Abstract
Background:
Continuous glucose monitoring (CGM) is revolutionizing diabetes care by giving both patients and the healthcare professionals unprecedented insights into glucose variability and patterns. It is established in National Institute for Health and Care Excellence (NICE) guidance as a standard of care for type 1 diabetes and diabetes in pregnancy under certain conditions. Diabetes mellitus (DM) is recognized as an important risk factor for chronic kidney disease (CKD). Around a third of patients receiving in-center haemodialysis as renal replacement therapy (RRT) have diabetes, either as a direct cause of renal failure or as an additional co-morbidity. Evidence of poor compliance with the current standard of care (self-monitoring of blood glucose [SMBG]) and overall greater morbidity and mortality, suggests this patient population as an ideal target group for CGM. However, there exists no strong published evidence showing the validity of CGM devices in insulin-treated diabetes patients requiring haemodialysis.
Methods:
We applied a Freestyle Libre Pro sensor to 69 insulin-treated diabetes haemodialysis (HD) patients on a dialysis day. Interstitial glucose levels were obtained, and time matched within 7 minutes to capillary blood glucose testing and any plasma blood glucose levels sent. Data cleansing techniques were applied to account for rapidly correcting hypoglycaemia and poor SMBG technique.
Results:
Clarke-error grid analysis showed 97.9% of glucose values in an acceptable range of agreement (97.3% on dialysis days and 99.1% on non-dialysis days).
Conclusions:
We conclude that the Freestyle Libre sensor is accurate in measuring glucose levels when compared to glucose as measured by capillary SMBG testing and laboratory obtained serum glucose in patients on HD.
Keywords: continuous glucose monitoring, haemodialysis, Libre, self-monitoring of blood glucose, glucose variability
Introduction
Diabetes mellitus (DM) is a leading cause of chronic kidney disease (CKD) worldwide. Progression of CKD can lead to end-stage renal failure (ESRF). To extend life, renal replacement therapies can be considered. These include renal transplantation, peritoneal dialysis, and haemodialysis (HD). Approximately one third of haemodialysis patients also have to manage their diabetes. 1 There is reduced survival for patients with diabetes commencing HD in comparison with those without, with a 1-year mortality rate of 17% compared to 11% for the population without diabetes. 2 The cause for this survival discrepancy is unclear with most of the mortality in the ESRF population attributed to cardiovascular complications, in particular sudden cardiac events. 3 The extra challenges related to the management of glycemia and glycemic variability in this patient population may be a strong contributing factor. Accurate glucose measurement and effective management of insulin in the dialysis patient population thus represents an important goal for health care professionals, with the potential for significant clinical consequences.
Insulin-treated people with diabetes who require HD face unique physiological challenges which can predispose to glycemic variability and difficulties in measuring glucose control, as dialysis changes insulin and glucose dynamics on HD days compared to non-HD days. 4 Dialysis days are associated with more glucose variability (and therefore hypoglycaemia risk) which could require a different treatment strategy for these days, adding complexity and potential for error into treatment regimes. Individual factors contributing to hyperglycaemia in renal patients include reduced insulin production, increased insulin resistance and direct effects during dialysis, and a glucose load from the dialysate itself. 5 Conversely, the potential for reduced insulin clearance on non-dialysis days, reduced renal gluconeogenesis and loss of glucose into the dialysate can all predispose to hypoglycaemia. All these features can be accentuated in times of infection or physiological stress. 6
Chronic hyperglycaemia has long been considered the primary pathological insult, being associated with endothelial dysfunction, oxidative stress, dysregulated inflammation, formation of advanced glycation products, and abnormal coagulation that are linked to multiorgan dysfunction. 7 However, recent evidence suggests that not just hyperglycaemia but glycemic variability is linked to cardiovascular disease (CVD) by an association with micro-vascular and macro-vascular complications.8,9 Glucose-level fluctuation ranging from hypo- to hyperglycaemia may, therefore, be more damaging than chronic hyperglycaemia alone.10,11 Historically such information has been difficult to assess due to the challenges in achieving the frequency of self-monitoring of blood glucose (SMBG) measurements required to describe this variability. 12 However, interstitial continuous glucose monitoring (CGM) offers the potential to identify such risks clearly (assuming its use can be validated in this patient group).
Yajima et al 13 conducted a study of 13 hospital inpatient HD patients with T2DM (11 males) comparing sensor data from both flash glucose monitors and CGM with blood samples taken prior to HD sessions. They found 100% of paired values fell within acceptable levels of agreement; however, the authors acknowledge that the number of study participants and the numbers of glucose levels compared were small, and additionally recognize that further studies are required including data from during and after HD sessions.
Local experience within our HD population with diabetes indicates poor concordance with SMBG (until now, the recommended standard of care for insulin-treated dialysis patients) most likely due to overall disease burden in this group with multiple co-morbidities. 14 Our study was therefore undertaken using a Pro (blinded) sensor to determine if such data collection was more acceptable, but this of course means that the clinical benefits of contemporary real-time CGM devices were not available.
The low level of SMBG use is associated with a lack of understanding and misperceptions regarding diabetes management, in a patient population where other methods for assessing glycemic control, such as HbA1c, glycated fructosamine and glycated albumin, are themselves unreliable. 15 Taken together, one can assume that difficulties in accurate glycemia measurement have contributed significantly to the paucity of evidence-based treatment targets for HD patients in the literature. 16
Interstitial fluid flash glucose sensors and CGM sensors have revolutionized type 1 diabetes monitoring and treatment through the increased ability to assess the impacts of insulin use. The technology saves data over a 10- to 14-day period and generates a graphical presentation of the data known as an ambulatory glucose profile, or AGP, providing far greater insight into an individual’s experience of hyperglycaemia exposure, hypoglycaemia exposure, between day glucose variability and within day glucose instability. This therefore facilitates more informed treatment decisions for both patients and healthcare teams. It has been previously used to demonstrate that HbA1c results are indeed unreliable and not indicative of overall risk in an insulin dependent, dialysis patient population, further advancing its own candidacy for routine use in the insulin-treated renal replacement therapy (RRT) patient population. 17 Therefore, the potential for benefit in this particularly challenging group of patients, who are prone to high levels of glucose variability and a strong association with adverse outcomes, is significant.
The DRIVE-HD study (Diabetes and Real-world investigation of glucose instability, variability, and exposure in haemodialysis) was a single center observational study by Wessex Kidney Center, based at Queen Alexandra hospital, Portsmouth (ISRCTN: 58101486). Interstitial glucose data collection by the Libre Freestyle Pro compared to blood glucose, either capillary by SMBG or serum laboratory results has allowed for the validation of this CGM device.
Methods
All male or female HD patients (who had been on renal replacement therapy for at least 3 months) aged 18 years or above with a diagnosis of DM (type 1 or type 2) treated with insulin, and who had access to a downloadable SMBG meter were invited to take part in this study. Table 1 (below) shows information on the study population.
Table 1.
Baseline Data on All Patients.
| Insulin-treated | 69 (100%) |
|---|---|
| Average age (range) | 64 (33-83) |
| % Male | 60.9% |
| Average BMI (range) | 29.4 (17-43.8) |
| T1DM | 14 |
| T2DM | 55 |
| Average length of diabetes in years (range) | 23 (3-50) |
| Average time on dialysis in months (range) | 30.9 (2-127) |
Abbreviations: BMI, body mass index; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
The exclusion criteria were acute prescription of medication that may increase serum glucose, planned change of RRT modality during study, planned imaging studies during study (due to requirement for sensor removal), co-enrolment in other studies related to glycemic control, and those unable to provide informed consent.
Study data analysis and (relevant to this manuscript) comparison analysis of interstitial glucose to blood glucose was carried out in all participants that achieved 7 or more days of consecutive CGM data collection in the in-center haemodialysis cohort.
Interstitial glucose levels (every 15 minutes) were obtained using the Libre Freestyle Pro (Abbott) CGM interstitial fluid (ISF) sensor. The sensors were applied on attendance at a dialysis session as per manufacturers guideline and were retained on removal after 14 days (or sooner if they became dislodged) to provide data for a retrospective analysis of the preceding 14 days.
Reference blood glucose levels utilized for comparison with the CGM data were from SMBG testing by the patients on review of personal meters, by capillary checks documented by dialysis staff at our haemodialysis units, and any plasma blood glucose level sent to the lab and available on the local renal data system. All such individual blood glucose results were matched to the nearest interstitial glucose (±7 minutes) from a Libre Pro sensor.
The data were reviewed by a diabetes specialist consultant who applied pre-established data cleaning criteria. These being (a) if SMBG value is in hypo range and delay to comparator CGM result is more than 2 minutes (when rescue treatment would have impacted on the second result), (b) if delay between measurements is > 15 minutes (eg, because of a sensor inactive at the time of the SMBG result), (c) if there was a comment in the clinical notes from either the dialysis nurse (if during a dialysis session) or from the person with diabetes (at other times) about an action which would have impacted either the SMBG or venous blood result, and (d) if there is evidence that the individual is poor at SMBG technique (as evidenced by wide variability between paired SMBG and venous blood results). Two patients removed were removed due to meeting criteria (d), with 59 total data pairs removed for hypoglycaemia. In all, 706 cleansed data points of a time matched interstitial and blood glucose measurements remain for comparison. Clarke Error gird analysis was applied to the overall data and data divided to that obtained on a dialysis versus a non-dialysis day.
Results
The DRIVE-HD study recruited a total of 89 eligible participants, from which 74 generated data for 7 or more consecutive days and as such were included in the data analysis; those with less than 7 days data from the CGM device have been excluded. Of the 74 participants with 7 to 14 days of continuous interstitial glucose monitoring 5 were undertaking home haemodialysis (HHD), therefore the in-center haemodialysis (ICHD) cohort for analysis comprised of 69 patients. No participants were captured over a planned switch between ICHD and HHD.
The average number of additional diagnoses in the study population was 8 per patient, with 13% being current smokers, 53% ex smokers, and 33% having never smoked. The study population was 96% of white/British ethnicity, reflecting underlying demographics of the area.
A total of 17 participants were on oral hypoglycemics in addition to their insulin (14 of these on Linagliptin) and the insulin regimes were split between basal-bolus (52%), basal (21%), and premixed (27%).
A total of 91,506 interstitial glucose levels were obtained in total, the In-center haemodialysis cohort contributed 85,672 and the home haemodialysis 5834. Continuous glucose monitoring sensor data capture was high (<0.05% of expected 15-minute records were lost). In all, 50% of all participants provided their personal meters for review; within the group that provided their meter some did so without any glucose data on them. All blood glucose levels, either from 37 participants own monitors (SMBG), dialysis unit recordings or laboratory glucose, (from whole group) have been paired to their nearest time point interstitial glucose. There is a total of 813 interstitial with blood glucose levels.
Clarke error grid analysis (EGA) based on 706 paired levels (from 69 ICHD patients) indicates 97.9% fall in the acceptable range of A and B (Figure 1). Error grid analysis has also been applied to pairs on a dialysis day (481) with 97.3% falling in range of A and B as seen in Figure 2, and to non-dialysis day pairs (225) with 99.1% falling in range A and B, as seen in Figure 3.
Figure 1.
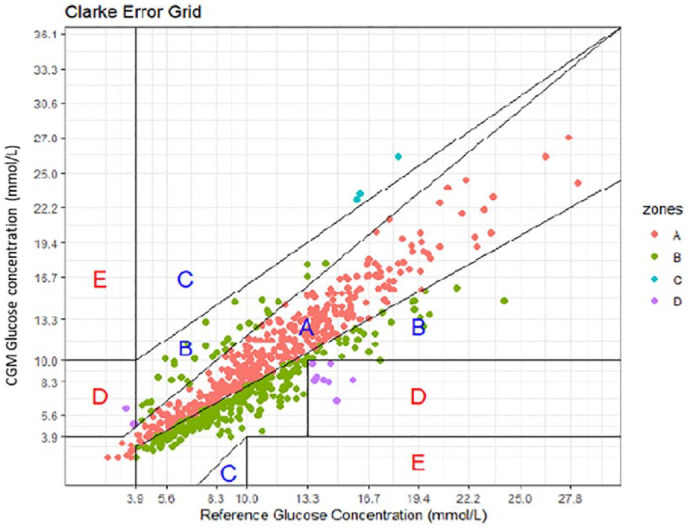
Clarke Error Grid for 706 interstitial glucose levels time matched to a blood glucose level. Results indicating 97.9% fall within A and B.
Figure 2.
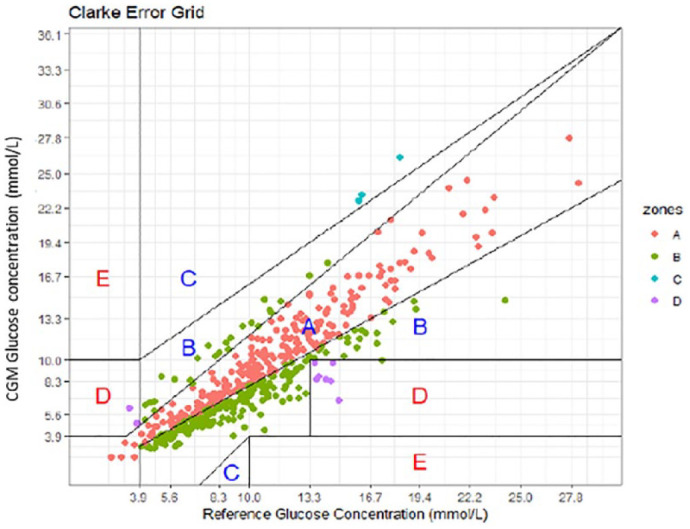
Clarke Error Grid for dialysis day interstitial glucose levels time matched to a blood glucose level. Results indicating 97.3% fall within A and B on a dialysis day.
Figure 3.
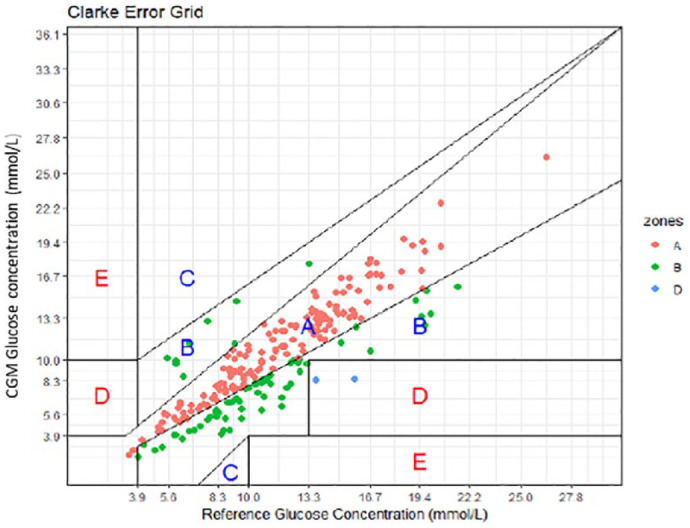
Clark Error Grid for non-dialysis day interstitial glucose levels time matched to a blood glucose level. Results indicating 99.1% fall within A and B on a non-dialysis day.
Conclusions/Discussion
Interstitial fluid glucose measurements derived from the Libre Pro CGM sensor show good correlation with the reference glucose results (a combination of patient-performed and HCP-performed capillary glucose testing, and lab based venous plasma glucose) at a level comparable with such validation and accuracy data from other populations. 18
This comparative validation dataset was recorded as a part of a wider survey of glucose control in dialysis subjects treated with insulin, the paired readings were thus achieved by retrospective data review, which required data cleansing for clinical circumstances during which approx. In all, 13% of the data pairs were excluded because of pre-agreed criteria (59 of which were for hypoglycaemia). While a stricter validation protocol may have reduced this number, it may have sacrificed the “real life” device accuracy measurement we were aiming for, and would have required the use of a real-time sensor which would have introduced other sources of error. The use of CGM sensors with real-time glucose reporting (as opposed to the “blinded” professional sensor used in this study) might impact on perceived/measured accuracy, by biasing the timing of the SMBG tests undertaken to periods when glucose levels are changing rapidly (and thus when concordance is lower). The good concordance between capillary and interstitial fluid glucose measurements in this study highlights this technique (interstitial CGM) as an appropriate strategy for glucose monitoring in this high-risk population who find SMBG concordance challenging. The theoretical impact on measured ISF glucose levels of ISF shifts associated with haemodialysis do not seem to make a significant impact on the reliability of such sensors in this study, and the application of sensors on a dialysis day which has been (anecdotally) associated with the potential for lower reliability of the sensor data over its life, in this survey using the FreeStyle LibrePro did not seem to impact on the accuracy. In light of these positive results, we recommend that, as has been recently adopted in the management of type 1 diabetes, that individuals with stage 5 CKD (with either type 1 or type 2 diabetes) who require insulin treatment of their diabetes should be offered access to interstitial CGM monitoring as a standard of care.
The limitations of this study include a partial reliance on glucose values from non-standardized home SMBG meters which were not subject to quality control testing. Further work to validate CGM accuracy and precision in an HD population using more controlled blood glucose measurements paired to the CGM data, while also being better able to account for periods of rapid glucose change (such as hypoglycaemia), would represent the logical next steps.
Footnotes
Abbreviations: AGP, ambulatory glucose profile; CGM, continuous glucose monitoring; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; EGA, Error Grid Analysis; ESRF, end-stage renal failure; HD, Haemodialysis; HHD, home haemodialysis; ICHD, in-center haemodialysis; ISF, interstitial fluid; NICE, National Institute for Health and Care Excellence; RRT, renal replacement therapy; SMBG, self-monitoring of blood glucose.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Cranston has received research grants, speaker fees or consultation advisory fees from the following: Eli Lilly, Boehringer-Ingelheim, NovoNordisk, Sanofi, Janssen, MSD, AstraZeneca, Napp, BMS, Roche Diagnostics, Johnson & Johnson, Animas, Abbott Diabetes Care, Takeda.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Libre Sensors provided by Abbott.
ORCID iD: Christopher Horne  https://orcid.org/0000-0002-7930-5266
https://orcid.org/0000-0002-7930-5266
References
- 1.UK Renal Registry. UK Renal Registry 23rd annual report—data to 31/12/19, Bristol, UK. https://ukkidney.org/audit-research/annual-report/23rd-annual-report-data-31122019. [Google Scholar]
- 2.UK Renal Registry. UK Renal Registry 21st annual report—data to 31/12/2017. https://ukkidney.org/sites/renal.org/files/publication/file-attachments/21st_UKRR_Annual_Report.pdf [Google Scholar]
- 3.Hayashi Shimizu N, Suzuki A, et al. Hemodialysis-related glycemic disarray proven by continuous glucose monitoring; glycemic markers and hypoglycemia. Diabetes Care. 2021;44(7):1647-1656. [DOI] [PubMed] [Google Scholar]
- 4.Williams ME, Garg R.Glycemic management in ESRD and earlier stages of CKD. Am J Kidney Dis. 2014;63(2):S22-S38. [DOI] [PubMed] [Google Scholar]
- 5.Asadollahi K, Beeching N, Gill G.Hyperglycaemia and mortality. J R Soc Med. 2007;100(11):503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abe M, Kalantar-Zadeh K.Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat Rev Nephrol. 2015;11(5):302-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada T, Nakao T, Matsumoto H, et al. Association between markers of glycemic control, cardiovascular complications and survival in type 2 diabetic patients with end-stage renal disease. Intern Med. 2007;46(12):807-814. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A, Monnier L, Owens D.Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019;7(3):221-230. [DOI] [PubMed] [Google Scholar]
- 9.Ceriello A.Glucose variability and diabetic complications: is it time to treat? Diabetes Care. 2020;43(6):1169-1171. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349-1354. [DOI] [PubMed] [Google Scholar]
- 11.Satya Krishna SV, Kota SK, Modi KD.Glycemic variability: clinical implications. Indian J Endocrinol Metab. 2013;17(4):611-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yajima T, Takahashi H, Yasuda K.Comparison of interstitial fluid glucose levels obtained by continuous glucose monitoring and flash glucose monitoring in patients with type 2 diabetes mellitus undergoing hemodialysis. J Diabetes Sci Technol. 2020;14(6):1088-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin EM. Self-monitoring of blood glucose: the basics. Clinical Diabetes. 2002;20(1):45-47. [Google Scholar]
- 15.Ling J, Ng JKC, Chan JCN, Chow E.Use of continuous glucose monitoring in the assessment and management of patients with diabetes and chronic kidney disease. Front Endocrinol. 2022;13:869899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frankel A, Kazempour-Ardebili S, Bedi R. Management of adults with diabetes on the haemodialysis unit. Joint British Diabetes Society for Inpatient Care. https://www.diabetes.org.uk/resources-s3/2017-09/JBDS%20Management%20of%20adults%20with%20diabetes%20on%20the%20haemodialysis%20unit%20%28full%20guideline%29.pdf. Published 2016. Accessed April 24, 2023. [DOI] [PubMed] [Google Scholar]
- 17.Vos FE, Schollum JB, Coulter CV, Manning PJ, Duffull SB, Walker RJ.Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology (Carlton). 2012;17(2):182-188. [DOI] [PubMed] [Google Scholar]
- 18.Kompala T, Neinstein A.Analysis of “accuracy of a 14-day factory calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes.” J Diabetes Sci Technol. 2022;16(1):78-80. [DOI] [PMC free article] [PubMed] [Google Scholar]


