Abstract
Background/Aim: Classic Hodgkin lymphoma (cHL) is a common B-cell malignancy. Despite the good prognosis, in some patients the standard chemotherapy and radiotherapy-based approach does not lead to long-term remission, and these patients eventually relapse. Moreover, the primary refractory disease is of major concern regarding prognosis.
Patients and Methods: We performed a retrospective analysis to evaluate PD-L1 expression in 120 patients with classic Hodgkin lymphoma (cHL).
Results: The median follow-up of the entire group of patients was 90 months. After initial therapy, complete remission was achieved in 113 (94.2%) patients. During the follow-up, cHL relapse/refractory disease was reported in 23 (19.2%) cases. A total of five patients died during the follow-up period, all from cHL progression. When determining PD-L1 expression on Hodgkin-Reed-Sternberg (HRS) cells, 37 cases (30.8%) were evaluated as negative, and 83 cases (69.2%) as positive. In the negative PD-L1 group of patients, no cHL relapse/refractory disease was observed during the follow-up period. However, out of 83 patients with positive PD-L1 expression on HRS cells, 23 (28%) showed relapse/refractory cHL.
Conclusion: A significantly higher relapse rate was observed in PD-L1-positive patients diagnosed with cHL.
Keywords: Classic Hodgkin lymphoma, PD-L1 expression, treatment, overall survival, relapse
Classic Hodgkin lymphoma (cHL) is a common B-cell malignancy with increased incidence in young adults and those over 55 years of age (1,2). Generally, cHL has a good prognosis; long-term remission is achieved in more than 80% of patients (3). Unfortunately, in some patients, the standard chemotherapy and radiotherapy-based approach does not lead to long-term remission, and these patients eventually relapse. Moreover, the primary refractory disease is of major concern regarding prognosis (4,5).
Compared to other lymphomas, cHL contains only a small portion of malignant cells in the tumor – Hodgkin-Reed-Sternberg (HRS) cells account for only 1% - 5% of all cells (6). The tumor microenvironment (TME) mostly consists of inflammatory cells, including macrophages, CD4+ and CD8+ T cells, plasma cells, eosinophils, and other immune cells (7). However, antitumor immune response is limited. Furthermore, studies show that HRS cells produce molecules that limit the efficacy of T cell–mediated antitumor immune responses (6). Primary HRS cells express programmed cell death-1 ligand 1 (PD-L1), while tumor-infiltrating T cells express the coinhibitory receptor, programmed death-1 (PD-1) (7).
The function of PD-1 signaling is to abrogate T cell–mediated immune responses (8). Normal antigen-presenting cells, dendritic cells, and macrophages express PD-1 ligands that bind PD-1 receptors on activated T cells (8,9). Then, PD-1 receptor recruits the Src homology 2 domain–containing protein tyrosine phosphatase-2 (SHP2) phosphatase to the immunoreceptor complex, resulting in attenuation of T cell receptor (TCR) signaling (8). PD-1 signaling results in “T-cell exhaustion”, temporary inhibition of activation and proliferation that can be reversed if the PD-1 signal is removed (2). Furthermore, PD-L1 promotes the induction and maintenance of PD-1+ T regulatory cells (10).
The PD-1 ligand genes, PD-L1 and PD-L2, are located on chromosome 9p24.1 and are separated by 42 kilobases (8). Interestingly, a regulatory loop with Janus kinase 2 (JAK2), located upstream from PD-L1 on 9p24.1, further augments PD-1 ligand expression in cHL (5).
PD-1 ligands are over-expressed and associated with an unfavorable prognosis in tumors, including malignant melanoma, colon, pancreatic, hepatocellular, and ovarian carcinomas (11–16). They are also associated with increased tumor invasiveness (17). In cHL, amplification of the PD-L1 and PD-L2 genes in HRS cells has been described (18). Subsequently, inhibition of PD-1 receptor binding to PD-L1 will potentiate the anti-tumor T-cell response and consequently reduce tumor growth (19) and thus may alter the current poor treatment outcomes in patients with relapsed/refractory cHL (20).
In our study, we analyzed the expression of PD-L1 on HRS cells and compared its level with treatment outcome of patients with cHL.
Patients and Methods
Clinical characteristics. Between 2003 and 2016, 713 individuals were diagnosed with cHL at the Department of Internal Medicine, Hematology and Oncology, University Hospital Brno, Czech Republic. Out of these, 120 patients were included into this retrospective analysis. Before entering the study, all patients signed the informed consent form approved by the Ethics committee of the hospital in accordance with the current version of the Helsinki Declaration.
The analyzed group of patients consisted of 52 (43.3%) men and 68 (56.7%) women treated and monitored by members of cHL group of our department. The median age at diagnosis was 30.5 years (range=14-76). Most patients were diagnosed at clinical stages I and II (78 patients, 65%), whereas 53 (35%) were diagnosed at stages III and IV. There were 85 cases of nodular sclerosis subtype (NSCHL), 27 cases of mixed cellularity subtype (MCCHL), 7 cases of lymphocyte-rich subtype (LRCHL), and 1 case of lymphocyte depleted subtype (LDCHL). The median follow-up period of our patients was 90 months (range=40-166 months). A total of five patients died during the follow-up of the group from cHL progression. All clinical characteristics are described in Table I.
Table I. Clinical characteristics of classic Hodgkin lymphoma (cHL) patients (N=120).
NSCHL: Nodular sclerosis cHL; MCCHL: mixed cellularity cHL; LRCHL: lymphocyte-rich cHL; LDCHL: lymphocyte depleted cHL.
Treatment protocols. Patients were treated with ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) or BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) chemotherapy regimens; 71 (59.2%) of the patients underwent radiotherapy as part of the initial treatment. cHL patients in localized stage I and II without risk factors were treated with 2 cycles of ABVD and radiotherapy (20-30 Gy IF). Patients with localized clinical stage I and II and risk factors were treated by 4 cycles of ABVD or 2 cycles of BEACOPP and 2 cycles of ABVD. After chemotherapy, all patients were treated with radiotherapy (30 Gy IF). Risk factors were defined based on current risk criteria (21). Patients in advanced stages III and IV were treated with 6-8 cycles of BEACOPP. Radiotherapy (30 Gy IF) was used only in cases when vital residuum remained after chemotherapy.
Immunohistochemistry. An antibody to PD-L1 (clone 28-8, dilution 1:100, Abcam, Cambridge, UK) was used in the immunohistochemical analysis. Clone 28-8 specifically reacts with extracellular domain of huPD-L1. The antibody was diluted using Ventana Antibody Diluent (Roche, Basel, Switzerland). Formalin-fixed, paraffin-embedded 4 μm thick sections from all tissue blocks were cut. The staining was performed using VENTANA BenchMark ULTRA (Roche). The protocol was based on heat induced epitope retrieval (HIER) in cell conditioning 1 (CC1), efficient heating time was 72 min. Then, slices were incubated for 16 min with the primary antibody and the linker for 4 min. OptiView (Ventana Medical Systems, Tucson, AZ, USA) was used as detection kit. Following dehydration in a series of concentrated ethanol baths and clearing in xylene, the preparations were mounted in Pentex (Pentex Labs, Kankakee, IL, USA). The expression of PD-L1 was evaluated by counting percent of positive cells to all neoplastic cells. Pathological evaluation was performed using a Nicon Eclipse 55i microscope (Nikon, Tokyo, Japan) equipped with 4×/0.10, 10×/0.30, 20×0.50, 40×/0.75 and 60×/0.85 objective lenses Positivity for PD-L1 was categorized into three and two groups according as follows: 0=“– negative” and 1–49=“+ slightly positive”, 50 or more=“++ strongly positive” ‘; or a rougher division into 0=“– negative” and 1 or more=“+ positive”.
Statistics. Standard descriptive statistics was applied in the analysis; absolute and relative frequencies for categorical variables and mean supplemented with median and min-max range for continuous variables. Statistical significance of differences was computed using Fisher exact test for categorical variables and Kruskal-Wallis test for continuous variables. Influence of PD-L1 positivity on disease status was analyzed using logistic regression and described using odds ratio, its 95% confidence interval and statistical significance. The level of statistical significance was set at α=0.05, all hypotheses were evaluated as two-sided. Analysis was computed using SPSS 24.0.0.1 (IBM Corporation, Armonk, NY, USA).
Results
Treatment. Complete remission after initial therapy was achieved in 113 (94.2%) patients, six patients progressed, one patient achieved only partial remission. During the follow-up, 23 (19.2%) patients relapsed; early relapse/refractory disease was observed in 10 (8.4%) patients and late relapse in 13 (10.8%) patients. In the group of patients with late relapse (≥12 months), the median relapse-free survival (RFS) was 36 months (13-68) (Table II).
Table II. Treatment response in classic Hodgkin lymphoma patients.
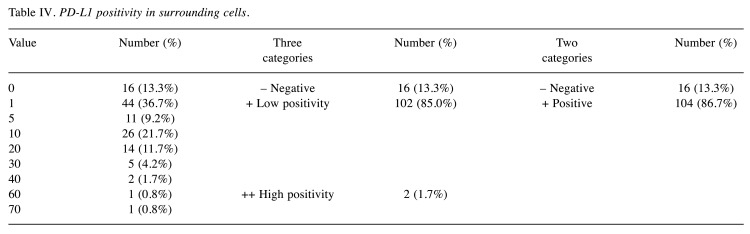
Immunohistochemistry. PD-L1 positivity was determined on HRS cells as well as on surrounding cells. When determining PD-L1 expression in HRS cells, a total of 37 cases (30.8%) were evaluated as negative and 83 (69.2%) evaluated as positive, of which 50 (41.7%) were slightly positive and 33 (27.5%) strongly positive (Table III). In surrounding cells, a total of 16 samples (13.3%) were assessed as PD-L1 negative, 104 (86.7%) as PD-L1 positive, of which 102 (85.0%) were slightly positive and 2 (1.7%) strongly positive (Table IV).
Table III. PD-L1 positivity in Hodgkin-Reed-Sternberg (HRS) cells.
Table IV. PD-L1 positivity in surrounding cells.
Correlation between PD-L1 status and relapse/progression. Analysis of PD-L1 expression in HRS cells indicated that out of the 120 cHL patients, 37 PD-L1 negative patients did not relapse during the follow-up period (100%). Furthermore, out of 83 PD-L1 positive patients, 60 patients did not relapse (72.3%) and 23 (27.7%) relapsed. Thus, a significantly higher relapse rate in patients with PD-L1 positive tumor cells was observed (p<0.001) (Figure 1).
Figure 1. Relapse-free survival of classic Hodgkin lymphoma patients according to PD-L1 positivity in Hodgkin-Reed-Sternberg cells.
Analysis of positivity of PD-L1 in surrounding cells indicated that there was no statistically significant difference between relapsed patients and patients without relapse (data not shown).
Furthermore, we divided the 83 PD-L1 positive patients into two groups based on PD-L1 expression: in the slightly positive (1%-50%) group, there were 50 patients; in the strongly positive group (51%-100%) there were 33 cases. We found that in the slightly positive group, 41 patients (82%) did not relapse, four (8%) relapsed early and five patients (10%) relapsed late. In the strongly positive group of patients, 19 patients (57.6%) did not relapse, six patients (18.2%) relapsed early and eight (24.2%) relapsed late (p<0.001) (Table V).
Table V. PD-L1 positivity in Hodgkin-Reed-Sternberg cells and treatment outcome (N=120).
We did not find a statistically significant correlation with cHL clinical stage in the evaluation of PD-L1 positivity in HRS cells (Figure 1). On the contrary, a correlation was found between PD-L1 positivity in surrounding cells and the clinical stage of cHL. Increased value of positivity correlated with increased stage (p=0.032) (Figure 2).
Figure 2. Relationship of PD-L1 positivity in background cells and stage of the disease (p<0.032).
Discussion
PD-1, also called CD279, was first identified in murine cell lines in 1992. PD-1 expression is induced in a variety of activated immune cells, such as macrophages, T cells, B cells, NKT cells and others. After PD-1 binds to its ligands (PD-L1 and PD-L2), the active immune cells are inhibited. In recent years, several studies have shown that tumor cells silence the immune system by expressing PD-L1. Thus, blockade of PD-1 and PD-L1 has been tested in ovarian, bladder, colorectal, and uterine cancers as well as Hodgkin lymphoma [reviewed in (22)]. Nivolumab, a human IgG4 monoclonal antibody blocking PD-1 expression, was approved by the FDA in 2014 for the treatment of metastatic melanoma.
Despite recent indisputable success of cHL treatment, approximately 20% of patients do not achieve long-term remission and must be treated for relapse or refractory cHL. However, the efficacy of this treatment is lower; approximately 50% of patients with relapsed or refractory disease following first-line treatment can be cured by high-dose chemotherapy (HDCT) and autologous stem cell transplantation (ASCT) and subsequent optional consolidation is performed in high-risk patients (23–29). In view of the new treatment options presented by PD-1 and PD-L1 inhibitors, many studies investigated the expression of PD-1/PD-L1 in cHL and its relevance to patients’ prognosis.
PD-L1 over-expression in cHL is likely to be more common than in other types of lymphomas. The comparison between cHL primary mediastinal large B-cell lymphoma (PMBL), and gray zone lymphoma (GZL) demonstrated over-expression of PD-L1 (≥70% of tumor cells) in 62% of cHL, 15% of PMBL and 46 % GZL cases (30).
In addition to the detection of PD-L1 expression in primary biopsies, the assessment of PD-L1 expression in relapsed or progressed cHL is clinically important. In the study of Vranic et al. (31), the expression of PD-L1 in 78 samples from patients with various treatment-resistant lymphomas was examined. The strongest and consistent expression (10/11 cases) was observed in cHL and PMBL (3/3). Diffuse large B-cell lymphomas (DLBCL) were often positive (13/26) regardless of sub-type. Follicular (1/8), peripheral T-lymphocytes (3/11) and mantle cells (1/8) were rarely positive in lymphomas, whereas small lymphoma lymphocytes/CLL and lymphomas in the peripheral zones were permanently negative (3/3) (31).
One study showed PD-1/PD-L1 expression on diagnostic samples of pediatric cHL patients at the time of diagnosis. This study included a group of patients in remission and a group of patients with relapsed/refractory cHL. Of the 42 patients analyzed, 31 achieved remission and the remaining 11 patients were classified as relapsing or refractory. At the time of diagnosis, low PD-1 expression was found in <20% of cases, while PD-L1 was expressed on tumor cells in all cases; most cases showed a strong PD-L1 positivity (≥50%). There were no significant differences in PD-1/PD-L1 expression levels between the groups (32).
The importance of PD-L1, PD-L2, and PD-1 expression in the prognosis of cHL patients was evaluated in a retrospective study involving a total of 109 cHL patients treated with ABVD. The median follow-up period was 4.91 years (range=0.17–17.33 years). Thirteen patients (11%) expressed PD-1 protein in the peritumoral microenvironment, which was associated with poor overall survival (OS). However, PD-L1 or PD-L2 expression level was not significant for OS. There was no correlation between PD-L1 and PD-1 expression or between PD-L2 and PD-1 expression. Multivariate analysis identified PD-1 protein as an independent prognostic factor for OS (33).
In another study, tissue samples of 87 cHL cases were analyzed. Co-expression of PD-1 and PD-L1 was found to be associated with shorter OS, although PD-1 or PD-L1 expression was not shown to be related to survival. In cases without PD-1 or PD-L1 expression, OS and disease-free survival (DFS) were 135 and 107 months, respectively. OS and DFS in cases expressing both PD-1 and PD-L1 were statistically significant at 24 and 20 months, respectively. Co-expression of PD-1 and PD-L1 was found to be an independent risk factor (34).
A study analyzing PD-L1/L2 genetic alterations in cHL evaluated a total of 108 biopsy samples from newly diagnosed cHL patients. It was shown that 97% of all evaluated cHL patients had identical changes in the PD-L1 and PD-L2 loci (5% polysomy, 56% copy number gain, 36% amplification). Progression-free survival (PFS) was significantly shorter in patients with 9p24.1 amplification, and the incidence of 9p24.1 amplification was higher in advanced stages of cHL (35).
It is also important to monitor the changes of PD-1 and PD-L1 expression before and during the treatment of cHL patients. In a group of 3 500 cHL patients, there were 11 patients with diagnostic cHL biopsy and a previous benign lymph node biopsy, which was later reclassified as cHL. These patients were marked as untreated. Thirty patients had a primary biopsy and a cHL relapse biopsy. These patients were described as treated. There was no statistically significant difference between biopsy 1 and 2 in untreated patients. In treated patients, a statistically significant increase in PD-1+ leukocytes, PD-L1+ leukocytes and PD-L1+ tumor cells was shown in cHL relapses (36).
In the CheckMate 205 study, the evaluation of the effect of the blockage of PD-1 receptor in patients with relapsed or refractory cHL who were treated with nivolumab was performed. Patients with higher 9p24.1 copies and over-expression of PD-L1 on HRS cells had better PFS. In HRS cells, expression of β2-microglobulin/MHC class I was not a significant predictive factor for complete remission or PFS on nivolumab therapy. On the contrary, MHC class II expression on HRS cells was a significant predictive factor for complete remission. In patients with an interval >12 months between ASCT and nivolumab therapy, MHC class II HRS expression was associated with prolonged PFS (37). High prevalence of PD-L1 and PD-L2 expression has also been demonstrated in patients with cHL in the KEYNOTE study (38).
In conclusion, negative expression of PD-L1 correlated with RFS of cHL patients in concordance with previously published studies.
Conflicts of Interest
The Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors’ Contributions
ZK designed the study, analyzed clinical data, and wrote the manuscript. LK performed immunohistochemistry. JM obtained clinical data. JM performed statistical analysis. TA and ZK obtained clinical data. VK performed immunohistochemistry. All Authors read and approved the final manuscript.
Acknowledgements
The Authors would like to thank all the patients for participating in this study.
References
- 1.Glaser S, Jarrett R. 1 The epidemiology of Hodgkin’s disease. Baillière’s Clinical Haematology. 2020;9(3):401–416. doi: 10.1016/S0950-3536(96)80018-7. [DOI] [PubMed] [Google Scholar]
- 2.Rozalli F, Chua S, Green D. Elucidation of acute renal failure due to recurrent non-Hodgkin lymphoma by F-18 FDG PET/CT. Clinical Nuclear Medicine. 2021;33(3):201–203. doi: 10.1097/RLU.0b013e318162ddc7. [DOI] [PubMed] [Google Scholar]
- 3.Ansell S. Hodgkin lymphoma: 2018 update on diagnosis, risk-stratification, and management. American Journal of Hematology. 2021;93(5):704–715. doi: 10.1002/ajh.25071. [DOI] [PubMed] [Google Scholar]
- 4.Longo D, Duffey P, Young R, Hubbard S, Ihde D, Glatstein E, Phares J, Jaffe E, Urba W, Devita V. Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin’s disease after combination chemotherapy: the low probability for cure. Journal of Clinical Oncology. 2022;10(2):210–218. doi: 10.1200/JCO.1992.10.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Master S, Koshy N, Wilkinson B, Rosen K, Mills G, Mansour R, Shi R. Effect of radiation therapy on survival in Hodgkin’s lymphoma: A SEER data analysis. Anticancer Res. 2017;37:3035–3043. doi: 10.21873/anticanres.11658. [DOI] [PubMed] [Google Scholar]
- 6.Küppers R, Engert A, Hansmann M-L. Hodgkin lymphoma. J Clin Invest. 2012;122:3439–3447. doi: 10.1172/JCI61245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, Kondo T, Ohmori K, Kurata M, Hayashi T, Uchiyama T. PD-1–PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 8.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman G, Minato N, Honjo T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunology Letters. 2023;84(1):57–62. doi: 10.1016/S0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 10.Francisco L, Salinas V, Brown K, Vanguri V, Freeman G, Kuchroo V, Sharpe A. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. Journal of Experimental Medicine. 2020;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2021;116(7):1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 12.Geng L, Huang D, Liu J, Qian Y, Deng J, Li D, Hu Z, Zhang J, Jiang G, Zheng S. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. Journal of Cancer Research and Clinical Oncology. 2019;134(9):1021–1027. doi: 10.1007/s00432-008-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Q, Wang X, Qiu S, Yamato I, Sho M, Nakajima Y, Zhou J, Li B, Shi Y, Xiao Y, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clinical Cancer Research. 2022;15(3):971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 14.Loos M, Giese N, Kleeff J, Giese T, Gaida M, Bergmann F, Laschinger M, Büchler M, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Letters. 2019;268(1):98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 15.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clinical Cancer Research. 2023;13(7):2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 16.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proceedings of the National Academy of Sciences. 2022;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Scientific Reports. 2023;5(1):13110. doi: 10.1038/srep13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett MT, Anderson KS, Lenkiewicz E, Andreozzi M, Cunliffe HE, Klassen CL, Dueck AC, McCullough AE, Reddy SK, Ramanathan RK, Northfelt DW, Pockaj BA. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget. 2015;6:26483–26493. doi: 10.18632/oncotarget.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sunshine J, Taube J. PD-1/PD-L1 inhibitors. Current Opinion in Pharmacology. 2020;23:32–38. doi: 10.1016/j.coph.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, De Boer JP, Kuruvilla J, Savage KJ, Trneny M, Shipp MA, Kato K, Sumbul A, Farsaci B, Ansell SM. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engert A, Franklin J, Eich HT, Brillant C, Sehlen S, Cartoni C, Herrmann R, Pfreundschuh M, Sieber M, Tesch H, Franke A, Koch P, de Wit M, Paulus U, Hasenclever D, Loeffler M, Müller RP, Müller-Hermelink HK, Dühmke E, Diehl V. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin’s lymphoma: final results of the GHSG HD7 trial. J Clin Oncol. 2007;25(23):3495–3502. doi: 10.1200/JCO.2006.07.0482. [DOI] [PubMed] [Google Scholar]
- 22.Salmaninejad A, Khoramshahi V, Azani A, Soltaninejad E, Aslani S, Zamani M, Zal M, Nesaei A, Hosseini S. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics. 2022;70(2):73–86. doi: 10.1007/s00251-017-1015-5. [DOI] [PubMed] [Google Scholar]
- 23.Engert A, Plütschow A, Eich H, Lohri A, Dörken B, Borchmann P, Berger B, Greil R, Willborn K, Wilhelm M, Debus J, Eble M, Sökler M, Ho A, Rank A, Ganser A, Trümper L, Bokemeyer C, Kirchner H, Schubert J, Král Z, Fuchs M, Müller-Hermelink H, Müller R, Diehl V. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. New England Journal of Medicine. 2020;363(7):640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 24.Von Tresckow B, Plütschow A, Fuchs M, Klimm B, Markova J, Lohri A, Kral Z, Greil R, Topp M, Meissner J, Zijlstra J, Soekler M, Stein H, Eich H, Mueller R, Diehl V, Borchmann P, Engert A. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin study group HD14 trial. Journal of Clinical Oncology. 2022;30(9):907–913. doi: 10.1200/JCO.2011.38.5807. [DOI] [PubMed] [Google Scholar]
- 25.Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, Zijlstra J, Král Z, Fuchs M, Hallek M, Kanz L, Döhner H, Dörken B, Engel N, Topp M, Klutmann S, Amthauer H, Bockisch A, Kluge R, Kratochwil C, Schober O, Greil R, Andreesen R, Kneba M, Pfreundschuh M, Stein H, Eich H, Müller R, Dietlein M, Borchmann P, Diehl V. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. The Lancet. 2018;379(9828):1791–1799. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- 26.Gordon LI, Hong F, Fisher RI, Bartlett NL, Connors JM, Gascoyne RD, Wagner H, Stiff PJ, Cheson BD, Gospodarowicz M, Advani R, Kahl BS, Friedberg JW, Blum KA, Habermann TM, Tuscano JM, Hoppe RT, Horning SJ. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31(6):684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Tresckow B, Engert A. The role of autologous transplantation in Hodgkin lymphoma. Current Hematologic Malignancy Reports. 2020;6(3):172–179. doi: 10.1007/s11899-011-0091-0. [DOI] [PubMed] [Google Scholar]
- 28.Morschhauser F, Brice P, Fermé C, Diviné M, Salles G, Bouabdallah R, Sebban C, Voillat L, Casasnovas O, Stamatoullas A, Bouabdallah K, André M, Jais J, Cazals-Hatem D, Gisselbrecht C. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. Journal of Clinical Oncology. 2022;26(36):5980–5987. doi: 10.1200/JCO.2007.15.5887. [DOI] [PubMed] [Google Scholar]
- 29.Moskowitz C, Nadamanee A, Masszi T, Agura E, Holowiecki J, Abidi M, Chen A, Stiff P, Gianni A, Carella A, Osmanov D, Bachanova V, Sweetenham J, Sureda A, Huebner D, Larsen E, Hunder N, Walewski J. The Aethera trial: Results of a randomized, double-blind, placebo-controlled phase 3 study of brentuximab vedotin in the treatment of patients at risk of progression following autologous stem cell transplant for Hodgkin lymphoma. Blood. 2019;124(21):673–673. doi: 10.1182/blood.V124.21.673.673. [DOI] [Google Scholar]
- 30.Tanaka Y, Maeshima A, Nomoto J, Makita S, Fukuhara S, Munakata W, Maruyama D, Tobinai K, Kobayashi Y. Expression pattern of PD-L1 and PD-L2 in classical Hodgkin lymphoma, primary mediastinal large B-cell lymphoma, and gray zone lymphoma. European Journal of Haematology. 2021;100(5):511–517. doi: 10.1111/ejh.13033. [DOI] [PubMed] [Google Scholar]
- 31.Vranic S, Ghosh N, Kimbrough J, Bilalovic N, Bender R, Arguello D, Veloso Y, Dizdarevic A, Gatalica Z. PD-L1 status in refractory lymphomas. PLoS One. 2016;11:e0166266–e0166266. doi: 10.1371/journal.pone.0166266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dilly-Feldis M, Aladjidi N, Refait JK, Parrens M, Ducassou S, Rullier A. Expression of PD-1/PD-L1 in children’s classical Hodgkin lymphomas. Pediatr Blood Cancer. 2019;66:e27571. doi: 10.1002/pbc.27571. [DOI] [PubMed] [Google Scholar]
- 33.Koh Y, Jeon Y, Yoon D, Suh C, Huh J. Programmed death 1 expression in the peritumoral microenvironment is associated with a poorer prognosis in classical Hodgkin lymphoma. Tumor Biology. 2019;37(6):7507–7514. doi: 10.1007/s13277-015-4622-5. [DOI] [PubMed] [Google Scholar]
- 34.Paydas S, Bağır E, Seydaoglu G, Ercolak V, Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Annals of Hematology. 2020;94(9):1545–1552. doi: 10.1007/s00277-015-2403-2. [DOI] [PubMed] [Google Scholar]
- 35.Roemer M, Advani R, Ligon A, Natkunam Y, Redd R, Homer H, Connelly C, Sun H, Daadi S, Freeman G, Armand P, Chapuy B, De Jong D, Hoppe R, Neuberg D, Rodig S, Shipp M. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. Journal of Clinical Oncology. 2022;34(23):2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollander P, Amini R, Ginman B, Molin D, Enblad G, Glimelius I. Expression of PD-1 and PD-L1 increase in consecutive biopsies in patients with classical Hodgkin lymphoma. PLOS ONE. 2018;13(9):e0204870. doi: 10.1371/journal.pone.0204870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roemer M, Redd R, Cader F, Pak C, Abdelrahman S, Ouyang J, Sasse S, Younes A, Fanale M, Santoro A, Zinzani P, Timmerman J, Collins G, Ramchandren R, Cohen J, De Boer J, Kuruvilla J, Savage K, Trneny M, Ansell S, Kato K, Farsaci B, Sumbul A, Armand P, Neuberg D, Pinkus G, Ligon A, Rodig S, Shipp M. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. Journal of Clinical Oncology. 2022;36(10):942–950. doi: 10.1200/JCO.2017.77.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armand P, Shipp M, Ribrag V, Michot J, Zinzani P, Kuruvilla J, Snyder E, Ricart A, Balakumaran A, Rose S, Moskowitz C. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. Journal of Clinical Oncology. 2022;34(31):3733–3739. doi: 10.1200/JCO.2016.67.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]