Abstract
Background/Aim: There seems to be a correlation between changes in movement patterns with aging and brain activation. In the preparation and execution of movements, neural oscillations play an important role. In this study, cortical high frequency brain oscillations were analyzed in 15 healthy young adults and 15 elderly adults who participated in eye-hand coordination tasks.
Patients and Methods: The brain activities of healthy young and older adults were recorded using electroencephalography (EEG).
Results: Elderly participants spent significantly more time completing the task than young participants. During eye-hand coordination in elderly groups, beta power decreased significantly in the central midline and parietal brain regions. The data suggest that healthy elderly subjects had intact cognitive performance, but relatively poor eye-hand coordination associated with loss of beta brain oscillation in the central midline and parietal cortex and reduced ability to attentional movement.
Conclusion: Beta frequency in the parietal brain sites may contribute to attentional movement. This could be an important method for monitoring cognitive brain function changes as the brain ages.
Keywords: Parietal cortex, beta, gamma, healthy elderly adult, attentional movement task
Physical degeneration and cognitive declines are common among the elderly. Furthermore, as people get older, their health begins to deteriorate, particularly motor skills (1-3), cognitive function (4,5) and attention (5,6). Previously, conclusive findings have been reported that body reaction, movement control, tapping speed and coordination of hands and feet were progressively decreased after 50 years old (7). These findings implied that motor control is clearly affected with increasing age. In particular, progression of age strongly affected motor skills and fine movement (8). The increased brain activities during task performance are associated with age particularly in the rostral ventral premotor cortex, intraparietal sulcus, caudal dorsal premotor cortex, caudal cingulate sulcus, intraparietal sulcus, insula, frontal operculum, and cerebellar vermis (9). Neural activities in cortical and subcortical regions during task performance were different between young and elderly people. This means that motor activities are regulated by multiple brain areas. The study on brain activity found that older participants had lower delta and theta frequency bands, as well as lower alpha frequency, while beta frequency increased. Furthermore, global activity in all bands decreased when the eyes were opened, but there was no difference between young and old people (10). Previous research discovered that the beta frequency of brain activity changes with increasing age (11). According to this study, neural synchronization of electroencephalography (EEG) in aging was maintained during rest with baseline visual perception. In addition, a previous study reported that alpha wave activity decreased in association with aging and age-related neuropathology (12).
Attention has been extensively studied as an essential function of the cognitive process in terms of learning and memory. It participates in the implementation of cognitive control as needed to reduce uncertainty in the learning context (13). For example, goal-directed attentional systems encourage motor performance efficiency (14). Selective attention has an impact on memory processing because it modulates the activity of cortical regions and stimulates hippocampus encoding mechanisms (15). Selective attention and memory performance were improved in people with moderate-intensity exercise (16). Furthermore, breath-focused yoga practice improved sustained attention (17). These findings appeared to confirm that healthiness is a factor that improves attentional performance. In general, there are correlations between attentional focus and motor skills. The influence of attention has a strong effect on movement execution and detection via motor cortex and supplementary motor area (18). Moreover, a focus on movement produced better motor performance (19). However, attentive function slowly decays with aging. Additionally, a previous research indicated that reaction times increase with age, meaning that elder people are slower at responding to stimuli (20). These findings suggest that attentiveness is declined in the elderly but can be enhanced through well-designed practices.
It is well known that cognitive function gradually declines and impacts daily performance. However, patterns of altered brain activity in older people during attentional performance remain to be clarified. Therefore, this study aimed to evaluate the responsiveness of brain activity in elders during goal-attentional motor performance using electroencephalography (EEG). In addition, comparing the EEG patterns of elderly and young people might explain the decline in physical performance efficiency and learning behavior.
Patients and Methods
Participants. Thirty healthy participants with right handedness (15 young healthy participants aged between 18-35 years and 15 elderly participants aged over 60 years) were included in this study. Young volunteers were recruited from Prince of Songkla University, Hat Yai, Thailand. Elderly subjects were members of the Center of Health Promotion and Rehabilitation of the elderly, Faculty of Nursing, Prince of Songkla University, Hat Yai, Thailand. Volunteers who had a history of epilepsy, post-stroke and neurological disorders were excluded. Prior to participating, each subject provided written informed consent, and the experimental protocol, which followed the Declaration of Helsinki, was approved by the Health Science Human Research Ethics Committee at Prince of Songkla University.
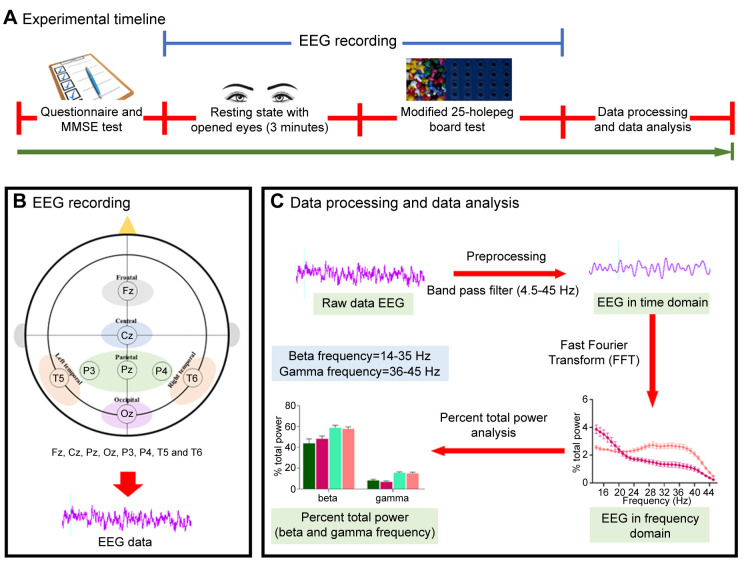
Procedures. A timeline of experimental procedures is shown in Figure 1A. Experimental protocols began with participants filling out the questionnaire to assess their health information and subjected to Mini-Mental State Examination (MMSE-Thai) to assess cognitive performance. The scalp of the participants was cleaned with alcohol around the electrode site as participants sat comfortably in a comfortable chair while wearing an EEG cap with eight electrodes (Fz, Cz, Pz, Oz, P3, P4, T5, and T6) fabricated by ANT Neuro Company (Enschelde, the Netherlands) according to the International 10/20 electrode placement system (Figure 1B). The EEG signals were then recorded for 3 minutes while the subjects were rested with their eyes open. Following that, participants were tested on their attentional movement and visual motor coordination by pinning a peg to a board. This experiment was created using small pegs (0.5 cm in diameter) and a 25-hole board. Subjects were instructed to pick and place pegs one by one into the holes on the board with their dominant hand as quickly as possible until all holes were filled. A simultaneous EEG recording was performed during the eye-hand coordination test. A record of the time taken to complete the eye-hand coordination test was measured.
Figure 1. A diagram of the experimental protocol. (A) Experimental timeline, (B) electroencephalography (EEG) recording and EEG electrode placement according to 10-20 international system, and (C) Data processing and data analysis.
EEG recording and data processing. EEG data were collected using the eego™ mini-series (ANT Neuro Company) and the ASAlab 4.10.2 acquisition system (Figure 1B). The electrode impedance was kept lower than 5 kΩ, the sampling rate was digitized at 2 kHz, and a band-pass filter of 4.5-45 Hz was used. A 50 Hz notch filter was applied to eliminate power line artifacts. After recording, the data were imported into LabChart software (ADInstruments, Bella Vista, NSW, Australia), and power spectral density (PSD) was generated by LabChart software using a Hanning window cosine with 50% window overlap and 2048 of FFT size. The Fast Fourier transform (FFT) algorithm was used for frequency power analysis. Power spectra were analyzed and divided into beta (14-35 Hz) and gamma (36-45 Hz) frequency ranges. EEG powers in each frequency band were averaged and expressed as a percentage of total power. Data processing and data analysis are shown in Figure 1C.
Statistical analysis. All data are expressed as mean±standard error of mean (S.E.M). For statistical analysis, the Thai-MMSE score and time to complete attentional movement time were recorded. The unpaired t-test was used to test for significant differences between two groups (young and elderly participants). The relationship between age and each activity was also examined using a simple linear regression test. Moreover, the influence of age on the EEG oscillations during resting state with eyes open and attentional movement test was analyzed using two-way repeated ANOVA followed by Tukey’s post hoc method for multiple comparisons. Statistical significance was accepted at p<0.05.
Results
Cognitive scores and eye-hand coordination. The average age of young healthy (7 men and 8 women) and elderly healthy (3 men and 12 women) subjects were 24.73±1.19 and 70.86±1.81 years, respectively. MMSE scores of young and elderly healthy participants were 29.20±0.23 and 28.67±0.22, respectively (Figure 2A). The simple linear regression between the scores and participants’ age did not show significant differences between age groups (Figure 2B). However, unpaired t-test analysis revealed the time to completion a modified 25-holepeg board test was dramatically different between the groups (t=4.590, df=28 and p<0.0001). Young participants completed the test in 57.60±3.35 s whereas the elderly subjects completed the test in 95.13±7.77 s (Figure 2C). Simple linear regression revealed the time increase to complete the task in elderly strongly related to their age [R2=0.5095, F(1,28)=29.08, p<0.0001] (Figure 2D).
Figure 2. Cognitive score and Modified 25-holepeg board test. (A) Violin plot of Thai- Mini-Mental State Examination (MMSE) score of young and elderly participants, (B) simple linear regression graph with age (x-axis) as the predictor variable and the Thai-MMSE score (Y-axis) as the dependent variable, (C) Box plot of the modified 25-holepeg board test completion time score, and (D) simple linear regression graph with age (xaxis) as the predictor variable and the time of completion (Y-axis) as the dependent variable. Data are expressed in mean±S.E.M, and unpaired ttest was used for statistical analysis (p<0.0001).
EEG patterns during resting state and attentional movement test. EEG signals of young and elderly participants were collected using Fz, Cz, Pz, Oz, P3, P4, T5 and T6 electrodes. EEG data were analyzed and are shown in the form of frequency range for each region. Figure 3 (A-H) shows the midline area (Fz, Cz, Pz, and Oz) of the percentage of total power during resting state and eye-hand coordination test in healthy young and elderly participants; meanwhile, Figure 4 (A-H) shows the activity of the right and left hemisphere of the parietal and temporal regions.
Figure 3. Percentage of total power of the electroencephalography (EEG) signal from the central midline during resting states and during task performance in young and elderly participants. Brain activities from the regions of Fz (A and E), Cz (B and F), Pz (C and G), and Oz (D and H) were recorded and analyzed. Data are expressed in mean±S.E.M.
Figure 4. Percentage of total power of the electroencephalography (EEG) signal in the left and right hemispheres during resting states and during task performance in young and elderly participants. Brain activities from the regions of P3 (A and E), P4 (B and F), T5 (C and G), and T6 (D and H) were recorded and analyzed. Data are expressed in mean±S.E.M.
A two-way repeated ANOVA revealed that there was significant interaction between age and percent total power in beta and gamma frequencies during rest and task performance in all brain regions including the frontal [Fz: F(3,112)=13.23, p<0.0001], central [Cz: F(3,112)=10.320, p<0.000], parietal [Pz: F(3,112)=19.73, p<0.0001, P3: F(3,112)=21.020, p<0.0001; P4: F(3,112)=17.180, p<0.0001], occipital [Oz: F(3,112)=18.370, p<0.0001], and temporal [T5: F(3,112)=18.930, p<0.0001; T6: F(3,112)=16.57, p<0.0001] brain regions (Figure 5). Multiple comparison analysis in the beta frequency band using a Tukey’s post-hoc test revealed that beta power in healthy elderly subjects showed significant increase during the eye-hand coordination test in Oz (p=0.0138), T5 (p=0.0143), and T6 (p=0.0098) in comparison to resting condition. Furthermore, a similar significant increase in beta-power activity was also found in healthy young participants in most brain regions, including the central [Cz (p=0.0009)], parietal [Pz (p<0.0001); P3 (p<0.0001); P4 (p<0.0001)], occipital [Oz (p<0.0001)], and temporal [T5 (p<0.0001); T6 (p<0.0001)] regions, except the frontal region (Fz). In addition, the activity of beta oscillation in the parietal area of the elderly was also significantly higher during the resting state than that of the young group [Pz (p=0.0119), P3 (p=0.0169) and P4 (p=0.0230)]. However, beta activity was not different between age groups during attentional movements. Tukey’s post hoc test also revealed the percent total powers of gamma frequency were significantly increased during a test session compared with the resting state (eyes open) in both age groups. During the attentional movement test, the gamma brain wave was significantly increased in Pz (p=0.0091), Oz (p=0.0234), P3 (p=0.0066), P4 (p=0.0140), T5 (p<0.0001), and T6 (p=0.0289) in younger group; Pz (p=0.0168), Oz (p=0.0028), P3 (p=0.0106), P4 (p=0.0143), T5 (p=0.0143), and T6 (p=0.0118) in the elderly group.
Figure 5. Beta and gamma band activities during the resting state and motor performance test in healthy young and aging participants. The data are shown for the following regions: Fz (A), Cz (B), Pz (C), Oz (D), P3 (E), P4 (F), T5 (G), and T6 (H). Data are expressed in mean±S.E.M, Twoway repeated ANOVA was used for statistical analysis followed by Tukey’s post hoc test (*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001).
Discussion
MMSE is a tool used worldwide to assess mental state and cognitive function (21). Basically, MMSE score below 24 indicates cognitive deficit (22). The present research demonstrated that MMSE scores of young and elderly healthy participants were higher than the mean score. Therefore, the healthy adult participants did not show cognitive decline. However, the present research found that the time to complete attentional eye-hand coordination test was significantly different between young and elderly volunteers. The elderly group took longer to finish the test. This finding is in agreement with that of a previous study that found less coordination and cognitive impairment in older people performing executive movements (23). In this study, the cognitive performance of elderly people remained intact, but motor functions declined. Cognitive and motor functions of the brain are affected differently through progressive brain degeneration in elderly people. Brain dysfunction caused by aging appears to promote several risk factors of cognitive decline (24). Acting in these areas is key to achieving “successful aging”. The degeneration process might affect the motor movement control even though the cognitive functions are fine.
A beta cortical rhythm was generated before movement and during motor execution (25,26) and during motor execution (25). Cortico-muscle network produced the synchronization of beta frequency in correlation with attention during sensorimotor performance (27). In addition, visual stimuli also provoked beta synchronization (28). Many performances including cognitive function, working memory or cognitive control seem to be enhanced by smart attention. Therefore, high quality of attention is likely to facilitate many activities in daily life. Correspondingly, beta power was produced during cognitive performance (29,30). Previously, many studies reported some of parietal cortex activity is associated with top-down attention (31-33). The parietal cortex is a region that projects information to several cortical and subcortical areas, especially sensory information. Moreover, the parietal region plays an important role in transforming sensory input into motor output and this region is associated with motor planning, which leads to motor performance (34).
The present study showed increases in cortical beta power in most regions during the test session that requires attentiveness in younger groups, except the frontal area. However, elderly participants showed significant differences only in the occipital and temporal regions. It can be suggested that in elderly participants neurons in the parietal and central regions might have started to deteriorate. There are many reports indicating that attentional motor movement is associated with cortical brain activity (35-37). A previous study revealed that the reflection of the sense of agency or sense of control, that is the feeling to control the action, is associated with parieto-occipital beta power (38). In addition, the processing of movement information is also controlled by the temporal brain region (39). Therefore, several brain regions are active during the attentional movement performance. The results of this study indicated the dominant activity of the cortical brain during attentional movement test in young participants. This discovery is in agreement with previous reports. Therefore, the data from the healthy young group was unsurprising; however, the activity of the central midline (Cz) and the parietal (Pz, P3, and P4) area in the elderly group was noteworthy because the central midline (Cz) and parietal (Pz, P3 and P4) regions did not show a significant increase in the elderly group. These results suggested that neural circuits that produce beta synchronization degenerate in the elderly, especially in the central midline (Cz) and parietal (Pz, P3, and P4) regions. These findings are consistent with a previous study that showed that the sensory motor processing was related with sensorimotor beta synchronization (37). Moreover, the midcentral brain region generates beta frequency after the imagination of movements (40). The central midline comprises the supplementary motor area (SMA) and this region is linked to movement. Moreover, it is involved in motor planning and coordinated movements. Therefore, there is a correlation between beta frequency and central midline region during motor performance (35). The parietal region is related to sensorimotor processing and movement. In addition, it is involved in the integration of sensory information, movement planning and execution. There is connectivity between the parietal region and the sensorimotor cortex; the beta oscillatory neurons were activated from the parietal cortex to the sensorimotor cortex during motor task (36). Therefore, parietal beta band is a dominant frequency during attentive motor task. These results suggested that neural circuits that produce beta synchronization degenerate in elderly people especially in the central midline and parietal regions. Thus, this attentional movement test appeared to induce cortical activity in the central midline parietal regions. Additionally, there was a positive correlation between brain activity and time to complete the test.
Previously, several studies have been conducted to elucidate the association between gamma power and motor movement. Primary motor cortex generated gamma oscillation during movement (41). Cued direction of attention stimulated neurons to produce gamma frequency (42). Furthermore, firing of neuronal populations in the parietal cortex that produces gamma rhythm was revealed during selective attention (43) or perception and cognition (44). Predominant gamma wave activities were observed during the test but not during resting state. The attentional movement test in this study was designed to examine eye-hand coordination activity. Therefore, this test was used not only to evaluate motor control but also to trigger top-down processes. It means that gamma rhythmic activity in several brain regions induced during the eye-hand coordination task is correlated with attentiveness.
In summary, cognitive performances in healthy young and healthy elderly subjects were not different according to Thai-MMSE test. However, relatively poor eye-hand coordination was observed in elderly groups with changes in beta synchronization especially in the parietal cortex. Therefore, the desynchronization of parietal beta frequency in elderly people during attentional movement activity represents neural processing associated with brain deterioration. Lastly, aging is normally associated with progressive neuronal deterioration that results in the decline of body function, particularly cognitive performance.
Conflicts of Interest
There are no conflicts of interest related to this study.
Authors’ Contributions
The study design was developed by RM and EK. The EEG procedures were performed by DC. The MMSE-test was prepared by PS. Data collection and analysis were carried out by RM and NS. Critical thinking was advised by EP. Final approval of the manuscript was obtained from all Authors after critical revision.
Acknowledgements
This research was financially supported by grants from the graduate school and Division of Health and Applied Sciences, faculty of Science, Prince of Songkla University, Songkhla, Thailand and the Education Hub for ASEAN Countries Scholarship was provided by the Graduate School, Prince of Songkla University, Songkhla, Thailand.
References
- 1.Hoogendam YY, van der Lijn F, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Ikram MA, van der Geest JN. Older age relates to worsening of fine motor skills: a population-based study of middle-aged and elderly persons. Front Aging Neurosci. 2014;6:259. doi: 10.3389/fnagi.2014.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curreri C, Trevisan C, Carrer P, Facchini S, Giantin V, Maggi S, Noale M, De Rui M, Perissinotto E, Zambon S, Crepaldi G, Manzato E, Sergi G. Difficulties with fine motor skills and cognitive impairment in an elderly population: The Progetto Veneto Anziani. J Am Geriatr Soc. 2018;66(2):350–356. doi: 10.1111/jgs.15209. [DOI] [PubMed] [Google Scholar]
- 3.Skedung L, El Rawadi C, Arvidsson M, Farcet C, Luengo GS, Breton L, Rutland MW. Mechanisms of tactile sensory deterioration amongst the elderly. Sci Rep. 2018;8(1):5303. doi: 10.1038/s41598-018-23688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peltzer K, Phaswana-Mafuya N. Cognitive functioning and associated factors in older adults in South Africa. S Afr J Psychiatr. 2012;18:157–163. [Google Scholar]
- 5.An Y, Feng L, Zhang X, Wang Y, Wang Y, Tao L, Lu Y, Qin Z, Xiao R. Patterns of cognitive function in middle-aged and elderly Chinese adults-findings from the EMCOA study. Alzheimers Res Ther. 2018;10(1):93. doi: 10.1186/s13195-018-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commodari E, Guarnera M. Attention and aging. Aging Clin Exp Res. 2008;20(6):578–584. doi: 10.1007/BF03324887. [DOI] [PubMed] [Google Scholar]
- 7.Kauranen K, Vanharanta H. Influences of aging, gender, and handedness on motor performance of upper and lower extremities. Percept Mot Skills. 1996;82(2):515–525. doi: 10.2466/pms.1996.82.2.515. [DOI] [PubMed] [Google Scholar]
- 8.Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, Markesbery WR, Zhang Z, Gerhardt GA, Kryscio RJ, Gash DM. Critical decline in fine motor hand movements in human aging. Neurology. 1999;53(7):1458–1461. doi: 10.1212/wnl.53.7.1458. [DOI] [PubMed] [Google Scholar]
- 9.Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126(Pt 4):873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barry RJ, De Blasio FM. EEG differences between eyes-closed and eyes-open resting remain in healthy ageing. Biol Psychol. 2017;129:293–304. doi: 10.1016/j.biopsycho.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Fan JC, Cheung RT, Chu LW, Fung PCW, Chang CQ, Sik HH, Zhang MM, Xie BJ, Hung YS, Gao JL. Age-related changes of EEG and its source in resting state. International Conference on Digital Signal Processing, Institute of Electrical and Electronics Engineers Inc. 2014:pp. 797–800. [Google Scholar]
- 12.Scally B, Burke MR, Bunce D, Delvenne JF. Resting-state EEG power and connectivity are associated with alpha peak frequency slowing in healthy aging. Neurobiol Aging. 2018;71:149–155. doi: 10.1016/j.neurobiolaging.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Mackie MA, Van Dam NT, Fan J. Cognitive control and attentional functions. Brain Cogn. 2013;82(3):301–312. doi: 10.1016/j.bandc.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coombes SA, Higgins T, Gamble KM, Cauraugh JH, Janelle CM. Attentional control theory: anxiety, emotion, and motor planning. J Anxiety Disord. 2009;23(8):1072–1079. doi: 10.1016/j.janxdis.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uncapher MR, Rugg MD. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. J Neurosci. 2009;29(25):8270–8279. doi: 10.1523/JNEUROSCI.1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman M, Offen K, Markant J. Exercise similarly facilitates men and women’s selective attention task response times but differentially affects memory task performance. Front Psychol. 2018;9:1405. doi: 10.3389/fpsyg.2018.01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmalzl L, Powers C, Zanesco AP, Yetz N, Groessl EJ, Saron CD. The effect of movement-focused and breath-focused yoga practice on stress parameters and sustained attention: A randomized controlled pilot study. Conscious Cogn. 2018;65:109–125. doi: 10.1016/j.concog.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Aliakbaryhosseinabadi S, Kamavuako EN, Jiang N, Farina D, Mrachacz-Kersting N. Influence of dual-tasking with different levels of attention diversion on characteristics of the movement-related cortical potential. Brain Res. 2017;1674:10–19. doi: 10.1016/j.brainres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Kal EC, van der Kamp J, Houdijk H. External attentional focus enhances movement automatization: a comprehensive test of the constrained action hypothesis. Hum Mov Sci. 2013;32(4):527–539. doi: 10.1016/j.humov.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Woods DL, Wyma JM, Yund EW, Herron TJ, Reed B. Factors influencing the latency of simple reaction time. Front Hum Neurosci. 2015;9:131. doi: 10.3389/fnhum.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philipps V, Amieva H, Andrieu S, Dufouil C, Berr C, Dartigues JF, Jacqmin-Gadda H, Proust-Lima C. Normalized Mini-Mental State Examination for assessing cognitive change in population-based brain aging studies. Neuroepidemiology. 2014;43(1):15–25. doi: 10.1159/000365637. [DOI] [PubMed] [Google Scholar]
- 22.Marioni RE, Chatfield M, Brayne C, Matthews FE, Medical Research Council Cognitive Function and Ageing Study Group The reliability of assigning individuals to cognitive states using the Mini Mental-State Examination: a population-based prospective cohort study. BMC Med Res Methodol. 2011;11:127. doi: 10.1186/1471-2288-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seidler RD, Alberts JL, Stelmach GE. Changes in multi-joint performance with age. Motor Control. 2002;6(1):19–31. doi: 10.1123/mcj.6.1.19. [DOI] [PubMed] [Google Scholar]
- 24.Granholm AC, Boger H, Emborg ME. Mood, memory and movement: an age-related neurodegenerative complex. Curr Aging Sci. 2008;1(2):133–139. doi: 10.2174/1874609810801020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13-35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 2005;25(34):7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalo E, Gilbertson T, Doyle L, Di Lazzaro V, Cioni B, Brown P. Phasic increases in cortical beta activity are associated with alterations in sensory processing in the human. Exp Brain Res. 2007;177(1):137–145. doi: 10.1007/s00221-006-0655-8. [DOI] [PubMed] [Google Scholar]
- 27.Kristeva-Feige R, Fritsch C, Timmer J, Lücking CH. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol. 2002;113(1):124–131. doi: 10.1016/s1388-2457(01)00722-2. [DOI] [PubMed] [Google Scholar]
- 28.Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bäuml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37(4):1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Lundqvist M, Rose J, Herman P, Brincat SL, Buschman TJ, Miller EK. Gamma and beta bursts underlie working memory. Neuron. 2016;90(1):152–164. doi: 10.1016/j.neuron.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundqvist M, Herman P, Warden MR, Brincat SL, Miller EK. Gamma and beta bursts during working memory readout suggest roles in its volitional control. Nat Commun. 2018;9(1):394. doi: 10.1038/s41467-017-02791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiol. 2003;13(2):187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 32.Han S, Jiang Y, Gu H, Rao H, Mao L, Cui Y, Zhai R. The role of human parietal cortex in attention networks. Brain. 2004;127(Pt 3):650–659. doi: 10.1093/brain/awh071. [DOI] [PubMed] [Google Scholar]
- 33.Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5(10):995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- 34.Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14(2):212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Jochumsen M, Rovsing C, Rovsing H, Cremoux S, Signal N, Allen K, Taylor D, Niazi IK. Quantification of movement-related EEG correlates associated with motor training: a study on movement-related cortical potentials and sensorimotor rhythms. Front Hum Neurosci. 2017;11:604. doi: 10.3389/fnhum.2017.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung JW, Ofori E, Misra G, Hess CW, Vaillancourt DE. Beta-band activity and connectivity in sensorimotor and parietal cortex are important for accurate motor performance. Neuroimage. 2017;144(Pt A):164–173. doi: 10.1016/j.neuroimage.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuper C, Wörtz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res. 2006;159:211–222. doi: 10.1016/S0079-6123(06)59014-4. [DOI] [PubMed] [Google Scholar]
- 38.Bu-Omer HM, Gofuku A, Sato K, Miyakoshi M. Parieto-occipital alpha and low-beta EEG power reflect sense of agency. Brain Sci. 2021;11(6):743. doi: 10.3390/brainsci11060743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noguchi Y, Kaneoke Y, Kakigi R, Tanabe HC, Sadato N. Role of the superior temporal region in human visual motion perception. Cereb Cortex. 2005;15(10):1592–1601. doi: 10.1093/cercor/bhi037. [DOI] [PubMed] [Google Scholar]
- 40.Pfurtscheller G, Neuper C, Brunner C, da Silva FL. Beta rebound after different types of motor imagery in man. Neurosci Lett. 2005;378(3):156–159. doi: 10.1016/j.neulet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 41.Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010;104(5):2873–2885. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- 42.Green JJ, Boehler CN, Roberts KC, Chen LC, Krebs RM, Song AW, Woldorff MG. Cortical and subcortical coordination of visual spatial attention revealed by simultaneous EEG-fMRI recording. J Neurosci. 2017;37(33):7803–7810. doi: 10.1523/JNEUROSCI.0326-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008;60(4):709–719. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann CS, Fründ I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev. 2010;34(7):981–992. doi: 10.1016/j.neubiorev.2009.09.001. [DOI] [PubMed] [Google Scholar]