Abstract
Background:
Glycemic outcomes during real-world hybrid closed-loop (HCL) system use by individuals with type 1 diabetes, in the United States, were retrospectively analyzed.
Methods:
Hybrid closed-loop system data voluntarily uploaded to Carelink™ personal software from March 2017 to November 2020 by individuals (aged ≥7 years) using the MiniMed™ 670G system and having ≥10 days of continuous glucose monitoring data after initiating Auto Mode were assessed. Glycemic outcomes including the mean glucose management indicator (GMI), sensor glucose (SG), percentage of time spent in (TIR), below (TBR), and above (TAR) target range (70-180 mg/dL) were analyzed. Outcomes were also analyzed in a subgroup of users per baseline GMI of <7% versus >8%.
Results:
The overall cohort (N = 123 355 users, with a mean of 87.9% of time in Auto Mode) had a GMI of 7.0% ± 0.4%, TIR of 70.4% ± 11.2%, TBR <70 mg/dL of 2.2% ± 2.1% and TAR>180 mg/dL of 27.5% ± 11.6%, post-Auto Mode initiation. Compared with pre-Auto Mode initiation, users (N = 52 941, 88.6% of time in Auto Mode) had a GMI that decreased from 7.3% ± 0.6% to 7.1% ± 0.5% (P < .001), TIR that increased from 61.5% ± 15.1% to 68.1% ± 11.9% (P < .001), TAR>180 mg/dL that decreased from 36.3% ± 15.7% to 29.8% ± 12.2% (P < .001) and TBR<70 mg/dL that decreased from 2.11 ± 2.4 to 2.07% ± 2.25% (P = .002). While all metrics statistically improved for the baseline GMI >8.0% group, the baseline GMI <7.0% group had unchanged TIR (77.4% ± 7.4% to 77.5% ± 8.0%, P = .456) and TAR>180 mg/dL that increased (19.2 ± 6.7 to 19.6 ± 7.9%, p < 0.001).
Conclusion:
Real-world HCL system use in the U.S. demonstrated overall glycemic control that trended similarly with the system pivotal trial outcomes and previous real-world system use analyses.
Keywords: automated insulin delivery, glucose management indicator, hybrid closed loop, real-world, time in range, type 1 diabetes
Introduction
From 2016-2018, continuous glucose monitoring (CGM) and insulin pump technology use in the United States increased from 7% to 30% and 57% to 63%, respectively. 1 However, only 17% of youth and 21% of adults achieved the American Diabetes Association (ADA) A1C goal of <7.5% and <7.0%, respectively. These rates, more than likely, did not reflect the true incidence of unmet glycemic goals by those with type 1 diabetes (T1D) in the United States, and even more so, now, as the recent ADA standards for medical care recommend an A1C of <7.0% for youth.2,3
Toward improved T1D glycemic goals and daily T1D management, innovation in automated insulin delivery has grown rapidly since the introduction of the first hybrid closed-loop (HCL) therapy. 4 The pivotal trials of this HCL system demonstrated improvements in A1C and time in target range (70-180 mg/dL) in adults, 5 adolescents 5 and children, 6 when compared with baseline run-in open-loop therapy. Although clinical trials can often involve a small number of study participants and selection bias, given specific trial eligibility criteria, assessments of real-world HCL therapy in larger T1D populations outside of clinical trial settings has increased.7-10
Prospective real-world HCL studies involving data collection in a clinical trial environment have demonstrated improvement (≥6 months) in glycemic outcomes that include A1C and time spent in range (TIR) in youth,11-13 but have primarily been single-center trials evaluating a selected cohort. Pragmatic evaluations of real-world HCL therapy through retrospective CGM data analyses7-10,14 have evolved to become an important method of outcomes assessment of more individuals for whom therapy was intended and within environments uninfluenced by highly controlled clinical trial settings. Indeed, the first real-world analysis of data from pediatric and adult users of the HCL system of interest 9 demonstrated findings that trended similarly with those observed in the system pivotal trials for the same age groups.5,6 The retrospective analysis, herein, reports on the real-world glycemic outcomes of over 100 000 individuals in the United States managing their T1D with this HCL system.
Methods
MiniMed™ 670G system (Medtronic, Northridge, CA) users with T1D living in the United States and reporting an age of ≥7 years voluntarily uploaded HCL system data to the CareLink™ personal software system (Medtronic) from March 2017 to November 2020. De-identified data comprising ≥10 days of CGM data underwent analysis, as previous report indicated this minimal duration of time sufficient for demonstrating 3 months of glycemic control. 15 In this study, the mean of the percentage of time spent in Auto Mode; mean and standard deviation (mean ± SD) of the glucose management indicator (GMI, 3.31 + 0.02392 × [mean SG]) 16 ; mean ± SD of the percentage of time spent in range (TIR, 70-180 mg/dL), below range (TBR<54 and TBR <70 mg/dL) and above range (TAR>180 and TAR>250 mg/dL); mean ± SD of SG; and mean of coefficient of variation of SG (CV) were determined after Auto Mode initiation for the 24-hour day, daytime (6:01AM to 11:59PM) and nighttime (12:00AM to 6:00AM) periods for the overall cohort who initiated Auto Mode for the first time.
The overall cohort data underwent a descriptive analysis which was followed by a subanalysis of similar metrics of users with ≥10 days of CGM data before and after Auto Mode initiation. These data were compared with t test (for normally distributed data) or Wilcoxon signed-rank test for nonparametric data. For some analyses, the average total daily dose of insulin (TDD) and the basal insulin (as a percentage of TDD) were determined, before and after Auto Mode initiation. A longitudinal analysis of data from users with ≥10 days of CGM data before Auto Mode initiation and at 30-day intervals (ie, first, sixth, and 12th months) after initiation was conducted with 1-way analysis of variance (ANOVA). A subgroup analysis of metrics (ie, percentage of mean time spent in Auto Mode; mean ± SD of GMI, SG, percentage of time at SG ranges and SD of SG; and the mean CV of SG) and insulin delivered before and after Auto Mode initiation was also conducted based on baseline glycemic control level (ie, GMI of <7.0% vs GMI of >8.0%, before Auto Mode initiation).
Hybrid closed-loop system use, glycemic outcomes and TDD before and 3 months after Auto Mode initiation in this study (N = 19 929 users) were compared with those in the first real-world HCL system use analysis (N = 3141 users) 9 and presented alongside the HCL system pivotal trial outcomes in adolescents and adults (N = 124 study participants). 5 All statistical analyses were conducted with SAS™ 9.4 (SAS Institute, Cary, NC). Given the large sample size and multiple comparisons in this retrospective analysis, a P value of < .001 was considered statistically significant.
Results
A total of 189 819 HCL system users in the United States uploaded data to the CareLink™ personal software system from March 2017 to November 2020. Initial retrospective analysis determined that an overall cohort of 123 555 users had ≥10 days of CGM data, after initiating Auto Mode for the first time. A summary of their percentage of time in Auto Mode and glycemic outcomes during the 24-hour day and the percentage of time spent at sensor glucose ranges for the daytime and nighttime periods are shown in Table 1.
Table 1.
Real-World HCL System Performance and Glycemic Outcomes After Auto Mode Initiation.
| Overall cohort | Before Auto Mode initiation | After Auto Mode initiation | P a | |
|---|---|---|---|---|
| n = 123 555 | n = 52 941 | |||
| 24-hour day | ||||
| Auto Mode, % | 87.9 | — | 88.6 | — |
| GMI, % | 7.0 ± 0.4 | 7.3 ± 0.6 | 7.1 ± 0.4 | <.001 |
| SG, mg/dL | 156 ± 56 | 168 ± 57 | 160 ± 54 | <.001 |
| CV, % | 36.1 | 34.0 | 33.7 | <.001 |
| Percentage of time spent at sensor glucose ranges | ||||
| <54 mg/dL | 0.59 ± 0.9 | 0.57 ± 1.0 | 0.58 ± 1.0 | <.001 |
| <70 mg/dL | 2.2 ± 2.1 | 2.1 ± 2.4 | 2.1 ± 2.3 | .002 |
| 70-180 mg/dL | 70.4 ± 11.2 | 61.5 ± 15.1 | 68.1 ± 11.9 | <.001 |
| >180 mg/dL | 27.5 ± 11.6 | 36.3 ± 15.7 | 29.8 ± 12.2 | <.001 |
| >250 mg/dL | 6.7 ± 6.2 | 10.7 ± 9.4 | 7.8 ± 7.1 | <.001 |
| Daytime | ||||
| Percentage of time spent at sensor glucose ranges | ||||
| <54 mg/dL | 0.59 ± 0.9 | 0.56 ± 1.0 | 0.57 ± 1.0 | <.001 |
| <70 mg/dL | 2.3 ± 2.3 | 2.2 ± 2.5 | 2.1 ± 2.4 | <.001 |
| 70-180 mg/dL | 68.0 ± 11.9 | 60.4 ± 15.4 | 66.0 ± 12.6 | <.001 |
| >180 mg/dL | 29.7 ± 12.5 | 37.5 ± 16.1 | 31.9 ± 13.1 | <.001 |
| >250 mg/dL | 7.4 ± 6.8 | 11.4 ± 10.0 | 8.5 ± 7.7 | <.001 |
| Nighttime | ||||
| Percentage of time spent at sensor glucose ranges | ||||
| <54 mg/dL | 0.58 ± 1.0 | 0.58 ± 1.2 | 0.58 ± 1.1 | .398 |
| <70 mg/dL | 2.0 ± 2.4 | 2.0 ± 2.8 | 2.0 ± 2.5 | <.001 |
| 70-180 mg/dL | 74.2 ± 12.4 | 63.5 ± 17.4 | 71.8 ± 13.5 | <.001 |
| >180 mg/dL | 23.8 ± 12.6 | 34.5 ± 18.0 | 26.3 ± 13.7 | <.001 |
| >250 mg/dL | 5.5 ± 6.0 | 9.5 ± 10.0 | 6.5 ± 7.1 | <.001 |
Data are shown as mean or mean ± SD. Daytime was 6:01 AM to 11:59 PM and nighttime was 12:00 AM to 6:00 AM. The percentage of time spent in Auto Mode and glycemic outcomes of the overall cohort of users (N = 123 555) with ≥10 days of CGM data after Auto Mode initiation and users (N = 52 941) with ≥10 days of CGM data before and after Auto Mode initiation are shown, for the 24-hour day, daytime and nighttime periods.
Abbreviations: HCL, hybrid closed loop; GMI, glucose management indicator; SG, sensor glucose; CV, coefficient of variation of SG; CGM, continuous glucose monitoring.
Comparison of before versus after Auto Mode initiation.
While the daytime TBR<54 mg/dL was comparable with that of the 24-hour day period, daytime TIR was lower (68.0% ± 11.9% vs 70.4% ± 11.2%), and daytime TBR<70 mg/dL (2.3% ± 2.3% vs 2.2% ± 2.1%), TAR>180 mg/dL (29.7% ± 12.5% vs 27.5% ± 11.6%), and TAR>250 mg/dL (7.4% ± 6.8% vs 6.7% ± 6.2%) were greater (P < .001 for all). In comparison with daytime outcomes, those during the nighttime period were statistically improved (P < .001 for all).
Impact of Real-World Auto Mode Initiation
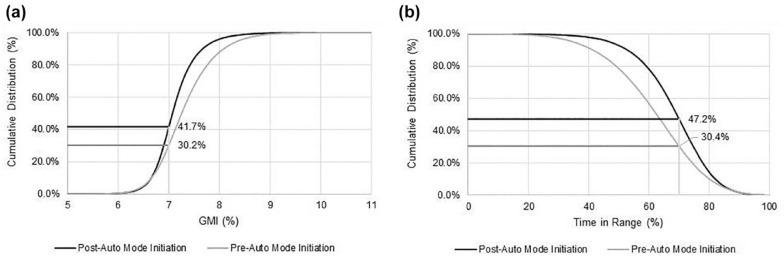
The glycemic outcomes of 52 941 users with ≥10 days of CGM data for both the pre-Auto Mode and post-Auto Mode initiation periods were also analyzed for the 24-hour day, daytime and nighttime (Table 1). Overall glycemic control (ie, GMI of <7.0%) for this group, before and after Auto Mode initiation, is shown in Figure 1a, where the proportion achieving the GMI of <7.0% increased from 30.2% to 41.7%. A similar increase in TIR of >70% was also observed (Figure 1b). Those achieving the recommended TIR goal, in addition to the <4% of time at TBR<70 mg/dL goal, also increased; from 24.6% pre-Auto Mode initiation to 40.7% post-Auto Mode initiation.
Figure 1.
Impact of hybrid closed-loop system Auto Mode initiation on achieved treatment goals for GMI and TIR. Comparison of the cumulative distribution of system users (N = 52 941) achieving the (a) GMI of <7.0% and (b) TIR of >70.0% goals, pre-Auto Mode and post-Auto Mode initiation. Abbreviation: GMI, glucose management indicator; TIR, time in range.
For 2627 users, and as early as 1 month after Auto Mode initiation (>80% of time in Auto Mode), GMI was reduced by 0.2% (P < .001) and SG was reduced by 10 mg/dL (P < .001), although CV increased by 1.0% (P < .001) (Table 2). These metrics remained relatively unchanged over time (ie, up to 12 months post-Auto Mode initiation). Compared with the period before Auto Mode initiation, the percentage of time spent at SG ranges was statistically improved with TIR increasing from 63.7% ± 15% to 72.6% ± 10.3% (P < .001) for the first month, to 71.8% ± 11.4% (P < .001) for the sixth month and to 72.0% ± 11.9% (P < .001) for the 12th month. At each of these time points, there was a trending increase in TDD (Table 2).
Table 2.
Real-World HCL System Performance, Glycemic Outcomes, and Insulin Delivered Before and up to 1 Year After Auto Mode Initiation.
| Before Auto Mode initiation | After Auto Mode initiation | ||||||
|---|---|---|---|---|---|---|---|
| First month | Sixth month | 12th month | |||||
| Metric | P a | Metric | P a | Metric | P a | ||
| Auto Mode, % | — | 95.8 | — | 92.4 | — | 91.6 | — |
| GMI, % | 7.2 ± 0.5 | 7.0 ± 0.3 | <.001 | 7.0 ± 0.4 | <.001 | 7.0 ± 0.4 | <.001 |
| SG, mg/dL | 164 ± 12.0 | 154 ± 11 | <.001 | 154 ± 12 | <.001 | 154 ± 12 | <.001 |
| CV, % | 32.0 | 33.0 | <.001 | 33.0 | <.003 | 33.0 | <.001 |
| Percentage of time spent at sensor glucose ranges | |||||||
| <54 mg/dL | 0.5 ± 0.8 | 0.4 ± 0.6 | <.995 | 0.5 ± 0.9 | <.002 | 0.6 ± 1.1 | <.001 |
| <70 mg/dL | 2.0 ± 2.2 | 1.8 ± 1.8 | <.518 | 2.1 ± 2.4 | <.096 | 2.2 ± 2.7 | <.006 |
| 70-180 mg/dL | 63.7 ± 15.0 | 72.6 ± 10.3 | <.001 | 71.8 ± 11.4 | <.001 | 72.0 ± 11.9 | <.001 |
| >180 mg/dL | 34.3 ± 15.6 | 25.7 ± 10.3 | <.001 | 26.1 ± 11.6 | <.001 | 25.8 ± 12.2 | <.001 |
| >250 mg/dL | 9.2 ± 8.6 | 5.6 ± 5.3 | <.001 | 6.0 ± 6.0 | <.001 | 6.0 ± 6.3 | <.001 |
| TDD, units | 50.1 ± 29.8 | 50.9 ± 30.2 | <.001 | 51.7 ± 30.8 | <.001 | 52.3 ± 31.0 | <.001 |
| Total basal insulin, b % | 50.5 ± 33.9 | 50.3 ± 34.0 | <.001 | 50.1 ± 34.6 | <.004 | 50.1 ± 34.4 | <.040 |
Data are shown as mean or mean ± SD. The percentage of time spent in Auto Mode, glycemic outcomes, and insulin delivered of users (N = 2627) with ≥10 days of continuous glucose monitoring data before Auto Mode initiation and at 1, 6, and 12 months after Auto Mode initiation are shown. The post-Auto Mode initiation total basal insulin, shown as a percentage of TDD, includes automated basal (micro-boluses) insulin and pre-programmed open-loop basal insulin.
Abbreviations: HCL, hybrid closed-loop; GMI, Glucose management indicator; SG, Sensor glucose; CV, Coefficient of variation of SG; TDD, total daily insulin dose.
Comparison of before versus after Auto Mode initiation.
Percentage of TDD.
The percentage of time in Auto Mode, glycemic outcomes, and insulin delivered of real-world users who had a GMI of <7.0% (n = 6388) and a GMI of >8.0% (n = 15 999) before Auto Mode initiation were also analyzed (Table 3). The group with higher baseline GMI showed statistically significant improvement in all glycemic metrics after Auto Mode initiation, with increased TDD and basal insulin. In contrast, the group with baseline GMI <7.0% had small but statistically significant increases in GMI and SG from 6.7% ± 0.2% to 6.8% ± 0.3% (P < .001) and 142 ± 47 mg/dL to 144 ± 46 mg/dL (P < .001), respectively, after Auto Mode initiation. The TIR of this group was unchanged (P = .456). While time spent in hypoglycemic ranges was reduced (P < .001), time spent at >180 and >250 mg/dL were increased. Although basal insulin for this group was somewhat decreased after Auto Mode initiation, the TDD increased by only 1.8 units, which was only one-third of the increase observed in TDD for the higher baseline GMI group.
Table 3.
Real-World HCL System Performance, Glycemic Outcomes, and Insulin Delivered Based on Glycemic Control Before Auto Mode Initiation.
| GMI of <7.0% before Auto Mode initiation (n = 6388) |
GMI of >8.0% before Auto Mode initiation (n = 15 999) |
|||||
|---|---|---|---|---|---|---|
| Before Auto Mode initiation | After Auto Mode initiation | P a | Before Auto Mode initiation | After Auto Mode initiation | P a | |
| Auto Mode, % | — | 87.1 | — | — | 84.4 | — |
| GMI, % | 6.7 ± 0.2 | 6.8 ± 0.3 | <.001 | 8.4 ± 0.4 | 7.7 ± 0.5 | <.001 |
| SG, mg/dL | 142.0 ± 46.5 | 144.4 ± 45.8 | <.001 | 213.6 ± 72.0 | 184.4 ± 66.1 | <.001 |
| CV, % | 32.8 | 31.7 | — | 36.0 | 34.0 | — |
| Percentage of time spent at sensor glucose ranges | ||||||
| <54 mg/dL | 0.9 ± 1.4 | 0.8 ± 1.4 | <.001 | 0.2 ± 0.4 | 0.4 ± 0.5 | <.001 |
| <70 mg/dL | 3.4 ± 3.3 | 2.9 ± 3.1 | <.001 | 0.8 ± 1.0 | 1.3 ± 1.3 | <.001 |
| 70-180 mg/dL | 77.4 ± 7.4 | 77.5 ± 7.9 | .456 | 34.9 ± 8.0 | 53.3 ± 12.5 | <.001 |
| >180 mg/dL | 19.2 ± 6.7 | 19.6 ± 7.9 | <.001 | 64.2 ± 8.4 | 45.4 ± 12.7 | <.001 |
| >250 mg/dL | 2.9 ± 2.1 | 3.2 ± 2.8 | <.001 | 29.6 ± 9.2 | 17.4 ± 10.4 | <.001 |
| TDD, units | 48.4 ± 27.6 | 50.2 ± 28.3 | <.001 | 55.7 ± 30.8 | 62.2 ± 35.8 | <.001 |
| Total basal insulin, b % | 49.3 ± 14.7 | 47.4 ± 14.7 | .199 | 49.7 ± 16.6 | 56.9 ± 21.6 | <.001 |
Data are shown as mean or mean ± SD. The percentage of time spent in Auto Mode, glycemic outcomes, and insulin delivered of users with ≥10 days of continuous glucose monitoring data before and after Auto Mode initiation, based on the average GMI <7.0% and GMI >8.0% before Auto Mode initiation are shown. The post-Auto Mode initiation total basal insulin, shown as a percentage of TDD, includes automated basal (micro-boluses) insulin and pre-programmed open-loop basal insulin.
Abbreviations: HCL, hybrid closed-loop; GMI, Glucose management indicator; SG, Sensor glucose; CV, Coefficient of variation of SG; TDD, total daily insulin dose.
Comparison of before versus after Auto Mode initiation.
Percentage of TDD.
Outcomes Comparison From the HCL System Pivotal Trial and the Real-World HCL System Use Analyses
The percentage of time in Auto Mode, glycemic outcomes, and total insulin delivered before and three months after Auto Mode initiation during the HCL system pivotal trial, 5 the first real-world analysis of the HCL system 9 and the present real-world analysis of the HCL system were qualitatively assessed (Table 4). For a mean of 82.2% of time in Auto Mode, the pivotal trial study phase demonstrated increased TIR from 67.1% to 72.4% and reductions in both TAR>180 and TBR < 70 mg/dL, compared with open-loop therapy during the run-in period. For the first and present real-world analyses, where mean time in Auto Mode was 80.8% and 89.4%, respectively, a statistically significant increase in TIR was also observed. Time spent in hyperglycemic ranges was statistically reduced and time spent in hypoglycemic ranges was reduced or unchanged, after Auto Mode initiation. For the pivotal trial and both real-world analyses, TDD was statistically increased during Auto Mode use.
Table 4.
Comparison of HCL System Pivotal Trial Outcomes and Real-World HCL System Use Analyses.
| HCL system pivotal trial
a
(N = 124) |
First real-world HCL system use analysis
a
(n = 3141) |
Present study real-world HCL system use analysis (n = 19 929) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Auto Mode initiation (Run-in) | After Auto Mode initiation (Study) | P b | Before Auto Mode initiation | 3 months after Auto Mode initiation | P b | Before Auto Mode initiation | 3 months after Auto Mode initiation | P b | |
| Auto Mode, % | — | 82.2 | — | — | 80.8 | — | — | 89.4 | — |
| SG, mg/dL | 150 | 151 | .267 | 159 | 152 | <.001 | 167 | 158 | <.001 |
| <54 mg/dL | 1.3 | 0.8 | <.001 | 0.6 | 0.5 | <.001 | 0.5 | 0.6 | <.017 |
| <70 mg/dL | 5.5 | 3.0 | <.001 | 2.7 | 2.1 | <.001 | 2.1 | 2.1 | .117 |
| 70-180 mg/dL | 67.1 | 72.4 | <.001 | 66.0 | 73.3 | <.001 | 62.4 | 69.3 | <.001 |
| >180 mg/dL | 27.4 | 24.5 | <.001 | 31.4 | 24.6 | <.001 | 35.6 | 28.7 | <.001 |
| >250 mg/dL | 6.9 | 5.6 | .007 | 8.1 | 5.4 | <.001 | 10.2 | 7.2 | <.001 |
| TDD, units | 47.5 | 50.9 | <.001 | 49.8 | 51.9 | <.001 | 50.1 | 51.1 | <.001 |
Data are shown as mean. The pivotal trial Run-in period (before Auto Mode initiation) was ~2 weeks and the Study period was 3 months. The mean percentage of time in Auto Mode, sensor glucose, percentage of time in sensor glucose ranges, and total daily insulin dose delivered before and 3 months after Auto Mode initiation are shown for the HCL system pivotal trial, the first real-world HCL system use analysis and the present real-world HCL system use analysis.
Abbreviations: HCL, hybrid closed-loop; SG, sensor glucose; TDD, total daily insulin dose.
As reported in Stone et al. 9
Comparison of before versus after Auto Mode initiation.
Discussion
This retrospective analysis of real-world data from more than 100 000 individuals with T1D in the United States, who initiated HCL system Auto Mode, demonstrates overall glycemic outcomes that met the recommended treatment goals for (estimated) A1C and time spent at sensor glucose ranges established by the American Diabetes Association2,3 and the international consensus for time at sensor glucose ranges. 17 In this study, the overall group initiating Auto Mode achieved a TIR of 70.4%, 68.0%, and 74.2% for the 24-hour, daytime, and nighttime periods, respectively, which were similar to the TIR of 72.0%, 70.1%, and 77.4% observed for these periods in the real-world analysis of HCL system use in Europe. 8
In this study, and compared with pre-Auto Mode initiation, Auto Mode afforded ~50 000 users improved mean SG that was reduced from 168 to 160 mg/dL, mean TIR that increased by 6.6%, mean TAR>180 mg/dL that reduced by 6.5% and mean TAR>250 mg/dL that reduced by 2.9%. With Auto Mode initiation, a greater proportion of system users achieved the recommended GMI of <7.0% and TIR of >70.0%, in addition to the combined targets of TIR >70% and TBR < 70 mg/dL of <4% (24.6% vs 40.7%). The use of this two-factor glycemic control assessment has been published in recent reports8,18,19 and provides a more comprehensive picture of glycemia with CGM-derived metrics. Recently, the combination of a safety metric (%TBR, low blood glucose index or LBGI, Hypoglycemia Index, GRADE hypoglycemia or frequency of hypoglycemic events per year) and an efficacy metric (mean glucose, HbA1C, %TIR, or %TAR) has been proposed to provide the essential data for the quality of glycemic control. 20
The recent report of real-world HCL system use in Europe demonstrated substantial benefits for diabetes management regardless of baseline glycemic control (ie, a GMI level of <7.0% vs >8.0%). 8 In that analysis, the impact of Auto Mode initiation appeared greater in the outcomes of the baseline GMI >8.0% group. The authors attributed this to the greater increase in TDD (10.1 units vs 5.7 units) and basal insulin (9.1 vs −1.8 units) delivered in this group, when compared with the TDD and basal insulin delivered in the baseline GMI <7.0% group, although more time was spent in Auto Mode (GMI <7.0%: 81.7% vs GMI >8.0%: 74.3%). In this study, there was a similar improvement in all glycemic metrics for the baseline GMI >8.0% group, after Auto Mode initiation. For the baseline GMI <7.0% group, however, the TIR (although a high 77.4% before Auto Mode initiation) did not change and the time spent at <70 mg/dL was significantly reduced by 0.5%. The different outcomes observed in these groups, post Auto Mode initiation, appeared partly due to insulin delivery, as well. The TDD was statistically increased for both, although by 12.7% (6.5 units) in the baseline GMI >8.0% group but by only 3.7% (1.8 units) in the baseline GMI <7.0% group.
For the Da Silva et al and present real-world analyses, basal insulin delivered after Auto Mode initiation (which included automated basal [micro-boluses] insulin and pre-programmed open-loop basal insulin) statistically increased by 14.5% and 19.1%, respectively, for the groups with less glycemic control at baseline. In contrast, and for both analyses, basal insulin reduced by 3.9% for the groups with a baseline GMI <7.0%. Thus, both real-world analyses reveal a significant role for bolus insulin delivered, alongside automated basal insulin delivery, for the baseline GMI <7.0% groups. While this study did not assess the number of daily insulin boluses delivered, Da Silva et al reported that the baseline GMI <7.0% group delivered more daily insulin boluses both before (6.3 ± 2.5 boluses vs 5.8 ± 2.4 boluses) and after (6.5 ± 2.1 boluses vs 5.5 ± 1.9 boluses) Auto Mode initiation, when compared with the baseline GMI >8.0% group. This finding provides insight into relevant real-world differences in dosing behaviors between groups with T1D that should be further investigated.
The lack of randomization, participant demographics, medical history (eg, baseline laboratory-determined A1C) and a control group (outside of the “before Auto Mode initiation” period) are limitations of this retrospective study. In addition, the personal information (eg, age and diabetes type) uploaded to the CareLink™ personal software at enrollment is based on user input and not verifiable. Like the longitudinal assessment of HCL system use in Da Silva et al, 8 this study observed high user attrition over time, for which all the causes are not known. These may include system upload cessation over time, given the duration of time that the HCL system has been an available diabetes technology therapy in the United States. Data upload frequency to the CareLink™ personal software system is highly dependent on the HCL system user, or family member caring for the HCL system user, and represents a confounding factor that would be minimized in a controlled clinical trial setting. Next-generation systems that automatically upload insulin delivery and CGM information (with system user consent), will mitigate upload burden and enable more complete data analyses over time. The sustained outcomes findings from the longitudinal analysis likely represent an adherence or selection bias as they are based on HCL system users who may have followed a routine upload regimen and/or other behaviors facilitating good glycemic control, in comparison with other users.
While discontinuation of system use in spite of improved glycemic outcomes has been a noted issue in trials or analyses of the HCL system when used ≥6 months,11,21-23 other prospective12,24 and retrospective25,26 studies have demonstrated improvements in glycemic control, while maintaining study participant enrollment. Despite the observed user attrition in this study, the time spent in Auto Mode during real-world HCL system use and the resultant glycemic outcomes demonstrate statistically improved or maintained glycemic control in the analyzed longitudinal cohort.
Strengths of this study include the large number of HCL system users in a before-and-after Auto Mode initiation comparison and a longitudinal analysis across 1 year of system use. While outcomes of the HCL system pivotal clinical trial and the first real-world cohort could not be compared statistically, this study shows that most glycemic outcomes results were like those observed in the pivotal trial. Overall, present study findings and those from the real-world analysis in Da Silva et al 8 allow generalizability of findings to a broader population of individuals who may use the HCL system in the at-home setting.
Conclusions
Real-world use of the HCL system Auto Mode in the United States showed improved GMI, TIR, and other glycemic metrics for most users. These data, alongside those from the first real-world HCL system analysis in 3141 users and those from the recent analysis of over 14 000 real-world HCL system users throughout Europe, validate the clinical outcomes from the HCL system pivotal trials. The study findings also demonstrate that Auto Mode allows a greater number of individuals with diabetes to achieve ADA and international consensus-recommended glycemic goals.
Acknowledgments
The authors thank Andrew R. Rhinehart, MD, and Richard A. M. Jonkers, PhD, for their review of the manuscript.
Footnotes
Abbreviations: CGM, Continuous Glucose Monitoring; GMI, Glucose Management Indicator; HCL, Hybrid Closed Loop; SG, Sensor Glucose; CV, Coefficient of Variation of Sensor Glucose; TIR, Time in Range; TBR, Time Below Range; TAR, Time Above Range; T1D, Type 1 Diabetes.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors are employees of Medtronic.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Medtronic.
ORCID iD: Toni L. Cordero  https://orcid.org/0000-0003-4597-4194
https://orcid.org/0000-0003-4597-4194
References
- 1.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S73-S84. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. 13. Children and adolescents: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S180-S199. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. MiniMed 670G system approval letter (P160017). Date unknown. http://www.accessdata.fda.gov/cdrh_docs/pdf16/P160017a.pdf. Accessed January 26, 2022.
- 5.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. Safety evaluation of the MiniMed 670G system in children 7-13 years of age with type 1 diabetes. Diabetes Technol Ther. 2019;21:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breton MD, Kovatchev BP.One year real-world use of the Control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021;23:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da Silva J, Bosi E, Jendle J, et al. Real-world performance of the MiniMed 670G system in Europe. Diabetes Obes Metab. 2021;23:1942-1949. [DOI] [PubMed] [Google Scholar]
- 9.Stone MP, Agrawal P, Chen X, et al. Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G system commercial launch. Diabetes Technol Ther. 2018;20:689-692. [DOI] [PubMed] [Google Scholar]
- 10.Tornese G, Buzzurro F, Carletti C, Faleschini E, Barbi E. Six-month effectiveness of advanced vs. standard hybrid closed-loop system in children and adolescents with type 1 diabetes mellitus. Front Endocrinol (Lausanne). 2021;12:766314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berget C, Messer LH, Vigers T, et al. Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020;21:310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrovski G, Al Khalaf F, Campbell J, et al. One-year experience of hybrid closed-loop system in children and adolescents with type 1 diabetes previously treated with multiple daily injections: drivers to successful outcomes. Acta Diabetol. 2021;58:207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messer LH, Berget C, Pyle L, et al. Real-world use of a new hybrid closed loop improves glycemic control in youth with type 1 diabetes. Diabetes Technol Ther. 2021;23:837-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Silva J, Lepore G, Battelino T, et al. Real-world performance of the MiniMed™ 780G system: first report of outcomes from 4120 users. Diabetes Technol Ther. 2022;24:113-119. doi: 10.1089/dia.2021.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddlesworth TD, Beck RW, Gal RL, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20:314-316. [DOI] [PubMed] [Google Scholar]
- 16.Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41:2275-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergenstal RM, Nimri R, Beck RW, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397:208-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson AL, Sherr JL, Shulman DI, et al. Safety and glycemic outcomes during the MiniMed Advanced Hybrid Closed-Loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2022;24:178-189. doi: 10.1089/dia.2021.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodbard D.Quality of glycemic control: assessment using relationships between metrics for safety and efficacy. Diabetes Technol Ther. 2021;23:692-704. [DOI] [PubMed] [Google Scholar]
- 21.Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21:319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong JC, Boyle C, DiMeglio LA, et al. Evaluation of pump discontinuation and associated factors in the T1D exchange clinic registry. J Diabetes Sci Technol. 2017;11:224-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM.One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019;42:2190-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAuley SA, Lee MH, Paldus B, et al. Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care. 2020;43:3024-3033. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz ME, Kaye WA, Pepper GM, et al. An analysis of Medtronic MiniMed 670G insulin pump use in clinical practice and the impact on glycemic control, quality of life, and compliance. Diabetes Res Clin Pract. 2021;177:108876. [DOI] [PubMed] [Google Scholar]
- 26.Lepore G, Scaranna C, Corsi A, Dodesini AR, Trevisan R.Switching from suspend-before-low insulin pump technology to a hybrid closed-loop system improves glucose control and reduces glucose variability: a retrospective observational case-control study. Diabetes Technol Ther. 2020;22:321-325. [DOI] [PubMed] [Google Scholar]