ABSTRACT
African swine fever (ASF) is an acute and highly contagious lethal infectious disease in swine that severely threatens the global pig industry. At present, a safe and efficacious vaccine is urgently required to prevent and control the disease. In this study, we evaluated the safety and immunogenicity of replication-incompetent type-2 adenoviruses carrying African swine fever virus (ASFV) antigens, namely CP204L (p30), E183L (p54), EP402R (CD2v), B646L (p72), and B602L (p72 chaperone). A vaccine cocktail delivered by simultaneous intramuscular (IM) and intranasal (IN) administration robustly elicited both systemic and mucosal immune responses against AFSV in mice and swine and provided highly effective protection against the circulating ASFV strain in farmed pigs. This multi-antigen cocktail vaccine was well tolerated in the vaccinated animals. No significant interference among antigens was observed. The combined IM and IN vaccination using this adenovirus-vectored antigen cocktail vaccine warrants further evaluation for providing safe and effective protection against ASFV infection and transmission.
KEYWORDS: African swine fever virus, adenovirus, infection, mucosal immunity, swine, vaccine
Introduction
African swine fever virus (ASFV) is the causative agent of African swine fever (ASF), a severe, extremely contagious, and fatal viral disease [1, 2]. ASFV is the only known DNA virus spread by arthropods, and it belongs to the Asfivirus genus within the Asfarviridae family [3, 4]. ASF symptoms and fatality rates differ depending on the animal species and virus strains involved. Acute ASFV infection has almost 100% mortality [5], whereas chronic infections are typically caused by less virulent strains that manifest as intermittent fever, weight loss, persistent skin ulcers, and arthritis [1]. Hence, ASF is recognized as a notifiable disease by the World Organization for Animal Health (WOAH) and continues to pose a serious threat to the global pig industry, food security, and economic trade. Currently, there is a lack of highly effective antiviral drugs, making the development of a safe and effective vaccine a pressing concern.
The ASFV genome is a double-stranded DNA molecule of 170–190 kbp with 151–167 open reading frames (ORFs), yet half of its genes have no known or predicted function [6, 7]. Although the development of live attenuated or subunit vaccines is the main focus of current ASF vaccine design approaches, various ASF vaccine approaches have been studied in recent decades. Live attenuated ASFV vaccines can confer protection of up to 100% [8–11], but they can also have side effects and possibly other safety concerns. Subunit vaccine strategies have only achieved limited success, primarily due to the selection of a delivery system and the lack of knowledge regarding protective antigens [12]. While the mechanisms behind protective immunity are not fully understood, both cellular and humoral immune responses induced by ASFV vaccines play crucial roles in providing host protection. Previous studies have shown that the viral-vectored ASFV vaccines are a viable approach to eliciting antigen-specific antibody and cellular immune responses, and that protection is associated with robust virus-specific T-cell responses [13–15]. Additionally, mucosal vaccination with adenovirus-vectored vaccines can effectively stimulate both systemic and mucosal immunity, preventing the transmission of exogenous pathogens [16–18]. Therefore, in addition to the choice of antigen, the administration route, immunization regimen, and dose are also crucial for the success of a viral-vectored ASFV vaccine.
In a previous study, vaccination using a pool of eight virally vectored ASFV antigens provided complete protection to all pigs when challenged with a lethal dose of virulent ASFV [13]. However, it is commercially infeasible to produce eight adenovirus vector vaccines, and more antigens may have the problem of antigen competition. Based on further analysis of these antigen-induced immune responses in this study, in combination with the architecture of the ASFV virion to determine which antigens may be more important for effective protection, we ultimately chose CP204L (p30), E183L (p54), EP402R (CD2v), B646L (p72), and B602L (p72 chaperone). Subsequently, we constructed and evaluated the immunogenicity of five replication-incompetent adenovirus type 2 (Ad2)-vectored ASFV vaccines in mice and swine. We also assessed the protective efficacy of adenovirus-vectored ASFV antigen cocktail vaccines against the ASFV prevalent strain in farmed pigs.
Materials and methods
Ethics statements
This study was conducted following the guidelines outlined in the Guide for the Care and Use of Laboratory Animals by the Ministry of Science and Technology of the People’s Republic of China. The vaccination experiments were approved by the Committee on the Ethics of Animal Experiments at the Guangzhou Institutes of Biomedicine and Health of the Chinese Academy of Sciences (No. 2020086 and 2022163 for mice and swine, respectively).
Virus infection assays
The adenovirus infection assays were conducted by infecting porcine kidney cells (PK-15, ATCC CCL-33) with Ad2-EGFP, Ad3-EGFP, Ad4-EGFP, Ad5-EGFP, Ad6-EGFP, Ad7-EGFP, Ad11-EGFP, Ad14-EGFP, and Ad55-EGFP at the desired doses at 37°C for 1 h. At 24 h post-infection, the cells were imaged using fluorescence microscopy and analyzed by an Accuri C6 flow cytometer. The Ad-EGFP vectors were purchased from Guangzhou nBiomed Ltd.
Adenovirus vectored vaccine
Replication-incompetent recombinant adenoviruses Ad2-p30, Ad2-p54, Ad2-p30-p54, Ad2-CD2v, and Ad2-p72-p72c were constructed as follows. In brief, the amino acid sequence of the ASFV antigen was obtained from Pig/Heilongjiang/2018 (HLJ/18) ASFV isolates (GenBank accession no. MK333180.1), which was designed as a synthetic gene codon to optimize protein expression in the porcine host (Genescript, China). To generate recombinant adenoviruses, these genes were amplified by PCR, subcloned into the shuttle plasmid pGA1, linearized, and subjected to homologous recombination with an E1- and E3-deleted pAd2ΔE1ΔE3 backbone in E. coli BJ5183 competent cells (Thermo Fisher) according to the manufacturer’s protocols [19]. These recombinant adenoviruses were then amplified, purified with a cesium chloride density gradient centrifuge, titrated, and stored at −80°C.
Immunocytochemistry
Immunocytochemistry was used to assess the protein expression of the recombinant adenoviruses. Briefly, the recombinant adenoviruses Ad2-p30, Ad2-p54, Ad2-p30-p54, Ad2-CD2v, and Ad2-p72-p72c were used to infect HEK-293 cell monolayers. The cells were fixed with 4% paraformaldehyde at 24 h after infection. The cells were blocked with 1× PBS containing 5% BSA at 37°C for 1 h and incubated with mouse anti-p30, anti-p54, anti-CD2v, and anti-p72 antibodies. These mouse antibodies against p30, p54, CD2v, or p72 were a gift from Prof Guangxia Gao. Following 3 washes, the cells were subsequently incubated for 1 h with a 1:5000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Beyotime, China). Following washes as described above, the cells were stained with 3,3-diaminobenzidine tetrahydrochloride and then observed under a confocal microscope. Mock-infected cells were used as negative controls.
Western blotting
Western blotting was further used to assess the protein expression of the recombinant adenoviruses. Briefly, the recombinant adenoviruses Ad2-p30, Ad2-p54, Ad2-p30-p54, Ad2-CD2v, Ad2-p72-p72c, and Ad2-empty were used to infect HEK-293 cell monolayers. 48 h post-infection, the cells were harvested and lysed, then resolved through SDS-PAGE and transferred to PVDF membranes. The membrane was blocked overnight at 4°C with 5% BSA and incubated with antibodies against p30, p54, CD2v, or p72 in PBST (PBS supplemented with 0.05% Tween-20 and 5% skim milk). Then, the membranes were incubated with HRP-conjugated secondary antibodies at room temperature for 1 h, and developed with chemiluminescent HRP substrate (Merck).
Mouse vaccination
Experiment 1
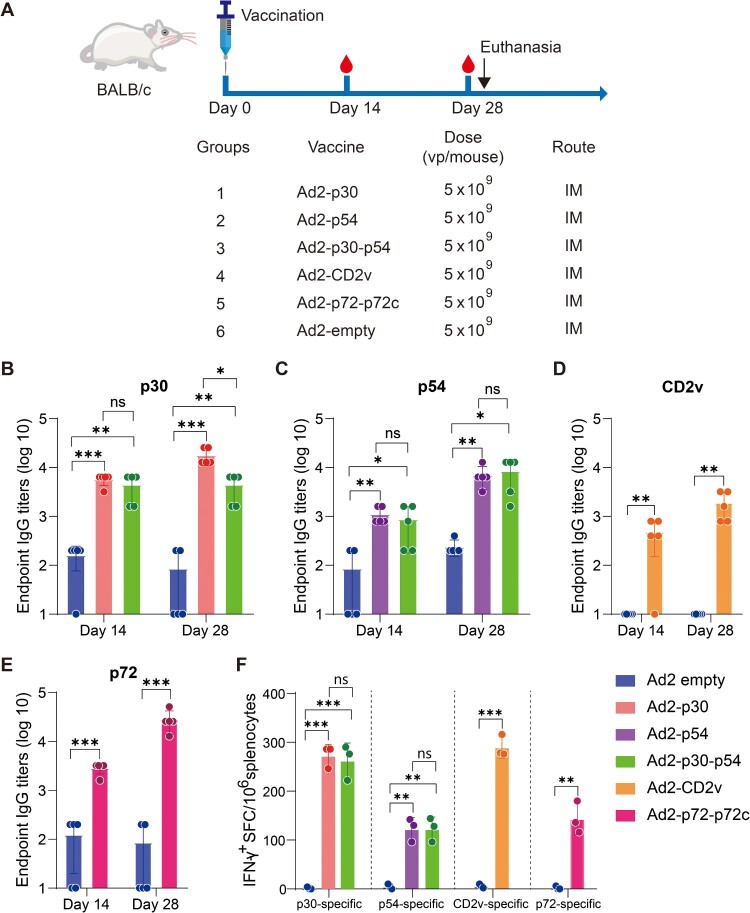
Female BALB/c mice that were six weeks old were randomly divided into 6 groups (n = 5). Figure 3(A) shows the vaccination regimen. One group of mice served as the control and received vaccination with 5 × 109 vp Ad2-empty via an IM route. Mouse sera were collected 14 days after vaccination. The mouse was euthanized on day 28 following vaccination, and the sera and splenocytes were collected and detected using an ELISA or ELISpot assay.
Figure 3.
The immunogenicity of Ad2-ASFV via intramuscular vaccination in mice. (A) Schematic of the vaccination schedule, dose, and route for the mouse experiment. Six-week-old BALB/c mice were vaccinated with Ad2-ASFV via the IM route at the indicated doses. The red blood drop symbols represent the time points when serum samples were collected. The mice were sacrificed 28 days after the prime vaccination. (B–E) ELISA was used to assess the binding antibodies to p30 (B), p54 (C), CD2v (D), and p72 (E) at the indicated time points following vaccination. (F) IFN-γ ELISpot was used to detect the number of antigen-specific IFN-γ-secreting cells per million splenic lymphocytes in mice. The data represent the results of three independent experiments. Comparisons using the student’s t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Experiment 2
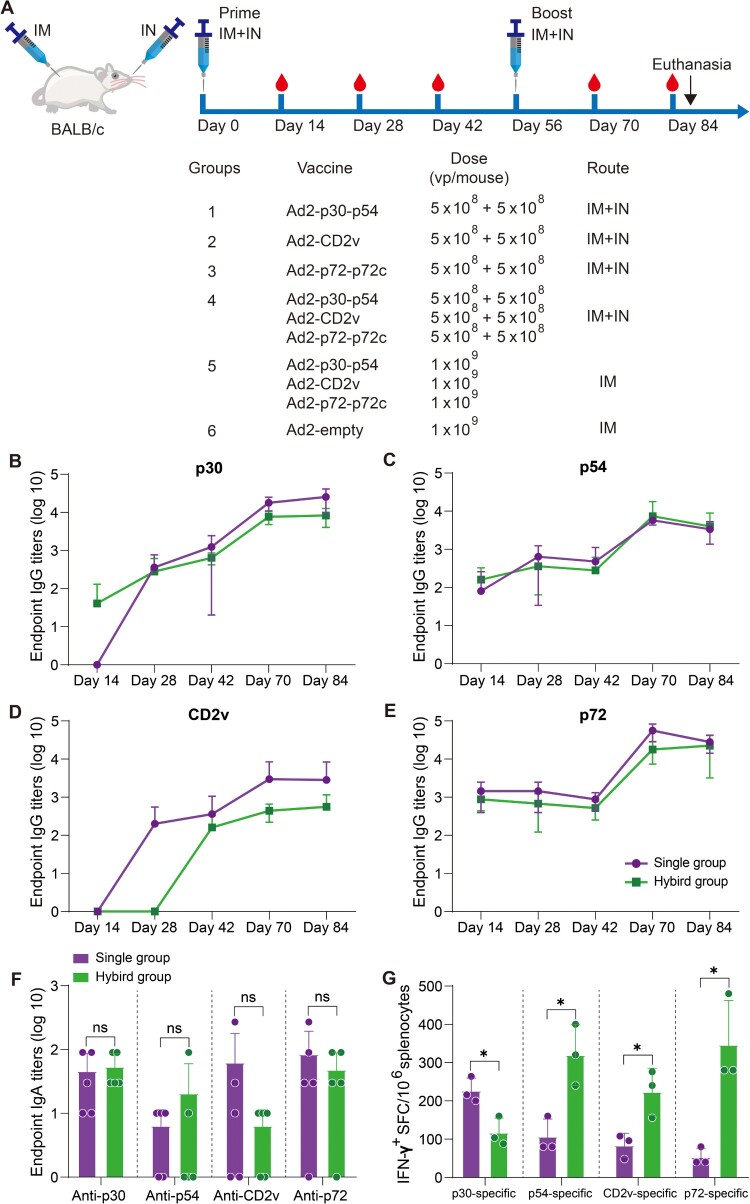
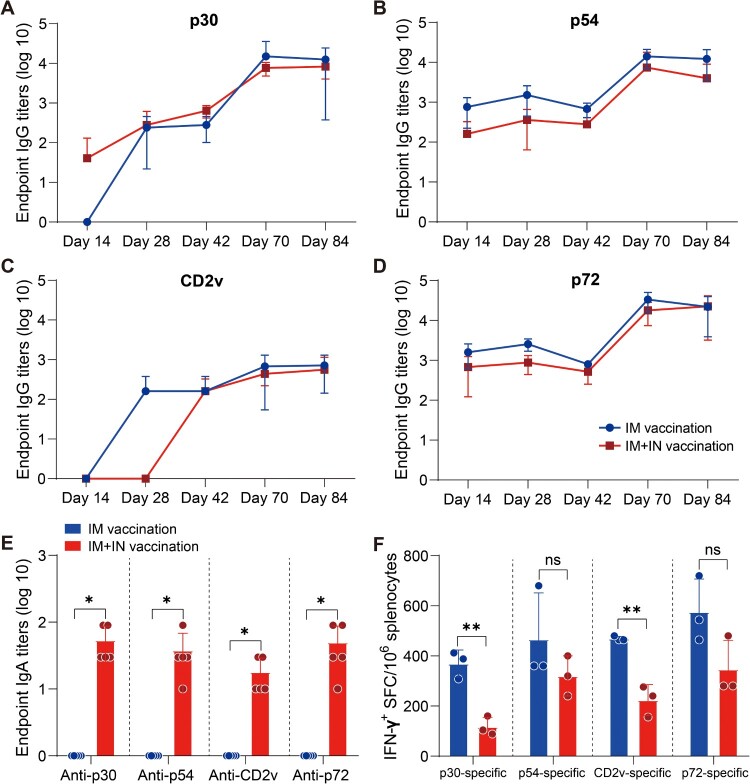
Female BALB/c mice that were six weeks old were randomly divided into 6 groups (n = 5). Figure 4(A) shows the vaccination regimen. The control group was vaccinated with 1 × 109 vp Ad2-empty by an IM route. Mouse sera were collected on days 14, 28, 42, and 70 after the vaccination. The mouse was euthanized on day 84 following vaccination, and the sera, BALFs, and splenocytes were collected and detected using an ELISA or ELISpot assay.
Figure 4.
The immunogenicity of Ad2-ASFV after prime-boost vaccination via IM + IN administration in mice. (A) Schematic of the prime-boost immunization scheme in mice. Mice received a second dose of the same vaccine 56 days later. The red blood drop symbols represent the time points when serum samples were collected. The mice were sacrificed 84 days following the prime vaccination. (B–E) The IgG antibodies against p30 (B), p54 (C), CD2v (D), and p72 (E) at the indicated time points postvaccination were assessed by ELISA. (F) ELISA was used to assess IgA antibodies against the p30, p54, CD2v, and p72 proteins in BALFs. (G) IFN-γ ELISpot was used to detect the number of antigen-specific IFN-γ-secreting cells per million splenic lymphocytes in mice. The data represent the results of three independent experiments. Comparisons using the student’s t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Swine vaccination
Experiment 1
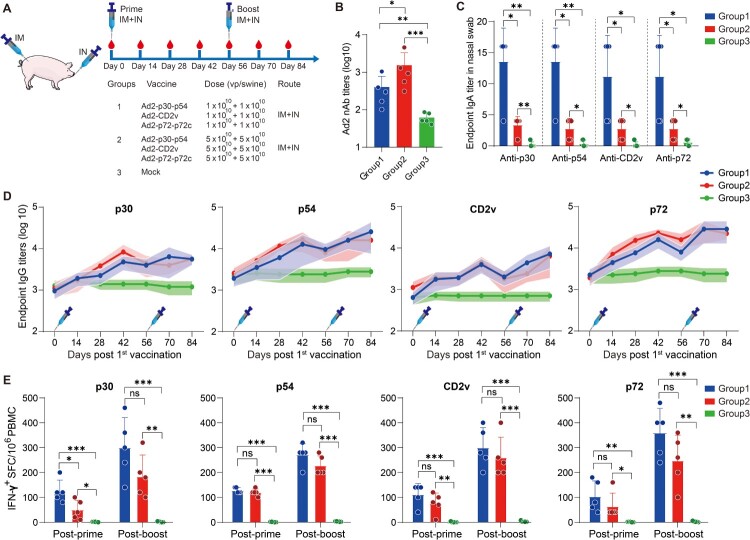
A total of 15 eight-week-old farm pigs (7 male, 8 female) were included in this study. Pigs were divided into 3 groups at random (n = 5). The non-vaccinated group served as the control. The vaccination regimen is shown in Figure 6(A). Serum samples, nasal swabs, and PBMCs were collected at multiple time points and detected using an ELISA or ELISpot assay.
Figure 6.
The systemic and mucosal antibody and T-cell immune responses to ASFV antigens after prime-boost vaccination via IM + IN administration in swine. (A) Schematic of the prime-boost vaccination scheme in swine. Swine received a second dose of the same vaccine 56 days later. The red blood drop symbols indicate the time points when blood samples and PBMCs were collected. (B) The nAb titres against Ad2 in different vaccination groups. (C) ELISA was used to assess IgA antibodies against the p30, p54, CD2v, and p72 proteins in nasal swabs. (D) The IgG antibodies to p30, p54, CD2v, and p72 at the indicated time points postvaccination were assessed by ELISA. (E) IFN-γ ELISpot was used to detect the number of antigen-specific IFN-γ-secreting cells per million PBMCs in swine. The data represent the results of three independent experiments. Comparisons using the student’s t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Experiment 2
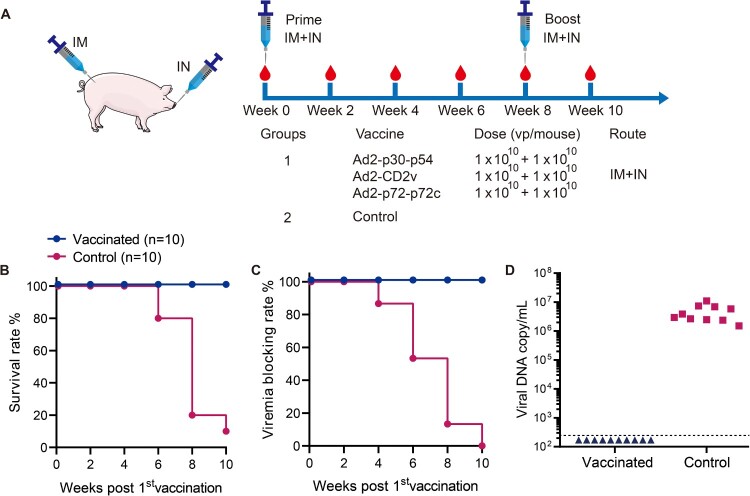
To assess the protective efficacy of the Ad2-ASFV vaccine in field farms, a total of 20 eight-week-old farm pigs (10 male, 10 female) were included in this study. Pigs were divided into 2 groups at random (n = 10) and cohoused in the same pens. The non-vaccinated group served as the control. The vaccination regimen is shown in Figure 7(A). Clinical signs were observed throughout the experiment. Blood samples from the dead or surviving pigs during the observation period were collected at multiple time points and detected using qPCR assays.
Figure 7.
Vaccine efficacy of Ad2-ASFV in commercial pigs. (A) Schematic of the prime-boost vaccination scheme in swine. Swine received a second dose of the same vaccine 56 days later. The red blood drop symbols indicate the time points when the blood samples were collected. (B) Survival rates of pigs. (C) Viremia blocking rates of pigs. (D) Viral DNA copies in blood samples of pigs. The viral p72 gene copies were detected using qPCR in blood samples collected from dead or surviving pigs. The lower limit of detection is indicated by the dashed lines.
ELISA
ELISA was used to quantify the antigen-specific IgG or IgA antibodies in the immunological sera, nasal swabs, and BALFs. Briefly, 0.05 μg of p30, p54, CD2v, or p72 protein (Msunflowers, China) were coated overnight at 4°C in 96-well plates and blocked with PBST and 5% skim milk for 2 h at room temperature. Serially diluted serum samples were added to each well and incubated at 37°C for 1 h. HRP-conjugated goat anti-mouse (Beyotime, China) or goat anti-swine IgG antibodies (Abbkine) were then added to the wells and incubated at 37°C for 1 h. The reaction was developed by TMB substrate and determined at 450 nm. The cut-off value was determined by calculating the mean optical density values at 450 nm (OD450) + 3 × standard derivations (SD) from the sera of nonvaccinated animals. The endpoint titres were then calculated as the reciprocal of the highest serum dilution at which the OD450 values were equal to or greater than the cut-off value. The levels of IgA antibodies were measured in mouse BALFs and swine nasal swabs using an HRP-conjugated polyclonal goat anti-mouse or anti-swine IgA antibody (Abcam), following a similar procedure.
ELISpot
ELISpot assays were performed to evaluate antigen-specific IFN-γ+ cell response using freshly isolated mouse splenic lymphocytes or swine PBMCs. In brief, the mouse or porcine IFN-γ coating antibody (Mabtech) was coated overnight at 4°C in sterile 96-well microtitre plates (Merck Millipore). Using a density gradient medium to isolate mouse splenic lymphocytes or swine PBMCs, 5 × 105 cells per well were seeded and stimulated with the corresponding peptide pool (p30, p54, CD2v, or p72) (Table S1) at 2 μg ml−1 per peptide (Genescript, China) for 24 h. The biotinylated detection antibodies (U-CyTech) were incubated and then developed with alkaline phosphatase-conjugated streptavidin (U-CyTech) and NBT/BCIP reagent (Pierce). Finally, the spots were counted with an ELISpot reader (Bioreader 4000, BIOSYS, Germany).
qPCR
ASFV genomic DNA quantity in the blood samples was determined by qPCR. QIAamp® DNA Mini Kit (Qiagen, Germany) was used to extract ASFV genomic DNA from EDTA-treated whole peripheral blood. qPCR was carried out on a Bio-Rad system (Bio-Rad, USA) according to the WOAH-recommended procedure as previously described [20].
Statistical analysis
An unpaired t test with Welch’s correction was used to analyze the differences in the mean antigen-specific antibody and IFN-γ responses between the treatment and control groups. A p < 0.05 was considered significant. GraphPad Prism Version 8 was used to construct data graphs.
Results
Construction of recombinant replication-incompetent adenoviruses for efficient delivery of ASFV antigens as vaccine candidates
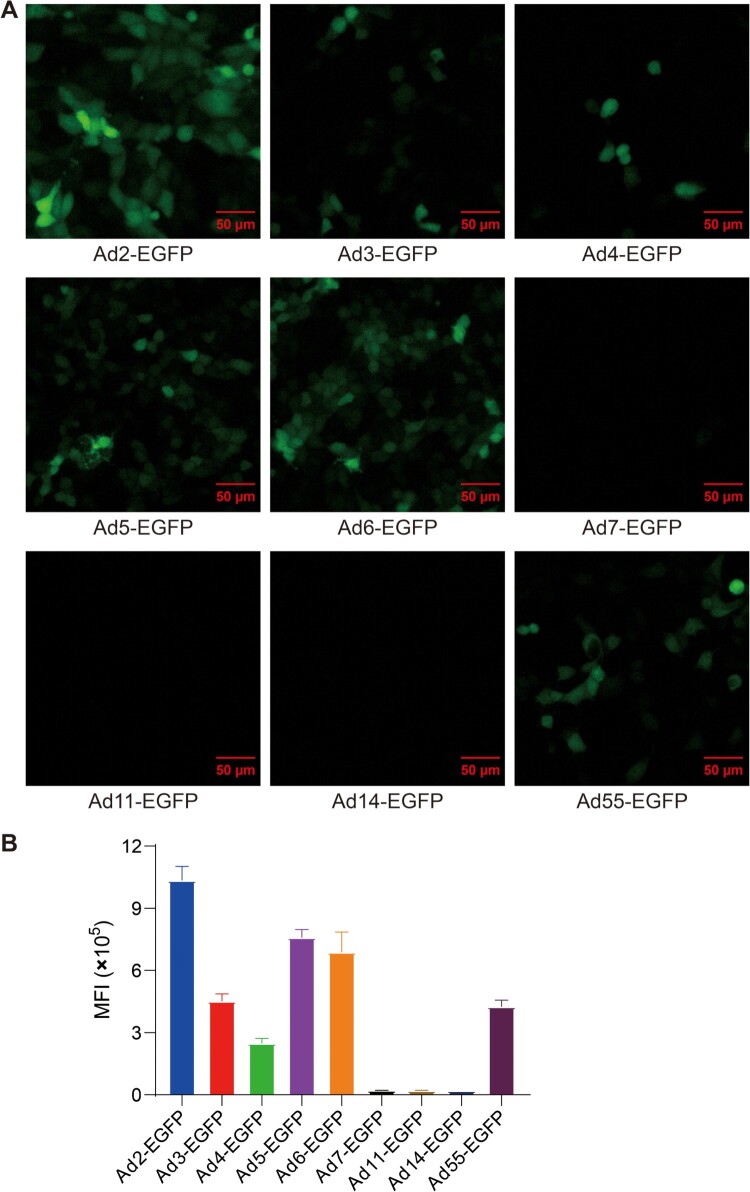
To develop a safe and effective vaccine against AFSV, the human replication-incompetent adenoviral vector was used as a platform for vaccine construction. To determine which type of human adenovirus can effectively mediate transgene expression in swine cells, PK-15 cells were infected with a series of human adenoviruses carrying EGFP gene at 500 viral particles (vp) per cell, including Ad2-EGFP, Ad3-EGFP, Ad4-EGFP, Ad5-EGFP, Ad6-EGFP, Ad7-EGFP, Ad11-EGFP, Ad14-EGFP, and Ad55-EGFP. EGFP signals from Ad-EGFP were detected by fluorescence microscopy and flow cytometry. Compared to other types of adenoviruses, Ad2 was the most effective at mediating transgene expression in pig-derived cells (Figure 1). Thus, we chose human replication-incompetent adenovirus type-2 as the vector to construct ASFV vaccine candidates.
Figure 1.
Infectivity of divergent human adenovirus serotypes in pig-derived cells as determined by a virus infection assay. (A) The infectivity of divergent serotypes of Ad-EGFP was determined in PK-15 cells. (B) The mean fluorescence intensity (MFI) of cells infected with divergent serotypes of Ad-EGFP. The cells were imaged under a fluorescence microscope at 24 h postinfection, and flow cytometry was used to examine the expression of EGFP. The data originated from three independent experiments. The MFI is represented by the mean ± SD.
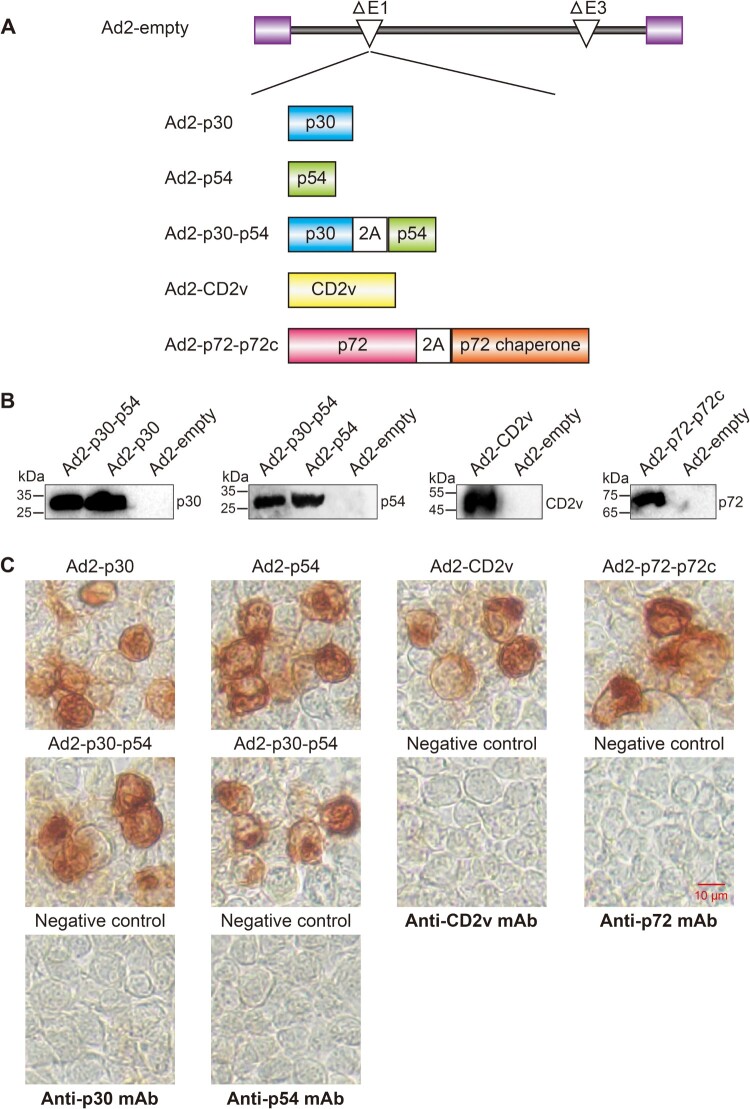
Five replication-incompetent recombinant adenovirus type 2, Ad2-p30, Ad2-p54, Ad2-CD2v, Ad2-p30-p54 and Ad2-p72-p72c, were constructed (Figure 2(A)). Genes encoding the ASFV antigens CP204L (p30), E183L (p54), EP402R (CD2v), B602L (p72 chaperone), and B646L (p72) were codon-optimized for optimal expression in swine cells. Previous studies have confirmed that the p72 chaperone protein is required as a molecular chaperone for the correct folded conformation of the p72 capsid protein [21]. To enable simultaneous expression of two proteins in the same cells, we inserted the “self-cleaving” 2A peptide between genes p72 and p72 chaperone, and between p30 and p54, to achieve expression of two genes in an expression cassette [22]. To confirm the expression of these ASFV antigens, these recombinant adenoviruses were used to infect HEK 293 cells, and subjected to Western blotting and immunocytochemistry analysis. Western blotting and Immunocytochemistry revealed that recombinant adenovirus-infected HEK-293 cells could efficiently express ASFV antigens (Figure 2(B,C)).
Figure 2.
Construction and characterization of the Ad2-ASFV vaccine. (A) Schematic representation of the Ad2-ASFV vaccine construction in this study. (B) Antigen expression in HEK293 cells infected with Ad2-ASFV was analyzed by Western blotting. (C) Antigen expression of the Ad2-ASFV vaccine was assessed by immunocytochemistry.
Ad2-ASFV administered intramuscularly elicits antibody and T-cell immune responses in mice
To evaluate the immunogenicity of Ad2-p30, Ad2-p54, Ad2-p30-p54, Ad2-CD2v, and Ad2-p72-p72c, five groups of 6-week-old female BALB/c mice were vaccinated intramuscularly (IM) with 5 × 109 vp Ad2-ASFV. The control group was vaccinated with 5 × 109 vp Ad2-empty by an IM route. (Figure 3(A)). The antigen-specific IgG antibody responses were analyzed on day 14 and day 28 after vaccination. The 5 × 109 vp Ad2-ASFV elicited significant serum IgG antibodies against the p30, p54, CD2v, and p72 on day 14 following IM vaccination (Figure 3(B–E)), whereas 1 × 109 vp Ad2-ASFV elicited lower serum IgG titres against the p30, p54, CD2v, and p72 (Figure 4(B–E)), demonstrating a dose-dependent response. The serum IgG antibodies against the p30, p54, CD2v, and p72 continued to increase until mice were sacrificed on day 28 (Figure 3(B–E)). In addition, the levels of serum IgG antibodies against p30 or p54 elicited by Ad2-p30-p54 were similar to those elicited by Ad2-p30 or Ad2-p54, demonstrating that Ad2-p30-p54 induces a balanced immune response with no signs of antigenic competition (Figure 3(B,C)).
Previous studies have shown that virus-specific T-cell immune responses are crucial for controlling ASFV infection [23, 24]. We performed interferon-γ (IFN-γ) enzyme-linked immune spot (ELISpot) assays to determine whether Ad2-AFSV elicit an antigen-specific T-cell immune response in mice using splenic lymphocytes. Splenocytes were stimulated with an ASFV peptide pool of p30, p54, CD2v, and p72. An IM vaccination with 5 × 109 vp Ad2-ASFV induced significant antigen-specific IFN-γ+ cell response, and high dosages of Ad2-ASFV elicited a higher T-cell immune response than low dosages (Figure 3(F,G)). Consistent with the antibody response, the Ad2-p30-p54 chimera elicited a similar level of p30- or p54-specific T-cell immune responses with Ad2-p30 or Ad2-p54.
Ad2-ASFV cocktail elicits systemic and mucosal antibody and T-cell immune responses in mice via a combined IM and IN vaccination regimen
Previous investigations on the ASFV vaccine have demonstrated that subunit vaccines based on one or two ASFV antigens have not been able to confer significant protection among vaccinees [24–26]. A multiantigen cocktail may successfully induce robust immunity to confer complete protection. ASFV infects through an aerosol route and replicates primarily in monocyte macrophages, and it is highly stable in the environment and can be easily transmitted orally through contaminated fomites. Thus, mucosal immunity would provide a powerful defence to prevent initial infection and subsequent spread. Next, we assessed the prime-boost vaccination regimen using an Ad2-ASFV cocktail via IM combined with intranasal (IN) routes in mice (Figure 4(A)). Female BALB/c mice that were six-week-old were divided into 6 groups at random: (1–3) 5 × 108 vp IM injection plus 5 × 108 vp IN instillation with Ad2-p30-p54, Ad2-CD2v, or Ad2-p72-p72c per mouse (n = 5); (4) A total of 1.5 × 109 vp IM injection plus 1.5 × 109 vp IN instillation with Ad2-p30-p54, Ad2-CD2v, and Ad2-p72-p72c cocktail per mouse (n = 5); (5) A total of 3 × 109 vp IM injection with Ad2-p30-p54, Ad2-CD2v, and Ad2-p72-p72c cocktail per mouse (n = 5); and (6) The control group was vaccinated with 1 × 109 vp Ad2-empty by an IM route (n = 5). On days 14, 28, and 42 post-priming vaccination, all vaccination groups generated antigen-specific IgG responses, which significantly increased after boost vaccination (Figure 4(B–E)). The Ad2-ASFV cocktail elicited a similar level of serum IgG antibodies against all antigens as the Ad2-ASFV single antigen groups, demonstrating that the Ad2-ASFV cocktail induces a balanced immune response without antigenic competition (Figure 4(B–E)). In addition, IM + IN vaccination also elicited a similar level of serum IgG antibodies against p30, p54, CD2v, and p72 antigens as IM vaccination (Figure 5(A–D)).
Figure 5.
The systemic and mucosal antibody and T-cell immune responses to ASFV antigens after prime-boost vaccination via IM or IM + IN administration in mice. (A–D) The binding antibodies to p30 (A), p54 (B), CD2v (C), and p72 (D) at the indicated time points postvaccination were assessed by ELISA. (E) ELISA was used to assess IgA antibodies against the p30, p54, CD2v, and p72 proteins in BALFs. (F) IFN-γ ELISpot was used to detect the number of antigen-specific IFN-γ-secreting cells per million splenic lymphocytes in mice. The data represent the results of three independent experiments. Comparisons using the student’s t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Mucosal vaccination induces potent mucosal IgA antibodies that provide the initial defence against infection by capturing and neutralizing respiratory pathogens at mucosal surfaces. Therefore, we assessed mucosal immunity by measuring ASFV antigen-specific IgA titres in bronchoalveolar lavage fluids (BALFs). Consistent with serum IgG responses, the Ad2-ASFV cocktail elicited a similar level of BALF IgA responses against p30, p54, CD2v, and p72 antigens as the Ad2-ASFV single antigen groups (Figure 4(F)). IM + IN vaccination elicited all antigen-specific IgA responses in BALFs, whereas IM vaccination did not (Figure 5(E)). Thus, the Ad2-ASFV cocktail can induce both systemic IgG and pulmonary IgA antibodies against ASFV antigen in mice by IM combined with IN.
We also evaluated ASFV antigen-specific IFN-γ-secreting cells in the splenic lymphocytes of the mouse. The data show that the Ad2-ASFV cocktail elicited a stronger splenic T-cell immune response against p30, p54, CD2v, and p72 antigens than the Ad2-ASFV single antigen groups in mice (Figure 4(G)). In addition, IM vaccination with the Ad2-ASFV cocktail elicited a higher splenic T-cell immune response to p30 and CD2v antigens than IM + IN vaccination in mice, but p54 and p72 antigens were not significantly different (Figure 5(F)). Taken together, our findings revealed that the IM + IN Ad2-ASFV cocktail vaccine induced robust systemic and mucosal immune responses.
Ad2-ASFV cocktail elicited systemic and mucosal antibody and T-cell immune responses in swine by a combined IM and IN vaccination regimen
Next, we assessed the immunogenicity in swine of the Ad2-ASFV cocktail administered with a prime-boost vaccination regimen followed by a booster of the same vaccine eight weeks after the prime vaccination via IM combined with IN (Figure 6(A)). Eight-week-old commercial swine were randomly divided into the following 3 groups: (1) A total of 3 × 1010 vp IM injection plus 3 × 1010 vp IN instillation with Ad2-p30-p54, Ad2-CD2v, and Ad2-p72-p72c cocktail per swine (n = 5); (2) A total of 1.5 × 1011 vp IM injection plus 1.5 × 1011 vp IN instillation with Ad2-p30-p54, Ad2-CD2v, and Ad2-p72-p72c cocktail per swine; and (3) swine without vaccination as a control group (Figure 6(A)). Consistent with the results in mice, at day 14 after priming, the Ad2-ASFV cocktail vaccine group generated antigen-specific IgG responses against p30, p54, CD2v, and p72 antigens and continuously increased until day 42 (Figure 6(D)). After a boost vaccination, the Ad2-ASFV cocktail vaccine group further increased the antibody titre against p30, p54, CD2v, and p72 antigens (Figure 6(D)). In addition, the nasal swabs were collected on day 14 postpriming vaccination and mucosal immunity was assessed by measuring ASFV-specific IgA titres. Compared with the control group, the Ad2-ASFV cocktail vaccine group elicited IgA responses against p30, p54, CD2v, and p72 antigens, whereas the IgA titres appeared to be higher at low dosages (Figure 6(C)). Furthermore, we ruled out the possibility of high- and low-dose confounding by measuring adenovirus neutralizing antibodies (Figure 6(B)).
To determine the T-cell immune response elicited by the Ad2-ASFV vaccine in swine, we examined ASFV-specific IFN-γ-secreting cells in the peripheral blood mononuclear cells (PBMCs) via ELISpot on days 14 and 70 post-priming vaccination. After the first vaccination, swine vaccinated with the Ad2-ASFV cocktail generated a strong T-cell immune response to p30, p54, CD2v, and p72 antigens, which was further enhanced after the boost vaccination, whereas high dosages of vaccination appeared to elicit a weaker systemic T-cell immune response, even though there was no significant difference (Figure 6(E)).
In addition, all pigs were active and healthy with a good appetite after vaccination with low and high dosages of the Ad2-ASFV multiantigen cocktail and remained so for the rest of the study period, indicating that the Ad2-ASFV cocktail vaccine was well tolerated in the swine. In conclusion, the Ad2-ASFV cocktail vaccine has good immunogenicity and safety when administered via IM + IN in a prime-boost regimen.
Ad2-ASFV confers protection against the current circulating ASFV strain in farmed pigs
Our results have shown that the Ad2-ASFV cocktail vaccine was immunogenic and safe in swine using a homologous prime-boost vaccination regimen. The next logical step is to assess whether the candidate vaccine Ad2-ASFV can confer protection against the current ASFV prevalent strain in field farms. To assess the protective efficacy of Ad2-ASFV, groups of 10 pigs were vaccinated with 3 × 1010 vp IM plus 3 × 1010 vp IN with Ad2-p30-p54, Ad2-CD2v, and Ad2-p72-p72c cocktail per swine, and a group of 10 nonvaccinated pigs was used as a control (Figure 7(A)). Blood samples were collected from each pig at different time points, and the viral p72 gene was detected by using qPCR according to the recommendation of WOAH to determine whether ASFV infection occurred in the pigs. Of note, it happened to have an ASFV China/GD/2019 outbreak on the swine farm. The 10 vaccinated pigs survived healthily and showed no clinical signs consistent with ASF during the 70-day observation period, and no viremia was detectable (Figure 7(B–D)). Of note, 9 out of the 10 control pigs developed clinical signs of ASFV disease including anorexia, depression, fever, purple skin discoloration, staggering gait, diarrhea, cough, and death within the 70-day observation period, and viremia was detected in all pigs (Figure 7(B–D)). These results indicate that two vaccinations with Ad2-ASFV could be highly effective against the circulating ASFV strain in farmed pigs.
Discussion
To date, ASFV continues to pose a serious threat to the global swine industry, and vaccination is still regarded as the most economical and effective means to control ASF epidemics. Several studies have reported that live attenuated virus vaccines have elicited robust immunity against related ASFV strains, but poor safety profile is still a concern [8–10, 27]. Thus, the ASFV subunit vaccine might be a better choice since it avoids potential safety issues, can distinguish infection from vaccinated animals (DIVA), and is easier to manufacture. Here, we screened a novel adenoviral vector Ad2 that can replicate more efficiently in pigs and evaluated the immunogenicity of the Ad2-ASFV cocktail vaccine in mice and swine via novel vaccination (IM + IN) route. Our studies showed that the Ad2-ASFV cocktail vaccine can elicit robust antigen-specific antibody, T-cell, and mucosal immune responses in mice and swine.
Several viral-vectored ASFV vaccines have been evaluated for immunogenicity in pigs, but only a few vaccines have been studied for protection against lethal ASFV challenges. A BacMam-vectored ASFV vaccine delivering the sHA/p54/p30 fusion construct protected 4 of 6 pigs against sublethal challenge, and this protection was associated with a strong antigen-specific T-cell response, as no specific antibody response was detected [24]. The ASFV gene pool was primed with the adenoviral vector Ad5 and boosted with a modified Ankara vaccinia virus (MVA) vector, which reduced clinical signs and viremia in pigs challenged with the virulent strain OUR T88/1 [28]. Another study showed that the adenovirus-vectored AFSV multiantigen combination formed in adjuvant elicited robust antibody responses but failed to confer protection against intranasal challenge with the Georgia 2007/1 strain [29]. However, a recent study showed that the use of rAd prime and MVA boost as a delivery system with an ASFV antigen pool provided 100% protection against lethal challenges with ASFV genotype I isolate [13]. These virus-vectored ASFV vaccines elicit not only antibody responses but also T-cell immune responses, which may act as an extra defence by eliminating virus-infected cells. Additionally, published data indicate that antibodies and T cells play crucial roles in the control of viruses, and protection associated with a robust specific T-cell immune response [23, 25, 30–32]. Here, an Ad2-ASFV multiantigen cocktail delivered intramuscularly combined with intranasal instillation not only generates superior antibody and T-cell immune responses but also potent mucosal immunity.
Since there is currently no clear dominant antigen for ASFV, an Ad2-ASFV cocktail vaccine with multiple protective antigens was developed in this study. It is generally recognized that the presence of multiple antigens may result in antigenic competition, which could lower the potential efficacy of vaccines. However, our results showed that mixing multiple ASFV antigens did not trigger antigenic competition. The underlying mechanism of antigenic competition is worthy of further investigation. Therefore, it will be necessary to detect the antigenic competition effect when developing multiple antigen vaccines in the future.
Most currently licensed vaccines are administered intramuscularly, but mucosal vaccination is superior in eliciting a protective mucosal immune response to prevent early viral infection and transmission [33, 34]. Recent research efforts have concentrated on developing intranasal COVID-19 vaccines in response to the ongoing evolution of SARS-CoV-2, and several intranasal vaccine candidates are currently undergoing clinical trials [35–38]. Direct contact with mucosal surfaces is one of the most common transmission routes for ASFV. Recent studies have also shown that live attenuated ASFV vaccines can confer protection against virulent challenges when administered intranasally [39, 40]. Additionally, adenovirus-vectored vaccines delivered intranasally may be a vaccination strategy that has less of an impact on anti-adenoviral immunity [37]. In our study, IM + IN vaccination induced more comprehensive local and systematic immunity than IM vaccination. Therefore, mucosal immunity plays a crucial role in protecting against ASFV infection and deserves further research in the development of ASFV vaccines.
Following nasal spray vaccination with Ad2-ASFV, the antigen is taken up by antigen-presenting cells in the nasopharynx and processed into antigenic peptides, and transported to lymphocytes. Subsequently, T and B cells are activated, proliferated, and differentiated to form immune effector cells after recognizing antigenic peptides. Immune effector cells are transported to other immune organs via lymphatic channels and blood vessels, or home to the nasal mucosal effect site, and collaborate with effector molecules to complete the mucosal immune response. Therefore, nasal vaccination can elicit mucosal IgA and resident memory B and T cell response in upper respiratory tract mucosa [41, 42]. Previous studies showed that mucosal IgA is a dimer form that is more potent than serum IgG antibodies against the same antigen [43]. Compared with systemic memory T cells, mucosal resident memory T cells have the functions of faster response, stronger antigen-binding ability, cytokine secretion, and cell-killing ability [44]. Therefore, intranasal Ad2-ASFV-induced mucosal IgA antibodies and resident memory T cells can recognize lower levels of antigens in the upper respiratory tract and exert a strong local immune response faster to defend against early ASFV infection.
Previous studies suggested that adenovirus-vectored vaccines based on various serotypes may have varying degrees of immunogenicity and protective efficacy [45, 46]. The various adenovirus serotypes have different cell tropisms, which affect target cell preferences and thus antigen expression [47]. Currently, most adenoviral-vectored vaccines have been constructed using Ad serotype 5 (Ad5) because of their remarkable potency in eliciting robust antibody and T-cell immune responses [37, 48, 49]. In this study, we found that Ad2 was the most effective in infecting pig-derived cells. It has been confirmed that Ad2 is a safe and efficient vector in several vaccine evaluations [19, 50]; thus, we used Ad2 to construct an adenoviral-vectored ASFV vaccine.
Strikingly, our findings defy conventional wisdom as we show that sometimes “less is more” regarding the immune system. In terms of antibody response, we observed that the low dosages elicited lower antibody responses than high dosages after a single prime vaccination; however, a lower dose of priming antigen induces a superior recall response later during boosting. Similar results have been reported for the Ad5-vectored COVID-19 vaccine, showing that limiting the priming dose improves the boosting capacity of antibody and CD8+ T-cell immune responses [51]. One possible explanation is that lower anti-vector immunity may promote transgene expression and antigen presentation as well as increase de novo priming after a boost. Concerning T-cell and mucosal immune responses, low dosages resulted in higher T-cell and mucosal immune responses compared with high dosages. A similar phenomenon was found in the adenovirus-vectored tuberculosis vaccine [52]. What is the possible mechanistic basis of this phenomenon observed in these studies? We speculate that the high dosage is an overdose that may lead to cell death of infected cells and inflammation at the vaccination site, which requires further investigation in the future.
In conclusion, the Ad2-ASFVcocktail vaccine has good immunogenicity and safety when administered via IM + IN in a homologous prime-boost regimen and can confer highly effective protection against the circulating ASFV strain in farmed pigs. We anticipate that this novel vaccine strategy will be crucial in the control of ASFV.
Supplementary Material
Acknowledgments
We thank Prof Guangxia Gao (Institute of Biophysics, Chinese Academy of Sciences) for providing antibodies against p30, p54, CD2v, and p72. We thank Yu Zhao, Du Wu, Yunpeng Ai, and Zhaoming Liu for their assistance with animal feeding and care.
Funding Statement
This work was supported by project of Youth Innovation Promotion Association of CAS [grant number 2022361]; Science and Technology Planning Project of Guangzhou [grant number 202201010658]; Guangdong Basic and Applied Basic Research Project [grant number 2021A1515110924]; State Key Laboratory of Respiratory Disease [grant number SKLRD-Z-202106, SKLRD-Z-202208].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Wardley RC, de Andrade CM, Black DN, et al. . African swine fever virus. Brief review. Arch Virol. 1983;76(2):73–90. doi: 10.1007/BF01311692 [DOI] [PubMed] [Google Scholar]
- 2.Dixon LK, Sun H, Roberts H.. African swine fever. Antiviral Res. 2019;165:34–41. doi: 10.1016/j.antiviral.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 3.Sánchez-Vizcaíno JM, Mur L, Bastos AD, et al. . New insights into the role of ticks in African swine fever epidemiology. Rev - Off Int Epizoot. 2015;34(2):503–511. doi: 10.20506/rst.34.2.2375 [DOI] [PubMed] [Google Scholar]
- 4.Alonso C, Borca M, Dixon L, et al. . ICTV virus taxonomy profile: Asfarviridae. J Gen Virol. 2018;99(5):613–614. doi: 10.1099/jgv.0.001049 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Kang W, Yang W, et al. . Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: a review. Front Immunol. 2021;12:715582–715582. doi: 10.3389/fimmu.2021.715582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yáñez RJ, Rodríguez JM, Nogal ML, et al. . Analysis of the complete nucleotide sequence of African swine fever virus. Virology. 1995;208(1):249–278. doi: 10.1006/viro.1995.1149 [DOI] [PubMed] [Google Scholar]
- 7.Dixon LK, Chapman DA, Netherton CL, et al . African swine fever virus replication and genomics. Virus Res. 2013;173(1):3–14. doi: 10.1016/j.virusres.2012.10.020 [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Zhao D, He X, et al. . A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci China Life Sci. 2020;63(5):623–634. doi: 10.1007/s11427-020-1657-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borca MV, Ramirez-Medina E, Silva E, et al. . Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J Virol. 2020;94(7):e02017–19. doi: 10.1128/JVI.02017-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teklue T, Wang T, Luo Y, et al. . Generation and evaluation of an African swine fever virus mutant with deletion of the CD2v and UK genes. Vaccines (Basel). 2020;8:763. doi: 10.3390/vaccines8040763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteagudo PL, Lacasta A, López E, et al. . BA71ΔCD2: a new recombinant live attenuated African swine fever virus with cross-protective capabilities. J Virol. 2017;91(21). doi: 10.1128/JVI.01058-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudreault NN, Richt JA.. Subunit vaccine approaches for African swine fever virus. Vaccines (Basel). 2019;7:56. doi: 10.3390/vaccines7020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goatley LC, Reis AL, Portugal R, et al. . A pool of eight virally vectored African swine fever antigens protect pigs against fatal disease. Vaccines (Basel). 2020;8(2):234. doi: 10.3390/vaccines8020234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lokhandwala S, Waghela SD, Bray J, et al. . Induction of robust immune responses in swine by using a cocktail of adenovirus-vectored African swine fever virus antigens. Clin Vacc Immunol. 2016;23(11):888–900. doi: 10.1128/CVI.00395-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lokhandwala S, Waghela SD, Bray J, et al. . Adenovirus-vectored novel African swine fever virus antigens elicit robust immune responses in swine. PloS One. 2017;12(5):e0177007. doi: 10.1371/journal.pone.0177007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czerkinsky C, Holmgren J.. Topical immunization strategies. Mucosal Immunol. 2010;3(6):545–555. doi: 10.1038/mi.2010.55 [DOI] [PubMed] [Google Scholar]
- 17.Patel A, Zhang Y, Croyle M, et al. . Mucosal delivery of adenovirus-based vaccine protects against Ebola virus infection in mice. J Infect Dis. 2007;196(Suppl 2):S413–SS20. doi: 10.1086/520603 [DOI] [PubMed] [Google Scholar]
- 18.Richardson JS, Pillet S, Bello AJ, et al. . Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J Virol. 2013;87(7):3668–3677. doi: 10.1128/JVI.02864-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Qu L, Ye X, et al. . Incorporation of NS1 and prM/M are important to confer effective protection of adenovirus-vectored Zika virus vaccine carrying E protein. NPJ Vacc. 2018;3:29. doi: 10.1038/s41541-018-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King DP, Reid SM, Hutchings GH, et al. . Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. J Virol Methods. 2003;107(1):53–61. doi: 10.1016/S0166-0934(02)00189-1 [DOI] [PubMed] [Google Scholar]
- 21.Cobbold C, Windsor M, Wileman T.. A virally encoded chaperone specialized for folding of the major capsid protein of African swine fever virus. J Virol. 2001;75(16):7221–7229. doi: 10.1128/JVI.75.16.7221-7229.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, Lee SR, Li LH, et al. . High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PloS One. 2011;6(4):e18556. doi: 10.1371/journal.pone.0018556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takamatsu H-H, Denyer MS, Lacasta A, et al. . Cellular immunity in ASFV responses. Virus Res. 2013;173(1):110–121. doi: 10.1016/j.virusres.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 24.Argilaguet JM, Pérez-Martín E, López S, et al. . BacMam immunization partially protects pigs against sublethal challenge with African swine fever virus. Antiviral Res 2013;98(1):61–65. doi: 10.1016/j.antiviral.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 25.Argilaguet JM, Pérez-Martín E, Nofrarías M, et al. . DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PloS One. 2012;7(9):e40942. doi: 10.1371/journal.pone.0040942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilan JG, Zsak L, Lu Z, et al. . Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology. 2004;319(2):337–342. doi: 10.1016/j.virol.2003.11.011 [DOI] [PubMed] [Google Scholar]
- 27.Borca MV, Rai A, Ramirez-Medina E, et al. . A cell culture-adapted vaccine virus against the current African swine fever virus pandemic strain. J Virol. 2021;95(14):e00123–e00121. doi: 10.1128/JVI.00123-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Netherton CL, Goatley LC, Reis AL, et al. . Identification and immunogenicity of African swine fever virus antigens. Front Immunol. 2019;10:1318–1318. doi: 10.3389/fimmu.2019.01318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lokhandwala S, Petrovan V, Popescu L, et al. . Adenovirus-vectored African swine fever virus antigen cocktails are immunogenic but not protective against intranasal challenge with Georgia 2007/1 isolate. Vet Microbiol. 2019;235:10–20. doi: 10.1016/j.vetmic.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 30.Dixon LK, Abrams CC, Bowick G, et al. . African swine fever virus proteins involved in evading host defence systems. Vet Immunol Immunopathol. 2004;100(3):117–134. doi: 10.1016/j.vetimm.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 31.Oura CAL, Denyer MS, Takamatsu H, et al. . In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J Gen Virol. 2005;86(9):2445–2450. doi: 10.1099/vir.0.81038-0 [DOI] [PubMed] [Google Scholar]
- 32.Escribano JM, Galindo I, Alonso C.. Antibody-mediated neutralization of African swine fever virus: Myths and facts. Virus Res. 2013;173(1):101–109. doi: 10.1016/j.virusres.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 33.Russell MW, Moldoveanu Z, Ogra PL, et al. . Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection. Front Immunol. 2020;11:611337. doi: 10.3389/fimmu.2020.611337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251 [DOI] [PubMed] [Google Scholar]
- 35.Ku MW, Bourgine M, Authié P, et al. . Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe. 2021;29(2):236-249.e6. doi: 10.1016/j.chom.2020.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan AO, Kafai NM, Dmitriev IP, et al. . A single-dose intranasal Chad vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184. doi: 10.1016/j.cell.2020.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng L, Wang Q, Shan C, et al. . An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat Commun. 2020;11(1):4207. doi: 10.1038/s41467-020-18077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S, Huang J, Zhang Z, et al. . Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21(12):1654–1664. doi: 10.1016/S1473-3099(21)00225-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sánchez-Cordón PJ, Chapman D, Jabbar T, et al. . Different routes and doses influence protection in pigs immunised with the naturally attenuated African swine fever virus isolate OURT88/3. Antiviral Res. 2017;138:1–8. doi: 10.1016/j.antiviral.2016.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barasona JA, Gallardo C, Cadenas-Fernández E, et al. . First oral vaccination of Eurasian wild boar against African swine fever virus genotype II. Front Vet Sci. 2019;6:137. doi: 10.3389/fvets.2019.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lund FE, Randall TD.. Scent of a vaccine. Science. 2021;373(6553):397–399. doi: 10.1126/science.abg9857 [DOI] [PubMed] [Google Scholar]
- 42.Mao T, Israelow B, Peña-Hernández MA, et al. . Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science. 2022;378(6622):eabo2523. doi: 10.1126/science.abo2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Lorenzi JCC, Muecksch F, et al. . Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021;13(577):eabf1555. doi: 10.1126/scitranslmed.abf1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin H, Iwasaki A.. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491(7424):463–467. doi: 10.1038/nature11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanley DA, Honko AN, Asiedu C, et al. . Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med. 2014;20(10):1126–1129. doi: 10.1038/nm.3702 [DOI] [PubMed] [Google Scholar]
- 46.Geisbert Thomas W, Bailey M, Hensley L, et al. . Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against Ebolavirus challenge. J Virol. 2011;85(9):4222–4233. doi: 10.1128/JVI.02407-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barouch DH, Pau MG, Custers JH, et al. . Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol (Baltimore, Md: 1950). 2004;172(10):6290–6297. doi: 10.4049/jimmunol.172.10.6290 [DOI] [PubMed] [Google Scholar]
- 48.Buchbinder SP, Mehrotra DV, Duerr A, et al. . Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu F, Li Y, Guan X, et al. . Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng Y, Li C, Hu P, et al. . An adenovirus serotype 2-vectored ebolavirus vaccine generates robust antibody and cell-mediated immune responses in mice and rhesus macaques. Emerg Microbes Infect. 2018;7(1):101. doi: 10.1038/s41426-018-0102-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez S, Palacio N, Dangi T, et al. . Fractionating a COVID-19 Ad5-vectored vaccine improves virus-specific immunity. Sci Immunol. 2021;6(66):eabi8635. doi: 10.1126/sciimmunol.abi8635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeyanathan M, Fritz DK, Afkhami S, et al. . Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight. 2022;7(3). doi: 10.1172/jci.insight.155655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.