Abstract
Exercise has many physical and psychological benefits and is recommended for people with type 1 diabetes; however, there are many barriers to exercise, including glycemic instability and fear of hypoglycemia. Closed-loop (CL) systems have shown benefit in the overall glycemic management of type 1 diabetes, including improving HbA1c levels and reducing the incidence of nocturnal hypoglycemia; however, these systems are challenged by the rapidly changing insulin needs with exercise. This commentary focuses on the principles, strengths, and challenges of CL in the management of exercise, and discusses potential approaches, including the use of additional physiological signals, to address their shortcomings in the pursuit of fully automated CL systems.
Keywords: closed loop, artificial pancreas, type 1 diabetes, exercise
Introduction
While regular physical activity promotes health in people living with type 1 diabetes (T1D), exercise may provoke hypoglycemia. 1 In the 100 years following the initial therapeutic use of insulin, several technological and pharmacological advances have made exercise safer. These advances include new insulin analogues, continuous glucose monitoring (CGM), and, most recently, closed-loop (CL) insulin delivery systems. This commentary reviews the benefits, and challenges, associated with CL insulin dosing for people with T1D who exercise and discusses potential technical advances which may address the limitations of current commercially available systems.
Benefits of Exercise
There have been a multitude of publications demonstrating an association between exercise and good health mediated by improvements to dyslipidemia, hypertension, visceral obesity, chronic inflammation, insulin resistance, and well-being. 2 This is of particular relevance to those with T1D with evidence confirming that exercise improves physical and psychological well-being in this group.3-5
In recognition of these benefits the American Diabetes Association advises that, in the absence of contraindications, adults with T1D should aim to perform at least 150 minutes of moderate- to vigorous-intensity aerobic exercise per week including two to three resistance sessions. 4 However, despite these benefits, people with T1D are often less active due to glycemic management challenges associated with exercise.6,7
Physiology of Exercise and T1D
Carbohydrate utilization as an energy substrate increases with exercise intensity. To maintain blood glucose homeostasis during prolonged exercise, counterregulatory hormone release (ie, glucagon, catecholamines, cortisol, and growth hormone) and sympathetic neural activity respond to progressively increase hepatic glucose production. During moderate-intensity exercise (MIE), where the counterregulatory response is modest, insulin requirements fall rapidly with physical activity as muscle contraction-mediated glucose uptake increases.8-10 In contrast, during predominately anaerobic or high-intensity exercise (HIE) and resistance exercise (RE), there is an augmented rise in counterregulatory hormones to increase endogenous glucose release and attenuate the exercise-associated rise in muscle insulin sensitivity. Consequentially, in individuals without diabetes, insulin secretion does not decrease as markedly during HIE and, in some instances, may increase to address a robust counterregulatory response. 11
The pharmacokinetics of exogenous subcutaneous insulin delivery brings significant limitations, namely the lack of direct secretion of insulin into the portal vein. Once infused into the subcutaneous tissue, circulating insulin levels cannot rapidly decrease, and may in fact rise because of increased subcutaneous blood flow with exercise. Consequently, for those with T1D undertaking MIE, glucose levels typically decrease, whereas during HIE or RE glucose levels can increase.9,12 Mixed (aerobic and anerobic) exercise may result in increased or decreased glucose levels depending upon the sequence and magnitude of MIE and HIE components.7,9,13 In addition, delayed increases in insulin sensitivity may vary with exercise intensity impacting glucose levels post-exercise. Other variables impacting glycemia in people with T1D undertaking exercise include the time of day, exercise duration, residual insulin activity, timing and composition of the last meal, antecedent hypoglycemia, competition stress, altitude, temperature, and site of insulin delivery.9,14
Published consensus statements and guidelines inform management decisions by health care providers and individuals with T1D using manual insulin dosing to maintain glycemia during and after exercise.9,15-17 While helpful, these strategies are complex, require preplanning, and responses can vary both within and between individuals making exercise a significant challenge. 9 To date, relatively few publications provide recommendations on how to best use CL systems with different forms of exercise. 17
Current CL Systems: Principles
CL systems have transformed the management of T1D improving glucose time-in-range and HbA1c, while minimizing hypoglycemia and improving quality of life.18,19 CL systems consist of a continuous glucose sensor, a control algorithm which estimates an individual’s insulin requirements using glucose sensor information and historical insulin delivery data, and a pump delivering rapid-acting insulin subcutaneously aiming to maintain glucose levels in a target range. Hybrid closed loop (HCL) refers to CL systems where users are still required to manually administer food boluses and corrections. At the time of publication, globally, there are three insulin-only HCL systems commercially available: the Medtronic MiniMed 670G/770G/780G Systems,18,20,21 the Tandem t:slim X2 with Control-IQ 22 and CamAPS fx23,24 with various other systems undergoing clinical trials.25-27
Current CL Systems and Exercise: Strengths
Unlike manually determined insulin dosing, HCL systems continuously adjust insulin delivery to address changing glucose levels. The devices are portable and robust, and experience to date indicates no significant degradation in performance with physical activity.28-30 Studies performed by our research group evaluating iterations of the Medtronic algorithm have shown excellent time-in-range during and up to four hours post-exercise.28-31 These findings are consistent across a range of CL systems that have shown improved time-in-range with CL insulin dosing compared with standard therapy.17,32-39
Current CL Systems and Exercise: Limitations
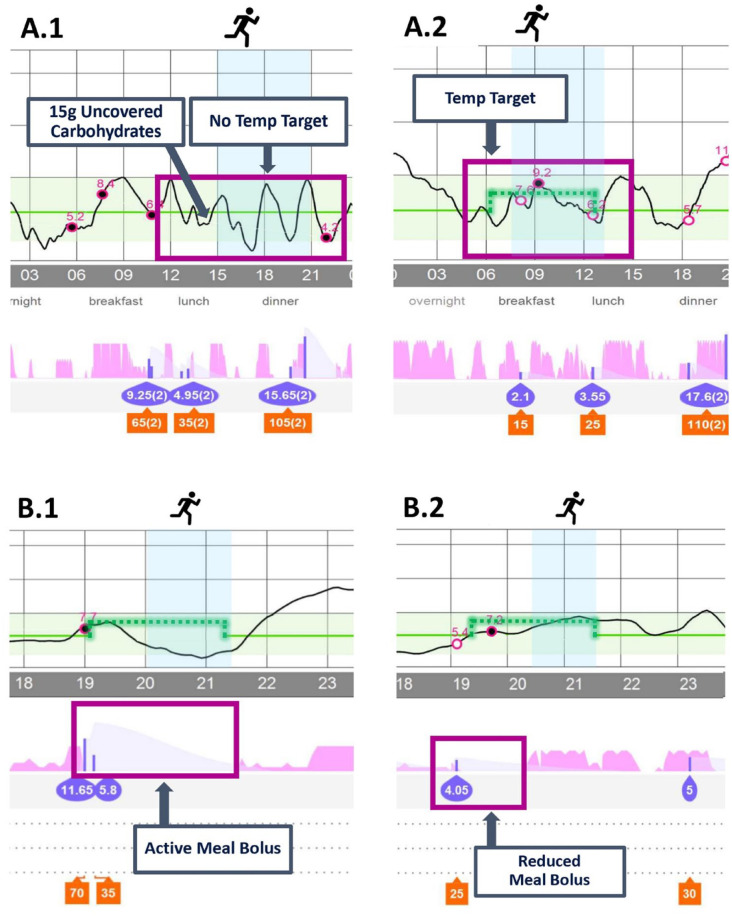
While CL insulin delivery is more responsive compared with manual insulin dosing, exercise still requires forward-planning. Most exercise interventions described in published studies have been performed in controlled clinic settings or have been preplanned with strict protocols requiring the individual to take pre-emptive action against hypoglycemia.9,17 These interventions include optimizing glucose levels to the upper half of the target range at exercise commencement (with an elevated glucose target implemented one to two hours prior to, and supplemental carbohydrate within ten minutes of exercise), minimizing insulin on board and, for exercise greater than one hour duration, supplemental carbohydrate.9,16,40 Examples of where these conditions have not been met and subsequent interventions are shown in Figure 1.
Figure 1.
Examples of Medtronic 670G/770G HCL insulin delivery without use of exercise interventions (A.1 and B.1) and effect of forward-planning interventions (A.2 and B.2). A.1: Moderate-intensity exercise (MIE) with no temporary target set and pre-exercise carbohydrates given prematurely, resulting in hypoglycemia. A.2: MIE with a temporary target set 90 minutes prior to exercise, no pre-exercise carbohydrates were required, reduced boluses were used for meals during prolonged exercise. B.1: MIE with a temporary target prior to exercise, however, a full meal bolus was given with no reduction resulting in hypoglycemia. B.2: MIE with a temporary target and reduction in pre-exercise meal bolus with avoidance of hypoglycemia.
These constraints result from three broad limitations associated with currently commercially available HCL systems:
The slower pharmacokinetics of subcutaneous insulin delivery: In individuals without diabetes, insulin is secreted directly into the portal circulation where it has an onset and offset in action of the order of minutes. 41 Current rapid-acting insulin formulations, such as insulin aspart and lispro, have significantly longer action offset mandating a reduction of insulin delivery approximately 90 minutes prior to exercise to minimize hypoglycemia risk. 42 The pharmacokinetics of “ultra-rapid”-acting insulin analogues, such as faster insulin aspart, while more favorable, are insufficient to meaningfully impact limitations associated with residual insulin action during exercise. 43
An absent glucagon response during exercise to address falling glucose levels: While, even in those with severely impaired hypoglycemia awareness, a significant counterregulatory response to exercise is observed with catecholamines, cortisol, and growth hormones, the response of glucagon to exercise and hypoglycemia remains severely impaired in the majority people with T1D.28,31 Current commercially available devices are single-hormone (insulin-only) systems lacking the facility to estimate the dose required for glucagon to avoid hypoglycemia and to deliver this hormone.
Current-generation commercially available HCL systems measure a single parameter, glucose, to determine insulin delivery. These systems lack the ability to automatically detect the onset, intensity, and duration of exercise, with rules governing insulin delivery fixed regardless of whether the individual is at rest or exercising. We recognize that additional inputs signaling exercise remain limited by the pharmacokinetics of subcutaneous insulin delivery. Even with complete suspension of insulin at the start of exercise, hypoglycemia may still occur. 44 Nevertheless, these potential additional signals may provide useful adjuncts reducing the risk for hypoglycemia.
These strengths and limitations of CL systems are summarized in Table 1.
Table 1.
Strengths and Limitations of Current Commercially Available CL Systems During Exercise.
| Strengths | Limitations |
|---|---|
| • Responsive to changes in glucose • Devices are portable • Improved time-in-range during exercise • Most systems are waterproof or water-resistant • Device performance is not significantly impacted by exercise |
• Preplanning required due to slow onset and offset of subcutaneously delivered insulin • CGM and cannula site adhesive may be affected by sweat • Inability to automatically recognize exercise versus rest • CGM accuracy can be impacted with rapid changes in glucose during exercise |
Abbreviations: CL, closed loop; CGM, continuous glucose monitoring.
Potential Strategies to Address HCL Limitations When Challenged by Exercise
The overarching goal for the future of CL technology is full automation that does not require user input for corrections, meals, or exercise. Potential strategies are summarized in Figure 2 and may include the following:
Figure 2.
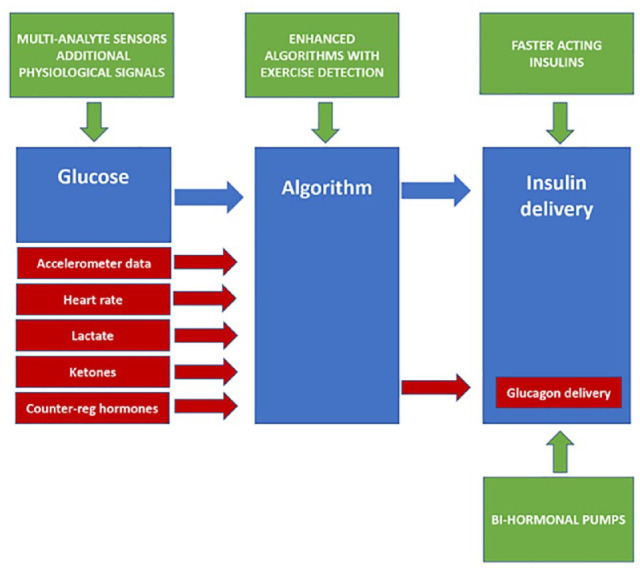
Potential strategies to address hybrid closed-loop (HCL) limitations when challenged by exercise.
(a) The development of insulin formulations and routes of delivery with more favorable pharmacokinetic characteristics: Insulins with significantly faster onset and offset in action are needed to overcome the limitations of subcutaneous insulin absorption. Intranasal insulin has a faster onset and offset of action 45 with the potential to reduce residual insulin activity associated with the pre-exercise meal bolus. However, inhaled insulin is not widely available, its long-term safety is unclear, and limitations associated with administration erode the benefits of CL. Continuous intraperitoneal insulin infusion (CIPII) also has a faster onset and offset of action compared with subcutaneous insulin. 46 CIPII improves HbA1c with less hypoglycemia compared with subcutaneous insulin delivery. 47 However, they are invasive, have technical limitations, carry risks of infection and increased immunogenicity. 48 Intravenous (IV)-delivered insulin permits rapid modulation of delivery with no absorption delay. IV CL insulin delivery models have been used for decades and would bypass the limitations of the subcutaneous route. However, they are invasive, prone to complications such as infections and thromboses, and are less feasible for ambulatory use. 49
(b) Bi-hormonal CL systems: CL systems delivering both insulin and glucagon may help offset the limitations of subcutaneous insulin delivery. Glucagon can minimize the impact of the delayed offset in insulin action by counteracting the persisting glucose-lowering effects of insulin. Several studies have shown that bi-hormonal CL systems result in improved time-in-range and less hypoglycemia during exercise when compared with insulin-only pumps.50-53 However, the stability and long-term safety of glucagon, cost, as well as the increased complexity of a bi-hormonal system also need to be considered.
(c) Additional signals: Pending the development of faster-acting insulins, a potential adjunct would be the use of physiological signals as additional inputs informing algorithms about the onset, offset, and intensity of physical activity.53-57 These include lactate, ketones, counterregulatory hormones, accelerometry, heart rate, galvanic skin response, skin temperature, and blood volume pulse. Counterregulatory hormones can differentiate exercise intensity12,28,31,30 but technical challenges associated with their measurement in real time preclude their use as additional signals in CL systems. Continuous ketone and lactate sensors are under development.58-61 Lactate profiles during exercise, like catecholamines, increase proportionally with exercise intensity and can differentiate MIE and HIE.12,28,30 Ketone responses to different types of exercise while mixed generally show a slow increase.12,28,30 Their use is unlikely to meaningfully inform insulin dosing with exercise due to their limited ability to distinguish exercise type though may play a role in ensuring safety during exercise. 59 Accelerometers and heart-rate sensors are already widely in use in the general population and coupled with lactate sensing would have the potential to inform CL on both exercise type (eg, running, swimming, or weights) and intensity (low, moderate, high, or interval).31,53 As with lactate and counterregulatory responses during exercise, the increase in heart rate is proportional to the exercise intensity; however, limitations include nonspecificity to exercise, with stress, illness, medications, and dehydration, all potential modulators.
Other wearable sensors combining galvanic skin response, accelerometry, blood volume pulse, heart, and skin temperature have been shown to classify physical states and exercise type. 56 These could potentially discriminate exercise and physiological stress (which can have polarizing effects on glucose metabolism) to enhance glucose estimation.57,62
To reduce the burden of additional sensor technology, these additional signals should be incorporated into a single sensing platform. Research is in progress for the development of wearable sensors that measure analytes such as glucose, lactate, accelerometry, and ketones.58-61,63-65
Subsequent in silico mathematical modeling and evaluation of the performance of CL algorithms incorporating these signals is then required to inform advances in next-generation CL systems. However, the impact of any additional signals influencing HCL insulin delivery would remain subject to the downstream influence of the pharmacokinetics of subcutaneous insulin. Nevertheless, additional signals may still have a role in post-exercise or prolonged exercise insulin dosing by predicting changes in insulin sensitivity depending on exercise performed.
(d) Enhanced algorithms: Some investigational HCL systems have incorporated heart rate and accelerometry,53,66 as well as energy expenditure and galvanic skin response 55 for physical activity detection with improvements in glycemic outcomes. However, these algorithms are reactive and remain subject to the slower pharmacokinetics of subcutaneous insulin absorption. Machine learning has also been considered for personalized tuning of CL systems 66-68 and the use of algorithms that can decipher habitual pattern recognition to trigger CL systems of an imminent exercise event is also possible. 69 While this approach may anticipate the likelihood of a metabolic challenge, and CL systems may be primed to respond to a likely event such as future exercise, there would be potential risks associated with insulin dosing determined upon the basis of the probability of an event occurring. This risk could, however, be mitigated by a system request of confirmation of a high likelihood of future exercise with clarification of exercise type.
(e) Improved usability of existing strategies: At present, existing strategies such as setting a temporary target one to two hours prior to aerobic exercise while minimizing the amount of “insulin on board” are the mainstay of exercise management in closed loop. These strategies rely upon pre-emptive action on the part of the person with T1D. The user-device interface could be further refined. An exercise button could be integrated into CL device activation which triggers a number of adaptive changes. These could include an automatic increase in the glucose target for a preset time. It could also modulate the algorithm so that it becomes less aggressive in minimizing rises in glucose levels and more aggressive in minimizing falls in glucose, eg, for a PID algorithm via detuning the Derivative component’s time constant (DT) to make the controller less aggressive in mitigating potential hyperglycemia when sensor glucose levels are rising (dSGdt > 0), but maintaining the same level of aggressiveness in mitigating potential hypoglycemia (dSGdt ≤ 0). Finally, a function to preprogram future recurrent scheduling for exercise (date and time) which could automatically set temporary targets 90 minutes prior.
Conclusions
Current commercially available CL systems improve glucose control and quality of life of people living with T1D, enabling them to exercise with greater safety and confidence. Nevertheless, there remains a need for forward-planning for those who exercise. The pharmacokinetics of subcutaneous insulin delivery remain the most significant limitation of CL systems when challenged by exercise. This limitation may in part be addressed by the co-infusion of glucagon though this incurs increased complexity and expense. Any additional signals informing a CL system about the onset and intensity of exercise cannot predict exercise and would remain subject to the limitations of subcutaneous insulin delivery. Machine learning algorithms may anticipate the likelihood and nature of an exercise event but the ability to respond in real time to rapid changes in insulin requirements would remain limited. Should subcutaneous insulin pharmacokinetics approach that of intravenous insulin delivery this may negate the need for the complexities detailed above. Ultimately, functionality mimicking the healthy beta cell is required.
Footnotes
Abbreviations: CL, closed loop; T1D, type 1 diabetes; CGM, continuous glucose monitoring; MIE, moderate-intensity exercise; HIE, high-intensity exercise; RE, resistance exercise; HCL, hybrid closed loop; CIPII, continuous intraperitoneal insulin infusion; IV, intravenous.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: B.P. and M.H.L. report speaker honoraria fees from Medtronic. D.P.Z. has received speaker honoraria fees from Medtronic Diabetes, Ascensia Diabetes, and Insulet. M.C.R. has served on advisory boards for Supersapiens, Zucara Therapeutics, Zealand Pharma, and Indigo Diabetes and has received speaker’s honoraria from Medtronic Diabetes, Insulet Corporation, Ascensia Diabetes, Novo Nordisk, Xeris Pharmaceuticals, Lilly Diabetes, and Lilly Innovation. D.N.O. has served on advisory boards for Abbott Laboratories, Medtronic, Merck Sharp & Dohme, Novo Nordisk, Roche, and Sanofi; received research support from Medtronic, Novo Nordisk, Roche, Eli Lilly and Company, and Sanofi; and received travel support from Novo Nordisk and Merck Sharp & Dohme.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: B.P. is supported by a University of Melbourne scholarship and research support from JDRF.
ORCID iDs: Barbora Paldus  https://orcid.org/0000-0002-4303-0125
https://orcid.org/0000-0002-4303-0125
Dessi P. Zaharieva  https://orcid.org/0000-0002-9374-8469
https://orcid.org/0000-0002-9374-8469
Michael C. Riddell  https://orcid.org/0000-0001-6556-7559
https://orcid.org/0000-0001-6556-7559
David N. O’Neal  https://orcid.org/0000-0002-0870-4032
https://orcid.org/0000-0002-0870-4032
References
- 1.Lawrence RD. The effect of exercise on insulin action in diabetes. Br Med J. 1926;1(3406):648-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Canadian Medical Association Journal. 2006;174(6):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pivovarov JA, Taplin CE, Riddell MC. Current perspectives on physical activity and exercise for youth with diabetes. Pediatr Diabetes. 2015;16(4):242-255. [DOI] [PubMed] [Google Scholar]
- 4.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohn B, Herbst A, Pfeifer M, et al. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross-sectional multicenter study of 18,028 patients. Diabetes Care. 2015;38(8):1536-1543. [DOI] [PubMed] [Google Scholar]
- 6.Czenczek-Lewandowska E, Leszczak J, Baran J, et al. Levels of physical activity in children and adolescents with type 1 diabetes in relation to the healthy comparators and to the method of insulin therapy used. Int J Environ Res Public Health. 2019;16(18):3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guelfi KJ, Ratnam N, Smythe GA, Jones TW, Fournier PA. Effect of intermittent high-intensity compared with continuous moderate exercise on glucose production and utilization in individuals with type 1 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E865-E870. [DOI] [PubMed] [Google Scholar]
- 8.Riddell MC, Zaharieva DP, Yavelberg L, Cinar A, Jamnik VK. Exercise and the development of the artificial pancreas: one of the more difficult series of hurdles. J Diabetes Sci Technol. 2015;9(6):1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5(5):377-390. [DOI] [PubMed] [Google Scholar]
- 10.Camacho RC, Galassetti P, Davis SN, Wasserman DH. Glucoregulation during and after exercise in health and insulin-dependent diabetes. Exerc Sport Sci Rev. 2005;33(1):17-23. [PubMed] [Google Scholar]
- 11.Fahey AJ, Paramalingam N, Davey RJ, Davis EA, Jones TW, Fournier PA. The effect of a short sprint on postexercise whole-body glucose production and utilization rates in individuals with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(11):4193-4200. [DOI] [PubMed] [Google Scholar]
- 12.Jayawardene DC, McAuley SA, Horsburgh JC, et al. Closed-loop insulin delivery for adults with type 1 diabetes undertaking high-intensity interval exercise versus moderate-intensity exercise: a randomized, crossover study. Diabetes Technol Ther. 2017;19(6):340-348. [DOI] [PubMed] [Google Scholar]
- 13.Yardley JE, Kenny GP, Perkins BA, et al. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care. 2012;35(4):669-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bally L, Laimer M, Stettler C. Exercise-associated glucose metabolism in individuals with type 1 diabetes mellitus. Curr Opin Clin Nutr Metab Care. 2015;18(4):428-433. [DOI] [PubMed] [Google Scholar]
- 15.Adolfsson P, Riddell MC, Taplin CE, et al. ISPAD Clinical Practice Consensus Guidelines 2018: exercise in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(suppl 27):205-226. [DOI] [PubMed] [Google Scholar]
- 16.Moser O, Riddell MC, Eckstein ML, et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia. 2020;63(12):2501-2520. [DOI] [PubMed] [Google Scholar]
- 17.Zaharieva DP, Messer LH, Paldus B, et al., Glucose control during physical activity and exercise using closed loop technology in adults and adolescents with type 1 diabetes. Canadian Journal of Diabetes. 2020;44(8):740-749. [DOI] [PubMed] [Google Scholar]
- 18.McAuley SA, Lee MH, Paldus B, et al. Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care. 2020;43(12):3024-3033. [DOI] [PubMed] [Google Scholar]
- 19.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. [DOI] [PubMed] [Google Scholar]
- 21.Collyns OJ, Meier RA, Betts ZL, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44(4):969-975. [DOI] [PubMed] [Google Scholar]
- 22.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392(10155):1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benhamou PY, Franc S, Reznik Y, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit Health. 2019;1(1):e17-e25. [DOI] [PubMed] [Google Scholar]
- 26.Blauw H, Onvlee AJ, Klaassen M, van Bon AC, DeVries JH. Fully closed loop glucose control with a bihormonal artificial pancreas in adults with type 1 diabetes: an outpatient, randomized, crossover trial. Diabetes Care. 2021;44(3):836-838. [DOI] [PubMed] [Google Scholar]
- 27.Burnside M, Lewis D, Crocket H, et al. CREATE (Community deRivEd AutomaTEd insulin delivery) trial. Randomised parallel arm open label clinical trial comparing automated insulin delivery using a mobile controller (AnyDANA-loop) with an open-source algorithm with sensor augmented pump therapy in type 1 diabetes. J Diabetes Metab Disord. 2020;19(2):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MH, Vogrin S, Paldus B, et al. Glucose and counterregulatory responses to exercise in adults with type 1 diabetes and impaired awareness of hypoglycemia using closed-loop insulin delivery: a randomized crossover study. Diabetes Care. 2020;43(2):480-483. [DOI] [PubMed] [Google Scholar]
- 29.Morrison D, Zaharieva DP, Lee MH, et al. Comparable glucose control with fast-acting insulin aspart versus insulin aspart using a second-generation hybrid closed-loop system during exercise. Diabetes Technol Ther. 2021;24:93-101. [DOI] [PubMed] [Google Scholar]
- 30.Paldus B, Lee MH, Morrison D, et al. First randomized controlled trial of hybrid closed loop versus multiple daily injections or insulin pump using self-monitoring of blood glucose in free-living adults with type 1 diabetes undertaking exercise. J Diabetes Sci Technol. 2021:15(6):1399-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paldus B, Morrison D, Zaharieva DP, et al. A randomized crossover trial comparing glucose control during moderate-intensity, high-intensity, and resistance exercise with hybrid closed-loop insulin delivery while profiling potential additional signals in adults with type 1 diabetes. Diabetes Care. 2022;45(1):194-203. [DOI] [PubMed] [Google Scholar]
- 32.Sherr JL, Cengiz E, Palerm CC, et al. Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care. 2013;36(10):2909-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breton MD, Cherñavvsky DR, Forlenza GP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40(12):1644-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dovc K, Macedoni M, Bratina N, et al. Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: a randomised controlled crossover trial. Diabetologia. 2017;60:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huyett LM, Ly TT, Forlenza GP, et al. Outpatient closed-loop control with unannounced moderate exercise in adolescents using zone model predictive control. Diabetes Technol Ther. 2017;19(6):331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinsker JE, Laguna Sanz AJ, Lee JB, et al. Evaluation of an artificial pancreas with enhanced model predictive control and a glucose prediction trust index with unannounced exercise. Diabetes Technol Ther. 2018;20(7):455-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekhlaspour L, Forlenza GP, Chernavvsky D, et al. Closed loop control in adolescents and children during winter sports: use of the Tandem Control-IQ AP system. Pediatric Diabetes. 2019;20(6):759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanaire H, Franc S, Borot S, et al. Efficacy of the Diabeloop closed-loop system to improve glycaemic control in patients with type 1 diabetes exposed to gastronomic dinners or to sustained physical exercise. Diabetes Obes Metabol. 2020;22(3):324-334. [DOI] [PubMed] [Google Scholar]
- 39.Eckstein ML, Weilguni B, Tauschmann M, et al. Time in range for closed-loop systems versus standard of care during physical exercise in people with type 1 diabetes: a systematic review and meta-analysis. J Clin Med. 2021;10(11):2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riddell MC, Scott SN, Fournier PA, et al. The competitive athlete with type 1 diabetes. Diabetologia. 2020;63(8):1475-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19-39. [PMC free article] [PubMed] [Google Scholar]
- 42.McAuley SA, Horsburgh JC, Ward GM, et al. Insulin pump basal adjustment for exercise in type 1 diabetes: a randomised crossover study. Diabetologia. 2016;59(8):1636-1644. [DOI] [PubMed] [Google Scholar]
- 43.Morrison D, Zaharieva DP, Lee MH, et al. Comparable glucose control with fast-acting insulin aspart versus insulin aspart using a second-generation hybrid closed-loop system during exercise. Diabetes Technol Ther. 2021:24(2):93-101. [DOI] [PubMed] [Google Scholar]
- 44.Zaharieva DP, McGaugh S, Pooni R, Vienneau T, Ly T, Riddell MC. Improved open-loop glucose control with basal insulin reduction 90 minutes before aerobic exercise in patients with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Care. 2019;42(5):824-831. [DOI] [PubMed] [Google Scholar]
- 45.Leary AC, Stote RM, Cussen K, O’Brien J, Leary WP, Buckley B. Pharmacokinetics and pharmacodynamics of intranasal insulin administered to patients with type 1 diabetes: a preliminary study. Diabetes Technol Ther. 2006;8(1):81-88. [DOI] [PubMed] [Google Scholar]
- 46.Giacca A, Caumo A, Galimberti G, et al. Peritoneal and subcutaneous absorption of insulin in type I diabetic subjects. J Clin Endocrinol Metab. 1993;77(3):738-742. [DOI] [PubMed] [Google Scholar]
- 47.van Dijk PR, Logtenberg SJJ, Gans ROB, Bilo HJG, Kleefstra N. Intraperitoneal insulin infusion: treatment option for type 1 diabetes resulting in beneficial endocrine effects beyond glycaemia. Clin Endocrinol. 2014;81(4):488-497. [DOI] [PubMed] [Google Scholar]
- 48.Bally L, Thabit H, Hovorka R. Finding the right route for insulin delivery—an overview of implantable pump therapy. Expert Opinion on Drug Delivery. 2017;14(9):1103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renard E. Insulin delivery route for the artificial pancreas: subcutaneous, intraperitoneal, or intravenous? pros and cons. J Diabetes Sci Technol. 2008;2(4):735-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haidar A, Rabasa-Lhoret R, Legault L, et al. Single- and dual-hormone artificial pancreas for overnight glucose control in type 1 diabetes. J Clin Endocrinol Metab. 2016;101(1):214-223. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs PG, Youssef JE, Reddy R, et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab. 2016;18(11):1110-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taleb N, Emami A, Suppere C, et al. Efficacy of single-hormone and dual-hormone artificial pancreas during continuous and interval exercise in adult patients with type 1 diabetes: randomised controlled crossover trial. Diabetologia. 2016;59(12):2561-2571. [DOI] [PubMed] [Google Scholar]
- 53.Castle JR, Youssef JE, Wilson LM, et al., Randomized outpatient trial of single- and dual-hormone closed-loop systems that adapt to exercise using wearable sensors. Diabetes Care. 2018;41(7):1471-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajizadeh I, Rashid M, Turksoy K, et al. Incorporating unannounced meals and exercise in adaptive learning of personalized models for multivariable artificial pancreas systems. J Diabetes Sci Technol. 2018;12(5):953-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turksoy K, Hajizadeh I, Hobbs N, et al. Multivariable artificial pancreas for various exercise types and intensities. Diabetes Technol Ther. 2018;20(10):662-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sevil M, Rashid M, Maloney Z, et al. Determining physical activity characteristics from wristband data for use in automated insulin delivery systems. IEEE Sens J. 2020;20(21):12859-12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sevil M, Rashid M, Hajizadeh I, et al. Discrimination of simultaneous psychological and physical stressors using wristband biosignals. Comput Meth Programs Biomed. 2021;199:105898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alva S, Castorino K, Cho H, Ou J. Feasibility of continuous ketone monitoring in subcutaneous tissue using a ketone sensor. J Diabetes Sci Technol. 2021;15(4):768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee MH, Paldus B, Krishnamurthy B, et al. The clinical case for the integration of a ketone sensor as part of a closed loop insulin pump system. J Diabetes Sci Technol. 2019;13(5):967-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang JY, Shang T, Koliwad SK, Klonoff DC. Continuous ketone monitoring: a new paradigm for physiologic monitoring. J Diabetes Sci Technol. 2021;15(4):775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dror N, Weidling J, White S, et al. Clinical evaluation of a novel subcutaneous lactate monitor. J Clin Monit Comput. 2021. doi: 10.1007/s10877-021-00685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sevil M, Rashid M, Hajizadeh I, et al. Physical activity and psychological stress detection and assessment of their effects on glucose concentration predictions in diabetes management. IEEE Trans Biomed Eng. 2021;68(7):2251-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graf A, McAuley SA, Sims C, et al. Moving toward a unified platform for insulin delivery and sensing of inputs relevant to an artificial pancreas. J Diabetes Sci Technol. 2016;11(2):308-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.University of California, Irvine. https://engineering.uci.edu/news/2021/4/botvinick-wins-grant-develop-advanced-monitor-type-1-diabetes. Accessed March 21, 2022.
- 65.PRWeb. http://www.prweb.com/releases/percusense_announces_project_with_the_university_of_melbourne_funded_by_the_helmsley_charitable_trust_to_develop_a_single_sensor_continuous_glucose_and_ketone_monitor/prweb17509712.htm. Accessed March 21, 2022.
- 66.Resalat N, Hilts W, Youssef JE, Tyler N, Castle JR, Jacobs PG. Adaptive control of an artificial pancreas using model identification, adaptive postprandial insulin delivery, and heart rate and accelerometry as control inputs. J Diabetes Sci Technol. 2019;13(6):1044-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor KA, Forlenza GP. Use of machine learning and hybrid closed loop insulin delivery at diabetes camps. Diabetes Technol Ther. 2020;22(7):535-537. [DOI] [PubMed] [Google Scholar]
- 68.Askari MR, Hajizadeh I, Rashid M, Hobbs N, Zavala VM, Cinar A. Adaptive-learning model predictive control for complex physiological systems: automated insulin delivery in diabetes. Annual Reviews in Control. 2020;50:1-12. [Google Scholar]
- 69.Garcia-Tirado J, Brown SA, Laichuthai NA, et al. Anticipation of historical exercise patterns by a novel artificial pancreas system reduces hypoglycemia during and after moderate-intensity physical activity in people with type 1 diabetes. Diabetes Technology & Therapeutics. 2020;23(4):277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]