ABSTRACT
Respiratory syncytial virus (RSV) is the leading cause of severe respiratory infections in children. In many countries, changes in RSV hospitalizations have occurred during COVID-19 restriction, with alterations in annual pre-pandemic trends. The objective of this retrospective study was to describe the epidemiology of RSV during the pandemic in Spain (2018–2021) through population-based estimates of hospitalization in children <2 years old. A total of 56,741 hospital discharges were identified with a 2.2% decrease between the beginning and the end of the COVID-19 pandemic resulting in a hospitalization rate of 1,915.89 (95% CI = 1,900.13–1,931.65) hospitalizations per 100,000 children. During the four-year period, a total of 34 deaths were recorded (males 63%, females 37%). The average annual cost to the National Health-Care System of bronchiolitis requiring hospitalization was €49,6 million with an average hospitalization cost per case of €3,054. RSV is a very frequent virus associated with community-acquired pneumonia (CAP) in children under 2 years old, so future preventive interventions should target this age group including vaccination programs.
KEYWORDS: Bronchiolitis, respiratory syncytial virus, COVID-19, epidemiology, Spain
Introduction
Human Respiratory Syncytial Virus (RSV) is a highly contagious pathogen associated with a high burden of acute, lower respiratory tract infections (LRTI), particularly in infants aged 2 years or less.1 RSV is the most common cause of bronchiolitis and pneumonia in children aged 1 year in the USA and is also recognized as a cause of illness in adults and high-risk adults, with a disease burden similar to seasonal influenza A and typically causative of seasonal outbreaks throughout the world.
The 2016 Global Burden of Disease (GBD) study estimated that RSV is responsible for 24.8 million acute respiratory infection (ARI) episodes and 76,600 deaths each year.2 By the age of 1, ~60%–70% of children have been infected with RSV, and 2%–3% of these infections result in hospitalization, making RSV a leading cause of mortality and morbidity in children age <5, particularly in low- and median-income countries.3,4 RSV is recognized as the primary cause of hospitalization for acute LRTI among infants worldwide5 and an important cause in the United States, creating a significant burden of disease in children under 5 years all over the world. A variety of factors, such as gender, chronological age at hospitalization, birth weight, and breast-feeding may affect the prevalence of RSV-related LRTI and, possibly, the risk of developing asthma-like symptoms during the school years.6 Additionally, gestational age, birth order, birth weight, and exposure to tobacco smoke affected the prevalence and severity of RSV-related LRTI.7 Premature of <32 gestational weeks, congenital heart disease, and atelectasis/condensation are the main risk factors for ICU admission in both RSV and non-RSV bronchiolitis.8 Each year, 4–5 million children younger than 4 years acquire an RSV infection, and more than 125,000 are hospitalized annually in the USA because of this. The impact of RSV infection in the USA is responsible for 177,000 hospitalizations and 14,000 deaths in the elderly ≥65 years of age.9,10 RSV circulation in the EU/EEA has recently increased, impacting the population with an earlier start of the season. This circulation raised in some EU/EEA countries, showing an increase in severe acute respiratory infections (SARI). It should be noted that 2022 showed more RSV activity that began earlier than in pre-COVID-19 seasons. However, the risk from RSV infection was estimated as low for the total population but high for infants under 6 months, adults 65 years and above and individuals with specific comorbidities. In Spain, since the end of October 2022 and as of week 47, RSV-related hospitalization rates remained high with an increase in >79 years. Furthermore, the Spanish Society of Paediatric Emergency Medicine published a statement expressing their concern about the increased volume of hospital admissions in pediatric emergencies.11
The Global Epidemiology of RSV (GERi) network was launched in 2019 to examine the global epidemiology and timing of RSV epidemics based on virological surveillance data.12 RSV infection has a substantial global impact, representing the second most frequent cause of death in infants (second only to malaria) and imparting annual global inpatient and outpatient costs of approximately €5 billion.13 The literature supports that RSV-positive ARI is associated with significant morbidity and mortality in at-risk adults. However, almost all previous studies assessed only short-term morbidity and mortality of medically attended RSV-positive ARI, typically in a hospital setting 7 to 28 days after RSV-positive ARI. Thus, long-term outcomes after RSV-positive ARI, including Quality of Life (QOL), in older adults are poorly understood.14 Childhood morbidity and mortality worldwide in children younger than 5 years of age are widely affected by RSV infections, especially before 6 months of life. In 2019, RSV infections were responsible for 33 million episodes, 3.6 million hospital admissions, 26300 in-hospital deaths, and 101,400 RSV-attributable overall deaths, of which more than 95% episodes occurred in low-income and middle-income countries.15 Ninety-nine percent of these deaths took place in developing countries, although the actual mortality rate due to RSV infection has been suggested to be higher than what was reported.16 In many countries, the majority of deaths occurred in hospitalized children with RSV pneumonia.17,18 Mortality estimates directly or indirectly attributable to RSV vary between 59,600 and 199,000 deaths per year, with almost all occurring in resource limited settings, where RSV testing is not routinely performed.19 RSV accounts for 22% of all ARI worldwide, and the majority (96%) of RSV illness occurs in low-resource countries. Incidence of RSV-associated illness is highest among infants <5 months of age when the presence of maternally derived antibodies prevents effective vaccination. However, incidence and mortality likely vary substantially from year to year and between settings.20 All hospitalizations related to RSV infection in children up to 5 years of age between 1997 and 2011 and up to 2 years between 2012 and 2017 studied in Spain showed that the highest incidence of hospitalization occurred during the first year of life with 80% of deaths in this age. In the first period, the average annual cost to the Spanish national health system for bronchiolitis requiring hospitalization was €47 million, with an average cost per hospitalization of €2,162 in children up to 5 years old.21
The Coronavirus disease 2019 (COVID-19) pandemic has drastically changed the epidemiology of other viral respiratory infections in both children and adults. Worldwide, the autumn and winter RSV epidemics have virtually disappeared and in some countries in the Southern Hemisphere like South Africa or Australia, the RSV season has moved to spring, marking an unprecedented phenomenon.22 COVID-19 had an impact on the epidemiology of RSV during the 2020–2021 winter season in the US. These epidemiologic changes could be related to interactions between respiratory viruses and/or ongoing COVID-19 mitigation efforts.23 In Spain, as in other European countries, the winter epidemic (November–February) has disappeared and moved to spring-summer.22
Additionally, as RSV immunization candidates reach the final stages of clinical development, the need for global monitoring of RSV molecular epidemiology becomes increasingly important to ensure their effectiveness during licensure and use. Evolutionary dynamics of RSV genotypes may correlate with transmission between seasons and disease severity among patient types.24 Despite the epidemiological importance, there is limited evidence on the impact of RSV-associated pneumonia in low-resource settings. Further, factors that predict etiological agents of community-acquired pneumonia are being explored in many sites as such information is beneficial to deliver appropriate therapy, reduce antibiotic consumption, and avoid costly microbiological tests.25 The development of vaccines against RSV is highly relevant for the control of associated respiratory diseases. However, it has a great limitation with the age at which vaccination is required, before 2 months of life, where there may be interference with vectors to which the mother has antibodies,26 and there is immaturity of the immune system. RSV infection is possibly the one that has the greatest impact on healthcare burden, so prevention is considered a priority. However, despite the development of this vaccine and the efforts made to achieve it, there is a still way to go.
It is important to consider the direct effects that sociological and economical changes attributable to COVID-19 may have on the epidemiology of RSV in the short, medium, and long term. As a result of changes in infection rates and contact patterns, it is likely that levels of immunity in the population are not reflective of recent years. Failure to consider these effects could have an impact on populations and health-care systems both globally.27 It is worth noting that, because of limited health care, more than 92% of all RSV-associated acute lower respiratory infections (ALRI) episodes and nearly 99% of the related fatalities occur in developing countries; this places a great burden on patients, parents, and broader society.28
During COVID-19 was declared a pandemic, much research regarding biological agents has grown. However, it is necessary to expand the study of the pandemic impact on the circulation of RSV and know the epidemiological implications that may have occurred as well as the consequences in the health field. Therefore, the present study describes the epidemiology of bronchiolitis during the Spain pandemic. Likewise, to estimate the change in RSV-attributable disease in the Spanish population in relation to the implementation of measures for the control of SARS-CoV-2. Additionally, we determine the burden of hospitalization due to RSV in children under 2 years old in Spain during 2018–2021.
Methods
Study design and population
We conducted a retrospective observational study to estimate the effect of COVID-19 public health measures on RSV using a time-series period. For this data were extracted from day month, year to day month, year (1 January 2018 to 31 December 2021). We study population of children up to 2 years old in Spain by reviewing data of the National Surveillance System for Hospital Data (CMBD), including all hospitalizations for 98% Spanish hospitals.29–31 Children were identified through the Spanish public health surveillance system that include the entire territory. The study is exempt from being reviewed by a research ethics committee. The personal information of each subject was delivered to the researchers anonymously, in strict compliance with the current Spanish and European legislation. This study complies with the Declaration of Helsinki.
Data sources
The Spanish Minimum Basic Data Set was implemented in 2016 as a new data model of the Minimum Basic Data Set for Hospital Discharges, extending the registry to other alternative areas to hospitalization (day hospital, highly complex techniques and procedures offices and emergencies) and to the private sector. We used discharge reports from the CMBD published annually by the Spanish Ministry of Health to retrospectively analyze hospital discharge data.
Clinical data
Spanish CMBD contains routinely hospital data contained a diagnosis of RSV infection, bronchitis, bronchiolitis, or pneumonia. All the cases confirmed RSV infection, RSV test, or RSV-associated respiratory disease. Since management and prognosis do not change, performing a rapid diagnostic test (RDT) for RSV is restricted to children, and the RDTs used are immediate (15–30 minutes). Likewise, real-time PCR molecular methods could be used, but they are more expensive and not available in all health centers. However, the SARS- CoV-2 pandemic has contributed to increasing their availability, to which must be added the tendency to use them more frequently due to their greater sensitivity and improvement over time to obtain results. Hospitalization principal reason was primary diagnosis code, and admission was considered as a single hospital episode. Double counting admissions that contained several of these diagnoses was avoid. The annual hospitalization rate, average length of hospitalization and case-fatality rate were calculated by using municipal register data. For each CMBD discharge record, we considered diagnoses coded on the basis of the International Classification of Diseases (ICD), 10th Revision, ICD-10-CM (B97.4, J12.1, J20.5, J20.9, J21.0, J21.9). For each case, specific data were gathered on age, sex, average length of hospitalization, hospitalization rate, and case fatality rate. Groups of age for study were <2 years old. First incidence hospitalizations with a diagnosis of RSV were included in the study. In rates by months of age, the number of total newborns and perinatal mortality in Spain was also considered through the Spanish Instituto Nacional de Estadística (INE). To associate the precise hospitalization date for the population at risk in any given month, we assumed a constant birth rate throughout the year for the population denominators by months of age. The case-fatality rate (CFR) was calculated by dividing the number of deaths by the total number of hospitalizations due to bronchiolitis (%). Additionally, the average length of stay at the hospital was obtained.32 The datasets generated and/or analyzed during the current study are available in the Hospital Discharge Records in the Spanish CMBD repository.
Hospitalization data
The cost of hospitalizations was provided by the Ministry of Health, based on the total cost, the number of discharges and diagnostic cost group. The last was based on the Diagnosis-Related Groups (DRG) for hospitalized patients depending on discharge ICD classification. DRG calculations were made by 3 MTM with Core Grouping System Software.
Statistical analysis
Two tests were used in the study: Student’s t-test was for comparing continuous variables and chi-square test to assess significant differences in proportions. Additionally, Poisson regression models were used to assess differences in the hospitalization rates by age group and sex. In all tests, the significance level used was p < .05. Statistical analyses were performed using R Software (version 3.4.3).
Results
For the 2018–2021 series, there were a total of 56,741 hospital discharges with a decrease of 12.3%. Table 1 shows the hospitalization rate for bronchiolitis at 0 and 1 years old during 2018–2021. The number of cases of bronchiolitis displayed a clear decrease in the third year of the pandemic, from 16,340 in 2018, 17,297 in 2019, 8,769 in 2020, and 14,335 in 2021. The male sex presented 57.3% of the cases and the female 42.7%. For the total number of years, in the monthly distribution, there was an approximate decrease in cases from January (12,753) to August (792), to go up to December (15,508). Throughout the 4 years of study, the average hospital length of stay was 5.21 days (SD 6.92) in both sexes, with a mean of 5.19 (SD 7.28) in males and 5.22 (SD 6.40) in females. Regarding the average length of stay by age group and year of admission, there was a slight decrease from 5.16 (SD = 5.66) to 4.94 (SD = 5.56) (p < .001). For <1 year, 52262 cases were identified, representing 92.1% of the total, with an average hospital length of stay of 5.32 days (SD 7.52). In this age group, males had a mean of 4.14 (SD 6.61) and females 4.16 (SD 3.66).
Table 1.
Hospitalization rate and case fatality rate.(a) Bronchiolitis by age in Spain (2018–2021).
| 2018 | 2019 | 2020 | 2021 | Total | |
|---|---|---|---|---|---|
| Hospitalization rates in 0 years old (95%CI) | 4042,13 (3979,34–4104,92) | 4483,75 (4416,32–4551,18) | 2346,88 (2296,79–2396,97) | 3672,69 (3609,56–3735,82) | 3650,29 (3619,57–3681,01) |
| Hospitalization rates in 1 years old (95%CI) | 260,68 (245,01–276,35) | 276,01 (259,51–292,51) | 143,89 (131,69–156,09) | 498,7 (475,79–521,61) | 292,77 (284,21–301,33) |
| Case-fatality rates in 0 years old (95%CI) | 0,07 (0,03–0,12) | 0,06 (0,03–0,11) | 0,06 (0,02–0,13) | 0,07 (0,04–0,13) | 0,07 (0,05–0,09) |
| Case-fatality rates in 1 years old (95%CI) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| (b) Bronchiolitis by month of age and sex in Spain (2018–2021). | |||||
| Age (in months) |
HR in males |
HR in females |
CFR in males |
CFR in females |
|
| 0 | 7975.58(7751.95–8199.21) | 7140.64(6923.26–7358.02) | 0.08 (0.03–0.19) | 0.05 (0.01–0.15) | |
| 1 | 12534.69(12254.33–12815.05) | 9814.29(9559.44–10069.14) | 0.07 (0.02–0.14) | 0 (0–0) | |
| 2 | 8447.32(8217.17–8677.47) | 6163.86(5961.89–6365.83) | 0.02 (0–0.09) | 0.08 (0.02–0.22) | |
| 3 | 5239.79(5058.52–5421.06) | 3882.99(3722.69–4043.29) | 0.03 (0–0.15) | 0.04 (0–0.21) | |
| 4 | 3924.13(3767.26–4081) | 2952.73(2812.94–3092.52) | 0.21 (0.08–0.46) | 0.12 (0.02–0.37) | |
| 5 | 2897.39(2762.6–3032.18) | 2156.84(2037.37–2276.31) | 0.17 (0.05–0.45) | 0.08 (0.01–0.37) | |
| 6 | 2264.05(2144.9–2383.2) | 1622.79(1519.16–1726.42) | 0.07 (0.01–0.34) | 0 (0–0) | |
| 7 | 1661.72(1559.64–1763.8) | 1180.06(1091.69–1268.43) | 0 (0–0) | 0.44 (0.12–1.16) | |
| 8 | 1320.56(1229.56–1411.56) | 1042.24(959.19–1125.29) | 0.25 (0.05–0.79) | 0 (0–0) | |
| 9 | 1096.93(1013.99–1179.87) | 795.89(723.32–868.46) | 0 (0–0) | 0 (0–0) | |
| 10 | 932.06(855.61–1008.51) | 714.93(646.15–783.71) | 0 (0–0) | 0 (0–0) | |
| 11 | 847.18(774.29–920.07) | 702.87(634.67–771.07) | 0 (0–0) | 0 (0–0) | |
| 12 | 605.54(545.97–665.11) | 526.41(469.27–583.55) | 0 (0–0) | 0 (0–0) | |
| 13 | 514.03(459.15–568.91) | 447.29(394.62–499.96) | 0 (0–0) | 0 (0–0) | |
| 14 | 408.78(359.84–457.72) | 350.4(303.78–397.02) | 0 (0–0) | 0 (0–0) | |
| 15 | 370.65(324.05–417.25) | 366.55(318.87–414.23) | 0 (0–0) | 0 (0–0) | |
| 16 | 324.89(281.26–368.52) | 306.8(263.18–350.42) | 0 (0–0) | 0 (0–0) | |
| 17 | 285.23(244.35–326.11) | 272.89(231.75–314.03) | 0 (0–0) | 0 (0–0) | |
| 18 | 263.88(224.56–303.2) | 259.98(219.82–300.14) | 0 (0–0) | 0 (0–0) | |
| 19 | 269.98(230.21–309.75) | 209.92(173.83–246.01) | 0 (0–0) | 0 (0–0) | |
| 20 | 184.56(151.67–217.45) | 198.61(163.51–233.71) | 0 (0–0) | 0 (0–0) | |
| 21 | 166.26(135.05–197.47) | 153.4(122.55–184.25) | 0 (0–0) | 0 (0–0) | |
| 22 | 147.95(118.51–177.39) | 142.1(112.41–171.79) | 0 (0–0) | 0 (0–0) | |
| 23 | 131.18(103.46–158.9) | 109.8(83.7–135.9) | 0 (0–0) | 0 (0–0) | |
| Total | 2136.37(2113.15–2159.59) | 1682.83(1661.64–1704.02) | 0.07 (0.04–0.1) | 0.05 (0.03–0.09) | |
In Spain, throughout the 4 years of the study, there was a hospitalization rate of 1,915.89 (95% CI = 1,900.13–1,931.65) hospitalizations per 100,000 children <2 years old. Males showed a higher hospitalization rate than females (2,136.37, 95% CI = 2,113.15–2,159.59 vs. 1,682.83; 95% CI = 1,661.64–1,704.02). Regarding the incidence of hospitalization by month and year, a lower one was identified in 2020 (1,214.51; 95% CI = 1,189.37–1,239.65) compared to 2018 (2,081. 95% CI = 2,081.51–2,110.59), 2019 (2,305.48; 95% CI = 2,271.69–2,339.27) and 2021 (2,035.3; 95% CI = 2,002.49–2,068.11). Therefore, there was a decrease of 2.2% in the period (p < .001).
Regarding the hospitalization rate by months of age (Figure 1a), it was notable how most cases occurred in infants under 3 months old. For the series, the highest number of cases per 100,000 infants of 0, 1, and 2 months of age was 13,526.78 (95% CI = 13,142.73–13,910.83), 9,288.68 (95% CI = 8,962.72–9,614.64) and 5,514.84 (95% CI = 5,258.51–5,771.17), respectively in 2019. The lowest values of incidence of hospitalization per 100,000 children were in the year 2020.
Figure 1.
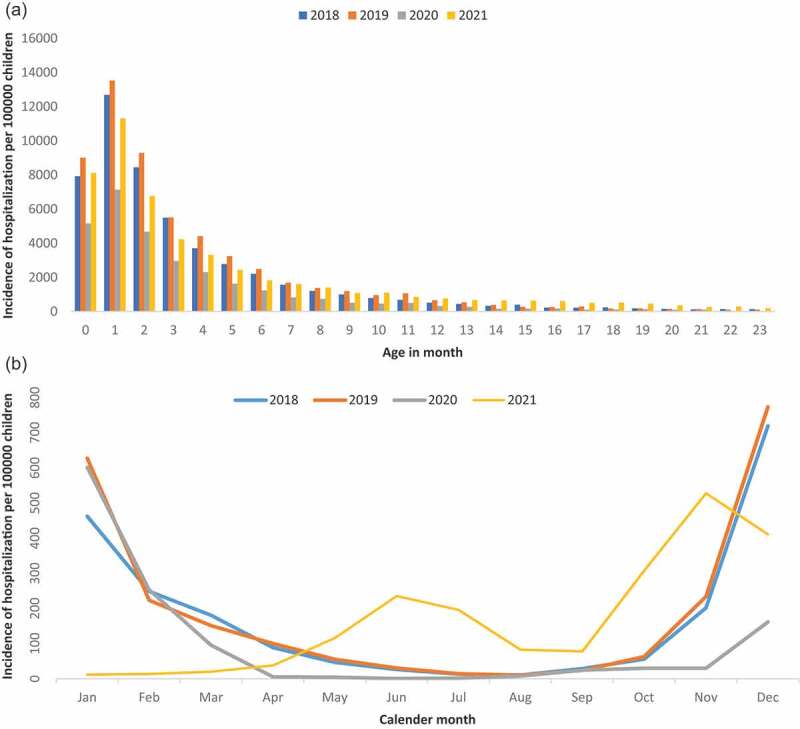
Incidence of hospitalization associated with bronchiolitis per 100,000 children. (a) by age in month in Spain. (b) by calendar month in Spain.
In relation to mortality 2018–2021, a total of 34 deaths were recorded (male 63%, female 37%). The year with the most deaths was 2018–2019 (10) and the fewest 2020 (5). The highest male:female ratio was in 2020 (4) and the lowest in 2018 (0.67). Regarding the total number of deaths from diseases, 9 (40.9%) were in children under 1 month, 10 (45.4%) in children under 3 months and 22 in children under 1 (100%) year. Deaths in females were 2 (16.7%) in children under 1 month, 5 (41.7%) in children under 3 months and 12 in children under 1 (100%) year. In both sexes, there were no deaths in the second year of life. The overall case-fatality rate was 0.06% (95% CI = 0.04–0.08), while for the years 2018, 2019, 2020, and 2021, it was also 0.06 (95% CI = 0.04–0.12; 95% CI = 0.03–0.1; 95% CI = 0.02–0.12; 95% CI = 0.03–0.11; respectively). For the period studied, the case-fatality rate according to sex was 0.07 (95% CI = 0.04–0.10) in males and 0.05 (95% CI = 0.03–0.09) in females. In the analysis of the cause of death, seven deaths confirmed to be RSV positive were identified (71.4% males-28.6% females) at first diagnosis. Regarding the first two diagnoses and according to sex, in the first four positions in diseases were as follows: A02.0-Infection or foodborne intoxication due to any Salmonella (12%), J21.0-Acute bronchiolitis due to RSV (8%), J21.8-Acute bronchiolitis due to other specified organisms (7%), and J11.1-Influenza with other respiratory manifestations, virus not identified (5%). In females, they were distributed as follows: A02.0-Infection or foodborne intoxication due to any Salmonella (38%), J21.0-Acute bronchiolitis due to RSV (13%), J21.8-Acute bronchiolitis due to other specified organisms (12%) and Q79.4-Prune belly syndrome (5%).
Hospitalization rate and case fatality rate of bronchiolitis by month of age and sex are shown in Table 1. The hospitalization rate was much higher in males than in females, 2,136.37 vs. 1,682.83 (p < .001), in which lower values are clearly appreciated in both at ages <2 years old. However, no major differences were observed in the case fatality rate with respect to sex, but there was difference regarding age (p < .001).
The incidence of hospitalization (Figure 1b) showed a similar seasonal pattern for the years 2019 and 2020, with an epidemic peak in winter and an absence of cases in summer. However, it is observed that in the pandemic period corresponding to 2021 the trend throughout the year is not conservative with the previous ones. It is in the summer months where it presents a first epidemic peak, unlike the other years, reaching the annual maximum in the pre-winter season.
The mean hospitalization cost per case that bronchiolitis caused to the National Health Care System of Spain was 3,054 (95% CI = 3,474–3,526), 3,481 (95% CI = 3,448–3,514) in males and 3,525 (95% CI = 3,484–3,566) in females. Regarding the annual hospitalization cost per case, the year 2019 presented the lowest value 3,133 (95% CI = 3,090–3,177), compared to 2018, 3,146 (95% CI = 3,108–3,184), 2020, 4,095 (95% CI = 4,007–4,181), and 2021, 3,983 (95% CI = 3,929–4,036) (p < .001). The annual average cost to the National Health-Care System for bronchiolitis requiring hospitalization was €49.6 million.
Discussion
This nationwide data-based study offers information on the health system in Spain. RSV-related hospitalization showed a huge public health burden. However, the hospitalization rates of RSV varied from 2018 to 2021. This study provided additional information regarding RSV supplementing previous national studies.15,18,32 In relation to RSV prevalence, some authors determined values of 15% (20% among infants) and 27% during epidemics (32% among infants), while other stipulated that the proportion of case patients aged 3 months was 65% and 43% for aged 6 months.33 In 2005, an estimated 33.8 (95% CI = 19.3–46.2) million new episodes of RSV-associated ALRI occurred worldwide in children younger than 5 years (22% of ALRI episodes), with at least 3.4 (2.8–4.3) million episodes representing severe RSV-associated ALRI necessitating hospital admission.34 In Spain, during the 2018–2021 triennium, there were 56.7% cases less of bronchiolitis among children under than 2 years hospitalized than 2012–2017. This could be due to the decrease in the birth rate in the period considered.
RSV-associated hospitalization increased among children and were a substantial burden in the world, especially among infants and young children.35 These could be due to increased knowledge about RSV or rapid RSV testing in clinical settings. RSV causes severe LRTI that require hospitalization, especially in children ≤2 years. Among children <5 years old, annual hospitalization rates in the United States is 3/1000 children, and rates in Canada and European countries are similar. Nevertheless, RSV in the United States is estimated to cause 1 of 334 hospitalizations. The global burden of RSV infection is unknown as few studies are from developing countries. Estimates indicate about one-fourth of all acute LRTI occur among children <5 years, and the greatest burden is among children in developing countries.5 Other estimates identified an average annual hospitalization rate of 293 hospitalizations per 100,000 children aged <5 years (95% CI = 271–371 hospitalizations per 100,000 children aged <5 years) and 1107 hospitalizations per 100,000 infants (95% CI = 1012–1211 hospitalizations per 100,000 infants). In low-income setting, rates of RSV hospital admission are substantial; they are comparable to estimates from the United States but considerably underestimate the burden in the full community.33 Hervás et al. calculated a 55/1,000 admission hospitalization rate, where rates of RSV-associated hospitalizations were highest among infants and young children, followed by the elderly.7 Holman et al.36 estimated 2700 RSV-associated hospitalizations occurred per 100,000 person-years among children aged <1 year. Zhou et al. showed 384 RSV-associated hospitalizations per 100,000 children aged <5 years similar to a rate of 290 obtained in a prospective study of laboratory-confirmed hospitalizations conducted from 2000 to 2001 through 2003–2004.37 Other studies have shown that RSV-SARI is associated with more severe disease than non-RSV ARI, resulting in a significant impact on healthcare resource utilization. On average, infants spend 2–11 days in hospital for RSV ARI. Bont et al. reported that infants younger than 6 months had the longest duration of average hospital length of stay.10 Infants <2 months with RSV infection had longer median hospital stay (6 vs. 5 days, p < .0001) and higher risk of ICU admission (OR 3.4; 95% CI = 2.5–4.6). In Spain, community-acquired RSV infection is a highly frequent cause of hospitalization in young children, especially in those aged less than 1 year. In this country, the hospitalization rate presented a considerable decrease in 2020 (almost half compared to the rates of the previous year) and returned to its initial values in 2021. Likewise, very high hospitalization rates were maintained at <2 months, that confirm what was presented by other authors.32 At the same time, it is evident that bronchiolitis has a much greater impact in <1 year; with a progressive decrease in the hospitalization rate from 8 months of age.
Ajayi-Obe et al. determined an RSV hospitalization incidence rates of 11/10,000 person-months. The RSV hospitalization rates were highest in children aged <24 months and <12 months (35.7/10,000 person-months). Infection rates were particularly high in those aged <6 months. The age-specific RSV admission rates were highest in those aged >6 months and those aged <12 months (43 and 92.5/10,000 person-months, respectively). However, few studies have determined the age-specific incidence of hospitalization in defined RSV populations.38 Svensson et al. described that the incidence under 1 year of age was 17.4/1,000/year and in children aged 1 to 4 years 0.6/1,000/year.39 Hacımustafaoğlu et al. showed that the annual incidences of hospitalization due to LRTI, acute bronchiolitis, and pneumonia were 20.5/1,000, 11.2/1,000, and 9.3/1,000, respectively, in children ≤2 years of age. The annual incidences of hospitalization due to RSV + LRTI, acute bronchiolitis, and pneumonia were found as 7.8/1,000, 4.6/1,000, and 3.2/1,000, respectively, in children ≤2 years of age. More than one-third of all children hospitalized with LRTI (38.3%) were in the 0–3 months age group. Additionally, compared to other age groups, RSV positivity was highest in that age group for acute bronchiolitis (57%).40 For this reason and according to what was presented in our study, RSV is a very important cause of lower respiratory infections in children <2 years of age and occurred most frequently in those 0–3 months of age in Spain. Several studies have researched the trends in RSV hospitalization rates among children over the last two decades. In 1980–1990 and the early 2000s, the RSV hospitalization rates in the USA and Canada showed a rise. However, from 1998 to 2009, a decrease in the incidence rate of RSV hospitalization was determined in the USA and France, while RSV hospitalization rates have remained relatively stable over similar time periods in the first and Europe.10 Therefore, it is estimated that it cannot be established a definitive conclusion on middle-term time trends, nor clearly in our study.
The impact of RSV on health systems and on the child population is a relevant topic today. Relative to mortality, Nair et al. estimated that 99% of deaths in children younger than 5 years from RSV-associated ALRI in 2005 occurred in developing countries. Nevertheless, incidence and mortality can vary substantially from year to year in any one setting.34 Additionally, preexisting disease/comorbidity, in particular, multiple preexisting diseases and cardiac anomaly, is associated with a significantly higher risk of death from severe RSV infection. Nosocomial/hospital-acquired RSV infection is an additional major risk factor for death in children with this infection.41 RSV infections affect 75% of infants in their first year of life, with a peak incidence between 2 and 3 months of age. Approximately 2–3% of children with a primary RSV infection in the first 12 months of life require hospitalization and 2–6% of them are admitted to Intensive Care. Mortality in previously healthy children hospitalized for bronchiolitis is very low in industrialized countries (0–1.5%), where access to mechanical ventilation and intensive care is easy. However, given its high frequency, every year 66,000–199,000 children die worldwide due to RSV infections, being the second cause of death after malaria in children between 1 and 12 months of age42 and occurred in 0.1% of cases.7 Regarding case-fatality in Spain, during the previous 6 years, 48 more deaths (58.5%) were registered than in 2018–2021. That is, mortality decreased for the last period considered.
Seasonal influenza viruses and RSV are primary causes of ARI in children. New respiratory viruses including human metapneumovirus (hMPV), human bocavirus (HBoV), and influenza 2009 A(H1N1) virus have a strong impact on the pediatric population.43 Some authors showed that the overall prevalence of RSV disease differed between seasons (27% vs. 37.7%, p < .01). The peak prevalence of RSV disease occurred in February, with the onset in November and the end in May.44 Hervás et al. estimated that bronchiolitis due to RSV was more frequent from November to March (97%). Therefore, its circulation is typically seasonal, with a peak of maximum incidence between the months of November and February, which coincides with what was described for 2018–2020 in the present study.7 Cattoir et al. estimated that RSV consistently peaked in November/December each year within a very narrow time frame and mostly detected in samples from infants <3 months. After the age of 1 year, RSV rapidly dropped and slightly increased again at older age (>50 years).45 Sometimes, when the epidemic outbreak begins sooner, the end is sooner as well. Bronchiolitis epidemics onset and conclusion varies along time years in hospitalized infants and showed circannual rhythmicity with a 12-months period. Nevertheless, when data were segregated by long and short hospital stay, no significant differences were found between the rhythms.46 In Spain, the study carried out shows that seasonality presents a general pattern with a high incidence in the winter months (November to January) and practically non-existent during the spring and summer months (April to August), mainly for the years 2018 and 2019. However, the year 2021 showed an increasing trend from the beginning of the year to June, to subsequently decrease until the end of summer and increase in winter. The overall incidence rate has an identical behavior from April to September in 2018 and 2019 (U-shape) and an epidemic peak at the end of the year. In 2020, it is totally in the form of a plateau, while in 2021 an increase is recognized as of September, as was the case in 2018 and 2019. It is estimated that the pandemic-induced alteration in behavior would have an impact in 2020. With the relaxation of restrictions in 2021, a return to normal seasonal RSV infection would be expected; especially since despite the “lockdown” the RSV never stopped circulating.
This particular behavior may be due to the relaxation in personal relationships after the period of isolation due to COVID-19. However, there could be more factors involved, such as climatic factors as those generated by the deep European storms of the 2020–2021 season. These events started as a system that later split in two and affected Spain and, to a lesser extent, Portugal from January 6 to 11, 2021.
Limitations in this study may be due to the fact that there is no microbiological certainty that all the cases considered have confirmation of RSV infection. Therefore, an overestimate could be produced in the RSV associated hospitalizations. However, the research reinforces the one carried out in Spain between 2012 and 201732 and contributes to better health decision-making based on the epidemiological field. Other potential limitation of this study is that it did not report the data regarding Palivizumab use, including the proportion of Palivizumab recipients.
Bacterial co-infection is the major confounder in the burden of disease analyses in RSV. The decision not to administer antibiotics to children hospitalized with RSV can be risky, particularly when there is considerable diagnostic uncertainty. Within current clinical practice, complications and deaths related to RSV are rare.47 Palivizumab was licensed in June 1998 by the Food and Drug Administration for the reduction of serious LRTI caused by RSV in children at increased risk of severe disease. Since that time, the American Academy of Pediatrics has updated its guidance for the use of palivizumab as additional data became available to provide a better understanding of infants and young children at greatest risk of hospitalization attributable to RSV infection.48 The introduction of palivizumab in premature diminished hospitalization for RSV bronchiolitis, oxygen need, hospital length of stay and mechanical ventilation. Currently, the only approved means of RSV prophylaxis is passive immunization with humanized F protein monoclonal antibody. Such prophylaxis, however, has limited availability, is expensive, and is recommended only for infants most at risk for severe RSV disease. Only widespread immunization of maternal and children is likely to diminish the current burden of RSV infection.5 Palivizumab is applied during the winter season. In our study, its consumption would suffer a decrease in the year 2020, since, as has been commented, the overall incidence rate has a plateau behavior for this year.
RSV infection is associated with substantial morbidity in children in both inpatient and outpatient settings. Most children with RSV infection are previously healthy, suggesting that control strategies targeting only high-risk children will have a limited effect on the total disease burden of RSV infection.49 To substantially reduce the burden of RSV hospitalizations, effective general preventive strategies will be required for all young infants, not just those with risk factors.50 Within public health, it is considered that RSV infection could be reduced through the development of new vaccines.51 An effective vaccine for children aged >2 months (outside the age group of poor responders) could prevent a large portion of RSV disease. Severity data suggest that the justification for RSV vaccination will be based on the prevention of morbidity, not mortality.33 Since “most cases occurred in infants under 3 months old,” a vaccine would need to be administered very early in life. Give the immaturity of the infant immune system, these data support maternal vaccination in Spain, where the highest percentage of children under 1 year of age who require hospitalization is among healthy infants under 3 months of age. This has been demonstrated in Spanish epidemiological studies, which show that risk groups are usually well protected against RSV infections, due to stricter hygiene measures, promotion of breastfeeding and administration of immunoprophylaxis such as palivizumab in infants at highest risk.52
Therefore, high pediatric RSV incidence rates can be used to inform subsequent RSV immunization in the future.38 Besides some authors support that RSV bronchiolitis seems to be a more severe disease than that caused by other viruses,7 therefore it is estimated the need to broaden the epidemiological knowledge of RSV.
Acknowledgments
The authors thank the Subdirección General del Instituto de Información Sanitaria for providing the information on which this study is based.
Funding Statement
Enrique Gea-Izquierdo received a María Zambrano Program fund from the European Union-Spain. This research study is part of the research activities of the “Cathedra in Vaccines Research from Rey Juan Carlos University”, which is sponsored by Sanofi.
Disclosure statement
RG-P has received travel and research grants and has participated in advisory boards from Sanofi, Merck, and Pfizer. AG-M has received travel and research grants and has participated in advisory boards from Sanofi, Merck, and Pfizer. EG-I and VH-B have no conflicts of interest.
Data availability statement
The datasets analyzed in the current study are publicly available in the Hospital Discharge Records in the Spanish National Health System (CMBD) repository at https://www.mscbs.gob.es/en/estadEstudios/estadisticas/cmbdhome.htm (accessed on 17 March 2023). The information contained in this repository can be accessed without the need for any administrative permissions.
Institutional review board statement
No formal ethics approval was required for this study.
References
- 1.Riccò M, Cerviere MP, Corrado S, Ranzieri S, Marchesi F.. Respiratory syncytial virus: an uncommon cause of febrile seizures-results from a systematic review and meta-analysis. Pediatr Rep. 2022;14(4):464–9. doi: 10.3390/pediatric14040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, Albertson SB, Deshpande A, Farag T, Abebe Z, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018;18(11):1191–210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, Rodríguez-Tenreiro C, Sly P, Ramilo O, Mejías A, Baraldi E, Papadopoulos NG, Nair H, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217:1356–64. doi: 10.1093/infdis/jiy056. [DOI] [PubMed] [Google Scholar]
- 4.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, et al. RSV global epidemiology network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–58. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets. 2012;12:92–7. doi: 10.2174/187152612800100099. [DOI] [PubMed] [Google Scholar]
- 6.Corsello G, Di Carlo P, Salsa L, Gabriele B, Meli L, Bruno S, Titone L. Respiratory syncytial virus infection in a Sicilian pediatric population: risk factors, epidemiology, and severity. Allergy Asthma Proc. 2008;29(2):205–10. doi: 10.2500/aap.2008.29.3101. [DOI] [PubMed] [Google Scholar]
- 7.Lanari M, Giovannini M, Giuffré L, Marini A, Rondini G, Rossi GE, Merolla R, Zuccotti GV, Salvioli GP. The investigators R.A.DA.R. study group. Prevalence of respiratory syncytial virus infection in Italian infants hospitalized for acute lower respiratory tract infections, and association between respiratory syncytial virus infection risk factors and disease severity. Pediatr Pulmonol. 2002;33(6):458–65. doi: 10.1002/ppul.10047. [DOI] [PubMed] [Google Scholar]
- 8.Hervás D, Reina J, Yañez A, Del Valle JM, Figuerola J, Hervás JA. Epidemiology of hospitalization for acute bronchiolitis in children: differences between RSV and non-RSV bronchiolitis. Eur J Clin Microbiol Infect Dis. 2012;31(8):1975–81. doi: 10.1007/s10096-011-1529-y. [DOI] [PubMed] [Google Scholar]
- 9.Respiratory Syncytial Virus Infection (RSV): Trends and Surveillance . Centers for disease control and prevention. [accessed 2023 Mar 13]. https://www.cdc.gov/rsv/research/us-surveillance.html.
- 10.Bont L, Checchia PA, Fauroux B, Figueras-Aloy J, Manzoni P, Paes B, Simões EA, Carbonell-Estrany X. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in Western Countries. Infect Dis Ther. 2016;5(3):271–98. doi: 10.1007/s40121-016-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control (ECDC) . Rapid risk assessment. Intensified circulation of respiratory syncytial virus (RSV) and associated hospital burden in the EU/EEA. 2022 Dec 12.
- 12.Bardsley M, Morbey RA, Hughes HE, Beck CR, Watson CH, Zhao H, Ellis J, Smith GE, Elliot AJ. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: a retrospective observational study. Lancet Infect Dis. 2023;23(1):56–66. doi: 10.1016/S1473-3099(22)00525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nivel . The GERI study. [accessed 2023 Jan 30]. https://www.nivel.nl/en/geri.
- 14.Juhn YJ, Wi CI, Takahashi PY, Ryu E, King KS, Hickman JA, Yao JD, Binnicker MJ, Natoli TL, Evans TK, et al. Incidence of respiratory syncytial virus infection in older adults before and during the COVID-19 pandemic. JAMA Netw Open. 2023;6(1):e2250634. doi: 10.1001/jamanetworkopen.2022.50634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heppe-Montero M, Gil-Prieto R, Del Diego Salas J, Hernández-Barrera V, Gil-de-Miguel Á. Impact of respiratory syncytial virus and influenza virus infection in the adult population in Spain between 2012 and 2020. Int J Environ Res Public Health. 2022;19(22):14680. doi: 10.3390/ijerph192214680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regassa BT, Gebrewold LA, Mekuria WT, Kassa NA. Molecular epidemiology of respiratory syncytial virus in children with acute respiratory illnesses in Africa: a systematic review and meta-analysis. J Glob Health. 2023;13:04001. doi: 10.7189/jogh.13.04001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitthikarnkha P, Uppala R, Niamsanit S, Sutra S, Thepsuthammarat K, Techasatian L, Niyomkarn W, Teeratakulpisarn J. Burden of respiratory syncytial virus related acute lower respiratory tract infection in hospitalized Thai children: a 6-year national data analysis. Children (Basel). 2022;91990(12). doi: 10.3390/children9121990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heppe-Montero M, Walter S, Hernández-Barrera V, Gil-Prieto R, Gil-de-Miguel Á. Burden of respiratory syncytial virus-associated lower respiratory infections in children in Spain from 2012 to 2018. BMC Infect Dis. 2022;22(1):315. doi: 10.1186/s12879-022-07261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ihling CM, Schnitzler P, Heinrich N, Mangu C, Sudi L, Souares A, Gies S, Sié A, Coulibaly B, Ouédraogo AT, et al. Molecular epidemiology of respiratory syncytial virus in children in Sub-Saharan Africa. Trop Med Int Health. 2021;26(7):810–22. doi: 10.1111/tmi.13573. [DOI] [PubMed] [Google Scholar]
- 20.Buchwald AG, Tamboura B, Tennant SM, Haidara FC, Coulibaly F, Doumbia M, Diallo F, Keita AM, Sow SO, Kotloff KL, et al. Epidemiology, risk factors, and outcomes of respiratory syncytial virus infections in newborns in Bamako, Mali. Clin Infect Dis. 2020;70(1):59–66. doi: 10.1093/cid/ciz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gil-Prieto R, Gonzalez-Escalada A, Marín-García P, Gallardo-Pino C, Gil-de-Miguel A. Respiratory syncytial virus bronchiolitis in children up to 5 years of age in Spain: epidemiology and comorbidities: an observational study. Medicine. 2015;94(21):e831. doi: 10.1097/MD.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández-Rivas L, Pedraz T, Calvo C, San Juan I, Mellado MJ, Robustillo A. Respiratory syncytial virus outbreak during the COVID-19 pandemic. How has it changed? Enferm Infecc Microbiol Clin. 2023;41(6):352–5. doi: 10.1016/j.eimc.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halabi KC, Saiman L, Zachariah P. The epidemiology of respiratory syncytial virus in New York City during the coronavirus disease-2019 pandemic compared with previous years. J Pediatr. 2022;242:242–4.e1. doi: 10.1016/j.jpeds.2021.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabor DE, Fernandes F, Langedijk AC, Wilkins D, Lebbink RJ, Tovchigrechko A, Ruzin A, Kragten-Tabatabaie L, Jin H, Esser MT, et al. Global molecular epidemiology of respiratory syncytial virus from the 2017-2018 INFORM-RSV study. J Clin Microbiol. 2020;59(1):e01828–20. doi: 10.1128/JCM.01828-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonapaladeniya M, Dissanayake T, Liyanage G. Burden of respiratory syncytial virus associated severe pneumonia in hospitalized children. Int J Pediatr. 2021;2021:8269400. doi: 10.1155/2021/8269400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31(2):B209–215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble M, Khan RA, Walker B, Bennett E, Gent N. Respiratory syncytial virus-associated hospitalisation in children aged =5 years: a scoping review of literature from 2009 to 2021. ERJ Open Res. 2022;8(2):00593–2021. doi: 10.1183/23120541.00593-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Z, Qin Q, Shen K, Fang C, Li Y, Deng T. The burden of respiratory syncytial virus associated with acute lower respiratory tract infections in Chinese children: a meta-analysis. Transl Pediatr. 2020;9(4):496–506. doi: 10.21037/tp-20-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ministerio de Sanidad y Consumo . Clasificación Internacional de Enfermedades. 10ª Revisión, Modificación Clínica. 2018.
- 30.Instituto Nacional de la Salud . Subdirección General de Coordinación administrativa. Conjunto Mínimo Básico de Datos. Hospitales de INSALUD 2001. Madrid; 2002. [accessed 2023 Feb 6]. https://ingesa.sanidad.gob.es/bibliotecaPublicaciones/publicaciones/internet/docs/CMBD-2001.pdf. [Google Scholar]
- 31.Ministerio de Sanidad y Consumo . Agencia de Calidad del Sistema Nacional de Salud. Instituto de Información Sanitaria. Metodología de análisis de la hospitalización en el sistema nacional de salud. Modelo de indicadores basado en el registro de altas (CMBD). 2008. [accessed 2023 Feb 6]. https://www.sanidad.gob.es/en/estadEstudios/estadisticas/docs/metod_modelo_cmbd_pub.pdf.
- 32.Heppe Montero M, Gil-Prieto R, Walter S, Aleixandre Blanquer F, Gil De Miguel Á. Burden of severe bronchiolitis in children up to 2 years of age in Spain from 2012 to 2017. Hum Vaccin Immunother. 2022;18(1):1883379. doi: 10.1080/21645515.2021.1883379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okes DJ, Ngama M, Bett A, Abwao J, Munywoki P, English M, Scott J, Cane P, Medley G. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis. 2009;49(9):1341–9. doi: 10.1086/606055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr Infect Dis J. 2012;31(1):5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 36.Holman RC, Curns AT, Cheek JE, Bresee JS, Singleton RJ, Carver K, Anderson LJ. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics. 2004;114(4):e437–444. doi: 10.1542/peds.2004-0049. [DOI] [PubMed] [Google Scholar]
- 37.Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng P-Y, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis. 2012;54(10):1427–36. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajayi-Obe EK, Coen PG, Handa R, Hawrami K, Aitken C, McIntosh EDG, BOOY R. Influenza a and respiratory syncytial virus hospital burden in young children in East London. Epidemiol Infect. 2008;136(8):1046–58. doi: 10.1017/S0950268807009557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svensson C, Berg K, Sigurs N, Trollfors B. Incidence, risk factors and hospital burden in children under five years of age hospitalised with respiratory syncytial virus infections. Acta Paediatr. 2015;104(9):922–6. doi: 10.1111/apa.13061. [DOI] [PubMed] [Google Scholar]
- 40.Hacimustafaoglu M, Celebi S, Bozdemir SE, Ozgür T, Ozcan I, Güray A, Cakır D. RSV frequency in children below 2 years hospitalized for lower respiratory tract infections. Turk J Pediatr. 2013;55:130–9. [PubMed] [Google Scholar]
- 41.Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch Dis Child. 2009;94(2):99–103. doi: 10.1136/adc.2008.139188. [DOI] [PubMed] [Google Scholar]
- 42.Luz García MA, Murua JK, Callejón Callejón A. Bronquiolitis aguda viral. AEP. 2017;1:85–102. [Google Scholar]
- 43.Zuccotti G, Dilillo D, Zappa A, Galli E, Amendola A, Martinelli M, Pariani E, Salvini F, Tanzi E, Riva E, et al. Epidemiological and clinical features of respiratory viral infections in hospitalized children during the circulation of influenza virus A(H1N1) 2009. Influenza Other Respir Viruses. 2011;5(6):e528–534. doi: 10.1111/j.1750-2659.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Constantopoulos AG, Kafetzis DA, Syrogiannopoulos GA, Roilides EJ, Malaka-Zafiriu EE, Sbyrakis SS, Marcopoulos M. Burden of respiratory syncytial viral infections on paediatric hospitals: a two-year prospective epidemiological study. Eur J Clin Microbiol Infect Dis. 2002;21(2):102–7. doi: 10.1007/s10096-001-0668-y. [DOI] [PubMed] [Google Scholar]
- 45.Cattoir L, Vankeerberghen A, Boel A, Van Vaerenbergh K, De Beenhouwer H. Epidemiology of RSV and hMPV in Belgium: a 10-year follow-up. Acta Clin Belgica Int J Clin Lab Med. 2019;74(4):229–35. doi: 10.1080/17843286.2018.1492509. [DOI] [PubMed] [Google Scholar]
- 46.Alonso A, Andres JM, Garmendia JR, Diez I, Gil JM, Ardura J. Bronchiolitis due to respiratory syncytial virus in hospitalized children: a study of seasonal rhythm. Acta Paediatr. 2007;96:731–5. doi: 10.1111/j.1651-2227.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- 47.Weigl JA, Puppe W, Rockahr S, Schmitt HJ. Burden of disease in hospitalized RSV-positive children in Germany. Klin Padiatr. 2002;214(6):334–42. doi: 10.1055/s-2002-35365. [DOI] [PubMed] [Google Scholar]
- 48.Brady MT, Byington CL, Davies HD, Edwards KM, Jackson MA, Maldonado YA, Murray DL, Orenstein WA, Rathore MH, Sawyer MH, et al. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):415–20. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 49.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, et al. Respiratory syncytial virus–associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132(2):e341–348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 51.Vicente D, Montes M, Cilla G, Perez-Yarza EG, Perez-Trallero E. Hospitalization for respiratory syncytial virus in the paediatric population in Spain. Epidemiol Infect. 2003;131(2):867–72. doi: 10.1017/s0950268803008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Luna M, Elola FJ, Fernandez-Perez C, Bernal JL, Lopez-Pineda A. Trends in respiratory syncytial virus bronchiolitis hospitalizations in children less than 1 year: 2004-2012. Curr Med Res Opin. 2016;32(4):693–8. doi: 10.1185/03007995.2015.1136606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in the current study are publicly available in the Hospital Discharge Records in the Spanish National Health System (CMBD) repository at https://www.mscbs.gob.es/en/estadEstudios/estadisticas/cmbdhome.htm (accessed on 17 March 2023). The information contained in this repository can be accessed without the need for any administrative permissions.


