Abstract
Diabetes Technology Society hosted its annual Diabetes Technology Meeting from November 3 to November 5, 2022. Meeting topics included (1) the measurement of glucose, insulin, and ketones; (2) virtual diabetes care; (3) metrics for managing diabetes and predicting outcomes; (4) integration of continuous glucose monitor data into the electronic health record; (5) regulation of diabetes technology; (6) digital health to nudge behavior; (7) estimating carbohydrates; (8) fully automated insulin delivery systems; (9) hypoglycemia; (10) novel insulins; (11) insulin delivery; (12) on-body sensors; (13) continuous glucose monitoring; (14) diabetic foot ulcers; (15) the environmental impact of diabetes technology; and (16) spinal cord stimulation for painful diabetic neuropathy. A live demonstration of a device that can allow for the recycling of used insulin pens was also presented.
Keywords: diabetes, digital health, glucose, continuous glucose monitor, insulin, ketones
Introduction
From November 3 to November 5, 2022, Diabetes Technology Society (DTS) gathered health care professionals (HCPs), industry representatives, academicians, researchers, and US regulatory officials for the Diabetes Technology Meeting (DTM). This three-day meeting included two workshops, 12 sessions, a live demonstration, and a keynote presentation by the director of the Center for Devices and Radiological Health (CDRH) at the US Food and Drug Administration (FDA), all covering current research and emerging topics in diabetes technology. Table 1 presents the agenda for the meeting, including a list of workshop and session topics. This meeting report summarizes the key points of each presentation.
Table 1.
Agenda for the 2022 Diabetes Technology Meeting, Including a List of Workshop and Session Topics.
| Thursday, November 3, 2022: workshops |
|---|
| Workshop A: Measurement of Glucose, Insulin, and Ketones. Panel 1: Measurement of Glucose. Panel 2: Measurement of Insulin and Ketones. Workshop B: Virtual Diabetes Care. Panel 1: Technical Barriers. Panel 2: Clinical Barriers. |
| Friday, November 4, 2022: general sessions |
| Keynote Presentation: FDA and Diabetes Technology. Session 1: Metrics for Managing Diabetes and Predicting Outcomes. Session 2: Integration of Continuous Glucose Monitor Data into the Electronic Health Record. Session 3: Regulation of Diabetes Technology. Session 4: Digital Health to Nudge Behavior. Session 5: Estimating Carbohydrates. Session 6: Algorithms for Fully Automated Insulin Delivery Systems. |
| Saturday, November 5, 2022: general sessions |
| Session 7: Technology for Diagnosing Hypoglycemia. Session 8: Novel Insulins and Insulin Delivery. Session 9: Novel On-Body Sensors for Diabetes. Session 10: Advances in Continuous Glucose Monitoring Technology. Session 11: Digital Health Tools to Prevent Diabetic Foot Ulcers: Achieving Success by Understanding Defeat. Session 12: Hot Topics. Live Demonstration. |
Source: Table courtesy of Jingtong Huang.
Abbreviation: FDA, US Food and Drug Administration.
Workshop A: Measurement of Glucose, Insulin, and Ketones—Panel 1: Measurement of Glucose
Moderators
John C. Pickup, MA, DPhil, FRCPath
King’s College London School of Medicine, London, UK
Howard Wolpert, MD
Boston Medical Center, Boston, USA
Self-powered Glucose Sensing Diaper Combining a Low-Power Wireless Transmission Device and a Paper Substrate Biofuel Cell
Isao Shitanda, PhD
Tokyo University of Science, Noda-shi, Chiba, Japan
A system for rapid monitoring of urinary glucose levels in people with diabetes (PWD) and those who may suffer from postprandial hyperglycemia is required.
A self-driven biosensor for monitoring urinary glucose levels is mounted on a diaper.
The biosensor can generate electricity from urinary glucose and has a low environmental impact because it is made of paper, carbon, and enzymes.
Traceability of Continuous Glucose Monitors
Guido Freckmann, MD
Institute for Diabetes Technology Research at Ulm University, Ulm, Germany
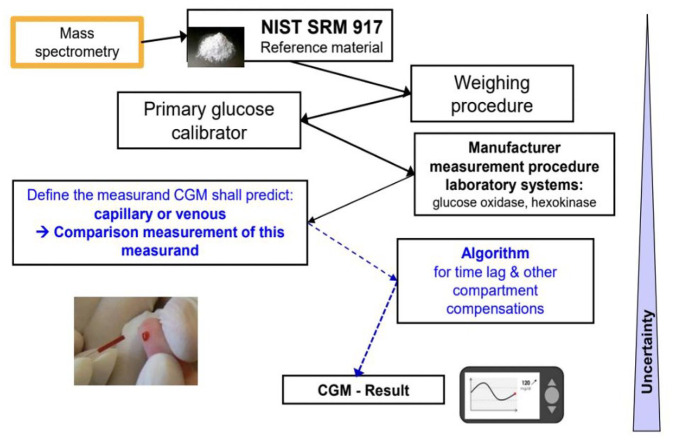
Metrological traceability is the property of a measurement result whereby the result can be related to a reference through a documented unbroken chain of calibrations, in which each link in the chain adds to measurement uncertainty.
For the establishment of a continuous glucose monitor (CGM) traceability chain, the measurand for calibration (capillary or venous blood) and the algorithm for compartment correction need to be considered because interstitial reference measurements are not currently feasible.
Traceability is a challenge for CGMs and needs to be better defined, especially when CGMs are used for clinical targets.
Advances in the Noninvasive Sensing of Glucose in People
Mark A. Arnold, PhD
The University of Iowa, Iowa City, IA, USA
The two most common approaches under development for the noninvasive measurement of glucose involve either direct analytical spectroscopy or indirect correlations associated with the outputs of optical sensors embedded within modern smartwatch technologies.
Accurate reference glucose measurements are of paramount importance for the development of viable noninvasive glucose sensing technology, which calls into question which type of reference measurement is best suited for advancing noninvasive technologies.
Multivariate calibration models are sensitive to small spectral variations, as can be illustrated with in vitro data collected for simple glucose measurements with a research-grade spectrometer.
Dynamic Interference Testing of Continuous Glucose Monitor Sensors
Andreas Pfützner, MD, PhD
Pfützner Science and Health Institute, Mainz, Germany
Interference is a major source of errors and misleading sensor readings for currently available CGM sensors.
Dynamic CGM in vitro interference testing can help to improve the safety and accuracy of currently used CGM sensors in daily life.
Dynamic in vitro interference testing offers an economically feasible way for effective interference testing of substance panels for current CGM sensors.
Continuous Glucose Monitor Accuracy—Clinical Trials versus Real-world Data
Jan S. Krouwer, PhD
Krouwer Counseling, Sherborn, MA, USA
Results from CGM clinical trials differ from real-world CGM use.
Reducing the number of adverse events will improve the health of PWD.
Achieving fewer adverse events requires analyzing adverse event data.
Novel technologies for noninvasive glucose measurement are actively being developed. A proof-of-concept biosensor for monitoring urinary glucose that could be mounted on a diaper has been tested, with data showing that the self-powered biosensor can generate electricity from urinary glucose. The biosensor further lowers environmental impact as it is made from paper. Despite the limitations of urinary glucose measurement, such as its commercial feasibility, other analytes such as ketones could potentially be monitored through applications of this platform. Other state-of-the-art noninvasive glucose measurement technologies include direct analytical spectroscopy and the use of outputs from optical sensors embedded within modern smartwatch devices. However, some important limitations of published smartwatch technologies include the accuracy of reference glucose standards and the need for the incorporation of multivariate calibration models to correct for the impact of background spectral variation on glucose measurements.
As the use of CGMs expands and this technology becomes established as the standard of care for all PWD, several important issues need to be addressed. A traceability chain has been outlined in Figure 1 with key steps listed to facilitate monitoring of the accuracy of factory-calibrated glucose sensors, analogous to the blood glucose monitor (BGM) surveillance system established by DTS. 1 In addition, there needs to be a formalized system for analyzing the adverse event data reported to the FDA. Some initial work in evaluating these data has been presented. To further prevent the occurrence of such adverse events, a novel in vitro system has been developed that offers an economic way to identify medications and other substances that can interfere with CGM sensor functioning and lead to misleading glucose readings.
Figure 1.
A traceability chain demonstrating the standardization process of continuous glucose monitoring.
Abbreviations: NIST, National Institute of Standards and Technology; SRM, standard reference material; CGM, continuous glucose monitor.
Source: Figure courtesy of Guido Freckmann.
Workshop A: Measurement of Glucose, Insulin, and Ketones—Panel 2: Measurement of Insulin and Ketones
Moderators
Peter G. Jacobs, PhD
Department of Biomedical Engineering, Oregon Health & Science University, Portland, Oregon, USA
Kirsten Nørgaard, MD, DMSc
Steno Diabetes Center Copenhagen, Herlev, Denmark
Current Technology Development to Realize Continuous Insulin Monitoring System
Koji Sode, PhD
The University of North Carolina at Chapel Hill; North Carolina State University, Chapel Hill, NC, USA
Continuous monitoring of insulin incorporates the principles of biorecognition and detection and is the most challenging task in biosensing related to diabetes.
An electrochemical insulin sensor was developed using anti-insulin antibodies as the biorecognition molecules and potentiometry principles.
The sensor covers continuous insulin concentration changes in the artificial serum within the physiological concentration range.
The Development of the Disposable and Highly Reproducible Insulin Sensing Point of Care Technology
Jeffrey T. La Belle, PhD
Grand Canyon University, Phoenix, AZ, USA
The development of a continuous insulin monitor from self-monitoring of blood insulin is made possible through the incorporation of electrochemical techniques, such as impedance spectroscopy, and the process serves as a framework for future sensor developments.
Saturation of antibody-based insulin sensors poses a challenge to insulin sensor development and promotes the development of other sensing modalities, such as the use of a redox probe.
A point-of-care insulin sensor has gone from ideation to the bench and into preclinical (animal) studies following the basic and required steps of the development and transition from self-monitoring of blood insulin to continuous insulin monitoring.
Microneedle Sensing of Insulin and Ketones
Hazhir Teymourian, PhD
AquilX Inc., San Diego, CA, USA
Microneedle-based transdermal sensors are in a prime position to be a key player in the future of medical wearables.
Microneedles synergize the advantages of dermal interstitial fluid (ISF) as a rich source of clinical indicators and painless skin pricking to allow for the collection of real-time diagnostic information.
While several continuous ketone monitor (CKM) products are expected to launch in the near future, more advances in chemical synthesis and molecular biology methods are needed to extend the success of continuous monitoring to low-concentration analytes such as insulin.
Continuous Ketone Monitoring Update
Shridhara Alva, PhD
Abbott Diabetes Care, Alameda, CA, USA
A factory-calibrated combined glucose and ketone sensor that is capable of continuously measuring glucose and ketones simultaneously will allow patients to detect rising ketone levels and intervene before diabetic ketoacidosis (DKA) develops.
Calibration of a ketone sensor by the patient is a challenge, unlike glucose sensors, because of very low ketone concentrations under normal physiological conditions.
Clinical performance of a factory-calibrated continuous ketone sensor in healthy participants on a low carbohydrate diet as well as while ingesting exogenous ketone drinks shows good correlation between blood ketones and ISF ketones.
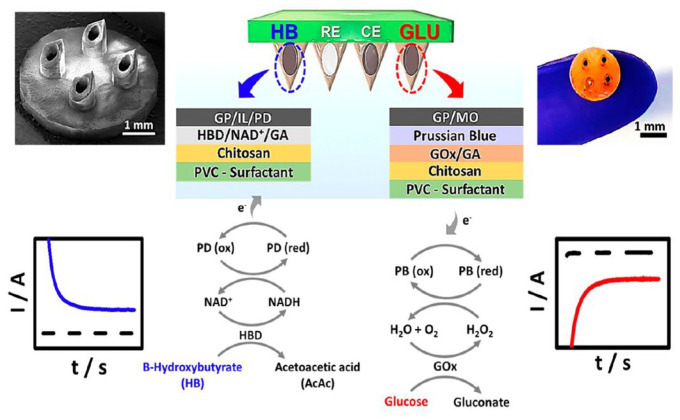
The latest research has demonstrated progress in the development of technologies for sensing insulin, ketones, and other analytes. Current technologies for sensing insulin include sensors that use aptamer-binding methodologies and affinity-based binding to field effect transistors and impose an analyte-dependent gating effect. A challenge of these types of sensors is that they often may not be used for continuous sensing and instead are better suited for one-time use as the molecule cannot be easily and quickly disengaged from the binding surface. To overcome the challenge of sensor saturation, a Nafion-coated insulin sensor for point-of-care applications has been developed. The sensor demonstrated linear behavior in vitro, and preliminary results in rats were promising. In the microneedle sensing field, the company AquilX is developing a multi-analyte sensor that uses a microneedle design for a combined ketone and glucose sensor as shown in Figure 2. Another microneedle design has been shown to be capable of simultaneously measuring glucose, lactate, and alcohol. The benefit of the microneedle design is that it is essentially painless because it only penetrates the upper layer of the skin (or the epidermis) without contacting nerves.
Figure 2.
Schematic representation of dual-marker HB/d-(+)-glucose anhydrous (GLU) sensing on microneedle sensor platform. Scanning electron microscope image of the computerized numerical control-fabricated microneedle showing a 2 × 2 array of hollow microneedles. Schematic illustration of the dual-analyte amperometric detection mechanism on multilayer modified sensors for HB (left) and GL (right). Also shown are typical amperograms obtained for HB (left) and GL (right) detection.
Abbreviations: HB, β-hydroxybutyrate; GLU, d-(+)-glucose anhydrous; RE, reference electrode; CE, counter electrode; GP, graphite powder; IL, ionic liquid; PD, phenanthroline dione; HBD, β-hydroxybutyrate dehydrogenase; GA, glutaraldehyde; PVC, polyvinyl chloride; MO, mineral oil; AcAc, acetoacetic acid; PB, Prussian blue.
Source: Reprinted with permission from Teymourian et al. 2 Copyright 2019 American Chemical Society.
Abbott presented results from a 14-day, factory-calibration clinical study in 36 humans on their ketone sensor. The sensor uses a very similar technology to their Libre sensor with only a change in the enzyme to β-hydroxybutyrate to measure ketones instead of glucose. Similar to the results reported on a single-calibration ketone sensor in Journal of Diabetes Science and Technology (JDST) recently, the factory-calibrated ketone sensor was stable over the 14 days and had 85.0% within 0.3 mM and a 0.3±0.32 mmol/L mean absolute difference from the reference. 3
Workshop B: Virtual Diabetes Care—Panel 1: Technical Barriers
Moderators
Wei-An (Andy) Lee, DO
Los Angeles County & University of Southern California Medical Center, Los Angeles, CA, USA
Andreas Pfützner, MD, PhD
Pfützner Science and Health Institute, Mainz, Germany
Bridging the Telemedicine Divide
Tejaswi Kompala, MD
Teladoc Health, Purchase, New York, USA; University of California, San Francisco, San Francisco, CA, USA
Patient preference should be considered regarding visit modality; the inclusion of multiple options for communication (telephone and video visits) is likely to increase the success of follow-up visits.
Establishing and supporting data-sharing practices at the outset, as well as encouraging the use of devices that minimize additional patient effort (cloud connected, cellularly connected), can optimize telehealth outcomes.
Virtual visits benefit from consideration of technical requirements and need not parallel in-person visit structure.
Technology Literacy
David Kerr, MBChB, DM, FRCP, FRCPE
Diabetes Technology Society, Santa Barbara, CA, USA
Technology literacy is multidimensional.
Technology illiteracy may worsen health disparities.
In the future, will artificial intelligence (AI) replace the need for diabetes education?
Secure Authentication for Diabetes Devices
Shahid N. Shah, MSc
Netspective Communications LLC, Landover, MD, USA
The CGMs and other personalized diabetes management devices should be treated as traditional, secure, Internet of things (IoT) devices like mobile phones.
Manufacturers need to consider identity management (IdM) and authentication models such as one-way, two-way, three-way, distributed, and centralized. No single model will work for all use cases, so devices need to support multiple IdM and authentication models.
Manufacturers should consider X.509 certificates, Transport Layer Security Certificates, the Hardware Security Module, the Trusted Platform Module, and the Symmetric Key Certification, or a mix of authentication standards. Buyers should not purchase devices that invent their own approaches and do not adhere to common IoT standards.
Cybersecurity of Diabetes Devices
David N. Kleidermacher, BS
Google, Mountain View, CA, USA
Unlike food and drug ingredient labels, consumers lack transparency of the security/privacy “ingredients” in digital products, including connected diabetes devices and other medical systems.
Recent work with the Institute of Electrical and Electronics Engineers (IEEE) and Connectivity Standards Alliance offers a path forward for multistakeholder, standards-based transparency of the security/privacy quality of digital products, including higher levels of assurance for safety-critical products, such as medical devices.
The unsolved challenge remaining is a lack of economic incentives for digital product developers to meet the standards and demonstrate compliance through certification and monitoring programs.
Privacy of Diabetes Devices
Randi E. Seigel, JD
Manatt, Phelps & Phillips LLP, New York, NY, USA
The laws and regulations that govern the privacy of virtual care are dependent, in part, on whether there is a covered entity collecting, using, or disclosing the individual’s health information.
Device manufacturers are not always covered entities that are subject to the Health Insurance Portability and Accountability Act (HIPAA). Similarly, HIPAA does not apply directly to many consumer-based digital health applications.
States are passing laws to fill this “privacy gap,” subjecting health care companies, including device manufacturers and digital health applications, to new consumer privacy laws.
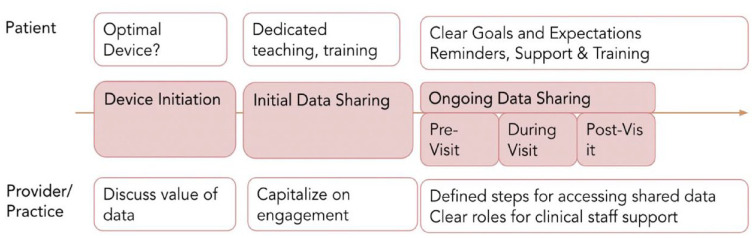
Virtual diabetes care (VDC) has significant technological barriers from the perspective of patients, application developers, and regulators. For PWD in vulnerable populations, digital inclusion, defined as activities necessary to ensure equitable access to and use of information and communication technologies, can be a “super” Social Determinants of Health (SDOH). 4
Bridging this digital divide should include ensuring equitable digital access, promoting efficient data sharing, and reimagining VDC visits, with a focus on serving the needs of patients. This process is outlined in Figure 3. Furthermore, poor technological literacy, in such domains as digital literacy and numeracy skills, is independently associated with poor glycemic control. 5 Technology disruptors may narrow this divide via AI-based real-time behavioral nudges shown in the framework for Just-in-Time Adaptive Interventions. 6
Figure 3.
Defined process to support early data sharing with patients to combat clinical inertia.
Source: Figure courtesy of Tejaswi Kompala.
For application developers, secure authentication and cybersecurity risk measurement standards are major obstacles. Niche authentication standards for medical devices exist despite commercially available industrial-grade IoT device standards. Moreover, there is a lack of transparent objective risk measurements and consumer communication regarding cybersecurity. Progress includes (1) IEEE 2621 adoption and update with the anticipated FDA recognition in 2023, 7 (2) IoT security/privacy labeling with quick response (QR) codes linked to real-time security states, and (3) better mobile applications disclosures for data collection and security.
HIPAA applies only to covered entities, which includes only providers who engage in certain electronic transactions; thus, HIPAA does not necessarily protect all data exchanged when providing virtual diabetic care (VDC). Some states, such as California, are attempting to close these privacy gaps; however, only a few other states are following California’s lead. So far national privacy legislation has not garnered national support, although the American Data Privacy and Protection Act has gone further than other previously introduced legislation.
Despite VDC technological challenges, there are promising solutions on the horizon offering improved digital inclusion, consumer technology literacy, secure authentication, cybersecurity standards, and legal protection for the patient’s privacy of their VDC information.
Workshop B: Virtual Diabetes Care—Panel 2: Clinical Barriers
Moderators
Andjela Drincic, MD
University of Nebraska Medical Center, Omaha, NE, USA
Rosemary G. Oshinsky, RN, CDE, MSN
New Life New U Holistic Diabetes Care, Bethesda, MD, USA
Delivering Diabetes Care in a Direct-to-Consumer Model
Erich S. Huang, MD, PhD
Verily Life Sciences, South San Francisco, CA, USA; Duke University School of Medicine, Durham, NC, USA
The question is as much about “Good Data” as “Big Data.” “We.” We should focus on building an ecosystem that supports high-quality data.
Data Cascades, an important Google Research paper 8 (https://research.google/pubs/pub49953/) talks about “an overall lack of recognition for the invisible, arduous, and taken-for-granted data work in AI [that has] led to poor data practices.” Talks about “an overall lack of recognition for the invisible, arduous, and taken-for-granted data work in AI [that has] led to poor data practices.”
To serve our diabetes patients best, we need to focus on the fundamentals of good data infrastructure and interoperability, and then good AI follows.
Big Data and Artificial Intelligence for Adaptive and Individualized Diabetes Management
Pavitra Krishnaswamy, PhD
Institute for Infocomm Research, Agency for Science Technology and Research (A*STAR), Singapore
Analysis of multimodal data from a tele-diabetes program highlights the characteristics of patients who could benefit from more adaptive approaches.
Novel technologies, such as interactive dialogue agents, can provide a resource-efficient means to inform and aid practitioners in adapting tele-support interventions for individual needs.
The design and evaluation of such technologies require an agile and participatory process that is tightly coupled with workflow considerations.
The Evolving Role of Virtual Care During the Pandemic
Ateev Mehrotra, MD, MPH
Harvard Medical School, Boston, MA, USA
Telemedicine uptake has substantially waned during the pandemic.
In contrast, there has been a rapid growth of remote patient monitoring (RPM) for diabetes.
There remains substantial uncertainty about the future of telemedicine reimbursement.
Delivering Telehealth to a County Hospital Population
Wei-An (Andy) Lee, DO
Los Angeles County & University of Southern California Medical Center, Los Angeles, CA, USA
Lack of health care system modernization and integration are major impediments to the streamlined delivery of diabetes care leading to increased, thereby increasing the overall cost and reduced quality of care experienced by low-income PWD.
Current suboptimal “patient engagement” strategies for reaching low-income diabetes patients that foster health equity and address SDOH should be recognized as major barriers that require significant attention and resourcing.
Preventing clinician burnout through reducing administrative burden and cognitive workload should be a perennial executive aim in all health care centers with clinicians managing diabetes.
The Payer Perspective on Telehealth
Jordan Silberman, MD, PhD
Elevance Health (formerly Anthem, Inc.), Palo Alto, CA, USA
Important barriers remain to be overcome for the deployment of digital health technologies to improve diabetes management. Key barriers include a lack of consensus around digital health evidence standards, inadequate adaptation of rigorous evidence assessment frameworks for digital health, and limited development of digital health interventions to meet the needs of underserved patients.
When developing an evidence-based strategy to drive the adoption of digital health technologies for PWD, details matter.
Through increased stakeholder collaboration around evidence standards, enhanced frameworks for digital health evidence assessment, and greater consideration of underserved populations, the digital health community may be able to provide greater value care to PWD.
Adoption of the direct-to-consumer model (DTC) in diabetes care offers an opportunity to restore the intimacy of individual patient care. This is achieved through enhancing access to care (care at any place and any time) and personalizing experiences through health care coaching. By using digital health, DTC facilitates instant access to large-end complex biometric data including CGM data needed to provide quality personalized care. The DTC is a privilege as we would be able to solicit health data directly from patients and the devices they use to help manage their disease. We should have our digital health platforms provide highly interoperable and rich data because we are not encumbered by the complexity of health information technology (IT).
A challenge to improving the quality of digital health, which relies on quality machine learning (ML) and AI-generated solutions to patient care, is poor data quality. If we do not have data excellence, we will not have clinical excellence. Fast Healthcare Interoperability Resource (FHIR) aims to address this problem. FHIR is an interoperability standard for the electronic exchange of health care information, providing a framework and standard for clinical data sharing, integration, and retrieval. By improving health information exchange, FHIR will facilitate the enhancement of AI and ML functions and augment clinicians’ capabilities to bring back the intimacy of individual patient care.
The concepts of Big Data analysis and of Individualization are usually considered to be at odds with each other. There was a presentation about an innovative approach that uses a combination of big data, behavioral science, and interactive language technology to enable the enhancement and individualization of virtual diabetes care models. The need for such an approach was born out of recognition that, while telemedicine has overall been shown to have a positive impact on clinical outcomes in diabetes, there is an unmet need to understand the characteristics of responders versus nonresponders and how to overcome gaps to better address individual patient needs. Furthermore, bridging these gaps requires systematic efforts to design technologies and workflows aimed at optimizing and personalizing telehealth delivery. To achieve this degree of personalization, the team of researchers at the Institute for Infocomm Research, A*STAR, Singapore have collated and processed a multimodal dataset comprising structured medical records alongside transcripts of telecare-patient conversations for a sizeable cohort of patients enrolled in the Telediabetes Program at Changi General Hospital during 2013-2019. The Program intervention consisted of nurse-led tele-support and self-management education delivered via phone. Outcomes included hemoglobin A1c, blood pressure, and appointment adherence. In addition, medical, psychosocial, and financial problems were tracked.
The research goal was to stratify and understand patient groups based on the hemoglobin A1c trajectories for targeted care and intervention. Sequence analysis of the hemoglobin A1c trajectories was performed, identifying clusters of patients with distinct health care outcomes. Analysis of the content of calls for patients from distinct clusters revealed specific characteristics and challenges for subgroups with limited hemoglobin A1c improvements. The results generated from such analyses are being used to further personalize telehealth interventions to meet individual patient needs, develop technology solutions to increase patient engagement, and optimize health care delivery via workflow changes. In particular, interactive dialogue technologies are being evaluated as enablers for a more personalized yet resource-efficient tele-support approach.
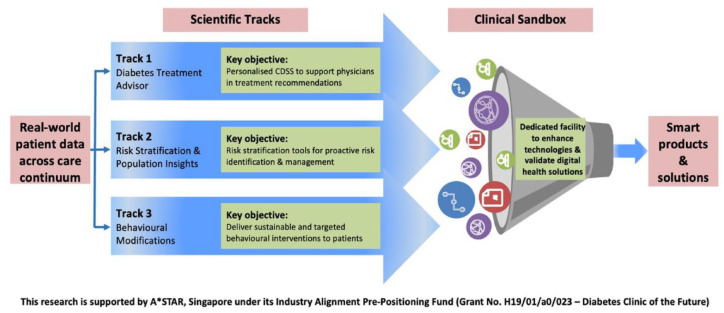
Finally, she shared on larger diabetes technology research and experimental development (R&D) efforts in Singapore. As shown in Figure 4, the Diabetes Clinic of the Future is a flagship program developed with the goal to move away from population-based care to individualized care and from episodic care to adaptive care. Beyond R&D initiatives, the Program also features a sandbox for working with industry and multidisciplinary stakeholders on new VDC models and solutions.
Figure 4.
Overview of the diabetes clinic of the future program and platforms.
Abbreviations: A*STAR, Agency for Science Technology and Research; CDSS, clinical decision support system.
Source: Figure courtesy of A*STAR and SingHealth, Singapore.
At the start of the pandemic, policymakers temporarily changed many policies to facilitate telemedicine use. Patients were able to access care in their homes (without limitations to rural residents), out-of-pocket costs were waived for patients, licensure requirements were waived for providers, and the types of providers and services available to patients were expanded. However, since its initial spike in March/April of 2020, the use of telemedicine, defined as synchronous video and phone visits for medical purposes, has been in steady decline in the United States and much of the world. 9 At the same time, there has been growth of other services including e-consults, RPM, and requests for asynchronous care via patient portal messaging. The following four key issues in clinical and policy debate are contributing to the uncertain future of telehealth: (1) impact on health care spending, (2) impact of telehealth on quality, (3) impact on health care disparities, and (4) limitations of state licensure requirements for clinicians. Regarding impact on health care spending, improved convenience and accessibility of telemedicine and telehealth have led to increased use of these services, raising concerns that improvement in care may come at the risk of increased spending. Regarding quality, concern remains that an increased number of visits via telehealth may not translate into improved health outcomes and that telemedicine may be offering low-value care and excessive spending. Current research has shown the feasibility of telemedicine and provided reassurance that the quality of care provided via telemedicine is comparable to standard care. Part of what is currently lacking and necessary for policymakers are data showing that the addition of telemedicine improves outcomes. Regarding health care disparities, a concern has been raised, with some supporting data, that telehealth is preferentially used by higher-income patients who already have access to care and good disease control. Finally, regarding limitations of state licensure requirements for clinicians, temporary regulatory changes allowing providers to provide care across state lines have now expired. Clinicians are discouraged from obtaining multiple licenses to practice telemedicine across state lines because of the time cost and administrative hassle. Multiple options for both federal and state-based reform exist to address this issue.
Three major sets of barriers have been encountered during pandemic-related telehealth adoption within the Los Angeles County hospital system. Los Angeles County health is the second largest health care municipal network in the United States representing four acute care hospitals a county-wide network of ambulatory care centers, serving patients of vastly different socioeconomic backgrounds. Implementing enterprise-level modern technology within such a diverse health care ecosystem has been very challenging. Integration of health care technology into a health care system faces a unique set of challenges related to the presence of fragmented internal electronic systems serving separate operational needs, including from billing services, security, and electronic health care records to name a few. The second barrier is related to patient engagement. The need for rapid deployment of telehealth during the pandemic highlighted the need but the unfortunate digital divide that marginalized patients with the Medicaid population. Digital barriers such as fiber optic high-speed internet availability for certain counties, defined as digital redlining, exist in many marginalized communities in Los Angeles County. There is a direct relationship between socioeconomic disparity and the availability of broadband decreases, limiting patient adoption of modern health care solutions evidenced by low patient use of video visits despite their availability. The third major barrier to the adoption of telehealth is related to workforce wellness. The burnout noticed in clinicians was a result of increased workload, time pressure, and administrative burden brought about by technology. Health care professionals themselves had a diverse degree of health IT skills. The chaotic environment that was created by rapid implementation resulted in increased stress and the perception that technology had a negative impact on the patient-physician relationship.
Many barriers exist to the adoption of digital health technologies. The focus was on digital health interventions delivered through mobile medical apps and wearables, designed to drive health behavior changes and thereby improve health outcomes. These types of interventions target modifications of behaviors such as self-monitoring of blood glucose (SMBG), medication adherence, nutrition, and physical activity. Assessing their true clinical value has been difficult due to a lack of consensus around digital health evidence standards and an inadequate adoption of a rigorous evidence assessment framework for digital health. To develop consensus, we need an interdisciplinary team approach and collaboration between various digital health tool developers and end users. Assessment across clinical and nonclinical domains is needed, including user experience, data privacy and security, capacity for integration with existing technology, and appropriateness across diverse populations. Public reporting of software changes that have been implemented after a digital health information intervention was trialed is also necessary. Last, to enhance the adaptation of these technologies for underserved patient groups, incorporating perspectives from these patients from day 1 in the development of digital health interventions is needed.
Keynote Presentation: FDA and Diabetes Technology
Jeffrey Shuren, MD, JD
Center for Devices and Radiological Health, United States Food and Drug Administration, Silver Spring, Maryland, USA
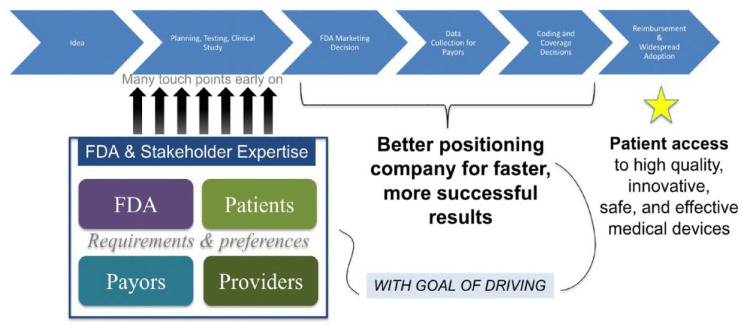
The FDA uses a patient-centered approach in its oversight of medical devices. It has approved many diabetes-related technologies, such as upgraded insulin pumps and their accessories, smart insulin pens, and software solutions that use CGMs for calculating insulin doses. The FDA will also support anticipated advances in the diabetes technology area such as broader access to CGMs, improved interoperability, use of automated insulin delivery (AID) systems in patients with type 2 diabetes (T2D), and fully closed-loop systems. The FDA is also advancing regulatory science and the development of tools to improve and more efficiently evaluate medical device performance, including AI-ML tools and the use of simulation data to enable innovation. Development of national-international consensus standards, in which the FDA is actively involved, will be transitioning its Accreditation Scheme for Conformity Assessment pilot to a full-fledged program. Several guidance and policies have been developed to advance innovation while providing appropriate patient safeguards. Examples include guidance for BGM systems used at point-of-care and over-the-counter, which are distinct for professional health care settings and lay users, guidance that provides recommendations about the feasibility clinical studies for medical devices intended to therapeutically improve glycemic control in patients with T2D, guidance for clinical decision support software, which provides clarity as to when these software products are not a medical device, and guidance for device cybersecurity. Finally, FDA is dedicated to ensuring that PWD should have access to their data, as well as access to health care, which will advance health equity. To foster responsible and high-quality digital health innovation, FDA has developed the Digital Health Center of Excellence as well as the Total Product Life Cycle Advisory Program, which includes a team of experts who provide dynamic strategic advice in real time to developers. As shown in Figure 5, the FDA also offers to developers the option to connect with patients, payers, and providers for faster and more successful results.
Figure 5.
Early, frequent, and coordinated stakeholder interaction speeds up patient access.
Abbreviation: FDA, US Food and Drug Administration.
Source: Figure courtesy of Jeffrey Shuren.
Session 1: Metrics for Managing Diabetes and Predicting Outcomes
Moderators
Viswanathan Mohan, MD, PhD, DSc
Dr. Mohan’s Diabetes Specialities Center & Madras Diabetes Research Foundation, Chennai, India
Jennifer L. Sherr, MD, PhD
Yale University, New Haven, CT, USA
Advances in Continuous Glucose Monitor Data Science
Boris Kovatchev, PhD
University of Virginia, Charlottesville, VA, USA
Artificial intelligence is rapidly entering the diabetes management field and is particularly relevant to decision support systems.
Machine learning classification of daily CGM profiles allows for a new clinical interpretation of glycemic volatility, as it progresses in time.
Most CGM-based metrics are highly inter-correlated, and virtually all metrics proposed to date are explained by only two essential patterns: exposure to hyperglycemia and risk for hypoglycemia.
The Glycemia Risk Index: A New Composite Metric for Assessing the Quality of Glycemia of a Continuous Glucose Monitor
David Klonoff, MD, FACP, FRCP (Edin), Fellow AIMBE
Mills-Peninsula Medical Center, San Mateo, CA, USA
The Glycemia Risk Index (GRI) is a composite metric of the seven most important features of an ambulatory glucose profile (AGP) report, weighted according to the opinions of 330 experienced clinicians.
The GRI weights time out-of-range hypoglycemia as more important than time out-of-range hyperglycemia and weights extremely abnormal values as more important than mildly abnormal values.
The GRI provides a single score that addresses how well a patient is doing and what should be done if the patient is not doing well.
Statistical Basis for the Glycemia Risk Index
Michael A. Kohn, MD, MPP
University of California, San Francisco, San Francisco, CA, USA
Principal component analysis of 225 fourteen-day CGM tracings revealed that two principal dimensions, one related to hyperglycemia and one related to hypoglycemia, explain almost 90% of the variability between tracings.
Of the seven AGP metrics, the four with the greatest explanatory power were %VLow (<54 mg/dL), %Low (54-70 mg/dL), %High (180-250 mg/dL), and %VHigh (>250 mg/dL).
Weights for these four metrics in the GRI were based on how clinicians weighted them in their rankings of the CGM tracings.
Continuous Glucose Monitor Data in People Without Diabetes
Viral N. Shah, MD
Barbara Davis Center for Diabetes, University of Colorado, Aurora, CO, USA
The normal reference range for sensor glucose in people without diabetes is 70 to 140 mg/dL with a mean glucose of 99 mg/dL. In this population, changes in glucose during and after exercise are minimal.
After meals, mean peak postprandial glucose increases to 130 ± 10 mg/dL from 93 ± 10 mg/dL prior to meals.
The glucose management indicator (GMI), a regression formula to estimate hemoglobin A1c from sensor glucose, should not be used in people without diabetes
With the wealth of data generated, CGM has altered the landscape of diabetes management. Thus, methods to assist with data interpretation have been of critical importance including practical metrics that can be implemented easily in clinical practice. Recently, application of glucose monitoring technology has been applied to people who do not have diabetes. The goal of the session was to provide insight into how CGM data will be interpreted in the future.
The CGM data have provided a nuanced understanding of the day-to-day lived experience of PWD. Yet, clinicians are faced with the onus of determining methods to review the vast amount of data generated and find actionable patterns on which to optimize therapy. In recent years, decision support tools have been developed using an AI approach to see whether algorithmic adjustments are feasible to optimize insulin doses. The ADVICE 4-U trial was a randomized controlled trial (RCT) involving 108 participants which showed noninferiority of the DreaMed Advisor Pro 2 automated artificial intelligence–based decision support system as compared with clinician-driven dose optimization. 10 An in-silico analysis of 52 weeks of data has shown that another decision support tool has 68% agreement with recommendations made by endocrinologists and allows for early identification of risky insulin regimens. 11
Furthermore, the use of Data Science methods offers the possibility for pattern recognition by which hidden structures of CGM performance are identified. 12 As numerous studies have created large data sets of CGM data in participants with both type 1 diabetes (T1D) and T2D, a pattern recognition approach using 200 000 daily CGM profiles in training, validation, and testing data sets identified 35 clinically significant clusters (CSCs). Following training, validation, and testing in independent data, these CSCs were fixed and do not need further adjustments for clinical applications. Each person’s CGM data fall within one CSC each day. The trajectory of a person across the CSCs over time indicates any daily changes in the degree of glycemic volatility. The number of unique CSCs visited over time differentiates states of health, types of diabetes, and treatment modalities. For example, healthy individuals may visit four CSCs, while the T2D population may visit eight CSCs on average, and those with T1D may visit 14 CSCs on average. It is also up to 14 clusters. Yet, it is critical to note that the number of CSCs visited will also depend on the treatment modality used. For example, those on automated insulin delivery (AID)/closed-loop systems may visit eight CSCs, while those treated with conventional pump therapy may visit 12 clusters, and those on multiple daily injections (MDIs) may visit 13 or 14 clusters on average. Thus, the clinical objective of glycemic optimization is partitioned into daily treatment adjustments aiming to reduce transitions from stable to volatile CSCs, thereby reducing the number of clusters visited and reducing overall glucose volatility.
Recognizing the potential inaccuracies of CGM data from events like compression lows and prediction methods allows for the detection of upcoming events through forecasting. The ML approaches have allowed for the possibility of predicting when compression lows will occur, which may impact sensor performance. Finally, classification metrics revealed that the geometry of diabetes optimization depends on two categories of variables, namely, metrics of hyperglycemia exposure (which include mean glucose, % time >180 mg/dL, and % time >250 mg/dL) as well as the risk of hypoglycemia (which is based on % time <70 mg/dL, % time <54 mg/dL, and coefficient of variation). 13 These two metrics together can accurately predict ~90% of the variance in training and data sets. 13
While the metrics described above are of great interest, it is important to consider how clinicians think about CGM data. Working on the premise that an item’s quality has many dimensions which can be captured by listing their performances individually or by using a composite score to summarize their features, Dr David Klonoff led an international group of 90 experts in the creation of the GRI. 14 It was recognized among this group that seven metrics are considered clinically important by clinicians, which include the time in range (TIR) (<54 mg/dL, <70 mg/dL, 70-180 mg/dL, >180 mg/dL, >250 mg/dL), mean glucose, and coefficient of variation as a measure of glycemic variability. To create the composite metric, four types of PWD generating CGM tracings were identified, which included people with (1) T1D on MDIs, (2) T2D on open-loop pump therapy, (3) T1D on closed-loop systems, and (4) T2D on MDIs. Using a total of 225 standardized tracings, 330 experienced clinicians were each asked to review 15 tracings, such that each tracing was reviewed by 22 people. Clinicians were asked to score each tracing on a scale from 0 to 100, where higher scores indicated more intense efforts to optimize therapy. For TIRs, it was clear that clinicians viewed time out of ranges depending on whether it was level 1 or level 2 hypoglycemia or hyperglycemia. (Level 1 [mild] hypoglycemia: glucose is <70 mg/dL but ≥54 mg/dL. Level 2 [moderate] hypoglycemia: glucose is <54 mg/dL. Level 1 [mild] hyperglycemia: glucose is >180 mg/dL but ≤250 mg/dL. Level 2 [moderate] hyperglycemia: glucose is >250 mg/dL). Thus, the GRI weights the four “out-of-range” metrics differently to reflect their clinical importance. The GRI allows multiple tracings to be visualized on a single figure to look at change in glucose control over time. This facilitates triage and risk stratification on a population scale as shown in Figure 6. Indeed, a recent report on the use of an AID system investigating participants with significant hypoglycemia at baseline noted that AID system use led to only a 5% increase in TIR; yet, the GRI provided a more nuanced view showing 13% improvement as it captured the 50% reduction in hypoglycemia achieved with system use. 15 A GRI calculator is now readily available from DTS at the following website (https://www.diabetestechnology.org/gri/). As a single number weighted according to risk for hypoglycemia and hyperglycemia based on the opinion of experienced clinicians, the GRI has the potential to become established as a useful metric for assessing and treating individuals, following the quality of glycemia in populations, and predicting long-term complications.
Figure 6.
(Left) A GRI grid showing the hyperglycemia component versus the hypoglycemia component for all 225 CGM tracings. The results for each of the four categories of patients are shown with different symbols. We highlighted individual data points for the CGM tracings from two persons (designated P1 and P2) with T1D receiving multiple daily insulin injections. (Right) The GRI over time for five different time periods. Legend: Between times 1 and 2, the TIR worsened by decreasing from 46% to 40%. However, the GRI improved from 90 to 75. For time 1, the hypoglycemia/hyperglycemia components are 16%/26%. For time 2, they are 6%/35%. Adjustments to reduce hypoglycemia could increase hyperglycemia.
Abbreviations: CGM, continuous glucose monitor); T1D, type 1 diabetes; TIR, time in range (70-180 mg/dL; 3.9-10.0 mmol/L; GRI, Glycemia Risk Index; MDI, multiple daily insulin injections; Pump, insulin infusion pump; HCL, hybrid closed loop; T2D, type 2 diabetes; Hyper, Hyperglycemia Component; Hypo, Hypoglycemia Component; VHigh, very-high-glucose hyperglycemia (>250 mg/dL; >13.9 mmol/L) (level 2 hyperglycemia); VLow, very-low-glucose hypoglycemia (180-250 mg/dL; >10.0-13.9 mmol/L) (level 1 hyperglycemia).
Source: Figure reproduced from Klonoff et al. 14
The next presentation provided the statistical underpinnings for the creation of the GRI. Of the seven metrics described above, the greatest explanatory power was described by % time <54 mg/dL, % time <70 mg/dL, % time >180 mg/dL, and % time >250 mg/dL. With these four metrics, one could then identify the time in the target range of 70 to 180 mg/dL. The principal component analysis identified two groups of metrics: those that cluster as hyperglycemia exposure (TIR, mean glucose, % time 180-250 mg/dL and % time >250 mg/dL) and those that cluster as hypoglycemia exposure (coefficient of variation, % time 54-70 mg<70 mg/dL and % time < 54 mg/dL). As both time in the target range and mean glucose correlate strongly with % time >250 mg/dL, these two variables were removed from the final model. The final equation to determine GRI is (3.0 × Very Low) + (2.5 × Low) + (1.6 × Very High) + (0.8 × High) or stated differently; the equation is as follows:
Thus, this new metric generates a single-number summary of the quality of glycemia. The plot of GRI is viewable on a two-dimensional grid for the hypoglycemia and hyperglycemia components, providing a useful metric for clinical practice.
To assess the glycemic profiles in healthy individuals, a multicenter clinical trial of 153 individuals aged seven to 80 years was conducted using the Dexcom G6 sensor in a blinded fashion for more than 10 days’ time. 16 Mean glucose was on average 99 mg/dL, with only those above 60 years of age found to have a slightly higher mean glucose of 104 mg/dL. The exquisite function of the pancreas demonstrated as a TIR of 70 to 140 mg/dL was noted to be 96% of the time for the overall cohort. 16 Furthermore, minimal change in glucose was noted even when examining periods of exercise. 17 When analyzing data on meals, mean glucose increased from 93 ± 10 mg/dL prior to the meal to 130 ± 13 mg/dL as the peak postprandial glucose. 17 As CGM is increasingly being applied in people without diabetes, a cautionary tale was provided on the use of the GMI, which is an estimated hemoglobin A1c from CGM data. 18 Importantly, GMI was derived from trials in those with diabetes using a linear regression method. What happens in someone who has normal glucose levels? There is no correlation between mean glucose and hemoglobin A1c in a healthy population and GMI overestimates hemoglobin A1c in those without diabetes. Thus, GMI should be interpreted with caution and not used in those who do not have diabetes, and the same may apply for those with very targeted glucose levels (defined as hemoglobin A1c <6.5%), wherein the GMI and hemoglobin A1c levels may diverge significantly.
In short, the session highlighted the analytical tools and advanced data science available due to CGM data. The session covered how the GRI, as a new composite metric, was developed and can be applied to clinical practice. It also covered the normative data available from the use of CGMs in those without diabetes. As CGMs are being more widely used in those without diabetes, this sets benchmarks and reference values for PWD to strive toward with future therapies created for those with diabetes to strive for as well as provides reference values as CGM is being more widely used in those who do not have diabetes. Undoubtedly, the learnings discussed in this session demonstrate we are emerging from our nascent phases of CGM data interpretation, and the future has much in store.
Session 2: Integration of Continuous Glucose Monitor Data into the Electronic Health Record
Moderator
Richard M. Bergenstal, MD
International Diabetes Center, HealthPartners Institute, Minneapolis, MN, USA
Priya Prahalad, MD, PhD
Stanford University, Stanford, CA, USA
The Stenopool Database
Christian Selmer, MD, PhD
Steno Diabetes Center Copenhagen, Herlev, Denmark
One platform for all diabetes device data is preferred by most diabetes clinics, and this can be developed and tailored for local needs based on open-source software.
Research and quality work can be performed based on the diabetes device data by cross-linking to other clinical registries.
There are numerous perspectives for decision support, user engagement, and personalized medicine when all diabetes device data are available in a single platform.
New Tools for Data Management
Mark A. Clements, MD, PhD, CPI, FAAP
Children’s Mercy Hospitals & Clinics, Kansas City, MO, USA
Managing “Big Data” is essential for the implementation of risk-based population health management approaches; such approaches improve clinical outcomes for youth and adults with diabetes.
The Diabetes Data Dock is a new kind of diabetes center-hosted cloud software that integrates data from the electronic health record (EHR), diabetes self-management devices, and other sources. This enables AI/ML, geocoding, population health dashboards, quality improvement (QI) tracking, RPM, intervention selection, and other population health functions.
The Diabetes Data Dock enables a new ecosystem of digital diabetes care characterized by prediction/prevention, precision medicine, and cost-efficient care.
Integration of Continuous Glucose Monitor Data into the Electronic Health Record (iCoDE): A New Standard for Integration of CGM DI Into the EHR
Juan Espinoza, MD, FAAP
Children’s Hospital Los Angeles, University of Southern California, Los Angeles, CA, USA
The CGM-EHR integration is critical for optimizing clinical workflows; making CGM data visible, measurable, and actionable can ultimately improve patient outcomes.
The 2022 iCoDE Report provides standards for CGM-EHR integration and recommendations for a comprehensive and practical guide to help stakeholders overcome barriers to integration through a standards-based approach.
The goal of iCoDE is to lower the barriers to integration and to democratize data access, benefiting all patients and health care organizations.
Management and integration of data from diabetes devices into the EHR is challenging for diabetes care teams. Steno Diabetes Center in Copenhagen, Denmark has adapted the open-source Tidepool platform to create Stenopool, a platform to facilitate the uploading of diabetes device data both in the clinic and from home. Device data are shared via a report that uploaded into the Epic EHR and are used by clinicians during routine clinical care. The tool will be the basis for population health management dashboards, QI, and personalized medicine. Another implementation of data management is the Rising Tide Alliance developed at Children’s Mercy Hospital in Kansas City, Missouri. To connect patient device data to medical record data, the Diabetes Data Dock has been developed to act as a piece of “middleware” to help connect patient-reported outcomes and experience data. Access to the data in one location can help clinical care teams develop population health management tools to facilitate RPM to identify individuals who would benefit from intervention and to help forecast risk. Despite these individual successes, there is no standard for the integration of CGM data into the EHR.
Dr Juan Espinoza from Children’s Hospital Los Angeles and Dr David Klonoff from DTS assembled a steering committee of more than 130 experts from 60 organizations in health care, manufacturing, IT, regulation, and policy create the 2022 iCoDE Report: CGM-EHR Integration Standards and Recommendations. The document contains 54 recommendations for facilitating the integration of CGM data into the EHR, along with extensive background, technical, and implementation information. The integration of patient data and device data promotes RPM and personalized medicine, both of which are important for improving the outcomes of PWD. The pipeline of CGM data delivery from patient to clinician and opportunities for standards development along the path are shown in Figure 7. Individual groups have already made great strides in integrating device data together; however, creating standards such as iCoDE can lead to more rapid innovation.
Figure 7.
Opportunities to adopt, adapt, or develop standards and best practices in the CGM data pipeline.
Abbreviations: CCD, Continuity of Care Documents; CDA, Clinical Document Architecture; CGM, continuous glucose monitor; CPT, Current Procedural Terminology; EHR, electronic health record; EMPI, Enterprise Master Patient Index; FHIR, Fast Healthcare Interoperability Resources; HIPAA, Health Insurance Portability and Accountability Act; HL7, Health Level 7; ICD-10, International Classification of Diseases 10th Revision; IEEE, Institute of Electrical and Electronics Engineers; LOINC, Logical Observation Identifiers Names and Codes; NIST CSF, National Institute of Standards and Technology Cybersecurity Framework; NPI, National Provider Identifier; OMOP, Observational Medical Outcomes Partnership; SMART, Substitutable Medical Applications, Reusable Technologies; SNOMED, Systemized Nomenclature of Medicine; SOC2, System and Organization Controls type 2 – Trust Services Criteria; UDI, Unique Device Identifier.
Source: Figure reproduced from Xu et al. 19
Session 3: Regulation of Diabetes Technology
Moderators
Alexander Fleming, MD
Kinexum, Harpers Ferry, WV, USA
Alberto Gutierrez, PhD
NDA Partners LLC, Bethesda, MD, USA
Regulation of Glucose Monitoring Systems
Yiduo Wu, PhD
United States Food and Drug Administration, Silver Spring, MD, USA
The CGM manufacturers may pursue the Class II integrated CGM (iCGM) pathway if their device meets all special controls in 862.1355.
The new Lancet Rules require that lancets used in new BGM submissions be 510(k) cleared.
Premarket approval (PMA) and De Novo can be potential options for noninvasive/minimally invasive glucose devices.
Regulation of Human-in-the-System Features of an Automated Insulin Delivery System
Zane Arp, PhD
United States Food and Drug Administration, Silver Spring, MD, USA
Fully interoperable systems, whether for diabetes control or other therapies, are complex systems that require adequate testing methodologies and tools to demonstrate reasonable assurance of safety and effectiveness.
Physiological models have the potential to be used as surrogates, mimicking either sensor outputs or therapeutic responses as supplements and alternatives to animal and patient testing.
The Office of Science and Engineering Laboratories is currently investigating regulatory science tools and frameworks that can be used to help support the advancement of interoperable systems for all medical device developers.
Trends in Digital Health Regulation
Bradley M. Thompson, JD, MBA, MADS
Epstein Becker Green P.C., Washington, D.C., USA
Now that the FDA’s precertification program has concluded, industry needs to think strategically about how it will get new digital products approved in the future.
There are significant implications for previously unregulated products because of the September 2022 changes in the FDA’s guidance documents on clinical decision support and on software generally, including mobile medical apps.
Diabetes-related products are still the subject of numerous medical device reports and recalls, and these data make it hard for industry to seek a less burdensome, more practical regulatory touch.
Regulation of Digital Health Tools
Brendan O’Leary, BSME
United States Food and Drug Administration, Silver Spring, MD, USA
CDRH Digital Health Center of Excellence was established to help develop, deploy, and use technologies responsibly in a patient-centric manner, allowing digital health to reach people who are underserved by the health care system today.
The FDA concluded the software precertification pilot program aimed at identifying regulatory approaches to software that can better promote and protect public health.
Patient-generated health data collected from digital health technologies improve our understanding of health and health interventions in the context of daily life.
In what has become a distinctive and high-value session of the Meeting, FDA officials and a regulatory expert provided a wide range of important information and updates about the regulation of medical devices. The Diabetes Diagnostics and Devices Branch is responsible for the regulation of insulin pumps, glucose monitoring, and other diabetes-related technology including CGMs. Historically, CGMs are class III medical devices that require a PMA submission to the FDA to be marketed. Earlier generations of CGMs were approved for detecting trends and tracking glucose patterns but not for replacing finger-stick BGMs to make diabetes treatment decisions. This is known as the adjunctive use of the CGM. Since 2016, the FDA has approved several PMAs for CGM devices that can replace BGMs to make treatment decisions. This new indication is called the nonadjunctive use of the CGM. With the rapid advances of CGM technology in PWD and with a better understanding of the benefit and risk profile from information gathered through our total product lifecycle approach, the FDA believes that a lower regulatory classification with proper special control would be sufficient to ensure the safety and effectiveness of CGMs for its intended use. Therefore, in 2018, the FDA created the class II integrated CGM (iCGM) regulation with various details, special controls, performance requirements, and interoperability that allows high-performance decisions to be cleared through the 510(k) pathway instead of with a PMA.
The Division of Biomedical Physics in the Office of Science and Engineering Laboratories at CDRH focuses on developing regulatory science tools. In the insulin pump space, AID systems are closed-loop systems at the forefront of interoperable systems among other potentially applicable uses, such as in ventilators, oxygen therapy, and anesthesia. There is some effort to release standards and guidance to help with the development of interoperable systems as such; however, safety and effectiveness remain as the major concerns. Many potential issues can occur at various points within a closed-loop system, and it is important to implement controls throughout the development of these systems. In addition, physiological models are difficult to design as they must be simple enough for the design of control but accurate enough to capture the physiology of interest, which is challenging especially in complex systems. Physiological systems may also have mechanisms that are unknown and difficult to model. Interoperable systems are still evolving in the space of AID. After developing tools to help lower the barrier to entry into this space, the ability to demonstrate the safety and effectiveness of these interoperable devices will improve.
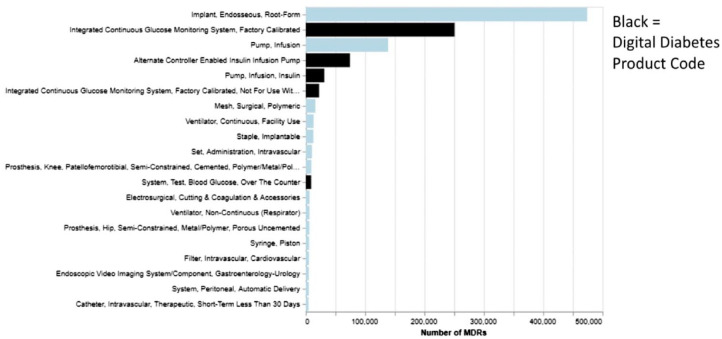
From a legal perspective, a wide range of FDA regulatory issues was discussed in relation to software, medical devices, pharmaceutical companies, and particularly, the conclusion of the pilot precertification program. A quantitative review of diabetes-related product codes showed that diabetes products, compared with the average product, generally take substantially longer than average to review in the 510(k) realms. In the calendar year 2021, of the 20 types of medical devices that had the greatest number of Medical Device Reports (MDRs) for adverse events and product problems, five of them were digital diabetes technology products (Figure 8). In September 2021, the FDA published the final report closing out the precertification program, concluding that the agency lacks statutory authority for the program. It is unclear how the FDA will modify its regulatory paradigm in the future to address software devices, especially those that employ machine learning.
Figure 8.
Twenty greatest number of class II product codes in the calendar year 2021 for MDRs.
Abbreviation: MDR, medical device report.
Source: Figure courtesy of Bradley M. Thompson.
The CDRH Digital Health Center of Excellence works to ensure that patients in the United States have access to safe and effective medical devices that include state-of-the-art technologies to improve their health. The FDA finalized the guidance on clinical decision support software, although the elements of that final guidance have proven to be controversial. The Center also launched the role of the Digital Health Policy Navigator, who would provide important context around that guidance. Most recently, they put out a spotlight on digital health, regulatory science, and research opportunities.
The FDA has extensive experience with the scientific evaluation of software-enabled medical devices, including the authorization of more than 500 AI- and ML-powered devices. These efforts led the FDA to issue their AI and ML Action Plan in 2019. They have worked internationally on this issue and have developed good ML practices and guiding principles with Canada and the United Kingdom. In addition, they explored new scientific approaches to evaluating these technologies, including using real-world performance data. They have supported the International Medical Device Regulators Forum in their development of key terms and definitions around AI- and ML-enabled devices.
Currently, CGMs do not meet the criteria for exclusion from the device definition, and technology that measures glucose is a medical device technology that FDA regulates. However, it seems reasonable to achieve safe and effective over-the-counter use of these devices for people with and without diabetes. The FDA believes that the regulatory tools, including special controls, will continue to ensure that these technologies are meeting an appropriate standard for accuracy as they consider additional patient populations. One challenge, however, is ensuring that no critical alarm is silenced inadvertently. The goal is to find a path forward that minimizes inadvertent deactivation and inadvertent failure to reactivate alarms, while also giving users who make a clear and conscious choice the flexibility to exercise more control over their devices.
Session 4: Digital Health to Nudge Behavior
Moderators
William T. Cefalu, MD
NIDDK/NIH, Bethesda, MD, USA
Victoria C. Hsiao, MD, PhD
University of California, San Francisco, San Francisco, CA, USA
Digital Health Tools to Improve Patient Engagement
Felix Lee, MPharm, MSc, MBA
Sanofi, Bridgewater, NJ, USA
Digital health tools have the potential to improve patient engagement; however, patient engagement is broad and multidimensional.
Patient engagement includes patient activation; activation interventions can improve physiological, psychological, and behavioral outcomes.
While some digital health tools and features have been associated with improved outcomes, the challenges remain in demonstrating causal relationships.
Better Behavior in Diabetes
Matthew E. Kahn, PhD
University of Southern California, Los Angeles, CA, USA
Individuals who have greater education are increasingly likely to take proactive steps to prevent T2D.
Technology such as CGMs can potentially help those at risk of developing T2D and those already diagnosed to engage in behavioral change.
Economic incentives should be piloted to encourage a more diverse set of individuals to experiment with adopting such technology.
New Metrics and Methods for Delivering Digital Health Interventions
Elizabeth Holt, MD, FACE
LifeScan Global Corporation, Malvern, PA, USA
The BGMs with apps that are connected to the cloud enable HCPs to have easy access to patient data, provide insightful reports, and are widely available.
Mobile apps allow for the collection of additional data beyond glucose readings, providing context that improves the ability of PWD to interpret and act on their results, which may lead to improved glycemic metrics.
Recent studies demonstrate the value of structured monitoring using BGMs (even when compared with CGMs) and show that PWD using the latest connected BGMs and diabetes apps significantly improved their glucose readings in range.
Behavioral Economics for Improving Adherence
Susana R. Patton, PhD, ABPP, CDCES
Nemours Children’s Health, Jacksonville, FL, USA
Applying behavioral economics principles offers an opportunity to rethink how we promote treatment engagement with PWD.
Early applications of behavioral economic theory in diabetes interventions show promise, but there also remain many gaps in knowledge.
Behavioral economics is adaptable to digital health applications for diabetes and diabetes prevention.
The term “patient engagement” includes various components such as patient activation, self-management, and shared decision-making (Figure 9). Improving patient activation not only enhances health outcomes and patient/provider experiences but also lowers costs. Digital tools achieve meaningful engagement by being clinically informed and data-driven. For example, average glucose levels improve when glucose measurements are tagged with information such as diet and exercise.
Figure 9.

Patient engagement: an umbrella concept to innovate health care.
Source: Figure reproduced from Graffigna et al 20 under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License.
Concepts on skill formation and determination to achieve long-term goals in diabetes control were presented. Field studies are needed to test whether access to technology will lower the risk of developing diabetes or complications. In addition, providing subsidies for technology like CGMs may reduce inequality in diabetes outcomes.
Another digital health tool developed to improve self-management of diabetes is the OneTouch Reveal app for blood glucose monitoring. Using the app more often by PWD improved glucose levels in PWD. Use of a BGM with color codes for low, in-range, and high glucose, in combination with the app and HCP interventions, improved hemoglobin A1c with high patient satisfaction. The HCPs can use the Reveal site to aid decision-making.
Behavioral economics can also be incorporated into patient adherence. Core concepts in behavioral economics include present bias, optimism bias, loss aversion, and default bias. A meta-analysis on varied incentives for lifestyle changes showed greater weight loss and lower blood pressure. One challenge is that the effects often wane when the incentive ends. Behavioral economics informs interventions to promote treatment engagement.
Session 5: Estimating Carbohydrates
Moderator
Thanh D. Hoang, DO, FACP, FACE
Walter Reed National Military Medical Center, Bethesda, MD, USA
Multimedia Data-Based Mobile Applications for Dietary Assessment
Stavroula G. Mougiakakou, PhD
University of Bern, Bern, Switzerland
The integration of computer vision and machine learning (ML), along with smartphone technologies, allows the translation of food multimedia data into nutrient content. Data acquisition takes place using the user’s smartphone.
The input variables may include food images from various angles, a video of the food, or information from depth sensors.
The challenges faced include the inability of the computer vision–based algorithms to identify mixed foods and account for various cooking methods that may affect the nutritional content of the food. A way to overcome these challenges is to collect more local food data and incorporate additional information about recipes, geolocation, and other sensors integrated into the smartphone, to improve identification and 3D reconstruction accuracy.
Predicting the Macronutrient Composition of Mixed Meals from Dietary Biomarkers
Ricardo Gutierrez-Osuna, PhD
Texas A&M University, College Station, TX, USA
Postprandial glucose response and dietary biomarkers for macronutrient intake were measured in ten participants in a study aimed to improve CGM accuracy.
Specific biomarkers for each macronutrient demonstrated a dose response and good prediction accuracy for the intake of various nutrients. Compared with the measurement of individual amino acid levels, CGMs showed greater error in predicting protein intake.
A combination of various dietary biomarkers including insulin, lysine, and triglycerides for the detection of carbohydrates, protein, and fat, respectively, yields the most accurate prediction of meal macronutrients.
Sensors for Detecting Food Intake
Samantha Kleinberg, PhD
Stevens Institute of Technology, Hoboken, NJ, USA
Meal detection from CGMs has achieved high accuracy in individuals with T1D. For the best results, data on physical activity are also needed.
Meal detection may be feasible from CGMs in individuals with T2D but is significantly more challenging and requires further investigation.
From CGM data, algorithms can estimate grams of carbohydrates. To estimate other macronutrients, other sensing modalities (audio, motion) may be needed.
Meal estimation is fundamental to diabetes self-management but requires extensive user training and input. Three different approaches were explored to accurately estimate macronutrient consumption while limiting user effort. Powered by artificial intelligence, food multimedia data can be translated into nutrient content through automatic food recognition and volume estimation as shown in Figure 10. Applications first require visual input captured through static photos or video. These videos and can be further augmented with depth sensors. These data are then processed using machine learning algorithms trained on food databases to estimate macronutrient composition. Four recent versions of the application have been validated in preclinical settings, in some cases outperforming an experienced dietician in macronutrient estimation. 21 Ongoing work needs to account for variability in food preparation, mixed foods, incomplete and inaccurate food databases, and incorporation of manual feedback for nonvisual ingredients, eg, usage of saturated versus unsaturated fat as the cooking method.
Figure 10.
Depth estimation using single red, green, and blue food images.
Source: Figure reproduced with permission from Lu et al. 22 Figure reproduced from Naaman et al 23 under a Creative Commons License.
A sensor-based approach for detecting food intake leverages wearable devices to passively identify unannounced meals. Using CGM data, AI models can more accurately predict meal timing and meal size in the T1D population. Predictions in T2D are more challenging given the presence of endogenous insulin but incorporating CGM data can improve precision. The current investigation explores combination sensing modalities (eg, audio sensors and motion sensors) to improve the accuracy of food type prediction.
Biomarker-based meal predictions have primarily focused on carbohydrate content and postprandial blood glucose (BG) based on CGM data alone. Ongoing investigation aims to expand prediction to the macronutrient composition of mixed meals from multiple dietary biomarkers in the blood. Predictive models were improved when incorporating insulin and glucose for carbohydrate content, lysine for protein content, and blood triglyceride for fat content.
Session 6: Algorithms for Fully Automated Insulin Delivery Systems
Moderators
Jeffrey Joseph, DO
Artificial Pancreas Center, Thomas Jefferson University, Philadelphia, PA, USA
Rayhan A. Lal, MD
Stanford University, Stanford, CA, USA
Dual Closed-Loop and SGLT2 Inhibitor Control
Ananda Basu, MD, FRCP
University of Virginia, Charlottesville, VA, USA
The use of low-dose sodium/glucose cotransporter-2 inhibitors (SGLT2i) as an adjunctive therapy with an AID system improves glucose control 24/7 in T1D.
SGLT2i increase TIR by 7% to 10% by lowering hyperglycemia and appear to lower glucose variability.
Ketoacidosis remains a challenge to the use of SGLT2i, and further evaluations are needed for the use of CKMs and a low dose of SGLT2i to improve patient outcomes.
Building a Full Closed-Loop Algorithm: Insulin Timing is the Key
Marc D. Breton, PhD
Center for Diabetes Technology, University of Virginia, Charlottesville, VA, USA
Current insulin analogues’ pharmacokinetics (PK) and pharmacodynamics (PD) impose front-loading on insulin in the early postprandial period.
In a fully closed-loop control paradigm, such preloading can be achieved in different ways, including insulin bolus priming, control algorithm design, and anticipation.
These techniques should be combined for optimal effectiveness.
RocketAP in Practice: Results From Pilot and Feasibility Trials
José F. García-Tirado, PhD
University of Virginia, Charlottesville, VA, USA
It is well known that the PK/PD of modern insulin analogues fails to match the rate of glucose appearance from carbohydrate consumption in T1D. Therefore, in the absence of faster insulin analogues, removing the user from the loop (decision-making process) necessitates additional strategies to mitigate large postprandial excursions.
Current commercial AID systems have shown the capability of coping with basal needs. However, physical activities (in many forms) and meals remain a challenge to achieve full AID operation.
Meal anticipation and/or rapid reaction to glycemic changes have shown promising results to help mitigate (unannounced) meal-related postprandial excursion in people with T1D.
Learning and Adaptation for Improved Control of the Artificial Pancreas
Francis J. Doyle III, PhD
Harvard University, Cambridge, MA, USA
Adaptation is more compelling than ever with the volume of data being generated from CGMs and AIDs.
A personalized approach is the most effective way to optimize the delivery of insulin.
Recent results from Bayesian optimization show promise (in silico) for improving the closed-loop performance of AIDs.
Fully Automated Insulin and Pramlintide Delivery System
Ahmad Haidar, PhD
McGill University, Montréal, QC, Canada
A fully closed-loop system with insulin-pramlintide co-formulation, together with timed pramlintide release based on a meal detection algorithm, can slow the absorption of meal glucose.
The time-in-target insulin-alone system and fully automated Fiasp-and-pramlintide system are comparable.
The fully automated insulin-pramlintide system does not lead to an increase in hypoglycemia. Gastrointestinal (GI) symptoms were rare with a 1:6 insulin to pramlintide ratio, while a 1:10 ratio resulted in a 15% to 20% report of non-mild GI symptoms.
The use of empagliflozin, an SGLT2i, as an adjunctive therapy to AIDs in T1D increases TIR by approximately 7% to 10% by lowering hyperglycemia and reducing glucose variability. Because euglycemic DKA remains a concern, the University of Virginia put together a grant proposal addressing the dosing of empagliflozin, algorithm modifications, and continuous ketone monitoring.
An in-silico strategy has been developed for addressing unannounced meal events. User-specific automated boluses within the first hour can limit postprandial excursions. Full closed-loop control may be achieved with anticipation of meal-like events, priming boluses, and designing an algorithm that balances bringing glucose to target against frequent dose adjustments based on total daily insulin with a wrapping paradigm like Model Predictive Control (MPC) (Figure 11).
Figure 11.
The algorithm balances bringing glucose to the target against frequent insulin dose adjustments.
Abbreviations: MPC, model predictive control; IOB, insulin on board; TDI, total daily insulin dose.
Source: Figure courtesy of Marc Breton.
Results of pilot and feasibility trials of the RocketAP algorithm were presented. These short, controlled studies of a general in-target population demonstrated safety and feasibility. Meal anticipation and/or a rapid reaction to glycemic changes have shown promising results to help mitigate unannounced meal-related postprandial excursions.
An AI-based approach demonstrated the personalization of insulin dosing using Bayesian optimization with safety guaranteed by the interior-points method. Points queried are always in the interior of the partially revealed safe region guaranteeing constraint satisfaction with high probability. The method was demonstrated in silico and on a personalized insulin dose guidance application.
A co-formulated pramlintide and insulin fully closed-loop system was developed to slow food absorption and suppress glucagon secretion without causing side effects such as frequent injections with every meal, GI side effects, and early postprandial hyperglycemia and late hypoglycemia. Results from a randomized crossover trial demonstrated noninferiority for 24 participants on a fully closed-loop bi-hormonal system (TIR 74%) versus an insulin-only system with carbohydrate announcements (TIR 78%). Plans are underway for further clinical investigations.
Session 7: Technology for Diagnosing Hypoglycemia
Moderators
Maya Fayfman, MD
Emory University School of Medicine, Atlanta, GA, USA
Brian M. Frier, BSc, MD, FRCPE
The Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, UK
Prevention of Rebound Hypoglycemia in an Advanced Automated Insulin Delivery System
Maria F. Villa-Tamayo, MSc
University of Virginia, Charlottesville, VA, USA
Full closed-loop AID systems can increase the risk of rebound hypoglycemia after reacting to rescue carbohydrates.
This work presents a controller-agnostic rebound hypoglycemia prevention layer (HypoSafe) that can be easily integrated into an AID system. It constrains the maximum allowable insulin delivery dose based on the minimum glucose measurement in the last hour and the current glucose concentration.
The proposed HypoSafe was shown to be effective in eliminating rebound hypoglycemia events without affecting TIR when combined with the full closed-loop AID system, RocketAP, previously developed at the University of Virginia.
The Role of Self-monitoring of Blood Glucose in Diagnosing Hypoglycemia
Michelle F. Magee, MD
Georgetown University, Washington, D.C., USA; MedStar Diabetes Institute, Washington, D.C., USA
Finger-stick blood glucose (FSBG) measurement plays an ongoing role in the evaluation of hypoglycemia and its urgent management. Only 3% to 4% of the US T2D population uses CGMs despite established benefits. Therefore, SMBG plays an important role in guiding diabetes management to enable the safe and effective attainment of individualized glycemic goals in this population.
In a diabetes “Boot Camp” approach using a mean of 1.5 connected FSBG daily, checked at varying times of day, reduction in hemoglobin A1c was safely accomplished with low hypoglycemia rates. 24
Hybrid systems using FSBG and software, such as mHealth Apps and logging with feedback, for hypoglycemia prevention or management are evolving rapidly and offer promise to further support self-care and improve glycemic outcomes.
The Hypo-RESOLVE Project
Bastiaan E. de Galan, MD, PhD
Maastricht University Medical Center, Maastricht, The Netherlands; Radboud University Medical Center, Nijmegen, The Netherlands
The Hypo-RESOLVE Project was designed to enhance the understanding of the clinical and psychological effects of hypoglycemia in PWD through a comprehensive multilevel approach in European centers.
A large analysis of RCTs has confirmed that antecedent hypoglycemia increases the risk of subsequent hypoglycemia. Data were presented from two recent Hypo-RESOLVE studies examining the effects of level 2 hypoglycemia (BG <3.0 mmol/L; 54 mg/dL) in people with insulin-treated diabetes on (1) domains of cognitive function and (2) pro-inflammatory responses.
These and other ongoing Hypo-RESOLVE studies are providing deeper insights into the impact of hypoglycemia and provide evidence to support the International Hypoglycaemia Study Group (IHSG)-proposed three-level classification of hypoglycemia.
Clinical Implications of Low-Sensor Glucose
Pratik Choudhary, FRCP, MD, MRCP, MBBS
University of Leicester, Leicester, UK
The widespread clinical use of CGMs is improving with a much greater understanding of glucose variability and hypoglycemia.
Continuous glucose monitoring identifies much more hypoglycemia than that identified by capillary BG monitoring or by patient report.
Many episodes of sensor-detected hypoglycemia are asymptomatic, and the HypoMETRICS study is assisting comprehension of the clinical implications of these episodes.
Electrocardiogram Diagnosis of Hypoglycemia
Sara Bachmann, MD
University Children’s Hospital Basel, Basel, Switzerland
Hypoglycemia induces changes in electrocardiogram (ECG) morphology and heart rate variability (HRV) secondary to sympathoadrenal activation.
QTc intervals are prolonged during episodes of hypoglycemia; the changes correlate with glucose levels.
The HRV changes occur early before the onset of nocturnal hypoglycemia, indicating that HRV might be useful in the detection, or even prediction, of hypoglycemia.
Hypoglycemia is a common and potentially deadly complication of antihyperglycemic treatment with implications for the intensity of therapy as well as patient quality of life. New technologies can help us to better understand and address hypoglycemia in clinical care. An algorithm has been developed to reduce rebound hypoglycemia that can occur with AID systems. Closed-loop algorithms are limited in their response to hypoglycemia by not factoring in the patient’s own response when taking in a glucose load, which leads to rapid glucose elevation, thus triggering increased insulin delivery and resulting in rebound hypoglycemia. The intervention, “HypoSafe,” is integrated into the AID algorithm and identifies when hyperglycemia occurs following the treatment of hypoglycemia. By employing constraints to both basal and bolus delivery and tamping down the AID response, the frequency and severity of rebound hypoglycemia following the initial episode were reduced.
The use of combined FSBG testing with remote monitoring can also improve glycemic control and reduce severe hypoglycemia. As shown in Figure 12, a mobile intervention called “Diabetes Boot Camp,” which is a cellular-enabled FSBG-driven monitoring system, can trigger hyperglycemia and hypoglycemia alerts to initiate clinician interventions. By providing weekly check-ins and adjustments to medical therapy in response to FSBG reports, participants in the program achieved not only marked hemoglobin A1c reductions from 11.2% to 8.1% on average but also reductions in severe hypoglycemia events that were driven by prompt intervention following episodes of hypoglycemia. The program highlights the utility of traditional validated measures such as FSBG combined with new technology to improve patient outcomes.
Figure 12.
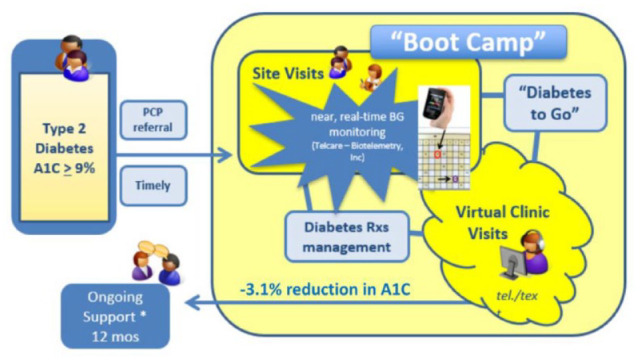
“Diabetes Boot Camp” is a mobile intervention program that incorporates FSBG monitoring.
Abbreviations: FSBG, finger-stick blood glucose; PCP, primary care provider; BG, blood glucose.
Source: Figure courtesy of Michelle Magee.
The Hypo-RESOLVE project 25 applies a multilevel approach, which will help to strengthen the IHSG classification of hypoglycemia (now accepted internationally) and understand its implications. A pooled analysis of 98 RCTs with more than 22 000 participants to predict the likelihood of recurrent hypoglycemia following an antecedent event, along with its clinical outcomes, was presented. Among patients with both T1D and T2D, following any hypoglycemia event, the likelihood of experiencing another event within 45 days was increased by 26%. Hypoglycemia, especially severe episodes involving cognitive effects, was linked to higher rates of cardiovascular events. Investigation of underlying mechanisms revealed associations between hypoglycemia and sustained pro-inflammatory responses and the profound effects that hypoglycemia has on cognitive function, mimicking alcoholic intoxication and sleep deprivation.
To help determine optimal parameters for CGM sensor-detected hypoglycemia, the HypoMETRICS study 26 aimed to correlate CGM sensors and patient-detected hypoglycemia. Preliminary data suggest that CGM-reported hypoglycemia occurred about 2 to 3 times more than patient-detected hypoglycemia. A longer duration of hypoglycemia was more likely to be recognized by patients, and patient-detected hypoglycemia had a greater impact on daily functioning than CGM-detected hypoglycemia.
Hypoglycemia can also be detected by monitoring ECG morphology and HRV. Hypoglycemia is linked to QTc prolongation as well as to changes in beat-to-beat HRV. Changes in HRV may even precede the onset of a hypoglycemia event allowing for early detection and prediction. This innovative approach of detecting hypoglycemia may have broad applications for the use of noninvasive wearables to alert individuals of the onset of hypoglycemia. In summary, the session’s presentations highlighted how evolving technology that aims to identify, treat, and prevent hypoglycemia will play a critical and expanding role in the clinical approach to treating insulin-treated diabetes.
Session 8: Novel Insulins and Insulin Delivery
Moderators
Barry H. Ginsberg, MD, PhD
Diabetes Technology Consultants, Arlington, VA, USA
Gerold M. Grodsky, PhD
University of California at San Francisco, San Francisco, CA, USA
Tirzepatide Physiology
William C. Roell, PhD
Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, IN, USA
Tirzepatide is a dual glucose-dependent insulinotropic polypeptide receptor (GIPR) and glucagon-like peptide 1 receptor (GLP-1R) agonist. As such, tirzepatide illustrates the benefits of multi-agonists engaging multiple tissues, resulting in the integration of multiple physiological pathways.
Tirzepatide improves glycemia both because of enhanced beta-cell function and increased insulin sensitivity.
Tirzepatide reduces body weight, which is associated with reduced food intake.
Ultralong and Ultrarapid Insulins
Tim Heise, MD
Profil, Neuss, Germany
Two insulins for once-weekly administration are currently in clinical development and have been shown to slightly improve hemoglobin A1c, TIR, or hypoglycemia rates in phase 2 studies in people with T2D.
In people with T1D, once-weekly insulins have been shown to be noninferior to second-generation basal analogues regarding hemoglobin A1c but might be associated with higher hypoglycemia rates.
Novel ultrarapid insulins are still at an early development stage (preclinical or phase 1) and show the potential of further accelerating insulin absorption compared with second-generation prandial insulins such as Fiasp or ultra-rapid lispro (URLi).
Intraperitoneal Insulin Delivery
Eric Renard, MD, PhD, FRCP (Edin)
Lapeyronie Montpellier University Hospital, University of Montpellier, Montpellier, France
Intraperitoneal (IP) insulin infusion allows for quicker “on” and “off” insulin action with lower peripheral insulinemia when compared with subcutaneous (SC) insulin infusion.
The IP insulin infusion is associated with a lower incidence of hypoglycemia and lower BG variability for similar insulin doses in comparison with SC insulin infusion.
Closed-loop trials with IP insulin infusion and SC glucose sensing have shown better glucose control with higher TIR and lower time above range (TAR) without meal announcement than closed-loop trials with SC insulin infusion and SC glucose sensing, demonstrating the feasibility of full closed loop while using IP insulin delivery.
Advances in Smart Pens and Caps for Insulin Delivery
Johan H. Jendle, MD, PhD
Örebro University, Örebro, Sweden
There is rapid development of insulin pens with connected caps, connected pens, and smart MDI systems, but the terminology still differs.
Smart insulin pens have been shown to improve glucose control and glucose metrics but might differ between systems.
Smart insulin pens have shown to be not only cost-effective but also cost saving.
Tirzepatide is a dual GIPR and GLP-1R agonist, used in the treatment of T2D. It was clinically studied in phase 3 SURPASS clinical trial program and compared with placebo, semaglutide 1.0 mg, insulin degludec, insulin glargine.27-29 At all doses studied (5, 10, 15 mg), it resulted in robust improvement in glycemic control and was superior to comparators in the reduction of hemoglobin A1c change from baseline. It also resulted in significant reductions in body weight, superior to comparators. Clamp studies demonstrated an increase in both first- and second-phase insulin secretion as well as whole-body insulin sensitivity. This may have contributed to both decreased glucose excursions in a standardized mixed meal test and reduced insulin secretion. Robust weight loss observed in this study was predominantly driven by a loss of fat mass and was associated with reduced appetite and reduced caloric intake.
Two insulins for once-weekly administration, icodec, with a half-life of about 8 days, and basal insulin Fc, with a half-life of 17 days, are currently in clinical development. In clinical studies, they are similar to current long-acting insulin, with slightly improved hemoglobin A1c, TIR, or hypoglycemia rates in people with T2D. In people with T1D, once-weekly insulins have been shown to be non-inferior to second-generation basal analogs regarding hemoglobin A1c but might be associated with higher hypoglycemia rates. Novel ultrarapid insulins are still at an early development stage (preclinical or phase 1) but show the potential of further accelerating insulin absorption compared with second-generation prandial insulins such as Fiasp or URLi, even at U500.
Intraperitoneal insulin infusion, compared with SC insulin infusion, is more rapid and displays more physiologic insulin action with lower peripheral insulinemia. IP insulin infusion has been studied for more than 40 years with both implantable infusion pumps and ports. The IP insulin delivery, compared with SC insulin infusion, is associated with better BG control, lower BG variability, and a lower incidence of hypoglycemia.
To prevent occlusion, implantable pumps would benefit from corticosteroid-coated catheters and insulin with a tension-active agent. When administered in closed-loop clinical trials using SC glucose sensing, the IP insulin infusion, compared with a closed-loop with SC insulin infusion, has shown better glucose control with higher TIR and lower TAR. Because of rapid insulin absorption, IP insulin can be used without meal announcement. New implantable insulin pumps are currently being developed.
There has been a rapid development of connected insulin delivery devices, including wireless insulin pens. Unfortunately, the terminology differs among providing companies. 30 Only Novo Nordisk has an open application programming interface, allowing it to interact with any compatible software. Smart insulin pens provide dosing information; their use leads to lower glucose variation and fewer missed and late doses of insulin. Hypoglycemia may be reduced after nine months of therapy by as much as 30% when using a smart insulin pen. Interestingly, smart insulin pens have been shown to be not only cost-effective but also cost saving (Figure 13).
Figure 13.
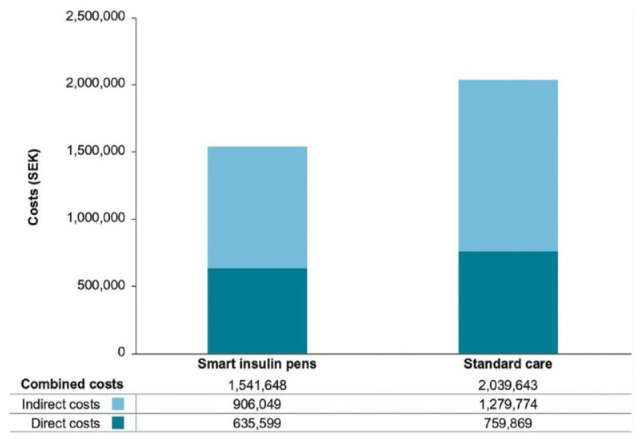
Combined (direct and indirect) costs of smart insulin pens over patients’ lifetimes. Base-case analysis, treatment effects were maintained for patient lifetimes.
Abbreviation: SEK, 2018 Swedish krona.
Source: Figure reproduced from Jendle et al 31 under the Creative Commons Attribution-Noncommercial 4.0 International License.
Session 9: Novel On-Body Sensors for Diabetes
Moderators
Rodolfo J. Galindo, MD, FACE
University of Miami Miller School of Medicine, Miami, FL, USA
Irl B. Hirsch, MD, MACP
University of Washington, Seattle, Washington, USA
Measurement of Sweat Glucose and Lactate
Joseph Wang, PhD
University of California, San Diego, San Diego, CA, USA
Sweat offers an attractive, reliable, and painless alternative for self-testing of BG.
Tracking BG through sweat is feasible in humans.
Sweat offers the possibility for pain-free, blood-free, simple, and rapid BG measurements.
Sleep Sensors for Monitoring Diabetes
Ali Cinar, PhD
Illinois Institute of Technology, Chicago, IL, USA
Collection of data during sleep through CGMs, wearables, or stationary sleep sensors may have several objectives for the treatment of diabetes, including prevention of hypoglycemia during sleep, BG concentration near target range at wakeup, and effects of sleep on glucose-insulin dynamics the next day.
Several alternative sensors and devices (CGM, accelerometers [Actigraph], multisensor wearables) are available for the collection of sleep-related data.
Processing of signals using modeling, statistical, and ML techniques is necessary to extract information from sleep data.
Cardiac Autonomic Neuropathy Sensors for Diabetes
Christian S. Hansen, MD, PhD
Steno Diabetes Center Copenhagen, Birkerød, Denmark
Screening PWD for cardiac autonomic neuropathy (CAN) is rarely implemented in clinical practice despite international recommendations and accessibility of point-of-care measuring devices.
The CAN screening can allow for the identification of PWD at risk of incident silent ischemic heart disease and future diabetic complications; it also allows for the opportunity to intervene early in the disease state.
People with T1D and CAN have increased glucose variability and might benefit from intensified use of CGMs and insulin pumps.
Electroencephalogram Findings in Hypoglycemia
Maria Rubega, PhD
University of Padova, Padova, Italy
The main complication of pharmacological treatment of people with T1D is hypoglycemia, an event that leads to both short-term and long-term automatic failure. Hypoglycemia can be life-threatening, especially when it occurs at night without subject awareness. The first organ affected by hypoglycemia is the brain.
In the last decade, alterations of cerebral functioning related to hypoglycemic events have been investigated through both linear and nonlinear analyses of the electroencephalogram (EEG) signal in patients with T1D.
Quantifying changes in the EEG signal due to hypoglycemia could help prevent hypoglycemia-induced neurological effects.
The most common wearable device today in diabetes is a CGM. However, new types of on-body sensors, as well as other measurements of interest (beyond glucose) for monitoring diabetes, are being actively developed.
The CGMs are being used by increasing numbers of people. These devices are still invasive, albeit minimally. Research toward noninvasive sensing of glucose in alternative biofluids (ie, sweat, saliva, tears, ISF) with wearable sensors have been actively pursued recently. In addition, attempts at measuring other diabetes-related biomarkers, such as lactate, ketones, alcohol, and cortisol, have been made. A promising blood-free and painless approach to reliably detect glucose (and other biomarkers) could be sweat. The human fingertip is an attractive site for rapid noninvasive sweat sampling. A personalized (via one-time calibration) sweat-to-blood translation algorithm is needed for the correction of inter-individual variability and to achieve a close correlation to BG.
Sleep cycles can be monitored with on-body sensors. While sleep is one of four major challenges in achieving 24/7 glucose regulation, it has not been sufficiently studied. The objective of sleep data collection is to assess sleep patterns and irregularities. Furthermore, one could look at the estimation of the effects of sleep on glucose-insulin dynamics as well as the prevention of hypoglycemia. Wearable devices for sleep are transitioning from accelerometer-based devices (Actigraphy) to multisensor systems (ie, from Apple, Google, Samsung, etc). Data are converted to understandable information such as light versus restful sleep as shown in Figure 14. Multiple-sensor wearables with ML and data analysis software can provide sleep information with appropriate accuracy for diabetes treatments. Integration of sleep information from wearable devices to AID systems would be feasible and useful in the future. It is important to note that there is a trend of wearable (consumer) device manufacturers, including Google, Apple, and Samsung, moving into the space of displaying comprehensive medical information.
Figure 14.
Screen capture from the Autosleep app of sleep data presentation. Qualities of sleep including awake, light, still, and deep sleep are presented in an easily understandable and user-friendly manner.
Abbreviation: bpm, beats per minute.
Source: Figure courtesy of Ali Cinar.
Diabetic CAN is a severe and prevalent complication in PWD. The CAN increases the risk of silent heart attacks and mortality. While CAN assessment allows for treatment and cardiovascular risk stratification, there is a lack of widespread implementation in clinical practice. 32 Point-of-care devices are available, allowing for easy and earlier detection of CAN, preventing progression toward advanced or symptomatic CAN. While reversibility is unlikely in advanced states, screening for CAN is crucial as progression can be stalled in both people with T1D or T2D and can provide a tool to stratify at-risk patients.
There is a correlation between hypoglycemia and its impact on cerebral functioning, measured by linear and nonlinear modeling via EEG. The EEG reflects the metabolic state of the brain. The brain is the first organ influenced by a fall in BG because glucose is its main source of energy and is not replaceable. During hypoglycemia, changes in temporal dynamics of microstates, (ie, quasi-stable spatial distributions of brain electric potential which last 80-100 ms) in particular a decrease of mapD, suggest that well-known theta- and alpha-power spectral increase and the drop in EEG complexity during hypoglycemia might be specific to a unique large-scale brain network.
Session 10: Advances in Continuous Glucose Monitor Technology
Moderators
J. Geoffrey Chase, PhD
University of Canterbury, Christchurch, New Zealand
Hubert W. Vesper, PhD
Centers for Disease Control and Prevention, Atlanta, GA, USA
Abbott Technology
Marc B. Taub, PhD
Abbott Diabetes Care, Alameda, CA, USA
The FreeStyle Libre 3 system is available in the United States and in ten countries across Europe and is the world’s most accurate, smallest, and thinnest CGM sensor.
There is a growing ecosystem of connected insulin delivery devices that work with FreeStyle Libre sensors.
Abbott is developing a combined continuous glucose and ketone sensor to help address the risks of DKA.
Dexcom Technology
Peter Simpson, MEng
Dexcom Inc., San Diego, CA, USA
Dexcom’s recently launched G7 is a significantly smaller, more accurate, and easier-to-use CGM system.
Dexcom’s expansive partnership ecosystem provides the best options and solutions to drive adoption and improved outcomes in diabetes care.
Dexcom continues to innovate on breakthrough technologies, including a fully closed-loop artificial pancreas system.
Medtronic Technology
Robert Vigersky, MD
Medtronic Diabetes, Northridge, CA, USA; Uniformed Services University of the Health Sciences, Bethesda, MD, USA
The real-world and pivotal trial glycemic outcomes of the noncalibration/nonadjunctive G4S sensor when used with the MiniMed 780G system are comparable to that of the GS3 sensor which requires calibration and is used adjunctively.
The next-generation sensor from Medtronic is disposable, requires no over-taping, and has a unique, user-friendly serter, which is the insertion device to be used with the MiniMed Quick-set infusion set.
The recently approved extended infusion set that lasts up to seven days has the potential for reducing patient burden by combining an infusion set and glucose sensor into a single device.
Senseonics Technology
Francine R. Kaufman, MD
Senseonics Inc., Germantown, MD, USA
The third generation fully implantable Eversense E3 CGM System has many unique attributes that include an up to six-month sensor duration with sustained excellent accuracy, a transmitter that elicits on-body vibratory alerts, use of a mild silicone-based adhesive, a removable transmitter, fluorescent technology, and others.
A robust innovative pathway has been developed for future Eversense CGM systems, including extending sensor duration to one year with reduced calibrations and adding a battery to the fully implantable sensor to allow for intermittent scanning when the transmitter is not worn.
To continue to innovate, feasibility studies are required. To facilitate rapid innovation, ML has allowed for the development of a conversion factor from finger-stick glucose values to comparable YSI values.
Ketones are a key element in DKA diagnosis and detection. Because elevated ketones also reduce insulin sensitivity, this metric could influence insulin delivery, particularly in people with insulin-dependent T2D, where intermittent fasting and timed eating are tools in glucose management. However, intermittent fasting can result in elevated ketone levels. Continuous ketone monitoring serves as a useful tool in this population, and some initial accuracy and trial results for ketone sensing have demonstrated good accuracy. The integration of smart Novo pen devices with the monitoring app for FreeStyle Libre CGMs has been accomplished in Europe. This integration demonstrates the increasing integration of care into single applications, in this case, combining the glucose measurements with insulin delivery to provide a far greater overall picture of care and outcomes.
There is a move toward the T2D market by Dexcom CGM technologies. Promising results were demonstrated from their first trials with insulin dependent T2D individuals. These results included measurable percentage reductions in hemoglobin A1c in this group, where every 1% reduction can significantly reduce complications, thus improving quality of life. Results also demonstrated that more accurate and more frequent measurements can improve glycemic control and outcomes. In addition, the Dexcom ONE sensor has been introduced for low-cost use in Europe. Although there is no specific date planned for its introduction to the United States, the increase in the availability of frontline care devices with a low-cost CGM will have a major impact and increase the reach of best-practice diabetes care.
The Medtronic G4 calibration-free sensor has shown equal performance to their calibration-based G3. This new device highlights the increasing growth toward calibration-free CGMs and the importance of minimizing patient burden and input. The “matching” new seven-day infusion sets were also demonstrated. While infusion sets are not CGMs or sensors, the ability to insert and remove both infusion sets and CGMs at the same time can lead to a significant reduction in patient overhead and burden. This approach represents a new take on how to integrate CGMs more seamlessly into diabetes care. Because of an ever-increasing CGM lifetime, an important question to investigate in the future is whether we will now begin to observe the same increase in the lifespan of infusion sets or patch pumps to reduce the cognitive burden on PWD.
Senseonics has taken a different approach with a (now) 180-day, fully implanted CGM, that unlike the other CGM products, is not transdermal, and it still requires regular daily calibration. The lack of changeover is also a means to significantly reduce the cognitive burden on patients and caregivers. There is also the future plan for 365-day sensors requiring only weekly calibration (Figure 15). At one year, this lifetime means that a glucose sensor demands much less attention and thus can be fully integrated into daily life more easily. The unique approach taken by Senseonics promotes very different use cases and benefits for at least some, if not many, groups of patients and caregivers.
Figure 15.

Product concepts of 365-day sensors requiring only weekly calibration.
Abbreviations: CGM, continuous glucose monitor; FGM, flash glucose monitor.
Source: Figure courtesy of Francine Kaufman.
Session 11: Digital Health Tools to Prevent Diabetic Foot Ulcers: Achieving Success by Understanding Defeat
Moderators
David G. Armstrong, DPM, MD, PhD
University of Southern California, Los Angeles, CA, USA
Bijan Najafi, PhD, MSc
Baylor College of Medicine, Houston, TX, USA
National Science Foundation’s Center to Stream Health Care in Place (C2SHIP.org)
David G. Armstrong, DPM, MD, PhD
University of Southern California, Los Angeles, CA, USA
Given the high recurrence of diabetic foot ulcer (DFUs), “healing” should be reframed as “remission.”
To measure the severity of DFUs, it is important to create a grand unifying metric system that takes into consideration ulcer-free days, hospital-free days, and activity-rich days.
Recurrence of DFUs is likely because there is a 40% recurrence rate at one-year post “healing.”
Digital Health-Led Decentralization for Managing Diabetic Foot Syndrome
Bijan Najafi, PhD, MSc
Baylor College of Medicine, Houston, TX, USA
Foot ulceration is the most common and costly late complication of diabetes with morbidity and mortality being worse than many cancers. It is estimated that three in five PWD will develop a DFU in their lifetime and that approximately one-third of cost associated with diabetes care is spent on diabetic foot syndrome, including DFUs.
Access to care, patient-clinician relationships, health literacy, and quality of preventive care and education prior to DFU onset are some of the major drivers for preventing and managing DFUs in a timely fashion.
The latest advances in digital health, wearables, RPM, and decentralized health care delivery models could address some of the barriers to improving health equity in people with DFU by engaging patients and their caregivers in the health ecosystem and empowering clinicians to provide personalized care. Some examples of the ideas and innovations discussed in this session include smart offloading, reinforcing adherence to these offloading devices, noninvasive monitoring tools to triage hard-to-heal wounds, and provision of timely referrals to the multidisciplinary wound care team to prevent amputation.
Noninvasive Monitoring for Tissue Oxygenation
Anuradha Godavarty, PhD
Florida International University, Miami, FL, USA
There is a need to complement the gold-standard visual assessment of DFUs using physiological assessment tools.
Noninvasive monitoring for tissue oxygenation in DFUs as mobile Health (mHealth) devices can augment remote care/telemedicine.
A smartphone-based device to assess tissue oxygenation has been developed and recently used to assess healing in DFUs.
Diabetic foot ulcers are costly and increasing in prevalence. Every 1.2 seconds, someone around the world develops a foot ulcer, often as a result of repetitive or constant stress on a neuropathic foot, leading to tissue breakdown.33-35 Every 20 seconds, someone with diabetes undergoes a limb amputation, of which half will become infected. 33 About 20% of those with infections are admitted to the hospital. Lower extremity complications due to diabetes are currently more expensive in terms of annual direct costs in billions of US dollars than each of the five most expensive cancers including breast, colorectal, lung, prostate, and leukemia. 36
There are several life-limiting aspects of DFUs in addition to increased chances of ulcer recurrence once developed. If one develops an ulcer, then there is a 2.6 times greater likelihood of death within the same year of development, and a >30% risk of death in five years. The risk of death significantly increases in the case of infection, and if one undergoes a limb-sparing amputation, then the risk of death increases to >50%. 36 Recurrence is common among persons with diabetes—upon undergoing the healing process, about 40% will develop another ulceration within one year, two-thirds within three years, and three-fourths within five years. 33 There are several life-limiting aspects of DFUs. If one develops an ulcer, one has a 2.6 times greater likelihood of death within the same year of development and a >30% risk of death in five years. The risk of death significantly increases in the case of infection. If one undergoes a limb-sparing amputation, the risk of death raises to >50%. 36 Recurrence is common among PWDs; during the healing process, about 40% will develop another ulcer at one year, 66% at three years, and 75% at five years. 33
There are three grand unifying metrics to measure and maximize: (1) ulcer-free days, (2) hospital-free days, and (3) activity-rich days.
Despite high rates of DFU development and corresponding limb amputation, 45% to 85% of amputations can be avoided, partially due to the current gap in health equity and access to care among populations.37,38 One study observed a higher likelihood of amputation if one lives >50 miles from an experienced hospital and these remote patients were older and less often Hispanic than other patients studied. 39
The Center to Stream Healthcare in Place (C2SHIP: https://c2ship.org/) aims to improve access to care in efforts to close the gap in health equity and care access while simultaneously empowering patients to be a part of the health ecosystem. 40 A component of C2SHIP’s vision is to change the mode of health care delivery from an in-hospital model to a decentralized health care model. A portion of the care can be carried out in patient homes. C2SHIP promotes a triaging system to differentiate between low-risk and high-risk cases to determine when a visit to the hospital is necessary.
Offloading, reducing high foot pressures to allow for wound healing, is widely considered the single most important intervention to manage DFUs. Special shoes can be costly, and while an irremovable boot addresses adherence, it remains far from ideal. C2SHIP supports research to promote adherence reinforcement through smart device notifications and remote monitoring of patients to personalize education and care for individuals. Smart offloading promotes high adherence with >90% accuracy. Smart offloading devices tally step count with >95% accuracy, measure cadence possesses high technological acceptance, visually indicate patient factors (ie, hemoglobin A1c levels, offloading adherence, successful DFU healing at 12 weeks, wound complexity, age, diabetes type, etc) using a data logger map, and provide physicians with rich information in minutes. 41 The effectiveness of patient engagement using smart offloading results shows how much technology impacts ulcer wound healing.
The latest advances in digital health, wearables, RPM, and a decentralized health care delivery model could address some of the barriers to improving health equity in people with DFUs through engaging patients and their caregivers in the health ecosystem and empowering clinicians in providing personalized care. Some examples of innovations discussed in this session include (1) smart offloading to reinforce adherence to offloading devices, and (2) noninvasive monitoring tools to facilitate triaging high-risk wounds and provision of timely referrals to multidisciplinary wound care teams to prevent amputation.
The DFU assessment often includes a visual inspection by a physician (ie, smell, touch) and is measured by the gold standard of wound healing, which consists of wound closure reaching more than >50% closure in a four-week span to be identified as a healed wound.
Several existing technologies for assessing factors that affect wound healing include skin perfusion pressure sensors, pulse volume recordings, transcutaneous oxygen measurements, thermal imaging, fluorescence imaging (ie, MolecuLight), multispectral and hyperspectral imaging for tissue oxygenation (ie, KENT, Oxy-View), among several others. Despite the myriad of technologies, there is an unmet need for mHealth devices to augment remote care.
Wound care is a nearly $30 billion industry, $10 billion of which is specific to the continuously growing DFU market. 42 There is a larger need to determine smartphone use and its integration into health care. The ideal smartphone technology characteristics for treating DFUs include (1) patient and clinician friendliness, (2) non-contact-based technique as wounds are potentially infectious and painful despite neuropathy onset, (3) 2D imaging, and (4) tissue oxygenation assessment maps to detect oxygenation signal below the wound and at the peripheral surfaces.
The SmartPhone Oxygenation Tool (SPOT) uses near-infrared optical imaging technology to measure tissue oxygenation and saturation beneath the skin’s surface in less than one minute to inform wound care clinicians of wound progression.43,44 The SPOT removes noise signals through singular value decomposition mathematics and overlays segmented and co-registered oxygenation maps measuring oxyhemoglobin (oxygen-rich) and total hemoglobin (oxygen-rich and deficient combined) concentrations onto real-life images of the wound sites across time to allow for physician monitoring of DFU wound healing (Figure 16). The SPOT is currently applied in clinical studies to differentiate between stable (low-risk) and chronic (high-risk) DFUs; however, machine learning (ML). However, ML algorithms are currently under testing to make SPOT oxygenation maps reliable for all skin tone types with varying melanin pigmentation levels.
Figure 16.
An illustration of the varying features of the SPOT device, (a) the SPOT device, (b) a comparison between the raw and filtered oxygen map imaging, and (c) oxygenation maps of DFUs across a four-week span, (d) segmented and co-registered oxygenation mapped onto color images of a DFU wound healing over time. Collaboration: University of Miami Hospital (Wound Center) & Department of Dermatology, Miami, Florida, Dr. Kirsner & Team.
Abbreviations: DFU, diabetic foot ulcer; HbO, oxy-hemoglobin; HbR, deoxy-hemoglobin; HbT, total hemoglobin; SPOT, SmartPhone Oxygenation Tool.
Source: Figure (a) adapted from Kaile et al 43 under the Creative Commons Attribution license (https://creativecommons.org/licenses/by/4.0/). Figure (b)-(d) courtesy of Anuradha Godavarty.
Session 12: Hot Topics
Moderators
Halis K. Aktürk, MD
Barbara Davis Center for Diabetes, University of Colorado, Aurora, CO, USA
Umesh Masharani, MB, BS
University of California, San Francisco, San Francisco, CA, USA
Diabetes and the Environment
Sultan A. Meo, MD, MBBS, PhD, FRCP
King Saud University College of Medicine, Riyadh, Saudi Arabia
A healthy environment plays a vital role in the normal developmental, biological, and metabolic processes of the human body.
The rapid growth in population and the fast pace of urbanization and industrialization reduces the natural green space environment and increases air pollution on the planet.
Countries with higher green space have lower levels of air pollution and lower prevalence of diabetes mellitus.
Diabetes Technology and the Environment
Lutz Heinemann, PhD
Science Consulting in Diabetes, Kaarst, Germany
Climate change represents a major health threat for everybody, especially PWD.
PWD have impaired heat regulation. Devices and drugs used for diabetes therapy are also impacted by heat.
Diabetes therapy has a considerable ecological footprint and generates a lot of plastic waste.
Spinal Cord Stimulation for Painful Diabetic Neuropathy
Erika A. Petersen, MD, FAANS, FACS
University of Arkansas for Medical Sciences, Little Rock, AR, USA
Spinal cord stimulation (SCS) is a safe, effective, and drug-free treatment option for many neurological and pain conditions.
Ten kilohertz SCS provides a safe, effective, and durable treatment for painful diabetic neuropathy (PDN) with the unique potential to improve neurological function.
Matching patients with PDN to appropriate interventions is essential to maximize benefit to patients.
Environmental factors have an impact on the prevalence of diabetes. The Environmental Performance Index (EPI) includes variables that assess environmental health (pollution) and ecosystem vitality (biodiversity and habitat). The EPI was used to classify 43 countries in Europe into three categories. Countries with an EPI >75 were considered to be highly green, 50 to 75 intermediately green, and <50 the least green. The prevalence of diabetes was lowest in the countries with EPI >75 and highest in countries with EPI <50. 45 The authors suggest that a polluted environment could impair beta-cell function and increase insulin resistance.
Health care leaves an environmental footprint. 46 The manufacturing of medical devices leaves a carbon footprint, and used medical products lead to waste. 46 Considering the large number of PWD who use various medicines and devices, it is likely that the ecological side effects of “diabetes treatment” are significant. There is also an impact of climate change, especially heat events, on diabetes treatment tools. There is little information about the impact of temperatures outside a certain range on glucose monitoring equipment such as glucose strips and CGMs. Extreme heat could also affect insulin stability. Devices such as smart pens and pumps could be developed to inform the user about possible degradation of insulin when the pens or pumps are exposed to high temperatures for a prolonged period.
Painful diabetic neuropathy affects the quality of life. Conventional medical treatments (pregabalin, duloxetine, and amitriptyline) have only limited efficacy. Spinal cord stimulation reduces pain by stimulating neurons with electrodes placed in the epidural space. As shown in Figure 17, clinical data showed that 10-kHz SCS has a long-lasting benefit and greater efficacy (~86%) compared with conventional medical therapy alone (39%-55%) or low frequency (up to 1200 Hz) SCS (35%-53%). 47
Figure 17.
Clinical evidence comparison for painful diabetic neuropathy.
Abbreviations: SCS, spinal cord stimulation; RCT, randomized controlled trial.
Source: Figure courtesy of Erika Petersen.
Live Demonstration
Moderators
Dorian Liepmann, PhD
University of California, Berkeley, Berkeley, CA, USA
Jane Jeffrie Seley, DNP, MPH, MSN, GNP, BC-ADM, CDCES, CDTC, FADCES
Weill Cornell Medicine, New York, NY, USA
A Novel Product for Greener Diabetes Technology
Brian Brandell, PhD, MBA
Hemingway Designs LLC, Oregon City, OR, USA
Colt Stuart, BSME
Hemingway Designs LLC, Oregon City, OR, USA
Tens of millions of PWD around the world enjoy the benefit and convenience of prefilled disposable insulin pens but at a significant environmental cost, with pens contributing an estimated 100 million pounds of single-use plastic to landfills and incinerators annually.
Hemingway Designs is developing a simple handheld mechanical device for home use that allows the used pen’s internal glass cartridge to be safely and easily removed in an intact manner for separate disposal from the surrounding plastic, which can then be recycled.
Hemingway Designs’ current prototype has performed successfully on disposable pens from Eli Lilly, Novo Nordisk, and Sanofi, opening a path to environmentally conscious use of disposable pens by giving users a safe, convenient home recycling solution for pennies.
Brian Brandell, PhD and Colt Stuart, BSME, co-founders of Hemingway Designs, LLC based in Oregon City, Oregon were troubled by the sheer volume of waste created by billions of disposable insulin pens. Although insulin pen manufacturers advise PWD to place their used pens in sharps containers, most dispose of them in their household trash. This practice translates into 60 to 100 million pounds of single-use plastic going to landfills or incinerators worldwide every year.
Upon investigation, Brandell and Stuart found that insulin pens cannot be recycled because they cannot be easily disassembled to allow the encaged inner glass cartridge containing residual insulin and potential medical and biological waste to be removed from the surrounding plastic. Using their engineering backgrounds and a large dose of creativity, they set out to design a recycling approach that would not be burdensome to PWD who were already juggling a plethora of self-management tasks on a daily basis.
To this end, Hemingway Designs is developing a simple, handheld mechanical device as shown in Figure 18 for home use that allows for the used insulin pen’s internal glass cartridge to be safely and easily removed in an intact manner for separate disposal from the surrounding plastic. The separation of the glass and plastic components of the pen would allow for the plastic to be recycled. The current prototype demonstrated at the virtual DTM has performed successfully on disposable pens from Eli Lilly, Novo Nordisk, and Sanofi, opening a pathway to environmentally conscious use of disposable pens by giving PWD a safe, convenient, and low-cost home recycling solution.
Figure 18.

Separation of the internal glass cartridge of a used insulin pen using a mechanical handheld device.
Source: Figure courtesy of Brian Brandell.
Conclusions
The DTM presented various perspectives on the current state of diabetes technology. The meeting’s presentations examined the development of diabetes technology through medical, scientific, regulatory, and engineering lenses.
Acknowledgments
For contributing key points to this report and reviewing the manuscript, we would like to thank Halis K. Aktürk, MD; Shridhara Alva, PhD; David G. Armstrong, DPM, MD, PhD; Mark A. Arnold, PhD; Zane Arp, PhD; Sara Bachmann, MD; Ananda Basu, MD, FRCP; Richard M. Bergenstal, MD; Brian Brandell, PhD, MBA; Marc D. Breton, PhD; William T. Cefalu, MD; Pratik Choudhary, FRCP, MD, MRCP, MBBS; Ali Cinar, PhD; Mark A. Clements, MD, PhD, CPI, FAAP; Bastiaan E. de Galan, MD, PhD; Francis J. Doyle III, PhD; Juan Espinoza, MD, FAAP; Guido Freckmann, MD; Brian M. Frier, BSc, MD, FRCP; José F. García-Tirado, PhD; Anuradha Godavarty, PhD; Gerold M. Grodsky, PhD; Ricardo Gutierrez-Osuna, PhD; Alberto Gutierrez, PhD; Ahmad Haidar, PhD; Christian S. Hansen, MD, PhD; Lutz Heinemann, PhD; Tim Heise, MD; Irl B. Hirsch, MD, MACP; Thanh D. Hoang, DO, FACP, FACE; Elizabeth Holt, MD, FACE; Erich S. Huang, MD, PhD; Johan H. Jendle, MD, PhD; Jeffrey Joseph, DO; Matthew E. Kahn, PhD; Francine R. Kaufman, MD; David Kerr, MBChB, DM, FRCP, FRCPE; David N. Kleidermacher, BS; Samantha Kleinberg, PhD; Michael A. Kohn, MD, MPP; Boris Kovatchev, PhD; Pavitra Krishnaswamy, PhD; Jan S. Krouwer, PhD; Jeffrey T. La Belle, PhD; Felix Lee, MPharm, MSc, MBA; Dorian Liepmann, PhD; Michelle F. Magee, MD; Ateev Mehrotra, MD, MPH; Sultan A. Meo, MD, MBBS, PhD, FRCP; Viswanathan Mohan, MD, PhD, DSc; Stavroula G. Mougiakakou, PhD; Kirsten Nørgaard, MD, DMSc; Brendan O’Leary, BSME; Rosemary G. Oshinsky, RN, CDE, MSN; Susana R. Patton, PhD, ABPP, CDCES; Erika A. Petersen, MD, FAANS, FACS; Andreas Pfützner, MD, PhD; John C. Pickup, MA, DPhil, FRCPath; Eric Renard, MD, PhD, FRCP (Edin); William C. Roell, PhD; Maria Rubega, PhD; Randi E. Seigel, JD; Christian Selmer, MD, PhD; Viral N. Shah, MD; Shahid N. Shah, MSc; Isao Shitanda, PhD; Jeffrey E. Shuren, MD, JD; Jordan Silberman, MD, PhD; Peter Simpson, MEng; Koji Sode, PhD; Colt Stuart, BSME; Marc B. Taub, PhD; Hazhir Teymourian, PhD; Bradley M. Thompson, JD, MBA, MADS; Hubert W. Vesper, PhD; Robert Vigersky, MD; Maria F. Villa-Tamayo, MSc; Joseph Wang, PhD; Yiduo Wu, PhD. We thank Annamarie Sucher-Jones for her expert editorial assistance.
Footnotes
Abbreviations: AcAc, acetoacetic acid; AGP, ambulatory glucose profile; AI, artificial intelligence; AID, automated insulin delivery; BG, blood glucose; BGM, blood glucose monitor; bpm, beats per minute; C2SHIP, Center to Stream Healthcare in Place; CAN, cardiac autonomic neuropathy; CCD, continuity of care documents; CDA, clinical document architecture; CDRH, Center for Devices and Radiological Health; CDSS, clinical decision support system; CE, counter electrode; CGM, continuous glucose monitor; CKM, continuous ketone monitor; CSC, clinically significant cluster; DFU, diabetic foot ulcer; DKA, diabetic ketoacidosis; DTC, direct-to-consumer model; DTM, Diabetes Technology Meeting; DTS, Diabetes Technology Society; ECG, electrocardiogram; EHR, electronic health record; EMPI, Enterprise Master Patient Index; EPI, Environmental Performance Index; FDA, US Food and Drug Administration; FGM, flash glucose monitor; FHIR, Fast Healthcare Interoperability Resource; FSBG, finger-stick blood glucose; GA, glutaraldehyde; GI, gastrointestinal; GIPR, glucose-dependent insulinotropic polypeptide receptor; GL, d-(+)-glucose anhydrous; GLP-1R, glucagon-like peptide 1 receptor; GLU, d-(+)-glucose anhydrous; GMI, glucose management indicator; GP, graphite powder; GRI, Glycemia Risk Index; HB, β-hydroxybutyrate; HBD, β-hydroxybutyrate dehydrogenase; HCL, hybrid closed loop; HCP, health care professional; HIPAA, Health Insurance Portability and Accountability Act; HL7, Health Level 7; HRV, heart rate variability; ICD-10, International Classification of Diseases, Tenth Revision; iCGM, class II integrated continuous glucose monitor; iCoDE, Integration of Continuous Glucose Monitor Data into the Electronic Health Record; IdM, identity management; IEEE, Institute of Electrical and Electronics Engineers; IHSG, International Hypoglycaemia Study Group; IL, ionic liquid; IOB, insulin on board; IoT, Internet of Things; IP, intraperitoneal; ISF, interstitial fluid; IT, information technology; JDST, Journal of Diabetes Science and Technology; LOINC, Logical Observation Identifiers Names and Codes; MDI, multiple daily injection; MDR, medical device report; mHealth, mobile Health; ML, machine learning; MO, mineral oil; MPC, model predictive control; NIST CSF, National Institute of Standards and Technology; NPI, National Provider Identifier; OMOP, Observational Medical Outcomes Partnership; PB, Prussian blue; PCP, primary care provider; PD, pharmacodynamics; PD, phenanthroline dione; PDN, painful diabetic neuropathy; PK, pharmacokinetics; PMA, premarket approval; PVC, polyvinyl chloride; Pump, insulin infusion pump; PWD, people with diabetes; QI, quality improvement; QR, quick response; RCT, randomized controlled trial; R&D, research and experimental development; RE, reference electrode; RGB, red, green, and blue; RPM, remote patient monitoring; SC, subcutaneous; SCS, spinal cord stimulation; SDOH, social determinants of health; SEK, 2018 Swedish krona; SGLT2i, sodium/glucose cotransporter-2 inhibitors; SMART, Substitutable Medical Applications, Reusable Technologies; SMBG, self-monitoring of blood glucose; SNOMED, Systematized Nomenclature of Medicine; SOC2, System and Organization Controls type 2—Trust Services Criteria; SPOT, SmartPhone Oxygenation Tool; SRM, standard reference material; TAR, time above range; TIR, time in range; TDI, total daily insulin dose; T1D, type 1 diabetes; T2D, type 2 diabetes; UDI, unique device identifier; URLi, ultra rapid lispro; US, United States; UK, United Kingdom; USA, United States of America; VDC, virtual diabetes care.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JH, AMY, ADubord, WL, ADrincic, PP, VCH, AS, JGC, and JJS have nothing to disclose.
WP was employed by Eli Lilly & Co from 2016 to 2021. PGJ is a co-founder, shareholder, and board member of Pacific Diabetes Technologies (this relationship is being managed by the OHSU conflict of interest office). EKS reports that this work was supported in part by a VA MERIT award from the US Department of Veterans Affairs Clinical Sciences Research and Development Service (1I01CX001825). He has received unrestricted research support from Dexcom (to the Baltimore VA Medical Center and to the University of Maryland) for the conduction of clinical trials. JLS is an Advisory Board member for Bigfoot Biomedical, Cecelia Health, Insulet, Medtronic, Vertex, and StartUp Health Diabetes Moonshot. She has also sponsored research for, has been a consultant for, and has received speaker fees from Insulet and Medtronic. She has been a consultant for and has received speaker fees from Lilly. She has received speaker fees from Zealand. AF is a paid consultant for 180 LS, A28, Adocia, Aerami, ALMS, Amolyt, Berg, Biocon, CarThera, Checkpoint, Diamyd, Diasome, Entera Bio, Enterin, Enzychem, Fortress, Glyscend, Hagar NIG, IM Therapeutics, I2o, Innoneo, Mars Symbioscience, Linear Therapies, MMD, Moelis, Northwest University, NuSirt, Oberland, Oramed, Orgenesis, Pano, Portal Insulin, RenovoRx, Rivus, Seraxis, SFC Fluidics, Stalicla, Surf Bio, TixiMED, Tyme, Unify, VeroScience, and Zucara. He is a member of the advisory board for Enterin, Hagar NIG, Oramed, and Pano and a member of the corporate board for Innoneo. TK is an employee and stockholder for Teladoc Health. RAL is a consultant for Abbott, Diabetes Care, Biolinq, Capillary Biomedical, Deep Valley Labs, Gluroo, Tidepool, and PhysioLogic Devices, as well as a member of the Advisory Board for Provention Bio. MF has aided in research for Dexcom (providing devices for NIH-funded study). BHG is a paid consultant for SFC Fluidics. RJG received research support from Novo Nordisk, Eli Lilly, Dexcom, and is a consultant for Sanofi, Eli Lilly, Weight Watchers, Pfizer, and Boehringer. BN received grants from BioSensics, LifeNet, AVAZZIA, EO2 Concepts, and NeuroMetrix. He is a consultant for BioSensics and Mölnlycke. UM was a former consultant for Ryse Health Inc and has received research funding from Clementia Pharmaceuticals. DCK is a consultant to EOFlow, Fractyl Health, Integrity, Lifecare, Rockley Photonics, and Thirdwayv.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This meeting described by the article was supported by educational grants from Medtronic, Novo Nordisk, Roche Diagnostics, and Sanofi and sponsorships from ARKRAY USA, Inc., Ascensia Diabetes Care, Dexcom, embecta, LifeScan, Lilly USA, MannKind, and Roche Diabetes Care GmbH. Corporate Sponsors of Diabetes Technology Society were Abbott, Glytec, and Nevro.
ORCID iDs: Jingtong Huang  https://orcid.org/0000-0002-3119-9361
https://orcid.org/0000-0002-3119-9361
Andrea M. Yeung  https://orcid.org/0000-0002-5592-453X
https://orcid.org/0000-0002-5592-453X
Ashley Y. DuBord  https://orcid.org/0000-0002-1478-7065
https://orcid.org/0000-0002-1478-7065
Howard Wolpert  https://orcid.org/0000-0001-9771-6344
https://orcid.org/0000-0001-9771-6344
Peter G. Jacobs  https://orcid.org/0000-0001-9897-4783
https://orcid.org/0000-0001-9897-4783
Wei-An Lee  https://orcid.org/0000-0002-2928-7338
https://orcid.org/0000-0002-2928-7338
Andjela Drincic  https://orcid.org/0000-0001-8365-7662
https://orcid.org/0000-0001-8365-7662
Elias K. Spanakis  https://orcid.org/0000-0002-9352-7172
https://orcid.org/0000-0002-9352-7172
Jennifer L. Sherr  https://orcid.org/0000-0001-9301-3043
https://orcid.org/0000-0001-9301-3043
Priya Prahalad  https://orcid.org/0000-0002-3894-4344
https://orcid.org/0000-0002-3894-4344
Alexander Fleming  https://orcid.org/0000-0002-6549-0288
https://orcid.org/0000-0002-6549-0288
Victoria C. Hsiao  https://orcid.org/0000-0001-5970-801X
https://orcid.org/0000-0001-5970-801X
Tejaswi Kompala  https://orcid.org/0000-0002-4492-5035
https://orcid.org/0000-0002-4492-5035
Rayhan A. Lal  https://orcid.org/0000-0002-8055-944X
https://orcid.org/0000-0002-8055-944X
Maya Fayfman  https://orcid.org/0000-0002-8733-3160
https://orcid.org/0000-0002-8733-3160
Barry H. Ginsberg  https://orcid.org/0000-0001-5404-826X
https://orcid.org/0000-0001-5404-826X
Rodolfo J. Galindo  https://orcid.org/0000-0002-9295-3225
https://orcid.org/0000-0002-9295-3225
Andreas Stuhr  https://orcid.org/0000-0002-8087-2436
https://orcid.org/0000-0002-8087-2436
J.Geoffrey Chase  https://orcid.org/0000-0001-9989-4849
https://orcid.org/0000-0001-9989-4849
Bijan Najafi  https://orcid.org/0000-0002-0320-8101
https://orcid.org/0000-0002-0320-8101
Umesh Masharani  https://orcid.org/0000-0002-3269-804X
https://orcid.org/0000-0002-3269-804X
Jane Jeffrie Seley  https://orcid.org/0000-0003-1582-4320
https://orcid.org/0000-0003-1582-4320
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
References
- 1.Klonoff DC.Postmarket surveillance of blood glucose monitor systems is needed for safety of subjects and accurate determination of effectiveness in clinical trials of diabetes drugs and devices. J Diabetes Sci Technol. 2019;13(3):419-423. doi: 10.1177/1932296819843398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teymourian H, Moonla C, Tehrani F, et al. Microneedle-based detection of ketone bodies along with glucose and lactate: toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal Chem. 2020;92(2):2291-2300. doi: 10.1021/acs.analchem.9b05109. [DOI] [PubMed] [Google Scholar]
- 3.Alva S, Castorino K, Cho H, Ou J.Feasibility of continuous ketone monitoring in subcutaneous tissue using a ketone sensor. J Diabetes Sci Technol. 2021;15(4):768-774. doi: 10.1177/19322968211008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr D, Aram M, Crosby KM, Glantz N.A persisting parallel universe in diabetes care within America’s capital. Eclinicalmedicine. 2022;43:101244. doi: 10.1016/j.eclinm.2021.101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr D.Poor numeracy: the elephant in the diabetes technology room. J Diabetes Sci Technol. 2010;4(6):1284-1287. doi: 10.1177/193229681000400601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-time adaptive interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Ann Behav Med. 2018;52(6):446-462. doi: 10.1007/s12160-016-9830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang O. Addressing the need for protecting cybersecurity in connected diabetes devices. IEEE Standards Association. https://standards.ieee.org/beyond-standards/addressing-the-need-for-protecting-cybersecurity-in-connected-diabetes-devices/. Published July 28, 2022. Accessed December 15, 2022. [Google Scholar]
- 8.Sambasivan N, Kapania S, Highfill H, Akrong D, Paritosh P, Aroyo LM. “Everyone wants to do the model work, not the data work”: Data Cascades in High-Stakes AI. In: Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems. CHI ’21. Association for Computing Machinery; 2021:1-15. doi: 10.1145/3411764.3445518. [DOI] [Google Scholar]
- 9.Huang J, Yeung AM, Eiland LA, Huang ES, Raymond JK, Klonoff DC.Telehealth fatigue: is it real? what should be done? J Diabetes Sci Technol. 2022. doi: 10.1177/19322968221127253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimri R, Battelino T, Laffel LM, et al. Insulin dose optimization using an automated artificial intelligence-based decision support system in youths with type 1 diabetes. Nat Med. 2020;26(9):1380-1384. doi: 10.1038/s41591-020-1045-7. [DOI] [PubMed] [Google Scholar]
- 11.Tyler NS, Mosquera-Lopez CM, Wilson LM, et al. An artificial intelligence decision support system for the management of type 1 diabetes. Nat Metab. 2020;2(7):612-619. doi: 10.1038/s42255-020-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobo B, Farhy L, Shafiei M, Kovatchev B.A data-driven approach to classifying daily continuous glucose monitoring (CGM) time series. IEEE Trans Biomed Eng. 2022;69(2):654-665. doi: 10.1109/TBME.2021.3103127. [DOI] [PubMed] [Google Scholar]
- 13.Montaser E, Fabris C, Kovatchev B.Essential continuous glucose monitoring metrics: the principal dimensions of glycemic control in diabetes. Diabetes Technol Ther. 2022;24(11):797-804. doi: 10.1089/dia.2022.0104. [DOI] [PubMed] [Google Scholar]
- 14.Klonoff DC, Wang J, Rodbard D, et al. A glycemia risk index (GRI) of hypoglycemia and hyperglycemia for continuous glucose monitoring validated by clinician ratings. J Diabetes Sci Technol. 2022. doi: 10.1177/19322968221085273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benhamou PY, Adenis A, Tourki Y, et al. Efficacy of a hybrid closed-loop solution in patients with excessive time in hypoglycaemia: a post hoc analysis of trials with DBLG1 system. J Diabetes Sci Technol. 2022. doi: 10.1177/19322968221128565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. doi: 10.1210/jc.2018-02763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DuBose SN, Li Z, Sherr JL, Beck RW, Tamborlane WV, Shah VN.Effect of exercise and meals on continuous glucose monitor data in healthy individuals without diabetes. J Diabetes Sci Technol. 2020;15(3):593-599. doi: 10.1177/1932296820905904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275-2280. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu NY, Nguyen KT, DuBord AY, et al. The launch of the iCoDE standard project. J Diabetes Sci Technol. 2022;16(4):887-895. doi: 10.1177/19322968221093662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graffigna G, Barello S, Triberti S. Giving (back) a role to patients in the delivery of healthcare services: theoretical roots of patient engagement. In: Graffigna G, Barello S, Triberti S, eds. Patient Engagement. Warsaw: De Gruyter Open Poland; 2016:13-26. doi: 10.1515/9783110452440-003. [DOI] [Google Scholar]
- 21.Vasiloglou MF, Marcano I, Lizama S, Papathanail I, Spanakis EK, Mougiakakou S.Multimedia data-based mobile applications for dietary assessment. J Diabetes Sci Technol. 2022. doi: 10.1177/19322968221085026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Stathopoulou T, Mougiakakou S. Partially supervised multi-task network for single-view dietary assessment. http://arxiv.org/abs/2008.00818. Published online July 15, 2020. Accessed December 12, 2022.
- 23.Naaman R, Parrett A, Bashawri D, et al. Assessment of dietary intake using food photography and video recording in free-living young adults: a comparative study. J Acad Nutr Diet. 2021;121(4):749-761. doi: 10.1016/j.jand.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magee MF, Baker KM, Fernandez SJ, et al. Redesigning ambulatory care management for uncontrolled type 2 diabetes: a prospective cohort study of the impact of a Boot Camp model on outcomes. BMJ Open Diabetes Res Care. 2019;7(1):e000731. doi: 10.1136/bmjdrc-2019-000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Galan BE, McCrimmon RJ, Ibberson M, et al. Reducing the burden of hypoglycaemia in people with diabetes through increased understanding: design of the Hypoglycaemia REdefining SOLutions for better liVEs (Hypo-RESOLVE) project. Diabet Med. 2020;37(6):1066-1073. doi: 10.1111/dme.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Divilly P, Zaremba N, Mahmoudi Z, et al. Hypo-METRICS: Hypoglycaemia—MEasurement, ThResholds and ImpaCtS—a multi-country clinical study to define the optimal threshold and duration of sensor-detected hypoglycaemia that impact the experience of hypoglycaemia, quality of life and health economic outcomes: the study protocol. Diabet Med. 2022;39(9):e14892. doi: 10.1111/dme.14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á.Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10(6):393-406. doi: 10.1016/S2213-8587(22)00070-5. [DOI] [PubMed] [Google Scholar]
- 28.Battelino T, Bergenstal RM, Rodríguez Á, et al. Efficacy of once-weekly tirzepatide versus once-daily insulin degludec on glycaemic control measured by continuous glucose monitoring in adults with type 2 diabetes (SURPASS-3 CGM): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10(6):407-417. doi: 10.1016/S2213-8587(22)00077-8. [DOI] [PubMed] [Google Scholar]
- 29.Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583-598. doi: 10.1016/S0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- 30.MacLeod J, Vigersky RA.A review of precision insulin management with smart insulin pens: opening up the digital door to people on insulin injection therapy. J Diabetes Sci Technol. 2022. doi: 10.1177/19322968221134546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jendle J, Ericsson Å, Gundgaard J, Møller JB, Valentine WJ, Hunt B.Smart insulin pens are associated with improved clinical outcomes at lower cost versus standard-of-care treatment of type 1 diabetes in Sweden: a cost-effectiveness analysis. Diabetes Ther. 2021;12(1):373-388. doi: 10.1007/s13300-020-00980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spallone V.Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019;43(1):3-30. doi: 10.4093/dmj.2018.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong DG, Boulton AJM, Bus SA.Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 34.Pitocco D, Spanu T, Di Leo M, et al. Diabetic foot infections: a comprehensive overview. Eur Rev Med Pharmacol Sci. 2019;23(suppl 2):26-37. doi: 10.26355/eurrev_201904_17471. [DOI] [PubMed] [Google Scholar]
- 35.Reardon R, Simring D, Kim B, Mortensen J, Williams D, Leslie A.The diabetic foot ulcer. Aust J Gen Pract. 2020;49(5):250-255. doi: 10.31128/AJGP-11-19-5161. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA.Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1):16. doi: 10.1186/s13047-020-00383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apelqvist J, Larsson J.What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev. 2000;16(suppl 1):S75-S83. doi: [DOI] [PubMed] [Google Scholar]
- 38.Lower Extremity Amputations Among Persons with Diabetes Mellitus—Washington, 1988. https://www.cdc.gov/mmwr/preview/mmwrhtml/00015500.htm. Published 1991. Accessed December 9, 2022. [PubMed]
- 39.Barshes NR, Uribe-Gomez A, Sharath SE, Mills JL, Sr, Rogers SO., Jr.Leg amputations among texans remote from experienced surgical care. J Surg Res. 2020;250:232-238. doi: 10.1016/j.jss.2019.09.074. [DOI] [PubMed] [Google Scholar]
- 40.Center to Stream Healthcare in Place. https://c2ship.org/. Accessed December 9, 2022.
- 41.Armstrong D. Improving the science of adherence reinforcement and safe mobility in people with diabetic foot ulcers using smart offloading. ClinicalTrials.gov; 2022. https://clinicaltrials.gov/ct2/show/NCT04460573. Accessed December 8, 2022.
- 42.Transparency Market Research. Diabetic foot ulcers treatment market. https://www.transparencymarketresearch.com/diabetic-foot-ulcers-treatment-market.html. Published 2020. Accessed December 9, 2022.
- 43.Kaile K, Trinidad A, Leiva K, et al. A stand-alone smartphone-based optical device to measure tissue oxygenation in diabetic foot ulcers. In: Biophotonics Congress: Biomedical Optics 2022. Optica Publishing Group; 2022. doi: 10.1364/TRANSLATIONAL.2022.TTu4B.7. [DOI] [Google Scholar]
- 44.Kwasinski R, Fernandez C, Leiva K, et al. Tissue oxygenation changes to assess healing in venous leg ulcers using near-infrared optical imaging. Adv Wound Care (New Rochelle). 2019;8(11):565-579. doi: 10.1089/wound.2018.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meo SA, Al-Khlaiwi T, Aqil M.Impact of the residential green space environment on the prevalence and mortality of type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2022;26(10):3599-3606. doi: 10.26355/eurrev_202205_28856. [DOI] [PubMed] [Google Scholar]
- 46.Lenzen M, Malik A, Li M, et al. The environmental footprint of health care: a global assessment. Lancet Planet Health. 2020;4(7):e271-e279. doi: 10.1016/S2542-5196(20)30121-2. [DOI] [PubMed] [Google Scholar]
- 47.Petersen EA, Stauss TG, Scowcroft JA, et al. Durability of high-frequency 10-kHz spinal cord stimulation for patients with painful diabetic neuropathy refractory to conventional treatments: 12-month results from a randomized controlled trial. Diabetes Care. 2022;45(1):e3-e6. doi: 10.2337/dc21-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]