Abstract
Background/Aim: COVID-19 has dramatically impacted non-pandemic-related care, including preventive medicine. Our objective was to quantify the alterations in the volume of screening tests for breast and cervical cancer during the COVID-19 era compared to pre-pandemic levels. Secondarily, we discussed the causes responsible for this change, presented suggestions for screening optimization and conducted a targeted search of the relevant literature for worsening of future mortality due to screening setback.
Materials and Methods: We systematically searched Pubmed, Google Scholar and Epistemonikos for articles in English or Greek, published from March 11th, 2020, until September 14th, 2022, that illustrated quantitative variations of mammograms or Pap/HPV tests. Preprint articles, editorials and speeches were excluded. Quality of included studies was assessed via the JBI critical appraisal checklist for studies reporting prevalence data. The evidence was narratively synthesized.
Results: A total of 56 articles were included, being either observational studies or reports from cancer registries. Large reductions were universally identified, peaked during the first wave but partially persisted after easing of the restrictions.
Conclusion: Our systematic review provides an updated record of the variations in screening volume and approaches screening neglect from a multidimensional perspective answering why it happened and how we could achieve recovery. A strong awareness campaign is proposed, in conjunction with triaging citizens more likely to benefit from screening. Cervical self-sampling is emphasized in the literature. Various studies displayed a potential increase in cancer mortality in the future based on predictive statistical models.
Keywords: COVID-19, pandemic, screening, cancer, mammography, Pap test, review
Since March 2020, when the Coronavirus disease 2019 (COVID-19) was officially declared a pandemic by the World Health Organization (WHO) (1), healthcare systems have been pushed to drastic adjustments in service provision and public health has been affected both directly and indirectly. By October 2022, more than 625 million people were infected, and total registered deaths had risen to over 6.5 million. Furthermore, major victims of this unprecedented health crisis have been non-emergency care and preventive medicine (2-4).
Malignant neoplasms are a major cause of morbidity and mortality with a global footprint, being the second leading cause of death worldwide with low- and middle-income countries already affected, partially due to the adoption of the western lifestyle (5).
Cancer screening is a method of secondary prevention and aims at detecting cancerous or precancerous lesions before they cause clinical symptoms. It is well established that screening of appropriate population groups can reduce the mortality of certain types of cancer, as early treatment can be more effective (6). The cancer types, in which screening has a proven benefit, include breast and cervical cancer. For breast cancer screening, mammography is mainly used, while regarding cervical cancer, Pap tests (cytological examination of cervical smear), possibly combined with HPV test, are the golden standard (6,7).
By 2030, breast cancer diagnoses are expected to exceed 2.4 million annually, demonstrating the enormous burden of the disease and its impact on global health. The screening of asymptomatic populations allows the detection of non-palpable tumors smaller than 15 millimeters. The method considered most sensitive and broadly used is screening mammography combined with clinical examination (8). The guidelines regarding at what age screening should start, at what age it should stop, and how often it should be repeated, are not unanimous (9,10). In many studies, it becomes clear that women who undergo regular screening have 10-25% lower chance of dying from breast cancer and thus demonstrate a net benefit in adopting screening guidelines (11).
Cervical cancer on the other hand is the most common malignancy of the female genital tract worldwide. A total of 85% of cases affect women in developing countries due to the lack of organized screening and vaccination programs for the HPV virus. Screening for the disease is carried out with a Pap test and/or an HPV test. (12). Thanks to the widespread acceptance of screening, the risk for a woman in the US of being diagnosed with cervical cancer in her lifetime is only 0.6% (13). According to the meta-analysis by Peirson et al., women who have had regular screening based on guidelines are 65 percent less likely to have developed cervical cancer (14).
As Primary Health Care was focusing on controlling the COVID-19 pandemic, cancer screening programs along with outpatient care for chronic disease suffered from defunding and restrictions imposed by the governments on a global scale (15). Our study aimed to quantify screening variations for breast and cervical cancer both during and after lockdown restrictions compared to the pre pandemic period.
Materials and Methods
Our systematic review was conducted in accordance with the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) of 2020. The review protocol is registered in PROSPERO and has been available online since January 17th, 2023 (CRD42023390559).
Search strategy and selection criteria. Our search strategy was generated by consensus among all researchers. Two authors independently identified records that reported on the change of the volume of screening tests performed during the COVID-19 pandemic, via systematically searching PubMed, Google Scholar and Epistemonikos databases for entries published from March 11th, 2020 (onset of the pandemic) until September 14th, 2022. Snowball sampling by searching reference lists and citation tracking was performed in each retrieved article. The following search terms were used: (“cancer screening” OR “mammography” OR “breast cancer screening” OR “Pap testing” OR “cervical cancer screening”) AND (“SARS-COV-2” OR “COVID-19” OR “pandemic”).
Criteria for inclusion followed the PICOT format. The population (P) of our study refers to female adults who meet the criteria of screening for breast or cervical cancer. Interventions (I) studied were mammography for breast cancer and Pap test or HPV test for cervical cancer, compared (C) with the same screening programs during the prepandemic period. The outcomes (O) were changes in number (or percentage variation) of screening tests performed and the type (T) of studies included were observational studies and data from cancer screening registries.
Only studies published in English or Greek were included. Articles referring to changes in test volume that did not distinguish pre-symptomatic from post-symptomatic control were excluded. Moreover, preprint articles published on Medrxiv and SSRN servers were excluded, as well as editorial type entries, commentaries, case reports, summaries of conference speeches, oral presentations, and posters.
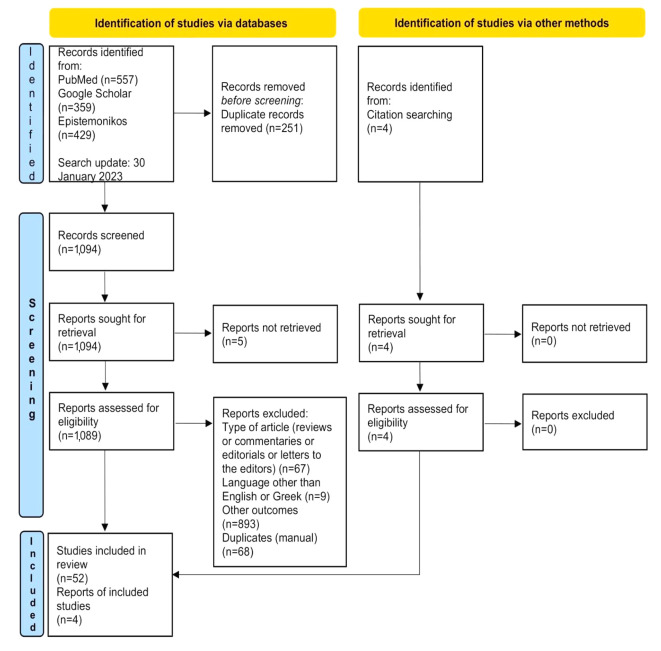
Data collection, quality assessment and analysis. Rayyan software was used to identify and remove duplicate entries among the three databases. Duplicate articles that could not be identified by the software were manually removed afterwards. Two authors independently reviewed the unique entries initially with title and abstract screening against eligibility criteria, followed by full-text reading of all potentially relevant publications. Further irrelevant articles were excluded at this second stage, and reasons for exclusion were recorded. The exact course of the procedure is presented in the flow chart on the 2020 PRISMA guidelines (Figure 1).
Figure 1. Flow diagram demonstrating the selection process of appropriate records based to PRISMA 2020 guidelines.
The following data were extracted from the included studies by two independent investigators: name of the first author, date of publication, country, design of the study, type of cancer, type of screening test and the changes in number of screening tests performed. The accuracy of the aforementioned data was confirmed by comparing the collection forms of the involved investigators.
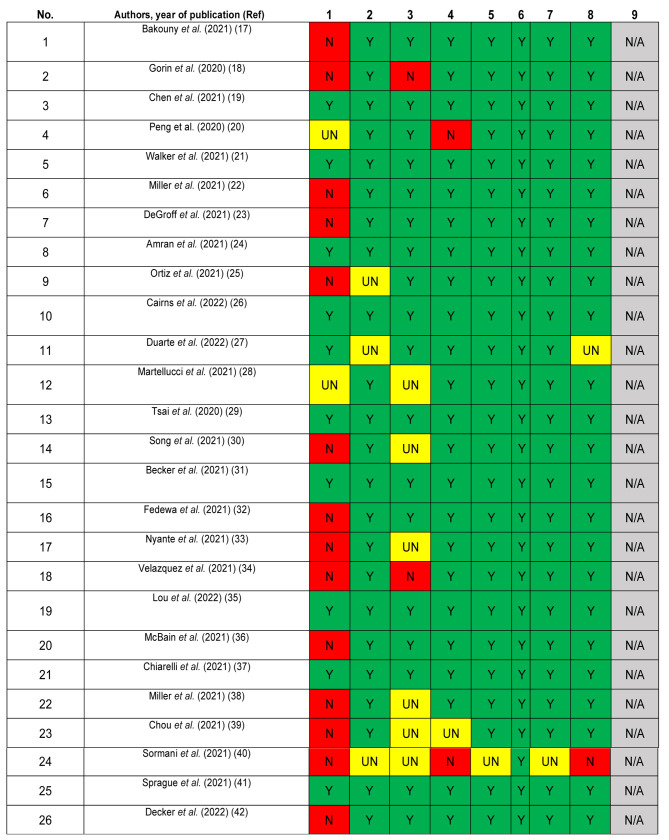
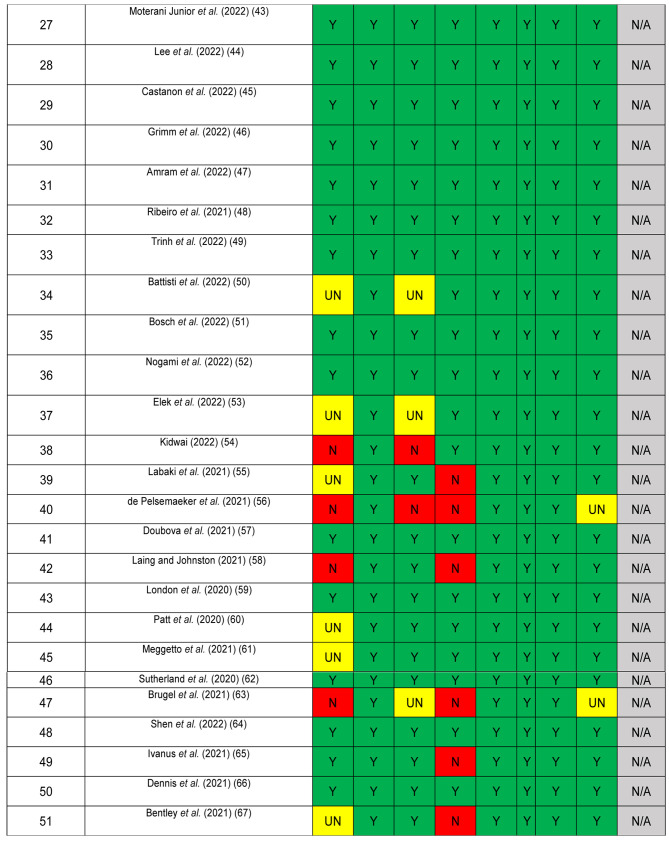
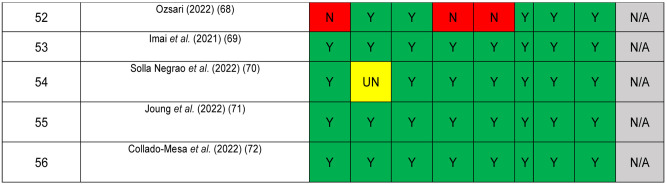
The quality of included studies was assessed using the JBI critical appraisal checklist for studies reporting prevalence data, a tool consisting of 9 criteria (Figure 2). Studies meeting a small proportion of these criteria are considered to be at high risk of bias and their results should be interpreted with caution (16). No article was excluded because of a low-quality score. Due to the high level of heterogeneity among the studies, a narrative synthesis of the evidence was conducted. Disagreements were resolved by discussion among all authors until a consensus was achieved.
Figure 2. Quality assessment of included articles using JBI checklist for prevalence studies. Y: Yes; N: no; UN: unclear; N/A: not applicable.
Results
Our search in PubMed, Google Scholar and Epistemonikos databases produced 1,094 articles after the deletion of 251 duplicate records. After reviewing the title and the abstract, 119 articles remained. Finally, 52 articles were selected after full text reading and further 4 articles were selected from the references of the included articles. The total number of the reviewed articles was 56 (17-72).
The 56 articles that were selected came from the following countries in descending order of frequency: USA (25 articles), Canada (6 articles), Taiwan (4 articles), Brazil (4 articles), Australia (2 articles), UK (2 articles), South Korea (2 articles), Bangladesh (1 article), France (1 article), Belgium (1 article), Puerto Rico (1 article), Cameroon (1 article), Mexico (1 article), Turkey (1 article), Slovenia (1 article), Spain (1 article), Italy (1 article), Hungary (1 article) and Japan (1 article).
During the COVID-19 pandemic, everyday life changed violently and non-emergency care, including cancer screening, was significantly disrupted. The review of the literature highlighted, in an overwhelming way, a decisive reduction in the number of screening tests performed for breast and cervical cancer, especially during the first months of the pandemic, when the fear of the unknown virus was evident, and the restrictive measures were notably strict on a global scale in order to flatten the transmission curve. Since the summer of 2020 there has been a recovery in numbers of screening tests performed, however not to a satisfactory extent that could make up lost ground (Table I).
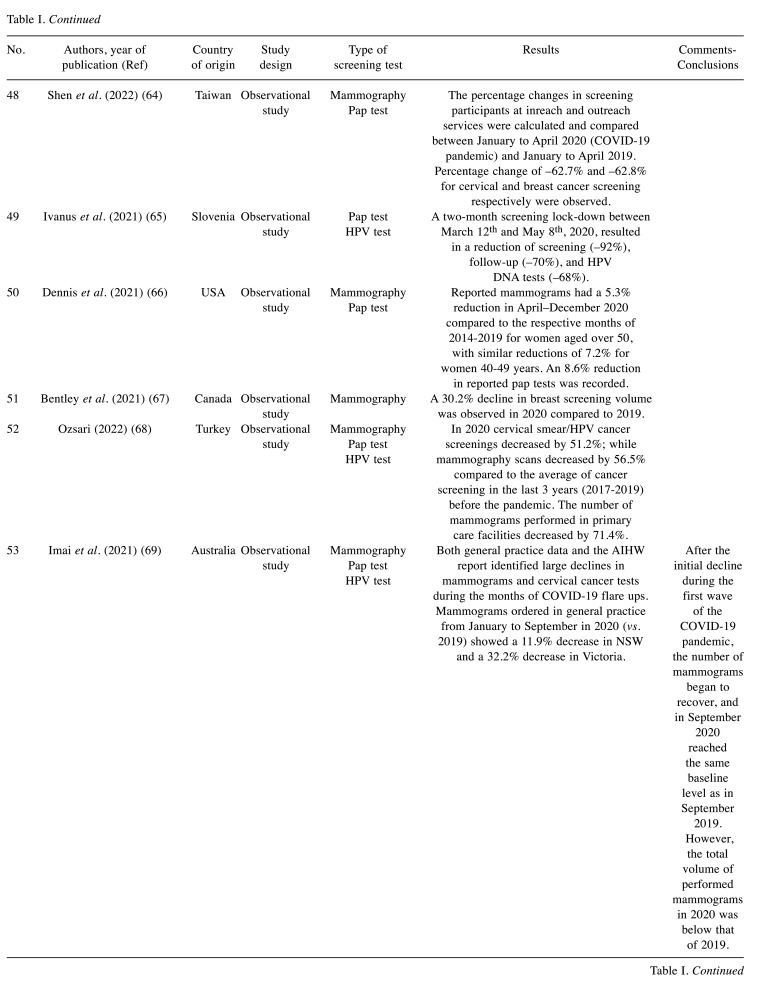
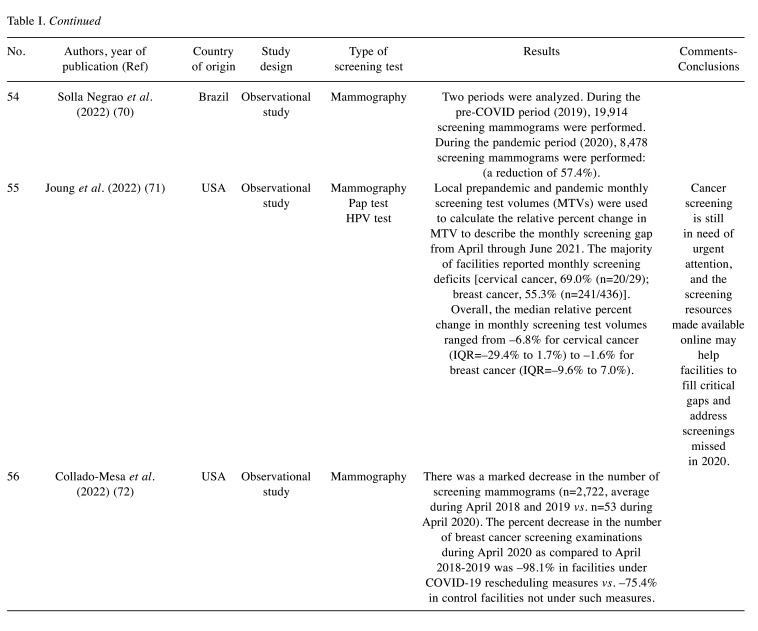
Table I. Screening volume alterations during COVID-19: studies characteristics and main findings.
Discussion
The present systematic review investigated the association of the COVID-19 pandemic with cancer screening and found evidence of a significant decline in the volume of screening tests performed for both breast and cervical cancer, especially during the early phase of the pandemic. The easing of restrictions coincided with a recovery in the number of screening tests, which, although in some studies it approached the pre-pandemic levels, in almost no case exceeded them, therefore the deficit of tests that had accumulated from the previous months did not shrink (19,22,55,67,69). These findings are in accordance with other relevant systematic reviews (2,73,74).
This decline appears to be attributed to a number of causes. Initially, during the first phase of the pandemic health policies around the globe focused on combating the COVID-19; resource allocation was drastically redesigned and material as well as human resources were shifted from non-COVID-19 services directly to the COVID-19 front, in order to reduce the extent of the unprecedented health crisis and to minimize its morbidity and mortality (18-20,22,30,33,37,38,54,56,60,61,75). As a result, many non-emergency chronic care services including cancer preventive programs had been marginalized (76,77).
Stay-at-home orders issued by almost every government globally, as well as recommendations by prestigious scientific organizations played a significant role in the screening setback; the Center for Disease Control and Prevention (CDC) and the Center for Medicare and Medicaid Services (CMS) but also many medical scientific societies such as the American College of Radiology, the Society of Breast Imaging and the American Cancer Society published guidelines urging the general public to comply with the government guidelines and suggested a temporary shutdown of non-emergency medical practices (33,78), not to mention people’s fear of the infection (79), reluctance to seek medical help and limited access to in-person medical examinations (18,20-22,62). Finally, another factor that disrupted the continuity of screening programs, especially in low-income countries, was the disruption of supply chains resulting in the unavailability of materials required for obtaining and processing cervical smears (40).
The most concerning potential adverse effect of cancer screening decline would be an increase in future cancer morbidity and mortality. The disruption of cancer screening programs could delay diagnosis of tumors, causing a shift to more advanced stages at diagnosis and fewer treatment options (e.g., surgical removal) resulting in a worse prognosis (19,30,32,59,60). Furthermore, this could potentially increase preventable deaths from cancer as well as healthcare spending (4,59,60,74).
The WHO, as early as 2019, had demonstrated the need to increase spending on PHC by at least 1% of GDP, a goal considered financially sustainable even for low-income countries (80). This need becomes even more urgent during pandemic times, when additional financial resources are required to cover the lost ground. Health facilities should increase the number of tests they process daily and temporarily achieve volumes higher than the pre-pandemic levels. This could be attainable by extending the operating hours and by upgrading the service capabilities of each unit, e.g., by hiring extra staff, creating new spaces, and meeting other needs (81,82).
Triaging eligible citizens seems of particular value, especially during times of limited resources, when maximal efficiency is desirable. The aim would be to prioritize those who are most likely to develop cancer and therefore screen positive. Examples of such could be women with a personal or family history of breast cancer, women with HIV infection, citizens with many years since their last check-up, or socially and economically marginalized individuals who face high barriers to healthcare access and are therefore less likely to self-seek participation (82-84).
Masson et al. stated that known and documented barriers to participation in screening related to insufficient information such as the fear that the procedure will be painful or the fear that the result may lead to the finding of cancer and the belief that the screening refers to specific categories of women, may have swelled during the pandemic (85). It is of paramount importance, awareness raising measures about the benefits of screening and the risks posed by neglecting it, to be massively promoted, in combination with building trust regarding the safety and hygiene measures that are taken in health facilities where the screening examinations are held, in order to limit patients’ fear of SARS-CoV-2 transmission and to increase the motivation of participating. The awareness campaign can use mass media, social media, but also personalized email messages or mobile phone messages. The existence of special support and information telephone lines as well as the creation of informative websites are considered necessary steps, while the opinion of expert scientists may play a decisive role, if it is communicated in a coordinated but at the same time comprehensible way (81,84).
Another possibility would be the transition from the classical cytological cervical smear (Pap test) to the HPV DNA test. The greatest difference lies in the fact that Pap tests are performed every three years while HPV DNA tests can be performed every five years, reducing the burden on health facilities. Despite the higher costs, their economic viability has been studied and proven even for low-income countries, while an additional advantage is the fact that the performance of the result is objective and does not depend on the skills of the performer (86).
The successful example of the fecal immunohistochemical (FIT) test could be cited as an alternative to screening colonoscopy, in which citizens collect a stool sample themselves and send it to the relevant laboratories. Cervical self-sampling could function similarly and constitutes an option suggested by several literature sources, as a solution that could reduce the risk of SARS-COV-2 transmission, while at the same time could diminish reluctance of many women to be examined in the genital area by a healthcare provider (81,83,86-88).
Strengths and limitations. A clear methodology was faithfully followed, described in detail previously. Our research being a systematic review, offers evidence ranked highly by the evidence hierarchy pyramid (89). We searched thoroughly three different databases (Pubmed, Google scholar and Epistemonikos) and the studies included in our review underwent quality assessment following the criteria of a widely recognized assessment checklist. However, the utility of our study could be partially limited by the fact that no meta-analysis was conducted to better quantify variations; studies included come from a small number of different countries, as most of them didn’t publish screening data for the time period we examined. Therefore, the conclusions we reached might not apply to each and every geographical region globally. Finally, most studies included did not offer screening data after the massive vaccinations for Covid-19 and the possibly milder course the pandemic started to follow.
Conclusion
The impact of the COVID-19 pandemic on breast and cervical cancer screening has been particularly significant. The unanimous conclusion from the entire literature of the large reduction in the number of tests performed combined with the statistical model predictions for an increase of avoidable advanced cancers and cancer-related deaths in the future, make it imperative to implement measures to reestablish cancer screening as the cornerstone of preventive medicine. As future pandemics caused by viral or other outbreaks are an unfortunate possibility, primary health care needs to ensure adequate coverage of the populations even in times of crisis by adopting the necessary steps towards that direction.
Conflicts of Interest
The Authors declare that there are no conflicts of interest.
Authors’ Contributions
Conceptualization: S.E., E.S.; literature search and data collection: S.E., P.S.; data analysis: S.E., P.S., A.-B. H., A.M., E.S.; writing original draft: S.E., P.S.; all authors critically revised the work; all authors have read and agreed to the published version of the manuscript.
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19. Available at: https://www.who.int/directorgeneral/speeches/detail/who-director-general-s-opening-remarksat-the-media-briefing-on-covid-19—-11-march-2020. [Last accessed on January 27, 2022]
- 2.Alkatout I, Biebl M, Momenimovahed Z, Giovannucci E, Hadavandsiri F, Salehiniya H, Allahqoli L. Has COVID-19 affected cancer screening programs? A Systematic Review. Frontiers in Oncology. 2021;11:675038. doi: 10.3389/fonc.2021.675038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell E. Declines in cancer screening during COVID-19 pandemic. Journal of the National Medical Association. 2020;112(6):563–564. doi: 10.1016/j.jnma.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO: COVID-19 significantly impacts health services for noncommunicable diseases. Available at: https://www.who.int/news/item/01-06-2020-covid-19-significantly-impactshealth-services-for-noncommunicable-diseases. [Last accessed on March 7, 2021]
- 5.Torre L, Siegel R, Ward E, Jemal A. Global cancer incidence and mortality rates and trends – an update. Cancer Epidemiology, Biomarkers & Prevention. 2022;25(1):16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 6.Pinsky P. Principles of cancer screening. Surgical Clinics of North America. 2018;95(5):953–966. doi: 10.1016/j.suc.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shieh Y, Eklund M, Sawaya G, Black W, Kramer B, Esserman L. Population-based screening for cancer: hope and hype. Nature Reviews Clinical Oncology. 2023;13(9):550–565. doi: 10.1038/nrclinonc.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman C. Early detection and screening for breast cancer. Seminars in Oncology Nursing. 2022;33(2):141–155. doi: 10.1016/j.soncn.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Senologic Hellenic Society Breast cancer prevention - breast cancer screening (in Greek) Available at: https://www.mastologia.gr/simvoules/91-prosimptomatikos-elegxos. [Last accessed on March 8, 2021]
- 10.Hellenic Society of Obstetrics and Gynecology Secondary prevention of breast cancer (in Greek) Available at: https://hsog.gr/wp-content/uploads/2017/11/defterogenis_prolipsi_karninou_tou_mastou.pdf. [Last accessed on May 16, 2023]
- 11.Jin J. Breast cancer screening: benefits and harms. JAMA. 2016;312(23):2585. doi: 10.1001/jama.2014.13195. [DOI] [PubMed] [Google Scholar]
- 12.Staples J, Duska L. Cancer screening and prevention highlights in gynecologic cancer. Obstetrics and Gynecology Clinics of North America. 2019;46(1):19–36. doi: 10.1016/j.ogc.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Sawaya G, Huchko M. Cervical cancer screening. Medical Clinics of North America. 2020;101(4):743–753. doi: 10.1016/j.mcna.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Systematic Reviews. 2019;2(1):35. doi: 10.1186/2046-4053-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stachteas P, Symvoulakis M, Tsapas A, Smyrnakis E. The impact of the COVID-19 pandemic on the management of patients with chronic diseases in Primary Health Care. Population Medicine. 2022;4(August):1–13. doi: 10.18332/popmed/152606. [DOI] [Google Scholar]
- 16.Critical Appraisal tools for use in JBI Systematic Reviews. Available at: https://jbi.global/critical-appraisal-tools. [Last accessed on May 16, 2023]
- 17.Bakouny Z, Paciotti M, Schmidt A, Lipsitz S, Choueiri T, Trinh Q. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncology. 2021;7(3):458. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorin S, Jimbo M, Heizelman R, Harmes K, Harper D. The future of cancer screening after COVID-19 may be at home. Cancer. 2021;127(4):498–503. doi: 10.1002/cncr.33274. [DOI] [PubMed] [Google Scholar]
- 19.Chen R, Haynes K, Du S, Barron J, Katz A. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncology. 2021;7(6):878. doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng S, Yang K, Chan W, Wang Y, Lin L, Yen A, Smith R, Chen T. Impact of the COVID-19 pandemic on a population-based breast cancer screening program. Cancer. 2020;126(24):5202–5205. doi: 10.1002/cncr.33180. [DOI] [PubMed] [Google Scholar]
- 21.Walker M, Meggetto O, Gao J, Espino-Hernández G, Jembere N, Bravo C, Rey M, Aslam U, Sheppard A, Lofters A, Tammemägi M, Tinmouth J, Kupets R, Chiarelli A, Rabeneck L. Measuring the impact of the COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: A provincial, population-based study. Preventive Medicine. 2023;151:106586. doi: 10.1016/j.ypmed.2021.106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller M, Xu L, Qin J, Hahn E, Ngo-metzger Q, Mittman B, Tewari D, Hodeib M, Wride P, Saraiya M, Chao C. Impact of COVID-19 on cervical cancer screening rates among women aged 21-65 years in a large integrated health care system - Southern California, January 1-September 30, 2019, and January 1-September 30, 2020. MMWR. Morbidity and Mortality Weekly Report. 2021;70(4):109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeGroff A, Miller J, Sharma K, Sun J, Helsel W, Kammerer W, Rockwell T, Sheu A, Melillo S, Uhd J, Kenney K, Wong F, Saraiya M, Richardson L. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January-June 2020, in the United States. Preventive Medicine. 2021;151:106559. doi: 10.1016/j.ypmed.2021.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amram O, Robison J, Amiri S, Pflugeisen B, Roll J, Monsivais P. Socioeconomic and racial inequities in breast cancer screening during the COVID-19 pandemic in Washington State. JAMA Network Open. 2021;4(5):e2110946. doi: 10.1001/jamanetworkopen.2021.10946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz A, Gierbolini-Bermúdez A, Ramos-Cartagena J, Colón-López V, Sonawane K, Deshmukh A, Ortiz-Ortiz K. Cervical cancer screening among medicaid patients during natural disasters and the COVID-19 pandemic in Puerto Rico, 2016 to 2020. JAMA Network Open. 2021;4(10):e2128806. doi: 10.1001/jamanetworkopen.2021.28806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cairns A, Jones V, Cronin K, Yocobozzi M, Howard C, Lesko N, Chiba A, Howard-McNatt M. Impact of the COVID-19 pandemic on breast cancer screening and operative treatment. The American Surgeon. 2022;88(6):1051–1053. doi: 10.1177/00031348221087920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duarte M, Argenton J, Carvalheira J. Impact of COVID-19 in cervical and breast cancer screening and systemic treatment in São Paulo, Brazil: an interrupted time series analysis. JCO Global Oncology. 2022;(8):e2100371. doi: 10.1200/GO.21.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acuti Martellucci C, Morettini M, Flacco M, Manzoli L, Palmer M, Giacomini G, Pasqualini F. Delivering cervical cancer screening during the COVID-19 emergency. BMJ Sexual & Reproductive Health. 2021;47(4):296–299. doi: 10.1136/bmjsrh-2021-201099. [DOI] [PubMed] [Google Scholar]
- 29.Tsai H, Chang Y, Shen C, Chung W, Tsai H, Chen F. Effects of the COVID-19 pandemic on breast cancer screening in Taiwan. The Breast. 2022;54:52–55. doi: 10.1016/j.breast.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song H, Bergman A, Chen AT, Ellis D, David G, Friedman AB, Bond AM, Bailey JM, Brooks R, Smith-McLallen A. Disruptions in preventive care: mammograms during the COVID-19 pandemic. Health Serv Res. 2021;56(1):95–101. doi: 10.1111/1475-6773.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker N, Moniz M, Tipirneni R, Dalton V, Ayanian J. Utilization of women’s preventive health services during the COVID-19 pandemic. JAMA Health Forum. 2021;2(7):e211408. doi: 10.1001/jamahealthforum.2021.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedewa S, Cotter M, Wehling K, Wysocki K, Killewald R, Makaroff L. Changes in breast cancer screening rates among 32 community health centers during the COVID-19 pandemic. Cancer. 2021;127(23):4512–4515. doi: 10.1002/cncr.33859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyante S, Benefield T, Kuzmiak C, Earnhardt K, Pritchard M, Henderson L. Population-level impact of coronavirus disease 2019 on breast cancer screening and diagnostic procedures. Cancer. 2021;127(12):2111–2121. doi: 10.1002/cncr.33460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velazquez A, Hayward J, Gregory B, Dixit N. Trends in breast cancer screening in a safety-net hospital during the COVID-19 pandemic. JAMA Network Open. 2021;4(8):e2119929. doi: 10.1001/jamanetworkopen.2021.19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou J, Kooragayala K, Williams J, Sandilos G, Butchy M, Yoon-Flannery K, Kwiatt M, Hong Y, Shersher D, Burg J. The early impact of the COVID-19 pandemic on lung, colorectal, and breast cancer screening and treatment at a tertiary cancer center. American Journal of Clinical Oncology. 2022;45(9):381–390. doi: 10.1097/COC.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBain R, Cantor J, Jena A, Pera M, Bravata D, Whaley C. Decline and rebound in routine cancer screening rates during the COVID-19 pandemic. Journal of General Internal Medicine. 2022;36(6):1829–1831. doi: 10.1007/s11606-021-06660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiarelli A, Walker M, Espino-Hernandez G, Gray N, Salleh A, Adhihetty C, Gao J, Fienberg S, Rey M, Rabeneck L. Adherence to guidance for prioritizing higher risk groups for breast cancer screening during the COVID-19 pandemic in the Ontario Breast Screening Program: a descriptive study. CMAJ Open. 2021;9(4):E1205–E1212. doi: 10.9778/cmajo.20200285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller M, Meneveau M, Rochman C, Schroen A, Lattimore C, Gaspard P, Cubbage R, Showalter S. Impact of the COVID-19 pandemic on breast cancer screening volumes and patient screening behaviors. Breast Cancer Research and Treatment. 2021;189(1):237–246. doi: 10.1007/s10549-021-06252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou C, Pan H, Yang T, Chiang C, Huang J, Tsai M. Impact of the COVID-19 pandemic on the volume of mammography examinations in Southern Taiwan. The Breast Journal. 2022;27(1):89–91. doi: 10.1111/tbj.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sormani J, Datchoua A, Petignat P, Kenfack B, Schmidt N. Effects of the COVID-19 pandemic on an urban cervical cancer screening program in West Cameroon. International Journal of Gynecologic Cancer. 2022;31(9):1297–1298. doi: 10.1136/ijgc-2021-002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sprague B, Lowry K, Miglioretti D, Alsheik N, Bowles E, Tosteson A, Rauscher G, Herschorn S, Lee J, Trentham-Dietz A, Weaver D, Stout N, Kerlikowske K. Changes in mammography use by women’s characteristics during the first 5 months of the COVID-19 pandemic. JNCI: Journal of the National Cancer Institute. 2021;113(9):1161–1167. doi: 10.1093/jnci/djab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Decker K, Feely A, Bucher O, Singh H, Turner D, Lambert P. Evaluating the impact of the COVID-19 pandemic on cancer screening in a central Canadian province. Preventive Medicine. 2022;155:106961. doi: 10.1016/j.ypmed.2022.106961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moterani Júnior N, Moterani V, Moterani L, Pimentel F, Reis F. Impact of Coronavirus disease 2019 pandemic on breast cancer screening and detection of high-risk mammographic findings. Revista da Associação Médica Brasileira. 2022;68(6):842–846. doi: 10.1590/1806-9282.20220182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee K, Lee Y, Suh M, Jun J, Park B, Kim Y, Choi K. Impact of COVID-19 on cancer screening in South Korea. Scientific Reports. 2022;12(1):11380. doi: 10.1038/s41598-022-15778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castanon A, Rebolj M, Pesola F, Pearmain P, Stubbs R. COVID-19 disruption to cervical cancer screening in England. Journal of Medical Screening. 2022;29(3):203–208. doi: 10.1177/09691413221090892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimm L, Lee C, Rosenberg R, Burleson J, Simanowith M, Fruscello T, Pelzl C, Friedewald S, Moy L, Zuley M. Impact of the COVID-19 pandemic on breast imaging: an analysis of the national mammography database. Journal of the American College of Radiology. 2022;19(8):919–934. doi: 10.1016/j.jacr.2022.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amram O, Amiri S, Robison J, Pflugeisen C, Monsivais P. COVID -19 and inequities in colorectal and cervical cancer screening and diagnosis in Washington State. Cancer Med. 2022;11(15):2990–2998. doi: 10.1002/cam4.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribeiro CM, Correa F de M, Migowski A. Short-term effects of the COVID-19 pandemic on cancer screening, diagnosis and treatment procedures in Brazil: a descriptive study, 2019-2020. Epidemiol Serv Saude. 2022;31(1):e2021405. doi: 10.1590/S1679-49742022000100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trinh T, Lee Y, Suh M, Jun J, Choi K. Changes in cancer screening before and during COVID-19: findings from the Korean National Cancer Screening Survey 2019 and 2020. Epidemiology and Health. 2022;44:e2022051. doi: 10.4178/epih.e2022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Battisti F, Falini P, Gorini G, Sassoli de Bianchi P, Armaroli P, Giubilato P, Giorgi Rossi P, Zorzi M, Battagello J, Senore C, Zappa M, Mantellini P. Cancer screening programmes in Italy during the COVID-19 pandemic: an update of a nationwide survey on activity volumes and delayed diagnoses. Ann Ist Super Sanita. 2022;58(1):16–24. doi: 10.4415/ANN_22_01_03. [DOI] [PubMed] [Google Scholar]
- 51.Bosch G, Posso M, Louro J, Roman M, Porta M, Castells X, Macia F. Impact of the COVID-19 pandemic on breast cancer screening indicators in a spanish population-based program: a cohort study. Elife. 2022;11:e77434. doi: 10.7554/eLife.77434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nogami Y, Makabe T, Komatsu H, Kawana K, Okamoto A, Mikami M, Katabuchi H. Impact of COVID-19 on cervical cancer screening in Japan: A survey of population-based screening in urban Japan by the Japan society of gynecologic oncology. J Obstet Gynaecol. 2022;48(3):757–765. doi: 10.1111/jog.15130. [DOI] [PubMed] [Google Scholar]
- 53.Elek P, Fadgyas-Freyler P, Váradi B, Mayer B, Zemplényi A, Csanádi M. Effects of lower screening activity during the COVID-19 pandemic on breast cancer patient pathways: evidence from the age cut-off of organized screening. Health Policy (New York) 2022;126(8):763–769. doi: 10.1016/j.healthpol.2022.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kidwai N. Routine cancer screening delays due to pandemic at veteran affairs. J Natl Med Assoc. 2022;114(1):12–15. doi: 10.1016/j.jnma.2021.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Labaki C, Bakouny Z, Schmidt A, Lipsitz SR, Rebbeck TR, Trinh QD, Choueiri TK. Recovery of cancer screening tests and possible associated disparities after the first peak of the COVID-19 pandemic. Cancer Cell. 2021;39(8):1042–1044. doi: 10.1016/j.ccell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Pelsemaeker MC, Guiot Y, Vanderveken J, Galant C, van Bockstal MR. The impact of the COVID-19 pandemic and the associated belgian governmental measures on cancer screening, surgical pathology and cytopathology. Pathobiology. 2021;88(1):46–55. doi: 10.1159/000509546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doubova SV, Leslie HH, Kruk ME, Pérez-Cuevas R, Arsenault C. Disruption in essential health services in Mexico during COVID-19: an interrupted time series analysis of health information system data. BMJ Glob Health. 2021;6(9):e006204. doi: 10.1136/bmjgh-2021-006204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laing S, Johnston S. Estimated impact of COVID-19 on preventive care service delivery: an observational cohort study. BMC Health Serv Res. 2021;21(1):1107. doi: 10.1186/s12913-021-07131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, Markward N, Sullivan M, Peng J, Zhou A. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clinical Cancer Informatics. 2022;(4):1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meggetto O, Jembere N, Gao J, Walker MJ, Rey M, Rabeneck L, Murphy KJ, Kupets R. The impact of the COVID-19 pandemic on the Ontario cervical screening program, colposcopy and treatment services in Ontario, Canada: a population-based study. BJOG. 2021;128(9):1503–1510. doi: 10.1111/1471-0528.16741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutherland K, Chessman J, Zhao J, Sara G, Went A, Dyson S, Levesque J. Impact of COVID-19 on healthcare activity in NSW, Australia. Public Health Research & Practice. 2020;30(4):3042030. doi: 10.17061/PHRP3042030. [DOI] [PubMed] [Google Scholar]
- 63.Brugel M, Carlier C, Essner C, Debreuve-Theresette A, Beck MF, Merrouche Y, Bouché O. Dramatic changes in oncology care pathways during the COVID-19 pandemic: the french ONCOCARE-COV Study. Oncologist. 2021;26(2):e338–e341. doi: 10.1002/onco.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen C, Hsieh H, Chang Y, Tsai H, Chen F. Different impacts of cancer types on cancer screening during COVID-19 pandemic in Taiwan. Journal of the Formosan Medical Association. 2022;121(10):1993–2000. doi: 10.1016/j.jfma.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ivanuš U, Jerman T, Gašper Oblak U, Meglič L, Florjančič M, Strojan Fležar M, Premru Sršen T, Smrkolj Š, Pakiž M, Primic Žakelj M, Kloboves Prevodnik V, Pogačnik A, Josipović I, Mate T, Gobec M. The impact of the COVID-19 pandemic on organised cervical cancer screening: The first results of the slovenian cervical screening programme and registry. Lancet Reg Health Eur. 2021;5:100101. doi: 10.1016/j.lanepe.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dennis L, Hsu C, Arrington A. Reduction in standard cancer screening in 2020 throughout the U.S. Cancers. 2021;13(23):5918. doi: 10.3390/cancers13235918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bentley H, Woods R, Mar C, Tang T, Raveinthiranathan N, Kellow Z, Yong-Ηing C. Hindsight is 2020: Understanding the impact of the COVID-19 pandemic on a provincial population-based breast screening program. Canadian Association of Radiologists Journal. 2022;73(3):589–591. doi: 10.1177/08465371211036902. [DOI] [PubMed] [Google Scholar]
- 68.Ozsari S. Cancer screening in the COVID-19 pandemic; Development of early diagnostic strategies. Exp Biomed Res. 2022;19(8):521–531. doi: 10.30714/j-ebr.2022275810. [DOI] [Google Scholar]
- 69.Imai C, Hardie R-A, Dai Z, Thomas J, Sezgin G, Li J, Georgiou A. The impact of the COVID-19 pandemic on cancer screening in general practice. COVID-19 General Practice Snapshot. 2021;7 doi: 10.25949/5Z8Y-2E49. [DOI] [Google Scholar]
- 70.Solla Νegrao E, Cabello C, Conz L, Mauad E, Zeferino L, Vale D. The impact of the COVID-19 pandemic on breast cancer screening and diagnosis in a Brazilian metropolitan area. Journal of Medical Screening. 2023;30(1):42–46. doi: 10.1177/09691413221122055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joung R, Nelson H, Mullett T, Kurtzman S, Shafir S, Harris J, Yao K, Brajcich B, Bilimoria K, Cance W. A national quality improvement study identifying and addressing cancer screening deficits due to the COVID-19 pandemic. Cancer. 2022;128(11):2119–2125. doi: 10.1002/cncr.34157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collado-Mesa F, Kaplan SS, Yepes MM, Thurber MJ, Behjatnia B, Kallos NPL. Impact of COVID-19 on breast imaging case volumes in south Florida: a multicenter study. Breast J. 2020;26(11):2316–2319. doi: 10.1111/tbj.14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mayo M, Potugari B, Bzeih R, Scheidel C, Carrera C, Shellenberger R. Cancer screening during the COVID-19 pandemic: a systematic review and meta-analysis. Mayo Clinic Proceedings: Innovations, Quality & Outcomes. 2021;5(6):1109–1117. doi: 10.1016/j.mayocpiqo.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teglia F, Angelini M, Astolfi L, Casolari G, Boffetta P. Global association of COVID-19 pandemic measures with cancer screening. JAMA Oncology. 2022;8(9):1287. doi: 10.1001/jamaoncol.2022.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Basu P, Lucas E, Zhang L, Muwonge R, Murillo R, Nessa A. Leveraging vertical COVID-19 investments to improve monitoring of cancer screening programme – A case study from Bangladesh. Preventive Medicine. 2023;151:106624. doi: 10.1016/j.ypmed.2021.106624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson R, Heesterbeek H, Klinkenberg D, Hollingsworth T. How will country-based mitigation measures influence the course of the COVID-19 epidemic. The Lancet. 2021;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z, Yao W, Wang Y, Long C, Fu X. Wuhan and Hubei COVID-19 mortality analysis reveals the critical role of timely supply of medical resources. Journal of Infection. 2021;81(1):147–178. doi: 10.1016/j.jinf.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prabhakar A, Glover M, Schaefer P, Brink J. Academic radiology departmental operational strategy related to the Coronavirus disease 2019 (COVID-19) pandemic. Journal of the American College of Radiology. 2020;17(6):730–733. doi: 10.1016/j.jacr.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vanni G, Materazzo M, Pellicciaro M, Ingallinella S, Rho M, Santori F, Cotesta M, Caspi J, Makarova A, Pistolese C, Buonomo O. Breast ψancer and COVID-19: The effect of fear on patients’ decision-making process. In Vivo. 2021;34(3 suppl):1651–1659. doi: 10.21873/invivo.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.World Health Organisation Primary health care on the road to universal health coverage: 2019 monitoring report, 2019. Available at: https://www.who.int/publications/i/item/9789240029040. [Last accessed on May 25, 2023]
- 81.Basu P, Alhomoud S, Taghavi K, Carvalho A, Lucas E, Baussano I. Cancer screening in the Coronavirus pandemic era: adjusting to a new situation. JCO Global Oncology. 2022;(7):416–424. doi: 10.1200/GO.21.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ajenifuja K, Belinson J, Goldstein A, Desai K, de Sanjose S, Schiffman M. Designing low-cost, accurate cervical screening strategies that take into account COVID-19: a role for self-sampled HPV typing. Infectious Agents and Cancer. 2021;15(1):61. doi: 10.1186/s13027-020-00325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corley D, Sedki M, Ritzwoller D, Greenlee R, Neslund-Dudas C, Rendle K, Honda S, Schottinger J, Udaltsova N, Vachani A, Kobrin S, Li C, Haas J. Cancer screening during the Coronavirus disease-2019 pandemic: a perspective from the National Cancer Institute’s PROSPR consortium. Gastroenterology. 2021;160(4):999–1002. doi: 10.1053/j.gastro.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Croswell J, Corley D, Lafata J, Haas J, Inadomi J, Kamineni A, Ritzwoller D, Vachani A, Zheng Y. Cancer screening in the U.S. through the COVID-19 pandemic, recovery, and beyond. Preventive Medicine. 2021;151:106595. doi: 10.1016/j.ypmed.2021.106595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masson H. Cervical pap smears and pandemics: The effect of COVID-19 on screening uptake & opportunities to improve. Women’s Health. 2021;17:174550652110170. doi: 10.1177/17455065211017070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woo Y, Gravitt P, Khor S, Ng C, Saville M. Accelerating action on cervical screening in lower- and middle-income countries (LMICs) post COVID-19 era. Preventive Medicine. 2021;144:106294. doi: 10.1016/j.ypmed.2020.106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim A. Will COVID-19 be the tipping point for primary HPV self-sampling. Cancer Epidemiology, Biomarkers & Prevention. 2022;30(2):245–247. doi: 10.1158/1055-9965.EPI-20-1538. [DOI] [PubMed] [Google Scholar]
- 88.Castanon A, Rebolj M, Burger EA, de Kok IMCM, Smith MA, Hanley SJB, Carozzi FM, Peacock S, O’Mahony JF. Cervical screening during the COVID-19 pandemic: optimising recovery strategies. Lancet Public Health. 2021;6(7):e522–e527. doi: 10.1016/S2468-2667(21)00078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murad M, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evidence Based Medicine. 2020;21(4):125–127. doi: 10.1136/ebmed-2016-110401. [DOI] [PMC free article] [PubMed] [Google Scholar]