Abstract
Background
The prevalence of sexually transmitted infections (STIs) in sub-Saharan Africa is poorly described. We aimed to determine the prevalence of five treatable STIs (Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma genitalium, Treponema pallidum) in a sample of Gambian women from the general population.
Methods
Archived specimens from 420 women aged 15 − 69 years living in The Gambia enrolled in a clinical trial of human papilloma virus vaccine schedules were tested in this study. Urine samples were tested for C. trachomatis, N. gonorrhoeae, T. vaginalis and M. genitalium using a commercially available, open-platform multiplex PCR kit. A fragment of the ompA gene was amplified from C. trachomatis-positive samples and sequenced. Serum samples were tested for T. pallidum using the Chembio DPP Syphilis Screen and Confirm test.
Results
Overall, 41/420 (9.8%) women tested positive for at least one STI. 32 (7.6%), 9 (2.1%), 1 (0.2%), 1 (0.2%) and 0 (0.0%) tested positive for T. vaginalis, C. trachomatis, N gonorrhoeae, M. genitalium and T. pallidum, respectively. ompA gene sequence was available from five C. trachomatis infections: four were genovar D,one was genovar G and one was genovar F.
Conclusions
STIs are endemic in The Gambia. Monitoring systems should be established.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-023-08399-2.
Keywords: Sexually transmitted infections, Gambia, Prevalence, Chlamydia trachomatis, Trichomonas vaginalis, Syphilis, Mycoplasma genitalium, Neisseria gonorrhoeae
Background
The World Health Organization (WHO) estimates that there are one million new cases of sexually transmitted infections (STIs) each day globally caused by a range of bacterial, viral and protozoan pathogens [1]. STIs can significantly impact reproductive health, can cause adverse outcomes during pregnancy and childbirth, and can be vertically transmitted to a new born [2]. Despite ongoing efforts to control transmission, the prevalence and incidence of many common STIs is thought to be stable or, for example in the case of Chlamydia trachomatis, increasing in several countries [3, 4]. Regional data synthesised from Africa suggest the prevalence of key curable STIs is particularly high and increasing [1]. However, available data come from a limited number of high-income countries, with data from low- and middle-income countries either lacking or coming from targeted or high-risk subgroups [1, 5]. The heterogeneity in data availability, combined with the variability in sampling and data collection methods can lead to uncertainty in disease rates which, in itself, can impair control efforts [6–8]. Case recording and management are often syndromic in resource-limited settings as molecular diagnostics are expensive, require specialist infrastructure and are hence unavailable [9]. However, since the majority of STI cases are asymptomatic, the lack of routine diagnostic testing is another barrier to understanding the global burden of infections [10]. There is an urgent need to generate more data on the epidemiology of STIs in low- and middle-income countries, first, to determine where interventions are needed and second, to provide baseline estimates against which future intervention efforts can be evaluated. STI prevalence is also known to vary widely within and between communities due to many sociocultural, behavioural and host − pathogen interactive factors. It is, therefore, critical to represent diverse populations in global STI surveillance efforts.
Studies in the early 1980s from antenatal clinics in Bakau, The Gambia, suggested that almost 50% of women attending had Neisseria gonorrhoeae, C. trachomatis, Trichomonas vaginalis or Treponema pallidum [11, 12]. In 2017, 14% of women attending antenatal clinics near Banjul, The Gambia, tested positive for N. gonorrhoeae, C. trachomatis, T. vaginalis or T. pallidum [13]. In rural communities in the Western Division of The Gambia in 1998, 0.5% and 4.4% of survey participants tested positive for C. trachomatis and T. pallidum, respectively [14]. Data on the prevalence of STIs among general populations are still uncommon, and no previous studies have been conducted using molecular techniques (the gold standard in terms of diagnostic accuracy) for C. trachomatis, N. gonorrhoeae, M. genitalium and T. vaginalis [15]. In this study, we used nucleic acid amplification tests for four prevalent treatable STIs (C. trachomatis, N. gonorrhoeae, T. vaginalis, Mycoplasma genitalium) and serological tests for T. pallidum to determine their prevalence in Gambian women from the general rural population.
Methods
Study setting and samples
The study used anonymised, archived urine and serum samples collected as part of an ongoing clinical trial of human papilloma virus (HPV) vaccine dosing schedules in The Gambia (the HANDS trial; https://clinicaltrials.gov/ct2/show/NCT03832049; date registered: 06/02/2019; clinicaltrials.gov registration reference: NCT03832049). The participants were recruited from Jarra Soma, a transit point on the trans-Gambian highway in the Lower River Region, and a further eight surrounding rural villages. Through community and school sensitisation, communities and school childrens’ guardians were made aware of the study, allowing interested potential participants to approach the study team. Eligible participants for the trial were females aged 4–26 years who lived in the study area. Potential participants were excluded if they had a significant chronic illness, known or suspected human immunodeficiency virus (HIV) infection, and if they were pregnant or planning to become pregnant in the six months of study participation. Participants were asked to complete a questionnaire to report their age, ethnic group, primary cooking fuel, water source, sanitation, education level, age at menarche, marriage status, occupation, and contraceptive use. Questionnaires included questions with categorial answers. Local knowledge was used to generate the categorical list of potential responses.
At the time of enrolment to the parent trial, before any trial interventions had taken place, urine and serum were collected from participants (≥ 15 years) or their mothers/female guardians (for participants < 15 years) for HPV genotypic and seroprevalence testing. The samples used in this study came from those who provided additional informed consent for the future use of retained samples for other ethically approved research. Specimens were collected between September 2019 and February 2021 and tested for the STI prevalence study in May 2021.
Specimen collection and processing
First-void urine (FVU) was self-collected directly into nucleic acid preservative using the Colli-Pee™ device (Novosanis, Antwerp, Belgium), [16] prior to aliquoting and storage at -80 °C before further processing. Blood samples for serum separation were collected by peripheral venepuncture into Serum Separation Tube™ (BD Biosciences, Wokingham, UK). Having been allowed to clot for at least 30 min at ambient temperature, serum was separated by centrifugation, aliquoted and stored at -80 °C before further processing.
Pathogen detection
DNA was extracted from 100 µL of FVU using the AmpliSens DNA-sorb-AM extraction kit (InterLabService Ltd, Moscow, Russia) and eluted into 100 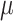 L of tris ethylenediaminetetraacetic acid (EDTA), according to manufacturer’s instructions. Presence of C. trachomatis, N. gonorrhoeae, T. vaginalis and M. genitalium was tested using a commercially available multiplex quantitative PCR assay (AmpliSens kit, multiprime-FRT variant [InterLabService, Moscow, Russia]) run on a BioRad CFX96 (Biorad, Hercules, CA, USA) platform, according to the manufacturer’s protocol [17]. Each sample was tested in a single well containing the reaction mix and 10
L of tris ethylenediaminetetraacetic acid (EDTA), according to manufacturer’s instructions. Presence of C. trachomatis, N. gonorrhoeae, T. vaginalis and M. genitalium was tested using a commercially available multiplex quantitative PCR assay (AmpliSens kit, multiprime-FRT variant [InterLabService, Moscow, Russia]) run on a BioRad CFX96 (Biorad, Hercules, CA, USA) platform, according to the manufacturer’s protocol [17]. Each sample was tested in a single well containing the reaction mix and 10 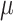 L of purified DNA. Manufacturer-provided positive exogenous control material (internal control) was added to each well, and manufacturer-provided positive and negative control samples were run on each plate. FVU sample test results were considered valid if they were run on a plate where the assay positive controls amplified, where no amplification was detected in negative controls and where the internal control amplified. Positive and negative results for C. trachomatis, N. gonorrhoeae, M. genitalium and T. vaginalis were called according to manufacturer’s recommendations [17].
L of purified DNA. Manufacturer-provided positive exogenous control material (internal control) was added to each well, and manufacturer-provided positive and negative control samples were run on each plate. FVU sample test results were considered valid if they were run on a plate where the assay positive controls amplified, where no amplification was detected in negative controls and where the internal control amplified. Positive and negative results for C. trachomatis, N. gonorrhoeae, M. genitalium and T. vaginalis were called according to manufacturer’s recommendations [17].
Serum samples were tested for T. pallidum using the Dual Path Platform (DPP) Syphilis Screen and Confirm kit test (Chembio, Hauppauge, NY, USA), [18, 19] according to manufacturer’s instructions. Serum tests for T. pallidum were considered valid if the control line was visible. An individual was considered to have a current syphilis infection if both treponemal (indicative of seropositivity for T. pallidum, equivalent to a positive T. pallidum particle agglutination test result) and non-treponemal (indicative of IgG or IgM anti-cardiolipin antibodies, equivalent to a high-titre Rapid Plasma Reagin test result) lines were visible, and a past syphilis infection if only the treponemal line was visible.
Chlamydia trachomatis ompA sequencing
The serotype of the C. trachomatis infections identified was determined by sequencing of the ompA gene and comparison to published sequences. A 972-bp fragment of ompA was amplified using a previously described nested PCR reaction [20]. Products were normalised to 10 ng/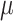 L, subjected to PCR using the BigDye Terminator v3.1 Cycle Kit (Applied Biosystems, Waltham, MA, USA) and sequenced using a 3730 DNA Analyser (Applied Biosystems, Walthm, MA, USA) by Source BioScience (Cambridge, UK).
L, subjected to PCR using the BigDye Terminator v3.1 Cycle Kit (Applied Biosystems, Waltham, MA, USA) and sequenced using a 3730 DNA Analyser (Applied Biosystems, Walthm, MA, USA) by Source BioScience (Cambridge, UK).
Data analysis
Proportions and confidence intervals (calculated with the Wilson method using the Hmisc::binconf function in R [21, 22]) were presented in crude form and not adjusted or post-stratified. Association between STI status, demographic and behavioural data were tested using logistic regression using the glm(family=”binomial”) function in R [22]. All variables were tested in univariable analyses. There was no evidence of an association between any variable and having any STI in univariable analyses, so no multivariable analyses were performed.
Raw sequence data were imported into R for analysis using the SangerSeqR package [23]. Read ends with a mean phred score < 20 across a 10-base sliding window were trimmed. Where the primary:secondary peak ratio was ≤ 0.33, the primary base was called, otherwise an N was assigned. The forward and reverse-complement of reverse reads were aligned using MUSCLE software to create consensus sequences. The consensus sequences were formed based on three rules: [1] where the base in both reads match, the base is retained; [2] where there are different bases in both reads, an N is assigned in the consensus sequence; [3] where there is a gap in one read and a base is called in the other, the base is called in the consensus sequence. Sequences were queried using the Basic Local Alignment Search Tool (BLAST) using a standard nucleotide query (BLASTN) against NCBI nucleotide databases and the serovar of the best reference strain match was assigned to the sample.
Study ethics
Both the HANDS trial (SCC1597) and the STI prevalence study (21700) were approved by the MRC Gambia Scientific Coordinating Committee, The Gambia Government/MRC Joint Ethics Committee and the London School of Hygiene & Tropical Medicine Observational Ethics Committee. The study adhered to the tenets of the Declaration of Helsinki.
Results
Participant overview
FVU, serum, and matched clinical data were available from 420 women. This included 212 15- to 26-year-old HANDS trial participants and 208 mothers/female guardians of younger participants. Of the available archived HANDS trial specimens from 427 women, there were five urine samples without paired serum samples, one urine sample where the internal control did not amplify and one serum DPP test where a control line was not visible so was considered invalid. These seven specimens were excluded from the analysis.
The median age of the study population was 25 years (range: 15 − 69 years; interquartile range: 19 − 36 years). The median age at menarche of the 375 study participants who could recall was 15 years (range: 10 − 20 years; interquartile range: 14 − 15 years). The majority (86%) of the women tested were of Mandinka ethnicity. One-third (33%) had never attended school. In total, 59% were married and 30% were currently using or had previously used contraception, predominantly injectables. A summary of the study population ethnicity, education status, marital status and contraception-use is shown in Table 1.
Table 1.
Descriptive characteristics of 420 women from The Gambia tested for five treatable sexually transmitted infections
| Variable | Level | Count (n = 420) (%) |
|---|---|---|
| Ethnicity | Mandinka | 362 (86) |
| Fula | 36 (9) | |
| Other (Jola, Serahule, Serere, Wolof, other) | 22 (5) | |
| Education level | None or can’t remember | 157 (37) |
| Lower Basic (grades 1 − 6) | 50 (12) | |
| Upper Basic (grades 7 − 9) | 103 (25) | |
| Senior Secondary (grades 10 − 12) | 101 (24) | |
| Diploma/equivalent | 9 (2) | |
| Marital Status | Married | 248 (59) |
| Single | 161 (38) | |
| Separated, divorced or widowed | 11 (3) | |
| Past or current contraceptive use | No | 293 (70) |
| Yes* | 127 (30) | |
| Injectable contraceptive | 106 | |
| Oral contraceptive | 26 | |
| Condom or other barrier method | 2 | |
| Intrauterine device | 2 |
* Some participants reported using more than one contraceptive method
Prevalence of sexually transmitted infections
Of the 420 women included, 41 (9.8%) tested positive for at least one STI. T. vaginalis was the most prevalent (7.6%), while there were no cases of T. pallidum infection. The proportion of tested women with each STI is shown in Table 2.
Table 2.
Proportion of 420 women from The Gambia with a positive sexually transmitted infection test result, presented by causative agent
| Infection | Negative | Positive | % (95% confidence interval) |
|---|---|---|---|
| Chlamydia trachomatis | 411 | 9 | 2.1 (0.9 − 4.0) |
| Neisseria gonorrhoeae | 419 | 1 | 0.2 (0.0 − 1.3) |
| Trichomonas vaginalis | 388 | 32 | 7.6 (5.3 − 10.6) |
| Mycoplasma genitalium | 419 | 1 | 0.2 (0.0 − 1.3) |
| Treponema pallidum | 420 | 0 | 0.0 (0.0 − 0.9) |
| Any infection | 379 | 41 | 9.8 (7.1 − 13.0) |
| One infection | 39 | - | |
| More than one infection | 2 | - |
There were three women (0.7%) who had a visible non-treponemal line and four (1.0%) who had a visible treponemal line on DPP testing. None had both lines.
STIs were most common in the 35 − 44-year-old age group, of whom 13% tested positive for at least one STI (Fig. 1A; Table 3). T. vaginalis was detected in participants of all age groups, whereas M. genitalium was only present in those aged 15–24 years, N. gonorrhoeae only in those aged 35–44, and C. trachomatis in those aged under 45 years (Fig. 1B; Table 3). There was no evidence of an association between any variable and STIs in univariable analysis (supplementary Table 1).
Fig. 1.
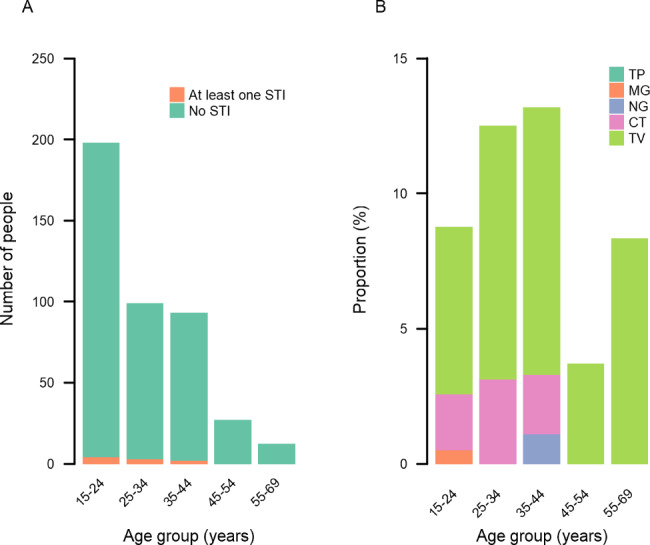
A. Number women from The Gambia testing negative and positive for at least one sexually transmitted infection, presented by age group. B. Proportion of women from The Gambia testing positive for syphilis (TP), Mycoplasma genitalium (MG), Neisseria gonorrhoeae (NG), Chlamydia trachomatis (CT) and Trichomonas vaginalis (TV), presented by age group
Table 3.
Age-specific prevalence of sexually transmitted infections in 420 women from The Gambia, presented in ten-year age groups
| Age group (years) | N |
T. vaginalis
% (95% CI) |
C. trachomatis
% (95% CI) |
N. gonorrhoeae
% (95% CI) |
M. genitalium
% (95% CI) |
T. pallidum
% (95% CI) |
Any infection % (95% CI) |
|---|---|---|---|---|---|---|---|
| 15 − 24 | 194 | 6.2 (2.8 − 9.6) | 2.1 (0.1 − 4.1) | 0.0 (0.0 − 1.9) | 0.5 (0.0 − 2.8) | 0.0 (0.0 − 1.9) | 8.2 (4.8 − 13.0) |
| 25 − 34 | 96 | 9.4 (3.6 − 15.2) | 3.1 (0.0 − 6.6) | 0.0 (0.0 − 3.8) | 0.0 (0.0 − 3.8) | 0.0 (0.0 − 3.8) | 11.5 (5.9 − 19.6) |
| 35 − 44 | 91 | 9.9 (3.8 − 16.0) | 2.2 (0.0 − 5.2) | 1.1 (0.0 − 6.0) | 0.0 (0.0 − 4.1) | 0.0 (0.0 − 4.1) | 13.2 (7.0 − 21.9) |
| 45 − 54 | 27 | 3.7 (0.0 − 10.8) | 0.0 (0.0 − 12.5) | 0.0 (0.0 − 12.5) | 0.0 (0.0 − 12.5) | 0.0 (0.0 − 12.5) | 3.7 (0.0 − 19.0) |
| 55 − 69 | 12 | 8.3 (0.0 − 23.9) | 0.0 (0.0 − 24.2) | 0.0 (0.0 − 24.2) | 0.0 (0.0 − 24.2) | 0.0 (0.0 − 24.2) | 8.3 (0.2 − 38.4) |
ompA gene fragments were amplified from six of the nine C. trachomatis-positive DNA extracts. The mean sequence length was 936 bases (range: 929 − 943). Four of these aligned most closely to published serovar D sequences, one aligned most closely to published serovar G sequences and one aligned most closely to serovar F sequences.
Discussion
We found approximately 10% of women in Lower River Region of The Gambia testing positive for at least one STI, with the highest prevalence being for T. vaginalis whilst no T. pallidum was detected.
The proportion of women testing positive for an STI was comparable to findings from other STI prevalence studies from the region. A meta-analysis of data from several countries estimated the WHO Africa region (AFRO) prevalence of C. trachomatis, N. gonorrhoeae, T. vaginalis and T. pallidum to be 5.0 (3.8–6.6), 1.9 (1.3–2.7), 11.7 (8.6–15.6) and 1.6% (1.2–2.0%), respectively, in 2016 [1]. Our findings are 2.1 (0.9 − 4.0), 0.2 (0.0 − 1.3), 7.6 (5.3 − 10.6) and 0% (0.0 − 0.9%), respectively, thus following a similar trend in relative prevalence. The WHO AFRO covers a large and diverse region, and the population of The Gambia only makes up a small proportion of the regional population, so comparison to these regional estimates should be considered accordingly.
Despite the variation in sampling and testing methods used in other studies, the similarity between these findings and previous studies was notable. At almost 8%, our data suggest T. vaginalis is the most prevalent STI in this population. Other studies in West Africa estimate the prevalence of T. vaginalis to range from 2 − 6%, although several used wet mount microscopy rather than molecular tests, [24–27] so the estimates are not directly comparable [28]. T. vaginalis is often found to be highly prevalent, particularly in Africa [1]. At < 1%, the proportion of women with N. gonorrhoeae in this population from The Gambia was low, and lower than several similar studies from the region, where estimates ranged from 1 − 4%, [26, 27, 29, 30] including a recent study from an urban coastal region in The Gambia where the prevalence of N. gonorrhoeae was 2% [13]. We found an M. genitalium prevalence of 0.2%. This is lower than expected, as from the limited population-based studies reported elsewhere, prevalence is estimated to be 3.9% across low-income settings [31]. The proportion of C. trachomatis-infected women in this study was 2.1% (0.9 − 4.0%). Although other estimates of C. trachomatis prevalence from the region show considerable heterogeneity, this is generally in keeping with prevalence estimates from neighbouring countries [26, 27, 29, 30]. To find no women with active T. pallidum infection was surprising given other estimates of T. pallidum prevalence from elsewhere in The Gambia. It is possible that the three positive non-treponemal test results were active cases of T. pallidum. However, because of the well-described risk of false positive non-treponemal test results from other non-treponemal infections, such as malaria, [32] in the absence of a positive treponemal test result we cannot say definitively that these were caused by T. pallidum. This diagnostic algorithm for T. pallidum is in keeping with recent guidelines from other organizations [33]. The four positive treponemal test results in the absence of positive non-treponemal test results suggests some historic exposure to T. pallidum but not active infection. Together, these data suggest some ongoing T. pallidum transmission in the population, albeit likely at a low level given the general low prevalence relative to estimates found elsewhere.
WHO identified strengthening STI surveillance and improving knowledge of prevalence (alongside symptom aetiology and antimicrobial resistance) as priorities in their STI strategy for 2016–2021 [34]. Sexual and reproductive health services in The Gambia should be strengthened to manage this burden of STIs. Controlling the prevalence of T. vaginalis should be a particular focus. Having T. vaginalis may increase risk of HIV acquisition [35] and may have adverse impact on pregnancy outcomes, [36] although the evidence is inconsistent [37]. Only 5% of T. vaginalis infections currently exhibit some resistance to first-line therapy, [38, 39] but there remain concerns of antimicrobial resistance (AMR) emerging [40] C. trachomatis is a pathogen which is generally thought to be increasing in some countries, [3, 4] so the low prevalence found here is notable. We found serovars D, G and F in this sample set, which were the three most common C. trachomatis serovars in a 2012 study from nearby Guinea Bissau [41]. The low N. gonorrhoeae prevalence is reassuring, given N. gonorrhoeae’s propensity to develop extensive drug resistance[42, 43] resulting in it being on WHO’s “high priority” list for new antibiotics [44]. M. genitalium is another pathogen where AMR is of increasing concern [43, 44]. However, in a systematic review of mutations associated with macrolide and fluoroquinolone resistance in M. genitalium, AMR data for the African region were only available from Kenya and South Africa [45]. Integrating AMR surveillance with prevalence and incidence monitoring would be valuable in this setting, particularly considering the amount of community-wide exposure to antimicrobials imparted by neglected disease mass drug administration campaigns in The Gambia and its neighbouring countries [46]. As part of this work, we attempted to amplify AMR-associated single nucleotide polymorphism-containing regions from the residual ex-diagnostic DNA eluate of positive samples. We found a product to amplify in < 50% samples and generated several low-quality sequences among the gene segments which did amplify. Therefore, more reproducible methods should be employed for future AMR surveillance.
The study has two key strengths. First, data in this study were collected from a sample of the general population in sub-Saharan Africa. General population samples are more generalizable to the wider population than some other common STI survey sampling methods, such as sampling of clinic attendees or high-risk groups. No published evidence of STI prevalence in this population were available at the time of the study, and very few data on STIs were available from The Gambia as a whole. Also, low- and middle-income countries in sub-Saharan Africa are under-represented in global estimates of STI burden. Second, recommended diagnostic approaches were used to detect STIs in this study: nucleic acid amplification tests for diagnosis of C. trachomatis, N. gonorrhoeae, M. genitalium and T. vaginalis and a specific serological diagnostic algorithm for detection of T. pallidum. These methods are essential for detecting asymptomatic carriage of STIs and offer improved sensitivity over culture or antigen detection methods.
There were also limitations to this study. First, for detection of C. trachomatis, N. gonorrhoeae, T. vaginalis and M. genitalium, we tested FVU. Despite its convenience as a sampling method, FVU is recognised as a difficult sample type to amplify DNA from and a less sensitive specimen type for detection of STIs than vaginal swab samples [47, 48]. This may be related to presence of inhibitors, or because of small proportion of the sample tested (in this case, 100 µL from several millilitres of FVU was tested). Second, the open-platform diagnostic test used in this study has not been as widely used as some of the other proprietary STI diagnostic platforms. However, the limited amount of performance data suggest good agreement with other platforms [17, 49] and we feel the flexible, transparent nature of open-platform multiplex PCR tests makes them the only viable option for this type of small scale, opportunistic study in resource-limited settings [27, 50]. Third, the participant sampling technique was not random, so we cannot assume the data are representative of the local or national Gambian populations. Although the samples were taken before any parent trial intervention, the trial was examining an HPV vaccine and recruitment relied on voluntary self-presentation to the study, both of which may have led to recruitment bias. Also, pregnant women and women who were known to be HIV positive, who may be at increased risk of STIs, were excluded. The influence of these factors should be considered when interpreting the results. Finally, as a result of the nature of the parent trial from which these specimens were taken, men were not included in our sampling frame.
Several unanswered questions remain. First, larger, randomly sampled surveys and longitudinal prevalence monitoring are needed to expand the map of STI prevalence in The Gambia. Compared to the general population, high-risk populations such as female sex workers often bear a disproportionately high proportion of STIs. Inclusion of these groups in future surveillance efforts is important. Men should also be included. Second, several key infections of the reproductive tract (for example, HIV, Herpes Simplex Virus, bacterial vaginosis, Candida albicans) were not tested for in this study. More data will be needed on their prevalence to ensure appropriate service provision. Third, prospectively designed STI surveys have increased scope to examine symptoms and behaviours related to STIs. Learning more about the epidemiology of these infections will be important in planning effective public health interventions. Finally, STIs, including T. vaginalis which was the most common in this study, are often asymptomatic, [33, 51] so proactive screening of asymptomatic men and women should be core to monitoring strategies. Given the documented limitations of urine testing, supplementing urine testing with swab sample testing in subsequent studies would be valuable. Several studies have demonstrated that self-collected vaginal swabs are acceptable and feasible [52, 53].
Our data, combined with those from elsewhere, will contribute to baseline STI prevalence estimates in The Gambia, laying a foundation for more systematic monitoring of the key challenges facing STI prevention, such as characterisation of antimicrobial susceptibility profiles among pathogen isolates and determining whether prevalence is changing over time. There is growing concern about STIs at the global level; the increasing evidence base in The Gambia should provide leverage for policy makers to ensure STIs are appropriately prioritised.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the participants who took part in the study and the field, laboratory and support teams who recruited the participants, collected and undertook initial processing of the samples.
Authors’ contributions
RB, EHE, MH and EC designed the study and obtained the funding. HV, HBH and SJ conducted the laboratory analyses. DO, AB, OA and LD collected the specimens. RB conducted the data analysis and wrote the manuscript. All authors reviewed and approved the manuscript.
Funding
This work was funded by the Wellcome Trust Institutional Strategic Support Fund (204928/Z/16/Z) and a grant from the Medical Research Council (UK), Wellcome Trust and UK Department for International Development (MC_EX_MR/N006070/1).
Availability of data and materials
Data available on request from corresponding author.
Declarations
Ethics approval and consent to participate
The study adhered to the tenets of the Declaration of Helsinki.
Individuals (or their parent/guardian if they were < 18 years) were required to provide informed consent to participate in the study before they could be enrolled. Both the HANDS trial (SCC1597) and the STI prevalence study (21700) were approved by the MRC Gambia Scientific Coordinating Committee, the Gambia Government/MRC Joint Ethics Committee and the London School of Hygiene & Tropical Medicine Observational Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rowley J, Hoorn S, Vander, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97(8):548–62. doi: 10.2471/BLT.18.228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Gerwen OT, Muzny CA, Marrazzo JM. Sexually transmitted infections and female reproductive health. Nat Microbiol. 2022;7(8):1116-1126. [DOI] [PMC free article] [PubMed]
- 3.US Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2020. 2021.
- 4.Public Health England. Sexually transmitted infections and screening for chlamydia in England, 2020: The annual official statistics data release [Internet]. Vol. GOV-9494. 2020.
- 5.Torrone EA, Morrison CS, Chen PL, Kwok C, Francis SC, Hayes RJ, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med. 2018;15(6):e1002608. doi: 10.1371/journal.pmed.1002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y, Wu J. Estimating the global burden of sexually transmitted infections – Authors’ reply. Lancet Infect Dis. 2022;22(8):1113–1114. doi: 10.1016/S1473-3099(22)00423-6. [DOI] [PubMed] [Google Scholar]
- 7.Peters RPH, Chico RM, Rowley J, Low N. Estimating the global burden of sexually transmitted infections. Lancet Infect Dis. 2022;22(8):1112–3. doi: 10.1016/S1473-3099(22)00415-7. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y, Yu Q, Lin Y, Zhou Y, Lan L, Yang S, et al. Global burden and trends of sexually transmitted infections from 1990 to 2019: an observational trend study. Lancet Infect Dis. 2022;22(4):541–51. [DOI] [PubMed]
- 9.WHO . Guidelines for the management of symptomatic sexually transmitted infections. [Internet] Geneva, Switzerland: World Health Organization; 2021. [PubMed] [Google Scholar]
- 10.Zemouri C, Wi TE, Kiarie J, Seuc A, Mogasale V, Latif A, et al. The performance of the vaginal discharge syndromic management in treating vaginal and cervical infection: a systematic review and meta-analysis. PLoS ONE. 2016;11(10):1–21. doi: 10.1371/journal.pone.0163365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mabey DCW, Lloyd-Evans NE, Conteh S, Forsey T. Sexually transmitted diseases among randomly selected attenders at an antenatal clinic in: the Gambia. Sex Transm Infect. 1984;60(5):331–6. doi: 10.1136/sti.60.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabey DCW, Whittle HC. Genital and neonatal chlamydial infection in a trachoma-endemic area. Lancet. 1982;2(8293):300–1. doi: 10.1016/S0140-6736(82)90273-2. [DOI] [PubMed] [Google Scholar]
- 13.Isara A, Baldeh AK. Prevalence of sexually transmitted infections among pregnant women attending antenatal clinics in west coast region of the Gambia. Afr Health Sci. 2021;21(2):585–92. doi: 10.4314/ahs.v21i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw M, Van der Sande M, West B, Paine K, Ceesay S, Bailey R, et al. Prevalence of herpes simplex type 2 and syphilis serology among young adults in a rural gambian community. Sex Transm Infect. 2001;77(5):358–65. doi: 10.1136/sti.77.5.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldeh AK, Isara AR. Knowledge of sexually transmitted infections amongst pregnant women attending antenatal clinics in west coast region of the Gambia. Afr J Reprod Health. 2019;23(3):49–56. doi: 10.29063/ajrh2019/v23i3.5. [DOI] [PubMed] [Google Scholar]
- 16.De Baetselier I, Smet H, Abdellati S, De Deken B, Cuylaerts V, Reyniers T et al. Evaluation of the “Colli-Pee”, a first-void urine collection device for self-sampling at home for the detection of sexually transmitted infections, versus a routine clinic-based urine collection in a one-to-one comparison study design: efficacy and accept. BMJ Open. 2019;9(4):e028145. [DOI] [PMC free article] [PubMed]
- 17.Rumyantseva T, Golparian D, Nilsson CS, Johansson E, Falk M, Fredlund H, et al. Evaluation of the new AmpliSens multiplex real-time PCR assay for simultaneous detection of Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis. Apmis. 2015;123(10):879–86. doi: 10.1111/apm.12430. [DOI] [PubMed] [Google Scholar]
- 18.Castro AR, Esfandiari J, Kumar S, Ashton M, Kikkert SE, Park MM, et al. Novel point-of-care test for simultaneous detection of nontreponemal and treponemal antibodies in patients with syphilis. J Clin Microbiol. 2010;48(12):4615–9. doi: 10.1128/JCM.00624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marks M, Yin YP, Chen XS, Castro A, Causer L, Guy R, et al. Metaanalysis of the performance of a combined Treponemal and Nontreponemal Rapid Diagnostic Test for Syphilis and Yaws. Clin Infect Dis. 2016;63(5):627–33. doi: 10.1093/cid/ciw348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreasen AA, Burton MJ, Holland MJ, Polley S, Faal N, Mabey DCW et al. Chlamydia trachomatis ompA variants in trachoma: what do they tell us? PLoS Negl Trop Dis 2008 Jan;2(9):e306. [DOI] [PMC free article] [PubMed]
- 21.Harrell F Jr. Hmisc: Harrell Miscellaneous. 2022.
- 22.R Core Team . R: a language and environment for statistical computing. [Internet] Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 23.Hill JT, Demarest BL, Bisgrove BW, Su Y-C, Smith M, Yost HJ. Poly peak parser: Method and software for identification of unknown indels using Sanger sequencing of PCR products. 2015;2(2):1632–6. [DOI] [PMC free article] [PubMed]
- 24.Tchelougou D, Karou D, Kpotsra A, Balaka A, Assih M, Bamoke M, et al. [Vaginal infections in pregnant women at the Regional Hospital of Sokode (Togo) in 2010 and 2011] Med Sante Trop. 2013;23(1):49–54. doi: 10.1684/mst.2013.0142. [DOI] [PubMed] [Google Scholar]
- 25.Olowe O, Makanjuola O, Olowe R, Adekanle D. Prevalence of vulvovaginal candidiasis, trichomoniasis and bacterial vaginosis among pregnant women receiving antenatal care in Southwestern Nigeria. Eur J Microbiol Immunol. 2014;4(4):193–7. doi: 10.1556/EUJMI-D-14-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Béhanzin L, Diabaté S, Minani I, Lowndes CM, Boily MC, Labbé AC et al. Decline in HIV Prevalence among Young Men in the General Population of Cotonou, Benin, 1998–2008. PLoS ONE. 2012;7(8):e43818. [DOI] [PMC free article] [PubMed]
- 27.Cowley G, Milne G, Teixeira Da Silva E, Nakutum J, Rodrigues A, Vasileva H, et al. Prevalence of and risk factors for curable sexually transmitted infections on Bubaque Island, Guinea Bissau. Sex Transm Infect. 2021;97(1):51–5. doi: 10.1136/sextrans-2019-054351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabweyambo S, Kakaire O, Sowinski S, Okeng A, Ojiambo H, Kimeze J, et al. Very low sensitivity of wet mount microscopy compared to PCR against culture in the diagnosis of vaginal trichomoniasis in Uganda: a cross sectional study. BMC Res Notes. 2017;10(1):4–9. doi: 10.1186/s13104-017-2581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yirenya-Tawiah D, Annang TN, Apea-Kubi KA, Lomo G, Mensah D, Akyeh L, et al. Chlamydia Trachomatis and Neisseria Gonorrhoeae prevalence among women of reproductive age living in urogenital schistosomiasis endemic area in Ghana. BMC Res Notes. 2014;7(1):1–7. doi: 10.1186/1756-0500-7-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diadhiou M, Ba Diallo A, Barry MS, Alavo SC, Mall I, Gassama O, et al. Prevalence and risk factors of Lower Reproductive Tract Infections in Symptomatic Women in Dakar, Senegal. Infect Dis Res Treat. 2019;12:117863371985182. doi: 10.1177/1178633719851825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumann L, Cina M, Egli-Gany D, Goutaki M, Halbeisen FS, Lohrer GR, et al. Prevalence of Mycoplasma genitalium in different population groups: systematic review andmeta-analysis. Sex Transm Infect. 2018;94(4):255–62. doi: 10.1136/sextrans-2017-053384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maves RC, Dean K, Gadea N, Halsey ES, Graf PCF, Lescanoa AG. False-positive rapid plasma reagin testing in patients with acute Plasmodium vivax malaria: a case control study. Travel Med Infect Dis. 2014;12(3):268–73. doi: 10.1016/j.tmaid.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and treatment of sexually transmitted infections: a review. JAMA - J Am Med Assoc. 2022;327(2):161–72. doi: 10.1001/jama.2021.23487. [DOI] [PubMed] [Google Scholar]
- 34.WHO Department of Reproductive Health and Research. Report on global sexually transmitted infection surveillance, 2018. World Health Organization. 2018. 6–7
- 35.Jackson DJ, Rakwar JP, Bwayo JJ, Kreiss JK, Moses S. Urethral trichomonas vaginalis infection and HIV-1 transmission. Lancet. 1997;350(9084):1076. doi: 10.1016/S0140-6736(05)70456-6. [DOI] [PubMed] [Google Scholar]
- 36.Cotch M, Pastorek J, 2nd, Nugent R, Hillier S, Gibbs R, Martin D, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Transm Dis. 1997;24(6):353–60. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Stringer E, Read JS, Hoffman I, Valentine M, Aboud S, Goldenberg RL. Treatment of trichomoniasis in pregnancy in sub-saharan Africa does not appear to be associated with low birth weight or preterm birth. South Afr Med J. 2010;100(1):58–64. [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkcaldy RD, Augostini P, Asbel LE, Bernstein KT, Kerani RP, Mettenbrink CJ, et al. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD surveillance network, 2009–2010. Emerg Infect Dis. 2012;18(6):939–43. doi: 10.3201/eid1806.111590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmid G, Narcisi E, Mosure D, Secor WE, Higgins J, Moreno H. Prevalence of metronidazole-resistant trichomonas vaginalis in a Gynecology Clinic. Obstet Gynecol Surv. 2001;56(11):693–4. doi: 10.1097/00006254-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Marques-Silva M, Lisboa C, Gomes N, Rodrigues AG. Trichomonas vaginalis and growing concern over drug resistance: a systematic review. J Eur Acad Dermatology Venereol. 2021;35(10):2007–21. doi: 10.1111/jdv.17461. [DOI] [PubMed] [Google Scholar]
- 41.Olsen B, Månsson F, Camara C, Monteiro M, Biai A, Alves A, et al. Phenotypic and genetic characterisation of bacterial sexually transmitted infections in Bissau, Guinea-Bissau, West Africa: a prospective cohort study. BMJ Open. 2012 Jan;2(2):e000636. [DOI] [PMC free article] [PubMed]
- 42.Wi T, Lahra MM, Ndowa F, Bala M, Dillon JAR, Ramon-Pardo P, et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med. 2017;14(7):1–16. doi: 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwuji C, Pillay D, Shamu P, Murire M, Nzenze S, Cox L-A et al. A systematic review of antimicrobial resistance in Neisseria gonorrhoeae and Mycoplasma genitalium in sub-saharan Africa. J Antimicrob Chemother. 2022;(May):2074–93. [DOI] [PMC free article] [PubMed]
- 44.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 45.Machalek DA, Tao Y, Shilling H, Jensen JS, Unemo M, Murray G, et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(11):1302–14. doi: 10.1016/S1473-3099(20)30154-7. [DOI] [PubMed] [Google Scholar]
- 46.Burr S, Sillah A, Sanou A, Wadagni A, Hart J, Harding-Esch E, et al. Cross-sectional surveys of the prevalence of follicular trachoma and trichiasis in the Gambia: has elimination been reached? PLoS Negl Trop Dis. 2016;10(9):e0004906. doi: 10.1371/journal.pntd.0004906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rönn MM, Mc Grath-Lone L, Davies B, Wilson JD, Ward H. Evaluation of the performance of nucleic acid amplification tests (NAATs) in detection of chlamydia and gonorrhoea infection in vaginal specimens relative to patient infection status: a systematic review. BMJ Open. 2019;9(1):1–9. doi: 10.1136/bmjopen-2018-022510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coorevits L, Traen A, Bingé L, Van Dorpe J, Praet M, Boelens J, et al. Identifying a consensus sample type to test for Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis and human papillomavirus. Clin Microbiol Infect. 2018;24(12):1328–32. doi: 10.1016/j.cmi.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Choe H-S, Lee D, Lee S-J, Hong S-H, Park DC, Lee M-K, et al. Performance of Anyplex™ II multiplex real-time PCR for the diagnosis of seven sexually transmitted infections: comparison with currently available methods. Int J Infect Dis. 2013 Dec;17(12):e1134–40. [DOI] [PubMed]
- 50.Karellis A, Naeem F, Nair S, Mallya SD, Routy JP, Gahagan J, et al. Multiplexed rapid technologies for sexually transmitted infections: a systematic review. The Lancet Microbe. 2022;3(4):e303–15. doi: 10.1016/S2666-5247(21)00191-9. [DOI] [PubMed] [Google Scholar]
- 51.Meites E, Gaydos CA, Hobbs MM, Kissinger P, Nyirjesy P, Schwebke JR, et al. A review of evidence-based care of symptomatic trichomoniasis and asymptomatic Trichomonas vaginalis infections. Clin Infect Dis. 2015;61(Suppl 8):837–48. doi: 10.1093/cid/civ738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paudyal P, Llewellyn C, Lau J, Mahmud M, Smith H. Obtaining self-samples to diagnose curable sexually transmitted infections: a systematic review of patients’ experiences. PLoS ONE. 2015;10(4):1–22. doi: 10.1371/journal.pone.0124310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sy F, Greuel M, Winkler V, Bussmann H, Bärnighausen T, Deckert A. Accuracy of HPV testing on self-collected and clinician-collected samples for different screening strategies in african settings: a systematic review and meta-analysis. Gynecol Oncol. 2022;166(2):358–68. doi: 10.1016/j.ygyno.2022.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from corresponding author.


