Abstract
Deep-sea polynoid scale worms endemic to hydrothermal vents have evolved an adaptive strategy to the chronically hypoxic environment, but its underlying molecular mechanisms remain elusive. Here, we assembled a chromosome-scale genome of the vent-endemic scale worm Branchipolynoe longqiensis (the first annotated genome in the subclass Errantia) and annotated two shallow-water polynoid genomes, aiming to elucidate the adaptive mechanisms. We present a genome-wide molecular phylogeny of Annelida which calls for extensive taxonomy revision by including more genomes from key lineages. The B. longqiensis genome with a genome size of 1.86 Gb and 18 pseudochromosomes is larger than the genomes of two shallow-water polynoids, possibly due to the expansion of various transposable elements (TEs) and transposons. We revealed two interchromosomal rearrangements in B. longqiensis when compared with the two shallow-water polynoid genomes. The intron elongation and interchromosomal rearrangement can influence a number of biological processes, such as vesicle transport, microtubules, and transcription factors. Furthermore, the expansion of cytoskeleton-related gene families may favor the cell structure maintenance of B. longqiensis in the deep ocean. The expansion of synaptic vesicle exocytosis genes has possibly contributed to the unique complex structure of the nerve system in B. longqiensis. Finally, we uncovered an expansion of single-domain hemoglobin and a unique formation of tetra-domain hemoglobin via tandem duplications, which may be related to the adaptation to a hypoxic environment.
Keywords: polynoid, hydrothermal vent, comparative genomics, phylogenomics, adaptation
Significance.
Deep-sea hydrothermal vent-endemic faunae have evolved unique adaptive strategies to their “extreme” environment. Here, we assembled a chromosome-level genome of the vent-endemic polynoid scale worm Branchipolynoe longqiensis and annotated two shallow-water polynoid genomes; these represent the first annotated genomes of the subclass Errantia. Through comparative genomic analyses, we provide molecular insights that enhance our understanding of adaptive strategies in hydrothermal vent organisms, especially Annelida for which such data are scarce.
Introduction
Deep-sea hydrothermal vent ecosystems harbor diverse endemic faunae that have evolved unique adaptive strategies to this “extreme” environment with high concentrations of heavy metals and low oxygen concentrations (Van Dover 2000). Among these vent-endemic species are scale worms in the genus Branchipolynoe (Annelida: Errantia: Polynoidae), which live inside the mantle cavity of bathymodioline mussels and feed both on host tissue and suspended organic matter (Britayev et al. 2007). These scale worms are thought to possess evolutionary adaptations to their chronically hypoxic environment, but little molecular evidence has been gathered to date. Hot vent polynoid scale worms represent a Cenozoic radiation (<66 Ma) and have shared similar ecology and adaptations since their last common ancestor (Projecto-Garcia et al. 2010). Currently, only two studies on the adaptive strategy of these polynoid scale worms have been published, with one focusing on the duplication of hemoglobin (Projecto-Garcia et al. 2010) and the other examining the use of amino acids (Zhang et al. 2017)—both reflecting their adaptive strategies using a limited number of genes. Furthermore, the phylum Annelida can be classified into three major groups: Errantia, Sedentaria, and the basal branching lineages (Rouse et al. 2022), but not a single annotated genome existed in Errantia, hindering the in-depth understanding of annelid molecular evolution. Here, we assembled and annotated a chromosome-scale genome of the vent-endemic Branchipolynoe longqiensis and annotated the genomes of two shallow-water polynoid scale worms, including Harmothoe impar (assembly wpHarImpa5.1, GCA_947462335.1) and Lepidonotus clava (Darbyshire et al. 2022), aiming to elucidate the adaptive strategies of vent scale worms at the genomic scale.
Results and Discussion
Genome Assembly
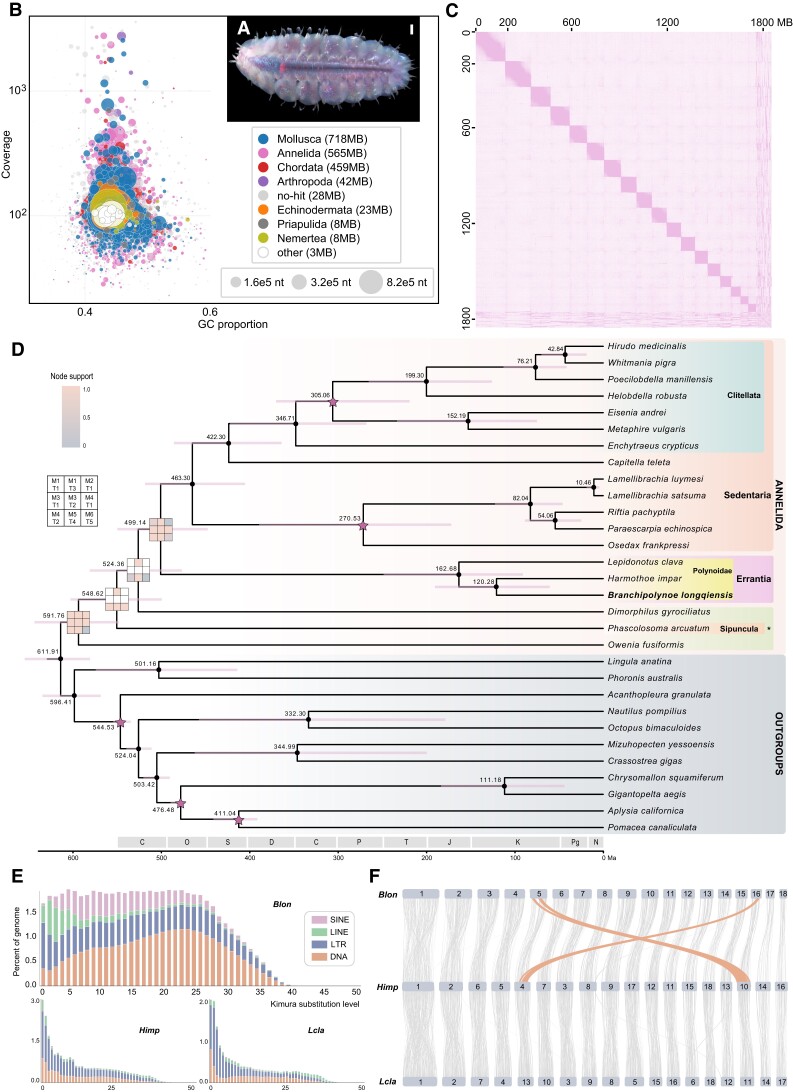
Branchipolynoe longqiensis lives in the mantle cavity (fig. 1A) of the mussel Bathymodiolus septemdierum at Indian Ocean hydrothermal vents (Zhou et al. 2017; Tunnicliffe and Breusing 2022). The genome size and heterozygosity of B. longqiensis were estimated to be 1.76 Gb and 1.08%, respectively, using 17-mer and 19-mer based on Illumina reads (supplementary figs. S1 and S2, Supplementary Material online). A merging method was applied with Quickmerge (Chakraborty et al. 2016) by combing the wtdbg2 assembly generated from the yacrd scrubbed Pacbio reads and a hybrid assembly generated by MaSURCA. Heterozygous contigs were removed by the Purge_dup pipeline v1.2.6 (Guan et al. 2020) and decontaminated using BlobTools (Challis et al. 2020) (fig. 1B). The Hi-C sequencing reads were used to scaffold these contigs (fig. 1C), and TGS-GapCloser v1.2.1 (Xu et al. 2020) was used to fill “N”-gap in the genome assembly. The final chromosome-scale genome included 4,255 scaffolds with an assembled size and N50 of 1.86 Gb and 97.05 Mb, respectively (table 1). The completeness of the genome is 89.3%, with 88.5% and 0.8% single and duplicated copies of BUSCOs when searching against metazoan odb10 database (Seppey et al. 2019). Though the genome heterozygosity was estimated to be 1.08%, the low duplicated BUSCO score suggests the successful removal of heterozygous allelic contigs from the primary assembly. The mapping rate of the Illumina paired-end rate is 98.65%, also indicating a high genome completeness. A total of 1.76 Gb contigs (94.7% of the total assembled genome) were anchored onto 18 pseudochromosomes, which is congruent with a former karyotyping study (Dixon et al. 2010) (fig. 1C).
Fig. 1.
Branchipolynoe longqiensis. (A) A photo of B. longqiensis collected from the Tiancheng vent field, scale bar: 1 mm. (B) BlobTools view on the mean GC content, sequencing coverage and NCBI species hit of the genomic assembly of B. longqiensis. (C) Hi-C interaction map illustrating a total of 18 chromosomes plus the unanchored contigs (bottom-right corner) of the genome. (D) Chronogram of annelids with available genomes plus a summary of phylogenetic inference from different matrices (M) and methods on protein sequences; *, Polychaeta incertae sedis; the star symbols indicate the calibration points for the phylogenetic reconstruction; small black dots indicate nodes with maximum support in all analyses. M1, 5,449 single-copy orthologous groups (OGs) with the occupancy of 50%; M2, 3,350 single-copy of OGs with an occupancy of 80%; M3, the best 1,200 OGs sorted by genesortR; M4, the best 900 OGs sorted by genesortR; M5, 500 randomly selected OGs from M1; and M6, the best 300 OGs sorted by genesortR. Tree(T)1, IQ-Tree with the MFP model; T2, IQ-Tree with the C60 site-heterogenous model; T3, ASTRAL tree; T4, Bayesian inference from Phylobayes; and T5, RaxML with the GTR + Γ model. Chronogram was inferred from MCMCtree on the most consistent tree topology and calibrated by fossil calibration points. (E) The TEs landscape of the three polynoid genomes. (F) Syntenic analysis among the three polynoid genomes indicating interchromosomal translocation.
Table 1.
Statistics of the Genome Assembly and Gene Models of Branchipolynoe longqiensis
| Category | Number | |
|---|---|---|
| Assembly | Genome size (Gb) | 1.86 |
| No. of scaffolds | 4255 | |
| Longest scaffold (Mb) | 189.33 | |
| N50 scaffold length (Mb) | 97.05 | |
| Complete BUSCO (%) | 89.3 (S: 88.5,D: 0.8) | |
| No. of chromosomes | 18 | |
| GC content (%) | 44.35 | |
| Repeat sequences (%) | 71.14 | |
| Gene model | No. of protein-coding genes | 21,235 |
| Complete BUSCO (%) | 84.9 (S: 84.4,D: 0.5) | |
| Average gene length (bp) | 18,117 | |
| Average exon length (bp) | 205 | |
| Average intron length (bp) | 3,150 | |
| Average exon per gene | 6.3 | |
| Average intergenic length | 66,883 |
Genome Annotation
Annotation of annelid genomes is challenging, even with multiple efforts applied to gene model predictions (Martín-Zamora et al. 2023). Here, we carefully curated the annotations with different combinations of annotation tools and their corresponding weights intending to achieve an annotation with the best BUSCO score without inflating the number of gene models. A total of 21,235, 28,143, and 23,457 gene models without gene isoform annotations with a BUSCO completeness score of 84.9% (84.4% single copy and 0.5% duplicated genes), 93.8% (88.9% single copy and 4.9% duplicated genes), and 96.4% (96.0% single copy and 0.4% duplicated genes), respectively, were obtained for B. longqiensis, H. impar, and L. clava, respectively (details are included in supplementary material S1 and supplementary tables S2 and S5, Supplementary Material online). Among the gene models annotated in these three species, 14,769 (69.6%), 22,928 (81.5%), and 19,300 (82.3%) gene models, respectively, could be functionally annotated by at least one protein database (supplementary tables S6–S8, Supplementary Material online). A former study analyzed the amino acid usage in deep-sea lineages via transcriptome comparison (Zhang et al. 2017), and we expanded this analysis to all polychaetes with available genomes. The results again showed that all deep-sea species, including B. longqiensis and deep-sea siboglinid tubeworms, have increased the use of the positively charged histidine (phylogenetic independent contrasts test, r = 0.95 and corrected P = 0.0001) and decreased the use of the negatively charged aspartic acid (r = 0.88 and corrected P = 0.004) and glutamic acid (r = 0.83 and corrected P = 0.015), indicating that the amino acid usage is convergently biased in deep-sea polychaetes (supplementary fig. S4 and table S9, Supplementary Material online).
Phylogenomic Analyses
Phylogenomic analyses based on a combination of different gene matrices and models resulted in two topologies differing only by one node among the species examined (fig. 1D). The current topology was selected because it was supported by multiple methods, while the other was only supported by IQ-Tree, though on various matrices (supplementary fig. S5 and table S3, Supplementary Material online). Our phylogeny of Annelida recovered similar paraphyletic issues in taxonomy as previously reported (Rouse et al. 2022), such as the paraphyly of polychaeteous Sedentaria (Sedentaria excluding Clitellata). The family Polynoidae represented by three scale worms here was monophyletic, in line with a former study (Zhang et al. 2018). The fossil record is rare across Annelida due to their soft body. Nevertheless, two fossil records could be used together with molluscan fossils for calibration: A hard minimum bound of 201.7 Ma for the divergence between Hirudinida (leeches) and other groups, as the first leech cocoon was discovered in the Late Triassic (Erséus et al. 2020); a hard minimum bound of 93.9 Ma for the divergence between Osedax and other siboglinid tubeworms taken from the first appearance of an Osedax-eaten bone in 100–93.9 Ma (Taboada et al. 2015). The divergence time between B. longqiensis and other polynoid scale worms was calculated to be 120.23 Ma (95% highest posterior density of 60.25–189.64 Ma), which predated the Cenozoic radiation event. To further decode the radiation and molecular evolution in the deep-sea hydrothermal vent polynoid worms, genomes of other genera, such as Branchinotogluma and Lepidonotopodium, are needed.
Annotation of Repeats
The genome size of B. longqiensis is larger than the two shallow-water polynoids H. impar and L. clava, which can be attributed to the larger amounts of repeat contents, including transposable elements (TEs) in B. longqiensis (1,323.7 Mb vs. 724.7 and 404.2 Mb; supplementary table S5, Supplementary Material online). Various studies have shown that deep-sea animals have their genome sizes inflated in the face of their “extreme” environments, with the gradual accumulation of TEs (Ritchie et al. 2017; Yuan et al. 2022); our result suggests a similar trend. We analyzed the distribution of TEs by calculating the Kimura substitution rate (K values) for all TEs to reveal their transposition ages, with lower K values indicating a more recent transposition and vice versa. The two shallow-water scale worms shared a similar pattern of TEs transposition age distribution, while for B. longqiensis, the transposition of TEs was initiated since the “middle-range” age, likely after the deep-sea colonization in this lineage (fig. 1E). The insertion of TEs remains very active for B. longqiensis —for instance, the long terminal repeat (LTR) retrotransposon insertion time analysis revealed that many repeats have uniquely expanded since the last one million years (supplementary fig. S3, Supplementary Material online), which is in concert with a former study on the deep-sea tubeworm Lamellibrachia luymesi (Aroh and Halanych 2021) and further corroborating their convergence. The accumulation of TEs in the B. longqiensis genome has resulted in longer intergenic regions and intron sizes (supplementary table S5, Supplementary Material online), and the expansion of the introns also led to a longer average gene length compared to H. impar and L. clava. Our gene ontology (GO) enrichment analysis of the top 10% genes with elongated length of B. longqiensis compared with two shallow-water scale worms revealed a series of molecular processes, including “vesicle-mediated transport” and “microtubule organizing center” (supplementary table S12, Supplementary Material online). Considering that introns could play a role in gene regulation by influencing alternative splicing (Rose 2019), these genes with the prolonged length of introns may have evolved different gene regulation mechanisms in the deep-sea scale worm compared with its shallow-water relatives.
Synteny Analysis
Genome assembly with Hi-C data on the three polynoid scale worms revealed 18 chromosomes for all three species investigated. The macrosynteny analysis among the three genomes indicated that the structure of the two shallow-water polynoid scale worm genomes was relatively conserved with nearly identical 1:1 chromosome-level correspondence (fig. 1F). However, two interchromosomal rearrangements were found between B. longqiensis and the two shallow-water polynoid genomes, corresponding to two chromosomes shuffling on Chr5 and Chr16 in B. longqiensis (fig. 1F and supplementary fig. S6, Supplementary Material online). We show that interchromosomal level rearrangements occur among cofamilial species even when the number of chromosomes remains the same. The two interchromosomal rearrangements lead to at least 151 genes with altered chromosome locations, including several homeodomain-containing transcription factors (e.g., two NKX1, NKX5, VAX, and EMX). These results may indicate a change in the gene expression levels, likely by reshuffling topologically associating domain (TAD) structures (Akdemir et al. 2020) in the deep-sea lineage due to the interchromosomal rearrangements, warranting further analyses.
Gene Family Analyses
Compared with the two shallow-water scale worms, 80 and 384 gene families were significantly expanded and contracted (Viterbi P < 0.01), respectively, in B. longqiensis. GO enrichment analysis revealed that at least “DNA polymerase complex”, “microtubule”, and “synaptic vesicle exocytosis” were expanded in the genome of B. longqiensis (supplementary fig. S7 and tables S10 and S11, Supplementary Material online). The “DNA polymerase complex” is represented by 26 transposons, in line with the large proportion of TEs in the B. longqiensis genome. High hydrostatic pressure can change the organization and structure of various cytoskeleton proteins, including microtubules (Bourns et al. 1988). The enrichment of genes related to microtubules in B. longqiensis may benefit its environmental adaptation to the high hydrostatic pressure in the deep-sea vent habitat. The expansion of “synaptic vesicle exocytosis” related to gene family may be linked to novel cell supporters found in the nervous system of vent scale worms, considered a novel adaptation of these organisms to thrive in hydrothermal vents (Shigeno et al. 2015).
Among the gene families expanded in B. longqiensis, the copy number of extracellular single-domain (SD) hemoglobin was increased compared with the two shallow-water polynoids (H. impar and L. clava). In addition, the tetra-domain (TD) hemoglobin was discovered in the B. longqiensis genome, which is consistent with its presence in two other deep-sea scale worms: Branchipolynoe symmytilida and Branchipolynoe seepensis (Projecto-Garcia et al. 2010). The TD hemoglobin in Branchipolynoe was suggested to be unique for this genus, being generated by two progressive duplications of one SD hemoglobin (Projecto-Garcia et al. 2010). A phylogenetic tree reconstructed based on the hemoglobin sequences in five polynoid scale worms indicated a species-/genus-restricted duplication event, and the duplication of hemoglobin in deep-sea Polynoidae appeared to be common and independent (supplementary fig. S8, Supplementary Material online). In the B. longqiensis genome, a TD hemoglobin was found in close proximity to an SD hemoglobin (BlonSD5)—the close location and the phylogenetic position of these two hemoglobins suggest that this TD hemoglobin could have been derived from the duplication of BlonSD5 (supplementary figs. S8 and S9, Supplementary Material online). Another TD hemoglobin and several copies of SD hemoglobin were also identified from the transcriptomic data of the congener, Branchipolynoe pettiboneae, underlining that the TD hemoglobin is common in this genus (Zhang et al. 2017) and could be an adaption to the hypoxic environment in the vent fields or the mantle cavity of bathymodioline mussels. None of the three polynoid genomes contained genes encoding hemerythrin, a non-heme protein that can also bind to oxygen and used by many annelids (Costa-Paiva et al. 2017), indicating that hemoglobin indeed plays a major role in oxygen binding in these scale worms.
In conclusion, by comparing the genomes of B. longqiensis and its two shallow-water relatives, we revealed several potential molecular adaptation processes to the deep-sea hydrothermal vent environment, including inflated genome size, the recent expansion of TEs, and interchromosomal rearrangements in this particular lineage. We show that the excessive intron length and interchromosomal shuffling likely have significant influences on various biological processes. The expanded transposons corroborate with the expansion of TEs and the expansion of “synaptic vesicle exocytosis” may be linked to the unique complex structure of nerve and sensory system in the deep-sea vent-endemic scale worms. Furthermore, the lineage-specific expansion of SD hemoglobins and the unique finding of a TD hemoglobin are potential adaptations to the hypoxic vent environment. Lastly, through phylogenomic analyses, we present a well-supported molecular phylogeny for Annelida using the available genomes—which calls for extensive revisions on the taxonomy of some major clades and warrants the sequencing of more genomes from key lineages.
Materials and Methods
The Supplementary material contains detailed information on Material and Methods. In brief, specimens were collected from the Tiancheng and Wocan hydrothermal vent fields in the Indian Ocean, by the remotely operated vehicle (ROV) Sea Dragon III and the human-occupied vehicle (HOV) Jiaolong on-board R/V Dayangyihao and Xiangyanghong 9 at a depth of 2,705 and 2,970 m in April 2019 and March 2017, respectively. The samples were flash-frozen in liquid nitrogen and then stored in −80 °C. Genome sequencing was performed by combining PacBio sequencing and Hi-C scaffolding as previously described (Sun et al. 2020). The PacBio reads were scrubbed by removing potentially chimeric reads (Marijon et al. 2020) and assembled by several assemblers (see Supplementary Materials for details). Heterozygous contigs were purged, and the resultant contigs were polished and decontaminated. Hi-C scaffolding was performed using the 3D-DNA pipeline with manual correction on the misassembled contigs and contig orientations. Genome annotation was applied using different annotation tools and merged by EVidenceModeler v2.0.0 (Haas et al. 2008).
Supplementary Material
Acknowledgments
This work was financially supported by the Science and Technology Innovation Project of Laoshan Laboratory (LSKJ202203104), National Key R&D Programs of China under the contract No. of 2022YFE0140200, National Natural Science Foundation of China (42106118 and 42176110), the Fundamental Research Funds for the Central Universities (202172002 and 202241002), the Young Taishan Scholars Program of Shandong Province (tsqn202103036), and the Collaborative Research Fund, University Grants Committee of Hong Kong (C2013-22GF). We thank the captain and crew of R/V Dayangyihao and the pilots of ROV Sea Dragon III as well as R/V Xiangyanghong 9 and pilots of HOV Jiaolong for their support during the cruise of DY-52III and DY-38I.
Contributor Information
Xing He, Institute of Evolution and Marine Biodiversity, Key Laboratory of Mariculture (Ministry of Education), Ocean University of China, Qingdao, China; Laoshan Laboratory, Qingdao, China.
Hui Wang, Institute of Evolution and Marine Biodiversity, Key Laboratory of Mariculture (Ministry of Education), Ocean University of China, Qingdao, China; Laoshan Laboratory, Qingdao, China.
Ting Xu, Department of Ocean Science, Hong Kong University of Science and Technology, Hong Kong, China; Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou, China.
Yanjie Zhang, School of Life Sciences, Hainan University, Haikou, China.
Chong Chen, X-STAR, Japan Agency for Marine-Earth Science and Technology (JAMSTEC), Yokosuka, Kanagawa, Japan.
Yanan Sun, Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou, China; Department of Biology, Hong Kong Baptist University, Hong Kong, China.
Jian-Wen Qiu, Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Guangzhou, China; Department of Biology, Hong Kong Baptist University, Hong Kong, China.
Yadong Zhou, Key Laboratory of Marine Ecosystem Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou, China.
Jin Sun, Institute of Evolution and Marine Biodiversity, Key Laboratory of Mariculture (Ministry of Education), Ocean University of China, Qingdao, China; Laoshan Laboratory, Qingdao, China.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Author Contributions
J.S., Y.Z., and J.-W.Q. conceived the project. Y.Z., J.S., and Y.S. collected the samples. X.H. performed the genome sequencing, amino acid usage, synteny analysis, and gene family analysis. X.H. and H.W. annotated the genome. X.H. and Y.Z. performed the phylogenomic analysis. X.H. and J.S. drafted the manuscript, and H.W., T.X., Y.Z., C.C., Y.S., J.-W.Q., and Y.Z. contributed to the interpretation and editing. All authors agreed with the submission and publication of the manuscript in its current form.
Data Availability
The raw data of B. longqiensis have been deposited in NCBI under the BioProject number PRJNA961926. The assembly of B. longqiensis has been deposited in NCBI with the accession number JASIRB000000000. The genome annotations of the three polynoid species have been deposited in Figshare under the DOI: 10.6084/m9.figshare.22707460.
Literature Cited
- Akdemir KC, et al. 2020. Disruption of chromatin folding domains by somatic genomic rearrangements in human cancer. Nat Genet. 52:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroh O, Halanych KM. 2021. Genome-wide characterization of LTR retrotransposons in the non-model deep-sea annelid Lamellibrachia luymesi. BMC Genomics 22:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourns B, Franklin S, Cassimeris L, Salmon ED. 1988. High hydrostatic pressure effects in vivo: changes in cell morphology, microtubule assembly, and actin organization. Cell Motility Cytoskeleton 10:380–390. [DOI] [PubMed] [Google Scholar]
- Britayev TA, Martin D, Krylova EM, Von Cosel R, Aksiuk TS. 2007. Life-history traits of the symbiotic scale-worm Branchipolynoe seepensis and its relationships with host mussels of the genus Bathymodiolus from hydrothermal vents. Mar Ecol. 28:36–48. [Google Scholar]
- Chakraborty M, Baldwin-Brown JG, Long AD, Emerson JJ. 2016. Contiguous and accurate de novo assembly of metazoan genomes with modest long read coverage. Nucleic Acids Res. 44:e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis R, Richards E, Rajan J, Cochrane G, Blaxter M. 2020. BlobToolKit—interactive quality assessment of genome assemblies. G3 10:1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Paiva EM, et al. 2017. Discovery and evolution of novel hemerythrin genes in annelid worms. BMC Evol Biol. 17:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbyshire T, Bishop J, Mieszkowska N, Adkins P, Holmes A. 2022. The genome sequence of the scale worm, Lepidonotus clava (Montagu, 1808). Wellcome Open Res. 7:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DR, Jolly MT, Vevers WF, Dixon LRJ. 2010. Chromosomes of Pacific hydrothermal vent invertebrates: towards a greater understanding of the relationship between chromosome and molecular evolution. J Mar Biol Assoc UK. 90:15–31. [Google Scholar]
- Erséus C, et al. 2020. Phylogenomic analyses reveal a Palaeozoic radiation and support a freshwater origin for clitellate annelids. Zool Scr. 49:614–640. [Google Scholar]
- Guan D, et al. 2020. Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics 36:2896–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B, et al. 2008. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 9:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijon P, Chikhi R, Varré J-S. 2020. yacrd and fpa: upstream tools for long-read genome assembly. Bioinformatics 36:3894–3896. [DOI] [PubMed] [Google Scholar]
- Martín-Zamora FM, et al. 2023. Annelid functional genomics reveal the origins of bilaterian life cycles. Nature 615:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projecto-Garcia J, et al. 2010. Origin and evolution of the unique tetra-domain hemoglobin from the hydrothermal vent scale worm Branchipolynoe. Mol Biol Evol. 27:143–152. [DOI] [PubMed] [Google Scholar]
- Ritchie H, Jamieson AJ, Piertney SB. 2017. Genome size variation in deep-sea amphipods. R Soc Open Sci. 4:170862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AB. 2019. Introns as gene regulators: a brick on the accelerator. Front Genet. 9:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse G, Pleijel F, Tilic E. 2022. Annelida. Oxford, UK: Oxford University Press. [Google Scholar]
- Seppey M, Manni M, Zdobnov EM. 2019. BUSCO: assessing genome assembly and annotation completeness. In: Kollmar M, editors. Gene prediction: methods and protocols. New York: (NY: ): Springer New York. p. 227–245. [DOI] [PubMed] [Google Scholar]
- Shigeno S, et al. 2015. Dual cellular supporters: multi-layer glial wrapping and the penetrative matrix specialized in deep-sea hydrothermal vent endemic scale-worms. Biol Bull. 228:217–226. [DOI] [PubMed] [Google Scholar]
- Sun J, et al. 2020. The scaly-foot snail genome and implications for the origins of biomineralised armour. Nat Commun. 11:1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboada S, et al. 2015. Bone-eating worms spread: insights into shallow-water Osedax (Annelida, Siboglinidae) from Antarctic, Subantarctic, and Mediterranean waters. PLoS One. 10:e0140341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnicliffe V, Breusing C. 2022. Redescription of Bathymodiolus septemdierum Hashimoto and Okutani, 1994 (Bivalvia, Mytilida, Mytilidae), a mussel broadly distributed across hydrothermal vent locations in the western Pacific and Indian oceans. Zootaxa 5214:337–364. [DOI] [PubMed] [Google Scholar]
- Van Dover CL. 2000. The ecology of deep-sea hydrothermal vents. New Jersey: Princeton University Press. [Google Scholar]
- Xu M, et al. 2020. TGS-GapCloser: a fast and accurate gap closer for large genomes with low coverage of error-prone long reads. GigaScience 9:giaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, et al. 2022. Genome of a giant isopod, Bathynomus jamesi, provides insights into body size evolution and adaptation to deep-sea environment. BMC Biol. 20:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. 2017. Adaptation and evolution of deep-sea scale worms (Annelida: Polynoidae): insights from transcriptome comparison with a shallow-water species. Sci Rep. 7:46205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. 2018. Phylogeny, evolution and mitochondrial gene order rearrangement in scale worms (Aphroditiformia. Annelida). Mol Phylogenet Evol. 125:220–231. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang D, Lu B, Wang C. 2017. Description of a new branchiate scale-worm (Polychaeta: Polynoidae) from the hydrothermal vent on Southwest Indian Ocean Ridge. Zootaxa 4282:123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of B. longqiensis have been deposited in NCBI under the BioProject number PRJNA961926. The assembly of B. longqiensis has been deposited in NCBI with the accession number JASIRB000000000. The genome annotations of the three polynoid species have been deposited in Figshare under the DOI: 10.6084/m9.figshare.22707460.