Abstract
Background
Characterisation of anatomical distribution and the clinical impact of middle cerebral artery M2 (MCA-M2) segment occlusion and its subsequent cortical branches (CBs) in acute ischaemic stroke patients (AIS).
Methods
Retrospective, monocentric study analysing radiological and clinical data of AIS patients with MCA-M2 segment occlusion with regard to the anatomic distribution of MCA-M2 occlusion and its subsequent CB.
Results
A total of 203 patients (median age 77 (IQR 66–83) years, 112 women) were included. There was an equal distribution of right-sided versus left-sided MCA-M2 vessel occlusions (right: n=97; left: n=106), as well as with a median number of affected MCA-M2 CBs of 4 (IQR, 3–6) and a median National Institute of Health Stroke Scale score (NIHSS) on admission of 9 (3–15). For both hemispheres, CBs of the inferior trunks were significantly more affected than the superior trunks. Endovascular treatment (EVT, n=94) was associated with a significant better outcome compared with patients with medical management alone (p=0.027).
Conclusion
In acute MCA-M2 segment occlusions, inferior trunks are significantly more affected compared with the superior trunks. The subsequent CBs of the paracentral region of both hemispheres are more commonly involved. In eloquent vascular territories, EVT was more often performed.
Keywords: ANATOMY, STROKE, CEREBROVASCULAR DISEASE, INTERVENTIONAL
WHAT IS ALREADY KNOWN ON THIS TOPIC.
High-quality data for medium-vessel occlusions (MeVO) endovascular treatment (EVT) is still lacking. For the optimisation of the treatment strategy for MeVO—especially in middle cerebral artery (MCA)-M2 occlusions—anatomical considerations are of utmost importance.
WHAT THIS STUDY ADDS
Anatomical distribution of MCA-M2 occlusions and its subsequent affected cortical branches of the downstream territories in acute ischaemic stroke patients both in patients with and without EVT.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Better understanding of anatomical constellation in MeVO patients with MCA-M2 occlusions.
Introduction
Medium-vessel occlusions (MeVO) accounts for 25%–40% of all acute ischaemic strokes (AIS).1 Among those, middle cerebral artery M2 (MCA-M2) segment occlusion is an important subgroup due to its arterial supply of several eloquent vascular territories such as the central region. Given the only weak evidence of efficacy and outcome, endovascular treatment (EVT) of (MCA-M2) occlusions have long been controversial.2 Whereas studies of natural course in patients with MCA-M2 occlusion revealed poor outcomes,3 subgroup meta-analysis from Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials (HERMES) collaboration group and recent data regarding EVT of AIS patients with MCA-M2 occlusions showed promising results regarding good clinical outcome and safety.4–6 However, high-quality data for MeVO EVT is still lacking.7 Beside technical issues, this is related to the clinical-anatomical difficulties about the operational definition of MCA-M2, the anatomical distribution of cortical branches (CB) and its potential clinical impact.8 9 For the optimisation of the treatment strategy for MeVO—especially in MCA-M2 occlusions—anatomical considerations are of utmost importance.
In this study, we aimed to analyse the anatomical distribution and the clinical impact of MCA-M2 occlusions and its subsequent affected CB of the downstream territories in AIS patients both in patients with and without EVT.
Material and methods
Patients
We retrospectively analysed the clinical and radiological data of all patients with an acute MCA-M2 occlusion at our tertiary stroke centre from 2014 to 2020 irrespective of the treatment modality. Patients had to be older than 18 years of age and have to have a radiologically proven MCA-M2 segment occlusion.
The decision and treatment modality (EVT vs medical management) was based on the respective joint decision between the treating neurologist and the interventionalist for each patient in the acute setting.
Methods
All acute vascular images (CT scans with CT angiography (n=196), digital subtraction angiography (n=120) or MRI scan with MRI angiography (time of flight, TOF; n=8) were reviewed and visually qualitatively assessed by experienced neuroradiologists. MCA-M2 segment was considered as the distal MCA branches in the Sylvian fissure arising from the main bifurcation (postbifurcation) of the MCA-M1 to the genu at the level of the operculum.4 The subsequent CBs were defined according to the anatomical definition from Gibo et al.10 MCA-M2 segments were considered as dominant (supplying >50% of vascular territory of the MCA), codominant (supplying 50% of vascular territory of the MCA) and non-dominant (supplying <50% of the vascular territory of the MCA).6
Statistical analysis
Statistical analysis was performed using Stata/IC V.14.2 (StataCorp). For group comparison, either t-test or Mann-Whitney U test was used. Univariate logistic regression analysis was performed for favourable (modified Rankin Score, mRS 0–2) and poor outcome (mRS 6). A p<0.05 was considered to be statistically significant.
Results
A total of 203 patients (median age 77 years (IQR 66–83 years), 112 (55%) women) were included in this study (table 1). The most common vascular risk factor was hypertension (n=150), followed by dyslipidaemia (n=100). Median National Institute of Health Stroke Scale (NIHSS) score on admission was 9 (IQR, 3–15). Median hospital stay was 8 days (IQR 6–11 days). On clinical follow-up, median mRS was 2 (IQR 1–5). Favourable outcome (mRS 0–2) was achieved in 57% of the patients (n=116), and 24% of the patients (n=48) died within follow-up.
Table 1.
Clinical and anatomical characteristics of the study population
| Characteristics | N=203 |
| Age in years—median (IQR) | 77 (66–83) |
| Women—no (%) | 112 (55) |
| Occlusion side—no (%) | R=97; L=106 |
| NIHSS on admission—median (IQR) | 9 (3–15) |
| Hospital stay in days—median (IQR) | 8 (6–11) |
| mRS on follow-up—median (IQR) | 2 (1–5) |
| Favourable outcome (mRS 0–2)—no (%) | 116 (57) |
| Mortality rate on follow-up—no (%) | 48 (24) |
| Hypertension—numbers (%) | 150 (74) |
| Dyslipidaemia—numbers (%) | 100 (49) |
| Atrial fibrillation—numbers (%) | 88 (43) |
| Nicotine abuse—numbers (%) | 46 (23) |
| Diabetes mellitus—numbers (%) | 35 (17) |
| Affected CB—median (IQR) | 4 (3-6) |
| Superior trunk—no | R: 45; L: 51 |
| Inferior trunk right side—no | R: 64; L: 69 |
| Dominant—no | R: 9; L: 15 |
| Codominant—no | R: 66; L: 75 |
| Non-dominant—no | R: 15; L: 22 |
| Endovascular treatment—no (%) | 94 (46) |
| Time from onset to groin-puncture in minutes—median (IQR; n=80) | 220 (175–304) |
| Time from onset to recanalisation in minutes—median (IQR, n=80) | 307 (223–410) |
| Excellent mTICI (3) after EVT |
CB, cortical branch; EVT, endovascular treatment; L, left; mRS, modified Rankin Scale Score; mTICI, modified Treatment in Cerebral Ischaemia Score; NIHSS, National Institute of Health Stroke Scale Score; R, right.
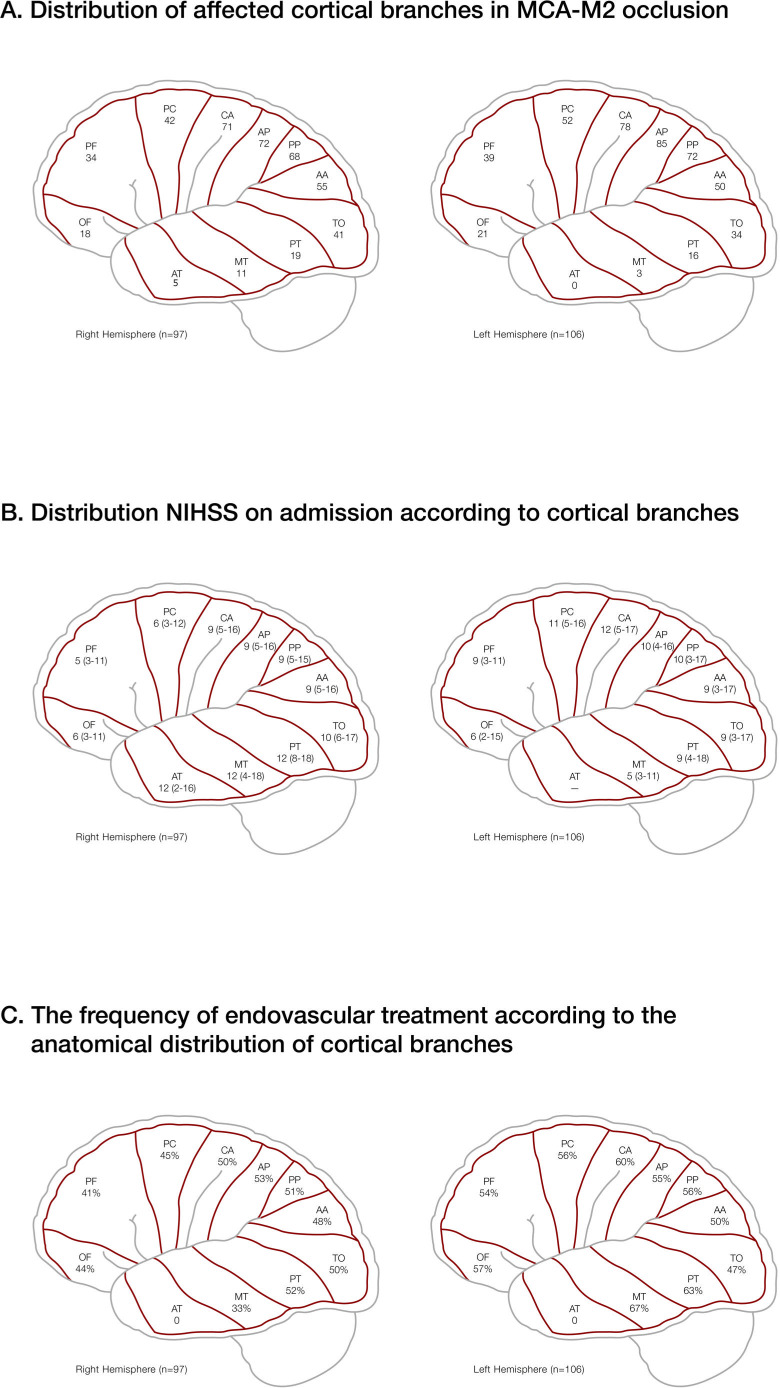
There was a nearly equal involvement for both hemispheres, with 97 MCA-M2 vessel occlusions on the right side and 107 MCA-M2 vessel occlusions on the left side. Median number of affected MCA-M2 CB was 4 (IQR, 3–6). For both hemispheres, the most commonly occluded MCA-M2 CB were located in the paracentral region of the MCA vascular territory, less commonly occluded MCA-M2 CB were located in the frontal and temporal region of the MCA vascular territory (figure 1A). The more paracentral the involved MCA-M2 CB, the higher the median NIHSS score on admission. However, there was a tendency for higher median NIHSS scores on admission on the left hemisphere (left: 10, IQR 4–16, right: 8, IQR 3–10; p=0.07), with a higher NIHSS scores on admission for the right-sided parietotemporal region, and a higher NIHSS score on admission for the paracentral region of the left side, respectively (figure 1B). For both hemispheres (left: p=0.046; right: p=0.036), CBs of the inferior trunks were significantly more occluded than the superior trunks.
Figure 1.
(A) Distribution of the affected downstream cortical branches in MCA-M2 segment occlusions, (B) NIHSS on admission and frequency of EVT according to anatomical distribution for both hemispheres. AA, angular artery; AP, anterior parietal artery; AT, anterior temporal artery; CA, central artery; EVT, endovascular treatmen; MCA, middle cerebral artery; MT, middle temporal artery; NIHSS, National Institute of Health Stroke Scale; OF, orbitofrontal artery; PC, precentral artery; PF, prefrontal artery; PP, posterior parietal artery; PT, posterior temporal artery; TO, temporo-occipital artery.
Irrespective of treatment modalities, a better outcome was observed when the left posterior parietal artery (p=0.02), the right temporo-occipital artery (p=0.03) and the right posterior temporal artery (p=0.014) were involved. However, there was no statistical difference regarding poor outcome for all subsequent MCA-M2 CB. No statistical differences regarding outcome were found for dominant, codominant or non-dominant MCA-M2 occlusions, irrespective of the affected side (data not shown). In the univariate logistic regression models, there was no association between favourable or poor outcome for any of the CBs. These results might reflect the relatively small subgroup sizes of each CB.
In 46% (n=93) of the cases, EVT was performed with median time from onset to groin puncture of 220 min (IQR 175–304 min, n=80) and median time from onset to recanalisation of 307 min (IQR 223–410 min, n=80), respectively. Of these cases, in 22% (n=20) briding intravenous thrombolysis was performed.
Furthermore, EVT was performed significantly more frequent if the following CB’s were involved: central artery (p=0.00001; anterior parietal artery (p=0.0004); posterior parietal artery (p=0.006) and anterior temporal artery (p=0.03), respectively. The frequency of EVT was higher for the central region in both hemispheres and the temporal region on the left side (figure 1C). However, there was no significant difference regarding the side of hemispheres (p=0.41). Patients treated with EVT (n=94) revealed a significantly better outcome (mRS 0–2; n=53%) compared with medical management alone (n=46; p=0.027). Of those, 91% (n=86) revealed an modified Treatment in Cerebral Ischaemia (mTICI) 2b-3 in the final angio-runs. Excellent angiographic results (mTICI 3) were achieved in 42% (n=40) of the patients. Among all EVT treated patients, only 2 (2%) patients had a severe adverse event. In one patient, a perforation of the common iliacal artery occurred during the access manoeuvre resulting in a fatal haemorrhagic shock despite emergency surgery. In the other patient, perforation of an intracranial vessel occurred that results in small non-fatal intracranial haematoma.
Discussion
In this study, CBs of the inferior trunk were significantly more affected than CB of the superior trunk in MCA-M2 occlusions. In addition, CBs of the paracentral region are more commonly affected compared with frontal and temporal region. In the right hemisphere, median NIHSS on admission was higher in the parietotemporal stroke, whereas in the left hemisphere the NIHSS on admission was higher in the paracentral stroke.
EVT is now the standard procedure in AIS patients with large vessel occlusions.11 However, it is still controversial whether and how to treat MeVO.1 These controversies are partially related to the clinical-anatomical difficulties of defining appropriate operational terms for the MCA-M2 segments and its subsequent CB, as well as its clinical relevance in context of EVT.12 Different classification systems are available such as the classic description of MCA-M2 segments proposed by Fisher with its anatomical boundaries related to the perisylvian region, and the classification system from the Interventional Management of Stroke Trial III Investigators proposed by Tomsick et al defining an M2 trunk as a continuation of the distal MCA-M1 segment.9 10 13 14 In this study, the MCA-M1 postbifurcation branches were defined as proximal MCA-M2 segments.4 The wide variation of the branching pattern has a strong impact on the vascular territory at risk and leads to various clinical presentations.15 Even small vessel occlusions can lead to severe neurological deficits depending on the eloquence of the affected vascular territory.16 Thus, we looked at the different MeVO of the MCA. We observed a more paracentral segment distribution of CB involvement in acute MCA-M2 occlusions compared with the frontal and temporal segments. This might be related to the anatomical MCA-M2 segment conditions, that favours clot migration into the inferior trunk due to its mostly straight course and larger diameter compared with the superior trunk.10 15 In addition, this supports that CBs of the inferior trunk are significantly more affected compared with the superior trunk for both cerebral hemispheres. Contrary to the findings of Seker et al,8 we did not observe any differences and associations regarding poor outcome in various anatomical constellations. There was also no clinical difference regarding dominant, codominant or non-dominant distribution of the MCA-M2 segments.
In this study, mostly four CBs have been involved in acute MCA-M2 segment occlusion that might be also reflected by the median NIHSS on admission of nine. This indicates that most of these patients presented with a truncal occlusion since the mean number of CB for superior or inferior trunks of the MCA-M2 are 4.5 and 4.0 CB, respectively.17 In addition, involvement of key CB—such as the paracentral region of the left hemisphere or the parietotemporal region of the right hemisphere—revealed higher NIHSS scores.
In line with several previous studies,4–6 AIS patients with MCA-M2 occlusion treated with EVT revealed a better outcome compared with the patients with medical management—including intravenous thrombolysis—suggesting that EVT is beneficial for AIS patients with MCA-M2 occlusions, especially if eloquent vascular territories are targeted. Thus, EVT was more commonly performed if CB of the paracentral region on both side and on the temporal region on the left side were involved presumably due to higher NIHSS on admissions and the eloquence vascular territories.
Limitations of this study include relatively small sample size, the retrospective nature of the data, single-centre experience and the lack of evaluation from an independent core laboratory and CT perfusion (CTP) data due to heterogeneity of the available CTP data. Also, we have done qualitative, not quantitative assessment of the data and our findings relate more to the location of the territory at risk than its volume.
Conclusion
In MCA-M2 segment occlusions, the inferior trunks are significantly more affected compared with the superior trunks. The subsequent CB of the paracentral region of both hemispheres are more commonly involved. EVT was more commonly performed, if eloquent vascular territories were involved.
Acknowledgments
We are grateful to Maria Castro for the artist drawing of figure 1.
Footnotes
Contributors: PG and LR contributed to conception and design of the study. PV and RS organised the database. JB performed the statistical analysis. PG and PV wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version. PG and PV shared first authorship. PG and LR both are the guarantors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethik Kommission Nordwest- und Zentralschweiz (EKNZ). It was a waived consent study approved by the local ethic committee (EKNZ) according to Swiss law.
References
- 1.Ospel JM, Goyal M. A review of Endovascular treatment for medium vessel occlusion stroke. J Neurointerv Surg 2021;13:623–30. 10.1136/neurintsurg-2021-017321 [DOI] [PubMed] [Google Scholar]
- 2.Cho YH, Choi JH. Mechanical Thrombectomy for acute ischemic stroke with occlusion of the M2 segment of the middle cerebral artery: A literature review. J Cerebrovasc Endovasc Neurosurg 2021;23:193–200. 10.7461/jcen.2021.E2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima FO, Furie KL, Silva GS, et al. Prognosis of untreated strokes due to anterior circulation proximal intracranial arterial Occlusions detected by use of computed tomography angiography. JAMA Neurol 2014;71:151–7. 10.1001/jamaneurol.2013.5007 [DOI] [PubMed] [Google Scholar]
- 4.Saber H, Narayanan S, Palla M, et al. Mechanical Thrombectomy for acute ischemic stroke with occlusion of the M2 segment of the middle cerebral artery: a meta-analysis. J Neurointerv Surg 2018;10:620–4. 10.1136/neurintsurg-2017-013515 [DOI] [PubMed] [Google Scholar]
- 5.Nakano T, Shigeta K, Ota T, et al. Efficacy and safety of mechanical Thrombectomy for occlusion of the second segment of the middle cerebral artery: retrospective analysis of the Tama-Registry of acute Endovascular Thrombectomy (TREAT). Clin Neuroradiol 2020;30:481–7. 10.1007/s00062-019-00810-3 [DOI] [PubMed] [Google Scholar]
- 6.Menon BK, Hill MD, Davalos A, et al. Efficacy of Endovascular Thrombectomy in patients with M2 segment middle cerebral artery Occlusions: meta-analysis of data from the HERMES collaboration. J Neurointerv Surg 2019;11:1065–9. 10.1136/neurintsurg-2018-014678 [DOI] [PubMed] [Google Scholar]
- 7.Shapiro M, Raz E, Nossek E, et al. Neuroanatomy of the middle cerebral artery: implications for Thrombectomy. J Neurointerv Surg 2020;12:768–73. 10.1136/neurintsurg-2019-015782 [DOI] [PubMed] [Google Scholar]
- 8.Seker F, Pfaff J, Neuberger U, et al. Comparison of superior and inferior division Occlusions treated with Endovascular Thrombectomy. Clin Neuroradiol 2020;30:339–43. 10.1007/s00062-019-00767-3 [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Menon BK, Krings T, et al. What constitutes the M1 segment of the middle cerebral artery J Neurointerv Surg 2016;8:1273–7. 10.1136/neurintsurg-2015-012191 [DOI] [PubMed] [Google Scholar]
- 10.Gibo H, Carver CC, Rhoton AL, et al. Microsurgical anatomy of the middle cerebral artery. J Neurosurg 1981;54:151–69. 10.3171/jns.1981.54.2.0151 [DOI] [PubMed] [Google Scholar]
- 11.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for Healthcare professionals from the American heart Association/American stroke Association. Stroke 2019;50:e344–418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 12.Coutinho JM, Liebeskind DS, Slater L-A, et al. Mechanical Thrombectomy for isolated M2 Occlusions: A post hoc analysis of the STAR, SWIFT, and SWIFT PRIME studies. AJNR Am J Neuroradiol 2016;37:667–72. 10.3174/ajnr.A4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomsick TA, Carrozzella J, Foster L, et al. Endovascular therapy of M2 occlusion in IMS III: role of M2 segment definition and location on clinical and Revascularization outcomes. AJNR Am J Neuroradiol 2017;38:84–9. 10.3174/ajnr.A4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer E. Die Lageabweichung der Vorderen Hirnarterien Im Gefässbild. Zentralbl Neurochir 1938;3:300–13. [Google Scholar]
- 15.Compagne KCJ, van der Sluijs PM, van den Wijngaard IR, et al. Endovascular treatment: the role of dominant caliber M2 segment occlusion in ischemic stroke. Stroke 2019;50:419–27. 10.1161/STROKEAHA.118.023117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan Y, Yang IH, Orru E, et al. Endovascular Thrombectomy for distal occlusion using a semi-deployed Stentriever: report of 2 cases and technical NOTE. Neurointervention 2019;14:137–41. 10.5469/neuroint.2019.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai SB, Varma RG, Kulkarni RN. Microsurgical anatomy of the middle cerebral artery. Neurol India 2005;53:186–90. 10.4103/0028-3886.16406 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.