Abstract
Background/Aim: Due to better career opportunities for women and a shift in sex roles, as well as improved reproductive medicine, the age of women who conceive children is rising. A variety of maternal risks and complications that may occur during pregnancy or childbirth in women with advanced maternal age has been examined and reported controversial results. The present study focused on controversial and debatable conclusions regarding the impact of advanced maternal age on maternal and neonatal outcomes.
Patients and Methods: Data from 8,523 patients, who gave singleton birth at the Women’s University Hospital Cologne between 2014 and 2018, were subdivided into two groups: those with maternal age ≥40 years and those <40, and analyzed.
Results: A significantly higher rate of C-section, more preterm births, more low birth weight, and higher incidence of retained placenta were observed in women older than or equal to 40. There were no significant differences regarding postpartum hemorrhage and fetal position. Younger patients tend to have more birth injuries and use more epidural administration. The evaluation of neonatal outcomes using fetal base-excess, birth pH, and Apgar score showed no significant clinical differences.
Conclusion: More antenatal complications could be identified in patients with advanced maternal age. Nonetheless, the neonatal outcomes were comparable and no severe complications in women with advanced maternal age were observed. These findings are due to a well standardized management system for women with risk pregnancies. This encourages better monitoring and care of pregnant women with risk factors.
Keywords: Advanced maternal age, antenatal complications, preterm birth, low birth weight, neonatal outcomes, birth injuries, postpartum hemorrhage, management system for women with highrisk pregnancies
Due to better career opportunities for women and a shift in sex roles, as well as improved reproductive medicine, such as assisted reproductive technology or social freezing, the age of women who are able to and intend to conceive children is rising. Both the number of live births from women younger than 18 years old and older than 40 years old has increased over the last decade. Thus, in 2019, of the 778,090 live births in Germany over 40,000 children were born to women over the age of 40 (1).
In the literature, a variety of maternal risks and complications that may occur during pregnancy or childbirth in women with advanced maternal age have been examined. These include an increased risk of developing gestational diabetes, gestational hypertension, preeclampsia, eclampsia and HELLP syndrome. The correlation of advanced maternal age with placental dysfunction such as placenta previa or premature placental abruption has also been frequently reported (2-11). Moreover, the increasing fetal risks such as increased number of chromosomal aberrations, low or high birth weight, and intrauterine fetal death are known to be correlated with advanced maternal age (3,8,9).
In contrast, there are studies on the impact of advanced maternal age that provide controversial results. Among them, studies examining influences of maternal age on delivery timing, birth injuries, risk of hemorrhage, fetal position, and fetal parameter with umbilical cord pH, base excess, and Apgar score often showed debatable conclusions (12–24).
In the present study, we focused on literature controversially discussed and debatable conclusions regarding the impact of advanced maternal age on maternal and neonatal outcomes.
Patients and Methods
Study design and population. In the present study, data from 8,523 patients, who gave birth at the Women’s Hospital of the University of Cologne between January 2014 and August 2018, were subdivided into two groups, those with maternal age <40 years and those ≥40, and retrospectively analyzed. To achieve unbiased cross-section data of the total population, all patients with a singleton pregnancy were included in the study, irrespective of the planned and unplanned mode of delivery. Parity was considered as an important influencing factor in the data analysis and a separate analysis was performed between nulliparous and multiparous women.
Data analysis. The delivery modes were categorized into caesarean section (C-section), vaginal-operative delivery, and spontaneous delivery. Furthermore, the rate of emergency C-section was analyzed. The gestational age at the time of delivery ranged from 22+0 to 42+0 weeks and was subdivided into extremely preterm birth (22+0-27+6 weeks), very preterm birth (28+0-33+6 weeks), and moderate preterm birth (34+0-36+6 weeks) according to the WHO definition and adjusted by the fact, that antenatal corticosteroids are administered in Germany until 33+6 weeks to prevent neonatal respiratory distress syndrome (25). The birth weight of a newborn was the first weight recorded after birth and was subdivided into extremely low (<1,000 g), very low (1,000 g-1,500 g), and low birth weight (LBW) (1,500 g-2,500 g) (26). Birth injuries were subdivided into perineal tears of I – IV degree and vaginal tears. Episiotomies were also counted here as birth injuries, as no prophylactic episiotomies were performed at the University of Cologne. Retained placenta was defined as an absent, delayed, and/or incomplete expulsion of the placenta such that the postpartum period was prolonged longer than 30 min, which is the second leading cause of postpartum hemorrhage, accounting for approximately 15% (27). According to the WHO, severe bleeding is defined as blood loss of more than 500 ml after vaginal delivery and more than 1,000 ml after C-section. Premature placental abruption is present when the placenta detaches from the uterus before delivery of the baby (28). Fetal position refers to the relationship between the long axis of the child and the long axis of the mother or uterus (29,30). To determine the neonatal outcomes and to monitor the child, various parameters can be used, which allow an objective assessment of the child’s condition. The guideline of the German Society of Gynecology and Obstetrics of 2012 recommends the pH - value and the base excess of the fetal blood as well as the fetal Apgar score (31), which were analyzed in the present study. A labor was called prolonged if any phase of labor exceeded in time and failed to progress (29). Finally, the rate of epidural administration was analyzed.
Statistical analysis. Nominal variables were analyzed using a chi-square test. For ordinal variables, the Mann-Whitney U-test was used. Interval-scaled variables were tested for differences in averages between the groups using a t-test for independent samples, with adjustment of degrees of freedom for inhomogeneous variances. All statistical tests were performed at a 5% significance level. When p<0.05 and <0.01, the results were declared to be statistically significant and highly significant, respectively. The statistical software package SPSS Statistics 26.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis.
Results
A total of 7,819 patients were younger than 40 years (group 1, 91.74%) and 704 patients were older than or equal to 40 (group 2, 8.26%). In group 1, 46.93% were nulliparous women, which were almost 10% more than those in group 2.
Regarding the rate of C-section, a significant difference was found between the groups, both dependent and independent of parity (p<0.001). The proportion of primary and secondary C-sections between the groups, however, showed no significant difference. A total of 3,358 patients in group 1 (42.9%) had C-section, and among them, 1,509 patients (19.3%) had a primary C- section and 1,849 patients (23.6%) had a secondary C-section. In group 2, 381 patients (54.1%) underwent C-section, of which 171 cases (24.3%) were primary C-section and 210 cases (29.8%) were secondary C-section. After accounting for parity, 1,790 nulliparous women underwent C-section in group 1 (44.8%) and 164 women in group 2 (62.4%). The rate of emergency C-sections showed no significant difference [group 1 with 158 (2.0%) and group 2 with 15 (2.1%)]. After considering parity, 87 nulliparous women in group 1 (2.2%) and 2 nulliparous women in group 2 (1%) had emergency C-section. Furthermore, our results showed that group 1 had significantly more spontaneous deliveries than group 2. A total of 3,632 women in group 1 (46.5%) and 269 women in group 2 (38.2%) delivered spontaneously (p<0.001). This significant difference remained even after parity was considered. A total of 1,577 nulliparous women in group 1 (39.4%) and 61 nulliparous women in group 2 (23.2%) delivered spontaneously (p<0.001). Regarding vaginal-operative delivery, we found a significantly increased rate in group 1; it was performed in 829 patients in group 1 (10.6%) and 54 patients in group 2 (7.7%) (p<0.001). After accounting for parity, there was also a significantly increased rate of vaginal - operative deliveries in nulliparous women of group 1 [group 1 with 633 (15.8%) vs. group 2 with 38 (14.4%), p<0.001] (Table I).
Table I. Delivery modes according to maternal age group.
Regarding the gestational age, patients in group 2 had significantly more preterm births than patients in group 1. The patients in group 2 presented significantly more preterm births than those of group 1 [extreme preterm birth 253 (3.2%) vs. 58 (8.2%), very preterm birth 361 (4.6%) vs. 117 (16.6%), moderate preterm birth 769 (9.8%) vs. 224 (31.8%), respectively group 1 vs. group 2]. Accordingly, the patients in group 2 had significantly more newborns with extremely low, very low, and low birth weight [250 (3.2%) vs. 62 (8.8%), 167 (2.1%) vs. 40 (5.7%), 800 (10.2%) vs. 205 (29.1%), respectively group 1 vs. group 2] (Table II).
Table II. Comparison of gestational age and birth weight according to maternal age group.
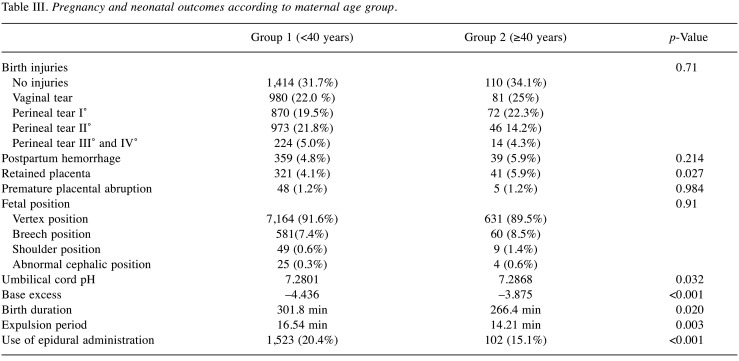
It was observed that younger nulliparous patients tended to have more birth injuries than patients with advanced maternal age. Up to 2nd degree perineal tears, which are considered as moderate birth injuries, the difference in both groups was statistically significant. In 416 nulliparous women of group 1 (10.4%) and in 13 nulliparous women of group 2 (4.6%), vaginal tears were observed. 1st degree perineal tears were found in 339 nulliparous women of group 1 (8.5%) and in 15 nulliparous women of group 2 (5.7%). 2nd degree perineal tears were found in 525 nulliparous women of group 1 (13.1%) and in 28 nulliparous women of group 2 (10.6%) (p<0.001). However, 3rd and 4th degree perineal tears did not show a significant difference [group 1, 224 (5.6%) vs. group 2, 14 (5.3%), for nulliparous women, group 1, 116 (2.9%) vs. group 2, 7 (2.7%)] (Table III and Table IV).
Table III. Pregnancy and neonatal outcomes according to maternal age group.
Table IV. Pregnancy outcomes according to maternal age group in nulliparous women.
Postpartum hemorrhage showed no significant difference between the groups. Regardless of parity, severe bleeding was found in 359 women of group 1 (4.8%) and in 39 women of group 2 (5.9%) (p=0.214). After adjusting for parity, bleeding was observed in 198 nulliparous women of group 1 (5.1%) and in 14 nulliparous women of group 2 (5.7%) (p=0.716). However, we found a significantly increased rate of retained placenta in group 2. Regardless of parity, 41 women in group 2 (5.9%) and 321 women in group 1 (4.1%) were documented to have retained placenta (p=0.027). After accounting for parity, there were 21 nulliparous women in group 2 (8.0%) and 188 nulliparous women in group 1 (4.7%) with retained placenta (p=0.017). Analysis regarding premature placental abruption showed no significant difference between the groups. Regardless of parity, 48 women in group 1 (1.2%) and 5 women in group 2 (1.2%) had premature placental abruption (p=0.984). After accounting for parity, the number of premature placental abruptions was observed in 21 nulliparous women of group 1 (0.9%) and 2 nulliparous women of group 2 (1.0%) (p=0.851) (Table III and Table IV).
Regarding different fetal positions, we did not find any significant difference between the groups. In group 1, 581 women (7.4%) had breech positions, 7,164 women (91.6%) had vertex positions, 49 women (0.6%) had shoulder positions, and 25 women (0.3%) had abnormal cephalic positions. In group 2, 60 women (8.5%) had breech positions, 631 women (89.6%) had vertex positions, 9 women (1.4%) had shoulder positions, and 4 women (0.6%) had abnormal cephalic positions. Even after parity was considered, the difference did not reach significance (Table III and Table IV).
Comparison of postpartum base-excess and birth pH showed significant differences in absolute values. The mean value of umbilical cord pH in newborns of group 2 was 7.2868 and of group 1 was 7.2801 (p=0.032). The mean value of base-excess of group 2 was –3.875 and of group 1 was –4.436 (p<0.001). After considering parity, a significant difference was still found with respect to base excess (group 1: –4.984 vs. group 2: –4.152; p<0.001). The difference in birth pH was no longer significant (p=0.071). Apgar score after 5 min did not show a significant difference independently of parity (p=0.058) or in nulliparous women (p=0.109) (Table III and Table IV).
Significant differences in birth duration were only found independent of parity. Here, we found a significant prolongation of both the expulsion period and the total duration of labor in group 1 (p=0.003). In group 1, the expulsion period was on average 16.54 min compared to 14.21 min in group 2. Total birth duration in group 1 was on average 301.8 min and 266.4 min in group 2 (p=0.020). After considering parity, the differences in nulliparous women in duration of birth (p=0.304) as well as expulsion period (p=0.857) were no longer significant (Table III and Table IV).
Our data show that, regardless of parity, women in group 1 used more epidural administration than in group 2. Overall, there were 1,523 women in group 1 (20.4%) and 102 women in group 2 (15.1%), who used epidural administration (p<0.001). After accounting for parity, the significant difference remained [1,040 women (27.6%) in group 1 versus 54 women (22.0%) in group 2, p<0.001] (Table III and Table IV).
Discussion
In the present study, a significantly higher rate of C-section was observed in women older than or equal to 40 years, regardless of parity. Our findings are consistent with the results from other studies (3-6,8,16-23,32-36). Pawde et al. found a higher rate of C-section in women older than 35 years, which was not, however, statistically significant. They discussed that these findings of a greater preference for C-section compared to vaginal birth were due to the higher age of the expectant mother (15). Ritzinger et al. and Usta et al. also attributed the increased rate of C-section to non-medical factors such as increased nervousness of physicians and mothers due to advanced age and in case of multiparous women to previous birth complications (10,16). In addition, according to Usta et al., the anxiety of expectant mothers for the unborn child plays a crucial role (16). Furthermore, medical indications are also frequently discussed. In the study of Goldmann et al., increased preterm births and complications in the advanced maternal age group were reported, thus indicating a C-section, which was also observed in our study. Advanced maternal age is also known to be correlated with an increase in placenta dysfunction, fetal malpresentations, previous C-section, and multiple pregnancies, which also indicate C-section (22,37). Furthermore, many studies explain the decrease of myometrial functioning with aging as a reason for the increased rate of C-section in women with advanced maternal age (17,20,22,36). Goldman et al. reported a decrease in the effectiveness of myometrial gap junctions as well as numerically fewer but also less sensitive myometrial oxytocin receptors, which subsequently decrease the effectiveness of labor (22). Roustaei et al. additionally referred to decreased uterine blood flow with increased uteroplacental blood flow with advanced age. Here, risk of hemorrhage increased and so that a C-section was indicated (3). Other studies also found an increased risk of severe bleeding, both intrapartum and postpartum, in women older than 40 years (16,19,20). Wang et al. reported an increased rate of postpartum hemorrhages in multiparous women older than 40 years, but no increased bleeding in nulliparous women. As a limiting factor, the authors pointed towards the study population, which consisted of patients with an increased risk profile (20). These results are not consistent with our findings. The same results were reported by Pawde et al., who also found no significant increase in intra- and postpartum bleeding in women with advanced maternal age (15). Regarding retained placenta, Lao et al. did not find an increased risk associated with advanced age. Their rationale lied in decreased perfusion of the uterus at advanced ages due to increased intramyometrial sclerotic lesions compared to younger ages, which, in turn, limits blood flow during labor (38). Hsieh et al. considered these same uteroplacental vasculopathies, which may cause problems in uterine vascularization due to sclerotic lesions in the myometrial arteries, to explain the increased risk of retained placenta in women with advanced age (35). Miller et al. and Usta et al. reported a higher rate of retained placenta in women with advanced age and found uterine scarring due to previous C-section as a possible risk factor (39,40). Usta et al. further explained hypertensive disorders as a reason for higher rate of retained placenta in women older than 35 years. The endothelial damage of uterine vessels due to hypertension may promote adhesion (40). Our results showed a significantly increased incidence of retained placenta in women older than or equal to 40 years, regardless of parity. This could be explained by the probable increased number of women with a history of surgery in uterus including C-section. However, it remained significant, even after adjusting for parity and became even more statistically significant with lower p-value. Therefore, it can be assumed that, independent of the previous mode of delivery, advanced maternal age is associated with an increased risk of retained placenta, which was also confirmed in other studies (35,39,40). Further controversial statements were also found regarding premature placental abruption in the existing literature. While some studies found an increased risk of premature placental abruption with advanced age (2,15,16,22,23,32,35), we did not find an increased risk of this serious complication in our study population and these findings are consistent with results of other studies (6, 18–20). Lean et al. explained the increased risk for premature placental abruption by existing pathologies of the placenta with advanced maternal age (2). Jahromi et al. and Usta et al. reported an association between increased hypertensive disease and the age and natural aging of the uterine vessels (16,23). Pawde et al. mentioned increased antepartum hemorrhage and the frequent occurrence of placental implantation disorder in women with advanced age as possible reasons (15). The differences in results of various studies could also be due to the preventive intervention of each clinic. The prenatal diagnostics can identify and prevent dangerous situations such as the risk of premature placental abruption at an early stage. It should be noted that especially women with preexisting disease or risk have a lower risk of major peripartum complications, which is probably due to the improved and intensive care in hospitals as well as a better self-perception of patients. We attribute the fact that no increased bleeding was observed despite an increased rate of retained placenta in women with advanced maternal age to a better antepartum and postpartum management in a level 1 prenatal center.
In our analysis, we found that women younger than 40 years delivered significantly more often vaginal - operative and spontaneously. Even after eradication of parity as an influencing factor, this significant difference remained. With increased use of epidural administration by women younger than 40 years, an association between increased vaginal - operative deliveries and increased use of epidural administration was observed in our study. This type of analgesia may prolong the total birth process via a concentration-dependent influence on the mother´s sensitivity, motor activity, and sympathetic nervous system activity (16). Thus, prolonged labor could be assumed as an indication for vaginal - operative delivery. In our study population, there was also a significant prolongation of the expulsion period and the total duration of labor in women younger than 40 years. This further strengthens the impression on the association between duration of labor, use of epidural administration and mode of delivery. There are also studies that found no age-dependent differences in birth duration (17,21,34). A possible reason could be age-related loosening of connective tissue, intensified by previous births, that leads to faster delivery in multiparous women with advanced age.
Regarding birth injuries, there was a significantly increased rate of birth injuries in nulliparous women younger than 40 years. Regardless of parity, however, there was no more evidence of an increased risk of birth injury. In a study of Hornemann et al., a proportional association between age and severity of birth injury was reported. Along with the report of Meister et al., they considered higher birth weight and vaginal - operative deliveries as risk factors (41,42). Soong et al. were able to identify first birth as a risk factor independent of age (43). Ogunyemi et al. mentioned younger age as a risk factor in addition to vaginal operative deliveries attributing these findings to tighter and more easily torn connective tissue (44). In our study, we also observed an increased number of vaginal - operative deliveries in younger nulliparous women. Since the risk of perineal and/or vaginal injuries increases during vaginal-operative deliveries, this is a plausible explanation for the increased number of birth injuries.
Many studies reported malpresentations of fetal positions in women with advanced maternal age (8,16-19). In particular, an increased number of breech presentations was observed. On the one hand, this is frequently being attributed to higher rates of uterine leiomyomata and anomalies of the uterus with increased age. On the other hand, it is being explained as a result of multiple pregnancies and an age-related decrease in skeletal muscle. In contrast, no significant correlation between maternal age and fetal malpresentations was found in our study. These results are consistent with studies by Wang et al. and Elser et al., which also showed no differences in fetal position in relation to maternal age (20,34).
Regarding preterm birth, several studies report that women with advanced age were more likely to deliver preterm and low birth weight newborns than younger women (45–47), which is also confirmed in our study. Due to lack of retrospective data evaluation, the reason for preterm birth could not always be analyzed in our study population. As far as can be determined, it showed that premature rupture of membranes was the most common cause, followed by placental dysfunction with or without preeclampsia and intrauterine growth retardation (IUGR). We see the plausible explanation of more preterm birth in women with advanced maternal age as increased pregnancy complication rate. Since advanced maternal age is known to be correlated with an increase in complications like placenta dysfunction, (pre-)eclampsia, IUGR and the incidence of preexisting disease rises with age, the preterm birth is often indicated to get the best outcome for the mother as well as the child (22,37). It thus leads to more newborns with LBW. However, the literature reports inconsistent results about the birth weight respective to maternal age. Some studies report increased age-related macrosomia as well as fetus with low birth weight (2, 5–8, 15, 16, 19, 22, 23, 32, 35, 48). In contrast, a variety of studies found no maternal age-dependent differences in size or weight (20,21). Jahromi et al. explained the increased incidence of LBW in nulliparous women older than 40 years by an increased incidence of certain diseases with age. For example, preexisting hypertensive disease can result in both LBW and IUGR. In addition, age-related changes in uterine vascularization may lead to poorer supply of the fetus, resulting in lower birth weight (23). Jolly et al. also hypothesized decreased perfusion capacity with advanced age. They suggested a decreased transplacental nutrient supply, which can lead to “small for gestational age” fetus (SGA). However, via a possible change in maternal metabolism with altered insulin resistance, an increased nutrient supply to the fetus occurs with hyperinsulinemia and increased hypertropic growth. Insulin resistance results in maternal hypertriglyceridemia, which leads to increased provision of free fatty acids to the fetus (19). Since obesity also occurs more frequently in older pregnant women, this could be a reason for increased obese newborns (9). Usta et al. postulated increased macrosomia especially in older multiparous women. This form of macrosomia is associated with obesity and untreated or poorly controlled diabetes mellitus (40). The deviating results of our study can be well explained by the size of our study population and the treatment of women with risk pregnancies in specialized prenatal centers in Germany.
Significant differences were found in both umbilical cord pH and base excess between women older and younger than 40 years. However, this difference in pH as well as base excess was, regardless of statistical significance, clinically not relevant, as the values are within the normal reference range. Maisonneuve et al. considered age over 35 as an independent risk factor for severe fetal acidosis. They postulated a previous C-section, abnormal fetal heart frequency during birth and uterine rupture as other risk factors (49). Ogawa et al. also found lower birth pH in newborns of women with advanced age (50). Gilbert et al. reported an increased risk of fetal asphyxia in women of advanced age and explained this by preexisting diseases such as hypertension or diabetes, which seem to correlate with age (51). However, in a meta-analysis, which examined 75 studies, no difference in pH was found (2). The 5-min Apgar, which was frequently reported in other studies (20,21,23,35), was also analyzed. In our analysis, there was no statistically significant difference of Apgar score after 5 min between the groups. In the literature, different results were found. In children from mother with advanced age, either no difference in Apgar score at 5 min was found (16,20) or a decreased Apgar score at 5 min was reported (21,23,35,52). Jahromi et al. found a significant decrease in 5 min-Apgar regardless of parity. This was attributed by the authors to preexisting maternal diseases, mainly hypertensive diseases, but also to extreme preterm births and fetal growth disorders (23). It seems noteworthy that studies reporting more complications and risks for older mothers show similar neonatal outcomes and NICU admission rates (15,23).
In the present study, we could identify more antenatal complications in patients older than or equal to 40 years than in younger patients. Nonetheless, the neonatal outcomes measured by umbilical pH value, base excess and Apgar score were comparable between the groups and no severe complications in women with advanced maternal age were observed. We attribute these findings to a well standardized management system in a specialized prenatal center for women with risk pregnancies, including advanced maternal age, where risks can be detected early and treated in time. This in turn encourages better monitoring and care of pregnant women with risk factors.
Conflicts of Interest
All Authors declare that they have no conflicts of interest in relation to this study.
Authors’ Contributions
DR designed the study and wrote the final manuscript. FS performed the data collection and wrote the draft of the manuscript. EG performed the statistical analysis. SL and JR contributed to the data analysis and interpretation. NM contributed to the data collection. BG was involved in conceptualizing the study. PM approved the final version. SB contributed to writing the final version of the manuscript and supervised the data analysis.
Acknowledgements
The Authors would like to thank all the patients that participated in the study.
References
- 1.Daten der Lebendgeborenen nach Altersgruppen der Mütter für die Jahre 2017 bis 2021. Statistisches Bundesamt. Available at: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Geburten/Tabellen/lebendgeborene-alter.html. [Last accessed on April 18, 2023]
- 2.Lean SC, Derricott H, Jones RL, Heazell AEP. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS One. 2017;12(10):e0186287. doi: 10.1371/journal.pone.0186287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roustaei Z, Vehviläinen-Julkunen K, Tuomainen TP, Lamminpää R, Heinonen S. The effect of advanced maternal age on maternal and neonatal outcomes of placenta previa: A register-based cohort study. Eur J Obstet Gynecol Reprod Biol. 2018;227:1–7. doi: 10.1016/j.ejogrb.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Osmundson SS, Gould JB, Butwick AJ, Yeaton-Massey A, El-Sayed YY. Labor outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2016;214(3):362.e1–362.e7. doi: 10.1016/j.ajog.2015.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zapata-Masias Y, Marqueta B, Gómez Roig MD, Gonzalez-Bosquet E. Obstetric and perinatal outcomes in women ≥40years of age: Associations with fetal growth disorders. Early Hum Dev. 2016;100:17–20. doi: 10.1016/j.earlhumdev.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Alves NCC, Feitosa KMA, Mendes MES, Caminha MFC. Complications in pregnancy in women aged 35 or older. Rev Gaucha Enferm. 2017;38(4):e2017–e2042. doi: 10.1590/1983-1447.2017.04.2017-0042. [DOI] [PubMed] [Google Scholar]
- 7.Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013;42(6):634–643. doi: 10.1002/uog.12494. [DOI] [PubMed] [Google Scholar]
- 8.Niessen K, Werner-Bierwisch T, Metzing S, Sayn-Wittgenstein FZ. [Motherhood at the age of 35 and over: the risk of advanced maternal age as perceived by women - a literature study] Z Geburtshilfe Neonatol. 2017;221(3):111–121. doi: 10.1055/s-0043-104864. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs F, Monet B, Ducruet T, Chaillet N, Audibert F. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS One. 2018;13(1):e0191002. doi: 10.1371/journal.pone.0191002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritzinger P, Dudenhausen JW, Holzgreve W. Späte Mutterschaft und deren Risiken. J Reproduktionsmed Endokrinol 8(2): 112-122, 2011. Available at: https://www.researchgate.net/publication/303646567_Spate_Mutterschaft_und_deren_Risiken. [Last accessed on April 23, 2023]
- 11.Prysak M, Lorenz RP, Kisly A. Pregnancy outcome in nulliparous women 35 years and older. Obstet Gynecol. 1995;85(1):65–70. doi: 10.1016/0029-7844(94)00330-g. [DOI] [PubMed] [Google Scholar]
- 12.Dulitzki M, Soriano D, Schiff E, Chetrit A, Mashiach S, Seidman DS. Effect of very advanced maternal age on pregnancy outcome and rate of cesarean delivery. Obstet Gynecol. 1998;92(6):935–939. doi: 10.1016/s0029-7844(98)00335-4. [DOI] [PubMed] [Google Scholar]
- 13.Benli AR, Cetin Benli N, Usta AT, Atakul T, Koroglu M. Effect of maternal age on pregnancy outcome and cesarean delivery rate. J Clin Med Res. 2015;7(2):97–102. doi: 10.14740/jocmr1904w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianco A, Stone J, Lynch L, Lapinski R, Berkowitz G, Berkowitz RL. Pregnancy outcome at age 40 and older. Obstet Gynecol. 1996;87(6):917–922. doi: 10.1016/0029-7844(96)00045-2. [DOI] [PubMed] [Google Scholar]
- 15.Pawde AA, Kulkarni MP, Unni J. Pregnancy in women aged 35 years and above: a prospective observational study. J Obstet Gynaecol India. 2015;65(2):93–96. doi: 10.1007/s13224-014-0616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usta IM, Nassar AH. Advanced maternal age. Part I: obstetric complications. Am J Perinatol. 2008;25(8):521–534. doi: 10.1055/s-0028-1085620. [DOI] [PubMed] [Google Scholar]
- 17.Ecker JL, Chen KT, Cohen AP, Riley LE, Lieberman ES. Increased risk of cesarean delivery with advancing maternal age: indications and associated factors in nulliparous women. Am J Obstet Gynecol. 2001;185(4):883–887. doi: 10.1067/mob.2001.117364. [DOI] [PubMed] [Google Scholar]
- 18.Ludford I, Scheil W, Tucker G, Grivell R. Pregnancy outcomes for nulliparous women of advanced maternal age in South Australia, 1998-2008. Aust N Z J Obstet Gynaecol. 2012;52(3):235–241. doi: 10.1111/j.1479-828X.2012.01442.x. [DOI] [PubMed] [Google Scholar]
- 19.Jolly M, Sebire N, Harris J, Robinson S, Regan L. The risks associated with pregnancy in women aged 35 years or older. Hum Reprod. 2000;15(11):2433–2437. doi: 10.1093/humrep/15.11.2433. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Tanbo T, Abyholm T, Henriksen T. The impact of advanced maternal age and parity on obstetric and perinatal outcomes in singleton gestations. Arch Gynecol Obstet. 2011;284(1):31–37. doi: 10.1007/s00404-010-1587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seoud MA, Nassar AH, Usta IM, Melhem Z, Kazma A, Khalil AM. Impact of advanced maternal age on pregnancy outcome. Am J Perinatol. 2002;19(1):1–8. doi: 10.1055/s-2002-20175. [DOI] [PubMed] [Google Scholar]
- 22.Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, Saade GR, Eddleman KA, Klugman S, Dugoff L, Timor-Tritsch IE, Craigo SD, Carr SR, Wolfe HM, Bianchi DW, D’Alton M, FASTER Consortium Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5 Pt 1):983–990. doi: 10.1097/01.AOG.0000158118.75532.51. [DOI] [PubMed] [Google Scholar]
- 23.Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321. doi: 10.1016/S1028-4559(08)60131-X. [DOI] [PubMed] [Google Scholar]
- 24.Milner M, Barry-Kinsella C, Unwin A, Harrison RF. The impact of maternal age on pregnancy and its outcome. Int J Gynaecol Obstet. 1992;38(4):281–286. doi: 10.1016/0020-7292(92)91019-k. [DOI] [PubMed] [Google Scholar]
- 25.Quinn JA, Munoz FM, Gonik B, Frau L, Cutland C, Mallett-Moore T, Kissou A, Wittke F, Das M, Nunes T, Pye S, Watson W, Ramos AA, Cordero JF, Huang WT, Kochhar S, Buttery J, Brighton Collaboration Preterm Birth Working Group Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. 2016;34(49):6047–6056. doi: 10.1016/j.vaccine.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutland CL, Lackritz EM, Mallett-Moore T, Bardají A, Chandrasekaran R, Lahariya C, Nisar MI, Tapia MD, Pathirana J, Kochhar S, Muñoz FM, Brighton Collaboration Low Birth Weight Working Group Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35(48 Pt A):6492–6500. doi: 10.1016/j.vaccine.2017.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO guidelines for the management of postpartum haemorrhage and retained placenta. [PubMed] [Google Scholar]
- 28.Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand. 2011;90(2):140–149. doi: 10.1111/j.1600-0412.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 29.Desseauve D, Fradet L, Lacouture P, Pierre F. Position for labor and birth: State of knowledge and biomechanical perspectives. Eur J Obstet Gynecol Reprod Biol. 2017;208:46–54. doi: 10.1016/j.ejogrb.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Pilliod RA, Caughey AB. Fetal malpresentation and malposition: diagnosis and management. Obstet Gynecol Clin North Am. 2017;44(4):631–643. doi: 10.1016/j.ogc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 31.AWMF Leitlinienregister. Available at: https://register.awmf.org/de/leitlinien/detail/024-005. [Last accessed on April 23, 2023]
- 32.Karl K, Lack N. Die ältere Erstgebärende – wie hoch ist das Risiko wirklich. Hebamme. 2009;22:234–237. doi: 10.1055/s-0029-1243146. [DOI] [Google Scholar]
- 33.Bayrampour H, Heaman M. Advanced maternal age and the risk of cesarean birth: a systematic review. Birth. 2010;37(3):219–226. doi: 10.1111/j.1523-536X.2010.00409.x. [DOI] [PubMed] [Google Scholar]
- 34.Elser H, Selbmann HK. [Influence of age and parity on risks during pregnancy labour and delivery and on the incidence of caesarean section and perinatal mortality (author’s transl)] Geburtshilfe Frauenheilkd. 1982;42(3):188–196. doi: 10.1055/s-2008-1037261. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh TT, Liou JD, Hsu JJ, Lo LM, Chen SF, Hung TH. Advanced maternal age and adverse perinatal outcomes in an Asian population. Eur J Obstet Gynecol Reprod Biol. 2010;148(1):21–26. doi: 10.1016/j.ejogrb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Mills T, Lavender T. Advanced maternal age. Obstetrics, Gynaecology & Reproductive Medicine. 2018;21(4):107–111. doi: 10.1016/j.ogrm.2010.12.003. [DOI] [Google Scholar]
- 37.Rydahl E, Declercq E, Juhl M, Maimburg RD. Cesarean section on a rise-Does advanced maternal age explain the increase? A population register-based study. PLoS One. 2019;14(1):e0210655. doi: 10.1371/journal.pone.0210655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lao TT, Sahota DS, Cheng YK, Law LW, Leung TY. Advanced maternal age and postpartum hemorrhage - risk factor or red herring. J Matern Fetal Neonatal Med. 2014;27(3):243–246. doi: 10.3109/14767058.2013.807240. [DOI] [PubMed] [Google Scholar]
- 39.Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177(1):210–214. doi: 10.1016/s0002-9378(97)70463-0. [DOI] [PubMed] [Google Scholar]
- 40.Usta IM, Hobeika EM, Musa AA, Gabriel GE, Nassar AH. Placenta previa-accreta: risk factors and complications. Am J Obstet Gynecol. 2005;193(3 Pt 2):1045–1049. doi: 10.1016/j.ajog.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 41.Hornemann A, Kamischke A, Luedders DW, Beyer DA, Diedrich K, Bohlmann MK. Advanced age is a risk factor for higher grade perineal lacerations during delivery in nulliparous women. Arch Gynecol Obstet. 2010;281(1):59–64. doi: 10.1007/s00404-009-1063-7. [DOI] [PubMed] [Google Scholar]
- 42.Meister MR, Cahill AG, Conner SN, Woolfolk CL, Lowder JL. Predicting obstetric anal sphincter injuries in a modern obstetric population. Am J Obstet Gynecol. 2016;215(3):310.e1–310.e7. doi: 10.1016/j.ajog.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 43.Soong B, Barnes M. Maternal position at midwife-attended birth and perineal trauma: is there an association. Birth. 2005;32(3):164–169. doi: 10.1111/j.0730-7659.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 44.Ogunyemi D, Manigat B, Marquis J, Bazargan M. Demographic variations and clinical associations of episiotomy and severe perineal lacerations in vaginal delivery. J Natl Med Assoc. 2006;98(11):1874–1881. [PMC free article] [PubMed] [Google Scholar]
- 45.Sohn K. The trend in the relationship of advanced maternal age to preterm birth and low birthweight. Eur J Contracept Reprod Health Care. 2017;22(5):363–368. doi: 10.1080/13625187.2017.1372569. [DOI] [PubMed] [Google Scholar]
- 46.Lu L, Li JH, Dai XF, Wei JB, Chen LH, Hu JF. Impact of advanced maternal age on maternal and neonatal outcomes in preterm birth. Ginekol Pol. 2022;93(2):134–141. doi: 10.5603/GP.a2021.0224. [DOI] [PubMed] [Google Scholar]
- 47.Waldenström U, Cnattingius S, Vixner L, Norman M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: a population-based register study. BJOG. 2017;124(8):1235–1244. doi: 10.1111/1471-0528.14368. [DOI] [PubMed] [Google Scholar]
- 48.Carolan M. Maternal age ≥45 years and maternal and perinatal outcomes: a review of the evidence. Midwifery. 2013;29(5):479–489. doi: 10.1016/j.midw.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Maisonneuve E, Audibert F, Guilbaud L, Lathelize J, Jousse M, Pierre F, Fraser W, Carbonne B. Risk factors for severe neonatal acidosis. Obstet Gynecol. 2011;118(4):818–823. doi: 10.1097/AOG.0b013e31822c9198. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa K, Urayama KY, Tanigaki S, Sago H, Sato S, Saito S, Morisaki N. Association between very advanced maternal age and adverse pregnancy outcomes: a cross sectional Japanese study. BMC Pregnancy Childbirth. 2017;17(1):349. doi: 10.1186/s12884-017-1540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilbert WM, Nesbitt TS, Danielsen B. Childbearing beyond age 40: pregnancy outcome in 24,032 cases. Obstet Gynecol. 1999;93(1):9–14. doi: 10.1016/s0029-7844(98)00382-2. [DOI] [PubMed] [Google Scholar]
- 52.Pinheiro RL, Areia AL, Mota Pinto A, Donato H. Advanced maternal age: Adverse outcomes of pregnancy, a meta-analysis. Acta Med Port. 2019;32(3):219–226. doi: 10.20344/amp.11057. [DOI] [PubMed] [Google Scholar]