SUMMARY
Background:
Oral pre-exposure prophylaxis (PrEP) for HIV prevention is highly effective and being scaled at health clinics throughout sub-Saharan Africa. However, barriers to clinic-based PrEP delivery such as high costs for clients and clinics remain. In a randomized implementation trial, we aimed to test whether semiannual PrEP clinic visits supported with interim home-based HIV self-testing (HIVST) would result in equivalent HIV testing, drug refilling, and adherence among PrEP clients compared to quarterly visits, the standard of care.
Methods:
In a randomized non-inferiority trial (ClinicalTrials.gov NCT03593629), we tested this novel model for PrEP delivery in Kenya. All participants were ≥18 years and had taken PrEP for one month. Participants were 2:1 randomized to: 1) six-month PrEP dispensing (with semiannual clinic visits, supported by HIVST conducted at home after three months) or 2) standard-of-care PrEP dispensing (three-month supply with quarterly clinic visits). Our primary outcomes, measured at six months, were HIV testing (any testing between enrollment and six-month visit), PrEP refilling, and PrEP adherence (detectable tenofovir-diphosphate in dried blood spots). We used binomial regression models to estimate risk differences (RDs) and interpreted one-sided 95% confidence interval (CI) lower bounds (LB) −10% as non-inferior.
Findings:
From May 2018 to February 2020, we enrolled and followed 495 participants: 165 men and 130 women in HIV serodifferent couples and 200 women not in known serodifferent couples. At six months, 83·3% (274/329) in the intervention arm tested for HIV compared to 84·3% (140/166) in the standard-of-care arm (RD −1·2%, one-sided 95% CI LB −6·9%). Among participants in the intervention arm, 78·1% (257/329) refilled PrEP, compared to 80·7% (134/166) in the standard-of-care arm (RD −2·6%, one-sided 95% CI LB −8·9%). In the intervention arm, 60·8% (200/329) were adherent to PrEP compared to 57·2% (95/166) in the standard-of-care arm (RD 2·4%, one-sided 95% CI LB −5·1%). No participants acquired HIV.
Interpretation:
Six-month PrEP dispensing with HIVST for interim testing reduces the number of PrEP clinic visits in half without compromising HIV testing, retention, or adherence.
Keywords: HIV self-testing, pre-exposure prophylaxis, adherence, women, Kenya
INTRODUCTION
Novel models for oral HIV pre-exposure prophylaxis (PrEP) delivery are needed in high prevalence settings to increase access and minimize the costs of delivery. In Kenya and other countries in sub-Saharan Africa, PrEP is primarily being delivered at public health clinics using a quarterly visit schedule, with three-month drug dispensing and clinic-based HIV testing.1 This model of PrEP delivery, while often successful, is limited by both client-level and health system barriers, including long travel distances and wait times at clinics for clients and high client volumes and costs for often already overstretched health systems.2–4 Efficient strategies for PrEP delivery are needed that could address these time and cost barriers, potentially improve client engagement in care, and increase the capacity of clinics to deliver care.
HIV self-testing (HIVST) is a new technology that has the potential to improve the efficiency of delivering HIV services, including PrEP.5–7 A critical pillar for PrEP delivery is HIV testing, which must occur regularly (every three months) following PrEP initiation to identify potential breakthrough HIV infections, reducing the risk of antiretroviral resistance and permitting rapid engagement in HIV care.8 Since clinic-based HIV testing is currently the standard for PrEP delivery in African settings, the maximum duration between PrEP clinic visits is thus three months.9 HIVST, which was introduced in Kenya in 2017, has the potential to shift HIV testing required for PrEP delivery from the clinic to the home, thus reducing the number of and expanding the period between clinic visits required for PrEP delivery.
Multinational studies have demonstrated high acceptability and uptake of HIVST among diverse populations in sub-Saharan Africa, including in Kenya,10–13 although those studies generally evaluated HIVST as a strategy to increase access to HIV testing, particularly for those unlikely to test otherwise. Relatively few studies have paired HIVST and PrEP: two pilot evaluations among Kenyan HIV serodifferent couples at HIV clinics and young women at family planning clinics found in-clinic HIVST to be an acceptable and feasible approach for HIV testing when done as part of routine PrEP delivery.5,7 Notably, the World Health Organization (WHO) does not currently support use of HIVST for PrEP delivery,14 citing the need for more evidence to understand the potential role that HIVST can play in PrEP delivery, including its ability to support PrEP continuation (i.e., retention and adherence) by reducing the burden of PrEP access for clients and delivery for clinics.
In a randomized implementation trial among PrEP users in Kenya, we tested whether HIVST could be used to decrease the number of PrEP clinic visits by half through a model of six-month PrEP dispensing supported with self-directed HIVST at home. We hypothesized home HIVST with six-monthly PrEP visits would result in equivalent HIV testing, drug refilling, and adherence among PrEP clients.
METHODS
Study design
The Jipime-JiPrEP (Kiswahili for “test yourself, PrEP yourself”) study was an open-label randomized, non-inferiority implementation trial testing the effect of six-month PrEP dispensing supported with HIVST (ClinicalTrials.gov NCT03593629).15 The trial was conducted at the Partners in Health and Research Development clinic in Thika, a peri-urban town in Kiambu County ~40 kilometers outside Nairobi.
Participants
We included individuals at risk of acquiring HIV who had recently started PrEP and returned for their first follow-up visit one month post-initiation, thus indicating interest in continued PrEP use. We recruited three different types of populations at HIV risk: men in HIV serodifferent couples, women in HIV serodifferent couples, and women not in known HIV serodifferent couples (i.e., singly enrolled).
We partnered with existing HIV counseling and testing (HCT) centers in Kiambu County and neighboring counties with population-level HIV prevalence rates ranging from 1.1% to 5.1%.16 We also partnered with local HIV clinics to identify individuals newly initiating PrEP and engaged community mobilization activities for HCT promotion to identify new individuals eligible for PrEP. For women singly enrolled, we used the Kenya Ministry of Health’s PrEP Rapid Assessment Screening Tool (RAST) to determine if they were eligible for PrEP; this is the tool used for risk assessment and PrEP eligibility at public clinics in Kenya. Other eligibility criteria for participants were: ≥18 years old, HIV uninfected (by rapid testing according to national guidelines),9 plans for continued PrEP use, no ongoing participation in another HIV prevention trial, and willingness to be randomized to the trial HIVST arm. Pregnant women were eligible for participation.
The study protocol15 was approved by the Scientific Ethics Review Unit at the Kenya Medical Research Institute and the Human Subjects Division at the University of Washington. All participants provided written informed consent. The study had a Data Safety Monitoring Board that met three times over the duration of the study.
Randomization
Randomization occurred individually at enrollment using opaque envelopes opened by the participant in the presence of the research staff. We randomized participants 1:1:1 to: 1) six-month PrEP dispensing + interim blood-based HIVST (with biannual clinic visits), 2) six-month PrEP dispensing + interim oral fluid-based HIVST (with biannual clinic visits), or 3) standard-of-care (SOC) PrEP delivery (three-month PrEP dispensing with quarterly clinic visits), Appendix: Page 1. A list of sequential randomization assignments was prepared for each study population (e.g., men in serodifferent relationships, women in serodifferent relationships, and women singly enrolled) using variable-block sizes by a study statistician not otherwise involved in participant contact. Due to the nature of the intervention, participants and research staff were unmasked to randomized assignment.
Procedures
Every six months, for a total of 12 months, participants randomized to one of the intervention arms were dispensed a six-month PrEP supply, received two HIV self-tests, and were scheduled to return to the clinic for follow-up six months later. Participants randomized to the SOC arm were dispensed a three-month PrEP supply, received no HIV self-tests, and were scheduled to return to the clinic for follow-up every three months. HIV self-tests were either oral-fluid (OraQuick Rapid HIV-1/2 Antibody Test, OraSure Technologies, USA) or blood-based (AtomoRapid HIV 1&2 Test, Atomo Diagnostics, Australia, rebranded as Mylan Rapid HIV 1&2 after acquisition by Mylan Lab Ltd.).
At the enrollment visit, we trained participants in the intervention arms on self-test use, including completing the assigned HIVST process, with interpretation of test results in the clinic with the guidance of a study staff member. All HIV self-tests delivered to participants included a pictorial informational brochure (translated into Kiswahili, the local language) with details on self-test use and interpretation; participants were instructed to reference this, as needed, during the testing process. Additionally, participants in the intervention arms received a toll-free 24-hour number that they were encouraged to call if they had any questions or tested HIV seropositive. We instructed participants to use a self-test for interim HIV testing three months following their enrollment visit and the other test as they wished (e.g., for additional testing, back-up testing, or testing for a sexual partner/friend).
We delivered PrEP following the 2018 Kenya national PrEP guidelines,9 which included HIV testing at all PrEP clinic visits (blood-based rapid testing following the national algorithm), serum creatinine clearance testing, syndromic assessment of sexually transmitted infections (STIs), urine pregnancy testing (for women only), counseling (on HIV risk reduction strategies and drug adherence), and assessment of potential PrEP side effects (follow-up visits only). Participants in HIV serodifferent couples received counseling on PrEP as a bridge to antiretroviral treatment (ART, i.e., PrEP use until the partner living with HIV is virally suppressed), and at six months, were recommended to discontinue PrEP if their partner had consistently been on ART for six months. 9
Participants in all arms were told that, if needed, they could contact or return to the research clinic at any point between scheduled PrEP clinic visits (e.g., to get a replacement for a lost HIV self-test, discuss a perceived change in HIV risk, etc.). To remind all participants of their scheduled follow-up visits, we followed standard clinical procedures for PrEP appointment reminders in Kenya: a phone call one day following a missed scheduled visit and a second phone call seven days following the first call.
At each study visit, participants completed a demographic and behavioral questionnaire, a physical exam, and received PrEP (if continuing PrEP use). All data were collected and stored using CommCare (Dimagi, Cambridge, USA), an electronic data collection platform. Physical exams included assessment of potential PrEP side effects and symptoms of STIs. We collected blood specimens at the six-month follow-up visit for dried blood spots (DBS) to measure PrEP adherence.
Outcomes
We had three pre-specified primary outcomes, all measured at the six-month follow-up visit: 1) HIV testing (any testing after the enrollment visit and before the six-month visit), 2) PrEP refilling (at the six-month visit), and 3) adherence to PrEP (detectable drug levels in DBS). Our HIV testing outcome was self-reported in questionnaires and included both clinic-based and at-home, self-testing. PrEP refilling was determined using dispensing records from the six-month clinic visit.
Adherence to PrEP was determined by measuring any detection of tenofovir-diphosphate (TFVDP) in DBS via liquid chromatography tandem mass spectrometry.17 We chose to measure any detection of TFV-DP in our primary analysis due to uncertainties around the definition of a protective PrEP threshold for cisgender African women. All outcomes were binary metrics that were dependent upon participants returning to their six-month follow-up visit.
All primary outcome measurements were dependent on retention (i.e., a participant returning for their six-month PrEP visit). We used inclusive retention windows, based on the scheduled PrEP follow-up visits for each arm, to capture any participants that returned for PrEP continuation during this follow-up period, including those who may have stopped and restarted PrEP based on their ongoing HIV risk.18 Our retention windows opened the day following enrollment and closed two weeks prior to the scheduled nine-month visit date for participants in the SOC arm (i.e., 256 days following randomization) and two weeks prior to the scheduled 12-month visit date for participants in the intervention arms (i.e., 346 days following randomization).
Statistical analysis
We based the sample size on the PrEP adherence primary outcome. We used a one-sided 95% confidence interval (CI) lower bound (LB), common for non-inferiority trials at the time of trial conception,19 and defined a non-inferiority margin of −10%. Assuming 80% PrEP adherence and 10% loss to follow-up, a sample size of 495 participants provided 80% power to rule out more than a 10% difference in PrEP adherence. In programmatic settings, however, nonattendance at clinic visits is overwhelmingly indicative of having stopped PrEP. Thus, after the study initiated, we performed additional power calculations at the recommendation of the trial’s Data Safety Monitoring Board that counted missing results, such as loss to follow-up, as not achieving the outcome (i.e., assuming 70% PrEP adherence in the intervention and SOC arms) and determined that with a sample size of 495 participants, we had 74% power to rule out more than a 10% difference in PrEP adherence.
Analyses were intention-to-treat, with missing results assigned as not achieving the study outcomes. Our primary pre-specified comparison was six-month PrEP dispensing + interim HIVST with semiannual clinic visits (i.e., combined intervention arms) versus SOC PrEP dispensing. We chose to analyze the two intervention arms together because we hypothesized that the effect of the intervention on PrEP adherence relates to the frequency of scheduled follow-up visits (and ability of six-month PrEP dispensing with interim HIVST to support that), not the HIVST modality. However, to understand how the HIVST modality (e.g., oral-fluid vs. blood-based) might affect the outcomes of interest, we also individually compared the intervention arms to the SOC arm, as well as to one another.
We measured the proportion of participants with each outcome by randomization group and estimated risk differences (RDs) between the groups. To estimate RDs and one-sided 95% CI LBs, we used binomial regression models with identity link, adjusting for study population (e.g., men in HIV serodifferent couples, women in HIV serodifferent couples, and women singly enrolled). We interpreted one-sided 95% CI LBs greater than or equal to −10% as indicating the intervention was non-inferior to SOC. Analyses were conducted using Stata/SE 16 (College Station, USA).
We pre-specified analyses among sub-groups, including HIV serodifferent couples, women (including both those enrolled in HIV serodifferent couples and those singly enrolled), and women singly enrolled. For women singly enrolled, we also planned a superiority analysis with the hypothesis that less frequent follow-up could be inferior to SOC due to the unique PrEP adherence challenges that this subpopulation experiences. In this superiority analysis among women singly enrolled, we interpreted two-sided 95% CIs that did not include zero as significant and estimated that we would have 80% power to a difference in PrEP adherence of 21% (49% vs. 70%) between those assigned to the intervention vs control arms. Additionally, we pre-specified measurement of study outcomes among participants <30 years and ≥30 years.
We completed two sensitivity analyses. First, we measured outcomes among participants that returned to the six-month follow-up visit “on-time”: any day after enrollment and no more than three weeks after the scheduled visit (i.e., 201 days following randomization). Second, we measured PrEP adherence using a “protective” level of TFV-DP that has commonly been used in other PrEP implementation studies (i.e., ≥700 fmol/punch).
In a post-hoc analysis requested by an independent reviewer, we also estimated the RD and one-sided 97.5% CL LB for each pre-specified outcome, a conservative approach recommended for evaluation of novel pharmaceutical agents,20 and similarly defined a non-inferiority margin of −10%.
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
RESULTS
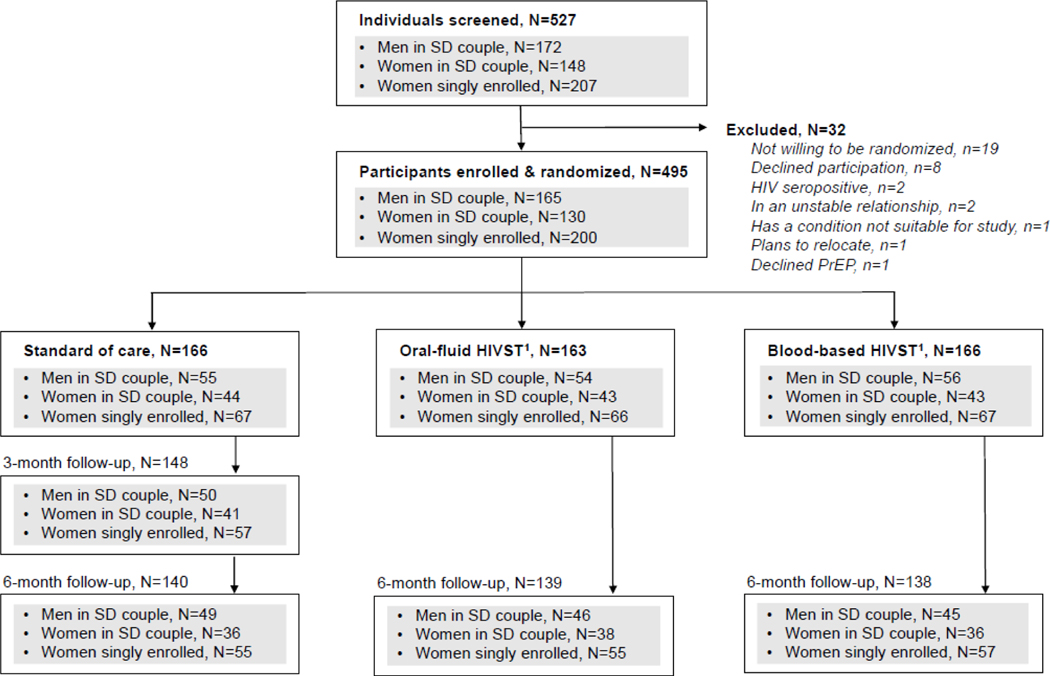
Between May 28, 2018 and February 24, 2020, we screened 527 individuals and enrolled 495: 165 men in HIV serodifferent couples, 130 women in HIV serodifferent couples, and 200 women singly enrolled (Figure 1); 166 were randomized to the SOC arm and 329 to the six-month PrEP dispensing + interim HIVST arms (163 to oral-fluid HIVST and 166 to blood-based HIVST).
Figure 1. CONSORT diagram.
Abbreviations: HIV self-testing (HIVST); HIV serodifferent (SD)
1Participants randomized to these intervention arms received six-month PrEP dispensing + interim HIVST (either oral-fluid or blood-based) and semiannual clinic visits.
Socio-demographic characteristics were similar across randomization arms. All were Black Africans. The median age was 33 years (interquartile range [IQR] 27–40), and most (67·1%) were ≥30 years, Table 1. The median years of education was nine (IQR 8–12), and most (77·2%) were married (95·6% in HIV serodifferent couples and 50·0% of women singly enrolled). All participants reported behaviors that indicated HIV risk, according to Kenya’s PrEP RAST;9 the vast majority (81·8%) reported inconsistent condom use (i.e., some condomless sex) in the past month. Nearly a quarter (24·0%) of women singly enrolled reported exchanging sex for money or goods in the past month, compared to 0·0% of men and 0·8% of women in HIV serodifferent couples.
Table 1.
Participants’ demographic characteristics at baseline, by study arm
| Characteristic | Standard-of-care | Oral-fluid HIVST1 | Blood-based HIVST1 | Overall |
|---|---|---|---|---|
| N | 166 | 163 | 166 | 495 |
| Study Population | ||||
| Men, in HIV serodifferent couple | 55 (33·1%) | 54 (33·1%) | 56 (33·7%) | 165 (33·3%) |
| Women, in HIV serodifferent couple | 44 (26·5%) | 43 (26·4%) | 43 (25·9%) | 130 (26·3%) |
| Women, singly enrolled | 67 (40·4%) | 66 (40·5%) | 67 (40·4%) | 200 (40·4%) |
| Age, median (IQR) | 33 (28–40) | 33 (28–40) | 32 (27–39) | 33 (27–40) |
| Age categories | ||||
| 18–24 years | 27 (16·3%) | 23 (14·1%) | 26 (15·7%) | 76 (15·4%) |
| 25–29 years | 25 (15·1%) | 31 (19·0%) | 31 (18·7%) | 87 (17·6%) |
| 30–34 years | 38 (22·9%) | 40 (24·5%) | 42 (25·3%) | 120 (24·2%) |
| ≥35 years | 76 (45·8%) | 69 (42·3%) | 67 (40·4%) | 212 (42·8%) |
| Travel time to clinic | ||||
| <30 minutes | 7 (4·2%) | 4 (2·5%) | 4 (2·4%) | 15 (3·0%) |
| 30–<60 minutes | 42 (25·3%) | 45 (27·6%) | 40 (24·1%) | 127 (25·7%) |
| 1–<2 hours | 36 (21·7%) | 34 (20·9%) | 37 (22·3%) | 107 (21·6%) |
| >2 hours | 80 (48·2%) | 80 (49·1%) | 85 (51·2%) | 245 (49·5%) |
| Years of education, median (IQR) | 10 (8–12) | 10 (8–12) | 8 (8–12) | 9 (8–12) |
| Age of sexual debut, median (IQR) | 18 (15–19) | 18 (16–20) | 17 (15–19) | 17 (16–19) |
| Married | 126 (75·9%) | 129 (79·1%) | 127 (76·5%) | 382 (77·2%) |
| Currently pregnant2,3 | 6 (5·4%) | 7 (6·4%) | 7 (6·4%) | 20 (6·1%) |
| Using modern method of contraception2,4 | 59 (53·2%) | 49 (45·0%) | 53 (48·2%) | 161 (48·8%) |
| Fully circumcised5 | 50 (90·9%) | 51 (94·4%) | 51 (91·1%) | 152 (92·1%) |
| Sexual Behaviors | ||||
| No. sex partners (past month), median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) |
| Frequency of sex (past month), median (IQR) | 8 (4–12) | 8 (4–12) | 8 (4–13) | 8 (4–12) |
| Inconsistent condom use (past month)6 | 144 (86·7%) | 139 (85·3%) | 122 (73·5%) | 405 (81·8%) |
| Exchanged sex for goods/money (past month)7,8 | 20 (12·0%) | 18 (11·0%) | 11 (6·6%) | 49 (9·9%) |
| PrEP disclosure to ≥1 person9 | 28 (16·9%) | 35 (21·5%) | 27 (16·3%) | 90 (18·2%) |
| Self-efficacy score, median (IQR)10,11 | 37 (29–40) | 38 (30–40) | 38 (30–40) | 37·5 (30–40) |
| Severity of depressive symptoms, median (IQR)11,12 | 1 (0–4) | 1 (0–4) | 1 (0–5) | 1 (0–4) |
| Any social harm, past 3 months13 | 10 (6·0%) | 12 (7·4%) | 18 (10·8%) | 40 (8·1%) |
Abbreviations:interquartile range (IQR)
Participants randomized to these intervention arms received six-month PrEP dispensing + interim HIVST (either oral-fluid or blood-based) with biannual clinic visits.
Outcome reported only among female participants (n=330).
Missing data on baseline pregnancy status for 19 participants.
Modern methods of contraception include oral, injectable, implants, and intrauterine devices (IUDs).
Outcome reported only among male participants (n=165); missing data for 10 participants.
Condom use categorized as inconsistent if condoms not used during every sex act in the past month; missing data for 28 participants.
Missing data for 24 participants.
Among females singly enrolled, 24% (n=48) reported exchanging sex for money/goods in past month.
Social harm includes any verbal, physical, or emotional abuse by a sexual partner.
Reported PrEP disclosure to at least one other person besides one’s main sexual partner in serodifferent couples.
Self-efficacy score calculated using the General Self-Efficacy Scale
Missing data for one participant.
Severity of depressive symptoms calculated using the Patient Health Questionnaire 9-item (PHQ-9) depression scale
Social harm includes any verbal, physical, or emotional abuse by a sexual partner.
At enrollment, all participants in the intervention arms received a six-month PrEP supply and two HIV self-tests, and all participants in the SOC arm received a three-month PrEP supply and no HIV self-tests. At three months, 89·2% (n=148) of participants in the SOC arm returned for their scheduled three-month follow-up visit, and 7.6% (n=25) of participants in the combined intervention arm returned to the clinic for a voluntary unscheduled follow-up visit. Reasons for voluntary unscheduled visits among participants in the combined intervention arm included: counseling (n=2), treatment (n=2), experienced social harm (n=1), clinic-based HIV testing (n=1), other laboratory testing or results (n=5), a medical visit unrelated to HIV testing (n=22), replacement of HIV self-test(s) that were lost/stolen (n=2), or PrEP drugs that were lost/stolen (n=1) or needed if the participant could not make their next scheduled visit (n=1). Some participants had more than one unscheduled visit. Among SOC arm participants who returned for a three-month follow-up visit, the median number of days since enrollment was 89 (IQR 84–91 days).
At six months, 84·3% of participants in the SOC arm (140/166) and 84·2% of participants in the combined intervention arm (277/329) returned for follow-up. Within the combined intervention arm, 85·3% (139/163) of participants in the oral-fluid HIVST arm and 83·1% (138/166) of participants in the blood-based HIVST arm returned for follow-up. The median number of days from enrollment to the six-month visit was 178 (IQR 168–181 days) for participants in the SOC arm and 178 (IQR 168–183 days) for participants in the combined intervention arm (oral-fluid HIVST arm: 179 days, IQR 169–187 days; blood-based HIVST arm: 174 days, IQR 168–182 days).
Among participants in the combined intervention arm, 81·2% (267/329) reported completing at least one of the HIV self-tests, and 41·9% (138/329) reported completing both of the HIV self-tests. The median time since enrollment that participants completed the first HIV self-test was 87 days (IQR 71–99 days) and second HIV self-test was 165 days (IQR 148–171 days), Appendix: Page 2. Among those who had one or more unused HIV self-tests at six months, 89·2% (124/139) reported still having the self-test(s), 5·8% (8/139) reported a lost or damaged self-test, 2·2% (3/139) reported giving the test to someone else, and 1·4% (2/139) reported using it to test someone else.
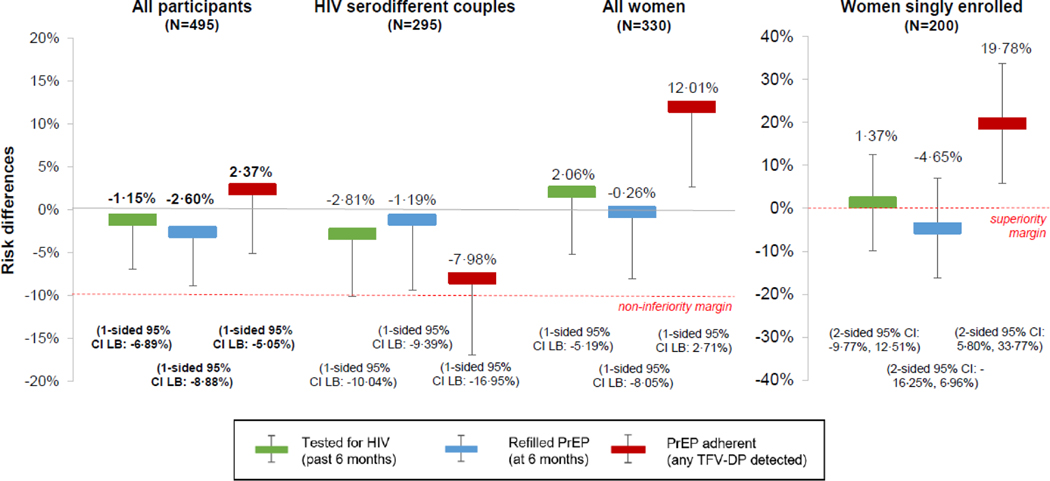
At six months, all of the primary outcomes – HIV testing (past six months), PrEP refilling (at the six-month visit), and PrEP adherence (any TFV-DP detected) – for the combined six-month PrEP dispensing + interim HIVST arm were non-inferior when compared to the SOC PrEP delivery arm, Table 2 and Figure 2: 83·3% (274/329) of participants in the combined intervention arm had tested for HIV in the prior six months compared to 84·3% (140/166) of participants in the SOC arm (RD −1·2%, one-sided 95% CI LB −6·9%); 78·1% (257/329) in the combined intervention arm refilled PrEP at their six-month visit compared to 80·7% (134/166) in the SOC arm (RD −2·6%, one-sided 95% CI LB −8·9%); and 60·8% (200/329) in the combined intervention arm were adherent to PrEP compared to 57·2% (95/166) in the SOC arm (RD 2·4%, one-sided 95% CI LB −5·1%).
Table 2.
Percentage of participants reporting primary outcomes, by study group and population
| Outcome | Standard of care | Oral-fluid + Blood-based HIVST1 | Oral-fluid HIVST1 | Blood-based HIVST1 | Overall |
|---|---|---|---|---|---|
| All participants | 166 | 329 | 163 | 166 | 495 |
|
| |||||
| Returned to clinic2 | 140 (84·3%) | 277 (84·2%) | 139 (85·3%) | 138 (83·1%) | 417 (84·2%) |
| Co-Primary: Tested for HIV* | 140 (84·3%) | 274 (83·3%) | 139 (85·3%) | 135 (81·3%) | 414 (83·6%) |
| Co-Primary: Refilled PrEP* | 134 (80·7%) | 257 (78·1%) | 130 (79·8%) | 127 (76·5%) | 391 (79·0%) |
| Co-Primary: Adherent (any TFV-DP detected)* | 95 (57·2%) | 200 (60·8%) | 94 (57·7%) | 106 (63·9%) | 295 (59·6%) |
| HIV serodifferent couples 3 | 99 | 196 | 97 | 99 | 295 |
|
| |||||
| Returned to clinic2 | 85 (85·9%) | 165 (84·2%) | 84 (86·6%) | 81 (81·8%) | 250 (84·8%) |
| Tested for HIV | 85 (85·9%) | 163 (83·2%) | 84 (86·6%) | 79 (79·8%) | 248 (84·1%) |
| Refilled PrEP | 79 (79·8%) | 154 (78·6%) | 78 (80·4%) | 76 (76·8%) | 233 (79·0%) |
| Adherent (any TFV-DP detected) | 74 (74·8%) | 132 (67·4%) | 65 (67·0%) | 67 (67·7%) | 206 (69·8%) |
| All women 3 | 111 | 219 | 109 | 110 | 330 |
|
| |||||
| Returned to clinic2 | 91 (82·0%) | 186 (84·9%) | 93 (85·3%) | 93 (84·6%) | 277 (83·9%) |
| Tested for HIV | 91 (82·0%) | 184 (84·0%) | 93 (85·3%) | 91 (82·7%) | 275 (83·3%) |
| Refilled PrEP | 88 (79·3%) | 173 (79·0%) | 87 (79·8%) | 86 (78·2%) | 261 (79·1%) |
| Adherent (any TFV-DP detected) | 50 (45·1%) | 125 (57·1%) | 57 (52·3%) | 68 (61·8%) | 175 (53·0%) |
| Women singly enrolled 3 | 67 | 133 | 66 | 67 | 200 |
|
| |||||
| Returned to clinic2 | 55 (82·1%) | 112 (84·2%) | 55 (83·3%) | 57 (85·1%) | 167 (83·5%) |
| Tested for HIV | 55 (82·1%) | 111 (83·5%) | 55 (83·3%) | 56 (83·6%) | 166 (83·0%) |
| Refilled PrEP | 55 (82·1%) | 103 (77·4%) | 52 (78·8%) | 51 (76·1%) | 158 (79·0%) |
| Adherent (any TFV-DP detected) | 21 (31·3%) | 68 (51·1%) | 29 (43·9%) | 39 (58·2%) | 89 (44·5%) |
Abbreviations:HIV self-testing (HIVST); pre-exposure prophylaxis (PrEP); tenofovir-diphosphate (TFP-DP).
Primary study outcomes were HIV testing (past six months), PrEP refilling (at six-month visit), and PrEP adherence (any TFV-DP detected) at six months among all participants, with the primary comparison being the pooled six-month PrEP dispensing + interim HIVST intervention arms vs. the standard-of-care PrEP dispensing arm.
Participants randomized to these intervention arms received six-month PrEP dispensing + interim HIVST (either oral-fluid or blood-based) with biannual clinic visits.
We included all follow-up visits assigned as six-month visits by study staff; the window for these visits closed 2 weeks prior to the next scheduled visit date, which as at nine months for participants randomized to the SOC group and 12 months for participants randomized to one of the intervention arms.
The sub-group HIV serodifferent couples included all men and women enrolled in HIV serodifferent couples (N=295). The sub-group all women included both women enrolled in HIV serodifferent couples and women singly enrolled (N=330). The sub-group women singly enrolled just included these women and not those enrolled in HIV serodifferent couples (N=200).
Figure 2. The effect of six-month PrEP dispensing + interim HIVST on recent HIV testing, PrEP refilling, and PrEP adherence at six months.
These risk differences (RDs) compare the combined six-month PrEP dispensing + interim HIV self-testing (HIVST, either oral-fluid or blood-based) arm with the SOC PrEP dispensing arm at six months.
The red lines indicate the marker of significance. The bolded RDs and 95% confidence interval (CI) lower bounds (LB) indicate the primary pre-specified outcomes: recent HIV testing (green bars), PrEP refilling (blue bars), and PrEP adherence (red bars) among all participants (N=495). We additionally pre-specified analysis of these outcomes among the following sub-groups: individuals taking PrEP who were members of HIV serodifferent couples (N=295), all women (N=330, including those enrolled in couples and those singly enrolled), and women singly enrolled (N=200).
To understand the impact of HIVST modality (e.g., oral-fluid vs. blood-based), we compared outcomes in the individual intervention arms to those in the SOC arm as well as to one another, Table 2 and Table 3. Outcomes were all non-inferior for participants randomized to the oral-fluid HIVST arm compared to the SOC arm. Additional findings showed that PrEP adherence for blood-based HIVST arm was non-inferior compared to the SOC arm, but we were not able to demonstrate non-inferiority in the HIV testing and PrEP refilling outcomes, the RDs of which were both below 0% and one-sided 95% CI LBs below −10%.
Table 3.
Effect size estimates for HIV testing, PrEP refilling, and PrEP adherence, by study population and un-pooled intervention groups
| Oral-fluid HIVST1 vs. Standard of care | Blood-based HIVST1 vs. Standard of care | Oral-fluid HIVST1 vs. Blood-based HIVST1 | |
|---|---|---|---|
| All participants (N=495) | RD (1-sided 95% CI lower bound) 4 | RD (1-sided 95% CI lower bound) 4 | RD (1-sided 95% CI lower bound) 4 |
| Returned to clinic2 | |||
| Tested for HIV | 0·72% (−5·74%) | −3·24% (−10·03%) | 3·96% (−2·78%) |
| Refilled PrEP | −1·18% (−8·39%) | −4·31% (−11·70%) | 3·07% (−4·39%) |
| Adherent (any TFV-DP detected) | −0·62% (−9·01%) | 4·67% (−3·78%) | −5·74% (−14·45%) |
| HIV serodifferent couples3 (N=295) | RD (1-sided 95% CI lower bound) 4 | RD (1-sided 95% CI lower bound) 4 | RD (1-sided 95% CI lower bound) 4 |
| Returned to clinic2 | |||
| Tested for HIV | 0·42% (−7·66%) | −6·43% (−15·18%) | 6·79% (−1·93%) |
| Refilled PrEP | 0·28% (−9·07%) | −3·13% (−12·76%) | 3·33% (−6·25%) |
| Adherent (any TFV-DP detected) | −8·37% (−18·88%) | −7·84% (−18·28%) | −0·63% (−11·65%) |
| All women3 (N=330) | RD (1-sided 95% CI lower bound) 4 | RD (1-sided 95% CI lower bound) 4 | RD (1-sided 95% CI lower bound) 4 |
| Returned to clinic2 | |||
| Tested for HIV | 3·51% (−4·65%) | 0·76% (−7·67%) | 2·74% (−5·39%) |
| Refilled PrEP | 0·35% (−8·59%) | −1·21% (−10·26%) | 1·53% (−7·49%) |
| Adherent (any TFV-DP detected) | 7·19% (−3·45%) | 16·29% (5·66%) | −9·26% (−20·09%) |
| Women singly enrolled3 (N=200) | RD (2-sided 95% CI) 5 | RD (2-sided 95% CI) 5 | RD (2-sided 95% CI) 5 |
| Returned to clinic2 | |||
| Tested for HIV | 1·24% (−11·61%, 14·09%) | 1·49% (−11·27%, 14·26%) | −0·25% (−12·88%, 12·38%) |
| Refilled PrEP | −3·30% (−16·78%, 10·17%) | −5·97% (−19·70%, 7·76%) | 2·67% (−11·53%, 16·86%) |
| Adherent (any TFV-DP detected) | 1·26% (−3·74%, 28·93%) | 26·87% (10·65%, 43·08%) | −14·27% (−31·09%, 2·55%) |
Risk differences (RDs) measured using binomial regression models with identity links, adjusted for study population at enrollment (e.g., men in HIV serodifferent couples, women in HIV serodifferent couples, and women singly enrolled).
Participants randomized to these intervention arms received six-month PrEP dispensing + interim HIVST (either oral-fluid or blood-based) with biannual clinic visits.
We included all follow-up visits assigned as six-month visits by study staff; the window for these visits closed 2 weeks prior to the next scheduled visit date, which as at nine months for participants randomized to the SOC arm and 12 months for participants randomized to one of the intervention arms.
The sub-group HIV serodifferent couples included all men and women enrolled in HIV serodifferent couples (N=295). The sub-group all women included both women enrolled in HIV serodifferent couples and women singly enrolled (N=330). The sub-group women singly enrolled just included these women and not those enrolled in HIV serodifferent couples (N=200).
In our non-inferiority analyses, we interpreted one-sided 95% CIs above −10% as non-inferior.
In our superiority analyses, we interpreted two-sided 95% CIs that did not include zero as significant.
CI: confidence interval; HIVST: HIV self-testing; PrEP: pre-exposure prophylaxis; RD: risk difference; TFV-DP: tenofovir-diphosphate
For those in HIV serodifferent couples, the only outcome that was non-inferior was PrEP refilling, which was 78·6% (154/196) in the combined intervention arm vs. 79·8% (79/99) in the SOC arm (RD −1·2%, one-sided 95% CI LB −9·4%). For both the HIV testing and PrEP adherence outcomes, we could not claim non-inferiority as HIV testing was 83·2% (163/196) in the combined intervention arm compared to 85·9% (85/99) in the SOC arm (RD −2·8%, one-sided 95% CI LB 10·0%) and PrEP adherence was 67·4% (132/196) in the combined intervention arm compared to 74·8% (74/99) in the SOC arm (RD −8·0%, one-sided 95% CI LB −17·0%). For descriptive outcomes disaggregated by men and women in HIV serodifferent couples, see Appendix: Page 3.
Among all women, while the study outcomes remained non-inferior for the combined intervention compared to the SOC arm, there was some indication that participants in the combined intervention arm had better PrEP adherence (57·1%, 125/219) than those in the SOC arm (45·1%, 50/111; RD 12·0%, one-sided 95% CI LB 2·7%). We could not, however, determine superiority because we did not pre-specify a superiority analysis for the all women sub-group.
Among women singly enrolled, PrEP adherence was 19·8% higher (two-sided 95% CI 5·8%, 33·8%) in the combined intervention arm (51·1%, 68/133) compared to the SOC arm (31·3%, 21/67), and this difference was statistically significant. For women singly enrolled, PrEP adherence was significantly higher in the blood-based HIVST intervention arm (58·2%, 39/67) compared to the SOC arm (31.3%, 21/67; RD 26·9%, two-sided 95% CI 10·7%, 43·1%), although there was no statistically significant difference in this outcome between the two intervention arms.
Among participants ≥30 years, all outcomes in the combined intervention arm were non-inferior compared to the SOC arm, but non-inferiority was not established for the smaller subgroup of those <30 years, Appendix: Page 4.
For the first sensitivity analysis that measured outcomes for the “on-time” retention window, PrEP adherence was non-inferior in the combined intervention arm compared to the SOC arm at 6 months, but we could not establish non-inferiority in the HIV testing and PrEP refilling outcomes, Appendix: Page 5. For the second sensitivity analysis that defined PrEP adherence using a TFV-DP threshold level of ≥700 fmol/punch, we were able to establish non-inferiority between the combined intervention arm and the SOC arm.
In the post-hoc analysis of study outcomes using a one-sided 97.5% CI LB, our findings remained largely the same, but the PrEP refilling outcome was no longer non-inferior in the combined intervention arm compared to the SOC arm at six months among all participants, Appendix: Page 6. Additionally, none of the outcomes among HIV serodifferent couples were non-inferior at six months in the combined intervention arm compared to the SOC arm, but all outcomes among all women remained non-inferior.
We did not observe any HIV seroconversions over the observation period.
DISCUSSION
In this randomized implementation trial, we found that six-month PrEP dispensing + interim HIVST reduced the number of PrEP clinic visits in half while maintaining equivalent HIV testing, PrEP refilling, and PrEP adherence at six months compared to SOC PrEP delivery. Additionally, we found that among women not in HIV serodifferent couples, this model of six-month PrEP dispensing with interim HIVST significantly increased PrEP adherence, measured objectively with blood sampling, compared to SOC PrEP delivery. These findings are important because they provide the first evidence on the use of HIVST to support PrEP continuation.
Our study demonstrates that at-home HIVST can replace some of the periodic routine HIV testing conducted at clinics for persons using PrEP, thereby reducing both client and clinic burdens associated with PrEP access and delivery without compromising adherence. This new evidence supports a differentiated model of PrEP delivery in which persons intending to use PrEP for a longer period could benefit from multi-month dispensing (greater than three months) with biannual clinic visits supported by interim HIVST. Future studies might even explore the potential benefits of spacing PrEP clinic visits greater than the six-month intervals tested in this trial and incorporate participants’ preferences for longer intervals of desired PrEP dispensing. These differentiated models of PrEP refilling could empower people to take charge of their own care (i.e., self-care). However, these models will rely on a robust supply chain of PrEP drugs, which has been a challenge in many settings and will need to be addressed to support longer periods of multi-month dispensing.21,22 Additionally, multi-month dispensing necessitates a trade-off of benefits associated with more frequent visits for PrEP users, specifically screening for and treating nonHIV STIs. At-home, self-administered STI testing kits being piloted in some settings could be provided together with HIV self-test kits to ensure this benefit is not lost with less frequent clinic visits.23
Several randomized trials and implementation studies have reported challenges with PrEP adherence among women in sub-Saharan Africa compared to their female counterparts in serodifferent relationships.24–27 These differences have been hypothesized to be a result of differences in risk perception. In our study, women singly enrolled also had lower PrEP adherence than men and women in HIV serodifferent couples. However, among women singly enrolled, we observed significantly higher PrEP adherence among those assigned to the intervention compared to SOC PrEP delivery, suggesting that six-month PrEP dispensing supported with interim HIVST may have helped overcome barriers to PrEP adherence for this population. This difference could be attributable to the intervention’s reduction in the frequency of PrEP clinic visits; women singly enrolled may have unique barriers to attending frequent clinic visits for PrEP access challenging. Additional explanations could include feelings of empowerment associated with HIVST28 and subsequent PrEP use; singly enrolled women’s unique periods of HIV risk and the intervention’s ability to support PrEP use at their best discretion; and singly enrolled women’s option to share the extra HIV self-test with sexual partners and then adapt their PrEP use based on their partner’s response to HIVST delivery.
In our study, we used both oral-fluid and blood-based HIV self-tests because these two modalities have different associated costs and cater to different participant preferences. Additional work from this trial will explore these costs and preferences. For our primary outcomes, we did not observe substantive differences between the two HIVST approaches and their support of PrEP delivery.
Among women singly enrolled, those randomized to the blood-based HIVST intervention arm had significantly higher PrEP adherence compared to those in the SOC arm; that outcome was not significantly different between the blood-based and oral-fluid HIVST intervention arms. In addition to the reasons hypothesized above about how the HIVST intervention in general may have improved PrEP adherence among women in this subgroup, it is possible that blood-based HIVST in particular increases confidence in a self-test result and thus has a stronger impact on behaviors (i.e., PrEP use). More research is needed to better understand how the HIVST might influence PrEP adherence among women.
Our study had limitations. First, our definitions of retention, PrEP refilling, and PrEP adherence in this study do not truly represent the overall clinical objective of preventing HIV acquisition, as HIV risk fluctuates over time and PrEP clients may choose to modify their treatment without compromising the effectiveness of their HIV prevention. Second, we did not measure outcomes for participants not retained in care, taking a conservative approach that missing data equated to failure for the outcome. Third, although participants were trained in the conduct of HIVST, we did not verify that the specific PrEP user actually conducted HIVST and relied on self-reported HIV testing data. Fourth, our trial did not incorporate participants’ HIVST modality preferences for interim HIV testing (participants were randomized to either blood-based or oral-fluid HIVST), and thus, we do not know the effect of selecting a preferred HIVST modality. Finally, our primary outcomes were measured at six months, and we do not yet know the impact of this intervention over longer periods.
Currently, the WHO does not recommend the use of HIVST for persons initiating or continuing PrEP because of concerns about self-test sensitivity, which could miss HIV seroconversions and put PrEP users at risk of HIV drug resistance. Compared to blood-based HIV self-tests and rapid diagnostic HIV tests, oral-fluid HIV self-test have a lower sensitivity,29 which in theory could contribute to missed or delayed HIV diagnoses. Delayed diagnosis may be more likely than missed, given our finding of non-inferior clinic-based HIV testing at six months with the intervention compared to SOC PrEP delivery. The extent of this risk is potentially mitigated by the rarity of breakthrough HIV infection on tenofovir/emtricitabine (FTC/TDF)-based PrEP, the even greater rarity of resistance to the tenofovir component, and the well-established successful treatment of individuals who acquired HIV.30 HIVST-supported PrEP delivery to reduce clinic and client burdens may be a pragmatic counterbalance to those potential risks.
Our findings are relevant for the era of COVID-19, where innovations to support multi-month PrEP dispensing are needed.31 To curb the spread of COVID-19, many countries including Kenya instituted restrictions on movement, curfews, and limitations on social gatherings. These measures impacted access to health services, including HIV testing, PrEP, and treatment.32 There have also been reports of patients avoiding clinics to reduce the risk of COVID-1933 acquisition associated with public transport and clinic visits. Multi-month dispensing and telemedicine are some of the adaptations being piloted to address the challenge of COVID-19; the findings from this study have relevance as we look towards developing models that support the continuation of service delivery during pandemics.33
Findings from this study could be used to motivate other simplified models of PrEP delivery that reduce the contact frequency with clinics and enhance elements of self-care (e.g., telehealth visits) or the evaluation of new community-based models of PrEP refilling supported with HIVST (e.g., PrEP refilling via sexual partners, mobile vans, or online platforms). Programs looking for ways to enhance the efficiency of PrEP delivery should consider evaluating the scale-up of this HIVST-supported model, which could reduce costs and other burdens for clients, providers, and health systems.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study:
Oral pre-exposure prophylaxis (PrEP) for HIV prevention and HIV self-testing (HIVST) are both new technologies in Kenya (both introduced in 2017) and other African countries with high HIV prevalence. Recent studies have found that among PrEP users, HIVST is acceptable and that HIVST use to support PrEP delivery may be feasible. We conducted a PubMed Search from January 1, 2012 (both PrEP and HIVST were approved by the US Food and Drug Administration in 2012) and August 22, 2021. Our search terms included “HIV,” “HIV self-testing,” “PrEP,” “pre-exposure prophylaxis,” “HIV self-testing and PrEP.” We did not find any published study that used HIVST to support PrEP delivery.
Added value of this study:
For persons using PrEP for HIV prevention, regular HIV testing at fixed-site clinics every three months has been the standard. We conducted a randomized non-inferiority trial to test the use of HIVST to decrease the frequency and burden of clinic visits of PrEP in Kenya. We found that six-month PrEP dispensing supported with interim HIVST reduced the number of PrEP clinic visits in half without impacting clients’ HIV testing, PrEP refilling, and PrEP adherence outcomes at six months, when compared to standard-of-care PrEP delivery (three-month PrEP dispensing with quarterly clinic visits).
Implications for all the available evidence:
Findings from our trial provides evidence for the use of HIVST to support PrEP continuation and suggest that HIVST can be used to support models of PrEP delivery requiring less frequent contact with the health system, including community-based models of PrEP delivery.
Acknowledgements
We would like to acknowledge all trial participants for taking time to contribute to this research. Additionally, we would like to acknowledge all the research staff at the Partners in Health and Research Development research and care clinic in Thika and University of Washington that made this trial possible. This trial was supported by the National Institute of Mental Health (R01 MH113572). KFO was additionally supported by the National Institutes of Mental Health (R34 MH120106 and K99 MH121166) and Allergy and Infectious Disease (P30 AI027757). ARB was additionally supported by the National Center for Advancing Translational Sciences (TL1 TR002318). The Atomo/Mylan blood-based HIVST kits were donated by Atomo Inc./Mylan Laboratories Ltd. The PrEP medication was provided by the Kenyan Ministry of Health.
Funding:
The US National Institute of Mental Health.
Footnotes
Declaration of interest
All other authors declare no completing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing
Deidentified data available upon author request and under appropriate data sharing agreements who provide a methodologically sound proposal.
Dr. Jared Baeten is a current employee of Gilead Sciences and has received consulting fees from Gilead Sciences and Merck in the past.
REFERENCES
- 1.Venter WDF. Pre-exposure Prophylaxis: The Delivery Challenge. Front Public Health 2018; 6: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mack N, Odhiambo J, Wong CM, Agot K. Barriers and facilitators to pre-exposure prophylaxis (PrEP) eligibility screening and ongoing HIV testing among target populations in Bondo and Rarieda, Kenya: results of a consultation with community stakeholders. BMC Health Serv Res 2014; 14: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel RC, Stanford-Moore G, Odoyo J, et al. “Since both of us are using antiretrovirals, we have been supportive to each other”: facilitators and barriers of pre-exposure prophylaxis use in heterosexual HIV serodiscordant couples in Kisumu, Kenya. J Int AIDS Soc 2016; 19(1): 21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigogo G, Audi A, Aura B, Aol G, Breiman RF, Feikin DR. Health-seeking patterns among participants of population-based morbidity surveillance in rural western Kenya: implications for calculating disease rates. Int J Infect Dis 2010; 14(11): e967–73. [DOI] [PubMed] [Google Scholar]
- 5.Ngure K, Heffron R, Mugo N, et al. Feasibility and acceptability of HIV self-testing among pre-exposure prophylaxis users in Kenya. J Int AIDS Soc 2017; 20(1): 21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortblad KF, Chanda MM, Musoke DK, et al. Acceptability of HIV self-testing to support pre-exposure prophylaxis among female sex workers in Uganda and Zambia: results from two randomized controlled trials. BMC Infect Dis 2018; 18(1): 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanga V, Omollo V, Bukusi EA, et al. Uptake and impact of facility-based HIV self-testing on PrEP delivery: a pilot study among young women in Kisumu, Kenya. J Int AIDS Soc 2020; 23(8): e25561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Public Health Service. “Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2017 Update.” pdf icon[PDF – 2 MB] Published March 2018. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf accessed on August 23, 2021.
- 9.Ministry of Health, National AIDS & STI Control Program. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2018 Edition. Nairobi, Kenya: NASCOP, August 2018. . [Google Scholar]
- 10.Hector J, Davies MA, Dekker-Boersema J, et al. Acceptability and performance of a directly assisted oral HIV self-testing intervention in adolescents in rural Mozambique. PLoS One 2018; 13(4): e0195391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dovel K, Shaba F, Offorjebe OA, et al. Effect of facility-based HIV self-testing on uptake of testing among outpatients in Malawi: a cluster-randomised trial. Lancet Glob Health 2020; 8(2): e276–e87. [DOI] [PubMed] [Google Scholar]
- 12.Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV 2016; 3(6): e266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masters SH, Agot K, Obonyo B, Napierala Mavedzenge S, Maman S, Thirumurthy H. Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial. PLoS Med 2016; 13(11): e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UNAIDS/WHO. Report on the global HIV/AIDS epidemic: December 1997. 1997. http://www.unaids.org/highband/document/epidemio/report97.html. [Google Scholar]
- 15.Ortblad KF, Kearney JE, Mugwanya K, et al. HIV-1 self-testing to improve the efficiency of pre-exposure prophylaxis delivery: a randomized trial in Kenya. Trials 2019; 20(1): 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National AIDS and STI Control Programme (NASCOP), Preliminary KENPHIA 2018 Report. Nairobi: NASCOP; 2020. [Google Scholar]
- 17.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother 2018; 62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed JB, Shrestha P, Were D, et al. HIV PrEP is more than ART-lite: Longitudinal study of real-world PrEP services data identifies missing measures meaningful to HIV prevention programming. J Int AIDS Soc 2021; 24(10): e25827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen E.Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol 2007; 46(5): 947–54. [DOI] [PubMed] [Google Scholar]
- 20.Althunian TA, de Boer A, Groenwold RHH, Klungel OH. Defining the noninferiority margin and analysing noninferiority: An overview. Br J Clin Pharmacol 2017; 83(8): 1636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Access to HIV medicines severely impacted by COVID‐19 as AIDS response stalls. https://www.who.int/news/item/06-07-2020-who-access-to-hiv-medicines-severely-impacted-by-covid-19-as-aids-response-stalls. Accessed on October 5, 2021.
- 22.Observer Star. PrEP shortages to be addressed within a week: https://www.starobserver.com.au/news/prep-shortages-to-be-addressed-within-a-week/200803.. Accessed on October 5, 2021 [Google Scholar]
- 23.Salow KR, Cohen AC, Bristow CC, McGrath MR, Klausner JD. Comparing mail-in self-collected specimens sent via United States Postal Service versus clinic-collected specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in extra-genital sites. PLoS One 2017; 12(12): e0189515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372(6): 509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367(5): 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367(5): 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heffron R, Ngure K, Odoyo J, et al. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa. Gates Open Res 2017; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wachinger J, Kibuuka Musoke D, Oldenburg CE, Bärnighausen T, Ortblad KF, McMahon SA. “But I Gathered My Courage”: HIV Self-Testing as a Pathway of Empowerment Among Ugandan Female Sex Workers. Qual Health Res 2021; 31(3): 443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaspard M, Le Moal G, Saberan-Roncato M, et al. Finger-stick whole blood HIV-1/−2 home-use tests are more sensitive than oral fluid-based in-home HIV tests. PLoS One 2014; 9(6): e101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh UM, Mellors JW. Should we fear resistance from tenofovir/emtricitabine preexposure prophylaxis? Curr Opin HIV AIDS 2016; 11(1): 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed 2020; 91(1): 157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pampati S, Emrick K, Siegler AJ, Jones J. Changes in sexual behavior, PrEP adherence, and access to sexual health services due to the COVID-19 pandemic among a cohort of PrEP-using MSM in the South. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoagland B, Torres TS, Bezerra DRB, et al. Telemedicine as a tool for PrEP delivery during the COVID-19 pandemic in a large HIV prevention service in Rio de Janeiro-Brazil. Braz J Infect Dis 2020; 24(4): 360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data available upon author request and under appropriate data sharing agreements who provide a methodologically sound proposal.
Dr. Jared Baeten is a current employee of Gilead Sciences and has received consulting fees from Gilead Sciences and Merck in the past.