Abstract
Myocardial infarction causes the loss of cardiomyocytes and the formation of cardiac fibrosis due to the activation of cardiac fibroblasts, leading to cardiac dysfunction and heart failure. Unfortunately, current therapeutic interventions can only slow the disease progression. Furthermore, they cannot fully restore cardiac function, likely because the adult human heart lacks sufficient capacity to regenerate cardiomyocytes. Therefore, intensive efforts have focused on developing therapeutics to regenerate the damaged heart. Several strategies have been intensively investigated, including stimulation of cardiomyocyte proliferation, transplantation of stem cell-derived cardiomyocytes, and conversion of fibroblasts into cardiac cells. Resident cardiac fibroblasts are critical in the maintenance of the structure and contractility of the heart. Fibroblast plasticity makes this type of cells be reprogrammed into many cell types, including but not limited to induced pluripotent stem cells, induced cardiac progenitor cells, and induced cardiomyocytes. Fibroblasts have become a therapeutic target due to their critical roles in cardiac pathogenesis. This review summarizes the reprogramming of fibroblasts into induced pluripotent stem cell-derived cardiomyocytes, induced cardiac progenitor cells, and induced cardiomyocytes to repair a damaged heart, outlines recent findings in utilizing fibroblast-derived cells for heart regeneration, and discusses the limitations and challenges.
Graphical Abstract
Introduction
More than 5 million Americans live with heart failure (HF), a devastating condition responsible for the high death rate and hospitalization [1]. One of the leading causes of HF is ischemic heart disease (IHD) caused by reduced blood flow to the heart [2]. Insufficient supply of oxygen and other nutrients in IHD causes the death of cardiomyocytes (CMs), weakening heart contraction. Myocardial infarction (MI) causes the death of CMs and the activation of cardiac fibroblasts (CFs) [3,4]. Activated CFs continuously proliferate and express extracellular matrix (ECM) proteins. Deposition of excessive ECM proteins results in cardiac fibrosis. CM loss and cardiac fibrosis synergistically impair cardiac function and structure. Around 40% of patients who suffer from MI eventually develop HF. CM loss is permanent because adult humans lack the sufficient regenerative capacity to remuscularization of infarct myocardium [5]. Advances in surgical and imaging techniques, pharmacotherapies, and implantable medical devices significantly improve outcomes and patient’s quality of life [6]. However, current interventions can only slow the disease progression but cannot regenerate damaged myocardium completely [7]. Various strategies for heart regeneration (e.g., stimulation of endogenous CM proliferation, transplantation of stem cell-derived CMs, and direct reprogramming of CFs into CMs) have been intensively investigated during the past decade [8].
Humans and mice have low rates of CM proliferation in adulthood [9-12]. Mice can regenerate their damaged hearts during a short time window after birth when CMs have high dividing rates [13]. Regeneration of the damaged heart by promoting CM proliferation attracts attention. After screening a human whole-genome microRNA (miRNA) library, Eulalio et al. identified two miRNAs (has-miR-590 and has-miR-199a) that facilitated the proliferation of both neonatal and adult CMs in vitro and in vivo [14]. After ligation of the left anterior descending coronary artery (LAD), adeno-associated virus (AAV) serotype 9 (AAV9) vectors expressing one miRNA (AAV9-miR-590 or AAV9-miR-199a) were injected into the peri-infarct area of adult mice. Overexpression of miR-590 or miR-199a in MI-hearts promoted CM proliferation, improved cardiac function, and decreased infarct size [14]. Gabisonia et al. examined the regenerative capacity of has-miR-199a in pigs [15]. Pigs were subjected to 90-min occlusion of the left anterior coronary artery followed by reperfusion. AAV6-miR-199a was injected into the left ventricle wall. Overexpression of miR-199a promoted CM proliferation, increased cardiac muscle mass, reduced scar size, and improved contractility of the heart one month after MI. However, continuous expression of miR-199a in the heart leads to sudden arrhythmic death of most pigs after 40 days [15], suggesting the detrimental effect of the over-proliferation of CMs. Inhibition of the Hippo pathway (e.g., by knockout or knockdown of Mst, sav, or Lats) leads to nuclear translocation of transcription factors Yap and Taz to stimulate CM proliferation in the mouse heart [16-19]. AAV9-Sav-shRNA viral particles were transendocardially injected into the border zone of the infarct pig heart using a catheter-based delivery method two weeks after ischemia/reperfusion (I/R) injury [20]. Knockdown of Sav leads to increased CM proliferation and reduced scar size in MI pigs. MI-pigs treated with AAV9-Sav-shRNA displayed a ~14% improvement in left ventricular ejection fraction three months after injection. The investigators did not observe adverse events, including uncontrolled CM proliferation, organ overgrowth, cardiac arrhythmias, and sudden cardiac death, possibly due to catheter-based gene delivery [20,21]. These studies in swine models [15, 20, 21] indicate that cardiac regeneration can be achieved by enhancement of CM proliferation if the proliferation event is tightly controlled.
It has been hypothesized that implanted stem cells can differentiate into CMs to regenerate the myocardium in the injured heart. However, clinical trials of stem cell therapy, including transplantation of mesenchymal stem cells, for heart regeneration have produced neutral or marginally positive outcomes [22-23]. The development of alternative approaches for heart regeneration is clinically urgent. Expression of lineage-specific factor cocktails or treatment with small molecules and/or growth factors can reprogram fibroblasts into many cell types, e.g., induced pluripotent stem cells or iPSCs [24, 25], induced CMs (iCMs) [26-28], induced cardiac progenitor cells (iCPCs) [29], induced cardiac tissue-like structures (rCVT) [30], skeletal muscles [31-33], induced neuronal cells [34], induced hepatocytes [35], and induced dendritic cells [36]. Fibroblasts exist in all organs, becoming an ideal cell resource to generate cardiac cells. Importantly, autologous cardiac cells generated by fibroblast reprogramming have the potential to overcome immune rejection and physiologically couple with surrounding cardiovascular tissue after they repopulate in the damaged heart. This review summarizes recent findings in the application of iPSC-derived CMs (iPSC-CMs), iCPCs, iCMs, and rCVT to repair the damaged heart and discusses the limitations and challenges of individual approaches (Figure 1).
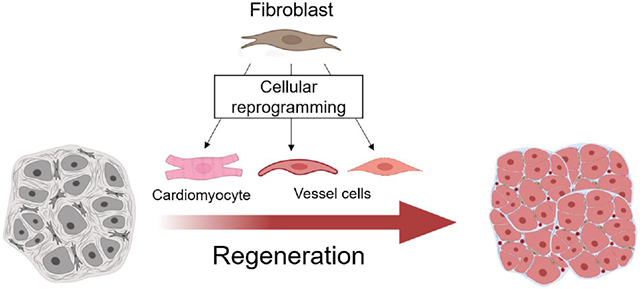
Figure 1. Regeneration of damaged heart by cellular reprogramming of fibroblasts.
Death of cardiomyocytes and fibrotic remodeling impair heart function. Cellular reprogramming of somatic cells for remuscularization and revascularization has the potential to avoid immune rejection. Reprogramming strategies and challenges are summarized. Fibroblasts can be reprogrammed into induced pluripotent stem cells (iPSCs) by Yamanaka factors containing OCT4, SOX2, KLF4, and c-MYC. Various small molecules can robustly induce the differentiation of human iPSCs into cardiomyocytes (iPSC-CMs). Recently, fibroblasts have been successfully reprogrammed into induced cardiomyocytes (iCMs), induced cardiac progenitor cells (iCPCs), and cardiovascular tissue-like structures (rCVT) containing induced cardiomyocytes (iCMs), induced smooth muscle cells (iSMCs), and induced endothelial cells (iECs). Transplantation of iPSC-CMs, iCPCs, or rCVT into rodent myocardium demonstrates the potential to facilitate heart regeneration. Many factors could affect the therapeutic outcome of engrafted cells, such as the purity of iPSC-CMs, retention of engrafted cells, electrical and physiological coupling of newly formed CMs with surrounding counterparts, potential tumorigenesis and arrhythmogenesis. Direct reprogramming of cardiac fibroblasts into induced cardiac lineage cells (iCLCs) such as iCMs, iSMCs, and iECs in the damaged heart has the potential to overcome the limitations of cell transplantation. However, direct reprogramming in situ is a new approach and needs to be optimized to enhance reprogramming efficiency, search for a specific gene delivery approach, and overcome the fibrotic environment that automatically inhibits direct reprogramming.
Transplantation of CMs derived from pluripotent stem cells into the injured heart
Takahashi et al. reprogrammed human and mouse fibroblasts into iPSCs by overexpressing pluripotent factors, Oct3/4, Sox2, Klf4, and c-Myc (also known as Yamanaka factors) (24, 25). Treatment with various combinations of small molecules robustly induces the differentiation of human pluripotent stem cells (PSC) such as iPSC into CMs (PSC-CMs) [37, 38]. The advantage is that self-renewal PSCs can produce an unlimited amount of CMs. Transplantation of human PSC-CMs to small animals' injured hearts shows encouraging heart repair results [39, 40]. In humans, one MI can cause the death of billions of CMs. Therefore, producing large-scale human PSC-CMs with high quality and purity is necessary for clinical applications, which becomes feasible with technological advances [41-46]. Chong et al. produced billions of human embryonic stem cell-derived CMs (hESC-CMs) and cryopreserved them with good viability [44]. Transplantation of 1 billion hESC-CMs into the injured heart of non-human primates (Macaca nemestrina) subjected to myocardial I/R revascularized the infarct heart. Engrafted hESC-CMs matured with time and electrically coupled with the host myocardium. All non-human primates receiving hESC-CMs displayed non-fatal ventricular arrhythmias. In this study, the investigators did not observe the improvement of cardiac function in monkeys receiving hESC-CMs [44]. However, transplanting fewer hESC-CMs (~750 million) into Macaca nemestrina with large myocardial infarctions leads to revascularization and remuscularization of the infarct heart and improved left ventricular function [45]. These studies in the same laboratory suggest that the transplantation of hESC-CMs for maximal heart regeneration must be optimized. The outcome could be affected by many factors, including infarct size, number of transplanted cells, delivery methods, timing and locations for cell transplantation.
The application of human ESCs faces ethical concerns, which can be avoided using the iPSC approach. Autologous transplantation of human iPSC-derived CMs (hiPSC-CMs) can also avoid immune rejection. Shiba et al. examine the therapeutic potential of iPSC-CMs in the cynomolgus monkey [46]. Given the essential role of major histocompatibility complex (MHC) in the immune response post-transplantation [47,48], Shiba et al. injected allogenic iPSC-CMs into the infarct zone and the border zone two weeks after MHC-matched monkeys were subjected to I/R injury. Transplanted iPSC-CMs survived for at least 12 weeks and electrically coupled with the surrounding myocardium, leading to partial remuscularization of the infarct heart and ejection fraction improvement. The occurrence of non-fatal arrhythmias in monkeys receiving iPSC-CMs peaked on day 14 post-transplantation and reduced over time, which might be due to the maturation and integration of iPSC-CMs and reduction in the portion of grafted iPSC-CM [46].
A primary barrier to cell therapy for heart regeneration is the low retention rate of transplanted cells in the myocardium [49]. Strategies to increase the retention rate have been intensively investigated [50]. For example, Park et al. adopted a combination approach by intramyocardial injection of human iPSC-derived CMs (hiPSC-CMs) and epicardial implantation of human mesenchymal stem cell-loaded patch made from the porcine heart-derived decellularized extracellular matrix (hMSC-PA) to the infarcted rat heart after LAD ligation [51]. Implanted hMSC-PA increased angiogenesis and improved the retention and maturation of hiPSC-CMs, ultimately preserving cardiac function. The study suggests that it is possible to increase the retention and survival rates of engrafted iPSC-CMs utilizing the bioengineering approach.
Reprogramming fibroblasts into induced cardiomyocytes
The adult mammalian heart is composed of ~30% CMs, ~40% endothelial cells, ~7% hematopoietic-derived cells, and ~ 10% CFs [10,52]. Resident CFs provide scaffold support and express ECM proteins to maintain the structure and mechanoproperties of the heart. In addition to CM necrosis, MI also causes the activation of CFs, which leads to CF proliferation and increases the expression of ECM proteins [53,54]. Activated CFs play an essential role in wound healing after acute MI to prevent heart rupture. Conversely, prolonged CF activation causes cardiac fibrosis and remodeling, deteriorating cardiac structure and function [3,55]. Given that activated CFs in a large number within damaged myocardium are primary cell sources for cardiac fibrosis, CFs have become a promising target for treating cardiovascular disease [54]. Direct reprogramming CFs into cardiac lineages such as induced CMs (iCMs) and blood vessel cells (cardiac reprogramming) has the potential to regenerate CMs, promote angiogenesis, and suppress cardiac fibrosis. If successful, cardiac reprogramming represents a promising therapeutic strategy for heart regeneration.
• Yamanaka factors- and compound-mediated cardiac reprogramming
Yamanaka factor-medicated iPSC reprogramming [24,25] is a process with many intermediate cell populations [56,57]. Efe et al. used Yamanaka factors to reprogram mouse fibroblasts into CMs without passing through the iPSC stage by modifying reprogramming conditions [58]. Mouse embryonic fibroblasts (MEFs) overexpressing Oct4, Sox2, and Klf4 were cultured in a LIF-free medium containing a JAK-STAT (Janus kinase-signal transducer and activator of transcription) inhibitor JI1. After being cultured for nine days, these cells were cultured in a chemically defined medium containing bone morphogenic protein 4 (BMP4). On days 9-10, cardiac progenitor markers, Flk1, Nkx2.5, and Gata4, were robustly activated. Many colonies began to contract by day 15 spontaneously. The addition of JI1 and BMP4 further increased the number of beating clusters. Most CMs exhibited atrial-like phenotype, assessed by the spontaneous action potentials [58]. Later, MEFs were also directly reprogrammed into cardiomyocyte-like cells by overexpression of Oct4 plus treatment with four compounds (SB431542 (ALK4/5/7 inhibitor), ChIR99021 (GSK3 inhibitor), Forskolin (adenylyl cyclase activator), and Parnate (LSD1/KDM1 inhibitor)) [59].
A compound cocktail CRFVPTZ composed of ChIR99021 (GSK3 inhibitor or Wnt signaling activator), RepSox (ALK5 inhibitor), Forskolin (Adenylyl cyclase inhibitor), VPA (an inhibitor of histone deacetylases), Parnate (or tranylcypromine, monoamine oxidase inhibitor), TTNPB (synthetic retinoic acid receptor ligand), and DZnep (S-adenosylhomocysteine hydrolase inhibitor) reprogramed MEFs into iPSCs [60]. Treating MEFs with CRFVPTZ, Fu et al. observed spontaneously beating CMs [61]. After optimizing the reprogramming conditions, such as the culture substrates and compound combinations, Fu et al. demonstrated that CRFVPT (ChIR99021, RepSox, Forskolin, Valproic acid (VPA, a histone deacetylase inhibitor), Parnate, and TTNPB) reprogramed mouse fibroblasts into iCMs through a transient intermediate/progenitor state [61]. CRFVPT treatment produced 15% of cells positive for α-actinin and 9% for α-MHC from MEFs on day 24. Approximately 20% of iCMs were ventricular-like myocytes, assessed by action potentials [61]. The efficiency of cardiac reprogramming induced by CRFVPT was further enhanced by growth factors such as neuregulin 1, G-CSF, and Rolipram (a selective phosphodiesterase-4 inhibitor) [61]. Expression of progenitor cell marker genes Flk1 and Mesp1 was activated during early reprogramming (e.g., on day 8) and decreased with time (e.g., on day 24). Therefore, CRFVPT-mediated reprogramming passes through an intermediate or progenitor stage [61]. Huang et al. examined whether the chemical cocktail CRFVPTM (ChIR99021, RepSox, Forskolin, VPA, Parnate, TTNPB, and Rolipram) could reprogram cardiac fibroblasts to iCM in vivo [62]. Fsp1-Cre/R26R-tdTomato mice were treated with CRFTM via oral and VP via intraperitoneal injection once a week for six weeks after MI. In Fsp1-Cre/R26R-tdTomato mice, tdTomato is expressed in fibroblasts, SMCs, ECs, and hematopoietic cells in the heart but not in CMs [63]. CRFVPTM treatment reprogramed ~ 1% of FSP1-tdTomato+ cells to iCMs. Compound-induced iCMs coupled with surrounding CMs via connexin 43 and N-cadherin. Isolated iCMs generated an atrial-like action potential. The CRFVPTM treatment also reduced cardiac fibrosis and improved cardiac function [62]. However, this study has not addressed the following questions, (1) whether the CRFVPTM treatment improves angiogenesis in the infarcted heart, (2) whether the systemic treatment with CRFVPTM causes adverse effects in other organs, and (3) how regenerated atrial-like iCMs improve ventricular remodeling.
The pluripotent factor Oct4 combined with the treatment with four compounds (SB431542, ChIR99021, Forskolin, and Parnate) reprogrammed MEFs into cardiomyocyte-like cells [59]. Cao et al. screened a pool of small molecules and identified nine compounds that induced ~ 7% of treated human foreskin fibroblasts (HFFs) positive for CM marker cardiac troponin T (cTnT) (ciCMs) on day 30 [64]. This nine-compound cocktail contains ChIR99021 (Wnt activator), A83-01 (TGFβ signaling inhibitor), BIX01294 (inhibitor of histone methyltransferase), AS8351 (KDM5B inhibitor), SC1 (inhibitor of extracellular signal-regulated kinase 1 and RasGAP), Y27632 (ROCK1 and ROCK2 inhibitor), OAC2 (OCT4 activator), SU16F (inhibitor of platelet-derived growth factor receptor β, PDGFRβ), and JNJ10198409 (Inhibitor of PDGFR) [63]. More than 90% of ciCMs spontaneously contracted. Most ciCMs displayed ventricular-like action potentials [64]. ciCMs and iPSC-CMs exhibited similarities in gene expression in this study. HFFs treated with nine compounds were transplanted into infarcted hearts of immunodeficient mice. After two weeks, transplanted compound-treated HFFs adopted CM phenotype, including expression of CM markers and assembling of sarcomeric structures [64]. The effects of ciCMs on heart function and cardiac remodeling were not reported in this study.
• microRNA-mediated cardiac reprogramming
Reprogramming of mouse and human fibroblasts into iPSCs can be achieved by microRNAs [65-67]. Overexpression of a microRNA cocktail (miRcombo containing miR-1, miR-133, miR-208, and miR-499) in CFs isolated from αMHC-CFP neonatal transgenic mice induced 1-5% of cells positive for αMHC-CFP [68]. The addition of JAK inhibitor 1 increased the reprogramming efficiency. miRcombo-induced cardiomyocyte-like cells expressed cardiac genes, such as Mef2C, Myh6, and Tnni3, and display spontaneous Ca2+ oscillations [68]. Four weeks after induction, the average frequency of spontaneous Ca2+ oscillations in αMHC-CFP+ cells is half that in cultured neonatal mouse cardiomyocytes [68]. Adult dermal fibroblasts overexpressing miRcombo barely displayed Ca2+ oscillations, despite expressing some cardiac markers. Lentiviral vectors encoding miRcombo were injected into infarcted zones of adult FSP1-Cre/R26R-tdTomato mice after permanent ligation of LAD [69]. By seven weeks after viral injection, tdTomato+ CMs with mature and immature morphologies were observed in the peri-infarct zone of miRcombo-injected mice. Most rod-shaped tdTomato+ CMs displayed normal Ca2+ transients and contractions in response to depolarization and fired action potentials similar to those of tdTomato− CMs. miRCombo-treatment also increased left ventricular fractional shortening and decreased cardiac fibrosis two months post-MI [69].
Compared to two-dimensional (2D) cell cultures, a three-dimensional (3D) culture system better mimics the native myocardial environment [70-73]. miRcombo converted mouse fibroblasts into iCMs in vitro and in vivo [68,69]. Hydrogel-based 3D culture enhanced cardiac gene expression in neonatal CFs expressing miRcombo [74]. The 3D environment increased the efficiency of reprogramming by ~ 4-fold, indicated by the percentage of cells positive for cTnT or α-actinin. Li et al. concluded that 3D hydrogel culture enhanced reprogramming efficiency by increasing the expression of matrix metalloproteinases (MMPs) [73]. Expression of miRcombo in adult human cardiac fibroblasts (AHCFs) induced ~11% of cells positive for cTnT on day 15 [75]. In addition, a fraction of iCMs (~38%) displayed spontaneous Ca2+ oscillations. ECM proteins extracted from human cardiac tissue improved the maturation of iPSC-CMs cultured in 3D hydrogels [76]. The addition of cardiac ECM proteins from AHCFs into 3D fibrin hydrogel (3D BM hydrogel) enhanced the efficiency of miRcombo-mediated reprogramming AHCFs into iCMs [77]. Culturing of AHCFs overexpressing miRcombo in 3D BM hydrogels increased the percentage of cTnT-positive cells and cells exhibiting Ca2+ transients. Functional parameters (e.g., spontaneous contraction and action potentials) of miRcombo-iCMs cultured in 3D remain elusive [75,77].
A combination MAB containing a single microRNA miR-208b-3p, ascorbic acid, and BMP4 simultaneously reprogram mouse tail-tip fibroblasts (TTFs) into three types of cardiac cells, functional CM-like cells (rCMs), endothelial-like cells (rECs), and smooth muscle-like cells (rSMCs) [30]. These cells assemble cardiac tissue-like structures (rCVT) in the culture dish. After MAB treatment, spontaneously beating cells were observed between day 4 and day 6. rCMs display myocyte sarcomeric structures and slow Ca2+ transients. However, rCMs are physiologically immature, indicated by nodal-like action potentials. rCVT is composed of approximately 17% rCMs, 28% rECs, and 19% rSMCs. rECs and rSMCs form vessel-like structures in rCVT. After permanent ligation of LAD, rCVT was transplanted to cover the heart's left ventricular anterior wall and apex. Reprogrammed cardiac cells migrated into damaged myocardium and contributed to neovascularization in the central and border zones of MI hearts. Migrated rCMs undergo maturation in the infarct heart over time. Engrafted rCVT enhanced cardiac function and decreased cardiac fibrosis after MI without triggering significant arrhythmias [30]. This study [30] demonstrates numerous benefits of transplantation of cardiovascular tissue-like structures reprogrammed from fibroblasts, including, but not limited to, increased survival of reprogrammed cells, simultaneous promotion of cardiomyogenesis and neovascularization, providing scaffold support, and activation of paracrine signaling to facilitate heart regeneration. In situ reprogramming of CFs into rCVT for cardiac regeneration needs to be examined.
• Cardiac lineage-specific transcription factor-mediated cardiac reprogramming
Overexpression of a single transcription factor MyoD reprograms fibroblasts into skeletal muscle cells [31-33], inspiring the search for transcription factors that can reprogram fibroblasts into CMs. By screening a pool of 14 transcription factors essential for cardiac development, Ieda et al. identified a combination of 3 transcription factors, Gata4, Mef2c, and Tbx5 (GMT) that reprogrammed mouse fibroblasts into iCMs [26]. Overexpression of GMT in neonatal cardiac fibroblasts isolated from αMHC-GFP transgenic mice converted ~4% of cells into iCMs positive for both αMHC-GFP and cTnT by one week without passing through a stem cell stage. After 2-4 weeks of culture, 30% of iCMs exhibited neonatal CM-like Ca2+ oscillations with variable frequency. A fraction of iCMs showed spontaneous contraction 4-5 weeks past-overexpression of GMT. Overall, GMT-induced iCMs are similar to neonatal CMs in global gene expression, epigenetic patterns, and electrical-physiological properties [26]. Some CFs transduced with GMT trans-differentiated into iCMs in the heart within two weeks after transplantation [26].
Qian et al. used a mouse reporter line Postn-Cre/R26R-LacZ wherein expression of β-galactosidase is controlled by the promoter of the fibroblast-enriched periostin (Postn) gene [78] to examine whether GMT reprogrammed CFs into iCMs in vivo [28]. Injection of retroviral particles carrying GMT into border zones of infarct hearts leads to the generation of ~30% βGal+ iCMs at the border zone. Around 50% of iCMs were similar to ventricular CMs in action potential properties. Injection of GMT also improved cardiac function and decreased fibrosis post-MI [28]. GMT reprogramed ~3% of adult TTFs into iCMs positive for both αMHC-GFP and cTnT by one week. The addition of Hand2 (GHMT) reprogramed ~7% of adult CFs and ~9% of adult TTFs into αMHC-GFP+ and cTnT+ double-positive iCMs [27]. Less than 1% of GHMT-induced iCMs display spontaneous contraction, Ca2+ transients, and spontaneous action potentials. In the heart of Tcf21-iCre/R26R-tdTomato mouse line, tdTomato is enriched in CFs with less expression in endothelial cells [79]. After retrovirus particles carrying GHMT were injected into border zones of Tcf21-iCre/R26R-tdTomato mice post-MI, GHMT-induced tdTomato+ iCMs showed similarities to endogenous CMs in gene expression, morphology, and electrical physiology. Expression of GHMT in infarct hearts improved cardiac function and reduced cardiac fibrosis [27]. Recently, transcription factor Ascl1 functioned as a pioneer factor to replace Gata4 and Tbx5 in GMT to generate more mature mouse iCMs [80], indicating that optimizing factor combinations represents an approach to enhance cardiac reprogramming.
GMT or GHMT only reprograms a small fraction of mouse fibroblasts into functional iCMs [26,27]. Since then, many efforts have focused on improving the efficiency of cardiac reprogramming by optimizing delivery methods and targeting various cell signaling pathways and epigenetic modifications. Reprogramming factors can be expressed in fibroblasts at much higher levels by nonintegrating Sendai virus (SeV) vectors, compared to retroviral vectors [81]. Consequently, SeV-GMT more efficiently reprogramed mouse fibroblasts into functional iCMs in vitro and in vivo [81,82]. Inhibition of profibrotic signaling such as TGFβ or ROCK pathway increased the efficiency of reprogramming MEFs into iCMs induced by GHMT or GHMT plus Nkx2.5 by > 3-fold [83-85]. Inhibition of the TGFβ pathway also enhanced GMT-mediated cardiac reprogramming in vitro and in vivo [86]. A combination of FGF2, FGF10, and VEGF increased the number of beating iCMs reprogrammed from MEFs by ~ 20-fold, further enhanced by Wnt inhibition [87]. Retinoic acid (RA) treatment combined with inhibition of histone deacetylases (HDAC) and Wnt signaling increased cardiac gene expression in rat cardiac fibroblasts induced by GMT [88].
Overexpression of constitutively active AKT1 increases GHMT (AGHMT)-mediated reprogramming of MEFs, CFs, and TTFs into beating iCMs. Activation of AKT signaling induced ~50% beating cells reprogrammed from MEFs by GHMT [89]. Suppressing inflammation signaling such as TNFα and NF-κB pathways, Cyclooxygenase-2 (COX-2) enhanced cardiac reprogramming induced by GHMT or GMT [90,91]. Cleavage of Notch by γ-Secretase, an intramembrane aspartyl protease, results in releasing and nuclear translocation of Notch intracellular domain (NICD). Nuclear NICD suppresses myogenesis by disrupting the ability of Mef2c to bind DNA [92]. Inhibition of γ-Secretase by DAPT increased Mef2c binding to promoters of TnnT2, Actc1, and Myh6 in fibroblasts expressing GHMT, thereby enhancing cardiac reprogramming [93].
Numerous studies have shown that macroautophagy is essential to maintain CM homeostasis, metabolism, survival, and function [94,95]. Atg5-dependent autophagy is critical for GMT-induced cardiac reprogramming [96]. However, the downregulation of Becn1, a component of the initial complex for autophagy, promoted GMT-mediated cardiac reprogramming in vitro and in vivo [96]. Thus, the roles of autophagy in cardiac reprogramming need further investigation.
Precise regulation of alternative splicing is required for cardiac reprogramming. Knockdown of a long non-coding RNA Trdn-as impaired the functional maturation of iCMs induced by GHMT via regulating alternative splicing of the Trdn gene that encodes a Ca2+ handling protein in CMs [97]. Knockdown of splicing factor Ptbp1 or Zrsr2, a component of U2 spliceosome, increased GMT-induced cardiac reprogramming, whereas knockdown of Sf3a1 and Sf3b1, members of U2 spliceosome impaired the reprogramming process [98,99]. Individual splicing factors may affect cardiac cell fate commitment by targeting distinguished molecules, which requires further investigation.
Epigenetic Repatterning is crucial for cell fate switch, which can be achieved via manipulating levels and behavior of epigenetic modification molecules. Epigenetic mechanisms in cardiac reprogramming have been comprehensively reviewed [100]. We briefly summarize recent advances here. Cell fate switch from fibroblasts to CMs must overcome suppressive epigenetic modifications at cardiac gene loci. For instance, inhibition of EZH2 (Enhancer Of Zeste 2 Polycomb Repressive Complex 2 Subunit) enhances cardiac reprogramming by reducing suppressive H3K27me3 (trimethylation of Lysine 27 on Histone H3) levels at cardiac gene loci in fibroblasts [101]. Chemical or genetic inhibition of the expression of Bmi1, a component of the polycomb group complex 1, reduces suppressive H2AK19ub (monoubiquitination of Lysine 119 on Histone H2A) and increases active H3K4me3 (trimethylation of Lysine 4 on Histone H3) at cardiac gene loci, thereby promoting cardiac reprogramming [102, 103].
Activation of profibrotic signaling pathways such as the TGFβ pathway inhibits cardiac reprogramming by altering the epigenetic landscape in fibroblasts. BRD4, a bromodomain and extraterminal (BET) family member, functions as a TGFβ effector and acetyl-lysine binding protein to facilitate fibrotic gene expression in CFs [104]. Dimethyl sulfoxide (DMSO) increased GHMT-mediated cardiac reprogramming, at least in part, via inhibiting histone acetyltransferase CBP/P300 bromodomain [105]. However, whether DMSO treatment alters the epigenetic landscape during cardiac reprogramming remains to be elucidated. The activation of TGFβ signaling causes the downstream effector Smad2 to compete with Gata4 to bind and recruit the Jumonji domain containing 3 (JMJD3 encoded by Lysine Demethylase 6B gene, KDM6B) to fibrotic gene loci to promote the removal of the transcriptional suppressor H3K27me3, which activates fibroblast gene expression and suppresses cardiac reprogramming [85]. Therefore, TGFβ inhibition facilitates the recruitment of JMJD3 to Gata4 binding sites to remove suppressive H3K27me3, thereby activating cardiac gene expression. In addition, JMJD3 also enhances the interaction between Gata4 and SWI/SNF chromatin remodeling complex to activate cardiac gene expression via an H3K27 demethylase activity-independent manner [85], suggesting that patterners of SWI/SNF chromatin remodeling complex have a potential to promote cardiac reprogramming. PHF7, a histone reader, increased AGHMT-induced cardiac reprogramming, as assessed by cardiac gene expression, the percentage of cells displaying Ca2+ flux, and the number of beating cells [106]. Through interaction with SWI/SNF complex, PHF7 increases chromatin accessibility around cardiac super enhancers and facilitates the recruitment of transcription factors to activate cardiac gene expression [106]. Understanding epigenetic mechanisms in response to cell signaling in cardiogenesis could further optimize cardiac reprogramming.
Substrate stiffness can change cell proliferation and differentiation [107,108]. Healthy myocardium is a soft tissue with an elastic modulus of 10-20 kPa in the transverse direction and 40-50 kPa in the longitudinal direction [109-111]. Culturing mouse fibroblasts overexpressing GHMT on 8 kPa hydrogels enhanced cardiac reprogramming, as shown in the percentage of cTnT+ cells, the percentage of αMHC-GFP+ cells, and the number of beating iCMs after four weeks [112]. A cell can sense matrix stiffness via mechanotransduction signaling pathways, such as a pathway mediated by Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding domain (TAZ) [113-115]. Mechanistically, a soft matrix comparable to the native myocardium in stiffness suppresses Rho/ROCK and YAP/TAZ mechanotransduction signaling, which contributes to enhancing cardiac reprogramming mediated by GHMT [112]. This finding is consistent with a previous study wherein inhibition of the Rho/ROCK pathway promotes cardiac reprogramming induced by GHMT on the stiff polystyrene dish [84]. These findings suggest that manipulating the microenvironment and its mechanosensing signaling represents an approach to enhance cardiac reprogramming.
• Human cardiac reprogramming
Mouse fibroblasts are reprogrammed into functional iCMs by various methods [116,117]. However, reprogramming human fibroblasts into iCMs is inefficient and takes longer. A combination of six factors containing GATA4, HAND2, TBX5, Myocardin, miR-1, and miR-133 (GHTM2miR) activated cardiac gene expression in ~10% of transduced human neonatal and adult fibroblasts [118]. After being cultured for four weeks, ~10% of transduced fibroblasts exhibited Ca2+ transients in response to potassium chloride stimulation. A low percentage of human iCMs displayed spontaneous Ca2+ flux eight weeks post-infection. After being cultured for 11 weeks, a small fraction of iCMs reprogrammed from adult human CFs by GHTM2miR spontaneously beat. However, GHTM2miR-iCMs derived from neonatal HFFs and adult human dermal fibroblasts did not spontaneously contract [118]. In another study, seven factors containing GMT, ESRRG, MESP1, Myocardin, and ZFPM2 (GMTEMMZ) induced 10% of human fibroblasts positive for cTnT two weeks after transduction [119]. Electrical field stimulation triggered Ca2+ transients in ~20% of GMTEMMZ-iCMs cultured for four weeks. Spontaneous contractions have not been observed after a long time in culture [119]. Five factors containing GMT, MESP1, and Myocardin (GMTMM) activated cTnT expression in ~6% of human cardiac fibroblasts [120]. Only ~1% of transduced cells exhibited spontaneous Ca2+ oscillations. Spontaneously beating iCMs induced by GMTMM have not been observed, despite being cultured for a long time [120]. RA treatment alongside inhibition of HDACs and Wnt signaling induced ~25% of human fibroblasts transduced with six factors containing GHMT, Myocardin, and miR-590 (GHMTMm) positive for cTnT [88]. However, spontaneously beating GHMTMm-iCMs were not observed. More recently, overexpression of a factor cocktail containing GMT, miR-133, and TBX20 (GMT133T) in human H9-derived fibroblasts (H9Fs) induced ~20% of cells positive for αMHC or α-actinin [121]. About 40% of GMT133T-iCMs exhibited visible sarcomere structures. Ca2+ flux and action potentials were observed in a fraction of GMT133T-iCMs. GMT133T-iCMs did not spontaneously beat. When cocultured with spontaneously contracting hiPSC-CMs, ~30% of GMT133T-iCMs spontaneously contracted [121]. Thus, reprogramming human fibroblasts into iCMs is relatively inefficient. The reprogramming approach needs to be optimized to efficiently obtain human iCMs showing mature functional phenotypes in bona fide human CMs, such as mature Ca2+ transients and action potentials.
Reprogramming fibroblasts into induced cardiac progenitor cells
Compared to terminally differentiated iCMs, cardiac progenitor cells (CPCs) are more scalable due to their self-renewal capacity. Furthermore, CPCs can be purified based on CPC markers, such as insulin gene enhancer protein 1 (Isl1), fetal liver kinase 1 (Flk-1), platelet-derived growth factor receptor (PdgfR) α, stage-specific embryonic antigen 1 (SSEA-1), and NK2 homeobox 5 (Nkx2.5) [122-125]. In addition, CPCs can differentiate into three types of cardiac-lineage cells (i.e., CMs, ECs, and SMCs), which are necessary to regenerate functional myocardium containing CMs and vessels. Thus, CPCs likely provide effective therapeutic applications because of their ability to differentiate into multiple cardiac lineages required for the complete regeneration of a damaged heart.
Menasche et al. differentiated human ESCs into CPCs using a cocktail containing bone morphogenetic protein 2 (BMP2) and SU5402 (an inhibitor of fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) receptor tyrosine kinases) [126]. Following differentiation, ISL1+/SSEA-1+ CPCs were purified and incorporated into a fibrin-thrombin patch. The CPC-loaded patch was transplanted onto the infarct area under the epicardial layer in a 68-year-old patient with severe heart failure. Three months later, the patient's left ventricular ejection fraction (LVEF) was increased from 26% to 36%. The onset of contraction of the infarct area was also noticed [126]. Later, the transplantation of human ESC-derived ISL1+/SSEA-1+ CPC-patch onto six patients with heart failure demonstrated short- and medium-term safety of CPC-loaded patches and symptomatic improvement [127]. These small clinical studies show promising clinical applications of CPCs to repair a damaged heart.
• Reprogramming of fibroblasts into induced CPCs by transcription factors
Overexpression of v-ets erythroblastosis virus E26 oncogene homolog 2 (ETS2) and mesoderm posterior BHLH transcription factor 1 (MESP1) in human dermal fibroblasts induced CPC-like phenotype [128]. However, differential potential and the impact of these CPC-like cells on heart regeneration have not been explored in this study [128].
From a pool of 22 genes encoding cardiac transcription factors, cardiac chromatin remodeling factors, and pluripotent transcription factors, Lalit et al. identified a combination of five transcription factors (MTGNB) that converted fibroblasts into iCPCs [29]. MTGNB containing Mesp1, Tbx5, Gata4, Nkx2.5, and Baf60c reprogramed adult CFs into NKX2.5-EYFP+ iCPCs at an efficiency of 7 colonies per 50,000 cells [29]. The Nkx2.5-EYFP was activated within 5 days after transduction. However, it took 40 days for iCPCs to reach a stable reprogramming state. These iCPCs could expand for up to 30 passages in the presence of BIO (6-bromoindirubin-3’-oxime, a canonical Wnt activator) and LIF (leukemia inhibitory factor to maintain pluripotency of mouse ESCs). Expanded iCPCs stably expressed key transcription factors, such as Nkx2.5, Tbx5, Mesp1, and Mef2c. iCPCs differentiated into three types of cardiac lineage cells, CMs, ECs, and SMCs In vitro. iCPCs also differentiated into CMs after being injected into the cardiac crescent of mouse embryos. However, it is unclear whether iCPCs can differentiate into ECs and SMCs in mouse embryos. iCPCs regenerated CMs, ECs, and SMCs in the MI-heart after injecting 1-1.5 million iCPCs into the border zone. Transplantation of iCPCs increased the survival rate of mice subjected to MI injury. Tumorigenesis was not observed during the four weeks of the study. However, the impact of engrafted iCPCs on cardiac function and remodeling remains elusive [29].
To avoid the detrimental effect of gene overexpression, Jiang et al. activated three cardiac transcription factor genes, Gata4, Nkx2.5, and Tbx5 in mouse TTFs utilizing an approach of CRISPRa (clustered regularly interspaced short palindromic repeats activation) [129]. CRISPRa-mediated activation of Gata4, Nkx2.5, and Tbx5 resulted in the expression of the reporter Nkx2.5-eGFP in over 80% of fibroblasts [129]. Nkx2.5-eGFP+ iCPCs maintained stemness and self-renewal for up to 20 passages in a defined medium containing LIF, basic FGF (bFGF), SB431542 (an inhibitor of TGFβ signaling), and ChIR99021 (an activator of Wnt signaling). Nkx2.5-eGFP+ iCPCs differentiated into αSMA+/SM-MHC+ SMCs, functional CD31+/vWF+ ECs, and CMs. iCPC-derived CMs exhibited Ca2+ transients and action potentials [129]. Around 60% of CMs showed ventricular-specific action potentials. iCPCs injected into the infarct heart differentiated into CMs, vascular ECs, and SMCs, indicating their capacity for cardiomyogenesis and angiogenesis in vivo. Engrafted iCPCs reduced cardiac remodeling and improved cardiac function [129]. However, only a few transplanted cells (<1%) survived in the heart 1-week post-injection [129].
• Reprogramming of fibroblasts into induced CPCs by small molecules
By modifying signaling pathways involved in cardiogenesis and heart development, Zhang et al. developed chemically defined conditions to reprogram fibroblasts into self-renewal iCPCs [130]. MEFs transiently expressing Oct4, Sox2, and Klf4 were cultured in a LIF-free medium containing JI1 for six days and in the medium supplemented with the Wnt activator ChIR99021 for another two days. Then, cells were continuously cultured in a medium containing BMP4, Activin A, ChIR99021, and SU5402. By one week later, most cells (> 70%) expressed the proliferative marker Ki-67 and CPC markers, Nkx2.5, Isl1, Gata4, Mef2c, Tbx5, Flk1, and PdgfRα, but did not express CM-specific genes (e.g., Tnnt2 and Myh6). Flk1+/PdgfRα+ iCPCs spontaneously differentiated into functional cardiovascular lineages (cTnT+/cTnI+ CMs, CD31+/VE-cadherin+ ECs, and αSMA+/calponin+ SMCs) and could be expanded for more than 18 passages without losing their cardiovascular stemness [130]. Transplanting iCPCs into the infarct mouse heart after LAD ligation promoted remuscularization and revascularization in damaged myocardium [130]. Engrafted iCPCs increased left ventricular ejection fraction and reduced cardiac remodeling over three months [130], indicating their regenerative impact on the damaged heart.
Reprogramming mouse and human fibroblasts into iCPC can be achieved via purely chemical treatment [131]. After being treated with six small molecules containing ChIR99021 (an activator of Wnt signaling), A83-01 (an inhibitor of TGFβ signaling), GSK126 (an inhibitor of Ezh2 histone methyltransferase), Forskolin (an adenylyl cyclase activator), CTPB (an activator of P300 histone acetyltransferase), and AM580 (a RARα activator) for eight days, and then cultured in a cardiogenic medium BACS (BMP4, Activin A, ChIR99021, and SU5402) identified previously [126], fibroblasts adopted a CPC cell fate. These chemically induced iCPCs (ciCPCs) differentiated into functional CMs, ECs, and SMCs in vitro [131]. In addition, Flk1+/PdgfRα+ ciCPC colonies picked on day 14 expanded for more than 20 passages without losing self-renewal and cardiovascular stemness. One million ciCPCs were intramyocardially injected into the infarcted heart after permanent ligation of LAD [131]. Two weeks post-transplantation, ciCPCs differentiated into CMs, ECs, and SMCs in the heart [131]. Transplanting ciCPCs into MI hearts improved cardiac function, decreased cardiac remodeling, and increased animal survival rate. Approximately 12% of transplanted ciCPCs survived in the infarct heart four weeks post-transplantation [131].
These studies [29, 129-131] demonstrate that the cardiac benefits of engrafted iCPCs are likely contributed by their capacity to promote myocardial regeneration, facilitate their autocrine/paracrine effects on angiogenesis, and enhance CM proliferation/survival. Even though the retention rate of engrafted iCPCs remains low, their regenerative impact on the damaged heart is apparent [129, 131]. Studies focusing on improving the retention rate have the potential to further boost the regenerative capacity of iCPCs in the heart.
Conclusion and Perspectives
Advances in cellular reprogramming make it possible to apply precision medicine approaches for heart regeneration. Patient-specific iPSC-CMs, iCPCs, iCMs, and rCVT derived from a patient’s fibroblasts can avoid immune rejection after they repopulate in the damaged heart. There are several ongoing clinical trials using human iPSC-CMs to treat heart failure (NCT04945018 and NCT04396899) or ischemic cardiomyopathy/heart failure (NCT04696328 and NCT05566600). The other reprogramming-based strategies are still at the stage of pre-clinical studies. The regenerative potential of iCPCs, iCMs, and rCVT needs to be examined in large animal models before they are translated into clinical applications. The potential challenges of these regenerative approaches are summarized in Figure 1.
Pre-clinical studies demonstrate that transplantation of PSC-CMs, iCPCs, and rCVT has the potential to regenerate the damaged heart [29, 30, 45, 129-131. However, only a small portion of engrafted cells can survive in the myocardium after transplantation [23,129,131,133], negatively impacting the therapeutic effects. For example, less than 1% of transplanted iCPCs could survive in the mouse heart after 1-week post-injection [129]. The retention of transplanted cells is affected by many factors, including but not limited to immune rejection, transplantation approaches, and receptors with acute or chronic MI. Utilizing patient-specific cardiac cells generated by cellular reprogramming approaches in combination with engineered biomaterials and pro-survival cocktails may resolve this issue. In addition, allogenic transplantation of iPSC-CMs and immaturity of engrafted CMs may lead to ventricular arrhythmias, which is common after non-human primates receive human PSC-CMs [45-47]. Pre-clinical studies focusing on autologous transplantation of cardiac cells and a combination with anti-arrhythmias drugs may provide new insights into preventing cardiac arrhythmias.
Compared to cell-based therapy, in situ cardiac reprogramming is a relatively new approach to heart repair. The efficacy of the method for heart regeneration is affected by many factors, including but not limited to the delivery methods for reprogramming factors, remuscularization and revascularization efficiency, and the microenvironment of damaged myocardium.
Therapeutic methods used to express reprogramming factors in the heart must meet the following criteria: (1) reprogramming factors can be efficiently and specifically expressed in CFs in injured myocardium, and (2) the delivery method is safe for humans. Lentiviral, retroviral, and adenoviral vectors have been used to express reprogramming factors in ischemic myocardium [27, 28, 69, 134, 135]. However, these viral vectors are toxic and cannot be utilized for clinical application. AAV-based vectors have emerged as a safe and effective delivery method for gene therapy [136]. Overexpression of GMT plus thymosin β4 using AAV-DJ in ischemic myocardium improved heart repair [137]. Although AAV vectors are safe for humans, identifying CF-specific AAV serotypes needs further investigation [138]. Francisco et al. used AAV-DJ/8 to express YAP driven by TCF21 promoter in CFs [139], which provides a safe platform for in situ cardiac reprogramming of CFs using CF-specific promoters. The efficiency of cardiac reprogramming correlates with high expression levels of reprogramming factors in fibroblasts [81]. Sendai viral vectors lead to higher expression of GMT and consequently induce higher efficiency of cardiac reprogramming and heart repair in vitro and in vivo, compared to retroviral vectors [81]. Sendai viral vector could be a good delivery tool for in situ reprogramming due to its safety in humans [140]. The success of modified mRNA (modRNA)-based vaccine in humans [141] raises the question of whether modRNA is robust for the expression of reprogramming factors in vivo. After LAD ligation in mice, Kaur et al. expressed GHMT with dominant negative (DN)-TGFβ, DN-Wnt8a, and acid ceramidase in the infarct zone using the 7G-modRNA platform [142]. 7G-modRNA-mediated expression of reprogramming factors induced angiogenesis and CM-like cells in the scar area, decreased cardiac fibrosis, improved cardiac function and long-term survival [142]. The modRNA-based delivery platform to achieve the highest regenerative capacity needs to be further optimized in large animal models.
One MI can cause the loss of billions of CMs in humans. Regeneration of a large amount of CMs requires high efficiency in reprogramming CFs into iCMs. However, the current approach in reprogramming human fibroblasts into iCMs in vitro remains inefficient, assessed by immaturity in gene expression, Ca2+ handling, action potentials, and contractile behavior [118-121]. Previous studies indicate that manipulating signaling pathways involved in cardiogenesis greatly enhances the reprogramming of mouse fibroblasts into cardiac lineages, including rCVT and iCMs [30, 116]. Future studies to gain mechanistic insights into human heart development and to determine reprogramming barriers will assist with increasing the efficiency of reprogramming human fibroblasts into cardiac lineages. In addition, the microenvironment should also be considered for optimizing human cardiac reprogramming. A culture condition (e.g., 3D culture and soft substrate) mimics the microenvironment in the native myocardium, which favors cardiac differentiation and CM maturation in vitro [77,116,143]. Unfortunately, adverse conditions such as MI dramatically alter the myocardial microenvironment, e.g., mechanical properties, the composition of soluble factors, the activation of profibrotic signaling pathways, the structure and composition of ECM, and the composition of cell types and their cell-cell communication. These changes in the myocardial microenvironment automatically inhibit cardiac reprogramming [84, 112, 116, 144]. Future studies revealing mechanisms and signaling pathways in the control of changes in the microenvironment in the damaged heart will allow discovering approaches to create a normal heart-like microenvironment, which benefits in situ reprogramming and regeneration.
Highlights.
Cellular reprogramming of fibroblasts to generate cardiac cells
Fibroblast-derived cardiac cells have the potential to regenerate a heart
Limitations and challenges in the reprogramming approach for heart repair
Acknowledgments:
This work was supported by NIH R01HL133230 and R01HL159086.
Footnotes
Declaration of Interests
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. , Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association, Circulation 145(8) (2022) e153–e639. [DOI] [PubMed] [Google Scholar]
- [2].Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. , Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study, J Am Coll Cardiol 76(25) (2020) 2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tallquist MD, Molkentin JD, Redefining the identity of cardiac fibroblasts, Nat Rev Cardiol 14(8) (2017) 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang H, Ren L, Shivnaraine RV, Targeting GPCRs to treat cardiac fibrosis, Front Cardiovasc Med 9 (2022) 1011176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vunjak-Novakovic G, Lui KO, Tandon N, Chien KR, Bioengineering heart muscle: a paradigm for regenerative medicine, Annu Rev Biomed Eng 13 (2011) 245–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ, Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis, Eur J Heart Fail 21(11) (2019) 1306–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. , Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015, J Am Coll Cardiol 70(1) (2017) 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sadek H, Olson EN, Toward the Goal of Human Heart Regeneration, Cell Stem Cell 26(1) (2020) 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, et al. , Evidence for cardiomyocyte renewal in humans, Science 324(5923) (2009) 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, et al. , Dynamics of Cell Generation and Turnover in the Human Heart, Cell 161(7) (2015) 1566–75. [DOI] [PubMed] [Google Scholar]
- [11].Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, et al. , Mammalian heart renewal by pre-existing cardiomyocytes, Nature 493(7432) (2013) 433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R, Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice, Proc Natl Acad Sci U S A 111(24) (2014) 8850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA, Transient regenerative potential of the neonatal mouse heart, Science 331(6020) (2011) 1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, et al. , Functional screening identifies miRNAs inducing cardiac regeneration, Nature 492(7429) (2012) 376–81. [DOI] [PubMed] [Google Scholar]
- [15].Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, et al. , MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs, Nature 569(7756) (2019) 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, et al. , Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size, Science 332(6028) (2011) 458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, et al. , A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice, Sci Transl Med 7(279) (2015) 279ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morikawa Y, Heallen T, Leach J, Xiao Y, Martin JF, Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation, Nature 547(7662) (2017) 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang J, Liu S, Heallen T, Martin JF, The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration, Nat Rev Cardiol 15(11) (2018) 672–684. [DOI] [PubMed] [Google Scholar]
- [20].Liu S, Li K, Wagner Florencio L, Tang L, Heallen TR, Leach JP, et al. , Gene therapy knockdown of Hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction, Sci Transl Med 13(600) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang S, Liu S, Leach JP, Li K, Perin EC, Martin JF, Gene Therapy Knockdown of Hippo Signaling Resolves Arrhythmic Events in Pigs After Myocardial Infarction, Circulation 146(20) (2022) 1558–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E, Cochrane Corner: stem cell therapy for chronic ischaemic heart disease and congestive heart failure, Heart 104(1) (2018) 8–10. [DOI] [PubMed] [Google Scholar]
- [23].Menasche P, Cell therapy trials for heart regeneration - lessons learned and future directions, Nat Rev Cardiol 15(11) (2018) 659–671. [DOI] [PubMed] [Google Scholar]
- [24].Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell 126(4) (2006) 663–76. [DOI] [PubMed] [Google Scholar]
- [25].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. , Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell 131(5) (2007) 861–72. [DOI] [PubMed] [Google Scholar]
- [26].Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. , Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors, Cell 142(3) (2010) 375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, et al. , Heart repair by reprogramming non-myocytes with cardiac transcription factors, Nature 485(7400) (2012) 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, et al. , In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes, Nature 485(7400) (2012) 593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lalit PA, Salick MR, Nelson DO, Squirrell JM, Shafer CM, Patel NG, et al. , Lineage Reprogramming of Fibroblasts into Proliferative Induced Cardiac Progenitor Cells by Defined Factors, Cell Stem Cell 18(3) (2016) 354–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cho J, Kim S, Lee H, Rah W, Cho HC, Kim NK, et al. , Regeneration of infarcted mouse hearts by cardiovascular tissue formed via the direct reprogramming of mouse fibroblasts, Nat Biomed Eng 5(8) (2021) 880–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Davis RL, Weintraub H, Lassar AB, Expression of a single transfected cDNA converts fibroblasts to myoblasts, Cell 51(6) (1987) 987–1000. [DOI] [PubMed] [Google Scholar]
- [32].Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, et al. , Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD, Proc Natl Acad Sci U S A 86(14) (1989) 5434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB, MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts, Science 242(4877) (1988) 405–11. [DOI] [PubMed] [Google Scholar]
- [34].Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M, Direct conversion of fibroblasts to functional neurons by defined factors, Nature 463(7284) (2010) 1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, et al. , Direct reprogramming of human fibroblasts to functional and expandable hepatocytes, Cell Stem Cell 14(3) (2014) 370–84. [DOI] [PubMed] [Google Scholar]
- [36].Rosa FF, Pires CF, Kurochkin I, Ferreira AG, Gomes AM, Palma LG, et al. , Direct reprogramming of fibroblasts into antigen-presenting dendritic cells, Sci Immunol 3(30) (2018). [DOI] [PubMed] [Google Scholar]
- [37].Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. , Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling, Proc Natl Acad Sci U S A 109(27) (2012) E1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, et al. , Chemically defined generation of human cardiomyocytes, Nat Methods 11(8) (2014) 855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, et al. , Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts, J Am Coll Cardiol 50(19) (2007) 1884–93. [DOI] [PubMed] [Google Scholar]
- [40].van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, et al. , Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction, Stem Cell Res 1(1) (2007) 9–24. [DOI] [PubMed] [Google Scholar]
- [41].Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, et al. , Formation of human myocardium in the rat heart from human embryonic stem cells, Am J Pathol 167(3) (2005) 663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ, Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview, Circ Res 111(3) (2012) 344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, et al. , Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes, Cell Stem Cell 12(1) (2013) 127–37. [DOI] [PubMed] [Google Scholar]
- [44].Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, et al. , Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts, Nature 510(7504) (2014) 273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, et al. , Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates, Nat Biotechnol 36(7) (2018) 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, et al. , Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts, Nature 538(7625) (2016) 388–391. [DOI] [PubMed] [Google Scholar]
- [47].Bach FH, Bach ML, Sondel PM, Differential function of major histocompatibility complex antigens in T-lymphocyte activation, Nature 259(5541) (1976) 273–81. [DOI] [PubMed] [Google Scholar]
- [48].Petersdorf EW, The major histocompatibility complex: a model for understanding graft-versus-host disease, Blood 122(11) (2013) 1863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li J, Hu S, Zhu D, Huang K, Mei X, Lopez de Juan Abad B, et al. , All Roads Lead to Rome (the Heart): Cell Retention and Outcomes From Various Delivery Routes of Cell Therapy Products to the Heart, J Am Heart Assoc 10(8) (2021) e020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Silver SE, Barrs RW, Mei Y, Transplantation of Human Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Regenerative Therapy, Front Cardiovasc Med 8 (2021) 707890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, et al. , Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction, Nat Commun 10(1) (2019) 3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, et al. , Revisiting Cardiac Cellular Composition, Circ Res 118(3) (2016) 400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Davis J, Molkentin JD, Myofibroblasts: trust your heart and let fate decide, J Mol Cell Cardiol 70 (2014) 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gourdie RG, Dimmeler S, Kohl P, Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease, Nat Rev Drug Discov 15(9) (2016) 620–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pesce M, Duda GN, Forte G, Girao H, Raya A, Roca-Cusachs P, et al. , Cardiac fibroblasts and mechanosensation in heart development, health and disease, Nat Rev Cardiol (2022). [DOI] [PubMed] [Google Scholar]
- [56].Stadtfeld M, Maherali N, Breault DT, Hochedlinger K, Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse, Cell Stem Cell 2(3) (2008) 230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Polo JM, Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM, et al. , A molecular roadmap of reprogramming somatic cells into iPS cells, Cell 151(7) (2012) 1617–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S, Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy, Nat Cell Biol 13(3) (2011) 215–22. [DOI] [PubMed] [Google Scholar]
- [59].Wang H, Cao N, Spencer CI, Nie B, Ma T, Xu T, et al. , Small molecules enable cardiac reprogramming of mouse fibroblasts with a single factor, Oct4, Cell Rep 6(5) (2014) 951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. , Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds, Science 341(6146) (2013) 651–4. [DOI] [PubMed] [Google Scholar]
- [61].Fu Y, Huang C, Xu X, Gu H, Ye Y, Jiang C, et al. , Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails, Cell Res 25(9) (2015) 1013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Huang C, Tu W, Fu Y, Wang J, Xie X, Chemical-induced cardiac reprogramming in vivo, Cell Res 28(6) (2018) 686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG, Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis, Am J Physiol Heart Circ Physiol 305(9) (2013) H1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cao N, Huang Y, Zheng J, Spencer CI, Zhang Y, Fu JD, et al. , Conversion of human fibroblasts into functional cardiomyocytes by small molecules, Science 352(6290) (2016) 1216–20. [DOI] [PubMed] [Google Scholar]
- [65].Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, et al. , Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency, Cell Stem Cell 8(4) (2011) 376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, et al. , Reprogramming of mouse and human cells to pluripotency using mature microRNAs, Cell Stem Cell 8(6) (2011) 633–8. [DOI] [PubMed] [Google Scholar]
- [67].Sandmaier SE, Telugu BP, MicroRNA-Mediated Reprogramming of Somatic Cells into Induced Pluripotent Stem Cells, Methods Mol Biol 1330 (2015) 29–36. [DOI] [PubMed] [Google Scholar]
- [68].Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, et al. , MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes, Circ Res 110(11) (2012) 1465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jayawardena TM, Finch EA, Zhang L, Zhang H, Hodgkinson CP, Pratt RE, et al. , MicroRNA induced cardiac reprogramming in vivo: evidence for mature cardiac myocytes and improved cardiac function, Circ Res 116(3) (2015) 418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liau B, Christoforou N, Leong KW, Bursac N, Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function, Biomaterials 32(35) (2011) 9180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Arhontoulis DC, Kerr CM, Richards D, Tjen K, Hyams N, Jones JA, et al. , Human cardiac organoids to model COVID-19 cytokine storm induced cardiac injuries, J Tissue Eng Regen Med 16(9) (2022) 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Swiatlowska P, Iskratsch T, Tools for studying and modulating (cardiac muscle) cell mechanics and mechanosensing across the scales, Biophys Rev 13(5) (2021) 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N, Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes, Biomaterials 34(23) (2013) 5813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li Y, Dal-Pra S, Mirotsou M, Jayawardena TM, Hodgkinson CP, Bursac N, et al. , Tissue-engineered 3-dimensional (3D) microenvironment enhances the direct reprogramming of fibroblasts into cardiomyocytes by microRNAs, Sci Rep 6 (2016) 38815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Paoletti C, Divieto C, Tarricone G, Di Meglio F, Nurzynska D, Chiono V, MicroRNA-Mediated Direct Reprogramming of Human Adult Fibroblasts Toward Cardiac Phenotype, Front Bioeng Biotechnol 8 (2020) 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Almeida HV, Tenreiro MF, Louro AF, Abecasis B, Santinha D, Calmeiro T, et al. , Human Extracellular-Matrix Functionalization of 3D hiPSC-Based Cardiac Tissues Improves Cardiomyocyte Maturation, ACS Appl Bio Mater 4(2) (2021) 1888–1899. [DOI] [PubMed] [Google Scholar]
- [77].Paoletti C, Marcello E, Melis ML, Divieto C, Nurzynska D, Chiono V, Cardiac Tissue-like 3D Microenvironment Enhances Route towards Human Fibroblast Direct Reprogramming into Induced Cardiomyocytes by microRNAs, Cells 11(5) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, et al. , Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload, J Clin Invest 120(1) (2010) 254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Acharya A, Baek ST, Banfi S, Eskiocak B, Tallquist MD, Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney, Genesis 49(11) (2011) 870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wang H, Keepers B, Qian Y, Xie Y, Colon M, Liu J, et al. , Cross-lineage potential of Ascl1 uncovered by comparing diverse reprogramming regulatomes, Cell Stem Cell 29(10) (2022) 1491–1504 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Miyamoto K, Akiyama M, Tamura F, Isomi M, Yamakawa H, Sadahiro T, et al. , Direct In Vivo Reprogramming with Sendai Virus Vectors Improves Cardiac Function after Myocardial Infarction, Cell Stem Cell 22(1) (2018) 91–103 e5. [DOI] [PubMed] [Google Scholar]
- [82].Isomi M, Sadahiro T, Fujita R, Abe Y, Yamada Y, Akiyama T, et al. , Direct reprogramming with Sendai virus vectors repaired infarct hearts at the chronic stage, Biochem Biophys Res Commun 560 (2021) 87–92. [DOI] [PubMed] [Google Scholar]
- [83].Ifkovits JL, Addis RC, Epstein JA, Gearhart JD, Inhibition of TGFbeta signaling increases direct conversion of fibroblasts to induced cardiomyocytes, PLoS One 9(2) (2014) e89678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhao Y, Londono P, Cao Y, Sharpe EJ, Proenza C, O'Rourke R, et al. , High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling, Nat Commun 6 (2015) 8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Riching AS, Danis E, Zhao Y, Cao Y, Chi C, Bagchi RA, et al. , Suppression of canonical TGF-beta signaling enables GATA4 to interact with H3K27me3 demethylase JMJD3 to promote cardiomyogenesis, J Mol Cell Cardiol 153 (2021) 44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mohamed TM, Stone NR, Berry EC, Radzinsky E, Huang Y, Pratt K, et al. , Chemical Enhancement of In Vitro and In Vivo Direct Cardiac Reprogramming, Circulation 135(10) (2017) 978–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yamakawa H, Muraoka N, Miyamoto K, Sadahiro T, Isomi M, Haginiwa S, et al. , Fibroblast Growth Factors and Vascular Endothelial Growth Factor Promote Cardiac Reprogramming under Defined Conditions, Stem Cell Reports 5(6) (2015) 1128–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Singh VP, Pinnamaneni JP, Pugazenthi A, Sanagasetti D, Mathison M, Wang K, et al. , Enhanced Generation of Induced Cardiomyocytes Using a Small-Molecule Cocktail to Overcome Barriers to Cardiac Cellular Reprogramming, J Am Heart Assoc 9(12) (2020) e015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhou H, Dickson ME, Kim MS, Bassel-Duby R, Olson EN, Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes, Proc Natl Acad Sci U S A 112(38) (2015) 11864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhou H, Morales MG, Hashimoto H, Dickson ME, Song K, Ye W, et al. , ZNF281 enhances cardiac reprogramming by modulating cardiac and inflammatory gene expression, Genes Dev 31(17) (2017) 1770–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Muraoka N, Nara K, Tamura F, Kojima H, Yamakawa H, Sadahiro T, et al. , Role of cyclooxygenase-2-mediated prostaglandin E2-prostaglandin E receptor 4 signaling in cardiac reprogramming, Nat Commun 10(1) (2019) 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wilson-Rawls J, Molkentin JD, Black BL, Olson EN, Activated notch inhibits myogenic activity of the MADS-Box transcription factor myocyte enhancer factor 2C, Mol Cell Biol 19(4) (1999) 2853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Abad M, Hashimoto H, Zhou H, Morales MG, Chen B, Bassel-Duby R, et al. , Notch Inhibition Enhances Cardiac Reprogramming by Increasing MEF2C Transcriptional Activity, Stem Cell Reports 8(3) (2017) 548–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lavandero S, Chiong M, Rothermel BA, Hill JA, Autophagy in cardiovascular biology, J Clin Invest 125(1) (2015) 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chi C, Leonard A, Knight WE, Beussman KM, Zhao Y, Cao Y, et al. , LAMP-2B regulates human cardiomyocyte function by mediating autophagosome-lysosome fusion, Proc Natl Acad Sci U S A 116(2) (2019) 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wang L, Ma H, Huang P, Xie Y, Near D, Wang H, et al. , Down-regulation of Beclin1 promotes direct cardiac reprogramming, Sci Transl Med 12(566) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zhao Y, Riching AS, Knight WE, Chi C, Broadwell LJ, Du Y, et al. , Cardiomyocyte-Specific Long Noncoding RNA Regulates Alternative Splicing of the Triadin Gene in the Heart, Circulation 146(9) (2022) 699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Liu Z, Wang L, Welch JD, Ma H, Zhou Y, Vaseghi HR, et al. , Single-cell transcriptomics reconstructs fate conversion from fibroblast to cardiomyocyte, Nature 551(7678) (2017) 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhou Y, Alimohamadi S, Wang L, Liu Z, Wall JB, Yin C, et al. , A Loss of Function Screen of Epigenetic Modifiers and Splicing Factors during Early Stage of Cardiac Reprogramming, Stem Cells Int 2018 (2018) 3814747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wang H, Yang Y, Liu J, Qian L, Direct cell reprogramming: approaches, mechanisms and progress, Nat Rev Mol Cell Biol 22(6) (2021) 410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tang Y, Zhao L, Yu X, Zhang J, Qian L, Jin J, et al. , Inhibition of EZH2 primes the cardiac gene activation via removal of epigenetic repression during human direct cardiac reprogramming, Stem Cell Res 53 (2021) 102365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhou Y, Wang L, Vaseghi HR, Liu Z, Lu R, Alimohamadi S, et al. , Bmi1 Is a Key Epigenetic Barrier to Direct Cardiac Reprogramming, Cell Stem Cell 18(3) (2016) 382–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Testa G, Russo M, Di Benedetto G, Barbato M, Parisi S, Pirozzi F, et al. , Bmi1 inhibitor PTC-209 promotes Chemically-induced Direct Cardiac Reprogramming of cardiac fibroblasts into cardiomyocytes, Sci Rep 10(1) (2020) 7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Stratton MS, Bagchi RA, Felisbino MB, Hirsch RA, Smith HE, Riching AS, et al. , Dynamic Chromatin Targeting of BRD4 Stimulates Cardiac Fibroblast Activation, Circ Res 125(7) (2019) 662–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lim CK, Efthymios M, Tan W, Autio MI, Tiang Z, Li PY, et al. , Dimethyl sulfoxide (DMSO) enhances direct cardiac reprogramming by inhibiting the bromodomain of coactivators CBP/p300, J Mol Cell Cardiol 160 (2021) 15–26. [DOI] [PubMed] [Google Scholar]
- [106].Garry GA, Bezprozvannaya S, Chen K, Zhou H, Hashimoto H, Morales MG, et al. , The histone reader PHF7 cooperates with the SWI/SNF complex at cardiac super enhancers to promote direct reprogramming, Nat Cell Biol 23(5) (2021) 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell 126(4) (2006) 677–89. [DOI] [PubMed] [Google Scholar]
- [108].Crowder SW, Leonardo V, Whittaker T, Papathanasiou P, Stevens MM, Material Cues as Potent Regulators of Epigenetics and Stem Cell Function, Cell Stem Cell 18(1) (2016) 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, et al. , Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance, Am J Physiol Heart Circ Physiol 290(6) (2006) H2196–203. [DOI] [PubMed] [Google Scholar]
- [110].Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, et al. , Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis, Nat Mater 15(6) (2016) 669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Montgomery M, Ahadian S, Davenport Huyer L, Lo Rito M, Civitarese RA, Vanderlaan RD, et al. , Flexible shape-memory scaffold for minimally invasive delivery of functional tissues, Nat Mater 16(10) (2017) 1038–1046. [DOI] [PubMed] [Google Scholar]
- [112].Kurotsu S, Sadahiro T, Fujita R, Tani H, Yamakawa H, Tamura F, et al. , Soft Matrix Promotes Cardiac Reprogramming via Inhibition of YAP/TAZ and Suppression of Fibroblast Signatures, Stem Cell Reports 15(3) (2020) 612–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. , Role of YAP/TAZ in mechanotransduction, Nature 474(7350) (2011) 179–83. [DOI] [PubMed] [Google Scholar]
- [114].Dupont S, Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction, Exp Cell Res 343(1) (2016) 42–53. [DOI] [PubMed] [Google Scholar]
- [115].Rausch V, Hansen CG, The Hippo Pathway, YAP/TAZ, and the Plasma Membrane, Trends Cell Biol 30(1) (2020) 32–48. [DOI] [PubMed] [Google Scholar]
- [116].Riching AS, Song K, Cardiac Regeneration: New Insights Into the Frontier of Ischemic Heart Failure Therapy, Front Bioeng Biotechnol 8 (2020) 637538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Garry GA, Bassel-Duby R, Olson EN, Direct reprogramming as a route to cardiac repair, Semin Cell Dev Biol 122 (2022) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, et al. , Reprogramming of human fibroblasts toward a cardiac fate, Proc Natl Acad Sci U S A 110(14) (2013) 5588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, et al. , Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state, Stem Cell Reports 1(3) (2013) 235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, et al. , Induction of human cardiomyocyte-like cells from fibroblasts by defined factors, Proc Natl Acad Sci U S A 110(31) (2013) 12667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Tang Y, Aryal S, Geng X, Zhou X, Fast VG, Zhang J, et al. , TBX20 Improves Contractility and Mitochondrial Function During Direct Human Cardiac Reprogramming, Circulation 146(20) (2022) 1518–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Burridge PW, Keller G, Gold JD, Wu JC, Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming, Cell Stem Cell 10(1) (2012) 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, et al. , Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification, Cell 127(6) (2006) 1151–65. [DOI] [PubMed] [Google Scholar]
- [124].Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, et al. , Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines, Cell Stem Cell 8(2) (2011) 228–40. [DOI] [PubMed] [Google Scholar]
- [125].Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. , Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages, Nature 433(7026) (2005) 647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, et al. , Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report, Eur Heart J 36(30) (2015) 2011–7. [DOI] [PubMed] [Google Scholar]
- [127].Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A, et al. , Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction, J Am Coll Cardiol 71(4) (2018) 429–438. [DOI] [PubMed] [Google Scholar]
- [128].Islas JF, Liu Y, Weng KC, Robertson MJ, Zhang S, Prejusa A, et al. , Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors, Proc Natl Acad Sci U S A 109(32) (2012) 13016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Jiang L, Liang J, Huang W, Ma J, Park KH, Wu Z, et al. , CRISPR activation of endogenous genes reprograms fibroblasts into cardiovascular progenitor cells for myocardial infarction therapy, Mol Ther 30(1) (2022) 54–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zhang Y, Cao N, Huang Y, Spencer CI, Fu JD, Yu C, et al. , Expandable Cardiovascular Progenitor Cells Reprogrammed from Fibroblasts, Cell Stem Cell 18(3) (2016) 368–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wang J, Gu S, Liu F, Chen Z, Xu H, Liu Z, et al. , Reprogramming of fibroblasts into expandable cardiovascular progenitor cells via small molecules in xeno-free conditions, Nat Biomed Eng 6(4) (2022) 403–420. [DOI] [PubMed] [Google Scholar]
- [132].Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, et al. , Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts, Nature 489(7415) (2012) 322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Penicka M, Lang O, Widimsky P, Kobylka P, Kozak T, Vanek T, et al. , One-day kinetics of myocardial engraftment after intracoronary injection of bone marrow mononuclear cells in patients with acute and chronic myocardial infarction, Heart 93(7) (2007) 837–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Inagawa K, Miyamoto K, Yamakawa H, Muraoka N, Sadahiro T, Umei T, et al. , Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5, Circ Res 111(9) (2012) 1147–56. [DOI] [PubMed] [Google Scholar]
- [135].Mathison M, Singh VP, Chiuchiolo MJ, Sanagasetti D, Mao Y, Patel VB, et al. , In situ reprogramming to transdifferentiate fibroblasts into cardiomyocytes using adenoviral vectors: Implications for clinical myocardial regeneration, J Thorac Cardiovasc Surg 153(2) (2017) 329–339 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kotterman MA, Schaffer DV, Engineering adeno-associated viruses for clinical gene therapy, Nat Rev Genet 15(7) (2014) 445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yoo SY, Jeong SN, Kang JI, Lee SW, Chimeric Adeno-Associated Virus-Mediated Cardiovascular Reprogramming for Ischemic Heart Disease, ACS Omega 3(5) (2018) 5918–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Liu Z, Klose K, Neuber S, Jiang M, Gossen M, Stamm C, Comparative analysis of adeno-associated virus serotypes for gene transfer in organotypic heart slices, J Transl Med 18(1) (2020) 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Francisco J, Zhang Y, Nakada Y, Jeong JI, Huang CY, Ivessa A, et al. , AAV-mediated YAP expression in cardiac fibroblasts promotes inflammation and increases fibrosis, Sci Rep 11(1) (2021) 10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Scaggs Huang F, Bernstein DI, Slobod KS, Portner A, Takimoto T, Russell CJ, et al. , Safety and immunogenicity of an intranasal sendai virus-based vaccine for human parainfluenza virus type I and respiratory syncytial virus (SeVRSV) in adults, Hum Vaccin Immunother 17(2) (2021) 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Chaudhary N, Weissman D, Whitehead KA, mRNA vaccines for infectious diseases: principles, delivery and clinical translation, Nat Rev Drug Discov 20(11) (2021) 817–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kaur K, Hadas Y, Kurian AA, Zak MM, Yoo J, Mahmood A, et al. , Direct reprogramming induces vascular regeneration post muscle ischemic injury, Mol Ther 29(10) (2021) 3042–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Branco MA, Cotovio JP, Rodrigues CAV, Vaz SH, Fernandes TG, Moreira LM, et al. , Transcriptomic analysis of 3D Cardiac Differentiation of Human Induced Pluripotent Stem Cells Reveals Faster Cardiomyocyte Maturation Compared to 2D Culture, Sci Rep 9(1) (2019) 9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Zhang Z, Zhang W, Blakes R, Sundby LJ, Shi Z, Rockey DC, et al. , Fibroblast fate determination during cardiac reprogramming by remodeling of actin filaments, Stem Cell Reports 17(7) (2022) 1604–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]



