Abstract
Background/Aim: A prospective randomized, open-label, single-blinded clinical trial was conducted to evaluate the efficacy of AFree on the symptoms and course of moderate and severe COVID-19 in the field hospital.
Patients and Methods: Two hundred hospitalized patients diagnosed with COVID-19 were enrolled. The patients were randomized into 100 patients in the interventional AFree group and 100 in the control group. The AFree group patients were treated with AFree oral spray in conjunction with the standard COVID-19 treatment protocol, while the control group of patients were treated with only standard care.
Results: Patients of the AFree group demonstrated a remarkedly faster improvement in all COVID-19-related symptoms, resulting in a shorter time for complete recovery than the control group. More importantly, they showed a shorter time for complete viral clearance. Adding AFree to the standard of care protocol also significantly improved the restoration of taste and smell and reduced lung infiltration. Additionally, the patients in the AFree group also exhibited fewer adverse effects related to treatment.
Conclusion: AFree oral spray is a simple-to-use, safe, and effective adjunctive treatment for moderate and severe COVID-19 cases. AFree oral spray was demonstrated to potentially be effective for COVID-19 prevention.
Keywords: COVID-19, Zinc, Iodide, dimethyl sulfoxide, DMSO, AFree, viral infection, SARS-CoV-2
The COVID-19 pandemic caused devastating damage to individual and community health with a disproportionately high number of hospitalizations and deaths. The symptoms of COVID-19 patients can vary from none, mild to life-threatening or even deaths in many cases. This clinical observation suggested the critical role of the host’s innate immunity in disease development and progression. As the first defense barrier against pathogens, the innate immune response determines the viral effectiveness, disease progression, and outcome (1,2). Using natural agents with mucosal and systemic immune-enhancing activities might play a vital role in preventing and treating COVID-19 (1).
Due to the airborne transmission of COVID-19 via exposure to infectious respiratory droplets, saliva, or direct contact, controlling the viral loads in the saliva and respiratory secretions is crucial. Thus, a cost-effective strategy to prevent cross-contamination and community transmission by implementing effective oral and throat hygiene has been recommended (2).
Several published reviews and meta-analyses have documented the importance of good oral hygiene and chemical antiseptic/disinfectant agents to prevent respiratory disorders and nosocomial infections (3-5). In our prior publication, we suggested that the implementation of zinc iodide in combination with dimethyl sulfoxide (DMSO) could provide a viable solution for preventing and treating COVID-19 (6-8). Zinc iodide and DMSO have been demonstrated in clinical and experimental studies as safe and effective antiviral, anti-inflammatory, antimicrobial, antifungal agents with mucosal, and systemic immune-enhancing activities (9,10).
AFree oral spray is registered in Vietnam as an oral sanitizer and disinfectant. The formulas were researched and developed by the Nimni-Cordoba Tissue Engineering and Drug Discovery Laboratory of the Department of Surgery at the Keck School of Medicine of the University of Southern California (Los Angeles, CA, USA) in cooperation with Thai Minh Pharmaceutical Company (Hanoi, Vietnam). AFree has been tested for acute and chronic toxicity, skin and mucosal irritation, and thyroid toxicity by the Pharmacology Department of the National Institute of Drug Quality Control of Vietnam. The results showed excellent tolerability and a safe drug profile. The key ingredients of AFree are zinc iodide, ginger extract, propolis extract, natural fruit flavor, xylitol, DMSO, and pure water.
This prospective randomized, open-label single-blinded parallel-group clinical trial was conducted to evaluate the efficacy of AFree oral spray in conjunction with standard of care on the symptoms and course of the disease in patients with moderate and severe COVID-19 in the resource-limited field hospital established by the Public Security Department of Vietnam.
Our study aimed to evaluate the efficacy and safety of AFree as an inexpensive and simple-to-use medication for treating and preventing COVID-19 in the field hospital. Due to the limited medical and human resources during the COVID-19 outbreak, we could only focus on studying and recording the most essential clinical and paraclinical data related to COVID-19 symptoms, disease progression, and substantiating a possible immediate implementation of AFree for COVID-19 treatment and prevention.
Patients and Methods
Study materials. AFree is registered and approved as an oral sanitizer and disinfectant medical device in Vietnam. Thai Minh Pharmaceutical Company manufactured and provided AFree for the field hospital as a donation. In Vietnam, AFree is distributed in the form of a medical device with the registration number 2100000725/PCBA-HN, and is used as an oral spray to help alleviate coughs, irritation, and sore throat and prevent respiratory infections caused by viruses and bacteria.
Study location. The study was conducted at Phuoc Loc field hospital, Ministry of Public Security (No. 17 Pham Hung, Phuoc Loc Commune, Nha Be District, Ho Chi Minh City, Vietnam).
Study design. The current study was prospective randomized, open-label, single-blinded (the investigators were blinded) using the parallel group method.
Inclusion criteria. COVID-19-positive hospitalized patients were laboratory-confirmed using RT- PCR with the sample collected within 72 h before the randomization. Male or non-pregnant females older than 15 years were enrolled. Subjects with clinical evidence of moderate to severe COVID-19 were defined according to the Vietnamese Health Ministry recommendations (Instruction 3416/QĐ-BYT dated 14/7/2021).
Exclusion criteria. A subject was not eligible for this study if any criteria below applied:
i) The physician decided that trial involvement was not in the patient’s best interest or that any condition did not allow the protocol to be followed safely.
ii) The patient was already in or had already been in, another clinical trial of an experimental treatment for COVID-19.
iii) The patient had a known medical history of allergy to zinc and iodine.
iv) The patient already received dialysis (either acute or chronic) or was imminent need of dialysis at the time of enrolment.
Study groups. Two hundred moderate and severe COVID-19 patients were randomized into the interventional group (AFree group) and the control group. The 100 patients in the AFree group received AFree oral spray 5 to 10 times daily, 5 sprays each time depending on the severity of the disease (moderate: 5 sprays, severe: 10 sprays) in conjunction with standard of care for COVID-19 and comorbidities. AFree oral spray was provided for 14 days. Meanwhile, 100 patients in the control group received only standard care protocol.
Severity classification. Moderate cases show lower respiratory disease during clinical assessment and chest X-ray examination with clinical features of dyspnea and/or hypoxia, SpO2 ≥94% on room air, and respiratory rate ≥24 and <30 breaths/min. Severe cases show lower respiratory disease during clinical assessment and chest X-ray examination with clinical features of dyspnea and hypoxia, SpO2 <94% on room air, and a respiratory rate ≥30 breaths/min.
Outcome assessments. The study outcome was assessed according to clinical symptomatology progress, time for recovery and symptom clearance, viral clearance rate, chest X-ray examination, and adverse effects of the treatment.
Statistical analysis. The statistical data analysis was performed using the software IBM SPSS Statistics 25.0 (IBM, Armonk, NY, USA) and other programs for medical research. Statistical tests were two-tailed, and a p-value <0.05 was considered to indicate significant differences.
Research ethics. Patients who were eligible as per the inclusion criteria were asked to consent to participate in the trial. Patients had the right to withdraw from the study at any time and for any reason. The Ministry of Health of Vietnam (Hanoi, Vietnam) has granted permission to distribute AFree Oral Spray. The study protocol 0016/QĐ-IMP was approved by the ethical committee of Thang Long Institute for Medical and Pharmaceutical Research (Hanoi, Vietnam).
Results
In our study, the proportions of males and females in each group were similar, with 57.0% of males and 43.0% of females in the control group and 58.0% males and 42.0% females in the AFree group.
As presented in Table I, there were no statistically significant differences in age between patients in AFree and control groups (p=0.1, p>0.05).
Table I. Age distribution of patients in AFree and control groups.
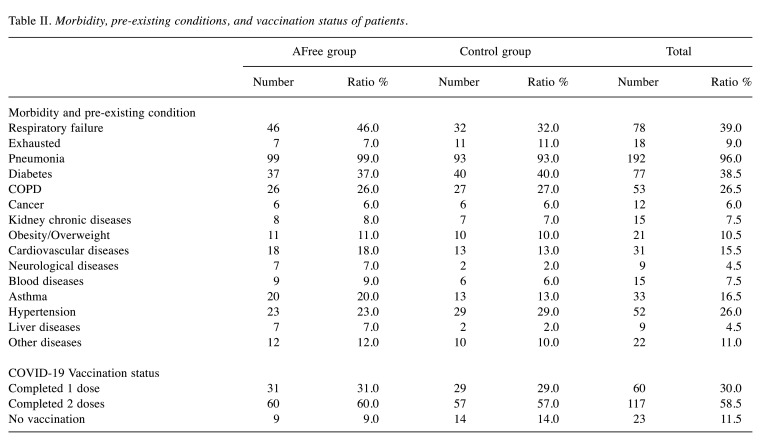
Table II lists the morbidity, pre-existing conditions, and vaccination status of the AFree and control groups. The most prevalent complications of COVID-19 in the studied patients were pneumonia (96.0%) and respiratory failure (39.5%). The common pre-existing comorbidities were diabetes (38.5%), chronic obstructive pulmonary disease (31.5%), hypertension (26.0%), bronchial asthma (16.5%), and cardiovascular diseases (15.5%). The complication of COVID-19 and concomitant diseases were comparable across the control and AFree groups with p>0.05. Based on the vaccination rates shown in Table II, the statistical data suggested a similarity between the two study groups with a p-value of >0.05.
Table II. Morbidity, pre-existing conditions, and vaccination status of patients.
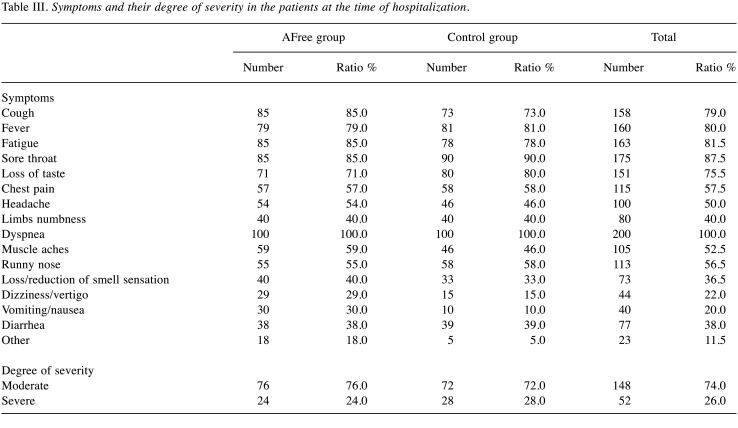
According to the data in Table III, patients in both groups had dyspnea (100.0%), sore throat (87.5%), excessive tiredness (81.5%), fever (80.0%), cough (79.0%), taste loss (75.5%), pain and chest tightness (57.5%), runny nose (56.5%), headache (50.0%), muscle pain (42.5%), numbness in limbs (40.0%), diarrhea (38.0%), loss/reduction of smell sensation (36.5%), dizziness/vertigo (22.0%) and vomiting/nausea (20.0%). Despite a higher number of patients in the AFree group presenting with vomiting and nausea, there were no noticeable differences in the symptomatology prevalence between the AFree and control groups at the time of admission. Also shown in Table III, the levels of severity were comparable in both groups, and the difference was not statistically significant (p>0.05).
Table III. Symptoms and their degree of severity in the patients at the time of hospitalization.
Altogether, Table I, Table II, and Table III show that the baseline demographic characteristics of all recruited patients in the study groups were compatible. The AFree and the control group can be considered statistically indistinguishable.
Treatment results. The treatment for COVID-19 patients in the study groups adhered to the Vietnamese Ministry of Health’s guidelines. As shown in Table IV, other than the administration of AFree in the AFree group patients, both study groups had the same medication profile following the standard of care protocol for COVID-19.
Table IV. Treatment medications for COVID-19 patients in the study groups.
Figure 1 shows that 15.5% of patients got oxygen via glasses, 57.0% received oxygen through masks, 25.0% received high-flow nasal cannula oxygen therapy, and 2.5% received non-invasive mechanical ventilation. The oxygen therapy usage in the AFree and the control groups was relatively similar (p>0.05).
Figure 1. Oxygen delivery methods to patients in the sudy groups.
Our study shows that the number of patients experiencing adverse effects in the AFree group was low. Only four patients suffered diarrhea, three had dizziness, and two had vomiting and nausea. Meanwhile, this rate was higher in the control group, where 18 patients experienced diarrhea, six had dizziness, and three had vomiting and nausea. Table V shows that more treatment adverse effects were observed in the control group than in the AFree group.
Table V. Undesirable side-effects of treatment in the study groups.
p=0.035, p<0.05; the p-value is for the total number of side effects.
The data in Figure 2 show a significant statistically significant difference in the ratio of patients with COVID-19-related symptoms in the AFree and control groups after four days of treatment with a p-value of <0.05 (p=0.03-0.01). Furthermore, on the fifth day of therapy, just 40.0% of patients in the AFree group exhibited clinical symptoms, while 60% had none. By the seventh day, only 1.0% of the patients in the AFree group showed clinical symptoms, while 99.0% were symptom-free. Compared to the control group, the improvement in COVID-19 symptomatology in the AFree group was highly significantly different starting from day five of treatment, and this trend continued to the end of the study.
Figure 2. Comparison of Covid-19 clinical symptoms improvement by treatment day.
The effect of AFree on restoring taste and smell. In the AFree group, after approximately four days of treatment, patients who had lost their sense of smell and taste could start to smell and taste food. In the control group, after at least seven days, patients were able to regain their sense of taste and smell after ten days.
In our study, the proportion of patients who recovered in less than 10 days was similar between the two groups (Figure 3). Most patients in both study groups needed treatment for 10-14 days, however a relatively low rate (6.0%) of patients in the AFree group and 27.0% of patients in the control group required more than 14 days of treatment. Patients in the AFree group had an average treatment duration of 10.6±2 days, whereas those in the control group had an average treatment duration of 13.5±2 days. This difference is statistically significant with a p-value of <0.05.
Figure 3. Treatment duration for Covid-19 of patients in the study groups.
Image examination and laboratory test results of study groups. Patients received a chest X-ray weekly. The number of patients who needed to take the second and third X-ray was 95 and 5 in the AFree group and 98 and 8 in the control group, respectively. The second and third chest X-rays, performed after the seventh day of treatment, showed signs of lung damage in 44.0% of patients in the AFree group and 66.0% of patients in the control group. The AFree group exhibited a remarkable reduction in lung infiltration (Figure 4).
Figure 4. Control chest X-ray results of patients in the study groups.
As shown in Table VI, the initial RT-PCR test showed 100% COVID-19 positivity at admission. The second test, carried out on the ninth to tenth day after admission, showed that 80% and 73.0% of patients in the AFree and control groups had negative results, respectively. To evaluate hospital discharges, the third RT-PCR test was performed 48 h following the second test. The AFree group reached 91.0% negative, whereas that of the control group was 81.0%. The remaining positive cases, whose cycle threshold was not assured as specified, were evaluated using RT-PCR for the fourth time after 24 h. The AFree group 98.0% of patients were negative compared to 91% of patients in the control group. The RT-PCR test results indicated patients in the AFree group had a faster viral clearance rate.
Table VI. RT-PCR test results of patients in the study groups.
p=0.047, p<0.05.
Discussion
Morbidity, mortality, infectiveness, and spread of SARS-CoV-2 depend on the host-pathogen relationship. Given the lack of inexpensive, effective, and safe antiviral drugs for COVID-19, especially in countries with limited resources, there is a need to focus on supporting the discovery and implementation of possible complementary therapeutic approaches. Additionally, pathological viruses such as SARS-CoV-2 are constantly mutated into different strains or variants that may limit the effectiveness of antiviral drugs. Therefore, research and development of holistic preventive and therapeutic strategies based on boosting the human body’s innate and adaptive immune defense mechanisms to combat COVID-19 should be encouraged, especially in a scenario where the virus may become endemic and seasonally recurrent (1,10,11).
Oral therapeutic intervention can substantially combat the COVID-19 pandemic, as intact oral mucosa circumvents the viral invasiveness and penetration (2,6,8). Additionally, the saliva secreted by the oral mucosal membrane is a primary transmission route of viruses and a potential source of recontamination and community spread of infection (3,4,12).
Viral agents such as SARS-CoV-2 can infect the upper respiratory tract for colonization and proliferation within the first few days after entering the body. Our study strongly indicated that effective therapeutics for preventing and treating the disease, including AFree oral spray, could be administered in the oral and pharyngeal cavities.
We acknowledge that our current study has limitations. This is a non-funded study conducted in a limited resource field hospital recently established during the emergency with COVID-19 breakout in Ho Chi Minh City. We could not prepare and use a placebo in the control group, hence we were not able to perform a double-blinded study. We could not monitor the dynamics of biomarkers for inflammation and coagulation abnormalities in the patients to document possible paraclinical evidence of additional substantial objective therapeutic benefits of AFree spray.
Despite the limitations mentioned above, the data of our present study clearly showed the robust therapeutic and possible preventive efficacy of AFree oral spray. Besides significant reduction in COVID-19 symptomatology, positive impact on the course of the disease, and viral clearance rate, our current study suggested using AFree early in the clinical practice may reduce the numbers of patients progressing to severe disease and transmission of COVID-19 in the community.
AFree is composed of well-characterized and proven natural and over-the-counter pharmaceutical ingredients with a safe profile. It is an exceptional advantage applying AFree as a therapeutic product against COVID-19 due to the considerable length of time required to research and develop a new drug.
A larger and well-designed clinical trial is warranted to explore more potential therapeutic activities of AFree and promote a possible, more comprehensive application of this simple-to-use, inexpensive, and effective oral spray for the treatment and prevention of COVID-19.
Conclusion
The current study results demonstrate that AFree oral spray is an inexpensive, simple-to-use, and effective treatment for moderate and severe COVID-19 cases. The data of our study also suggested that AFree can potentially be effective as primary and secondary prevention of COVID-19.
Conflicts of Interest
The Authors declare no conflicts of interest regarding the data presented in this publication.
Authors’ Contributions
DTT: Investigation, data curation, writing – original draft, TNP: Investigation, data curation, writing – review & editing, NHTN: Investigation, data curation, HDT: Conceptualization, visualization, writing – original draft, HQH: Writing – review & editing, AKN: Writing-review & editing, BH: Conceptualization, prepared the tables, figure, writing – review & editing, BXH: Methodology, conceptualization, writing-original draft-reviewing & editing. All Authors read and approved the manuscript prior to submission.
References
- 1.Han B, Hoang B. Opinions on the current pandemic of COVID-19: Use functional food to boost our immune functions. Journal of Infection and Public Health. 2022;13(12):1811–1817. doi: 10.1016/j.jiph.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chopra A, Sivaraman K, Radhakrishnan R, Balakrishnan D, Narayana A. Can povidone iodine gargle/mouthrinse inactivate SARS-CoV-2 and decrease the risk of nosocomial and community transmission during the COVID-19 pandemic? An evidence-based update. Japanese Dental Science Review. 2022;57:39–45. doi: 10.1016/j.jdsr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. Journal of Medical Virology. 2022;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.To K, Tsang O, Yip C, Chan K, Wu T, Chan J, Leung W, Chik T, Choi C, Kandamby D, Lung D, Tam A, Poon R, Fung A, Hung I, Cheng V, Chan J, Yuen K. Consistent detection of 2019 novel Coronavirus in saliva. Clinical Infectious Diseases. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. International Journal of Oral Science. 2023;12(1):9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoang B, Hoang H, Han B. Zinc Iodide in combination with Dimethyl Sulfoxide for treatment of SARS-CoV-2 and other viral infections. Med Hypotheses. 2020;143:109866. doi: 10.1016/j.mehy.2020.109866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoang C, Nguyen A, Nguyen T, Fang W, Han B, Hoang B, Tran H. Application of dimethyl sulfoxide as a therapeutic agent and drug vehicle for eye diseases. Journal of Ocular Pharmacology and Therapeutics. 2021;37(8):441–451. doi: 10.1089/jop.2021.0043. [DOI] [PubMed] [Google Scholar]
- 8.Hoang B, Han B. A possible application of hinokitiol as a natural zinc ionophore and anti-infective agent for the prevention and treatment of COVID-19 and viral infections. Medical Hypotheses. 2021;145:110333. doi: 10.1016/j.mehy.2020.110333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang L, Roth M, S’ng C, Tamm M, Han B, Hoang B. Zinc salicylate reduces airway smooth muscle cells remodelling by blocking mTOR and activating p21(Waf1/Cip1) The Journal of Nutritional Biochemistry. 2021;89:108563. doi: 10.1016/j.jnutbio.2020.108563. [DOI] [PubMed] [Google Scholar]
- 10.Hoang B, Han B. Micronutrient zinc roles in adjunctive therapy for COVID-19 by enhancing patients immunoregulation and tolerance to the pathogen. Reviews in Medical Microbiology. 2022;32(3):149–157. doi: 10.1097/MRM.0000000000000263. [DOI] [Google Scholar]
- 11.Hoang B, Shaw G, Fang W, Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. Journal of Global Antimicrobial Resistance. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. International Journal of Oral Science. 2023;12(1):11. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]