Abstract
Background/Aim: Cancer mortality has decreased due to the contribution of extensive research on cancer treatment, including chemotherapy, radiation, and immunotherapy. However, histopathologically similar tumors originating from the same organ are treated with identical or similar chemotherapeutic regimens regardless of patient characteristics or cancer subtypes. The aim of this study was to evaluate the utility of organoids in predicting responses to chemotherapeutic agents.
Patients and Methods: This study retrospectively reviewed patient-derived organoids (PDOs) from 10 colorectal cancer patients to compare chemotherapy responses. Drug sensitivities for 5-fluorouracil (5-FU), cisplatin, oxaliplatin, and irinotecan were compared using GI50 (concentration that inhibits cancer cell growth by 50%).
Results: When organoids were treated with 5-FU, GI50 was the lowest compared to the other three chemotherapeutic agents (cisplatin, oxaliplatin, and irinotecan). The responsiveness to chemotherapeutic agents differed depending on specific patient characteristics including age, tumor location, stage, and gross type. The response of the patients’ organoids to chemotherapeutic agents was consistent with the response to chemotherapy actually performed in those patients with cancer recurrence after surgery.
Conclusion: PDOs may be useful as a preclinical model in predicting chemotherapy responses in cancer patients.
Keywords: Colorectal neoplasms, organoids, drug therapy
In 2018, the International Agency for Research on Cancer reported that colorectal cancer (CRC) was the third most commonly diagnosed cancer (10.20% of the total cases) and is still one of the leading causes of cancer-related mortality (9.20% of the total cases) (1). Current conventional chemotherapy guidelines for colorectal cancer include drugs such as 5-fluorouracil (5-FU), cisplatin, oxaliplatin, and irinotecan (2). These drugs are used without considering individual characteristics in most patients. Although chemotherapy is one of the most important treatments of colorectal cancer, the outcome may differ by patient due to tumor heterogeneity (3,4). Therefore, finding a way to predict chemotherapy response in each patient is crucial in developing personalized cancer treatment (5).
Up to now, animal models or cancer cell lines were commonly used to test chemotherapy responses. However, these models have limitations in reflecting cancer progression in the human body. The limitations include low success rate, inefficient generation from primary patient cells, high cost, and long-time treatment (6,7). Patient-derived organoids (PDOs) have emerged as a more accurate and elaborate new model for cancer treatment research (8), and many studies have been conducted on their development and culturing methodologies (9,10). Organoids, a three-dimensional in vitro culture system (11), contain self-renewing stem cells that differentiate into various organ-specific cell types and tissues that assume an organization and functionality similar to that of an organ (12).
Drost and Clevers (13) reviewed the potential values of organoids in both basic and translational cancer research. They especially emphasized on the utilization of organoids for drug screening and toxicity testing, which enables the development of personalized cancer treatment regimens. Many studies have shown that PDOs can effectively predict responses to systemic chemotherapy (14,15). Luo et al. (16) reported that using organoids overcomes the previously discussed limitations because of their higher similarity to native tissues in aspects of cell composition, behavior, physiology, and stable genomic structures.
In this study, we focused on comparing chemotherapy responses on colorectal cancer by using organoids derived from 10 patients. Specifically, this study was conducted to achieve the following objectives. First, we compared the efficacy of 5-FU, cisplatin, oxaliplatin, and irinotecan in colorectal cancer patients using PDOs. Second, we evaluated the relationship between the sensitivity to chemotherapeutic agents and specific patient characteristics. Third, we investigated the relationship between response to chemotherapy actually performed in patients with cancer recurrence after surgery and response to those PDOs. Overall, we examined the potential of organoids in predicting individual responses to various chemotherapies, which would open the possibility to personalized cancer treatment.
Patients and Methods
Patients. This study includes 10 organoids derived from patients who underwent surgical resection for colorectal cancer at Pusan National University Hospital between February 2020 and March 2021. All patient data were collected retrospectively by reviewing medical records. The inclusion criteria were patients aged 18 years or older with histologically diagnosed colorectal cancer. Patients with Eastern Cooperative Oncology Group (ECOG) performance status 3-4, and those who refused chemotherapy, were under 18 years of age, or had an intellectual disability were excluded.
Informed consent was obtained from all participants. The study protocol was registered at the Clinical Research Information Service (CRIS registration number: KCT0003511). The study protocol was approved by the Institutional Review Board of Pusan National University Hospital (IRB number: 1801-020-062).
Establishment of patient-derived organoids. The colorectal cancer tissues obtained from surgery were cultured as organoids within 2 h. First, the tissue was washed twice with phosphate-buffered saline (Welgene, Daegu, Republic of Korea) containing 2% penicillin and streptomycin (Gibco, Langley, OK, USA), and chopped to pieces of 2 mm or less. The tissue pieces were collected in a 50 ml conical tube containing 30 ml of phosphate buffered saline and centrifuged at 1,977×g for 10 min at 4˚C. After removal of the supernatant, 20 μg/ml collagenase type Ⅺ (Sigma-Aldrich, St. Louis, MO, USA) containing 0.1 M DL-dithiothreitol solution (Sigma-Aldrich) and 10 mg/ml dispase2 (Sigma-Aldrich) were added to the tissue pellet and dissociated at 37˚C for 30 min in 5% CO2 incubator. After incubation, the suspensions were repeatedly minced by pipetting and passed through a 100 μm cell strainer (BD Falcon, Franklin Lakes, NJ, USA), and centrifuged at 494×g for 5 min at 4˚C. The supernatant was removed and red blood cell lysis buffer (Biolegend, San Diego, CA, USA) was added to the pellet at 37˚C for 3 min. To stop the reaction, DMEM medium (Welgene) containing 10% FBS (Gibco) was added and centrifuged at 494×g for 5 min. The cell pellet was resuspended in 20 μl of organoid complete medium, added 20 μl of matrigel matrix (Corning, Corning, NY, USA), and plated in ultra-low attachment plate (24-well, Corning) to solidify at 37˚C for 25 min in a 5% CO2 incubator. After complete solidification, 500 μl of warm organoid complete medium was added per well. The organoid complete medium is Advanced DMEM/F12 medium (50%, Gibco) containing L-WRN medium (50%, L-WRN cell cultured medium, ATCC, Manassas, VA, USA), 1 mM nicotinamide (Sigma-Aldrich), 1× B-27 (Gibco), 2 mM Gluta-max (Gibco), 10 nM Gastrin (Sigma-Aldrich), 10 μM Y-27632 (Sigma-Aldrich), 10 μM SB202190 (Sigma-Aldrich), 500 nM A83-01 (Sigma-Aldrich), 50 ng/ml EGF (Peprotech, Rocky Hill, NJ, USA), 1× N2 solution (Gibco), 100 μg/ml primocin™ (Invitrogen, Carlsbad, CA, USA) 2 μg/ml heparin (Sigma-Aldrich), 1 mM N-acetyl-L-cysteine (Sigma-Aldrich), 10 mM HEPES (Gibco) and 1 μM LY-2157299 (Peprotech).
Drug screening of patient-derived organoids. Organoids were plated at 4,000 organoids per well in 96-well ultra-low attachment plates (Corning) and cultured for 3 days. Next, 50 μl of organoid complete medium containing 7 different doses of 5-FU, cisplatin, oxaliplatin, or irinotecan were added. After another 3 days, organoids to which 100 μl/well of CellTiter-Glo (Promega, Madison, WI, USA) were added were reacted for 25 min at 37˚C. Then, the supernatant was transferred to a 96-well black plate (SPL Life sciences, Gyeonggi-do, Republic of Korea) and the viability of the organoids was measured as a luminescent signal using a Hybrid Multi-Mode Microplate reader (Synergy H1, BioTek, Winooski, VA, USA).
Statistical analysis. A one-way analysis of variance was used to find differences between various groups within the sample. p-Values <0.05 were considered statistically significant. All statistical analyses were conducted using IBM SPSS statistics, version 25.
Results
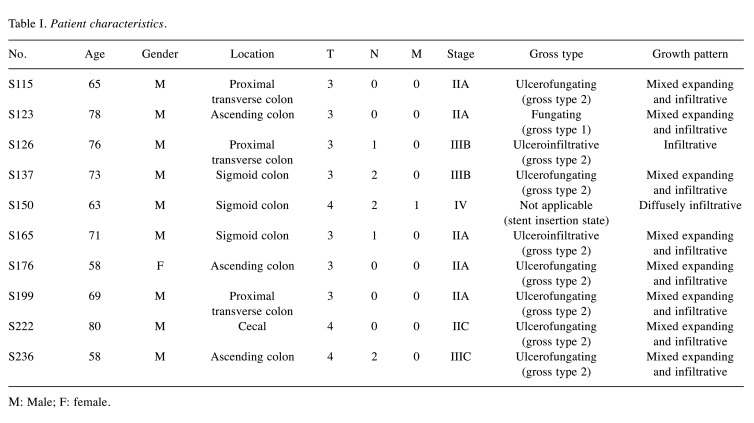
Patient characteristics. This study compared the efficacy of standard chemotherapeutics in organoids derived from 10 colorectal cancer patients that underwent surgery at our institution. The standard chemotherapeutic agents included 5-FU, cisplatin, oxaliplatin, and irinotecan, which interfere with DNA synthesis. The characteristics of the patients are listed in Table I. All patients underwent surgery. The age of the 10 patients ranged from 58 to 80 years with the median age of 69. Of all the patients, nine were male and one was female; five were between stages I-IIA and five were stages IIB-IV; one was fungating, six were ulcerofungating, two were ulceroinfiltrative, and one was not applicable to being categorized into a gross type due to stent insertion; eight showed a growth pattern of mixed expanding and infiltrative, one was infiltrative, and one was diffusely infiltrative.
Table I. Patient characteristics.
Μ: Male; F: female.
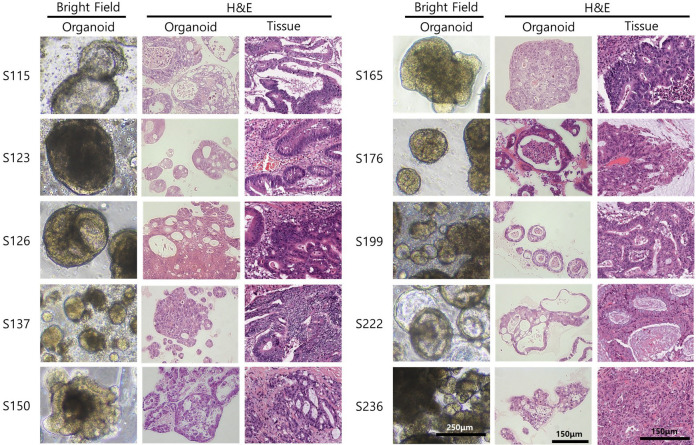
Patient-derived organoids reflect the patients’ primary tumor morphology. The morphology of the organoid can be confirmed with bright field imaging after culturing the patient’s tissue for about a week. The organoid image was different for each patient. Similarities of each patient’s organoid and tissue could be confirmed using Hematoxylin & Eosin staining. It was confirmed that the patient’s organoid and tissue showed similar morphologies of glands, mucin inclusions, and histological characteristics of the cancer (Figure 1).
Figure 1. Bright-field images of 10 established patient-derived organoids and hematoxylin and eosin (H&E) staining of the organoids and patients’ tissues. Scale bar of bright-field image indicates 250 μm, scale bar of H&E image indicates 150 μm.
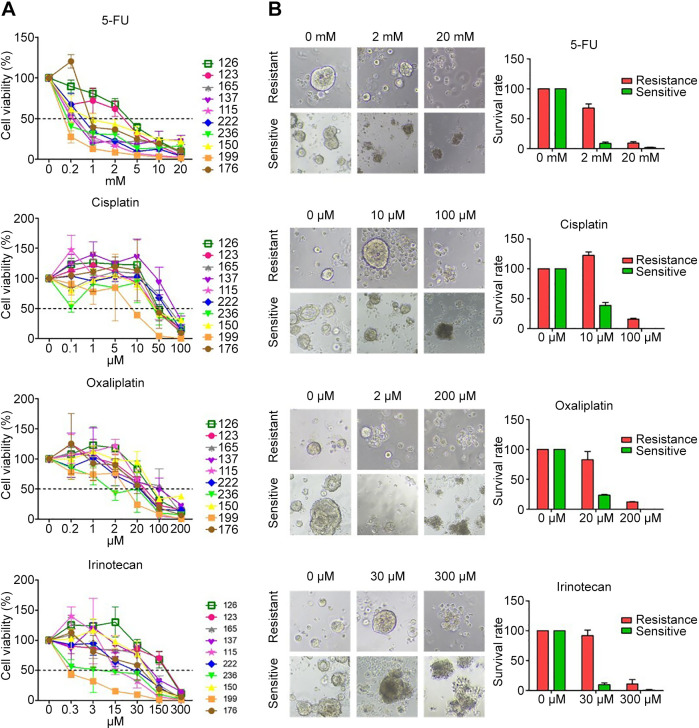
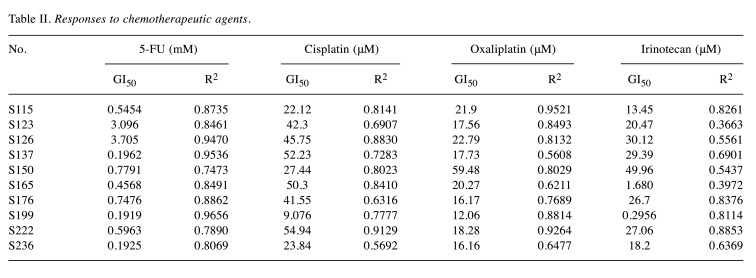
Responses of organoids to 5-FU, cisplatin, oxaliplatin, and irinotecan. The cell viability of 10 organoids was checked following treatment with different concentrations of each chemotherapeutic agent (Figure 2). The GI50 (concentration that inhibits cancer cell growth by 50%) and R2 were calculated and listed in Table II. Interestingly, the 10 PDOs were most sensitive to 5-FU than the other chemotherapeutic agents. However, PDOs derived from different patients showed various responses to the same chemotherapeutic agent due to the heterogeneity of the tumors. For example, organoids from S236 were more sensitive to 5-FU (GI50=0.1925 mM) than those from S123 (GI50=3.096 mM).
Figure 2. Response of colorectal organoids to drugs. (A) Cell viability following treatment with different concentrations of chemotherapeutic agents. (B) The image and survival rate on day 3 after drug treatment. Drug-resistant organoid 126 and drug-sensitive organoid 199 showed a 59% difference in cell viability following treatment with 2 mM 5-FU, 83% difference following treatment with 10 μM cisplatin, 58% following treatment with 20 μM oxaliplatin, and 81% following treatment with 30 μM irinotecan.
Table II. Responses to chemotherapeutic agents.
Relationships between organoids’ sensitivity to chemotherapeutic agents (5-FU, cisplatin, oxaliplatin, and irinotecan) and patient characteristics. We evaluated the relationship between the sensitivity to chemotherapeutic agents and specific patient characteristics including age, tumor location, stage, and gross type.
Age. The patients were categorized into two groups by the age of 69, which was the median age of the 10 patients. A one-way ANOVA revealed a significant difference between the two groups of age when using cisplatin [F(1,8)=18.289, p<0.01]. Patients who were under the age of 69 showed a lower GI50 to cisplatin (Munder-69=24.81, SD=11.65 vs. Mover-69=49.10, SD=5.07). But there were no significant differences between the two groups of age for 5-FU, oxaliplatin, and irinotecan.
Tumor location. The patients were categorized into two groups according to the tumor location; seven originated in the right colon and three in the left colon. A one-way ANOVA revealed a meaningful difference between the two groups of tumor location when using oxaliplatin [F(1,8)=3.065, p=0.118]. Patients who had tumors in the right colon showed a lower GI50 to oxaliplatin (Mright-colon=17.85, SD=3.66 vs. Mleft-colon=32.49, SD=23.41). But there were no significant differences for 5-FU, cisplatin, and irinotecan.
Stage. According to the Korean Health Insurance Review & Assessment Service, adjuvant chemotherapy may be administered when the risk of recurrence is high in patients with stage II after surgery. Therefore, we categorized the 10 patients into the following two groups; the first group included stages I-IIA and the second group included stages IIB-IV. A one-way ANOVA revealed a significant difference between the two groups of stage when using irinotecan [F(1,8)=6.321, p<0.05]. Patients with stages I-IIA showed a lower GI50 to irinotecan (Mstage I-IIA=12.52, SD=11.53 vs. Mstage IIB-IV=30.95, SD=11.64). But there were no significant differences between the two groups of stage when using 5-FU, cisplatin, and oxaliplatin.
Gross type. One of the patients was excluded from the analysis because the gross type was unknown due to stent insertion. Nine patients were categorized into three groups according to the tumor gross type, and only one patient was gross type 2. A one-way ANOVA revealed a meaningful difference across the three groups for 5-FU [F(2,6)=4.701, p=0.059]. Patients who had a gross type 2 (ulcerofungating) showed the lowest GI50 to 5-FU (Mgross type 2=0.41, SD=0.25 vs. Mgross type 1=3.10 vs. Mgross type 3=2.08, SD=2.30). But there were no significant differences among the three groups of tumor gross type for cisplatin, oxaliplatin, and irinotecan.
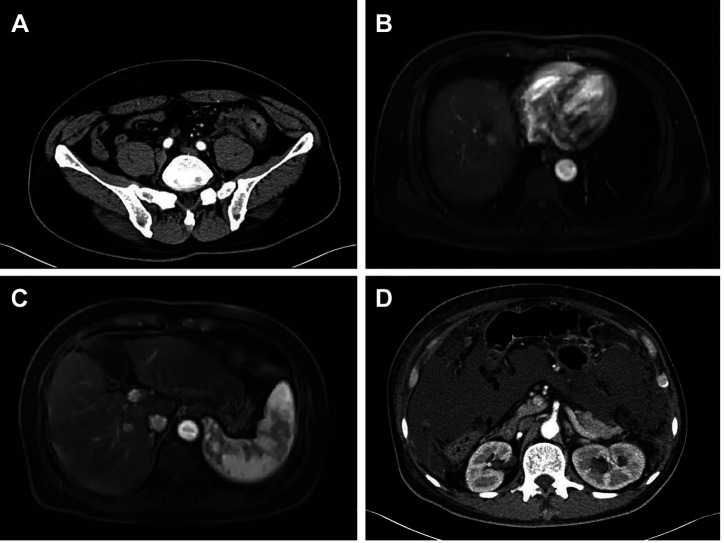
Comparison between drug response of organoids and actual patients. S150, a 63-year-old male, presented to our hospital with constipation and melena that started 20 days ago. Initial carcinoembryonic antigen (CEA) level was higher than normal (20.4 ng/ml) and abdominal computed tomography (CT) showed focal increased pericolic fat stranding in the sigmoid colon with multiple lymph node enlargements, suggesting the presence of sigmoid colon cancer (Figure 3A). An eight cm uncovered self-expandable metallic stent was inserted to relieve the obstruction. MRI showed a 1.4 cm sized LR-4 lesion in the liver segment VIII and 1.1 cm sized LR-3 lesion in the liver segment VI (Figure 3B and C). He underwent laparoscopic anterior resection. Pathology confirmed the patient had moderately differentiated adenocarcinoma. The tumor showed a high level of epidermal growth factor receptor (EGFR) expression and no mutations in K-Ras genes and microsatellite-stable (MSS) phenotype. Follow up CT performed one month after surgery showed carcinomatosis peritonei (Figure 3D). He underwent the first cycle of Erbitux+FOLFIRI+Ferbon. About one week later, he was readmitted due to abdominal distention and diarrhea. Sepsis and hepatic encephalopathy did not show improvement and the patient expired. The S150 organoid of this patient showed very high GI50s for irinotecan (49.96 μM) and oxaliplatin (59.48 μM) and therefore expected to be resistant to these drugs. It can be considered that this is consistent with the clinical results.
Figure 3. Clinical course of S150. (A) Initial abdominal computed tomography scan showing pericolic fat stranding in the sigmoid colon, suggesting sigmoid colon cancer. (B) Magnetic resonance imaging (MRI) showing an 1.4 cm sized LR-4 lesion in the liver segment VIII. (C) MRI showing a 1.1 cm sized LR-3 lesion in the liver segment VI. (D) Follow up CT performed one month after surgery showing carcinomatosis peritonei.
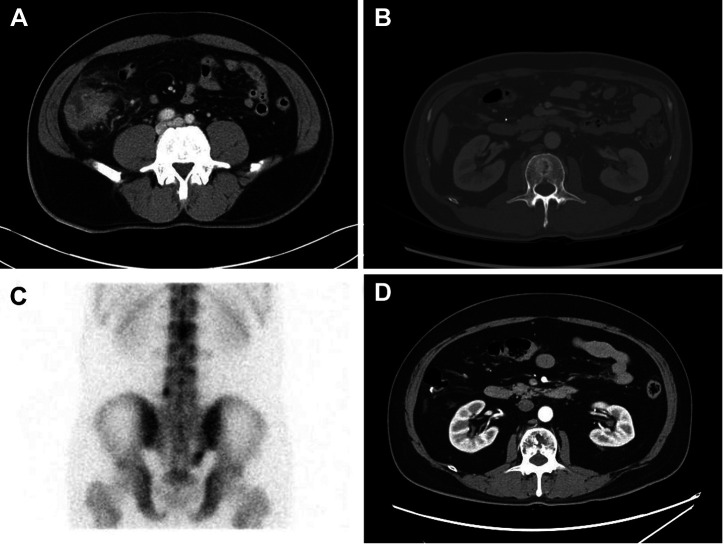
S236, a 58-year-old male, had right lower quadrant abdominal pain. Initial CEA level was very high (104.0 ng/ml) and abdominal CT showed probable cecal cancer with paraaortic lymph node metastasis and colonoscopy showed a fungating mass at the cecum (Figure 4A). He underwent laparoscopic right hemicolectomy and was diagnosed with stage IIIC (T4N3N0). Pathology confirmed the patient had moderately differentiated adenocarcinoma. The tumor showed a high level of epidermal growth factor receptor (EGFR) expression, a mutation in K-Ras gene, and MSS phenotype. He underwent four cycles of adjuvant XELOX. However, follow-up abdominal CT showed finding suggestive of new appearance of bone metastasis on L2 (Figure 4B). Bone scan confirmed bone metastases in T11, L2, sacrum (right ala), and L5 (Figure 4C). Due to cancer progression, a second-line chemotherapy with FOLFIRI+avastin was initiated. He received 20 cycles of chemotherapy and is still under treatment. He showed stable response until the recent CT follow-up (Figure 4D). The S236 organoid showed relatively lower GI50s of 5-FU (0.1925 mM), oxaliplatin (16.16 μM), and irinotecan (18.2 μM). The patient presented a continuous anticancer response after chemotherapy, which was consistent with the anticancer drug response of organoids.
Figure 4. Clinical course of S236. (A) Initial abdominal computed tomography (CT) scan showing probable cecal cancer with paraaortic lymph node metastasis. (B) CT after surgery showing bone metastasis in L2. (C) Bone scan showing bone metastases in T11, L2, sacrum (right ala), and L5. (D) Follow-up CT performed after second-line chemotherapy showing bone metastases had not progressed any further, suggesting that the response was stable.
Discussion
Colon cancer is still one of the leading causes of cancer-related mortality (1). In order to prolong life expectancy of patients with cancer, the development and introduction of various cytotoxic drugs and targeted agents for cancer treatment has been actively investigated and is still ongoing. Many researches mention CEA levels and MS instability status as validated prognostic factors for colorectal cancer (17). Although mutational status of K-Ras and N-Ras is used to predict responses to immunotherapy such as cetuximab and panitumumab, the factors for predicting responses to chemotherapy are unknown (18). Lately, PDOs, which overcome many of the previously mentioned limitations of animal models or cancer cell lines (6), are increasingly used to develop personalized cancer treatments. Wang et al. (19) evaluated the predictive accuracy of a patient-derived tumor organoid (PDTO) culture model for response to chemotherapy regimens in stage IV colorectal cancer. In addition, a pilot study of 43 PDTO culture samples from 30 patients was performed to define the half-maximal inhibitory concentration of the response to chemotherapeutic agents. Then, they conducted a blind study containing 96 samples from 71 patients, of which 64 samples from 45 patients were eligible for evaluation. Their study reported a sensitivity of 63.33%, specificity of 94.12%, and accuracy of 79.69% in the PDTO model for predicting responses to chemotherapy regimens. Similarly, Vlachogiannis et al. (20) reported that PDOs showed a sensitivity of 100%, specificity of 93%, positive predictive value of 88%, and negative predictive value of 100% in predicting responses to targeted agents or chemotherapeutic agents.
In metastatic colorectal cancer patients, standard-of-care drug therapy includes 5-FU or capecitabine in combination with either oxaliplatin or irinotecan (21,22). However, up to now there is no effective method in foreknowing which patients may respond to the treatment. Therefore, recent studies focus on finding a way to predict chemotherapy responses in individual patients. Ooft et al. (23) suggested that PDOs could be used to prevent cancer patients from receiving ineffective irinotecan-based chemotherapy. Furthermore, Luo et al. (16) examined the application of colorectal cancer organoids in disease model construction, basic biological research, organoid biobank construction, drug screening and personalized medicine, drug development, drug toxicity and safety, and regenerative medicine. They also showed the limitations and challenges of organoids and examined further directions of development of organoids. In this stream of research, our study also compared the efficacy of standard chemotherapeutic agents for colorectal cancer using PDOs. Furthermore, we investigated the relationship between the sensitivity to chemotherapeutic agents and specific patient characteristics including age, tumor location, stage, growth type, and growth pattern.
In summary, the first results of this study showed lowest GI50 when the organoids were treated with 5-FU. Secondly, based on patient characteristics, those under the age 69 showed highest sensitivity to cisplatin. Patients with cancer located in the right colon showed highest sensitivity to oxaliplatin. Patients with stage I-IIA were most responsive to irinotecan. Patients with gross type 2 were most responsive to 5-FU. Thirdly, responses to chemotherapy in the two patients with cancer recurrence after surgery were compared with those on organoids. One patient expired after one cycle of chemotherapy while the other patient is still receiving chemotherapy after 20 cycles. These clinical outcomes were consistent with the drug responses of their organoids.
This study included a small sample size of 10 colorectal cancer patients that were treated in a single institution. Therefore, there might be some limitations in generalizing the results derived from this study. Because the total sample size was small, it was difficult to make detailed groupings according to patient characteristics. Significant patient characteristics affecting the response to chemotherapy could not be investigated statistically. Future investigations should be performed with multicenter and large sized samples. Establishing a control group such as patient-derived tumor xenografts or patient-derived normal organoids could help validate the predictive values of PDTOs. Overall, this study showed that PDOs have great potential as an in vitro model that can predict the response of patients prior to the administration of anticancer drugs, enabling the development of personalized anticancer therapies.
Conflicts of Interest
The Authors disclose no conflicts of interest in relation to this study.
Authors’ Contributions
Conceptualization: K.Y., S.H.P., D.U.K, B.C.L.; Data curation: S.H.P., D.Y.J., H.J.L., D.U.K.; Formal analysis: K.Y., S.H.P., D.U.K.; Funding acquisition: D.U.K.; Investigation: K.Y., S.H.P., D.U.K.; Methodology: K.Y., S.H.P., D.U.K.; Supervision: D.U.K, B.C.L.; Visualization: K.Y., S.H.P., D.Y.J., H.J.L.; Roles/Writing - original draft: K.Y., S.H.P., D.U.K.; Writing – review & editing: K.Y., S.H.P., D.U.K, B.C.L., G.A.S., H.J.J., D.H.B., J.H.H. Approval of final manuscript: all Authors.
Acknowledgements
This work was supported by a 2-year Research Grant of Pusan National University, Busan, Republic of Korea.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2019;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Carlsen L, Schorl C, Huntington K, Hernandez-Borrero L, Jhaveri A, Zhang S, Zhou L, El-Deiry W. Pan-drug and drug-specific mechanisms of 5-FU, irinotecan (CPT-11), oxaliplatin, and cisplatin identified by comparison of transcriptomic and cytokine responses of colorectal cancer cells. Oncotarget. 2021;12(20):2006–2021. doi: 10.18632/oncotarget.28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagle P, Plukker J, Muijs C, Van Luijk P, Coppes R. Patient-derived tumor organoids for prediction of cancer treatment response. Seminars in Cancer Biology. 2020;53:258–264. doi: 10.1016/j.semcancer.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Hao M, Cao Z, Wang Z, Xin J, Kong B, Xu J, Zhang L, Chen P. Patient-derived organoid model in the prediction of chemotherapeutic drug response in colorectal cancer. ACS Biomaterials Science & Engineering. 2023;8(8):3515–3525. doi: 10.1021/acsbiomaterials.2c00354. [DOI] [PubMed] [Google Scholar]
- 5.Flood M, Narasimhan V, Wilson K, Lim W, Ramsay R, Michael M, Heriot A. Organoids as a robust preclinical model for precision medicine in colorectal cancer: a systematic review. Annals of Surgical Oncology. 2023;29(1):47–59. doi: 10.1245/s10434-021-10829-x. [DOI] [PubMed] [Google Scholar]
- 6.Ben-David U, Ha G, Tseng Y-Y, Greenwald NF, Oh C, Shih J, McFarland JM, Wong B, Boehm JS, Beroukhim R. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat Genet. 2017;49(11):1567–1575. doi: 10.1038/ng.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasch C, Favreau P, Yueh A, Babiarz C, Gillette A, Sharick J, Karim M, Nickel K, Dezeeuw A, Sprackling C, Emmerich P, Destefanis R, Pitera R, Payne S, Korkos D, Clipson L, Walsh C, Miller D, Carchman E, Burkard M, Lemmon K, Matkowskyj K, Newton M, Ong I, Bassetti M, Kimple R, Skala M, Deming D. Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation. Clinical Cancer Research. 2022;25(17):5376–5387. doi: 10.1158/1078-0432.CCR-18-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramzy G, Koessler T, Ducrey E, McKee T, Ris F, Buchs N, Rubbia-Brandt L, Dietrich P, Nowak-Sliwinska P. Patient-derived in vitro models for drug discovery in colorectal carcinoma. Cancers. 2020;12(6):1423. doi: 10.3390/cancers12061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sailer V, Pauli C, Merzier E, Mosquera J, Beltran H, Rubin M, Rao R. On-site cytology for development of patient-derived three-dimensional organoid cultures - a pilot study. Anticancer Research. 2018;37(4):1569–1573. doi: 10.21873/anticanres.11486. [DOI] [PubMed] [Google Scholar]
- 10.Ishida Y, Tsunoda T, Hamada Y, Tsuchiya N, Koga T, Kitaguchi T, Matsumoto K, Matsumoto S, Sasaki T, Nakashima R, Ishii F, Kajiwara M, Shirasawa S, Hasegawa S, Hirai F. Standardized methods using EUS-guided fine-needle biopsy and a minimal medium creates three pancreatic cancer organoids. Anticancer Research. 2022;42(8):4103–4109. doi: 10.21873/anticanres.15908. [DOI] [PubMed] [Google Scholar]
- 11.Kiwaki T, Kataoka H. Patient-derived organoids of colorectal cancer: a useful tool for personalized medicine. Journal of Personalized Medicine. 2022;12(5):695. doi: 10.3390/jpm12050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, McLaren-Douglas A, Blokker J, Jaksani S, Bartfeld S, Volckman R, van Sluis P, Li VS, Seepo S, Sekhar Pedamallu C, Cibulskis K, Carter SL, McKenna A, Lawrence MS, Lichtenstein L, Stewart C, Koster J, Versteeg R, van Oudenaarden A, Saez-Rodriguez J, Vries RG, Getz G, Wessels L, Stratton MR, McDermott U, Meyerson M, Garnett MJ, Clevers H. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drost J, Clevers H. Organoids in cancer research. Nature Reviews Cancer. 2021;18(7):407–418. doi: 10.1038/s41568-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 14.Aberle M, Burkhart R, Tiriac H, Olde Damink S, Dejong C, Tuveson D, Van Dam R. Patient-derived organoid models help define personalized management of gastrointestinal cancer. British Journal of Surgery. 2021;105(2):e48–e60. doi: 10.1002/bjs.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho YW, Min DW, Kim HP, An Y, Kim S, Youk J, Chun J, Im JP, Song SH, Ju YS, Han SW, Park KJ, Kim TY. Patient‐derived organoids as a preclinical platform for precision medicine in colorectal cancer. Mol Oncol. 2022;16(12):2396–2412. doi: 10.1002/1878-0261.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo L, Ma Y, Zheng Y, Su J, Huang G. Application progress of organoids in colorectal cancer. Frontiers in Cell and Developmental Biology. 2022;10:815067. doi: 10.3389/fcell.2022.815067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousová M, Holubec L, Sturgeon C. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. International Journal of Cancer. 2022;134(11):2513–2522. doi: 10.1002/ijc.28384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winder T, Lenz H. Molecular predictive and prognostic markers in colon cancer. Cancer Treatment Reviews. 2021;36(7):550–556. doi: 10.1016/j.ctrv.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Pan W, Zheng H, Zheng H, Wang Z, Li J, Deng C, Yan J. Accuracy of using a patient-derived tumor organoid culture model to predict the response to chemotherapy regimens in stage IV colorectal cancer. Diseases of the Colon & Rectum. 2021;64(7):833–850. doi: 10.1097/DCR.0000000000001971. [DOI] [PubMed] [Google Scholar]
- 20.Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, Rata M, Koh DM, Tunariu N, Collins D, Hulkki-Wilson S, Ragulan C, Spiteri I, Moorcraft SY, Chau I, Rao S, Watkins D, Fotiadis N, Bali M, Darvish-Damavandi M, Lote H, Eltahir Z, Smyth EC, Begum R, Clarke PA, Hahne JC, Dowsett M, de Bono J, Workman P, Sadanandam A, Fassan M, Sansom OJ, Eccles S, Starling N, Braconi C, Sottoriva A, Robinson SP, Cunningham D, Valeri N. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920–926. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll H, Tveit K, Gibson F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clinical Colorectal Cancer. 2022;14(1):1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Mohelnikova-Duchonova B. FOLFOX/FOLFIRI pharmacogenetics: The call for a personalized approach in colorectal cancer therapy. World Journal of Gastroenterology. 2019;20(30):10316. doi: 10.3748/wjg.v20.i30.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E, Schipper L, Hoes L, Vis DJ, van de Haar J. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Science Transl Med. 2019;11(513):eaay2574. doi: 10.1126/scitranslmed.aay2574. [DOI] [PubMed] [Google Scholar]