Abstract
Context
Type 1 diabetes (T1D) is associated with alterations of the immune response which persist even after the autoimmunity aspect is resolved. Clinical factors that cause dysregulation, however, are not fully understood.
Objective
To identify clinical factors that affect immune dysregulation in people with longstanding T1D.
Design
In this cross-sectional study, 243 participants with longstanding T1D were recruited between February 2016 and June 2017 at the Radboudumc, the Netherlands. Blood was drawn to determine immune cell phenotype and functionality, as well as circulating inflammatory proteome. Multivariate linear regression was used to determine the association between glycated hemoglobin (HbA1c) levels, duration of diabetes, insulin need, and diabetes complications with inflammation.
Results
HbA1c level is positively associated with circulating inflammatory markers (P < .05), but not with immune cell number and phenotype. Diabetes duration is associated with increased number of circulating immune cells (P < .05), inflammatory proteome (P < .05), and negatively associated with adaptive immune response against Mycobacterium tuberculosis and Rhizopus oryzae (P < .05). Diabetes nephropathy is associated with increased circulating immune cells (P < .05) and inflammatory markers (P < .05)
Conclusion
Disease duration and chronic complications associate with persistent alterations in the immune response of individuals with long standing T1D.
Keywords: diabetes, diabetes complications, inflammation, proteomics
Diabetes is a highly prevalent disease accompanied by chronically elevated plasma glucose levels. Hyperglycemia is the driver of microvascular complications associated with diabetes and has also been implied to be a risk factor for macrovascular complications like stroke (1).
It has been well established that the presence of diabetes impacts the immune system (2). First, the immune system is involved in the development of the disease, particularly in individuals with type 1 diabetes (T1D), an autoimmune disorder. Activation of the immune system leads to the destruction of beta-cell function and loss of insulin production. In addition, many diabetes complications seem to be driven by altered responses of the immune system. For example, the presence of diabetes is accompanied by an increased number of white blood cells and by increased circulating levels of inflammatory proteins suggestive of a chronic immune activation (3, 4). This proinflammatory phenotype has been associated with accelerated vascular inflammation (5, 6), leading to cardiovascular morbidity and mortality, and may also play a role in the development of microvascular complications (7).
Interestingly, diabetes, particularly poorly controlled diabetes, is associated with an increased susceptibility to infections in general (8, 9), and some fatal infections in particular, as recently again demonstrated by the COVID-19 pandemic (10). These findings imply not only a chronic activation of the immune system, yet also the inability to respond maximally to external stimuli, including pathogens during infection.
While altered responses of the immune system play an important role in various complications associated with diabetes, the underlying mechanisms involved are not completely understood. For example, how the level of glycemia or disease duration would impact various parameters of the immune system is unknown. Identification of factors that drive immune dysfunction is imperative to develop potential treatment strategies to prevent complications.
The proinflammatory phenotype has been observed both for type 1 and type 2 diabetes; while people with type 2 diabetes are often obese and obesity has also been identified as a low-grade inflammatory state, there is no doubt that hyperglycemia itself is an important inflammatory driver (11). Hence, T1D, in essence a state of “pure” chronic hyperglycemia, represents a good model to study the interaction between hyperglycemia and the inflammatory system.
In the present study, we performed multidimensional immunophenotyping using a functional immunology approach (12) in a large cohort of individuals with longstanding T1D, to determine associations between the clinical factors associated with diabetes and various immune readouts.
Methods
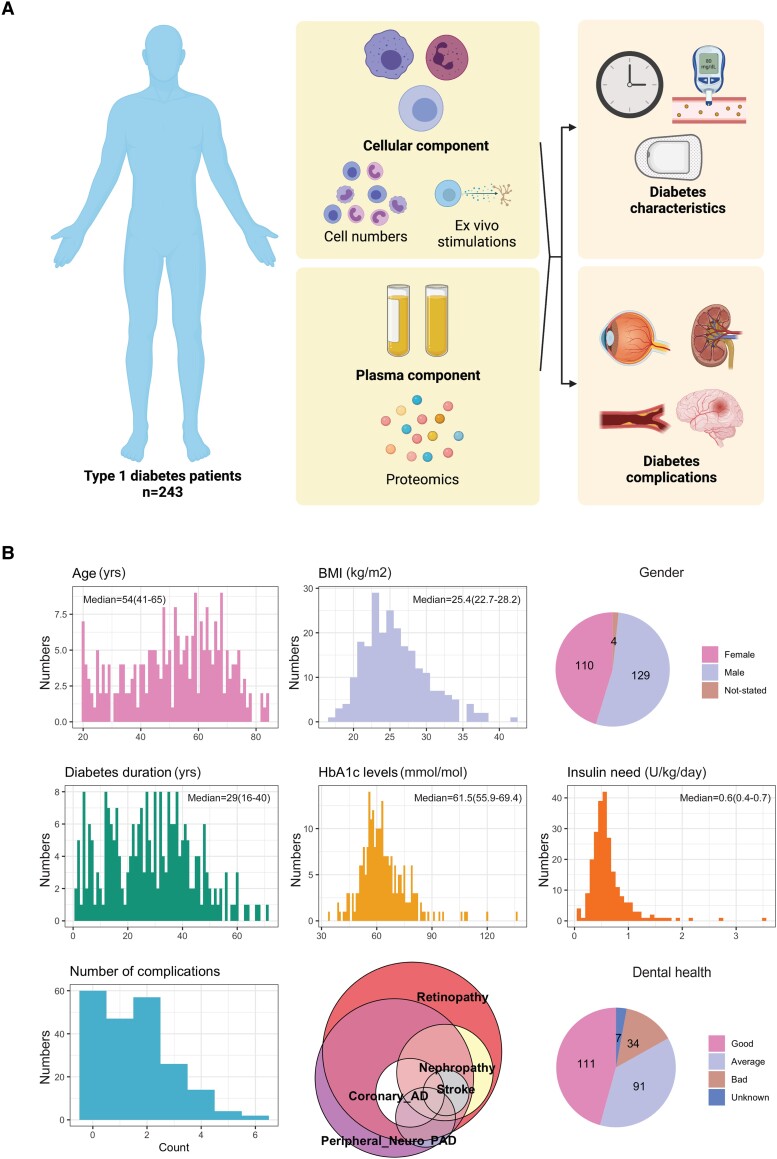
We performed a multidimensional characterization of immune function in our cohort (outline of measurements: Fig. 1A). This included determination of immune cell compositions, measurements of immune cell function through ex vivo stimulation, and quantification of circulating inflammatory proteome, as described in detail below.
Figure 1.
A, Study design and methods. B, distribution of the clinical data.
Study Population
The cohort consists of people with T1D older than 18 years. Patients were recruited from the outpatient clinic of Radboudumc, Nijmegen, The Netherlands. All participants have a positive T1D diagnosis based on all accepted clinical criteria, with or without anti-GAD positivity. Exclusion criteria are 1) history of infectious disease 4 weeks prior to the study inclusion and 2) pregnancy. If a participant had a fever in the week before the appointment date, the tests were rescheduled. During the appointment, participants were asked to fill in a questionnaire about their general health data such as age, gender, body mass index (BMI), smoking status, dental health, age of diabetes onset, duration of diabetes, and insulin dose. Additional information which includes glycated hemoglobin (HbA1c) and history of diabetes-related complications were obtained from clinical records. This study was approved by the institutional review board of the Radboud University Medical Centre and was conducted in accordance with the principles of human experimentation as stated in the Declaration of Helsinki. No formal sample size calculation was performed due to the multidimensionality of the outcomes measured (immune cell phenotype, proteome, and ex vivo stimulations). Thus, sample size was decided based on previous studies from the human functional genomics, which the cohort is a part of (http://www.humanfunctionalgenomics.org/site/) (13).
In total, 243 T1D patients were recruited in the study in the period from February 2016 to June 2017. An overview of the characteristics of the study participants can be found in Table 1 and in Fig. 1B. The median of diabetes duration in our cohort was 29 (14-38) years and the median HbA1c level of the study cohort was 61.5 (55.9-69.4 mmol/mol. A total of 161 participants were diagnosed with at least one diabetes complication, including microvascular complications such as retinopathy and macrovascular complications such as coronary artery disease and stroke (Fig. 1B).
Table 1.
Clinical characteristics of the cohort
| General characteristics | Median (IQR) |
|---|---|
| Age, yrs | 54 (41-65) |
| Sex, female, % | 44.70% |
| BMI, kg/m2 | 25.4 (22.7-28.2) |
| HbA1c (%) HbA1c, mmol/mol |
7.8% (7.3-8.5) 61.5 (55.9-69.4) |
| Diabetes duration, yrs | 29 (16-40) |
| Insulin need, IU/kg | 0.6 (0.4-0.7) |
| Diabetes complications, % | |
| Retinopathy | 56% |
| Peripheral neuropathy | 45% |
| Nephropathy | 17% |
| Coronary arterial disease | 10% |
| Stroke | 6.2% |
| Peripheral arterial disease | 6.6% |
| Immune cell count, × 103/uL) | |
| Total white blood cells | 5.7 (4.9-7.0) |
| Neutrophils | 3.3 (2.7-4.4) |
| Lymphocytes | 1.6 (1.3-2.0) |
| Monocytes | 0.5 (0.4-0.6) |
Blood Collection and Isolation of Peripheral Blood Mononuclear Cells
Venous blood of the participants was drawn into 10 mL EDTA tubes (Vacutainer system; Becton Dickinson, Franklin Lakes, New Jersey) between 08:00 and 11:00 Am. White blood cell numbers and composition were measured from the whole blood using Sysmex XN-450 Hematology Analyzer (Sysmex Corp., Kobe, Japan). Peripheral blood mononuclear cells (PBMCs) were isolated from the whole blood using the Ficoll-Paque density gradient centrifugation method (GE Healthcare, Amersham, UK). The isolated PBMCs were washed twice with phosphate-buffered saline to remove platelets and other impurities. The cells were then resuspended in Roswell Park Memorial Institute (RPMI) 1640 Dutch-modified culture medium (Gibco/Invitrogen, Breda, The Netherlands), supplemented with 50 mg/L gentamycin, 1 mM pyruvate (Gibco/Invitrogen), and 2 mM L-glutamine (Gibco/Invitrogen). The composition of the isolated PBMCs was determined using Sysmex XN-450 Hematology Analyser.
Ex Vivo Mononuclear Function
The PBMCs (500 000 cells) were seeded in round-bottom 96-well plates and stimulated using the stimulation panel as previously reported (39). In short, the cells were stimulated using, among other things, Toll-like receptor ligands (eg, lipopolysaccharide, pam3cys) and pathogenic stimuli (eg, Mycobacterium tuberculosis lysate, heat-inactivated Candida albicans hyphae) for 24 hours or 7 days. At the end of the incubation period, supernatant was collected and frozen at −20C for the measurement of cytokine production. The production of innate (tumor necrosis factor [TNF], interleukin [IL]-1β, and IL-6) and adaptive (interferon [IFN]γ, IL-17, IL-22) cytokines was measured in the supernatants using R&D Duoset enzyme-linked immunosorbent assay (R&D Duoset ELISA Systems) according to the manufacturers protocol. The research resource identifier (RRID) for all antibodies used in this study can be found in Table 2. If the measured response fell outside of the ELISA threshold, response was maximized at the threshold value. Stimulations in which >50% of the ELISA readings fell outside of the threshold were excluded from the final analysis.
Table 2.
Research resource identifier (RRID) of the antibodies used for cytokine measurements
| Antibody name | Antibody registry ID |
|---|---|
| Human IL-6 DuoSet ELISA Kit | RRID:AB_2814717 |
| Human IL-1beta DuoSet ELISA Kit | RRID:AB_2848158 |
| Human TNF-alpha DuoSet ELISA Kit | RRID:AB_2848160 |
| Human IL-17 DuoSet ELISA Kit | RRID:AB_2928042 |
| Human IL-22 DuoSet ELISA Kit | RRID:AB_2928043 |
| Human IFN-gamma DuoSet ELISA Kit | RRID:AB_2928044 |
| HumanIL-18bpa DuoSet ELISA Kit | RRID:AB_2928087 |
| Human CRP DuoSet ELISA Kit | RRID:AB_2928088 |
Circulating Inflammatory Marker Measurements
Circulating plasma inflammatory proteins were measured using the commercially available Olink Proteomics AB Inflammation Panel (92 inflammatory proteins) (Uppsala Sweden). Proteins are recognized by antibody pairs coupled to cDNA strands which bind in close proximity and extend by a polymerase reaction. Proteins were excluded for analysis when the detection level of 75% was not met. Quality control was performed by Olink Proteomics with 6 samples not passing the quality control and subsequently excluded from the analysis. Overall, 75 of the 92 (81.5%) proteins were detected in at least 75% of the plasma samples and included in the analysis.
The levels of IL-18 binding protein, IL-18, and high-sensitivity C-reactive protein (hsCRP) in the plasma were determined using sandwich ELISA (R&D Duoset ELISA Systems) according to the manufacturer's protocol. Three controls of pooled plasma from the cohort were included in each ELISA plate to enable cross-plate comparisons.
Statistical Analysis
All statistical analyses were performed using R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). Data distribution of the dataset was checked using the Shapiro-Wilk test. Multivariate linear regression was performed to determine the associations between the predictors and the outcomes in all parts of the study. The effect of different clinical factors on immune cell subsets, circulating inflammatory markers, and cytokine production upon ex vivo stimulations were determined using the following formula:
Outcome = (intercept) + age + BMI + sex + duration of diabetes + mean HbA1c + insulin need (+ seasonality, for ex vivo stimulation)
The effect of diabetes complications on immune phenotype was determined and corrected for age, BMI, sex, and diabetes-related factors (duration of diabetes, mean HbA1c levels, and insulin need). Missing data were handled for each comparison separately. Outliers in the dataset were removed before performing statistical analyses to minimize type 1 error. P values were adjusted for multiple testing using Benjamini-Hochberg correction with false discovery rate (FDR) of <.05 used as a threshold for statistical significance. Propensity score–matching was done using the matchit function from the “MatchIt” package from R with age, duration of diabetes, and HbA1c level used as predictors of the phenotype. Details of the statistical analysis can be found in Supplementary Tables 1-6 (40)
Results
Immune Cell Quantity
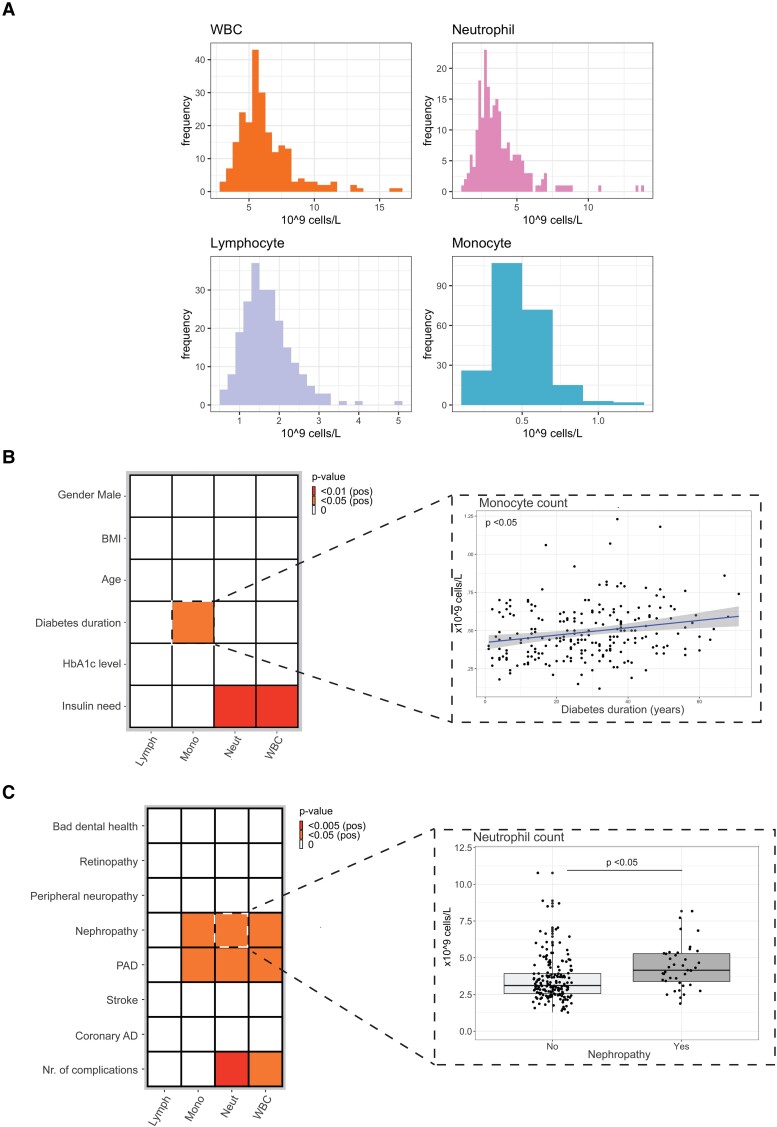
In total, 243 participants were included in the study. Using a flow cytometry-based method, we first determined the composition of immune cells in our cohort (Table 1). The average total white blood cell count within our cohort was 6.16 (±2.00) × 103 cells/uL. Neutrophil count was 3.74 (±1.72) × 103 cells/uL, while monocyte and lymphocyte counts were 0.49(±0.18) × 103 cells/uL and 1.69(±0.61) × 103 cells/uL, respectively (Table 1).
We then set out to associate diabetes duration, HbA1c level, and insulin need with circulating immune cell numbers, corrected for age, sex, and BMI. Our multivariate linear model revealed that total insulin need (U/kg/day) and duration of diabetes were positively associated with circulating immune cell counts (Fig. 2A and 2B). Whereas insulin need was positively associated with both neutrophil and total white blood cell counts (P value <.01 for both), duration of diabetes was associated with increased circulating monocyte counts (P value <.05) (Fig. 2A).
Figure 2.
A, The effect of diabetes duration, glycemia, and insulin need on circulating immune cells. B, Representative scatter plot showing the association between diabetes duration and monocyte counts. C, The effect of diabetes complications on circulating immune cells. D, Representative boxplot showing the association between peripheral arterial disease (PAD) with neutrophil counts. The exact P value of all statistical tests done in this study can be found in Supplementary Tables 1-6 (40).
In addition, we observed positive associations between the presence of diabetes complications and number of circulating immune cells (Fig. 2C). The presence of peripheral arterial disease (PAD) was particularly associated with circulating monocyte, neutrophils, and total white blood cell counts (P value <.05 for all) (Fig. 2C and 2D). The cumulative number of diabetes complications was also strongly associated with neutrophil and total white blood cell counts (P value <.005 and <.05, respectively).
Immune Cell Function
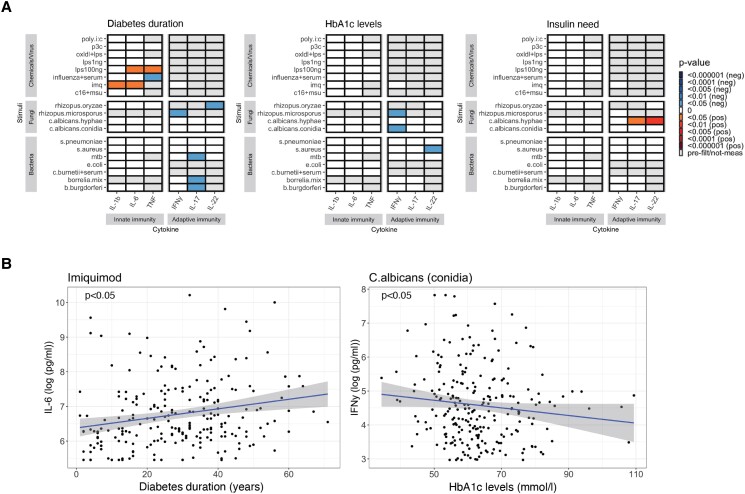
In addition to changes in immune cell numbers, we determined potential changes in immune cell functionality using cytokine release upon ex vivo stimulations as a readout. Duration of diabetes was associated with cytokine production upon Toll-like receptor (TLR) activation (Fig. 3A). Specifically, the productions of IL-6 and TNFα after TLR4 stimulation using lipopolysaccharide (100 ng) were positively associated with the duration of diabetes (P value <.05), as were TLR7 responses reflected by IL-1β and IL-6 after stimulation using imiquimod (P value <.05 for both) (Fig. 3A). Diabetes duration was also negatively associated with TNFα production against Influenza virus (P value <.05). Moreover, duration of diabetes was also negatively associated with adaptive immune response against Rhizopus microsporus, Rhizopus oryzae, M. tuberculosis, and Borrelia (P value <.05 for all).
Figure 3.
A, The effect of diabetes duration, glycemia, and insulin need on the immune cell response against pathogenic stimulations. B, 2 representative scatterplots of the associations between duration of diabetes and ex vivo IL6 production upon TLR7 activation and, glucose control with ex vivo IFNγ production in response to C. albicans.
Glycemia (mean HbA1c levels) and insulin need also impacted immune cell functionality, albeit to a lesser extent than diabetes duration. Cytokine responses against C. albicans and Staphylococcus aureus were linked with glycemia and insulin need (Fig. 3); glycemia was negatively associated with the production of IFNγ upon stimulation with C. albicans and R. microsporus, while insulin need is positively associated with the production of IL-17 and IL-22 against C. albicans.
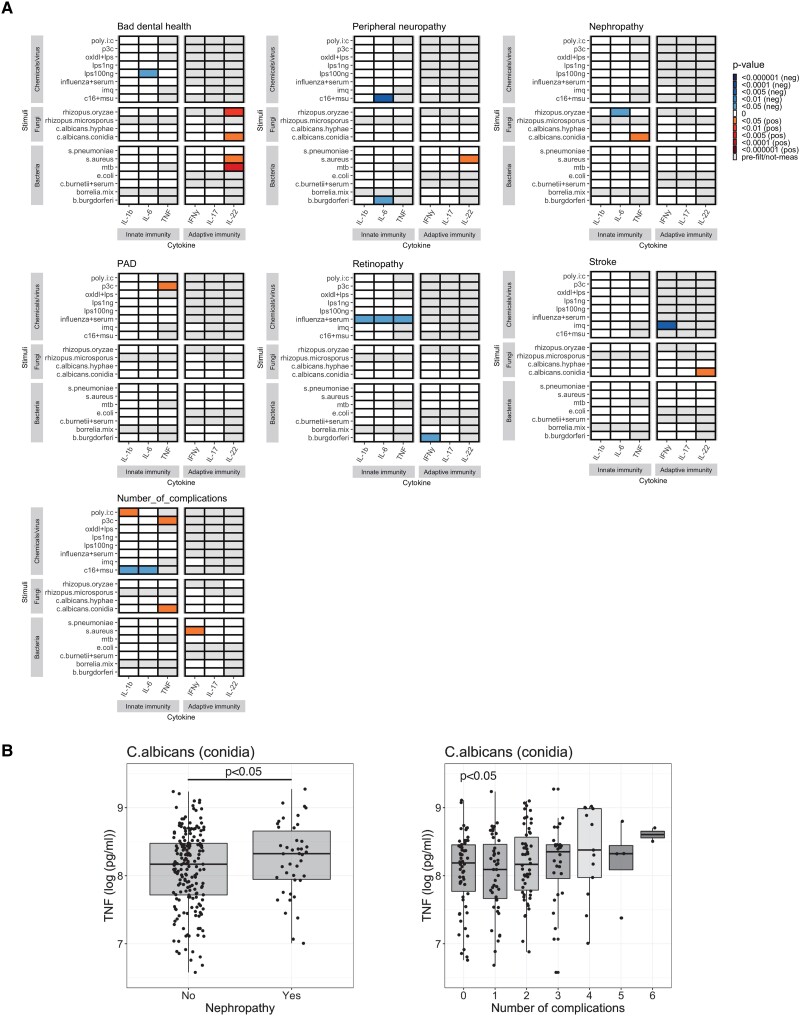
We observed a number of associations between diabetes complications and ex vivo immune cell function. Worsening of dental health status was positively associated with the response against C. albicans conidia, R. oryzae, S. aureus, and M. tuberculosis (P value <.05 for all) (Fig. 4). Limited associations were observed between nephropathy and peripheral artery disease (PAD) against cytokine responses to C. albicans and TLR2 agonist pam3cys, respectively (P value <.05 for both). Retinopathy was negatively associated with innate immune response against influenza and IFNγ production against Borrelia burgdorferi (P value .05 for all). And again, the number of complications was associated with the ex vivo cytokine response toward inflammasome activator (MSU crystal + palmitic acid), TLR1/2 agonist pam3cys, C. albicans, and S. aureus (P value <.05 for all) (Fig. 4).
Figure 4.
A, The link between diabetes complications and elevated immune cell response toward pathogenic stimulations. B, Representative boxplots of ex vivo TNFα production in response to C. albicans and number of complications.
Circulating Inflammatory Markers
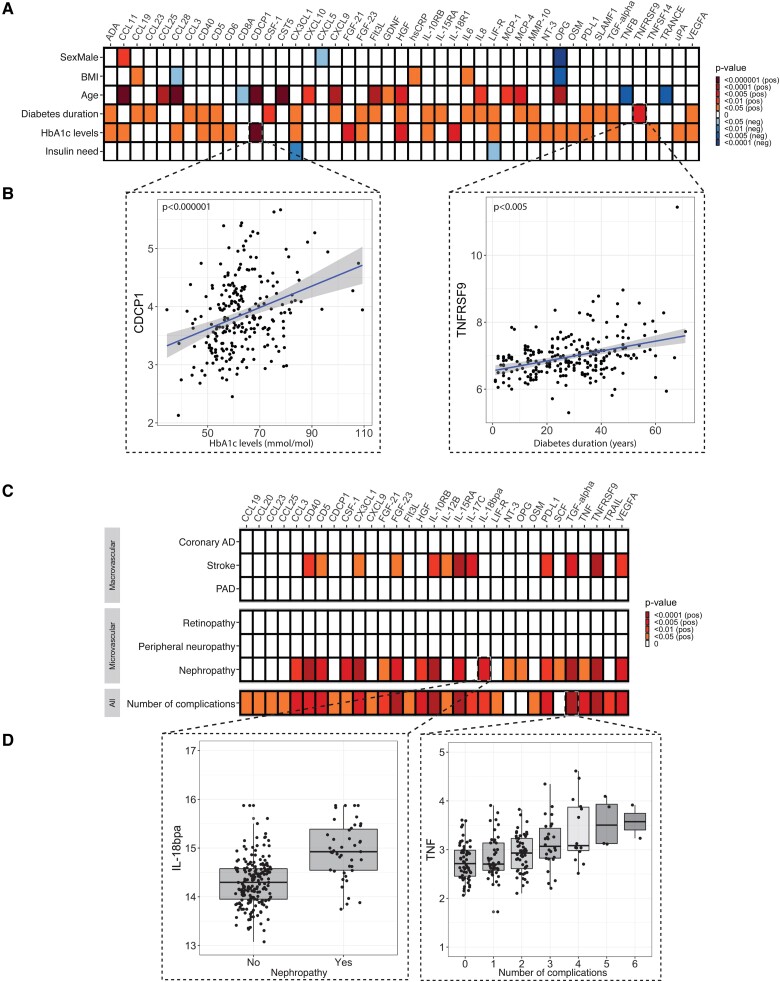
We also determined circulating inflammatory protein levels using a proteomics approach. Our targeted proteomics approach revealed that 24 out of 75 inflammatory proteins were positively associated with diabetes duration (Fig. 5A). Diabetes duration was associated with circulating chemokine ligands (eg, CCL19 and CCL23), inflammatory cytokines (eg, IL-6 and IL-8), and markers of immune cell activation (eg, PD-L1). Glycemia was associated with 23/75 proteins, eg, CDCP1, fibroblast growth factor (FGF)-21, hepatocyte growth factor (HGF) and TNFSF14. Insulin need was negatively associated with the circulating levels of CX3CL1 and LIF-R.
Figure 5.
A, The effect of diabetes duration, glycemia, and insulin need on circulating inflammatory markers. B, Representative scatterplots showing the associations between glycemic control (left) and diabetes duration (right) with inflammatory proteome. C, The effect of diabetes complications on circulating inflammatory markers and D, Representative boxplots of the associations between nephropathy and circulating IL-18 bp, and number of complications with TNF.
Two of the recorded diabetes complications, namely, nephropathy and stroke, were positively associated with circulating inflammatory markers (Fig. 5C). Nephropathy was positively associated with 19/75 proteins. Stroke was positively associated with 12/75 proteins, 10 of which (eg, CD5, CD40, FGF-21, and FGF-23) overlapped with the ones that associated with nephropathy. Other diabetes complications, both microvascular and macrovascular, as well as bad dental health did not associate with increased circulating inflammatory proteins (Fig. 5C). However, cumulative numbers of complications are highly associated with circulating inflammatory markers (28/75 proteins). Additional analysis on a propensity score–matched case-control group between participants that had at least one diabetes complication and those who did not (n = 118, Supplementary Fig. 1A) revealed that people with diabetes complication(s) had higher levels of circulating inflammatory markers than people who did not have complications (Supplementary Fig. 1B). Diabetes-related characteristics, like insulin need, also had a more robust association with inflammatory markers in T1D patients with diabetes complications than those without (Supplementary Fig. 1C) (14)
Discussion
The present study reveals a clear association between duration of diabetes with inflammation, as well as a relation between the presence of complications and inflammation. The impact of chronic glycemia (HbA1c) on inflammation is present, but less apparent. Together these findings suggest that diabetes leads to a proinflammatory state, and that this contributes to diabetes-related complications.
First, we observed positive, albeit limited, associations between glycemia and inflammation. It has been well established that chronically elevated levels of glucose are a strong risk factor for the development of various complications associated with diabetes (15). People with T1D have been shown to have a higher activated immune cell subsets than nondiabetic individuals (16). Our results reveal that level of glycemia does not linearly associate with circulating immune cell numbers and function, in agreement with earlier studies (17). This may suggest that any level of glycemia, regardless of its magnitude, is already a trigger for altering immune cell proliferation and/or demargination. The same holds true for the immune cell functionality, as shown by the ex vivo stimulation results: although we previously observed that people with T1D produce a less robust immune response against pathogen stimulation compared to healthy controls (18), here we show that this impairment is not linearly associated with glycemia. As such, changes in immune response in T1D may be influenced more by other factors such as the duration of chronic hyperglycemia, rather than the magnitude of the glycemia itself, as we will discuss later.
Our analysis did reveal a positive association between level of glycemia and circulating inflammatory proteome levels, including proteins that are associated with altered tissue functions such as hepatocyte growth factor (for liver abnormality) and FGF-21 (adipose and endothelial tissue activation) (19, 20). The relation between glycemia and tissue damage may be related to a number of underlying mechanisms. First, glycemia could lead to a higher production of inflammatory markers that subsequently lead to tissue damage. However, glycemia in itself may also cause tissue damage that subsequently leads to a higher production of proinflammatory markers. It may very well be that both mechanisms work in tandem, creating a negative feedback loop that worsens tissue damage and immune dysregulation. Additionally, glycemia may also not just result in tissue damage but may also change the baseline inflammatory capacity of the tissues. Other studies have also shown that, in vitro, hyperglycemia exposure modulates epigenetic reprogramming on endothelial cells and adipose progenitor cells (21, 22).
Second, we found diabetes duration to be associated with several immune-related parameters. In terms of immune cell composition, the number of circulating monocytes is positively associated with the duration of diabetes. A number of animal studies have shown that long exposure toward diabetes mellitus changes the proliferative and migratory capacity of immune progenitor cells (23, 24). These results suggest that it is not the degree of hyperglycemia itself that primes the changes in progenitor cells, but rather the duration of the exposure.
Our ex vivo stimulation model also showed a relation between diabetes duration and both innate and adaptive immune response. A positive relation between diabetes duration and prevalence of infectious disease such as tuberculosis has been previously described (25). Here, we found that diabetes duration affects both innate and adaptive immunity, albeit in the opposite manners; while diabetes duration was positively associated with innate immune response, the opposite can be observed with regard to adaptive immunity. We found lower productions of IFNγ, IL-17, and IL-22 when cells were stimulated with R. microsporus, M. tuberculosis, and R. oryzae, respectively. Both monocytes and lymphocytes express RAGE, receptor of advanced glycated products (AGE) that can induce proinflammatory responses (26, 27). However, hyperglycemia-induced metabolic stress has also been shown to impair antigen presenting capacity, leading to dampened proliferation of CD4+ T-cells (28). Thus, hyperglycemic exposure can have effects on different immune cell subsets depending on the signaling cascades it triggers. These weakened adaptive responses against pathogenic stimulations in people with longer diabetes duration may explain why people with longstanding diabetes have a significantly higher risk for developing diseases like mucormycosis and tuberculosis (9, 25, 29).
The fact that we could observe associations between immune cell functionality and diabetes duration in our ex vivo model, which was done under standard cell culture condition, shows that prolonged hyperglycemic exposure can have a long-term impact on the immune response. This notion also comes in agreement with other in vitro and in vivo studies on the effect of long-term hyperglycemia on inflammation (30, 31). Moreover, we also found associations between diabetes duration and proteome markers produced by the bone marrow, such as FGF-23. It is therefore likely that prolonged exposure of hyperglycemia results in the reprogramming of immune progenitor cells through epigenetic changes, altering the immune response.
Third, we observed limited associations between insulin need and inflammation. Insulin need is a surrogate marker of insulin resistance and was associated with circulating neutrophil numbers, adaptive immune response against C. albicans, and with hsCRP but not with the other circulating inflammatory proteins. The effect of insulin resistance on inflammation in T1D may be less robust than in type 2 diabetes. The association we found between insulin need and inflammation may be driven by confounders such as sedentary lifestyle that we did not correct for in our model. However, insulin has also been shown to directly alter immune cell phenotype and functionality (32). The activation of insulin receptors in immune cells results in increased migration of monocytes (32), T-cell proliferation (33), and organ infiltration of different immune cell subsets (34). Our results show that the relation between immune cell functionality and insulin may be chronic, as we could observe limited but consistent positive associations between insulin need and adaptive immune response against Candida in our ex vivo model, even though we did not add insulin into the cell culture. This finding highlights the possibility of a sustained effect of insulin on immunity that may be driven by epigenetic changes in the progenitor cells.
Last, we found several associations between the presence of diabetes complications and changes in immune phenotypes. Independent of glycemia and diabetes duration, nephropathy and number of complications were positively associated with the levels of circulating monocytes and neutrophils. The association between aberrant immune system and diabetes complications has been previously explored (35, 36). Increases in circulating immune cell counts have been shown to occur during the onset of stroke and also early-stage diabetic nephropathy (35, 36), which highlight the potentials of using cell counts as a marker for the progression of diabetes complications (37). Here, we show that the increase is sustained after the disease onset, which may result in a further progression in tissue damage.
At the functional level, we found that the number of complications was positively associated with both adaptive and innate immune response against a number of ex vivo stimulations. This may be caused by the overactivation of immune cells and their progenitors by damage-associated molecular patterns (DAMPs) released from damaged tissue. This notion is supported by our findings on the positive associations between several circulating inflammatory proteins and chemokines like CCL3 and CCL20 with diabetes complications. Thus, theoretically, this would imply that normalization of immune response during the early stage of diabetes complications may potentially halt the progression of diabetes complications.
We also found MMP-10, a protein that is associated with early signs of chronic kidney damage (38), to be associated with nephropathy in our cohort. As diabetic nephropathy significantly decreases the life expectancy of diabetic individuals, utilization of biomolecules or other markers to detect early signs of chronic kidney disease can be useful in preventing the progression of this disease. Future studies should focus on assessing the utilization of MMP-10 in detecting early signs of chronic kidney disease in patients with diabetes.
The relation between T1D and inflammation has previously been described (31, 41-43). Type 1 diabetes is associated with an increase in ligands of Toll-like receptors (TLR) 2 and 4 (41), soluble intracellular adhesion molecule type 1 (sICAM-1), soluble intracellular adhesion molecule type 1 (sICAM-1) (31), and high-sensitivity C-reactive proteins (hsCRP) (31, 42, 43) in either children or adults. The driving factors behind the increase of these inflammatory markers, however, have not been extensively described. In this study, we performed multidimensional phenotyping and patient characterization to determine how diabetes duration, HbA1c levels, insulin need, and presence of diabetes complications can affect the degree of inflammation in adults with longstanding T1D.
Previous studies have shown the association between HbA1c levels and BMI on a limited numbers of inflammatory markers such as sICAM-1 and hsCRP (31, 42). Our extensive analysis, however, suggests that duration of diabetes, and therefore exposure time toward abnormal glucose levels, plays a more important role in altering immune responses, rather than glycemia itself. Moreover, we also confirmed previous findings on the relation between diabetes complications with inflammation. Measuring circulating white blood cells may thus provide an inexpensive way to determine the degree of chronic inflammation due to its association with different complications we found. It can potentially serve as a predictive value, as changes in immune cells may precede the development of complications. It would therefore be interesting to study how management of chronic inflammation alongside glucose control can affect the development of complications in people with longstanding T1D. Future studies should also focus on elucidating the effect of duration of glycemia exposure toward epigenetic rewiring on the immune response of immune progenitor and other inflammatory cells.
This study has a number of limitations and strengths. One of the limitations of our study is the cross-sectional design, which makes it difficult to infer causality between immune system dysregulation and the different factors we studied. Secondly, the ex vivo model that we used did not emulate the physiological condition in T1D, as we did not culture the immune cells in the presence of insulin or excess glucose.
This study has also strengths. First, we performed multidimensional immunophenotype characterization on a large cohort. The alterations of immune system in people with diabetes mellitus involve both long-term alterations that result in low-grade chronic inflammation, as well as changes in the acute immune response against pathogens. By studying both the baseline inflammatory status through circulating immune cell numbers, and inflammatory markers, as well as immune cell functionality, we could get a better understanding on how longstanding T1D affects the immune response. Second, this study was performed in a cohort of people with longstanding T1D. Many immunological studies in T1D have focused on the early stage of the disease, due to the important role autoimmunity plays during the disease onset. However, dysregulation of the immune system in T1D continues, even after the beta cells are diminished. Our study offers a glimpse on how long-term exposure toward T1D affect the immune system at multiple levels. Third, by performing the study on T1D, in essence a state of chronic hyperglycemia, we focus on the effect of glucose control on the immune system with less interference from other metabolic alterations like dyslipidemia, which occur in type 2 diabetes.
In summary, the present study reveals a clear association between duration of diabetes and presence of complications and several readouts of immune activity. The impact of chronic glycemia (HbA1c) is present, but less apparent. Together these findings suggest that activation of the immune system plays a role in diabetes-associated long-term complications.
Acknowledgments
The authors thank all the participants for their contributions in the study. The authors would also thank Helga Dijkstra and Heidi Lemmers for their assistance during the experimental bench work.
Abbreviations
- BMI
body mass index
- ELISA
enzyme-linked immunosorbent assay
- FGF
fibroblast growth factor
- HbA1c
glycated hemoglobin
- hsCRP
high-sensitivity C-reactive protein
- IFN
interferon
- IL
interleukin
- PBMC
peripheral blood mononuclear cell
- T1D
type 1 diabetes
- TLR
toll-like receptor
- TNF
tumor necrosis factor
Contributor Information
Mandala Ajie, Department of Internal Medicine, Radboud University Medical Centre, 6525 GA Nijmegen, The Netherlands.
Julia I P van Heck, Department of Internal Medicine, Radboud University Medical Centre, 6525 GA Nijmegen, The Netherlands.
Anna W M Janssen, Department of Internal Medicine, Radboud University Medical Centre, 6525 GA Nijmegen, The Netherlands.
Rick I Meijer, Department of Internal Medicine, Radboud University Medical Centre, 6525 GA Nijmegen, The Netherlands.
Cees J Tack, Department of Internal Medicine, Radboud University Medical Centre, 6525 GA Nijmegen, The Netherlands.
Rinke Stienstra, Department of Internal Medicine, Radboud University Medical Centre, 6525 GA Nijmegen, The Netherlands; Division of Human Nutrition and Health, Wageningen University, 6708 PB Wageningen, The Netherlands.
Funding
Part of this project is funded by the collaborative TIMID project (LSHM18057-SGF) financed by the PPP allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen (SGF) to stimulate public–private partnerships and co-financing by health foundations that are part of the SGF, and by Perspectief Biomarker Development Center Research, which is partly funded by the Netherlands Organization for Scientific Research (NWO). The study funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Author Contributions
R.S. and C.J.T. designed the study. A.W.J. recruited the participants. M.A. and J.I.P.H. performed the experiments. M.A., J.I.P.H., and R.M. analyzed the data. M.A. performed the statistical analyses and wrote the first version of the manuscript. All authors discussed the results and implications, commented on the manuscript, and approved the final version of the manuscript.
Disclosures
All of the authors declared no conflict of interest.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Guarantor Statement
R.S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
A part of this study was presented in the European Association for the Study of Diabetes (EASD) Annual Meeting 2021.
References
- 1. Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res. 2016;118(11):1771‐1785. [DOI] [PubMed] [Google Scholar]
- 2. Thiem K, van Dierendonck XAMH, Janssen AWM, et al. A high glycemic burden relates to functional and metabolic alterations of human monocytes in patients with type 1 diabetes. Diabetes. 2020;69(12):2735‐2746. [DOI] [PubMed] [Google Scholar]
- 3. Hu FB, Meigs JB, Li TY, Rifai N, Manson JAE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693‐700. [DOI] [PubMed] [Google Scholar]
- 4. Aulich J, Cho YH, Januszewski AS, et al. Associations between circulating inflammatory markers, diabetes type and complications in youth. Pediatr Diabetes. 2019;20(8):1118‐1127. [DOI] [PubMed] [Google Scholar]
- 5. Mokgalaboni K, Dludla PV, Nyambuya TM, Yakobi SH, Mxinwa V, Nkambule BB. Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus: a systematic review and meta-analysis of pre-clinical and clinical studies. JRSM Cardiovasc Dis. 2020;9:204800401990074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nwadiugwu MC. Inflammatory activities in type 2 diabetes patients with co-morbid angiopathies and exploring beneficial interventions: a systematic review. Front Public Heal. 2021;8:600427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coca SG, Nadkarni GN, Huang Y, et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol. 2017;28(9):2786‐2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunachie S, Chamnan P. The double burden of diabetes and global infection in low and middle-income countries. Trans R Soc Trop Med Hyg. 2019;113(2):56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corzo-León DE, Chora-Hernández LD, Rodríguez-Zulueta AP, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;56(1):29‐43. [DOI] [PubMed] [Google Scholar]
- 10. Saha S, Al-Rifai RH, Saha S. Diabetes prevalence and mortality in COVID-19 patients: a systematic review, meta-analysis, and meta-regression. J Diabetes Metab Disord. 2021;20(1):939‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wróblewski A, Strycharz J, Świderska E, et al. Chronic and transient hyperglycemia induces changes in the expression patterns of IL6 and ADIPOQ genes and their associated epigenetic modifications in differentiating human visceral adipocytes. Int J Mol Sci. 2021;22(13):6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ter Horst R, van den Munckhof ICL, Schraa K, et al. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler Thromb Vasc Biol. 2020;40(7):1787‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Netea MG, Joosten LAB, Li Y, et al. Understanding human immune function using the resources from the Human Functional Genomics Project. Nat Med. 2016; 22(8):831‐833. [DOI] [PubMed] [Google Scholar]
- 14. Ajie M, van Heck JI, Janssen AWM, Meijer R, Tack CJ, Stienstra R. Supplementary figure 1: Data from disease duration and chronic complications associate with immune activation in individuals with longstanding type 1 diabetes. Figshare. Deposited February 1, 2023. 10.6084/m9.figshare.21988586.v1. [DOI] [PMC free article] [PubMed]
- 15. Lachin JM, Nathan DM ; DCCT/EDIC Research Group . Understanding metabolic memory: the prolonged influence of glycemia during the diabetes control and complications trial (DCCT) on future risks of complications during the study of the epidemiology of diabetes interventions and complications (EDIC). Diabetes Care. 2021;44(10):2216‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menart-Houtermans B, Rütter R, Nowotny B, et al. Leukocyte profiles differ between type 1 and type 2 diabetes and are associated with metabolic phenotypes: results from the German Diabetes Study (GDS). Diabetes Care. 2014;37(8):2326‐2333. [DOI] [PubMed] [Google Scholar]
- 17. Lachmandas E, Vrieling F, Wilson LG, et al. The effect of hyperglycaemia on in vitro cytokine production and macrophage infection with Mycobacterium tuberculosis. PLoS One. 2015;10(2):e0117941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janssen AWM, Stienstra R, Jaeger M, et al. Understanding the increased risk of infections in diabetes: innate and adaptive immune responses in type 1 diabetes. Metabolism. 2021;121:154795. [DOI] [PubMed] [Google Scholar]
- 19. Ebrahimi F, Urwyler SA, Betz MJ, et al. Effects of interleukin-1 antagonism and corticosteroids on fibroblast growth factor-21 in patients with metabolic syndrome. Sci Rep. 2021;11(1):7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliveira AG, Araújo TG, Carvalho BM, Rocha GZ, Santos A, Saad MJA. The role of hepatocyte growth factor (HGF) in insulin resistance and diabetes. Front Endocrinol (Lausanne). 2018;9:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El-Osta A, Brasacchio D, Yao D, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409‐2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rønningen T, Shah A, Reiner AH, Collas P, Moskaug JØ. Epigenetic priming of inflammatory response genes by high glucose in adipose progenitor cells. Biochem Biophys Res Commun. 2015;467(4):979‐986. [DOI] [PubMed] [Google Scholar]
- 23. Hazra S, Jarajapu YPR, Stepps V, et al. Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model. Diabetologia. 2013;56(3):644‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santopaolo M, Sullivan N, Thomas AC, et al. Activation of bone marrow adaptive immunity in type 2 diabetes: rescue by co-stimulation modulator Abatacept. Front Immunol. 2021;12:609406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoo JE, Kim D, Han K, Rhee SY, Shin DW, Lee H. Diabetes status and association with risk of tuberculosis among Korean adults. JAMA Netw Open 2021;4(9):e2126099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xanthis A, Hatzitolios A, Fidani S, Befani C, Giannakoulas G, Koliakos G. Receptor of advanced glycation end products (RAGE) positively regulates CD36 expression and reactive oxygen species production in human monocytes in diabetes. Angiology. 2009;60(6):772‐779. [DOI] [PubMed] [Google Scholar]
- 27. Reed JC, Preston-Hurlburt P, Philbrick W, et al. The receptor for advanced glycation endproducts (RAGE) modulates T cell signaling. PLoS One. 2020;15(9):e0236921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clement CC, Nanaware PP, Yamazaki T, et al. Pleiotropic consequences of metabolic stress for the major histocompatibility complex class II molecule antigen processing and presentation machinery. Immunity. 2021;54(4):721‐736.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLoS One. 2017;12(11):e0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suzuki T, Yamashita S, Hattori K, Matsuda N, Hattori Y. Impact of a long-term high-glucose environment on pro-inflammatory responses in macrophages stimulated with lipopolysaccharide. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(10):2129‐2139. [DOI] [PubMed] [Google Scholar]
- 31. Schaumberg DA, Glynn RJ, Jenkins AJ, et al. Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the diabetes control and complications trial. Circulation. 2005;111(19):2446‐2453. [DOI] [PubMed] [Google Scholar]
- 32. Tsai S, Clemente-Casares X, Zhou AC, et al. Insulin receptor-mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 2018;28(6):922‐934.e4. [DOI] [PubMed] [Google Scholar]
- 33. Ratter JM, van Heck JIP, Rooijackers HMM, et al. Insulin acutely activates metabolism of primary human monocytes and promotes a proinflammatory phenotype. J Leukoc Biol. 2021;110(5):885‐891. [DOI] [PubMed] [Google Scholar]
- 34. Ferreira SS, Oliveira MA, Tsujita M, et al. Insulin modulates the immune cell phenotype in pulmonary allergic inflammation and increases pulmonary resistance in diabetic mice. Front Immunol. 2020;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bettermann K, Sinha K, Kumari R, Fox C, Simpson IA. The peripheral immune response in hyperglycemic stroke. Clin Neurol Neurosurg. 2020;195:106061. [DOI] [PubMed] [Google Scholar]
- 36. Chang TT, Chen JW. The role of chemokines and chemokine receptors in diabetic nephropathy. Int J Mol Sci. 2020;21(9):3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu Y, Lin Q, Ye D, et al. Neutrophil count as a reliable marker for diabetic kidney disease in autoimmune diabetes. BMC Endocr Disord. 2020;20(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mora-Gutiérrez JM, Rodríguez JA, Fernández-Seara MA, et al. MMP-10 is increased in early stage diabetic kidney disease and can be reduced by renin-angiotensin system blockade. Sci Rep. 2020;10(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ter Horst R, Jaeger M, Smeekens SP, et al. Host and environmental factors influencing individual human cytokine responses. Cell. 2016;167(4):1111‐1124.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ajie M, van Heck JIP, Jansen AW, Meijer R, Tack C, Stienstra R. Supplementary table 1-6. Data from disease duration and chronic complications associate with immune activation in individuals with longstanding type 1 diabetes. Figshare. Deposited February 1, 2023. 10.6084/m9.figshare.21988586.v1. [DOI] [PMC free article] [PubMed]
- 41. Devaraj S, Dasu MR, Park SH, Jialal I. Increased levels of ligands of toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia. 2009;52(8):1665‐1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pérez-Segura P, De Dios O, Herrero L, et al. Children with type 1 diabetes have elevated high-sensitivity C-reactive protein compared with a control group. BMJ Open Diabetes Res Care. 2020;8(1):e001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seo Y-H, Shin H-Y. Relationship between hs-CRP and HbA1c in diabetes mellitus patients: 2015–2017 Korean National Health and Nutrition Examination Survey. Chonnam Med J. 2021;57(1):62‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.