Abstract
Objectives:
Achieving optimal glycemic outcomes in young children with type 1 diabetes (T1D) is challenging. This study examined the durability of continuous glucose monitoring (CGM) coupled with a family behavioral intervention (FBI) to improve glycemia.
Study Design:
This one-year study included an initial 26-week randomized controlled trial of CGM with FBI (CGM+FBI) and CGM alone (Standard-CGM) compared with blood glucose monitoring (BGM), followed by a 26-week extension phase wherein the BGM Group received the CGM+FBI (BGM-Crossover) and both original CGM groups continued this technology.
Results:
Time in range (70-180 mg/dL) did not improve with CGM use (CGM+FBI: baseline 37%, 52 weeks 41%; Standard-CGM: baseline 41%, 52 weeks 44%; BGM-Crossover: 26 weeks 38%, 52 weeks 40%). All three groups sustained decreases in hypoglycemia (<70 mg/dL) with CGM use (CGM+FBI: baseline 3.4%, 52 weeks 2.0%; Standard-CGM: baseline 4.1%, 52 weeks 2.1%; BGM-Crossover: 26 weeks 4.5%, 52 weeks 1.7%, P-values <.001). Hemoglobin A1c was unchanged with CGM use (CGM+FBI: baseline 8.3%, 52 weeks 8.2%; Standard-CGM: baseline 8.2%, 52 weeks 8.0%; BGM-Crossover: 26 weeks 8.1%, 52 weeks 8.3%). Sensor use remained high (52-week study visit: CGM+FBI 91%, Standard-CGM 92%, BGM-Crossover 88%).
Conclusion:
Over 12 months young children with T1D using newer CGM technology sustained reductions in hypoglycemia and, in contrast to prior studies, persistently wore CGM. However, pervasive hyperglycemia remained unmitigated. This indicates an urgent need for further advances in diabetes technology, behavioral support, and diabetes management educational approaches to optimize glycemia in young children.
Keywords: young children, continuous glucose monitoring, glycemic control, hypoglycemia, behavioral intervention
Introduction
Achieving and maintaining target glycemic outcomes in very young children with type 1 diabetes (T1D) is challenging due to their unpredictable eating behaviors, erratic physical activity and moods, inability to articulate symptoms of hypo- and hyperglycemia,1,2 frequent intercurrent illnesses, and complete reliance on caregivers for diabetes management. Hence, young children with T1D spend little time in optimal glycemic range 3 and are at risk for both severe hypoglycemia and hyperglycemia.
Clinical trials of continuous glucose monitoring (CGM) devices have been shown to improve glycemic control in older children and adults4,5; however, data on CGM effectiveness in very young children are limited.6-8 CGMs provide real-time glucose data and alerts, as well as comprehensive retrospective glucose patterns and trends to inform diabetes management decisions for caregivers of young children with T1D. Recent improvements in CGM technology including smaller device size, enhanced accuracy, and ability to monitor data remotely offer great promise for leveraging CGM to optimize glycemic outcomes.
The Strategies to Enhance New CGM use in Early childhood (SENCE) study investigated the effects of CGM combined with family behavioral intervention (FBI) and CGM alone on glycemic outcomes compared with blood glucose monitoring (BGM) in young children with T1D in a 26-week randomized controlled trial. The SENCE study included 143 youth aged two to less than eight years and did not show a significant change in time in target glucose range (70-180 mg/dL) in either the CGM+FBI Group or Standard-CGM Group compared with BGM in the initial 26-week randomized phase. However, it did show significant reductions in time spent in hypoglycemia (<70 and <54 mg/dL) for both CGM groups compared to BGM during the 26-week randomized control period. 9
Subsequently, the current analysis was undertaken to examine the durability of CGM use and glycemic outcomes in a subsequent 26-week extension period. Specifically, the two initial CGM groups continued CGM use with less intensive follow-up, whereas the original BGM group crossed over to use CGM in combination with FBI training (BGM-Crossover Group) during the 26-week extension. Herein, we present results from the full 52-week study in all three treatment groups.
Methods
The study was conducted at 14 pediatric endocrinology practices in the United States with enrollment visits taking place between February 2017 and August 2018. The protocol and informed consent forms were approved by institutional review boards. Written informed consent was obtained from the parent or guardian of each participant and assent was obtained from each participant when applicable. Major eligibility criteria included clinical diagnosis of T1D for at least three months, age two to less than eight years, total daily insulin requirement ≥0.3 units/kg/d, no use of real-time CGM in 30 days prior to enrollment, and hemoglobin A1c (HbA1c) 7.0% to <10.0% (53-<86 mmol/mol). The full eligibility criteria have been published previously, 3 the protocol is available at https://public.jaeb.org/datasets, and details are provided on clinicaltrials.gov (NCT02912728).
26-Week Randomized Clinical Trial
All participants completed a two- to three-week prerandomization screening period using masked CGM (G4 Platinum® Professional; Dexcom, San Diego, CA) to collect baseline glucose data. Following successful completion of the screening period, participants were randomized to one of three groups: (1) CGM+FBI Group used the Dexcom G5 real-time CGM with standardized training and a family behavioral intervention, (2) Standard-CGM Group used the Dexcom G5 real-time CGM with standardized training without the family-based intervention, and (3) fingerstick BGM control group without CGM for a period of 26 weeks. 9 During the 26-week randomized phase, all study groups were matched for attention with a similar visit schedule. All groups were provided the same chart of glycemic targets (Supplemental Appendix). At four time points, BGM control group participants wore masked Dexcom G4 Platinum Professional CGM for one week in order to collect sensor glucose data.
26-Week Extension Phase
Following completion of the randomized trial, participants randomized to the BGM control group crossed over to initiate real-time CGM (BGM-Crossover Group) and received the standardized CGM training and FBI. All three groups were provided real-time Dexcom G5 CGM during this 26-week extension phase to wear through completion of the 52-week study (Figure 1). Of note, the Dexcom G5 CGM required calibrations every 12 hours, hence the study teams recommended participants perform fingerstick blood glucose checks at least three times a day to prevent overnight calibration alerts.
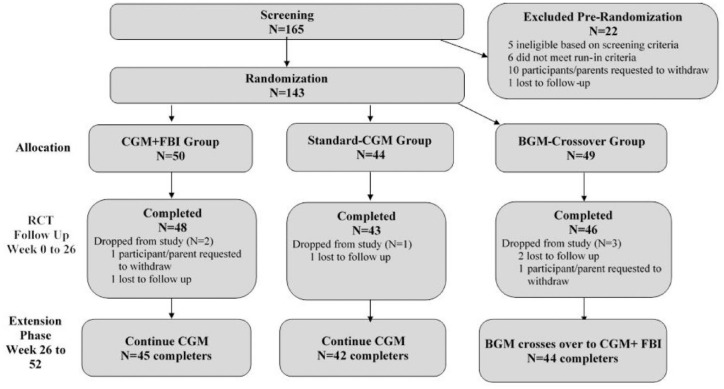
Figure 1.
Flowchart of study.
Abbreviations: CGM, continuous glucose monitoring; FBI, family behavioral intervention; BGM, blood glucose monitoring; RCT, randomized controlled trial.
In the 26-week extension phase, study contact was reduced in the CGM+FBI Group and Standard CGM Group to in-clinic visits at 39 and 52 weeks. The BGM-Crossover Group, who initiated real-time CGM at the 26-week visit, had a phone call at 27 weeks and in-clinic visits at 28, 39, and 52 weeks to receive standardized CGM education and FBI.
Training and Education
The standardized CGM education provided to the two CGM groups during the first 26 weeks and the BGM-Crossover Group in the extension phase involved five 30-minute standardized sessions of CGM training. These sessions were delivered by experienced diabetes educators and focused on how CGM works, CGM basics, troubleshooting and nonadjunctive use of CGM, using CGM to minimize highs and lows, and evaluating CGM data. Low glucose alerts were initially set to alarm when sensor glucose reached <80 mg/dL. The high alert was initially set to 400 mg/dL and was then lowered over the course of the CGM training sessions to 300 mg/dL at week 3 unless the family preferred not to make this change. The BGM-Crossover Group received the standardized CGM education sessions at the screening, 26-, 27-, 28-, and 39-week visits (Supplemental Table 1). Caregivers were also trained on use of remote CGM data monitoring through the Dexcom SHARE/FOLLOW applications.
The FBI provided to the CGM+FBI Group during the initial 26 weeks of the randomized trial and to the BGM-Crossover Group in the extension phase was developed based on survey and qualitative data from interviews with caregivers of young children with T1D.10-13 The BGM-Crossover Group received the FBI sessions at 26, 27, 28, and 39 weeks, with a review session at 52 weeks (Supplemental Table 1). Sessions were ~30 minutes, delivered via telephone or in person by a trained research assistant. The standardized intervention materials addressed obstacles to CGM use and caregiver feelings, attitudes, and behaviors related to effective and sustained CGM use, and interventionists taught caregivers relaxation, problem-solving, and communication strategies. Specifically, sessions included strategies to minimize CGM insertion pain and wearability concerns, discussion of parental expectations of CGM use, and education about glucose variability and links between glucose levels and mood. The FBI also covered discussions about parents’ emotional reactions to above and below range CGM data, review of how to treat above and below range glucose, and reframing attention to benefits of CGM alerts for optimizing diabetes management. Caregivers were counseled in seeking social support, communicating with children about their devices, and teaching other adults about CGM in planning for leaving the child with another caregiver.
Adverse Events
Reportable adverse events included severe hypoglycemia (defined as an event that required assistance from another person due to altered consciousness), hyperglycemia resulting in treatment at a health care facility or that involved diabetic ketoacidosis (as defined by the Diabetes Control and Complications Trial), 14 device-related events with potential effects on participant safety, and all serious adverse events regardless of causality.
Statistical Analysis
Glycemic outcomes included percent of time in target range (70-180 mg/dL), mean glucose, coefficient of variation, percent of time in hyperglycemia (>180, >250, and >300 mg/dL), percent of time in hypoglycemia (<70 and <54 mg/dL), rate of hypoglycemic events per week, and HbA1c. 15
CGM-measured outcomes were calculated at baseline using the masked data collected during the screening phase. For the CGM+FBI Group and Standard-CGM Group, CGM outcomes were calculated at each follow-up visit by pooling data from the four weeks prior. For the BGM control group, CGM outcomes were calculated using data from the masked CGM at each study visit in the initial 26 weeks of the randomized trial and then by pooling real-time CGM data from four weeks prior to study visits in the extension phase (BGM-Crossover Group).
HbA1c was measured by the central lab at randomization and 13, 26, 39, and 52 weeks at the University of Minnesota using the Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer. If the central lab result was missing, values were imputed using local results (lab or point of care), if available.
A paired t-test, signed rank test, or McNemar’s test, as appropriate, was used to evaluate the change in each glycemic outcome between baseline and 52 weeks in the CGM+FBI Group and Standard-CGM Group and between 26 and 52 weeks in the BGM-Crossover Group. In addition, a linear or logistic regression model was used to compare each glycemic outcome between the CGM+FBI Group and Standard-CGM Group at 52 weeks. The model was adjusted for baseline value of the outcome, baseline HbA1c, and clinical site as a random effect.
P-values were two-sided and adjusted for multiple comparisons to control the false discovery rate using the adaptive Benjamini-Hochberg procedure. 16 All analyses included participants with available data who completed the 52-week visit. Baseline refers to prerandomization measurements. Analyses were conducted with SAS software version 9.4 (SAS Institute Inc., Cary, NC).
Results
The full 52-week trial (RCT + extension) was completed by 131 of the 143 participants (92%), including 45 of 50 in the CGM+FBI Group, 42 of 44 in the Standard-CGM Group, and 44 of 49 in the BGM-Crossover Group. Six participants dropped from the study during the initial 26 weeks (two in the CGM +FBI Group, one in the Standard-CGM Group, and three in the BGM control group) and six dropped during the extension phase (three in the CGM+FBI Group, one in the Standard-CGM Group, and two in the BGM-Crossover Group) (Figure 1).
At baseline, the 131 children were 5.7 ± 1.8 years of age, with 2.3 ± 1.9 years duration of diabetes. Participants were 50% female; 69% identified as non-Hispanic white, 13% as non-Hispanic Black, 12% as Hispanic or Latino, <1% as Asian, and 5% as another or more than one race/ethnicity. Sixty-two percent had private insurance. Thirty-five percent were using an insulin pump and 87% were CGM naïve at baseline. Baseline participant characteristics according to treatment randomization group are shown in Table 1.
Table 1.
Participant Characteristics at Baseline.
| Overall
a
(N = 131) |
CGM + FBI Group (N = 45) |
Standard-CGM Group (N = 42) |
BGM-Crossover Group (N = 44) |
|
|---|---|---|---|---|
| Age (years), mean ± SD | 5.7 ± 1.8 | 5.7 ± 1.6 | 5.1 ± 1.8 | 6.1 ± 1.7 |
| Duration of T1D (years), mean ± SD | 2.3 ± 1.9 | 2.4 ± 1.9 | 1.8 ± 1.7 | 2.7 ± 2.0 |
| Prior CGM use, N (%) | ||||
| Yes, but no recent use | 17 (13%) | 5 (11%) | 5 (12%) | 7 (16%) |
| Never | 114 (87%) | 40 (89%) | 37 (88%) | 37 (84%) |
| Insulin pump use, N (%) | 46 (35%) | 14 (31%) | 13 (31%) | 19 (43%) |
| Sex: Female, N (%) | 65 (50%) | 26 (58%) | 17 (40%) | 22 (50%) |
| Race/ethnicity, N (%) | ||||
| White, non-Hispanic | 88 (69%) | 30 (68%) | 31 (76%) | 27 (63%) |
| Black, non-Hispanic | 17 (13%) | 7 (16%) | 4 (10%) | 6 (14%) |
| Hispanic or Latino | 15 (12%) | 5 (11%) | 5 (12%) | 5 (12%) |
| Asian | 1 (<1%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Other/more than one race | 7 (5%) | 2 (5%) | 1 (2%) | 4 (9%) |
| Annual household income, N (%) | ||||
| <$35 000 | 21 (17%) | 6 (14%) | 10 (25%) | 5 (13%) |
| $35 000 to <$75 000 | 52 (43%) | 18 (43%) | 18 (45%) | 16 (41%) |
| ≥$75 000 | 48 (40%) | 18 (43%) | 12 (30%) | 18 (46%) |
| Highest parent education, N (%) | ||||
| High school or less | 28 (23%) | 10 (24%) | 9 (24%) | 9 (20%) |
| Some college/Associates degree | 42 (34%) | 11 (26%) | 14 (37%) | 17 (39%) |
| Bachelor’s degree or higher | 54 (44%) | 21 (50%) | 15 (39%) | 18 (41%) |
| Health insurance, N (%) | ||||
| Private | 80 (62%) | 27 (60%) | 26 (62%) | 27 (64%) |
| Other | 47 (36%) | 17 (38%) | 15 (36%) | 15 (36%) |
| None | 2 (2%) | 1 (2%) | 1 (2%) | 0 (0%) |
| HbA1c (%), mean ± SD | 8.2 ± 0.8 | 8.3 ± 0.8 | 8.2 ± 0.8 | 8.2 ± 0.7 |
| Total daily insulin units per kg, mean ± SD | 0.68 ± 0.22 | 0.68 ± 0.23 | 0.65 ± 0.23 | 0.69 ± 0.20 |
| BMI Percentile, median (IQR) | 74 (53, 92) | 70 (55, 90) | 77 (59, 95) | 72 (50, 90) |
Abbreviations: CGM, continuous glucose monitoring; FBI, family behavioral intervention; BGM, blood glucose monitoring; BMI, body mass index; IQR, interquartile range.
Missing data: race/ethnicity 3 (2%), income 10 (8%), parent education 7 (5%), health insurance 2 (2%), total daily insulin 1 (<1%).
Glycemic Outcomes in the Standard-CGM Group and CGM+FBI Group
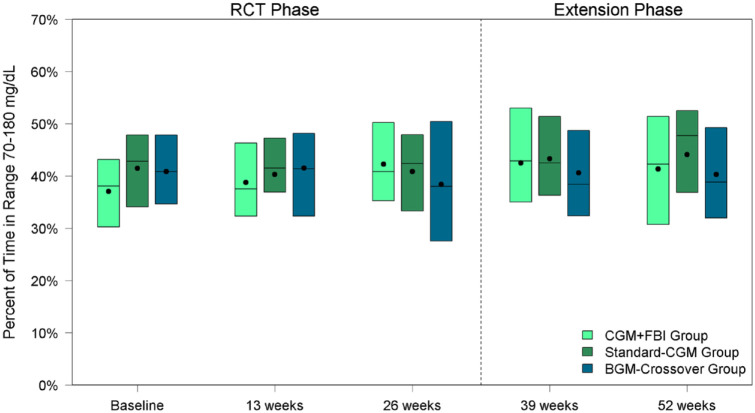
Time in target range (70-180 mg/dL) was low at baseline and did not significantly change over the study. The mean time in target range was 37% ± 12% at baseline and 41% ± 12% at 52 weeks for the CGM+FBI Group and 41% ± 10% at baseline and 44% ± 13% at 52 weeks for the Standard-CGM Group. Neither change was statistically significant within groups (P-value baseline vs 52 weeks >.05 for both groups) nor was there a difference between treatment groups at 52 weeks (P = 0.82) (Table 2, Figure 2). Similarly, there were no statistically significant changes in mean glucose or HbA1c from baseline to 52 weeks during the study period in either group.
Table 2.
Glycemic Outcomes in the CGM Groups.
| CGM + FBI Group | Standard-CGM Group | P-value for treatment group difference at 52 weeksb,c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 26 weeks | 52 weeks |
P-value Baseline vs 52 weeksa,b |
Baseline | 26 weeks | 52 weeks |
P-value Baseline vs 52 weeksa,b |
||
| CGM-measured outcomes | |||||||||
| N | 45 | 45 | 43 | 42 | 42 | 42 | |||
| Hours of data | 307 (282, 352) |
629 (579, 642) |
626 (535, 648) |
301 (282, 345) |
609 (545, 640) |
626 (571, 646) |
|||
| Glucose control | |||||||||
| % Time in range 70-180 mg/dL | 37 ± 12 | 42 ± 11 | 41 ± 12 | .09 | 41 ± 10 | 41 ± 10 | 44 ± 13 | .14 | .82 |
| % Time in range 70-180 mg/dL >60% | 1 (2%) | 2 (4%) | 3 (7%) | .32 | 1 (2%) | 0 (0%) | 5 (12%) | .10 | .98 |
| Mean glucose (mg/dL) | 213 ± 37 | 203 ± 26 | 204 ± 27 | .26 | 198 ± 28 | 203 ± 25 | 198 ± 30 | .97 | .82 |
| Coefficient of variation (%) | 43 ± 7 | 40 ± 4 | 40 ± 5 | .002 | 44 ± 7 | 39 ± 5 | 39 ± 6 | <.001 | .82 |
| Hyperglycemia | |||||||||
| % Time > 180 mg/dL | 58 ± 14 | 55 ± 12 | 56 ± 12 | .59 | 53 ± 13 | 57 ± 11 | 53 ± 14 | .89 | .82 |
| % Time > 250 mg/dL | 33 (26, 40) | 29 (17, 36) | 27 (21, 37) | .05 | 26 (22, 33) | 26 (19, 33) | 22 (14, 34) | .22 | .82 |
| % Time > 300 mg/dL | 18 (11, 24) | 14 (7, 21) | 13 (9, 18) | .004 | 13 (10, 18) | 12 (6, 17) | 10 (5, 16) | .14 | .82 |
| Hypoglycemia | |||||||||
| % Time <70 mg/dL | 3.4 (1.8, 7.1) |
1.9 (1.2, 3.1) |
2.0 (1.2, 3.7) |
<.001 | 4.1 (1.7, 8.4) |
1.7 (0.8, 3.1) |
2.1 (0.9, 4.2) |
<.001 | .90 |
| Meeting target of <4% time <70 mg/dL | 25 (56%) | 35 (78%) | 33 (77%) | .02 | 20 (48%) | 33 (79%) | 31 (74%) | .005 | .98 |
| % Time <54 mg/dL | 1.2 (0.4, 2.8) |
0.3 (0.2, 0.7) |
0.5 (0.2, 0.8) |
<.001 | 1.4 (0.2, 3.1) |
0.5 (0.1, 0.9) |
0.5 (0.1, 1.1) |
<.001 | .82 |
| Meeting target of <1% time <54 mg/dL | 20 (44%) | 34 (76%) | 34 (79%) | <.001 | 18 (43%) | 33 (79%) | 31 (74%) | .003 | .98 |
| Hypoglycemic events per week d | 1.7 (1.0, 3.1) |
0.7 (0.3, 2.0) |
1.0 (0.3, 1.5) |
<.001 | 2.3 (0.6, 3.6) |
1.0 (0.3, 2.2) |
0.9 (0.3, 1.9) |
.002 | .98 |
| HbA1c | |||||||||
| N | 45 | 45 | 45 | 42 | 42 | 42 | |||
| HbA1c | 8.3 ± 0.8 | 8.1 ± 0.8 | 8.2 ± 0.9 | .63 | 8.2 ± 0.8 | 8.2 ± 0.8 | 8.0 ± 0.9 | .20 | .82 |
Data are mean ± SD, median (IQR), or N (%).
Abbreviations: CGM, continuous glucose monitoring; FBI, family behavioral intervention; IQR, interquartile range.
P-value from a paired t-test, signed rank test, or McNemar’s test, as appropriate. Only includes participants who had values at both time points.
P-values are adjusted for multiple comparisons to control the false discovery rate (FDR).
P-values for continuous variables are calculated from a longitudinal model of baseline, 26 weeks, and 52 weeks adjusted for baseline HbA1c and site as a random effect. P-values for binary variables are calculated from a logistic regression model at 52 weeks adjusted for baseline value of the continuous metric, baseline HbA1c, and site as a random effect.
A CGM-measured hypoglycemic event was defined as 15 consecutive minutes with a sensor glucose value <54 mg/dL. The end of the hypoglycemic event was defined as a minimum of 15 consecutive minutes with a sensor glucose concentration >70 mg/dL.16
Figure 2.
Percent of time in range 70 to 180 mg/dL by treatment group and visit.
Abbreviations: CGM, continuous glucose monitoring; FBI, family behavioral intervention; BGM, blood glucose monitoring; RCT, randomized controlled trial.
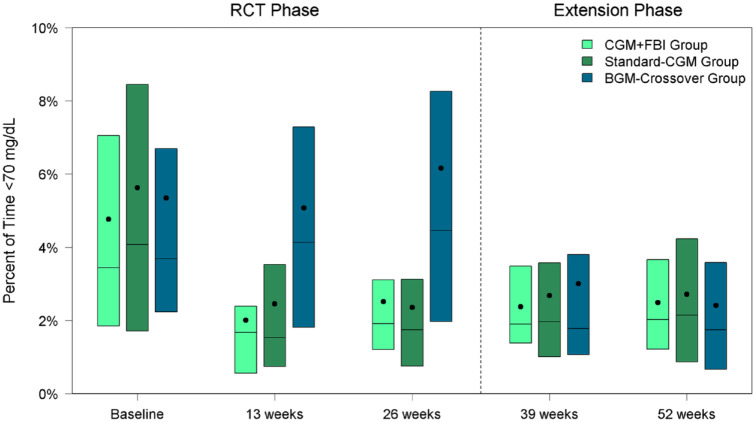
In both the Standard-CGM Group and CGM+FBI Group, median percent time <70 mg/dL decreased within six weeks of starting real-time CGM and remained significantly lower than baseline throughout the entire one-year of the study. The reduction in hypoglycemia was from 3.4% (50 min/d) at baseline to 2.0% (29 min/d) at 52 weeks in the CGM+FBI Group (P-value baseline vs 52 weeks <.001) and from 4.1% (59 min/d) at baseline to 2.1% (31 min/d) at 52 weeks in the Standard-CGM Group (P-value baseline vs 52 weeks <.001) (Table 2, Figure 3). There was no treatment group difference in median percent time <70 mg/dL between CGM+FBI Group and Standard-CGM Group at 52 weeks (P = .90). Median percent time <54 mg/dL also decreased within six weeks of starting real-time CGM and remained significantly lower than baseline in both initial CGM groups (P-value baseline vs 52 weeks <.001 for both groups) but was not different between groups at 52 weeks (P = .82). Moreover, median percent time <54 mg/dL was reduced for both daytime (6 am-<10 pm) and nighttime (10 pm-6 am) hours in both groups (Supplemental Table 2).
Figure 3.
Percent of time <70 mg/dL by treatment group and visit.
Center line represents median, center dot represents mean.
Abbreviations: CGM, continuous glucose monitoring; FBI, family behavioral intervention; BGM, blood glucose monitoring; RCT, randomized controlled trial.
There were no statistically significant changes from baseline in mean percent time >180 mg/dL in either group, but there was a reduction in the CGM+FBI Group in median percent time >250 mg/dL (baseline 33% vs 52 weeks 27%, P-value .05) and median percent time >300 mg/dL (baseline 18% vs 52 weeks 13%, P-value .004) (Table 2). The Standard-CGM Group also observed a reduction in these hyperglycemia metrics, but it did not reach the threshold for statistical significance (median percent time >250 mg/dL: baseline 26% vs 52 weeks 22%, P-value .22; median percent time >300 mg/dL: baseline 13% vs 52 weeks 10%, P-value .14). There were no treatment group differences in hyperglycemia metrics between the CGM+FBI Group and Standard CGM Group at 52 weeks.
Glycemic Outcomes in the BGM-Crossover Group
As described above, upon completion of the initial 26-week randomized trial, the BGM-Crossover Group initiated real-time CGM along with the FBI during the 26-week extension phase. CGM use in the BGM-Crossover Group did not result in a significant change in mean time in range from 26 to 52 weeks (38% at 26 weeks vs 40% at 52 weeks, P-value .31) (Table 3, Figure 2). There was also no significant change in mean glucose. Mean HbA1c increased from 8.1% at 26 weeks to 8.3% at 52 weeks (P-value .01).
Table 3.
Glycemic Outcomes in the BGM-Crossover Group.
| Baseline | 26 weeks | 52 weeks |
P-value 26 vs 52 weeks a |
|
|---|---|---|---|---|
| CGM-measured outcomes | ||||
| N | 44 | 43 | 39 | |
| Hours of data | 315 (263, 362) |
150 (131, 159) |
612 (517, 636) |
|
| Glucose control | ||||
| % Time in range 70-180 mg/dL | 41 ± 10 | 38 ± 13 | 40 ± 12 | .31 |
| % Time in range 70-180 mg/dL >60% | 1 (2%) | 1 (2%) | 3 (8%) | .16 |
| Mean glucose (mg/dL) | 199 ± 25 | 203 ± 35 | 205 ± 26 | .84 |
| Coefficient of variation (%) | 44 ± 7 | 44 ± 8 | 39 ± 6 | <.001 |
| Hyperglycemia | ||||
| % Time > 180 mg/dL | 54 ± 12 | 55 ± 16 | 57 ± 13 | .45 |
| % Time > 250 mg/dL | 27 (22, 34) | 30 (19, 40) | 26 (20, 37) | .18 |
| % Time > 300 mg/dL | 14 (9, 19) | 15 (9, 26) | 14 (8, 19) | .040 |
| Hypoglycemia | ||||
| % Time < 70 mg/dL | 3.7 (2.2, 6.7) | 4.5 (2.0, 8.3) | 1.7 (0.7, 3.6) | <.001 |
| Meeting target of <4% time <70 mg/dL | 23 (52%) | 21 (49%) | 32 (82%) | .003 |
| % Time < 54 mg/dL | 1.0 (0.4, 3.6) | 1.3 (0.4, 4.3) | 0.3 (0.1, 1.0) | .007 |
| Meeting target of <1% time <54 mg/dL | 22 (50%) | 17 (40%) | 30 (77%) | .002 |
| Hypoglycemic events per week b | 1.5 (0.8, 4.1) | 1.7 (1.0, 4.6) | 0.7 (0.0, 1.6) | .002 |
| HbA1c | ||||
| N | 44 | 44 | 44 | |
| HbA1c, mean ± SD | 8.2 ± 0.7 | 8.1 ± 0.8 | 8.3 ± 0.9 | 0.014 |
Data are mean ± SD, median (IQR), or N (%).
Abbreviations: BGM, blood glucose monitoring; CGM, continuous glucose monitoring; IQR, interquartile range.
P-value from a paired t-test, signed rank test, or McNemar’s test, as appropriate. Only includes participants who had values at both time points. P-values are adjusted for multiple comparisons to control the false discovery rate (FDR).
A CGM-measured hypoglycemic event was defined as 15 consecutive minutes with a sensor glucose value <54 mg/dL. The end of the hypoglycemic event was defined as a minimum of 15 consecutive minutes with a sensor glucose concentration >70 mg/dL. 16
On the contrary, median percent time <70 mg/dL decreased from 4.5% (64 min/d) at 26 weeks to 1.7% (25 min/d) at 52 weeks (P-value <.001) and median percent time <54 mg/dL decreased from 1.3% (18 min/d) at 26 weeks to 0.3% (5 min/d) at 52 weeks (P-value .007) (Table 3, Figure 3). Again, the reductions in median percent time <54 mg/dL were observed during both daytime and nighttime hours (Supplemental Table 3).
There were no significant changes in mean percent time >180 mg/dL or median percent time >250 mg/dL, but there was a small reduction in median percent time >300 mg/dL from 15% at 26 weeks to 14% at 52 weeks (P-value .04) (Table 3).
Sensor Use
Sensor use was high and sustained throughout the extension phase. During month 12, median sensor use was 91% in the CGM+FBI Group, 92% in the Standard-CGM Group, and 88% in the BGM-Crossover Group (Table 4).
Table 4.
CGM Use and Device Feature Use.
| CGM + FBI Group | Standard-CGM Group | BGM-Crossover Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Randomized trial | Extension phase | Randomized trial | Extension phase | Extension phase | ||||||
| 13 weeks | 26 weeks | 39 weeks | 52 weeks | 13 weeks | 26 weeks | 39 weeks | 52 weeks | 39 weeks | 52 weeks | |
| CGM use (%) | ||||||||||
| N a | 45 | 45 | 44 | 43 | 42 | 42 | 41 | 41 | 44 | 41 |
| Median (Q1, Q3) | 91% (77%, 96%) |
94% (86%, 95%) |
91% (80%, 95%) |
91% (78%, 96%) |
90% (81%, 95%) |
91% (82%, 95%) |
92% (84%, 95%) |
92% (85%, 96%) |
84% (68%, 94%) |
88% (72%, 94%) |
| 0% | 1 (2%) | — | — | 2 (5%) | — | — | 1 (2%) | — | 4 (9%) | 4 (10%) |
| <50% | 4 (9%) | 2 (4%) | 2 (5%) | 4 (9%) | 2 (5%) | 2 (5%) | 1 (2%) | — | 5 (11%) | 4 (10%) |
| 50%-<60% | 2 (4%) | — | 1 (2%) | — | 1 (2%) | 1 (2%) | — | 3 (7%) | 1 (2%) | 1 (2%) |
| 60%-<70% | 1 (2%) | 2 (4%) | 2 (5%) | 1 (2%) | 1 (2%) | 1 (2%) | — | 1 (2%) | 1 (2%) | 1 (2%) |
| 70-%<80% | 5 (11%) | 5 (11%) | 6 (14%) | 5 (12%) | 4 (10%) | 4 (10%) | 5 (12%) | 5 (12%) | 4 (9%) | 6 (15%) |
| 80%-<90% | 8 (18%) | 10 (22%) | 11 (25%) | 8 (19%) | 12 (29%) | 9 (21%) | 12 (29%) | 6 (15%) | 11 (25%) | 5 (12%) |
| 90%-≤100% | 24 (53%) | 26 (58%) | 22 (50%) | 23 (53%) | 22 (52%) | 25 (60%) | 22 (54%) | 26 (63%) | 18 (41%) | 20 (49%) |
| Device feature use | ||||||||||
| N b | 44 | 45 | 44 | 41 | 42 | 42 | 40 | 41 | 39 | 37 |
| SHARE feature | 18 (41%) | 23 (51%) | 22 (50%) | 21 (51%) | 17 (40%) | 17 (40%) | 19 (48%) | 23 (56%) | 10 (26%) | 9 (24%) |
| Dosing insulin using CGM without BG confirmation | 33 (75%) | 37 (82%) | 34 (77%) | 35 (85%) | 34 (81%) | 41 (98%) | 37 (93%) | 37 (90%) | 31 (79%) | 31 (84%) |
Abbreviations: CGM, continuous glucose monitoring; FBI, family behavioral intervention; BGM, blood glucose monitoring; BG, blood glucose.
If a participant reported using a non-study device, they were excluded from the calculation of sensor use.
Only participants who had non-zero and non-missing CGM use are included in the tabulation of feature use.
Of those who were using the CGM at 52 weeks, 51% of the CGM+FBI Group, 56% of the Standard-CGM Group, and 24% of the BGM-Crossover Group reported using the SHARE feature of the mobile app to allow remote monitoring of real-time CGM data with another person. In addition, 85% of the CGM+FBI Group, 90% of the Standard-CGM Group, and 84% of the BGM-Crossover Group reported using CGM glucose values to dose insulin without fingerstick blood glucose confirmation. In all three groups, the median number of fingerstick blood glucose checks was ~6 per day prior to initiation of real-time CGM and decreased to ~4 per day by the end of the study.
Severe Hypoglycemia and Diabetic Ketoacidosis
During the extension phase, severe hypoglycemic events involving impaired cognition requiring the assistance of another person were reported for two participants in the CGM+FBI Group and four participants in the BGM-Crossover Group. Of these events, two in the CGM+FBI Group and one in the BGM-Crossover Group resulted in seizure or loss of consciousness. No severe hypoglycemic events during the extension phase were reported in the Standard-CGM Group. One participant in each group reported an episode of diabetic ketoacidosis during the extension phase.
Discussion
This one-year study of CGM use in early childhood demonstrated durability of real-time CGM use and a sustained reduction in hypoglycemia throughout the study, an important clinical benefit of CGM use in this age range given the frequency of hypoglycemia, risk of severe hypoglycemia events, and potential for long-term impact on cognitive development.3,10,17 However, CGM use was not associated with improved time in target glucose range or HbA1c.
The inability of real-time CGM to improve time in range parallels results from former trials of families with young children with T1D carried out by the DirecNet research group, using earlier and less accurate CGM devices.1,18 Even in those early studies, parents appeared to primarily use CGM to avoid hypoglycemia even at the expense of spending time in the hyperglycemic range. Despite improvements in CGM technology, taken together these data indicate that different or additional interventions are needed to help families not only reduce hypoglycemia, but to more effectively reduce time in hyperglycemic ranges.
Furthermore, the findings that children in all groups maintained >50% time in hyperglycemia (>180 mg/dL) throughout the entire 52-week study period are consistent with data from the T1D Exchange Clinic Registry,19,20 which reveal high mean HbA1c and a low percentage of young children achieving glycemic targets; only 24% of those two to less than six years achieve HbA1c <7.5% and only 7% achieve HbA1c <7.0%. Notably, the American Diabetes Association and International Society of Pediatric and Adolescent Diabetes have lowered the HbA1c target to <7.0% 21 based on emerging evidence that exposure to hyperglycemia negatively impacts central nervous system structure and function.21-27 Therapeutic advances, such as automated insulin delivery systems, can improve time in range in younger and older children with T1D by diminishing both hyper- and hypoglycemia through algorithms working 24 hours a day and only requiring intermittent intervention by caregivers.28-31 Additional research is needed to inform content focused on simultaneously addressing fear of hypoglycemia in caregivers and proactively treating or preventing hyperglycemia in their young children.
In the setting of standardized education, real-time CGM wear remained high throughout the extension phase for all groups in this population of late adopters to diabetes device technology who had either previously discontinued or were naïve to CGM. In fact, CGM use in this study was far above what has been previously reported using earlier CGM systems in young children with T1D.8,18 The DirecNet group performed the prior large study in this age group and found that only 41% of children were wearing CGM at least six days a week at six months, 1 declining further to 33% at 12 months. 18 Improvements in CGM (eg, fewer required fingersticks, remote monitoring capabilities, smaller device size) that reduce the burden and enhance the benefits of use for parents (eg, less worry, improved sleep) 11 likely contributed to 80% to 95% of young children wearing CGM at least six days a week during the extension phase and are mirrored in reports of real-world use.6,7 The high CGM wear rates over 52 weeks in this study are important, as consistent CGM wear is a critical component of advancing diabetes technologies including automated insulin delivery systems. Moreover, the durability of CGM wear underscores the importance of trained personnel to provide effective and long-lasting education, a valuable tool for future generalizability of device use in young children with T1D.
This study was strengthened by the involvement of 14 centers throughout the United States who recruited a large, racially, ethnically, and socioeconomically diverse cohort of young children with T1D and maintained a high retention rate over one full year. Notably, our cohort uniquely included a large portion of youth not using insulin pumps and without private health insurance. The results suggest that with standardized education, even late adopters to CGM who previously declined use of or lacked access to CGM can durably use diabetes technology. Hence, this intervention may be generalizable to the greater population of young children with T1D. This is of key importance to ensure that young children from structurally disadvantaged families have access to the glycemic benefits of both CGM and automated insulin delivery systems.
Due to the rapid pace of CGM advances, factory-calibrated systems with less complicated insertion requirements became commercially available in the United States during the study; however, these were not available to participants. Hence, the study was limited by use of older technology requiring twice daily fingerstick calibration, and clinical outcomes may have been more favorable with the use of newer systems. CGM devices were supplied by the study, removing barriers of insurance coverage and paperwork often experienced by families and diabetes clinical teams. This, in addition to the run-in eligibility criteria of at least 200 hours of masked CGM wear time over a two- to three-week period and screening HbA1c of 7.0% to 9.9%, may limit the generalizability of findings.
In summary, reduction in hypoglycemia and high rates of CGM use in young children with T1D were sustained over 12 months of CGM wear; however, improvements in time in target range and HbA1c were not achieved. Real-time CGM is a vital component of diabetes care in young children with T1D. Further advances in diabetes technology, behavioral supports, and education in diabetes management in young children are needed to shift the paradigm away from avoiding hypoglycemia at the expense of hyperglycemia to optimizing time in range.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968221084667 for Long-term Continuous Glucose Monitor Use in Very Young Children With Type 1 Diabetes: One-Year Results From the SENCE Study by Michelle A. Van Name, Lauren G. Kanapka, Linda A. DiMeglio, Kellee M. Miller, Anastasia Albanese-O’Neill, Persis Commissariat, Sarah D. Corathers, Kara R. Harrington, Marisa E. Hilliard, Barbara J. Anderson, Jennifer C. Kelley, Lori M. Laffel, Sarah A. MacLeish, Brandon M. Nathan, William V. Tamborlane, R. Paul Wadwa, Steven M. Willi, Kristen M. Williams, Kupper A. Wintergerst, Stephanie Woerner, Jenise C. Wong and Daniel J. DeSalvo in Journal of Diabetes Science and Technology
Acknowledgments
SENCE Study Group: Joslin Diabetes Center- Pediatric, Boston, MA Lori Laffel MD, MPH (PI); Kara Harrington PhD, MS (I); Anat Hanono MD (I); Nisha Naik (PC); Louise Ambler-Osborn MS, RN, CPNP (C); Alan Schultz MSN, CPNP (C); Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN Linda DiMeglio MD, MPH (PI); Stephanie Woerner RN, MSN, FNP-C CDE (I); Heather Jolivette RN, CPNP, CDE (I); Heba Ismail MB BCh, MSc, PhD (I); Megan Tebbe RN, BSN, CCRP, CDE (PC); America Newnum (C); Megan Legge BA CCRP (C); Yale Pediatric Diabetes Program, New Haven, CT William Tamborlane MD (PI); Michelle Van Name MD (I); Kate Weyman MSN, FNP-C, APRN, CDE (I); Jennifer Finnegan (PC); Amy Steffen BSN (C); Melinda Zgorski BSN (C); Baylor College of Medicine/Texas Children’s Hospital, Houston, TX Daniel DeSalvo MD (PI); Marisa Hilliard PhD (I); Kylie DeLaO, RN, CDE (C); Cicilyn Xie, BA (PC); Wendy Levy LCSW (C); Barbara Davis Center for Diabetes, Aurora, CO R. Paul Wadwa MD (PI); Greg Forlenza MD (I); Shideh Majidi MD (I); Guy Alonso MD (I); Isabel Weber MSc (PC); Michelle Clay RN, BSN, CDE (C); Emily Simmons BA (C); University of Minnesota, Minneapolis, MN Brandon Nathan MD (PI); Muna Sunni MBBCh, MS(I); Jessica Sweet (PC); Beth Pappenfus (C); Anne Kogler BSN, RN, CDE (C); Marrissa Ludwig, BSN, RN (C); Brittney Nelson (C); Anne Street RN (C); Darcy Weingartner BSN, RN (C); University of Florida, Gainesville, FL Anastasia Albanese-O’Neill PhD, APRN, CDE (PI); Michael Haller MD, MS-CI (I); Janey Adams (PC); Miriam Cintron (C); Nicole Thomas (C); Vanderbilt University Medical Center, Nashville, TN Jennifer Kelley MD, MSCE (PI); Jill Simmons MD (I); George William RN, CDE (PC); Faith Brendle RN (C); Naomi Berrie Diabetes Center, Columbia University Medical Center, New York, NY Robin Goland MD (PI): Kristen Williams MD (I); Rachelle Gandica MD (I); Sarah Pollak RN, MSN (PC); Emily Casciano RD, CDN, CDE (C); Elizabeth Robinson (C); Children’s Hospital of Philadelphia, Philadelphia, PA Steven Willi MD (PI); Pantea Minnock RN, CPNP, CCRP (I); Diana Olivos MS (PC); Cathy Carchidi RN, MS, CDE, CPT, CCRC(C); Brian Grant RN, CDE (C); University of California San Francisco and the Madison Clinic for Pediatric Diabetes, San Francisco, CA Jenise C. Wong MD, PhD (PI); Saleh Adi MD (I); Cincinnati Children’s Hospital Medical Center and University of Cincinnati, College of Medicine, Cincinnati, OH Sarah Corathers MD (PI); Nicole Sheanon MD, MS (I); Cathy Fox MS, RD, LD, CDE (PC); Tammy Weis BSN, RN, CCRP (C); Rainbow Babies and Children’s Hospital Cleveland Medical Center, Cleveland, OH Sarah MacLeish DO (PI); Jamie Wood MD (I); Terri Casey RN, BSN (PC); Wendy Campbell RN, BSN (C); Paul McGuigan RN, BSN (C); Wendy Novak Diabetes Center, University of Louisville, Norton Children’s Hospital, Louisville, KY Kupper Wintergerst MD (PI); Sara Watson MD (I); Suzanne Kingery MD (I); Gwen Pierce (PC); Heather Rush (C); Lauren Rayborn (C); Manuel Rodriguez-Luna (C); Amy Deuser (C)
Footnotes
Abbreviations: BGM, blood glucose monitoring; CGM, continuous glucose monitoring; FBI, family behavioral intervention; HbA1c, hemoglobin A1c; SENCE, Strategies to Enhance New CGM use in Early childhood; T1D, type 1 diabetes.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.A.V.N., L.G.K., L.A.D., K.M.M., A.A.-O., P.C., S.D.C., K.R.H., M.E.H., B.J.A., J.C.K., S.A.M., B.M.N., W.V.T., K.M.W., K.A.W., S.W., and J.C.W. have no disclosures to report. L.M.L. reports grants and personal fees from Dexcom, outside the submitted work. R.P.W. reports grants from Dexcom, Eli Lilly, and Tandem Diabetes Care and personal fees from Dompe and Tandem Diabetes Care outside the submitted work. S.M.W. reports personal fees from Roche Diagnostics and Boehringer Ingelheim, outside the submitted work. D.J.D. reports research support from Insulet and personal fees from Dexcom outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the Leona M. and Harry B. Helmsley Trust, Dexcom Inc provided nonfinancial support.
ORCID iDs: Linda A. DiMeglio  https://orcid.org/0000-0003-4440-5168
https://orcid.org/0000-0003-4440-5168
Anastasia Albanese-O’Neill  https://orcid.org/0000-0001-8219-6059
https://orcid.org/0000-0001-8219-6059
Persis Commissariat  https://orcid.org/0000-0002-6964-1223
https://orcid.org/0000-0002-6964-1223
Lori M. Laffel  https://orcid.org/0000-0002-9675-3001
https://orcid.org/0000-0002-9675-3001
Kristen M. Williams  https://orcid.org/0000-0002-8464-4537
https://orcid.org/0000-0002-8464-4537
Jenise C. Wong  https://orcid.org/0000-0003-0573-6650
https://orcid.org/0000-0003-0573-6650
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Mauras N, Beck R, Xing D, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care. 2012;35:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundberg F, Forsander G. Detection and treatment efficacy of hypoglycemic events in the everyday life of children younger than 7 yr. Pediatr Diabetes. 2014;15:34-40. [DOI] [PubMed] [Google Scholar]
- 3.DiMeglio LA, Kanapka LG, DeSalvo DJ, et al. Time spent outside of target glucose range for young children with type 1 diabetes: a continuous glucose monitor study. Diabet Med. 2020;37:1308-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323:2388-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323:2397-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dovc K, Van Name M, Jenko Bizjan B, et al. Continuous glucose monitoring use and glucose variability in very young children with type 1 diabetes (VibRate): a multinational prospective observational real-world cohort study. Diabetes Obes Metab. 2021. doi: 10.1111/dom.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundberg F, Forsander G. Continuous glucose monitoring in healthy children aged 2-8 years. Diabetes Technol Ther. 2018;20:113-116. [DOI] [PubMed] [Google Scholar]
- 8.Tsalikian E, Fox L, Weinzimer S, et al. Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr Diabetes. 2012;13:301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strategies to Enhance New CGMUiECSG. A randomized clinical trial assessing Continuous Glucose Monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabetes Care. 2021;44:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Name MA, Hilliard ME, Boyle CT, et al. Nighttime is the worst time: parental fear of hypoglycemia in young children with type 1 diabetes. Pediatr Diabetes. 2018;19:114-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21:493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commissariat PV, Harrington KR, Whitehouse AL, et al. “I’m essentially his pancreas”: parent perceptions of diabetes burden and opportunities to reduce burden in the care of children <8 years old with type 1 diabetes. Pediatr Diabetes. 2020;21:377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Commissariat PV, Whitehouse AL, Hilliard ME, et al. Sources and valence of information impacting parents’ decisions to use diabetes technologies in young children <8 years old with type 1 diabetes. Diabetes Technol Ther. 2020;22:697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group DR. DCCT Protocol (Publication No PB 88-116462-AS). Springfield, VA: US Department of Commerce, National Technical Information Service; 1988. [Google Scholar]
- 15.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. Journal of Educational and Behavioral Statistics. 2000;25:60-83. [Google Scholar]
- 17.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perantie DC, Lim A, Wu J, et al. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:87-95. [DOI] [PubMed] [Google Scholar]
- 19.Tansey M, Weinzimer S, Beck R, et al. Extended 6-month follow-up of a randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care. 2013;36:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971-978. [DOI] [PubMed] [Google Scholar]
- 21.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes A. 13. Children and adolescents: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S163-S182. [DOI] [PubMed] [Google Scholar]
- 23.DiMeglio LA, Acerini CL, Codner E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(suppl 27):105-114. [DOI] [PubMed] [Google Scholar]
- 24.Barnea-Goraly N, Raman M, Mazaika P, et al. Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care. 2014;37:332-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marzelli MJ, Mazaika PK, Barnea-Goraly N, et al. Neuroanatomical correlates of dysglycemia in young children with type 1 diabetes. Diabetes. 2014;63:343-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foland-Ross LC, Reiss AL, Mazaika PK, et al. Longitudinal assessment of hippocampus structure in children with type 1 diabetes. Pediatr Diabetes. 2018. doi: 10.1111/pedi.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazaika PK, Weinzimer SA, Mauras N, et al. Variations in brain volume and growth in young children with type 1 diabetes. Diabetes. 2016;65:476-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox LA, Hershey T, Mauras N, et al. Persistence of abnormalities in white matter in children with type 1 diabetes. Diabetologia. 2018;61:1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44:1630-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forlenza GP, Ekhlaspour L, DiMeglio LA, et al. Glycemic outcomes of children 2-6 years of age with type 1 diabetes during the pediatric MiniMed 670G system trial. Pediatr Diabetes. 2022. doi: 10.1111/pedi.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanapka LG, Wadwa RP, Breton MD, et al. Extended use of the control-iq closed-loop control system in children with type 1 diabetes. Diabetes Care. 2021;44:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ware J, Allen JM, Boughton CK, et al. Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med. 2022;386:209-219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968221084667 for Long-term Continuous Glucose Monitor Use in Very Young Children With Type 1 Diabetes: One-Year Results From the SENCE Study by Michelle A. Van Name, Lauren G. Kanapka, Linda A. DiMeglio, Kellee M. Miller, Anastasia Albanese-O’Neill, Persis Commissariat, Sarah D. Corathers, Kara R. Harrington, Marisa E. Hilliard, Barbara J. Anderson, Jennifer C. Kelley, Lori M. Laffel, Sarah A. MacLeish, Brandon M. Nathan, William V. Tamborlane, R. Paul Wadwa, Steven M. Willi, Kristen M. Williams, Kupper A. Wintergerst, Stephanie Woerner, Jenise C. Wong and Daniel J. DeSalvo in Journal of Diabetes Science and Technology