Abstract
The colon mucus layer is organized with an inner colon mucus layer that is impenetrable to bacteria and an outer mucus layer that is expanded to allow microbiota colonization. A major component of mucus is MUC2, a glycoprotein that is extensively decorated especially with O-glycans. In the intestine, goblet cells are specialized in controlling glycosylation and making mucus. Some microbiota members are known to encode multiple proteins predicted to bind and/or cleave mucin glycans. The interactions between commensal microbiota and host mucins drive intestinal colonization, while at the same time the microbiota can utilize the glycans on mucins and affect the colonic mucus properties. This review will examine this interaction between commensal microbes and intestinal mucins and discuss how this interplay impacts health and disease.
In Brief
This review discusses how commensal bacteria can be selected by and how they can utilize the complex O-glycans of the colonic mucins while kept at a distance by the inner mucus layer. This interplay is discussed in relation to normal health and disease.
Introduction
The human gastrointestinal tract is extensively colonized by a diverse microbial community composed of ~1013 bacteria with an important role for human health.1 The commensal microbiota have co-evolved with the host to confer resistance to pathogens.2 These bacteria are also required for immune system development, the synthesis of vitamins and degradation of complex carbohydrates available in food where it provides up to 10% of food energy consumed.3,6 Complex glycans in dietary fibers are only accessible for bacterial degradation and thus host diet significantly impacts in the composition and function of the microbiota.7, 8 Human populations consuming fiber rich diets have higher microbiota diversity, relative to Western populations where the intake of fiber is lower.9, 10 In mice models with a low fiber diet, this decrease in diversity is associated with the extinction of bacterial taxa over multiple generations.11 Generally, fiber rich diets associate with a higher abundance of Prevotella spp., while a protein and fat rich diet (low fiber) enriches Bacteroides spp.9, 12 These shifts in microbiota composition reflect the metabolic capacity of the microbiota.13 In the absence of dietary fibers, some bacteria utilize host glycans, such as, the O-glycans decorating the MUC2 mucin, the main component of colonic mucus.14 Interestingly, changing to a low fiber diet that alters the microbiota composition and mucus barrier function, has been associated with multiple diseases such as cancer, obesity, and inflammatory bowel diseases (IBD).14, 15
The discovery that colonic bacteria are separated from epithelial cells by an inner mucus layer and the advent of next-generation DNA-sequencing of complex bacterial communities has helped to drive the current interest in intestinal bacteria and their role in health and disease.16, 17 The commensal colonic bacteria colonizes the outer and surface of the inner mucus layer, the primary site of interaction between bacteria and host. In the small intestine, the bacteria stay short periods due to peristalsis and, although the mucus is penetrable, the bacteria are kept away from the epithelial cells by continuous mucus secretion and antibacterial peptides.18 The intestinal mucus is built around the highly glycosylated MUC2 mucin. Some commensal bacteria are specialized in binding and degrading glycans on these mucins.19 In fact, bacteria approaching the intestinal surface from the lumen will largely encounter a landscape made up of only glycans that are likely to have a key role in microbial intestinal colonization. Despite the ability of some commensal bacteria to utilize mucin O-glycans, in a healthy steady-state status the balance between bacterial degradation and the turn-over of mucins is kept tightly controlled providing the ideal environment for the microbiota to thrive while having access to a huge range of available dietary and host glycans. This review will focus on commensal bacteria and their interaction and utilization of the mucins and their glycans.
Organization of intestinal mucus
The major building block of colonic mucus is the MUC2 mucin, a glycoprotein with about 5,200 amino acids and around 80% glycans, mostly O-glycans. The MUC2 protein has three von Willebrand D domains (VWD), a central proline-threonine-serine rich (PTS) sequence interrupted by two CysD domains and a C-terminal with one VWD and 3.5 von Willebrand C (VWC) domains followed by a cystine-knot (CK) domain (Figure 1).20 The MUC2 gene is localized in a gene cluster together with the other three gel-forming mucins on the short arm of chromosome 11. Due to the repetitive nature of the MUC2 central PTS sequence, there are only a few assembled MUC2 genes available, all showing variable sequences.21 The central PTS sequence is encoded in a single large exon, made up of 98-105 imperfect repeats of 23 amino acids. The hydroxy amino acids (threonine and serine) are typically O-glycosylated to form mucin domains that form long, extended, stiff rods. A MUC2 mucin monomer will contain up to 1,600 O-glycans and 30 N-glycans giving more than 3,300 terminal sugar residues, a massive glycan array for commensal bacteria to interact with and utilize.
Figure 1.

The main building block of intestinal mucus and bacterial source of O-glycans, the MUC2 mucin.
The CK-domain of MUC2 forms a dimer via disulfide bonds between three cysteine amino acids using the endoplasmic reticulum machinery. The N-terminus is kept separate while the dimer passes the Golgi apparatus and becomes fully O-glycosylated. In the trans-Golgi network, MUC2 is sorted to secretory vesicles and condensed due to decreased pH. During this process, the third VWD forms two disulfide bonds to an N-terminal dimer.22 In addition, trimeric forms have been observed.23 During processing in goblet cells (GC), additional cross-links are formed by the sidechains on glutamine and lysine amino acid, called isopeptide bonds.24 The appearance of these bonds coincides with MUC2 mucin becoming insoluble in water and in larger polymers in chaotropic salts.25, 26
MUC2 mucin is sorted to and stored in the large granules of the GC together with other major mucus proteins like FCGBP, CLCA1, and ZG16. In the specialized GC found at the colon surface, the intercrypt goblet cells (icGC), the mucus is only stored for a short period in small granules and is continuously secreted27. The crypt GCs in both the colon and small intestine have relatively large storage granules. In the small intestine, the mucus granules are emptied by a novel secretory mechanism, the expanding secretion. In this type of secretion, the mucus granules expand and rupture inside the cell and the slightly expanded mucus fills up the apical part of the GC until its apical membrane ruptures.28 These processes are controlled by intracellular Ca2+-levels and interestingly these GCs are dependent on a functional Cystic fibrosis transmembrane conductance regulator (CFTR) channel. Well out in the crypt lumen, more salt and liquid are provided by adjacent epithelial cells to allow further expansion, while a mucus plug effectively clears the small intestinal crypt from the bottom. In the colon one specialist GC called the sentinel goblet cell (senGC) guards the crypt entrance.29 The senGC is activated by increased levels of ligands for TLR receptors, especially TLR4, when bacteria come close to the colonic crypts openings. By activating a ring of GCs at the top of the crypt, the senGC coordinate formation of a mucus plume that clears the bacteria from the crypt opening. In conclusion, both the small and large intestine have efficient, although different, methods for keeping the crypts free from bacteria.
The colon inner mucus layer is formed by fusing the mucus plumes from the crypt opening GC and the surface icGC mucus (Figure 2).27 These two mucus types are initially separated, but quickly combine to form the stratified and attached inner mucus layer. This layer is self-organized and further stabilized by the action of transglutaminase 3 (TGM3) that forms covalent isopeptide bonds between MUC2 glycoproteins.30 When studied without external pressure, the inner colon mucus layer is around 50 μm in mice and 200 μm in humans.31 Typically, this layer is not penetrable to bacteria or beads the size of bacteria. Interestingly, the penetrability of this inner mucus layer is highly dependent of the microbiota as this varies with bacterial composition.32 The normal inner colon mucus layer acts as a filter and is impenetrable to non-pathogenic bacteria (Figure 2).16 Therefore, this mucus layer is instrumental for separating luminal bacteria from the host-epithelium creating a barrier that prevents the bacterial contact with and activation of the immune system.
Figure 2. The mucus layers of small and large intestine.
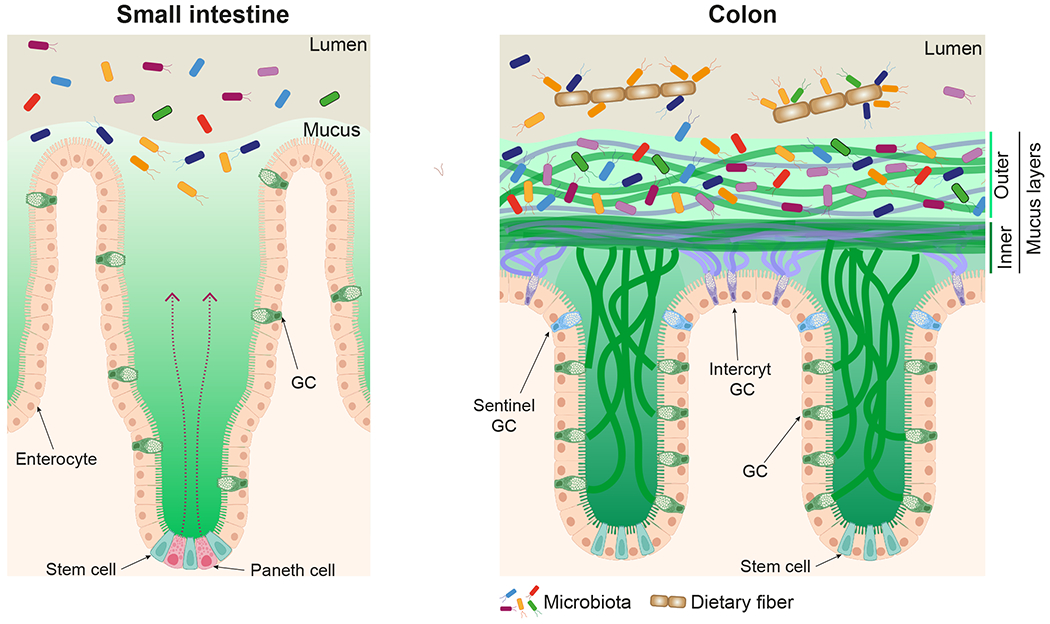
Anti-bacterial compounds secreted by the Paneth cells together with the mucus keep bacteria from the epithelium in the small intestine. The inner mucus layer of colon is formed by mucins and other molecules from the icGC and crypt GC to generate a bacterial barrier. The commensal bacteria live and utilize the mucins of the outer colon mucus layer. Created with BioRender.com
The colon outer mucus layer is formed from the inner mucus layer by still unknown mechanisms that include host-controlled proteolytic processes. These mechanisms cause the mucus to detach and become movable. Importantly the mucus network expansion allows bacteria to enter and colonize this environment, allowing commensals to start utilizing the mucin glycans. As will be discussed in detail, specific bacteria can sequentially release and utilize monosaccharides or short oligosaccharides to obtain energy to thrive in the intestine.
The small intestine has only one type of mucus that is non-attached and readily penetrable by bacteria as it is not as dense and well-structured as in colon (Figure 2).18 This may be caused by the different anatomical organization where the mucus is secreted in different directions, but could be due to differently assembled and cross-linked MUC2 mucins or by the presence of additional unknown mucus accessory proteins. Despite this single penetrable mucus layer, the bacteria are kept at distance and do not reach the epithelial cells. This is accomplished by the secretion of antimicrobial peptides by the Paneth cells and mucus from the GCs, continuously renewing the mucus. The milieu in the mucus, for example pH, is controlled by the host and relatively independent of the luminal milieu.33 The small intestinal mucus has a diffuse outer surface where the non-attached mucus will follow intestinal peristalsis.
Goblet cell – specialized machine for mucin glycosylation
Mucins are biosynthesized, glycosylated, and stored in GC. As mucins and other components of the mucus are characterized by their glycans, the glycosylation machinery within the GC is very important. The N-glycan core is added in the endoplasmic reticulum. On the other hand, O-glycans are built by adding one monosaccharide at a time by specific glycosyltransferases in the Golgi apparatus. In order to achieve a controlled sequential extension of the glycans, the glycosyltransferases are geographically arranged according to the order of monosaccharide addition (Figure 3A).34 The O-glycosylation is initiated by one of the twenty peptidyl-acetylgalactosamine transferases that add N-acetylgalactosamine (GalNAc) to the serine or threonine amino acid residues in the MUC2 PTS sequences.35 These transferases have different specificities, but require unfolded protein regions and are most active in the neighborhood of proline amino acids making the mucin PTS sequences ideal targets. A few of these enzymes also require adjacent, already attached GalNAc further showing their specialization for mucin glycosylation. Next, the addition of galactose (Gal) or GlcNAc to the C-3 or C-6 of the peptide-bound GalNAc generate one of the main core structures, called Core1-Core4.36 While passing through the Golgi apparatus (Figure 3A), these cores will be elongated by the addition of Gal and GlcNAc residues (Figure 1). Importantly, the order in which the glycans meet the different glycosyltransferases has a critical role in O-glycosylation since specific monosaccharides, such as fucose (Fuc) or N-acetylneuraminic acid (NeuAc), cap and limit further elongation. An interesting difference is found in the human colon that has an early active ST6-sialyltransferase adding NeuAc to the C-6 of the peptide-bound GalNAc, a main O-glycan type in the human colon.36 The outcome of the Golgi glycosylation is basically dependent on the glycosyltransferases expressed in each cell explaining the high variability between different cells, organs, and species. As glycan biosynthesis is sequential, the activity of the individual glycosyltransferases will affect multiple glycans. Additionally, the transferase mRNA expression levels are not the only control level, as this alone cannot explain the often fast and dynamic glycosylation alterations observed.
Figure 3. The goblet cell mucin biosynthesis and glycosylation.
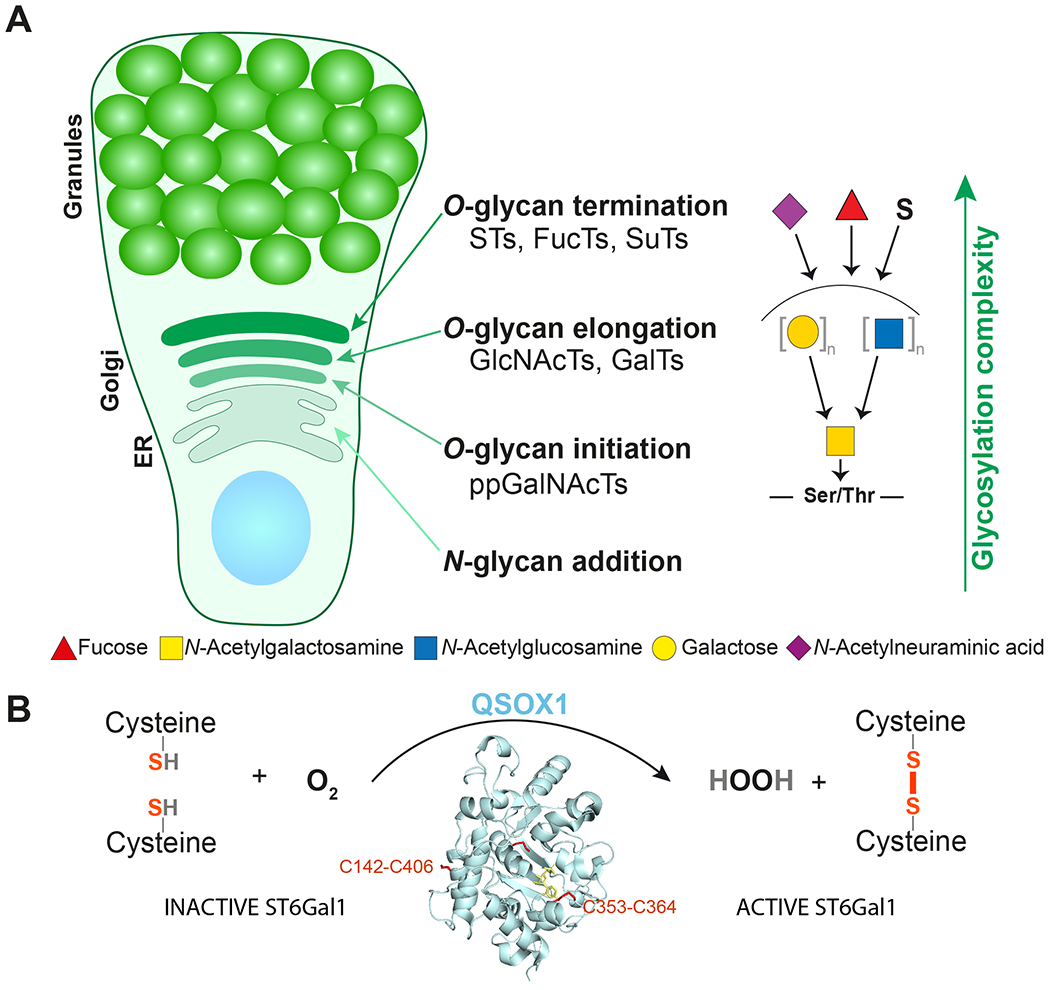
(A) Goblet cell with organization of the glycosylation machinery.
(B) QSOX1 activates sialyltransferase ST6Gal1 by oxidation of cysteines forming two disulfide bonds.
It was previously observed that the glycan structural outcome changed when the pH gradient over the Golgi apparatus and secretory pathway was altered or eliminated.37 The glycosyltransferase localization changed as a decreased pH gradient moved the transferases forward in the Golgi stack. More recently, Kellokumpu and coworkers38 revealed that hypoxia inhibited terminal sialylation. They further observed that hypoxia was accompanied by the loss of two surface disulfide bonds in a terminal sialyltransferase enzyme, α-2,6-sialyltransferase (ST6Gal1). Reduction of these disulfide bonds or removal by mutation made the enzyme inactive. Recently, Ilani et al. showed that the Golgi-localized oxido-reductase QSOX1 was required to reduce the two disulfide bonds in ST6Gal1 and that mice lacking QSOX1 had lower sialylation of MUC2 mucin.39 Thus, an active QSOX1 is required for ST6Gal1 sialyltransferase activity. Interestingly, QSOX1 uses molecular oxygen to generate disulfide bonds with the release of hydrogen peroxide (Figure 3B). This suggests that QSOX1 can sense oxygen tension and by this control and regulate specific glycosyltransferase activities.
Glycan analyses of colonic mucins reveal that there is less sialylation and more sulfation of the MUC2 glycans towards the distal end of the large intestine in both mouse and humans.40, 41 However, this is not reflected in the levels of glycosyltransferases as the protein levels of both sialyl- and sulfotransferases increase in the distal direction42. The luminal colon has an oxygen free atmosphere, a milieu important to maintain and control a healthy anaerobic microbiota. This raises the possibility that decreased sialylation allows more sulfation in the distal colon as a reflection of the anaerobic colon lumen as sensed by QSOX1. This might reflect that the host requires mechanisms to control the bacterial composition required for an anaerobic milieu.
Commensal bacteria are mucin-utilizers and O-glycan degraders
The commensal non-pathogenic microbiota colonize the colonic outer mucus layer where they can access all the glycans available on the MUC2 mucin. Mucins are decorated with complex O-glycans that protect this glycoprotein from being easily degraded. The repertoire of glycan degrading enzymes of the commensal microbiota allows them to cleave and utilize mucin O-glycans as an energy source. Therefore, commensal bacteria should be described as O-glycan degraders or mucin-utilizers, the latter term not necessarily meaning that the bacteria can metabolize the glycans. This terminology should be preferred instead of the mucus or mucin degraders, since mucin degrader implies that the commensals can breakdown the mucin network and, if this is the case, these bacteria should cause the collapse of the colonic mucus barrier. It is important to point out that commensals, like Akkermansia muciniphila (A. muciniphila) and Bacteroides thetaiotaomicron (B. theta), have glycoproteases that can degrade the protein core of the mucin domains with shortened O-glycans, but it remains unclear if any of these proteases can cleave intact MUC2. On the other hand, pathogens have proteases able to efficiently degrade and dissolve the protective colon inner mucus layer by degrading the MUC2 mucin polymer. For example, the enterotoxigenic Escherichia coli EatA protease and the homologue from Shigella have been shown to quickly breakdown human MUC2 mucin.43 Therefore, pathogens can be described as true mucin degraders due their ability to breakdown the mucin polymeric network causing the collapse of the mucus structure to allow bacterial tissue invasion. In contrast, commensal bacteria have evolved to utilize mucins by degrading O-glycans without disrupting the inner mucus layer barrier.
It is unlikely that any single commensal bacterium encodes all of the required enzymes to fully degrade all O-glycan structures since this could lead to complete degradation of the protective colonic mucus layer. Complete degradation of mucin O-glycans is likely achieved through stepwise removal of one monosaccharide or short oligosaccharide at a time. This sequential degradation likely takes time, allowing the inner mucus layer to renew. The renewal time for the inner mucus layer has been estimated to be one hour in mice.44 Understanding the dynamic balance between mucus renewal and commensal bacteria glycan utilization will be most important for understanding the symbiotic relation between commensal bacteria and host.
The role of microbiota in promoting a protective colonic mucus barrier
The microbiota community has a key role in the development and maintenance of a healthy mucus layer. In contrast to colonized mice, germ-free animals have an attached small intestinal mucus layer. During the first weeks after colonization, there is a shift in bacteria from dominance by Firmicutes as in the donor microbes to Bacteroidetes. When the mucus is normalized and becomes detached after 3-4 weeks, the bacterial composition reverts to the normal dominance by Firmicutes. In the colon, germ-free mice have an inner and outer mucus layers. However, in contrast to colonized mice, the inner mucus layer of germ-free mice in penetrable to beads the size of bacteria.45 Colonizing germ-free mice with bacteria leads to the development of an impenetrable inner mucus layer, a process that can take up to seven weeks. Interestingly, this microbiota protective capacity is dependent on the bacterial composition as the mucus penetrability recapitulates that observed in the donor mice.32 The importance of the microbiota composition in the development of an impenetrable inner colonic mucus is illustrated in wild mice that have an inner mucus layer thicker and denser than experimental animals32. Additionally, it is important to point out that mucus is >95% water and its properties can only be estimated on live tissue, best using an ex vivo explant system.27 It is common that researchers measure mucus thickness on fixed tissue sections. This approach has multiple limitations as the observed mucus is mixed with mucus from the proximal intestine and tissue fixation leads to artefacts such as mucus shrinking.16, 46 The mucus layer in fixed tissues will thus not represent the in vivo mucus situation. However, fixed mucus thickness indirectly reflects the mucus properties as a more penetrable mucus shrinks more and becomes thinner on tissue sections, but thinner mucus layer does not imply that the commensal bacteria have degraded the mucins of the inner mucus layer.
The mechanisms for how the bacteria influence the development of the impenetrable colonic mucus layer remain largely unknown, although it is likely to depend of the microbiota signaling to the host using small molecules. Such molecules are often amino acid metabolites, that in some cases are known to have distant effects, for example the brain.47,49 Increased amounts of mucus provide an improved energy source for the bacteria and guarantee an ideal environment for bacterial colonization at the same time as an impenetrable barrier keeps the host protected. The production of highly glycosylated mucins represents a major energy burden for the host, although this provides an ideal environment for the microbiota that will supply the host with energy in the form of short fatty acids.
The bacterial composition is also determined by diet. A fiber-rich diet contributes to the selection of a microbiota community that can access the complex plant cell wall polysaccharides. On the other hand, a low-fiber diet leads to a decrease in fiber degrading bacteria and an expansion of mucin-utilizing bacteria.50 The shift in microbiota by Western-diet (high fat, low fiber) is associated with alterations in the inner colon mucus layer that become more penetrable to bacteria-sized beads after only 3-7 days.51 This is associated with a decrease in mucus growth rate and decrease in thickness of the inner mucus layer, all measured ex-vivo on live tissue. Interestingly, the alterations in mucus-growth rate can be reverted by the probiotic Bifidobacterium longum and the mucus penetrability by the prebiotic inulin.51, 52 Overtime, the host partly compensates the alterations in the mucus barrier properties, but the increased penetrability remains. Diet alterations also have an impact on the small intestine where Western-type diet leads to alterations in the jejunal mucus and disturbs this essential microbiota habitat.53 The shifts in diet lead to a fast alteration of the microbiota community. As mucus production is controlled by the host, alterations in bacterial metabolism associated with increased utilization of mucin O-glycans likely affect bacterial signaling to the host, which leads to alterations in the inner mucus layer penetrability. However, despite clear evidence that diet has a key role in shaping the microbiota composition and metabolism it remains unclear how these key factors for the symbiotic interdependences determine the host mucus barrier status.
Glycans - a key factor that shapes intestinal colonization
The host mechanisms for selecting its normal commensal microbiota is poorly understood, although early experiments with bacterial transfer between different species show strong host selective effects.54 In both the small and large intestine, the exposed surface is almost exclusively glycans, which varies between species arguing that glycans play a major role in the bacterial selection process. In the mucus, the highly glycosylated mucins present multiple O-glycan epitopes that can act as attachment points for commensal bacteria. Some microbiota members express proteins that recognize O-glycans and these interactions are likely to have an important role in colonization. Indeed, alterations in O-glycosylation have been associated with changes in microbiota composition. Mice deficient of C1galtc1, the chaperone required for adding Gal to the GalNAc of the protein backbone, lack the main Core1/Core2 glycan structures found in mouse mucin O-glycans.52, 55 Microbiota composition analysis have shown that C1galtc1 knock-out mice have an increase in Bacteroidetes and a decrease in Firmicutes.56 Additionally, the lack of active fucosyltransferase 2 (FUT2), as in human non-secretors, results in absence of terminal α1,2-fucosylation and alterations in microbiota composition in mice and humans.57,59 Together these studies point to an active role of mucin glycans in defining bacterial composition.
Intestinal colonization is initiated at birth, which likely has a major role in determining the composition of the adult microbiota. In breastfed infants, the human milk oligosaccharides (HMOs) make up to 18% of human milk60 including more than 100 different HMOs glycans.61 Due to the lack of human intestinal enzymes able to cleave the sugar linkages in these complex oligosaccharides, HMOs cannot be degraded or absorbed in the intestinal tract and thus provide a continuous carbon source for the intestinal microbiota. Bacteria known to utilize HMOs, such as Bifidobacterium, dominates the microbiota of breastfed infants.62, 63 However, known mucin O-glycan utilizers associated with adult microbiota, such as, A. muciniphila, B. theta and Bacteroides fragilis (B. fragilis), are also found in the infant intestine.64 These mucin-utilizers are also able to grow on HMOs and utilize the same enzymes for breaking down HMO and mucin O-glycans.65, 66 This is due to the structural similarities between these glycans (Figure 4A). Additionally, the utilization of O-glycans can have a direct impact on colonization. In B. theta, the deletion of five sensors that activate specific mucin utilization loci did not show any defect in competitive colonization in vivo, but this mutant failed to colonize the pups when competing with wild-type bacteria.67 This suggests that in B. theta the transgenerational transmission is dependent on the mucin utilization genes that allow the bacteria to access available host glycans.
Figure 4. Commensal bacteria utilization of host O-glycans.

(A) Human milk oligosaccharides (HMOs) are structurally similar to mucin O-glycans. Some commensal bacteria evolved to utilize HMOs and O-glycans as a carbon source. (B) Schematic representation of five B. theta polysaccharide utilization loci (PULs) upregulated during growth on HMOs and mucin O-glycans.
(C) Representation of the specificity of characterized O-glycan active enzymes. The arrows point to the linkage cleaved by the different enzymes. Glycoside hydrolases, sulfatases, and carbohydrate esterase are shown inside blue, green and gray boxes, respectively. Sugars are shown according to the Symbol Nomenclature for Glycan System. GHXXX, glycoside hydrolase family XXX.
Despite the structural complexity of HMOs, the similarities between these glycans and mucin O-glycans suggests that these host glycans co-evolved to allow infant colonization with mucin-utilizers. After the introduction of solid food, there is a marked shift in microbiota community composition with an increase in Bacteroidetes and Firmicutes and bacteria able to degrade complex fibers and dietary glycans.68, 69 At the same time there is a decrease in Bifidobacterium. Although it is known that diet has a huge impact in shaping the microbiota composition, the colonization of the infant intestine is likely to be determined by key interactions with the host that allow bacterial persistence on the mucus layer and the continuous re-population of the intestinal lumen. At steady state, the mucins O-glycan utilization by the microbiota is limited by the continuous generation of new outer mucus layer formed from the turnover of the inner mucus layer. At the same time, this turnover also provides a continuous source of O-glycans that can be utilized by the gut microbiota.
Importantly, it is likely that the capacity of bacteria to bind to the attached inner mucus layer has a critical role in gut colonization by allowing the bacteria to remain in the colon and avoid removal by peristalsis. The mucin O-glycans located on this outer rim of the colonic inner mucus layer display the controlled and complete glycan structures biosynthesized by the host GCs and are likely important for bacterial selection. The molecular details of this bacterial-mucin interaction remain unexplored, but may include binding to complex glycan surfaces made up by the numerous oligosaccharides arranged in glycan patches on mucin glycosylated PTS domains.
Microbiota-mucin interactions
The commensal bacteria have evolved to stably colonize the colonic mucus. These bacteria encode an enormous repertoire of proteins that allow recognition and utilization of available glycans. A human microbiome with only 177 reference genomes encodes almost 10,000 enzymes dedicated to the utilization of glycans, while the human genome encodes less than 17 such enzymes.70 This indicates that the intestinal bacteria are extremely well adapted to utilize glycans not affected by the host digestive system. Some microbiota members have evolved to utilize a limited number of glycans. One example of such a specialist bacterium is A. muciniphila, which only grows on mucins and HMOs.65, 71 Specialist species encode only a limited number of enzymes that target a specific substrate. Other bacteria are more versatile and able to rely on multiple glycan sources. Such a generalist is B. theta, able to degrade and utilize complex plant cell wall polysaccharides found in our diet as well as different host glycans.67, 72 A generalist species encodes a large repertoire of proteins that adapt the bacteria to utilize different glycans as a carbon source and to access different metabolic niches.70 The genes required to recognize, degrade, take up, and sense a specific glycan are often encoded in specific genomic regions known as polysaccharide utilization loci (PUL) (Figure 4B).69, 73 A single bacterium can encode multiple PULs each targeting complex glycans. B. theta has dedicated 18% of its genome to 88 PULs, of which at least 16 PULs are targeting the utilization of mucin O-glycans.67 Characterization of different PULs targeting plant cell wall polysaccharides and host glycans such as N-glycans and glycosaminoglycans have shown how intestinal bacteria evolved to target available glycans.74,76 However, detailed mechanisms of mucin utilization by commensal bacteria remains largely unexplored.
To recognize and degrade complex glycans, the bacteria express enzymes such as, sulfatases, glycoside hydrolases (GH), carbohydrate esterases (CE) and proteases (Figure 4C). Enzymes can also contain carbohydrate binding modules (CBMs).77 These different proteins are classified in families based on amino acid sequence similarities as in databases such as SulfAtlas (sulfatases) and CAZy (GHs, CE and CBMs).78, 79 Importantly, this classification in families does not consider their specificities and a single family can contain enzymes and binding proteins that target multiple linkages found in different complex glycans. This causes a significant challenge in predicting the function of annotated genes. In the last few years, our understanding of the role of different enzymes and binding proteins in the utilization of dietary and host glycans by the human microbiota has increased.74,76,80,83 However, the rate of protein characterization has not followed the exponential increase in genomic sequences and annotated proteins in metagenomic and metaproteomic datasets.84 Additionally, the study of bacterial proteins that interact with mucin O-glycans is further limited by the complexity of this substrate. Significantly, the lack of structural and organizational insight of O-glycans on the mucin domains and the limited availability of human colon mucins provide significant barriers for understanding microbiota-mucin interactions.
Microbiota binding proteins
The intestinal bacteria encodes multiple binding proteins that can interact with O-glycans.79,85 These interactions can be to single glycan epitopes or more likely, to glycan surfaces formed by the arranged glycans in mucin domains, termed glycan patches (Figure 5). Bacterial binding is likely driven by multiple proteins that recognize the glycosylated mucin domains, allowing for targeted bacterial selection while giving access the host glycans. Binding proteins expressed by pathogenic bacteria have been extensively characterized and shown to play a key role in invasion and disease.86,87 However, the understanding and importance of commensal bacterial binding proteins interacting with mucin domains and O-glycans is more limited (Table 1). An example of such a bacteria is Lactobacilus rhammnosus that encodes mucin binding proteins recognizing epitopes on gastric mucins.88 Adhesins that interact with mucin epitopes, such as binding proteins, flagellum, and pili, have been described for Bifidobacterium and E. coli Nissle (Table 1).89 However, these proteins were characterized in vitro and the glycans recognized by the majority of these adhesins remain unexplored.
Figure 5.
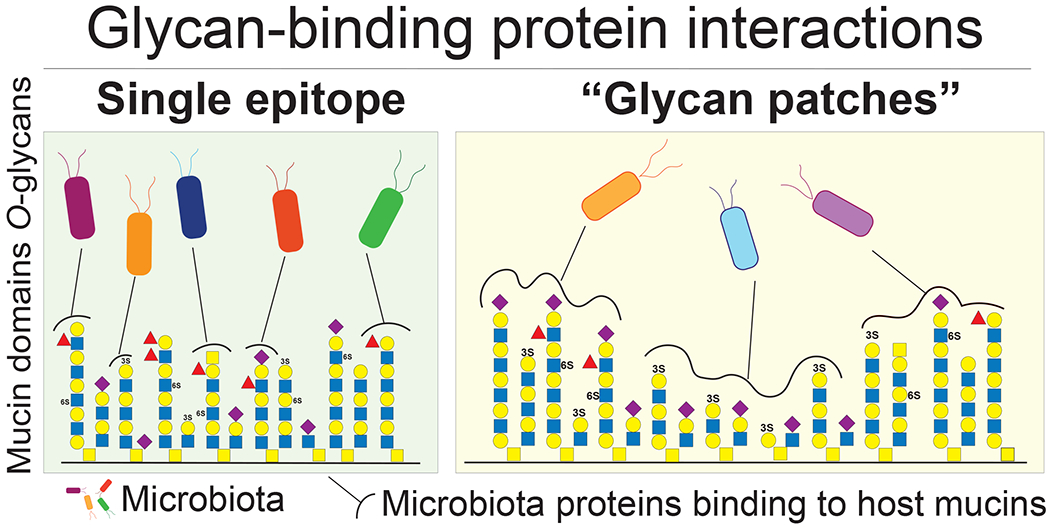
Bacterial binding proteins will recognize single glycan epitopes or more likely mucin glycan patches.
Table 1.
Intestinal microbiota adhesion proteins that bind to mucin-type glycan epitopes
| Commensal bacteria | Proteina | Target substrateb | Glycan epitopec |
|---|---|---|---|
| Bifidobacterium longum BBMN68114 | FimM | Human epithelial cell lines, PGM | |
| Bifidobacterium bifidum ATCC 15696115 | Sialidase (SiaBb2) | PCM, mice colonic tissue sections | α2,6-linked sialic acid, Blood group A |
| Bifidobacterium bifidum JCM 1254116 | CBM32 domain of sulfoglycosidase (BbhII) | In vitro assays, PGM, PCM | N-acetylglucosamine-6-sulfate |
| Bifidobacterium bifidum A8117 | Transaldolase (Tal) | Human intestinal epithelial cell lines, PGM | |
| Bifidobacterium longum NCC2705118 | GroEL, Transaldolase (Tal) | PGM | |
| Bifidobacterium longum subsp.longum119 | Fimbrial protein | PCM | |
| Bifidobacterium longum subsp. Infantis120 | Blon_0343 and Blon_2202 (F1SBPs) | Mammalian glycan array | Blood group type H, 2’-fucosyllactose |
| Blon_0883 (F1SBPs) | Mammalian glycan array | Type 1 glycans, Lewis a, Blood group H type 1 | |
| Blon_2177 (F1SBPs) | Mammalian glycan array | Type 1 glycans | |
| Escherichia coli Nissle 1917 (EcN)121 | flagellum | Human epithelial cell lines, porcine MUC2, human colonic mucus | |
| Lactobacillus fermentum 104R122 | Mucin-binding protein (MucBP) | Porcine small intestinal mucus, PGM | |
| Lactococcus lactis (TIL448)123 | Pilin and mucus-binding protein | PGM | Neutral oligosaccharides |
| Lactobacillus rhamnosus 124 | Pilin | Colonic mucus from colorectal cancer patients | |
| Lactobacillus johnsonii NCC533 (La1)125, 126 | EF-Tu and GroEL | Human intestinal epithelial cell lines, HCM | |
| Lactobacillus paracasei subsp. paracase127 | EF-Tu and GroEL | Micro Arrays | α2,3-linked sialic acid, α1,2-linked fucose |
| Lactobacillus reuteri 1063128 | Mub | Porcine gastric and colonic mucus | |
| Ruminococcus gnavus ATCC 29149129 | CBM40 domain of trans-sialidase (RgNanH) | In vitro glycan arrays, mouse colonic tissue sections and binding assays | α2,3/2,6-sialyllactose |
Only proteins with described binding to mucins or mucus were included in the table.
CBM, carbohydrate binding module; PGM, porcine gastric mucin; PCM, porcine colonic mucin; HCM, human colonic mucus; F1SBPs, Family 1 of solute binding proteins; MUC2, Mucin 2.
When reported, the described target glycans are shown on the column on the Glycan epitope column.
Mucin utilizers, such as B. theta, B. fragilis and A. muciniphilia, are known to upregulate multiple genes encoding putative binding proteins such as CBMs and surface glycan binding proteins (SGBP) in vitro when grown on mucin glycans or in vivo in animals fed a low fiber diet.50, 67, 90 Several of these putative binding proteins are predicted to be located at the bacterial cell surface where they can interact with mucin O-glycans. Although the function of these proteins remains largely unknown, it is likely that bacterial surface binding proteins have a major role in recognizing clustered glycan patches on mucin domains. B. fragilis encodes a commensal colonization factor (ccf) locus containing a putative binding protein (CBM32) that is critical for the colonization of the colonic crypts in gnotobiotic mice.91 The ccf locus is highly expressed in an in vivo context and is critical to the resilient colonization of B. fragilis following antibiotics treatment or infection with Citrobacter rodentium.91 Despite the critical role of the B. fragilis ccf locus in colonization of a specific niche that allows bacterial repopulation, it is unclear which specific mucin glycans or glycan surfaces are targeted. Recently it was shown that in an in vivo context, a high-fat/high-sugar diet promotes a rapid adaptation of B. theta by selecting mutants with an increased ability to utilize mucin O-glycans.92 Interestingly, some of these advantageous mutations were described in a B. theta PUL homologue to the ccf locus described in B. fragilis.92 This suggests that the ccf locus is not only associated with colonization, but may also have a direct impact in mucin utilization. B. theta mutations associated with a fast growth on O-glycans were described in the SusD-like protein, a transmembrane protein implicated in uptake of oligosaccharides into the periplasm. However, the mechanisms behind the B. theta increased utilization of O-glycans in the SusD mutants and why this confers an advantage within the colonic mucus environment is not understood.
Mucins O-glycans – an available carbon source for specific bacteria
Several microbiota members have been described as able to degrade mucin O-glycans.19, 50, 93 To degrade complex mucin O-glycans with hundreds of different glycan structures, the mucin-utilizing bacteria express multiple enzymes that target single, specific linkages.19, 94 Indeed, in Bacteroides there is a strain dependence in utilization of O-glycans with some strains growing better on certain substrates than others.93 This suggests that different mucin-utilizers evolved to target different mucin O-glycans. Additionally, it was recently observed that A. municiphila and Bacteroides massiliensis bacteria, previously described as mucin-utilizers, grow on porcine gastric mucins, but cannot utilize released colonic O-glycans.95 Since mucin O-glycosylation varies along the gastrointestinal tract, one possible explanation is that these bacteria have evolved to target mucin O-glycans commonly found in salivary, lung, or stomach mucins. These are swallowed and transported to the colon without degradation by host digestive enzymes. Supplementing the diet with O-glycans from porcine gastric mucin in vivo led to an increase in A. muciniphila, suggesting that this bacterium is particularly well equipped to degrade gastric mucin O-glycans.96 Since O-glycosylation is also species specific, it remains unclear if A. muciniphila or other microbiota members are able to grow on human mucin O-glycans. Although in a seminal study, A. muciniphila was able to grow on human MUC2 isolated from patients with artificial urinary bladder constructed from the large intestine.97,98 Therefore, it is clear that the capacity of commensal microbiota to degrade and utilize different human mucin O-glycans is very complex and likely very dynamic.
Role of O-glycan-active enzymes in colonization
The O-glycosylation of colonic mucins is highly variable due to the presence of different Core glycans, variable side chain sizes, branches, and multiple capping structures, such as, sialic acid, Fuc, sulfate, and acetyl groups. These capping structures protect the mucin O-glycans from being easily degraded.36, 94 Several bacterial enzymes active on different O-glycan structures have been characterized over the years and it is clear that the human microbiota encodes all the required enzymes to break down mucin O-glycans (Figure 4C).19, 94 Recently, the first endoactive enzymes active on O-glycans were characterized. Interestingly, these enzymes are only active after the sialic acid has been removed, illustrating that capping sugars limit the access of the bacteria to mucin O-glycans.99 The biochemical characterization of O-glycan-active enzymes has often been limited to synthetic aryl substrates or short oligosaccharide substrates that do not reflect the complexity of linkages found in colonic mucins.
Mucin O-glycans can be sulfated on positions C3, C4, and C6 of Gal and C6 of GlcNAc. The sulfation on Gal is always found at the non-reducing end, while sulfation on GlcNAc can be found internally or at the non-reducing end.95 These sulfate groups act as blocking units that prevent the degradation of the mucin O-glycans. However, several microbiota members are known to encode putative sulfatases that can act on these linkages. For example, the A. muciniphila and B. theta genome encode 11 and 28 sulfatases, respectively.78 The sulfatase activity in B. theta was previously shown to be required for the development of colitis in a susceptible animal model.100 Recently, the first sulfatases active on colonic mucin O-glycans were described showing that 11 of 28 B. theta sulfatases can act on all sulfated linkages found on O-glycans.95 B. theta sulfatases have evolved to target sulfated linkages in different glycan contexts, suggesting that this bacterium is well adapted to utilize many different sulfated O-glycans.95,101 Moreover, a single sulfatase that specifically targets 3-sulfated Gal is required for the utilization of porcine colonic O-glycans showing that complex host glycan degradation is dependent of key enzymes.95 In colonization of gnotobiotic mice, the key sulfatase is an important fitness factor in competitive colonization experiments. In this in vivo system, sulfatases targeting GlcNAc-6-sulfate, a common linkage found in the mouse colon, also had an important role in intestinal colonization.95 A key role of sulfatases in in vivo colonization was also recently described in B. fragilis. In this species, a sulfatase, homologue to the B. theta GlcNAc-6-sulfatase, was shown to be an important in vivo fitness factor in the presence of an invading bacteria.102 Therefore, bacterial sulfatases may be important factors to allow access to specific colonization niches within the mucus layer.
Sialylation is a common terminal epitope found on mucins. To cleave sialic acid, bacteria typically encode one or multiple sialidases (neuraminidases) that can be trans-sialidases or hydrolytic enzymes. Trans-sialidase activity, was first described on Ruminococcus gnavus, where the released sialic acid is modified into a 2,7-anhydro product that can be specifically metabolized by this bacterium conferring an in vivo fitness advantage.103, 104 Indeed, the deletion of the R. gnavus trans-sialidase was associated with impaired in vitro growth on sialylated glycans.103 Interestingly, the trans-sialidase allowed R. gnavus to colonize the colonic mucus layer while the mutant lacking this enzyme is unable to access this niche.103 This suggests that R. gnavus deploys a unique strategy to utilize sialylated O-glycans that allows its access to specific colonization niches within the mucus layer.
Hydrolytic sialidases release sialic acid that can be used by the bacteria itself or shared with other microbiota. For example, B. theta encodes a single sialidase active on all linkages found in mucins.105 However, this bacterium lacks the operon encoding the enzymes required for metabolizing sialic acid. Although it remains unclear if sharing sialic acids between different bacteria affects commensal microbiota communities, the free sialic acid can be used by pathogens such as, Salmonella typhimurium and Clostridium difficile leading to an expansion of these species after antibiotic treatment.106 Sialidases are thus key enzymes for commensal bacteria, but if not controlled, increased levels of sialic acids may also promote pathogens.
Bacterial removal of sialic acids is limited by the acetylation of sialic acid, a common substitution found in human colonic mucins.41 Little is known about microbial esterases removing acetyl groups on mucin O-glycans. It has been shown that the growth on mucins by commensal and pathogenic E. coli is dependent on sialic acid acetyl-esterases expressed by B. theta and B. fragilis.107. However, further studies are required to understand the role of esterases in intestinal colonization and O-glycan utilization by commensal bacteria.
The foraging of O-glycan monosaccharides by pathogenic bacteria was previously observed for fucose. B. theta encodes multiple fucosidases of the GH29 and GH95 families that together are predicted to cleave all Fuc linkages found in O-glycans.94 While B. theta can metabolize Fuc, pathogens such as enterohaemorrhagic E. coli can scavenge the released Fuc leading to increased virulence and colonization by this pathogen.108 This highlights that O-glycan degradation by commensals can have key roles for pathogenic bacteria colonization. Less understood is the importance of mucin-utilization in defining the commensal microbiota community in a healthy steady-state. R. gnavus ATCC29149 endo-β1,4-galactosidase activity allows R. gnavus E1 to grow on a blood group A tetrasaccharide, but also on the O-glycans exposed by this enzymatic activity.109 Additionally, co-cultures A. muciniphila fucosidase and sialidase activities are required to promote butyrate production by Clostridia.110 This clearly demonstrates how monosaccharide sharing can affect the gut microbiota composition and metabolism. Together, it is suggested that mucin O-glycan utilization by the commensal microbiota is likely to be initiated by specific bacteria that remove capping groups such as, sulfate and sialic acid, something that allows other bacteria to access and utilize protected O-glycans. However, further understanding of how the microbiota cooperate to degrade O-glycans is required before we better understand the role of mucin utilization for healthy microbiota colonization.
Mucus in disease
Inflammatory bowel diseases are on the rise in industrialized countries implicating the involvement of intestinal bacteria and their interaction with the intestinal mucus. Whereas Crohn’s disease is more genetically influenced by its relation to intracellular handling of bacteria, ulcerative colitis (UC) shows a defined link to the colonic mucus. Early studies showed bacteria in close proximity to the epithelium in UC111, which after the observation of an impenetrable inner mucus layer16, it became obvious that breaches and structural weakening of this layer can initiate UC.112,113 This weakening is the result of increased emptying of stored goblet cell mucus112, misunderstood as ‘goblet cell depletion’, loss of sentinel goblet cells at the crypt opening29, 113, and as recently shown, loss of intercrypt goblet cells at the surface periphery of the crypt opening.27 The critical nature of mucus and its relation to the commensal microbiota as discussed in this review show the need for a deeper understanding that can open the door for specific interventions.
Future perspectives
The commensal microbiota encodes an immense repertoire of binding proteins and enzymes that allow these bacteria to bind and access the variable and complex world of glycans. Some bacteria have evolved to utilize complex mucin O-glycans and others the glycan fibers of the plant world. The O-glycan linkage diversity is overcome by mucin-utilizing bacteria expressing multiple enzymes that work in tandem. Numerous putative mucin O-glycan degrading enzymes have been predicted from genomic sequences, but the characterization of these enzymes remains a challenge. Even less is known about the attachment and selection of commensal bacteria in the intestine, both with regard to the nature of the bacterial adhesins and the organization and surface patterns generated by the dense glycans on the mucin domains. There are presently no tools for revealing the surface structure of such glycan patches, but a combined effort on bacterial adhesins and mucin domains will unravel new mechanisms for bacterial selection. For progress in understanding microbial glycan degradation, it is critical that future studies of enzymes and binding proteins use mucins and bacteria from the same origin.
The separation of bacteria from the host epithelium is now known to be important for a healthy intestine. How the mucus properties that allow this separation is generated and controlled is poorly understood. Little is also known on how the commensal bacteria and their metabolic machinery generate signals that are sensed by the host epithelium. Once we know how such signals are controlled by different bacterial species, bacterial metabolism, and availability of food polysaccharides or host glycans, we will be able to target dysbiosis, IBD and colon cancer development. Furthermore, understanding how different commensal microbiota metabolizes and interact host glycans, will disclose important key elements of commensal microbiota-human mucin symbiotic interactions. This is of highest importance as more and more emerging diseases in the Western world and now also in the developing world can be attributed to intestinal microbiota.
Acknowledgments
This work was supported by the The Knut and Alice Wallenberg Foundation (2017.0028), European Research Council ERC (694181), National Institute of Allergy and Infectious Diseases (U01AI095473) and National Institute of Health (R01 DK125445)-the contents of these are solely the responsibility of the authors and does not necessarily represent the official views of the NIH), Swedish Research Council (2017-00958, 2021-01409), IngaBritt and Arne Lundberg Foundation (2018-0117), Wilhelm and Martina Lundgren’s Foundation, Svenska Sällskapet Medicinsk Forskning (S21-0026), Sahlgrenska Academy International Starting Grant (2021/1070) and Jeanssons Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
REFERENCES
- 1.Sender R, Fuchs S, and Milo R (2016). Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 164, 337–340. [DOI] [PubMed] [Google Scholar]
- 2.Pickard JM, Zeng MY, Caruso R, and Nunez G (2017). Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunological Reviews 279, 70–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caruso R, Lo BC, and Nunez G (2020). Host—microbiota interactions in inflammatory bowel disease. Nat Rev Immunol 20, 411–426. [DOI] [PubMed] [Google Scholar]
- 4.Cheng HY, Ning MX, Chen DK, and Ma WT (2019). Interactions Between the Gut Microbiota and the Host Innate Immune Response Against Pathogens. Frontiers in Immunology 10, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, and Tuohy K. (2018). Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition 57, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNeil NI (1984). The contribution of the large intestine to energy supplies in man. The American Journal of Clinical Nutrition 39, 338–342. [DOI] [PubMed] [Google Scholar]
- 7.Groussin M, Mazel F, Sanders JG, Smillie CS, Lavergne S+, Thuiller W, and Alm EJ. (2017). Unraveling the processes shaping mammalian gut microbiomes over evolutionary time. Nature Communications 8, 14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonz+ílez A, Fontana L, Henrissat B, Knight R, and Gordon JI. (2011). Diet Drives Convergence in Gut Microbiome Functions Across Mammalian Phylogeny and Within Humans. Science 332, 970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, and Lionetti P (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. PNAS 107, 14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP et al. (2012). Human gut microbiome viewed across age and geography. Nature 486, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, and Sonnenburg JL (2016). Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R et al. (2011). Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 334, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens EC, Neumann M, and Desai MS (2018). Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nature Reviews Microbiology 16, 457–470. [DOI] [PubMed] [Google Scholar]
- 15.Turnbaugh PJ, Backhed F, Fulton L, and Gordon JI (2008). Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson MEV, Phillipson M, Petersson J, Holm L, Velcich A, and Hansson GC (2008). The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 105, 15064–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley RE, Lozupone CA, Hamady M, Knight R, and Gordon JI (2008). Worlds within worlds: evolution of the vertebrate gut microbiota. Nature Reviews Microbiology 6, 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ermund A, Schutte A, Johansson MEV, Gustafsson JK, and Hansson GC (2013). Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol. Gastroint. Liver Physiol 305, G341–G347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tailford LE, Crost EH, Kavanaugh D, and Juge N (2015). Mucin glycan foraging in the human gut microbiome. Frontiers in Genetics 6, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallego P, Garcia-Bonete MJ, Trillo-Muyo S, Recktenwald CV, Johansson MEV, and Hansson GC (2023). The intestinal MUC2 mucin C-terminus is stabilized by an extra disulfide bond in comparison to von Willebrand factor and other gel-forming mucins. Nature Communications 14, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svensson F, Lang T, Johansson MEV, and Hansson GC (2018). The central exons of the human MUC2 and MUC6 mucins are highly repetitive and variable in sequence between individuals. Scientific Reports 8, 17503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javitt G, Khmelnitsky L, Albert L, Bigman LS, Elad N, Morgenstern D, Ilani T, Levy Y, Diskin R, and Fass D (2020). Assembly Mechanism of Mucin and von Willebrand Factor Polymers. Cell 183, 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambort D, Johansson MEV, Gustafsson JK, Nilsson H, Ermund A, Johansson BR, Kock P, Hebert H, and Hansson GC (2012). Calcium and pH-dependent Packing and Release of the Gel-forming MUC2 Mucin. Proc. Natl. Acad. Sci. USA 109, 5645–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recktenwald CV and Hansson GC (2016). The reduction-insensitive bonds of the MUC2 mucin are isopeptide bonds. J. Biol. Chem 291, 13580–13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Axelsson MAB, Asker N, and Hansson GC (1998). O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J. Biol. Chem 273, 18864–18870. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann A, Lindell G, Nordman H, and Carlstedt I (1995). The insoluble glycoprotein complex from human colon contains two MUC2 subunits of different size. Biochemical Society Transactions 23, S535. [DOI] [PubMed] [Google Scholar]
- 27.Nystrom EEL, Martinez-Abad B, Arike L, Birchenough GMH, Nonnecke EB, Castillo PA, Svensson F, Bevins CL, Hansson GC, and Johansson MEV (2021). An intercrypt subpopulation of goblet cells are essential for colonic mucus barrier function. Science 372, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolan B, Ermund A, Martinez-Abad B, Johansson MEV, and Hansson GC (2022). Clearance of small intestinal crypts involves goblet cell mucus secretion by intracellular granule rupture and enterocyte ion transport. Sci. Signal 15, eabl5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birchenough GMH, Nystrom ELN, Johansson MEV, and Hansson GC (2016). A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion . Science 352, 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpen JDA, Dolan B, Nystrom ELN, Birchenough GMH, Arike L, Martinez-Abad B, Johansson MEV, Hansson GC, and Recktenwald CV (2022). Transglutaminase 3 crosslinks the secreted MUC2 mucin and stabilizes the colonic mucus layer. Nature Commun. 13, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustafsson JK, Ermund A, Johansson MEV, Schutte A, Hansson GC, and Sjovall H (2012). An ex vivo method for studying mucus formation, properties and thickness in human colonic biopsies and mouse small and large intestinal explants. Am. J. Physiol 302, G430–G438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakobsson HE, Rodriguez-Pineiro AM, Schütte A, Ermund A, Boysen P, Sommer F, Bäckhed F, Hansson GC, and Johansson MEV (2015). The gut microbiota composition impairs the colon inner mucus layer barrier. EMBO Reports 16, 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgetti M, Klymiuk N, Bahr A, Hemmerling M, Jinton L, Tarran R, Malmgren A, Astrand A, Hansson GC, and Ermund A (2021). New generation ENaC inhibitors detach cystic fibrosis airway mucus bundles via sodium/hydrogen exchanger inhibition. Eur. J. Pharmacol 904, 174123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger EG, Warren G, and Nilsson T (1995). Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci 108, 1617–1627. [DOI] [PubMed] [Google Scholar]
- 35.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, and Tabak LA (2012). Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansson GC (2020). Mucins and the Microbiome. Annu. Rev. Biochem 89, 769–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Axelsson MAB, Karlsson NG, Steel D, Ouwendijk J, Nilsson T, and Hansson GC (2001). Neutralization of the pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology 11, 633–644. [DOI] [PubMed] [Google Scholar]
- 38.Hassinen A, Khoder-Agha F, Khosrowabadi E, Mennerich D, Harrus D, Noel M, Dimova EY, Glumoff T, Harduin-Lepers A, Kietzmann T et al. (2019). A Golgi-associated redox switch regulates catalytic activation and cooperative functioning of ST6Gal-I with B4GalT-I. Redox Biology 24, 101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilani T, Reznik N, Yeshaya N, Feldman T, Vilela P, Lansky Z, Javitt G, Shemesh M, Brenner O, Elkis Y et al. (2022). The QSOX1 Disulfide Catalyst Maintains the Colon Mucosal Barrier by Regulating Golgi Glycosyltransferases. EMBO J. 42, e111869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmen-Larsson JM, Thomsson KA, Rodriguez-Pineiro AM, Karlsson H, and Hansson GC (2013). Studies of mucus in mouse stomach, small intestine, and colon. III. Gastrointestinal Muc5ac and Muc2 mucin O-glycan patterns reveal a regiospecific distribution. Am. J. Physiol. Gastroint. Liver Physiol 305, G357–G363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbe C, Capon C, Maes E, Rousset M, Zweibaum A, Zanetta JP, and Michalski JC (2003). Evidence of Regio-specific Glycosylation in Human Intestinal Mucins: Presence of an acidic gradient along the gastrointestinal tract. J. Biol. Chem 278, 46337–46348. [DOI] [PubMed] [Google Scholar]
- 42.van der Post S and Hansson GC (2014). Membrane protein profiling of human colon reveals distinct regional differences. Mol. Cell. Proteomics 13, 2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheikh A, Wangdi T, Vickers TJ, A. B, Palmer M, Miller MJ, Kim S, Herring C, Simoes R, Crainic JA et al. (2022). Enterotoxigenic Escherichia coli degrades the host MUC2 mucin barrier to facilitate critical pathogen-enterocyte interactions in human small intestine. Infection and Immunity 90, e00572–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson MEV (2012). Fast Renewal of the Distal Colonic Mucus Layers by the Surface Goblet Cells as Measured by In Vivo Labeling of Mucin Glycoproteins. PLoS ONE 7, e41009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johansson MEV, Jacobsson HE, Holmén-Larsson J, Schütte A, Ermund A, Rodriguez-Pineiro A, Arike L, Wising C, Svensson F, Bäckhed F et al. (2015). Normalization of the host intestinal mucus systems requires long-term colonization. Cell Host Microbe 18, 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergstrom K, Shan X, Casero D, Batushansky A, Lagishetty V, Jacobs JP, Hoover C, Kondo Y, Shao B, Gao L et al. (2020). Proximal colon–derived O-glycosylated mucus encapsulates and modulates the microbiota. Science 370, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wlodarska M, Luo C, Kolde R, Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA et al. (2017). Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host & Microbe 22, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donia MS and Fischbach MA (2015). Small molecules from the human microbiota. Science 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Needham BD, Funabashi M, Adame MD, Wang Z, Boktor JC, Haney J, Wu WL, Rabut C, Ladinsky MS, Hwang SJ et al. (2022). A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 602, 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A et al. (2016). A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 167, 1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeder BO, Birchenough GMH, Stahlman M, Arike L, Johansson MEV, Hansson GC, and Backhed F (2018). Bifidobacterium or fiber protect against diet-induced deterioration of the inner colonic mucus layer. Cell Host & Microbe 23, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomsson KA, Holmen-Larsson J, Angstrom J, Johansson MEV, Xia L, and Hansson GC (2012). Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology 22, 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birchenough GMH, Schroeder BO, Sharba S, Arike L, Recktenwald CV, Puertolas-Balint F, Subramani MV, Hansson KT, Yilmaz B, Linden SK et al. (2023). Muc2-dependent microbial colonization of the jejunal mucus layer is diet sensitive and confers local resistance to enteric pathogen infection. Cell Reports 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rawls JF, Mahowald MA, Ley RE, and Gordon JI (2006). Reciprocal Gut Microbiota Transplants from Zebrafish and Mice to Germ-free Recipients Reveal Host Habitat Selection. Cell 127, 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu J, Wei B, Wen T, Johansson MEV, Xiaowei L, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M et al. (2011). Loss of intestinal core 1-derived O-glycans causes spontaneous colitis. J. Clin. Invest 121, 1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sommer F, Adam N, Johansson ME, Xia L, Hansson GC, and Backhed F (2014). Altered mucus glycosylation in core 1 o-glycan-deficient mice affects microbiota composition and intestinal architecture. PLoS One. 9, e85254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kashyap PC, Marcobal A, Ursell LK, Smits SA, Sonnenburg ED, Costello EK, Higginbottom SK, Domino SE, Holmes SP, Relman DA et al. (2013). Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc. Natl. Acad. Sci. USA 110, 17059–17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rausch P, Rehman A, Kunzel S, Hasler R, Ott SJ, Schreiber S, Rosenstiel P, Franke A, and Baines JF (2011). Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc. Natl. Acad. Sci. USA 108, 19030–19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tong M, McHardy I, Ruegger P, Goudarzi M, Kashyap PC, Haritunians T, Li X, Graeber TG, Schwager E, Huttenhower C et al. (2014). Reprograming of gut microbiome energy metabolism by the FUT2 Crohns disease risk polymorphism. The ISME Journal 8, 2193–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smilowitz JT, O’sullivan A, Barile D, German JB, L+Ânnerdal B, and Slupsky CM (2013). The Human Milk Metabolome Reveals Diverse Oligosaccharide Profiles. The Journal of Nutrition 143, 1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobata A (2010). Structures and application of oligosaccharides in human milk. Proc. Japan Academy, series B 86, 731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma J, Li Z, Zhang W, Zhang C, Zhang Y, Mei H, Zhuo N, Wang H, Wang L, and Wu D (2020). Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Scientific Reports 10, 15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sela DA and Mills DA (2010). Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol 18, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H et al. (2015). Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 17, 690–703. [DOI] [PubMed] [Google Scholar]
- 65.Kostopoulos I, Elzinga J, Ottman N, Klievink JT, Blijenberg B, Aalvink S, Boeren S, Mank M, Knol J, de Vos WM et al. (2020). Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Scientific Reports 10, 14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marcobal A, Barboza M, Sonnenburg E, Pudlo N, Martens E, Desai P, Lebrilla C, Weimer B, Mills D, German J et al. (2011). Bacteroides in the Infant Gut Consume Milk Oligosaccharides via Mucus-Utilization Pathways. Cell Host & Microbe 10, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martens EC, Chiang HC, and Gordon JI (2008). Mucosal Glycan Foraging Enhances Fitness and Transmission of a Saccharolytic Human Gut Bacterial Symbiont. Cell Host & Microbe 4, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, and Ley RE (2011). Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 108 Suppl. 1, 4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koropatkin NM, Cameron EA, and Martens EC (2012). How glycan metabolism shapes the human gut microbiota. Nature Rev. Microbiol 10, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaoutari AE, Armougom F, Gordon JI, Raoult D, and Henrissat B (2013). The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nature Reviews Microbiology 11, 497–504. [DOI] [PubMed] [Google Scholar]
- 71.Derrien M, Vaughan EE FAU - Plugge C, Plugge CM FAU - de Vos W, and de Vos WM. (2004). Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Biol 54, 1469–1476. [DOI] [PubMed] [Google Scholar]
- 72.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN et al. (2011). Recognition and Degradation of Plant Cell Wall Polysaccharides by Two Human Gut Symbionts. PLOS Biology 9, e1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Terrapon N, Lombard V, Gilbert HJ, and Henrissat B (2015). Automatic prediction of polysaccharide utilization loci in Bacteroidetes species. Bioinformat. 31, 647–655. [DOI] [PubMed] [Google Scholar]
- 74.Briliute J, Urbanowicz PA, Luis AS, Basle A, Paterson N, Rebello O, Hendel J, Ndeh DA, Lowe EC, Martens EC et al. (2019). Complex N-glycan breakdown by gut Bacteroides involves an extensive enzymatic apparatus encoded by multiple co-regulated genetic loci. Nature Microbiology 4, 1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cartmell A, Lowe EC, Basle A, Firbank SJ, Ndeh DA, Murray H, Terrapon N, Lombard V, Henrissat B, Turnbull JE et al. (2017). How members of the human gut microbiota overcome the sulfation problem posed by glycosaminoglycans. Proc. Natl. Acad. Sci. USA 114, 7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ndeh D, Basle A, Strahl H, Yates EA, McClurgg UL, Henrissat B, Terrapon N, and Cartmell A (2020). Metabolism of multiple glycosaminoglycans by Bacteroides thetaiotaomicron is orchestrated by a versatile core genetic locus. Nature Communications 11, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wardman JF, Bains RK, Rahfeld P, and Withers SG (2022). Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nature Reviews Microbiology 20, 542–556. [DOI] [PubMed] [Google Scholar]
- 78.Barbeyron T, Brillet-Gueguen L, Carre W, Carriere C, Caron C, Czjzek M, Hoebeke M, and Michel G (2016). Matching the Diversity of Sulfated Biomolecules: Creation of a Classification Database for Sulfatases Reflecting Their Substrate Specificity. PLoS ONE 11, e0164846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drula E, Garron ML, Dogan S, Lombard V, Henrissat B, and Terrapon N (2022). The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Research 50, D571–D577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rogowski A, Briggs JA, Mortimer JC, Tryfona T, Terrapon N, Lowe EC, Basle A, Morland C, Day AM, Zheng H et al. (2015). Glycan complexity dictates microbial resource allocation in the large intestine. Nature Communications 6, 7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cartmell A, Munoz-Munoz J, Briggs JA, Ndeh DA, Lowe EC, Basle A, Terrapon N, Stott K, Heunis T, Gray J et al. (2018). A surface endogalactanase in Bacteroides thetaiotaomicron confers keystone status for arabinogalactan degradation. Nature Microbiology 3, 1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ndeh D, Rogowski A, Cartmell A, Luis AS, Basle A, Gray J, Venditto I, Briggs J, Zhang X, Labourel A et al. (2017). Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luis AS, Briggs J, Zhang X, Farnell B, Ndeh D, Labourel A, Basle A, Cartmell A, Terrapon N, Stott K et al. (2018). Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nature Microbiology 3, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garron ML and Henrissat B (2019). The continuing expansion of CAZymes and their families. Current Opinion in Chemical Biology 53, 82–87. [DOI] [PubMed] [Google Scholar]
- 85.Ficko-Blean E and Boraston AB (2012). Insights into the recognition of the human glycome by microbial carbohydrate-binding modules. Curr. Opin. Struct. Biol 22, 570–577. [DOI] [PubMed] [Google Scholar]
- 86.Juge N (2012). Microbial adhesins to gastrointestinal mucus. Trends microbiol. 20, 30–39. [DOI] [PubMed] [Google Scholar]
- 87.McGuckin MA, Linden SK, Sutton P, and Florin TH (2011). Mucin dynamics and enteric pathogens. Nat Rev Micro 9, 265–278. [DOI] [PubMed] [Google Scholar]
- 88.Roos S and Jonsson H (2002). A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148, 433–442. [DOI] [PubMed] [Google Scholar]
- 89.Sicard JF, Le Bihan G, Vogeleer P, Jacques M, and Harel J (2017). Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Frontiers in Cellular and Infection Microbiology 7, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pudlo NA, Urs K, Kumar SS, German JB, Mills DA, and Martens EC (2015). Symbiotic Human Gut Bacteria with Variable Metabolic Priorities for Host Mucosal Glycans. mBio 6, e01282–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, and Mazmanian SK (2013). Bacterial colonization factors control specificity and stability of the gut microbiota. Nature 501, 426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dapa T, Ramiro RS, Pedro MF, Gordo I, and Xavier KB (2022). Diet leaves a genetic signature in a keystone member of the gut microbiota. Cell Host & Microbe 30, 183–199. [DOI] [PubMed] [Google Scholar]
- 93.Pudlo NA, Urs K, Crawford R, Pirani A, Atherly T, Jimenez R, Terrapon N, Henrissat B, Peterson D, Ziemer C et al. (2022). Phenotypic and Genomic Diversification in Complex Carbohydrate-Degrading Human Gut Bacteria. mSystems 7, e00947–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raba G and Luis AS (2023). Mucin utilization by gut microbiota: recent advances on characterization of key enzymes. Essays in Biochemistry EBC20220121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luis AS, Jin C, Pereira G, Glowaki R, Gugel S, Sing S, Byrne DP, Pudlo N, London J, Basle A et al. (2021). A single sulfatase is required for metabolism of colonic mucin O-glycans and intestinal colonization by a symbiotic human gut bacterium. Nature 598, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pruss KM, Marcobal A, Southwick AM, Dahan D, Smits SA, Ferreyra JA, Higginbottom SK, Sonnenburg ED, Kashyap PC, Choudhury B et al. (2021). Mucin-derived O-glycans supplemented to diet mitigate diverse microbiota perturbations. The ISME Journal 15, 577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, and Florin THJ (2010). Mucolytic Bacteria With Increased Prevalence in IBD Mucosa AugmentIn VitroUtilization of Mucin by Other Bacteria. Am. J. Gastroenterology 105, 2420–2428. [DOI] [PubMed] [Google Scholar]
- 98.Robbe-Masselot C, Herrmann A, Carlstedt I, Michalski JC, and Capon C (2008). Glycosylation of the two O -glycosylated domains of human MUC2 mucin in patients transposed with artificial urinary bladders constructed from proximal colonic tissue. Glycoconjugate Journal 25, 213–224. [DOI] [PubMed] [Google Scholar]
- 99.Crouch LI, Liberato MV, Urbanowicz PA, Basl+® A, Lamb CA, Stewart CJ, Cooke K, Doona M, Needham S, Brady RR et al. (2020). Prominent members of the human gut microbiota express endo-acting O-glycanases to initiate mucin breakdown. Nature Communications 11, 4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hickey CA, Kuhn KA, Donermeyer DL, Porter NT, Jin C, Cameron EA, Jung H, Kaiko GE, Wegorzewska M, Malvin NP et al. (2015). Colitogenic Bacteroides thetaiotaomicron Antigens Access Host Immune Cells in a Sulfatase-Dependent Manner via Outer Membrane Vesicles. Cell Host Microbe 17, 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luis AS, Basle A, Byrne DP, Wright GSA, London JA, Jin C, Karlsson NG, Hansson GC, Eyers PA, Czjzek M et al. (2022). Sulfated glycan recognition by carbohydrate sulfatases of the human gut microbiota. Nature Chemical Biology 18, 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Donaldson GP, Chou WC, Manson AL, Rogov P, Abeel T, Bochicchio J, Ciulla D, Melnikov A, Ernst PB, Chu H et al. (2020). Spatially distinct physiology of Bacteroides fragilis within the proximal colon of gnotobiotic mice. Nature Microbiology 5, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bell A, Brunt J, Crost E, Vaux L, Nepravishta R, Owen CD, Latousakis D, Xiao A, Li W, Chen X et al. (2019). Elucidation of a sialic acid metabolism pathway in mucus-foraging Ruminococcus gnavus unravels mechanisms of bacterial adaptation to the gut. Nature Microbiology 4, 2393–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tailford LE, Owen CD, Walshaw J, Crost EH, Hardy-Goddard J, Le GG, de Vos WM, Taylor GL, and Juge N (2015). Discovery of intramolecular trans-sialidases in human gut microbiota suggests novel mechanisms of mucosal adaptation. Nat Commun. 6, 7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park KH, Kim MG, Ahn HJ, Lee DH, Kim JH, Kim YW, and Woo EJ (2013). Structural and biochemical characterization of the broad substrate specificity of Bacteroides thetaiotaomicron commensal sialidase. Biochim. Biophys. Acta 1834, 1510–1519. [DOI] [PubMed] [Google Scholar]
- 106.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM et al. (2013). Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502, 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Robinson LS, Lewis WG, and Lewis AL (2017). The sialate O-acetylesterase EstA from gut Bacteroidetes species enables sialidase-mediated cross-species foraging of 9-O-acetylated sialoglycans. J. Biol. Chem 292, 11861–11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, and Sperandio V (2012). Fucose sensing regulates bacterial intestinal colonization. Nature 492, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu H, Crost EH, Owen CD, van Bakel W, Martinez Gascuena A, Latousakis D, Hicks T, Walpole S, Urbanowicz PA, Ndeh D et al. (2021). The human gut symbiont Ruminococcus gnavus shows specificity to blood group A antigen during mucin glycan foraging: Implication for niche colonisation in the gastrointestinal tract. PLOS Biology 19, e3001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shuoker B, Pichler MJ, Jin C, Sakanaka H, Wu H, Gascue+╎a A.M.n., Liu J, Nielsen TS, Holgersson J, Nordberg Karlsson E et al. (2023). Sialidases and fucosidases of Akkermansia muciniphila are crucial for growth on mucin and nutrient sharing with mucus-associated gut bacteria. Nature Communications 14, 1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, and Dorffel Y (2007). Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56, 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johansson MEV, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H et al. (2014). Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and in patients with ulcerative colitis. Gut 213, 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van der Post S, Jabbar KS, Birchenough GMH, Arike L, Akhtar N, Sjovall H, Johansson MEV, and Hansson GC (2019). Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 68, 2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xiong Y, Zhai Z, Lei Y, Xiao B, and Hao Y (2020). A Novel Major Pilin Subunit Protein FimM Is Involved in Adhesion of Bifidobacterium longum BBMN68 to Intestinal Epithelial Cells. Frontiers in Microbiology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishiyama K, Yamamoto Y, Sugiyama M, Takaki T, Urashima T, Fukiya S, Yokota A, Okada N, and Mukai T (2017). Bifidobacterium bifidum Extracellular Sialidase Enhances Adhesion to the Mucosal Surface and Supports Carbohydrate Assimilation. mBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Katoh T, Yamada C, Wallace MD, Yoshida A, Gotoh A, Arai M, Maeshibu T, Kashima T, Hagenbeek A, Ojima MN et al. (2023). A bacterial sulfoglycosidase highlights mucin O-glycan breakdown in the gut ecosystem. Nature Chemical Biology. [DOI] [PubMed] [Google Scholar]
- 117.Gonzalez-Rodriguez I, Sanchez B, Ruiz L, Turroni F, Ventura M, Ruas-Madiedo P, Gueimonde M, and Margolles A (2012). Role of Extracellular Transaldolase from Bifidobacterium bifidum in Mucin Adhesion and Aggregation. Applied and Environmental Microbiology 78, 3992–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nishiyama K, Takaki T, Sugiyama M, Fukuda I, Aiso M, Mukai T, Odamaki T, Xiao J, Osawa R, and Okada N (2020). Extracellular Vesicles Produced by Bifidobacterium longum Export Mucin-Binding Proteins. Applied and Environmental Microbiology 86, e01464–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suzuki K, Nishiyama K, Miyajima H, Ro O, Yamamoto Y, and Mukai T (2016). Adhesion properties of a putative polymorphic fimbrial subunit protein from Bifidobacterium longum subsp. longum. Biosci. Microbiota Food Health 35, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garrido D, Kim JH, German JB, Raybould HE, and Mills DA (2011). Oligosaccharide Binding Proteins from Bifidobacterium longum subsp.infantis Reveal a Preference for Host Glycans. PLoS ONE 6, e17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Troge A, Scheppach W, Schroeder BO, Rund SA, Heuner K, Wehkamp J, Stange EF, and Oelschlaeger TA (2012). More than a marine propeller - the flagellum of the probiotic Escherichia coli strain Nissle 1917 is the major adhesin mediating binding to human mucus. International Journal of Medical Microbiology 302, 304–314. [DOI] [PubMed] [Google Scholar]
- 122.Rojas M, Ascencio F, and Conway PL (2002). Purification and characterization of a surface protein from Lactobacillus fermentum 104R that binds to porcine small intestinal mucus and gastric mucin. Appl. Environ. Microbiol 68, 2330–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Le DTL, Tran TL, Duviau MP, Meyrand M, Guerardel Y, Castelain M, Loubiere P, Chapot-Chartier MP, Dague E, and Mercier-Bonin M (2013). Unraveling the Role of Surface Mucus Binding Protein and Pili in Muco-Adhesion of Lactococcus lactis. PLoS ONE 8, e79850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx APA, Lebeer S et al. (2009). Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc. Natl. Acad. Sci. USA 106, 17193–17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, and Corth+®sy-Theulaz I.n.E. (2006). GroEL of Lactobacillus johnsonii La1 (NCC 533) Is Cell Surface Associated: Potential Role in Interactions with the Host and the Gastric Pathogen Helicobacter pylori. Infection and Immunity 74, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Granato D, Bergonzelli GE, Pridmore RD, Marvin L, Rouvet M, and Corthesy-Theulaz IE (2004). Cell Surface-Associated Elongation Factor Tu Mediates the Attachment of Lactobacillus johnsonii NCC533 (La1) to Human Intestinal Cells and Mucins. Infection and Immunity 72, 2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Houeix B, Synowsky S, Cairns MT, Kane M, Kilcoyne M, and Joshi L (2019). Identification of putative adhesins and carbohydrate ligands of Lactobacillus paracasei using a combinatorial in silico and glycomics microarray profiling approach. Integr. Biol. (Camb) 11, 315–329. [DOI] [PubMed] [Google Scholar]
- 128.Roos S and Jonsson H (2002). A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148, 433–442. [DOI] [PubMed] [Google Scholar]
- 129.Owen CD, Tailford LE, Monaco S, Suligoj T, Vaux L, Lallement R, Khedri Z, Yu H, Lecointe K, Walshaw J et al. (2017). Unravelling the specificity and mechanism of sialic acid recognition by the gut symbiont Ruminococcus gnavus. Nature Communications 8, 2196. [DOI] [PMC free article] [PubMed] [Google Scholar]


