Abstract
Background
Previous studies challenge the impact of obstructive sleep apnea (OSA) once patients are diagnosed with Alzheimer’s disease (AD). Nevertheless, OSA recognizably disrupts sleep, and relevant associations between sleep, AD pathological markers, and cognition have been demonstrated. We aimed to further explore this, evaluating the associations between each breathing cessation event that compose the apnea–hypopnea index (AHI) and the sleep structure to finally investigate whether this was related to increased levels of AD markers and higher cognitive decline.
Methods
Observational, prospective study, including consecutive patients diagnosed with mild-moderate AD. The participants were submitted to overnight polysomnography followed by a cerebrospinal fluid collection for AD pathological markers levels determination. Neuropsychological assessment was performed at baseline and after 12 months of follow-up.
Results
The cohort was composed of 116 patients (55.2% females) with a median [p25;p75] age of 76.0 [72.0;80.0] years and an AHI of 25.9 [15.1;48.5], which was mainly defined by the presence of hypopneas and obstructive apneas. These were distinctively associated with the sleep structure, with obstructive apneas being related to arousals and sleep lightening and hypopneas being related to an increased number of arousals only. Despite having a lower frequency, mixed and central apneas also presented associations with the sleep structure, particularly increasing the time spent in the lighter sleep stages. In relation to AD pathological markers, obstructive and mixed apneas were related to an augment in neurofilament light levels while hypopneas were associated with a higher phosphorylated-tau/amyloid-beta protein ratio. Hypopneas were the most important event for an increased cognitive decline at the 12-month follow-up.
Conclusions
Our findings highlight the importance of a patient-centered approach, with a comprehensive and detailed analysis of the AHI to effectively predict the different outcomes and tailor the appropriate therapeutic strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-023-01266-x.
Keywords: Alzheimer’s disease, Obstructive sleep apnea, Apnea–hypopnea index, Hypopneas, Cognitive decline
Background
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder in the world, currently affecting more than 55 million people [1]. Female sex and the presence of the apolipoprotein E (APOE) ε4 allele are two non-modifiable features associated with an increased risk of developing the disease [2]. Given that aging is also a relevant non-modifiable risk factor, estimations are that the prevalence of AD will increase in the next decades, especially considering the growing number of older adults in the nowadays society [1, 3]. Aiming to prevent this and/or reduce the progression of the disease, considerable attention has been dedicated to the modifiable factors associated with increased risk of AD such as hypertension [4], diabetes [5], obesity [6], and obstructive sleep apnea (OSA) [7], among others.
The presence of OSA among cognitively healthy subjects is associated with atypical levels of AD pathological markers [8–10], increased cognitive decline [11, 12], and a higher risk of mild cognitive impairment/dementia [13–16]. Nevertheless, previous studies suggest a different scenario once patients are diagnosed with AD. Accordingly, brain amyloid burden or cerebrospinal fluid (CSF) levels of phosphorylated-tau (P-tau) and total-tau (T-tau) presented similar alterations among AD patients with and without OSA after 2.52 ± 0.51 years of follow-up [17]. In addition, the presence of OSA was not associated with an increase in the magnitude of cognitive decline among patients with mild-moderate AD after three years of follow-up [18]. Yet, OSA recognizably disrupts the sleep structure, and previous research reported relevant associations between sleep lightening, atypical levels of AD pathological markers, and increased cognitive decline [19, 20].
OSA is currently diagnosed based on the apnea–hypopnea index (AHI), a metric composed of distinct events which are not properly evaluated for their individual contribution in terms of adverse outcomes. To address this and improve the understanding concerning the impact of OSA among patients diagnosed with AD, we first performed a comprehensive characterization of the breathing cessation events that compose the AHI among our cohort of consecutive patients with mild-moderate AD. In the sequence, we evaluated the impact of each of these events on sleep structure. Finally, we investigated whether the events associated with increased disruption of the sleep structure would be the ones related to atypical levels of AD pathological markers and higher cognitive decline.
Methods
Study population
This is an ancillary study of a prospective trial designed to evaluate the influence of OSA on the cognitive decline of AD patients after one year of follow-up (NCT02814045). The recruitment started in 2015 and finished in 2019. We included acetylcholinesterase inhibitor-naïve individuals aged over 60 years who were diagnosed with AD according to the National Institute on Aging and Alzheimer’s Association (NIA-AA) clinical criteria [21] and presented a Mini-Mental State Examination (MMSE) score ≥ 20. We excluded individuals presenting at least one of the following characteristics: (i) presence of visual and/or communication difficulties that could influence the compliance with the procedures of the study; (ii) presence of a previously diagnosed sleep disturbance; (iii) comorbidities such as cancer, severe renal or hepatic insufficiency, severe cardiac or respiratory failure; (iv) excessive alcohol intake (> 280 g/week); (v) MRI evidence of hydrocephalus, stroke, space-occupying lesion, or any clinically relevant disease of the central nervous system other than AD; (vi) presence of mental disorders according to DSM-V-TR™ criteria; (vii) use of medications under investigation or use of beta-blockers, antidepressants, neuroleptics, or hypnotics during the 15 days previous to polysomnography (PSG); (viii) presence of untreated (or treated for less than 3 months prior to the screening visit) vitamin B12 or folate deficiency; and (ix) presence of untreated thyroid disease.
The study was approved by the ethics committee of the Hospital Universitari Arnau de Vilanova-Santa Maria (Lleida, Spain) (CE-1218) and conducted according to the Declaration of Helsinki. The patient, the responsible caregiver, and the legal representative (when different from the responsible caregiver) signed an informed consent form.
Study design
Consecutive patients arrived at the Cognitive Disorders Unit of the Hospital Universitari de Santa Maria (Lleida, Spain) and were assessed for their eligibility. A clinical evaluation was performed to investigate associated comorbidities and to collect sociodemographic and anthropometric data. Blood and CSF were obtained to respectively determine ApoE genotypes and the levels of AD pathological markers. Sleep-related evaluations included the application of the Epworth Sleepiness Scale (ESS) and an overnight PSG. Neuropsychological assessment was performed at baseline and after 12 months of follow-up.
Clinical variables
The following variables were collected: sex, age, years of education, comorbidities (hypertension, diabetes mellitus, cardiopathy, periodic limb movements), and personal and family psychiatric and neurological history. Body mass index (BMI) was calculated as body weight (in kg)/height (in m2).
Apolipoprotein E (ApoE) genotype
DNA was extracted from buffy coat cells using a Maxwell® RCS blood DNA kit (Promega, USA). Twenty microliters of DNA were used for ApoE genotyping by polymerase chain reaction (PCR). ApoE genotype was dichotomized as ApoE-ε4 homozygous or heterozygous carrier (ApoEε4 +) or not (ApoEε4 −).
CSF biomarkers
The CSF samples were collected at baseline between 8:00 and 10:00 a.m. They were placed in polypropylene tubes, centrifuged at 2000 × g for 10 min at 4 °C, immediately frozen, and stored within 4 h in a − 80 °C freezer. The measurement of amyloid-beta protein (Aß42), T-tau, and P-tau was performed using commercial kits (Innotest® β-Amyloid-42; Innotest® hTAU Ag; and Innotest® Phospho-TAU181P, Fujirebio-Europe, Gent, Belgium). Neurofilament light (NF-L) was measured by commercial ELISA kit (Quidel, San Diego, CA). All determinations were performed in duplicate and in one round of experiments using one batch of reagents by board-certified laboratory technicians who were blinded to the clinical data. The intra-assay coefficients of variation were lower than 10% for internal quality control samples (two per plate). Based on previous data obtained by the research group, Aβ42 levels < 600 pg/ml were considered pathological [22].
Epworth Sleepiness Scale (ESS)
Excessive daytime somnolence was assessed by the ESS. This questionnaire is composed of eight questions to assess the chance of falling asleep during different daily situations. Each question is rated on a 3-point scale, in which 0 represents no chance of occurrence, and 3 indicates a high chance of occurrence. The overall score ranges from 0 to 24 points. Higher scores represent increased daytime somnolence [23–25].
Polysomnography (PSG)
The overnight PSG (Philips Respironics Alice 6 LDx, Philips, Murrysville, USA) was performed to assess the following variables: time spent in bed (in hours), total sleep time (in hours), sleep efficiency (in %, defined as the ratio between total sleep time and the time spent in bed), latency to N1 (in minutes, defined as the time spent awake until the first sleep episode), latency to REM sleep (in minutes, defined as the time spent asleep until the first REM sleep episode), the time spent in N1 stage (in %, defined as the percentage of time spent in N1 while asleep), the time spent in N2 stage (in %, defined as the percentage of time spent in N2 while asleep), the time spent in slow-wave sleep (SWS, also known as N3) (in %, defined as the percentage of time spent in SWS while asleep), the time spent in REM sleep (in %, defined as the percentage of time spent in REM sleep while asleep), the arousals index (defined as the mean number of awakening events per hour after the sleep onset), and the AHI (defined as the mean number of apnea and hypopnea events per hour during the time spent asleep).
The sleep staging and classification of the breathing cessation events that compose the AHI were performed according to the American Academy of Sleep Medicine (AASM) manual [26] by an experienced sleep technician who was blinded to this study. In this study, hypopneas were defined as the reduction of airflow that lasted more than 10 s leading to arousal or oxygen desaturation (represented by a decrease in the oxygen saturation greater than 3%).
The indexes of each one of the breathing cessation events that compose the AHI such as obstructive apneas, central apneas, mixed apneas, and hypopneas were calculated in three different ways: (i) considering the total sleep time ([number of the event of interest × 60]/total sleep time), (ii) considering only NREM sleep ([number of the event of interest during NREM sleep × 60]/time spent in NREM sleep), and (iii) considering only REM sleep ([number of the event of interest during REM sleep × 60]/time spent in REM sleep).
Neuropsychological assessment
Patients underwent a neuropsychological evaluation through the MMSE at the beginning of the study and after 12 months of follow-up. The MMSE includes questions to evaluate different domains, such as attention, time and place orientation, and word recall. The scores of this test range from 0 to 30, and a higher score indicates better cognitive function [27, 28].
Statistical analysis
Descriptive statistics were performed to report sociodemographic, clinical, and AD- and sleep-related data. Absolute and relative frequencies were used for qualitative data. The means (standard deviation (SD)) and medians (25th percentile; 75th percentile [p25;p75]) were estimated for quantitative variables with normal and nonnormal distributions, respectively. The normality of the distribution was assessed by the Shapiro–Wilk test.
The associations between the breathing cessation events that compose the AHI, sleep structure, and AD pathological markers were evaluated through linear regression models adjusted by age, sex, and BMI. Given the high collinearity among the breathing cessation events, we performed additional methods of data integration using partial least squares (PLS) regression to identify the pattern, in terms of AHI events, that better explained the relationship among the variables related to each outcome (sleep structure and AD pathological markers).
The associations between the breathing cessation events that compose the AHI and the cognitive decline at 12-month follow-up were evaluated through linear regression models adjusted by age, sex, BMI, years of education, and MMSE at baseline. Additionally, we performed multivariate analyses using PLS regression to identify the pattern, in terms of AHI events, most associated with the cognitive decline. The association between the first component of the PLS regression and cognitive decline was evaluated with Generalized Additive Model (GAM) adjusted for age, sex, BMI, years of education, and MMSE at baseline.
The p-value threshold defining statistical significance was set at < 0.05. Data management and statistical analyses were performed using R (version 4.0.1).
Results
Baseline characteristics
The population was composed of 116 patients diagnosed with mild-moderate AD (Table 1). Accordingly, the median [p25;p75] score of the MMSE at baseline was 23.0 [22.0;25.0] points. With 55.2% of females, the cohort presented a median age of 76.0 [72.0;80.0] years and a BMI of 27.7 [25.3;30.2] kg·m−2. Hypertension, periodic limb movements, and depression were the most frequent comorbidities with a prevalence of 62.9%, 44.7%, and 25.9%, respectively. Furthermore, the median AHI was 25.9 [15.1;48.5], with a sleep efficiency of 68.0% [53.7;78.2], and no daytime sleepiness according to the ESS (5.00 [3.00;8.00]).
Table 1.
Baseline characteristics of the cohort
| Global | |
|---|---|
| n = 116 | |
| n (%), mean (SD) or median [p25;p75] | |
| Sociodemographic data | |
| Sex, female | 64 (55.2%) |
| Age, years | 76.0 [72.0;80.0] |
| BMI, kg·m−2 | 27.7 [25.3;30.2] |
| Education, years | 7.0 [7.0;7.0] |
| Comorbidities | |
| Hypertension | 73 (62.9%) |
| Diabetes mellitus | 23 (19.8%) |
| Cardiopathy | 25 (21.6%) |
| Depression | 30 (25.9%) |
| Periodic limb movements | 46 (44.7%) |
| Alzheimer’s disease parameters | |
| Cognitive function | |
| MMSE | 23.0 [22.0;25.0] |
| Biomarkers levels | |
| Aβ42, pg/ml | 527 [415;643] |
| T-tau, pg/ml | 464 [324;618] |
| P-tau, pg/ml | 73.4 [52.3;94.4] |
| Genetic risk | |
| ApoEε4 + | 60 (53.1%) |
| Sleep parameters | |
| Questionnaire | |
| Epworth sleepiness scale | 5.00 [3.00;8.00] |
| Polysomnography | |
| Total sleep time, hours | 4.57 [3.51;5.38] |
| Sleep efficiency, % | 68.0 [53.7;78.2] |
| N1, % | 12.1 [8.14;18.4] |
| N2, % | 24.3 (11.3) |
| SWS, % | 17.2 [8.27;24.5] |
| REM sleep, % | 6.74 [2.48;11.2] |
| Latency to N1, minutes | 21.0 [9.26;41.8] |
| Latency to REM, minutes | 161 [99.6;250] |
| AHI | 25.9 [15.1;48.5] |
AHI apnea–hypopnea index, ApoEε4 + apolipoprotein E carrier, Aβ42, amyloid-beta protein, BMI body mass index, MMSE Mini-Mental State Examination, n number, p percentile, P-tau phosphorylated-tau, SD standard deviation, SWS slow-wave sleep, T-tau total-tau. Missings: biomarkers, 12; ApoEε4 + , 3
Frequency of the breathing cessation events
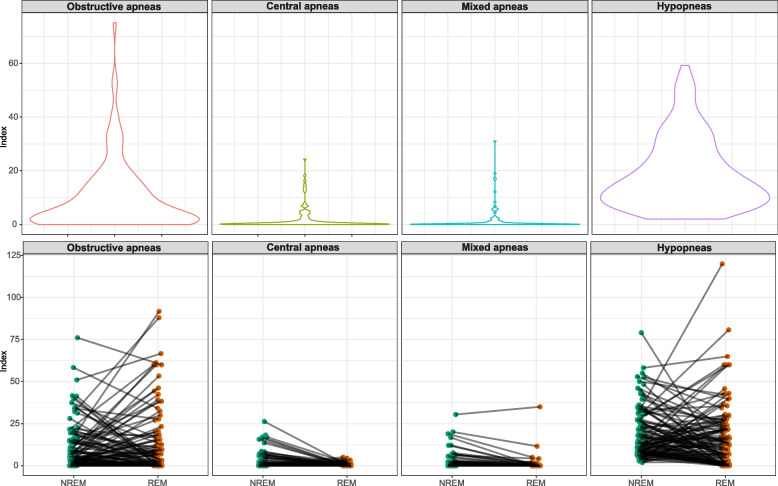
The breathing cessation events composing the AHI were evaluated to determine their frequency considering the total sleep time and each sleep stage individually. Hypopneas were the most frequent event considering the total sleep time with a median index of 14.7 [8.32;25.8], followed by the obstructive apneas (median index of 5.00 [0.86;14.1]) (Table S1). Such scenario was maintained considering each sleep stage individually, with central and mixed apneas presenting a modest frequency. Furthermore, comparisons between NREM and REM sleep demonstrated that the distribution of hypopneas and obstructive apneas among these sleep stages varied significantly according to each patient (Fig. 1).
Fig. 1.
Frequency of the breathing cessation events composing the AHI among mild-moderate Alzheimer’s disease patients. AHI, apnea–hypopnea index
Breathing cessation events and the sleep structure
Linear regression models adjusted by age, sex, and BMI revealed distinct patterns of associations between the breathing cessation events composing the AHI and the sleep structure (Table 2). The index of obstructive apneas presented positive associations with the arousals index (effect size [95% CI] of 0.78 [0.651 to 0.909]) and the percentage of time in N1 (0.334 [0.165 to 0.503]) in detriment of the time spent in SWS (− 0.227 [− 0.405 to − 0.049]). Higher central and mixed apneas indexes were related to an increased percentage of time in N1 (central: 0.289 [0.120 to 0.457]; mixed: 0.199 [0.024 to 0.373]), but no compensatory association with a decrease in the percentage of time in SWS was observed. Differently, the hypopnea index was only associated with the arousals index (0.521 [0.356 to 0.685]), and no alterations in relation to the sleep stages were detected.
Table 2.
Sleep structure according to the breathing cessation events
| Effect size (95% CI) | p-value | |
|---|---|---|
| Obstructive apnea index | ||
| N1 | 0.334 (0.165 to 0.503) | < 0.001 |
| N2 | 0.104 (− 0.084 to 0.292) | 0.281 |
| SWS | − 0.227 (− 0.405 to − 0.049) | 0.014 |
| REM sleep | − 0.059 (− 0.251 to 0.133) | 0.549 |
| Arousals | 0.78 (0.651 to 0.909) | < 0.001 |
| Central apnea index | ||
| N1 | 0.289 (0.120 to 0.457) | 0.001 |
| N2 | − 0.151 (− 0.337 to 0.035) | 0.113 |
| SWS | − 0.082 (− 0.262 to 0.098) | 0.377 |
| REM sleep | − 0.063 (− 0.253 to 0.127) | 0.518 |
| Arousals | 0.173 (− 0.013 to 0.359) | 0.071 |
| Mixed apnea index | ||
| N1 | 0.199 (0.024 to 0.373) | 0.026 |
| N2 | 0.145 (− 0.041 to 0.331) | 0.128 |
| SWS | − 0.053 (− 0.233 to 0.127) | 0.565 |
| REM sleep | 0.071 (− 0.119 to 0.261) | 0.464 |
| Arousals | 0.425 (0.254 to 0.595) | < 0.001 |
| Hypopnea index | ||
| N1 | 0.029 (− 0.149 to 0.207) | 0.747 |
| N2 | 0.135 (− 0.051 to 0.321) | 0.158 |
| SWS | − 0.123 (− 0.305 to 0.059) | 0.188 |
| REM sleep | − 0.094 (− 0.286 to 0.098) | 0.335 |
| Arousals | 0.521 (0.356 to 0.685) | < 0.001 |
Linear regression models adjusted by age, sex, and BMI representing the sleep structure according to the breathing cessation events that compose the AHI
AHI apnea–hypopnea index, BMI body mass index, CI confidence interval, SWS slow-wave sleep
Additional analyses were performed to investigate whether the pattern of associations between the components of the AHI and the sleep structure would be distinct considering the events occurring in each sleep stage (NREM or REM sleep) separately (Table S2). Contemplating the events occurring during NREM sleep only, there was a similar pattern of associations compared to those previously observed. Nevertheless, such pattern was not detected in the analysis considering exclusively the events occurring during REM sleep.
To counteract the high collinearity among the breathing cessation events and among the sleep stages, we performed methods of data integration based on PLS regression. The findings corroborated those previously observed, revealing that the most relevant pattern among the sample was mainly characterized by the presence of obstructive apneas and an increased number of arousals (Fig. S1).
Breathing cessation events and the pathological markers of Alzheimer’s disease
Linear regression models adjusted by age, sex, and BMI revealed that a higher hypopnea index was associated with an increased P-tau/Aβ42 ratio with an effect size of 0.217 (0.017 to 0.417) (Table 3). In addition, higher indexes of obstructive and mixed apneas were related to increased levels of NF-L (obstructive: 0.252 [0.013 to 0.491]; mixed: 0.229 [0.043 to 0.415]). A similar pattern of associations between the breathing cessation events and the pathological markers of AD was observed when contemplating the events occurring during NREM sleep only (Table S3). Nevertheless, no associations were detected in the analysis considering exclusively the events occurring during REM sleep.
Table 3.
Pathological markers of Alzheimer’s disease according to the breathing cessation events
| Effect size (95% CI) | p-value | |
|---|---|---|
| Obstructive apnea index | ||
| Aβ42 | 0.108 (− 0.086 to 0.302) | 0.276 |
| T-tau | 0.035 (− 0.161 to 0.231) | 0.723 |
| P-tau | 0.011 (− 0.185 to 0.207) | 0.915 |
| NF-L | 0.252 (0.013 to 0.491) | 0.042 |
| P-tau/Aβ42 | − 0.028 (− 0.226 to 0.17) | 0.782 |
| Central apnea index | ||
| Aβ42 | 0.022 (− 0.19 to 0.234) | 0.84 |
| T-tau | 0.148 (− 0.064 to 0.36) | 0.172 |
| P-tau | 0.052 (− 0.162 to 0.266) | 0.634 |
| NF-L | 0.09 (− 0.126 to 0.306) | 0.414 |
| P-tau/Aβ42 | 0.023 (− 0.193 to 0.239) | 0.832 |
| Mixed apnea index | ||
| Aβ42 | 0.019 (− 0.171 to 0.209) | 0.848 |
| T-tau | − 0.017 (− 0.207 to 0.173) | 0.859 |
| P-tau | 0.027 (− 0.163 to 0.217) | 0.785 |
| NF-L | 0.229 (0.043 to 0.415) | 0.019 |
| P-tau/Aβ42 | 0.013 (− 0.179 to 0.205) | 0.898 |
| Hypopnea index | ||
| Aβ42 | − 0.109 (− 0.309 to 0.091) | 0.289 |
| T-tau | − 0.033 (− 0.235 to 0.169) | 0.748 |
| P-tau | 0.2 (0.002 to 0.398) | 0.051 |
| NF-L | − 0.08 (− 0.310 to 0.150) | 0.49 |
| P-tau/Aβ42 | 0.217 (0.017 to 0.417) | 0.035 |
Linear regression models adjusted by age, sex, and BMI representing the pathological markers of AD according to the breathing cessation events that compose the AHI
AD Alzheimer’s disease, AHI apnea–hypopnea index, Aβ42 amyloid-beta protein, BMI body mass index, CI confidence interval, NF-L neurofilament light, P-tau phosphorylated-tau, T-tau total-tau
The PLS regression analysis to counteract the high collinearity among the breathing cessation events and among the pathological markers of AD revealed that the component with the most relevance was characterized by the number of obstructive apneas and hypopneas mostly, without a clear distinctiveness among the AD pathological markers (Fig. S2).
Breathing cessation events and the cognitive decline
Linear regression models adjusted by age, sex, BMI, years of education, and MMSE at baseline did not reveal substantial associations between the breathing cessation events composing the AHI and the cognitive decline (Table S4). We performed an additional analysis to evaluate whether distinct patterns in terms of AHI events would be related to a differential cognitive decline. The results revealed that the pattern that better explained the variability of the sample in terms of cognitive decline was characterized by the presence of hypopneas, possibly associated with the presence of mixed and obstructive apneas and inversely related to the presence of central apneas (Fig. 2). Accordingly, such pattern was associated with an increased cognitive decline at the 12-month follow-up (0.509 [0.025 to 0.992]) (Fig. 3).
Fig. 2.
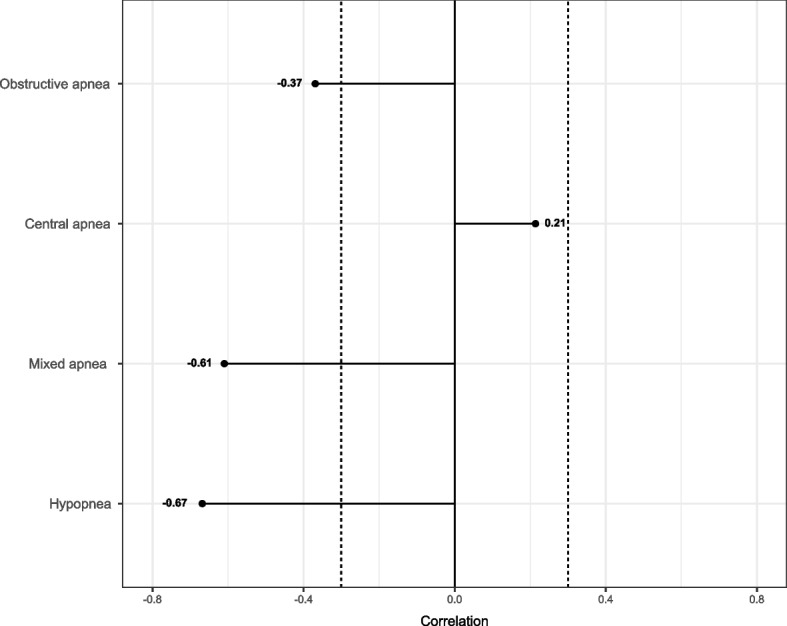
PLS regression analysis (cognitive decline). The findings reveal that the pattern of breathing cessation events that better explains the variability of the sample in terms of cognitive decline is mainly characterized by the presence of hypopneas. PLS, partial least squares
Fig. 3.
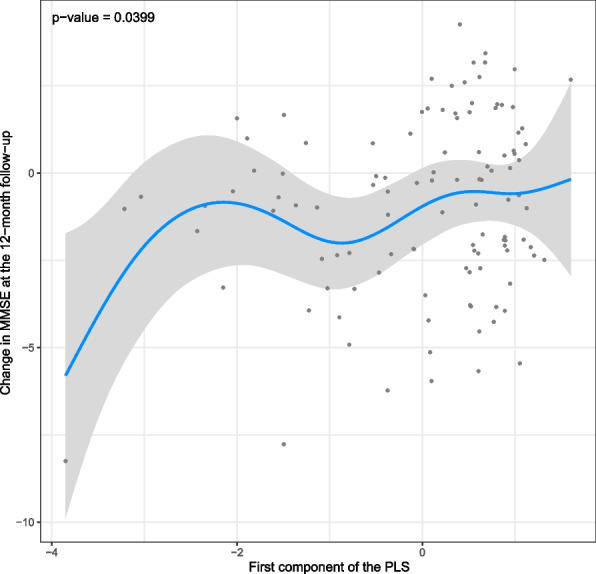
Cognitive decline according to the first component of the PLS regression analysis. Lower values of the first component of the PLS (X-axis) represent a higher number of hypopneas. Lower values of change in MMSE at the 12-month follow-up (Y-axis) represent a greater decrease in MMSE at the 12-month follow-up (i.e., higher cognitive decline). MMSE, Mini-Mental State Examination; PLS, partial least squares
Given that the previous analyses suggested a negligible influence of the events occurring during REM sleep, we performed an additional PLS regression considering only the events occurring during NREM sleep. Similar findings were obtained, with the pattern that better explained the variability of the sample in terms of cognitive decline being characterized by the presence of hypopneas (Fig. S3). Furthermore, such pattern was associated with a higher cognitive decline after 12 months of follow-up, with an effect size of 0.476 (− 0.001 to 0.952) after adjusting for age, sex, BMI, years of education, and MMSE at baseline.
Discussion
In the current study, we first characterized the breathing cessation events composing the AHI among our cohort of mild-moderate AD patients. Such analysis revealed a high frequency of hypopneas followed by the presence of obstructive apneas. The distribution of the events along the sleep stages presented substantial variability among the patients. Furthermore, each event presented a distinct pattern of associations with sleep. Apneas were associated with arousals and sleep lightening whereas hypopneas, despite being related to an increased number of arousals, did not affect the sleep structure. Nevertheless, hypopneas were associated with the levels of the classical markers of AD. More importantly, the hypopnea index was the most relevant factor to predict an increased cognitive decline at the 12-month follow-up.
The inclusion of apneas and hypopneas in the same metric to diagnose and establish the severity of the so-called OSA generates an implicit assumption that these events are similar in relation to their clinical impact. While some studies corroborate this line [29], others challenge the absence of distinctiveness among them [30, 31]. Kulkas and collaborators (2017) demonstrated that obstructive apneas are associated with more severe SpO2 desaturation compared to hypopneas, suggesting a higher relevance of the first when estimating the severity of OSA and associated long-term cardiovascular outcomes [30]. Several studies also reveal distinct outcomes according to the used criteria for the classification of hypopneas, highlighting the relevance of a detailed analysis of the events composing the AHI [31–36]. To our knowledge, this is the first study to address this matter, demonstrating the distinctiveness between hypopneas and obstructive apneas in terms of associated clinical outcomes among AD patients.
We observed that obstructive apneas were related to an increase in the time spent in N1 in detriment of the time spent in SWS, besides an association with an increased number of arousals. Also, these events were related to increased levels of NF-L, a marker for the axonal damage and cognitive decline of patients with AD [37–39]. Accordingly, we have previously demonstrated that patients with a propensity to spend most of the time in the lighter sleep stage (light sleepers) present an increased probability of having high NF-L levels compared with those individuals with a propensity to deepen their sleep (deep sleepers) [40]. Differently, despite the association with an increased number of arousals, the hypopneas were not related to alterations in the sleep structure. Nevertheless, the hypopnea index was associated with the levels of classical markers of AD and was the most relevant breathing cessation event to predict an increased cognitive decline at the 12-month follow-up. This suggests a possible contribution of arousals within this context regardless of whether alterations in the sleep structure are present or detected.
Based on the guidelines of the American Academy of Sleep Medicine (AASM), hypopneas and obstructive apneas are differentiated by the degree of airflow decrease (30% to 89% versus ≥ 90%, respectively) [26]. In consequence, obstructive apneas are associated with higher oxygen desaturation, which suggests a greater clinical impact in comparison to hypopneas [30]. Similarly, our findings revealed a stronger association between obstructive apneas and arousals than that involving hypopneas, which reinforces the greater risk of clinical consequences related to the apneic events. Still, our data indicate a distinguished role of hypopneas on the cognitive decline after 12 months of follow-up. A possible explanation for such outcome could be related to the 3-times higher frequency of hypopneas among our population compared to that of obstructive apneas. Future studies will be necessary to evaluate such interesting finding, confirming whether there are relationships of causality in this regard. Also, larger cohorts will provide the required variability to investigate differences between patients who have a pattern of breathing cessation events mostly composed of hypopneas and those in whom obstructive apneas are the most prevalent.
Limitations
The current findings should be interpreted in light of some aspects: (i) due to the sample size and the exploratory nature of this study, our data were not adjusted for multiple comparisons; (ii) given previous demonstrations of a differential effect of OSA on cognitive function depending on the cognitive status of the subjects and/or presence of AD pathology [8, 12, 17, 18], the associations herein observed cannot be extrapolated to cognitively healthy subjects or cognitively unimpaired patients with AD pathology; (iii) the sleep evaluation revealed a low percentage of time spent in REM sleep, which might have prevented the observation of associations in this regard; (iv) the discreet prevalence of central and mixed apneas in addition to the high collinearity among the components of the AHI may have led to associations influenced by the most prevalent events such as hypopneas and obstructive apneas; (v) although the objective of this study was limited to investigate whether the AHI components associated with increased sleep disruption would be the ones linked to worse outcomes, the influence of the respiratory burden related to each event should not be ruled out; (vi) given the observational design of this study, it is not possible to confirm causality and directionality between the associations of interest.
Conclusions
In summary, we demonstrated that the hypopneas and the obstructive apneas are the components that define the AHI among consecutive patients with mild-moderate AD. These events were distinctively associated with the sleep structure, the levels of pathological markers of AD, and cognitive decline, suggesting that the OSA does have an influence once patients are diagnosed with symptomatic disease up to a certain extent. This altogether highlights the importance of a patient-centered approach, with a comprehensive and detailed analysis of the AHI to effectively predict the different outcomes and tailor the appropriate therapeutic strategies.
Supplementary Information
Additional file 1: Table S1. Frequency of the breathing cessation events.
Additional file 2: Table S2. Sleep structure according to the breathing cessation events in each sleep stage.
Additional file 3: Table S3. Pathological markers of Alzheimer’s disease according to the breathing cessation events in each sleep stage.
Additional file 4: Table S4. Cognitive decline according to breathing cessation events.
Additional file 5: Figure S1. PLS regression analysis (sleep structure). The findings reveal that the most relevant pattern in relation to the breathing cessation events and sleep structure is mainly characterized by the presence of obstructive apneas and an increased number of arousals. PLS, partial least squares; SWS, slow wave sleep.
Additional file 6: Figure S2. PLS regression analysis (pathological markers of Alzheimer’s disease). The findings reveal that the most relevant pattern in relation to the breathing cessation events and pathological markers of AD is characterized by the number of obstructive apneas and hypopneas mostly, without a clear distinctiveness among the Alzheimer’s disease pathological markers. AD, Alzheimer’s disease; Aβ42, amyloid-beta protein; NF-L, neurofilament light; P-tau, phosphorylated-tau; PLS, partial least squares; T-tau, total-tau.
Additional file 7: Figure S3. PLS regression analysis (cognitive decline). The findings reveal that the pattern of breathing cessation events occurring during NREM sleep that better explains the variability of the sample in terms of cognitive decline is mainly characterized by the presence of hypopneas. PLS, partial least squares.
Acknowledgements
We would like to express our sincere gratitude to all of the patients and to the professionals of both Sleep and Dementia Unit at the Hospital Universitari Santa Maria. We were supported by the IRBLleida Biobank (B.0000682) and PLATAFORMA BIOBANCOS PT17/0015/0027/.
Abbreviations
- AD
Alzheimer’s disease
- AHI
Apnea-hypopnea index
- ApoE
Apolipoprotein E
- Aβ42
Amyloid-beta protein
- BMI
Body mass index
- CSF
Cerebrospinal fluid
- ESS
Epworth sleepiness scale
- MMSE
Mini-Mental State Examination
- NF-L
Neurofilament light
- OSA
Obstructive sleep apnea
- P-tau
Phosphorylated-tau
- PSG
Polysomnography
- SWS
Slow-wave sleep
- T-tau
Total-tau
Authors’ contributions
AT, IB, MSdlT, FB, and GPR designed the study; FD, OM, RV, MD, and GPR collected the data; AT, IB, and AMM were responsible for the data management; IB and AT analyzed the data; AT, IB, and GPR interpreted the data; AT wrote the manuscript draft. All authors revised the manuscript and approved it for submission.
Funding
Generalitat of Catalonia, Department of Health (PERIS 2019 SLT008/18/00050) and “Fundació La Marató TV3” (464/C/2014) to GPR. Co-financed by FEDER funds from the European Union (‘A way to build Europe’). IRBLleida is a CERCA Programme/Generalitat of Catalonia. FD was supported by Agency for Management of University and Research Grants (FI_B100153). MSdT has received financial support from the “Ramón y Cajal” grant (RYC2019-027831-I) from the “Ministerio de Ciencia e Innovación—Agencia Estatal de Investigación” cofunded by the European Social Fund (ESF)/ “Investing in your future.”
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the Hospital Universitari Arnau de Vilanova-Santa Maria (Lleida, Spain) (CE-1218) and conducted according to the Declaration of Helsinki. The patient, the responsible caregiver, and the legal representative (when different from the responsible caregiver) signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adriano D. S. Targa, Email: adrianotargads@gmail.com
Gerard Piñol-Ripoll, Email: gerard_437302@hotmail.com.
References
- 1.International AD, University M. World Alzheimer Report 2021. Alzheimer’s Dis Int. 2021;2–314. Available from: https://www.alzint.org/resource/world-alzheimer-report-2021/.
- 2.Reitz C, Mayeux R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014;88(4):640–51. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021;17:327–406. [DOI] [PubMed]
- 4.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat. Rev. Nephrol. 2021;17(10):639–54. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018;14(10):591–604. doi: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picone P, Di Carlo M, Nuzzo D. Obesity and Alzheimer’s disease: Molecular bases. Eur. J. Neurosci. 2020;52(8):3944–50. doi: 10.1111/ejn.14758. [DOI] [PubMed] [Google Scholar]
- 7.Bubu OM, Andrade AG, Umasabor-Bubu OQ, Hogan MM, Turner AD, de Leon MJ, et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research. Sleep Med Rev. 2020;50:101250. [DOI] [PMC free article] [PubMed]
- 8.Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly: A longitudinal study. Am J Respir Crit Care Med. 2018;197(7):933–43. doi: 10.1164/rccm.201704-0704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liguori C, Mercuri NB, Izzi F, Romigi A, Cordella A, Sancesario G, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep. 2017;40(5). [DOI] [PubMed]
- 10.Bubu OM, Umasabor-Bubu OQ, Turner AD, Parekh A, Mullins AE, Kam K, et al. Self-reported obstructive sleep apnea, amyloid and tau burden, and Alzheimer’s disease time-dependent progression. Alzheimer’s Dement. 2021;17(2):226–45. doi: 10.1002/alz.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackwell T, Yaffe K, Laffan A, Redline S, Ancoli-Israel S, Ensrud KE, et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: The osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2015;63(3):453–61. doi: 10.1111/jgs.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin M Saint, Sforza E, Roche F, Barthélémy JC, Thomas-Anterion C. Sleep breathing disorders and cognitive function in the elderly: An 8-year follow-up study. The proof-synapse cohort. Sleep. 2015;38(2):179-87. [DOI] [PMC free article] [PubMed]
- 13.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaffe K, Nettiksimmons J, Yesavage J, Byers A. Sleep Quality and Risk of Dementia among Older Male Veterans. Am J Geriatr Psychiatry. 2015;23(6):651–4. doi: 10.1016/j.jagp.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Lutsey PL, Misialek JR, Mosley TH, Gottesman RF, Punjabi NM, Shahar E, et al. Sleep characteristics and risk of dementia and Alzheimer’s disease: The Atherosclerosis Risk in Communities Study. Alzheimer’s Dement. 2018;14(2):157–66. doi: 10.1016/j.jalz.2017.06.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, et al. Sleep Apnea and the Risk of Dementia: A Population-Based 5-Year Follow-Up Study in Taiwan. PLoS One. 2013;8(10). [DOI] [PMC free article] [PubMed]
- 17.Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, et al. Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep. 2019;42:1–13. doi: 10.1093/sleep/zsz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorge C, Targa A, Benítez ID, Dakterzada F, Torres G, Minguez O, et al. Obstructive sleep apnoea and cognitive decline in mild-to-moderate Alzheimer’s disease. Eur Respir J. 2020;56:2000523. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.00523-2020. [DOI] [PubMed]
- 19.Targa ADS, Benítez ID, Dakterzada F, Carnes A, Pujol M, Jorge C, et al. Sleep profile predicts the cognitive decline of mild-moderate Alzheimer’s disease patients. Sleep. 2021;44(10):zsab117. [DOI] [PubMed]
- 20.Targa A, Dakterzada F, Benítez I, López R, Pujol M, Dalmases M, et al. Decrease in sleep depth is associated with higher cerebrospinal fluid neurofilament light levels in patients with Alzheimer’s disease. Sleep. 2021;44:1–9. doi: 10.1093/sleep/zsaa147. [DOI] [PubMed] [Google Scholar]
- 21.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Targa A, Dakterzada F, Benítez ID, de Gonzalo-Calvo D, Moncusí-Moix A, López R, et al. Circulating MicroRNA Profile Associated with Obstructive Sleep Apnea in Alzheimer’s Disease. Mol Neurobiol. 2020;57(11):4363–72. doi: 10.1007/s12035-020-02031-z. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 24.Chiner E, Arriero JM, Signes-Costa J, Marco J, Fuentes I. Validación de la versión española del test de somnolencia Epworth en pacientes con síndrome de apnea de sueño. Arch Bronconeumol. 1999;35(9):422–7. doi: 10.1016/S0300-2896(15)30037-5. [DOI] [PubMed] [Google Scholar]
- 25.Ferrer M, Vilagut G, Monasterio C, Montserrat JM, Mayos M, Alonso J. Measurement of the perceived impact of sleep problems: the Spanish version of the functional outcomes sleep questionnaire and the Epworth sleepiness scale. Med Clin (Barc). 1999;113(7):250–5. [PubMed] [Google Scholar]
- 26.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. J. Clin. Sleep Med. 2007;1-49.
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-98. [DOI] [PubMed]
- 28.Folstein MF, Folstein SE, McHugh PR, Fanijang G. MMSE - Examen Cognoscitivo Mini-mental. J Psychiatr Res. 1975;12:189–98. Available from: https://linkinghub.elsevier.com/retrieve/pii/0022395675900266. [DOI] [PubMed]
- 29.Spector AR, Loriaux D, Farjat AE. The Clinical Significance of Apneas Versus Hypopneas: Is There Really a Difference? Cureus. 2019;11(4):e4560. [DOI] [PMC free article] [PubMed]
- 30.Kulkas A, Duce B, Leppänen T, Hukins C, Töyräs J. Severity of desaturation events differs between hypopnea and obstructive apnea events and is modulated by their duration in obstructive sleep apnea. Sleep Breath Sleep Breathing. 2017;21:829–835. doi: 10.1007/s11325-017-1513-6. [DOI] [PubMed] [Google Scholar]
- 31.Budhiraja R, Javaheri S, Parthasarathy S, Berry RB, Quan SF. The association between obstructive sleep apnea characterized by a minimum 3 percent oxygen desaturation or arousal hypopnea definition and hypertension. J Clin Sleep Med. 2019;15:1261–1270. doi: 10.5664/jcsm.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho V, Crainiceanu CM, Punjabi NM, Redline S, Gottlieb DJ. Calibration Model for Apnea-Hypopnea Indices: Impact of Alternative Criteria for Hypopneas. Sleep. 2015;38:1887–1892. doi: 10.5665/sleep.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J Am Coll Cardiol. 2017;69(7):841–58. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra A, Ayappa I, Ayas N, Collop N, Kirsch D, Mcardle N, et al. Metrics of sleep apnea severity: Beyond the apnea-hypopnea index. Sleep. 2021;44:1–16. doi: 10.1093/sleep/zsab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra RK, Kirsch DB, Kristo DA, Olson EJ, Aurora RN, Carden KA, et al. Polysomnography for Obstructive Sleep Apnea Should Include Arousal-Based Scoring: An American Academy of Sleep Medicine Position Statement. J Clin Sleep Med. 2018;14:1245–1247. doi: 10.5664/jcsm.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budhiraja R, Quan SF. Sleepiness in obstructive sleep apnea using hypopneas defined by a 3% oxygen desaturation or arousal but not by 4% or greater oxygen desaturation. Sleep Breath. 2021;1–5. 10.1007/s11325-021-02494-x. [DOI] [PubMed]
- 37.Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 38.Moscoso A, Grothe MJ, Ashton NJ, Karikari TK, Lantero Rodríguez J, Snellman A, et al. Longitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain with Neurodegeneration in Alzheimer Disease. JAMA Neurol. 2021;78(4):396–406. doi: 10.1001/jamaneurol.2020.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cullen NC, Leuzy A, Janelidze S, Palmqvist S, Svenningsson AL, Stomrud E, et al. Plasma biomarkers of Alzheimer’s disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun. 2021;12(1):3555. doi: 10.1038/s41467-021-23746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Targa A, Dakterzada F, Benítez I, López R, Pujol M, Dalmases M, et al. Decrease in sleep depth is associated with higher cerebrospinal fluid neurofilament light levels in Alzheimer’s disease patients. Sleep. 2020;44(2):zsaa147. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Frequency of the breathing cessation events.
Additional file 2: Table S2. Sleep structure according to the breathing cessation events in each sleep stage.
Additional file 3: Table S3. Pathological markers of Alzheimer’s disease according to the breathing cessation events in each sleep stage.
Additional file 4: Table S4. Cognitive decline according to breathing cessation events.
Additional file 5: Figure S1. PLS regression analysis (sleep structure). The findings reveal that the most relevant pattern in relation to the breathing cessation events and sleep structure is mainly characterized by the presence of obstructive apneas and an increased number of arousals. PLS, partial least squares; SWS, slow wave sleep.
Additional file 6: Figure S2. PLS regression analysis (pathological markers of Alzheimer’s disease). The findings reveal that the most relevant pattern in relation to the breathing cessation events and pathological markers of AD is characterized by the number of obstructive apneas and hypopneas mostly, without a clear distinctiveness among the Alzheimer’s disease pathological markers. AD, Alzheimer’s disease; Aβ42, amyloid-beta protein; NF-L, neurofilament light; P-tau, phosphorylated-tau; PLS, partial least squares; T-tau, total-tau.
Additional file 7: Figure S3. PLS regression analysis (cognitive decline). The findings reveal that the pattern of breathing cessation events occurring during NREM sleep that better explains the variability of the sample in terms of cognitive decline is mainly characterized by the presence of hypopneas. PLS, partial least squares.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.