCT) with the carbazolide-nitrogen significantly contributing to the ligand π-orbital.265 The ligand π-orbital in turn donates into the metal’s dz2-orbital.
Scheme 53. Wingtip Sterics Influencing the Coordination Environment around a Nickel(II) Complex.
Additional calculations on a model complex (the tert-butyl groups at the carbazole backbone were omitted during the calculations) evidenced the sought-after influence exhorted by the NNN-pincer ligand’s wingtip sterics on the electronic and coordination properties around the four-coordinate NiII complexes (ii, Scheme 53).265 Increasing the steric bulk of the wingtip substituents resulted in an increase in the steric repulsion between the wingtip substituents and chloride ligand. This in turn allowed for the N(carbazole)–Ni–Cl bond angle to decrease from the ideal square planar angle. The trans influence exhorted on the carbazole-nitrogen by the chloride ligand diminishes due to the chloride ligand being forced out of the carbazole plane, resulting in an increase in the N(carbazole)–Ni bond length. Calculations suggested that these changes in both the bond length and bond angle leads to the complex’s singlet state becoming energetically less competitive with the triplet state, favoring the triplet state which would yield a complex higher in reactivity due to the electrons being unpaired. Therefore, manipulating the wingtip steric bulk can have a significant impact on both the electronic and coordination environment around a metal center, affecting its kinetic stability.
In an effort to gain a better understanding of the role that vanadium fulfills in vanadium nitrogenase, Lee and co-workers coordinated the bis(pyrazole)carbazolide pincer to vanadium(III).266 Similar to the preparation of the corresponding iron263 and nickel complexes,265350 could be synthesized by deprotonation of 291 with LDA followed by addition to a slurry of VCl3 in THF (Scheme 54).266 Reacting the dichloride with excess sodium azide yielded the bis(azido) vanadium complex 351, which yields the dimeric vanadium(IV)-nitride complex 352 after reduction with potassium graphite. The dimeric nitride 352 could alternatively be prepared through reduction of the dichloro complex 350 with KC8 under a nitrogen gas atmosphere. 352 was determined to be highly stable, as evidenced by reactions with various redox, proton, and hydrogen atom transfer reagents. The reactivity studies were corroborated by DFT calculations, attributing the stability of 352 to both steric protection by the ligand’s bulky wingtip substituents and strong metal-nitrogen π-bonds.
Scheme 54. Bis(pyrazole)carbazolide Vanadium Complexes.
4.3. Cooperativity of Wingtips for Bond Manipulation
4.3.1. Protic NHC Wingtips
Implication of protic N-heterocyclic carbenes (PNHCs) as ligands for metal-ligand bifunctional cooperative catalysis has increased interest in this class of NHCs, although synthetic access to PNHC complexes are generally considered challenging.267−269 Grotjahn et al. introduced PNHCs as flanking groups to bis(imidazol-2-ylidene)carbazole CNC-pincer ligand 353 by a three-step synthesis (Scheme 55) from carbazole,239 by modifying the method reported by Kunz et al. for aprotic BIMCA analogues (vide supra, section 2.3).140,190 Direct metalation of 353 with the group 10 metal (Ni, Pd, and Pt) precursors afforded the formation of two carbon-metal bonds from the unfunctionalized C–H bonds of the PNHC moieties in a single synthetic step (Scheme 55).239 The chloride complexes 354–356 obtained were further converted into acetate and triflate analogues, 357–359 and 360–362, respectively, by using the corresponding silver salts (Scheme 55). NMR and IR spectroscopic investigations indicated intramolecular hydrogen bonding in 357–362. Solid state structures of 358 and 361 confirmed this by revealing interaction between the acetato ligand and one NH wingtip in 358, while in 361 a simultaneous interaction between the triflato ligand and two NH wingtips were observed.
Scheme 55. Synthesis of Bis(PNHC)-Carbazole and Its Group 10 Metal Complexes.
Triflate complexes 360–362 were subjected to reactivity tests with CO and ethylene to yield complexes 363–365 and 366, respectively (Scheme 55).239 CO binding was shown to be reversible in the nickel and palladium complexes 363 and 364, respectively, suggesting a weaker M–CO bond than in the platinum complex 365. In the case of ethylene, only the formation of Pt complex 366 (Scheme 55) was observed. Spectroscopic and structural studies of 363–365 evidenced intermolecular hydrogen bonding between the uncoordinated triflate anion and two NH wingtips. Further reactivity tests were performed on 362, which showed anti-Markovnikov selectivity in O–H addition to alkynes. Stoichiometric reaction of 362 with 3-butyn-1-ol resulted in the formation of 367 featuring a cyclic carbene as a coligand, while the reaction with 13C-labeled alkyne yielded the acyl complex 368 (Scheme 55).
With the chloride complexes 355 and 356, Grotjahn and his group targeted partial deprotonation of the ligand to obtain complexes that simultaneously contain both the bond-activating imidazol-2-ylidene unit and the PNHC proton donor.270 For this, they first treated 355 and 356 with sodium tert-butoxide in dichloromethane, which instead of the targeted monodeprotonation led to the formation of the dimers 369 and 370 (Scheme 56). The crystal structure of 369 showed a dimeric structure consisting of two metal complexes coordinated to each other via the nitrogen atoms of the imidazolyl moieties following deprotonation of the NH wingtips. Dimer formation was presumably due to the loss of NaCl. Hence, the reaction conditions were adjusted and 355 and 356 were treated with a stronger base LiN(iPr)2 in the more polar solvent THF. One base equivalent in the reactions resulted in the formation of 371 and 372 (Scheme 56), in which one of the PNHC moieties was deprotonated. The addition of the second base equivalent led to the deprotonation of the second NH wingtip to yield the bis(imidazolyl) complexes 373 and 374 (Scheme 56). Dimer formation was not observed in these reactions and prompted reactivity tests with H2, ethylene, and 1-heptene. However, the substrates did not displace the chlorido ligand without dimer formation, which appears to dominate the reactivity of the bis-PNHC complexes under basic conditions.
Scheme 56. Formation of Dimers and Mono and Di-Lithium Adducts of Bis(PNHC)-Carbazolide Group 10 Metal Complexes.
4.3.2. Tethered NHC Wingtips
Modification of the BIMCA ligand was done to include a pentamethylene tether connecting the two NHC donors in the ligand precursors 199 or 375 with bromide or PF6– counterions, respectively (Scheme 57).134 If the deprotonated precursor 375 was coordinated to a pentamethylcyclopentadienyl (Cp*) ruthenium(II) precursor, a rare example of the carbazole-based pincer ligand with facial coordination in complex 378 is obtained, similar to the RuCp* complex of the BIMCA ligand with homoallyl wingtip groups (vide infra, section 4.3.3).271 Besides the presence of the coligand Cp*, the hindered rotation of the carbene moieties in the tethered pincer ligand were concluded to enable the observed fac coordination of the BIMCA ligand, contrary to the conventional mer geometry as demonstrated for example, by a bis(triazolylidene)carbazolide ruthenium(II) complex.272 Not only the d6-metal but also the alkali metal complexes 376 and 377, obtained from the reaction of the protioligand 199 or 375 with LiHMDS or KHMDS, respectively, display this fac-geometry (Scheme 57).134 Crystal structure analysis of the lithium complex 376 revealed a dimeric structure in the solid state in which the coordination of the ligand can be described as both chelating and bridging, as the ligand is coordinated to lithium via the carbazolide-nitrogen atom and two NHC donor moieties, one of which also coordinates the lithium atom of the other lithium pincer complex monomer to form the observed dimer. In contrast, in the case of the potassium complex 377, the solid-state structure showed that the BIMCA ligand coordinates to potassium only in a facial manner. The NHC moieties of the ligand coordinate to potassium while the nitrogen of the carbazolide spacer coordinates to the potassium of the next monomeric complex, thus forming a polymeric structure.
Scheme 57. Synthesis of Ruthenium(II) and Palladium(II) Complexes Bearing a Macrocyclic CNC Ligand.
The properties of transition metal complexes of macrocyclic CNC ligands containing alkyl tethers of 8–16 C atoms have been found to depend on ring size, even though the tether itself shows no reactivity.273−278 It was anticipated that the smaller ring size macrocyclic BIMCA ligand derivative with a pentamethyl tether could induce a stronger interaction with the metal due to the proximity and influence its reactivity by steric restrictions when coordinated in traditional meridional geometry.134 Indeed, the ruthenium complex 379 obtained from the reaction of in situ deprotonated bis(imidazolium) salt 199 with [Ru(PPh3)3Cl2] showed that intramolecular C–H activation occurred resulting in the formation of an olefinic donor site (Scheme 57), with the pentamethylene-tethered BIMCA ligand acting as a monoanionic tetradentate macrocyclic ligand. Another proof for intramolecular C–H activation induced by the proximity of the alkyl tether to the metal center was provided in the form of a palladium complex 380 from the reaction of in situ deprotonated 199 with Pd(OAc)2 (Scheme 57). As was shown by NMR experiments, intramolecular C–H activation led to the deprotonation of the alkyl chain and the formation of a carbanionic donor site, resulting in the formation of a dianionic tetradentate macrocyclic ligand. The observed C–H activation was rationalized by the proximity of pentamethylene in the first formed CNC pincer complex, which enables its intramolecular deprotonation by the acetate ligand.
4.3.3. Hemilabile Allyl-Functionalized NHC Wingtips
The influence of not only the L groups but also the R wingtips on the electronic environment is well illustrated by the modification of the flanking NHC donors’ wingtips from methyl to coordinating allyl groups, in the rhodium complexes of BIMCA.229,279 Increased control of the rhodium coordination sphere is hereby obtained and allows not only for increased electron density at the metal (by replacement of the carbonyl ligand in 194 (section 3.2 and Scheme 30) with a coordinating allyl moiety) but also for ligand-assisted metal reactivity in catalytic conversions. The additional nucleophilicity of the rhodium complexes was exploited to improve catalytic performance in the isomerization of terminal epoxides to methyl ketones. The rearrangement of epoxides, the so-called Meinwald reaction,280 is most commonly catalyzed by Lewis acids leading to the formation of aldehydes,281−293 whereas rarer examples of catalytic isomerization involve the use of a nucleophilic catalyst together with a Lewis acid cocatalyst to yield methyl ketones (Scheme 58).294−298
Scheme 58. Nucleophilic and Lewis Acid Catalyzed Isomerization of Terminal Epoxides.
Rhodium complex 194 (section 3.2) was shown to catalyze the regioselective rearrangement of terminal alkyl epoxides into methyl ketones under mild conditions, i.e., in a reaction that was carried out in the presence of 5 mol % of 194 along with 20 mol % of the Lewis acid additive LiNTf2 at 60 °C in benzene-d6.229 Near to full conversion of monoalkylated epoxides into their respective methyl ketones with no detection of the respective aldehydes was achieved. Further studies with other terminal epoxide derivatives showed a high functional group tolerance for a wide variety of substrates with different functionalities; however, a notable decrease in conversion and/or yield was observed.229,279 To improve the efficiency of the catalyst, attention was shifted to the N-homoallyl substituted ligand 198 to access a rhodium complex that is CO-free, thereby increasing the electron density at the metal center, while the olefinic units coordinated to the rhodium center stabilizes the complex intramolecularly during catalysis, thus increasing its lifetime.279 For this, in situ deprotonation of bis(imidazolium) salt 198 with KHMDS or LiHMDS, followed by transmetalation with [Rh(μ-Cl)(cod)]2, afforded 383 or 381, respectively, the latter of which was isolated with lithium salts, LiBr and LiCl (Scheme 59). The syntheses of the analogous cobalt and iridium complexes were also reported (Scheme 59).299 Iridium complexes 384 and 382 were obtained by following the synthetic method of Rh complexes 383 and 381. For the preparation of the cobalt complex 389, two methods were reported: both direct transmetalation (Scheme 59, route b) and a two-step synthesis (Scheme 59, route a), where the transmetalation was followed by the reduction of the formed CoII complex 388 to the target complex 389.
Scheme 59. Synthesis of Rhodium, Iridium, And Cobalt Complexes of N-Homoallyl-substituted Bis(NHC) Pincer Ligand.
The catalytic activity of rhodium complexes 383 and 381 was further tested in the isomerization of terminal epoxides.279 For 381 including LiBr and LiCl salts, full conversion into methyl ketones was observed. The lithium salt free 383, on the other hand, did not catalyze the rearrangement, indicating that a weak Lewis acid cocatalyst in the reaction is necessary for catalytic activity. 381 proved to be catalytically more active than 194, catalyzing the nucleophilic Meinwald reaction at a lower temperature and in the absence of an additional Lewis acid. With a catalyst loading of 5 mol % at room temperature in benzene-d6, 381 catalyzed the isomerization of various functionalized terminal epoxides into the respective methyl ketones almost quantitatively and with full regioselectivity, thus showing a high functional group tolerance. Even for aryl oxiranes, almost full conversion into methyl ketones was achieved with excellent chemo- and regioselectivity, thus emphasizing the crucial role of the higher nucleophilicity of the complex 381 in the rearrangement reaction. In contrast, the iridium and cobalt complexes 384 and 389, respectively, showed lower catalytic activity than their Rh analogue 383 in the presence of Lewis acid additive LiBr.299 The lower catalytic activity of the iridium and cobalt complexes was justified by the differences in their oxidation potentials compared to that of 383. In the case of 389, the lower oxidation potential is less favorable toward the nucleophilic opening of the epoxide ring, decreasing the reactivity of the compound. On the other hand, the oxidation potential of 384 is higher than that of 383 leading to an increasing metallacyclopropane character, translating to higher stability observed for the coordinated N-homoallyl moieties. This hampers the dissociation of the second allyl moiety, which is a prerequisite for the formation of a nucleophilic active species, thus reducing the activity of complex 384. However, 389 and 384 showed increased catalytic activation under H2 gas (1 bar), although it also led to side reactions. To evaluate whether N-homoallyl moieties are susceptible to hydrogenation under catalytic conditions, additional hydrogenation experiments were performed for the iridium and cobalt complexes 384 and 389, respectively, but also for the rhodium complex 383 by exposing them to H2 gas.299 In the case of rhodium and iridium, complete hydrogenation of N-homoallyl moieties into N-n-butyl groups and the formation of dimeric hydrido complexes 385 and 386 (Scheme 59) were observed, as verified by single crystal X-ray structure analysis.
Capitalizing on an even more nucleophilic metal center to yield a more active catalyst, the catalytic system could be further improved by introducing the 16-electron rhodium complex 392 with only one N-homoallyl substituent in the ligand scaffold (Scheme 60).300 The ligand precursor 391 was synthesized by selective monoalkylation of 91 with methyl iodide followed by N-allylation to afford the targeted bis(imidazolium) salt 391 (Scheme 60). In situ deprotonation and subsequent transmetalation of 391 produced the rhodium complex 392. Attempts to isolate 392 by chromatography unexpectedly led to the isomerization of the homoallyl double bond and the formation of compound 393, which was confirmed by NMR spectroscopy. 392 catalyzed the isomerization of aryl oxiranes to the corresponding methyl ketones in high yields with excellent chemo- and regioselectivity at room temperature with lower catalyst loading (1 mol %) and shorter reaction time, thus surpassing 381 in terms of catalytic activity. Moreover, 392 also showed excellent functional group tolerance for a wide range of epoxides. However, the reaction still needed a Lewis acid additive to preactivate the substrate.
Scheme 60. Synthesis of Unsymmetrically N-Homoallyl-Substituted BIMCA Ligand and Its Rhodium Complexes.
A possible catalytic cycle was proposed for the Meinwald isomerization of epoxides catalyzed by nucleophilic rhodium complexes 383 and 392 (Scheme 61).300 In the first step (a), the Lewis acid cocatalyst preactivates the epoxide substrate by coordination. In the case of catalyst 383, the dissociation of the second homoallyl group is a prerequisite for the formation of the catalytically active complex. This is followed by a nucleophilic attack of the RhI center on the most electrophilic and usually the least substituted site of the epoxide ring (step b), resulting in the opening of the epoxide ring and the formation of the RhIII intermediate 394 or 395. From this point forward, intermediate 394 or 395 can react in two different reaction pathways. In pathway 1, the intermediate 394 or 395 can release the methyl ketone directly by a concerted 1,2-hydride transfer proceeding through the transition state 396 or 397, respectively. Alternative reaction pathway 2 involves β-hydride migration (step d) leading to the formation of the RhIII hydrido complex 398 or 399, followed by reductive elimination (step e) under which the methyl ketone is released and the catalytically active Rh(I) complex 383 or 392 is regenerated. Formation of the RhIII hydrido complex 398 or 399 could not be verified in practice.
Scheme 61. Proposed Catalytic Cycle for 383 and 392, Demonstrating Catalyst Deactivation Product Formation.
Further insight into the mechanistic details of the catalytic cycle was obtained by D/H exchange experiments.300 D/H exchange was observed in experiments carried out under catalytic and stoichiometric conditions with the deuterated phenyloxirane substrate and N-homoallyl-substituted 381 (containing lithium salts, see Scheme 59) and 392, as in all cases the deuterium had been replaced by hydrogen in 5–7% of the formed methyl ketones. This confirmed that the reaction pathway 2 is more plausible for the rhodium catalysts and the homoallyl substituent of the ligand plays a role in the catalytic cycle, inserting into intermediate 398 or 399 to form a rhodium alkyl intermediate 400 or 401 which can further undergo D/H exchange if a deuterated substrate is present in the reaction.
In connection with the isomerization of epoxides catalyzed by 392, the complex 404 was also isolated, which is the probable deactivation product of the catalyst (Scheme 61).300 The crystal structure of the compound showed the formation of a bromido-RhIII complex 404 where the N-homoallyl substituent is η3-allyl coordinated to the metal center. It was speculated that 404 was formed after catalysis and is a result of the C–H activation of the allylic position yielding an allylhydride complex followed by replacement of the hydride with a bromido ligand.
The catalytic activity of nucleophilic rhodium complexes was applied also in the selective isomerization of terminal aziridines (analogues of epoxides) to enamides.301 As before, the most nucleophilic complex 392 exceeded the catalytic performance of 194 and 383, while a Lewis acid cocatalyst remains essential. 392 catalyzes the transformation of various N-Boc terminal aziridines into the corresponding enamides at 1 mol % catalyst loading (i, Scheme 62). Isomerization of aziridines with electron-donating substituents was found to proceed faster than aziridines with electron-withdrawing substituents. In these cases, the formation of an intermediate 406 with a terminal C=C double bond was observed, which eventually rearranged to the target enamide.
Scheme 62. (i) Catalyzed Isomerization of N-Boc Terminal Aziridines and (ii) Proposed Mechanism for the Isomerization of Terminal Aziridines Catalyzed by 392.
A mechanism analogous to the nucleophilic dual-activation pathway proposed for the rearrangement of epoxides was also proposed for the isomerization of aziridines (ii, Scheme 62).301 In the first step (a), the Lewis acid coordinates to the aziridine, preactivating it. In the next step (b), the nucleophilic attack of the catalyst 392 on the most electrophilic site of the aziridine results in the opening of the aziridine ring and the formation of an intermediate 407 that is probably Lewis acid stabilized. The following β-hydride elimination (step c) can lead to the formation of the RhIII complex 408, which in the subsequent reductive elimination (step d) reforms the catalytically active compound 392 and releases the intermediate 406. This further isomerizes to the target enamide 405 (step e), but it is noteworthy that the rate of this isomerization is substrate-dependent. If aziridine has electron-donating substituents, the intermediate hydrido complex 408 can also isomerize to intermediate 409 (step f), which in a further reductive elimination (step g) releases the target enamide 405 and reconstitutes the active catalyst 392.
4.4. Introducing Chiral Peripheries at the Wingtip Positions
Surprisingly, the inclusion of chiral wingtip groups on molecular catalysts for asymmetric transformations has been scarcely explored for monoanionic PNP pincer ligands and is limited to pincer scaffolds with an aliphatic backbone.302,303 A report by Gade et al. describes the incorporation of chiral 1,3-diphenylphospholane donor moieties with methylene bridges linking to the carbazole backbone.154 The dibromomethylcarbazole 34 is the key intermediate to both the achiral 36 and chiral 414 protioligands, the latter of which is obtained by treatment of 34 with the enantiopure lithium (2R,5R)-2,5-diphenylphospholanide-borane complex,304 followed by deprotection with diethylamine. The position and orientation of the phosphine units render these tridentate ligands as ideal for meridional coordination to d-block metals, and the coordination chemistry of a range of transition metals to both achiral 36 (i, Scheme 63) and chiral 414 (ii, Scheme 63) was explored.154 Deprotonation of 36 with a lithium base followed by metalation with either [PdCl2(cod)] or [NiX2(dme)] (dme = dimethoxyethane) yielded the square planar complexes 412 or 410 and 411, respectively (i, Scheme 63). A similar treatment of 414, with chiral phospholane wingtips, afforded complexes 415, 418, and 419 (ii, Scheme 63). Alternatively, oxidative addition of the carbazole N–H to a Ni0 precursor yields the nickel(II) hydrido complexes 413 (i, Scheme 63) and 416 (ii, Scheme 63), with distorted square planar geometry as evidenced by the N(carbazolide)–Ni–H bond angle of 169(1)° and P–Ni–P bond angles 164.12 (3)° from the crystal structure of 413. The use of acac or acetato metal precursors that can act as an internal base is an alternative route for complex formation, whereby reaction of [Rh(acac)(CO)2] with 36 results in formation of 190 (i, Scheme 63) and treatment of 414 with Ni(OAc)2 yields 417 (ii, Scheme 63). The effect of the chiral wingtip groups is most pronounced in the molecular structures obtained for the bromido-substituted 419 and acetato-complex 417. The square planar nickel center is shielded by one phenyl group of each phospholane which are facing in opposite directions, with the coordination plane twisted with respect to the carbazole ring. The two phospholane groups point to opposite faces of the carbazole plane in 419. This helical twist between the carbazolide ring and the plane spanned by the PNP-ligating atoms is slightly larger in 417, inferring that the approach of substrates to the metal center can be manipulated by the chiral wingtip groups, as demonstrated for NNN-pincer carbazolide complexes discussed below.
The carbazole backbone forms the basis of the well-known bis(oxazolinyl)carbazole ligand,305 which garnered success in the chromium-catalyzed Nozaki-Hiyama reaction306 due to the high selectivity imposed by the ligand on the catalyzed reaction.99,100,305,307,308 The C–C bond forming reaction is catalyzed by the in situ formed bis(oxazolinyl)carbazolide chromium complex and various cocatalyst and/or additives. Indeed, the asymmetric allylation,309,310 allenylation,311 silylallenylation,312,313 and propargylation314 of aldehydes can be catalyzed with the in situ formed NNN-pincer ligated chromium complex yielding products with high enantiomeric excess and high yields. Furthermore, various other reactions can also be catalyzed with the in situ formed bis(oxazolinyl)carbazolide chromium complex, including among others the selective functionalization of halide-substituted olefins with aldehydes or ketones, respectively, yielding the corresponding secondary315−317 or tertiary318 alcohols, with high enantioselectivities. Access to targeted compounds with excellent selectivity renders this chromium-catalyzed protocol with high relevance, justifying the segments in various reviews regarding the use of the bis(oxazolinyl)carbazole ligand in the Nozaki-Hiyama reaction.99,100,305,307,308,319,320 Hence, the topic will only be highlighted in this communication with the inclusion of selected examples illustrating the synthetic potential of the catalytic reaction when catalyzed with the in situ formed bis(oxazolinyl)carbazolide chromium complex.
In a continuation of the work by Fürstner et al.,321,322 Nakada and co-workers sought to decrease the stoichiometric use of chromium in the Nozaki-Hiyama C–C bond-forming reaction to catalytic amounts.305,309,310 The authors accomplished this by using the bis(oxazolinyl)carbazole ligand 423, which was synthesized from l-valinol in the presence of ZnCl2 and the in situ prepared 1,8-dicyano-3,6-diphenylcarbazole, obtained through reacting 422 with CuCN in DMF (i, Scheme 64).309,310 Coordination of the NNN-pincer ligand to chromium leads to a stable, yet coordinatively unsaturated complex able to accommodate an incoming substrate. These attributes allowed for catalytically active chromium complex 424 to mediate the asymmetric allylation of aldehydes, with high enantiomeric excess (i, Scheme 64). It was further demonstrated that the ligand could be recycled several times, with retention of selectivity and catalytic activity.309 The same group further demonstrated the underlying potential of this synthetic protocol while preparing FR901512 (430, ii, Scheme 64), an effective HMG-CoA reductase inhibitor.323 The optimized chromium catalyzed Nozaki-Hiyama reaction was exploited en route to 430, yielding both 427 and 429 from the allylation of aldehydes 426 and 428, respectively, with high yields and excellent selectivities. Nakada et al. proved the applicability of the catalyzed reaction through the efficient, concise, and protecting-group-free enantioselective synthesis of biologically relevant compounds, while further modification to the ligand’s steric map or the reaction conditions added to the scope of this highly selective transformation protocol (vide infra).
Scheme 64. (i) Chiral Carbazole Ligand for Asymmetric Nozaki-Hiyama Allylation and (ii) Preparation of HMG-CoA Reductase Inhibitor FR901512.
Turning their attention to the optimization of the selectivity of the asymmetric Nozaki-Hiyama reaction, Nakada et al. leveraged the steric handle provided by the modulation of the ligand’s wingtips. Facile tailoring of the wingtip moieties is paramount to the formation of a stable yet catalytically active complex that can dictate the selectivity for the catalyzed reaction.305,314 As such, the decreased steric bulk at 431, featuring either a methyl or isopropyl wingtip substituent, allowed for coordination of the aldehyde at the equatorial position, leading to si-face reactivity (Figure 6). Increasing the steric bulk at the wingtips from methyl and isopropyl to tert-butyl groups at 432 directs coordination of the aldehyde at the apical position due to increased steric strain between the wingtip substituents and the incoming aldehyde. With aldehyde coordination at the apical position, re-face reactivity ensues and leads to reversal of the observed selectivity. Figure 6 depicts the two different proposed transition states, leading to si- or re-face reactivity.
Figure 6.
Wingtip sterics dictating selectivity by directing re- or si-face reactivity.
Enantioenriched α-exo-methylene-γ-butyrolactones, structural motifs commonly encountered in natural products, can be accessed through asymmetric chromium-catalyzed allylation of aldehydes followed by lactonization.324 The lactones were prepared from the selective chromium-catalyzed allylation of the aldehyde with an acrylate, followed by treatment of the resulting homoallylic alcohol with trifluoroacetic acid leading to the targeted lactone. Ligand fine-tuning through facile steric modification from 423 and 433–437 yielded 447 with increased ee when employing ligand 433 in the catalytic process, while the requirement for the rigid carbazole backbone was evidenced when compared against the bis(oxazolinylphenyl)amine (BOPA) 440 (Scheme 65). Even though the ee of the catalyzed reaction was excellent when using ligand 433, the yield was unsatisfactory. However, optimization of the reaction conditions improved the catalytic activity, while the ee of the reaction remained unchanged. The rate of the reaction could be increased through the addition of cobalt(II) phthalocyanine (CoPc), which facilitates the formation of an allyl radical that coordinates the chromium carbazolide 442. The rate of transmetalation to the carbazolide chromium complex could also be increased, in this case by the addition of LiCl. Aldehyde coordination at 443 ensues, leading to the alkoxide 445 via the proposed transition state 444 (Scheme 65). Dissociation of the allenic alkoxide from 445 was favored by the addition of ZrCp2Cl2 or TMSCl. As a proof of concept, the synthesis of (+)-methylenolactocin was successfully explored, yielding 449 with a high degree of selectivity (i, Scheme 66).324
Scheme 65. Enantioselective Chromium-Catalyzed Aldehyde Functionalization to Lactones.
Scheme 66. Enantioenriched (i) Lactones and (ii) Homoallylic Alcohols.
Enantioselective three-component coupling of 1,3-butadienes with aldehydes and alkyl halides (ii, Scheme 66), either fluorinated or nonfluorinated, was also catalyzed employing similar reaction conditions as used toward 447 (Scheme 65).325 Various ligands were used during the catalytic preparation of the homoallylic alcohol, with ligand 433 again demonstrating superior selectivity toward 450 when compared against 435, 436, and 438–441. A similar reaction mechanism was suggested, with the notable difference being the incorporation of butadiene, which reacts first with the alkyl radical leading to a π-allyl radical species. The allyl radical is trapped by the CrII intermediate with subsequent isomerization and carbonyl coordination. The reaction then ensues as above, via a transition state proposed to be similar to 444. This further demonstrates the selectivity and the broad substrate scope compatibility of the finely tuned bis(oxazolinyl)carbazole ligand in conjunction with the optimized reaction conditions.
The catalyzed dearomatization of aromatic substrates with aldehydes is also feasible with chromium salts, proton sponge (PS), manganese, and ZrCp2Cl2 (Scheme 67).326 Not surprising then is the addition of a bis(oxazolinyl)carbazole ligand that significantly improves the yield and selectivity of this catalyzed transformation. Variation of the steric bulk at the wingtip provides significant control over the catalyzed process. High ee was reported when using ethyl-substituted ligand 439, with a slight decrease in ee noted when increasing the wingtip bulk to the iPr moieties of 433. Further increasing the wingtip steric bulk with tBu groups resulted in reduced yield and selectivities, while the benzyl-substituted pincer ligand 435 did not fare a lot better. The catalytic strategy was extended to include bromomethylnaphthalenes leading to optically pure products with multiple stereogenic centers (ii, Scheme 67).327 Similar to the results obtained for heteroarenes,326 the catalyzed reaction with ligand 433 furnished the desired products with remarkable enantio- and diastereoselectivities, allowing access to optically pure compounds primed for further manipulation leading to more elaborate molecules.327 For both catalyzed processes, a transition state was proposed, as depicted by 452 and 454 in Scheme 67.326,327
Scheme 67. Catalytic Dearomatization of (i and ii) Aromatic Substrates with Aldehydes.
The reaction conditions described by Zhang et al.324 were further implemented by the group of Nagasawa during the preparation of calcitriol lactone, a major metabolite of vitamin D3.328 Under the prescribed reaction conditions with ligand 433, stereochemistry at C23 was introduced by crotylation of aldehyde with 2-(bromomethyl)acrylate, yielding the corresponding homoallylic alcohol 456 with a 7:1 diastereomeric ratio. The impact of the wingtip substituents was demonstrated when accessing homoallylic alcohol 457, simply by inverting the stereochemistry at the wingtips of ligand 433 to 455 (i, Scheme 68). Paraconic acids, structural motifs widely encountered in natural products and medicinally relevant compounds, could also be prepared with stereochemistry being introduced through the chromium-catalyzed aldehyde functionalization, as disclosed by Winssinger and co-workers.329 Ligand 433 was again utilized toward the preparation of 459, precursor to the targeted paraconic acid 460, from the corresponding bromolactone and aldehyde with near perfect selectivity for 459 (ii, Scheme 68).
Scheme 68. Synthesis of (i) Calcitriol Lactone and (ii) Paraconic Acids.
5. Applications of LNL-Carbazolide Pincer Complexes
5.1. Facilitation of Redox Activity and Redox Noninnocence
For LNL-pincer metal complexes (L = N, P, C) containing an electrochemically active carbazole spacer,330,331 the ligand-based redox chemistry is observed in a different potential range (oxidations with E1/2 +0.4 to +0.7 V263,332−335 compared to other related LNL- type pincers, with E1/2 = −0.5 to +0.3 V)336−339 and includes the advantages of being reversible and readily tunable by carbazole-substituent modification.335,340 In the case of bi- and trinuclear dyads aimed at light harvesting, photovoltaic, and molecular electronic applications, this means that the redox activity can be tuned in the potential range outside that of commonly employed metalloporphyrins used in these applications.341 So-called “Pacman” porphyrins342−345 were prepared by Kadish et al., so that two porphyrins are linked in a cofacial arrangement by a rigid carbazole bridge to control the distance and the degree of opening of the two porphyrin macrocycles via changes in the extent of π–π interaction.333 The precursor bis(porphyrin)carbazole 463 was prepared by Schiff base condensation of the amino porphyrin 461 and the tert-butyl diformylcarbazole 462 (Scheme 69). Metalation with copper(II) acetate yielded the homonuclear trimetallic dyad 464, or sequential metalation of the porphyrins with Zn(OAc)2 (465) followed by metalation with Cu(OAc)2 yielded the heteronuclear trimetallic copper, dizinc dyad 466 (Scheme 69). Without the presence of the third metal (CuII) in the dyads (as represented by bimetallic dyad 465), the two redox centers are noninteracting, as confirmed by cyclic voltammetry and ESR data. Consecutive oxidations of the two porphyrin zinc units involve one-electron abstractions at each metal center at the same potential to give a dyad with two linked porphyrin π-cation radicals, which is converted to another dyad with two linked porphyrin dications after the second one-electron oxidation at both metal centra, again at the same potential. If a CuII ion is coordinated to the central carbazole in the pincer pocket, however, an enhanced π–π interaction between the porphyrins of the tris-metal dyad results in a splitting of both the first two one-electron oxidations, as well as the second two one-electron oxidations, into two well-defined processes, respectively. Trimetallic dyad 464 similarly shows an interaction upon oxidation, which is only possible if a significant structural rearrangement occurs after the abstraction of one electron. A further consequence of the third metal (copper) presence in the central pincer pocket is the reduction of binding ability of the zinc porphyrins with axial ligands, as measured from the binding constants of 466 with chloride and acetate, compared to 465.
Scheme 69. Synthesis of Pacman Diporphyrins Linked by a Rigid Copper(II) NNN-Carbazolide Bridge.
The persistence of organic aminyl radical cations is essential for their performance in organic electronic devices.346 The stabilization of the radical cations obtained from the oxidation of the arylamine derivatives can be achieved by coordination with transition metals, while providing the simultaneous opportunity for tailoring the electronic structure. In the context of this review, a carbazole-based pincer ligand coordinated to a low-spin palladium(II) would provide a planar system to prevent disruption of potential conjugation and resulting radical stabilization, while the coordinational lability of a Pd–Cl ligand would provide for potential further metal functionalization.347 With the introduction of a second metal center connected via a more extended conjugated backbone in a Janus-type pincer system, the redox sites would additionally be connected. The p-type organic semiconductor indolo[3,2-b]carbazole was used as the central core, provisioning for two NNN-pincers on either end. Following the bromination and protection of the N atoms of the precursor 467, the fused carbazole precursor 468 can be functionalized with thiazole (Scheme 70). The resultant ligand precursor 469 features the pincer cores facing in opposite directions of the fused carbazole scaffold. Metalation of 469 with Pd(cod)Cl2 requires thermolysis of the N–Me bonds to obtain the bimetallic Janus pincer complex 470. Two reversible oxidation processes are observed in the cyclic voltammetry study. The relation of the ΔE values with the comproportionation constant Kc classifies the complexes as belonging to the Robin-Day class III mixed valence compounds, with intermediate oxidation state exhibited by each redox site bridged by a scaffold very efficient in electron transfer.348 Surprisingly, the ΔE value (0.52 V) of 470 proved smaller than that of the bimetallic analogue (ΔE = 0.68 V) based on a diarylamido bisphosphino Janus pincer complex 471 with significantly smaller degree of coplanarity and conjugation (Scheme 70).347 This observation was rationalized as a consequence of significant electron delocalization in 470 compared to redox events that can be viewed as more “concentrated” in the central-diaminobenzene unit of the unfused analogue 471. Visualization of the calculated SOMOs illustrates this conclusion, while the LUMO corresponds to a π* orbital of the fused ligand backbone in conjugation with the electron-deficient central thiazoles, leading to the lower orbital energies of the π-system.
Scheme 70. Synthesis of Palladium(II) Janus Pincers Containing Fused Indolo[3,2-b]carbazole Scaffold and Its Diphenylamide Bisphosphino Analogue.
The redox noninnocence of the carbazole ligand, in conjunction with oxazoline donors, can be extrapolated to unusually facilitate both oxidation of monomeric ytterbium(II) pincer complexes, as well as metal reduction of a trivalent ytterbium complex analogue349 as a promising indicator. The combination of both central carbazole and donor group contribution to the redox versatility of the carbazole platform is particularly demonstrated in the report of a manganese complex coordinated to a bis(triazolylidene)carbazolide pincer ligand 234 (vide supra, Scheme 36).350 The ligand contains sterically undemanding N-fused triazolylidene wingtips that allow for variable oxidation state stabilization351 and homoleptic binding to a manganese precursor, in the synthesis of a rare example of an air-stable MnIV complex 472 (Scheme 71). Coupled with the redox-activity of the carbazole spacer group, the pincer scaffold provides for the observation of five different oxidation states, as observed by cyclic voltammetry and computational (DFT and CASSCF) calculations. Four reversible redox processes were observed, although only the isolation of the first reduced state could be accomplished by chemical reduction of 472 with KC8 to form the monocationic MnIII473 (Scheme 71).350 SEC-EPR spectroscopy (SEC = spectroelectrochemistry) of the oxidized species 474 and 475, as well as magnetometric measurements and magnetic circular dichroism measurements of the isolated 472 and 473, gave experimental insight into their electronic structures, while the quantum chemical calculations elucidated the electronic structure of the entire series. Metal-based reduction of dication MnIV472 with S = 3/2 to an uncommon low-spin d4 configuration (S = 1) was confirmed with the unpaired electrons occupying the degenerate dxz and dyz orbitals for 473 (Scheme 71). A second metal-based reduction yields MnII474 with an overall spin of S = 1/2. The stepwise one-electron oxidations of 472 to 475 and 476 proceed via the consecutive removal of two electrons from the axial carbazolide π-donor ligands, leaving the manganese in a formal oxidation state of +IV. For 476, a high-spin configuration of the MnIV leads to antiferromagnetic coupling of the two unpaired electrons to the two unpaired electrons located at the carbazolide-nitrogen atoms. The ligand-centered oxidations that give rise to (light-sensitive) MnIV organic radicals are particularly appealing for potential photoredox catalytic processes.
Scheme 71. Synthesis of a Homoleptic Bis(triazolylidene)carbazolide Manganese Complex and the Electronic Structure of the Complex in Five Oxidation States.
In another example of the use of the bis(oxazolinyl)carbazole ligand in asymmetric catalysis by the group of Nakada (section 4.4), the preparation of a redox noninnocent NNN-carbazolide iron complex 477 (Scheme 72) was reported for oxidation reactions that parallel iron porphyrin complex reactivity.352 The similarity of 477 (Scheme 72) to the well-known iron porphyrin complex was drawn from the planar aromatic structure featuring an extended π-conjugation, in addition to the anionic amido moiety being a strong σ-donor. The asymmetric epoxidation of alkenes was investigated, taking advantage of the chiral wingtip sterics to control the selectivity of the resulting epoxides. It was found that 477, in conjunction with catalytic amounts of NaBArF4 (ArF = 3,5-bis(trifluoromethyl)phenyl), could catalyze the oxidation reaction yielding the epoxide with ee generally higher than 80% (Scheme 72). The requirement of the carbazole’s planar aromatic scaffold was evidenced when compared to the biphenylamide coordinated iron complex, which yielded only trace amounts of the epoxide. It was further established that addition of a catalytic amount of SIPrAgCl (SIPr = N,N′-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazol-2-ylidene) improved the catalyst activity, leading to an increased yield of the targeted epoxide. In an effort to elucidate the reaction mechanism, a solution of 477 with NaBArF4 and SIPrAgCl was analyzed, leading to formation of the in situ characterized dicationic iron complex 478 with an intermediate spin state of S = 3/2. Oxidation of 478 with iodosobenzene yields the proposed iron(IV) oxo complex 479 with a π-cationic radical and low spin state of S = 1/2, suggested to be a catalytic intermediate turning over alkenes to asymmetric epoxides. While the coordination number differs, the electronic structure of 479 is similar to that of iron porphyrin. The NNN-carbazolide therefore facilitates a two-electron oxidation which leads to a one electron event on both the ligand and the metal, while the chiral wingtip substituents dictate the selectivity of the catalyzed reaction.
Scheme 72. Asymmetric Epoxidation Catalyzed by a Porphyrin-Like Bis(oxazolinyl)carbazolide Iron Complex.
5.2. Photophysical Properties and Photoredox Catalysis
The combination of the extended aromatic heterocycle of carbazole, and its easily accessible functionalization at positions 1, 8, 3, 6, and 9 (Figure 1), has ensured its popularity for photophysical and -chemical applications. Both its light harvesting properties (absorber in pigments, biodetectors, and bioimaging)1,10,353−356 and its light emission properties (OLED applications,25,357−359 fluorescence, and phosphorescence in photonic applications)41,360,361 are well explored. More recent, but already widespread, is the use of carbazole derivatives as organic photoinitiators to activate radical precursors in photocatalysis.32,45−47,361,362 Introduction of transition metals to carbazole-based photosystems allow for the development of organic electronic materials with a range of properties perhaps not attainable with organic molecules alone.347 Certainly, binding of a d-metal in the pincer pocket of a functionalized carbazole can significantly influence the photophysical behavior to either enhance photoluminescence as a result of decreased nonradiative decay in a less strained coordination environment of six-membered chelate rings,363,364 modulate emission color or mechanism, or the inverse effect: quenching emission.365 The fluorescence enhancement by ligand exchange and metal ion removal lends itself for the design of highly sensitive and selective ion sensing, as demonstrated for cyanide detection by a copper(II) bis(triazolyl)carbazolide pincer complex 482 (Scheme 73).366 The ligand precursor 481 was prepared from the copper-catalyzed alkyne-azide click-reaction (CuAAC)367−369 of the bis(alkynyl)carbazole precursor 104 and acts as fluorophore with fluorescence emission at ca. 385 nm.366 Coordination to a Cu2+ ion yields the stable, nonfluorescent 482. If 482 is treated incrementally with tetrabutylammonium cyanide (TBACN), a two-step change in the UV–vis spectrum is observed, dependent on the stoichiometry of the reaction. After addition of 1 equiv of TBACN, ligand exchange at the copper is complete to form the weakly fluorescent copper(II) cyano complex 483 (Scheme 73). When excess cyanide is added, a marked increase in the fluorescence emission intensity is indicative of demetalation of 483 to form the stable Cu(CN)2 or Cu(CN)4 complexes with recovery of fluorophore 481.
Scheme 73. Proposed Cyanide Sensing Mechanism by Bis(triazolyl)carbazolide Copper(II).
To achieve the opposite effect, namely preventing the quenching of photoluminescence, the BIMCA pincer ligand 92(140) was chosen for complexation to platinum(II) with a series of ancillary isonitrile (484),370 alkynyl (485),371 and borane-substituted alkynyls (486)372 (Figure 7). The choice of BIMCA as CNC-pincer ligand was rationalized by its known stability toward hemilability and fluxionality to suppress potential nonradiative decay, while the steric influence of the flanking imidazolylidene wingtips were anticipated to prevent aggregation of the square planar PtII complexes in the ground state or in excited states as the cause of low emission yields and short lifetimes.370 In the first series of PtII emitters (484), π-accepting isonitriles were employed as ancillary ligands for charge transfer luminescence. The molecular structures obtained from single crystal X-ray diffraction confirmed the distorted square planar geometries of the complexes, with the isonitrile moieties protruding from the Pt-CNC plane. Moderate solid state emission was observed (emission maxima 460–475 nm), assigned as a LMCT, with absolute quantum yields up to 22% and millisecond decay lifetimes. Expanding their range of d8 triplet emitters, phenylacetylide BIMCA-PtII complexes 485 (Figure 7) were prepared to comprise of ancillary carbon-donor ligands to destabilize σ-antibonding ligand-field states, for increased intersystem crossing (ISC) from singlet excited states to longer-lived emissive triplet state deriving from the spin–orbit coupling of the heavy metal.371 The same rationale as for 484 was followed for the use of the BIMCA pincer ligands in 485; the nonplanar chelation geometry and bulky substituents of the CNC-pincer scaffold, as well as the ligand-field strength of the ligand, allowed for the marked increase in emission quantum yield and lifetimes as solids (up to 93% with an 11 ms lifetime at 298 K). No evidence for π-stacking or metallophilic interactions was observed in the solid state structures, with the alkynyl ligand displaced from the BIMCA ligand plane. From the time-dependent DFT calculations performed, the phosphorescence was found to arise from a state with mixed 3(MLCT/LL) character, with the BIMCA ligand dominating the frontier orbitals. Deactivation by ligand-field excited states is inhibited by the combined ligand fields of the BIMCA and alkynyl ligand to banish the σ-antibonding dx2–y2 orbital 1.5 eV above the HOMO.
Figure 7.
BIMCA-Pt(II) complexes investigated for their photophysical properties.
To promote intramolecular charge transfer luminescence between the d8 emitter and the alkynyl ancillary ligand, different borane-substituted alkynyls were employed as ancillary ligands in the series 486 (Figure 7).372 In complex series 484(370) and 485,371 emission is localized on the BIMCA core. Incorporation of the borane substituents were proposed to combine the strongly absorbing BIMCA core with efficient spin–orbit coupling from PtII and the Lewis acidity of three-coordinate boron as electron-accepting units.373−375 Visualization of the calculated Kohn-Sham orbitals of 486 suggests charge transfer between the BIMCA-Pt localized HOMO and the alkynylboron ligand centered LUMO was achieved, to provide for charge separation that enables ligand/metal-to-ligand charge transfer (LMLCT).372 The resulting emission of 486 is longer lived (up to 43 ms) than observed for 484 or 485, but the emission is comparatively weaker. Possible sources of thermal vibrational energy dissipation were proposed to be either the methyl wingtips of the BIMCA ligand or the alkynyl ligands.
Three-coordinate complex geometries can be tailored by variation of the metal-ligand dihedral angles to demonstrate tunable behavior from pure phosphorescence to thermally activated delayed fluorescence (TADF) for the d10 coinage metals,376 although few ligand scaffolds are available for photophysical tuning of the triplet-singlet gap with ground state Jahn-Teller distorted T-shapes.377 Beyond the steric directing effect employed in the above-mentioned examples to ensure nonplanarity, the employment of CNC-pincer ligands based on a carbazole scaffold can also be used to dictate the coordination geometry of photoluminescent d10 coinage metals, while the electronic effect of the carbazole amido allows for postcomplexation modification of the metal center to modulate the photophysical behavior.197 The combination of a carbazolide with two flanking 1,2,3-triazol-5-ylidenes yields such T-shape geometries for all three of the coinage metals CuI (110),193 AgI (111),197 and AuI (113)186 (vide supra, Scheme 17), whereby three of the design principles outlined in this review (electronic effect of metal-amide bond, mesoionic nature of flanking carbene L-donors, and steric directing bulky wingtip R-groups) are applied. The unique reactivity of 113 with electrophiles provides the opportunity to modify the gold(I) complex by electrophilic attack either at the amide to yield the cationic linear AuI complex 117(186) or at the nucleophilic metal to furnish the AuIII–F square planar complex 121 (Scheme 18).197 Emissions extending from the blue (copper) to green (gold) to orange (silver) spectrum originate from metal-perturbed π(carbazolide)−π*(carbene) 3ILCT excited states. However, low quantum yields were observed, with the highest quantum yield (ΦPL = 14%) for the linear AuI complex 117. Reverse intersystem crossing (rISC) is prohibited by a larger triplet-singlet gap to suppress TADF, with greater phosphorescence contributions to the photoluminescence leading to longer decay times in THF at room temperature. On the other hand, the lifetime of CuI complex 110 is too short (subnanosecond) to be determined with certainty, and emission may be fluorescent in nature. For the AgI111 and AuI113 analogues, decay lifetimes in the microsecond range increase to millisecond lifetimes upon cooling to 77 K. This increase is in line with the assignment of a change in emission origin from 3ILCT to 3IL excited state and may suggest TADF.
If the modified bis(triazolylidene)carbazolide pincer ligand precursor 234 (see section 3.2 and 5.1) is employed, where the carbene donor heterocycles are connected to the carbazole via the N1-triazole atom instead of the C4-triazole atom, isolation of a photoactive lithium dimeric structure (487), bridged by iodo- and lithium iodide adducts, can be achieved.241487 is the precursor for the mononuclear magnesium bromide complex 488 (Figure 8). The use of these (earth) alkali metals prevents ligand distortion following excitation to avoid nonradiative deactivation, and blue-green and intense lime-green luminescence in solution were observed for 487 and 488 (ΦPL = 16% and 14%, respectively) at room temperature, rationalized by ILCT from the carbazolide to the mesoionic carbenes as derived from the quantum chemical calculations.
Figure 8.
Photoactive bis(triazolylidene)carbazolide complexes of the s-block metals.
Two highly successful examples of ligand design for tuning of the photophysical properties of 3d metals were recently reported by Wenger and co-workers.378,379 The advantages of carbazole-based pincer ligands were exploited, including the π-donating character of the carbazolide-nitrogen, the use of either π-accepting pyridines378 or strong σ-donating carbenes379 as flanking donors, with rigid meridional geometry allowing for bite-angle optimized 6-membered chelated metallacycles to avoid nonradiative relaxation, and low steric bulk of the wingtips on the donor moieties to facilitate homoleptic complex formation. For the first-row metal ion chromium(III), design strategies for emissive complexes have focused on increasing the energy gap between the emissive 2E and the 4T2 excited state by enhancing the ligand field strength,378 to minimize nonradiative relaxation from the higher-lying state and, consequently, optimizing luminescence quantum yields (iii, Figure 9). Lowering of the 2E state energy, however, directly affects the 2E → 4A2 spin-flip transition of CrIII to tune the emission color and is more susceptible to electron-electron repulsion than ligand field strength. For metal complexes with more covalent metal-ligand bonds, d-electrons are spatially more distributed and less confined to the metal core, in the so-called nephelauxetic effect.380,381 The mutual repulsion between the d-electrons is quantified by the Racah B parameter, with a decrease in B-values indicating that the repulsion diminishes. By complexation of a bis(pyridine)carbazolide pincer ligand to yield the (NNN)2CrIII complex 489 (i, Figure 9), Wenger et al. accomplished a shift in the 2E emission to 1067 nm at 77 K (compared to the range of 727–778 nm reported for typical CrIII polypyridine complexes in the red to NIR-I spectral region), classifying 489 as the first example of a CrIII NIR-II emitter.378 This large red-shift to the NIR-II region was attributed to the unusually strong metal–ligand bond covalence facilitated by π-electron density donated from the ligands to the metal in the axial direction, whereas in the equatorial plane π-electron density flows from the metal toward the pyridine π-acceptors. These push-pull interactions induce the strong nephelauxetic effect observed.
Figure 9.
Homoleptic complexes of the high-valent 3d metals (i) chromium(III) and (ii) cobalt(III). Configurational coordinate diagram (iii) for Oh symmetry energy states of 489(378) and (iv) key electronic transitions occurring in 490, with configurational coordinate diagram of energetically low-lying charge transfer and MC excited states with time constants of two relaxation processes.379
Similarly for the 3d6 metal CoIII, the choice of a CNC-pincer ligand based on the carbazole spacer group allows for the isolation of a low-spin configuration identical to that of IrIII emitters.379 The BIMCA protioligand 92(140) was utilized as a precursor. The basic imidazolylidenes as strong σ-donor flanking groups, promote stabilization of the high-valent central metal against ligand dissociation upon photoexcitation of homoleptic 490 (ii, Figure 9), while the increased covalency of the carbazolide-metal bond was once again exploited to effect a decrease in the Racah B parameter determining the metal-centered (MC) states.379 In this way, 490 was demonstrated to feature a photoactive excited state with substantial MLCT character, in contrast to the typically observed low-lying LMCT states.382,383 The photoactive excited state of 490 has an intraligand contribution, resembling the photoactive excited states with mixed MLCT/ILCT character of cyclometalated iridium(III) emitters (iv, Figure 9).379 The excited state decays to the electronic ground state without a noticeable population of any MC state, rationalizing the lack of photoluminescence in 490. UV-vis transient absorption spectroscopy and spectro-electrochemical investigations revealed the unexpected photostability of 490 and its participation in photoinduced electron transfer reactions. Both the photophysical and -chemical behavior of 490 were emphasized to depend on a ligand design rationale comprising of ligand field strength optimization (σ-donor and π-acceptor properties) and increased metal-ligand bond covalence, accessible by using π-donor ligands for enabling a lowest excited state with MLCT character that is not efficiently depopulated by MC (metal centered) states shifted to high energies (iv, Figure 9).
Facile preparation of carbamate-protected primary amines from the corresponding carbamate and the unactivated secondary alkyl halides was made possible through the use of a copper-coordinated PNP-carbazolide complex under blue LED irradiation.384 The group of Peters and Fu, focusing on photoinduced copper-mediated coupling processes,385−393 prepared the photocatalyst 492 for the coupling reaction in the presence of CuBr and LiOtBu, at room temperature under light irradiation (i, Scheme 74).384 On the basis of the results obtained, a mechanistic pathway was proposed whereby irradiation of the copper carbazolide 492 by blue LED lamps generated the excited state intermediate 493 (ii, Scheme 74). Reduction of the electrophile follows, leading to the alkyl radical and a copper(II) intermediate 494. This SET process was supported by EPR spectroscopy, which confirmed the in situ formation of the paramagnetic 494. The CuII494 oxidizes a copper(I)-nucleophile complex 497, leading to the copper(I) carbazolide 492 and the oxidized 495. An out-of-cage coupling reaction between the alkyl radical and the CuII495 yields the cross-coupled product and 496, in turn, reacting with a nucleophile leading to 497 via a ligand substitution reaction.
Scheme 74. (i) Direct Synthesis of Carbamate-Protected Primary Amines with (ii) Supplementary Proposed Catalytic Mechanism.
5.3. Beyond 3-Coordinate Pincers: Expansion of the LNL-Carbazolide Pincer Motif to Polydentate Systems
Expansion of the tridentate binding motif of the carbazole-based pincer ligands under review is a topic with surprisingly few examples. In section 4.3.3, the introduction of one or two homoallyl groups tethered to the carbene-donor imidazolylidene-nitrogen of the BIMCA ligand resulted in tetra- or pentacoordinate ligand coordination, whereby the introduction of the olefinic donors not only yielded more nucleophilic metal centers (383, Scheme 59 and 392, Scheme 60) with enhanced catalytic performance but also demonstrated cooperative or chemically noninnocent metal-ligand reactivity in the proposed catalytic pathways.299−301 In a related example, the BIMCA ligand was modified to contain a pentamethylene tether connecting the two imidazolylidene donor ligands (199), to facilitate dehydrogenation (379) and C–H activation (380) reactivity in their binding to the central metal atom (see Scheme 57).134
Utilization of the diformylcarbazole precursor 462 and 498 paves the way for introduction of ancillary imine groups by way of Schiff base condensation, for the synthesis of macrocyclic ligands with up to six ligating nitrogen atoms.394,395 Changing the ligand scaffold to comprise of a carbazole head unit instead of the previously studied pyrrole of diphenylamine based head units in the Schiff base macrocycles leads to (i) modification of the pKa (diphenylamine pKa = 25, pyrrole pKa = 23, and carbazole pKa = 20 in solvent dimethylsulfoxide),396,397 (ii) structural changes including decreased flexibility and bite angle changes, and (iii) variation of the delocalized electron density on the head unit. The group of Brooker et al. prepared the [1 + 1] macrocycles 499 and 500 directly by refluxing of the respective dialdehyde precursors 498 or 462 and diethylenetriamine in ethanol, followed by addition of the metal(II) acetate salt in acetonitrile solution (Scheme 75).394 In the case of Cu(OAc)2, five coordinate square pyramidal complexes 501 and 502 with an ancillary aqua ligand in the axial position were isolated, while the diamagnetic nickel(II) complexes 503 and 504 featured square planar geometry as a result of the strong ligand field imposed by the macrocycle.
Scheme 75. Synthesis of Mono- And Dinuclear Macrocyclic Bis(imine)carbazolide Metal Complexes.
In a similar metal-free condensation procedure, the [2 + 2] Schiff base macrocycles 505 and 506, containing two carbazole units tethered by two diamine units (Scheme 75), were prepared using ethylene diamine, followed by metalation with zinc(II) acetate.395 The resulting dizinc complexes 507 and 508 retain the stepped conformation of the metal-free macrocycles 505 and 506, despite deprotonation and binding of two ZnII centers in the two tridentate pockets, with one acetate anion displaying μ1,1-bridging and the other μ1,3-bridging. 507 and 508 are strongly blue fluorescent (λmax = 460 nm). Subsequent fluorescence studies demonstrated the strong selectivity of 505 and 506 for ZnII ions over metal(II) ions (Ca, Mg, Mn–Cu) in a turn-on fluorescence to highlight potential applications for these Schiff base carbazole-based macrocycles.
The resemblance of carbazole-based macrocycles to porphyrinoids portends well for their diverse application. A calix[4]pyrrole[2]carbazole macrocycle was prepared nearly two decades ago and employed in anion sensing applications via fluorescence quenching means; however, no metalation of the macrocycle was reported.398 The first example of a porphyrin-related macrocycle coordinated to the transition metal cobalt was only reported in 2011, with the aromatic carbazole and pyridine blocks connected exclusively via aryl-aryl bonds.399 Four-fold cross-coupling of the diboronic ester carbazole precursor 509 with 2,6-dibromopyridine was achieved by Suzuki-Miyaura coupling to yield the macrocycle 510 (Scheme 76). The macrocycle displays a saddle-like conformation with the pyridine moieties twisted “up” and the carbazoles twisted “down”. The ligand design permits the formation of a cavity big enough to complex cobalt(II) to form a distorted octahedral complex 511, with solvent molecule thf and a water molecule axially coordinated. The saddle conformation is maintained upon metal binding.
Scheme 76. Synthesis and Metalation of Porphyrin-Like Macrocycles Based on Carbazole and Pyridine Units.
The interest in carbazole incorporation into fused porphyrins lies in the consequent electronic delocalization over the carbazole and pyrroles to promote conjugation and intramolecular electronic communication. By employing Suzuki-Miyaura cross-coupling reaction conditions with dibromocarbazole 32 and diboryltripyrrane, macrocycle 512 could be synthesized (Scheme 77).400 Selective bromination with NBS yields 516 and 517, for subsequent boryl functionalization to 518 and 519. A follow-up coupling reaction of 516 and 517 with 518 and 519 yields the dimeric 520 and trimeric 521. Metalation of 512 with the divalent metal salts (Pd2+, Zn2+, or Ni2+) yields the complexes 513–515. Similarly, reaction of zinc(II) acetate with 520 or 521 leads to bi- and trimetallic complexes 522 and 523, respectively (Scheme 77). NMR spectroscopic studies yielded no evidence for a global and macrocyclic aromaticity in the macrocycles 512, 520, or 521. The HOMO and LUMO showed relative delocalization over the pyrrole and carbazole units for the metal compounds compared to the metal-free compounds, with insertion of the metal ion into the cavity proposed to strengthen the conjugation. The photophysical behavior of the ZnII porphyrins 514, 522, and 523 was studied and the exciton coupling strength between the carbazole-tripyrrole units determined to be stronger than that of linear Zn-porphyrin arrays. The greater degree of torsional freedom between the constituent units of the dimer 522 and 523 allowed for torsional relaxation in the excited state dynamics, with the mixing of the higher-lying charge transfer state with an exciton coupled state leading to a reduction of the fluorescence properties of the carbazole-tripyrrolic arrays.
Scheme 77. Synthesis and Metalation of Porphyrin-Like Macrocycles Based on Carbazole and Pyrrole Units.
A recent addition to the class of carbazole-based porphyrinoid ligands is the carbenaporphyrin reported by Kunz.401 The synthesis of the carbenaphorphyrin is dependent on the substitution of the pyrrole units not with imidazole-based NHCs but with triazole-based carbenes accessible by the facile click reaction (CuAAC)367−369 of the bis(alkynyl)- and bis(azido)carbazole precursors. Optimization of the 1,3-dipolar cycloaddition to obtain the carbazole macrocycle 524, followed by alkylation of the triazole rings with Meerwein’s salt, yielded the dicationic macrocycle 525 (Scheme 78).401525 is colorless, indicating that it lacks antiaromatic (20 e–) or a macrocyclic aromatic π-system like porphyrin (18 e–). This was supported by visualization of the calculated frontier orbitals, where the individual aromatic character of the contributing ring-units is retained and the HOMO almost fully localized on one carbazole unit. Deprotonation with a lithium base forms the corresponding dilithio-carbenaporphyrin complex 526, with a N,C,N-η3-coordination of each lithium atom supported by DFT calculations, in addition to the coordination of two solvent thf molecules. Transmetalation with scandium trichloride yields the scandium porphyrin complex 527, with the η4-coordination of the carbenaporphyrin ligand in the basal plane confirmed by X-ray structure analysis while the additional chloride and solvent thf molecule are coordinated cis above the plane. To probe for potential ring current in 527, it was reacted with CpLi (Scheme 78). The 1H NMR spectrum of 528 displayed Cp–H signals in the expected region, and the lack of shielding effect observed precludes any macrocyclic aromatic or antiaromatic ring effect. Nevertheless, the ligand scaffold provides the geometric features common to porphyrins upon complexation with the metals. In addition, the mesoionic nature of the triazolylidenes provides for stronger electron-donor properties of the carbenaporphyrin ligand compared to porphyrins, as evaluated by the calculated Mulliken population analyses of the scandium metal center.
Scheme 78. Synthesis, Deprotonation, And Metalation of Carbazole-Triazolylidene Porphyrin.
6. Outlook
6.1. Combinatorial Effect
The combination of more than one of the building-up strategies, incorporating multiple control points (with carbazole-N as prerequisite, in combination of E, L, R, and Z, Figure 1) listed progressively in the preceding sections 2–5, generates exciting possibilities for multifunctional and adaptive molecules in different disciplines. As a summative perspective to the scope of this combinatorial effect for guiding future synthetic methodologies, we wanted to highlight “case studies” as illustrative examples of the potential of the LNL-carbazolide pincer, taking advantage of the inherent attributes of carbazole which has accounted for its success in medicinal, redox-, and photoactive applications.
In the area of anticancer therapeutics based on metallodrugs that can overcome the challenges presented by platinum-based compounds, gold-based anticancer agents can induce cytotoxicity either by targeting DNA (or other intracellular proteins) via direct covalent binding or noncovalently via intercalation if stabilized as AuIII in an appropriate multidentate ligand scaffold.402 The tendency of the gold(III) complexes to be reduced to gold(I) or metallic gold under physiological conditions, as well as the nonselectivity of DNA-binding, limit their usefulness as chemotherapeutics. Appropriate choice of a CNC-pincer ligand that provisions a planar aromatic backbone for cytotoxicity against cancer cells by the inhibition of DNA topoisomerases, with simultaneous stability against intracellular reductants, is found by modifying the bis(triazolylidene)carbazolide scaffold (see section 2.3). The 3,6-positions of carbazole is thus unsubstituted for DNA intercalation, while the combination of the donor (L) mesoionic carbenes and remote basicity effected by the carbazolide-gold(I) interaction186 is required for both the preparation and proven stabilization of the CNC-AuIII complexes as potential anticancer agents with multicellular targets.403 In this case study, the triazolylidene wingtip groups also played a significant role in the isolation of well-defined mononuclear pincer complexes.
The precursor triazolium salts 531 and 532 were prepared from 1,4-disubstituted 1,2,3-triazoles 529 and 530, followed by alkylation of both triazoles on their N3-positions (i, Scheme 79).403 The kinetic instability of the N3-alkylated triazolylidenes during direct metalation with in situ generated free carbenes compelled the use of transmetalation techniques for gold(I) complex formation from the alkylated protioligands 531 and 532. The ligands were first metalated with Ag2O in the presence of excess KCl, yielding tetranuclear monocarbene silver(I) complexes 533 and 534 as intermediates (i, Scheme 79). However, when the tert-butyl analogue was further reacted with the gold(I) metal precursor, the reaction unexpectedly afforded the tetranuclear zwitterionic AuI bis(carbene) complex 535, which was not stable in solution and decomposes to the cationic dinuclear AuI bis(carbene) complex 536 within a week.
Scheme 79. Synthesis of (i) Alkylated Triazolium Salts and Their Polynuclear Silver(I) and Gold(I) Complexes, as well as (ii) Bis(triazolylidene)carbazolide Coordinated Cationic AuIII Chloride Complexes.
To circumvent multinuclear complex formation, 1,2,3-triazolium salts with two bulky aryl (Dipp) substituents on the triazole rings, 106(193) and the corresponding unsubstituted analogue featuring hydrogens instead of tBu groups on the carbazole scaffold403 were prepared using the previously reported methodology193 to facilitate mononuclear complexation with the desired pincer-coordination via deprotonation and in situ carbene formation.403 The reaction of the in situ deprotonated ligand with gold(I) precursor yielded the gold(I) complex 537. As for 113,186 the complex 537 also has a three-coordinated gold center with a T-shaped geometry.403 The demonstrated reactivity of the T-shaped gold(I) complexes with electrophiles186 was employed in the oxidation with iodobenzene dichloride to their respective cationic gold(III) complexes 538 and 539.403 The resulting monomeric, square planar complexes feature a labile chloride ligand available for covalent DNA-bonding, thus furnishing another avenue to target cancer DNA (ii, Scheme 79).
When investigating the cytotoxicity of the AuIII complexes 538 and 539, as well as the AuI complexes 537 and 113 against the breast cancer cell line MDS-MB-231, notable cytotoxicity of the AuIII complex 538 was observed (IC50 = 2.3 ± 0.8 μM), although the ligand precursor was even more cytotoxic (IC50 = 0.4 ± 0.1 μM).403 However, the selectivity of the ligand precursor was found to be lower compared to 538. The underlying factors of the cytotoxicity shown by 538 were clarified in further studies. The interaction between 538 and DNA was investigated using several techniques, which suggested that the complex behaves as a partial DNA intercalator. In addition, in silico screening indicated that 538 can target DNA three-way junctions and several other forms of β- and Z-DNA with good specificity. The redox stability of 538 under physiological conditions was evaluated in the presence of the intracellular reductant glutathione. The complex was found to be stable and neither reduction of the 538 to 537 nor demetalation was observed in the presence of excess glutathione. The redox stability of 538 combined with its DNA affinity are attributed as the key factors for its cytotoxicity in vitro. The synthetic feasibility of 538, in turn, is reliant on the combination of donor oxidation state tolerance, backbone, and wingtip sterics as well as the carbazolide electronics for the needed remote basicity of gold.
The advantage of the redox-stability attainable by the use of the LNL-carbazolide, in combination with 3,6-carbazole backbone modification and judicious choice of anionic O-donors as the flanking L groups, is similarly well-illustrated in the preparation of a composite molecular anode for water oxidation catalysis (WOC).404 Despite the evident utility of a trianionic ONO-pincer ligand based on a carbazole scaffold as demonstrated in section 2.4, very few examples of this type of pincer ligand are known, in contrast to the well-explored NNN, PNP, and CNC-analogues. In this review, only two examples of ONO-carbazolide pincers are noted, including precursor 122(205) (vide supra) and 541,404 the analogue of 45 with the carbazole backbone containing methyl groups in the 3,6-positions of the carbazole. The dicarboxylic acid-functionalized 541 was prepared stepwise from 32 by cyano-substitution of the bromines followed by hydrolyzation (i, Scheme 80). Synthesis of the ruthenium(III) complex 542 coordinated by the dicarboxylate-carbazole ONO3– ligand was done by reaction of 541 with [Ru(dmso)4Cl2] (dmso = dimethylsulfoxide) under basic conditions, followed by addition of excess 4-picoline. The choice of ligand was rationalized with the target of preparing a water oxidation catalyst that can access high oxidation states and form Ru-oxo species across a narrow potential gap by a proton-coupled electron transfer (PCET) pathway,405 while maintaining a low catalytic overpotential to match the oxidation potential of photosensitizers for effective light-driven water oxidation. A trianionic pincer ligand based on a conjugated carbazole scaffold would permit a sufficiently low redox potential to allow for water oxidation to be driven by visible light because of its strongly electron-donating character derived from the three anionic donor moieties (central carbazole-N and flanking carboxylates), as well as the rigid planar structure to stabilize the ruthenium center in high redox states.404 From the single crystal X-ray analysis of the molecular structure of 542, occupation of the two axial positions and one equatorial position trans to the carbazolide-nitrogen by the coordinating 4-picoline ligands was confirmed. The longest Ru–N bond length was observed for the picoline group trans to the carbazole-amido, indicating that ligand exchange of 4-picoline with water would most likely occur at that position. The improved donating ability of the ONO-carbazolide ligand was demonstrated by the three oxidation potentials observed for the RuII/RuIII, RuIII/RuIV, and RuIV/RuV oxidation processes (namely, E = 0.20, 0.63, and 0.99 V, respectively, vs NHE), at oxidation potentials markedly lower than those reported not only for WOCs containing neutral ligands but also for Ru-based WOC with anionic ligands.406−408542 proved an efficient WOC catalyst in the CeIV-driven process with an initial TOF of 0.28 s–1.404 The active intermediate involved in the catalytic cycle was determined by high-resolution mass spectrometry as being the RuIV-OH species 544 (shown in the proposed catalytic cycle ii, Scheme 80), where ligand exchange of 4-picoline with water already occurred in the RuIII-state (542 + H2O-4-picoline → 543). The PCET process of 542 was monitored by evaluating the dependence of the potentials on pH, indicating typical one-electron, one-proton PCET processes for the three oxidation steps, corresponding to the H-RuII-picoline/RuIII-OH2 (543), RuIII-OH2/RuIV-OH (544), and RuIV/RuV=O (545). These experimental results form the basis for the proposed catalytic cycle of water oxidation as catalyzed by 542 (ii, Scheme 80), with the critical O–O bond formation step occurring via the nucleophilic attack of water to form RuIV-O-OH (546) which releases O2 via a reductive elimination step to regenerate 543. The low onset potential (1.24 V vs NHE) measured under neutral conditions suggested that 542 could catalyze water oxidation driven by visible light with a photosensitizer. Very good activity with an initial TOF of 5 min–1 was observed in this three-component system.
Scheme 80. (i) Synthesis of Charge-Neutral ONO-Ru Complex As Water Oxidation Catalyst, (ii) Proposed Mechanism for the Catalytic Cycle of Water Oxidation Mediated by 542 or 551, and (iii) Composite Molecular Anode 551/MWCNTsCOOH/GC Constructed in the Electrochemical Cell for Efficient Water Splitting409.
The performance of the mononuclear RuIII catalyst 542 was significantly improved by the same group, with the expedient of first modifying the carbazole-backbone to contain two dodecyl chains instead of tert-butyl groups on the 3,6-positions (548, i, Scheme 80).409 Functionalization of the 1,8-positions to contain two carboxylic groups proceeded in the same way as for ligand 541,404 to yield the ONO-ligand precursor 550 for complexation to ruthenium in preparation of the charge-neutral mononuclear RuIII551 (i, Scheme 80).409 The hydrophobic features of the dodecyl chains allow for easy binding to carbon nanotubes and immobilization of 551 on COOH-functionalized multiwalled carbon nanotubes (MWCNTsCOOH) followed by loading onto the surface of a glassy carbon electrode yielded the composite molecular anode 551/MWCNTsCOOH/GC illustrated in iii, Scheme 80. Following the same mechanistic route as proposed for the previously reported 542 (ii, Scheme 80), the charge-neutral intermediates 543–546 were proposed to stabilize the catalytic center and promote the durability of the assembled molecular anode. With this assembly, excellent catalytic activity for the oxygen evolution reaction (OER) is reported, with an onset overpotential of only 380 mV (compared to the 1.24 V for 542)404 and a steady catalytic current density of 1.25 mA/cm2 at the overpotential of 580 mV.409 A Faradaic efficiency of 96% and a TOF of 10.3 s–1 for OER could be achieved from this molecular device as a demonstration of its high efficiency and durability in electrochemical catalytic water oxidation.
For the realization of stereoselective cross-coupling catalyzed reactions, as exemplified by the work of Zhang410−413 and Liu et al.,414−417 the requirement is an anionic multidentate ligand to enhance the reducing ability of an in situ formed copper complex which reduces carboelectrophiles under mild reaction conditions. Furthermore, a demand for stereoselective control on the formed products necessitates the introduction of chiral wingtip R groups on the multidentate ligand. These prerequisites, specifically for the asymmetric alkyl and aryl azolation of alkenes, were met by the bis(oxazolinyl)carbazolide scaffold (see sections 4.4 and 5.1).418 The authors reported the selective three-component coupling of benzoxazole, styrene, and ethyl 2-bromo-2-methylpropanoate, promoted by the in situ formed copper carbazolide complex in the presence of tBuOLi (i, Scheme 81). Mediating the reaction with ligands 554–556 and 557–558 resulted in a significant reduction in both catalytic yield and enantioselectivities (iii, Scheme 81). The results evidenced the requirement for both an anionic coordination site and the rigid carbazole backbone. Moreover, the sterically bulky 434 exhorted the highest degree of control over the catalyzed reaction when compared against the analogues 433, 435, and 436. It was further determined that a range of different heterocyclic systems, alkenes, and alkyl halohydrocarbons could be tolerated while retaining good yields and selectivities (i, Scheme 81). Similarly, the carbazolide-coordinated copper catalyst furnished the three-component coupling between benzoxazole, styrene, and diaryliodonium trifluoromethyl sulfonates with retention of the enantioselectivities and yields (ii, Scheme 81).
Scheme 81. Copper Carbazolide-Mediated Asymmetric Alkyl and Aryl Azolation of Alkenes.
A plausible catalytic reaction mechanism could be elucidated based on various control experiments (Scheme 82).418 Base facilitated copper carbazolide formation is followed by coordination of the in situ deprotonated azole, leading to intermediate 560. It was proposed that this intermediate serves as the reductive species, which reacts with either alkyl halide or the diaryliodonium salt leading to a SET event, yielding the oxidized copper(II) intermediate 561 and the alkyl or aryl radical, respectively. Subsequent addition of the alkyl/aryl radical to the alkene furnishes the corresponding radical, which in turn reacts with and results in the oxidized CuIII intermediate 562. Two proposed CuIII transition states account for the observed selectivity, again dictated by the wingtip steric bulk as has been noted throughout this contribution. Both aryl and alkyl groups experience decreased steric repulsion with si-face attack at the metal center, favoring the observed (S)-product. The re-face transition state 563 is disfavored due to steric repulsion between the substrate and the tert-butyl wingtip groups. Finally, reductive elimination ensues, leading to the catalytically active 559 and the targeted product 564.
Scheme 82. Proposed Catalytic Cycle for the Copper Catalyzed Asymmetric Alkyl and Aryl Azolation of Alkenes.
In the final case study, the group of Zhang also reported on the blue LED-promoted asymmetric-catalyzed alkylation of azoles with aryl-substituted secondary alkyl bromides, yielding the chiral alkyl azoles in high yields and moderate to excellent enantioselectivity (Scheme 83).419 The carbazole ligand 434 proved to be the best suited for the catalytic transformation out of the range of ligands tested, which included 433, 436, 439, 440, 556, and 558. It was further reported that the use of green instead of blue LEDs was detrimental to the product yield, but the selectivity remained unchanged. UV–visible light absorption experiments recorded the absence of light absorption of benzoxazole, CuI, and 434 at a wavelength over 400 nm. On the contrary, the copper carbazolide 559 and the intermediate anionic complex 560 both absorbed in the 400–500 nm wavelength range. The excited states of the in situ formed 559 and 560 were quenched by benzylic bromide, as determined through Stern–Volmer experiments. The Stern–Volmer constants for 559 and 560 were reported to be 8.8 mM–1 and 47 mM–1, respectively. The larger Stern–Volmer constants for 560 suggest that it is the photoactive species when irradiated with blue LEDs, while a radical-chain process was ruled out. On the basis of these results, in addition to various control experiments which included deuterium reactions, a catalytic mechanism was proposed (Scheme 83). The in situ formation of the copper carbazolide 559 is followed by transmetalation with [Li-azole], obtained from the deprotonation of the azole with tBuOLi. This results in the photoactive species 560, subsequently engaged in photoexcitation which leads to the excited intermediate 565. Electron transfer to the alkyl bromide ensues, with oxidation of 565 to the CuII intermediate 566. Enantioselective radical trapping results in rapid oxidation of 566 to the alkyl coordinated CuIII567, followed by reductive elimination yielding the optically pure benzylic azole and the copper carbazolide 559 (Scheme 83).419 The demonstration of a single 3d-transition metal base catalytic system promoting a mild asymmetric coupling reaction via light irradiation is made possible by the use of a redox-stabilizing and photoactive carbazole based pincer ligand for the preparation of the T-shaped catalyst precursor.
Scheme 83. Enantioselective Alkylation of Azoles and Proposed Reaction Mechanism.
6.2. Conclusion
In the examples above, the bottom-up approach to assembling a smart (switchable, multifunctional, adaptable or tunable)81 pincer ligand was shown to provide access to functional molecules, tailored according to the specific requirements for a targeted application. The systematic incorporation of the various design principles available to the LNL-carbazolide scaffold, based on the modulation of the steric and electronic effects furnished by the planar, rigid carbazole backbone and the central amido moiety, the flanking donor ligands and their wingtips, provides for a combinatorial effect to address challenges in a wide variety of chemical applications. Particularly the application of this class of pincer ligands in the fast burgeoning area of small molecule activation by the strategies of element-ligand cooperativity420 or geometrically constrained elements of the p-block421−423 is a promising prospect with main group elements coordinated by carbazole-pincer ligands (section 3.3.1) hardly explored.
Acknowledgments
Financial support by the Jane and Aatos Erkko Foundation, Finland (D.B., 200030), the Finnish Cultural Foundation (S.T., 00231142), and the University of Oulu, Faculty of Technology, is greatly appreciated.
Glossary
Abbreviations
- [H-9BBN]2
9-borabicyclo[3.3.1]nonane dimer
- [PPN]Cl
bis(triphenylphosphine)iminium chloride
- acac
acetyl acetonato
- Ad
adamantyl
- BIMCA
3,6-di-tert-butyl-1,8-bis(imidazole-2-ylidene-1-yl)carbazolide
- bpy
2,2′-bipyridine
- BOPA
bis(oxazolinylphenyl)amine
- CASSCF
complete active space self-consistent field
- CHC
cyclohexene carbonate
- CHO
cyclohexene oxide
- CL
caprolactone
- COA
cyclooctane
- cod
1,5-cyclooctadiene
- COE
cyclooctene
- CoPc
cobalt(II) phthalocyanine
- Cp
cyclopentadienyl
- Cp*
pentamethylcyclopentadienyl
- CuAAC
copper-catalyzed azide-alkyne cycloaddition
- dba
dibenzylideneacetone
- DCE
1,2-dichloroethane
- de
diastereomeric excess
- DFT
density functional theory
- DIPEA
N,N-diisopropylethylamine
- Dipp
2,6-diisopropylphenyl
- DMAP
dimethylaminopyridine
- dme
dimethoxyethane
- DMF
dimethylformamide
- DMPA
2,2-dimethoxy-2-phenylacetophenone
- DMSO
dimethyl sulfoxide
- DOSY
diffusion ordered spectroscopy
- dppf
1,1′-bis(diphenylphosphino) ferrocene
- dr
diastereoselectivity
- ee
enantiomeric excess
- HOMO
highest occupied molecular orbital
- HMDS
hexamethyldisilazane
- ILCT
intraligand-charge transfer
- IP
isoprene
- ISC
intersystem crossing
- LDA
lithium diisopropylamide
- LMCT
ligand-to-metal charge transfer
- LMLCT
ligand/metal-to-ligand charge transfer
- LUMO
lowest unoccupied molecular orbital
- MBEF
2-(2-methylidenebut-3-enyl)furan
- MC
metal-centered
- Mes
2,4,6-trimethylphenyl
- Mes*
2,4,6-tri-tert-butylphenyl
- MHD
3-methylenehepta-1,6-diene
- MIC
mesoionic carbene
- MLCT
metal-to-ligand charge transfer
- MPEP
2-(3-methylidenepent-4-en-1-yl)pyridine
- Nacnac
β-diketiminate
- NBS
N-bromosuccinimide
- NHC
N-heterocyclic carbene
- NHE
normal hydrogen electrode
- NIR
near-infrared spectroscopy
- OAc
acetate
- OER
oxygen evolution reaction
- OLED
organic light emitting diode
- OTf
trifluoromethanesulfonate
- PCET
proton-coupled electron transfer
- PCHC
poly(cyclohexene carbonate)
- PCL
polycaprolactone
- pet.
petroleum
- phen
1,10-phenenthroline
- PIP
polyisoprene
- Pipp
para-isopropylphenyl
- PNHC
protic N-heterocyclic carbene
- PS
proton sponge
- rISC
reverse intersystem crossing
- SEC
spectroelectrochemistry
- SET
single electron transfer
- SIPr
N,N′-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazol-2-ylidene
- SOMO
singly occupied molecular orbital
- TADF
thermally activated delayed fluorescence
- TBA
tert-butylethane
- TBACN
tetrabutylammonium cyanide
- TBAF
tetrabutylammonium fluoride
- TBE
tert-butylethylene
- tBoc
tert-butoxycarbonyl
- TBS
tert-butyldimethylsilyl
- TBTA
tris(benzyltriazolylmethyl)amine
- TEMPO
2,2,6,6-tetramethylpiperidinyloxyl
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- tht
tetrahydrothiophene
- TIPS
triisopropylsilyl
- TMEDA
tetramethylethylenediamine
- TMS
trimethylsilyl
- TOF
turnover frequency
- Trip
2,4,6-triisopropylphenyl
- WOC
water oxidation catalysis
Biographies
George Kleinhans completed his Ph.D. in 2018 at the University of Pretoria under the supervision of Daniela Bezuidenhout, focusing on organometallic complexes toward small molecule activation and homogeneous catalysis. After his postgraduate studies, he moved to industry as a research and development scientist. In 2021, he moved to the University of Oulu as a postdoctoral fellow where he completed his fellowship in 2023. He focused on tailored ligand design for coordination to 3d transition metals to be used as homogeneous catalyst for CO2 and NH3 functionalization.
Aino Karhu obtained her Ph.D. at University of Oulu in 2016 under the supervision of Prof. Risto S. Laitinen, focusing on preparation, characterization, and coordination chemistry of imido-selenium compounds. She is currently working as a postdoctoral researcher in Assoc. Prof. Daniela Bezuidenhout’s research group, where her research focus is on developing compounds based on carbenes and main group elements that can be utilized as transition metal replacements for small molecule activation and catalysis.
Hugo Boddaert graduated in 2020 with an MSc. in Organic Synthesis from the University of Lyon 1 and a Chemical and Process Engineering degree from the engineering school Chimie Physique Electronique (CPE Lyon). After completing his master’s thesis on nickel/photoredox acylation of C(sp3) moieties in the group of Abderrahmane Amgoune, he is now working under the supervision of Assoc. Prof. Daniela Bezuidenhout, developing new carbene complexes designed for small molecule activation and the development of new catalytical processes.
Sadia Tanweer studied chemistry at the Aligarh Muslim University, where she obtained her B.Sc. (Hons), M.Sc., and M.Phil. In 2017, she joined the University of Eastern Finland for the international master’s degree programme for Research Chemists. There she worked under the supervision of Dr. Igor Koshevoy for her master’s thesis. In 2021, she joined the research group of Dr. Daniela Bezuidenhout as a doctoral researcher. Her doctoral thesis is focused on the synthesis of mesoionic carbene complexes of manganese and their utilization for the (catalytic) transformation of carbon dioxide and other unsaturated hydrocarbons.
David Wunderlin studied chemistry at the University of Zürich, where he graduated with his MSc. in Chemistry in 2019 after completing his master’s thesis on synthetic approaches toward platinum-photosensitizer conjugates for photodynamic therapy in the group of Prof. Dr. Bernhard Spingler. In 2021, he joined the research group of Dr. Daniela Bezuidenhout as a doctoral researcher with his thesis focused on the synthesis and development of mesoionic carbene nickel complexes for small molecule activation and utilization in catalytic transformations.
Daniela Bezuidenhout studied chemistry at the University of Pretoria, where she obtained her Ph.D. under the direction of Prof. Simon Lotz. She joined the group of Prof. Guy Bertrand at the University of California, San Diego, as a Fulbright postdoctoral scholar. Her independent career started with a return to the University of Pretoria, followed by a position at the University of the Witwatersrand, South Africa. In 2019, she was appointed at the University of Oulu, Finland, as an associate professor. Her research focuses on the development of new carbene ligands and complexes for the activation of enthalpically strong bonds in small molecules and for the mediation of new template chemical reactions and catalytic transformations. She is especially interested in the role of noninnocent and cooperative ligand behavior in these transformations.
Author Contributions
H.B., S.T., and D.W. contributed equally. CRediT: George Kleinhans conceptualization, supervision, visualization, writing-original draft, writing-review & editing; Aino J Karhu supervision, visualization, writing-original draft, writing-review & editing; Hugo Boddaert data curation, writing-review & editing; Sadia Tanweer data curation, writing-review & editing; David Wunderlin data curation, writing-review & editing; Daniela Ina Bezuidenhout conceptualization, funding acquisition, project administration, resources, supervision, writing-original draft, writing-review & editing.
The authors declare no competing financial interest.
References
- Yin J.; Ma Y.; Li G.; Peng M.; Lin W. A Versatile Small-Molecule Fluorescence Scaffold: Carbazole Derivatives for Bioimaging. Coord. Chem. Rev. 2020, 412, 213257. 10.1016/j.ccr.2020.213257. [DOI] [Google Scholar]
- Manickam M.; Iqbal P.; Belloni M.; Kumar S.; Preece J. A. A Brief Review of Carbazole-Based Photorefractive Liquid Crystalline Materials. Isr. J. Chem. 2012, 52, 917–934. 10.1002/ijch.201200058. [DOI] [Google Scholar]
- Mitra A. K. Sesquicentennial Birth Anniversary of Carbazole, a Multifaceted Wonder Molecule: A Revisit to Its Synthesis, Photophysical and Biological Studies. J. Iran. Chem. Soc. 2022, 19, 2075–2113. 10.1007/s13738-021-02444-0. [DOI] [Google Scholar]
- Grigalevicius S. 3,6(2,7),9-Substituted Carbazoles as Electroactive Amorphous Materials for Optoelectronics. Synth. Met. 2006, 156, 1–12. 10.1016/j.synthmet.2005.10.004. [DOI] [Google Scholar]
- Li C.; Liu M.; Pschirer N. G.; Baumgarten M.; Müllen K. Polyphenylene-Based Materials for Organic Photovoltaics. Chem. Rev. 2010, 110, 6817–6855. 10.1021/cr100052z. [DOI] [PubMed] [Google Scholar]
- Karon K.; Lapkowski M. Carbazole Electrochemistry: A Short Review. J. Solid State Electrochem. 2015, 19, 2601–2610. 10.1007/s10008-015-2973-x. [DOI] [Google Scholar]
- Sathiyan G.; Sivakumar E. K. T.; Ganesamoorthy R.; Thangamuthu R.; Sakthivel P. Review of Carbazole Based Conjugated Molecules for Highly Efficient Organic Solar Cell Application. Tetrahedron Lett. 2016, 57, 243–252. 10.1016/j.tetlet.2015.12.057. [DOI] [Google Scholar]
- Wang G.; Sun S.; Guo H. Current Status of Carbazole Hybrids as Anticancer Agents. Eur. J. Med. Chem. 2022, 229, 113999. 10.1016/j.ejmech.2021.113999. [DOI] [PubMed] [Google Scholar]
- Trosien S.; Böttger P.; Waldvogel S. R. Versatile Oxidative Approach to Carbazoles and Related Compounds Using MoCl5. Org. Lett. 2014, 16, 402–405. 10.1021/ol403304t. [DOI] [PubMed] [Google Scholar]
- Kato S. I.; Shimizu S.; Taguchi H.; Kobayashi A.; Tobita S.; Nakamura Y. Synthesis and Electronic, Photophysical, and Electrochemical Properties of a Series of Thienylcarbazoles. J. Org. Chem. 2012, 77, 3222–3232. 10.1021/jo202625p. [DOI] [PubMed] [Google Scholar]
- Ledwon P.; Motyka R.; Ivaniuk K.; Pidluzhna A.; Martyniuk N.; Stakhira P.; Baryshnikov G.; Minaev B. F.; Ågren H. The Effect of Molecular Structure on the Properties of Quinoxaline-Based Molecules for OLED Applications. Dyes Pigm. 2020, 173, 108008. 10.1016/j.dyepig.2019.108008. [DOI] [Google Scholar]
- Prusty N.; Banjare S. K.; Mohanty S. R.; Nanda T.; Yadav K.; Ravikumar P. C. Synthesis and Photophysical Study of Heteropolycyclic and Carbazole Motif: Nickel-Catalyzed Chelate-Assisted Cascade C-H Activations/Annulations. Org. Lett. 2021, 23, 9041–9046. 10.1021/acs.orglett.1c03234. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Jia D.; Zeng J.; Liu Y.; Bu X.; Yang X. Benzocarbazole Synthesis via Visible-Light-Accelerated Rh(III)-Catalyzed C-H Annulation of Aromatic Amines with Bicyclic Alkenes. Org. Lett. 2021, 23, 7740–7745. 10.1021/acs.orglett.1c02709. [DOI] [PubMed] [Google Scholar]
- Li J.; Grimsdale A. C. Carbazole-Based Polymers for Organic Photovoltaic Devices. Chem. Soc. Rev. 2010, 39, 2399–2410. 10.1039/b915995a. [DOI] [PubMed] [Google Scholar]
- Blouin N.; Leclerc M. Poly(2,7-Carbazole) s: Structure-Property Relationships. Acc. Chem. Res. 2008, 41, 1110–1119. 10.1021/ar800057k. [DOI] [PubMed] [Google Scholar]
- Wang H. Y.; Liu F.; Xie L. H.; Tang C.; Peng B.; Huang W.; Wei W. Topological Arrangement of Fluorenyl-Substituted Carbazole Triads and Starbursts: Synthesis and Optoelectronic Properties. J. Phys. Chem. C 2011, 115, 6961–6967. 10.1021/jp200433e. [DOI] [Google Scholar]
- Vlasselaer M.; Dehaen W. Synthesis of Linearly Fused Benzodipyrrole Based Organic Materials. Molecules 2016, 21, 785. 10.3390/molecules21060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z. C.; Lou Q. X.; Niu Y.; Yang S. D. Temporary (P = O) Directing Group Enabled Carbazole Ortho Arylation Via Palladium Catalysis. Chem. Commun. 2021, 57, 2021–2024. 10.1039/D0CC07596E. [DOI] [PubMed] [Google Scholar]
- Bera S. S.; Bahukhandi S. B.; Empel C.; Koenigs R. M. Catalyst-Controlled Site-Selective N-H and C3-Arylation of Carbazole Via Carbene Transfer Reactions. Chem. Commun. 2021, 57, 6193–6196. 10.1039/D1CC01863A. [DOI] [PubMed] [Google Scholar]
- Kaur R.; Banga S.; Babu S. A. Construction of Carbazole-Based Unnatural Amino Acid Scaffolds Via Pd(II)-Catalyzed C(Sp3)-H Functionalization. Org. Biomol. Chem. 2022, 20, 4391–4414. 10.1039/D2OB00658H. [DOI] [PubMed] [Google Scholar]
- Sharma V. S.; Sharma A. S.; Ganga V. S. R.; Shrivastav P. S.; Shah P. A.; Agarwal N. Room-Temperature Blue-Light-Emitting Liquid Crystalline Materials Based on Phenanthroimidazole-Substituted Carbazole Derivatives. New J. Chem. 2021, 45, 22193–22201. 10.1039/D1NJ04234C. [DOI] [Google Scholar]
- Maeda B.; Mori G.; Sakakibara Y.; Yagi A.; Murakami K.; Itami K. Photo-Induced Arylation of Carbazoles With Aryldiazonium Salts. Asian J. Org. Chem. 2021, 10, 1428–1431. 10.1002/ajoc.202100191. [DOI] [Google Scholar]
- Ledwon P. Recent Advances of Donor-Acceptor Type Carbazole-Based Molecules for Light Emitting Applications. Org. Electron. 2019, 75, 105422. 10.1016/j.orgel.2019.105422. [DOI] [Google Scholar]
- Li J.; Grimsdale A. C. Carbazole-based polymers for organic photovoltaic devices. Chem. Soc. Rev. 2010, 39, 2337–2732. 10.1039/b915995a. [DOI] [PubMed] [Google Scholar]
- Nguyen Q. P. B.; Baek S. J.; Kim M. J.; Shin N. Y.; Kim G. W.; Choe D. C.; Kwon J. H.; Chai K. Y. Novel Hole Transporting Materials Based on 4-(9H-Carbazol-9-Yl) Triphenylamine Derivatives for OLEDs. Molecules 2014, 19, 14247–14256. 10.3390/molecules190914247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlińska-Nȩcek K.; Starowicz Z.; Tavgeniene D.; Krucaite G.; Grigalevicius S.; Schab-Balcerzak E.; Lipiński M. A Solution-Processable Small-Organic Molecules Containing Carbazole or Phenoxazine Structure as Hole-Transport Materials for Perovskite Solar Cells. Opto-Electron. Rev. 2019, 27, 137–142. 10.1016/j.opelre.2019.04.003. [DOI] [Google Scholar]
- Sharma A.; Balasaravanan R.; Thomas K. R. J.; Ram M.; Dubey D. K.; Yadav R. A. K.; Jou J. H. Tuning Photophysical and Electroluminescent Properties of Phenanthroimidazole Decorated Carbazoles with Donor and Acceptor Units: Beneficial Role of Cyano Substitution. Dyes Pigm. 2021, 184, 108830. 10.1016/j.dyepig.2020.108830. [DOI] [Google Scholar]
- Vogel A.; Schreyer T.; Bergner J.; Rominger F.; Oeser T.; Kivala M. A Symmetrically π-Expanded Carbazole Incorporating Fluoranthene Moieties. Chem.—Eur. J. 2022, 28, e202201424 10.1002/chem.202201424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golba S. Electrochemical and Opto-Electronic Properties of Carbazole-Based Derivatives with Symmetric A-CZ-A Architecture. Russ. J. Electrochem. 2018, 54, 567–584. 10.1134/S1023193518070030. [DOI] [Google Scholar]
- Konidena R. K.; Thomas K. R. J. Star-Shaped Asymmetrically Substituted Blue Emitting Carbazoles: Synthesis, Photophyscial, Electrochemical and Theoretical Investigations. ChemistrySelect 2017, 2, 7514–7524. 10.1002/slct.201701336. [DOI] [Google Scholar]
- Bagdžiunas G.; Palinauskas D.; Ramanavičius A. Towards Colourless-to-Green Electrochromic Smart Glass Based on a Redox Active Polymeric Semiconductor Containing Carbazole Moiety. Dyes Pigm. 2020, 177, 108328. 10.1016/j.dyepig.2020.108328. [DOI] [Google Scholar]
- Wex B.; Kaafarani B. R. Perspective on Carbazole-Based Organic Compounds as Emitters and Hosts in TADF Applications. J. Mater. Chem. C 2017, 5, 8622–8653. 10.1039/C7TC02156A. [DOI] [Google Scholar]
- Tao Y.; Yang C.; Qin J. Organic Host Materials for Phosphorescent Organic Light-Emitting Diodes. Chem. Soc. Rev. 2011, 40, 2943–2970. 10.1039/c0cs00160k. [DOI] [PubMed] [Google Scholar]
- Krucaite G.; Grigalevicius S. 2,7(3,6)-Diaryl(Arylamino)-Substituted Carbazoles as Components of OLEDs: A Review of the Last Decade. Materials 2021, 14, 6754. 10.3390/ma14226754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigot C.; Noirbent G.; Bui T. T.; Péralta S.; Duval S.; Gigmes D.; Nechab M.; Dumur F. Synthesis, and the Optical and Electrochemical Properties of a Series of Push-Pull Dyes Based on the 4-(9-Ethyl-9H-Carbazol-3-Yl)-4-Phenylbuta-1,3-Dienyl Donor. New J. Chem. 2021, 45, 5808–5821. 10.1039/D1NJ00275A. [DOI] [Google Scholar]
- Li Z.; Pei Y.; Wang Y.; Lu Z.; Dai Y.; Duan Y.; Ma Y.; Guo H. Blue-/NIR Light-Excited Fluorescence Switch Based on a Carbazole-Dithienylethene-BF2bdk Triad. J. Org. Chem. 2019, 84, 13364–13373. 10.1021/acs.joc.9b01508. [DOI] [PubMed] [Google Scholar]
- Dumur F. Carbazole-Based Polymers as Hosts for Solution-Processed Organic Light-Emitting Diodes: Simplicity, Efficacy. Org. Electron. 2015, 25, 345–361. 10.1016/j.orgel.2015.07.007. [DOI] [Google Scholar]
- Bekkar F.; Bettahar F.; Moreno I.; Meghabar R.; Hamadouche M.; Hernáez E.; Vilas-Vilela J. L.; Ruiz-Rubio L. Polycarbazole and Its Derivatives: Synthesis and Applications. A Review of the Last 10 Years. Polymers 2020, 12, 2227. 10.3390/polym12102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi K.; Takao H.; Nakagawa T. Synthesis and Optical Properties of Conjugated Polymers Bearing a 1,8-Difunctionalized Carbazole Unit. Polym. J. 2013, 45, 396–400. 10.1038/pj.2012.153. [DOI] [Google Scholar]
- Dumur F. Recent Advances on Carbazole-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 125, 109503. 10.1016/j.eurpolymj.2020.109503. [DOI] [Google Scholar]
- Grazulevicius J. V.; Strohriegl P.; Pielichowski J.; Pielichowski K. Carbazole-Containing Polymers: Synthesis, Properties and Applications. Prog. Polym. Sci. 2003, 28, 1297–1353. 10.1016/S0079-6700(03)00036-4. [DOI] [Google Scholar]
- Zheng Q.; Chen S.; Zhang B.; Wang L.; Tang C.; Katz H. E. Highly Soluble Heteroheptacene: A New Building Block for p-Type Semiconducting Polymers. Org. Lett. 2011, 13, 324–327. 10.1021/ol102806x. [DOI] [PubMed] [Google Scholar]
- Kang B. G. Well-Defined ABA Triblock Copolymers Containing Carbazole and Ethynyl Groups: Living Anionic Polymerization, Postpolymerization Modification, and Thermal Cross-Linking. Macromol. Res. 2021, 29, 470–476. 10.1007/s13233-021-9061-0. [DOI] [Google Scholar]
- Wei X.; Zhu M.; Cheng Z.; Lee M.; Yan H.; Lu C.; Xu J. Aggregation-Induced Electrochemiluminescence of Carboranyl Carbazoles in Aqueous Media. Angew. Chem., Int. Ed. 2019, 131, 3194–3198. 10.1002/ange.201900283. [DOI] [PubMed] [Google Scholar]
- Xu J.; Cao J.; Wu X.; Wang H.; Yang X.; Tang X.; Toh R. W.; Zhou R.; Yeow E. K. L.; Wu J. Unveiling Extreme Photoreduction Potentials of Donor-Acceptor Cyanoarenes to Access Aryl Radicals from Aryl Chlorides. J. Am. Chem. Soc. 2021, 143, 13266–13273. 10.1021/jacs.1c05994. [DOI] [PubMed] [Google Scholar]
- Speckmeier E.; Fischer T. G.; Zeitler K. A Toolbox Approach to Construct Broadly Applicable Metal-Free Catalysts for Photoredox Chemistry: Deliberate Tuning of Redox Potentials and Importance of Halogens in Donor-Acceptor Cyanoarenes. J. Am. Chem. Soc. 2018, 140, 15353–15365. 10.1021/jacs.8b08933. [DOI] [PubMed] [Google Scholar]
- Shang T. Y.; Lu L. H.; Cao Z.; Liu Y.; He W. M.; Yu B. Recent Advances of 1,2,3,5-Tetrakis(Carbazol-9-Yl)-4,6-Dicyanobenzene (4CzIPN) in Photocatalytic Transformations. Chem. Commun. 2019, 55, 5408–5419. 10.1039/C9CC01047E. [DOI] [PubMed] [Google Scholar]
- Han M.; Wang C.; Ji Y.; Song Z.; Xing L.; Su Y.; Wang X.; Zhang A.; Ai J.; Geng M. Metabolism-Based Structure Optimization: Discovery of a Potent and Orally Available Tyrosine Kinase ALK Inhibitor Bearing the Tetracyclic Benzo[b]Carbazolone Core. Bioorg. Med. Chem. Lett. 2016, 26, 5399–5402. 10.1016/j.bmcl.2016.10.039. [DOI] [PubMed] [Google Scholar]
- Liger F.; Popowycz F.; Besson T.; Picot L.; Galmarini C. M.; Joseph B. Synthesis and Antiproliferative Activity of Clausine E, Mukonine, and Koenoline Bioisosteres. Bioorg. Med. Chem. 2007, 15, 5615–5619. 10.1016/j.bmc.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Gluszynska A. Biological Potential of Carbazole Derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. 10.1016/j.ejmech.2015.02.059. [DOI] [PubMed] [Google Scholar]
- Woodward R. B.; Iacobucci G. A.; Hochstein I. A. The Synthesis of Ellipticine. J. Am. Chem. Soc. 1959, 81, 4434–4435. 10.1021/ja01525a085. [DOI] [Google Scholar]
- Sakamoto H.; Tsukaguchi T.; Hiroshima S.; Kodama T.; Kobayashi T.; Fukami T. A.; Oikawa N.; Tsukuda T.; Ishii N.; Aoki Y. CH5424802, a Selective ALK Inhibitor Capable of Blocking the Resistant Gatekeeper Mutant. Cancer Cell 2011, 19, 679–690. 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kodama T.; Tsukaguchi T.; Yoshida M.; Kondoh O.; Sakamoto H. Selective ALK Inhibitor Alectinib with Potent Antitumor Activity in Models of Crizotinib Resistance. Cancer Lett. 2014, 351, 215–221. 10.1016/j.canlet.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Gadgeel S. M.; Gandhi L.; Riely G. J.; Chiappori A. A.; West H. L.; Azada M. C.; Morcos P. N.; Lee R. M.; Garcia L.; Yu L.; et al. Safety and Activity of Alectinib Against Systemic Disease and Brain Metastases in Patients with Crizotinib-Resistant ALK-Rearranged Non-Small-Cell Lung Cancer (AF-002JG): Results from the Dose-Finding Portion of a Phase 1/2 Study. Lancet Oncol. 2014, 15, 1119–1128. 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- Gu W.; Wang S. Synthesis and Antimicrobial Activities of Novel 1H-Dibenzo[a,c]Carbazoles from Dehydroabietic Acid. Eur. J. Med. Chem. 2010, 45, 4692–4696. 10.1016/j.ejmech.2010.07.038. [DOI] [PubMed] [Google Scholar]
- Clausen J. D.; Kjellerup L.; Cohrt K. O. H.; Hansen J. B.; Dalby-Brown W.; Winther A. M. L. Elucidation of Antimicrobial Activity and Mechanism of Action by N-Substituted Carbazole Derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 4564–4570. 10.1016/j.bmcl.2017.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana Y.; Kikuzaki H.; Lajis N. H.; Nakatani N. Antioxidative Activity of Carbazoles from Murraya Koenigii Leaves. J. Agric. Food Chem. 2001, 49, 5589–5594. 10.1021/jf010621r. [DOI] [PubMed] [Google Scholar]
- Bandgar B. P.; Adsul L. K.; Chavan H. V.; Jalde S. S.; Shringare S. N.; Shaikh R.; Meshram R. J.; Gacche R. N.; Masand V. Synthesis, Biological Evaluation, and Docking Studies of 3-(Substituted)-Aryl-5-(9-Methyl-3-Carbazole)-1H-2-Pyrazolines as Potent Anti-Inflammatory and Antioxidant Agents. Bioorg. Med. Chem. Lett. 2012, 22, 5839–5844. 10.1016/j.bmcl.2012.07.080. [DOI] [PubMed] [Google Scholar]
- Caruso A.; Ceramella J.; Iacopetta D.; Saturnino C.; Mauro M. V.; Bruno R.; Aquaro S.; Sinicropi M. S. Carbazole Derivatives as Antiviral Agents: An Overview. Molecules 2019, 24, 1912. 10.3390/molecules24101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D. Y.; Chan Y. Y.; Hwang T. L.; Juang S. H.; Huang S. C.; Kuo P. C.; Thang T. D.; Lee E. J.; Damu A. G.; Wu T. S. Constituents of the Roots of Clausena Lansium and Their Potential Anti-Inflammatory Activity. J. Nat. Prod. 2014, 77, 1215–1223. 10.1021/np500088u. [DOI] [PubMed] [Google Scholar]
- Börger C.; Brütting C.; Julich-Gruner K. K.; Hesse R.; Kumar V. P.; Kutz S. K.; Rönnefahrt M.; Thomas C.; Wan B.; Franzblau S. G.; et al. Anti-Tuberculosis Activity and Structure-Activity Relationships of Oxygenated Tricyclic Carbazole Alkaloids and Synthetic Derivatives. Bioorg. Med. Chem. 2017, 25, 6167–6174. 10.1016/j.bmc.2016.12.038. [DOI] [PubMed] [Google Scholar]
- Xie Y. P.; Sangaraiah N.; Meng J. P.; Zhou C. H. Unique Carbazole-Oxadiazole Derivatives as New Potential Antibiotics for Combating Gram-Positive and -Negative Bacteria. J. Med. Chem. 2022, 65, 6171–6190. 10.1021/acs.jmedchem.2c00001. [DOI] [PubMed] [Google Scholar]
- The Privileged Pincer-Metal Platform: Coordination Chemistry & Applications; Van Koten G., Gossage R. A., Eds.; Springer International Publishing, 2015, 10.1007/978-3-319-22927-0. [DOI] [Google Scholar]
- Morales-Morales D.The Chemistry of PCP Pincer Phosphinite Transition Metal Complexes. In The Chemistry of Pincer Compounds; Elsevier: Amsterdam, 2007; pp 151–179, 10.1016/B978-044453138-4/50010-6. [DOI] [Google Scholar]
- Albrecht M.; Lindner M. M. Cleavage of Unreactive Bonds with Pincer Metal Complexes. Dalton Trans. 2011, 40, 8733–8744. 10.1039/c1dt10339c. [DOI] [PubMed] [Google Scholar]
- Choi J.; MacArthur A. H. R.; Brookhart M.; Goldman A. S. Dehydrogenation and Related Reactions Catalyzed by Iridium Pincer Complexes. Chem. Rev. 2011, 111, 1761–1779. 10.1021/cr1003503. [DOI] [PubMed] [Google Scholar]
- Murugesan S.; Kirchner K. Non-Precious Metal Complexes with an Anionic PCP Pincer Architecture. Dalton Trans. 2016, 45, 416–439. 10.1039/C5DT03778F. [DOI] [PubMed] [Google Scholar]
- Alig L.; Fritz M.; Schneider S. First-Row Transition Metal (De) Hydrogenation Catalysis Based on Functional Pincer Ligands. Chem. Rev. 2019, 119, 2681–2751. 10.1021/acs.chemrev.8b00555. [DOI] [PubMed] [Google Scholar]
- Annibale V. T.; Song D. Multidentate Actor Ligands as Versatile Platforms for Small Molecule Activation and Catalysis. RSC Adv. 2013, 3, 11432–11449. 10.1039/c3ra40618k. [DOI] [Google Scholar]
- Mata J. A.; Poyatos M.; Peris E. Structural and Catalytic Properties of Chelating Bis- and Tris-N-Heterocyclic Carbenes. Coord. Chem. Rev. 2007, 251, 841–859. 10.1016/j.ccr.2006.06.008. [DOI] [Google Scholar]
- Pugh D.; Danopoulos A. A. Metal Complexes With ’Pincer’-Type Ligands Incorporating N-Heterocyclic Carbene Functionalities. Coord. Chem. Rev. 2007, 251, 610–641. 10.1016/j.ccr.2006.08.001. [DOI] [Google Scholar]
- Valdés H.; García-Eleno M. A.; Canseco-Gonzalez D.; Morales-Morales D. Recent Advances in Catalysis with Transition-Metal Pincer Compounds. ChemCatChem. 2018, 10, 3136–3172. 10.1002/cctc.201702019. [DOI] [Google Scholar]
- Peris E.; Crabtree R. H. Key Factors in Pincer Ligand Design. Chem. Soc. Rev. 2018, 47, 1959–1968. 10.1039/C7CS00693D. [DOI] [PubMed] [Google Scholar]
- Asay M.; Morales-Morales D. Non-Symmetric Pincer Ligands: Complexes and Applications in Catalysis. Dalton Trans. 2015, 44, 17432–17447. 10.1039/C5DT02295A. [DOI] [PubMed] [Google Scholar]
- Benito-Garagorri D.; Kirchner K. Modularly Designed Transition Metal PNP and PCP Pincer Complexes Based on Aminophosphines: Synthesis and Catalytic Applications. Acc. Chem. Res. 2008, 41, 201–213. 10.1021/ar700129q. [DOI] [PubMed] [Google Scholar]
- Abbenseth J.; Goicoechea J. M. Recent Developments in the Chemistry of Non-Trigonal Pnictogen Pincer Compounds: From Bonding to Catalysis. Chem. Sci. 2020, 11, 9728–9740. 10.1039/D0SC03819A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishna M. S. Unusual and Rare Pincer Ligands: Synthesis, Metallation, Reactivity and Catalytic Studies. Polyhedron 2018, 143, 2–10. 10.1016/j.poly.2017.07.026. [DOI] [Google Scholar]
- Kumar A.; Gao C. Homogeneous (De) Hydrogenative Catalysis for Circular Chemistry - Using Waste as a Resource. ChemCatChem. 2021, 13, 1105–1134. 10.1002/cctc.202001404. [DOI] [Google Scholar]
- Li H.; Zheng B.; Huang K. W. A New Class of PN3-Pincer Ligands for Metal-Ligand Cooperative Catalysis. Coord. Chem. Rev. 2015, 293–294, 116–138. 10.1016/j.ccr.2014.11.010. [DOI] [Google Scholar]
- Van Der Vlugt J. I. Cooperative Catalysis with First-Row Late Transition Metals. Eur. J. Inorg. Chem. 2012, 2012, 363–375. 10.1002/ejic.201100752. [DOI] [Google Scholar]
- Peris E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. 10.1021/acs.chemrev.6b00695. [DOI] [PubMed] [Google Scholar]
- Bezuidenhout D. I.; Kleinhans G.; Karhu A. J.. Nonclassical Carbenes as Noninnocent Ligands. In Synthesis, Structure, and Bonding in Inorganic Molecular Systems; Laitinen, R. S.; in Comprehensive Inorganic Chemistry III; Reedijk J.; Poeppelmeier K. R., Eds.; Oxford: Elsevier, 2023; pp 234–314.. [Google Scholar]
- Luca O. R.; Crabtree R. H. Redox-Active Ligands in Catalysis. Chem. Soc. Rev. 2013, 42, 1440–1459. 10.1039/C2CS35228A. [DOI] [PubMed] [Google Scholar]
- Singh K.; Kundu A.; Adhikari D. Ligand-Based Redox: Catalytic Applications and Mechanistic Aspects. ACS Catal. 2022, 12, 13075–13107. 10.1021/acscatal.2c02655. [DOI] [Google Scholar]
- Van der Vlugt J. I. Radical-Type Reactivity and Catalysis by Single-Electron Transfer to or from Redox-Active Ligands. Chem.—Eur. J. 2019, 25, 2651–2662. 10.1002/chem.201802606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broere D. L. J.; Plessius R.; Van der Vlugt J. I. New Avenues for Ligand-Mediated Processes-Expanding Metal Reactivity by the Use of Redox-Active Catechol, o-Aminophenol and o-Phenylenediamine Ligands. Chem. Soc. Rev. 2015, 44, 6886–6915. 10.1039/C5CS00161G. [DOI] [PubMed] [Google Scholar]
- Dzik W. I.; Zhang X. P.; De Bruin B. Redox Noninnocence of Carbene Ligands: Carbene Radicals in (Catalytic) C-C Bond Formation. Inorg. Chem. 2011, 50, 9896–9903. 10.1021/ic200043a. [DOI] [PubMed] [Google Scholar]
- Allgeier A. M.; Mirkin C. A. Ligand Design for Electrochemically Controlling Stoichiometric and Catalytic Reactivity of Transition Metals. Angew. Chem., Int. Ed. 1998, 37, 894–908. . [DOI] [PubMed] [Google Scholar]
- Van Der Vlugt J. I.; Reek J. N. H. Neutral Tridentate PNP Ligands and Their Hybrid Analogues: Versatile Non-Innocent Scaffolds for Homogeneous Catalysis. Angew. Chem., Int. Ed. 2009, 48, 8832–8846. 10.1002/anie.200903193. [DOI] [PubMed] [Google Scholar]
- Li H.; Gonçalves T. P.; Lupp D.; Huang K. W. PN3(P)-Pincer Complexes: Cooperative Catalysis and Beyond. ACS Catal. 2019, 9, 1619–1629. 10.1021/acscatal.8b04495. [DOI] [Google Scholar]
- Gunanathan C.; Milstein D. Bond Activation and Catalysis by Ruthenium Pincer Complexes. Chem. Rev. 2014, 114, 12024–12087. 10.1021/cr5002782. [DOI] [PubMed] [Google Scholar]
- Khusnutdinova J. R.; Milstein D. Metal-Ligand Cooperation. Angew. Chem., Int. Ed. 2015, 54, 12236–12273. 10.1002/anie.201503873. [DOI] [PubMed] [Google Scholar]
- Gunanathan C.; Milstein D. Metal-Ligand Cooperation by Aromatization-Dearomatization: A New Paradigm in Bond Activation and “Green” Catalysis. Acc. Chem. Res. 2011, 44, 588–602. 10.1021/ar2000265. [DOI] [PubMed] [Google Scholar]
- Shimbayashi T.; Fujita K. I. Recent Advances in Homogeneous Catalysis via Metal-Ligand Cooperation Involving Aromatization and Dearomatization. Catalysts 2020, 10, 635. 10.3390/catal10060635. [DOI] [Google Scholar]
- Milstein D. Discovery of Environmentally Benign Catalytic Reactions of Alcohols Catalyzed by Pyridine-Based Pincer Ru Complexes, Based on Metal-Ligand Cooperation. Top. Catal. 2010, 53, 915–923. 10.1007/s11244-010-9523-7. [DOI] [Google Scholar]
- Gonçalves T. P.; Dutta I.; Huang K. W. Aromaticity in Catalysis: Metal Ligand Cooperation via Ligand Dearomatization and Rearomatization. Chem. Commun. 2021, 57, 3070–3082. 10.1039/D1CC00528F. [DOI] [PubMed] [Google Scholar]
- Feichtner K. S.; Gessner V. H. Cooperative Bond Activation Reactions with Carbene Complexes. Chem. Commun. 2018, 54, 6540–6553. 10.1039/C8CC02198H. [DOI] [PubMed] [Google Scholar]
- Kuwata S.; Ikariya T. Metal-Ligand Bifunctional Reactivity and Catalysis of Protic N-Heterocyclic Carbene and Pyrazole Complexes Featuring β-NH Units. Chem. Commun. 2014, 50, 14290–14300. 10.1039/C4CC04457F. [DOI] [PubMed] [Google Scholar]
- McManus H. A.; Guiry P. J. Recent Developments in the Application of Oxazoline-Containing Ligands in Asymmetric Catalysis. Chem. Rev. 2004, 104, 4151–4202. 10.1021/cr040642v. [DOI] [PubMed] [Google Scholar]
- Hargaden G. C.; Guiry P. J. The Development of the Asymmetric Nozaki-Hiyama-Kishi Reaction. Adv. Synth. Catal. 2007, 349, 2407–2424. 10.1002/adsc.200700324. [DOI] [Google Scholar]
- Wang H.; Wen J.; Zhang X. Chiral Tridentate Ligands in Transition Metal-Catalyzed Asymmetric Hydrogenation. Chem. Rev. 2021, 121, 7530–7567. 10.1021/acs.chemrev.1c00075. [DOI] [PubMed] [Google Scholar]
- Janssen-Müller D.; Schlepphorst C.; Glorius F. Privileged Chiral N-Heterocyclic Carbene Ligands for Asymmetric Transition-Metal Catalysis. Chem. Soc. Rev. 2017, 46, 4845–4854. 10.1039/C7CS00200A. [DOI] [PubMed] [Google Scholar]
- Fliedel C.; Labande A.; Manoury E.; Poli R. Chiral N-Heterocyclic Carbene Ligands with Additional Chelating Group(s) Applied to Homogeneous Metal-Mediated Asymmetric Catalysis. Coord. Chem. Rev. 2019, 394, 65–103. 10.1016/j.ccr.2019.05.003. [DOI] [Google Scholar]
- César V.; Bellemin-Laponnaz S.; Gade L. H. Chiral N-Heterocyclic Carbenes as Stereodirecting Ligands in Asymmetric Catalysis. Chem. Soc. Rev. 2004, 33, 619–636. 10.1039/B406802P. [DOI] [PubMed] [Google Scholar]
- Gade L. H.; Bellemin-Laponnaz S.. Chiral N-Heterocyclic Carbenes as Stereodirecting Ligands in Asymmetric Catalysis. N-Heterocyclic Carbenes in Transition Metal Catalysis. Topics in Organometallic Chemistry; Glorius F., Ed.; Topics in Organometallic Chemistry; Springer: Berlin, 2006; pp 117–157, 10.1007/3418_028. [DOI] [Google Scholar]
- Perry M. C.; Burgess K. Chiral N-Heterocyclic Carbene-Transition Metal Complexes in Asymmetric Catalysis. Tetrahedron: Asymmetry 2003, 14, 951–961. 10.1016/S0957-4166(03)00037-5. [DOI] [Google Scholar]
- Wang F.; Liu L.-J.; Wang W.; Li S.; Shi M. Chiral NHC-Metal-Based Asymmetric Catalysis. Coord. Chem. Rev. 2012, 256, 804–853. 10.1016/j.ccr.2011.11.013. [DOI] [Google Scholar]
- Zhong R.; Lindhorst A. C.; Groche F. J.; Kühn F. E. Immobilization of N-Heterocyclic Carbene Compounds: A Synthetic Perspective. Chem. Rev. 2017, 117, 1970–2058. 10.1021/acs.chemrev.6b00631. [DOI] [PubMed] [Google Scholar]
- Ranganath K. V. S.; Onitsuka S.; Kumar A. K.; Inanaga J. Recent Progress of N-Heterocyclic Carbenes in Heterogeneous Catalysis. Catal. Sci. Technol. 2013, 3, 2161–2181. 10.1039/c3cy00118k. [DOI] [Google Scholar]
- Román-Martínez M. C.; Salinas-Martínez de Lecea C.. Heterogenization of Homogeneous Catalysts on Carbon Materials. In New and Future Developments in Catalysis: Hybrid Materials, Composites, and Organocatalysts; Steven S. L., Ed.; Elsevier, 2013; pp 55–78, 10.1016/B978-0-444-53876-5.00003-9. [DOI] [Google Scholar]
- Cazin C. S. J. Recent Advances in the Design and Use of Immobilised N-Heterocyclic Carbene Ligands for Transition-Metal Catalysis. C R Chim. 2009, 12, 1173–1180. 10.1016/j.crci.2008.10.016. [DOI] [Google Scholar]
- Esfandiari M.; Havaei G.; Zahiri S.; Mohammadnezhad G. Pincer Complex Immobilization onto Different Supports: Strategies and Applications. Coord. Chem. Rev. 2022, 472, 214778. 10.1016/j.ccr.2022.214778. [DOI] [Google Scholar]
- Chapple P. M.; Kahlal S.; Cartron J.; Roisnel T.; Dorcet V.; Cordier M.; Saillard J. Y.; Carpentier J. F.; Sarazin Y. Bis(Imino) Carbazolate: A Master Key for Barium Chemistry. Angew. Chem., Int. Ed. 2020, 59, 9120–9126. 10.1002/anie.202001439. [DOI] [PubMed] [Google Scholar]
- Nolla-Saltiel R.; Geer A. M.; Lewis W.; Blake A. J.; Kays D. L. Dehydrogenation of Dimethylamine-Borane Mediated by Group 1 Pincer Complexes. Chem. Commun. 2018, 54, 1825–1828. 10.1039/C7CC08385H. [DOI] [PubMed] [Google Scholar]
- Nolla-Saltiel R.; Geer A. M.; Taylor L. J.; Churchill O.; Davies E. S.; Lewis W.; Blake A. J.; Kays D. L. Hydrophosphination of Activated Alkenes by a Cobalt(I) Pincer Complex. Adv. Synth. Catal. 2020, 362, 3148–3157. 10.1002/adsc.202000514. [DOI] [Google Scholar]
- Hill M. S.; Liptrot D. J.; Weetman C. Alkaline Earths as Main Group Reagents in Molecular Catalysis. Chem. Soc. Rev. 2016, 45, 972–988. 10.1039/C5CS00880H. [DOI] [PubMed] [Google Scholar]
- Avent A. G.; Crimmin M. R.; Hill M. S.; Hitchcock P. B. Kinetic Stability of Heteroleptic (β-Diketiminato) Heavier Alkaline-Earth (Ca, Sr, Ba) Amides. Dalton Trans. 2005, (2), 278–284. 10.1039/B415468A. [DOI] [PubMed] [Google Scholar]
- Westerhausen M.; Koch A.; Görls H.; Krieck S. Heavy Grignard Reagents: Synthesis, Physical and Structural Properties, Chemical Behavior, and Reactivity. Chem.—Eur. J. 2017, 23, 1456–1483. 10.1002/chem.201603786. [DOI] [PubMed] [Google Scholar]
- Sarazin Y.; Carpentier J. F. Calcium, Strontium and Barium Homogeneous Catalysts for Fine Chemicals Synthesis. Chem. Rec. 2016, 16, 2482–2505. 10.1002/tcr.201600067. [DOI] [PubMed] [Google Scholar]
- Chapple P. M.; Cordier M.; Dorcet V.; Roisnel T.; Carpentier J. F.; Sarazin Y. A Versatile Nitrogen Ligand for Alkaline-Earth Chemistry. Dalton Trans. 2020, 49, 11878–11889. 10.1039/D0DT02236E. [DOI] [PubMed] [Google Scholar]
- Chapple P. M.; Roisnel T.; Cordier M.; Carpentier J. F.; Sarazin Y. Heteroleptic Carbazolato-Barium Hydroborates and a Related Separated Ion Pair. Polyhedron 2022, 217, 115731. 10.1016/j.poly.2022.115731. [DOI] [Google Scholar]
- Chapple P. M.; Hamdoun G.; Roisnel T.; Carpentier J. F.; Oulyadi H.; Sarazin Y. Bis(Imino) Carbazolate Lead(II) Fluoride and Related Halides. Dalton Trans. 2021, 50, 9021–9025. 10.1039/D1DT01615F. [DOI] [PubMed] [Google Scholar]
- Jana A.; Sarish S. P.; Roesky H. W.; Leusser D.; Objartel I.; Stalke D. Pentafluoropyridine as a Fluorinating Reagent for Preparing a Hydrocarbon Soluble β-Diketiminatolead(Ii) Monofluoride. Chem. Commun. 2011, 47, 5434–5436. 10.1039/C1CC11310K. [DOI] [PubMed] [Google Scholar]
- Jana A.; Sarish S. P.; Roesky H. W.; Schulzke C.; Doring A.; John M. Facile Access of Well-Defined Stable Divalent Lead Compounds with Small Organic Substituents. Organometallics 2009, 28, 2563–2567. 10.1021/om801167c. [DOI] [Google Scholar]
- Murso A.; Straka M.; Kaupp M.; Bertermann R.; Stalke D. A Heterotopically Chelated Low-Valent Lead Amide. Organometallics 2005, 24, 3576–3578. 10.1021/om0501677. [DOI] [Google Scholar]
- Pu L.; Twamley B.; Power P. P. Terphenyl Ligand Stabilized Lead(II) Derivatives of Simple Organic Groups: Characterization of Pb(R) C6H3–2,6-Trip2 (R = Me, t-Bu, or Ph; Trip = C6H2–2,4,6-i-Pr3), {Pb(μ-Br) C6H3–2,6-Trip2}2, Py·Pb(Br) C6H3–2,6-Trip2 (Py = Pyridine), and the Bridged Plumbyly. Organometallics 2000, 19, 2874–2881. 10.1021/om0001624. [DOI] [Google Scholar]
- Kaupp M.; Schleyer P. V. R. Ab Initio Study of Structures and Stabilities of Substituted Lead Compounds. Why Is Inorganic Lead Chemistry Dominated by PbII but Organolead Chemistry by PbIV?. J. Am. Chem. Soc. 1993, 115, 1061–1073. 10.1021/ja00056a034. [DOI] [Google Scholar]
- Tokitoh N.; Mizuhata Y.. Low-Coordinate Main Group Compounds - Group 14 (Sn, Pb). In Comprehensive Inorganic Chemistry II: From Elements to Applications; Reedijk J.; Poeppelmeier K., Eds.; Elsevier Ltd, 2013; Vol. 1, pp 579–585, 10.1016/B978-0-08-097774-4.00150-9. [DOI] [Google Scholar]
- Jana A.; Roesky H. W.; Schulzke C.; Samuel P. P.; Döring A. Synthesis and Reaction of Monomeric Germanium(II) and Lead(II) Dimethylamide and the Synthesis of Germanium(II) Hydrazide by Clevage of One N-H Bond of Hydrazine. Inorg. Chem. 2010, 49, 5554–5559. 10.1021/ic1003808. [DOI] [PubMed] [Google Scholar]
- Asay M.; Jones C.; Driess M. N-Heterocyclic Carbene Analogues with Low-Valent Group 13 and Group 14 Elements: Syntheses, Structures, and Reactivities of a New Generation of Multitalented Ligands. Chem. Rev. 2011, 111, 354–396. 10.1021/cr100216y. [DOI] [PubMed] [Google Scholar]
- Wang S.; Liu K. Y.; Li H. J.; Lee W. C.; Huang S. L.; Wu W. C.; Shi F. K.; Cheng Y. S.; Lu I. C.; Liu H. J. Access to Monomeric Lead(II) Hydrides with Remarkable Thermostability and Their Use in Catalytic Hydroboration of Carbonyl Derivatives. Inorg. Chem. 2022, 61, 13096–13103. 10.1021/acs.inorgchem.2c01658. [DOI] [PubMed] [Google Scholar]
- Fang H.; Wang Z.; Fu X. Beyond Carbocations: Synthesis, Structure and Reactivity of Heavier Group 14 Element Cations. Coord. Chem. Rev. 2017, 344, 214–237. 10.1016/j.ccr.2016.11.017. [DOI] [Google Scholar]
- van Koten G. Tuning the Reactivity of Metals Held in a Rigid Ligand Environment. Pure Appl. Chem. 1989, 61, 1681–1694. 10.1351/pac198961101681. [DOI] [Google Scholar]
- Jordan R.; Kunz D. The Fascinating Flexibility and Coordination Modes of a Pentamethylene Connected Macrocyclic CNC Pincer Ligand. Molecules 2021, 26, 1669. 10.3390/molecules26061669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüger N.; Wadepohl H.; Gade L. H. Iridium Complexes Containing a PNP Pincer Ligand: Syntheses, Structural Chemistry, and Bond Activations. Eur. J. Inorg. Chem. 2013, 2013, 5358–5365. 10.1002/ejic.201300857. [DOI] [Google Scholar]
- Britovsek G. J. P.; Gibson V. C.; Hoarau O. D.; Spitzmesser S. K.; White A. J. P.; Williams D. J. Iron and Cobalt Ethylene Polymerization Catalysts: Variations on the Central Donor. Inorg. Chem. 2003, 42, 3454–3465. 10.1021/ic034040q. [DOI] [PubMed] [Google Scholar]
- Kanemasa S.; Oderaotoshi Y.; Yamamoto H.; Tanaka J.; Wada E.; Curran D. P. Cationic Aqua Complexes of the C2-Symmetric Trans-Chelating Ligand (R,R)-4,6-Dibenzofurandiyl-2,2′-Bis(4- Phenyloxazoline). Absolute Chiral Induction in Diels-Alder Reactions Catalyzed by Water-Tolerant Enantiopure Lewis Acids. J. Org. Chem. 1997, 62, 6454–6455. 10.1021/jo970906w. [DOI] [Google Scholar]
- Adams G. M.; Weller A. S. POP-Type Ligands: Variable Coordination and Hemilabile Behaviour. Coord. Chem. Rev. 2018, 355, 150–172. 10.1016/j.ccr.2017.08.004. [DOI] [Google Scholar]
- Gaunt J. A.; Gibson V. C.; Haynes A.; Spitzmesser S. K.; White A. J. P.; Williams D. J. Bis(Imino) Carbazolide Complexes of Rhodium: Highly Nucleophilic Ligands Exerting a Dramatic Accelerating Effect on MeI Oxidative Addition. Organometallics 2004, 23, 1015–1023. 10.1021/om034309d. [DOI] [Google Scholar]
- Moser M.; Wucher B.; Kunz D.; Rominger F. 1,8-Bis(Imidazolin-2-Yliden-1-Yl) Carbazolide (Bimca): A New CNC Pincer-Type Ligand with Strong Electron-Donating Properties. Facile Oxidative Addition of Methyl Iodide to Rh(Bimca)(CO). Organometallics 2007, 26, 1024–1030. 10.1021/om060952z. [DOI] [Google Scholar]
- Haynes A.; Mann B. E.; Maitlis P. M.; Morris G. E. Mechanistic Studies on Rhodium-Catalyzed Carbonylation Reactions: Spectroscopic Detection and Reactivity of a Key Intermediate, [MeRh(CO)2I3]–. J. Am. Chem. Soc. 1993, 115, 4093–4100. 10.1021/ja00063a030. [DOI] [Google Scholar]
- Gonsalvi L.; Gaunt J. A.; Adams H.; Castro A.; Sunley G. J.; Haynes A. Quantifying Steric Effects of α-Diimine Ligands. Oxidative Addition of MeI to Rhodium(I) and Migratory Insertion in Rhodium(III) Complexes. Organometallics 2003, 22, 1047–1054. 10.1021/om020777w. [DOI] [Google Scholar]
- Gonsalvi L.; Adams H.; Sunley G. J.; Ditzel E.; Haynes A. A Dramatic Steric Effect on the Rate of Migratory CO Insertion on Rhodium Ligand Steric and Electronic Effects Play a Key Role in Determining Organometallic Reactivity Trends and Catalytic Behav- Ior. For Monodentate Ligands, a Number of Quantitative Pa. J. Am. Chem. Soc. 1999, 121, 11233–11234. 10.1021/ja992891k. [DOI] [Google Scholar]
- Gonsalvi L.; Adams H.; Sunley G. J.; Ditzel E.; Haynes A. Steric and Electronic Effects on the Reactivity of Rh and Ir Complexes Containing P-S, P-P, and P-O Ligands. Implications for the Effects of Chelate Ligands in Catalysis. J. Am. Chem. Soc. 2002, 124, 13597–13612. 10.1021/ja0176191. [DOI] [PubMed] [Google Scholar]
- Rankin J.; Benyei A. C.; Poole D.; Cole-hamilton D. J. Joanne Rankin 1999, 2, 3771–3782. 10.1039/a905308e. [DOI] [Google Scholar]
- Gupta M.; Hagen C.; Flesher R. J.; Kaska W. C.; Jensen C. M. A Highly Active Alkane Dehydrogenation Catalyst: Stabilization Of Dihydrido Rhodium And Iridium Complexes By A P-C-P Pincer Ligand. Chem. Commun. 1996, 17, 2083–2084. 10.1039/CC9960002083. [DOI] [Google Scholar]
- Wang D. Y.; Choliy Y.; Krogh-Jespersen K.; Hartwig J. F.; Goldman A. S.. Abstracts of Papers. In Proceedings of the 240th ACS National Meeting, ACS, Boston, MA, 2010; p INOR-5.
- Wang D. Y.; Krogh-Jespersen K.; Goldman A. S.. Abstracts of Papers. In Proceedings of the 244th ACS National Meeting and Exposition, Philadelphia, PA; ACS: Washington DC, 2012; p INOR-587.
- Cheng C.; Kim B. G.; Guironnet D.; Brookhart M.; Guan C.; Wang D. Y.; Krogh-Jespersen K.; Goldman A. S. Synthesis and Characterization of Carbazolide-Based Iridium PNP Pincer Complexes. Mechanistic and Computational Investigation of Alkene Hydrogenation: Evidence for an Ir(III)/Ir(V)/Ir(III) Catalytic Cycle. J. Am. Chem. Soc. 2014, 136, 6672–6683. 10.1021/ja501572g. [DOI] [PubMed] [Google Scholar]
- Ozerov O. V.; Guo C.; Papkov V. A.; Foxman B. M. Facile Oxidative Addition of N-C and N-H Bonds to Monovalent Rhodium and Iridium. J. Am. Chem. Soc. 2004, 126, 4792–4793. 10.1021/ja049659l. [DOI] [PubMed] [Google Scholar]
- Weng W.; Guo C.; Moura C.; Yang L.; Foxman B. M.; Ozerov O. V. Competitive Activation of N-C and C-H Bonds of the PNP Framework by Monovalent Rhodium and Iridium. Organometallics 2005, 24, 3487–3499. 10.1021/om050346o. [DOI] [Google Scholar]
- Gatard S.; Çelenligil-Çetin R.; Guo C.; Foxman B. M.; Ozerov O. V. Carbon-Halide Oxidative Addition and Carbon-Carbon Reductive Elimination at a (PNP) Rh Center. J. Am. Chem. Soc. 2006, 128, 2808–2809. 10.1021/ja057948j. [DOI] [PubMed] [Google Scholar]
- Weng W.; Guo C.; Çelenligil-Çetin R.; Foxman B. M.; Ozerov O. V. Skeletal Change in the PNP Pincer Ligand Leads to a Highly Regioselective Alkyne Dimerization Catalyst. Chem. Commun. 2006, (2), 197–199. 10.1039/B511148J. [DOI] [PubMed] [Google Scholar]
- Grüger N.; Rodríguez L. I.; Wadepohl H.; Gade L. H. Achiral and Chiral PNP-Pincer Ligands with a Carbazole Backbone: Coordination Chemistry with D8 Transition Metals. Inorg. Chem. 2013, 52, 2050–2059. 10.1021/ic302454n. [DOI] [PubMed] [Google Scholar]
- Rossi A. R.; Hoffmann R. Transition Metal Pentacoordination. Inorg. Chem. 1975, 14, 365–374. 10.1021/ic50144a032. [DOI] [Google Scholar]
- Fan L.; Parkin S.; Ozerov O. V. Halobenzenes and Ir(I): Kinetic C-H Oxidative Addition and Thermodynamic C-Hal Oxidative Addition. J. Am. Chem. Soc. 2005, 127, 16772–16773. 10.1021/ja0557637. [DOI] [PubMed] [Google Scholar]
- Gupta M.; Hagen C.; Kaska W. C.; Cramer R. E.; Jensen C. M. Catalytic Dehydrogenation of Cycloalkanes to Arenes by a Dihydrido Iridium P-C-P Pincer Complex. J. Am. Chem. Soc. 1997, 119, 840–841. 10.1021/ja962560x. [DOI] [Google Scholar]
- Bézier D.; Guan C.; Krogh-Jespersen K.; Goldman A. S.; Brookhart M. Experimental and Computational Study of Alkane Dehydrogenation Catalyzed by a Carbazolide-Based Rhodium PNP Pincer Complex. Chem. Sci. 2016, 7, 2579–2586. 10.1039/C5SC04794C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Y.; Choliy Y.; Haibach M. C.; Hartwig J. F.; Krogh-Jespersen K.; Goldman A. S. Assessment of the Electronic Factors Determining the Thermodynamics of “Oxidative Addition” of C-H and N-H Bonds to Ir(I) Complexes. J. Am. Chem. Soc. 2016, 138, 149–163. 10.1021/jacs.5b09522. [DOI] [PubMed] [Google Scholar]
- Xu W.-W.; Rosini G. P.; Gupta M.; Jensen C. M.; Kaska W. C.; Krogh-Jespersen K.; Goldman A. S. Thermochemical Alkane Dehydrogenation Catalyzed In Solution Without The Use Of A Hydrogen Acceptor. Chem. Commun. 1997, 1997, 2273–2274. 10.1039/a705105k. [DOI] [Google Scholar]
- Collman J. P. Patterns Of Organometallic Reactions Related To Homogeneous Catalysis. Acc. Chem. Res. 1968, 1, 136–143. 10.1021/ar50005a002. [DOI] [Google Scholar]
- Higuchi J.; Kuriyama S.; Eizawa A.; Arashiba K.; Nakajima K.; Nishibayashi Y. Preparation and Reactivity of Iron Complexes Bearing Anionic Carbazole-Based PNP-Type Pincer Ligands Toward Catalytic Nitrogen Fixation. Dalton Trans. 2018, 47, 1117–1121. 10.1039/C7DT04327A. [DOI] [PubMed] [Google Scholar]
- Yang L.; Powell D. R.; Houser R. P. Structural Variation In Copper(I) Complexes With Pyridylmethylamide Ligands: Structural Analysis With A New Four-Coordinate Geometry Index, Τ4. Dalton Trans. 2007, (9), 955–964. 10.1039/B617136B. [DOI] [PubMed] [Google Scholar]
- Kuriyama S.; Arashiba K.; Nakajima K.; Matsuo Y.; Tanaka H.; Ishii K.; Yoshizawa K.; Nishibayashi Y. Catalytic Transformation Of Dinitrogen Into Ammonia And Hydrazine By Iron-Dinitrogen Complexes Bearing Pincer Ligand. Nat. Commun. 2016, 7, 12181. 10.1038/ncomms12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J. C.; Bürgy D.; Guan H.; Gade L. H. 3d Metal Complexes in T-Shaped Geometry as a Gateway to Metalloradical Reactivity. Acc. Chem. Res. 2022, 55, 857–868. 10.1021/acs.accounts.1c00737. [DOI] [PubMed] [Google Scholar]
- Ott J. C.; Wadepohl H.; Enders M.; Gade L. H. Taking Solution Proton NMR to Its Extreme: Prediction and Detection of a Hydride Resonance in an Intermediate-Spin Iron Complex. J. Am. Chem. Soc. 2018, 140, 17413–17417. 10.1021/jacs.8b11330. [DOI] [PubMed] [Google Scholar]
- Ott J. C.; Blasius C. K.; Wadepohl H.; Gade L. H. Synthesis, Characterization, and Reactivity of a High-Spin Iron(II) Hydrido Complex Supported by a PNP Pincer Ligand and Its Application as a Homogenous Catalyst for the Hydrogenation of Alkenes. Inorg. Chem. 2018, 57, 3183–3191. 10.1021/acs.inorgchem.7b03227. [DOI] [PubMed] [Google Scholar]
- Ott J. C.; Wadepohl H.; Gade L. H. Opening up the Valence Shell: A T-Shaped Iron(I) Metalloradical and Its Potential for Atom Abstraction. Angew. Chem., Int. Ed. 2020, 59, 9448–9452. 10.1002/anie.202003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J. C.; Wadepohl H.; Gade L. H. Metalloradical Reactivity, Charge Transfer, and Atom Abstractions in a T-Shaped Iron(I) Complex. Inorg. Chem. 2021, 60, 3927–3938. 10.1021/acs.inorgchem.0c03724. [DOI] [PubMed] [Google Scholar]
- Chirik P. J. Iron- and Cobalt-Catalyzed Alkene Hydrogenation: Catalysis with Both Redox-Active and Strong Field Ligands. Acc. Chem. Res. 2015, 48, 1687–1695. 10.1021/acs.accounts.5b00134. [DOI] [PubMed] [Google Scholar]
- Fong H.; Moret M. E.; Lee Y.; Peters J. C. Heterolytic H2 Cleavage And Catalytic Hydrogenation By An Iron Metallaboratrane. Organometallics 2013, 32, 3053–3062. 10.1021/om400281v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kismartoni L. C.; Weitz E.; Cedeño D. L. Density Functional Study Of Fe(CO)3 And Fe(CO)3(L) With H2 And C2H4, Where L = H2 Or C2H4: Reactions Relevant To Olefin Hydrogenation. Organometallics 2005, 24, 4714–4720. 10.1021/om0499411. [DOI] [Google Scholar]
- Plundrich G. T.; Wadepohl H.; Gade L. H. Synthesis and Reactivity of Group 4 Metal Benzyl Complexes Supported by Carbazolide-Based PNP Pincer Ligands. Inorg. Chem. 2016, 55, 353–365. 10.1021/acs.inorgchem.5b02498. [DOI] [PubMed] [Google Scholar]
- Ott J. C.; Suturina E. A.; Kuprov I.; Nehrkorn J.; Schnegg A.; Enders M.; Gade L. H. Observability of Paramagnetic NMR Signals at over 10 000 Ppm Chemical Shifts. Angew. Chem., Int. Ed. 2021, 60, 22856–22864. 10.1002/anie.202107944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingleson M. J.; Fullmer B. C.; Buschhorn D. T.; Fan H.; Pink M.; Huffman J. C.; Caulton K. G. Influence Of The D-Electron Count On CO Binding By Three-Coordinate [(TBu2PCH2SiMe2)2N]Fe, -Co, And -Ni. Inorg. Chem. 2008, 47, 407–409. 10.1021/ic7023764. [DOI] [PubMed] [Google Scholar]
- MacLeod K. C.; Dimucci I. M.; Zovinka E. P.; McWilliams S. F.; Mercado B. Q.; Lancaster K. M.; Holland P. L. Masked Radicals: Iron Complexes of Trityl, Benzophenone, and Phenylacetylene. Organometallics 2019, 38, 4224–4232. 10.1021/acs.organomet.9b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeaux D.; Lajzerowicz-Bonneteau J.; Briere R.; Lemaire H.; Rassat A. Détermination Des Axes Propres et Des Valeurs Principales Du Tenseur g Dans Deux Radicaux Libres Nitroxydes Par Étude de Monocristaux. Org. Magn. Reson. 1973, 5, 47–52. 10.1002/mrc.1270050111. [DOI] [Google Scholar]
- Ott J. C.; Isak D.; Melder J. J.; Wadepohl H.; Gade L. H. Single or Paired? Structure and Reactivity of PNP-Chromium(II) Hydrides. Inorg. Chem. 2020, 59, 14526–14535. 10.1021/acs.inorgchem.0c02315. [DOI] [PubMed] [Google Scholar]
- Fryzuk M. D.; Leznoff D. B.; Rettig S. J.; Thompson R. C. Magnetic Exchange in Dinuclear Chromium(II) Complexes: Effect of Bridging Chlorides and Bridging Hydrides in Antiferromagnetic Coupling. Inorg. Chem. 1994, 33, 5528–5534. 10.1021/ic00102a029. [DOI] [Google Scholar]
- Rozenel S. S.; Chomitz W. A.; Arnold J. Chromium Complexes Supported by the Multidentate Monoanionic N2P2 Ligand: Reduction Chemistry and Reactivity with Ethylene. Organometallics 2009, 28, 6243–6253. 10.1021/om900695s. [DOI] [Google Scholar]
- Heintz R. A.; Haggerty B. S.; Rheingold A. L.; Theopold K. H.; Wan H. [{Cp*Cr(Μ3-H)}4]—a Paramagnetic Chromium Hydride with a Cubane Structure. Angew. Chem., Int. Ed. Engl. 1992, 31, 1077–1079. 10.1002/anie.199210771. [DOI] [Google Scholar]
- Heintz R. A.; Koetzle T. F.; Ostrander R. L.; Rheingold A. L.; Theopold K. H.; Wu P. Unusually Strong Intramolecular Magnetic Coupling in a Chromium Hydride Cluster. Nature 1995, 378, 359–362. 10.1038/378359a0. [DOI] [Google Scholar]
- Hung Y. T.; Yap G. P. A.; Theopold K. H. Unexpected Reactions of Chromium Hydrides with a Diazoalkane. Polyhedron 2019, 157, 381–388. 10.1016/j.poly.2018.10.030. [DOI] [Google Scholar]
- MacAdams L. A.; Buffone G. P.; Incarvito C. D.; Golen J. A.; Rheingold A. L.; Theopold K. H. A Stable Alkyl Hydride of a First Row Transition Metal. Chem. Commun. 2003, 3, 1164–1165. 10.1039/b301943h. [DOI] [PubMed] [Google Scholar]
- Seyboldt A.; Wucher B.; Hohnstein S.; Eichele K.; Rominger F.; Törnroos K. W.; Kunz D. Evidence for the Formation of Anionic Zerovalent Group 10 Complexes as Highly Reactive Intermediates. Organometallics 2015, 34, 2717–2725. 10.1021/om500836m. [DOI] [Google Scholar]
- Kleinhans G.; Hansmann M. M.; Guisado-Barrios G.; Liles D. C.; Bertrand G.; Bezuidenhout D. I. Nucleophilic T-Shaped (LXL) Au(I)-Pincer Complexes: Protonation and Alkylation. J. Am. Chem. Soc. 2016, 138, 15873–15876. 10.1021/jacs.6b11359. [DOI] [PubMed] [Google Scholar]
- Poyatos M.; Mata J. A.; Peris E. Complexes with Poly(N-Heterocyclic Carbene) Ligands: Structural Features and Catalytic Applications. Chem. Rev. 2009, 109, 3677–3707. 10.1021/cr800501s. [DOI] [PubMed] [Google Scholar]
- Andrew R. E.; González-Sebastián L.; Chaplin A. B. NHC-Based Pincer Ligands: Carbenes with a Bite. Dalton Trans. 2016, 45, 1299–1305. 10.1039/C5DT04429D. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang B.; Guo S. Transition Metal Complexes Supported by N-Heterocyclic Carbene-Based Pincer Platforms: Synthesis, Reactivity and Applications. Eur. J. Inorg. Chem. 2021, 2021, 188–204. 10.1002/ejic.202000911. [DOI] [Google Scholar]
- Jürgens E.; Buys K. N.; Schmidt A. T.; Furfari S. K.; Cole M. L.; Moser M.; Rominger F.; Kunz D. Optimised Synthesis of Monoanionic Bis(NHC)-Pincer Ligand Precursors and Their Li-Complexes. New J. Chem. 2016, 40, 9160–9169. 10.1039/C6NJ01941B. [DOI] [Google Scholar]
- Hartmann D.; Wadepohl H.; Gade L. H. Synthesis and Structural Characterization of Group 10 Metal Complexes Bearing an Amidodiphosphine Pincer Ligand. Z. Anorg. Allg. Chem. 2018, 644, 1011–1017. 10.1002/zaac.201800173. [DOI] [Google Scholar]
- Jürgens E.; Back O.; Mayer J. J.; Heinze K.; Kunz D. Synthesis of Copper(II) and Gold(III) Bis(NHC)-Pincer Complexes. Z. Naturforsch. B 2016, 71, 1011–1018. 10.1515/znb-2016-0158. [DOI] [Google Scholar]
- Bezuidenhout D. I.; Kleinhans G.; Guisado-Barrios G.; Liles D. C.; Ung G.; Bertrand G. Isolation of a Potassium Bis(1,2,3-Triazol-5-Ylidene) Carbazolide: A Stabilizing Pincer Ligand for Reactive Late Transition Metal Complexes. Chem. Commun. 2014, 50, 2431–2433. 10.1039/c3cc49385g. [DOI] [PubMed] [Google Scholar]
- Vivancos A.; Segarra C.; Albrecht M. Mesoionic and Related Less Heteroatom-Stabilized N-Heterocyclic Carbene Complexes: Synthesis, Catalysis, and Other Applications. Chem. Rev. 2018, 118, 9493–9586. 10.1021/acs.chemrev.8b00148. [DOI] [PubMed] [Google Scholar]
- Guisado-Barrios G.; Soleilhavoup M.; Bertrand G. 1 H-1,2,3-Triazol-5-Ylidenes: Readily Available Mesoionic Carbenes. Acc. Chem. Res. 2018, 51, 3236–3244. 10.1021/acs.accounts.8b00480. [DOI] [PubMed] [Google Scholar]
- Kleinhans G.; Guisado-Barrios G.; Liles D. C.; Bertrand G.; Bezuidenhout D. I. A Rhodium(I)-Oxygen Adduct as a Selective Catalyst for One-Pot Sequential Alkyne Dimerization-Hydrothiolation Tandem Reactions. Chem. Commun. 2016, 52, 3504–3507. 10.1039/C6CC00029K. [DOI] [PubMed] [Google Scholar]
- Kleinhans G.; Chan A. K. W.; Leung M. Y.; Liles D. C.; Fernandes M. A.; Yam V. W. W.; Fernández I.; Bezuidenhout D. I. Synthesis and Photophysical Properties of T-Shaped Coinage-Metal Complexes. Chem.—Eur. J. 2020, 26, 6993–6998. 10.1002/chem.202000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerabek P.; Vondung L.; Schwerdtfeger P. Tipping the Balance Between Ligand and Metal Protonation Due to Relativistic Effects: Unusually High Proton Affinity in Gold(I) Pincer Complexes. Chem.—Eur. J. 2018, 24, 6047–6051. 10.1002/chem.201800755. [DOI] [PubMed] [Google Scholar]
- Joost M.; Zeineddine A.; Estévez L.; Mallet-Ladeira S.; Miqueu K.; Amgoune A.; Bourissou D. Facile Oxidative Addition of Aryl Iodides to Gold(I) by Ligand Design: Bending Turns on Reactivity. J. Am. Chem. Soc. 2014, 136, 14654–14657. 10.1021/ja506978c. [DOI] [PubMed] [Google Scholar]
- Rodriguez J.; Bourissou D. Chirale Gold(III)-Complexes: New Opportunities in Asymmetric Catalysis. Angew. Chem. 2018, 130, 392–394. 10.1002/ange.201710105. [DOI] [PubMed] [Google Scholar]
- Wu C. Y.; Horibe T.; Jacobsen C. B.; Toste F. D. Stable Gold(III) Catalysts by Oxidative Addition of a Carbon-Carbon Bond. Nature 2015, 517, 449–454. 10.1038/nature14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J.; Munz D.; Jazzar R.; Melaimi M.; Bertrand G. Synthesis of Hemilabile Cyclic (Alkyl)(Amino) Carbenes (CAACs) and Applications in Organometallic Chemistry. J. Am. Chem. Soc. 2016, 138, 7884–7887. 10.1021/jacs.6b05221. [DOI] [PubMed] [Google Scholar]
- O’Reilly M. E.; Ghiviriga I.; Abboud K. A.; Veige A. S. A New ONO 3-Trianionic Pincer-Type Ligand for Generating Highly Nucleophilic Metal-Carbon Multiple Bonds. J. Am. Chem. Soc. 2012, 134, 11185–11195. 10.1021/ja302222s. [DOI] [PubMed] [Google Scholar]
- Gonsales S. A.; Pascualini M. E.; Ghiviriga I.; Abboud K. A.; Veige A. S. Fast “Wittig-Like” Reactions As a Consequence of the Inorganic Enamine Effect. J. Am. Chem. Soc. 2015, 137, 4840–4845. 10.1021/jacs.5b01599. [DOI] [PubMed] [Google Scholar]
- Mandal U.; Venkatramani S.; Ghiviriga I.; Abboud K. A.; Veige A. S. Synthesis and Characterization of Tungsten Alkylidene and Alkylidyne Complexes Featuring a New Carbazole-Based Rigid Trianionic ONO3-Pincer-Type Ligand. Organometallics 2020, 39, 2207–2213. 10.1021/acs.organomet.0c00150. [DOI] [Google Scholar]
- de Miguel Y. R. Supported Catalysts and Their Applications in Synthetic Organic Chemistry. J. Chem. Soc., Perkin Trans. 1 2000, 2000, 4213–4221. 10.1039/a906850c. [DOI] [Google Scholar]
- Addison A. W.; Rao T. N.; Reedijk J.; van Rijn J.; Verschoor G. C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen-Sulphur Donor Ligands ; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2’-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc., Dalton Trans. 1984, 7, 1349–1356. 10.1039/DT9840001349. [DOI] [Google Scholar]
- O’Reilly M. E.; Ghiviriga I.; Abboud K. A.; Veige A. S. Unusually Stable Tungstenacyclobutadienes Featuring an ONO Trianionic Pincer-Type Ligand. Dalton Trans. 2013, 42, 3326–3336. 10.1039/c2dt32653a. [DOI] [PubMed] [Google Scholar]
- Spielvogel K. D.; Stumme N. C.; Fetrow T. V.; Wang L.; Luna J. A.; Keith J. M.; Shaw S. K.; Daly S. R. Quantifying Variations in Metal-Ligand Cooperative Binding Strength with Cyclic Voltammetry and Redox-Active Ligands. Inorg. Chem. 2022, 61, 2391–2401. 10.1021/acs.inorgchem.1c03014. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Rettenmeier C. A.; Plundrich G. T.; Wadepohl H.; Enders M.; Gade L. H. Borane-Bridged Ruthenium Complex Bearing a PNP Ligand: Synthesis and Structural Characterization. Organometallics 2015, 34, 5113–5118. 10.1021/acs.organomet.5b00699. [DOI] [Google Scholar]
- Ledger A. E. W.; Ellul C. E.; Mahon M. F.; Williams J. M. J.; Whittlesey M. K. Ruthenium Bidentate Phosphine Complexes For The Coordination And Catalytic Dehydrogenation Of Amine- And Phosphine-Boranes. Chem.—Eur. J. 2011, 17, 8704–8713. 10.1002/chem.201100101. [DOI] [PubMed] [Google Scholar]
- Tang C. Y.; Thompson A. L.; Aldridge S. Dehydrogenation Of Saturated CC And BN Bonds At Cationic N-Heterocyclic Carbene Stabilized M(III) Centers (M = Rh, Ir). J. Am. Chem. Soc. 2010, 132, 10578–10591. 10.1021/ja1043787. [DOI] [PubMed] [Google Scholar]
- Johnson K. R. D.; Hayes P. G. Cyclometalative C-H Bond Activation in Rare Earth and Actinide Metal Complexes. Chem. Soc. Rev. 2013, 42, 1947–1960. 10.1039/C2CS35356C. [DOI] [PubMed] [Google Scholar]
- Wang L.; Cui D.; Hou Z.; Li W.; Li Y. Highly Cis-1,4-Selective Living Polymerization of 1,3-Conjugated Dienes and Copolymerization with ε-Caprolactone by Bis(Phosphino) Carbazolide Rare-Earth-Metal Complexes. Organometallics 2011, 30, 760–767. 10.1021/om1009395. [DOI] [Google Scholar]
- Zhang L.; Suzuki T.; Luo Y.; Nishiura M.; Hou Z. Cationic Alkyl Rare-Earth Metal Complexes Bearing an Ancillary Bis(Phosphinophenyl) Amido Ligand: A Catalytic System for Living Cis-1,4-Polymerization and Copolymerization of Isoprene and Butadiene. Angew. Chem., Int. Ed. 2007, 46, 1909–1913. 10.1002/anie.200604348. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Ghebremeskel G. N. A Review of Some of the Factors Affecting Fracture and Fatigue in SBR and BR Vulcanizates. Rubber Chem. Technol. 2001, 74, 409–427. 10.5254/1.3547645. [DOI] [Google Scholar]
- Dong J. Y.; Hu Y. Design and Synthesis of Structurally Well-Defined Functional Polyolefins via Transition Metal-Mediated Olefin Polymerization Chemistry. Coord. Chem. Rev. 2006, 250, 47–65. 10.1016/j.ccr.2005.05.008. [DOI] [Google Scholar]
- Li L.; Li S.; Cui D. Highly Cis-1,4-Selective Living Polymerization of 3-Methylenehepta-1,6-Diene and Its Subsequent Thiol-Ene Reaction: An Efficient Approach to Functionalized Diene-Based Elastomer. Macromolecules 2016, 49, 1242–1251. 10.1021/acs.macromol.5b02654. [DOI] [Google Scholar]
- Long S.; Lin F.; Yao C.; Cui D. Highly Cis-1,4 Selective Living Polymerization of Unmasked Polar 2-(2-Methylidenebut-3-Enyl) Furan and Diels-Alder Addition. Macromol. Rapid Commun. 2017, 38, 1700227. 10.1002/marc.201700227. [DOI] [PubMed] [Google Scholar]
- Cai L.; Long S.; Wu C.; Li S.; Yao C.; Hua X.; Na H.; Liu D.; Tang T.; Cui D. Highly Selective: Cis −1,4 Copolymerization of Dienes with Polar 2-(3-Methylidenepent-4-En-1-Yl) Pyridine: An Approach for Recyclable Elastomers. Polym. Chem. 2020, 11, 1646–1652. 10.1039/C9PY01811E. [DOI] [Google Scholar]
- Leicht H.; Göttker-Schnetmann I.; Mecking S. Stereoselective Copolymerization of Butadiene and Functionalized 1,3-Dienes. ACS Macro Lett. 2016, 5, 777–780. 10.1021/acsmacrolett.6b00321. [DOI] [PubMed] [Google Scholar]
- Johnson K. R. D.; Hayes P. G. Synthesis and Reactivity of Dialkyl Lutetium Complexes Supported by a Novel Bis(Phosphinimine) Carbazole Pincer Ligand. Organometallics 2009, 28, 6352–6361. 10.1021/om900731x. [DOI] [Google Scholar]
- Johnson K. R. D.; Hayes P. G. Yttrium and Scandium Complexes of a Bulky Bis(Phosphinimine) Carbazole Ligand. Inorg. Chim. Acta 2014, 422, 209–217. 10.1016/j.ica.2014.05.045. [DOI] [Google Scholar]
- Johnson K. R. D.; Hayes P. G. Kinetic and Mechanistic Investigation of Metallacycle Ring Opening in an Ortho-Metalated Lutetium Aryl Complex. Organometallics 2011, 30, 58–67. 10.1021/om100814h. [DOI] [Google Scholar]
- Johnson K. R. D.; Kamenz B. L.; Hayes P. G. Ligand Influence on Intramolecular Cyclometalation in Bis(Phosphinimine) Rare Earth Alkyl Complexes. Can. J. Chem. 2016, 94, 330–341. 10.1139/cjc-2015-0368. [DOI] [Google Scholar]
- Johnson K. R. D.; Hayes P. G. A Cascade Reaction: Ring-Opening Insertion of Dioxaphospholane into Lutetium Alkyl Bonds. Dalton Trans. 2014, 43, 2448–2457. 10.1039/C3DT52790E. [DOI] [PubMed] [Google Scholar]
- Johnson K. R. D.; Hayes P. G. Organolutetium-Mediated Dearomatization and Functionalization of Pyrimidine Rings. Organometallics 2013, 32, 4046–4049. 10.1021/om400413e. [DOI] [Google Scholar]
- Johnson K. R. D.; Kamenz B. L.; Hayes P. G. Bis(Pyrazolyl) Carbazole as a Versatile Ligand for Supporting Lutetium Alkyl and Hydride Complexes. Organometallics 2014, 33, 3005–3011. 10.1021/om500226t. [DOI] [Google Scholar]
- Jürgens E.; Wucher B.; Rominger F.; Törnroos K. W.; Kunz D. Selective Rearrangement of Terminal Epoxides into Methylketones Catalysed by a Nucleophilic Rhodium-NHC-Pincer Complex. Chem. Commun. 2015, 51, 1897–1900. 10.1039/C4CC07154A. [DOI] [PubMed] [Google Scholar]
- Li H.; He Y.; Liu C.; Tan G. A Bis(Imino) Carbazolate Pincer Ligand Stabilized Mononuclear Gallium(I) Compound: Synthesis, Characterization, and Reactivity. Dalton Trans. 2021, 50, 12674–12680. 10.1039/D1DT02209A. [DOI] [PubMed] [Google Scholar]
- Wucher B.; Moser M.; Schumacher S. A.; Rominger F.; Kunz D. First X-Ray Structure Analyses of Rhodium(III) Η1-Allyl Complexes and a Mechanism for Allylic Isomerization Reactions. Angew. Chem., Int. Ed. 2009, 48, 4417–4421. 10.1002/anie.200805899. [DOI] [PubMed] [Google Scholar]
- Seyboldt A.; Wucher B.; Alles M.; Rominger F.; Maichle-Mössmer C.; Kunz D. Synthesis and Reactivity of an Ir(I) Carbonyl Complex Bearing a Carbazolide-Bis(NHC) Pincer Ligand. J. Organomet. Chem. 2015, 775, 202–208. 10.1016/j.jorganchem.2014.10.045. [DOI] [Google Scholar]
- Trost B. M.; Lee C.. Asymmetric Carbon-Carbon Bond-Forming Reactions: Asymmetric Allylic Alkylation Reactions. In Catalytic Asymmetric Synthesis; John Wiley & Sons, Inc., 2005; pp 593–649, 10.1002/0471721506.ch19. [DOI] [Google Scholar]
- Barloy L.; Ramdeehul S.; Osborn J. A.; Carlotti C.; Taulelle F.; De Cian A.; Fischer J. Η1- and Η3-Allylpalladium(II) Complexes Bearing Potentially Tridentate Ligands: Synthesis, Solution Dynamics, and Crystal Structures. Eur. J. Inorg. Chem. 2000, 2000, 2523–2532. . [DOI] [Google Scholar]
- Kollmar M.; Helmchen G. An (Η1-Allyl) Palladium Complex of a Chiral Bidentate Ligand: Crystallographic and NMR Studies on a (Η1–3,3-Diphenylallyl)(Phosphinooxazoline) Palladium Complex. Organometallics 2002, 21, 4771–4775. 10.1021/om020323z. [DOI] [Google Scholar]
- Filipuzzi S.; Pregosin P. S.; Albinati A.; Rizzato S. Palladium-Allyl Phosphoramidite Complexes: Solid-State Structures and Solution Dynamics. Organometallics 2006, 25, 5955–5964. 10.1021/om060726p. [DOI] [Google Scholar]
- Maulbetsch T.; Jürgens E.; Kunz D. Deoxygenation of Epoxides with Carbon Monoxide. Chem.—Eur. J. 2020, 26, 10634–10640. 10.1002/chem.202002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. Y.; Lin Y. J.; Chang Y. Z.; Huang L. S.; Ko B. T.; Huang J. H. Nickel-Catalyzed Coupling of Carbon Dioxide with Cyclohexene Oxide by Well-Characterized Bis(N-Heterocyclic Carbene) Carbazolide Complexes. Organometallics 2017, 36, 291–297. 10.1021/acs.organomet.6b00756. [DOI] [Google Scholar]
- Marelius D. C.; Darrow E. H.; Moore C. E.; Golen J. A.; Rheingold A. L.; Grotjahn D. B. Hydrogen-Bonding Pincer Complexes with Two Protic N-Heterocyclic Carbenes from Direct Metalation of a 1,8-Bis(Imidazol-1-Yl) Carbazole by Platinum, Palladium, and Nickel. Chem.—Eur. J. 2015, 21, 10988–10992. 10.1002/chem.201501945. [DOI] [PubMed] [Google Scholar]
- Watt F. A.; Sieland B.; Dickmann N.; Schoch R.; Herbst-Irmer R.; Ott H.; Paradies J.; Kuckling D.; Hohloch S. Coupling of CO2 and Epoxides Catalysed by Novel N-Fused Mesoionic Carbene Complexes of Nickel(II). Dalton Trans. 2021, 50, 17361–17371. 10.1039/D1DT03311E. [DOI] [PubMed] [Google Scholar]
- Pinter P.; Schüßlbauer C. M.; Watt F. A.; Dickmann N.; Herbst-Irmer R.; Morgenstern B.; Grünwald A.; Ullrich T.; Zimmer M.; Hohloch S.; et al. Bright Luminescent Lithium and Magnesium Carbene Complexes. Chem. Sci. 2021, 12, 7401–7410. 10.1039/D1SC00846C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plundrich G. T.; Wadepohl H.; Clot E.; Gade L. H. n6-Arene-Zirconium-PNP-Pincer Complexes: Mechanism of Their Hydrogenolytic Formation and Their Reactivity as Zirconium(II) Synthons. Chem.—Eur. J. 2016, 22, 9283–9292. 10.1002/chem.201601213. [DOI] [PubMed] [Google Scholar]
- Kurogi T.; Ishida Y.; Kawaguchi H. Synthesis of Titanium and Zirconium Complexes Supported by a P-Terphenoxide Ligand and Their Reactions with N2, CO2 and CS2. Chem. Commun. 2013, 49, 11755–11757. 10.1039/c3cc47284a. [DOI] [PubMed] [Google Scholar]
- Semproni S. P.; Knobloch D. J.; Milsmann C.; Chirik P. J. Redox-Induced N2 Hapticity Switching in Zirconocene Dinitrogen Complexes. Angew. Chem., Int. Ed. 2013, 52, 5372–5376. 10.1002/anie.201301800. [DOI] [PubMed] [Google Scholar]
- Gilbert Z. W.; Hue R. J.; Tonks I. A. Catalytic Formal [2 + 2+1] Synthesis of Pyrroles from Alkynes and Diazenes via TiII/TiIV Redoxcatalysis. Nat. Chem. 2016, 8, 63–68. 10.1038/nchem.2386. [DOI] [PubMed] [Google Scholar]
- Kawashima T.; Takao T.; Suzuki H. Dehydrogenative Coupling of 4-Substituted Pyridines Catalyzed by Diruthenium Complexes. J. Am. Chem. Soc. 2007, 129, 11006–11007. 10.1021/ja074224u. [DOI] [PubMed] [Google Scholar]
- Takao T.; Kawashima T.; Kanda H.; Okamura R.; Suzuki H. Synthesis of Triruthenium Complexes Containing a Triply Bridging Pyridyl Ligand and Its Transformations to Face-Capping Pyridine and Perpendicularly Coordinated Pyridyl Ligands. Organometallics 2012, 31, 4817–4831. 10.1021/om300379d. [DOI] [Google Scholar]
- Nagaoka M.; Kawashima T.; Suzuki H.; Takao T. Dehydrogenative Coupling of 4-Substituted Pyridines Catalyzed by a Trinuclear Complex of Ruthenium and Cobalt. Organometallics 2016, 35, 2348–2360. 10.1021/acs.organomet.6b00277. [DOI] [Google Scholar]
- Merz L. S.; Wadepohl H.; Clot E.; Gade L. H. Dehydrogenative Coupling of 4-Substituted Pyridines Mediated by a Zirconium(II) Synthon: Reaction Pathways and Dead Ends. Chem. Sci. 2018, 9, 5223–5232. 10.1039/C8SC01025K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. J.; Lutz S.; O’Kane C.; Zeller M.; Chen C. H.; Al Assil T.; Lee W. T. Synthesis and Characterization of an Iron Complex Bearing a Hemilabile NNN-Pincer for Catalytic Hydrosilylation of Organic Carbonyl Compounds. Dalton Trans. 2018, 47, 3243–3247. 10.1039/C7DT04928E. [DOI] [PubMed] [Google Scholar]
- Rimola A.; Sodupe M.; Ros J.; Pons J. A Theoretical Study on PdII Complexes Containing Hemilabile Pyrazole-Derived Ligands. Eur. J. Inorg. Chem. 2006, 2006 (2), 447–454. 10.1002/ejic.200500794. [DOI] [Google Scholar]
- Mancano G.; Page M. J.; Bhadbhade M.; Messerle B. A. Hemilabile and Bimetallic Coordination in Rh and Ir Complexes of NCN Pincer Ligands. Inorg. Chem. 2014, 53, 10159–10170. 10.1021/ic501158x. [DOI] [PubMed] [Google Scholar]
- Bailey W. D.; Luconi L.; Rossin A.; Yakhvarov D.; Flowers S. E.; Kaminsky W.; Kemp R. A.; Giambastiani G.; Goldberg K. I. Pyrazole-Based PCN Pincer Complexes of Palladium(II): Mono- and Dinuclear Hydroxide Complexes and Ligand Rollover C-H Activation. Organometallics 2015, 34, 3998–4010. 10.1021/acs.organomet.5b00355. [DOI] [Google Scholar]
- Ghannam J.; Sun Z.; Cundari T. R.; Zeller M.; Lugosan A.; Stanek C. M.; Lee W. T. Intramolecular C-H Functionalization Followed by a [2σ + 2π] Addition via an Intermediate Nickel-Nitridyl Complex. Inorg. Chem. 2019, 58, 7131–7135. 10.1021/acs.inorgchem.9b00168. [DOI] [PubMed] [Google Scholar]
- Alamo D. C.; Cundari T. R. DFT and TDDFT Study of the Reaction Pathway for Double Intramolecular C-H Activation and Functionalization by Iron, Cobalt, and Nickel-Nitridyl Complexes. Inorg. Chem. 2021, 60, 12299–12308. 10.1021/acs.inorgchem.1c01507. [DOI] [PubMed] [Google Scholar]
- DeMott J. C.; Dekarske J. R.; McCulloch B. J.; Ozerov O. V. Cyclometallation of the NNN Pincer Ligand in Complexes of Platinum. Inorg. Chem. Front. 2015, 2, 912–916. 10.1039/C5QI00102A. [DOI] [Google Scholar]
- Merz L. S.; Blasius C. K.; Wadepohl H.; Gade L. H. Square Planar Cobalt(II) Hydride versus T-Shaped Cobalt(I): Structural Characterization and Dihydrogen Activation with PNP-Cobalt Pincer Complexes. Inorg. Chem. 2019, 58, 6102–6113. 10.1021/acs.inorgchem.9b00384. [DOI] [PubMed] [Google Scholar]
- Kuriyama S.; Arashiba K.; Tanaka H.; Matsuo Y.; Nakajima K.; Yoshizawa K.; Nishibayashi Y. Direct Transformation of Molecular Dinitrogen into Ammonia Catalyzed by Cobalt Dinitrogen Complexes Bearing Anionic PNP Pincer Ligands. Angew. Chem., Int. Ed. 2016, 55, 14291–14295. 10.1002/anie.201606090. [DOI] [PubMed] [Google Scholar]
- Guard L. M.; Hebden T. J.; Linn D. E.; Heinekey D. M. Pincer-Supported Carbonyl Complexes of Cobalt(I). Organometallics 2017, 36, 3104–3109. 10.1021/acs.organomet.7b00434. [DOI] [Google Scholar]
- Krishnan V. M.; Arman H. D.; Tonzetich Z. J. Preparation and Reactivity of a Square-Planar PNP Cobalt(II)-Hydrido Complex: Isolation of the First {Co-NO}8-Hydride. Dalton Trans. 2018, 47, 1435–1441. 10.1039/C7DT04339B. [DOI] [PubMed] [Google Scholar]
- Ingleson M.; Fan H.; Pink M.; Tomaszewski J.; Caulton K. G. Three-Coordinate Co(I) Provides Access to Unsaturated Dihydrido-Co(III) and Seven-Coordinate Co(V). J. Am. Chem. Soc. 2006, 128, 1804–1805. 10.1021/ja0572452. [DOI] [PubMed] [Google Scholar]
- Gibson V. C.; Spitzmesser S. K.; White A. J. P.; Williams D. J. Synthesis and Reactivity of 1,8-Bis(Imino) Carbazolide Complexes of Iron, Cobalt and Manganese. Dalton Trans. 2003, (13), 2718. 10.1039/b301902k. [DOI] [Google Scholar]
- Lugosan A.; Todtz S. R.; Alcázar A.; Zeller M.; Devery J. J.; Lee W. T. Synthesis and Characterization of Trigonal Bipyramidal FeIII Complexes and Their Solution Behavior. Polyhedron 2021, 208, 115384. 10.1016/j.poly.2021.115384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugosan A.; Kawamura A.; Dickie D. A.; Zeller M.; Anderson J. S.; Lee W.-T. Magnetically Coupled Iron Azide Chains. Inorg. Chim. Acta 2021, 516, 120150. 10.1016/j.ica.2020.120150. [DOI] [Google Scholar]
- Ghannam J.; Al Assil T.; Pankratz T. C.; Lord R. L.; Zeller M.; Lee W.-T. A Series of 4- and 5-Coordinate Ni(II) Complexes: Synthesis, Characterization, Spectroscopic, and DFT Studies. Inorg. Chem. 2018, 57, 8307–8316. 10.1021/acs.inorgchem.8b00958. [DOI] [PubMed] [Google Scholar]
- Lugosan A.; Cundari T.; Fleming K.; Dickie D. A.; Zeller M.; Ghannam J.; Lee W.-T. Synthesis, Characterization, DFT Calculations, and Reactivity Study of a Nitrido-Bridged Dimeric Vanadium(<scp>iv<scp>) Complex. Dalton Trans. 2020, 49, 1200–1206. 10.1039/C9DT04544A. [DOI] [PubMed] [Google Scholar]
- Kuwata S.; Ikariya T. β-Protic Pyrazole and N-Heterocyclic Carbene Complexes: Synthesis, Properties, and Metal-Ligand Cooperative Bifunctional Catalysis. Chem.—Eur. J. 2011, 17, 3542–3556. 10.1002/chem.201003296. [DOI] [PubMed] [Google Scholar]
- Hahn F. E. Substrate Recognition and Regioselective Catalysis with Complexes Bearing NR,NH-NHC Ligands. ChemCatChem. 2013, 5, 419–430. 10.1002/cctc.201200567. [DOI] [Google Scholar]
- Kuwata S.; Hahn F. E. Complexes Bearing Protic N-Heterocyclic Carbene Ligands. Chem. Rev. 2018, 118, 9642–9677. 10.1021/acs.chemrev.8b00176. [DOI] [PubMed] [Google Scholar]
- Marelius D. C.; Moore C. E.; Rheingold A. L.; Grotjahn D. B. Reactivity Studies of Pincer Bis-Protic N-Heterocyclic Carbene Complexes of Platinum and Palladium Under Basic Conditions. Beilstein J. Org. Chem. 2016, 12, 1334–1339. 10.3762/bjoc.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens E.; Kunz D. A Rigid CNC Pincer Ligand Acting as a Tripodal Cp Analogue. Eur. J. Inorg. Chem. 2017, 2017 (2), 233–236. 10.1002/ejic.201601008. [DOI] [Google Scholar]
- Kleinhans G.; Guisado-Barrios G.; Peris E.; Bezuidenhout D. I. Ruthenium(II) Pincer Complexes Featuring an Anionic CNC Bis(1,2,3-Triazol-5-Ylidene) Carbazolide Ligand Coordinated in a Meridional Fashion. Polyhedron 2018, 143, 43–48. 10.1016/j.poly.2017.07.010. [DOI] [Google Scholar]
- Andrew R. E.; Chaplin A. B. Synthesis, Structure and Dynamics of NHC-Based Palladium Macrocycles. Dalton Trans. 2014, 43, 1413–1423. 10.1039/C3DT52578C. [DOI] [PubMed] [Google Scholar]
- Andrew R. E.; Chaplin A. B. Synthesis and Reactivity of NHC-Based Rhodium Macrocycles. Inorg. Chem. 2015, 54, 312–322. 10.1021/ic5024828. [DOI] [PubMed] [Google Scholar]
- Andrew R. E.; Storey C. M.; Chaplin A. B. Well-Defined Coinage Metal Transfer Agents for the Synthesis of NHC-Based Nickel, Rhodium and Palladium Macrocycles. Dalton Trans. 2016, 45, 8937–8944. 10.1039/C6DT01263A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey C. M.; Gyton M. R.; Andrew R. E.; Chaplin A. B. Terminal Alkyne Coupling Reactions through a Ring: Mechanistic Insights and Regiochemical Switching. Angew. Chem., Int. Ed. 2018, 57, 12003–12006. 10.1002/anie.201807028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leforestier B.; Gyton M. R.; Chaplin A. B. Oxidative Addition of a Mechanically Entrapped C(Sp)-C(Sp) Bond to a Rhodium(I) Pincer Complex. Angew. Chem., Int. Ed. 2020, 59, 23500–23504. 10.1002/anie.202009546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey C. M.; Gyton M. R.; Andrew R. E.; Chaplin A. B. Terminal Alkyne Coupling Reactions Through a Ring: Effect of Ring Size on Rate and Regioselectivity. Chem.—Eur. J. 2020, 26, 14715–14723. 10.1002/chem.202002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y.; Jürgens E.; Kunz D. Regio- and Chemoselective Rearrangement of Terminal Epoxides into Methyl Alkyl and Aryl Ketones. Chem. Commun. 2018, 54, 11340–11343. 10.1039/C8CC06503A. [DOI] [PubMed] [Google Scholar]
- Meinwald J.; Labana S. S.; Chadha M. S. Peracid Reactions. III. The Oxidation of Bicyclo [2.2.1]Heptadiene. J. Am. Chem. Soc. 1963, 85, 582–585. 10.1021/ja00888a022. [DOI] [Google Scholar]
- Miyashita A.; Shimada T.; Sugawara A.; Nohira H. Nickel-Catalyzed Ring-Opening Reactions of Epoxides and Their Regioselectivities. Chem. Lett. 1986, 15, 1323–1326. 10.1246/cl.1986.1323. [DOI] [Google Scholar]
- Ranu B. C.; Jana U. Indium(III) Chloride-Promoted Rearrangement of Epoxides: A Selective Synthesis of Substituted Benzylic Aldehydes and Ketones. J. Org. Chem. 1998, 63, 8212–8216. 10.1021/jo980793w. [DOI] [Google Scholar]
- Kulasegaram S.; Kulawiec R. J. Palladium-Catalyzed Isomerization of Aryl-Substituted Epoxides: A Selective Synthesis of Substituted Benzylic Aldehydes and Ketones. J. Org. Chem. 1997, 62, 6547–6561. 10.1021/jo970743b. [DOI] [Google Scholar]
- Kulasegaram S.; Kulawiec R. J. On the Mechanism of the Palladium(0)-Catalyzed Isomerization of Epoxides to Carbonyl Compounds. Tetrahedron 1998, 54, 1361–1374. 10.1016/S0040-4020(97)10363-5. [DOI] [Google Scholar]
- Desnoyer A. N.; Geng J.; Drover M. W.; Patrick B. O.; Love J. A. Catalytic Functionalization of Styrenyl Epoxides via 2-Nickela(II) Oxetanes. Chem.—Eur. J. 2017, 23, 11509–11512. 10.1002/chem.201702824. [DOI] [PubMed] [Google Scholar]
- Suda K.; Baba K.; Nakajima S.-I.; Takanami T. Metalloporphyrin-Catalyzed Regioselective Rearrangement of Monoalkyl-Substituted Epoxides into Aldehydes. Tetrahedron Lett. 1999, 40, 7243–7246. 10.1016/S0040-4039(99)01363-5. [DOI] [Google Scholar]
- Anderson A. M.; Blazek J. M.; Garg P.; Payne B. J.; Mohan R. S. Bismuth(III) Oxide Perchlorate Promoted Rearrangement of Epoxides to Aldehydes and Ketones. Tetrahedron Lett. 2000, 41, 1527–1530. 10.1016/S0040-4039(99)02330-8. [DOI] [Google Scholar]
- Banerjee M.; Roy U. K.; Sinha P.; Roy S. Tuning the Reactivity of Organotin(IV) by LiOH: Allylation and Propargylation of Epoxides via Redox Transmetalation. J. Organomet. Chem. 2005, 690, 1422–1428. 10.1016/j.jorganchem.2004.12.008. [DOI] [Google Scholar]
- Robinson M. W. C.; Pillinger K. S.; Mabbett I.; Timms D. A.; Graham A. E. Copper(II) Tetrafluroborate-Promoted Meinwald Rearrangement Reactions of Epoxides. Tetrahedron 2010, 66, 8377–8382. 10.1016/j.tet.2010.08.078. [DOI] [Google Scholar]
- Umeda R.; Muraki M.; Nakamura Y.; Tanaka T.; Kamiguchi K.; Nishiyama Y. Rhenium Complex-Catalyzed Meinwald Rearrangement Reactions of Oxiranes. Tetrahedron Lett. 2017, 58, 2393–2395. 10.1016/j.tetlet.2017.05.018. [DOI] [Google Scholar]
- Chang C.-L.; Kumar M. P.; Liu R.-S. A Highly Efficient Ruthenium-Catalyzed Rearrangement of α,β-Epoxyketones to 1,2-Diketones. J. Org. Chem. 2004, 69, 2793–2796. 10.1021/jo0303867. [DOI] [PubMed] [Google Scholar]
- Vyas D. J.; Larionov E.; Besnard C.; Guénée L.; Mazet C. Isomerization of Terminal Epoxides by a [Pd-H] Catalyst: A Combined Experimental and Theoretical Mechanistic Study. J. Am. Chem. Soc. 2013, 135, 6177–6183. 10.1021/ja400325w. [DOI] [PubMed] [Google Scholar]
- Humbert N.; Vyas D. J.; Besnard C.; Mazet C. An Air-Stable Cationic Iridium Hydride as a Highly Active and General Catalyst for the Isomerization of Terminal Epoxides. Chem. Commun. 2014, 50, 10592–10595. 10.1039/C4CC05260A. [DOI] [PubMed] [Google Scholar]
- Rickborn B.; Gerkin R. M. The Lithium Salt-Catalyzed Epoxide-Carbonyl Rearrangement. J. Am. Chem. Soc. 1968, 90, 4193–4194. 10.1021/ja01017a071. [DOI] [Google Scholar]
- Rickborn B.; Gerkin R. M. Lithium Salt Catalyzed Epoxide-Carbonyl Rearrangement. I. Alkyl-Substituted Epoxides. J. Am. Chem. Soc. 1971, 93, 1693–1700. 10.1021/ja00736a021. [DOI] [Google Scholar]
- Kulasegaram S.; Kulawiec R. J. Chemo- and Regioselective Isomerization of Epoxides to Carbonyl Compounds via Palladium Catalysis. J. Org. Chem. 1994, 59, 7195–7196. 10.1021/jo00103a005. [DOI] [Google Scholar]
- Prandi J.; Namy J. L.; Menoret G.; Kagan H. B. Selective Catalyzed-Rearrangement of Terminal Epoxides to Methyl Ketones. J. Organomet. Chem. 1985, 285, 449–460. 10.1016/0022-328X(85)87389-7. [DOI] [Google Scholar]
- Lamb J. R.; Jung Y.; Coates G. W. Meinwald-Type Rearrangement of Monosubstituted Epoxides to Methyl Ketones Using an [Al Porphyrin]+[Co(CO)4]- Catalyst. Org. Chem. Front. 2015, 2, 346–349. 10.1039/C4QO00324A. [DOI] [Google Scholar]
- Tian Y.; Maulbetsch T.; Jordan R.; Törnroos K. W.; Kunz D. Synthesis and Reactivity of Cobalt(I) and Iridium(I) Complexes Bearing a Pentadentate N-Homoallyl-Substituted Bis(NHC) Pincer Ligand. Organometallics 2020, 39, 1221–1229. 10.1021/acs.organomet.0c00018. [DOI] [Google Scholar]
- Tian Y.; Jürgens E.; Mill K.; Jordan R.; Maulbetsch T.; Kunz D. Nucleophilic Isomerization of Epoxides by Pincer-Rhodium Catalysts: Activity Increase and Mechanistic Insights. ChemCatChem. 2019, 11, 4028–4035. 10.1002/cctc.201900594. [DOI] [Google Scholar]
- Tian Y.; Kunz D. Nucleophilic RhI Catalyzed Selective Isomerization of Terminal Aziridines to Enamides. ChemCatChem. 2020, 12, 4272–4275. 10.1002/cctc.202000597. [DOI] [Google Scholar]
- Burk M. J.; Feaster J. E.; Harlow R. L. New Chiral Phospholanes; Synthesis, Characterization, and Use in Asymmetric Hydrogenation Reactions. Tetrahedron: Asymmetry 1991, 2, 569–592. 10.1016/S0957-4166(00)86109-1. [DOI] [Google Scholar]
- Abdur-Rashid K.Transfer Hydrogenation Processes and Catalysts. US Patent 7291753B2, 2007.
- Guillen F.; Rivard M.; Toffano M.; Legros J.-Y.; Daran J.-C.; Fiaud J.-C. Synthesis and First Applications of a New Family of Chiral Monophosphine Ligand: 2,5-Diphenylphosphospholanes. Tetrahedron 2002, 58, 5895–5904. 10.1016/S0040-4020(02)00554-9. [DOI] [Google Scholar]
- Inoue M.; Suzuki T.; Kinoshita A.; Nakada M. Catalytic Asymmetric Nozaki-Hiyama Reactions with a Tridentate Bis(Oxazolinyl) Carbazole Ligand. Chem. Rec. 2008, 8, 169–181. 10.1002/tcr.20148. [DOI] [PubMed] [Google Scholar]
- Okude Y.; Hirano S.; Hiyama T.; Nozaki H. Grignard-Type Carbonyl Addition of Allyl Halides by Means of Chromous Salt. A Chemospecific Synthesis of Homoallyl Alcohols. J. Am. Chem. Soc. 1977, 99, 3179–3181. 10.1021/ja00451a061. [DOI] [Google Scholar]
- Connon R.; Roche B.; Rokade B. V.; Guiry P. J. Further Developments and Applications of Oxazoline-Containing Ligands in Asymmetric Catalysis. Chem. Rev. 2021, 121, 6373–6521. 10.1021/acs.chemrev.0c00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmark S. E.; Fu J. Catalytic Enantioselective Addition of Allylic Organometallic Reagents to Aldehydes and Ketones. Chem. Rev. 2003, 103, 2763–2794. 10.1021/cr020050h. [DOI] [PubMed] [Google Scholar]
- Inoue M.; Suzuki T.; Nakada M. Asymmetric Catalysis of Nozaki-Hiyama Allylation and Methallylation with a New Tridentate Bis(Oxazolinyl) Carbazole Ligand. J. Am. Chem. Soc. 2003, 125, 1140–1141. 10.1021/ja021243p. [DOI] [PubMed] [Google Scholar]
- Suzuki T.; Kinoshita A.; Kawada H.; Nakada M. A New Asymmetric Tridentate Carbazole Ligand: Its Preparation and Application to Nozaki-Hiyama Allylation. Synlett 2003, 4, 0570–0572. 10.1055/s-2003-37535. [DOI] [Google Scholar]
- Inoue M.; Nakada M. Studies into Asymmetric Catalysis of the Nozaki-Hiyama Allenylation. Angew. Chem., Int. Ed. 2006, 45, 252–255. 10.1002/anie.200502871. [DOI] [PubMed] [Google Scholar]
- Durán-Galván M.; Connell B. T. Asymmetrie Synthesis of (1,3-Butadien-2-Yl) Methanols from Aldehydes via [1-(Silylmethyl) Allenyl]Methanols. Eur. J. Org. Chem. 2010, 2010 (13), 2445–2448. 10.1002/ejoc.201000199. [DOI] [Google Scholar]
- Durán-Galván M.; Worlikar S. A.; Connell B. T. Enantioselective Synthesis of Butadien-2-Ylcarbinols via (Silylmethyl) Allenic Alcohols from Chromium-Catalyzed Additions to Aldehydes Utilizing Chiral Carbazole Ligands. Tetrahedron 2010, 66, 7707–7719. 10.1016/j.tet.2010.07.065. [DOI] [Google Scholar]
- Inoue M.; Nakada M. Studies on Catalytic Asymmetric Nozaki–Hiyama Propargylation. Org. Lett. 2004, 6, 2977–2980. 10.1021/ol048826j. [DOI] [PubMed] [Google Scholar]
- Ji H. T.; Tian Q. S.; Xiang J. N.; Zhang G. Z. Chromium-Catalyzed Asymmetric Synthesis of 1, 3-Diols. Chin. Chem. Lett. 2017, 28, 1182–1184. 10.1016/j.cclet.2017.03.016. [DOI] [Google Scholar]
- Bai J.; Chen B.; Zhang G. Enantioselective Synthesis of Cis-2,6-Disubstituted-4-Methylene Tetrahydropyrans via Chromium Catalysis. Chin. J. Chem. 2020, 38, 1642–1646. 10.1002/cjoc.202000296. [DOI] [Google Scholar]
- Guo R.; Yang Q.; Tian Q.; Zhang G. Barbier-Type Anti-Diastereo- and Enantioselective Synthesis of β-Trimethylsilyl, Fluorinated Methyl, Phenylthio Homoallylic Alcohols. Sci. Rep. 2017, 7, 4873. 10.1038/s41598-017-04986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán-Galván M.; Hemmer J. R.; Connell B. T. Synthesis of Tertiary 1,3-Butadien-2-Ylcarbinols from Chromium-Catalyzed Addition of (4-Bromobut-2-Ynyl) Trimethylsilane to Ketones. Tetrahedron Lett. 2010, 51, 5080–5082. 10.1016/j.tetlet.2010.07.104. [DOI] [Google Scholar]
- Melen R. L.; Gade L. H.. New Chemistry with Anionic NNN Pincer Ligands. In The Privileged Pincer-Metal Platform: Coordination Chemistry & Applications; Springer International Publishing, 2015; pp 179–208, 10.1007/3418_2015_114. [DOI] [Google Scholar]
- Malthus S. J.; Cameron S. A.; Brooker S. First Row Transition Metal Complexes of Di-o-Substituted-Diarylamine-Based Ligands (Including Carbazoles, Acridines and Dibenzoazepines). Coord. Chem. Rev. 2016, 316, 125–161. 10.1016/j.ccr.2016.02.006. [DOI] [Google Scholar]
- Fürstner A.; Shi N. Nozaki–Hiyama–Kishi Reactions Catalytic in Chromium. J. Am. Chem. Soc. 1996, 118, 12349–12357. 10.1021/ja9625236. [DOI] [Google Scholar]
- Fürstner A. Carbon-Carbon Bond Formations Involving Organochromium(III) Reagents. Chem. Rev. 1999, 99, 991–1045. 10.1021/cr9703360. [DOI] [PubMed] [Google Scholar]
- Inoue M.; Nakada M. Structure Elucidation and Enantioselective Total Synthesis of the Potent HMG-CoA Reductase Inhibitor FR901512 via Catalytic Asymmetric Nozaki-Hiyama Reactions. J. Am. Chem. Soc. 2007, 129, 4164–4165. 10.1021/ja070812w. [DOI] [PubMed] [Google Scholar]
- Chen W.; Yang Q.; Zhou T.; Tian Q.; Zhang G. Enantioselective Synthesis of α-Exo-Methylene γ-Butyrolactones via Chromium Catalysis. Org. Lett. 2015, 17, 5236–5239. 10.1021/acs.orglett.5b02597. [DOI] [PubMed] [Google Scholar]
- Xiong Y.; Zhang G. Enantioselective 1,2-Difunctionalization of 1,3-Butadiene by Sequential Alkylation and Carbonyl Allylation. J. Am. Chem. Soc. 2018, 140, 2735–2738. 10.1021/jacs.7b12760. [DOI] [PubMed] [Google Scholar]
- Tian Q.; Bai J.; Chen B.; Zhang G. Chromium-Catalyzed Asymmetric Dearomatization Addition Reactions of Halomethyl Heteroarenes. Org. Lett. 2016, 18, 1828–1831. 10.1021/acs.orglett.6b00559. [DOI] [PubMed] [Google Scholar]
- Chen W.; Bai J.; Zhang G. Chromium-Catalysed Asymmetric Dearomatization Addition Reactions of Bromomethylnaphthalenes. Adv. Synth. Catal. 2017, 359, 1227–1231. 10.1002/adsc.201600962. [DOI] [Google Scholar]
- Nagata A.; Akagi Y.; Masoud S. S.; Yamanaka M.; Kittaka A.; Uesugi M.; Odagi M.; Nagasawa K. Stereoselective Synthesis of Four Calcitriol Lactone Diastereomers at C23 and C25. J. Org. Chem. 2019, 84, 7630–7641. 10.1021/acs.joc.9b00403. [DOI] [PubMed] [Google Scholar]
- Liu W.; Yu Z.; Winssinger N. Total Syntheses of Paraconic Acids and 1,10-Seco-Guaianolides via a Barbier Allylation/Translactonization Cascade of 3-(Bromomethyl)-2(5H)-Furanone. Org. Lett. 2021, 23, 969–973. 10.1021/acs.orglett.0c04165. [DOI] [PubMed] [Google Scholar]
- Ambrose J. F.; Carpenter L. L.; Nelson R. F. Electrochemical and Spectroscopic Properties of Cation Radicals: III. Reaction Pathways of Carbazolium Radical Ions. J. Electrochem. Soc. 1975, 122, 876. 10.1149/1.2134365. [DOI] [Google Scholar]
- Ambrose J. F.; Nelson R. F. Anodic Oxidation Pathways of Carbazoles: I. Carbazole and N-Substituted Derivatives. J. Electrochem. Soc. 1968, 115, 1159. 10.1149/1.2410929. [DOI] [Google Scholar]
- Hollas A. M.; Gu W.; Bhuvanesh N.; Ozerov O. V. Synthesis and Characterization of Pd Complexes of a Carbazolyl/Bis(Imine) NNN Pincer Ligand. Inorg. Chem. 2011, 50, 3673–3679. 10.1021/ic200026p. [DOI] [PubMed] [Google Scholar]
- Barbe J. M.; Habermeyer B.; Khoury T.; Gros C. P.; Richard P.; Chen P.; Kadish K. M. Three-Metal Coordination by Novel Bisporphyrin Architectures. Inorg. Chem. 2010, 49, 8929–8940. 10.1021/ic101170k. [DOI] [PubMed] [Google Scholar]
- Samanta S.; Zheng C.; Gajecki L.; Berg D. J.; Oliver A. G.; Crosby T.; Godin L.; Sandhu J. Carbazolyl-Bis(Triazole) and Carbazolyl-Bis(Tetrazole) Complexes of Palladium(II) and Platinum(II). J. Coord. Chem. 2021, 74, 983–1008. 10.1080/00958972.2021.1882674. [DOI] [Google Scholar]
- Bennington M. S.; Feltham H. L. C.; Buxton Z. J.; White N. G.; Brooker S. Tuneable Reversible Redox of Cobalt(III) Carbazole Complexes. Dalton Trans. 2017, 46, 4696–4710. 10.1039/C7DT00295E. [DOI] [PubMed] [Google Scholar]
- Adhikari D.; Basuli F.; Fan H.; Huffman J. C.; Pink M.; Mindiola D. J. P = N Bond Formation via Incomplete N-Atom Transfer from a Ferrous Amide Precursor. Inorg. Chem. 2008, 47, 4439–4441. 10.1021/ic800182m. [DOI] [PubMed] [Google Scholar]
- Radosevich A. T.; Melnick J. G.; Stoian S. A.; Bacciu D.; Chen C. H.; Foxman B. M.; Ozerov O. V.; Nocera D. G. Ligand Reactivity in Diarylamido/Bis(Phosphine) PNP Complexes of Mn(CO)3 and Re(CO)3. Inorg. Chem. 2009, 48, 9214–9221. 10.1021/ic9010218. [DOI] [PubMed] [Google Scholar]
- Wanniarachchi S.; Liddle B. J.; Toussaint J.; Lindeman S. V.; Bennett B.; Gardinier J. R. Chemical Switching Behaviour of Tricarbonylrhenium(I) Complexes of a New Redox Active “Pincer” Ligand. Dalton Trans. 2010, 39, 3167–3169. 10.1039/c001344g. [DOI] [PubMed] [Google Scholar]
- Harkins S. B.; Mankad N. P.; Miller A. J. M.; Szilagyi R. K.; Peters J. C. Probing the Electronic Structures of [CU2(μ-XR 2)]N+ Diamond Cores as a Function of the Bridging X Atom (X = N or P) and Charge (n = 0, 1, 2). J. Am. Chem. Soc. 2008, 130, 3478–3485. 10.1021/ja076537v. [DOI] [PubMed] [Google Scholar]
- Pryjomska-Ray I.; Zornik D.; Pätzel M.; Krause K. B.; Grubert L.; Braun-Cula B.; Hecht S.; Limberg C. Comparing Isomeric Tridentate Carbazole-Based Click Ligands: Metal Complexes and Redox Chemistry. Chem.—Eur. J. 2018, 24, 5341–5349. 10.1002/chem.201704858. [DOI] [PubMed] [Google Scholar]
- Kadish K. M.; Smith K. M.; Guilard R.. The Porphyrin Handbook; Elsevier, 2003; Vol. 11, 10.1016/C2009-0-22714-0. [DOI] [Google Scholar]
- Chang C. J.; Loh Z. H.; Deng Y.; Nocera D. G. The Pacman Effect: A Supramolecular Strategy for Controlling the Excited-State Dynamics of Pillared Cofacial Bisporphyrins. Inorg. Chem. 2003, 42, 8262–8269. 10.1021/ic034750w. [DOI] [PubMed] [Google Scholar]
- Collman J. P.; Hutchison J. E.; Lopez M. A.; Tabard A.; Guilard R.; Seok W. K.; Ibers J. A.; L’Her M. Synthesis and Characterization of a Superoxo Complex of the Dicobalt Cofacial Diporphyrin [(.Mu.-O2) Co2(DPB)(1,5-Diphenylimidazole)2][PF6], the Structure of the Parent Dicobalt Diporphyrin Co2(DPB), and a New Synthesis of the Free-Base Cofacial Diporphyri. J. Am. Chem. Soc. 1992, 114, 9869–9877. 10.1021/ja00051a020. [DOI] [Google Scholar]
- Guilard R.; Lopez M. A.; Tabard A.; Richard P.; Lecomte C.; Brandes S.; Hutchison J. E.; Collman J. P. Synthesis and Characterization of Novel Cobalt Aluminum Cofacial Porphyrins. First Crystal and Molecular Structure of a Heterobimetallic Biphenylene Pillared Cofacial Diporphyrin. J. Am. Chem. Soc. 1992, 114, 9877–9889. 10.1021/ja00051a021. [DOI] [Google Scholar]
- Rosenthal J.; Nocera D. G. Role of Proton-Coupled Electron Transfer in O-O Bond Activation. Acc. Chem. Res. 2007, 40, 543–553. 10.1021/ar7000638. [DOI] [PubMed] [Google Scholar]
- Hioe J.; Šakić D.; Vrček V.; Zipse H. The Stability of Nitrogen-Centered Radicals. Org. Biomol. Chem. 2015, 13, 157–169. 10.1039/C4OB01656D. [DOI] [PubMed] [Google Scholar]
- Yu C.-H.; Zhu C.; Ji X.; Hu W.; Xie H.; Bhuvanesh N.; Fang L.; Ozerov O. V. Palladium Bis-Pincer Complexes with Controlled Rigidity and Inter-Metal Distance. Inorg. Chem. Front. 2020, 7, 4357–4366. 10.1039/D0QI01111H. [DOI] [Google Scholar]
- Robin M. B.; Day P. Mixed Valence Chemistry-A Survey and Classification. Adv. Inorg. Chem. Radiochem. 1968, 10, 247–422. 10.1016/S0065-2792(08)60179-X. [DOI] [Google Scholar]
- Zou J.; Berg D. J.; Oliver A.; Twamley B. Unusual Redox Chemistry of Ytterbium Carbazole-Bis(Oxazoline) Compounds: Oxidative Coupling of Primary Phosphines by an Ytterbium Carbazole-Bis(Oxazoline) Dialkyl. Organometallics 2013, 32, 6532–6540. 10.1021/om400861d. [DOI] [Google Scholar]
- Wittwer B.; Dickmann N.; Berg S.; Leitner D.; Tesi L.; Hunger D.; Gratzl R.; van Slageren J.; Neuman N. I.; Munz D.; et al. Mesoionic Carbene Complex of Manganese in Five Oxidation States. Chem. Commun. 2022, 58, 6096–6099. 10.1039/D2CC00097K. [DOI] [PubMed] [Google Scholar]
- Krüger A.; Albrecht M. Abnormal N-Heterocyclic Carbenes: More than Just Exceptionally Strong Donor Ligands. Aust. J. Chem. 2011, 64, 1113–1117. 10.1071/CH11265. [DOI] [Google Scholar]
- Niwa T.; Nakada M. A Non-Heme Iron(III) Complex with Porphyrin-like Properties That Catalyzes Asymmetric Epoxidation. J. Am. Chem. Soc. 2012, 134, 13538–13541. 10.1021/ja304219s. [DOI] [PubMed] [Google Scholar]
- Mamgain R.; Singh F. V. Selenium-Based Fluorescence Probes for the Detection of Bioactive Molecules. ACS Org. Inorg. Au 2022, 2, 262–288. 10.1021/acsorginorgau.1c00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K. [2.2]Paracyclophane-Based Chiral Platforms for Circularly Polarized Luminescence Fluorophores and Their Chiroptical Properties: Past and Future. Front. Chem. 2020, 8, 700. 10.3389/fchem.2020.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J. M.; Hur S. H.; Pathak A.; Jeong J.-E.; Woo H. Y. Recent Advances in Organic Luminescent Materials with Narrowband Emission. NPG Asia Mater. 2021, 13, 53. 10.1038/s41427-021-00318-8. [DOI] [Google Scholar]
- McClenaghan N. D.; Passalacqua R.; Loiseau F.; Campagna S.; Verheyde B.; Hameurlaine A.; Dehaen W. Ruthenium(II) Dendrimers Containing Carbazole-Based Chromophores as Branches. J. Am. Chem. Soc. 2003, 125, 5356–5365. 10.1021/ja021373y. [DOI] [PubMed] [Google Scholar]
- Romanov A. S.; Yang L.; Jones S. T. E.; Di D.; Morley O. J.; Drummond B. H.; Reponen A. P. M.; Linnolahti M.; Credgington D.; Bochmann M. Dendritic Carbene Metal Carbazole Complexes as Photoemitters for Fully Solution-Processed OLEDs. Chem. Mater. 2019, 31, 3613–3623. 10.1021/acs.chemmater.8b05112. [DOI] [Google Scholar]
- Bettington S.; Tavasli M.; Bryce M. R.; Beeby A.; Al-Attar H.; Monkman A. P. Tris-Cyclometalated Iridium(III) Complexes of Carbazole(Fluorenyl) Pyridine Ligands: Synthesis, Redox and Photophysical Properties, and Electrophosphorescent Light-Emitting Diodes. Chem.—Eur. J. 2007, 13, 1423–1431. 10.1002/chem.200600888. [DOI] [PubMed] [Google Scholar]
- Percino M. J.; Chapela V. M.; Cerón M.; Soriano-Moro G.; Castro M. E.; Melendez F. J. Molecular Packing and Solid-State Fluorescence of Conjugated Compounds of Carbazole-Acrylonitrile Derivatives. Curr. Phys. Chem. 2014, 4, 137–150. 10.2174/1877946803666131213231346. [DOI] [Google Scholar]
- Comerford T. A.; Zysman-Colman E. Supramolecular Assemblies Showing Thermally Activated Delayed Fluorescence. Small Sci. 2021, 1, 2100022. 10.1002/smsc.202100022. [DOI] [Google Scholar]
- Prentice C.; Morrisson J.; Smith A. D.; Zysman-Colman E. Recent Developments in Enantioselective Photocatalysis. Beilstein J. Org. Chem. 2020, 16, 2363–2441. 10.3762/bjoc.16.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg-Douglas N.; Nicewicz D. A. Photoredox-Catalyzed C-H Functionalization Reactions. Chem. Rev. 2022, 122, 1925–2016. 10.1021/acs.chemrev.1c00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudadu M. S.; Singh A. N.; Thummel R. P. Preparation and Study of 1,8-Di(Pyrid-2′-Yl) Carbazoles. J. Org. Chem. 2008, 73, 6513–6520. 10.1021/jo801132w. [DOI] [PubMed] [Google Scholar]
- Maeda C.; Yoshioka N. Synthesis and Characterization of Novel Fused Porphyrinoids Based on Cyclic Carbazole[2]Indolones. Org. Lett. 2012, 14, 2122–2125. 10.1021/ol300585v. [DOI] [PubMed] [Google Scholar]
- Schulze B.; Friebe C.; Jäger M.; Görls H.; Birckner E.; Winter A.; Schubert U. S. PtII Phosphors with Click-Derived 1,2,3-Triazole-Containing Tridentate Chelates. Organometallics 2018, 37, 145–155. 10.1021/acs.organomet.7b00777. [DOI] [Google Scholar]
- Gee H.-C.; Lee C.-H.; Jeong Y.-H.; Jang W.-D. Highly Sensitive and Selective Cyanide Detection via Cu2+ Complex Ligand Exchange. Chem. Commun. 2011, 47, 11963–11965. 10.1039/c1cc14963f. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Chan T. R.; Hilgraf R.; Fokin V. V.; Sharpless K. B.; Finn M. G. Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- Tornøe C. W.; Christensen C.; Meldal M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- Meldal M.; Tornøe C. W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- Li M.; Liska T.; Swetz A.; Ayoub N.; Lai P.-N.; Zeller M.; Gray T. G. (Isonitrile) Platinum(II) Complexes of an Amido Bis(N-Heterocyclic Carbene) Pincer Ligand. Organometallics 2020, 39, 1667–1671. 10.1021/acs.organomet.0c00065. [DOI] [Google Scholar]
- Liska T.; Swetz A.; Lai P.-N.; Zeller M.; Teets T. S.; Gray T. G. Room-Temperature Phosphorescent Platinum(II) Alkynyls with Microsecond Lifetimes Bearing a Strong-Field Pincer Ligand. Chem.—Eur. J. 2020, 26, 8417–8425. 10.1002/chem.202000500. [DOI] [PubMed] [Google Scholar]
- Liska T.; Li M.; Cañada L. M.; Yoon S.; Teets T. S.; Zeller M.; Gray T. G. Enhancing Charge Transfer in (BIMCA) Pt(II) Alkynyls Through the Use of Substituted Boranes. Organometallics 2021, 40, 1555–1559. 10.1021/acs.organomet.1c00115. [DOI] [Google Scholar]
- Liu X. Y.; Bai D. R.; Wang S. Charge-Transfer Emission in Nonplanar Three-Coordinate Organoboron Compounds for Fluorescent Sensing of Fluoride. Angew. Chem., Int. Ed. 2006, 45, 5475–5478. 10.1002/anie.200601286. [DOI] [PubMed] [Google Scholar]
- Zhao C.-H.; Sakuda E.; Wakamiya A.; Yamaguchi S. Highly Emissive Diborylphemlene-Containing Bis(Phenylethynyl) Benzenes: Structure-Photophysical Property Correlations and Fluoride Ion Sensing. Chem.—Eur. J. 2009, 15, 10603–10612. 10.1002/chem.200900864. [DOI] [PubMed] [Google Scholar]
- Xu W.-J.; Liu S.-J.; Zhao X.-Y.; Sun S.; Cheng S.; Ma T.-C.; Sun H.-B.; Zhao Q.; Huang W. Cationic Iridium(III) Complex Containing Both Triarylboron and Carbazole Moieties as a Ratiometric Fluoride Probe That Utilizes a Switchable Triplet-Singlet Emission. Chem.—Eur. J. 2010, 16, 7125–7133. 10.1002/chem.201000362. [DOI] [PubMed] [Google Scholar]
- Leitl M. J.; Krylova V. A.; Djurovich P. I.; Thompson M. E.; Yersin H. Phosphorescence Versus Thermally Activated Delayed Fluorescence. Controlling Singlet-Triplet Splitting in Brightly Emitting and Sublimable Cu(I) Compounds. J. Am. Chem. Soc. 2014, 136, 16032–16038. 10.1021/ja508155x. [DOI] [PubMed] [Google Scholar]
- Barakat K. A.; Cundari T. R.; Omary M. A. Jahn-Teller Distortion in the Phosphorescent Excited State of Three-Coordinate Au(I) Phosphine Complexes. J. Am. Chem. Soc. 2003, 125, 14228–14229. 10.1021/ja036508u. [DOI] [PubMed] [Google Scholar]
- Sinha N.; Jiménez J.-R.; Pfund B.; Prescimone A.; Piguet C.; Wenger O. S. A Near-Infrared-II Emissive Chromium(III) Complex. Angew. Chem., Int. Ed. 2021, 60, 23722–23728. 10.1002/anie.202106398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N.; Pfund B.; Wegeberg C.; Prescimone A.; Wenger O. S. Cobalt(III) Carbene Complex with an Electronic Excited-State Structure Similar to Cyclometalated Iridium(III) Compounds. J. Am. Chem. Soc. 2022, 144, 9859–9873. 10.1021/jacs.2c02592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen C. K. The Interelectronic Repulsion and Partly Covalent Bonding in Transition-Group Complexes. Discuss. Faraday Soc. 1958, 26, 110–115. 10.1039/DF9582600110. [DOI] [Google Scholar]
- Schaffer C. E.; Klixbull Jørgensen C. The Nephelauxetic Series of Ligands Corresponding to Increasing Tendency of Partly Covalent Bonding. J. Inorg. Nucl. Chem. 1958, 8, 143–148. 10.1016/0022-1902(58)80176-1. [DOI] [Google Scholar]
- Pal A. K.; Li C.; Hanan G. S.; Zysman-Colman E. Blue-Emissive Cobalt(III) Complexes and Their Use in the Photocatalytic Trifluoromethylation of Polycyclic Aromatic Hydrocarbons. Angew. Chem., Int. Ed. 2018, 57, 8027–8031. 10.1002/anie.201802532. [DOI] [PubMed] [Google Scholar]
- Wenger O. S. A Bright Future for Photosensitizers. Nat. Chem. 2020, 12, 323–324. 10.1038/s41557-020-0448-x. [DOI] [PubMed] [Google Scholar]
- Ahn J. M.; Peters J. C.; Fu G. C. Design of a Photoredox Catalyst That Enables the Direct Synthesis of Carbamate-Protected Primary Amines via Photoinduced, Copper-Catalyzed N-Alkylation Reactions of Unactivated Secondary Halides. J. Am. Chem. Soc. 2017, 139, 18101–18106. 10.1021/jacs.7b10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz S. E.; Lotito K. J.; Fu G. C.; Peters J. C. Photoinduced Ullmann C-N Coupling: Demonstrating the Viability of a Radical Pathway. Science 2012, 338, 647–651. 10.1126/science.1226458. [DOI] [PubMed] [Google Scholar]
- Tan Y.; Muñoz-Molina J. M.; Fu G. C.; Peters J. C. Oxygen Nucleophiles as Reaction Partners in Photoinduced, Copper-Catalyzed Cross-Couplings: O-Arylations of Phenols at Room Temperature. Chem. Sci. 2014, 5, 2831–2835. 10.1039/C4SC00368C. [DOI] [Google Scholar]
- Bissember A. C.; Lundgren R. J.; Creutz S. E.; Peters J. C.; Fu G. C. Transition-Metal-Catalyzed Alkylations of Amines with Alkyl Halides: Photoinduced, Copper-Catalyzed Couplings of Carbazoles. Angew. Chem., Int. Ed. 2013, 52, 5129–5133. 10.1002/anie.201301202. [DOI] [PubMed] [Google Scholar]
- Do H.-Q.; Bachman S.; Bissember A. C.; Peters J. C.; Fu G. C. Photoinduced, Copper-Catalyzed Alkylation of Amides with Unactivated Secondary Alkyl Halides at Room Temperature. J. Am. Chem. Soc. 2014, 136, 2162–2167. 10.1021/ja4126609. [DOI] [PubMed] [Google Scholar]
- Ratani T. S.; Bachman S.; Fu G. C.; Peters J. C. Photoinduced, Copper-Catalyzed Carbon-Carbon Bond Formation with Alkyl Electrophiles: Cyanation of Unactivated Secondary Alkyl Chlorides at Room Temperature. J. Am. Chem. Soc. 2015, 137, 13902–13907. 10.1021/jacs.5b08452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D. T.; Choi J.; Muñoz-Molina J. M.; Bissember A. C.; Peters J. C.; Fu G. C. A Versatile Approach to Ullmann C-N Couplings at Room Temperature: New Families of Nucleophiles and Electrophiles for Photoinduced, Copper-Catalyzed Processes. J. Am. Chem. Soc. 2013, 135, 13107–13112. 10.1021/ja4060806. [DOI] [PubMed] [Google Scholar]
- Uyeda C.; Tan Y.; Fu G. C.; Peters J. C. A New Family of Nucleophiles for Photoinduced, Copper-Catalyzed Cross-Couplings via Single-Electron Transfer: Reactions of Thiols with Aryl Halides under Mild Conditions (O °C). J. Am. Chem. Soc. 2013, 135, 9548–9552. 10.1021/ja404050f. [DOI] [PubMed] [Google Scholar]
- Johnson M. W.; Hannoun K. I.; Tan Y.; Fu G. C.; Peters J. C. A Mechanistic Investigation of the Photoinduced, Copper-Mediated Cross-Coupling of an Aryl Thiol with an Aryl Halide. Chem. Sci. 2016, 7, 4091–4100. 10.1039/C5SC04709A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J. M.; Ratani T. S.; Hannoun K. I.; Fu G. C.; Peters J. C. Photoinduced, Copper-Catalyzed Alkylation of Amines: A Mechanistic Study of the Cross-Coupling of Carbazole with Alkyl Bromides. J. Am. Chem. Soc. 2017, 139, 12716–12723. 10.1021/jacs.7b07052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthus S. J.; Wilson R. K.; Vikas Aggarwal A.; Cameron S. A.; Larsen D. S.; Brooker S. Carbazole-Based N4-Donor Schiff Base Macrocycles: Obtained Metal Free and as Cu(II) and Ni(II) Complexes. Dalton Trans. 2017, 46, 3141–3149. 10.1039/C6DT04598G. [DOI] [PubMed] [Google Scholar]
- Malthus S. J.; Cameron S. A.; Brooker S. Improved Access to 1,8-Diformyl-Carbazoles Leads to Metal-Free Carbazole-Based [2 + 2] Schiff Base Macrocycles with Strong Turn-On Fluorescence Sensing of Zinc(II) Ions. Inorg. Chem. 2018, 57, 2480–2488. 10.1021/acs.inorgchem.7b02763. [DOI] [PubMed] [Google Scholar]
- Bordwell F. G.; Drucker G. E.; Fried H. E. Acidities of Carbon and Nitrogen Acids: The Aromaticity of the Cyclopentadienyl Anion. J. Org. Chem. 1981, 46, 632–635. 10.1021/jo00316a032. [DOI] [Google Scholar]
- Gimbert C.; Moreno-Mañas M.; Pérez E.; Vallribera A. Tributylphosphine, Excellent Organocatalyst for Conjugate Additions of Non-Nucleophilic N-Containing Compounds. Tetrahedron 2007, 63, 8305–8310. 10.1016/j.tet.2007.05.088. [DOI] [Google Scholar]
- Pia̧tek P.; Lynch V. M.; Sessler J. L. Calix[4]Pyrrole[2]Carbazole: A New Kind of Expanded Calixpyrrole. J. Am. Chem. Soc. 2004, 126, 16073–16076. 10.1021/ja045218q. [DOI] [PubMed] [Google Scholar]
- Arnold L.; Norouzi-Arasi H.; Wagner M.; Enkelmann V.; Müllen K. A Porphyrin-Related Macrocycle from Carbazole and Pyridine Building Blocks: Synthesis and Metal Coordination. Chem. Commun. 2011, 47, 970–972. 10.1039/C0CC03052J. [DOI] [PubMed] [Google Scholar]
- Wu T.; Kim T.; Yin B.; Wang K.; Xu L.; Zhou M.; Kim D.; Song J. Carbazole-Containing Porphyrinoid and Its Oligomers. Chem. Commun. 2019, 55, 11454–11457. 10.1039/C9CC06114B. [DOI] [PubMed] [Google Scholar]
- Maulbetsch T.; Kunz D. Carbenaporphyrins: No Longer Missing Ligands in N-Heterocyclic Carbene Chemistry. Angew. Chem., Int. Ed. 2021, 60, 2007–2012. 10.1002/anie.202013434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Westhuizen D.; Bezuidenhout D. I.; Munro O. Q. Cancer Molecular Biology and Strategies for the Design of Cytotoxic Gold(I) and Gold(III) Complexes: A Tutorial Review. Dalton Trans. 2021, 50, 17413–17437. 10.1039/D1DT02783B. [DOI] [PubMed] [Google Scholar]
- Westhuizen D.; Slabber C. A.; Fernandes M. A.; Joubert D. F.; Kleinhans G.; Westhuizen C. J.; Stander A.; Munro O. Q.; Bezuidenhout D. I. A Cytotoxic Bis(1,2,3-Triazol-5-Ylidene) Carbazolide Gold(III) Complex Targets DNA by Partial Intercalation. Chem.—Eur. J. 2021, 27, 8295–8307. 10.1002/chem.202100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Gao Y.; Chen H.; Liu Z.; Sun L. Water Oxidation Catalyzed by a Charge-Neutral Mononuclear Ruthenium(III) Complex. Dalton Trans. 2017, 46, 1304–1310. 10.1039/C6DT04160D. [DOI] [PubMed] [Google Scholar]
- Meyer T. J.; Huynh M. H. V. The Remarkable Reactivity of High Oxidation State Ruthenium and Osmium Polypyridyl Complexes. Inorg. Chem. 2003, 42, 8140–8160. 10.1021/ic020731v. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Ng S.-M.; Yiu S.-M.; Lam W. W. Y.; Wei X.-G.; Lau K.-C.; Lau T.-C. Catalytic Water Oxidation by Ruthenium(II) Quaterpyridine (Qpy) Complexes: Evidence for Ruthenium(III) Qpy-N,N’’-Dioxide as the Real Catalysts. Angew. Chem., Int. Ed. 2014, 53, 14468–14471. 10.1002/anie.201408795. [DOI] [PubMed] [Google Scholar]
- Duan L.; Xu Y.; Tong L.; Sun L. CeIV- and Light-Driven Water Oxidation by [Ru(Terpy)(Pic)3]2+ Analogues: Catalytic and Mechanistic Studies. ChemSusChem 2010, 4, 238–244. 10.1002/cssc.201000313. [DOI] [PubMed] [Google Scholar]
- Duan L.; Xu Y.; Gorlov M.; Tong L.; Andersson S.; Sun L. Chemical and Photochemical Water Oxidation Catalyzed by Mononuclear Ruthenium Complexes with a Negatively Charged Tridentate Ligand. Chem.—Eur. J. 2010, 16, 4659–4668. 10.1002/chem.200902603. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Wei Y.; Lu Z.; Chen X.; Wang D. A Steady Composite Molecular Anode RuI/MWCNTsCOOH/GC for Robust Catalytic Water Oxidation. J. Energy Chem. 2019, 35, 49–54. 10.1016/j.jechem.2018.10.013. [DOI] [Google Scholar]
- Mo X.; Chen B.; Zhang G. Copper-Catalyzed Enantioselective Sonogashira Type Coupling of Alkynes with α-Bromoamides. Angew. Chem., Int. Ed. 2020, 59, 13998–14002. 10.1002/anie.202000860. [DOI] [PubMed] [Google Scholar]
- Lei G.; Zhang H.; Chen B.; Xu M.; Zhang G. Copper-Catalyzed Enantioselective Arylalkynylation of Alkenes. Chem. Sci. 2020, 11, 1623–1628. 10.1039/C9SC04029C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X.; Huang H.; Zhang G. Tetrasubstituted Carbon Stereocenters via Copper-Catalyzed Asymmetric Sonogashira Coupling Reactions with Cyclic Gem-Dihaloketones and Tertiary α-Carbonyl Bromides. ACS Catal. 2022, 12, 9944–9952. 10.1021/acscatal.2c01973. [DOI] [Google Scholar]
- Zhang Y.; Sun Y.; Chen B.; Xu M.; Li C.; Zhang D.; Zhang G. Copper-Catalyzed Photoinduced Enantioselective Dual Carbofunctionalization of Alkenes. Org. Lett. 2020, 22, 1490–1494. 10.1021/acs.orglett.0c00071. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-F.; Dong X.-Y.; Cheng J.-T.; Yang N.-Y.; Wang L.-L.; Wang F.-L.; Luan C.; Liu J.; Li Z.-L.; Gu Q.-S.; et al. Enantioconvergent Cu-Catalyzed Radical C-N Coupling of Racemic Secondary Alkyl Halides to Access α-Chiral Primary Amines. J. Am. Chem. Soc. 2021, 143, 15413–15419. 10.1021/jacs.1c07726. [DOI] [PubMed] [Google Scholar]
- Dong X.-Y.; Li Z.-L.; Gu Q.-S.; Liu X.-Y. Ligand Development for Copper-Catalyzed Enantioconvergent Radical Cross-Coupling of Racemic Alkyl Halides. J. Am. Chem. Soc. 2022, 144, 17319–17329. 10.1021/jacs.2c06718. [DOI] [PubMed] [Google Scholar]
- Su X. L.; Ye L.; Chen J. J.; Liu X. D.; Jiang S. P.; Wang F. L.; Liu L.; Yang C. J.; Chang X. Y.; Li Z. L.; et al. Copper-Catalyzed Enantioconvergent Cross-Coupling of Racemic Alkyl Bromides with Azole C(Sp2)–H Bonds. Angew. Chem., Int. Ed. 2021, 60, 380–384. 10.1002/anie.202009527. [DOI] [PubMed] [Google Scholar]
- Zhou H.; Li Z. L.; Gu Q. S.; Liu X. Y. Ligand-Enabled Copper(I)-Catalyzed Asymmetric Radical C(Sp3)-C Cross-Coupling Reactions. ACS Catal. 2021, 11, 7978–7986. 10.1021/acscatal.1c01970. [DOI] [Google Scholar]
- Ma X.; Zhang G. Asymmetric Alkyl and Aryl/Azolation of Alkenes via a Single Cu(I) Complex. ACS Catal. 2021, 11, 5108–5118. 10.1021/acscatal.0c05576. [DOI] [Google Scholar]
- Li C.; Chen B.; Ma X.; Mo X.; Zhang G. Light-Promoted Copper-Catalyzed Enantioselective Alkylation of Azoles. Angew. Chem., Int. Ed. 2021, 60, 2130–2134. 10.1002/anie.202009323. [DOI] [PubMed] [Google Scholar]
- Greb L.; Ebner F.; Ginzburg Y.; Sigmund L. M. Element-Ligand Cooperativity with p-Block Elements. Eur. J. Inorg. Chem. 2020, 2020, 3030–3047. 10.1002/ejic.202000449. [DOI] [Google Scholar]
- Kundu S. Pincer-Type Ligand-Assisted Catalysis and Small-Molecule Activation by Non-VSEPR Main-Group Compounds. Chem. - An Asian J. 2020, 15, 3209–3224. 10.1002/asia.202000800. [DOI] [PubMed] [Google Scholar]
- Weetman C.; Inoue S. The Road Travelled: After Main-Group Elements as Transition Metals. ChemCatChem. 2018, 10, 4213–4228. 10.1002/cctc.201800963. [DOI] [Google Scholar]
- Yadav S.; Saha S.; Sen S. S. Compounds with Low-Valent p-Block Elements for Small Molecule Activation and Catalysis. ChemCatChem. 2016, 8, 486–501. 10.1002/cctc.201501015. [DOI] [Google Scholar]