Abstract
Background:
Accuracy of a seventh-generation “G7” continuous glucose monitoring (CGM) system was evaluated in children and adolescents with type 1 diabetes (T1D).
Methods:
Sensors were worn on the upper arm and abdomen. The CGM data were available from 127 of 132 participants, ages 7 to 17 years, across 10.5 days of use, various glucose concentration ranges, and various rates of glucose change for comparisons with temporally matched venous blood glucose measurements (YSI). Data were also available from 28 of 32 participants, ages 2 to 6 years, for whom capillary (fingerstick) blood provided comparator glucose values. Accuracy metrics included the mean absolute relative difference (MARD) between CGM and comparator glucose pairs, the proportion of CGM values within 15 mg/dL or 15% of comparator values <100 or ≥100 mg/dL, respectively, and the analogous %20/20 and %30/30 agreement rates.
Results:
For participants aged 7 to 17, a total of 15 437 matched pairs were obtained from 122 arm-placed and 118 abdomen-placed sensors. For arm-placed sensors, the overall MARD was 8.1% and overall %15/15, %20/20, and %30/30 agreement rates were 88.8%, 95.3%, and 98.7%, respectively. For abdomen-placed sensors, the overall MARD was 9.0% and overall %15/15, %20/20, and %30/30 agreement rates were 86.0%, 92.9%, and 97.7%, respectively. Good accuracy was maintained across wear days, glucose ranges, and rates of glucose change. Among those aged 2 to 6, a total of 343 matched pairs provided an overall MARD of 9.3% and an overall %20/20 agreement rate of 91.5%.
Conclusions:
The G7 CGM placed on the arm or abdomen was accurate in children and adolescents with T1D. NCT#: NCT04794478
Keywords: continuous glucose monitoring, pediatrics, Dexcom, G7, accuracy, MARD
Introduction
Continuous glucose monitoring (CGM) systems are approved in many countries to inform diabetes treatment decisions in children 2 years and older. Advancements in CGM technology over the past decade (eg, better accuracy, predictive alerts, automated insertions, data sharing capabilities, resistance to acetaminophen interference, optional calibrations) 1 have likely contributed to increased CGM use in the pediatric population. The CGM technology has helped children and adolescents with type 1 diabetes (T1D) improve their glycemic control,2-4 and studies have demonstrated the importance of early CGM initiation 5 and frequent sensor usage 6 on glycemic outcomes.
Here we describe the performance of a seventh-generation “G7” CGM system (Dexcom, Inc., San Diego, CA). The G7 is a disposable, single-use device that features a smaller wearable profile and shorter warm-up period than the company’s earlier sixth-, fifth-, and fourth-generation systems (G6, G5, and G4). The G7 system also differs from these earlier systems in that the transmitter and sensor are provided as an integrated unit, avoiding the need to attach and remove a transmitter from different sensors during the transmitter’s working life. The G7 system displays glucose values and trending information over a 10-day wear session that can be extended for an additional 12-hour “grace period,” allowing users to continue monitoring their glucose levels while starting a new sensor. In this study, we evaluated the accuracy of the G7 CGM in children and adolescents 2 to 17 years of age. Accuracy metrics of G7 in adults are reported elsewhere. 7
Methods
Study procedures and methods were similar to those used to assess G7 accuracy in adults. 7 Briefly, a prospective, multicenter, single-arm study (clinicaltrials.gov, NCT04794478) was conducted from February to June 2021 to evaluate the accuracy and performance of G7 sensors. Participants were enrolled at six US clinical sites and included older (ages 7-17 years) and younger (ages 2-6) cohorts.
The older cohort wore up to three G7 sensors on the back of the upper arm and abdomen, with sensor insertions performed at the clinic by participants and/or caregivers. Clinic sessions of varying duration were scheduled on days 1 or 2, days 4 or 7, and the second half of day 10 or the first half of day 11 of sensor wear. Participants 13 to 17 years old had two clinic sessions, whereas participants 7 to 12 years old had one clinic session. During clinic sessions, members of the older cohort had arterialized venous blood drawn from an intravenous catheter; blood glucose was measured with the YSI 2300 STAT PLUS analyzer (YSI, Inc., Yellow Springs, OH). Blood glucose determinations were made at 15±5-minute intervals for YSI values in the 80 to 300 mg/dL range and at 10±5-minute intervals for YSI values that were <80 or >300 mg/dL. Under close supervision and per protocol, subjects aged 13 years and older underwent deliberate insulin and meal challenges to induce mild to moderate hyperglycemia and hypoglycemia. Accuracy metrics included mean absolute relative difference (MARD), as well as %15/15 (the proportion of CGM values within 15% of the YSI comparator for glucose >100 mg/dL or within 15 mg/dL of the YSI comparator for glucose ≤100 mg/dL) and the analogous %20/20 and %30/30 agreement rates.
The younger cohort (ages 2-6 years) wore up to two G7 sensors on the arm, abdomen, or upper buttocks and participated in one clinic session lasting approximately 4 hours that did not involve intentional glucose manipulations. Capillary (not venous) blood glucose measurements (Ascencia CONTOUR NEXT; Ascensia Diabetes Care, Parsippany, NJ) were collected approximately every 30 minutes and used as comparator values. Surveys regarding the ease of sensor insertion and comfort were completed by participants or caregivers.
Results
Study Population
A summary of baseline demographics for 164 participants is shown in Table 1, 132 of whom were aged 7 to 17 and 32 of whom were aged 2 to 6 years. Of those in the older cohort, one withdrew from the study before sensor insertion, one did not participate in any clinic sessions, and three had both arm and abdomen devices fail prior to clinic sessions. The remaining 127 participants had 122 arm and 118 abdomen devices with valid CGM readings and provided 15 809 matched pairs for accuracy analysis. Of these matched pairs, 15 437 (97.6%) included CGM values in the reportable range of 40 to 400 mg/dL and were used to calculate accuracy metrics; 8068 pairs were from arm-placed sensors and 7369 pairs were from abdomen-placed sensors.
Table 1.
Baseline Demographics (N = 164).
| Demographic | Value |
|---|---|
| Age, years | |
| Mean (SD) | 12.2 (4.1) |
| Median | 12.9 |
| Min, Max | 2.7, 17.9 |
| Gender, n (%) | |
| Female | 78 (47.6%) |
| Male | 86 (52.4%) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 21 (12.8%) |
| Not Hispanic or Latino | 143 (87.2%) |
| Race, n (%) | |
| American Indian or Alaska Native | 0 (0.0%) |
| Asian | 1 (0.6%) |
| Black; African American; or of African Heritage | 8 (4.9%) |
| Native Hawaiian or Pacific-Islander | 0 (0.0%) |
| White | 142 (86.6%) |
| Other | 13 (7.9%) |
There were 32 participants aged 2 to 6 years, with 28 participants having CGM and capillary blood glucose matched pairs available from 14 arm, 5 abdomen, and 13 upper buttocks devices. A total of 343 matched pairs from this younger cohort included CGM values in the reportable range and were available for accuracy analysis.
Accuracy Metrics
In the older cohort, data from 122 arm-placed sensors showed an overall MARD of 8.1% and overall %15/15, %20/20, and %30/30 agreement rates of 88.8%, 95.3%, and 98.7%, respectively; 114 (93.4%) of the arm sensors provided >80% of readings that were within the %20/20 agreement limits. Data from 118 abdomen-placed sensors showed an overall MARD of 9.0% and overall %15/15, %20/20, and %30/30 agreement rates of 86.0%, 92.9%, and 97.7%, respectively; 102 (86.4%) of the abdomen sensors provided >80% of readings that were within the %20/20 agreement limits.
Table 2 summarizes accuracy results across the clinic session day for the older cohort. Accuracy was good in both arm- and abdomen-placed sensors across the 10.5-day wear sessions. Accuracy improved after day 1 and remained good through the 12-hour grace period. On day 1 of sensor wear, the %20/20 agreement rate and MARD were 87.7% and 11.7% for arm-placed sensors, and 88.6% and 11.1% for abdomen-placed sensors. By day 10.5 of sensor wear (grace period), the %20/20 agreement rate and MARD had improved to 94.1% and 7.3% (arm), and 92.9% and 8.8% (abdomen).
Table 2.
Accuracy of CGM vs YSI by Clinic Session Day, Participants Aged 7 to 17 Years.
| Placement | Clinic day | Matched pairs (n) | %15/15 (%) | %20/20 (%) | %30/30 (%) | MARD (%) |
|---|---|---|---|---|---|---|
| Arm (N=122) |
Day 1 | 1741 | 74.6 | 87.7 | 95.7 | 11.7 |
| Day 2 | 1637 | 88.6 | 95.5 | 99.0 | 7.9 | |
| Day 4 | 1764 | 93.9 | 98.4 | 99.8 | 6.8 | |
| Day 7 | 1577 | 95.4 | 99.1 | 99.9 | 6.8 | |
| Day 10 | 669 | 96.6 | 99.3 | 100.0 | 6.3 | |
| Day 10.5 | 680 | 89.4 | 94.1 | 98.7 | 7.3 | |
| Overall | 8068 | 88.8 | 95.3 | 98.7 | 8.1 | |
| Abdomen (N=118) |
Day 1 | 1758 | 77.3 | 88.6 | 96.6 | 11.1 |
| Day 2 | 1625 | 89.2 | 94.3 | 97.9 | 8.6 | |
| Day 4 | 1726 | 94.1 | 97.5 | 98.6 | 7.1 | |
| Day 7 | 1480 | 82.4 | 90.6 | 97.9 | 9.0 | |
| Day 10 | 442 | 88.5 | 95.5 | 98.6 | 9.2 | |
| Day 10.5 | 338 | 87.0 | 92.9 | 96.4 | 8.8 | |
| Overall | 7369 | 86.0 | 92.9 | 97.7 | 9.0 |
Abbreviations: CGM, continuous glucose monitoring; MARD, mean absolute relative difference.
Accuracy was also good across glucose ranges for arm- and abdomen-placed sensors (Table 3). MARD is provided for glucose levels >80 mg/dL while MAD is provided for glucose ≤80 mg/dL. Of the 240 sensors used in the study, 11 (4.6%) had MARD values greater than 20 and 177 (73.8%) had MARD values 10% or less (Figure 1). The average per-sensor MARD was 8.7% ± 5.8%. To assess G7 accuracy within biologically relevant glucose ranges, matched pairs of YSI and CGM data were analyzed. As shown in Table 4, concurrence of CGM and YSI values for CGM-based concentration ranges of <70, 70 to 180, and >180 mg/dL was 73.5%, 89.6%, and 96.2% in arm-placed sensors and 63.6%, 89.0%, and 95.5% for abdomen-placed sensors. Table 5 summarizes accuracy across rates of glucose change. Accuracy was consistent across various rate of change (ROC) categories analyzed. During rapid rates of change (RoC <-2 and >2 mg/dL/min), the %20/20 agreement rate in arm and abdomen devices was no less than 90.3%, and MARD was no more than 9.7%.
Table 3.
Accuracy of CGM vs YSI Across CGM Glucose Ranges, Participants Aged 7 to 17 Years.
| Placement | CGM glucose range (mg/dL) | Matched pairs (n) | %15/15 (%) | %20/20 (%) | %30/30 (%) | MARD (%) | MAD (mg/dL) |
|---|---|---|---|---|---|---|---|
| Arm (N = 122) |
40-60 | 402 | 74.4 | 85.3 | 92.0 | NA | 11.3 |
| 61-80 | 1089 | 93.0 | 95.5 | 97.8 | NA | 6.4 | |
| 81-180 | 3386 | 86.5 | 94.1 | 98.7 | 8.4 | NA | |
| 181-300 | 2029 | 88.5 | 97.0 | 99.8 | 7.6 | NA | |
| 301-400 | 1162 | 96.9 | 99.4 | 100.0 | 5.4 | NA | |
| Abdomen (N = 118) |
40-60 | 439 | 56.0 | 73.1 | 86.8 | NA | 15.6 |
| 61-80 | 865 | 85.9 | 90.6 | 96.0 | NA | 9.0 | |
| 81-180 | 3046 | 84.1 | 91.2 | 97.8 | 9.2 | NA | |
| 181-300 | 1874 | 90.4 | 97.4 | 99.6 | 7.1 | NA | |
| 301-400 | 1145 | 95.4 | 99.6 | 100.0 | 5.7 | NA |
Abbreviations: CGM, continuous glucose monitoring; MARD, mean absolute relative difference; MAD, mean absolute difference.
Figure 1.
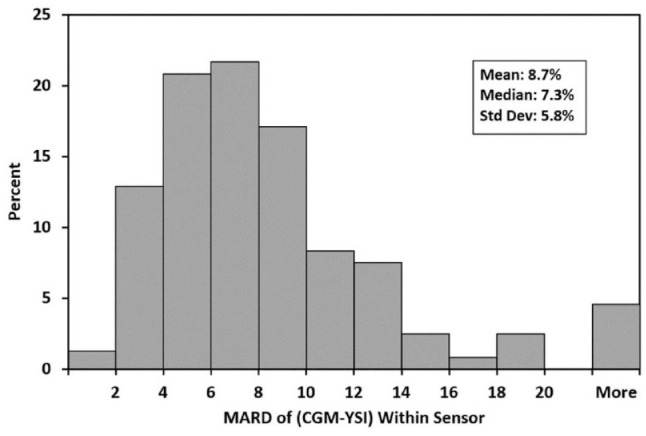
Per-sensor MARD distribution. Abbreviations: MARD, mean absolute relative difference; CGM, continuous glucose monitoring.
Table 4.
Concurrence of CGM and YSI Values by CGM Glucose Range.
| Placement | CGM glucose range (mg/dL) | Comparator glucose values (mg/dL) | |||
|---|---|---|---|---|---|
| <70 n (%) |
70-180 n (%) |
>180 n (%) |
Overall N |
||
| Arm (N = 122) |
<70 | 702 (73.5) | 253 (26.5) | 0 (0.0) | 955 |
| 70-180 | 227 (5.7) | 3582 (89.6) | 187 (4.7) | 3996 | |
| >180 | 0 (0.0) | 123 (3.8) | 3146 (96.2) | 3269 | |
| Abdomen (N = 118) |
<70 | 625 (63.6) | 354 (36.0) | 3 (0.3) | 982 |
| 70-180 | 197 (5.6) | 3133 (89.0) | 189 (5.4) | 3519 | |
| >180 | 0 (0.0) | 138 (4.5) | 2950 (95.5) | 3088 | |
Abbreviation: CGM, continuous glucose monitoring.
Table 5.
Accuracy Across Glucose Rates of Change.
| Placement | CGM rate range (mg/dL/min) | Matched pairs (n) | %20/20 (%) | MARD (%) |
|---|---|---|---|---|
| Arm (N = 122) |
<−2 | 280 | 91.1 | 9.6 |
| −2 to <−1 | 896 | 94.8 | 8.3 | |
| −1 to <0 | 2864 | 96.2 | 7.7 | |
| 0 to 1 | 2461 | 96.4 | 7.9 | |
| >1 to 2 | 813 | 94.5 | 8.3 | |
| >2 | 547 | 91.6 | 9.4 | |
| Abdomen (N = 118) |
<−2 | 268 | 90.3 | 9.7 |
| −2 to <−1 | 854 | 91.8 | 8.7 | |
| −1 to <0 | 2675 | 92.6 | 9.0 | |
| 0 to 1 | 2219 | 94.4 | 8.9 | |
| >1 to 2 | 677 | 94.1 | 8.6 | |
| >2 | 474 | 91.8 | 9.6 |
Abbreviations: CGM, continuous glucose monitoring; MARD, mean absolute relative difference.
For CGM versus capillary blood glucose comparisons of data from children aged 2 to 6 years, the overall MARD was 9.3%, and the %15/15, %20/20, and %30/30 agreement rates were 83.4%, 91.5%, and 95.9%, respectively.
Adverse Events, Ease-of-Use, and Comfort
No serious adverse events were reported. Eight participants reported mild-to-moderate device-related adverse events corresponding to pain during sensor insertion, discomfort/pain during sensor removal, or skin irritation at the adhesive site. The insertion process was rated as “somewhat easy” or “very easy” by 157 (96.4%) of the 163 participants who responded to the survey question. When asked to compare G7 to their previous system, 113 (71.5%) of 158 responders rated the insertion process as “slightly easier” or “much easier,” and 117 (74.5%) of 157 responders rated the comfort of G7 as “slightly better” or “much better.”
Discussion
As described here, the G7 CGM has good accuracy in children and adolescents aged 7 to 17 across days of wear, glucose ranges, and rates of glucose change. For arm- and abdomen-placed sensors, overall G7 MARD values were 8.1% and 9.0%, respectively, and overall %20/20 values were 95.3% and 92.9%, respectively. G7 was also accurate in young children aged 2 to 6 with overall MARD and %20/20 agreement rates of 9.3% and 91.5%, respectively. These results are consistent with those of the G6 system in children and adolescents with T1D 8 and with an earlier study of the G5 system that evaluated accuracy at different insertion sites. 9
The G7 system shares several features with the earlier CGM systems that have proven to be helpful in managing pediatric diabetes. The Share/Follow feature, first introduced with the G4 system, allows parents and caregivers to monitor a child’s glucose levels and receive alerts for existing or impending out-of-range values. It is used more often for younger patients 10 and has been associated with improved glycemic parameters in youth. 11 Other studies have demonstrated that data sharing improves the quality of life and reduces fear of hypoglycemia for parents and caregivers.12-14
Several new features were introduced with the G7. Compared with the currently available G6, the G7 has a shorter warm-up period (27 minutes vs 2 hours), making it possible for youth and caregivers of young children to obtain glucose data more quickly for diabetes management decisions. While day 1 accuracy tends to be lower across CGM devices, 15 G7’s high day 1 accuracy coupled with its shorter warm-up period should improve the sensor experience in young users. Accuracy remained high through the 12-hour grace period at the end of the 10-day sensor session (MARD of 7.3% in the arm and 8.8% in the abdomen). Families and G7 users can continue receiving accurate glucose measurements for half a day while a new session is started. This additional sensor life may be of use during months with 31 days in people with insurance that provides only three sensors per month.
Barriers to sustained CGM use among children and adolescents include discomfort during insertions or daily wear, inconvenience, disruptive alerts, gaps in data, poor between-device connections, and difficulty attaching multiple devices to the body.16-18 These and other barriers likely contribute to high rates of therapy abandonment, as documented in a recent study from Australia were 59% of patients aged 15 to 21 stopped using the devices within 6 months, despite the presence of universal coverage. 16 Design improvements to the G7 that enhance the usability of the device may address some of these concerns. For example, the G7 has a shorter introducer needle and a smaller implanted sensor, which may be associated with less discomfort (rather than pain) during insertion and wear. The on-body components of G7 are smaller than those of G6; users may find this to be more discrete and appreciate that less skin is subject to potential irritation from the adhesive patch. The G7 startup sequence involves fewer steps than that of G6, and G7 provides 24 hours of data backfill (compared with 3 hours for G6) to allow for better data coverage. Audible alerts may also be temporarily silenced (up to 6 hours) to reduce alarm nuisance.
Strengths of this study include the large number of matched pairs, the evaluation of accuracy metrics in different glycemic ranges, and assessment of sensor accuracy at different anatomical locations. The inclusion of very young participants is an additional strength of the study. Because of the need to limit study burden in very young children, they were not subjected to venous blood sampling or intentional glucose manipulations; therefore, metrics for this population are based on a different comparator method and do not include accuracy at different glucose concentrations or glucose rates of change. Further studies are warranted to evaluate the clinical and psychosocial outcomes associated with G7 use in clinical practice and to establish the suitability of G7 data for use in automated insulin delivery systems.
Conclusions
The G7 CGM system provided accurate glucose concentration estimates in children and adolescents with T1D, whether placed on the abdomen or arm. Good accuracy was observed across the sensors’ working life and across a range of glucose concentrations and rates of glucose concentration change, suggesting that the data can be used safely as the basis for diabetes management decisions. The G7’s on-body components are smaller than those of G6, and the integration of the G7 sensor and transmitter allows for a simplified initialization process. These attributes, combined with overall ease-of-use and comfort, may contribute to higher adoption and durable utilization rates for CGM in children and adolescents with diabetes.
Acknowledgments
The authors thank participants and their families and the staff at the investigational sites. The authors also thank Michelle Zhang, Ping Gao, Christy Chao, and John Welsh, employees of Dexcom, Inc. for data analysis and manuscript preparation. Dexcom is a registered trademark of Dexcom, Inc. in the United States and may be in other countries.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; MAD, mean absolute difference; MARD, mean absolute relative difference; T1D, type 1 diabetes.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LML reports grant support to her institution from NIH, JDRF, Helmsley Charitable Trust, Eli Lilly and Company, Insulet, Dexcom, and Boehringer Ingelheim; she receives consulting fees unrelated to the current report from Johnson & Johnson, Sanofi, Novo Nordisk, Roche, Insulet, Boehringer Ingelheim, ConvaTec, Medtronic, Lifescan, Laxmi, and Insulogic. TB conducts research sponsored by Abbott, Capillary Biomedical, Dexcom, Diasome, Eli Lilly, Kowa, Lexicon, Medtronic, Medtrum, Novo Nordisk, REMD, Sanofi, Senseonics, Viacyte, vTv Therapeutics, Zealand Pharma, and has been a consultant, speaker, and/or advisory board member for Abbott, LifeScan, Metronom Health, Novo, Sanofi. MPC conducts research sponsored by Abbott, Biolinq, Dexcom, Eli Lilly, Novo Nordisk, Medtronic, Insulet, Sanofi Aventis, Merck, and Senseonics. JLR and SEB are employees and stockholders of Dexcom, Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This was funded byDexcom, Inc.
ORCID iDs: Lori M. Laffel  https://orcid.org/0000-0002-9675-3001
https://orcid.org/0000-0002-9675-3001
Timothy Bailey  https://orcid.org/0000-0003-4178-3462
https://orcid.org/0000-0003-4178-3462
References
- 1.Welsh JB, Gao P, Derdzinski M, et al. Accuracy, utilization, and effectiveness comparisons of different continuous glucose monitoring systems. Diabetes Technol Ther. 2019;21(3):128-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thabit H, Prabhu JN, Mubita W, et al. Use of factory-calibrated real-time continuous glucose monitoring improves time in target and HbA1c in a multiethnic cohort of adolescents and young adults with type 1 diabetes: the MILLENNIALS study. Diabetes Care. 2020;43(10):2537-2543. [DOI] [PubMed] [Google Scholar]
- 3.Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group. A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with finger-stick blood glucose monitoring in very young children with type 1 diabetes. Diabetes Care. 2021;44(2):464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prahalad P, Addala A, Scheinker D, Hood KK, Maahs DM. CGM initiation soon after type 1 diabetes diagnosis results in sustained CGM use and wear time. Diabetes Care. 2020;43(1):e3-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Buckingham B, et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32(11):1947-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg S, Kipnes MS, Castorino K, et al. Accuracy and safety of Dexcom G7 continuous glucose monitoring in adults with diabetes [published online ahead of print February2022]. Diabetes Technol Ther. doi: 10.1089/dia.2022.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welsh JB, Zhang X, Puhr SA, et al. Performance of a factory-calibrated, real-time continuous glucose monitoring system in pediatric participants with type 1 diabetes. J Diabetes Sci Technol. 2019;13(2):254-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faccioli S, Del Favero S, Visentin R, et al. Accuracy of a CGM sensor in pediatric subjects with type 1 diabetes. comparison of three insertion sites: arm, abdomen, and gluteus. J Diabetes Sci Technol. 2017;11(6):1147-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akturk HK, Dowd R, Shankar K, Derdzinski M. Real-world evidence and glycemic improvement using Dexcom G6 features. Diabetes Technol Ther. 2021;23(suppl 1):S21-S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welsh JB, Derdzinski M, Parker AS, Puhr S, Jimenez A, Walker T. Real-time sharing and following of continuous glucose monitoring data in youth. Diabetes Therapy. 2019;10(2):751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polonsky WH, Fortmann AL. Impact of real-time CGM data sharing on quality of life in the caregivers of adults and children with type 1 diabetes. J Diabetes Sci Technol. 2022;16:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burckhardt MA, Roberts A, Smith GJ, Abraham MB, Davis EA, Jones TW. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care. 2018;41(12):2641-2643. [DOI] [PubMed] [Google Scholar]
- 14.Tanenbaum ML, Zaharieva DP, Addala A, et al. “I was ready for it at the beginning”: parent experiences with early introduction of continuous glucose monitoring following their child’s type 1 diabetes diagnosis. Diabet Med. 2021;38:e14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freckmann G, Link M, Kamecke U, Haug C, Baumgartner B, Weitgasser R. Performance and usability of three systems for continuous glucose monitoring in direct comparison. J Diabetes Sci Technol. 2019;13(5):890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MA, Holmes-Walker DJ, Farrell K, Clark-Lucitti A. The impact of continuous glucose monitoring in youth with type I diabetes aged 15-21 [published online ahead of print May2021]. Intern Med J. doi: 10.1111/imj.15347. [DOI] [PubMed] [Google Scholar]
- 17.Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engler R, Routh TL, Lucisano JY. Adoption barriers for continuous glucose monitoring and their potential reduction with a fully implanted system: results from patient preference surveys. Clin Diabetes. 2018;36(1):50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]


