Abstract
Background/Aim: Catastrophic antiphospholipid syndrome (CAPS) may be the first manifestation (“de novo”) of antiphospholipid syndrome (APS) or a complication in the clinical course of patients known to have this syndrome. Approximately 40% of patients had an associated autoimmune disease, mainly, systemic lupus erythematosus (SLE). The trigger can be one of the following: infections, surgical interventions, neoplasms, pregnancy, discontinuation of anticoagulant treatment, and others. CAPS is a medical emergency in which early identification and prompt initiation of aggressive therapy is extremely important. According to the Guidelines for the use of Therapeutic Apheresis in Clinical Practice developed by the American Society for Apheresis (ASFA), last updated in April 2023, in CAPS, the indication for therapeutic plasma exchange (TPE) is category I, grade 2C.
Case Report: We present a case of probable CAPS secondary to systemic lupus erythematosus (SLE) in an elderly patient in whom clinical and biological improvement was achieved through a multidisciplinary approach and prompt initiation of TPE. Because TPE is considered first-line therapy in CAPS, we initiated the procedure as soon as the attending rheumatologist raised this suspicion. Four plasmapheresis sessions were performed in the Intensive Care Unit. We used TPE by membrane filtration. Following the therapeutic intervention with TPE, corticotherapy (Solumedrol in puls-therapy), cyclophosphamide and anticoagulant treatment, the evolution was favourable, with clinical and biological improvement.
Conclusion: The prompt initiation of TPE, because of the suspicion of CAPS, increases the chances of a favourable evolution.
Keywords: Catastrophic antiphospholipid syndrome, CAPS, therapeutic apheresis, therapeutic plasma exchange, TPE, systemic lupus erythematosus, SLE
Therapeutic apheresis is an extracorporeal treatment that selectively removes abnormal cells or substances from the blood that are associated with or cause health problems. The treated disease determines which elements are removed from the blood and which replacement fluid to use. Depending on this, the procedure used may be: therapeutic plasma exchange (TPE; removing the patient’s plasma and replacing it with replacement fluid), cytapheresis (removing certain pathological or excess cells), immunoadsorption (removing immunoglobulins) and lipoprotein apheresis (removing lipoprotein particles). TPE is most commonly used in order to remove a specific pathogenic substance from the plasma (e.g., cryoglobulins, autoantibodies, immunoglobulins) quickly and efficiently. Ideal characteristics of such substances include high molecular weight, greater than 15,000 Da, slow rate of formation, prolonged half-life, a high percentage of intravascular distribution, and a low turnover rate. Autoimmune diseases including catastrophic antiphospholipid syndrome, are autoantibody-associated conditions, that are ideal potential candidates for TPE (1-11).
Catastrophic antiphospholipid syndrome (CAPS) is defined by the presence of antiphospholipid antibodies and the sudden onset of multiple thromboses in at least 3 organ systems occurring within a short period of time (days or weeks). It usually affects the small vessels of the kidneys, lungs, brain, heart, and skin, although large vessels can also be involved (2,3,12-15).
The exact mechanism by which TPE has a beneficial role in CAPS is unknown, although removal of antiphospholipid antibodies, cytokines, pulmonary necrosis factor and the complement components likely play an important role. The optimal treatment in CAPS is unknown because there are no prospective studies due to the low incidence of this disease (584 patients in the CAPS Registry as of December 2021) (3,16-18).
According to the Guidelines for the use of therapeutic apheresis in clinical practice developed by the American Society for Apheresis (ASFA), last updated in 2023, in CAPS, the indication for TPE is category I, grade 2C (3).
TPE uses centrifugation or membrane separation technologies. Apheresis using centrifugation requires blood flows of 50-120 ml/min and that using membrane filtration requires higher flows of 150-200 ml/min. By centrifugation (2,000-2,500 revolutions/min) the anticoagulated blood elements are separated, depending on the density. In TPE by centrifugation the blood is removed from the patient and anticoagulated before centrifugation, and then the plasma is separated. Plasma is discarded, and red blood cells and replacement fluid are returned to the patient. In TPE by membrane filtration the blood is perfused through a hollow fiber filter through. The replacement fluid is infused after filtration back into the patient. Anticoagulation is used to prevent clotting in the extracorporeal circuit. There are two agents used to prevent clotting in the extracorporeal circuit, heparin and citrate (1,5,6).
We used TPE by membrane filtration. Approximately 90% of TPE treatments are performed by membrane plasma separation in some countries, while in the United States most TPE procedures are centrifugation-based (1).
In TPE by membrane filtration, the plasma constituents are removed non-selectively through a semi-permeable membrane (e.g., propylene with ethylene oxide sterilization or polyethylene with gamma ray sterilization). The separation efficiency depends on the filtration rates, the membrane properties (pore size, surface area) and the sieving coefficient. The sieving coefficients are a measure of the equilibration of a particular solute between the filtrate and the blood, on either side of the membrane, indicating how efficiently a molecule is moved through the filter. Sieving coefficient depends on the characteristics of the molecule (size, binding to proteins, charge). Adsorption of serum proteins on the membrane will lead to filter clogging and decreased separation. High ultrafiltration rates lead to high filtration fractions, red cell damage and blood coagulation as the hematocrit value increases. Therefore, in TPE, membrane filtration is limited to a fraction of 30-35% of processed plasma, requiring longer treatment sessions, higher blood flow rates and, consequently, central venous access, in contrast to TPE by centrifugation where a peripheral venous approach can be used (1,5,6).
More recently, immunoadsorption is gaining more and more ground, and is indicated for autoimmune diseases. Immunoadsorption (IA) is a selective method of therapeutic apheresis in with patient plasma, after separation from blood, is passed through an adsorber column which has a capacity to remove immunoglobulins and immune complexes by binding them to select ligands. The advantage of IA compared to TPE is that there is no need for substitution with human plasma, thus avoiding the adverse effects of plasma administration (allergic reactions, intolerance, transmission of infections, alteration of coagulation). In both TPE and IA, it is necessary to monitor fibrinogen when the procedure is performed daily, because fibrinogen is removed by these procedures and it takes 48 hours to return to normal values (2,3).
There are several well-documented case reports about the beneficial effects of TPE in SLE associated with thrombotic thrombocytopenic purpura (TTP), CAPS, hyperviscosity, myasthenia gravis, and cryoglobulinemia. There are data on the use of double filtration plasmapheresis (DFPP) and immuno-adsorption (IA) in severe, treatment-refractory cases (2).
The volume of filtered plasma should generally be 1-1.5 plasma volume per session and the number and frequency of sessions depends on the evolution of the disease. The number of sessions can vary from minimum of 3-5 to several procedures. The replacement fluid is plasma or plasma in combination with 5% human albumin. Since plasma provides antithrombin that is required for heparin anticoagulation, using albumin alone as a replacement fluid may prevent the beneficial effect of heparin anticoagulation. A combination of plasma and albumin as a replacement fluid would provide the necessary benefit of TPE and minimize the potential adverse effects of using excess plasma. Discontinuation of TPE sessions is based on clinical response, no single laboratory parameter is considered to stop treatment (2,3,5,16-18).
TPE is rarely the only way to treat the patient. TPE must be coordinated with the other therapies so as not to affect their effectiveness.
This article discusses the place of TPE in CAPS from the perspective of an intensive care physician responsible for prescribing and managing TPE. CAPS is a medical emergency and the prompt initiation of TPE increases the chances of a favourable evolution.
Case Report
We present a case of probable CAPS secondary to SLE in an elderly patient in whom clinical and biological improvement was achieved through a multidisciplinary approach.
The patient was a 67-year-old Caucasian female with a body mass index of 23.78 kg/m2, registered in the rheumatological records of the Medical and Rheumatology Clinics with Sibiu County Emergency Clinical Hospital for about 2 years. It was known to have SLE, secondary severe antiphospholipid syndrome, and being under chronic corticosteroid treatment (Medrol 16 mg × 2/day), anticoagulant (Eliquis), and other drug combinations (gluconic calcium, Detrical, Concor, Atorvastatin, Controloc, Vessel due). The patient presented to Sibiu County Clinical Emergency Hospital for vascular complaints, peripheral and visceral Raynaud’s syndrome (intestinal malabsorption syndrome, necrosis of the distal phalanx of the 3rd finger of the right hand, ascito-edematous syndrome due to protein deficiency, inappetence, nausea, epigastric pains), severe anemia (Hb 6 g/dl), acute renal failure stage I (creatinine 1.36 mg/dl), thrombocytopenia (122,000/mm3). Chest x-ray upon admission showed dextroconvex dorsal scoliosis and absence of pulmonary lesions. During the cardiology consultation and Doppler ultrasound, no thrombosis was detected in the large vessels; normal valves. It was decided to administer Solumedrol in pulse therapy and cyclophosphamide.
Because during hospitalization, under treatment, the evolution in the last week was unfavourable with rapid progression (in days) towards probable CAPS (microthrombosis in the fingers and toes, manifested by new skin necrosis with changes in coagulation tests and thrombocytopenia), hemodynamic instability and psychomotor agitation, the attending rheumatologist requested an intensive care consultation in order to perform TPE.
Thus, the patient was transferred to the intensive care ward where a large-bore, double-lumen central venous catheter (12 French) was mounted on the left femoral vein and an access catheter on the right femoral vein with the initiation of TPE. Four plasmapheresis sessions were performed in the intensive care unit. We used membrane therapeutic plasma exchange on a Prismaflex System (Baxter International Inc., Deerfield, IL, USA).
The four TPE sessions were performed daily, the processed plasma was 1.5 of the total plasma volume. The total plasma volume (in liters) was calculated according to the formula 0.07 × weight (kg) × (1-hematocrit). Plasma was used as a replacement fluid. Heparin was used as an anticoagulant during the procedures. The filtered plasma varied between 3,200-3,500 ml.
Upon admission to the intensive care unit, the patient was conscious and difficult to cooperate, and had periods of psycho-motor agitation. She was subfebrile, had pale skin and mucous membranes, leg edema, necrosis in the fingers and toes, pain in the front right thigh, joint pain, epigastralgia, and tachipneea. In addition, she was stable from the respiratory point of view but hemodynamically unstable requiring vasoactive support (noradrenaline), and tachycardic (110-120 b/min). Continuous electronic monitoring of diuresis recorded a urine output over 0.5 ml/kg/h. ASTRUP test revealed metabolic acidosis (pH 7.22).
Both during the plasmapheresis sessions and between them, the patient required vasoactive support of noradrenaline to maintain the blood pressure values (0.68-0.84 mcg/kg/min) (Figure 1), transfusions and supplementation with iron therapy. After the plasmapheresis sessions, the patient was weaned off the vasoactive support, and the systolic blood pressure values remained at 100/120 mm Hg. There was also an improvement in symptoms (epigastralgias and digestive intolerance) including the neuro-psychiatric status, with the disappearance of episodes of psychomotor agitation. No other areas of skin necrosis appeared. Anemia, thrombocytopenia and coagulation disorders were corrected, as well as water-electrolyte and acid-base imbalances (pH 7.37). Since the procedure was performed daily, fibrinogen was monitored. Given the hypoproteinemia, medication with Albumin 20% was administered, with edema relief. Recurrent infections due to iatrogenic immunosuppression, urinary tract infections with E coli, Cl Difficile GDH+ and fungal enterocolitis were identified and treated. Parenteral nutrition solutions (Nutriflex) were administered due to episodes of severe digestive intolerance, which subsequently improved. The anticoagulant administered was enoxparin in a therapeutic dose (60 mg every 12 h). After four TPE sessions, the patient presented a clinical-biological improvement and she and her family decided to be discharged on their own responsibility.
Figure 1. Vasoactive support (noradrenaline) necessary pre- and posttherapeutic plasma exchange.

Following the therapeutic intervention with TPE, corticotherapy (Solumedrol in pulse-therapy), cyclophospha-mide and anticoagulant treatment, the evolution was favourable, with clinical and biological improvement (Figure 1, Figure 2, Figure 3, Figure 4, and Figure 5).
Figure 2. PaO2/FiO2 ratio obtained on arterial gases.

Figure 3. Evolution of creatinine level during hospitalization.
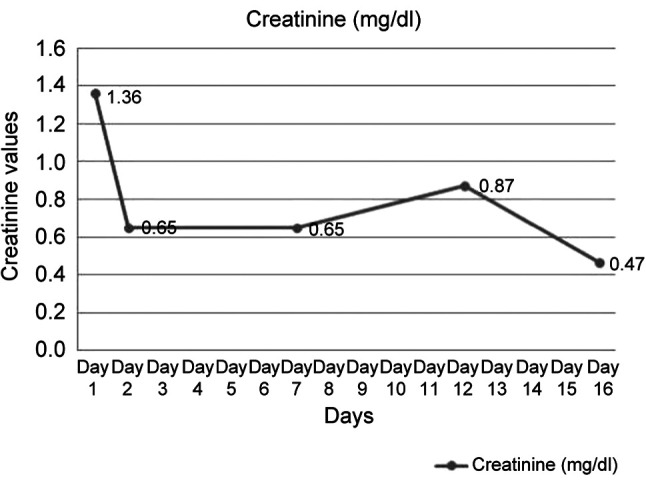
Figure 4. Evolution of hemoglobin (Hb) level during hospitalization.

Figure 5. Evolution of platelets number during hospitalization.

Discussion
CAPS may be the first manifestation of antiphospholipid syndrome (APS), a complication in the clinical course of patients known to have this syndrome. Approximately 40% of patients had an associated autoimmune disease, mainly SLE (75%) (17). Our patient was known to have SLE and antiphospholipid syndrome.
CAPS is a severe and rare condition with an incidence of 5 cases per 10,000,000 persons per year. A total of 584 patients were in CAPS Registry as of December 2021 (18).
CAPS is defined as the acute onset of tromboses in at least 3 organ systems over a period of days or weeks. CAPS was suspected based on multiorgan involvement in a patient known to have SLE and APS. The main organs affected in CAPS are the kidneys (73%), lungs (60%), brain (56%), heart (50%) and skin (47%) (3,17,18). Our patient had acute renal insufficiency upon admission, developing skin necrosis and psychomotor agitation. She also had digestive intolerance, with epigastralgias and vomiting.
In CAPS, the trigger can be one of the following: infections (49%), surgical interventions (17%), malignancy (16%), contraceptives (10%), obstetric complications (8%) and others (23%) (17). Our patient had an E. Coli urinary infection at admission.
CAPS in young patients is frequently associated with an infectious trigger whereas cases triggered by a neoplasm tended to occur in older patients (17). Laboratory features of CAPS include thrombocytopenia (67%) (2,3,18). Our patient presented thrombocytopenia at admission. The treatment of CAPS includes the combination of high doses of glucocorticoids, anticoagulation, TPE and/or intravenous immunoglobulins. Cyclophosphamide is used for severe complications of SLE. Rituximab might be useful in cases refractory to first line therapy (18).
A review of 26 patients with SLE and neuropsychiatric involvement who were treated with TPE or TPE/ cyclophosphamide mentioned that 74% of patients showed an improvement of the conditions (19). In our case, under the therapeutic intervention with corticotherapy, cyclophosphamide, TPE and anticoagulants, the evolution was favourable, and the neuro-psychiatric status improved.
In CAPS, the indication for TPE, according to the 2023 ASFA guidelines, is of category I, grade 2C (3). Because TPE is considered first-line therapy in CAPS, we initiated the procedure as soon as the attending rheumatologist raised this suspicion.
The replacement fluid we used was plasma, the alternative would have been plasma in combination with albumin. Plasma provides antithrombin, which is required to mediate heparin anticoagulation. A combination of plasma and albumin as a replacement fluid would minimize the potential adverse effects of using excess plasma (2,16). Our patient did not experience adverse reactions to plasma administration.
The mortality rate in CAPS is 37%, with cerebral damage being the main cause of death (27% of cases). CAPS associated with SLE is more likely to have severe brain or cardiac involvement and a higher mortality rate (48%). The highest recovery rate was found in patients receiving a combination of anticoagulant, corticosteroid, and plasma exchange therapy (17,20-22). The mortality rate in patients who received triple therapy (glucocorticosteroids, anticoagulants, TPE) was 29% (22).
Although the mortality rate of this condition has decreased during the last two decades from 50% to 30%, it remains high (18). The prompt initiation of TPE when CAPS is suspected increases the chances of a favourable evolution.
Conclusion
TPE is a safe and well tolerated invasive procedure. CAPS is a medical emergency in which early identification and prompt initiation of aggressive therapy is extremely important. The diagnosis of CAPS should be considered whenever clinicians are dealing with patients presenting with sudden-onset, multi-organ acute thrombosis and especially in patients known to have APS and SLE.
Following the therapeutic intervention with TPE, corticotherapy (Solumedrol in pulse-therapy), cyclophosphamide and anticoagulant treatment, the evolution was favourable, with clinical and biological improvement.
The prompt initiation of TPE, together with the suspicion of the diagnosis of CAPS increases the chances of a favourable evolution.
Conflicts of Interest
There are no conflicts of interest related to this study.
Authors’ Contributions
Concept – ASB; Design – ASB, TP, RB; Supervision – CC, MS; Resources – ASB, TP, BV, LC, IC; Data collection and/or processing – ASB, TP, RB, BV; Analysis and/or interpretation – ASB, TP, RB; Literature search – AB, RB, SC, OC; Writing manuscript – AB, RB; Critical review – MS.
References
- 1.Cervantes C, Bloch E, Sperati C. Therapeutic plasma exchange: Core curriculum 2023. American Journal of Kidney Diseases. 2023;81(4):475–492. doi: 10.1053/j.ajkd.2022.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Padmanabhan A, Connelly-Smith L, Aqui N, Balogun R, Klingel R, Meyer E, Pham H, Schneiderman J, Witt V, Wu Y, Zantek N, Dunbar N, Schwartz G. Guidelines on the use of therapeutic apheresis in clinical practice -evidence- based approach from the Writing Committee of the American Society for Apheresis: the eighth special issue. Journal of Clinical Apheresis. 2022;34(3):171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 3.Connelly-Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, Patriquin CJ, Pham HP, Sanchez AP, Schneiderman J, Witt V, Zantek ND, Dunbar NM. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing committee of the American Society for Apheresis: The Ninth Special Issue. J Clin Apher. 2023;38(2):77–278. doi: 10.1002/jca.22043. [DOI] [PubMed] [Google Scholar]
- 4.Fridey JL, Kaplan AA. Therapeutic apheresis (plasma exchange or cytapheresis): Indications and technology. UpToDate, 2023. Available at: https://www.uptodate.com/contents/therapeuticapheresis-plasma-exchange-or-cytapheresis-indications-andtechnology. [Last accessed on May 30, 2023]
- 5.Gounder D. Guidelines for the use of therapeutic plasma exchange (TPE) in renal disease. National, Manual(s), Clinical Comp. 2020:pp. 1–5. [Google Scholar]
- 6.Ahmed S, Kaplan A. Therapeutic plasma exchange using membrane plasma separation. Clinical Journal of the American Society of Nephrology. 2023;15(9):1364–1370. doi: 10.2215/CJN.12501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguirre-Valencia D, Naranjo-Escobar J, Posso-Osorio I, Macía-Mejía MC, Nieto-Aristizábal I, Barrera T, Obando MA, Tobón GJ. Therapeutic plasma exchange as management of complicated systemic lupus erythematosus and other autoimmune diseases. Autoimmune Dis. 2019;2019:5350960. doi: 10.1155/2019/5350960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bambauer R, Latza R, Bambauer C, Burgard D, Schiel R. Therapeutic apheresis in autoimmune diseases. Open Access Rheumatol. 2013;13(5):93–103. doi: 10.2147/OARRR.S34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanatta E, Cozzi M, Marson P, Cozzi F. The role of plasma exchange in the management of autoimmune disorders. British Journal of Haematology. 2022;186(2):207–219. doi: 10.1111/bjh.15903. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz J, Winters J, Padmanabhan A, Balogun R, Delaney M, Linenberger M, Szczepiorkowski Z, Williams M, Wu Y, Shaz B. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. Journal of Clinical Apheresis. 2021;28(3):145–284. doi: 10.1002/jca.21276. [DOI] [PubMed] [Google Scholar]
- 11.Roman-Filip C. Therapeutic plasma exchange and double filtration plasmapheresis in severe neuroimmune disorders. Acta Clinica Croatica. 2020;58:621–626. doi: 10.20471/acc.2019.58.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uthman I, Noureldine M, Ruiz-Irastorza G, Khamashta M. Management of antiphospholipid syndrome. Annals of the Rheumatic Diseases. 2022;78(2):155–161. doi: 10.1136/annrheumdis-2018-213846. [DOI] [PubMed] [Google Scholar]
- 13.Sciascia S, Lopez-Pedrera C, Roccatello D, Cuadrado MJ. Catastrophic antiphospholipid syndrome (CAPS) Best Pract Res Clin Rheumatol. 2012;26(4):535–41. doi: 10.1016/j.berh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Kazzaz NM, McCune WJ, Knight JS. Treatment of catastrophic antiphospholipid syndrome. Curr Opin Rheumatol. 2016;28(3):218–227. doi: 10.1097/BOR.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legault K, Schunemann H, Hillis C, Yeung C, Akl E, Carrier M, Cervera R, Crowther M, Dentali F, Erkan D, Espinosa G, Khamashta M, Meerpohl J, Moffat K, O’Brien S, Pengo V, Rand J, Rodriguez Pinto I, Thom L, Iorio A. McMaster RARE-Bestpractices clinical practice guideline on diagnosis and management of the catastrophic antiphospholipid syndrome. Journal of Thrombosis and Haemostasis. 2023;16(8):1656–1664. doi: 10.1111/jth.14192. [DOI] [PubMed] [Google Scholar]
- 16.Marson P, Bagatella P, Bortolati M, Tison T, De Silvestro G, Fabris F, Pengo V, Ruffatti A. Plasma exchange for the management of the catastrophic antiphospholipid syndrome: importance of the type of fluid replacement. Journal of Internal Medicine. 2021;264(2):201–203. doi: 10.1111/j.1365-2796.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Pinto I, Moitinho M, Santacreu I, Shoenfeld Y, Erkan D, Espinosa G, Cervera R, CAPS Registry Project Group (European Forum on Antiphospholipid Antibodies) Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the International CAPS Registry. Elsevier. Autoimmun Rev. 2016;15(12):1120–1124. doi: 10.1016/j.autrev.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Benjume B, Rodriguez-Pinto I, Amigo MC, Erkan D, Shoenfeld Y, Cervera R, Espinosa G. Eculizumab use in catastrophic antiphospholipid syndrome (CAPS): descriptive analysis from the “CAPS Registry”. Autoimmun Rev. 2022;21(4):103055. doi: 10.1016/j.autrev.2022.103055. [DOI] [PubMed] [Google Scholar]
- 19.Neuwelt C. The role of plasmapheresis in the treatment of severe central nervous system neuropsychiatric systemic lupus erythematosus. Therapeutic Apheresis and Dialysis. 2023;7(2):173–182. doi: 10.1046/j.1526-0968.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 20.Velasquez-Rimachi V, Palma-García L, Pacheco-Barrios K, Alva-Díaz C. Successful treatment of catastrophic antiphospholipid syndrome with therapeutic plasma exchange: a case report. Hematol Transfus Cell Ther. 2020;42(3):287–291. doi: 10.1016/j.htct.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu Sayf A, Virk F, Tatem G. A case of catastrofic antiphospholipid antibody syndrome. Chest J 150. 2016;(4):233. doi: 10.1016/j.chest.2016.08.246. [DOI] [Google Scholar]
- 22.Rodriguez-Pinto I, Espinosa G, Erkan D, Shoenfeld Y, Cervera R, CAPS Registry Project Group The effect of triple therapy on the mortality of catastrophic anti-phospholipid syndrome patients. Br J Rheumatol. 2018;57(7):1264–1270. doi: 10.1093/rheumatology/key082. [DOI] [PubMed] [Google Scholar]


