Abstract
Background/Aim: The Philadelphia chromosome-negative (Ph-) myeloproliferative neoplasms (MPNs) are a group of blood cancers that arise from abnormal growth of blood cells in the bone marrow. Patients with MPNs are at increased risk for life-threatening thromboembolic complications. The detection of JAK2V617F in endothelial cells (ECs) brought a new perspective to the research of thromboembolic events. However, the mechanisms by which the mutation contributes to risk have yet to be entirely understood. Consequently, the objective of this study was to investigate how JAK2V617F impacts endothelial cells by considering thermoregulation.
Materials and Methods: We applied our previously created model for EC that was genetically modified with JAK2 wild type (WT)-GFP and JAK2V617F-GFP lentiviruses; the cells were cultured for 48 h at 37˚C for normothermia and 32˚C for mild hypothermia. We examined the effect of thermoregulation on infection efficiency and the expression of cell surface markers, including endothelial protein C receptor (EPCR), thrombomodulin (TM), and tissue factor (TF), which are related to the coagulation pathways. Furthermore, the microparticle production from the genetically modified EC (EMPs) was analyzed.
Results: We found suppression of the expression of coagulation factors, including EPCR, TM, and TF in JAK2V617F positive ECs under mild hypothermia. JAK2V617F-positive ECs showed slightly higher EMP production under mild hypothermia.
Conclusion: Although the molecular mechanisms of the thermal effects on the tumor microenvironment with JAK2V617F and its effect on EMP production and coagulation are not known yet, the therapy-oriented effect of thermoregulation might be considered in future studies.
Keywords: JAK2V617F, extracellular vesicles, microparticles, hypothermia, thrombosis, endothelial cells
Myeloproliferative neoplasms (MPN) are acquired disorders of hematopoietic progenitor stem cells that give rise to the abnormal proliferation of one or more terminal myeloid cell lines in the peripheral blood; they include polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF) (1). Although the exact etiology of MPNs is not known, some driver mutations have been identified in Janus kinase 2 (JAK2) (2-5), calreticulin (CALR) (6) and myeloproliferative leukemia virus oncogene (MPL) (7) genes and patients with such mutations are associated with a higher risk of developing MPNs. The most frequent mutation is JAK2V617F. It is detected in 95% of PV and 50% of ET and PMF (8) and has been shown to activate three myeloid cytokine receptors, including the erythropoietin receptor, respectively, MPL receptor and colony-stimulating factor receptor (9).
MPNs contribute to cardiovascular complications such as arterial/venous thrombosis and thromboembolic complications, which might manifest as stroke, pulmonary embolism, myocardial infarction, hemorrhage, pulmonary hypertension, accelerated atherosclerosis, etc. (10). The microenvironment of vessels plays a crucial role in controlling blood hemostasis. Endothelial cells (ECs) are the critical elements of vessels and the leading players in the microenvironment (11,12). The discovery of the JAK2V617F mutation in ECs raised the question of the possible role of the endothelium in the pathogenesis of the disease since there is a high incidence of vascular complications in patients with MPN (13,14). Also, several studies have shown the role of thrombotic events of JAK2V617F-positive endothelium in the MPN (15-18). Hemostasis is greatly influenced by cell-to-cell communication via direct contact between cells, soluble molecules, and extracellular vesicles (EVs). Microparticles (MPs) are EVs released from healthy or diseased cells through the budding of the plasma membrane during several biological processes, including cellular activation, senescence, and apoptosis (19). MPs have diameters ranging from 100 to 1,000 nm and might carry proteins and genetic material of the parental cells (20). The phospholipid vesicle structure of such nanoscale MP may be prominent vehicles of metastasis (21). Numerous studies have revealed the role of MPs in cancer and thrombosis-associated complications (22,23). A study by Zhang et al. revealed that MPs are elevated for PV, ET, and MF patients with thrombotic complications (24). Furthermore, we have previously shown that genetically modified ECs with JAK2V617F mutation release EC-originated MPs (EMPs), which carry the JAK2V617F mutation in their cargo (25). Yet, little is known about the molecular mechanisms through which EMPs play a role in thrombosis and metastasis in MPNs.
Thermoregulation is essential for maintaining the balance of cellular functions and biological reactions to produce biomolecules, such as nucleic acids and proteins, and EVs. Body temperature is tightly controlled and has a narrow range between 36.5 and 37˚C, which is referred to as normothermia (26). A body temperature below 36˚C is defined as hypothermia and is further classified for clinical purposes as mild (35˚C to 32˚C), moderate (26˚C to 31˚C), deep (25˚C to 20˚C), and profound (19˚C to 14˚C) hypothermia (27). Since ancient times, hypothermia has been applied as a therapeutic agent to treat hemorrhage and traumatic brain injury (28,29). In addition, hypothermia has been utilized as a cancer cell therapy. In a study by Fay Temple in 1940, hypothermia therapy resulted in a remarkable recovery from breast carcinoma (30). Since then, various studies concluded that hypothermia suppressed cancer cell proliferation, adhesion, and metastasis to neighboring cells (31-34). In addition, blood regulation is greatly influenced by hypothermia. Platelets, platelet function, fibrinogen, and enzymes in the clotting cascade are deregulated under hypothermic conditions (35).
No study has been available regarding JAK2V617F-dependent coagulation abnormalities under mild hypothermia. In this study, we investigated the impact of mild hypothermia on EC functions relative to coagulation and its subsequent effects on EMP secretion. We applied thermoregulation to our previously created model for ECs that were genetically modified with lentiviruses; JAK2 wild type (WT)- encoding green fluorescent protein (GFP) and JAK2V617F-GFP into the human umbilical vein EC. We examined the possible effect of mild hypothermia on infection efficiency. We questioned the role of mild hypothermia both on the expressions of cell surface markers, including endothelial protein C receptor (EPCR), thrombomodulin (TM), and tissue factor (TF) in JAK2V617F-positive ECs and EMPs.
We discovered the temperature-dependent secretion of EMPs and suppression of the expression of coagulation factors, including EPCR, TM, and TF, both on JAK2V617F positive ECs and EMPs under mild hypothermia. Despite the importance of its clinical application in cancer therapy, little is known about the molecular mechanisms of EMPs and the thermal effects on tumor microenvironment.
Materials and Methods
Plasmids. The feline immunodeficiency virus-based lentivirus plasmids (FIV) (System Biosciences, Palo Alto, CA, USA), pCDF1-JAK2WT-GFP and pCDF1-JAK2V617F-GFP and helper vectors, including pCI-VSVG, pCPRDEnv have been reported elsewhere (36).
Cell lines. A derivative of the human embryonic kidney 293 cell line containing the SV40 T-antigen (HEK 293T) and human umbilical vein endothelial cells (HUVECs) were purchased from ATCC (Manassas, VA, USA). The cell lines were cultured in high-glucose Dulbecco’s modified Eagle’s medium (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Gibco, Thermo Fisher Scientific, Inc.).
Lentivirus infection of endothelial cells. A detailed method for generating genetically modified ECs with the expression vectors, including pCDF1-JAK2WT-GFP and pCDF1-JAK2V617F-GFP, has been reported elsewhere (36). The genetically modified HUVECs are referred to as JAK2 wild-type (JAK2WT) and JAK2V617F mutation (JAK2V617F) throughout the manuscript.
Thermoregulation of genetically engineered endothelial cells. HUVECs were grown under normothermia (37˚C) for 24 before lentivirus infections. After adding 5 mg Polybrene (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and lentiviruses, HUVECs were incubated either at normothermia (37˚C) or mild hypothermia (32˚C) for 48 h.
Endothelial cell surface and microparticle marker analysis using flow cytometry. After 48 h of incubation at 32˚C or 37˚C in an atmosphere of 5% CO2 and ≥95% humidity with the lentiviruses, HUVECs were detached using 0.25% trypsin-EDTA (Sigma-Aldrich, Merck KGaA). The genetically modified HUVECs were stained for the EPCR, also known as activated protein C receptor (CD201-PE; BD Pharmingen, San Diego, CA, USA), thrombomodulin (TM) (CD141-PE; BD Pharmingen, San Diego, CA,) and tissue factor (TF) (CD142-APC; Biolegend, San Diego, CA, USA) as previously described (25). The acquisition of data and analysis were performed using FACSCalibur (BD Biosciences, San Jose, CA, USA) instrument.
EMPs released from the genetically modified JAK2WT and JAK2V617F HUVECs were collected from supernatants of cultured cells using a centrifugation protocol initially optimized for MP isolation elsewhere (37). The size of MPs was determined using latex microbeads of various-size ranges (Megamix-Plus FSC, Biocytex, Marseille, France) and FACSCalibur (BD Biosciences). The MPs were further labeled using PECAM-1 (CD31-PE; Biolegend) and TF (CD142-APC; Biolegend) antibodies for the characterization and analysis of EMPs. The percentage of GFP-positive EMPs was determined in PECAM-1 or TF-positive EMPs under normothermic and mild hypothermic conditions.
Statistical analyses. All values are displayed as mean±standard deviation of the mean (STDV) using GraphPad Prism v.8 (GraphPad Prism Inc., San Diego, CA, USA). Statistical analyses were performed using a paired t-test to determine statistical significance. The accepted significant p-values of p<0.05* p<0.01**, p<0.001***, and p<0.0001**** are shown in the figures. The exact statistical significance of each experiment is presented in the figure legends and in the text.
Results
The infection efficiency of JAK2WT and JAK2V617F lentiviruses under mild hypothermic conditions shows a statistically significant decrease compared to normothermia. To understand the possible effect of mild hypothermia on infection efficiency, we produced genetically modified HUVECs using JAK2WT or JAK2V617F lentiviruses. The HUVECs were incubated at 37˚C or 32˚C for 48 h. Infection efficiency was examined using the flow cytometer analysis. The summary of the experimental layout is presented in Figure 1A. The data revealed that mild hypothermia negatively affected the infection efficiency. Under normothermia conditions, the infection percentage of JAK2WT-HUVECs was 97.17%±1.45, whereas, in mild hypothermia, the rate was 0.45%±0.43 (p=0.001) (Figure 1B). In addition, the infection percentage of JAK2V617F-HUVECs under normothermia was 43.25%±4.40, and under mild hypothermia 2.97%±1.52 (p=0.002) (Figure 1B).
Figure 1. Thermoregulation model for microparticle analysis. A) Schematic of the experimental design and the lentiviral vectors used in the study. B) Lentiviral infection efficiency decreases under mild hypothermic conditions. The infection efficiency of JAK2WT and JAK2V617F lentiviruses was analyzed under normothermia and mild hypothermia. Error bars show standard deviation. ***p<0.001; **p<0.01.
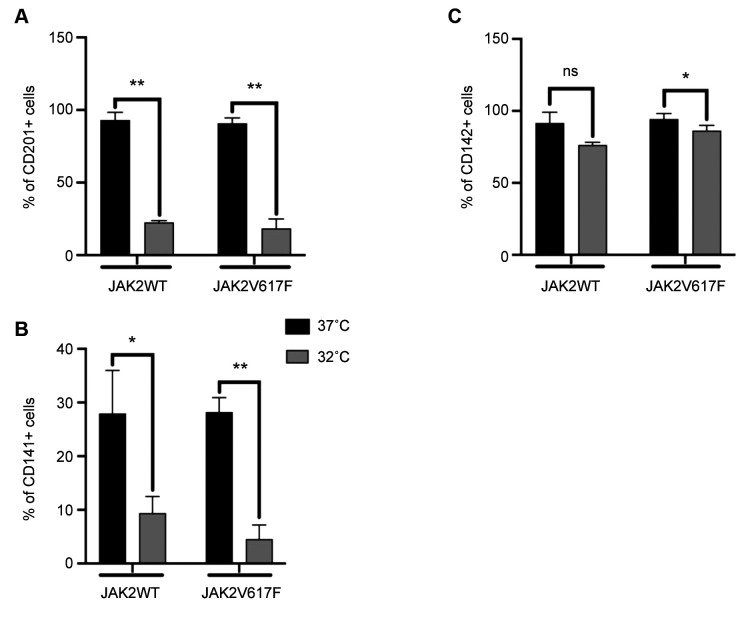
Key players of hemostasis show a statistically significant decrease under mild hypothermic conditions. Next, to understand the role of thermoregulation, we performed cell surface analyses of EPCR, TM, and TF expression on genetically modified HUVECs. JAK2WT- or JAK2V617F-HUVECs were stained using EPCR, TM, or TF antibodies. Stained HUVECs were analyzed using a flow cytometer by gating on GFP positive signals.
The expression of EPCR in JAK2WT-HUVECs under normothermia was 93.04%±5.45, whereas, under mild hypothermia, the expression was decreased to 22.5%±11.33 (p=0.0025). The expression of EPCR in JAK2V617F-HUVECs under normothermia was 90.85%±3.72 and decreased to 18.49%±6.47 (p=0.0031) under mild hypothermia (Figure 2A).
Figure 2. Cell surface expression analysis of genetically modified HUVECs under normothermia and mild hypothermia. A) The cell surface expression of EPCR on genetically modified HUVECs under normothermia and mild hypothermia: EPCR expression in JAK2WTand JAK2V617F-HUVECs under normothermia and mild hypothermia. Error bars show standard deviation. **p<0.01. B) The cell surface expression of TM on genetically modified HUVECs under normothermia and mild hypothermia conditions: TM expression in JAK2WT- and JAK2V617F-HUVECs under normothermia and mild hypothermia. Error bars show standard deviation. *p<0.05, **p<0.01. C) The Cell Surface expression of TF on genetically modified HUVECs under normothermia and mild hypothermia conditions: TF expression in JAK2WT- and JAK2V617F-HUVECs under normothermia and mild hypothermia. Error bars show standard deviation. *p<0.05.
The expression of TM in JAK2WT-HUVECs under normothermia was 27.96%±8.02, whereas under mild hypothermia, the expression was decreased to 9.37%±3.13 (p=0.0231). The expression of TM in JAK2V617F-HUVECs under normothermia was 28.21%±2.71 and was reduced to 4.53%±2.64 (p=0.0016) under mild hypothermia (Figure 2B).
The expression of TF in JAK2WT-HUVECs under normothermia was 91.60%±13.77, whereas, under mild hypothermia the expression was decreased to 76.27%±1.91. No statistical significance was detected. The expression of TF in JAK2V617F-HUVECs under normothermia was 94.26%±3.99 and decreased to 86.35%±3.63 under mild hypothermia (p=0.0133) (Figure 2C).
Overall, we observed a statistically significant decrease in the expressions of all three coagulation factors in JAK2V617F–HUVECs under mild hypothermia compared to normothermia. Only TF did not show statistically significant change in JAK2WT-HUVECs under mild hypothermia compared to normothermia.
Evaluation of microparticles released from JAK2WT- and JAK2V617F-HUVECs under mild hypothermic conditions shows a statistically significant decrease. EMPs released from JAK2WT- or JAK2V617F-HUVECs were collected from the supernatant of the cells. EMPs were stained using PECAM-1 or TF antibodies. The analyses were performed using the flow cytometer.
The expression of PECAM-1 on the EMPs of JAK2WT-HUVECs under normothermia was 73.30%±1.97 whereas, under mild hypothermia, was decreased to 11.96%±1.70 (p=0.0001). The expression of PECAM-1 on the EMPs of JAK2V617F-HUVECs under normothermia was 60.87%±4.63 and was decreased to 21.13%±2.93 under mild hypothermia (p=0.0110) (Figure 3A).
Figure 3. The microparticle surface expression analysis under normothermia and mild hypothermia. A) The expression of PECAM-1 on EMPs under normothermia and mild hypothermia conditions. The expression of PECAM-1 in JAK2WT- and JAK2V617F-positive endothelial microparticles (EMP) collected from JAK2WT- and JAK2V617F- HUVECs. The analysis was performed under normothermic and mild hypothermic conditions. Error bars show standard deviation. ****p<0.0001, *p<0.05. B) The expression of TF on EMPs under normothermia and mild hypothermia conditions. Cytosolic TF e levels in JAK2WT- and JAK2V617F- positive endothelial microparticles (EMP) collected from JAK2WT- and JAK2V617F-HUVECs. The analysis was performed under normothermic and mild hypothermic conditions. Error bars show standard deviation. ***p<0.0001, *p<0.05.
The expression of TF on the EMPs of JAK2WT-HUVECs under normothermia was 76.21%±0.77 and decreased to 43.97% 2.05 under mild hypothermia (p=0.0006). The expression of TF on the EMPs for JAK2V617F-HUVECs under normothermia was 70.69%±4.48 and decreased to 52.99%±6.58 under mild hypothermia (p=0.0434) (Figure 3B).
Discussion
The research on JAK2V617F mutation in ECs was initiated in 2009 with the original finding of this mutation in ECs of MPN patients with Budd-Chiari Syndrome (13). Although detecting a hematopoietic stem cell-originated mutation in ECs might initiate a debate about the origin of the mutation, the consequence of its presence in EC might also provide a connection with the pathophysiological processes that lead to many detected complications in MPNs. ECs, the largest organ in the body covering blood vessels, are the leading player in managing blood hemostasis together with EPCR, TM, and TF and play crucial roles in the coagulation cascade.
We have previously applied a model to study the JAK2V617F mutation in ECs where genetically modified HUVECs with JAK2WT or JAK2V617F using a feline-based lentivirus system (36). Although the presence of JAK2V617F mutation did not have a significant effect on the expression of EPCR, TM, and TF, all of which have roles in managing blood hemostasis together, on HUVECs, surprisingly, we detected the presence of microparticles generated from JAK2V617F-HUVECs (25). EMP production from JAK2V617F-HUVECs might have a profound effect on hemostasis. Blood regulation is greatly influenced by thermoregulation. In this study, we examined the effect of normothermia and mild hypothermia on the infection of HUVECs with JAK2WT and JAK2V617F lentiviruses. We found that under mild hypothermia, the infection efficiency decreased significantly for both JAK2WT and JAK2V617F. The comparison of the rates of infection with JAK2WT and JAK2V617F indicate that infection efficiency is not affected by the mutation because both have a similar response to the two conditions (JAK2WT, p=0.001; JAK2V617F, p=0.002) (Figure 1B).
We observed a significant decrease in the EPCR expression in both JAK2WT- and JAK2V617F- HUVECs under mild hypothermia compared to normothermia (Figure 2A). EPCR is a cell surface receptor that initiates anticoagulation activity along with the TM-TF complex that serves as a mediator of the binding to protein C. As the complex forms, the anticoagulation protein C becomes active (38). This might indicate that temperature affects the production of the EPCR protein, which is supported by a previous in-vivo study by Gong et al., who found a decrease of EPCR in the blood of a pig that was exposed to hypothermia (39). No difference was detected between the expression of EPCR in JAK2WT- and JAK2V617F-HUVECs, which might suggest that the mutation does not have an effect additional to that of thermoregulation on EPCR expression.
TM is a type 1 transmembrane protein predominantly expressed in the endothelium. It has a high binding affinity for TF and protein C and promotes anticoagulant, anti-fibrinolytic, and anti-inflammatory responses (40). In our study, the expression of TM decreased under mild hypothermia in both JAK2WT- and JAK2V617F-HUVECs. There might be different theories about the effects of hypothermia on the TM. TM is converted to a soluble form (sTM), which is as the principal circulating TM generated by either enzymatic or chemical cleavage of the intact protein (41). A study showed lower TM levels in the serum and urine of persons died from hypothermia than in other non-cold-related deaths at the mRNA and protein levels (42). Although the impact of thermoregulation was not examined, another study revealed that the mutation JAK2V617F resulted in higher sTM levels in the plasma of patients with MPN compared to those of healthy controls (43). However, in our study, it might be valuable to notice the more significant decrease in the expression of TM in JAK2V617F-HUVECs (p=0.0016) compared to JAK2WT-HUVECs (p=0.0231) under mild hypothermia (Figure 2B). This could be a direct effect of JAK2V617F on TM protein expression via thermoregulation-mediated control of TM gene transcription.
TF is a type 1 integral membrane receptor for factor (F) VII/VIIa. The TF–FVIIa complex is involved in thrombosis by activating the extrinsic coagulation pathway and inflammation (44). A study by Johnson et al. revealed that hypothermia (25˚C) reversibly inhibited the transcription and expression but not the induction of TF in ECs (45). In this study, TF expression in JAK2V617F-HUVECs decreased under mild hypothermia (p=0.0133). There was a slight, not statistically significant decrease in TF expression in JAK2WT-HUVECs (Figure 2C). Considering the treatment modalities of mild hypothermia established as a protective therapeutic strategy for patients having coronary artery surgery (46), cardiac arrest (47), and coma (48), this result might indicate that the mechanisms causing temperature-dependent TM and TF suppression, especially in a hypothermic environment, help reduce the thrombotic events better in the JAK2V617F positive patients than in JAK2V617F negative patients with MPN.
Surprising results were obtained with MP analysis. MPs are extracellular vesicles produced by all cell types under normal and pathological conditions. It is well established that under low temperatures, cellular metabolic activity is also decreased (49), and the quantity of MPs released from the cells is expected to decrease. In support, a study found the temperature-dependent secretion of the smaller EVs called exosomes (100-150 nm diameter) and identified the responsible gene, low-density lipoprotein receptor (LDLR), whose expression is also thermally controlled (22). Our results also indicate that the number of PECAM-1-positive EMPs released from either JAK2WT- or JAK2V617F-HUVECs decreased under mild hypothermic conditions (p=0.0001 and p=0.0110, respectively) (Figure 3A).
However, the high MP production capacity of JAK2V617F-HUVECs might be related to the effect of JAK2V617F mutation on EV production under mild hypothermic conditions. Similarly, higher levels of TF-positive MPs were released from JAK2V617F-HUVECs under mild hypothermic conditions compared to JAK2WT-HUVECs (p=0.0434 and p=0.0006, respectively) (Figure 3B). Although, there is no known connection between JAK2V617F mutation and the LDLR gene, the considerably higher release of EMPs from JAK2V617F-HUVECs might also be related to the indirect JAK2V617F effect on LDLR function.
Comparison of the levels of PECAM-1- and TF-positive MPs between JAK2WT- and JAK2V617F-HUVECs revealed a more significant decrease in MPs released from JAK2WT-HUVECs under mild hypothermia. It is notable that a study revealed that the levels of tumor TF and TF-positive EVs were associated with venous thromboembolism and disseminated intravascular coagulation in patients with pancreatic cancer (50). Since thermoregulation affects the production of MPs, controlling EVs using hypothermia might impact therapy.
Here, we investigated the possible effects of hypothermia and normothermia on the genetically modified HUVECs. We also analyzed the expression of EC surface markers and the levels of microparticles that have essential roles in managing blood hemostasis under thermoregulation. Overall, JAK2V617F mutation was found to have a profound effect on infection capacity and MP production under mild hypothermic conditions compared to normothermia. TM and TF levels were decreased in JAK2V617F-HUVECs compared to JAK2WT-HUVECs under mild hypothermia. This might indicate that the coagulation cascade might have diminished influence under hypothermia on JAK2V617F-HUVECs. A detailed analysis of EVs, including EMPs, is required to clarify further the molecular mechanisms linking EVs and thermal changes in tumor microenvironments and the importance of its clinical application in cancer therapy.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
Study design: Selcuk Sozer, Hilal Hekimoğlu; Data collection: Hilal Hekimoğlu, Selcuk Sozer; Statistical analysis: Hilal Hekimoğlu; Data interpretation: Selcuk Sozer; Manuscript preparation: Hilal Hekimoğlu, Esra Nur Demirtaş, Selcuk Sozer; Literature search: Hilal Hekimoğlu, Selcuk Sozer; Funds collection: Selcuk Sozer.
Acknowledgements
The first Author of this manuscript, Hilal Hekimoğlu, is supported by the 100/2000 YÖK Ph.D. scholarship program. The Research Fund of Istanbul University (Project No: 58164) and The Research Fund of TUBITAK (Project No. 111S173) supported the present work.
References
- 1.Arber D, Orazi A, Hasserjian R, Thiele J, Borowitz M, Le beau M, Bloomfield C, Cazzola M, Vardiman J. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2019;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couédic J, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval J, Constantinescu S, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2023;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Baxter E, Scott L, Campbell P, East C, Fourouclas N, Swanton S, Vassiliou G, Bench A, Boyd E, Curtin N, Scott M, Erber W, Green A. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. The Lancet. 2020;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 4.Levine R, Wadleigh M, Cools J, Ebert B, Wernig G, Huntly B, Boggon T, Wlodarska I, Clark J, Moore S, Adelsperger J, Koo S, Lee J, Gabriel S, Mercher T, D’Andrea A, Fröhling S, Döhner K, Marynen P, Vandenberghe P, Mesa R, Tefferi A, Griffin J, Eck M, Sellers W, Meyerson M, Golub T, Lee S, Gilliland D. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2020;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Kralovics R, Passamonti F, Buser A, Teo S, Tiedt R, Passweg J, Tichelli A, Cazzola M, Skoda R. A gain-of-function mutation of JAK2 in myeloproliferative disorders. New England Journal of Medicine. 2020;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 6.Klampfl T, Gisslinger H, Harutyunyan A, Nivarthi H, Rumi E, Milosevic J, Them N, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer G, Bagienski K, Milanesi C, Casetti I, Sant’Antonio E, Ferretti V, Elena C, Schischlik F, Cleary C, Six M, Schalling M, Schönegger A, Bock C, Malcovati L, Pascutto C, Superti-Furga G, Cazzola M, Kralovics R. Somatic mutations of calreticulin in myeloproliferative neoplasms. New England Journal of Medicine. 2017;369(25):2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 7.Guglielmelli P, Calabresi L. The MPL mutation. Cellular and Molecular Aspects of Myeloproliferative Neoplasms - Part. 2023;A:163–178. doi: 10.1016/bs.ircmb.2021.09.003. [DOI] [Google Scholar]
- 8.James C, Ugo V, Casadevall N, Constantinescu S, Vainchenker W. A JAK2 mutation in myeloproliferative disorders: pathogenesis and therapeutic and scientific prospects. Trends in Molecular Medicine. 2019;11(12):546–554. doi: 10.1016/j.molmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Guijarro-Hernandez A, Vizmanos JL. A broad overview of signaling in ph-negative classic myeloproliferative neoplasms. Cancers (Basel) 2021;13(5):984. doi: 10.3390/cancers13050984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leiva O, Hobbs G, Ravid K, Libby P. Cardiovascular disease in myeloproliferative neoplasms: Jacc: Cardiooncology state-of-the-art review. JACC CardioOncol. 2022;4(2):166–182. doi: 10.1016/j.jaccao.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruger-Genge A, Blocki A, Franke RP, Jung F. Vascular endothelial cell biology: An update. Int J Mol Sci. 2019;20(18):4411. doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farina M, Russo D, Hoffman R. The possible role of mutated endothelial cells in myeloproliferative neoplasms. Haematologica. 2021;106(11):2813–2823. doi: 10.3324/haematol.2021.278499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sozer S, Fiel M, Schiano T, Xu M, Mascarenhas J, Hoffman R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2019;113(21):5246–5249. doi: 10.1182/blood-2008-11-191544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sozer S, Ishii T, Fiel M, Wang J, Wang X, Zhang W, Godbold J, Xu M, Hoffman R. Human CD34+ cells are capable of generating normal and JAK2V617F positive endothelial like cells in vivo. Blood Cells, Molecules, and Diseases. 2019;43(3):304–312. doi: 10.1016/j.bcmd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Farina M, Russo D, Hoffman R. The possible role of mutated endothelial cells in myeloproliferative neoplasms. Haematologica. 2022;106(11):2813–2823. doi: 10.3324/haematol.2021.278499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teofili L, Martini M, Iachininoto M, Capodimonti S, Nuzzolo E, Torti L, Cenci T, Larocca L, Leone G. Endothelial progenitor cells are clonal and exhibit the JAK2V617F mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood. 2019;117(9):2700–2707. doi: 10.1182/blood-2010-07-297598. [DOI] [PubMed] [Google Scholar]
- 17.Oppliger Leibundgut E, Horn MP, Brunold C, Pfanner-Meyer B, Marti D, Hirsiger H, Tobler A, Zwicky C. Hematopoietic and endothelial progenitor cell trafficking in patients with myelo-proliferative diseases. Haematologica. 2006;91(11):1465–1472. [PubMed] [Google Scholar]
- 18.Yoder M, Mead L, Prater D, Krier T, Mroueh K, Li F, Krasich R, Temm C, Prchal J, Ingram D. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2023;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101(3):439–451. [PubMed] [Google Scholar]
- 20.Mause S, Weber C. Microparticles. Circulation Research. 2022;107(9):1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 21.Voloshin T, Fremder E, Shaked Y. Small but mighty: microparticles as mediators of tumor progression. Cancer Microenvironment. 2020;7(1-2):11–21. doi: 10.1007/s12307-014-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owens A, Mackman N. Microparticles in hemostasis and thrombosis. Circulation Research. 2022;108(10):1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwicker J, Liebman H, Neuberg D, Lacroix R, Bauer K, Furie B, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clinical Cancer Research. 2022;15(22):6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Qi J, Zhao S, Shen W, Dai L, Han W, Huang M, Wang Z, Ruan C, Wu D, Han Y. Clinical significance of circulating microparticles in Ph- myeloproliferative neoplasms. Oncology Letters. 2019;14(2):2531–2536. doi: 10.3892/ol.2017.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hekimoglu H, Toprak SF, Sozer S. Jak2v617f-positive endothelial cells induce apoptosis and release jak2v617f-positive microparticles. Turk J Haematol. 2022;39(1):13–21. doi: 10.4274/tjh.galenos.2021.2021.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall JE, Hall ME, Hall J. Guyton and hall textbook of medical physiology. Fourteenth edition. edn. Philadelphia, PA, USA, Elsevier. 2021 [Google Scholar]
- 27.Wong KC. Physiology and pharmacology of hypothermia. West J Med. 1983;138(2):227–232. [PMC free article] [PubMed] [Google Scholar]
- 28.Vaghebin R, Khalili M, Amiresmaili S, Namdar H, Javad Mousavi M. Treatment of traumatic brain injury from the viewpoint of Avicenna (Ibn Sina): A historical review. Interdisciplinary Neurosurgery. 2022;28:101498. doi: 10.1016/j.inat.2022.101498. [DOI] [Google Scholar]
- 29.Wang H, Olivero W, Wang D, Lanzino G. Cold as a therapeutic agent. Acta Neurochirurgica. 2019;148(5):565–570. doi: 10.1007/s00701-006-0747-z. [DOI] [PubMed] [Google Scholar]
- 30.Fay T. Observations on prolonged human refrigeration. Anesthesiology. 2021;2(3):347–347. doi: 10.1097/00000542-194105000-00018. [DOI] [Google Scholar]
- 31.Zhang XM, Lv YG, Chen GB, Zou Y, Lin CW, Yang L, Guo P, Lin MP. Effect of mild hypothermia on breast cancer cells adhesion and migration. Biosci Trends. 2012;6(6):313–324. [PubMed] [Google Scholar]
- 32.Kalamida D, Karagounis I, Mitrakas A, Kalamida S, Giatromanolaki A, Koukourakis M. Fever-range hyperthermia vs. hypothermia effect on cancer cell viability, proliferation and HSP90 expression. PLOS ONE. 2020;10(1):e0116021. doi: 10.1371/journal.pone.0116021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulbert C, Gaude C, Sulpice E, Chabardès S, Ratel D. Moderate hypothermia inhibits both proliferation and migration of human glioblastoma cells. Journal of Neuro-Oncology. 2020;144(3):489–499. doi: 10.1007/s11060-019-03263-3. [DOI] [PubMed] [Google Scholar]
- 34.Condon H. Protective hypothermia for cancer chemotherapy. British Journal of Anaesthesia. 2022;39(10):806–812. doi: 10.1093/bja/39.10.806. [DOI] [PubMed] [Google Scholar]
- 35.Wallner B, Schenk B, Hermann M, Paal P, Falk M, Strapazzon G, Martini W, Brugger H, Fries D. Hypothermia-associated coagulopathy: a comparison of viscoelastic monitoring, platelet function, and real time live confocal microscopy at low blood temperatures, an in vitro experimental study. Frontiers in Physiology. 2020;11:843. doi: 10.3389/fphys.2020.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Göksu A, Hekimoğlu H, Sözer Tokdemir S. The effect of jak2v617f mutation to the endothelial cells and the expression profiles of socs1-4 genes in myeloproliferative neoplasms. J Adv Health Sci Res. 2020;3(3):135–147. doi: 10.26650/JARHS2020-790117. [DOI] [Google Scholar]
- 37.Van Ierssel S, Van Craenenbroeck E, Conraads V, Van Tendeloo V, Vrints C, Jorens P, Hoymans V. Flow cytometric detection of endothelial microparticles (EMP): Effects of centrifugation and storage alter with the phenotype studied. Thrombosis Research. 2020;125(4):332–339. doi: 10.1016/j.thromres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein c activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci USA. 1996;93(19):10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong P, Zhang M, Zhao H, Tang Z, Hua R, Mei X, Cui J, Li C. Effect of mild hypothermia on the coagulation-fibrinolysis system and physiological anticoagulants after cardiopulmonary resuscitation in a porcine model. PLoS ONE. 2019;8(6):e67476. doi: 10.1371/journal.pone.0067476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boehme MW, Deng Y, Raeth U, Bierhaus A, Ziegler R, Stremmel W, Nawroth PP. Release of thrombomodulin from endothelial cells by concerted action of tnf-alpha and neutrophils: In vivo and in vitro studies. Immunology. 1996;87(1):134–140. [PMC free article] [PubMed] [Google Scholar]
- 41.Scharbert G, Kalb M, Essmeister R, Kozek-Langenecker S. Mild and moderate hypothermia increases platelet aggregation induced by various agonists: a whole blood in vitro study. Platelets. 2020;21(1):44–48. doi: 10.3109/09537100903420269. [DOI] [PubMed] [Google Scholar]
- 42.Pakanen L, Kaija H, Kortelainen M, Särkioja T, Porvari K. Victims of lethal hypothermia have decreased levels of thrombomodulin in myocardium and urine. International Journal of Legal Medicine. 2022;129(2):289–296. doi: 10.1007/s00414-014-1138-2. [DOI] [PubMed] [Google Scholar]
- 43.Arellano-Rodrigo E, Alvarez-Larrán A, Reverter J, Colomer D, Villamor N, Bellosillo B, Cervantes F. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: Relationship with thrombosis occurrence and JAK2 V617F allele burden. American Journal of Hematology. 2021;84(2):102–108. doi: 10.1002/ajh.21338. [DOI] [PubMed] [Google Scholar]
- 44.Ollivier V, Bentolila S, Chabbat J, Hakim J, de Prost D. Tissue factor-dependent vascular endothelial growth factor production by human fibroblasts in response to activated factor vii. Blood. 1998;91(8):2698–2703. [PubMed] [Google Scholar]
- 45.Johnson M, Haddix T, Pohlman T, Verrier ED. Hypothermia reversibly inhibits endothelial cell expression of e-selectin and tissue factor. J Card Surg 10(4. 1995;Suppl):428–435. doi: 10.1111/j.1540-8191.1995.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 46.Nathan H, Wells G, Munson J, Wozny D. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass. Circulation. 2016;104(suppl 1):I–85-I-91. doi: 10.1161/hc37t1.094710. [DOI] [PubMed] [Google Scholar]
- 47.Dzięcioł M, Kacprzak M, Goleniewska B, Zielińska M. Osborn wave in patients with st-elevation myocardial infarction undergoing mild therapeutic hypothermia after cardiac arrest. Acta Cardiologica. 2014;69(5):532–540. doi: 10.1080/AC.69.5.3044880. [DOI] [PubMed] [Google Scholar]
- 48.Dell’Anna AM, Taccone FS, Halenarova K, Citerio G. Sedation after cardiac arrest and during therapeutic hypothermia. Minerva anestesiologica. 2014;80(8):954–962. [PubMed] [Google Scholar]
- 49.Wang J, Wei Y, Zhao S, Zhou Y, He W, Zhang Y, Deng W. The analysis of viability for mammalian cells treated at different temperatures and its application in cell shipment. PLOS ONE. 2020;12(4):e0176120. doi: 10.1371/journal.pone.0176120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hisada Y, Mackman N. Tissue factor and extracellular vesicles: activation of coagulation and impact on survival in cancer. Cancers. 2021;13(15):3839. doi: 10.3390/cancers13153839. [DOI] [PMC free article] [PubMed] [Google Scholar]