Abstract
Background/Aim: To evaluate retrospectively whether bland embolization using microspheres is safe and useful for relieving pain in patients with painful malignant musculoskeletal (MSK) tumors.
Patients and Methods: Bland embolization using microspheres was performed for 20 patients (11 women/9 men) with a median age of 69 years (range=40-89 years) who had 22 painful malignant MSK tumors. The maximum tumor diameters were 2.4-13.8 cm (median, 7.5 cm). Pain was evaluated using the visual analog scale. A decrease of this score by 2 or more after embolization was defined as clinically effective pain relief. Adverse events (AEs) were evaluated using CTCAE v5.0. Objective response, disease control rates, and overall survival were also evaluated.
Results: Effective pain relief was achieved in 18 patients (90.0%, 18/20). Grade-3 AEs developed in four patients (20.0%, 4/20): skin ulcer (n=2), skin ulcer and pain (n=1), and muscle weakness with dysesthesia (n=1). No grade-4 or grade-5 AEs developed. Objective response and disease control rates were 26.7% (4/15) and 86.7% (13/15), respectively. The 1-year survival rate was 43.8%, with median survival of 9.2 months (range=0.5-41.0 months).
Conclusion: Although the survival benefit is equivocal, bland embolization is acceptably safe and useful for relieving pain by controlling tumor growth in patients with painful malignant MSK tumors.
Keywords: Bland embolization using microspheres, painful malignant musculoskeletal (MSK) tumors, effective pain relief
Malignant musculoskeletal (MSK) tumors, whether primary or metastatic, can degrade a patient’s quality of life, especially because of pain (1,2). Surgical resection is the gold standard local treatment for painful malignant MSK tumors (3). When surgical resection is difficult, palliative radiotherapy is performed (4). However, pain relief can be achieved in only 60-70% of patients after palliative radiotherapy, which takes 4-6 weeks to become effective (5,6). Percutaneous ablation therapy, such as radiofrequency ablation and cryoablation, is another local treatment applied for painful MSK tumors (7-9). However, the indications of percutaneous ablation therapy are limited by tumor size and location (10). Opioids are widely used in case of contraindication for those local treatments. However, the effects of opioids are sometimes unsatisfactory. More than 30% of patients experience persistent pain even after the initiation of opioids (11). Therefore, other less invasive and equally or more effective therapeutic options for managing painful malignant MSK tumors have been sought.
A few reports of earlier studies have described artery embolization as a useful therapeutic option for managing painful malignant MSK tumors (10,12). However, embolization in these earlier studies was performed mainly using gelatin sponge (GS) particles. Moreover, reports of embolization using microspheres are few (10). The longer-term utility of artery embolization for painful malignant MSK tumors remains uncertain.
Therefore, this study evaluated whether bland embolization using microspheres is safe and useful for relieving pain in patients with painful malignant MSK tumors.
Patients and Methods
Patients. Our institutional review board approved this retrospective study. Written informed consent to participate in this study was waived because of its retrospective nature.
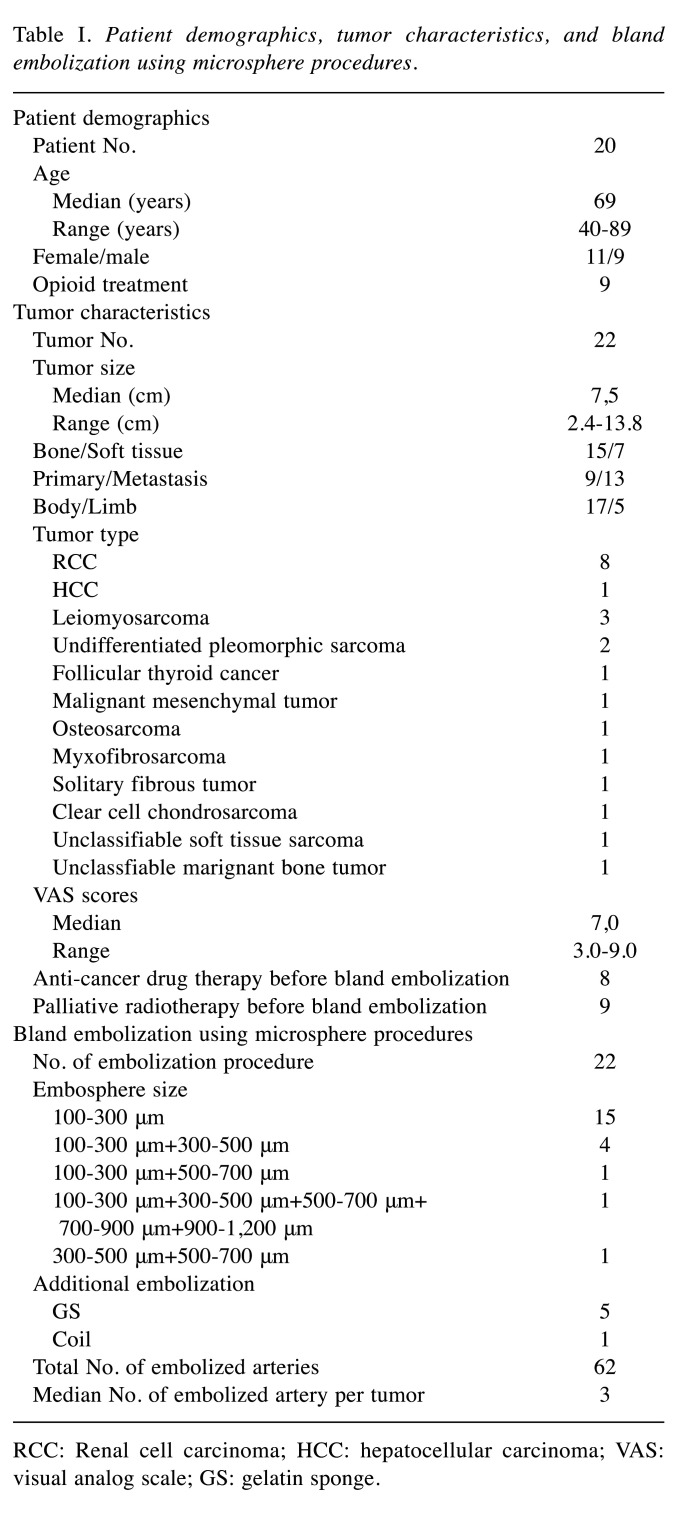
Between April 2016 and April 2021, 27 consecutive patients underwent bland embolization using microspheres for the treatment of unresectable MSK tumors. Among them, patients with painless tumors (n=5) and patients who received combination therapy of bland embolization using microspheres and other local treatments (n=2) were excluded (Figure 1). Therefore, 20 patients (74.1%, 20/27) were included in this study. The study subjects were 11 women (55.0%, 11/20) and 9 men (45.0%, 9/20) with median age of 69 years (range=40-89 years). Of them, 9 (45.0%, 9/20) had primary and 11 (55.0%, 11/20) had metastatic MSK tumors. Also, 18 patients (90.0%, 18/20) had a single tumor, the remaining two patients (10.0%, 2/20) had two tumors. Therefore, a total of 22 painful malignant MSK tumors (15 bone tumors and 7 soft tissue tumors) with median size of 7.5 cm (range=2.4-13.8 cm) were treated by bland embolization using microspheres. These tumors were, respectively, in the body trunk (n=17) and the limb (n=5). Thirteen patients (65.0%, 13/20) had received earlier treatment including anti-cancer drug therapy (n=4), palliative radiotherapy (n=5), or both (n=4) before embolization. Patient information and tumor characteristics are presented in Table I.
Figure 1. Flowchart of this study.

Table I. Patient demographics, tumor characteristics, and bland embolization using microsphere procedures.
RCC: Renal cell carcinoma; HCC: hepatocellular carcinoma; VAS: visual analog scale; GS: gelatin sponge.
Pretreatment work-up. All patients received routine physical examinations, laboratory tests, a chest X-ray, an electrocardiogram test, and computed tomography (CT) or magnetic resonance (MR) imaging within 1 month before bland embolization using microspheres. Diagnoses of painful malignant MSK tumors were confirmed histopathologically for 9 primary and 3 metastatic tumors. For the remaining 10 metastatic tumors, the diagnosis was made using CT and/or MR imaging.
The visual analog scale (VAS) score was used to evaluate the pain severity. The baseline VAS score was evaluated within a week before bland embolization using microspheres. The median baseline visual analog scale (VAS) score was 7.0 (range=3.0-9.0) on a tumor basis. Palliative radiotherapy had been performed for nine painful tumors (31.8%, 9/22) before embolization. The 13 patients had already taken an opioid (n=7), a nonsteroidal anti-inflammatory drug (NSAID) (n=4), or both (n=2).
Procedures for bland embolization using microspheres. Bland embolization using microspheres was performed percutaneously under local anesthesia using lidocaine (Xylocaine; AstraZeneca K.K., Osaka, Japan). After a 4-French (Fr) catheter was introduced into a femoral artery, angiography was performed to confirm the arteries feeding the target tumor. Then a 1.7-Fr microcatheter (Progreatλ17®; Terumo Clinical Supply Co., Ltd., Gifu, Japan) was inserted superselectively into the feeding arteries. In principle, embolization was begun with 100-300 μm diameter tris-acryl gelatin microspheres (Embosphere®; Nippon Kayaku Ltd, Tokyo, Japan) to target the small distal arteries. Then, the particle size was increased gradually to embolize the proximal arteries efficiently. During five embolization sessions, the appearance of vascular lakes was observed during bland embolization using microspheres. Then, GS particles (Serescue®; Nihon-kayaku Ltd., Tokyo, Japan) were added to embolize these vascular lakes. Coil embolization was added to an embolization session to avoid non-targeting embolization. The disappearance of tumor enhancement was regarded as an endpoint of embolization (Figure 2). In all, 22 embolization sessions were performed for the treatment of 22 painful malignant MSK tumors, for which 62 arteries were embolized (median, 3 arteries per tumor; range=1-6 arteries per tumor). Details of procedures used for bland embolization using microspheres are presented in Table I.
Figure 2. A woman in her 70s with a painful metastasis from uterine leiomyosarcoma in the left thigh. A) Angiography showed a hypervascular tumor in her left thigh. B) Angiography immediately after embolization showed the disappearance of tumor enhancement. C) Contrast-enhanced magnetic resonance (MR) imaging showed an enhanced tumor measuring 10.4 cm in her left thigh before embolization. D) Contrast-enhanced computed tomography at 1 month after embolization demonstrated the complete disappearance of tumor enhancement. E) Contrast-enhanced MR imaging at 18 months after embolization demonstrated tumor shrinkage.

Follow up. The follow-up protocol included routine physical examinations, laboratory tests, and imaging studies. Contrast-enhanced CT or MR imaging was performed 1-3 months after embolization and every 3-6 months thereafter. Follow-up was closed at the time of patient death or at their last visit up to May 31st, 2022. The median follow-up period was 9 months (range=1-41 months).
Assessment and statistical analysis. Pain severity was evaluated at one day, 1-2 weeks, and 3-4 weeks after embolization. For two patients, assessment of cancer pain could not be performed at 3-4 weeks after embolization because of the loss of follow-up (n=1) and death (n=1). Effective pain relief was defined as a decrease in the VAS score of 2 or more compared to the baseline. A decreased VAS score increased again by 2 or more was defined as a relapse of pain. Changes in the dose of analgesia were also evaluated.
Adverse events (AEs) within 1 month after embolization were recorded and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) ver. 5.0 on a patient basis.
Among the 20 patients, baseline contrast-enhanced CT or MR imaging was available in 15 patients (75.0%, 15/20) with 15 painful malignant MSK tumors (68.2%, 15/22). Therefore, evaluation of treatment response was performed for these 15 patients. These patients underwent follow-up contrast-enhanced CT or MR imaging at 1-3 months after embolization. The treatment response was evaluated using modified response evaluation criteria in solid tumors (mRECIST) (13). The objective response rate was defined as the percentage of tumors with complete response (CR) and partial response (PR). The disease control rate was defined as the percentage of tumors with CR, PR, and stable disease (SD). Overall survival was calculated from the date of embolization to the date of death or last follow-up.
All continuous data are expressed as a median with a range (min-max). The VAS scores before and after embolization were compared using the Wilcoxon’s signed rank test. Survival curves were estimated using the Kaplan-Meier method. All statistical analyses were conducted using SAS software (SAS, release 9.1; SAS Institute Inc., Cary, NC, USA).
Results
Pain. Median VAS scores decreased significantly from the baseline at 1-2 weeks (median, 4.0; range=0-9.0; p<0.001) and at 3-4 weeks (median, 2.0; range=0-8.0; p=0.001) after bland embolization using microspheres, but they were not significantly different on the following day (median, 5.5; range=1.0-10.0; p=0.23) (Figure 3). Effective pain relief was found for 18 patients (90.0%, 18/20) with 20 tumors (90.9%, 20/22). Pain relief was insufficient for two patients (10.0%, 2/20): one patient had right clavicle metastasis from renal cell carcinoma, which was associated with pathological fracture; the other patient had a malignant mesenchymal tumor of the hip, which was complicated by skin ulcer. Among the 18 patients found to have effective pain relief after embolization, 7 patients (38.9%, 7/18) developed a relapse of pain with a median interval of 3 months (range=1-8 months). The remaining 11 patients (61.1%, 11/18) had no relapse of pain during the median follow-up period of 8 months (range=1-41 months). The dose of analgesia was reduced in one patient.
Figure 3. Changes in VAS scores before and a day, 1-2 weeks, and 3-4 weeks after embolization. After embolization, median VAS scores decreased significantly from 7.0 (range=3.0-9.0) to 4.0 (range=0-9.0) (p<0.001) at 1-2 weeks and to 2.0 (range=0-8.0) (p=0.001) at 3-4 weeks. However, median VAS scores had not decreased significantly from 7.0 (range=3.0-9.0) to 5.5 (range=1.0-10.0) (p=0.23) a day later.

Safety. No grade-4 or grade-5 AE occurred. Grade-3 AEs occurred in four patients: thigh or scapula skin ulcer (n=2), buttock skin ulcer and pain (n=1), and distal lower extremity muscle weakness with dysesthesia (n=1). In the patient who developed muscle weakness and dysesthesia, thoracic spinal MR imaging showed hyperintensity foci in the spinal cord on diffusion-weighted (DWI) and T2-weighted imaging (T2WI), suggesting spinal cord infarction. Symptoms became less severe after rehabilitation. The patient was discharged from the hospital with a crutch, walking at 1 month after embolization. Grade 1 or 2 AEs include pain (n=15) and fever (n=4). The AEs are presented in Table II.
Table II. Adverse events (Aes) after bland embolization using microsphere.
Treatment response. The treatment response at 1-3 months after embolization was CR in one patient (6.7%, 1/15), PR in 3 patients (20.0%, 3/15), and SD in 9 patients (60.0%, 9/15) according to mRECIST criteria. Therefore, the objective response rate was 26.7% (4/15). The disease control rate was 86.7% (13/15).
Overall survival. The cumulative overall survival rates were 43.8% [95% confidence interval (CI)=20.5-65.1%] at one year and 14.6% (95%CI=1.0-44.9%) at three years with median survival time of 9.2 months (range=0.5-41.0 months) (Figure 4). Twelve patients had died (60.0%, 12/20) by the end of the follow-up. Causes of death were cancer progression in 11 patients (55.0%, 11/20) and pneumonia in one patient (5.0%, 1/20).
Figure 4. Cumulative overall survival rates were 44% [95% confidence interval (CI)=21-65%] at 1 year and 15% (95%CI=1-45%) at three years, with a median survival time of 9.2 months (range=0.5-41.0 months).

Discussion
This study showed that bland embolization using microspheres is an acceptably safe and useful therapeutic option for managing painful MSK tumors.
In this study, a decrease in the VAS score of 2 or more was observed in 90.9% of patients after bland embolization using microspheres. This effect was continued during the median follow-up period of 8 months in 65.0% of patients. Pain relief after palliative radiotherapy has been reported as observed in 68-71% of patients. The palliative effect reportedly lasts for about 5 months (5,6). Therefore, the results of this study suggest that embolization, like palliative radiotherapy, is a useful option to achieve pain relief for patients with painful MSK tumors. Effects of pain relief by embolization were apparently similar to those of embolization with GS particles, for which pain relief was observed in 87% of patients (10).
A significant decrease in the VAS score was observed at 1-2 weeks after embolization. Reportedly it takes 4-6 weeks to achieve significant pain relief after radiotherapy. Therefore, rapid pain relief can be regarded as an advantage of embolization.
However, after embolization two patients (10.0%, 2/20) were not found to have had significant pain relief. One patient had a tumor associated with pathological fracture. The other patient had a complicated grade 3 skin ulcer after embolization. The patients’ associated pathological fracture and skin ulcer might have hindered pain relief after embolization.
In this study, no grade-4 or greater AE was observed. Therefore, embolization can be regarded as an acceptably safe procedure. The most frequent grade-3 AE after embolization was skin ulcer (13.6%, 3/20). However, in earlier reports describing embolization performed by GS, the complication of skin ulcers was not reported (10). For this study, microspheres of 100-300 μm were used in most cases. The use of such small particles might induce skin ischemia and ulcer formation by distal embolization. Further research is needed to optimize the size of the microspheres for the treatment of MSK tumors. Grade-3 muscle weakness and dysesthesia were observed in one patient. These complications are most likely caused by non-target embolization of spinal branches from intercostal arteries. Therefore, careful injection is necessary, especially when bland embolization using microspheres is performed from intercostal arteries.
Earlier reports describing the oncologic efficacy of artery embolization for MSK tumors are few. Ni et al. reported the objective response and disease control rates at 4-8 weeks after artery embolization using drug-eluting beads as 30.0% and 79.0%, respectively, with a 1-year overall survival rate of 90.0% and median survival time of 21 months (14). The objective response and disease control rates at 1–3 months (26.7% and 86.7%, respectively) found in this study were similar to those reported by Ni et al., but survival results were worse than those of their study (43.8% 1-year overall survival rate and 9.2 months median survival time). Given that most tumors included in this study were refractory to conventional anti-cancer drug and/or radiotherapy, bland embolization using microspheres can be regarded as an option not only for pain management but also for achieving local tumor control.
This study includes several limitations. The retrospective study design examining small numbers of patients, their inhomogeneous backgrounds, and the short follow-up periods are apparent limitations. Further evaluation with more patients and a longer follow-up time are expected to be necessary to confirm the results of this study.
In conclusion, although the survival benefit is equivocal, bland embolization is acceptably safe and useful for relieving pain by controlling tumor growth in patients with painful malignant MSK tumors.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Conflicts of Interest
The Authors declare that they have no conflicts of interest in relation to this study.
Authors’ Contributions
J.T. was involved in the methodology, investigation, data curation, and writing - original draft. H.T. was involved in the conceptualization, methodology, formal analysis, writing-review & editing, and project administration supervision. H.K. was involved in the conceptualization, methodology, and formal analysis. T.K., M.T., A.O., Y.K., K.K., T.M., and H.F. were involved in the formal analysis. K.Y. was involved in the supervision. All Authors critically revised the manuscript, approved it for publication, and agreed to accept responsibility for all aspects.
References
- 1.Buga S, Sarria J. The management of pain in metastatic bone disease. Cancer Control. 2021;19(2):154–166. doi: 10.1177/107327481201900210. [DOI] [PubMed] [Google Scholar]
- 2.Veselis C, Awan O, Thomas A, Ling S, Jonnalagadda P, Aneja A, Ali S. Bone tumors occurring in the soft tissues: a review of the clinical, imaging, and histopathologic findings. Current Problems in Diagnostic Radiology. 2022;50(3):419–429. doi: 10.1067/j.cpradiol.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Zubairi A, Hasan O, Mustafa M, Umer M. Musculoskeletal tumors throughout history and beyond: clinical features, imaging, staging and biopsy. Journal of the Pakistan Medical Association. 2020;(0):1. doi: 10.5455/JPMA.11954. [DOI] [PubMed] [Google Scholar]
- 4.Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, Howell D, Konski A, Kachnic L, Lo S, Sahgal A, Silverman L, Von Gunten C, Mendel E, Vassil A, Bruner D, Hartsell W. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. International Journal of Radiation Oncology*Biology* Physics. 2022;79(4):965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Steenland E, Leer J, Van Houwelingen H, Post W, Van den Hout W, Kievit J, De Haes H, Martijn H, Oei B, Vonk E, Van der Steen-Banasik E, Wiggenraad R, Hoogenhout J, Wárlám-Rodenhuis C, Van Tienhoven G, Wanders R, Pomp J, Van Reijn M, Van Mierlo T, Rutten E. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiotherapy and Oncology. 2019;52(2):101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 6.Chow E, Van der Linden Y, Roos D, Hartsell W, Hoskin P, Wu J, Brundage M, Nabid A, Tissing-Tan C, Oei B, Babington S, Demas W, Wilson C, Meyer R, Chen B, Wong R. Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. The Lancet Oncology. 2019;15(2):164–171. doi: 10.1016/s1470-2045(13)70556-4. [DOI] [PubMed] [Google Scholar]
- 7.Locklin LK, Mannes A, Berger A, Wood BJ. Palliation of soft tissue cancer pain with radiofrequency ablation. J Support Oncol. 2004;2(5):439–445. [PMC free article] [PubMed] [Google Scholar]
- 8.Callstrom M, Atwell T, Charboneau J, Farrell M, Goetz M, Rubin J, Sloan J, Novotny P, Welch T, Maus T, Wong G, Brown K. Painful metastases involving bone: percutaneous image-guided cryoablation-prospective trial interim analysis. Radiology. 2016;241(2):572–580. doi: 10.1148/radiol.2412051247. [DOI] [PubMed] [Google Scholar]
- 9.Nakatsuka A, Yamakado K, Uraki J, Takaki H, Yamanaka T, Fujimori M, Hasegawa T, Sakuma H. Safety and clinical outcomes of percutaneous radiofrequency ablation for intermediate and large bone tumors using a multiple-electrode switching system: a phase II clinical study. Journal of Vascular and Interventional Radiology. 2018;27(3):388–394. doi: 10.1016/j.jvir.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Koike Y, Takizawa K, Ogawa Y, Muto A, Yoshimatsu M, Yagihashi K, Nakajima Y. Transcatheter arterial chemoembolization (TACE) or embolization (TAE) for symptomatic bone metastases as a palliative treatment. CardioVascular and Interventional Radiology. 2019;34(4):793–801. doi: 10.1007/s00270-010-0031-8. [DOI] [PubMed] [Google Scholar]
- 11.Sima L, Fang W, Wu X, Li F. Efficacy of oxycodone/paracetamol for patients with bone-cancer pain: a multicenter, randomized, double-blinded, placebo-controlled trial. Journal of Clinical Pharmacy and Therapeutics. 2022;37(1):27–31. doi: 10.1111/j.1365-2710.2010.01239.x. [DOI] [PubMed] [Google Scholar]
- 12.Marciel A, Van Zandt B, Baxter A. Transcatheter arterial embolization for the palliation of painful bone lesions. Techniques in Vascular and Interventional Radiology. 2018;14(3):141–149. doi: 10.1053/j.tvir.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Llovet J. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Seminars in Liver Disease. 2017;30(01):052–060. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni J, Sun H, Chen Y, Luo J, Wang W, Jiang X, Chen D, Xu L. Drug-eluting bead transarterial chemoembolization in the treatment for unresectable soft tissue sarcoma refractory to systemic chemotherapy: a preliminary evaluation of efficacy and safety. Journal of Cancer Research and Clinical Oncology. 2018;144(1):157–163. doi: 10.1007/s00432-017-2530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]