Abstract
Elevated eosinophil counts are implicated in multiple diseases, from relatively prevalent organ-specific disorders such as severe eosinophilic asthma, to rare multisystem disorders such as hypereosinophilic syndrome (HES) and eosinophilic granulomatosis with polyangiitis (EGPA). Patients with these multisystem diseases, often associated with markedly elevated eosinophil counts, have a substantial risk of morbidity and mortality due to delayed diagnosis or inadequate treatment. A thorough work-up of symptomatic patients presenting with elevated eosinophil counts is essential, although in some cases the differential diagnosis may remain difficult due to overlapping presentations between HES and EGPA. Notably, first- and second-line treatment options and response to therapy may differ for specific HES and EGPA variants. Oral corticosteroids (OCS) are the first line of treatment for HES and EGPA, except when HES is the result of specific mutations driving clonal eosinophilia that are amenable to targeted treatment with a kinase inhibitor. Cytotoxic or immunomodulatory agents may be required for those with severe disease. Novel eosinophil-depleting therapies, such as those targeting interleukin-5 or its receptor, have shown great promise in reducing blood eosinophil counts, and reducing disease flares and relapses in patients with HES and EGPA. Such therapies could reduce the side effects associated with long-term OCS or immunosuppressant use. This review provides a pragmatic guide to approaching the diagnosis and clinical management of patients with systemic hypereosinophilic disorders. We highlight practical considerations for clinicians and present cases from real-world clinical practice to illustrate the complexity and challenges associated with diagnosing and treating patients with HES and EGPA.
Introduction
Eosinophils are granulocytes that play a diverse range of roles in human health and disease.1 Eosinophil counts may be elevated in blood and/or tissue in the setting of numerous diseases including severe eosinophilic asthma, chronic rhinosinusitis with nasal polyposis, and atopic dermatitis, as well as in rare, systemic diseases often associated with marked blood hypereosinophilia such as hypereosinophilic syndrome (HES) and eosinophilic granulomatosis with polyangiitis (EGPA).1, 2 Other potential causes of markedly elevated blood eosinophil counts include parasitic infections, cancer and adverse drug reactions.3
An absolute eosinophil count (AEC) above 0.5×109 cells/L is considered elevated in most laboratories, while hypereosinophilia is defined as AEC ≥1.5×109 cells/L.4 HES is characterized by persistent hypereosinophilia and evidence of eosinophil-mediated end-organ damage.5 Patients who have HES may present with involvement of a range of organ systems and have diverse associated symptoms.6 While the clinical presentation of EGPA can also be heterogeneous, patients typically present with asthma and high-grade eosinophilia, and may show various systemic manifestations of small vessel vasculitis and granulomatous eosinophilic inflammation.7 There is considerable overlap between certain clinical presentations of HES and EGPA, and diagnosis can be difficult to reach in the absence of hallmarks of vasculitis.7 Determining a correct diagnosis is important as treatment options and response to therapy differ for specific disease variants. Ultimately, HES and EGPA are both associated with significant morbidity and mortality if uncontrolled or suboptimally treated.8, 9
This review provides practical insight into the diagnosis and treatment of patients with HES or EGPA, with discussion of example cases, including HES/EGPA overlap. We focus on the identification and treatment of disease variants that have distinguishing features and require specific approaches to clinical management. Methods for distinguishing between HES and EGPA, when possible, are also discussed.
Clinical presentation and diagnosis
Clinical presentation and classification of HES
HES is a heterogeneous disorder, with varied organ system involvement and diverse symptoms that range in severity (Table 1).6, 7, 9–16 The most common presenting signs and symptoms reflect cutaneous, pulmonary, and gastrointestinal involvement, though any organ can be involved.13 Although present in a smaller proportion of patients, cardiovascular and neurologic complications are associated with significant morbidity and mortality.8 Disease course in HES is variable; some patients present with persistent or progressive disease, while others experience fluctuating disease activity (flares) with episodic symptom worsening.17 Several variants of HES have been defined, including idiopathic HES (I-HES), in which the mechanisms resulting in eosinophil expansion remain unknown; lymphocytic HES (L-HES), in which hypereosinophilia is driven by a clonal population of activated T cells that overproduce eosinophilopoietic cytokines, most notably interleukin (IL)-5; and myeloid HES (M-HES), predominantly associated with the FIP1-like 1 (FIP1L1) platelet-derived growth factor receptor alpha (PDGFRA) fusion gene.5, 6
Table 1.
| ORGAN SYSTEMS AFFECTED IN HES AND EGPA | ||
|---|---|---|
| HES | EGPA | |

|
Relative frequency: + Important cause of morbidity and mortality. eg, eosinophilic (necrotizing) myocarditis, mural thrombus formation, endomyocardial fibrosis |
Relative frequency: + eg, eosinophilic cardiomyopathy, endomyocardial fibrosis, coronary vasculitis, pericarditis |

|
Relative frequency: +++ Most common clinical manifestation of L-HES. eg, eczema, urticaria, erythematous papules or plaques, erythroderma, mucosal ulceration (M-HES), purpura |
Relative frequency: ++ eg, urticaria, purpura |

|
Relative frequency: ++ eg, eosinophilic gastrointestinal involvement, cholangitis, hepatitis |
Relative frequency: + eg, eosinophilic gastrointestinal involvement, ischemia, perforation (vasculitic involvement) |

|
Relative frequency: + Presence suggestive of underlying myeloid disease/neoplasm. eg, anemia, thrombocytopenia, splenomegaly |
Relative frequency: (+) (unusual) |
|
Relative frequency: (+) (rarely reported in HES) eg, interstitial nephritis, eosinophilic cystitis |
Relative frequency: + More common in ANCA-positive patients who have vasculitic manifestations eg, glomerulonephritis, interstitial nephritis |

|
Relative frequency: + to ++ eg, cerebral vessel thrombosis, stroke (embolism from intracardiac thrombus), encephalopathy, peripheral neuropathy |
Relative frequency: ++ eg, peripheral neuropathy, mononeuritis multiplex, stroke |
|
Relative frequency: ++ More common in I-HES and M-HES. eg, asthma, interstitial pulmonary infiltrates, eosinophilic bronchitis, pleural effusion |
Relative frequency: ++++ (almost all patients with EGPA) eg, asthma, interstitial pulmonary infiltrates, alveolar hemorrhage |
|
Relative frequency: + eg, arthritis, tenosynovitis, fasciitis |
Relative frequency: + to ++ eg, arthralgia, arthritis |
|
Relative frequency: + to ++ eg, chronic rhinosinusitis |
Relative frequency: +++ Nasal polyps are common in EGPA. eg, chronic rhinosinusitis with or without polyposis |

|
Relative frequency: + Hypercoagulability and/or endovascular damage contribute to vascular events/complications. eg, arterial or venous thrombosis, microvascular damage, digital gangrene |
Relative frequency: +++ Hypercoagulability and/or small vessel vasculitis contribute to vascular events/complications (depending on affected organ system) eg, arterial or venous thrombosis, necrotizing vasculitis |
Relative frequencies are based on figures reported in the published literature or estimated based on the clinical observations of the authors: +, 0–<25% of patients; ++, ≥25–<50%; +++ ≥50–<75%; ++++ ≥75–100%.
ANCA, antineutrophil cytoplasmic antibody; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; I-HES, idiopathic HES; L-HES, lymphocyte-variant HES; M-HES, myeloid/lymphoid neoplasm HES.
Clinical presentation and classification of EGPA
In many but not all cases, EGPA begins with a prodromal phase, typically including asthma and/or chronic rhinosinusitis (with/without polyposis), with some degree of blood eosinophilia.7 Marked hypereosinophilia with tissue infiltrates (typically pulmonary but may include other organs) (Table 1)14 heralds disease progression, and if untreated, patients may develop necrotizing vasculitis with complications such as mononeuritis multiplex or purpura; extravascular eosinophil-rich granulomas may also be observed.14 Of note, the mean interval between initial presentation with adult asthma and vasculitic manifestations is 9 years.9 However, this can vary between patients and may be influenced by the use of systemic corticosteroid and/or immunomodulatory treatments.18
EGPA is classified as a small-vessel, anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), although only approximately 30%–47% of patients with EGPA are ANCA-positive, with most of those exhibiting anti-myeloperoxidase (MPO) ANCA positivity.7 ANCA-positive and ANCA-negative presentations of EGPA have recently been shown to be associated with distinct genetic polymorphisms suggesting distinct underlying pathogenic mechanisms.19 Hence, MPO ANCA-positive EGPA may be considered an eosinophilic autoimmune disease associated with human leukocyte antigen polymorphisms similar to other types of AAV; in contrast, ANCA-negative EGPA appears linked to mucosal/barrier function pathways and type 2 inflammation.19 These differences are consistent with prior observations that ANCA status is associated with different clinical presentations. In general, patients with ANCA-positive EGPA appear more at risk of developing a vasculitic phenotype (necrotizing vasculitis and necrotizing glomerulonephritis), whereas patients with ANCA-negative EGPA appear more likely to develop the direct consequences of eosinophilic organ infiltration (such as endomyocardial involvement).20 However, ANCA status alone is neither sufficiently sensitive nor specific to predict clinical presentation in a particular patient, and cannot guide treatment decisions.21
Diagnosis of HES and EGPA
When a patient presents with hypereosinophilia, other underlying causes should first be excluded (Figure 1).4, 6, 22–25 Patients should also be tested for evidence of myeloid neoplasms associated with eosinophilia using cytogenetic and molecular testing, which can identify those with M-HES requiring molecular-targeted therapy. In particular, patients should be tested for PDGFRA, PDGFRB, FGFR1, or JAK rearrangements or mutations.4, 26 Some patients may present with features of myeloid disease such as splenomegaly and high serum tryptase and/or vitamin B12 levels without the presence of the above genetic rearrangements or mutations; further in-depth molecular testing is warranted and, if unrevealing, an empirical therapeutic trial with imatinib mesylate (imatinib) may be considered.27 Determining if myeloid disease is present is paramount, even in cases where manifestations are suggestive of other variants of HES or EGPA (Case 1).
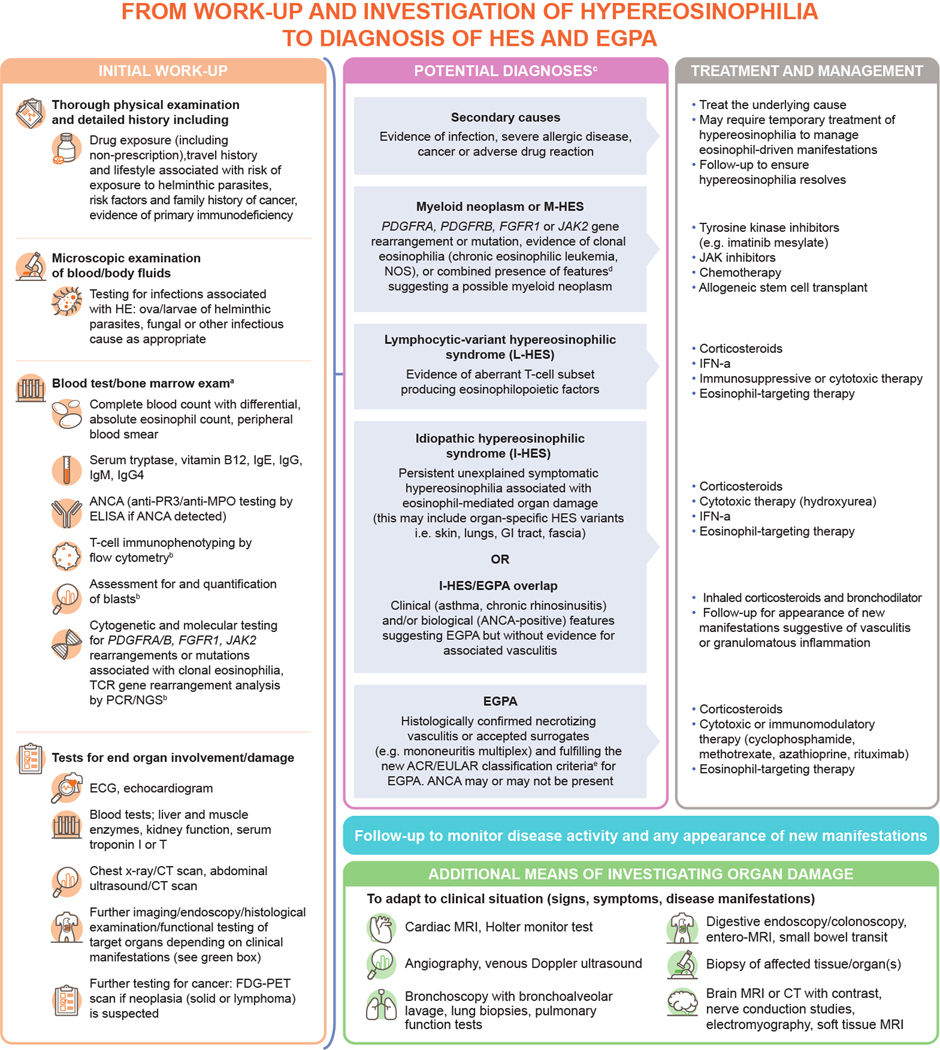
Figure. Diagnostic work-up for identifying patients with HES and EGPA4, 6, 22–24.
aBone marrow exam should be considered in the following situations: phenotyping and/or cytogenetic tests suggesting myeloid or lymphoid variant HES, blood eosinophilia >5000/mm3, elevated serum tryptase, lack of response to systemic corticosteroid treatment; btesting which can be performed on bone marrow; ca further category of potential diagnoses includes HE of undetermined significance, where unexplained hypereosinophilia is present but patients do not have associated eosinophil-related end organ damage or complications; this is not discussed further as it lies outside the scope of this review; deg, anemia/thrombocytopenia, splenomegaly, elevated serum vitamin B12/tryptase or lack of response to systemic corticosteroid therapy; ecurrent ACR/EULAR EGPA classification criteria require a total score ≥6 across the following seven criteria: obstructive airway disease (+3), nasal polyps (+3), mononeuritis multiplex (+1), blood eosinophil count ≥1 × 109cells/L (+5), extravascular eosinophilic-predominant inflammation on biopsy (+2), positive test for cytoplasmic ANCA or anti-PR3 antibodies (−3), hematuria (−1).36
ACR/EULAR, American college of rheumatology/European alliance of associations for rheumatology; ANCA, antineutrophil cytoplasmic antibody; CT, computed tomography; ECG, electrocardiogram; EGPA, eosinophilic granulomatosis with polyangiitis; FDG-PET, fluorodeoxyglucose-positron emission tomography; FGFR1, fibroblast growth factor receptor 1; HE, hypereosinophilia; HES, hypereosinophilic syndrome; IFN, interferon; Ig, immunoglobulin; JAK, Janus kinase; MPO, myeloperoxidase; MRI, magnetic resonance imaging; NGS, next-generation sequencing; NOS, not otherwise specified; PCR, polymerase chain reaction; PDGFRA, platelet-derived growth factor receptor alpha; PDGFRB, platelet-derived growth factor receptor beta; PR3, proteinase 3; TCR, T-cell receptor.
Case 1
A 40-year-old man was consented on an IRB approved natural history study to evaluate eosinophilia (NCT00001406). He presented with an erythematous pruritic lesion and an AEC of 3.3×109 cells/L and was treated with antibiotics; symptoms did not resolve. Medical history included a diagnosis of asthma in adolescence, treated with inhaled corticosteroids (ICS), which improved by adulthood. At age 30, he was diagnosed with psoriasis and developed sinusitis (managed with intranasal corticosteroids and antihistamines). At age 35, sinusitis worsened, and he was treated with repeated courses of antibiotics. The patient’s cutaneous lesions progressed; a diagnosis of folliculitis was made based on a skin biopsy showing perivascular eosinophils. One year after presentation, he developed shortness of breath; pulmonary function tests demonstrated an obstructive pattern with normal airway diffusion. He was prescribed combination ICS and long-acting beta-2-agonist (LABA); however, symptoms progressed, leading to an emergency department visit where wheezing, a skin rash, and mild splenomegaly were noted. An echocardiogram was normal, while a chest computed tomography (CT) scan showed interstitial infiltrates in bilateral mid-/upper lungs, mediastinal and hilar lymphadenopathy, and borderline splenomegaly. Sinus CT scan demonstrated diffuse mucosal thickening. White blood count was 21.9×109 cells/L, with absolute neutrophil count of 3.5×109 cells/L and AEC of 15.3×109 cells/L. Common causes of hypereosinophilia were ruled out. Serum ANCA and anti-nuclear antibody testing was negative; rheumatoid factor levels were normal. Serum IgE (4 KU/L) and tryptase (3.9 ng/mL) were within normal limits, while vitamin B12 levels were elevated (>2000 pg/mL). A bronchoscopy with endobronchial lymph node biopsy showed fibrosis with eosinophilia and granulomatous inflammation; no acid-fast bacillus or fungal pathogens were detected. High-dose oral corticosteroid (OCS) therapy was initiated, and he was discharged. Despite treatment, 4 weeks later his AEC remained high (5.2×109 cells/L). A chest CT scan showed improvement in airspace opacities; however, lymphadenopathy and splenomegaly remained, prompting referral to a hematologist. A bone marrow biopsy and aspirate demonstrated hypercellularity with focal areas of marked fibrosis and no increase in mast cells. Molecular testing revealed a FIP1L1::PDGFRA rearrangement; imatinib was initiated, and he achieved complete remission.
Final diagnosis:
FIP1L1::PDGFRA+ M-HES presenting with respiratory and cutaneous symptoms.
Key lessons:
Eosinophilic presentations with elevated vitamin B12 or serum tryptase, other hematologic abnormalities (anemia, thrombocytosis), splenomegaly, and/or lack of response to high-dose OCS warrant further work-up for myeloid neoplasms, particularly in male patients.
During work-up, patients should also be tested to confirm whether eosinophilia is caused by T cells by performing T cell immunophenotyping using flow cytometry to detect aberrant subsets (most commonly CD3−CD4+), as well as TCRβ/γ chain gene rearrangement pattern analysis by polymerase chain reaction (PCR) or next generation sequencing (NGS) to detect clonality although demonstration of T cell clonality is associated with low sensitivity and specificity for a diagnosis of L-HES.28, 29 In the presence of IL-5-producing phenotypically abnormal T cells, a diagnosis of L-HES can be made.28 The identification of patients with L-HES has prognostic and therapeutic implications, as they are at risk for the development of lymphoma, and their treatment responses differ from those with I-HES.30 When phenotyping and/or cytogenetic tests on peripheral blood suggest M-HES or L-HES, or when a patient has blood eosinophilia >5000/mm3, elevated serum tryptase or lack of response to corticosteroid treatment, bone marrow testing should be conducted for morphological (including blast cell) assessment, T-cell immunophenotyping, and further cytogenetic and molecular testing.4, 26
For patients with clinical manifestations that suggest EGPA, namely asthma and sinonasal abnormalities, ANCA serology and testing for anti-MPO and anti-PR3 are warranted (Case 2). The presence of ANCA is highly suggestive of EGPA, however, rare cases of ANCA-positive HES have been reported,31 and up to 70% of EGPA cases are ANCA-negative. For patients with strongly suspected or confirmed EGPA, utilization of the revised five factor scoring system (FFS) can assist in determining severity of disease and help guide treatment.32
Case 2
A 61-year-old man initially presented with a cough. Treatment for gastroesophageal reflux disease (proton pump inhibitor) then for presumptive bronchitis (antibiotics) failed to improve his condition. After 4 months, asthma was suspected because of night-time and exercise-related episodes of breathlessness; severe asthma was confirmed by a pulmonologist. He had no sinonasal complaints. Combination ICS and ultra-LABA was prescribed; blood work revealed hypereosinophilia (5.9×109 cells/L) and increased serum IgE (667 KU/L). Parasitic infection and drug allergy were ruled out. Testing for FIP1L1::PDGFRA was negative. A clonal T-cell receptor (TCR) gene rearrangement pattern was observed, but lymphocyte phenotyping did not reveal an aberrant T-cell subset. Thoracic CT scan revealed ground-glass opacities and bronchiectasis, while bronchoalveolar lavage demonstrated 72% eosinophils. ANCA testing was negative. Within weeks, AEC increased (12.89 × 109 cells/L), and the patient developed new symptoms including myalgia, purpura, fever, sweating, and severe fatigue. Shortly thereafter, he reported sudden and severe pain in his right forearm followed by weakness and complete loss of sensation in the 4th and 5th digits of the right hand; electromyography showed fibrillation and fasciculations in the first interosseous muscles. Biopsy of a purpuric lesion showed leukocytoclastic vasculitis with neutrophils and eosinophils, and necrosis. OCS was started and symptoms, other than the loss of sensation, dissipated within days. After 2 months, the OCS dose was reduced; within a week the patient reported the sudden appearance of right lower leg and foot pain, accompanied by foot drop. Nerve conduction studies showed axonal degeneration involving both fibular nerves. Together with the history of ulnar nerve damage, mononeuritis multiplex was diagnosed. The OCS dose was increased, and intravenous cyclophosphamide was initiated; pain subsided within days. Intravenous cyclophosphamide continued for 6 months, at which point complete reversal of motor symptoms was observed. Maintenance immunosuppressive therapy combined OCS first with azathioprine then with methotrexate, providing prolonged remission. Tapering off OCS entirely while maintaining methotrexate resulted in disease recurrence (asthma and AEC above 1.5 × 109 cells/L). He remains OCS-dependent.
Final diagnosis:
ANCA-negative EGPA.
Key lessons:
Presence of asthma, marked blood hypereosinophilia, and vasculitic clinical complications (leukocytoclastic vasculitis with purpura and mononeuritis multiplex) is consistent with a diagnosis of EGPA, despite ANCA negativity. Immunosuppressive treatment was initiated because of clear evidence of vasculitis. As typically encountered in this disorder, the asthmatic component is often the limiting factor for OCS-tapering once remission has been induced with immunosuppressive treatment. Eosinophil-lowering targeted treatment may be considered at this stage for better asthma control.
HES/EGPA overlap
Patients with hypereosinophilia and systemic manifestations in whom testing for well-characterized myeloid and lymphocytic HES variants and for ANCA is unrevealing may have either I-HES or ANCA-negative EGPA. Some patients present with overlapping symptoms, making this distinction challenging (Case 3).18, 25 Importantly, despite several studies being undertaken, there are currently no validated disease-specific or blood-based biomarkers that reliably differentiate between ANCA-negative EGPA and I-HES,18, 33 although one recent study proposed that low serum C-reactive protein levels in patients with eosinophilia and asthma at diagnosis may be suggestive of the latter.34 Mediastinal lymphadenopathy has also been identified as a sign of systemic inflammation associated with EGPA; but further work is warranted to determine its clinical potential.35
Case 3
A 62-year-old woman developed periorbital edema following spa treatments and presented at an emergency department with tongue swelling. Despite no significant history of allergy or asthma, respiratory symptoms and cough persisted for several months. Subsequently, progressive severe dyspnea and fatigue developed over the course of 1 week, resulting in an emergency department visit. AEC was 10.6×109 cells/L and she developed respiratory failure, requiring intensive care unit (ICU) admission and intubation. Parasitic infection and drug-induced hypereosinophilia were ruled out. Chest CT scan showed prominent airway thickening and mediastinal lymphadenopathy. She was treated with high-dose intravenous corticosteroids; AEC peaked at 23.5×109 cells/L, normalizing following 1 week of treatment, and she was extubated. Due to the acute presentation, bronchoalveolar lavage fluid (BALF) examination could not be performed ahead of corticosteroid initiation. Testing for ANCA was negative; serum vitamin B12 (>2000 pg/mL), and immunoglobulin E (28,019 KU/L) were elevated while serum tryptase (2.5 ng/mL) was within normal limits. The TCR gene rearrangement pattern was polyclonal; bone marrow biopsy showed hypercellularity with marked eosinophilia. Testing for myeloid neoplasms (FIP1L1::PDGFRA, BCR-ABL) was normal. A lymph node biopsy demonstrated no evidence of lymphoma but showed follicular hyperplasia. The patient was able to taper off OCS over the following months, but subsequently developed shortness of breath and hypoxemia, and was once again admitted to the ICU. Sinus CT revealed pan-sinusitis. AEC peaked at 5.0×109 cells/L prior to restarting high-dose OCS. Mepolizumab, 300 mg every 4 weeks, was initiated and OCS successfully tapered. Respiratory symptoms resolved within 1 month with no relapses over 10 months of follow-up.
Final diagnosis:
HES/EGPA overlap.
Key lessons:
This case with severe respiratory symptoms and sinus involvement in the setting of marked blood hypereosinophilia illustrates the difficulty in distinguishing I-HES from ANCA-negative EGPA. As such, long-term follow-up is essential. Should this patient’s disease progress with development of vasculitic manifestations, a revised diagnosis of EGPA would be appropriate.
Ongoing assessment and re-diagnosis
In patients with overlapping manifestations, regular ongoing assessment and periodic re-evaluation for new symptoms is important to better classify patients if the disease progresses. Indeed, some disease features more characteristic of vasculitis may appear later, or with OCS tapering, and could warrant modification of the treatment strategy. For example, in the presence of disease manifestations such as purpura, mononeuritis multiplex, glomerulonephritis, or new histopathologic findings showing necrotizing trans-mural vasculitic and/or granulomatous inflammation, a more formal classification as EGPA is appropriate, provided American College of Rheumatology/European Alliance of Associations for Rheumatology (ACR/EULAR) criteria are met (≥6-point score).36 Furthermore, some therapies may mask the development of clinical signs or pathologic findings that would otherwise point towards a specific diagnosis.
For both EGPA and HES, there are currently no reliable biomarkers to assess disease activity or relapse.33, 37, 38 Although blood eosinophil counts are commonly used in clinical practice as their levels can follow disease activity, this is not always the case and discriminating between disease activity and worsening of underlying asthma or sinusitis in the case of EGPA is challenging.37 Exploration of predictive biomarkers is currently ongoing, such as testing for autoantibodies in EGPA and severe eosinophilic asthma (NCT04671446), and predicting renal flares in AAV by monitoring urinary T-cell populations (NCT04428398). More work is needed to develop tools to facilitate clinical treatment decisions.2, 14, 39
Treatment options
Oral corticosteroids
OCS are used as first-line therapy for both HES and EGPA (Table 2)4, 6, 13, 26, 27, 38, 40–68, with some exceptions as described below. For the majority of patients, initiation of OCS typically leads to rapid reductions in blood eosinophil counts,4 although response to OCS varies depending on the HES variant; patients with I-HES, particularly those with organ-restricted disease, have been shown to have significantly better responses as compared with M-HES or L-HES.30 In EGPA, the clinical response to induction treatment with OCS is generally rapid, with the exception of specific disease complications such as mononeuritis multiplex that may take weeks or months to recover, or may cause irreversible neurological damage if treatment is delayed.30 For patients with HES and EGPA, maintenance therapy often includes long-term OCS use, and clinical management should include approaches to reduce OCS exposure.4, 6, 25
Table 2.
Treatment options for patients with HES and EGPA
| Treatment | Disease and approval status | Indications | Considerations | |
|---|---|---|---|---|
| Steroids | OCS 6, 38, 40, 68 | HES, EGPA | Appropriate first-line therapy for many patients with HES or EGPA, with exception of patients with suspected or confirmed eosinophil clonality such as FIP1L1::PDGFRA fusion. Typically leads to rapid reductions in eosinophil counts. Patients with EGPA should initially be treated with systemic CS with the aim of achieving remission; severe manifestations warrant early initiation of an immunosuppressant. | Long-term use is associated with substantial toxicity. Initiate empirical/preventive anti-parasite treatment in patients with a travel history suggesting possible strongyloides exposure to avoid hyperinfestation syndrome. |
| Kinase inhibitors | Imatinib mesylate (tyrosine kinase inhibitor) 6, 13, 26, 27, 40, 41, 43–45, 64 | M-HES (Approved) | First-line therapy for patients with M-HES associated with rearrangements involving PDGFRA/PDGFRB. Initial recommended dose is 100–400 mg daily for patients with M-HES depending on the cytogenetic rearrangements, followed by lowering for maintenance. May also be considered for patients with myeloid features in the absence of a detectable mutation, with initial recommended dose of 400–800 mg daily. | The low-dose maintenance regimen required for FIP1L1::PDGFRA fusion kinase-positive HES (ie, 100 mg/day) is well tolerated. Molecular remission is the goal of treatment and is achieved rapidly in most cases. Several studies indicate a potential for a cure in patients with FIP1L1::PDGFRA fusion kinase-positive HES if treatment is maintained for several years. Higher doses may cause side effects. Should be used in combination with OCS for a brief period at treatment initiation to prevent potential cardiac toxicity, likely related to eosinophil destruction. |
| Ruxolitinib (JAK1/2 kinase inhibitor) 4, 47, 86 | M-HES (In development) | Phase II trials are ongoing for patients with M-HES associated with rearrangements involving JAK2 (PCM1-JAK2, ETV6-JAK2, BCR-JAK2); two trials are currently recruiting patients (NCT03801434 and NCT00044304). | Further studies are needed to confirm efficacy and safety. Allogeneic HSCT may be suitable for some patients depending on co-morbidities and risk/benefit considerations. | |
| Tofacitinib (JAK1/3 kinase inhibitor) 46, 47 | L-HES and I-HES (In development) | A small case study showed gain of function mutations in STAT3 in 1 patient with L-HES and over-expression of STAT-3 dependent genes in a further 2 patients which suggested disruption of the JAK/STAT pathway could be effective in treating patients with HES. A follow-up small therapeutic trial of tofacitinib or ruxolitinib in 2 patients with L-HES and 3 patients with I-HES, all with cutaneous involvement, showed efficacy in reducing blood eosinophil counts and OCS-sparing capability. | Further studies are required to confirm efficacy and safety. | |
| Cytotoxic/immunomodulatory agents | Interferon-α (IFN-α) 6, 13, 40, 48 | HES | Second-line option for patients with HES, with case reports and case series showing efficacy in I-HES, M-HES, and L-HES. Pegylated form seems equally effective and is both more convenient (weekly dosing) and better tolerated. Response rates vary. Preferred second-line option when available for L-HES. |
Frequent requirement for incremental increase in dosing for improved tolerance. May be associated with poor tolerance (flu-like symptoms) and/or significant toxicity. Often discontinued. Secondary resistance has been reported. |
| Hydroxyurea (HU) 6, 13, 40, 49 | HES | For patients with I-HES, combination of low-dose OCS and HU following induction of remission has shown success in stabilizing disease. | Often discontinued due to lack of efficacy or hematologic/gastrointestinal side effects. | |
| Cyclophosphamide 38, 50, 68 | EGPA | Used at treatment initiation in combination with OCS to induce remission for patients with severe organ-or life-threatening manifestations of EGPA. Can be used in either continuous oral or pulsed intravenous dosing regimens. Treatment typically has a duration of 3–6 months. | Associated with significant treatment-related urinary tract toxicity and decreased fertility. May require fertility preservation considerations. | |
| Rituximab 50–54, 68 | EGPA | Demonstrated efficacy as maintenance therapy in observational studies of patients with EGPA. | Possible infusion reactions (can be avoided with systematic preventive measures). Associated with immunosuppression and reduced responses to vaccination. | |
| Methotrexate 38, 50, 68 | EGPA | Used in combination with OCS as maintenance therapy | To be used after the successful induction of remission. Case reports and small series in HES did not show efficacy. |
|
| Azathioprine 38, 50, 68 | EGPA | Used in combination with OCS as maintenance therapy | To be used after the successful induction of remission. Not typically used for treatment of HES, with the exception of rare cases with liver involvement. |
|
| Novel eosinophil-targeting therapies | Mepolizumab (Anti–IL-5) 6, 55–60 | HES, EGPA (Approved for use in patients with EGPA or HES in multiple regions worldwide) | HES: Reduced eosinophil count and clinical benefit (reduced flares and reduction in maintenance OCS therapy) shown in Phase III trials. An expanded access program ongoing since 2005 has treated and shown efficacy in hundreds of patients with HES (mepolizumab exposure >1500 patient years). EGPA: In a Phase III trial in EGPA, treatment increased the proportion of patients in remission and reduced the relapse rate. Although remission and relapse definitions included a vasculitis component, efficacy on vasculitis in EGPA remains to be determined. |
Approved dose for HES and EGPA is 300 mg given as subcutaneous injections every 4 weeks. |
| Reslizumab (Anti–IL-5) 61–63 | HES, EGPA (Studied) | Reduced eosinophil counts and clinical benefit (reduced OCS dose and/or symptoms) shown in patients with EGPA and HES in small open-label studies. | Given intravenously with dosing according to weight, in contrast to mepolizumab and benralizumab. May be interesting to determine efficacy in patients with high BMI who fail to respond to other IL-5(R)–targeted therapies. | |
| Benralizumab (Anti–IL-5R) 64, 65 | HES, EGPA (In development) | Reduced blood eosinophil counts and tissue eosinophilia with clinical improvement shown in patients with PDGFRA-negative HES and reduced OCS use and exacerbation rates shown in patients with EGPA in Phase II trials. | Yet to be confirmed whether beneficial in HES/EGPA in the setting of Phase III studies (ongoing: NCT04191304 and NCT04157348). | |
| Dexpramipexole (small molecule) 66 | HES (In development) | OCS-sparing capability in a subset of patients with HES shown in proof-of-principle investigator-initiated Phase II study. | N/A | |
| Lirentelimab (Anti–Siglec-8) 67 | HES (In development) | Not yet explored for use in HES or EGPA but shown to deplete eosinophils in Phase II trial in patients with peripheral blood eosinophilia and eosinophilic gastritis and/or eosinophilic duodenitis. | N/A |
ALS, amyotrophic lateral sclerosis; BCR, Bruton’s tyrosine; BMI, body mass index; CS, corticosteroids; EGPA, eosinophilic granulomatosis with polyangiitis; ETV6, ETS-variant6; FIP1L1, factor interacting with PAPOLA And CPSF1; HES, hypereosinophilic syndrome; HU, hydroxyurea; HSCT, hematopoietic stem cell transplant; IFN-α, interferon-alpha; IL, interleukin; JAK, Janus activated kinase; M-HES, myeloid hypereosinophilic syndrome; OCS, oral corticosteroids; PCM1, pericentriolar material gene 1; PDGFRA, platelet-derived growth factor receptor alpha; Siglec-8, sialic acid-binding immunoglobulin-like lectin 8; STAT, signal transducer and activation of transcription.
Kinase inhibitors
HES with suspected/confirmed mutations driving clonal eosinophilia (eg, PDGFRA/PDGFRB/JAK2) should be treated with a targeted kinase inhibitor (Table 2).4, 25, 26 M-HES associated abnormalities involving PDGFRA are extremely sensitive to treatment with imatinib, with patients typically showing a rapid and sustained response.26 Some patients presenting with features of myeloid disease such as splenomegaly, high serum tryptase or vitamin B12 levels but without detectable mutations using standard PCR/fluorescence in situ hybridization (FISH) testing have responded to imatinib.27 For these patients, NGS approaches may identify novel targets that are treatable with imatinib or other agents.41 Several Janus kinase (JAK) inhibitors are also in development and been effective in small numbers of patients with specific HES subtypes (Table 2)4, 46, 47, however further studies are ongoing to determine the efficacy and safety in patients with HES.
Cytotoxic/immunomodulatory agents
For patients with HES, hydroxyurea (HU) and/or interferon-α (IFN-α) may be used in combination with OCS (Table 2). HU is a cytotoxic agent that acts centrally to lower eosinophil numbers,6, 13 while IFN-α is an immunomodulatory agent that affects both eosinophils and T cells. Although some patients respond well to these agents, response rates vary, and tolerance is often poor, frequently leading to treatment discontinuation.6, 13, 48, 49
For patients with EGPA who have organ-/life-threatening manifestations, or those who have severe disease based on the revised FFS, combination high-dose corticosteroids (CS) and immunosuppressive therapy (ie, cyclophosphamide or rituximab) should be administered initially (Table 2).32, 38, 68 Other agents that can be used in combination with CS include azathioprine or methotrexate, although these are generally used as maintenance therapy once induction of remission has been achieved (Table 2).38, 50, 68 Rituximab (anti-CD20 monoclonal antibody) has shown efficacy in observational studies of patients with EGPA51–53 and a placebo-controlled Phase IV trial (NCT03164473) comparing the efficacy of rituximab versus azathioprine in maintaining remission is ongoing. While a recent Phase III trial (NCT02807103) reported that rituximab was not superior to conventional therapy in inducing remission in patients with EGPA,69 it can be considered as an immunosuppressive therapy option in patients with severe disease.69
Novel eosinophil-targeting therapies
Due to side effects associated with long-term OCS and immunomodulatory agent use, therapies directly targeting eosinophils have been explored as treatments for patients with HES and EGPA.70 The cytokine interleukin-(IL)-5 is a key mediator in eosinophil biology and drives eosinophil proliferation, maturation, recruitment, activation and survival.71 Mepolizumab and reslizumab are humanized monoclonal antibodies that target IL-5.60, 72 Benralizumab is a monoclonal antibody targeting the IL-5 receptor α subunit that depletes eosinophils in blood, bone marrow, and tissue via antibody-dependent cellular cytotoxicity.42
Mepolizumab has been shown to reduce disease activity and OCS use in patients with HES in several clinical studies and is approved for use in multiple countries worldwide.55, 57, 59, 60, 73–75 Reslizumab reduced eosinophil counts and clinical symptoms in a small open-label study in four patients with HES,63 and benralizumab reduced blood eosinophil counts compared with placebo in a Phase II study in patients with PDGFRA-negative HES;64 a Phase III study is currently recruiting (NCT04191304). PDGFRA-associated M-HES is not treated with mepolizumab due to the availability of effective targeted therapy with imatinib.13, 26
Mepolizumab is also approved in multiple countries for the treatment of EGPA based on data from the Phase III MIRRA study.58–60 This showed increased duration of remission, a higher proportion of participants in remission, reduced relapse rates, and greater OCS dose reductions with mepolizumab compared with placebo.56, 76 For reslizumab, a Phase II study (NCT02947945) demonstrated reductions in the required dose of OCS in patients with EGPA.62 A Phase III non-inferiority trial of benralizumab versus mepolizumab in relapsing or refractory EGPA is currently recruiting patients (MANDARA, NCT04157348). It is not yet clear whether targeted eosinophil depletion will have a beneficial effect on vasculitic manifestations of EGPA, as only 10% of patients recruited in the MIRRA trial were ANCA-positive at baseline and detailed clinical data on proven vasculitis were not collected; this should become clear in the ongoing MANDARA trial and real-world use of anti–IL-5 therapies. Mepolizumab, in combination with corticosteroids, is conditionally recommended for remission induction in patients with active, non-severe EGPA in the ACR/Vasculitis Foundation 2021 guideline for the management of ANCA-associated vasculitis.68
While dupilumab (anti–interleukin-4 receptor α monoclonal antibody) has been approved for severe type 2 asthma and would be expected to help stabilize this component of EGPA, it should be used with caution because of the frequent increase in blood eosinophil counts that occurs following its initiation77, 78 and the occasional observation of exacerbation of pulmonary eosinophilic conditions in this setting. Currently, there is no published data for tezepelumab (anti-thymic stromal lymphopoietin monoclonal antibody) in EGPA; it is highly efficacious in severe asthma and may theoretically improve this disease component in EGPA. While there are case reports of successful use of dupilumab in EGPA,79–81 further studies for both dupilumab and tezepelumab are required.
Several other eosinophil-targeting therapies are currently in development for use in HES and other eosinophil-driven diseases. Following the fortuitous observation that the small-molecule drug dexpramipexole reduced eosinophil counts in trials enrolling patients with amyotrophic lateral sclerosis, a small Phase II open-label study (NCT02101138) was conducted in HES. This showed marked reductions in eosinophil counts in a subset of patients, together with OCS-sparing capability.66 Another therapy under investigation is the monoclonal antibody lirentelimab (AK002). Lirentelimab targets sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8), a transmembrane protein specifically expressed on the surface of eosinophils and mast cells.82 Whilst lirentelimab has not yet been explored for use in systemic HES or EGPA, it was shown to deplete blood and tissue eosinophils in patients with eosinophilic gastritis or duodenitis in a Phase II placebo-controlled trial (NCT03496571).67
Cardiac disease in HES/EGPA
Cardiac involvement has previously been shown to be the main cause of mortality in patients with HES8 and EGPA83. Management of cardiac involvement in HES depends on the disease subtype. For patients with myeloid disease with known imatinib-sensitive mutations receiving imatinib mesylate, OCS should be added for the first few days if there is evidence of cardiac involvement to prevent acute myocardial necrosis.84 For patients with I-HES or L-HES, treatment selection is not altered based on the presence or absence of cardiac disease; however, different monitoring requirements (eg troponin) and evaluation (cardiac magnetic resonance imaging) are implemented and attempts to suppress eosinophilia are more urgent. For patients with EGPA and cardiac involvement, a combination of OCS and cyclophosphamide can be given as induction treatment85 (FFS ≥1).
Conclusion
With advances in our understanding of HES and EGPA, patients with these rare diseases can be more easily identified and effectively treated. However, considerable gaps concerning upstream disease mechanisms remain, and further insight is required to determine how polymorphisms conferring genetic susceptibility and environmental triggers may interact to induce the inflammatory patterns that characterize these disorders. Furthermore, the distinction between these disorders may be challenging for patients with predominant respiratory presentations. Until then, optimizing therapeutic regimens so that patients are at reduced risk for treatment-related toxicity is crucial. Given the important role played by eosinophils in certain disease manifestations/complications of HES and EGPA, eosinophil-targeting therapy may help achieve this goal for a significant proportion of patients. As use of monoclonal anti–IL-5 therapies for the treatment of HES and EGPA becomes more widespread following recent approvals for these indications, it is essential that investigators monitor patients closely when tapering background CS and/or immunosuppressive therapy. Any changes in disease expression may reflect non–eosinophil-mediated features of these diseases warranting other/additional therapies. There is a need for multicenter studies on the real-world use of and responses to eosinophil-targeting therapeutics, together with large-scale mechanistic studies are needed to provide valuable insight, particularly in regard to outcomes of patients with life-threatening complications who were excluded from pre-approval clinical trials. These data may further our understanding of HES and EGPA pathogenesis, help to identify new biomarkers facilitating diagnosis and monitoring, and further refine and personalize treatment approaches for patients with systemic hypereosinophilic syndromes.
Article highlights.
Elevated eosinophil counts may indicate the presence of rare systemic disorders such as hypereosinophilic syndrome (HES) and eosinophilic granulomatosis with polyangiitis (EGPA).
Patients with HES or EGPA can display a variety of clinical manifestations involving various organ systems.
While some variants of HES or EGPA can be distinguished on the basis of a combination of clinical features and investigations, diagnosis may prove difficult in overlap cases and in the early stages of disease presentation or may be confounded by the use of systemic corticosteroids.
Although corticosteroid treatment is effective in the majority of patients with HES and EGPA, management may differ in the choice of first line agents in certain subtypes of HES and of second-line immunosuppressive or cytotoxic therapy.
The advent of novel eosinophil-targeting therapies may enable tapering of corticosteroid and/or second line agents in subsets of patients with these diseases, reducing long-term toxicity and improving outcomes.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating, and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Laura Murch, PhD, and Laura Gardner, PhD, CMPP, at Fishawack Indicia, a part of Fishawack Health, and was funded by GSK. The Division of Intramural Research, NIAID, NIH funded time spent on this article by one of the authors (PK).
Financial support:
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Laura Murch, PhD, and Laura Gardner, PhD, CMPP, at Fishawack Indicia, a part of Fishawack Health, and was funded by GlaxoSmithKline. The Division of Intramural Research, NIAID, NIH funded time spent on this article by one of the authors (PK). All authors approved the manuscript for submission and made the final decision to submit.
Abbreviations
- AAV
anti-neutrophil cytoplasmic antibody-associated vasculitis
- AEC
absolute eosinophil count
- ANCA
anti-neutrophil cytoplasmic antibody
- BALF
bronchoalveolar lavage fluid
- CT
computed tomography
- EGPA
eosinophilic granulomatosis with polyangiitis
- FFS
five factor scoring system
- FGFR1
fibroblast growth factor receptor 1
- FIP1L1
FIP1-like 1
- FISH
fluorescence in situ hybridization
- HES
hypereosinophilic syndrome
- HU
hydroxyurea
- ICS
inhaled corticosteroids
- ICU
intensive care unit
- IFN-α
interferon-α
- I-HES
idiopathic HES
- IL
interleukin
- IRB
institutional review board
- JAK
Janus kinase
- LABA
long-acting beta-2-agonist
- L-HES
lymphocytic HES
- M-HES
myeloid HES
- MPO
myeloperoxidase
- MRI
magnetic resonance imaging
- NGS
next generation sequencing
- OCS
oral corticosteroid
- PCR
polymerase chain reaction
- PDGFRA
platelet-derived growth factor receptor alpha
- PDGFRB
platelet-derived growth factor receptor beta
- TCR
T-cell receptor
Footnotes
Conflict of interest disclosure: All authors had access to clinical data, take responsibility for the accuracy of data and had authority over manuscript preparation. PK reports employer and salary support for time spent on this article from the Division of Intramural Research, NIAID, NIH, and grant funding from APFED. PA reports advisory board membership, research funding and personal fees from GSK, advisory board membership, research funding and personal fees from AstraZeneca, research funding from Regeneron, research funding from NIH, personal fees from Sanofi, honoraria from AHK, MJH Lifesciences, Prime CME, Projects in Knowledge, Rockpointe, Vindico CME and WebMD/Medscape, royalties from UpToDate and is an Assembly Program Committee Volunteer for the American Thoracic Society. NK and JS were employees of GSK at the time of writing and own stocks/shares. FR reports advisory board membership, consultancy fees and honoraria from AstraZeneca and GSK and grants from Fonds de la Recherche Scientifique (FNRS) for time spent writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wechsler ME, Munitz A, Ackerman SJ, et al. Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clinic proceedings. 2021;96:2694–2707. [DOI] [PubMed] [Google Scholar]
- 2.Klion AD, Ackerman SJ, Bochner BS. Contributions of Eosinophils to Human Health and Disease. Annual review of pathology. 2020;15:179–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovalszki A, Weller PF. Eosinophilia. Prim Care. 2016;43:607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94:1149–1167. [DOI] [PubMed] [Google Scholar]
- 5.Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. The Journal of allergy and clinical immunology. 2012;130:607–612.e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis C, Ogbogu P. Hypereosinophilic Syndrome. Clin Rev Allergy Immunol. 2016;50:240–251. [DOI] [PubMed] [Google Scholar]
- 7.Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy. 2013;68:261–273. [DOI] [PubMed] [Google Scholar]
- 8.Podjasek JC, Butterfield JH. Mortality in hypereosinophilic syndrome: 19 years of experience at Mayo Clinic with a review of the literature. Leuk Res. 2013;37:392–395. [DOI] [PubMed] [Google Scholar]
- 9.Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis and rheumatism. 2013;65:270–281. [DOI] [PubMed] [Google Scholar]
- 10.Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994;83:2759–2779. [PubMed] [Google Scholar]
- 11.Marques CC, Fernandes EL, Miquelin GM, Colferai MMT. Cutaneous manifestations of Churg-Strauss syndrome: key to diagnosis. An Bras Dermatol. 2017;92:56–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durel CA, Berthiller J, Caboni S, Jayne D, Ninet J, Hot A. Long-Term Followup of a Multicenter Cohort of 101 Patients With Eosinophilic Granulomatosis With Polyangiitis (Churg-Strauss). Arthritis care & research. 2016;68:374–387. [DOI] [PubMed] [Google Scholar]
- 13.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. The Journal of allergy and clinical immunology. 2009;124:1319–1325.e1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raffray L, Guillevin L. Treatment of Eosinophilic Granulomatosis with Polyangiitis: A Review. Drugs. 2018;78:809–821. [DOI] [PubMed] [Google Scholar]
- 15.Carpentier C, Verbanck S, Schandené L, et al. Eosinophilia Associated With CD3(−)CD4(+) T Cells: Characterization and Outcome of a Single-Center Cohort of 26 Patients. Frontiers in immunology. 2020;11:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohmer J, Couteau-Chardon A, Trichereau J, et al. Epidemiology, clinical picture and long-term outcomes of FIP1L1-PDGFRA-positive myeloid neoplasm with eosinophilia: Data from 151 patients. Am J Hematol. 2020;95:1314–1323. [DOI] [PubMed] [Google Scholar]
- 17.Kahn JE, Groh M, Lefèvre G. (A Critical Appraisal of) Classification of Hypereosinophilic Disorders. Frontiers in medicine. 2017;4:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury P, Zagallo P, Talar-Williams C, et al. Serum biomarkers are similar in Churg-Strauss syndrome and hypereosinophilic syndrome. Allergy. 2012;67:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons PA, Peters JE, Alberici F, et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat Commun. 2019;10:5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang HC, Chou PC, Lai CY, Tsai HH. Antineutrophil Cytoplasmic Antibodies and Organ-Specific Manifestations in Eosinophilic Granulomatosis with Polyangiitis: A Systematic Review and Meta-Analysis. The journal of allergy and clinical immunology. In practice. 2021;9:445–452. [DOI] [PubMed] [Google Scholar]
- 21.Moiseev S, Bossuyt X, Arimura Y, et al. International Consensus on ANCA Testing in Eosinophilic Granulomatosis with Polyangiitis. American journal of respiratory and critical care medicine. 2020: 10.1164/rccm.202005-201628SO. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg CE, Khoury P. Approach to Eosinophilia Presenting With Pulmonary Symptoms. Chest. 2021;159:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen RL, Savage NM. How I investigate Eosinophilia. Int J Lab Hematol. 2019;41:153–161. [DOI] [PubMed] [Google Scholar]
- 24.Khoury P, Bochner BS. Consultation for Elevated Blood Eosinophils: Clinical Presentations, High Value Diagnostic Tests, and Treatment Options. The journal of allergy and clinical immunology. In practice. 2018;6:1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klion AD. How I treat hypereosinophilic syndromes. Blood. 2015;126:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. [DOI] [PubMed] [Google Scholar]
- 27.Khoury P, Desmond R, Pabon A, et al. Clinical features predict responsiveness to imatinib in platelet-derived growth factor receptor-alpha-negative hypereosinophilic syndrome. Allergy. 2016;71:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roufosse F, Cogan E, Goldman M. Lymphocytic variant hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:389–413. [DOI] [PubMed] [Google Scholar]
- 29.Carpentier C, Schandené L, Dewispelaere L, Heimann P, Cogan E, Roufosse F. CD3(−)CD4(+) Lymphocytic Variant Hypereosinophilic Syndrome: Diagnostic Tools Revisited. The journal of allergy and clinical immunology. In practice. 2021;9:2426–2439.e2427. [DOI] [PubMed] [Google Scholar]
- 30.Khoury P, Abiodun AO, Holland-Thomas N, Fay MP, Klion AD. Hypereosinophilic Syndrome Subtype Predicts Responsiveness to Glucocorticoids. The journal of allergy and clinical immunology. In practice. 2018;6:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ZwerinJ, StrehD, BeyeC, Schet G. Can ANCA differentiate eosinophilic granulomatosis with polyangiitis (Churg-Strauss) from idiopathic hypereosinophilic syndrome? Clin Exp Rheumatol. 2013;31:989–990. [PubMed] [Google Scholar]
- 32.Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Toumelin PL. The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine. 2011;90:19–27. [DOI] [PubMed] [Google Scholar]
- 33.Pagnoux C, Nair P, Xi Y, et al. Serum cytokine and chemokine levels in patients with eosinophilic granulomatosis with polyangiitis, hypereosinophilic syndrome, or eosinophilic asthma. Clin Exp Rheumatol. 2019;37 Suppl 117:40–44. [PMC free article] [PubMed] [Google Scholar]
- 34.Leurs A, Chenivesse C, Lopez B, et al. C-Reactive protein as a diagnostic tool in differential diagnosis of hypereosinophilic syndrome and antineutrophil cytoplasmic antibody-negative eosinophilic granulomatosis with polyangiitis. The journal of allergy and clinical immunology. In practice. 2019;7:1347–1351.e1343. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki H, Miyata J, Suematsu R, et al. Radiological significance of mediastinal lymphadenopathy in eosinophilic granulomatosis with polyangiitis. Allergol Int. 2022;71:536–538. [DOI] [PubMed] [Google Scholar]
- 36.Grayson PC, Ponte C, Suppiah R, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis With Polyangiitis. Arthritis & rheumatology (Hoboken, N.J.). 2022;74:386–392. [DOI] [PubMed] [Google Scholar]
- 37.Dejaco C, Oppl B, Monach P, et al. Serum biomarkers in patients with relapsing eosinophilic granulomatosis with polyangiitis (Churg-Strauss). PloS one. 2015;10:e0121737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groh M, Pagnoux C, Baldini C, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. European journal of internal medicine. 2015;26:545–553. [DOI] [PubMed] [Google Scholar]
- 39.Kovacs N, Benjamin K, Holland-Thomas N, et al. Symptom assessment in hypereosinophilic syndrome: Toward development of a patient-reported outcomes tool. The journal of allergy and clinical immunology. In practice. 2020;8:3209–3212.e3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275–289; quiz 290. [DOI] [PubMed] [Google Scholar]
- 41.Helbig G Imatinib for the treatment of hypereosinophilic syndromes. Expert review of clinical immunology. 2018;14:163–170. [DOI] [PubMed] [Google Scholar]
- 42.Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. The Journal of allergy and clinical immunology. 2010;125:1344–1353.e1342. [DOI] [PubMed] [Google Scholar]
- 43.Pardanani A, Reeder T, Porrata LF, et al. Imatinib therapy for hypereosinophilic syndrome and other eosinophilic disorders. Blood. 2003;101:3391–3397. [DOI] [PubMed] [Google Scholar]
- 44.Müller AM, Martens UM, Hofmann SC, Bruckner-Tuderman L, Mertelsmann R, Lübbert M Imatinib mesylate as a novel treatment option for hypereosinophilic syndrome: two case reports and a comprehensive review of the literature. Annals of hematology. 2006;85:1–16. [DOI] [PubMed] [Google Scholar]
- 45.FDA. Imatinib Mesylate (Gleevec) Prescribing Information 2016. [Google Scholar]
- 46.Walker S, Wang C, Walradt T, et al. Identification of a gain-of-function STAT3 mutation (p.Y640F) in lymphocytic variant hypereosinophilic syndrome. Blood. 2016;127:948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King B, Lee AI, Choi J. Treatment of Hypereosinophilic Syndrome with Cutaneous Involvement with the JAK Inhibitors Tofacitinib and Ruxolitinib. The Journal of investigative dermatology. 2017;137:951–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butterfield JH, Weiler CR. Use of pegylated interferon in hypereosinophilic syndrome. Leuk Res. 2012;36:192–197. [DOI] [PubMed] [Google Scholar]
- 49.Dahabreh IJ, Giannouli S, Zoi C, Zoi K, Voulgarelis M, Moutsopoulos HM. Management of hypereosinophilic syndrome: a prospective study in the era of molecular genetics. Medicine. 2007;86:344–354. [DOI] [PubMed] [Google Scholar]
- 50.Nozaki Y New Insights Into Novel Therapeutic Targets in ANCA-Associated Vasculitis. Frontiers in immunology. 2021;12:631055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohammad AJ, Hot A, Arndt F, et al. Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis (Churg-Strauss). Ann Rheum Dis. 2016;75:396–401. [DOI] [PubMed] [Google Scholar]
- 52.Teixeira V, Mohammad AJ, Jones RB, Smith R, Jayne D. Efficacy and safety of rituximab in the treatment of eosinophilic granulomatosis with polyangiitis. RMD Open. 2019;5:e000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canzian A, Venhoff N, Urban ML, et al. Use of Biologics to Treat Relapsing and/or Refractory Eosinophilic Granulomatosis With Polyangiitis: Data From a European Collaborative Study. Arthritis & rheumatology (Hoboken, N.J.). 2021;73:498–503. [DOI] [PubMed] [Google Scholar]
- 54.Houot R, Levy R, Cartron G, Armand P. Could anti-CD20 therapy jeopardise the efficacy of a SARS-CoV-2 vaccine? European journal of cancer (Oxford, England : 1990). 2020;136:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roufosse F, Kahn JE, Rothenberg ME, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial. The Journal of allergy and clinical immunology. 2020;146:1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med. 2017;376:1921–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–1228. [DOI] [PubMed] [Google Scholar]
- 58.PMDA. Mepolizumab (Nucala) Prescribing Information (Japan) 2020. [Google Scholar]
- 59.EMA. Mepolizumab (Nucala) Summary of Product Characteristics2021. [Google Scholar]
- 60.FDA. Mepolizumab (Nucala) Prescribing Information2022. [Google Scholar]
- 61.Kim YJ, Prussin C, Martin B, et al. Rebound eosinophilia after treatment of hypereosinophilic syndrome and eosinophilic gastroenteritis with monoclonal anti-IL-5 antibody SCH55700. The Journal of allergy and clinical immunology. 2004;114:1449–1455. [DOI] [PubMed] [Google Scholar]
- 62.Mank LA, Guntur VP, Denson JL, et al. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2021;126:696–701.e691. [DOI] [PubMed] [Google Scholar]
- 63.Klion AD, Law MA, Noel P, Kim YJ, Haverty TP, Nutman TB. Safety and efficacy of the monoclonal anti-interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood. 2004;103:2939–2941. [DOI] [PubMed] [Google Scholar]
- 64.Kuang FL, Legrand F, Makiya M, et al. Benralizumab for PDGFRA-Negative Hypereosinophilic Syndrome. N Engl J Med. 2019;380:1336–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guntur VP, Manka LA, Denson JL, et al. Benralizumab as a Steroid-Sparing Treatment Option in Eosinophilic Granulomatosis with Polyangiitis. The journal of allergy and clinical immunology. In practice. 2021;9:1186–1193. [DOI] [PubMed] [Google Scholar]
- 66.Panch SR, Bozik ME, Brown T, et al. Dexpramipexole as an oral steroid-sparing agent in hypereosinophilic syndromes. Blood. 2018;132:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dellon ES, Peterson KA, Murray JA, et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N Engl J Med. 2020;383:1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chung SA, Langford CA, Maz M, et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis & rheumatology (Hoboken, N.J.). 2021;73:1366–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terrier B, Pugnet G, de Moreuil C, et al. Rituximab versus Conventional Therapeutic Strategy for Remission Induction in Eosinophilic Granulomatosis with Polyangiitis: A Double-blind, Randomized, Controlled Trial [abstract]. Arthritis & rheumatology (Hoboken, N.J.). 2021;73 (suppl. 10). [Google Scholar]
- 70.Wechsler ME, Fulkerson PC, Bochner BS, et al. Novel targeted therapies for eosinophilic disorders. The Journal of allergy and clinical immunology. 2012;130:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ackerman SJ, Bochner BS. Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol Allergy Clin North Am. 2007;27:357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.FDA. Rezslizumab (Cinqaero) Prescribing Information2019. [Google Scholar]
- 73.Roufosse F, de Lavareille A, Schandene L, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. The Journal of allergy and clinical immunology. 2010;126:828–835 e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roufosse FE, Kahn JE, Gleich GJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. The Journal of allergy and clinical immunology. 2013;131:461–467 e461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gleich GJ, Roufosse F, Chupp G, et al. Safety and efficacy of mepolizumab in hypereosinophilic syndrome: an open-label extension study. The journal of allergy and clinical immunology. In practice. 2021;9:4431–4440. [DOI] [PubMed] [Google Scholar]
- 76.Steinfeld J, Bradford ES, Brown J, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. The Journal of allergy and clinical immunology. 2019;143:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castro M, Corren J, Pavord ID, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018;378:2486–2496. [DOI] [PubMed] [Google Scholar]
- 78.Wechsler ME, Klion AD, Paggiaro P, et al. Effect of Dupilumab on Blood Eosinophil Counts in Patients With Asthma, Chronic Rhinosinusitis With Nasal Polyps, Atopic Dermatitis, or Eosinophilic Esophagitis. The journal of allergy and clinical immunology. In practice. 2022;10:2695–2709. [DOI] [PubMed] [Google Scholar]
- 79.Galant-Swafford J, Geng B, Leibel S, et al. Two pediatric cases of ANCA-negative eosinophilic granulomatosis with polyangiitis successfully treated with dupilumab. The journal of allergy and clinical immunology. In practice. 2020;8:3643–3646 e3641. [DOI] [PubMed] [Google Scholar]
- 80.Adachi S, Oshikata C, Kaneko T, Tsurikisawa N. Rituximab and dupilumab improve eosinophilic granulomatosis with polyangiitis with multiple pulmonary thrombi. Allergy Asthma Clin Immunol. 2022;18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matucci A, Bormioli S, Bercich L, et al. Effect of dupilumab treatment in a severe asthma patient with EGPA. The journal of allergy and clinical immunology. In practice. 2021;9:3824–3825. [DOI] [PubMed] [Google Scholar]
- 82.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacology & therapeutics. 2012;135:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Groh M, Masciocco G, Kirchner E, et al. Heart transplantation in patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33:842–850. [DOI] [PubMed] [Google Scholar]
- 84.Klion A Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology. American Society of Hematology. Education Program. 2018;2018:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y, Guo X, Zhou J, et al. Cardiac Involvement in Eosinophilic Granulomatosis With Polyangiitis: A Retrospective Study in the Chinese Population. Frontiers in medicine. 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reiter A, Gotlib J. Myeloid neoplasms with eosinophilia. Blood. 2017;129:704–714. [DOI] [PubMed] [Google Scholar]