Abstract
Preeclampsia (PE) is a serious hypertensive complication of pregnancy and is a leading cause of maternal death and major contributor to maternal and perinatal morbidity, including establishment of long-term complications. The continued prevalence of PE stresses the need for identification of novel treatments which can target prohypertensive factors implicated in the disease pathophysiology, such as soluble fms-like tyrosine kinase 1 (sFlt-1). To identify novel compounds to reduce placental sFlt-1 and determine whether this occurs via hypoxia- inducible factor (HIF)-1α inhibition. We utilized a commercially available library of natural compounds to assess their ability to reduce sFlt-1 release from primary human placental cytotrophoblast cells (CTBs). Human placental explants from normotensive (NT) and preeclamptic (PE) pregnancies were treated with varying concentrations of luteolin. Protein and mRNA expression of sFlt-1 and upstream mediators were evaluated using ELISA, Western blot, and real-time PCR. Of the natural compounds examined, luteolin showed the most potent inhibition of sFlt-1 release, with >95% reduction compared to vehicle-treated. Luteolin significantly inhibited sFlt-1 in cultured placental explants compared to vehicle-treated in a dose- and time-dependent manner. Additionally, significant decreases in HIF-1α expression were observed in luteolin-treated explants, suggesting a mechanism for sFlt-1 downregulation. The ability of luteolin to inhibit HIF-1α may be mediated through the Akt pathway, as inhibitors to Akt and its upstream regulator phosphatidylinositol-3 kinase (PI3K) resulted in significant HIF-1α reduction. Luteolin reduces anti-angiogenic sFlt-1 through inhibition of HIF-1α, making it a novel candidate for treatment of PE.
Keywords: Preeclampsia, flavones, hypoxia, placenta, sFlt-1
Graphical Abstract
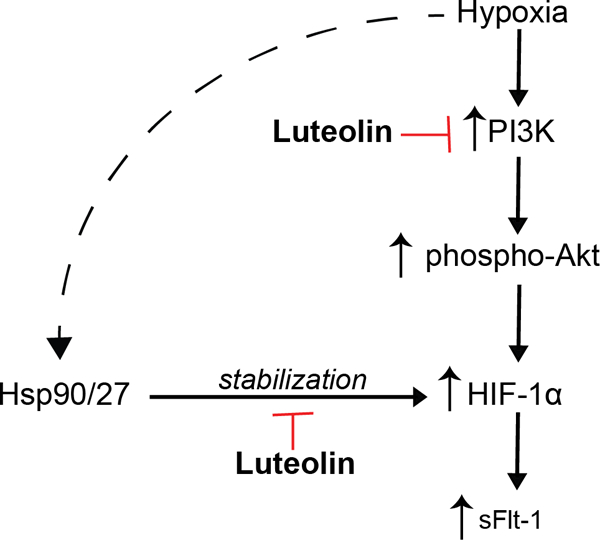
Proposed mechanisms of action of luteolin to suppress HIF-1α and sFlt-1 expression: The hypoxic placenta activates pathways to increase expression of HIF-1α, including the PI3K/ Akt pathway. Our work suggests that luteolin inhibits the actions of PI3K, leading to downstream inhibition of HIF-1α, and subsequently, sFlt-1. Hsp90 and Hsp27 have previously been shown to stabilize HIF-1α protein, but whether luteolin prevents this stabilization has yet to be explored.
INTRODUCTION
Preeclampsia (PE) is a common pregnancy disorder occurring after 20 weeks of gestation characterized by new-onset hypertension, proteinuria, and end-organ damage linked to endothelial and vascular dysfunction (1). Worldwide, PE affects ~7 million women each year resulting in over 500,000 fetal deaths and 70,000 maternal deaths, with a higher mortality rate in the United States compared to other developed countries (2–6). Major risk factors associated with PE occurrence include a history of PE, chronic hypertension, obesity, diabetes mellitus, multiple gestations, and antiphospholipid syndrome (7, 8). Moreover, PE is associated with substantial long-term risk for cardiovascular disease, cerebrovascular disease (9, 10), and renal dysfunction (11) in mothers and increased risk for cardiovascular disease and metabolic syndrome in their children (12, 13). Currently, there are no pharmacological treatments, and the only effective treatment strategy for PE requires delivery of the baby and removal of the placenta, which often occurs prematurely (1).
The current paucity of effective treatments for PE likely is due to the complex pathophysiology of the disease. Disease pathogenesis is believed to progress in two stages beginning with abnormal formation of the placental vasculature in the first trimester resulting in placental ischemia and hypoxia. Abnormal placental development drives the systemic vascular dysfunction through release of anti-angiogenic factors, such as soluble fms-like tyrosine kinase 1 (sFlt-1), ultimately leading to the clinical manifestations of PE during the second stage in the late second and third trimesters (7, 14, 15).
sFlt-1 is the soluble receptor for vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), which are important for maintenance of vascular health. Thus, sFlt-1 exerts its pathological actions by quenching bioavailable levels of VEGF and PlGF, causing endothelial and vascular dysfunction culminating in systemic vasoconstriction and hypertension. sFlt-1 is produced and secreted by the placenta in normal pregnancy, with inappropriate upregulation in PE such that circulating levels are elevated leading up to PE onset (16–19). Its direct ability to promote hypertension in pregnancy has been demonstrated by overexpression of sFlt-1 producing PE-like symptoms in animal models (17, 20–22). Similarly, hypoxia-inducible factor 1α (HIF-1α) is highly expressed in preeclamptic placentas (23), and HIF-1α overexpression in pregnant mice is associated with hypertension and increased sFlt-1 expression (24–26). Studies have shown that sFlt-1 antagonism or depletion in cell culture (27) as well in vivo animal models of PE led to improved clinical symptoms (28–30). Removal of sFlt-1 using apheresis recently demonstrated promise in reducing maternal blood pressure as well as prolong pregnancies in women with preterm PE (31, 32), suggesting that sFlt-1 reduction may provide relief from PE symptoms.
Currently, no approved treatments can safely prolong pregnancies or reduce dysregulated sFlt-1 levels in women with PE, making the delivery the sole treatment option, contributing to adverse neonatal outcomes. Thus, identifying a safe therapeutic for PE that can target anti-angiogenic factors implicated in the pathogenesis of PE is a major unmet need for women worldwide. Bioflavonoids are present in many plants, including their fruits and vegetables, and have been well-established for their antioxidant and anti-inflammatory effects (33–35). Regular consumption of bioflavonoids or their sources is associated with a reduced risk of chronic cardiovascular and neurodegenerative diseases (36). While flavonoids, such as quercetin and puerarin, have also been studied for their potential to reduce blood pressure in PE animal models, it is unclear whether any of these compounds are inhibitors of sFlt-1 (37–39). Therefore, the aim of this study was to identify if bioflavonoids are inhibitors of sFlt-1 and determine its mode of action, such as inhibition of the HIF-1α pathway.
MATERIALS AND METHODS
Protocol for obtaining human placentas
Placental tissue was collected from normotensive (NT) or preeclamptic patients delivered at the University of Chicago Medical Center. PE was diagnosed according to the American College of Obstetricians and Gynecologists (ACOG) guidelines (1). Patients with a history of diabetes, chronic hypertension, renal disease, or multiple gestations were excluded from this study. The Institutional Review Board approved using all study-related materials at the University of Chicago (Institutional Review Board No. #14–1532).
Primary cytotrophoblasts isolation and culture for initial screening of natural compounds to inhibit sFlt-1
Primary cytotrophoblast cells (CTBs) were isolated from placentas collected from NT and PE patients and cryopreserved for subsequent culture experiments as described (40). CTBs were cultured in medium 199 (Corning; Cat# 10–060-CV; Manassas, VA) with 5% fetal bovine serum (FBS) (Corning; Cat# 35–015-CV; Manassas, VA) and 1% penicillin-streptomycin (Corning; Cat# 30–002-CI; Manassas, VA). For screening experiments, cells were thawed and plated in a 96-well flat-bottom plate (Microtest 96; Becton Dickinson; Franklin Lakes, NJ). Prior to screening experiments, the medium was removed and replaced with fresh medium with either a drug library (Enzo Life Sciences; Cat# BML-2865; Farmington, NY) compound or vehicle dimethyl sulfoxide (DMSO) (Sigma-Aldrich; Cat# D8418; St. Louis, MO) and incubated in standard culture conditions, with 5% CO2–95% room air (21% O2) at 37°C, for 72 h, as previously described (41). The compounds of the library were provided at a stock concentration of 2mg/mL, which was diluted 1:100 in culture medium for a concentration of 20μg/mL. Additional information about the product library can be seen in Supplemental Table 1. At the end of the experiment, cell culture supernatant was collected for analysis.
Placental villous explant cultures
Placental villous explant tissues were cultured in a complete medium, followed by RNA and protein extraction as described previously (41, 42). Villous biopsies (2 cm3) were excised from the maternal surface, midway between the chorionic and basal plates, within 30 min of delivery, and decidual layers were carefully removed. Tissue was dissected into 0.5 cm3 explants and thoroughly rinsed with phosphate-buffered saline (PBS) to ensure removal of maternal blood and placed in a 24-well flat-bottom plate (Falcon multi-well tissue culture plate; Becton Dickinson) containing 1 mL of conditioned medium 199 for 72 h under standard tissue culture conditions (room air with 5% CO2) or hypoxia (5% CO2, 2% O2, 93% N2) in a humidified cell culture incubator with varying concentrations of luteolin (1, 5,10 μM) (Sigma-Aldrich; Cat# L9283; St. Louis, MO), Akt IV inhibitor (Santa Cruz Biotechnology; Cat# sc-203809; Dallas, TX), LY294002 (Sigma-Aldrich; Cat# 440202; Temecula, CA), or DMSO control. Concentrations of luteolin for these experiments was based on published in vitro experiments (43, 44). After 72 h, the explants were removed, blotted with sterile cotton gauze, and flash-frozen along with corresponding conditioned media for storage at −80°C. Experiments were duplicated on explants from each NT and PE patient.
Enzyme-linked immunosorbent assay (ELISA) for sFlt-1
Collected media was applied to a human VEGF receptor 1 (VEGFR1) Quantikine ELISA Kit (R&D Systems; DVR 100B; Minneapolis, MN, USA) to quantify sFlt-1 secreted by primary trophoblast cells in culture. The manufacturer’s specifications approve of using culture media on this assay, and the instructions were followed. The sensitivity of this ELISA to detect sFlt-1 was reported to be 5 pg/mL, with an intra-assay coefficient of variation of 2.6–3.8% and an inter-assay coefficient of variation of 7.0–8.1%.
Immunoblotting
Tissue from placental explants (described above) were homogenized, and total protein was collected (45, 46). Briefly, protein quantification was assessed using Pierce BCA Protein Assay Kit (ThermoFisher; Cat# 23225; Scientific, Waltham, MA), and equal quantities (50 μg total protein) were resolved on a 4–20% SDS gel and transferred to a nitrocellulose membrane. Western blots of sFlt-1 in culture medium were performed using equal amounts of heparin agarose-enriched medium as previously described (41) and similarly transferred to a nitrocellulose membrane. Membranes were blocked with 5% milk in tris-buffered saline-tween (TBS-T) (0.05% tween) for one hour and incubated with the primary antibodies against phosphorylated (ser473) Akt (Cell Signaling; Cat# 4051S; Danvers, MA), total Akt (Cell Signaling; Cat# 4685; Danvers, MA), HIF-1α (BD Biosciences; Cat# 610958; Sparks, MD), VEGFR1 (sFlt-1) (Abcam; ab32152; Waltham, MA) and β-Actin (BD Biosciences; Cat# 612656; Sparks, MD) at 1:1000 in 1% milk in TBS-T overnight at 4°C. After washing the membrane with TBS-T, goat anti-mouse (ThermoFisher Scientific; Cat# 31430; Waltham, MA) or goat anti-rabbit (ThermoFisher Scientific; Cat# 31460; Waltham, MA) secondary antibodies (1:5000) in 1% milk in TBS-T were added for one hour at room temperature. Proteins were detected using enhanced chemiluminescent reagents and quantified using ImageJ (NIH) to collect densitometry data.
Quantitative real-time PCR
RNA was extracted from placental explants (n = 5) using TRIzol (Invitrogen, Carlsbad, CA) and transcribed into cDNA using a high-capacity cDNA kit (Applied Biosystems, Carlsbad, CA). Reverse transcription–PCR was performed as previously described (47) and using a custom primer (Applied Biosystems; Carlsbad, CA) for sFlt-1 (Forward primer: TCAGAGGTGAGCACTGCAACA; reverse primer: CATTCCTTGTGCTTTTAAATTTGGA) and a commercially available primer for HIF-1α (Catalog# Hs00936371; Life Technologies, Carlsbad, CA). Relative mRNA gene expression was calculated using the 2-ΔΔCt method as described (48).
Statistical Analysis
Data from control DMSO (NT or normoxic) samples were averaged and used for normalization. Data are presented as means ± standard error of the mean (SEM), and analyses were completed using Prism (GraphPad; San Diego, CA). Statistically significant differences between means were assessed by t-test and two-way analysis of variance (ANOVA). The Tukey post-hoc test was applied for ANOVA models. Differences were considered significant at P <0.05.
RESULTS
Natural library screen for sFlt-1 inhibitors
A library of 502 natural compounds, including 11 bioflavonoids, was screened using primary placental CTB cells treated with the compounds (20 μg/mL) or vehicle (DMSO) for 72 h. Several compounds reduced sFlt-1 protein expression released into the medium (Figure 1). Of these, luteolin was the most effective at reducing sFlt-1 expression at 97%, followed by other flavonoids apigenin (89%), naringenin (82%), and hesperetine (79%), relative to the vehicle control. In previous studies, luteolin has been shown to provide protective effects using in vitro, and in vivo models of coronary artery disease, atherosclerosis, and heart failure (49), thus, we focused on luteolin to further investigate its potential in mediating the pathophysiology of PE.
Figure 1: Natural product library to reduce sFlt-1 release from primary placental CTBs.
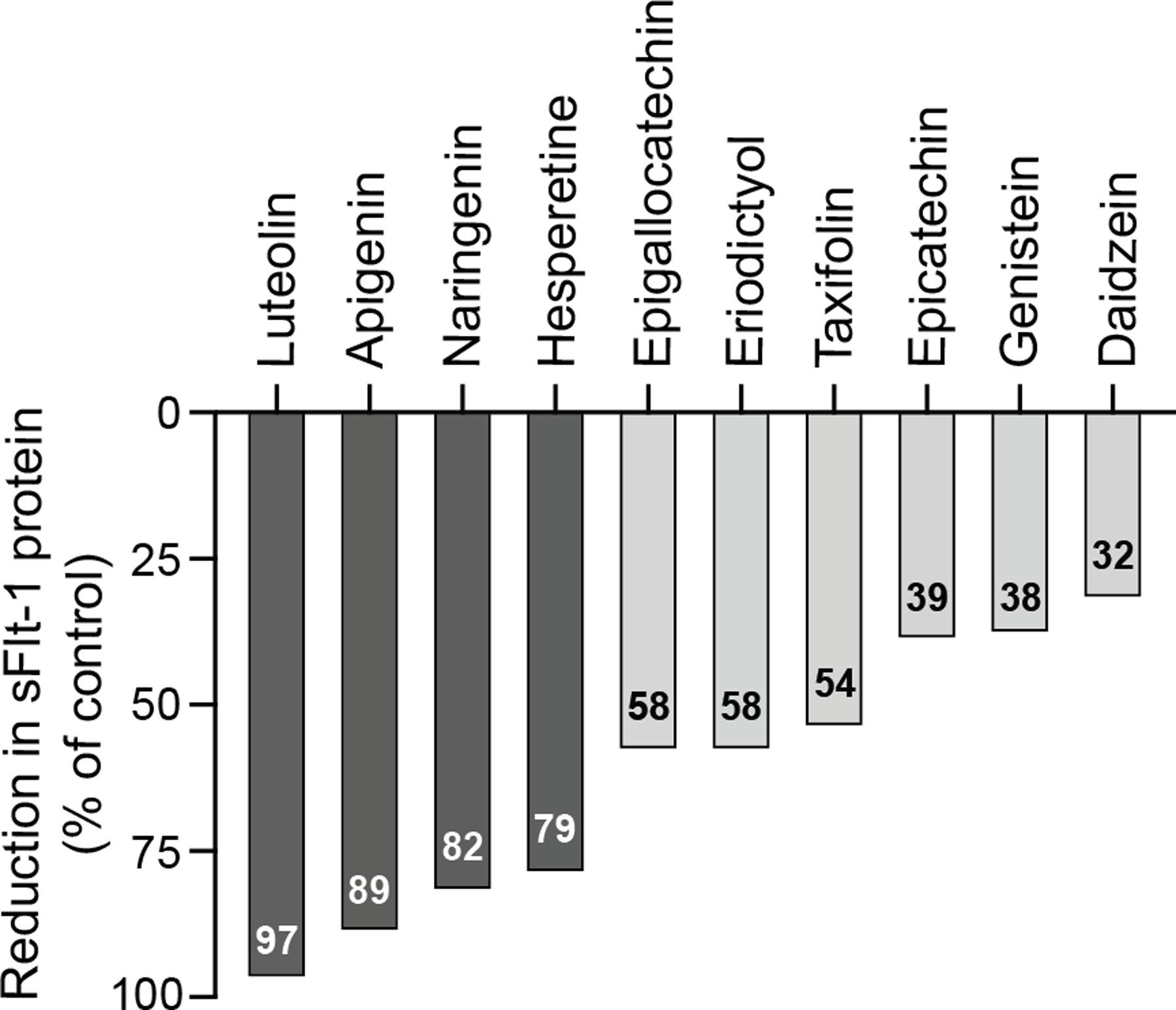
CTBs were isolated from normal placentas and plated in 96-well plates. CTBs were treated with each bioflavonoid at 20 μg/mL (~10–20 μM) or DMSO vehicle for 72 h in normoxia. sFlt-1 protein was quantified in the conditioned medium using ELISA. Data are the percentage of the sFlt-1 reduction compared to the control (vehicle-treated) conditioned medium.
Dose- and time-dependent effects of luteolin on sFlt-1 expression in NT and PE placenta
Placental explants collected from NT and PE patients at delivery were treated with 0–10 μM luteolin for 72 h to determine the dose-response of luteolin in reducing sFlt-1. In both NT and PE samples, luteolin significantly decreased sFlt-1 protein expression at 5 and 10 μM by Western blot (Figure 2A, B). Similarly, a time-course study of placental explants treated with 5 μM luteolin for 0–72 h demonstrated that placental sFlt-1 increases over time in control samples, and this increase is diminished with luteolin treatment after 72 hours (P<0.001; Figure 2C). Further, sFlt-1 secretion in conditioned medium from both NT and PE placental explants was significantly reduced (P<0.0001 and P<0.01, respectively) following luteolin treatment (5 μM, 72 h) compared with control explants as detected by western blot (Figure 2D, E).
Figure 2: Luteolin inhibits sFlt-1 production in placental explants.
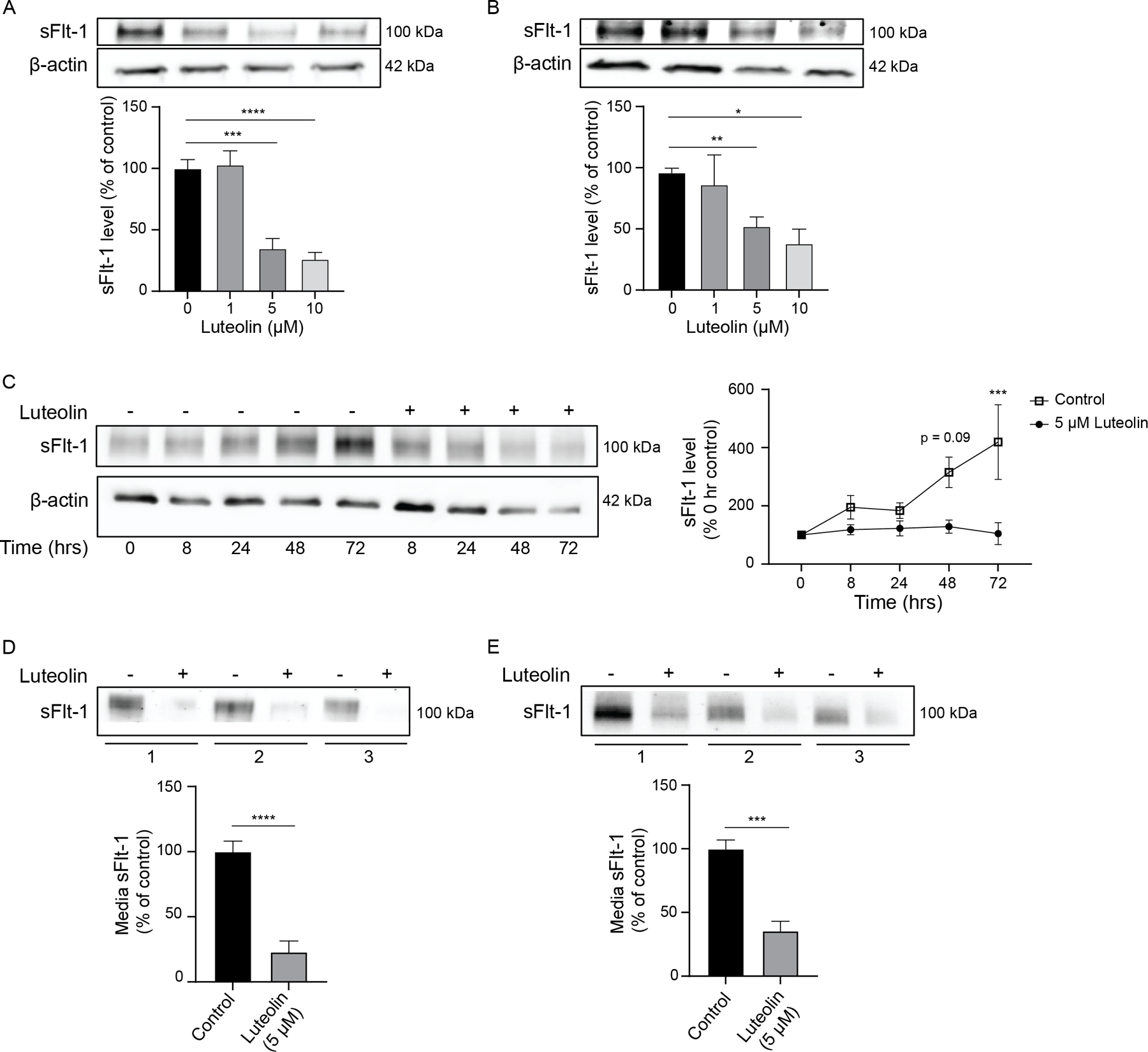
Representative Western blots of placental explants from normotensive (A) and preeclamptic (B) patients treated with luteolin for 72 hours show a dose-dependent decrease in sFlt-1, demonstrating significance with 5–10μM treatment compared to DMSO control. Over time, explants from normotensive patients have significantly increased sFlt-1 production when treated with DMSO control, but levels of sFlt-1 remain unchanged over time when treated with 5μM luteolin (C). Similarly, sFlt-1 released into the media from normotensive (D) and preeclamptic (E) explants are significantly decreased with 5μM luteolin treatment compared to DMSO control. N=6; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Effect of luteolin on sFlt-1 and HIF-1α protein and mRNA expression
Previous work has suggested that sFlt-1 expression is regulated by HIF-1α (23, 50); thus, we evaluated whether the reduction in sFlt-1 expression by luteolin was associated with a decrease in HIF-1α. Placental explants from NT and PE patients were treated with 5 μM luteolin for 72 h. sFlt-1 and HIF-1α protein expression were significantly higher in placental explants from PE patients as compared to tissue from NT patients (P <0.05; Figure 3A), which is consistent with previous work (17, 51). PE placental tissue treated with luteolin had significantly decreased expression in HIF-1α and sFlt-1 (P<0.01) relative to control. NT placental tissue treated with luteolin had significantly reduced HIF-1α expression (P<0.05). Luteolin treatment significantly reduced sFlt-1 mRNA (P<0.05); however, no significant differences were observed in HIF-1α mRNA (P=0.15; Figure 3B). These results suggest that luteolin reduces HIF-1α protein expression but not transcription, thereby decreasing sFlt-1 transcription and protein expression.
Figure 3: Luteolin decreases placental HIF-1α and sFlt-1 expression.
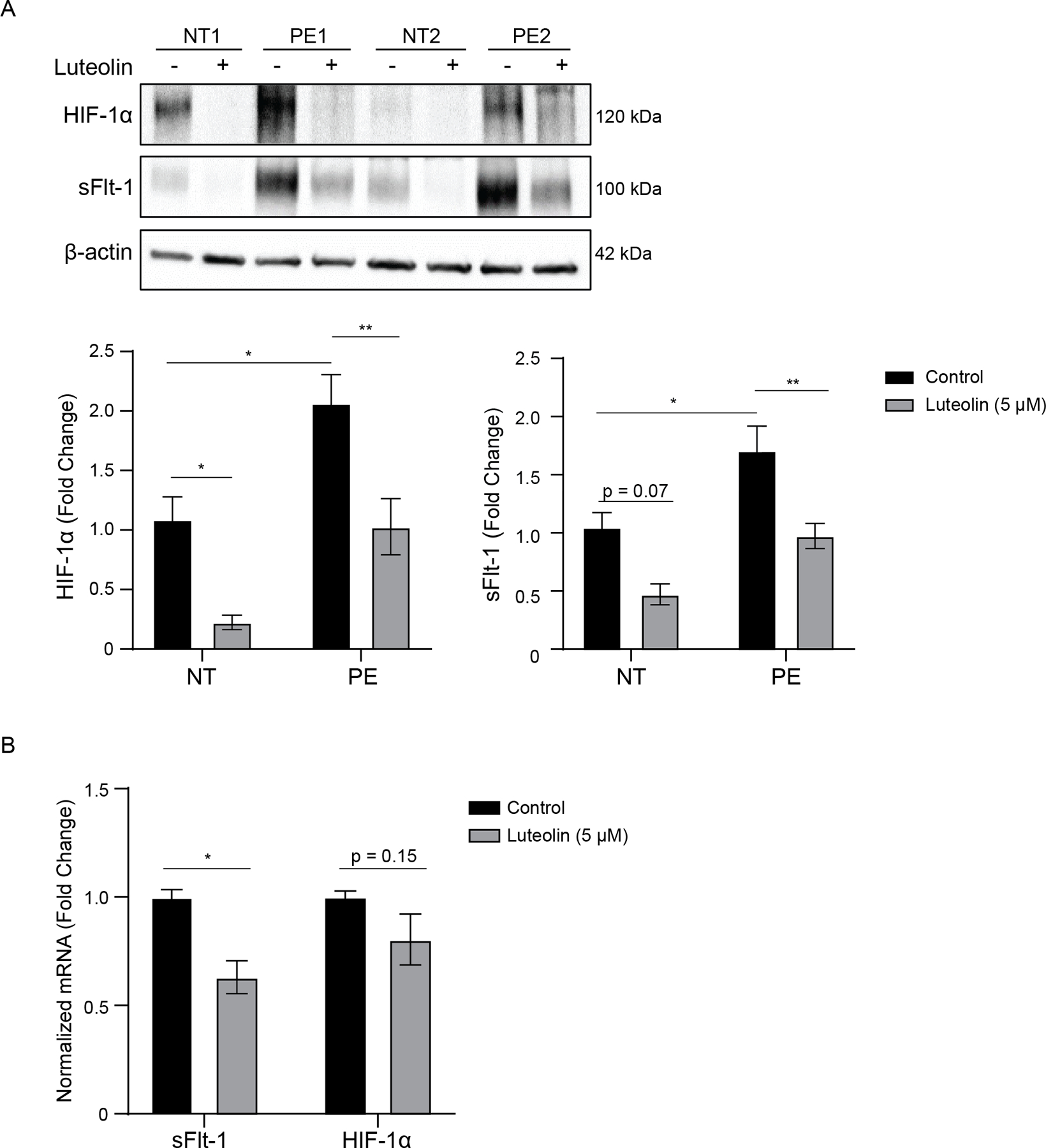
Representative Western blots of placental explants from normotensive (NT) or preeclamptic (PE) patients have significantly reduced levels of HIF-1α expression when treated with 5μM luteolin compared to DMSO control. This decrease in HIF-1α is associated with a near-significant decrease in sFlt-1 in normotensive explants and a significant sFlt-1 decrease in preeclamptic explants (A). RT-PCR of mRNA from NT explants treated with luteolin demonstrates a significant reduction in sFlt-1 expression and a non-significant decrease in HIF-1α (B). N=5; *p<0.05, **p<0.01.
Examining the impact of luteolin on upstream mediators of HIF-1α expression
sFlt-1 regulation may occur through upstream regulation by mTOR and downstream effector, p70S6K, which is mediated upstream by Akt signaling and epidermal growth factor receptor (EGFR) (52). Luteolin treatment (5 μM, 72 h) had a non-significant trend to decrease phospho-Akt (Ser473) expression in both NT and PE explants (p=0.07 and p=0.053, respectively; Figure 4). To further evaluate this pathway, we assessed HIF-1α expression after treatment with Akt inhibitors and luteolin. We utilized placental explants from NT patients in normoxia (21% O2) and hypoxia (2% O2) to mimic the conditions of PE, and increased HIF-1α expression in hypoxia was confirmed in all experiments. Decreased HIF-1α protein expression was observed with treatment using a direct Akt inhibitor (5μM) compared with control (P<0.05; Figure 5A). The Akt inhibitor combined with luteolin demonstrated a reduction in HIF-1α similar to 10μM luteolin, suggesting that luteolin is potentially capable of inhibiting this pathway through additional mechanisms. Similarly, treatment with an inhibitor of upstream phosphoinositide 3 kinase (PI3K) (LY294002, 10 and 50μM) resulted in significantly decreased HIF-1α expression compared with control under hypoxic conditions (P<0.01 and P<0.001, respectively; Figure 5B). No changes were observed in normoxic samples.
Figure 4: The effect of luteolin on phospho-Akt and total Akt expression.
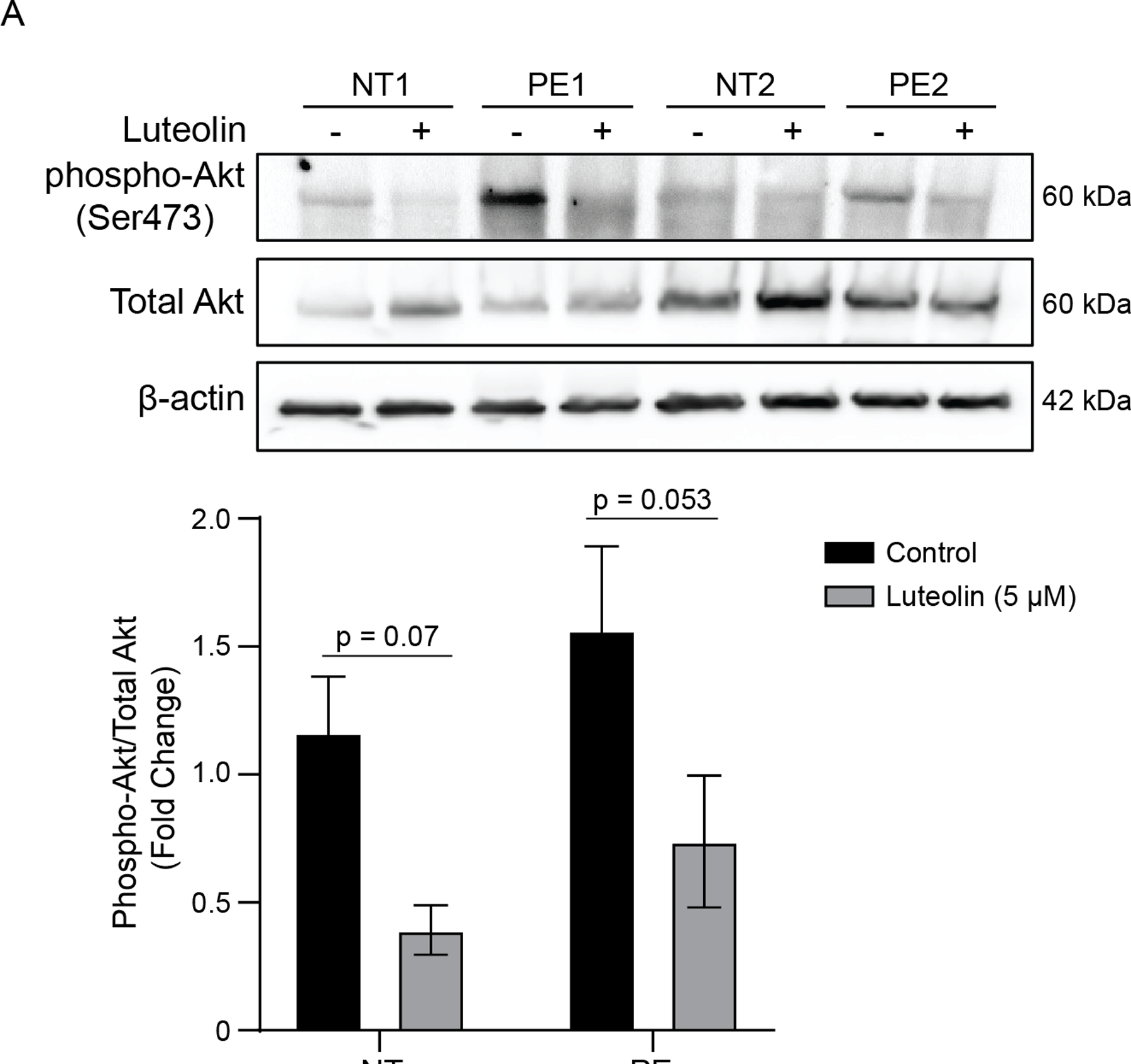
Representative Western blots of placental explants from normotensive and preeclamptic patients treated with luteolin show a near-significant decrease in the ratio of Phospho-Akt to total Akt, suggesting that this pathway may be relevant in the inhibition of HIF-1α and sFlt-1. N=5.
Figure 5: Luteolin decreases HIF-1α through Akt Pathway.
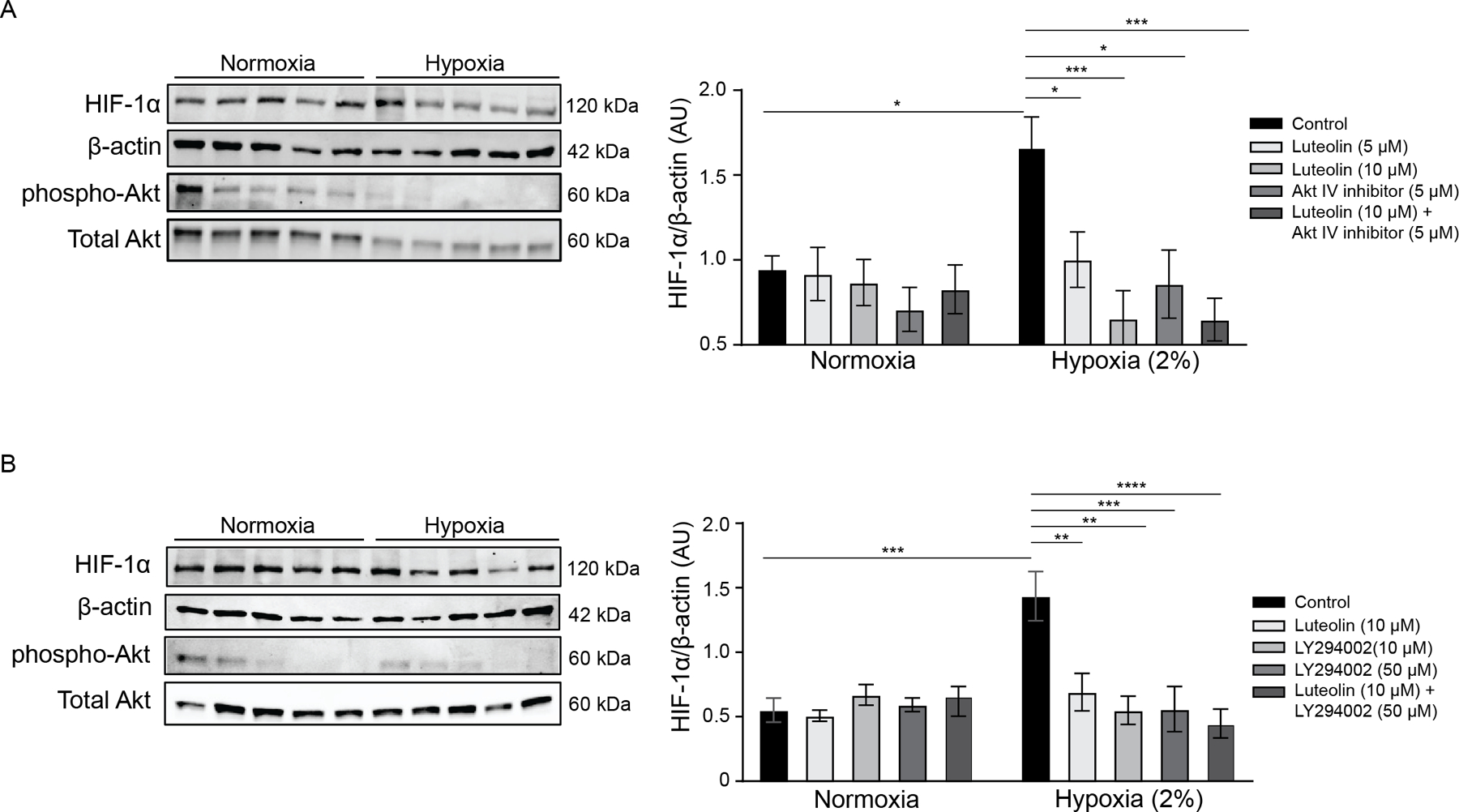
Representative Western blots of explants from normotensive patients were treated in normoxic and hypoxic conditions with luteolin, Akt Inhibitor IV, or a combination of the two (A). We also compared luteolin treatment with PI3K inhibitor LY294002 or a combination of the two (B). We confirmed that phospho-Akt was decreased as a result of the treatment, and HIF-1α expression was measured and normalized to β-Actin (samples in blots are in the same order as listed in the graph). As expected, there was a significant increase in HIF-1α in hypoxia. However, treatment with luteolin, Akt inhibitor, and both concentrations of LY294002 resulted in a significant decrease of HIF-1α expression in hypoxia. Of note, samples in blots are in the same order as listed in the figures, and separate blots were used for the measurement of phospho-Akt and total Akt. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; N=5.
DISCUSSION
In this study exploring novel compounds to target sFlt-1 production in PE, we report several key findings: 1) we identified that luteolin, a naturally occurring bioflavonoid, can reduce sFlt-1 protein levels, 2) luteolin also regulates HIF-1α expression, and 3) luteolin decreases HIF-1α through the Akt pathway. Given the evidence that sFlt-1 plays a key role in PE pathogenesis and that inhibition of sFlt-1 has been shown to improve PE symptoms and signs in human pregnancies, our data suggest that luteolin can potentially be used as a therapy for PE.
PE is a multi-system disease, and in this study, we aimed to see how luteolin would impact the factors leading to endothelial and vascular dysfunction in PE. Increased activation of HIF-1α leading to inappropriate upregulation of sFlt-1 is a common pathway observed in PE (23, 50). Increased sFlt-1 antagonizes VEGF and PlGF, creating angiogenic imbalance, causing maternal hypertension and end-organ damage (17). Reduction of sFlt-1 from maternal circulation by plasmapheresis resulted in decreased blood pressure and proteinuria and extended human pregnancy before delivery (31). These findings highlight the importance of targeting the sFlt-1 pathway. However, apheresis is an expensive and invasive procedure that requires extensive expertise and increases the risk for complications, such as uncontrolled bleeding and infection, thus limiting its utility in most clinical settings. Although other strategies, such as siRNA, are being developed as a therapy for PE (53), natural compounds offer an advantage in their inexpensive and stable capsule formulations. Upon screening over 500 natural compounds to reduce sFlt-1 expression, we identified luteolin as having the most robust inhibition, prompting our study.
Luteolin is a natural bioflavonoid found in many plants, fruits, vegetables, teas, and herbs and is thus consumed regularly (54). It has been shown to protect against reactive oxygen species, restoring normal nitric oxide production and mitochondrial function (55), as well as nuclear factor-κB-mediated inflammation (56). Several flavonoids have been studied in pathological settings, including cardiovascular disease and hypertension (44, 57, 58), infections (54, 59), and mitigating the negative effects of chemotherapies (60, 61). Given the broad spectrum of beneficial effects that flavonoids have been found to exert, they have also been recently studied for the potential treatment of PE (37–39).
In placentas from both NT and PE pregnancies, we evaluated the ability of luteolin to prevent sFlt-1 expression under various conditions. Luteolin significantly attenuated sFlt-1 production at 5μM and 10μM, which is consistent with previously reported in vitro doses of luteolin (43, 44). Although the half-life of luteolin is approximately 5 hours in vivo (62), we observed that luteolin inhibited sFlt-1 production over time and significantly inhibited sFlt-1 after 72 hours, suggesting that the mechanisms of luteolin degradation are not present in human placental tissue in vitro. Because sFlt-1 production has been linked to HIF-1α expression (50), we also assessed whether this was a pathway through which luteolin acted. Placental tissue from NT and PE patients had significantly decreased HIF-1α expression with luteolin. Several pathways upregulate HIF-1α, including Akt (63), and our study shows decreased phospho-Akt in placental samples treated with luteolin, suggesting that luteolin inhibits this pathway.
Utilizing NT placentas, we examined the effect of Akt pathway inhibitors compared to luteolin treatment in normoxia and hypoxia, allowing us to examine samples from the same tissue under conditions mimicking NT and PE, respectively. Treatment with either luteolin or Akt IV inhibitor significantly decreased HIF-1α expression. Luteolin’s ability to reduce HIF-1α expression more than the Akt IV inhibitor under hypoxia suggests that luteolin potentially inhibits HIF-1α through additional pathways. Similar reductions in HIF-1α were observed when treated with the upstream PI3K inhibitor, LY294002. In addition to the PI3K/ Akt pathway, luteolin may inhibit HIF-1α expression through alternate mechanisms. Our group previously showed that ouabain inhibits HIF-1α expression by preventing its stabilization by heat shock proteins (41). Along with pathways promoting the degradation of HIF-1α, these are potential mechanisms whereby luteolin might regulate the expression of HIF-1α, which will be explored further in future experiments.
Our current studies are limited to luteolin treatment in vitro to determine the effects on the anti-angiogenic protein sFlt-1 and its regulators. Luteolin has shown promise in reducing reactive oxygen species and inflammation. Still, its ability to mitigate these factors in PE has not been explored and will be a focus of future experiments. Although we have previously shown that luteolin causes vasodilation of uterine arteries in pregnant rats (64), in vivo animal studies are ongoing to evaluate the effect of luteolin on sFlt-1 production, uteroplacental perfusion, blood pressure, and safety in pregnancy. In summary, these experiments provide compelling evidence that luteolin is a potent inhibitor of sFlt-1 production and secretion in the human placenta.
CONCLUSION
Bioflavonoids, such as luteolin, are readily available and inexpensive compounds and represent an exciting potential therapeutic for PE. Here, we observed significant decreases in sFlt-1, HIF-1α, and phospho-Akt with luteolin treatment in human placental explants, suggesting promise for its use. However, future studies are necessary to determine the ability of luteolin to target these pathways and reduce PE symptoms in preclinical animal models.
Supplementary Material
ACKNOWLEDGMENTS
This study was primarily funded by SR (NIH/NHLBI: 1R56HL157579-01).
JPG is funded through several NIH awards (NIH/NIGMS: U54GM115428; NIH/NHLBI: T32HL105324; NIH/ NIGMS: 1P20 GM104357; NIH/NHLBI: R01HL148191). FTS is funded through NIH/NHLBI R00130577; NIH/NIGMS P20 GM121334; Industry: AWD-001111.
We want to acknowledge Dr. Abir Mukherjee for his contributions in conducting experiments, and Dr. Ernst Lengyel for use of laboratory space and equipment.
ABBREVIATIONS
- ANOVA
Analysis of variance
- CTB
Cytotrophoblast
- DMSO
Dimethyl sulfoxide
- ELISA
Enzyme linked immunosorbent assay
- EGFR
Epidermal growth factor receptor
- FBS
Fetal bovine serum
- HIF-1α
Hypoxia-inducible factor-1α
- Hsp
Heat shock protein
- mTOR
Mammalian target of rapamycin
- NT
Normotensive
- p70S6K
p70S6 kinase
- PE
Preeclampsia
- phospho
Phosphorylated
- PlGF
Placental growth factor
- sFlt-1
Soluble fms-like tyrosine kinase 1
- SEM
Standard error of the mean
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
Footnotes
CONFLICT OF INTEREST - SR reports serving as a consultant for Roche Diagnostics, Thermo Fisher, Beckman Coulter, Siemens and has received research funding from Roche Diagnostics and Siemens for work related to angiogenic biomarkers unrelated to this work. All other authors have no conflict of interest.
DATA AVAILABILITY
The data that support the findings of this study are available in the Methods section of this article.
REFERENCES
- 1.(2019)ACOG Practice Bulletin No. 202 Summary: Gestational Hypertension and Preeclampsia. Obstet. Gynecol 133, 211–214 [DOI] [PubMed] [Google Scholar]
- 2.Betrán AP, Wojdyla D, Posner SF, and Gülmezoglu AM (2005) National estimates for maternal mortality: an analysis based on the WHO systematic review of maternal mortality and morbidity. BMC Public Health 5, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, Lopez AD, Lozano R, and Murray CJ (2010) Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 375, 1609–1623 [DOI] [PubMed] [Google Scholar]
- 4.Wanderer JP, Leffert LR, Mhyre JM, Kuklina EV, Callaghan WM, and Bateman BT (2013) Epidemiology of obstetric-related ICU admissions in Maryland: 1999–2008*. Crit Care Med 41, 1844–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuklina EV, Ayala C, and Callaghan WM (2009) Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 113, 1299–1306 [DOI] [PubMed] [Google Scholar]
- 6.Prevention, C. f. D. C. a. (2018) Pregnancy Mortality Surveillance System [Google Scholar]
- 7.Rana S, Lemoine E, Granger J, and Karumanchi SA (2019) Preeclampsia. Circ. Res 124, 1094–1112 [DOI] [PubMed] [Google Scholar]
- 8.Bartsch E, Medcalf KE, Park AL, and Ray JG (2016) Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 353, i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutinho T, Lamai O, and Nerenberg K (2018) Hypertensive Disorders of Pregnancy and Cardiovascular Diseases: Current Knowledge and Future Directions. Curr Treat Options Cardiovasc Med 20, 56. [DOI] [PubMed] [Google Scholar]
- 10.Tooher J, Thornton C, Makris A, Ogle R, Korda A, and Hennessy A (2017) All Hypertensive Disorders of Pregnancy Increase the Risk of Future Cardiovascular Disease. Hypertension 70, 798–803 [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand AM, Hladunewich MA, and Garg AX (2017) Preeclampsia and the Long-term Risk of Kidney Failure. Am J Kidney Dis 69, 487–488 [DOI] [PubMed] [Google Scholar]
- 12.Gluckman PD, and Hanson MA (2004) The developmental origins of the metabolic syndrome. Trends Endocrinol Metab 15, 183–187 [DOI] [PubMed] [Google Scholar]
- 13.Osmond C, Kajantie E, Forsén TJ, Eriksson JG, and Barker DJ (2007) Infant growth and stroke in adult life. Stroke 38, 264–270 [DOI] [PubMed] [Google Scholar]
- 14.Redman CW, and Sargent IL (2005) Latest advances in understanding preeclampsia. Science 308, 1592–1594 [DOI] [PubMed] [Google Scholar]
- 15.Romero R, and Chaiworapongsa T (2013) Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest 123, 2775–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, and Karumanchi SA (2004) Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350, 672–683 [DOI] [PubMed] [Google Scholar]
- 17.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, and Karumanchi SA (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111, 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, and Karumanchi SA (2012) Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 125, 911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, and Taketani Y (2003) Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 88, 2348–2351 [DOI] [PubMed] [Google Scholar]
- 20.Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, and Granger JP (2009) Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens 22, 564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, and Saade GR (2007) The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol 196, 396 e391–397; discussion 396 e397 [DOI] [PubMed] [Google Scholar]
- 22.Mateus J, Bytautiene E, Lu F, Tamayo E, Betancourt A, Hankins G, M ML, and Saade G (2011) Endothelial growth factor therapy improves preeclampsia-like manifestations in a murine model induced by overexpression of sVEGFR-1. Am J Physiol Heart Circ Physiol 301, 1781–1788 [DOI] [PubMed] [Google Scholar]
- 23.Rajakumar A, Brandon HM, Daftary A, Ness R, and Conrad KP (2004) Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 25, 763–769 [DOI] [PubMed] [Google Scholar]
- 24.Yousefzadeh Y, Soltani-Zangbar MS, Kalafi L, Tarbiat A, Shahmohammadi Farid S, Aghebati-Maleki L, Parhizkar F, Danaii S, Taghavi S, Jadidi-Niaragh F, Samadi Kafil H, Mahmoodpoor A, Ahmadian Heris J, Hojjat-Farsangi M, and Yousefi M (2022) Evaluation of CD39, CD73, HIF-1α, and their related miRNAs expression in decidua of preeclampsia cases compared to healthy pregnant women. Mol Biol Rep 49, 10183–10193 [DOI] [PubMed] [Google Scholar]
- 25.Albers RE, Kaufman MR, Natale BV, Keoni C, Kulkarni-Datar K, Min S, Williams CR, Natale DRC, and Brown TL (2019) Trophoblast-Specific Expression of Hif-1α Results in Preeclampsia-Like Symptoms and Fetal Growth Restriction. Sci Rep 9, 2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tal R, Shaish A, Barshack I, Polak-Charcon S, Afek A, Volkov A, Feldman B, Avivi C, and Harats D (2010) Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: possible implications for preeclampsia and intrauterine growth restriction. Am J Pathol 177, 2950–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad S, and Ahmed A (2004) Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95, 884–891 [DOI] [PubMed] [Google Scholar]
- 28.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves A, Grone HJ, Ahmed A, and Weich HA (2010) Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med 14, 1857–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Zhang Y, Ma JY, Kapoun AM, Shao Q, Kerr I, Lam A, O’Young G, Sannajust F, and Stathis P (2007) Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50, 686–692 [DOI] [PubMed] [Google Scholar]
- 30.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, and Okabe M (2011) Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A 108, 1451–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, Wenger J, Lucchesi KJ, Tamez H, Lindner T, Fridman A, Thome U, Kribs A, Danner M, Hamacher S, Mallmann P, Stepan H, and Benzing T (2016) Removal of Soluble Fms-Like Tyrosine Kinase-1 by Dextran Sulfate Apheresis in Preeclampsia. J Am Soc Nephrol 27, 903–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, Jank A, Kribs A, Cornely OA, Kreyssig C, Hemphill L, Rigby AC, Khedkar S, Lindner TH, Mallmann P, Stepan H, Karumanchi SA, and Benzing T (2011) Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 124, 940–950 [DOI] [PubMed] [Google Scholar]
- 33.Aziz N, Kim MY, and Cho JY (2018) Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol 225, 342–358 [DOI] [PubMed] [Google Scholar]
- 34.Lin Y, Shi R, Wang X, and Shen H-M (2008) Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 8, 634–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu JF, Ma Y, Wang Y, Du ZY, Shen JK, and Peng HL (2011) Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stress. Phytother. Res 25, 588–596 [DOI] [PubMed] [Google Scholar]
- 36.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, and Etherton TD (2002) Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med 113 Suppl 9B, 71s–88s [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Yin L, Si Y, Zhang C, Meng Y, and Yang W (2020) The bioflavonoid quercetin improves pathophysiology in a rat model of preeclampsia. Biomed Pharmacother 127, 110122. [DOI] [PubMed] [Google Scholar]
- 38.Sun X, Zhang S, and Song H (2020) Quercetin attenuates reduced uterine perfusion pressure -induced hypertension in pregnant rats through regulation of endothelin-1 and endothelin-1 type A receptor. Lipids Health Dis 19, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang X, Liu Y, Chen L, and Chen S (2021) The natural compound puerarin alleviates inflammation and apoptosis in experimental cell and rat preeclampsia models. Int Immunopharmacol 99, 108001. [DOI] [PubMed] [Google Scholar]
- 40.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, and Strauss JF 3rd. (1986) Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118, 1567–1582 [DOI] [PubMed] [Google Scholar]
- 41.Rana S, Rajakumar A, Geahchan C, Salahuddin S, Cerdeira AS, Burke SD, George EM, Granger JP, and Karumanchi SA (2014) Ouabain inhibits placental sFlt1 production by repressing HSP27-dependent HIF-1alpha pathway. FASEB J 28, 4324–4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajakumar A, Doty K, Daftary A, Harger G, and Conrad KP (2003) Impaired oxygen-dependent reduction of HIF-1alpha and −2alpha proteins in pre-eclamptic placentae. Placenta 24, 199–208 [DOI] [PubMed] [Google Scholar]
- 43.Seo Y, Ryu K, Park J, Jeon DK, Jo S, Lee HK, and Namkung W (2017) Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLoS One 12, e0174935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su J, Xu H-T, Yu J-J, Gao J-L, Lei J, Yin Q-S, Li B, Pang M-X, Su M-X, and Mi W-J (2015) Luteolin ameliorates hypertensive vascular remodeling through inhibiting the proliferation and migration of vascular smooth muscle cells. Evidence-Based Complementary and Alternative Medicine 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, and Lengyel E (2011) Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med 17, 1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenny HA, Kaur S, Coussens LM, and Lengyel E (2008) The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J. Clin. Invest 118, 1367–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, and Lengyel E (2008) Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res 68, 2329–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shell S, Park SM, Radjabi AR, Schickel R, Kistner EO, Jewell DA, Feig C, Lengyel E, and Peter ME (2007) Let-7 expression defines two differentiation stages of cancer. Proc. Natl. Acad. Sci. U. S. A 104, 11400–11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo Y, Shang P, and Li D (2017) Luteolin: A Flavonoid that Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol 8, 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S, and Caniggia I (2006) Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. American Journal of Physiology-Regulatory, Integrative and Comparative Physiolog y 291, R1085–R1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korkes HA, De Oliveira L, Sass N, Salahuddin S, Karumanchi SA, and Rajakumar A (2017) Relationship between hypoxia and downstream pathogenic pathways in preeclampsia. Hypertens Pregnancy 36, 145–150 [DOI] [PubMed] [Google Scholar]
- 52.Knuth A, Liu L, Nielsen H, Merril D, Torry DS, and Arroyo JA (2015) Placenta growth factor induces invasion and activates p70 during rapamycin treatment in trophoblast cells. Am. J. Reprod. Immunol 73, 330–340 [DOI] [PubMed] [Google Scholar]
- 53.Turanov AA, Lo A, Hassler MR, Makris A, Ashar-Patel A, Alterman JF, Coles AH, Haraszti RA, Roux L, Godinho B, Echeverria D, Pears S, Iliopoulos J, Shanmugalingam R, Ogle R, Zsengeller ZK, Hennessy A, Karumanchi SA, Moore MJ, and Khvorova A (2018) RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat Biotechnol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rungsung S, Singh TU, Rabha DJ, Kumar T, Cholenahalli Lingaraju M, Parida S, Paul A, Sahoo M, and Kumar D (2018) Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine 110, 333–343 [DOI] [PubMed] [Google Scholar]
- 55.Chen HI, Hu WS, Hung MY, Ou HC, Huang SH, Hsu PT, Day CH, Lin KH, Viswanadha VP, Kuo WW, and Huang CY (2020) Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr Metab Cardiovasc Dis 30, 1032–1043 [DOI] [PubMed] [Google Scholar]
- 56.Dong J, Xu O, Wang J, Shan C, and Ren X (2021) Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-κB pathway in allergic rhinitis rats. Immunopharmacol Immunotoxicol 43, 319–327 [DOI] [PubMed] [Google Scholar]
- 57.Oyagbemi AA, Omobowale TO, Ola-Davies OE, Asenuga ER, Ajibade TO, Adejumobi OA, Afolabi JM, Ogunpolu BS, Falayi OO, Saba AB, Adedapo AA, and Yakubu MA (2018) Luteolin-mediated Kim-1/NF-kB/Nrf2 signaling pathways protects sodium fluoride-induced hypertension and cardiovascular complications. Biofactors 44, 518–531 [DOI] [PubMed] [Google Scholar]
- 58.Wu YT, Chen L, Tan ZB, Fan HJ, Xie LP, Zhang WT, Chen HM, Li J, Liu B, and Zhou YC (2018) Luteolin Inhibits Vascular Smooth Muscle Cell Proliferation and Migration by Inhibiting TGFBR1 Signaling. Front Pharmacol 9, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang ZH, Yang HX, Jin Q, Wu YL, Cui ZY, Shang Y, Liu J, Zhan ZY, Lian LH, and Nan JX (2021) Luteolin attenuates hepatic injury in septic mice by regulating P2X7R-based HMGB1 release. Food Funct 12, 10714–10727 [DOI] [PubMed] [Google Scholar]
- 60.Kang KP, Park SK, Kim DH, Sung MJ, Jung YJ, Lee AS, Lee JE, Ramkumar KM, Lee S, Park MH, Roh SG, and Kim W (2011) Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol Dial Transplant 26, 814–822 [DOI] [PubMed] [Google Scholar]
- 61.Boeing T, Speca S, de Souza P, Mena AM, Bertin B, Desreumax P, Mota da Silva L, Faloni de Andrade S, and Dubuqoy L (2022) The PPARγ-dependent effect of flavonoid luteolin against damage induced by the chemotherapeutic irinotecan in human intestinal cells. Chem Biol Interact 351, 109712. [DOI] [PubMed] [Google Scholar]
- 62.Luo Y, Shang P, and Li D (2017) Luteolin: A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Frontiers in Pharmacology 8, 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, and Vogt PK (2001) Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ 12, 363–369 [PubMed] [Google Scholar]
- 64.Yang W, Li Q, Duncan JW, Bakrania BA, Bradshaw JL, Granger JP, Rana S, and Spradley FT (2021) Luteolin-induced vasorelaxation in uterine arteries from normal pregnant rats. Pregnancy Hypertens 23, 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in the Methods section of this article.


