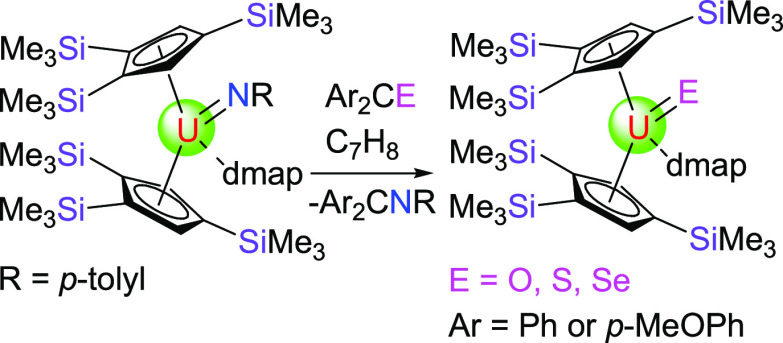
Abstract
Terminal uranium oxido, sulfido, and selenido metallocenes were synthesized, and their reactivity was comprehensively studied. Heating of an equimolar mixture of [η5-1,2,4-(Me3Si)3C5H2]2UMe2 (2) and [η5-1,2,4-(Me3Si)3C5H2]2U(NH-p-tolyl)2 (3) in the presence of 4-dimethylaminopyridine (dmap) in refluxing toluene forms [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4), which is a useful precursor for the preparation of the terminal uranium oxido, sulfido, and selenido metallocenes [η5-1,2,4-(Me3Si)3C5H2]2U=E(dmap) (E = O (5), S (6), Se (7)) employing a cycloaddition–elimination methodology with Ph2C=E (E = O, S) or (p-MeOPh)2CSe, respectively. Metallocenes 5–7 are inert toward alkynes, but they act as nucleophiles in the presence of alkylsilyl halides. The oxido and sulfido metallocenes 5 and 6 undergo [2 + 2] cycloadditions with isothiocyanate PhNCS or CS2, while the selenido derivative 7 does not. The experimental studies are complemented by density functional theory (DFT) computations.
Introduction
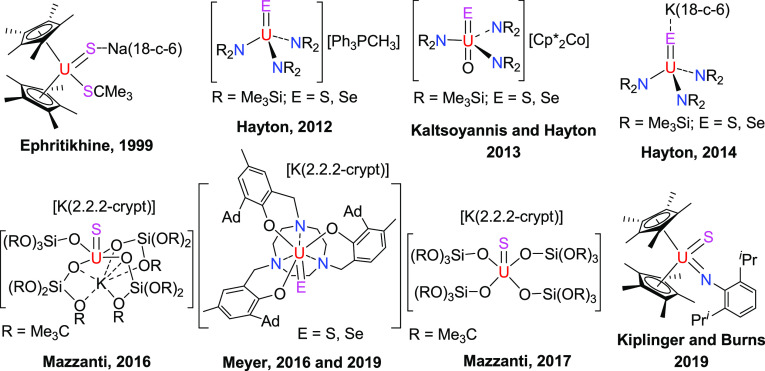
Attributed to their unique structural properties and their potential applications in group transfer and catalysis, organoactinide complexes featuring terminal metal–ligand multiple bonds have been intensively studied over the past two decades.1 These research activities focused on oxido and chalcogenido organoactinide complexes in particular1−5 due to the ubiquity of these functionalities in actinide chemistry, as shown by the prevalence of binary oxides and sulfides in the solid state.6 In this context, well-defined molecular structures will not only advance our understanding of the bonding and reactivity of the An=E (O, S, Se, Te) functional groups but also enable us to uncover novel and potentially useful transformations. For example, chlorocarbons convert on solid U3O8 to yield COx and HCl.7 This unusual reaction occurs at the U–O moieties on the U3O8 surface. Over the years, many oxido organouranium complexes have been prepared, which certainly enhanced the structural library, but their intrinsic reactivity was rather limited.2,3 One notable exception, however, constitutes [η5-1,2,4-(Me3C)3C5H2]2U=O whose reactivity toward alkyl halides was studied in more detail.3e Moving to the heavier chalcogenides S and Se, structurally authenticated terminal sulfido complexes have so far been limited to the derivatives, such as [Na(18-crown-6)][(η5-Me5C5)2UIV(=S)(SCMe3)],5a [Ph3PMe][UIV(=S)[N(SiMe3)2]3],5b [(η5-C5Me5)2Co][UVI(=S)(=O)[N(SiMe3)2]3],5d [K(18-crown-6)][UIV(=S)[N(SiMe3)2]3],5e [(S=)UIV(OSi(OtBu)3)4K][K(2.2.2-crypt)],5h [((Ad,MeArO)3tacn)UIV(=S)][K(2.2.2-crypt)],5i [K(2.2.2-cryptand)][UV(=S){OSi(OtBu)3}4],5k and (η5-C5Me5)2UVI(=S)( =N-2,6-iPr2C6H3) (Figure 1),5l and only a few examples of the uranium complexes containing a terminal selenido functionality, such as [Ph3PMe][UIV(=Se)[N(SiMe3)2]3],5b [(η5-C5Me5)2Co][UVI(=Se)( =O)[N(SiMe3)2]3],5d [K(18-crown-6)][UIV(=Se)(NR2)3],5f and [K(2.2.2-crypt)][((Ad,MeArO)3tacn)UIV(=Se)],5m have been reported (Figure 1). Moreover, the reactivity of the terminal sulfido and selenido actinide complexes has so far remained elusive and this also extends to the underlying structure–reactivity relationship.5 This renders the synthesis of novel actinide oxido and chalcogenido metallocenes an interesting but still challenging synthetic target for which bulky ligands are indispensable.3e Besides structural aspects and the reactivity of organoactinide complexes in small molecule activation,8 research at the bottom of the periodic table is also driven by the more fundamental question dealing with the impact of the 6d and 5f orbitals on structure, bonding, and reactivity.9 Following up on these research lines, we have investigated actinide complexes featuring terminal metal–ligand multiple bonds and explored their reactivity.10 For these studies, the sterically encumbered cyclopentadienyl ligand, 1,2,4-(Me3C)3C5H2, has been our ligand of choice stabilizing the terminal actinide oxido metallocenes [η5-1,2,4-(Me3C)3C5H2]2AnIV=O(dmap) (An = Th, U), which readily react with small molecules such as Me3SiCl, Ph2CE (E = O, S) and CS2.3e,10b Furthermore, only two examples of terminal actinide sulfido metallocenes and no examples of terminal actinide selenido metallocenes have been structurally authenticated,5a,5l so we decided to extend these investigations to terminal uranium oxido and chalcogenido metallocenes featuring two 1,2,4-(Me3Si)3C5H2 ligands. Although 1,2,4-(Me3Si)3C5H2 is closely related to its counterpart 1,2,4-(Me3C)3C5H2, this ligand is less sterically demanding and more electron-deficient than 1,2,4-(Me3C)3C5H2. In this contribution, we detail the synthesis of the terminal uranium oxido, sulfido, and selenido complexes, [η5-1,2,4-(Me3Si)3C5H2]2UIV=E(dmap) (E = O (5), S (6), Se (7)), and their reactivity. We also compare the reactivity of these derivatives to those of thorium(IV) and group 4 metallocenes.
Figure 1.
Selected crystallographically authenticated terminal uranium sulfido and selenido species.
Results and Discussion
Synthesis of [η5-1,2,4-(Me3Si)3C5H2]2UIV=E(dmap) (E = O (5), S (6), Se (7))
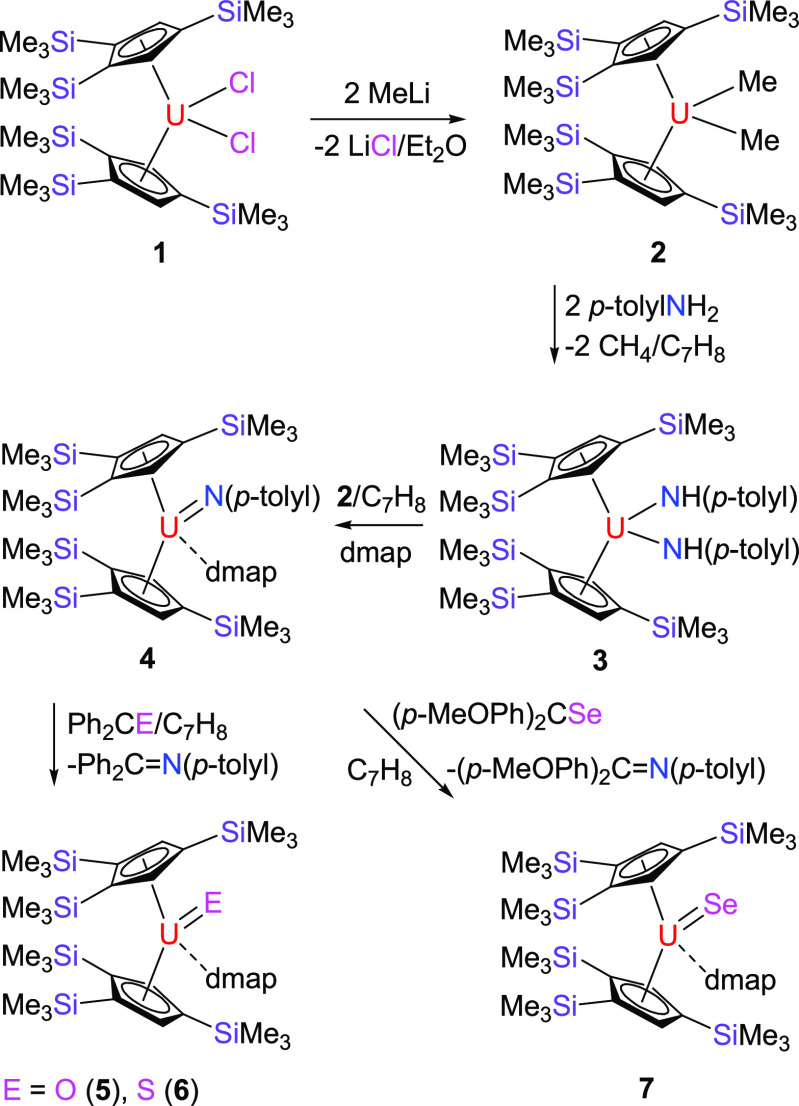
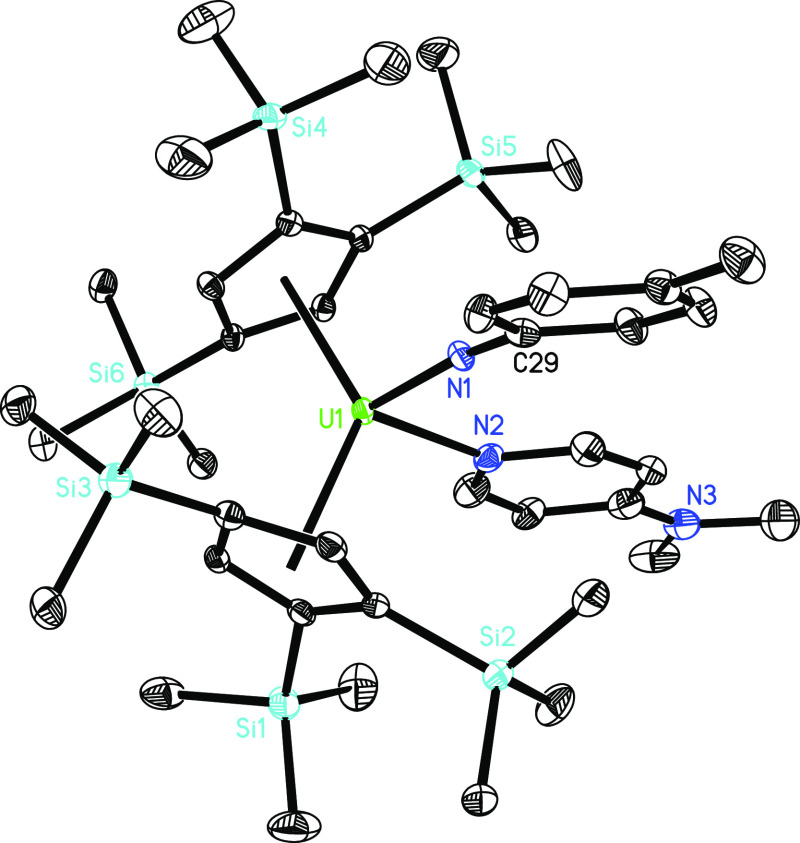
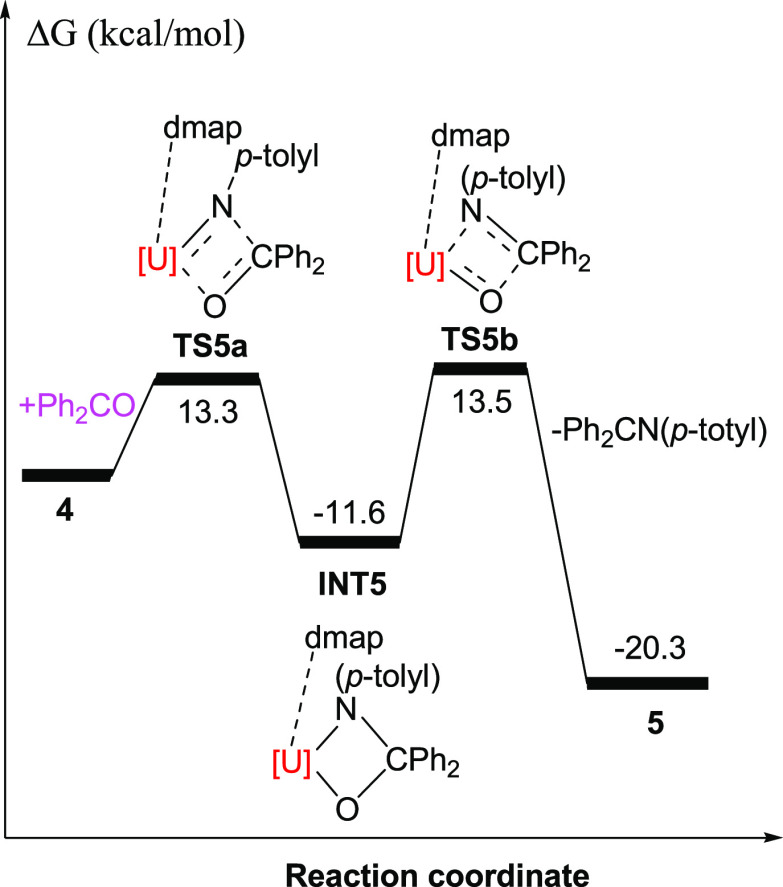
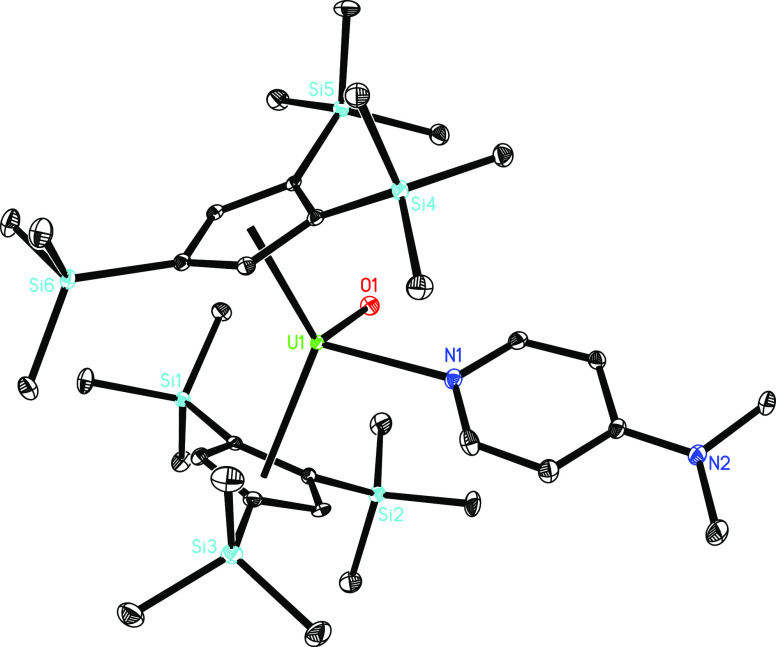
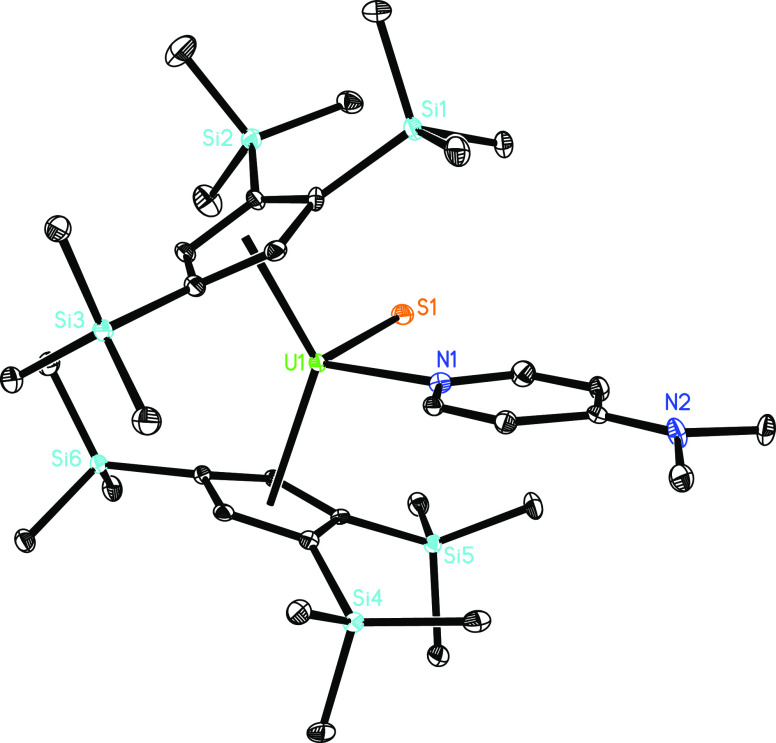
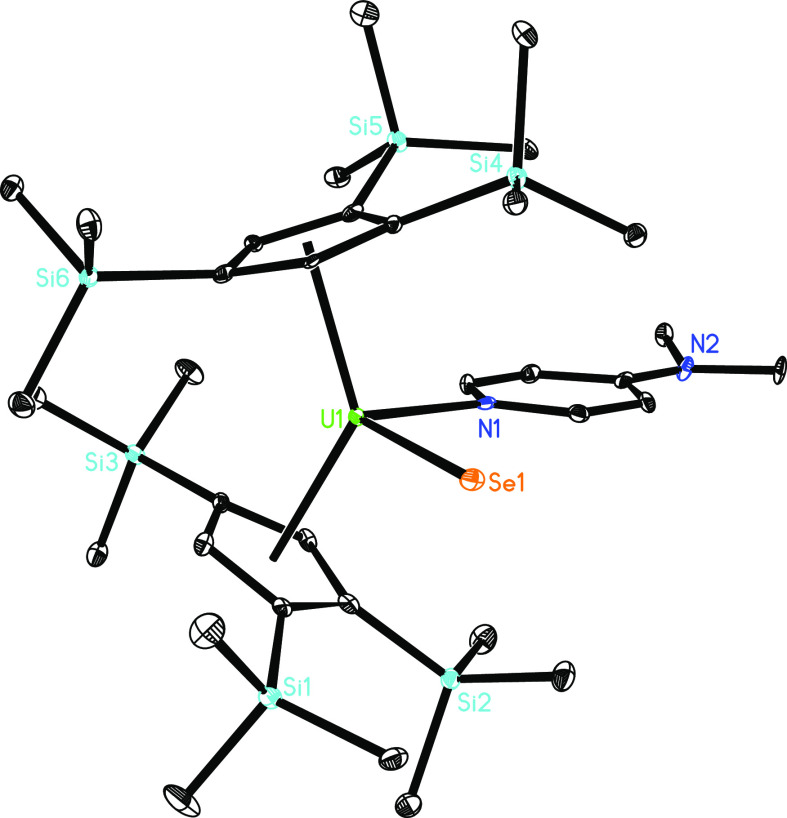
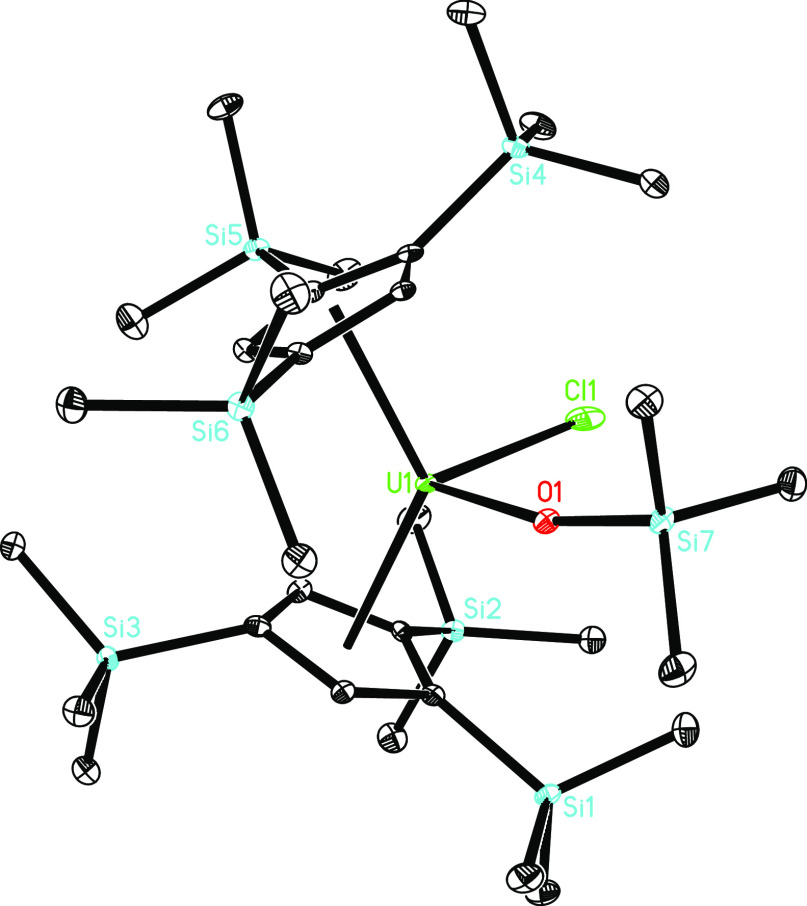
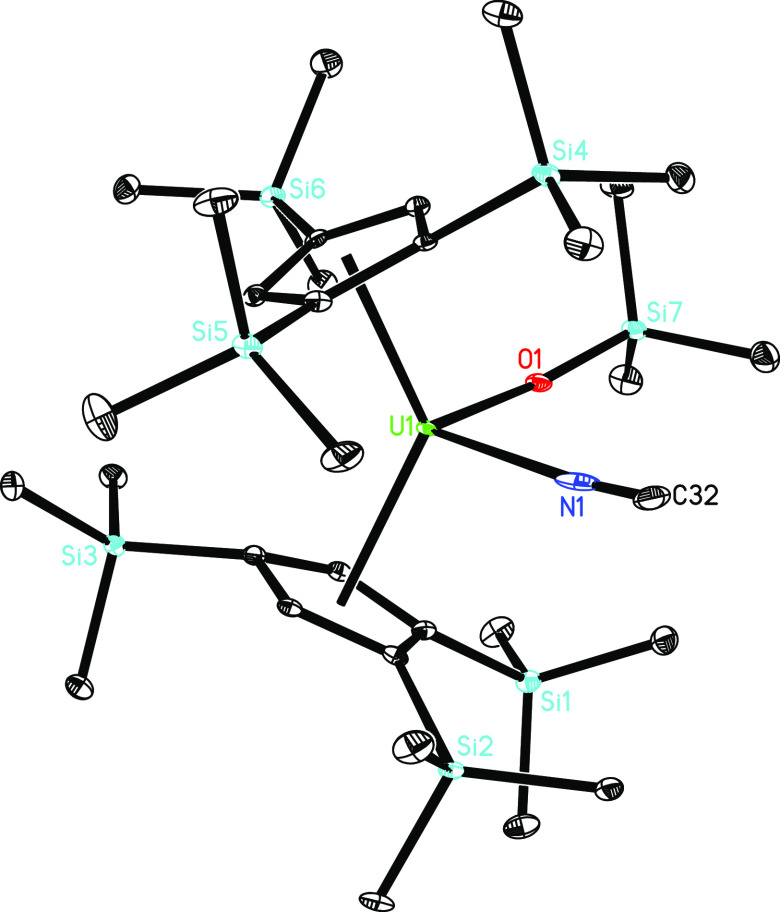
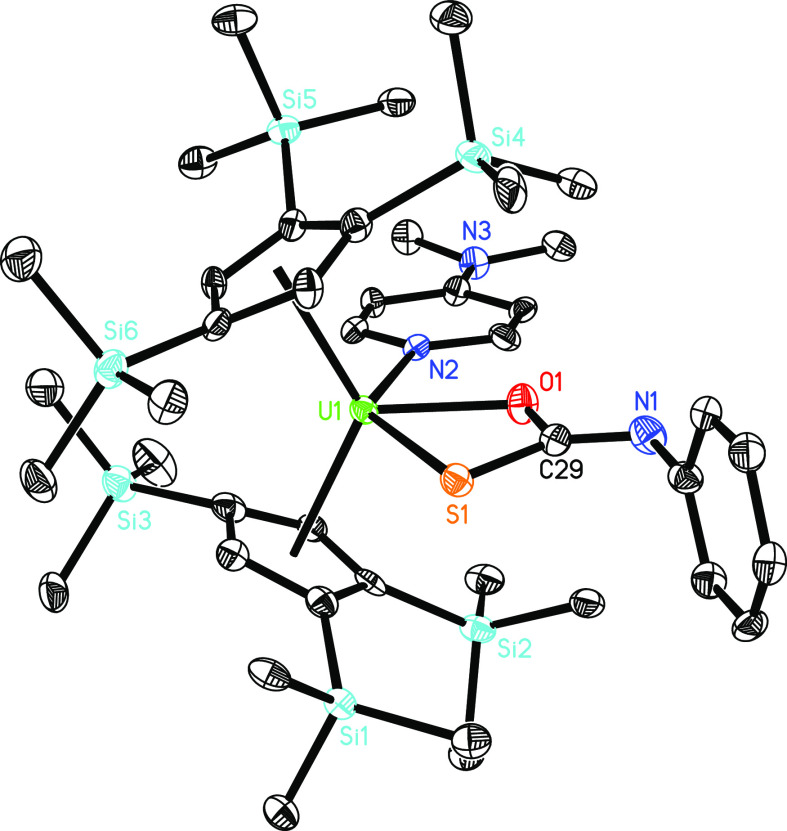
Salt metathesis between [η5-1,2,4-(Me3Si)3C5H2]2UCl2 (1) and 2 equiv of MeLi in diethyl ether affords [η5-1,2,4-(Me3Si)3C5H2]2UMe2 (2) in 82% yield. The subsequent reaction of 2 with 2 equiv of p-toluidine in toluene gives [η5-1,2,4-(Me3Si)3C5H2]2U(NH-p-tolyl)2 (3) in excellent yield (93%). Heating of an equimolar mixture of 2 and 3 in the presence of 4-dimethylaminopyridine (dmap) in refluxing toluene proceeds cleanly furnishing the uranium imido complex [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4) in 87% yield (Scheme 1). The molecular structure of 4 is shown in Figure 2; for selected bond distances and angles, refer to Table 1. Complex 4 features two very different U–N bond distances of 2.426(6) and 2.021(5) Å for U-N(2) and U-N(1), respectively, which are consistent with a dative bond between the dmap ligand and the U(IV) atom and the formation of a uranium–imido complex, respectively. A similar uranium–imido bond distance of 1.988(5) Å was found in the closely related derivative [η5-1,2,4-(Me3C)3C5H2]2U=N(p-tolyl).3e In line with the reaction of the actinide imidos [η5-1,2,4-(Me3C)3C5H2]2An=N(p-tolyl) (An = Th, U) with Ph2CO,3e,10b treatment of 4 with 1 equiv of Ph2CO results in the isolation of the terminal oxido derivative, [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5), in good yield (Scheme 1). DFT investigations imply that 4 initially engages in a [2 + 2] cycloaddition with Ph2C=O to give the heterocyclic intermediate INT5. The formation of INT5 is energetically favorable (ΔG(298 K) = −11.6 kcal/mol) and proceeds via the transition state TS5a with a reaction barrier of ΔG‡(298 K) = 13.3 kcal/mol (Figure 3). However, the degradation of INT5 to form the oxido compound 5 and Ph2C=N(p-tolyl) is more thermodynamically preferred (ΔG(298 K) = −20.3 kcal/mol) and proceeds via the transition state TS5b with a reaction barrier of ΔG‡(298 K) = 25.1 kcal/mol (Figure 3). This profile is consistent with the experimentally observed formation of 5 at ambient temperature. The molecular structure of 5 is provided in Figure 4; for selected bond distances and angles, see Table 1. The U–O and U–N distances are 1.873(2) and 2.526(3) Å, respectively, which are comparable to those established for [η5-1,2,4-(Me3C)3C5H2]2U=O(dmap) with a U–O distance of 1.860(3) Å and U–N distance of 2.535(4) Å3e and [η5-1,2,4-(Me3C)3C5H2]2U=O(py) with a U–O distance of 1.874(4) Å and U–N distance of 2.589(5) Å.11 Nevertheless, the Cp(cent)–U–Cp(cent) angle is 128.2(1)°, which is smaller than those found in [η5-1,2,4-(Me3C)3C5H2]2U=O(dmap) (141.7(4)°)3e and [η5-1,2,4-(Me3C)3C5H2]2U=O(py) (139.2(2)°).11 This suggests a much more open coordination sphere at the uranium atom in 5, which allows stabilizing ligands to coordinate, and therefore this difference reflects the reactivity of these complexes.3e In contrast to the reaction of the thorium derivative [η5-1,2,4-(Me3C)3C5H2]2Th=N(p-tolyl) with Ph2CS in the presence of dmap,10b the terminal uranium sulfido metallocene, [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6), can be isolated from the reaction of 4 with 1 equiv of Ph2CS in good yield (Scheme 1). Figure 5 shows the molecular structure of 6 and selected bond distances and angles are provided in Table 1. Complex 6 is the third representative of structurally authenticated actinide sulfido metallocenes and is therefore a significant addition to the other two actinide sulfido derivatives, [Na(18-crown-6)][(η5-Me5C5)2UIV(=S)(SCMe3)] and (η5-C5Me5)2UVI(=S)(=N-2,6-iPr2C6H3).5a,5l The U–N distance of 2.509(5) Å is comparable to those found for compounds 4 and 5 (Table 1). The U–S distance of 2.437(1) Å can be related to those found in [Na(18-crown-6)][(η5-Me5C5)2UIV(=S)(SCMe3)] (2.477(2) and 2.462(2) Å),5a [Ph3PMe][UIV(=S)[N(SiMe3)2]3] (2.4805(5) Å),5b [(C5Me5)2Co][UVI(=S)(=O)[N(SiMe3)2]3] (2.390(8) Å),5d [K(18-crown-6)][UIV(=S)[N(SiMe3)2]3] (2.4463(6) Å),5e [(S=)UIV(OSi(OtBu)3)4K][K(2.2.2-crypt)] (2.5220(14) Å),5h [((Ad,MeArO)3tacn)UIV(=S)][K(2.2.2-crypt)] (2.536(2) Å),5i [K(2.2.2-cryptand)][UV(=S){OSi(OtBu)3}4] (2.376(5) Å),5k and (η5-C5Me5)2UVI(=S)(=N-2,6-iPr2C6H3) (2.363(1) Å),5l further supporting the formation of a uranium metallocene featuring a terminal U=S bond. Moreover, treatment of 4 with 1 equiv of (p-MeOPh)2CSe yields the terminal selenido derivative, [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7) (Scheme 1). Figure 6 shows the molecular structure of 7; for selected bond distances and angles, see Table 1. To the best of our knowledge, 7 constitutes the first structurally authenticated terminal selenido actinide metallocene, and it also expands the limited family of structurally characterized actinide selenido complexes, comprising [Ph3PMe][UIV(=Se)[N(SiMe3)2]3],5b [(C5Me5)2Co][UVI(=Se)( =O)[N(SiMe3)2]3],5d [K(18-crown-6)][UIV(=Se)(NR2)3],5f and [K(2.2.2-crypt)][((Ad,MeArO)3tacn)UIV(=Se)].5m The U–N distance of 2.507(4) Å is unremarkable and close to those found for compounds 4–6 (Table 1). Furthermore, the U–Se distance of 2.583(1) Å is in the range established for [Ph3PMe][UIV(=Se)[N(SiMe3)2]3] (2.6463(7) Å),5b [(C5Me5)2Co][UVI(=Se)( =O)[N(SiMe3)2]3] (2.533(1) Å),5d [K(18-crown-6)][UIV(=Se)(NR2)3] (2.585(1) and 2.595(1) Å),5f and [K(2.2.2-crypt)][((Ad,MeArO)3tacn)UIV(=Se)] (2.695(2) Å).5m
Scheme 1. Synthesis of Complexes 2–7.
Figure 2.
Molecular structure of 4 (thermal ellipsoids drawn at the 35% probability level).
Table 1. Selected Distances (Å) and Angles (°) for Compounds 4–10, 12–15, and 17a.
| compound | C(Cp)–Ub | C(Cp)–Uc | Cp(cent)–Ub | U–X | Cp(cent)–U–Cp(cent) | X–U–X/Y |
|---|---|---|---|---|---|---|
| 4 | 2.809(6) | 2.745(6) to 2.864(6) | 2.533(6) | N(1) 2.021(5), N(2) 2.426(6) | 130.8(2) | 93.2(2) |
| 5 | 2.836(3) | 2.741(3) to 2.945(3) | 2.564(3) | O(1) 1.873(2), N(1) 2.526(3) | 128.2(1) | 87.2(1) |
| 6 | 2.788(7) | 2.741(6) to 2.824(6) | 2.511(6) | S(1) 2.437(1), N(1) 2.509(5) | 134.0(2) | 95.9(1) |
| 7 | 2.784(4) | 2.741(5) to 2.827(5) | 2.505(5) | Se(1) 2.583(1), N(1) 2.507(4) | 135.2(2) | 96.5(1) |
| 8 | 2.758(4) | 2.714(4) to 2.803(4) | 2.476(4) | O(1) 2.071(3), Cl(1) 2.820(1) | 129.2(1) | 91.8(1) |
| 9 | 2.776(6) | 2.727(6) to 2.823(5) | 2.493(3) | O(1) 2.077(3), I(1) 2.963(1) | 128.3(2) | 93.3(1) |
| 10 | 2.757(4) | 2.698(3) to 2.807(4) | 2.474(3) | O(1) 2.067(2), N(1) 2.525(4) | 130.1(1) | 92.3(1) |
| 12 | 2.816(5) | 2.745(5) to 2.905(5) | 2.541(5) | O(1) 2.114(4), O(2) 2.109(3) | 125.2(2) | 91.7(1) |
| 13 | 2.770(13) | 2.737(13) to 2.803(12) | 2.496(12) | O(1) 2.214(9), S(1) 2.748(3) N(2) 2.530(10) | 127.9(3) | 61.2(2)d |
| 14 | 2.737(5) | 2.683(4) to 2.783(5) | 2.453(4) | S(1) 2.608(2), I(1) 2.944(1) | 130.3(2) | 100.2(1) |
| 15 | 2.769(7) | 2.722(7) to 2.833(7) | 2.489(6) | S(1) 2.755(1), S(2) 2.724(1) N(2) 2.565(5) | 131.0(2) | 65.1(1)e |
| 17 | 2.784(7) | 2.684(7) to 2.892(7) | 2.506(7) | U(1) Se(1) 2.763(1), Se(2) 2.758(1) U(2) Se(1) 2.761(1), Se(2) 2.758(1) | 119.5(2) | 79.7(1) |
Cp = cyclopentadienyl ring.
Average value.
Range.
The angle of O(1)-U(1)-S (1).
The angle of S(1)-U(1)-S(2).
Figure 3.
Energy profile (kcal/mol) for the reaction of 4 + Ph2CO (computed at T = 298 K). [U] = [η5-1,2,4-(Me3Si)3C5H2]2U.
Figure 4.
Molecular structure of 5 (thermal ellipsoids drawn at the 35% probability level).
Figure 5.
Molecular structure of 6 (thermal ellipsoids drawn at the 35% probability level).
Figure 6.
Molecular structure of 7 (thermal ellipsoids drawn at the 35% probability level).
Bonding Studies
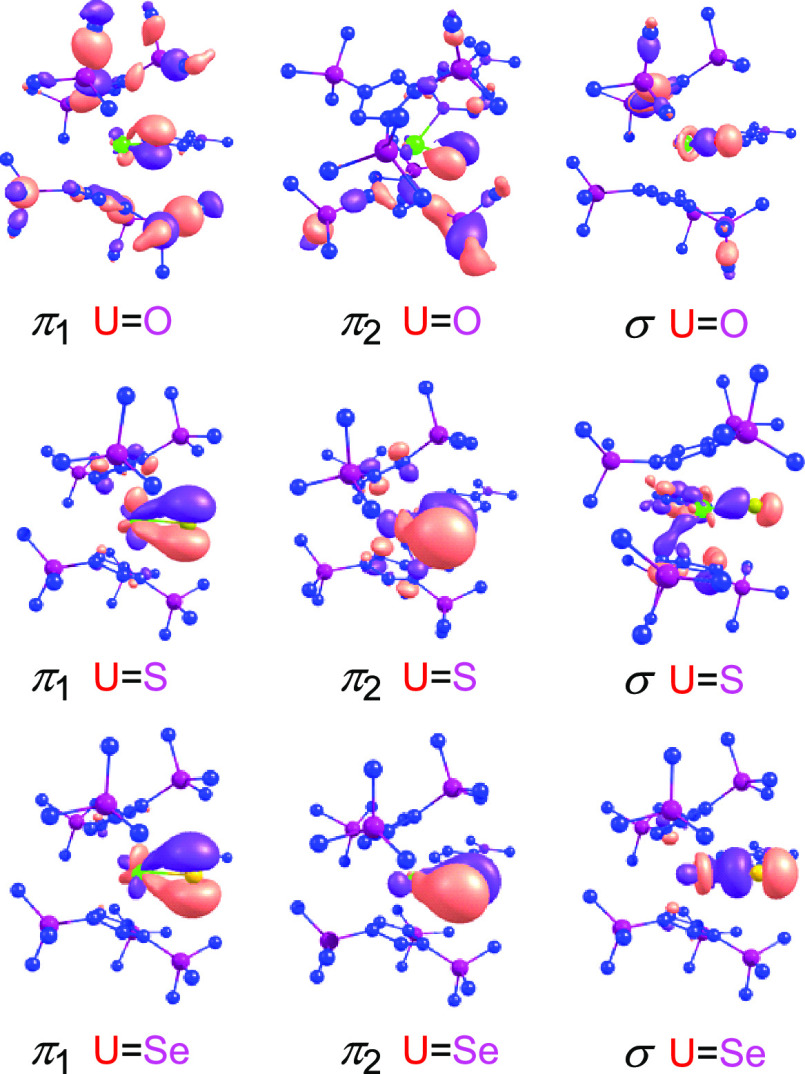
To further probe the interaction between the uranium atom and the chalcogenides, oxygen, sulfur, or selenium, density functional theory (DFT) computations at the B3PW91 level of theory were undertaken. While the computed structures of 5–7 are in excellent agreement with the experimental data, computations reveal that the [E]2– fragment is coordinated to the {[η5-1,2,4-(Me3Si)3C5H2]2(dmap)U}2+ moiety by one U–E σ-bond and two U–E π-bonds, as illustrated in Figure 7. The bonding in [η5-1,2,4-(Me3Si)3C5H2]2U=E(dmap) was analyzed by a natural localized molecular orbital (NLMO) approach, and the results are summarized in Table 2. The U–E σ-bond comprises a chalcogenide hybrid orbital and a uranium hybrid orbital. Moving from E = O to E = Se, the uranium contribution to the σ-bond increases from 14.1% to 25.7%, while that of the chalcogenide decreases from 85.9% to 74.3%. More importantly, the 6d orbital contribution declines from 69.0% to 59.2%. Notably, the 5f orbital contribution reaches a maximum for E = S (25.8%) but decreases again when moving to E = Se (20.0%). The π bonds (π1 and π2) are composed of pure chalcogenide-based p orbitals and uranium hybrid orbitals. The U–E π bonds (π1 and π2) also experience an increased uranium contribution (16.5% to 27.2% for π1 and 18.5% to 32.2% for π2) when moving from O to Se, while the chalocogenide contribution (83.5% to 72.8% for π1 and 81.5% to 67.8% for π2) becomes smaller. This is consistent with the increased π interaction between {[η5-1,2,4-(Me3Si)3C5H2]2(dmap)U}2+ and [E]2– fragments from O to Se. Differences, however, arise in the contributions of 6d and 5f orbitals to the π1 and π2 bonds. Whereas the 6d orbital contributions rises slightly (54.2% to 57.4%), the 5f orbital contribution (42.6% to 37.7%) declines for the π1 bond. A different trend is, however, observed for the π2 bond. Here, the 6d orbital contribution reaches a maximum (38.3%) at E = S, while the 5f orbital contribution is at its minimum (59.9%). Overall, the sum of the 6d and 5f contributions varies only slightly between 98.5% and 98.7%, regardless of the chalogenide. However, the electrostatic charge separation between the two fragments {[η5-1,2,4-(Me3Si)3C5H2]2(dmap)U}2+ and [E]2– becomes progressively smaller along the series from O to Se, that is, 1.76 (for E = O (5)), 0.84 (for E = S (6)), and 0.72 (for E = Se (7)) (Table 2), implying that the electrostatic (ionic) interaction between the chalocogenide atom and the uranium atom is reduced from E = O to E = Se. This is correlated to an increase in the Wiberg U=E bond order from 1.80 (for 5) to 2.24 (for 6) to 2.25 (for 7) (Table 2). This also points to an increased orbital overlap and therefore more covalent bonding between the chalocogenide-based valence orbitals and the uranium atom transitioning from E = O to E = Se. These computations indicate a significant involvement of the uranium 5f orbitals in the bonding between the metallocene {[η5-1,2,4-(Me3Si)3C5H2]2(dmap)U}2+ and [E]2– fragments, consistent with previous conclusions that the 5f orbitals play a distinct role in the bonding of actinide complexes and that the U=E bonds are generally more polarized than those in d-transition metals.9c This difference carries on in the reactivity of the uranium complexes 5–7 when compared to their group 4 relatives.12,13
Figure 7.
Plots of HOMOs for 5–7 (the hydrogen atoms have been omitted for clarity).
Table 2. Natural Localized Molecular Orbital (NLMO) Analysis of U=E Bonds,a Bond Order, and the Natural Charges for the [Cp2U(dmap)] and [E] Units.
| 5 (O) | 6 (S) | 7 (Se) | ||
|---|---|---|---|---|
| σ U-E | %U | 14.1 | 24.1 | 25.7 |
| %s | 9.5 | 11.6 | 15.7 | |
| %p | 3.6 | 3.6 | 5.1 | |
| %d | 69.0 | 59.0 | 59.2 | |
| %f | 17.9 | 25.8 | 20.0 | |
| %E | 85.9 | 75.9 | 74.3 | |
| %s | 45.9 | 48.9 | 47.6 | |
| %p | 54.1 | 51.0 | 52.4 | |
| π1 U=E | %U | 16.5 | 26.5 | 27.2 |
| %p | 3.2 | 3.5 | 4.9 | |
| %d | 54.2 | 56.2 | 57.4 | |
| %f | 42.6 | 40.3 | 37.7 | |
| %E | 83.5 | 73.5 | 72.8 | |
| %p | 100 | 100 | 100 | |
| π2 U=E | %U | 18.5 | 29.5 | 32.2 |
| %p | 1.5 | 1.8 | 2.3 | |
| %d | 37.3 | 38.3 | 35.6 | |
| %f | 61.2 | 59.9 | 63.1 | |
| %E | 81.5 | 70.5 | 67.8 | |
| %p | 100 | 100 | 100 | |
| Wiberg bond order | 1.80 | 2.24 | 2.25 | |
| (U=E) | ||||
| NBO charge | 0.88 | 0.42 | 0.36 | |
| (Cp2(dmap)U) | ||||
| NBO charge (E) | –0.88 | –0.42 | –0.36 |
The contributions by atom and orbital are averaged over all the ligands of the same character and over alpha and beta orbital contributions.
To further assess the U–E bonding, the quantum theory of atoms in molecules (QTAIM) that focuses on the topology of the electron density rather than the orbital structure was adopted to probe the extent of covalency of actinide-ligand bonds, utilizing diagnostic properties such as the electron density (ρb) and energy density (Hb) at the actinide-ligand bond critical points (BCPs) and the delocalization indices (DI) between two atoms.5d,5j ρb and Hb data for the U–E at the BCPs and DI data for the U–E bonds in 5–7 are collected in Table 3: ρb values greater than 0.2 eÅ–3 are typically associated with covalent bonds and smaller density values are indicative of closed-shell (ionic) interactions;5d,5j the more stabilizing covalent interaction, the more negative energy density Hb,5d,5j whereas the DIs integrate the electron density in the bonding region between two atoms and its value is closely related to the bond order. However, the computed DI values are always smaller than those expected from the Lewis structures and this difference is often attributed to bond polarity.5j According to the QTAIM analysis, the U–E bonds in the complexes 5–7 are rather polar and exhibit an appreciable ionic character (Table 3). Moreover, the calculated QTAIM DIs indicate a significant electron density accumulation in the U–E bonding region of the complexes 5–7 (Table 3). In addition, the QTAIM analysis suggests that the overlap decreases down the group 16 from O to Se, whereas the energy matching increases (Table 3). Hence, contrary to the NLMO analyses (Table 2), the computed ρb, Hb, and DI values also indicate reduced covalency when moving down in group 16 from O to Se (Table 3), which is also observed for the uranium complexes [{(Me3Si)2N}2U(O)(E)]− (E = O, S, Se).5j This is chemically counterintuitive, but as stated by Kaltsoyannis and coworkers, the QTAIM parameters evaluated at the BCPs have to be treated with caution,5c,14 when a series with different bond distances are compared. The U–E distances (computed U–E distance of 1.847, 2.422, and 2.573 Å for 5, 6, and 7, respectively) vary significantly and therefore caution should be exercised in concluding that the U–E covalence for complexes 5–7 decreases as group 16 is descended.
Table 3. Electron Densities (ρb) and Energy Densities (Hb) at Selected Bond Critical Points of 5–7 (H in Bold, Both in Atomic Units) and Delocalization Indices (DI) for U=E Bonds.
| 5 (O) | 6 (S) | 7 (Se) | |
|---|---|---|---|
| electron densities (ρ) | 0.122 | 0.089 | 0.018 |
| energy densities (H) | –0.118 | –0.019 | –0.010 |
| delocalization indices (DI) | 2.470 | 2.312 | 2.286 |
Reactivity of Oxido Complex [η5-1,2,4-(Me3Si)3C5H2]2UO(dmap) (5)
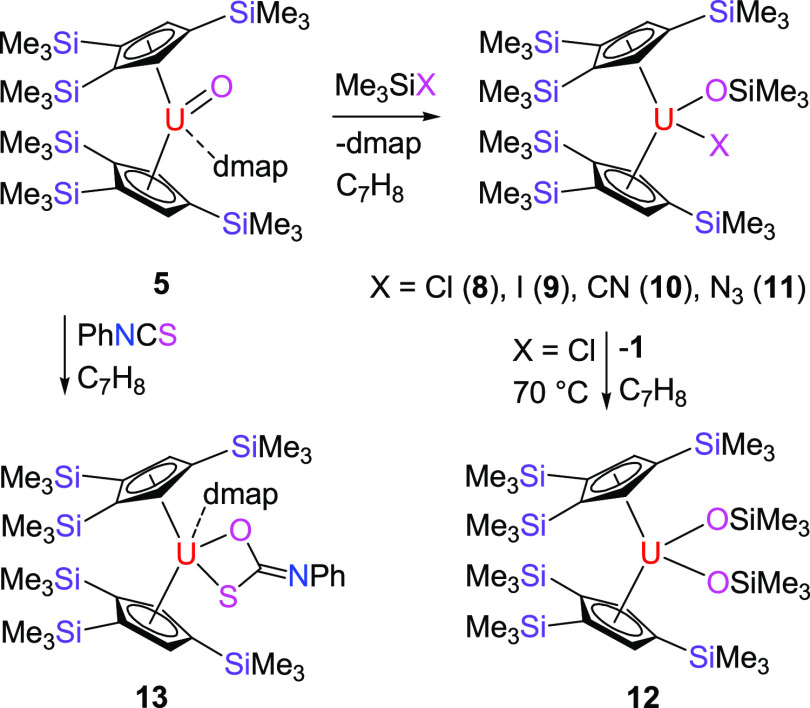
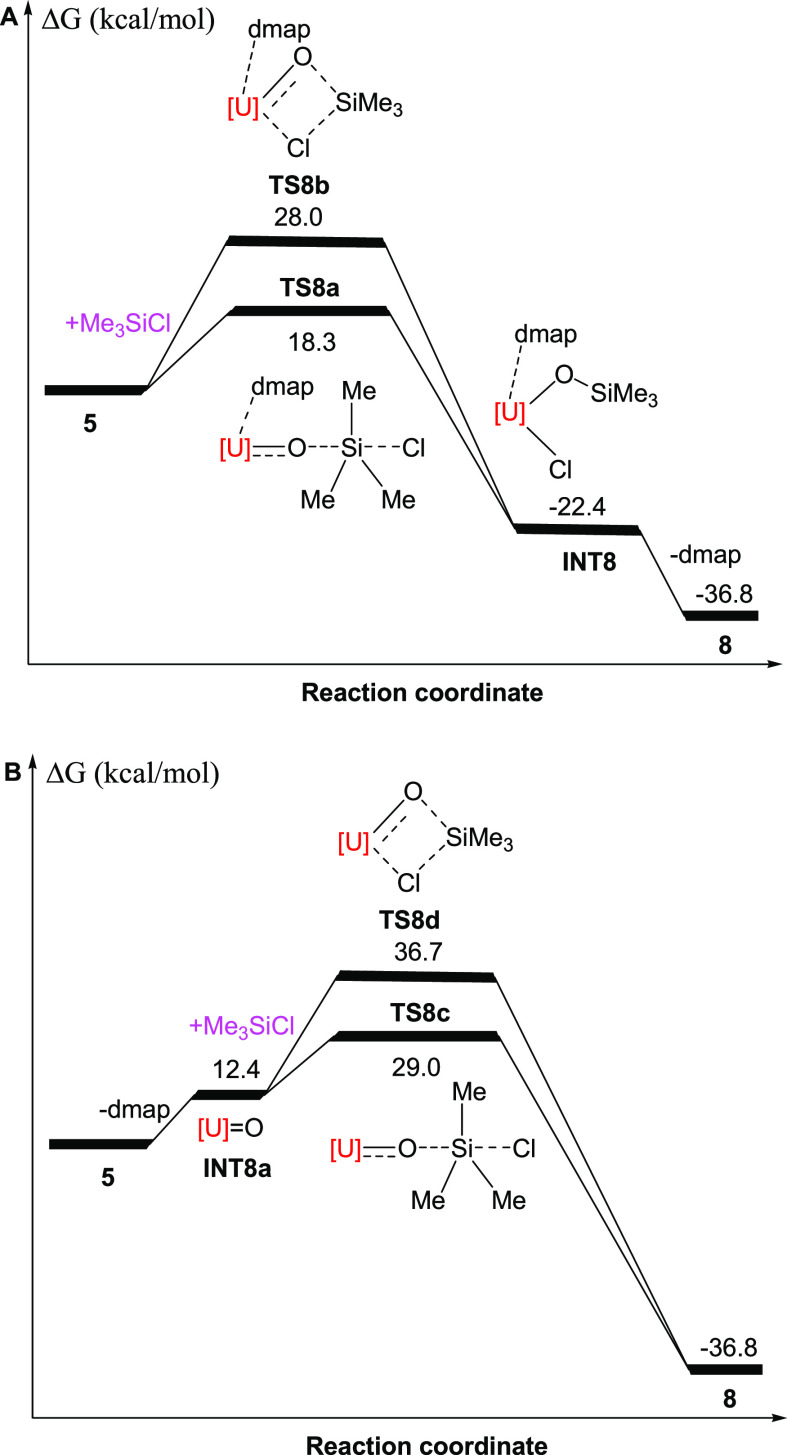
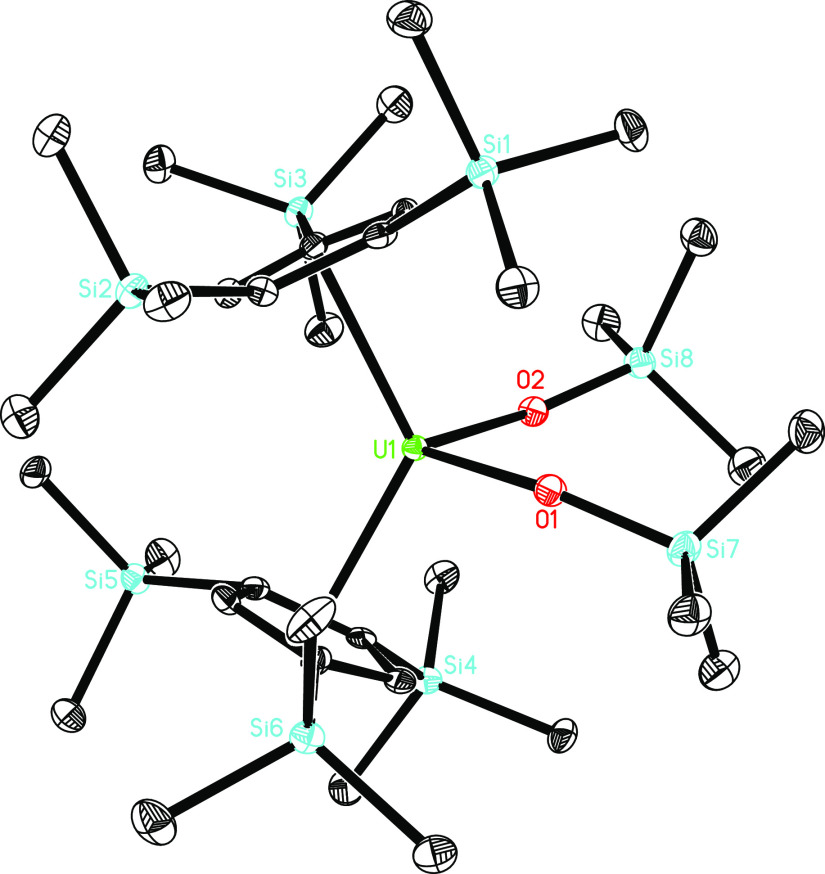
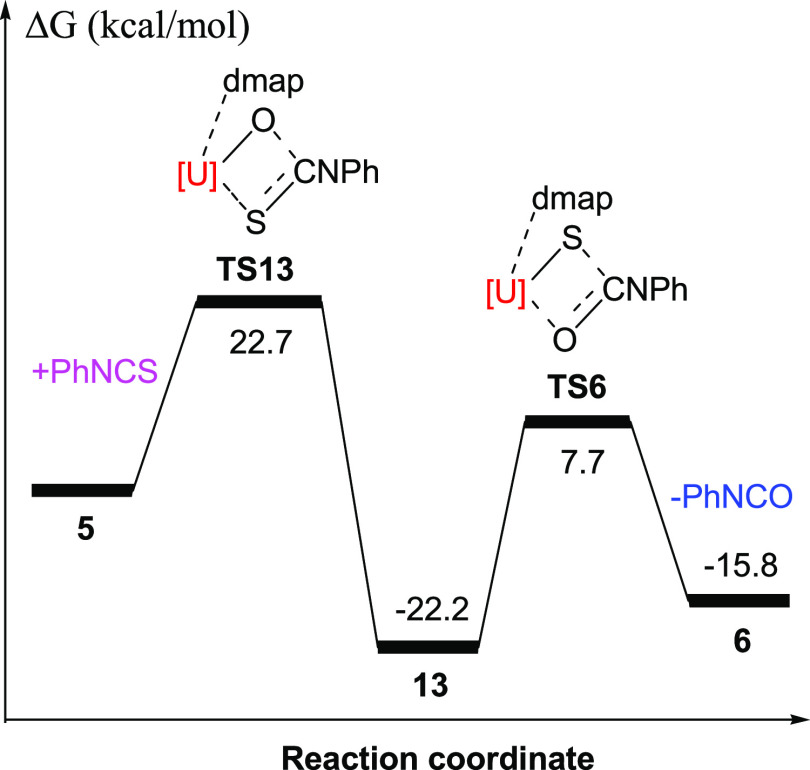
The more polarized nature of the moiety U=O may also impact its reactivity. Nevertheless, in contrast to [η5-1,2,4-(Me3C)3C5H2]2U=O(dmap),3e the dmap ligand in [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5) cannot be removed by Ph3B addition to yield a base free oxido complex [η5-1,2,4-(Me3Si)3C5H2]2UO, presumably due to the more electron-deficient and less sterically demanding ligand 1,2,4-(Me3Si)3C5H2, which renders the uranium atom more electrophilic and renders a more open coordination sphere at the uranium atom, therefore binding the dmap more strongly. Nevertheless, in analogy to the oxido compounds [η5-1,2,4-(Me3C)3C5H2]2An=O(dmap) (An = Th, U),3e,10b [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5) forms with Me3SiX the metallocenes, [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(X) (X = Cl (8), I (9), NC (10), and N3 (11)), concomitant with dmap loss (Scheme 2). DFT studies suggest that the reaction of 5 with Me3SiCl may proceed via two different ways, i.e., an SN2 (TS8a) or an addition (TS8b) mechanism (Figure 8A). The optimized bond distances of O–Si and Si–Cl in TS8a are 2.479 and 2.245 Å (see the Supporting Information for details), respectively, indicating that the O–Si bond is formed, while the Si–Cl bond is broken simultaneously, and the resulting Cl– anion migrates to the U atom to establish a U–Cl bond when it leaves the Si atom. The Si atom in Me3SiCl can readily approach the O atom, and the energy barrier for the SN2 process is only ΔG‡(298 K) = 18.3 kcal/mol (Figure 8A), implying that the reaction readily proceeds at ambient temperature. In contrast, the addition reaction occurs via the concerted transition state TS8b, in which the two forming bond distances of U–Cl and Si–O are 4.254 and 2.263 Å, respectively (see the Supporting Information for details). In combination with the Si–Cl distance of 2.167 Å, these metric parameters imply that the O–Si and U–Cl bond formations and Si–Cl bond breakage occur simultaneously, in which the formation of the Si–O bond drives the Si–Cl bond breakage. Nevertheless, the activation barrier of ΔG‡(298 K) = 28.0 kcal/mol for the addition reaction of 5 with Me3SiCl (Figure 8A) is energetically less favorable than that of the SN2 reaction. However, the formation of INT8 is thermodynamically energetically favorable (ΔG(298 K) = −22.4 kcal/mol), but driven by dmap loss, INT8 immediately converts to the even more thermodynamically stable product 8 (ΔG(298 K) = −36.8 kcal/mol) (Figure 8A). Moreover, it is reasonable to assume that the U–Cl and Si–S bond formation energies in the reaction of [η5-1,2,4-(Me3Si)3C5H2]2US(dmap) (6) with Me3SiCl favor the formation of [η5-1,2,4-(Me3Si)3C5H2]2UCl2 and (Me3Si)2S (Scheme 3), whereas the reaction of 5 with Me3SiCl stops with the formation of [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)Cl (8) attributed to the strong U–O bond (Scheme 2). We also considered that dmap dissociation initiates the formation of 8via either an SN2 (TS8c) or an addition (TS8d) reaction with Me3SiCl. However, the activation barriers for these processes of ΔG‡(298 K) = 29.0 and = 36.7 kcal/mol (Figure 8B), respectively, are significantly larger than that for the SN2 reaction illustrated in Figure 8A, whose computed reaction profile is in agreement with 8 being formed at ambient temperature. The molecular structures of 8 and 10 are presented in Figures 9 and 10, respectively, and ORTEP of 9 is shown in the Supporting Information. In complex 8, the U–O distance of 2.071(3) Å is close to that in 9 (2.077(3) Å). The U–Cl distance in 8 is 2.820(1) Å, whereas the U–I distance amounts to 2.963(1) Å in 9. In contrast to uranium cyanide complex [η5-1,2,4-(Me3C)3C5H2]2U(OSiMe3)(CN)3e but analogous to uranium isocyanide complex [(Me3Si)2N]3U(NC) and the thorium isocyanide complex [η5-1,2,4-(Me3C)3C5H2]2Th(OSiMe3)(NC),10b,15 the CN– ligand in 10 coordinates to the U4+ ion by its nitrogen atom instead of the carbon atom, presumably due to the electron-deficient nature of 1,2,4-(Me3Si)3C5H2, which increases the Lewis acidity of the metal ion. DFT computations also predict the isocyanide isomer to be energetically more favorable than the cyanide one [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(CN) (ΔG(298 K) = −0.8 kcal/mol) (see the Supporting Information for details), which is in line with the experiment. The U–O distance of 2.067(2) Å is shorter than those in 8 and 9 (Table 1), whereas the U–N distance is 2.525(4) Å. Nevertheless, complexes 8–10 are unstable toward ligand redistribution at high temperature in toluene solution. For example, when heated at 70 °C, 8 yields 1 and [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)2 (12) (Scheme 2). The molecular structure of 12 is provided in Figure 11; for selected bond distances and angles, consult Table 1. The U–O distances are 2.114(4) and 2.109(3) Å, and the angle of O–U–O is 91.7(1)°. Moreover, in analogy to the thorium oxido [η5-1,2,4-(Me3C)3C5H2]2Th=O(dmap),10b [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5) is reactive toward unsaturated organic substrates. For example, treatment of 5 with PhNCS in toluene rapidly forms the four-membered metallocene [η5-1,2,4-(Me3Si)3C5H2]2U[OC(=NPh)S)(dmap) (13) (Scheme 2). DFT investigations imply that the formation of 13 from 5 + PhNCS is exergonic with ΔG(298 K) = −22.2 kcal/mol and proceeds via a transition state TS13 with a low reaction barrier of ΔG‡(298 K) = +22.7 kcal/mol (Figure 12). Moreover, DFT computations also predict that the degradation of 13 to 6 + PhNCO is energetically unfavorable (ΔG(298 K) = 6.4 kcal/mol) and proceeds via a transition state TS6 with a high reaction barrier of ΔG‡(298 K) = +29.9 kcal/mol (Figure 12). These results are consistent with the experiment in which complex 13 is formed at ambient temperature. The molecular structure of 13 is shown in Figure 13; for selected bond distances and angles, consult Table 1. The U–N distance of 2.530(10) Å is in line with those found in 4–7 (Table 1). The U–O distance is 2.214(9) Å, whereas the U–S distance of 2.748(3) Å is longer than that found in 6 (2.437(1) Å). However, like for the actinide oxidos [η5-1,2,4-(Me3C)3C5H2]2An=O(dmap) (An = Th, U)3e,10b but in contrast to the group 4 derivatives (η5-C5Me5)2Ti=O(py) and (η5-C5Me5)2Zr=O,12b,12c,13 no reaction occurs between complex 5 and internal alkynes RC≡CR (R = Ph, Me, p-tolyl) even when heated to 100 °C for 1 week, presumably attributed to the more polarized nature of the actinide oxido bond An+-O–.16
Scheme 2. Synthesis of Complexes 8–13.
Figure 8.
Energy profile (kcal/mol) for the reaction of 5 + Me3SiCl (computed at T = 298 K). [U]=[η5-1,2,4-(Me3Si)3C5H2]2U.
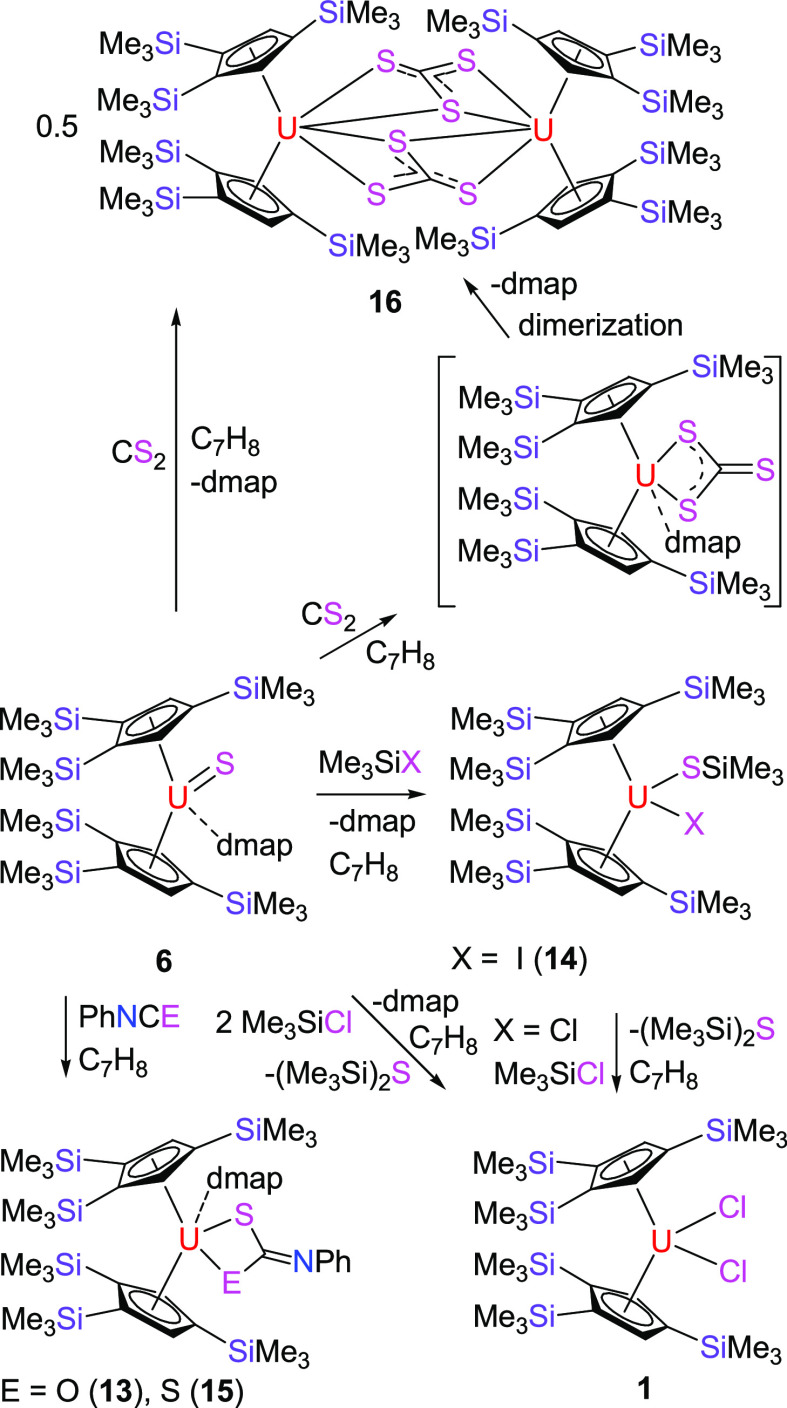
Scheme 3. Synthesis of Complexes 13–16.
Figure 9.
Molecular structure of 8 (thermal ellipsoids drawn at the 35% probability level).
Figure 10.
Molecular structure of 10 (thermal ellipsoids drawn at the 35% probability level).
Figure 11.
Molecular structure of 12 (thermal ellipsoids drawn at the 35% probability level).
Figure 12.
Energy profile (kcal/mol) for the reaction of 5 + PhNCS (computed at T = 298 K). [U]=[η5-1,2,4-(Me3Si)3C5H2]2U.
Figure 13.
Molecular structure of 13 (thermal ellipsoids drawn at the 35% probability level).
Reactivity of Sulfido Complex [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6)
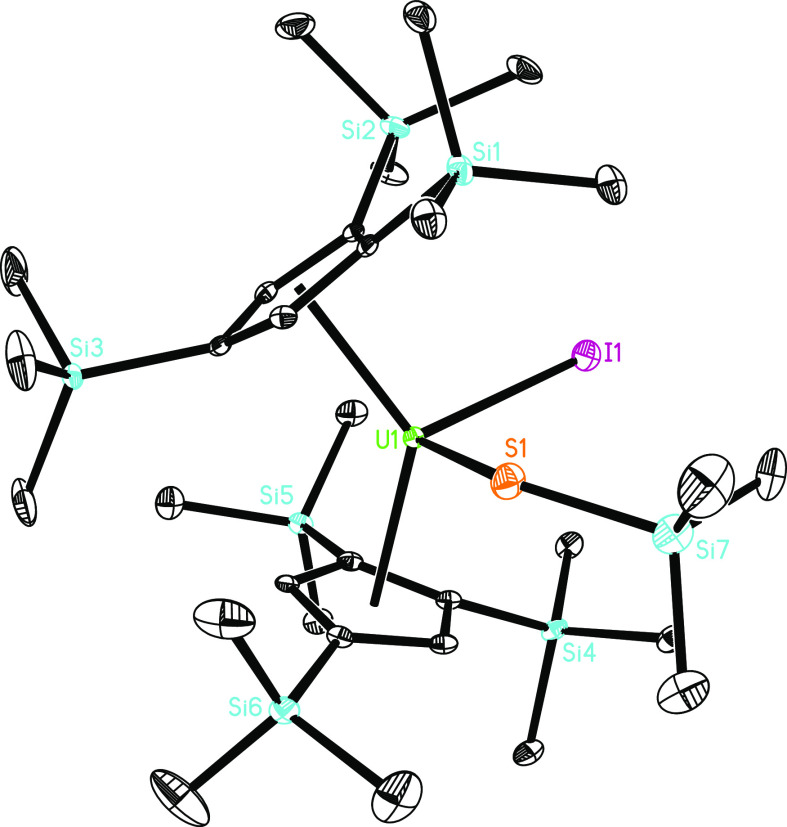
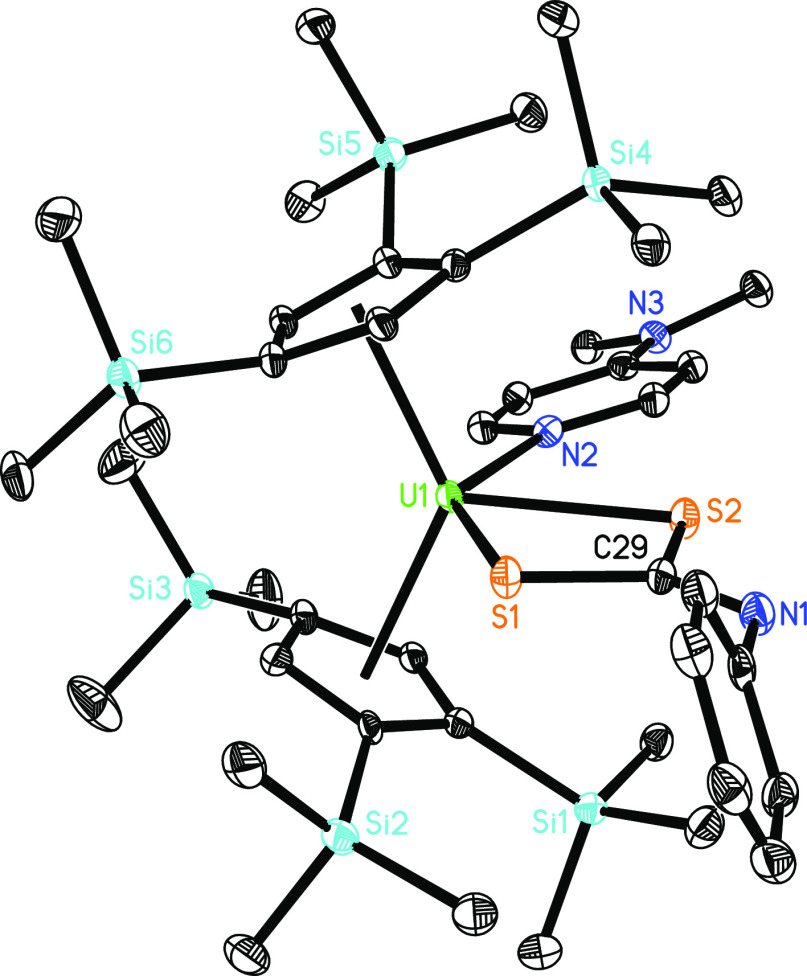
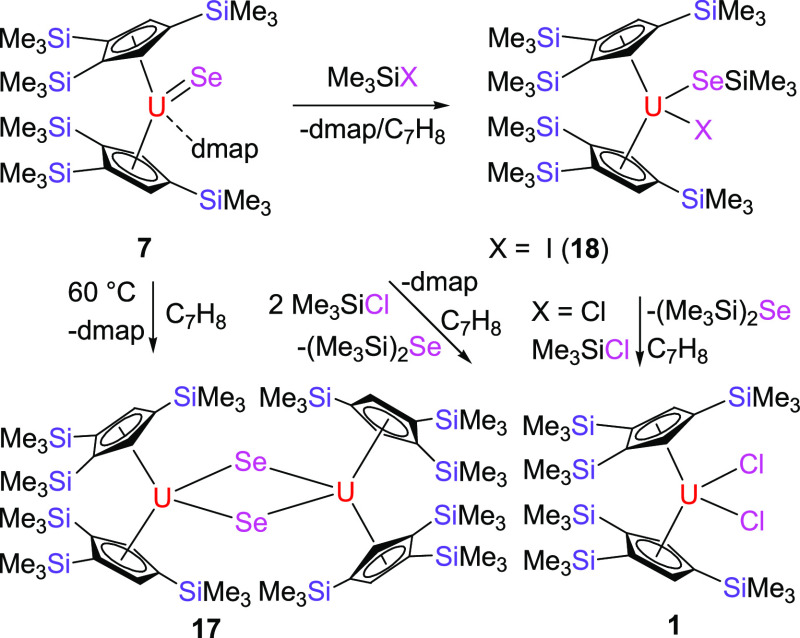
In contrast to the uranium sulfido complex [η5-1,2,4-(Me3C)3C5H2]2U=S that readily reacts with one equivalent of Ph2CS to form [η5-1,2,4-(Me3C)3C5H2]2U(S2CPh2) even in the presence of a Lewis base such as dmap,11 the dmap-stabilized terminal uranium sulfido [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6) is formed from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4) and Ph2CS at room temperature (Scheme 1). We attribute this difference to a combination of steric and electronic effects. The 1,2,4-(Me3Si)3C5H2 is less bulky and therefore accommodates dmap coordination, but it is also more electron-deficient and hence increases the Lewis acidity of the uranium ion. In analogy to its oxido counterparts [η5-1,2,4-(Me3E)3C5H2]2U=O(dmap) (E = C,3e Si (5)), at room temperature, complex 6 reacts immediately upon mixing with Me3SiI to release the dmap ligand and to give the addition product [η5-1,2,4-(Me3Si)3C5H2]2U(SSiMe3)(I) (14) (Scheme 3). The molecular structure of 14 is depicted in Figure 14; for selected bond distances and angles, see Table 1. The U–I distance of 2.944(1) Å is comparable to that found in 9 (2.963(1) Å), whereas the U–S distance of 2.608(2) Å is longer than that found in 6 (2.437(1) Å). Nevertheless, when Me3SiCl is used as a substrate, dichlorido complex [η5-1,2,4-(Me3Si)3C5H2]2UCl2 (1) is formed along with dmap and (Me3Si)2S loss (Scheme 3). Most likely, this reaction proceeds via a monochlorido intermediate [η5-1,2,4-(Me3Si)3C5H2]2U(SSiMe3)(Cl) but it readily converts in the presence of Me3SiCl via (Me3Si)2S loss to the product 1 (Scheme 3). This contrasts to the reactivity of 5 with Me3SiCl yielding as a stable product [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(Cl) (8) (Scheme 2). Moreover, like its oxido counterpart [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5), 6 reacts with unsaturated organic substrates. For example, treatment of 6 with PhNCO or PhNCS in toluene forms the four-membered metallocenes, [η5-1,2,4-(Me3Si)3C5H2]2U[EC(=NPh)S)(dmap) (E = O (13), S (15)) (Scheme 3). The formation of 13 from 6 + PhNCO is consistent with the reaction energy profile in Figure 12. The molecular structure of 15 is provided in Figure 15; for selected bond distances and angles, refer to Table 1. The U–N distance is 2.565(5) Å, whereas the U–S distances are 2.755(1) and 2.724(1) Å. Moreover, complex 6 transforms in the presence of CS2 to a uranium trithiocarbonato intermediate [η5-1,2,4-(Me3Si)3C5H2]2U(CS3)(dmap), which readily dimerizes to the trithiocarbonato-bridged complex {[η5-1,2,4-(Me3Si)3C5H2]2U}2(μ-CS3)2 (16)17 concomitant with dmap release (Scheme 3). However, in analogy to the thorium sulfido [η5-1,2,4-(Me3C)3C5H2]2Th=S10b but in contrast to the group 4 derivatives (η5-C5Me5)2TiS(py)12f and (η5-C5Me5)2ZrS,12b,13 no [2 + 2] cycloaddition products [η5-1,2,4-(Me3Si)3C5H2]2U[SC(R)=C(R)] are obtained between complex 6 and internal alkynes RC≡CR (R = Ph, Me, p-tolyl) even when the mixture is heated at 100 °C for 1 week, again, presumably due to the more polarized nature of the actinide sulfido bond An+–S–.16
Figure 14.
Molecular structure of 14 (thermal ellipsoids drawn at the 35% probability level).
Figure 15.
Molecular structure of 15 (thermal ellipsoids drawn at the 35% probability level).
Reactivity of Selenido Complex [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7)
In contrast to the oxido and sulfido derivatives, [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5) and [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6), the isolated selenido adduct [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7) degrades in toluene solution at 60 °C to the dimer {[η5-1,2,4-(Me3Si)3C5H2]2U}2(μ-Se)2 (17) (Scheme 4), presumably due to the larger ionic radius of the Se2– anion. The molecular structure of 17 is provided in Figure 16; for selected bond distances and angles, see Table 1. The U–Se distances within the U2Se2-core are essentially identical and vary between 2.758(1) and 2.763(1) Å. This also extends to the Se–U(1)–Se angles and the Se–U(2)–Se angles; both are 79.7(1)°. Like the oxido [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5) and sulfido [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6), complex 7 immediately reacts upon mixing with Me3SiI at room temperature to give the addition product [η5-1,2,4-(Me3Si)3C5H2]2U(SeSiMe3)(I) (18) concomitant with dmap loss (Scheme 4). Moreover, analogous to the sulfido derivative [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6), when Me3SiCl is used as a substrate, the dichlorido complex [η5-1,2,4-(Me3Si)3C5H2]2UCl2 (1) is formed along with dmap and (Me3Si)2Se (Scheme 4). Again, this reaction proceeds via a monochlorido intermediate [η5-1,2,4-(Me3Si)3C5H2]2U(SeSiMe3)(Cl), which readily converts in the presence of Me3SiCl releasing (Me3Si)2Se to give complex 1 (Scheme 4). However, unlike the oxido [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5) and sulfido [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6), complex 7 shows no reaction with PhNCS, presumably attributed to the large size of the Se2– anion, which hinders the approach to the uranium atom. Moreover, like the oxido [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5) and sulfido [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6), also in the case of 7, no reaction is observed in the presence of internal alkynes RC≡CR (R = Ph, Me, p-tolyl)..
Scheme 4. Synthesis of Complexes 17 and 18.
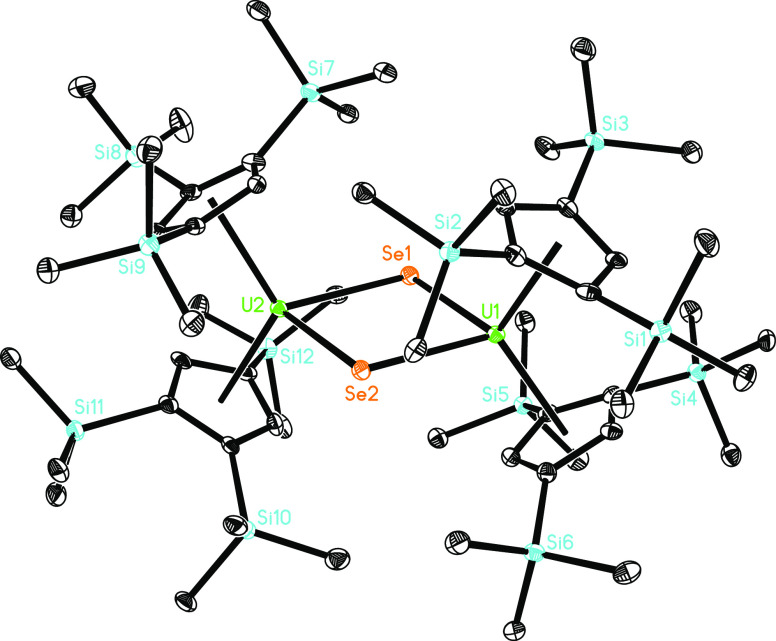
Figure 16.
Molecular structure of 17 (thermal ellipsoids drawn at the 35% probability level).
Conclusions
The uranium oxido metallocenes [η5-1,2,4-(Me3E)3C5H2]2U=O(dmap) (E = C,3e Si (5)) and the thorium derivative [η5-1,2,4-(Me3C)3C5H2]2Th=O(dmap)10b react as nucleophiles toward alkylsilyl halides mimicking the reactivity of (η5-C5Me5)2Zr=O(py),12e but they do not undergo cycloaddition reactions with alkynes in contrast to (η5-C5Me5)2Ti=O(py)12c and (η5-C5Me5)2Zr=O.12b Moreover, both the uranium sulfido [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6) and its thorium relative [η5-1,2,4-(Me3C)3C5H2]2Th=S are inert toward internal alkynes in contrast to (η5-C5Me5)2Ti=S(py)12f and (η5-C5Me5)2Zr=S, which participate in cycloaddition reactions.12b We propose that these differences arise from the more polarized nature of the actinide oxido and sulfido bonds An+–E– (E = O, S).9c
Moreover, uranium sulfido and selenido metallocenes exhibit distinctly different reactivity patterns, e.g., whereas the sulfido derivative [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6) is stable in toluene solution, its selenido counterpart [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7) degrades to the dimer {[η5-1,2,4-(Me3Si)3C5H2]2U}2(μ-Se)2 (17). Furthermore, while 6 undergoes a cycloaddition with PhNCS, 7 remains inert toward PhNCS. This shows that the reactivity of actinide metallocenes carrying a terminal An=E (E = heteroatom) functional group is influenced by the size of the heteroatom as well as the polarization of the An=X bond.
Furthermore, this study also illustrates that small variations in the supporting cyclopentadienyl ligand modulate the reactivity of these uranium metallocenes. For example, the dmap ligand in complex [η5-1,2,4-(Me3C)3C5H2]2U=O(dmap) can be readily removed by Ph3B,3e whereas this is not possible for [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5). Moreover, in contrast to the uranium cyanide complex [η5-1,2,4-(Me3C)3C5H2]2U(OSiMe3)(CN),3e the uranium isocyanide complex [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(NC) (10) is energetically more favorable than its cyanide isomer [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(CN). Furthermore, while [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6) is stable, the complex [η5-1,2,4-(Me3C)3C5H2]2U=S(dmap) is not.11 Electronic effects of the cyclopentadienyl ligands might also influence the reactivity of these complexes, whereas steric effects seem to prevail in all of these systems, dictating the reaction products being formed. Since the ligand 1,2,4-(Me3Si)3C5H2 is less sterically demanding than 1,2,4-(Me3C)3C5H2, this renders the open wedge at the uranium atom more open, inducing smaller Cp(cent)-U-Cp(cent) angles, which allows additional stabilizing ligands to coordinate, which enables the isolation of reaction products and intermediates inaccessible to uranium fragments bearing the more sterically encumbered 1,2,4-(Me3C)3C5H2 derivative.
In conclusion, the imido uranium metallocene [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4) is a useful precursor for the synthesis of the terminal oxido, sulfido, and selenido uranium metallocenes, which enabled us to systematically probe the intrinsic reactivity of U=E (E = O, S, Se) moieties. This allowed us to map the space of chemical transformations accessible to organoactinide oxido and chalcogenido complexes, which may also extend to solid-state actinide metal oxides and chalcogenides. Further investigations on the intrinsic reactivity of terminal chalcogenido actinide metallocenes and the uranium imido metallocene 4 are ongoing and will be communicated in due course.
Experimental Section
General Procedures
All reactions and product manipulations were carried out under an atmosphere of dry dinitrogen with rigid exclusion of air and moisture using standard Schlenk or cannula techniques, or in a glove box. All organic solvents were freshly distilled from sodium benzophenone ketyl immediately prior to use. [η5-1,2,4-(Me3Si)3C5H2]2UCl2 (1)17 and (p-MeOPh)2CSe18 were prepared according to previously reported procedures. All other chemicals were purchased from Aldrich Chemical Co. and Beijing Chemical Co. and used as received unless otherwise noted. Infrared spectra were recorded in KBr pellets on an Avatar 360 Fourier transform spectrometer. 1H and 13C{1H} NMR spectra were recorded on a Bruker AV 400 spectrometer at 400 and 100 MHz, respectively. 29Si{1H} NMR spectra were recorded on a JEOL 600 spectrometer at 119.2 MHz. All chemical shifts are reported in δ units with reference to the residual protons of the deuterated solvents, which served as internal standards, for proton and carbon chemical shifts, and to external Me4Si (0.00 ppm) for silicon chemical shifts. Melting points were obtained on X-6 melting point apparatus and were uncorrected. Elemental analyses were performed on a Vario EL elemental analyzer.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2UMe2 (2)
A diethyl ether (30.7 mL) solution of MeLi (0.15 M in diethyl ether; 4.6 mmol) was slowly added to a diethyl ether (25 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2UCl2 (1; 2.00 g, 2.3 mmol) with stirring at room temperature. After the solution was stirred for 1 h at room temperature, the solvent was removed. The residue was extracted with n-hexane (15 mL × 3) and filtered. The volume of the filtrate was reduced to 10 mL, and orange microcrystals of 2 formed when this solution was kept at −20 °C for 2 days. Microcrystals of 2 were isolated by filtration, quickly washed with cooled n-hexane (5 mL), and dried at 50 °C under vacuum overnight. Yield: 1.57 g (82%). M.p.: 150–152 °C (dec.). 1H NMR (C6D6): δ −0.07 (36H, Si(CH3)3), −2.32 (18H, Si(CH3)3), −52.98 (6H, CH3) ppm; protons of the ring CH were not observed. 13C{1H} NMR (C6D6): δ 273.9 (UCH3), 246.3 (ring C), 193.0 (ring C), 3.1 (Si(CH3)3), −1.9 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −39.7, −52.8 ppm. IR (KBr, cm–1): ν 2958 (s), 1253 (s), 1095 (s), 835 (s). Anal. calcd for C30H64Si6U: C, 43.34; H, 7.76. Found: C, 43.36; H, 7.74.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U(NH-p-tolyl)2 (3)
A toluene (10 mL) solution of p-toluidine (0.26 g, 2.4 mmol) was added to a toluene (10 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2UMe2 (2; 1.00 g, 1.2 mmol) with stirring at room temperature. After the solution was stirred at 60 °C overnight, the solvent was removed. The residue was extracted with n-hexane (10 mL × 3) and filtered. The volume of the filtrate was reduced to 10 mL, and brown microcrystals of 3 formed when this solution was kept at −20 °C for 1 day. Microcrystals of 3 were isolated by filtration, quickly washed with cooled n-hexane (5 mL), and dried at 50 °C under vacuum overnight. Yield: 1.13 g (93%). M.p.: 170–172 °C (dec.). 1H NMR (C6D6): δ 5.58 (d, J = 5.6 Hz, 4H, phenyl), 5.20 (s, 6H, CH3), 3.30 (s, 18H, Si(CH3)3), −1.12 (s, 36H, Si(CH3)3), −12.48 (s, 4H, phenyl) ppm; protons of Cp-ring CH and NH were not observed. 13C{1H} NMR (C6D6): δ 269.8 (phenyl C), 226.3 (phenyl C), 185.8 (ring C), 184.4 (ring C), 148.0 (phenyl C), 105.3 (phenyl C), 9.2 (CH3), 5.3 (Si(CH3)3), −0.0 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −62.4, −64.0 ppm. IR (KBr, cm–1): ν 2960 (s), 1514 (s), 1383 (s), 1248 (s), 1095 (m), 831 (s). Anal. calcd for C42H74N2Si6U: C, 49.77; H, 7.36; N, 2.76. Found: C, 49.75; H, 7.38; N, 2.78.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4)
A toluene (10 mL) solution of 4-dimethylaminopyridine (dmap; 0.25 g, 2.05 mmol) was added to a toluene (10 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2UMe2 (2; 0.82 g, 0.99 mmol) and [η5-1,2,4-(Me3Si)3C5H2]2U(NH-p-tolyl)2 (3; 1.0 g, 0.99 mmol) with stirring at room temperature. After the solution was stirred at 60 °C overnight, the solution was filtered. The volume of the filtrate was reduced to 5 mL, and brown crystals of 4 formed when this solution was kept at −20 °C for 2 days. Crystals of 4 were isolated by filtration, quickly washed with cooled n-hexane (5 mL), and dried at 50 °C under vacuum overnight. Yield: 1.77 g (87%). M.p.: 202–204 °C (dec.). 1H NMR (C6D6): δ 53.98 (s, 2H, phenyl), 41.91 (s, 2H, phenyl), 30.98 (t, J = 12 Hz, 3H, CH3), 25.68 (s, 2H, ring CH), 5.61 (s, 18H, Si(CH3)3), −3.26 (s, 18H, Si(CH3)3), −8.45 (s, 6H, N(CH3)2), −9.18 (s, 18H, Si(CH3)3), −9.26 (s, 4H, py), −32.81 (s, 2H, ring CH) ppm. 13C{1H} NMR (C6D6): δ 196.1 (py C), 151.3 (py C), 150.6 (py C), 149.8 (phenyl C), 142.6 (phenyl C), 141.2 (phenyl C), 117.0 (phenyl C), 116.4 (ring C), 112.4 (ring C), 111.0 (ring C), 102.6 (ring C), 91.1 (ring C), 31.4 (N(CH3)2), 31.2 (N(CH3)2), 5.3 (CH3), −5.8 (Si(CH3)3), −17.2 (Si(CH3)3), −60.4 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −93.9, −97.4, −115.4 ppm. IR (KBr, cm–1): ν 2958 (s), 1616 (s), 1384 (s), 1249 (s), 1095 (m), 1006 (s), 829 (s). Anal. calcd for C42H75N3Si6U: C, 49.04; H, 7.35; N, 4.09. Found: C, 49.03; H, 7.36; N, 4.06.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap)·C6H6 (5·C6H6)
Method A
A toluene (10 mL) solution of Ph2CO (91 mg, 0.50 mmol) was added to a toluene (10 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4; 514 mg, 0.50 mmol) with stirring at room temperature. After this solution was stirred at room temperature overnight, the solvent was removed. The residue was extracted with benzene (10 mL × 3) and filtered. The volume of the filtrate was reduced to 5 mL, and orange crystals of 5·C6H6 formed when this solution was kept at 10 °C for 2 days. Crystals of 5·C6H6 were isolated by filtration, quickly washed with cooled n-hexane (5 mL), and dried at 50 °C under vacuum overnight. Yield: 432 mg (85%). M.p.: 164–166 °C (dec.). 1H NMR (C6D6): δ 20.19 (s, 18H, Si(CH3)3), 7.15 (s, 6H, C6H6), −2.26 (s, 18H, Si(CH3)3), −7.49 (s, 6H, N(CH3)2), −9.38 (s, 18H, Si(CH3)3) ppm; other protons were not observed. 13C{1H} NMR (C6D6): δ 200.5 (py C), 130.7 (py C), 129.6 (py C), 129.3 (ring C), 128.5 (C6H6), 121.3 (ring C), 119.2 (ring C), 96.2 (ring C), 55.0 (ring C), 32.2 (N(CH3)2), 22.2 (Si(CH3)3), −5.3 (Si(CH3)3), −22. 1 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −35.8, −122.4, −144.9 ppm. IR (KBr, cm–1): ν 2953 (s), 1616 (s), 1533 (s), 1444 (s), 1384 (s), 1244 (s), 1095 (s), 1006 (s), 937 (s), 833 (s). Anal. calcd for C41H74N2OSi6U: C, 48.39; H, 7.33; N, 2.75. Found: C, 48.42; H, 7.36; N, 2.76.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of Ph2CO (3.6 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4; 20.6 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 5 and those of Ph2C=N(p-tolyl) (1H NMR (C6D6): δ 7.97 (m, 2H, aryl), 7.12 (m, 3H, aryl), 6.98 (m, 2H, aryl), 6.89 (m, 3H, aryl), 6.77 (m, 4H, aryl), 1.97 (s, 3H, CH3) ppm)10b were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap)·0.5C6H6 (6·0.5C6H6)
Method A
This compound was prepared as orange crystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4; 514 mg, 0.50 mmol) and Ph2CS (99 mg, 0.50 mmol) in toluene (15 mL) at room temperature and recrystallization from a benzene solution by a similar procedure to that in the synthesis of 5. The product was isolated by filtration, quickly washed with cooled n-hexane (5 mL), and dried at 50 °C under vacuum overnight. Yield: 417 mg (84%). M.p.: 175–177 °C (dec.). 1H NMR (C6D6): δ 9.30 (s, 18H, Si(CH3)3), 7.15 (s, 3H, C6H6), −0.83 (s, 18H, Si(CH3)3), −8.45 (s, 6H, N(CH3)2), −9.13 (s, 18H, Si(CH3)3) ppm; other protons were not observed. 13C{1H} NMR (C6D6): δ 196.9 (py C), 185.2 (py C), 159.8 (py C), 130.7 (ring C), 129.7 (ring C), 129.6 (ring C), 129.4 (ring C), 128.5 (C6H6), 121.4 (ring C), 31.9 (N(CH3)2), 5.9 (Si(CH3)3), −0.7 (Si(CH3)3), −8.8 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −61.6, −81.6, −111.5 ppm. IR (KBr, cm–1): ν 2957 (s), 1614 (s), 1535 (s), 1444 (s), 1384 (s), 1244 (s), 1095 (s), 1006 (s), 833 (s). Anal. calcd for C38H71N2SSi6U: C, 45.89; H, 7.20; N, 2.82. Found: C, 45.92; H, 7.18; N, 2.84.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of Ph2CS (4.0 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4; 20.6 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 6 and those of Ph2C=N(p-tolyl) were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap)·0.5C6H6 (7·0.5C6H6)
Method A
This compound was prepared as brown crystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4; 514 mg, 0.50 mmol) and (p-MeOPh)2CSe (153 mg, 0.50 mmol) in toluene (15 mL) at room temperature and recrystallization from a benzene solution by a similar procedure to that in the synthesis of 5. The product was isolated by filtration, quickly washed with cooled n-hexane (5 mL), and dried at room temperature under vacuum overnight. Yield: 453 mg (87%). M.p.: 153–155 °C (dec.). 1H NMR (C6D6): δ 7.15 (s, 3H, C6H6), 5.18 (s, 18H, Si(CH3)3), 0.92 (s, 18H, Si(CH3)3), −7.62 (s, 18H, Si(CH3)3), −8.71 (s, 6H, N(CH3)2) ppm; other protons were not observed. 13C{1H} NMR (C6D6): δ 158.6 (py C), 137.4 (py C), 133.5 (py C), 133.0 (ring C), 132.9 (ring C), 132.0 (ring C), 129.3 (ring C), 128.5 (C6H6), 121.7 (ring C), 31.9 (N(CH3)2), −0.5 (Si(CH3)3), −0.7 (Si(CH3)3), −2.2 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −74.2, −75.6, −103.9 ppm. IR (KBr, cm–1): ν 2955 (m), 2835 (m), 1604 (s), 1510 (s), 1249 (s), 1170 (s), 1031 (s), 837 (s). Anal. calcd for C38H71N2SeSi6U: C, 43.82; H, 6.87; N, 2.69. Found: C, 43.79; H, 6.88; N, 2.73.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of (p-MeOPh)2CSe (6.1 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=N(p-tolyl)(dmap) (4; 20.6 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 7 and those of (p-MeOPh)2C=N(p-tolyl) (1H NMR (C6D6): δ 7.99 (s, 2H, aryl), 7.20 (s, 2H, aryl), 7.02 (s, 2H, aryl), 6.84 (s, 2H, aryl), 6.78 (s, 2H, aryl), 6.63 (s, 2H, aryl), 3.21 (s, 3H, OCH3), 3.16 (s, 3H, OCH3), 2.01 (s, 3H, CH3) ppm)19 were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(Cl) (8)
Method A
A toluene (10 mL) solution of Me3SiCl (28 mg, 0.25 mmol) was added to a toluene (10 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 235 mg, 0.25 mmol) with stirring at room temperature. After the solution was stirred at room temperature for 1 h, the solvent was removed. The residue was extracted with n-hexane (10 mL × 3) and filtered. The volume of the filtrate was reduced to 3 mL, and orange crystals of 8 formed when this solution was kept at −20 °C for 2 days. Crystals of 8 were isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 199 mg (86%). M.p.: 175–177 °C (dec.). 1H NMR (C6D6): δ 31.85 (s, 9H, Si(CH3)3), 8.03 (s, 18H, Si(CH3)3), −7.63 (s, 18H, Si(CH3)3), −9.78 (s, 18H, Si(CH3)3) ppm; the protons of Cp-ring CH were not observed. 13C{1H} NMR (C6D6): δ 165.3 (ring C), 157.7 (ring C), 121.3 (ring C), 83.6 (ring C), 82.1 (ring C), 11.1 (Si(CH3)3), −6.2 (Si(CH3)3), −17.7 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −31.5, −86.4, −118.4 ppm. IR (KBr, cm–1): ν 2957 (s), 1248 (s), 1089 (s), 991 (s), 839 (s). Anal. calcd for C31H67ClOSi7U: C, 40.21; H, 7.29. Found: C, 40.18; H, 7.31.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of Me3SiCl (2.2 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 18.8 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 8 and those of dmap were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(I) (9)
Method A
This compound was prepared as orange crystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 235 mg, 0.25 mmol) and Me3SiI (50 mg, 0.25 mmol) in toluene (15 mL) at room temperature and recrystallization from an n-hexane solution by a similar procedure to that in the synthesis of 8. The product was isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 214 mg (84%). M.p.: 152–156 °C (dec.). 1H NMR (C6D6): δ 36.48 (s, 9H, Si(CH3)3), 10.00 (s, 18H, Si(CH3)3), −8.13 (s, 18H, Si(CH3)3), −10.42 (s, 18H, Si(CH3)3) ppm; the protons of Cp-ring CH were not observed. 13C{1H} NMR (C6D6): δ 157.9 (ring C), 155.6 (ring C), 112.6 (ring C), 82.4 (ring C), 81.4 (ring C), 13.8 (Si(CH3)3), −6.6 (Si(CH3)3), −18.1 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −35.3, −97.5, −132.9 ppm. IR (KBr, cm–1): ν 2958 (s), 1248 (s), 1087 (s), 989 (s), 837 (s). Anal. calcd for C31H67IOSi7U: C, 36.60; H, 6.64. Found: C, 36.58; H, 6.66.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of Me3SiI (4.0 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 18.8 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 9 and those of dmap were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(NC) (10)
Method A
This compound was prepared as yellow crystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 235 mg, 0.25 mmol) and Me3SiCN (25 mg, 0.25 mmol) in toluene (15 mL) at room temperature and recrystallization from an n-hexane solution by a similar procedure to that in the synthesis of 8. The product was isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 177 mg (78%). M.p.: 163–165 °C (dec.). 1H NMR (C6D6): δ 36.11 (s, 9H, Si(CH3)3), 6.76 (s, 18H, Si(CH3)3), −8.16 (s, 18H, Si(CH3)3), −10.77 (s, 18H, Si(CH3)3) ppm; the protons of Cp-ring CH were not observed. 13C{1H} NMR (C6D6): δ 180.7 (CN), 175.8 (ring C), 136.6 (ring C), 121.4 (ring C), 80.5 (ring C), 79.3 (ring C), 9.5 (Si(CH3)3), −7.4 (Si(CH3)3), −20.1 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −48.5, −100.1, −125.1 ppm. IR (KBr, cm–1): ν 2954 (s), 2036 (w), 1612 (s), 1249 (s), 1087 (s), 840 (s). Anal. calcd for C32H67NOSi7U: C, 41.94; H, 7.37; N, 1.53. Found: C, 41.92; H, 7.38; N, 1.56.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of Me3SiCN (2.0 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 18.8 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 10 and those of dmap were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(N3) (11)
Method A
This compound was prepared as brown microcrystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 235 mg, 0.25 mmol) and Me3SiN3 (29 mg, 0.25 mmol) in toluene (15 mL) at room temperature and recrystallization from an n-hexane solution by a similar procedure to that in the synthesis of 8. The product was isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL) and, dried at 50 °C under vacuum overnight. Yield: 172 mg (74%). M.p.: 168–170 °C (dec.). 1H NMR (C6D6): δ 28.67 (s, 9H, Si(CH3)3), 6.97 (s, 18H, Si(CH3)3), −7.51 (s, 18H, Si(CH3)3), −9.25 (s, 18H, Si(CH3)3) ppm; the protons of Cp-ring CH were not observed. 13C{1H} NMR (C6D6): δ 178.1 (ring C), 163.6 (ring C), 137.0 (ring C), 132.5 (ring C), 130.6 (ring C), 20.8 (Si(CH3)3), −0.7 (Si(CH3)3), −1.3 (Si(CH3)3), −17.0 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ 15.8, −33.7, −83.7, −109.1 ppm. IR (KBr, cm–1): ν 2955 (s), 2083 (s), 1612 (s), 1249 (s), 1003 (s), 833 (s). Anal. calcd for C31H67N3OSi7U: C, 39.93; H, 7.24; N, 4.51. Found: C, 39.92; H, 7.26; N, 4.49.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of Me3SiN3 (2.3 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 18.8 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 11 and those of dmap were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)2 (12)
Method A
After a toluene (10 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(Cl) (8; 185 mg, 0.2 mmol) was stirred at 70 °C overnight, the solution was filtered. The volume of the filtrate was reduced to 5 mL and cooled to −20 °C, yielding red crystals [η5-1,2,4-(Me3Si)3C5H2]2UCl2 (1), which were isolated by decantation of the supernatant. Yield: 75 mg (43% based on 8). After the red crystals of 1 were isolated, the solvent of the mother liquid was removed. The residue was extracted with n-hexane (5 mL × 3) and filtered. The volume of the filtrate was reduced to 4 mL, yellow crystals of 12 formed when this solution was kept at −40 °C for 2 days. Crystals of 12 were isolated by decantation of the supernatant, quickly washed with n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 74 mg (38% based on 8). M.p.: 135–137 °C (dec.). 1H NMR (C6D6): δ 36.12 (s, 18H, Si(CH3)3), 8.91 (s, 18H, Si(CH3)3), −7.81 (s, 18H, Si(CH3)3), −10.15 (s, 18H, Si(CH3)3) ppm; the protons of Cp-ring CH were not observed. 13C{1H} NMR (C6D6): δ 121.4 (ring C), 120.3 (ring C), 120.2 (ring C), 11.1 (Si(CH3)3), −6.5 (Si(CH3)3), −7.4 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −31.5, −32.2, −91.4 ppm. IR (KBr, cm–1): ν 2957 (s), 1614 (m), 1248 (s), 1093 (m), 831 (s). Anal. calcd for C34H76O2Si8U: C, 41.68; H, 7.82. Found: C, 41.71; H, 7.79.
Method B: NMR Scale
After a C6D6 (0.5 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2U(OSiMe3)(Cl) (8; 185 mg, 0.2 mmol) was kept at 70 °C overnight, resonances of 12 and those of [η5-1,2,4-(Me3Si)3C5H2]2UCl2 (1) (1 H NMR (C6D6): δ 13.70 (br s, 4H, ring CH), 2.79 (s, 36H, Si(CH3)3), −13.39 (s, 18H, Si(CH3)3) ppm)17 were observed by 1H NMR spectroscopy (100% conversion).
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U[OC(=NPh)S)(dmap)·0.5C6H6 (13·0.5C6H6)
Method A
This compound was prepared as orange crystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 235 mg, 0.25 mmol) and PhNCS (34 mg, 0.25 mmol) in toluene (15 mL) at room temperature and recrystallization from a benzene solution by a similar procedure as that in the synthesis of 8. The product was isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 239 mg (86%). M.p.: 159–161 °C (dec.). 1H NMR (C6D6): δ 16.09 (s, 2H, phenyl), 8.83 (s, 2H, phenyl), 7.15 (s, 3H, C6H6), 6.39 (br s, 18H, Si(CH3)3), 2.47 (s, 2H, ring CH), 1.45 (s, 2H, py H), 0.06 (br s, 18H, Si(CH3)3), −2.61 (s, 2H, py H), −3.54 (s, 1H, phenyl), −4.48 (s, 6H, N(CH3)2), −7.55 (s, 18H, Si(CH3)3) ppm; two Cp-ring CH were not observed. 13C{1H} NMR (C6D6): δ 177.2 (CN), 167.3 (py C), 149.5 (py C), 145.3 (py C), 140.3 (phenyl C), 137.0 (phenyl C), 132.5 (phenyl C), 130.6 (phenyl C), 129.6 (ring C), 129.5 (ring C), 129.4 (ring C), 128.5 (C6H6), 128.4 (ring C), 127.1 (ring C), 34.7 (N(CH3)2), 10.7 (Si(CH3)3), 6.7 (Si(CH3)3), −2.2 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −67.9, −97.1, −99.7 ppm. IR (KBr, cm–1): ν 2954 (s), 2900 (s), 1612 (s), 1573 (s), 1249 (s), 1095 (s), 995 (s), 840 (s). Anal. calcd for C45H76N3OSSi6U: C, 48.53; H, 6.88; N, 3.77. Found: C, 48.52; H, 6.86; N, 3.79.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of PhNCS (2.7 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=O(dmap) (5; 18.8 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 13 were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U(SSiMe3)(I) (14)
Method A
This compound was prepared as orange crystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6; 239 mg, 0.25 mmol) and Me3SiI (50 mg, 0.25 mmol) in toluene (15 mL) at room temperature and recrystallization from an n-hexane solution by a similar procedure to that in the synthesis of 8. The product was isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 222 mg (86%). M.p.: 156–158 °C (dec.). 1H NMR (C6D6): δ 13.24 (s, 18H, Si(CH3)3), 12.98 (s, 2H, ring CH), −2.00 (s, 18H, Si(CH3)3), −3.48 (s, 2H, ring CH), −7.66 (s, 9H, Si(CH3)3), −13.46 (s, 18H, Si(CH3)3) ppm. 13C{1H} NMR (C6D6): δ 130.6 (ring C), 129.7 (ring C), 129.6 (ring C), 129.4 (ring C), 121.4 (ring C), 28.6 (Si(CH3)3), 16.8 (Si(CH3)3), 13.8 (Si(CH3)3), 5.5 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −11.4, −52.4, −86.6, −114.4 ppm. IR (KBr, cm–1): ν 2955 (s), 1400 (s), 1244 (s), 1087 (s), 989 (s), 929 (s), 823 (s). Anal. calcd for C31H67ISSi7U: C, 36.03; H, 6.53. Found: C, 36.05; H, 6.56.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of Me3SiI (4.0 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6; 19.1 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 14 and those of dmap were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6) with Me3SiCl
NMR Scale
A C6D6 (0.3 mL) solution of Me3SiCl (2.2 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6; 19.1 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 1 along with those of unreacted 6, (Me3Si)2S (1H NMR (C6D6): δ 0.29 (s, 18H, Si(CH3)3) ppm), and dmap were observed by 1H NMR spectroscopy (50% conversion based on 6) when this solution was kept at room temperature overnight. Nevertheless, when 2 equiv of Me3SiCl (4.4 mg, 0.04 mmol) was added to a C6D6 (0.5 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6; 19.1 mg, 0.02 mmol), resonances of 1 along with those of (Me3Si)2S and dmap were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6) with PhNCO
NMR Scale
A C6D6 (0.3 mL) solution of PhNCO (2.4 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6; 19.1 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 13 were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U[SC(=NPh)S)(dmap) (15)
Method A
This compound was prepared as brown crystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6; 239 mg, 0.25 mmol) and PhNCS (34 mg, 0.25 mmol) in toluene (15 mL) at room temperature and recrystallization from a benzene solution by a similar procedure to that in the synthesis of 8. The product was isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 224 mg (82%). M.p.: 165–167 °C (dec.). 1H NMR (C6D6): δ 9.13 (s, 2H, phenyl), 6.59 (s, 2H, phenyl), 2.41 (s, 18H, Si(CH3)3), 1.38 (s, 18H, Si(CH3)3), −0.84 (s, 2H, py H), −2.59 (s, 18H, Si(CH3)3), −3.75 (s, 6H, N(CH3)2), −4.94 (s, 2H, ring CH), −8.72 (s, 1H, phenyl), −9.02 (s, 2H, py H) ppm; two Cp-ring CH protons were not observed. 13C{1H} NMR (C6D6): δ 144.7 (CN), 136.6 (py C), 134.8 (py C), 133.4 (py C), 129.5 (phenyl C), 127.2 (phenyl C), 127.1 (phenyl C), 125.7 (phenyl C), 122.5 (ring C), 95.5 (ring C), 35.8 (N(CH3)2), 14.0 (Si(CH3)3), 12.3 (Si(CH3)3), 3.8 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −46.9, −56.4, −110.6 ppm. IR (KBr, cm–1): ν 2954 (m), 1612 (s), 1535 (s), 1249 (s), 1087 (s), 995 (s), 833 (s). Anal. calcd for C42H73N3S2Si6U: C, 46.25; H, 6.75; N, 3.85. Found: C, 46.22; H, 6.76; N, 3.87.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of PhNCS (2.7 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6; 19.1 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 15 were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of {[η5-1,2,4-(Me3Si)3C5H2]2U}2(μ-CS3)2 (16)
Method A
This compound was prepared as brown crystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6; 239 mg, 0.25 mmol) and CS2 (19 mg, 0.25 mmol) in toluene (15 mL) at room temperature and recrystallization from a benzene solution by a similar procedure to that in the synthesis of 8. The product was isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 205 mg (90%). 1H NMR (C6D6): δ 20.21 (s, 9H, Si(CH3)3), 14.23 (br s, 9H, Si(CH3)3), −7.42 (s, 9H, Si(CH3)3), −7.68 (s, 9H, Si(CH3)3), −10.16 (s, 9H, Si(CH3)3), −12.98 (s, 9H, Si(CH3)3) ppm; the protons of the Cp-ring CH were not observed. These spectroscopic data agreed with those reported in the literature.17 Furthermore, this complex was also characterized by X-ray diffraction analysis and its molecular structure is shown in the Supporting Information.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of CS2 (1.5 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=S(dmap) (6; 19.1 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 16 and those of dmap were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Preparation of {[η5-1,2,4-(Me3Si)3C5H2]2U}2(μ-Se)2 (17)
After a toluene (10 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7; 193 mg, 0.2 mmol) was stirred at 60 °C overnight, the solvent was removed. The residue was extracted with benzene (5 mL × 3) and filtered. The volume of the filtrate was reduced to 4 mL, and brown microcrystals of 17 formed when this solution was kept at 10 °C for 2 days. The product was isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 144 mg (82%). M.p.: 183–185 °C (dec.). 1H NMR (C6D6): δ 36.44 (s, 18H, Si(CH3)3), 13.92 (s, 36H, Si(CH3)3) ppm; the protons of Cp-ring CH were not observed. 13C{1H} NMR (C6D6): δ 150.1 (ring C), 139.0 (ring C), 137.4 (ring C), 4.6 (Si(CH3)3), 2.7 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −74.4, −75.8, −104.0 ppm. IR (KBr, cm–1): ν 2957 (s), 1595 (s), 1510 (s), 1249 (s), 1172 (s), 1030 (s), 831 (s). Anal. calcd for C56H116Se2Si12U2: C, 38.20; H, 6.64. Found: C, 38.23; H, 6.61. Brown crystals of 17·0.5C6H14 suitable for X-ray structural analysis were isolated from a mixture of benzene and n-hexane (4:1) solution.
Preparation of [η5-1,2,4-(Me3Si)3C5H2]2U(SeSiMe3)(I) (18)
Method A
This compound was prepared as orange crystals from the reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7; 241 mg, 0.25 mmol) and Me3SiI (50 mg, 0.25 mmol) in toluene (15 mL) at room temperature and recrystallization from an n-hexane solution by a similar procedure as that in the synthesis of 8. The product was isolated by decantation of the supernatant, rapidly washed with cooled n-hexane (2 mL), and dried at 50 °C under vacuum overnight. Yield: 221 mg (85%). M.p.: 164–166 °C (dec.). 1H NMR (C6D6): δ 14.03 (s, 18H, (CH3)3Si), −1.25 (s, 18H, (CH3)3Si), −9.50 (s, 9H, (CH3)3Si), −13.57 (s, 18H, (CH3)3Si) ppm; the protons of Cp-ring CH were not observed. 13C{1H} NMR (C6D6): δ 131.4 (ring C), 129.5 (ring C), 121.7 (ring C), 113.7 (ring C), 113.6 (ring C) 21.7 (Si(CH3)3), 13.8 (Si(CH3)3), 8.4 (Si(CH3)3), 6.2 (Si(CH3)3) ppm. 29Si{1H} NMR (C6D6): δ −11.5, −35.3, −53.5 ppm. IR (KBr, cm–1): ν 2954 (s), 1597 (m), 1512 (s), 1381 (s), 1249 (s), 1172 (m), 1087 (m), 1026 (m), 833 (s). Anal. calcd for C31H67ISeSi7U: C, 34.46; H, 6.25. Found: C, 34.45; H, 6.26. This complex was also characterized by X-ray diffraction analysis and its molecular structure is shown in the Supporting Information. Nevertheless, the quality of the data was rather poor because of crystal twinning, but sufficient to at least establish the overall connectivity.
Method B: NMR Scale
A C6D6 (0.3 mL) solution of Me3SiI (4.0 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7; 19.3 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 18 and those of dmap were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
Reaction of [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7) with Me3SiCl
NMR Scale
A C6D6 (0.3 mL) solution of Me3SiCl (2.2 mg, 0.02 mmol) was slowly added to a J. Young NMR tube charged with [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7; 19.3 mg, 0.02 mmol) and C6D6 (0.2 mL). Resonances of 1 along with those of unreacted 7, (Me3Si)2Se (1 H NMR (C6D6): δ 0.36 (s, 18H, Si(CH3)3) ppm), and dmap were observed by 1H NMR spectroscopy (50% conversion based on 7) when this solution was kept at room temperature overnight. Nevertheless, when 2 equiv of Me3SiCl (4.4 mg, 0.04 mmol) was added to a C6D6 (0.5 mL) solution of [η5-1,2,4-(Me3Si)3C5H2]2U=Se(dmap) (7; 19.3 mg, 0.02 mmol), resonances of 1 along with those of (Me3Si)2Se and dmap were observed by 1H NMR spectroscopy (100% conversion) when this solution was kept at room temperature overnight.
X-ray Crystallography
Single-crystal X-ray diffraction measurements were carried out on a Rigaku Saturn CCD diffractometer at 100(2) K using Mο Kα radiation (λ = 0.71073 Å) or Cu Kα radiation (λ = 1.54184 Å). An empirical absorption correction was applied using the SADABS program.20 All structures were solved by direct methods and refined by full-matrix least squares on F2 using the SHELXL program package.21 All the hydrogen atoms were geometrically fixed using the riding model. The crystal data and experimental data for 4–10 and 12–17 are summarized in the Supporting Information. Selected bond lengths and angles are listed in Table 1.
Computational Methods
All calculations were carried out with the Gaussian 09 program (G09),22 employing the B3PW91 functional, plus a polarizable continuum model (PCM) (denoted as B3PW91-PCM), with standard 6-31G(d) basis set for C, H, N, O, S, Se, Si, and Cl elements and a quasi-relativistic 5f-in-valence effective-core potential (ECP60MWB or ECP80MWB) treatment with 60 or 80 electrons in the core region for U and the corresponding optimized segmented ((14s13p10d8f6g)/[10s9p5d4f3g]) basis set for the valence shells of U,23 to fully optimize the structures of reactants, complexes, transition state, intermediates, and products and also to mimic the experimental toluene-solvent conditions (dielectric constant ε = 2.379). All stationary points were subsequently characterized by vibrational analyses, from which their respective zero-point (vibrational) energy (ZPE) was extracted and used in the relative energy determinations; in addition, frequency calculations were also performed to ensure that the reactant, complex, intermediate, product, and transition state structures resided at minima and 1st-order saddle points on their potential energy hypersurfaces. Please note that to properly describe the reaction profile for the reaction of 4 + Ph2CO, dispersion effects (D3)24 had to be considered for the sterically encumbered uranium complexes since dispersion contributes significantly to the overall energy profile (see the Supporting Information for details, Figure S4 and Tables S6 and S7) and therefore single-point B3PW91-PCM-D324 calculations based on B3PW91-PCM geometries were performed for this transformation. Bader’s quantum theory of atoms-in-molecules (QTAIM)25 analyses were performed using the Multiwfn program.26
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant nos. 22271017, 21871029, and 21672024) and the Deutsche Forschungsgemeinschaft (DFG) through the Heisenberg program (WA 2513/8).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c03753.
The authors declare no competing financial interest.
Supplementary Material
References
- For selected recent reviews, see:; a Fox A. R.; Bart S. C.; Meyer K.; Cummins C. C. Towards uranium catalysts. Nature 2008, 455, 341–349. 10.1038/nature07372. [DOI] [PubMed] [Google Scholar]; b Hayton T. W. Metal-ligand multiple bonding in uranium: structure and reactivity. Dalton Trans. 2010, 39, 1145–1158. 10.1039/B909238B. [DOI] [PubMed] [Google Scholar]; c Fortier S.; Hayton T. W. Oxo ligand functionalization in the uranyl ion (UO22+). Coord. Chem. Rev. 2010, 254, 197–214. 10.1016/j.ccr.2009.06.003. [DOI] [Google Scholar]; d Hayton T. W. Recent developments in actinide-ligand multiple bonding. Chem. Commun. 2013, 49, 2956–2973. 10.1039/c3cc39053e. [DOI] [PubMed] [Google Scholar]; e Andrews M. B.; Cahill C. L. Uranyl Bearing Hybrid Materials: Synthesis, Speciation, and Solid-State Structures. Chem. Rev. 2013, 113, 1121–1136. 10.1021/cr300202a. [DOI] [PubMed] [Google Scholar]; f Jones M. B.; Gaunt A. J. Recent Developments in Synthesis and Structural Chemistry of Nonaqueous Actinide Complexes. Chem. Rev. 2013, 113, 1137–1198. 10.1021/cr300198m. [DOI] [PubMed] [Google Scholar]; g Zi G. Organothorium complexes containing terminal metal-ligand multiple bonds. Sci. China: Chem. 2014, 57, 1064–1072. 10.1007/s11426-014-5094-y. [DOI] [Google Scholar]; h Ephritikhine M. Molecular actinide compounds with soft chalcogen ligands. Coord. Chem. Rev. 2016, 319, 35–62. 10.1016/j.ccr.2016.04.020. [DOI] [Google Scholar]; i Cowie B. E.; Purkis J. M.; Austin J.; Love J. B.; Arnold P. L. Thermal and Photochemical Reduction and Functionalization Chemistry of the Uranyl Dication, [UVIO2]2+. Chem. Rev. 2019, 119, 10595–10637. 10.1021/acs.chemrev.9b00048. [DOI] [PubMed] [Google Scholar]; j Leduc J.; Frank M.; Jürgensen L.; Graf D.; Raauf A.; Mathur S. Chemistry of Actinide Centers in Heterogeneous Catalytic Transformations of Small Molecules. ACS Catal. 2019, 9, 4719–4741. 10.1021/acscatal.8b04924. [DOI] [Google Scholar]
- For selected papers on uranium non-metallocenes containing terminal oxo groups, see:; a Andersen R. A. Tris((hexamethyldisilyl)amido)uranium(III): preparation and coordination chemistry. Inorg. Chem. 1979, 18, 1507–1509. 10.1021/ic50196a021. [DOI] [Google Scholar]; b Docrat T. I.; Mosselmans J. F. W.; Charnock J. M.; Whiteley M. W.; Collison D.; Livens F. R.; Jones C.; Edmiston M. J. X-ray Absorption Spectroscopy of Tricarbonatodioxouranate(V), [UO2(CO3)3]5–, in Aqueous Solution. Inorg. Chem. 1999, 38, 1879–1882. 10.1021/ic9814423. [DOI] [PubMed] [Google Scholar]; c Duval P. B.; Burns C. J.; Buschmann W. E.; Clark D. L.; Morris D. E.; Scott B. L. Reaction of the Uranyl(VI) Ion (UO22+) with a Triamidoamine Ligand: Preparation and Structural Characterization of a Mixed-Valent Uranium(V/VI) Oxo–Imido Dimer. Inorg. Chem. 2001, 40, 5491–5496. 10.1021/ic010155n. [DOI] [PubMed] [Google Scholar]; d Duval P. B.; Burns C. J.; Clark D. L.; Morris D. E.; Scott B. L.; Thompson J. D.; Werkema E. L.; Jia L.; Andersen R. A. Synthesis and structural characterization of the first uranium cluster containing an isopolyoxometalate core. Angew. Chem., Int. Ed. 2001, 40, 3358–3361. . [DOI] [PubMed] [Google Scholar]; e Roussel P.; Boaretto R.; Kingsley A. J.; Alcock N. W.; Scott P. Reactivity of a triamidoamine complex of trivalent uranium. J. Chem. Soc., Dalton Trans. 2002, 1423–1428. 10.1039/b108584k. [DOI] [Google Scholar]; f Berthet J.-C.; Nierlich M.; Ephritikhine M. Isolation of a Uranyl [UO2]+ Species: Crystallographic Comparison of the Dioxouranium(V) and (VI) Compounds [UO2(OPPh3)4](OTf)n (n=1, 2). Angew. Chem., Int. Ed. 2003, 42, 1952–1954. 10.1002/anie.200250506. [DOI] [PubMed] [Google Scholar]; g Burdet F.; Pecaut J.; Mazzanti M. Isolation of a Tetrameric Cation–Cation Complex of Pentavalent Uranyl. J. Am. Chem. Soc. 2006, 128, 16512–16513. 10.1021/ja067445t. [DOI] [PubMed] [Google Scholar]; h Berthet J.-C.; Siffredi G.; Thuery P.; Ephritikhine M. Easy access to stable pentavalent uranyl complexes. Chem. Commun. 2006, 3184–3186. 10.1039/B605710A. [DOI] [PubMed] [Google Scholar]; i Natrajan L.; Burdet F.; Pécaut J.; Mazzanti M. Synthesis and Structure of a Stable Pentavalent-Uranyl Coordination Polymer. J. Am. Chem. Soc. 2006, 128, 7152–7153. 10.1021/ja0609809. [DOI] [PubMed] [Google Scholar]; j Hayton T. W.; Boncella J. M.; Scott B. L.; Batista E. R. Exchange of an Imido Ligand in Bis(imido) Complexes of Uranium. J. Am. Chem. Soc. 2006, 128, 12622–12623. 10.1021/ja064400j. [DOI] [PubMed] [Google Scholar]; k Hayton T. W.; Wu G. Synthesis, Characterization, and Reactivity of a Uranyl β-Diketiminate Complex. J. Am. Chem. Soc. 2008, 130, 2005–2014. 10.1021/ja077538q. [DOI] [PubMed] [Google Scholar]; l Arnold P. L.; Patel D.; Wilson C.; Love J. B. Reduction and selective oxo group silylation of the uranyl dication. Nature 2008, 451, 315–317. 10.1038/nature06467. [DOI] [PubMed] [Google Scholar]; m Hayton T. W.; Wu G. Mixed-Ligand Uranyl(V) β-Diketiminate/β-Diketonate Complexes: Synthesis and Characterization. Inorg. Chem. 2008, 47, 7415–7423. 10.1021/ic800778j. [DOI] [PubMed] [Google Scholar]; n Bart S. C.; Anthon C.; Heinemann F. W.; Bill E.; Edelstein N. M.; Meyer K. Carbon Dioxide Activation with Sterically Pressured Mid- and High-Valent Uranium Complexes. J. Am. Chem. Soc. 2008, 130, 12536–12546. 10.1021/ja804263w. [DOI] [PubMed] [Google Scholar]; o Nocton G.; Horeglad P.; Pécaut J.; Mazzanti M. Polynuclear Cation–Cation Complexes of Pentavalent Uranyl: Relating Stability and Magnetic Properties to Structure. J. Am. Chem. Soc. 2008, 130, 16633–16645. 10.1021/ja804766r. [DOI] [PubMed] [Google Scholar]; p Hayton T. W.; Wu G. Exploring the Effects of Reduction or Lewis Acid Coordination on the U=O Bond of the Uranyl Moiety. Inorg. Chem. 2009, 48, 3065–3072. 10.1021/ic802360y. [DOI] [PubMed] [Google Scholar]; q Berthet J.-C.; Siffredi G.; Thuéry P.; Ephritikhine M. Synthesis and crystal structure of pentavalent uranyl complexes. The remarkable stability of UO2X (X = I, SO3CF3) in non-aqueous solutions. Dalton Trans. 2009, 3478–3494. 10.1039/b820659g. [DOI] [PubMed] [Google Scholar]; r Cantat T.; Graves C. R.; Scott B. L.; Kiplinger J. L. Challenging the Metallocene Dominance in Actinide Chemistry with a Soft PNP Pincer Ligand: New Uranium Structures and Reactivity Patterns. Angew. Chem., Int. Ed. 2009, 48, 3681–3684. 10.1002/anie.200806115. [DOI] [PubMed] [Google Scholar]; s Horeglad P.; Nocton G.; Filinchuk Y.; Pécaut J.; Mazzanti M. Pentavalent uranyl stabilized by a dianionic bulky tetradentate ligand. Chem. Commun. 2009, 1843. 10.1039/b821398d. [DOI] [PubMed] [Google Scholar]; t Fortier S.; Wu G.; Hayton T. W. Synthesis of a Nitrido-Substituted Analogue of the Uranyl Ion, [N=U=O]+. J. Am. Chem. Soc. 2010, 132, 6888–6889. 10.1021/ja101567h. [DOI] [PubMed] [Google Scholar]; u Kraft S. J.; Walensky J.; Fanwick P. E.; Hall M. B.; Bart S. C. Crystallographic Evidence of a Base-Free Uranium(IV) Terminal Oxo Species. Inorg. Chem. 2010, 49, 7620–7622. 10.1021/ic101136j. [DOI] [PubMed] [Google Scholar]; v Brow J. L.; Wu G.; Hayton T. W. Oxo Ligand Silylation in a Uranyl β-Ketoiminate Complex. J. Am. Chem. Soc. 2010, 132, 7248–7249. 10.1021/ja1013739. [DOI] [PubMed] [Google Scholar]; w Tourneux J.-C.; Berthet J.-C.; Cantat T.; Thuéry P.; Mézailles N.; Ephritikhine M. Exploring the Uranyl Organometallic Chemistry: From Single to Double Uranium–Carbon Bonds. J. Am. Chem. Soc. 2011, 133, 6162–6165. 10.1021/ja201276h. [DOI] [PubMed] [Google Scholar]; x Schnaars D. D.; Wu G.; Hayton T. W. Borane-Mediated Silylation of a Metal–Oxo Ligand. Inorg. Chem. 2011, 50, 4695–4697. 10.1021/ic2008649. [DOI] [PubMed] [Google Scholar]; y Brown J. L.; Mokhtarzadeh C. C.; Lever J. M.; Wu G.; Hayton T. W. Facile Reduction of a Uranyl(VI) β-Ketoiminate Complex to U(IV) Upon Oxo Silylation. Inorg. Chem. 2011, 50, 5105–5112. 10.1021/ic200387n. [DOI] [PubMed] [Google Scholar]; z Fortier S.; Kaltsoyannis N.; Wu G.; Hayton T. W. Probing the Reactivity and Electronic Structure of a Uranium(V) Terminal Oxo Complex. J. Am. Chem. Soc. 2011, 133, 14224–14227. [DOI] [PubMed] [Google Scholar]; aa Arnold P. L.; Pécharman A.-F.; Love J. B. Oxo Group Protonation and Silylation of Pentavalent Uranyl Pacman Complexes. Angew. Chem., Int. Ed. 2011, 50, 9456–9458. 10.1002/anie.201104359. [DOI] [PubMed] [Google Scholar]; ab Kosog B.; La Pierre H. S.; Heinemann F. W.; Liddle S. T.; Meyer K. Synthesis of Uranium(VI) Terminal Oxo Complexes: Molecular Geometry Driven by the Inverse Trans-Influence. J. Am. Chem. Soc. 2012, 134, 5284–5289. 10.1021/ja211618v. [DOI] [PubMed] [Google Scholar]; ac Mills D. P.; Cooper O. J.; Tuna F.; McInnes E. J. L.; Davies E. S.; McMaster J.; Moro F.; Lewis W.; Blake A. J.; Liddle S. T. Synthesis of a Uranium(VI)-Carbene: Reductive Formation of Uranyl(V)-Methanides, Oxidative Preparation of a [R2C=U=O]2+ Analogue of the [O=U=O]2+ Uranyl Ion (R = Ph2PNSiMe3), and Comparison of the Nature of UIV=C, UV=C, and UVI=C Double Bonds. J. Am. Chem. Soc. 2012, 134, 10047–10054. 10.1021/ja301333f. [DOI] [PubMed] [Google Scholar]; ad Fortier S.; Brown J. L.; Kaltsoyannis N.; Wu G.; Hayton T. W. Synthesis, Molecular and Electronic Structure of UV(O)[N(SiMe3)2]3. Inorg. Chem. 2012, 51, 1625–1633. 10.1021/ic201936j. [DOI] [PubMed] [Google Scholar]; ae Lewis A. J.; Carroll P. J.; Schelter E. J. Reductive Cleavage of Nitrite to Form Terminal Uranium Mono-Oxo Complexes. J. Am. Chem. Soc. 2013, 135, 511–518. 10.1021/ja311057y. [DOI] [PubMed] [Google Scholar]; af Kiernicki J. J.; Cladis D. P.; Fanwick P. E.; Zeller M.; Bart S. C. Synthesis, Characterization, and Stoichiometric U–O Bond Scission in Uranyl Species Supported by Pyridine(diimine) Ligand Radicals. J. Am. Chem. Soc. 2015, 137, 11115–11125. 10.1021/jacs.5b06217. [DOI] [PubMed] [Google Scholar]; ag Halter D. P.; Heinemann F. W.; Maron L.; Meyer K. The role of uranium-arene bonding in H2O reduction catalysis. Nat. Chem. 2018, 10, 259–267. 10.1038/nchem.2899. [DOI] [PubMed] [Google Scholar]; ah Rice N. T.; McCabe K.; Bacsa J.; Maron L.; La Pierre H. S. Two-Electron Oxidative Atom Transfer at a Homoleptic, Tetravalent Uranium Complex. J. Am. Chem. Soc. 2020, 142, 7368–7373. 10.1021/jacs.0c02693. [DOI] [PubMed] [Google Scholar]; ai Assefa M. K.; Wu G.; Hayton T. W. Uranyl Oxo Silylation Promoted by Silsesquioxane Coordination. J. Am. Chem. Soc. 2020, 142, 8738–8747. 10.1021/jacs.0c00990. [DOI] [PubMed] [Google Scholar]; aj Hartline D. R.; Löffler S. T.; Fehn D.; Kasper J. M.; Heinemann F. W.; Yang P.; Batista E. R.; Meyer K. Uranium-Mediated Peroxide Activation and a Precursor toward an Elusive Uranium cis-Dioxo Fleeting Intermediate. J. Am. Chem. Soc. 2023, 145, 8927–8938. 10.1021/jacs.2c12868. [DOI] [PubMed] [Google Scholar]
- For selected papers about uranium metallocenes containing terminal oxo groups, see:; a Villiers C.; Adam R.; Ephritikhine M. Deoxygenation of carbon monoxide by triscyclopentadienyluranium alkyl complexes giving alkylbenzene molecules. J. Chem. Soc., Chem. Commun. 1992, 1555–1556. 10.1039/c39920001555. [DOI] [Google Scholar]; b Arney D. S. J.; Burns C. J. Synthesis and structure of high-valent organouranium complexes containing terminal monooxo functional groups. J. Am. Chem. Soc. 1993, 115, 9840–9841. 10.1021/ja00074a077. [DOI] [Google Scholar]; c Arney D. S. J.; Burns C. J. Synthesis and Properties of High-Valent Organouranium Complexes Containing Terminal Organoimido and Oxo Functional Groups. A New Class of Organo-f-Element Complexes. J. Am. Chem. Soc. 1995, 117, 9448–9460. 10.1021/ja00142a011. [DOI] [Google Scholar]; d Evans W. J.; Kozimor S. A.; Ziller J. W. Bis(pentamethylcyclopentadienyl) U(III) oxide and U(IV) oxide carbene complexes. Polyhedron 2004, 23, 2689–2694. 10.1016/j.poly.2004.08.007. [DOI] [Google Scholar]; e Zi G.; Jia L.; Werkema E. L.; Walter M. D.; Gottfriedsen J. P.; Andersen R. A. Preparation and Reactions of Base-free Bis(1,3,4-tri-t-butylcyclopentadienyl)uranium Oxide, Cp’2UO. Organometallics 2005, 24, 4251–4264. 10.1021/om050406q. [DOI] [Google Scholar]
- For selected papers about thorium complexes containing terminal oxo groups, see:; a Smiles D. E.; Wu G.; Kaltsoyannis N.; Hayton T. W. Thorium-ligand multiple bonds via reductive deprotection of a trityl group. Chem. Sci. 2015, 6, 3891–3899. 10.1039/C5SC01248A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rupasinghe D. M. R. Y. P.; Baxter M. R.; Gupta H.; Poore A. T.; Higgins R. F.; Zeller M.; Tian S.; Schelter E. J.; Bart S. C. Actinide-Oxygen Multiple Bonds from Air: Synthesis and Characterization of a Thorium Oxo Supported by Redox-Active Ligands. J. Am. Chem. Soc. 2022, 144, 17423–17431. 10.1021/jacs.2c04947. [DOI] [PubMed] [Google Scholar]
- For selected papers about actinide complexes containing terminal chalcogenides, see:; a Ventelon L.; Lescop C.; Arliguie T.; Leverd P. C.; Lance M.; Nierlich M.; Ephritikhine M. Synthesis and X-ray crystal structure of [Na(18-crown-6)][U(Cp*)2(SBut)(S)], the first f-element compound containing a metal-sulfur double bond. Chem. Commun. 1999, 659–660. 10.1039/a900466a. [DOI] [Google Scholar]; b Brown J. L.; Fortier S.; Lewis R. A.; Wu G.; Hayton T. W. A Complete Family of Terminal Uranium Chalcogenides, [U(E)(N-{SiMe3}2)3]− (E = O, S, Se, Te). J. Am. Chem. Soc. 2012, 134, 15468–15475. 10.1021/ja305712m. [DOI] [PubMed] [Google Scholar]; c Behrle A. C.; Barnes C. L.; Kaltsoyannis N.; Walensky J. R. Systematic Investigation of Thorium(IV)– and Uranium(IV)–Ligand Bonding in Dithiophosphonate, Thioselenophosphinate, and Diselenophosphonate Complexes. Inorg. Chem. 2013, 52, 10623–10631. 10.1021/ic401642a. [DOI] [PubMed] [Google Scholar]; d Brown J. L.; Fortier S.; Wu G.; Kaltsoyannis N.; Hayton T. W. Synthesis and Spectroscopic and Computational Characterization of the Chalcogenido-Substituted Analogues of the Uranyl Ion, [OUE]2+ (E=S, Se). J. Am. Chem. Soc. 2013, 135, 5352–5355. 10.1021/ja402068j. [DOI] [PubMed] [Google Scholar]; e Smiles D. E.; Wu G.; Hayton T. W. Synthesis of Uranium-Ligand Multiple Bonds by Cleavage of a Trityl Protecting Group. J. Am. Chem. Soc. 2014, 136, 96–99. 10.1021/ja411423a. [DOI] [PubMed] [Google Scholar]; f Smiles D. E.; Wu G.; Hayton T. W. Synthesis of Terminal Monochalcogenide and Dichalcogenide Complexes of Uranium Using Polychalcogenides, [En]2– (E=Te, n = 2; E=Se, n = 4), as Chalcogen Atom Transfer Reagents. Inorg. Chem. 2014, 53, 10240–10247. 10.1021/ic501267f. [DOI] [PubMed] [Google Scholar]; g Smiles D. E.; Wu G.; Hayton T. W. Reversible Chalcogen-Atom Transfer to a Terminal Uranium Sulfide. Inorg. Chem. 2014, 53, 12683–12685. 10.1021/ic502500z. [DOI] [PubMed] [Google Scholar]; h Andrez J.; Pécaut J.; Scopelliti R.; Kefalidis C. E.; Maron L.; Rosenzweig M. W.; Meyer K.; Mazzanti M. Synthesis and reactivity of a terminal uranium(IV) sulfide supported by siloxide ligands. Chem. Sci. 2016, 7, 5846–5856. 10.1039/C6SC00675B. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Rosenzweig M. W.; Scheurer A.; Lamsfus C. A.; Heinemann F. W.; Maron L.; Andrez J.; Mazzanti M.; Meyer K. Uranium(IV) terminal hydrosulfido and sulfide complexes: insights into the nature of the uranium-sulfur bond. Chem. Sci. 2016, 7, 5857–5866. 10.1039/C6SC00677A. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Smiles D. E.; Wu G.; Hrobárik P.; Hayton T. W. Use of 77Se and 125Te NMR Spectroscopy to Probe Covalency of the Actinide-Chalcogen Bonding in [Th(En){N(SiMe3)2}3]− (E=Se, Te; n = 1, 2) and Their Oxo-Uranium(VI) Congeners. J. Am. Chem. Soc. 2016, 138, 814–825. 10.1021/jacs.5b07767. [DOI] [PubMed] [Google Scholar]; k Kelly R. P.; Falcone M.; Lamsfus C. A.; Scopelliti R.; Maron L.; Meyer K.; Mazzanti M. Metathesis of a UV imido complex: a route to a terminal UV sulfide. Chem. Sci. 2017, 8, 5319–5328. 10.1039/C7SC01111C. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Pagano J. K.; Arney D. S. J.; Scott B. L.; Morris D. E.; Kiplinger J. L.; Burns C. J. A sulphur and uranium fiesta! Synthesis, structure, and characterization of neutral terminal uranium(VI) monosulphide, uranium(IV) η2-disulphide, and uranium(IV) phosphine sulphide complexes. Dalton Trans. 2019, 48, 50–57. 10.1039/C8DT02932F. [DOI] [PubMed] [Google Scholar]; m Rosenzweig M. W.; Hümmer J.; Scheurer A.; Lamsfus C. A.; Heinemann F. W.; Maron L.; Mazzanti M.; Meyer K. A complete series of uranium(IV) complexes with terminal hydrochalcogenido (EH) and chalcogenido (E) ligands E=O, S, Se, Te. Dalton Trans. 2019, 48, 10853–10864. 10.1039/C9DT00530G. [DOI] [PubMed] [Google Scholar]
- Morss L. R.; Edelstein N. M.; Fuger J.; Katz J. J. Eds. The Chemistry of the Actinide and Transactinide Elements; 3rd edition, Volumes 1-5, Springer: Dordrecht, 2006, 10.1007/1-4020-3598-5. [DOI] [Google Scholar]
- Hutchings G. J.; Heneghan C. S.; Hudson I. D.; Taylor S. H. Uranium-oxide-based catalysts for the destruction of volatile chloro-organic compounds. Nature 1996, 384, 341–343. 10.1038/384341a0. [DOI] [Google Scholar]
- For selected reviews on activation of small molecules by organoactinides, see:; a Barnea E.; Eisen M. S. Organoactinides in catalysis. Coord. Chem. Rev. 2006, 250, 855–899. 10.1016/j.ccr.2005.12.007. [DOI] [Google Scholar]; b Ephritikhine M. The vitality of uranium molecular chemistry at the dawn of the XXIst century. Dalton Trans. 2006, 2501–2516. 10.1039/b603463b. [DOI] [PubMed] [Google Scholar]; c Summerscales O. T.; Cloke F. G. N. Activation of small molecules by U(III) cyclooctatetraene and pentalene complexes. Struct. Bonding 2008, 127, 87–117. 10.1007/430_2007_078. [DOI] [Google Scholar]; d Meyer K.; Bart S. C. Tripodal carbene and aryloxide ligands for small-molecule activation at electron-rich uranium and transition metal centers. Adv. Inorg. Chem. 2008, 60, 1–30. 10.1016/S0898-8838(08)00001-9. [DOI] [Google Scholar]; e Andrea T.; Eisen M. S. Recent advances in organothorium and organouranium catalysis. Chem. Soc. Rev. 2008, 37, 550–567. 10.1039/B614969N. [DOI] [PubMed] [Google Scholar]; f Lam O. P.; Anthon C.; Meyer K. Influence of steric pressure on the activation of carbon dioxide and related small molecules by uranium coordination complexes. Dalton Trans. 2009, 9677–9691. 10.1039/b905537a. [DOI] [PubMed] [Google Scholar]; g Eisen M. S.; Catalytic C.-N. C-O, and C-S Bond Formation Promoted by Organoactinide Complexes. Top. Organomet. Chem. 2010, 31, 157–184. 10.1007/978-3-642-12073-2_7. [DOI] [Google Scholar]; h Lam O. P.; Meyer K. Hydrogenation of CO at a Uranium(III) Center. Angew. Chem., Int. Ed. 2011, 50, 9542–9544. 10.1002/anie.201104157. [DOI] [PubMed] [Google Scholar]; i Arnold P. L. Uranium-mediated activation of small molecules. Chem. Commun. 2011, 47, 9005–9010. 10.1039/c1cc10834d. [DOI] [PubMed] [Google Scholar]; j Lam O. P.; Meyer K. Uranium-mediated carbon dioxide activation and functionalization. Polyhedron 2012, 32, 1–9. 10.1016/j.poly.2011.07.015. [DOI] [Google Scholar]; k Johnson K. R. D.; Hayes P. G. Cyclometalative C-H bond activation in rare earth and actinide metal complexes. Chem. Soc. Rev. 2013, 42, 1947–1960. 10.1039/C2CS35356C. [DOI] [PubMed] [Google Scholar]; l Ephritikhine M. Recent Advances in Organoactinide Chemistry As Exemplified by Cyclopentadienyl Compounds. Organometallics 2013, 32, 2464–2488. 10.1021/om400145p. [DOI] [Google Scholar]; m Hayton T. W. An actinide milestone. Nat. Chem. 2013, 5, 451–452. 10.1038/nchem.1643. [DOI] [PubMed] [Google Scholar]; n Gardner B. M.; Liddle S. T. Small-Molecule Activation at Uranium(III). Eur. J. Inorg. Chem. 2013, 3753–3770. 10.1002/ejic.201300111. [DOI] [Google Scholar]; o La Pierre H. S.; Meyer K. Activation of Small Molecules by Molecular Uranium Complexes. Prog. Inorg. Chem. 2014, 58, 303–415. 10.1002/9781118792797.ch05. [DOI] [Google Scholar]; p Arnold P. L.; McMullon M. W.; Rieb J.; Kühn F. E. C-H Bond Activation by f-Block Complexes. Angew. Chem., Int. Ed. 2015, 54, 82–100. 10.1002/anie.201404613. [DOI] [PubMed] [Google Scholar]; q Liddle S. T. The Renaissance of Non-Aqueous Uranium Chemistry. Angew. Chem., Int. Ed. 2015, 54, 8604–8641. 10.1002/anie.201412168. [DOI] [PubMed] [Google Scholar]; r Ortu F.; Formanuik A.; Innes J. R.; Mills D. P. New vistas in the molecular chemistry of thorium: low oxidation state complexes. Dalton Trans. 2016, 45, 7537–7549. 10.1039/C6DT01111J. [DOI] [PubMed] [Google Scholar]; s Zi G. Recent developments in actinide metallacycles. Chem. Commun. 2018, 54, 7412–7430. 10.1039/C8CC01516C. [DOI] [PubMed] [Google Scholar]; t Schüdle D.; Anwander R. Rare-earth metal and actinide organoimide chemistry. Chem. Soc. Rev. 2019, 48, 5752–5805. 10.1039/C8CS00932E. [DOI] [PubMed] [Google Scholar]; u Boreen M. A.; Arnold J. The synthesis and versatile reducing power of low-valent uranium complexes. Dalton Trans. 2020, 49, 15124–15138. 10.1039/D0DT03151H. [DOI] [PubMed] [Google Scholar]; v Revathi S.; Raja P.; Saha S.; Eisen M. S.; Ghatak T. Recent developments in highly basic N-heterocyclic iminato ligands in actinide chemistry. Chem. Commun. 2021, 57, 5483–5502. 10.1039/D1CC00933H. [DOI] [PubMed] [Google Scholar]
- Selected papers about the bonding of organoactinide complexes, see:; a Barros N.; Maynau D.; Maron L.; Eisenstein O.; Zi G.; Andersen R. A. Single but Stronger UO, Double but Weaker UNMe Bonds: The Tale Told by Cp2UO and Cp2UNR. Organometallics 2007, 26, 5059–5065. 10.1021/om700628e. [DOI] [Google Scholar]; b Cantat T.; Graves C. R.; Jantunen K. C.; Burns C. J.; Scott B. L.; Schelter E. J.; Morris D. E.; Hay P. J.; Kiplinger J. L. Evidence for the Involvement of 5f Orbitals in the Bonding and Reactivity of Organometallic Actinide Compounds: Thorium(IV) and Uranium(IV) Bis(hydrazonato) Complexes. J. Am. Chem. Soc. 2008, 130, 17537–17551. 10.1021/ja8067287. [DOI] [PubMed] [Google Scholar]; c Yahia A.; Maron L. Is Thorium a d Transition Metal or an Actinide? An Answer from a DFT Study of the Reaction between Pyridine N-Oxide and Cp2M(CH3)2 with M = Zr, Th, and U. Organometallics 2009, 28, 672–679. 10.1021/om800943a. [DOI] [Google Scholar]; d Walensky J. R.; Martin R. L.; Ziller J. W.; Evans W. J. Importance of Energy Level Matching for Bonding in Th3+-Am3+ Actinide Metallocene Amidinates, (C5Me5)2[iPrNC(Me)NiPr]An. Inorg. Chem. 2010, 49, 10007–10012. 10.1021/ic1013285. [DOI] [PubMed] [Google Scholar]; e Ren W.; Deng X.; Zi G.; Fang D.-C. The Th=C double bond: an experimental and computational study of thorium poly-carbene complexes. Dalton Trans. 2011, 40, 9662–9664. 10.1039/c1dt11149c. [DOI] [PubMed] [Google Scholar]; f Seaman L. A.; Pedrick E. A.; Tsuchiya T.; Wu G.; Jakubikova E.; Hayton T. W. Comparison of the Reactivity of 2-Li-C6H4CH2NMe2 with MCl4 (M = Th, U): Isolation of a Thorium Aryl Complex or a UraniumBenzyne Complex. Angew. Chem., Int. Ed. 2013, 52, 10589–10592. 10.1002/anie.201303992. [DOI] [PubMed] [Google Scholar]; g Kaltsoyannis N. Does Covalency Increase or Decrease across the Actinide Series? Implications for Minor Actinide Partitioning. Inorg. Chem. 2013, 52, 3407–3413. 10.1021/ic3006025. [DOI] [PubMed] [Google Scholar]; h Neidig M. L.; Clark D. L.; Martin R. L. Covalency in f-element complexes. Coord. Chem. Rev. 2013, 257, 394–406. 10.1016/j.ccr.2012.04.029. [DOI] [Google Scholar]; i Gardner B. M.; Cleaves P. A.; Kefalidis C. E.; Fang J.; Maron L.; Lewis W.; Blake A. J.; Liddle S. T. The role of 5f-orbital participation in unexpected inversion of the σ-bond metathesis reactivity trend of triamidoamine thorium(IV) and uranium(IV) alkyls. Chem. Sci. 2014, 5, 2489–2497. 10.1039/C4SC00182F. [DOI] [Google Scholar]; j Fang B.; Ren W.; Hou G.; Zi G.; Fang D.-C.; Maron L.; Walter M. D. An Actinide Metallacyclopropene Complex: Synthesis, Structure, Reactivity, and Computational Studies. J. Am. Chem. Soc. 2014, 136, 17249–17261. 10.1021/ja509770t. [DOI] [PubMed] [Google Scholar]; k Bell N. L.; Maron L.; Arnold L. L. Thorium Mono- and Bis(imido) Complexes Made by Reprotonation of cyclo-Metalated Amides. J. Am. Chem. Soc. 2015, 137, 10492–10495. 10.1021/jacs.5b06630. [DOI] [PubMed] [Google Scholar]; l Fang B.; Hou G.; Zi G.; Fang D.-C.; Walter M. D. A thorium metallacyclopentadiene complex: a combined experimental and computational study. Dalton Trans. 2015, 44, 7927–7934. 10.1039/C5DT00838G. [DOI] [PubMed] [Google Scholar]; m Fang B.; Zhang L.; Hou G.; Zi G.; Fang D.-C.; Walter M. D. Experimental and Computational Studies on an Actinide Metallacyclocumulene Complex. Organometallics 2015, 34, 5669–5681. 10.1021/acs.organomet.5b00923. [DOI] [Google Scholar]; n Zhang L.; Hou G.; Zi G.; Ding W.; Walter M. D. Influence of the 5f Orbitals on the Bonding and Reactivity in Organoactinides: Experimental and Computational Studies on a Uranium Metallacyclopropene. J. Am. Chem. Soc. 2016, 138, 5130–5142. 10.1021/jacs.6b01391. [DOI] [PubMed] [Google Scholar]; o Browne K. P.; Maerzke K. A.; Travia N. E.; Morris D. E.; Scott B. L.; Henson N. J.; Yang P.; Kiplinger J. L.; Veauthier J. M. Synthesis, Characterization, and Density Functional Theory Analysis of Uranium and Thorium Complexes Containing Nitrogen-Rich 5-Methyltetrazolate Ligands. Inorg. Chem. 2016, 55, 4941–4950. 10.1021/acs.inorgchem.6b00492. [DOI] [PubMed] [Google Scholar]; p Zhang L.; Fang B.; Hou G.; Zi G.; Ding W.; Walter M. D. Experimental and Computational Studies of a Uranium Metallacyclocumulene. Organometallics 2017, 36, 898–910. 10.1021/acs.organomet.6b00936. [DOI] [Google Scholar]; q Zhang C.; Wang Y.; Hou G.; Ding W.; Zi G.; Walter M. D. Experimental and computational studies on a three-membered diphosphido thorium metallaheterocycle [η5-1,3-(Me3C)2C5H3]2Th[η2-P2(2,4,6-iPr3C6H2)2]. Dalton Trans. 2019, 48, 6921–6930. 10.1039/C9DT01160A. [DOI] [PubMed] [Google Scholar]; r Kelley M. P.; Popov I. A.; Jung J.; Batista E. R.; Yang P. δ and φ back-donation in AnIV metallacycles. Nat. Commun. 2020, 11, 1558. 10.1038/s41467-020-15197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; s Kloditz R.; Radoske T.; Schmidt M.; Heine T.; Stumpf T.; Patzschke M. Comprehensive Bonding Analysis of Tetravalent f-Element Complexes of the Type [M(salen)2]. Inorg. Chem. 2021, 60, 2514–2525. 10.1021/acs.inorgchem.0c03424. [DOI] [PubMed] [Google Scholar]; t Wang D.; Ding W.; Hou G.; Zi G.; Walter M. D. Uranium versus Thorium: Synthesis and Reactivity of [η5-1,2,4-(Me3C)3C5H2]2U[η2-C2Ph2]. Chem. – Eur. J. 2021, 27, 6767–6782. 10.1002/chem.202100089. [DOI] [PMC free article] [PubMed] [Google Scholar]; u Nguyen T. H.; Paul E. L.; Lukens W. W.; Hayton T. W. Evaluating f-Orbital Participation in the UV=E Multiple Bonds of [U(E)(NR2)3] (E=O, NSiMe3, NAd; R = SiMe3). Inorg. Chem. 2023, 62, 6447–6457. 10.1021/acs.inorgchem.3c00455. [DOI] [PubMed] [Google Scholar]
- a Zi G.; Blosch L. L.; Jia L.; Andersen R. A. Preparation and Reactions of Base-free Bis(1,2,4-tri-t-butylcyclopentadienyl)uranium Methylimide, Cp’2U=NMe, and Related Compounds. Organometallics 2005, 24, 4602–4612. 10.1021/om050427k. [DOI] [Google Scholar]; b Ren W.; Zi G.; Fang D.-C.; Walter M. D. Thorium Oxo and Sulfido Metallocenes: Synthesis, Structure, Reactivity, and Computational Studies. J. Am. Chem. Soc. 2011, 133, 13183–13196. 10.1021/ja205280k. [DOI] [PubMed] [Google Scholar]; c Ren W.; Zi G.; Fang D.-C.; Walter M. D. A Base-Free Thorium-Terminal-Imido Metallocene: Synthesis, Structure, and Reactivity. Chem. – Eur. J. 2011, 17, 12669–12682. 10.1002/chem.201101972. [DOI] [PubMed] [Google Scholar]; d Ren W.; Zhou E.; Fang B.; Zi G.; Fang D.-C.; Walter M. D. Si-H addition followed by C-H bond activation induced by a terminal thorium imido metallocene: a combined experimental and computational study. Chem. Sci. 2014, 5, 3165–3172. 10.1039/c4sc00576g. [DOI] [Google Scholar]; e Ren W.; Zhou E.; Fang B.; Hou G.; Zi G.; Fang D.-C.; Walter M. D. Experimental and Computational Studies on the Reactivity of a Terminal Thorium Imidometallocene towards Organic Azides and Diazoalkanes. Angew. Chem., Int. Ed. 2014, 53, 11310–11314. 10.1002/anie.201406191. [DOI] [PubMed] [Google Scholar]; f Zhou E.; Ren W.; Hou G.; Zi G.; Fang D.-C.; Walter M. D. Small Molecule Activation Mediated by a Thorium Terminal Imido Metallocene. Organometallics 2015, 34, 3637–3647. 10.1021/acs.organomet.5b00454. [DOI] [Google Scholar]; g Yang P.; Zhou E.; Hou G.; Zi G.; Ding W.; Walter M. D. Experimental and Computational Studies on the Formation of Thorium-Copper Heterobimetallics. Chem. – Eur. J. 2016, 22, 13845–13849. 10.1002/chem.201603519. [DOI] [PubMed] [Google Scholar]; h Zhang C.; Yang P.; Zhou E.; Deng X.; Zi G.; Walter M. D. Reactivity of a Lewis Base Supported Thorium Terminal Imido Metallocene toward Small Organic Molecules. Organometallics 2017, 36, 4525–4538. 10.1021/acs.organomet.7b00212. [DOI] [Google Scholar]; i Zhang C.; Hou G.; Zi G.; Ding W.; Walter M. D. A Base-Free Terminal Actinide Phosphinidene Metallocene: Synthesis, Structure, Reactivity and Computational Studies. J. Am. Chem. Soc. 2018, 140, 14511–14525. 10.1021/jacs.8b09746. [DOI] [PubMed] [Google Scholar]; j Zhang C.; Hou G.; Zi G.; Walter M. D. A base-free terminal thorium phosphinidene metallocene and its reactivity toward selected organic molecules. Dalton Trans. 2019, 48, 2377–2387. 10.1039/C9DT00012G. [DOI] [PubMed] [Google Scholar]; k Zhang C.; Hou G.; Ding W.; Walter M. D. An Alkali-Metal Halide-Bridged Actinide Phosphinidiide Complex. Inorg. Chem. 2019, 58, 1571–1590. 10.1021/acs.inorgchem.8b03091. [DOI] [PubMed] [Google Scholar]; l Wang Y.; Zhang C.; Zi G.; Ding W.; Walter M. D. Preparation of a potassium chloride bridged thorium phosphinidiide complex and its reactivity towards small organic molecules. New J. Chem. 2019, 43, 9527–9539. 10.1039/C9NJ02269D. [DOI] [Google Scholar]; m Wang D.; Ding W.; Hou G.; Zi G.; Walter M. D. Experimental and Computational Studies on a Base-Free Terminal Uranium Phosphinidene Metallocene. Chem. – Eur. J. 2020, 26, 16888–16899. 10.1002/chem.202003465. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Wang D.; Wang S.; Hou G.; Zi G.; Walter M. D. A Lewis Base Supported Terminal Uranium Phosphinidene Metallocene. Inorg. Chem. 2020, 59, 14549–14563. 10.1021/acs.inorgchem.0c02363. [DOI] [PubMed] [Google Scholar]; o Wang D.; Hou G.; Zi G.; Walter M. D. (η5-C5Me5)2U(=P-2,4,6-tBu3C6H2)(OPMe3) Revisited – Its Intrinsic Reactivity towards Small Organic Molecules. Organometallics 2020, 39, 4085–4101. 10.1021/acs.organomet.0c00640. [DOI] [Google Scholar]; p Wang D.; Hou G.; Zi G.; Walter M. D. Influence of the Lewis Base Ph3PO on the Reactivity of the Uranium Phosphinidene (η5-C5Me5)2U(=P-2,4,6-iPr3C6H2)(OPPh3). Organometallics 2021, 40, 383–396. 10.1021/acs.organomet.0c00716. [DOI] [Google Scholar]; q Wang D.; Wang S.; Li T.; Heng Y.; Hou G.; Zi G.; Walter M. D. Reactivity Studies Involving a Lewis Base Supported Terminal Uranium Phosphinidene Metallocene [η5-1,3-(Me3C)2C5H3]2U(=P-2,4,6-iPr3C6H2)(OPMe3). Dalton Trans. 2021, 50, 8349–8363. 10.1039/D1DT00742D. [DOI] [PubMed] [Google Scholar]; r Wang S.; Li T.; Heng Y.; Hou G.; Zi G.; Walter M. D. Influence of the 1,3-Bis(trimethylsilyl)cyclopentadienyl Ligand on the reactivity of the Uranium Phosphinidene [η5-1,3-(Me3Si)2C5H3]2U(=P-2,4,6-iPr3C6H2)(OPPh3). Organometallics 2021, 40, 2149–2165. 10.1021/acs.organomet.1c00303. [DOI] [Google Scholar]; s Wang S.; Heng Y.; Li T.; Hou G.; Zi G.; Walter M. D. Synthesis and reactivity of the uranium phosphinidene metallocene [η5-1,3-(Me3Si)2C5H3]2U(=P-2,4,6-iPr3C6H2)(OPMe3): Influence of the coordinated Lewis base. Dalton Trans. 2021, 50, 12502–12516. 10.1039/D1DT02149D. [DOI] [PubMed] [Google Scholar]
- Li T.; Wang D.; Heng Y.; Hou G.; Zi G.; Walter M. D. Influence of the 1,2,4-Tri-tert-butylcyclopentadienyl Ligand on the Reactivity of the Uranium Bipyridyl Metallocene [η5-1,2,4-(Me3C)3C5H2]2U(bipy). Organometallics 2023, 42, 392–406. 10.1021/acs.organomet.2c00637. [DOI] [Google Scholar]
- For selected papers on oxido, sulfido, and selenido group 4 metallocenes, see:; a Tainturier G.; Fahim M.; Trouvé-Bellan G.; Gautheron B. Thermal stability and reaction of chalcogen-containing metallocenic compounds with elemental chalcogens. J. Organomet. Chem. 1989, 376, 321–332. 10.1016/0022-328X(89)85142-3. [DOI] [Google Scholar]; b Carney M. J.; Walsh P. J.; Bergman R. G. Room temperature generation of reactive intermediates Cp*2Zr=O and Cp*2Zr=S: trapping reactions with unsaturated organic molecules and dative ligands. J. Am. Chem. Soc. 1990, 112, 6426–6428. 10.1021/ja00173a057. [DOI] [Google Scholar]; c Polse J. L.; Andersen R. A.; Bergman R. G. Cycloaddition and Cycloreversion Reactions of a Monomeric Ti(IV) Oxo Complex with Terminal and Internal Alkynes. A Reversible Oxametallacyclobutene/Hydroxoacetylide Interconversion. J. Am. Chem. Soc. 1995, 117, 5393–5394. 10.1021/ja00124a036. [DOI] [Google Scholar]; d Howard W. A.; Parkin G. Terminal oxo, sulfido, selenido, and tellurido complexes of zirconium, (η5-C5Me4R)2Zr(E)(NC5H5): comparison of terminal Zr-E single and Zr=E double-bond lengths. J. Am. Chem. Soc. 1994, 116, 606–615. 10.1021/ja00081a022. [DOI] [Google Scholar]; e Howard W. A.; Trnka T. M.; Waters M.; Parkin G. Terminal chalcogenido complexes of zirconium: Syntheses and reactivity of Cp2*Zr(E)(NC5H5) (E=O, S, Se, Te). J. Organomet. Chem. 1997, 528, 95–121. 10.1016/S0022-328X(96)06584-9. [DOI] [Google Scholar]; f Sweeney Z. K.; Polse J. L.; Andersen R. A.; Bergman R. G. Cycloaddition and Nucleophilic Substitution Reactions of the Monomeric Titanocene Sulfido Complex (η5-C5Me5)2(C5H5N)Ti=S. J. Am. Chem. Soc. 1998, 120, 7825–7834. 10.1021/ja980877m. [DOI] [Google Scholar]
- Parkin G. Terminal chalcogenido complexes of the transition metals. Prog. Inorg. Chem. 1998, 47, 1–165. 10.1002/9780470166482.ch1. [DOI] [Google Scholar]
- Jones M. B.; Gaunt A. J.; Gordon J. C.; Kaltsoyannis N.; Neu M. P.; Scott B. L. Uncovering f-element bonding differences and electronic structure in a series of 1:3 and 1:4 complexes with a diselenophosphinate ligand. Chem. Sci. 2013, 4, 1189–1203. 10.1039/c2sc21806b. [DOI] [Google Scholar]
- Hervé A.; Bouzidi Y.; Berthet J.-C.; Belkhiri L.; Thuéry P.; Boucekkine A.; Ephritikhine M. UIII-CN versus UIV-NC Coordination in Tris(silylamide) Complexes. Inorg. Chem. 2015, 54, 2474–2490. 10.1021/acs.inorgchem.5b00034. [DOI] [PubMed] [Google Scholar]
- 5f orbitals play a key role in the bonding in actinide complexes, which leads to a very polarized M=E bond, whereas, this does not occur for a group 4 metal complex,9c e.g., the M=O is a single bond with an electrostatic interaction for actinide metals (i.e., M+–O-), while it is a double bond for group 4 metals.9a,c
- Wang S.; Wang D.; Li T.; Heng Y.; Hou G.; Zi G.; Walter M. D. Synthesis, Structure and Reactivity of the Uranium Bipyridyl Complex [{η5-1,2,4-(Me3Si)3C5H2}2U(bipy)]. Organometallics 2022, 41, 1543–1557. 10.1021/acs.organomet.2c00175. [DOI] [Google Scholar]
- Hock R.; Hillenbrand S.; Erker G.; Krüger C.; Werner S. Dihydroselenapyrans by [4+2] Cycloaddition of Diary1 Selenoketones. Chem. Ber. 1993, 126, 1895–1903. 10.1002/cber.19931260821. [DOI] [Google Scholar]
- Kitamura M.; Tokuda Y.; Tashiro N.; Okauchi T. Cu(OAc)2-Mediated Cross-Coupling Reaction of Benzophenone N,N,N-Trimethylhydrazonium Salts and Aryl Boronic Acids. Aust. J. Chem. 2012, 65, 1687–1690. 10.1071/CH12350. [DOI] [Google Scholar]
- Sheldrick G. M.SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick G. M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford CT, 2009.
- a Küchle W.; Dolg M.; Stoll H.; Preuss H. Energy-adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J. Chem. Phys. 1994, 100, 7535–7542. 10.1063/1.466847. [DOI] [Google Scholar]; b Cao X.; Dolg M.; Stoll H. Valence basis sets for relativistic energy-consistent small-core actinide pseudopotentials. J. Chem. Phys. 2003, 118, 487–496. 10.1063/1.1521431. [DOI] [Google Scholar]; c Cao X.; Dolg M. Segmented contraction scheme for small-core actinide pseudopotential basis sets. J. Mol. Struct.: THEOCHEM 2004, 673, 203–209. 10.1016/j.theochem.2003.12.015. [DOI] [Google Scholar]
- Grimme S.; Antony J.; Ehrlich S.; Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
- a Bader R. W. B.Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, U.K., 1990. [Google Scholar]; b Matta C. F.; Boyd R. J.. The Quantum Theory of Atoms in Molecules; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Lu T.; Chen F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.