Abstract
Context
Type 2 diabetes mellitus (T2D) negatively affects muscle mass and function throughout life. Whether adult muscle stem cells contribute to the decrease in muscle health is not clear and insights into the stem cell niche are difficult to obtain.
Objective
To establish the upstream signaling pathway of microRNA (miR)-501, a marker of activated myogenic progenitor cells, and interrogate this pathway in muscle biopsies from patients with T2D.
Methods
Analysis of primary muscle cell cultures from mice and 4 normoglycemic humans and muscle biopsies from 7 patients with T2D and 7 normoglycemic controls using gene expression, information on histone methylation, peptide screening, and promoter assays.
Results
miR-501 shares the promoter of its host gene, isoform 2 of chloride voltage-gated channel 5 (CLCN5-2), and miR-501 expression increases during muscle cell differentiation. We identify platelet-derived growth factor (PDGF) as an upstream regulator of CLCN5-2 and miR-501 via Janus kinase/signal transducer and activator of transcription. Skeletal muscle biopsies from patients with T2D revealed upregulation of PDGF (1.62-fold, P = .002), CLCN5-2 (2.85-fold, P = .03), and miR-501 (1.73-fold, P = .02) compared with normoglycemic controls. In addition, we observed a positive correlation of PDGF and miR-501 in human skeletal muscle (r = 0.542, P = .045, n = 14).
Conclusions
We conclude that paracrine signaling in the adult muscle stem cells niche is activated in T2D. Expression analysis of the PDGF–miR-501 signaling pathway could represent a powerful tool to classify patients in clinical trials that aim to improve muscle health and glucose homeostasis in patients with diabetes.
Keywords: PDGF, microRNA, skeletal muscle, type 2 diabetes mellitus
Preservation of skeletal muscle mass and function is a prerequisite for an independent, healthy life and upkeep of metabolic control. Diabetes mellitus is a condition in which skeletal muscle health is progressively affected over the life time (1). Fibro-fatty degeneration and muscle atrophy are characteristics of the diabetic muscle (2-5), partly related to increased lipid exposure of the muscle tissue (6) and partly to other unknown factors. Observational studies suggest that patients with diabetes have a 1.95-fold increased odds ratio for sarcopenia (7), the generalized and accelerated loss of muscle mass during the aging process. Importantly, the loss of muscle mass in diabetes can be observed even in the absence of late complications such as diabetic polyneuropathy (8). The molecular underpinnings for the marked changes of muscle health in diabetes are less clear but they could be related to altered paracrine signaling (4) and impaired muscle regeneration (9, 10).
Muscle tissue possesses remarkable dynamic and plastic properties, allowing for adaptation to nutrition, exercise, and injury (11-14). The capacity of skeletal muscle to regenerate arises from adult tissue–resident stem cells termed satellite cells or myogenic progenitors (MPs), characterized by the stem cell marker paired box-7 (Pax7) (15, 16). Myogenic differentiation recapitulates embryonic development, in which the normally quiescent cells are activated, vastly proliferate, and eventually fuse with each other or with existing muscle fibers (17). In adult skeletal muscle, MPs are an absolute requirement for muscle adaptation to environmental stimuli, and mouse models in which MPs have been deleted show an age-dependent decline in response to overload-induced hypertrophy (18) and a complete failure to regenerate with widespread fibrosis following injury (19, 20). Skeletal muscle can respond to exercise with a marked hypertrophic response even in the absence of MPs, but MP depletion alters the transcriptional response to exercise and muscle adaptation is ultimately attenuated (21, 22). During the aging process, loss of MPs might be dispensable for general maintenance of muscle mass but contributes to fibrosis, particularly in periods of remodeling (23, 24). The role of MPs in skeletal muscle from patients with type 2 diabetes mellitus (T2D) is not clear, but MPs isolated from these patients maintain impaired glucose metabolism in vitro (25, 26).
Maintenance of skeletal muscle tissue, where injured fibers are continuously repaired and replaced, is mediated by a complex paracrine signaling network consisting of hormones, cytokines, and growth factors acting on the MPs. In healthy muscle, for instance, the network involves signaling molecules such as insulin-like growth factor (IGF) and hepatocyte growth factor to activate satellite cells (27, 28) as well as cell-intrinsic regulatory pathways, such as notch signaling, to maintain quiescence of satellite cells (29). Wnt signaling on the other hand promotes satellite cell proliferation and self-renewal (30). The spatial and temporal activation of such signaling cascades is crucial to ensure proper regeneration of skeletal muscle.
An additional level of intracellular signaling is mediated through microRNAs (miRNAs). The expression of miRNAs can be highly tissue specific, such as miR-122 for liver (31) or miR-375 for the pancreatic beta cell (32). miRNAs that are specific for muscle tissue are referred to as myomiRs. Some myomiRs are capable of inducing transdifferentiation of fibroblasts into muscle lineage cells (33). In addition, the myomiR miR-133 promotes proliferation of myoblasts (34), whereas miR-1 and miR-206 affect the stemness of MPs by inhibiting Pax7 (35). Genetic ablation of miR-206 delays the regeneration of skeletal muscle without affecting fiber size once regeneration is completed (36). Other myomiRs such as miR-208b and miR-499 regulate fiber type composition within the muscle (37).
In the present study, we assessed the upstream signaling of a novel myomiR, miR-501-3p, which we have previously identified in activated MPs (38). Pharmacological inhibition of miR-501 during regeneration leads to a reduction in the size of newly formed myofibers (38). miR-501 is located in an intron of isoform 2 of chloride voltage-gated channel 5 (CLCN5). While isoform 1 of Clcn5 (CLCN5-1) is found predominantly in the kidney, isoform 2 of Clcn5 (CLCN5-2) is preferentially expressed in skeletal muscle (38). Here, we identify that platelet-derived growth factor (PDGF) activates both CLCN5-2 and miR-501 in muscle cells via Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling. Using expression analysis of the PDGF–miR-501 pathway in human muscle biopsies, we provide evidence that signaling in the adult stem cell niche is activated in skeletal muscle from patients with T2D.
Materials and Methods
Human Biopsy Acquisition
Human biopsies of the tensor fasciae latae muscle were obtained during elective hip replacement surgery as previously described (39). Biopsies were used to isolate total RNA and to isolate human MPs for in vitro culture (described later). The young group included 3 females and 2 males <55 years (36.0 ± 7.2) and the aged group included 3 females and 3 males >75 years (79.3 ± 1.6; P ≤ .001, Student’s t test compared with young). Cell cultures used in the present study comprised 2 females and 2 males of the young group. Human biopsies of the musculus vastus lateralis were obtained using a Bergström needle biopsy technique. The sampling was performed under local anesthesia on the morning after an overnight fast. In total, biopsies from 46 male subjects were analyzed. Insulin sensitivity was determined by the euglycemic–hyperinsulinemic clamp, as described previously (40). For the comparison between normal glucose tolerant participants and participants with T2DM, 7 lean metabolically healthy males (age, 38.4 ± 5.8 years; body mass index [BMI], 23.3 ± 2.0 kg m−2; M-value/insulin, 0.150 ± 0.067 mg kg−1 min−1 per μU mL−1 of insulin) and 7 overweight/obese T2DM male patients (age, 43.6 ± 5.7 years; BMI, 30.8 ± 2.2 kg m−2; M-value/insulin, 0.054 ± 0.032 mg kg−1 min−1 per μU mL−1 of insulin) were evaluated. For the extended analysis muscle samples from 23 metabolically healthy males (age, 43.8 ± 7.8 years; BMI, 27.0 ± 3.8 kg m−2; M-value/insulin, 0.121 ± 0.053 mg kg−1 min−1 per μU mL−1 of insulin), 12 patients with prediabetes (age, 44.8 ± 10.0 years; BMI, 28.4 ± 3.2 kg m−2; M-value/insulin, 0.077 ± 0.040 mg kg−1 min−1 per μU mL−1 of insulin) and 11 newly diagnosed yet untreated male patients with T2DM (age, 49.0 ± 8.3 years; BMI, 31.6 ± 3.9 kg m−2; M-value/insulin, 0.056 ± 0.039 mg kg−1 min−1 per μU mL−1 of insulin) were analyzed. Muscle samples were immediately blotted from excessive blood, frozen in liquid nitrogen, and stored at −80 °C. All studies involving human subjects were performed in accordance with the principles set out in the Declaration of Helsinki and were approved by the institutional ethics review committees. All study participants provided written informed consent prior to entering the studies.
Mice
All animals used housed at 2 to 5 littermates per cage in individually ventilated cages under conditions of controlled temperature (22 °C) and illumination (12-hour light/12-hour dark cycle; light off at 6 Pm) with ad libitum access to chow and water. All experiments were approved by the Veterinary office of the Canton of Zurich (License number 061/2019) and health status of all mouse lines were monitored on a regular basis according to FELASA guidelines. Serum from heart blood was collected directly after the mice were sacrificed.
Cell Preparation and fluorescence-activated cell sorting (FACS)
Procedures for isolation of primary muscle cells from human and mouse skeletal muscle were performed as previously described (41). Mouse skeletal muscle tissue was excised from the hind limbs. Muscles were minced and digested with 2 mg mL–1 collagenase type II (Gibco) in collagenase buffer (1.5% bovine serum albumin in Hanks’ balanced salt solution) for 1 hour at 37 °C. Muscle slurries were filtered through a 100-µm cell strainer (BD Biosciences), followed by treatment with erythrocyte lysis buffer (154 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and filtration using 40 µm cell strainers (BD Biosciences). Cells were resuspended in washing buffer consisting of phosphate-buffered saline with 0.5% bovine serum albumin and stained with antibodies for 1 hour at 4 °C. Cell sorting was performed on a FACSAria III 4L (BD Biosciences). Mouse MPs were isolated from the live cell population (7-AAD−; Sigma) based on positive staining for α7-integrin (R and D Systems Cat# FAB3518P, RRID:AB_2249296) and absence of Sca1 (BioLegend Cat# 108111, RRID:AB_313348), CD31 (BioLegend Cat# 102414, RRID:AB_493408), and CD45 (BioLegend Cat# 103121, RRID:AB_493532) staining, while fibro/adipogenic progenitors were sorted based on positive staining for Sca1 and absence of staining for α7-integrin, CD31, and CD45. Human MPs were sorted by excluding CD31 (BioLegend Cat# 303103, RRID:AB_314329)/CD45 (BioLegend Cat# 304019, RRID:AB_493033) and based on positive CD56 (BioLegend Cat# 318305, RRID:AB_604093) staining and absence of CD15 (BioLegend Cat# 323007, RRID:AB_756013).
Cell Culture
Primary human and mouse myoblasts were cultured on collagen-coated plates in 1:1 v/v Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F-10 Nutrient Mix (Gibco) containing 20% fetal bovine serum, 1% penicillin/streptomycin (P/S) and 5 ng mL–1 recombinant human fibroblast growth factor (FGF)-2 (Gibco). C2C12 and human embryonic kidney 293 T/17 cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% P/S. Differentiation of MPs was initiated when myoblasts reached subconfluency by changing the medium to DMEM containing 2% horse serum (Gibco) and 1% P/S. Differentiation of primary mouse Pax7CreERDgcr8flox/flox myoblasts was induced after recombination with 4-hydroxytamoxifen (Sigma, 20 nM) administered in growth medium for 48 hours, as previously described (42). 3T3-L1 cells and were cultured in DMEM supplemented with 10% fetal bovine serum and 1% P/S on collagen-coated plates. For adipogenic differentiation, cells were grown to confluence, and IBMX (500 mM), dexamethasone (1 μM), and insulin (100 nM, all purchased from Sigma) were added to full medium, marking day 0. At day 2, the medium was changed to maintenance medium (100 nM insulin in full medium) and renewed every 48 hours thereafter. All cell cultures were incubated in a 37 °C 5% CO2 water-jacketed incubator. Cell culture media were collected at the time points indicated in the figure legends to assess secretion of PDGF using PDGF-AA enzyme-linked immunosorbent assay (Cloud-Clone Corp, Cat# SEA523Mu, RRID:AB_2924845). Transfections of oligonucleotides were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Insulin (100 nM) or recombinant proteins were added to cell culture media for 2 × 24 hours and inhibitors for 24 hours. All concentrations are listed elsewhere (Table 1 (43)). MG-132 (Merck Millipore) was administered to medium for 6 hours at 10 μM.
RNA Extraction, cDNA Synthesis, Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated using TRIzol Reagent (Invitrogen) according to the manufacturer’s instruction. Traces of genomic DNA were removed using the DNA-free DNA Removal Kit (Invitrogen). Equal amounts of RNA were reverse-transcribed with random hexamer primers using SuperScript III First-Strand Synthesis System (Invitrogen). For miRNA quantitative reverse transcription polymerase chain reaction (RT-PCR), 10 ng of total RNA was reverse-transcribed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). Quantitative RT-PCR for miRNA and mRNA levels was performed on a Quant Studio 5 Real-time PCR system (Applied Biosystems) using TaqMan Fast Universal PCR Master Mix, no AmpErase UNG (Applied Biosystems), and PowerUp SYBR Green Master Mix (Applied Biosystems), respectively. Gene expression was calculated using the relative standard curve method and 18S rRNA, as indicated, for normalization. All primer sequences are listed elsewhere (Table 2 (43)). The levels of miRNA were calculated using the ΔΔCt method and snoRNA234 or U6 snRNA for normalization. TaqMan assays for miR-501-3p (human, TM 002435; mouse, TM001651), miR-206 (TM 000510), snoRNA234 (TM 001234), and U6 snRNA (TM 001973) were purchased from Applied Biosystems. The extended cohort analysis was performed using the Qiagen miScript primer assay (miR-501, MS00009828; snord44, MS00007518).
Protein Extraction and Western Blot
Cells were lysed in RIPA Buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Triton X100, 0.5% sodium deoxycholate) supplemented with protease (Complete, Roche) and phosphatase (PhosSTOP, Roche) inhibitor cocktails. Lysates were cleared by centrifugation at 14 000g for 20 minutes at 4 °C. Protein concentrations were determined using BCA Assay. Equal amounts of protein (20 µg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto Protran nitrocellulose membranes (GE Healthcare) using eBlot L1 Fast Wet Transfer System (GenScript), followed by incubation with the indicated primary antibodies against Phospho-Stat3 (Cell Signaling Technology Cat# 9131, RRID:AB_331586; 1:1000), and γ-tubulin (Sigma-Aldrich Cat# T5326, RRID:AB_532292; 1:1000). Signals of antirabbit (Millipore Cat# 401393-2ML, RRID:AB_437797) and antimouse (Millipore Cat# 401253, RRID:AB_437779) IgG horseradish peroxidase–conjugated secondary antibody (1:10 000) were visualized on a LAS-3000 Luminescent Image Analyzer (Fujifilm) using Lumi-Light Western Blotting Substrate (Roche).
Plasmid Construction
The regions 1.5 kb upstream of the transcriptional start site of both CLCN5-1 and CLCN5-2 were PCR-amplified from human cDNA using Phusion High-Fidelity DNA Polymerase (Invitrogen) and cloned into pCR2.1-TOPO using TOPO TA Cloning Kit (Invitrogen). Primers used for plasmid construction and sequences are listed elsewhere (43). Subsequently, constructs were subcloned into pGL3-Enhancer-Vector (Promega). The following plasmids were purchased from Addgene: MyoD (#8398; (44)), Myogenin (#78341; by M. Alexander and L. Kunkel), MEF2A (#118354; (45)), and Stat3 (#8722; (46)). To generate the pAAV2/1-Stat3 plasmid, the sequence was recloned into the AAV-CAG-GFP (Addgene plasmid #28014), replacing the eGFP element. All constructs were verified by Sanger Sequencing (Microsynth, Switzerland).
Luciferase Assay
For promoter assays, promoter constructs and plasmids were cotransfected with pRL-TK (Promega) for internal control of Renilla luciferase expression. After 24 hours, the medium was changed to growth or differentiation medium. Luciferase reporter activity was measured after 48 hours using the Dual-Luciferase Reporter Assay (Promega) on an Infinite 200 PRO plate reader and i-control software (Tecan). Promoter activity was calculated by normalizing Firefly luciferase counts to Renilla values.
Histology
Histological sections of human tensor fasciae latae were performed as previously described (39). Briefly, muscles biopsies were fixed in 4% formalin, and embedded in paraffin and cut into sections 4 µm thick. Subsequently, stained with wheat germ agglutinin (Alexa Fluor 594, Invitrogen, 5 μg mL–1 in phosphate-buffered saline) followed by blocking and permeabilizing (0.5% Triton-X 100 in phosphate-buffered saline) and staining with DAPI (Invitrogen, 1:1000) for 15 minutes at room temperature each. Sections were scanned using an Axio Scan.Z1 slide scanner (Zeiss) equipped with ZEN Imaging software v3.1 and analyzed using ImageJ (v. 1.53c).
Data Accession
ENCODE H3K4me3 ChIP-seq datasets (47) were obtained with the following identifiers: mouse kidney (ENCFF177YGH), C2C12 myoblasts (ENCFF436ZSV), C2C12 cells differentiated for 60 hours (ENCFF518CXM), and human primary myoblasts (ENCFF700DPA).
Statistical Analysis
Numerical values are reported as mean ± SEM. Sample sizes were determined on the basis of previous experiments and publications using similar methodologies as well as on observed effect sizes. Cell culture experiments were performed with 2 technical replicates, and reproduced using independent cell cultures. Numerical values are shown as mean ± SEM. Statistical significance (*P ≤ .05; **P ≤ .01; ***P ≤ .001) was evaluated as specified in the figure legends using GraphPad Prism v9.1.0.
Results
miR-501 and its Host Gene Clcn5-2 are Regulated During Muscle Differentiation in Mice and Humans
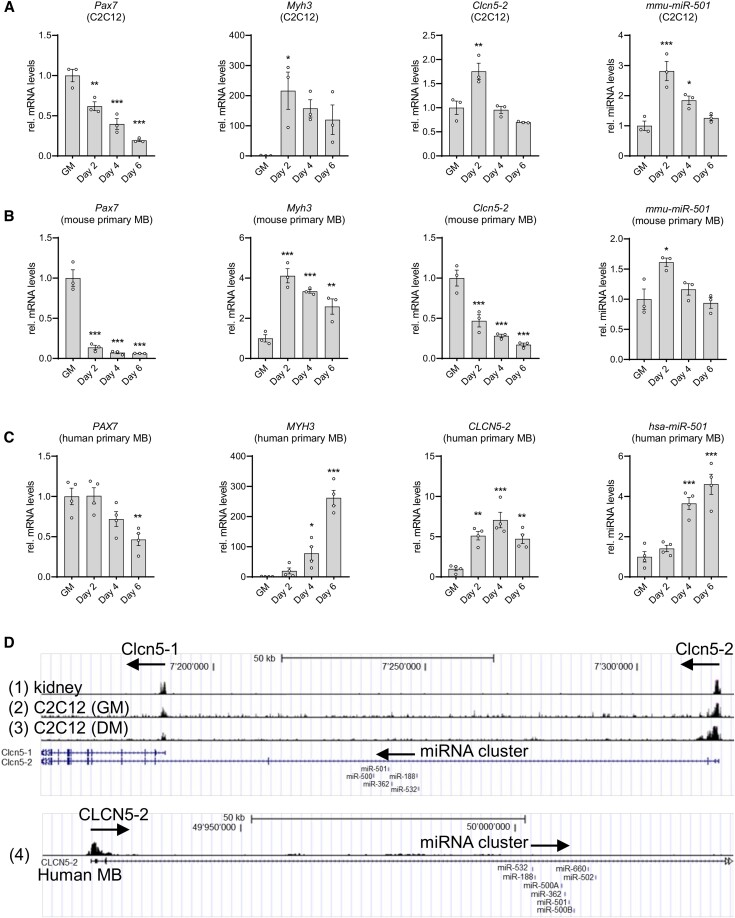
miR-501 expression in myoblasts decreases after deletion of Dgcr8, an RNA binding protein critical for miRNA synthesis (42), indicating that generation of miR-501 uses the canonical miRNA pathway in muscle cells (Fig. 1 (43)). Seeking to understand the mechanisms that drive expression of miR-501 and its host gene Clcn5-2 specific to the regenerating muscle and activated MPs (38), we followed their expression during muscle cell differentiation in 3 model systems: C2C12 myoblasts, and primary myoblasts from mouse and human (Fig. 1A-1C). As expected, during the 6 days of differentiation, Pax7 expression decreased, while levels of the differentiation marker myosin heavy chain-3, Myh3, increased in all 3 model systems. This regulation was fastest in mouse muscle cells (Fig. 1A and 1B), and slowest in differentiating human primary myoblasts (Fig. 1C). In C2C12 and human myoblasts, miR-501 levels always followed the expression of its host gene Clcn5 (Fig. 1A-1C). In mouse primary myoblasts, the onset of differentiation was the fastest and the initial induction of Clcn5 was not observed (Fig. 1B). Notably, the expression profile of the miRNA in mouse and human myoblasts differed, where miR-501 peaked at day 2 in the 2 mouse models (Fig. 1A and 1B) while its expression levels continuously increased until day 6 in the human muscle cells (Fig. 1C). However, in all 3 model systems maximum miR-501 levels correlated with maximum levels of Myh3 expression. We have previously reported gigaxonin (Gan) as a target gene of miR-501 (38). The expression of Gan increased during muscle differentiation, but did not decrease as expected for a miR-501 target (Fig. 2 (43)). The target genes of miR-501 that are downregulated during muscle differentiation remain to be elucidated.
Figure 1.
Regulation of Clcn5-2 and miR-501 during muscle cell differentiation. A-C) Expression of Pax7, Myh3, Clcn5-2, and miR-501 in C2C12 (A), mouse primary myoblasts (B), and human primary myoblasts (C) during differentiation; n = 3-4. (D) Schematic representation of ENCODE H3K4me3 ChIP-seq data at the 5′-UTR of the mouse Clcn5 gene in C57BL/6 kidney (1), C2C12 myoblasts (2), and C2C12 differentiated for 60 hours (3), as well as the human CLCN5-2 gene in adult (22 years) human skeletal muscle myoblasts (4). All data are plotted as mean ± SEM; qPCR values were normalized to snoRNA234 (mmu-miR-501), U6 small nuclear RNA (hsa-miR-501) or 18S ribosomal RNA. Significance was evaluated vs growth medium (GM) by 1-way analysis of variance with multiple comparisons test; *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Next, we analyzed the expression of miR-501 during muscle differentiation using histone methylation analysis. Trimethylation at the fourth lysine residue of the H3 histone protein (H3K4me3) is an epigenetic modification at promoter elements associated with activation of transcription (48). Accordingly, we analyzed ENCODE H3K4me3 ChIP-seq datasets (47) of mouse kidney, C2C12 myoblasts, C2C12 cells differentiated for 60 hours, and human primary myoblasts for methylation peaks at the Clcn5 gene (Fig. 1D). Although methylation peaks were observed at the transcription start sites (TSSs) of both isoforms in kidney and muscle, the methylation of Clcn5-2 in muscle cells was greater than that of Clcn5-1 in the kidney (Fig. 1D). Furthermore, there was a substantial increase in methylation upon differentiation of the muscle cells. Methylation peaks were additionally present upstream of the TSS of CLCN5-2, but not CLCN5-1, in human primary myoblasts, indicating a conserved regulatory mechanism across species for the host gene (Fig. 1D). Importantly, no methylation peaks were observed downstream of the TSS and near the miRNA cluster that contains miR-501 (Fig. 1D), indicating that miR-501 expression is driven by the promoter region of its host gene Clcn5-2. In contrast to Clcn5-2, expression of the kidney-specific isoform Clcn5-1 was not induced during differentiation in mouse or human muscle cells (Fig. 2b (43)). Together, the expression analysis during muscle cell differentiation and the study of H3K4me3 modification demonstrate that Clcn5-2 and miR-501 are restricted to muscle cells and that miR-501 shares the Clcn5-2 promoter. miR-501 levels increase during differentiation of muscle cells from mouse and human and peak at maximum levels of Myh3 expression.
Our next aim was to clone the promoter region of CLCN5-2 to identify the regulation of miR-501 in muscle cells. To this end, we cloned the promoter region for CLCN5-1, which has been described for both mouse (49) and human (50) at 1.5 kb upstream of the TSS, and accordingly we cloned 1.5 kb upstream of the TSS of the second isoform, CLCN5-2. The activities of these 2 bona fide promoter regions were measured using dual luciferase assays (Fig. 3a (43)). In line with the methylation peaks, activity in the promoter region of CLCN5-2 increased when differentiation was induced in C2C12 cells through serum starvation, but not in serum-starved human embryonic kidney cells nor during differentiation of 3T3-L1 cells into adipocytes (Fig. 3b (43)). The correlation of miR-501 levels with Myh3 expression during muscle cell differentiation raised the possibility that miR-501 might be regulated similarly by myogenic regulatory factors (MRFs), such as MyoD, myogenin, or the myogenic cofactor MEF2A (51). Indeed, expression levels of myogenin and Mef2a, but not MyoD, correlate with miR-501 expression during C2C12 cell differentiation (Fig. 3c (43)). MYOD was able to activate the myogenin promoter as previously described (42) (Fig. 3d (43)) confirming the validity of our luciferase assays. However, none of the MRFs were able to activate the promoter activity of CLCN5-2 (Fig. 3e (43)). In addition to the biogenesis pathway, degradation by the proteasome plays a key role in regulating the biological action and half-life of miRNAs (52, 53). We hypothesized that this process might cause enrichment of miR-501 in MPs compared with other precursor cells such as fibro/adipogenic progenitors (FAPs) (38). However, treatment using the proteasomal inhibitor MG-132 in FAPs did not increase expression of miR-501 in the nonmyogenic cells in contrast to p21 expression, which served as positive control (54) (Fig. 3f (43)). Taken together, these data show that the transcription of miR-501 in muscle cells is driven by the promoter of Clcn5-2, but that this promoter is not directly regulated by MRFs.
PDGF Signaling Regulates miR-501 Expression in Muscle Cells
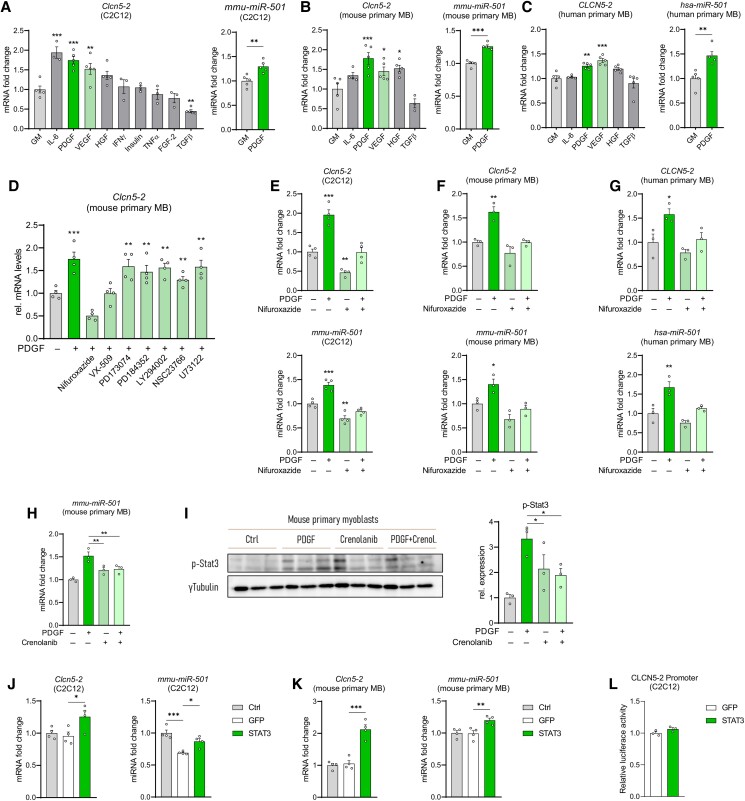
Additionally to the cell-intrinsic transcriptional network, growth factors and cytokines act in the adult muscle stem cell niche to modulate the activation of MPs (55). We therefore tested the effect of several signaling molecules that are relevant in the adult muscle stem cell niche on the expression of Clcn5-2 and miR-501. Importantly, only PDGF-AA induced Clcn5-2 and miR-501 in C2C12 myoblasts as well as in primary myoblasts from mouse and human (Fig. 2A-2C). We therefore continued to investigate the numerous intracellular pathways downstream of PDGF. Only the inhibitors of JAK3 (VX-509) and its downstream target STAT3 (nifuroxazide) prevented the induction of Clcn5-2 caused by PDGF (Fig. 2D). Nifuroxazide treatment also blocked the induction of miR-501 expression and its host gene in the presence of PDGF in all mouse and human muscle model systems (Fig. 2E-2G). In C2C12 cells, nifuroxazide reduced Clcn5-2 and miR-501 expression levels also in the absence of PDGF, indicating that STAT3 signaling might be already activated at baseline in this cell line (Fig. 2E). The PDGF receptor inhibitor Crenolanib was able to block the induction of miR-501 and the phosphorylation of P-STAT3 in response to PDGF (Fig. 2H and 3I). Crenolanib, however, was not able to decrease miR-501 levels without PDGF (Fig. 2H) indicating the absence of endogenous PDGF signaling in the MP cells. Indeed, we were also not able to detect PDGF protein in the supernatant of cultured mouse myoblasts (Fig. 4 (43)).
Figure 2.
PDGF signaling regulates expression levels of miR-501 in muscle cells. (A-C) Clcn5-2 and miR-501 expression in C2C12 myoblasts (A), mouse primary myoblasts (B), and human primary myoblasts (C) after treatment with recombinant proteins for 2 × 24 hours; n = 3-5. (D) Clcn5-2 expression after treatment with PDGF and inhibitors against STAT3 (Nifuroxazide), JAK3 (VX-509), FGFR1 (PD173074), MEK1/2 (PD184352), PI3Kα/δ/β (LY294002), Rac GTPase (NSC23766), or PLC-γ (U73122); n = 4. (E-G) Clcn5 and miR-501 expression in C2C12 myoblasts (E), mouse primary myoblasts (F), and human primary myoblasts (G) after treatment with PDGF and inhibition of STAT3 signaling (Nifuroxazide); n = 3-4. (H, I) Effect of PDGF inhibitor Crenolanib in mouse primary myoblasts on miR-501 expression (H) and p-Stat3 as determined by immunoblot and quantified relative to γ-tubulin (I); n = 3. (J-L) Clcn5-2 and miR-501 expression in C2C12 myoblasts (J), and mouse primary myoblasts (K) upon overexpression of STAT3; n = 4. (L) miR-501 promoter activity upon overexpression of STAT3; n = 3. All data are plotted as mean ± SEM; qPCR values were normalized to snoRNA234 (mmu-miR-501), U6 small nuclear RNA (hsa-miR-501), or 18S ribosomal RNA. All data are plotted as mean ± SEM. Significance was evaluated vs control by t-test (A, B, C: miRNA) and 1-way analysis of variance with the multiple comparisons test (A, B, C: mRNA; D, E, F, H, I, J, K); *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Figure 3.
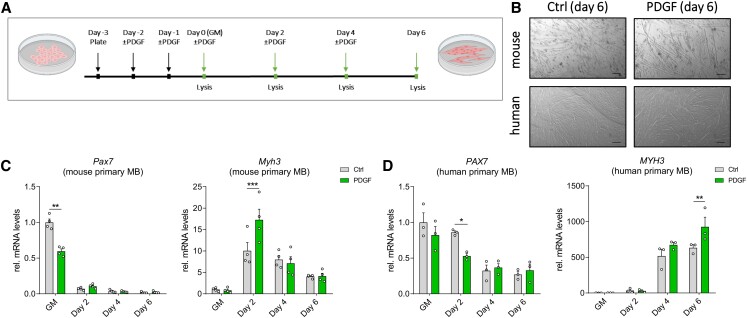
PDGF treatment during myogenic differentiation affects gene expression profile. (A) mouse and human primary myoblasts were cultured in growth medium with added PDGF-AA for 2 × 24 hours prior to differentiation, during which cell culture medium ± PDGF was exchanged every 2 days. Gene expression was assessed at indicated time points (last four arrows). (B) Light microscopy of mouse (top) and human (bottom) primary myofibers at day 6 of differentiation ± PDGF. Scale bar = 200 μm. (C, D) Pax7 and Myh3 expression in mouse primary myoblasts (C; n = 4 independent cell cultures), and human primary myoblasts (D; n = 3 independent cell cultures). All data are plotted as mean ± SEM; qPCR values were normalized to 18S ribosomal RNA. All data are plotted as mean ± SEM. Significance was evaluated vs control 2-way analysis of variance with the multiple comparisons test; *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Next, we assessed if activation of STAT3 would be sufficient to mimic the induction of miR-501 by PDGF. Indeed, overexpression of STAT3 as shown by immunoblot (Fig. 5a (43)) resulted in an increase of Clcn5-2 and miR-501 in C2C12 cells (Fig. 2J) and mouse primary myoblasts (Fig. 2K). Transfection efficiency of primary mouse myoblasts was at least 20% as estimated by flow cytometry for GFP (Fig. 5b (43)). Interestingly, overexpression of STAT3 was not able to activate our Clcn5-2 promotor assay (Fig. 2L), indicating that induction of Clcn5-2 by PDGF might either be cis-acting outside its 1.5 kb upstream sequence or through a trans-acting factor. We conclude that the expression of miR-501 is controlled through a PDGF–JAK/STAT–Clcn5-2 signaling pathway.
PDGF Regulates Genes Involved in Myogenic Differentiation in Mouse and Human Myoblasts
Next, we analyzed the effect of PDGF on myogenic differentiation. We incubated mouse and human primary myoblasts with recombinant PDGF-AA and induced differentiation by serum withdrawal as indicated in Fig. 3A. PDGF did not alter muscle cell differentiation morphologically (Fig. 3B), but PDGF incubation downregulated the expression of Pax7, while Myh3 was induced, in both mouse and human cells (Fig. 3C and 3D). We conclude that PDGF is not only able to activate the expression of miR-501, but is sufficient to alter the expression of genes that are involved in myogenic differentiation in mouse and human myoblasts.
The PDGF–Clcn5-2–miR-501 Axis Is Active in Human Skeletal Muscle and Induced in Type 2 Diabetes Mellitus
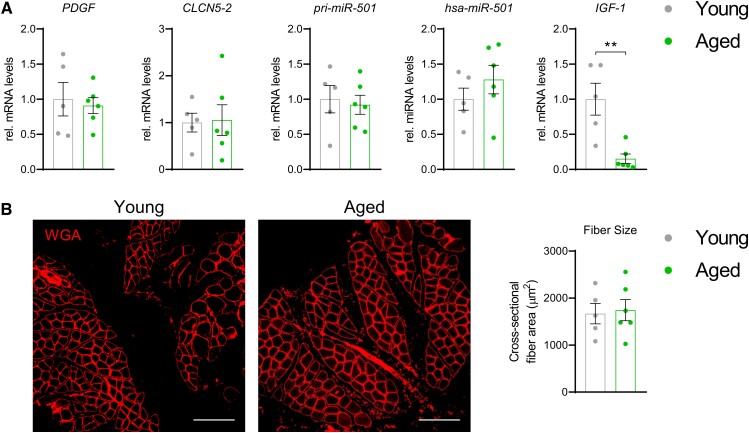
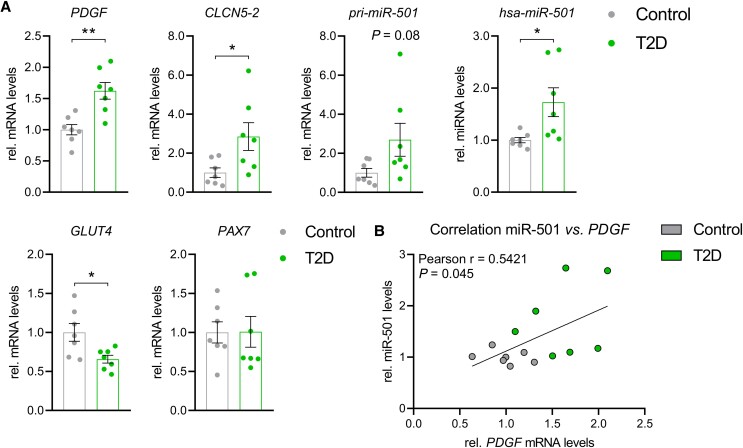
Having identified the upstream signaling pathway that induces miR-501 in muscle cells, our next aim was to investigate whether this pathway is regulated in skeletal muscle in humans. First, we tested the effect of aging on the expression of the PDGF-miR-501 axis in a group of young and aged subjects. We have previously shown that in these muscle samples the aged group have increased amounts of intramuscular adipose tissue formation and activation of FGF-2 signaling (56). However, no changes in PDGF, CLCN5-2, pri-miR-501, or miR-501 levels were detected in the aged group compared with the young (Fig. 4A). Downregulation of IGF-1 characterizes aged skeletal muscle (57) and was also noticeable in our aged subjects (Fig. 4A). However, we did not observe differences in fiber size between young and aged subjects, indicating that our study did not recapitulate all hallmarks of the aged skeletal muscle (Fig. 4B). Next, we turned to the effects of diabetes mellitus on the PDGF signaling axis. Intriguingly, PDGF, CLCN5-2, pri-miR-501, and miR-501 were all significantly increased in muscle biopsies from patients with T2D compared with control subjects (Fig. 5A). Downregulation of the expression of the glucose transporter GLUT4 confirmed that the biopsies adequately reflect common transcriptional changes in skeletal muscle during T2D (58). Moreover, the expression of the adult muscle stem cell marker PAX7 was unchanged in line with previous observations that the number of MP cells is not altered in T2D (4). Importantly, PDGF expression positively correlated with miR-501 levels in skeletal muscle from T2D and controls combined (Fig. 5B). Together, our results provide evidence that PDGF signaling controls the expression of miR-501 in skeletal muscle from humans and is activated in T2D. To begin to understand whether the increase in miR-501 expression in T2D is age dependent we reanalyzed miR-501 expression in an extended cohort of 46 male subjects, including 12 glucose intolerant subjects with prediabetes (pre-T2D), 16 additional metabolically healthy subjects (controls), and 4 additional patients with T2D ( Fig. 6 (43)). Results were stratified into 3 age groups: <40 years (range 28-37 years), 40-50 years (range 42-48 years), and >50 years (range 50-63 years). Interestingly, a significant induction of miR-501 was only observed between controls and the subjects with prediabetes/diabetes in the oldest group (Fig. 6 (43)). We conclude that the activation of miR-501 in skeletal muscle of glucose-intolerant patients is age dependent.
Figure 4.
miR-501 levels are not altered in aged compared with young human skeletal muscle. (A) Expression levels of PDGF, CLCN5-2, pri-miR-501, miR-501, and IGF-1; n = 5 (young) vs 6 (aged). (B) Immunofluorescence (wheat germ agglutinin) in aged human muscle and quantification of average fiber size; n = 5 vs 6. All data are shown as mean ± SEM; qPCR data was normalized to U6 small nuclear RNA (hsa-miR-501) or 18S ribosomal RNA. Significance was evaluated by the t-test; **P < .01.
Figure 5.
miR-501 levels are increased in skeletal muscle from humans with type 2 diabetes. (A) Expression levels of PDGF, CLCN5-2, pri-miR-501, miR-501, GLUT4, and PAX7 in muscle from subjects with T2D and control; n = 7. (B) Correlation between miR-501 and PDGF levels as shown in A; n = 7. All data are shown as mean ± SEM; qPCR data were normalized to U6 small nuclear RNA (hsa-miR-501) or 18S ribosomal RNA. Significance was evaluated by the t-test (A) and Pearson correlation (B); *P < .05, **P < .01.
Discussion
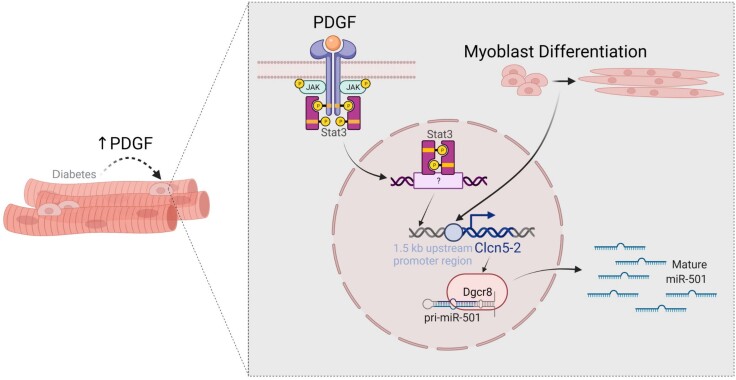
Differentiation of adult skeletal muscle stem cells to form myofibers relies on a tightly controlled network of extracellular and intracellular cues. The mechanisms that affect muscle health in patients with diabetes are difficult to assess in muscle biopsies and only poorly understood. Here, we demonstrate that the expression of the muscle stem cell–specific miR-501 is mediated through paracrine signaling downstream of PDGF. Using expression analysis of this signaling pathway in muscle biopsies, we provide evidence for activation of PDGF signaling in the adult stem cell niche in skeletal muscle from humans with T2D (Fig. 6).
Figure 6.
Graphical representation of the regulation of miR-501 in myogenic progenitor cells by differentiation and PDGF signaling and its activation in T2D.
Paracrine signaling is an important regulator of glycemia and muscle composition (59). For example, FGF-1, -4, and -21 have potent antihyperglycemic activity by stimulating glucose uptake in muscle tissue (60-62), and FGF-2-dependent signaling is associated with intramuscular adipose tissue formation in human skeletal muscle (56). Muscle expression of hepatocyte growth factor also improves glucose uptake into muscle (63), while transforming growth factor-beta signaling in muscle could further impinge on insulin signaling in humans with an impaired response to exercise (64). How PDGF signaling, which we show here to be activated in skeletal muscle from people with T2D, affects glucose metabolism in the muscle is not known, but upregulation of PDGF signaling in the adult muscle stem cell niche could impact both muscle composition and muscle cell differentiation. PDGF is an important growth factor for FAPs, which support muscle regeneration by deposition of extracellular matrix (ECM) (65, 66). In line with our results, increased expression of the receptor for PDGF (PDGFRA) has recently been reported in skeletal muscle from patients with T2D and correlated with intramuscular fibrosis (67). It was shown that PDGF acts as a key FAP regulator and stimulates cell division and collagen production of FAPs while the ability of FAPs to form adipocytes was reduced (4). PDGF activation in the adult muscle stem cell niche could therefore be involved in the accumulation of FAPs and the fibro-fatty degeneration observed in skeletal muscle in T2D (2-5).The induction of miR-501 in our study indicates that PDGF might not only affect FAPs but also muscle regeneration through direct effects on MPs where miR-501 is expressed (38). We show that PDGF altered the expression of genes involved in myogenesis in both mouse and human muscle cells. Upregulation of PDGF signaling could therefore also directly participate in the altered regenerative capacity in diabetic muscle. The defects of satellite cells and muscle regeneration and diabetes are currently not well established. In general, diabetes, high-fat diet feeding, and hyperglycemia are linked to the reduced regenerative capacity (both proliferation and differentiation) of skeletal muscle and muscle satellite cells (68, 69). However, the findings on the direct impact of diabetes on myoblast differentiation capacity in vitro are not consistent. We (70) and others (71) did not observe alterations in differentiation markers in cell cultures derived from skeletal muscle of individuals with T2D. However, Iovino et al showed in a cellular model of human muscle insulin resistance normal myogenic differentiation morphologically, but altered expression of genes involved in myogenesis (72). Davegardh et al pointed at reduced myogenic potential in myoblasts from individuals with T2D (73).
It is a limitation of our study that we were not able to show activation of PDGF signaling directly in the MP cells, which is technically challenging. However, the expression of the adult muscle stem cell marker PAX7 was unchanged between controls and patients with T2D, in line with a previous report that the number of MPs in skeletal muscle from T2D is unaffected (4). Since miR-501 is specifically expressed in MPs (38) we conclude that increased expression of miR-501 provides strong evidence for activation of PDGF-dependent signaling in the adult muscle stem cell niche rather than reflecting changed numbers of MPs. PDGF has been described to be secreted by various different cell types such as endothelial or immune cells (74); however, the source for increased PDGF expression in diabetic muscles is not known. The study by Farup et al showed that mature muscle fibers are particularly high in PDGFA expression in human skeletal muscle and also pointed to smooth muscle cells/pericytes as a potential source of PDGFA (4). These findings are in line with our results showing no evidence for endogenous PDGF signaling in the MP cells. Furthermore, it has been shown that insulin stimulates the expression of FGF-21 (75) and the FGF receptor 2 (76) in human skeletal muscle. Whether insulin signaling is also involved in activation of PDGF-dependent signaling in muscle deserves further investigation.
The impact of increased miR-501 levels on differentiation in MP cells has not been explored in detail so far, although miR-501 upregulation was able to increase myogenic differentiation in the C2C12 myoblast cell line (77). We therefore regard the upregulation of miR-501 in T2D as a biomarker for activation of MP cells (38). Interestingly, miR-501 levels in skeletal muscle correlated negatively with insulin sensitivity (Fig. 6 (43)). Whether miR-501 contributes causally to the insulin responsiveness of muscle tissue deserves further investigation. Moreover, the preferential induction of miR-501 in the oldest group of subjects with prediabetes/type 2 diabetes suggests that upregulation of miR-501 might be related to the preferential muscle loss in this patient group. Expression of the PDGF–CLCN5–miR-501 pathway was not altered in skeletal muscle from aged vs young humans. However, in aged mice, PDGF levels are downregulated in serum and Pdgf expression is decreased in several tissues including skeletal muscle (78). These discrepancies could be explained by differences between species or between muscle groups. In this regard, the skeletal muscle biopsy site might be critical. Indeed, we did not observe changes in muscle fiber diameter in our samples, which would normally be indicative of aged muscle tissue (79, 80). In line, the site of biopsy in our study, the tensor fasciae latae, has been shown to be affected by atrophy to a much lesser extent than other hip muscles in osteoarthritis (81). It remains to be seen whether PDGF-dependent signaling is affected in aged human skeletal muscle in other muscle groups.
Interestingly, we also observed differences between species in terms of the expression of miR-501 during muscle cell differentiation. The induction of miR-501 was considerably stronger in human muscle cells than in mouse (4-fold vs 1.5-fold, respectively). These differences are likely explained by differences in differentiation dynamics. The half-life of Pax7 mRNA in differentiating human muscle cells was 4 days vs less than 12 hours in mouse cells, while Myh3 expression peaked at day 6 in humans vs day 2 in mouse cells. Therefore, primary muscle cells from humans seem to be a more suitable model to study the regulation of miR-501. Accordingly, the small induction of miR-501 during differentiation of mouse muscle cells was not caught in our previous study (38).
Our results demonstrate that the Clcn5-2 promoter is responsible for the expression of miR-501 in muscle cells from both mouse and humans. miR-501 followed the expression of Clcn5-2 during muscle cell differentiation, and this was mirrored by an increase in histone methylation at the TSS of Clcn5-2 as well as in the activity of the Clcn5-2 promoter. Our results are further supported by a global bioinformatics screen on putative miRNA TSSs (82). Interestingly, we did not observe a direct regulation of the Clcn5-2 promoter by MRFs, which have previously been shown to control the expression of myomiRs (83). The specific factors that drive the expression of miR-501 and its host gene during muscle cell differentiation remain to be determined.
Changes within the stem cell niche greatly affect the capacity of stem cells to contribute to tissue repair (84). However, the assessment of the regulation of the niche is hard to obtain from tissue biopsies. miRNAs have emerged as promising biomarkers for skeletal muscle disease (85), but the myomiRs described to date are not specific to MPs in contrast to miR-501 (38). miR-501 is predominantly expressed by activated muscle stem cells, rather than quiescent MPs (38), rendering it the perfect marker to interrogate the stem cell niche in various pathologies. Taken together, our data provide evidence for the activation of paracrine PDGF signaling in the adult muscle stem cell niche in patients with T2D, which contributes to our understanding of the impaired muscle health in this patient group. Determining the activity of PDGF signaling in the stem cell niche via the expression of miR-501 and its host gene CLCN5-2 might prove powerful classifiers of patients in future clinical trials that aim to improve skeletal muscle mass and function in patients with diabetes.
Acknowledgments
We thank E. Luca for assistance with flow cytometry and critical discussion of the manuscript, C. Ghirlanda-Keller for performing the paraffin sections and K. Rerkova (BMC SAS) for technical assistance. We further acknowledge the assistance and support of the Center for Microscopy and Image Analysis and the Flow Cytometry Facility of the University of Zurich. The cartoon in Fig. 5 was created with BioRender.com.
Abbreviations
- BMI
body mass index
- CLCN5-2
isoform 2 of chloride voltage-gated channel 5
- DMEM
Dulbecco’s modified Eagle’s medium
- FAP
fibro/adipogenic progenitor
- FGF
fibroblast growth factor
- IGF
insulin-like growth factor
- JAK
Janus kinase
- miRNA/miR-
microRNA
- MP
myogenic progenitor
- MRF
myogenic regulatory factor
- Pax7
paired box-7
- PDGF
platelet-derived growth factor
- RT-PCR
Reverse Transcription Polymerase Chain Reaction
- STAT
signal transducer and activator of transcription
- T2D
type 2 diabetes mellitus
- TSS
transcription start site
Contributor Information
Alexandra Fahrner, Division of Endocrinology, Diabetes, and Clinical Nutrition, University Hospital Zurich, 8091 Zurich, Switzerland; Life Science Zurich Graduate School, Biomedicine, University of Zurich, 8057 Zurich, Switzerland.
Nikoleta Alchus Laiferová, Department of Metabolic Disease Research, Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, 84505 Bratislava, Slovakia.
Barbara Ukropcová, Department of Metabolic Disease Research, Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, 84505 Bratislava, Slovakia; Institute of Pathophysiology, Faculty of Medicine, Comenius University, 81108 Bratislava, Slovakia.
Jozef Ukropec, Department of Metabolic Disease Research, Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, 84505 Bratislava, Slovakia.
Jan Krützfeldt, Division of Endocrinology, Diabetes, and Clinical Nutrition, University Hospital Zurich, 8091 Zurich, Switzerland; Life Science Zurich Graduate School, Biomedicine, University of Zurich, 8057 Zurich, Switzerland.
Funding
This study was supported by a grant from the Swiss National Science Foundation (SNF, 182716) to J.K., and unrestricted grants from the Vontobel and Philhuman foundations to J.K, and the Josef Huwyler Ruth Bernet-Engeli Foundation and Candoc Grant of the University of Zurich (FK-21-023) to A.F. Part of the study performed in Bratislava was supported by Slovak Research and Development Agency, SRDA/APVV 20/0466 (BU) 19/0411 (JU) and Grant Agency of the Slovak Academy of Sciences VEGA 2/0076/22 (BU) 2/0164/20 (JU).
Author Contributions
A.F. designed, performed and analyzed experiments, and wrote the manuscript. N.A.L. performed miRNA analysis in the extended cohort. B.U and J.U. designed and performed the clinical study in patients with type 2 diabetes and collected muscle biopsies. J.K. supervised the study, designed and analyzed experiments, and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Disclosures
The authors declare that no financial interests exist that relate to the research described in this paper.
Data Availability
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885‐892. [DOI] [PubMed] [Google Scholar]
- 3. Moore CW, Allen MD, Kimpinski K, Doherty TJ, Rice CL. Reduced skeletal muscle quantity and quality in patients with diabetic polyneuropathy assessed by magnetic resonance imaging. Muscle Nerve. 2016;53(5):726‐732. [DOI] [PubMed] [Google Scholar]
- 4. Farup J, Just J, de Paoli F, et al. Human skeletal muscle CD90+ fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients. Cell Metab. 2021;33(11):2201‐2214. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rasmussen DGK, Hansen TW, von Scholten BJ, et al. Higher collagen VI formation is associated with all-cause mortality in patients with type 2 diabetes and microalbuminuria. Diabetes Care. 2018;41(7):1493‐1500. [DOI] [PubMed] [Google Scholar]
- 6. Richardson DK, Kashyap S, Bajaj M, et al. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem. 2005;280(11):10290‐10297. [DOI] [PubMed] [Google Scholar]
- 7. Qiao YS, Chai YH, Gong HJ, et al. The association between diabetes mellitus and risk of sarcopenia: accumulated evidences from observational studies. Front Endocrinol (Lausanne). 2021;12:782391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leenders M, Verdijk LB, van der Hoeven L, et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013;14(8):585‐592. [DOI] [PubMed] [Google Scholar]
- 9. D'Souza DM, Zhou S, Rebalka IA, et al. Decreased satellite cell number and function in humans and mice with type 1 diabetes is the result of altered notch signaling. Diabetes. 2016;65(10):3053‐3061. [DOI] [PubMed] [Google Scholar]
- 10. Fu X, Zhu M, Zhang S, Foretz M, Viollet B, Du M. Obesity impairs skeletal muscle regeneration through inhibition of AMPK. Diabetes. 2016;65(1):188‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caiozzo VJ. Plasticity of skeletal muscle phenotype: mechanical consequences. Muscle Nerve. 2002;26(6):740‐768. [DOI] [PubMed] [Google Scholar]
- 12. Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin IH, Chang J-L, Hua K, Huang W-C, Hsu M-T, Chen Y-F. Skeletal muscle in aged mice reveals extensive transformation of muscle gene expression. BMC Genet. 2018;19(1):55‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim K-S, Park K-S, Kim M-J, Kim S-K, Cho Y-W, Park SW. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr Gerontol Int. 2014;14(S1):115‐121. [DOI] [PubMed] [Google Scholar]
- 15. Bazgir B, Fathi R, Rezazadeh Valojerdi M, Mozdziak P, Asgari A. Satellite cells contribution to exercise mediated muscle hypertrophy and repair. Cell J. 2017;18(4):473‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23(16):3430‐3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmidt M, Schüler SC, Hüttner SS, von Eyss B, von Maltzahn J. Adult stem cells at work: regenerating skeletal muscle. Cell Mol Life Sci. 2019;76(13):2559‐2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murach KA, White SH, Wen Y, et al. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet Muscle. 2017;7(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lepper C, Partridge TA, Fan C-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138(17):3639‐3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138(17):3625‐3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masschelein E, D’Hulst G, Zvick J, et al. Exercise promotes satellite cell contribution to myofibers in a load-dependent manner. Skelet Muscle. 2020;10(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Englund DA, Figueiredo VC, Dungan CM, et al. Satellite cell depletion disrupts transcriptional coordination and muscle adaptation to exercise. Function (Oxf). 2021;2(1):zqaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee JD, Fry CS, Mula J, et al. Aged muscle demonstrates fiber-type adaptations in response to mechanical overload, in the absence of myofiber hypertrophy, independent of satellite cell abundance. J Gerontol A Biol Sci Med Sci. 2016;71(4):461‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fry CS, Lee JD, Mula J, et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21(1):76‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ciaraldi TP, Abrams L, Nikoulina S, Mudaliar S, Henry RR. Glucose transport in cultured human skeletal muscle cells. Regulation by insulin and glucose in nondiabetic and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1995;96(6):2820‐2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaster M, Petersen I, Hojlund K, Poulsen P, Beck-Nielsen H. The diabetic phenotype is conserved in myotubes established from diabetic subjects: evidence for primary defects in glucose transport and glycogen synthase activity. Diabetes. 2002;51(4):921‐927. [DOI] [PubMed] [Google Scholar]
- 27. Serra C, Bhasin S, Tangherlini F, et al. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology. 2011;152(1):193‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wozniak AC, Anderson JE. Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn. 2007;236(1):240‐250. [DOI] [PubMed] [Google Scholar]
- 29. Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30(2):232‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807‐810. [DOI] [PubMed] [Google Scholar]
- 31. Jopling C. Liver-specific microRNA-122: biogenesis and function. RNA Biol. 2012;9(2):137‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432(7014):226‐230. [DOI] [PubMed] [Google Scholar]
- 33. Jayawardena TM, Egemnazarov B, Finch EA, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110(11):1465‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen J-F, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen J-F, Tao Y, Li J, et al. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010;190(5):867‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu N, Williams AH, Maxeiner JM, et al. microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J Clin Invest. 2012;122(6):2054‐2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Rooij E, Quiat D, Johnson BA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17(5):662‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mizbani A, Luca E, Rushing EJ, Krutzfeldt J. MicroRNA deep sequencing in two adult stem cell populations identifies miR-501 as a novel regulator of myosin heavy chain during muscle regeneration. Development. 2016;143(22):4137‐4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mathes S, Fahrner A, Ghoshdastider U, et al. FGF-2-dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis. Proc Natl Acad Sci U S A. 2021;118(37):e2021013118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kurdiova T, Balaz M, Vician M, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592(5):1091‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Galimov A, Merry TL, Luca E, et al. MicroRNA-29a in adult muscle stem cells controls skeletal muscle regeneration during injury and exercise downstream of fibroblast growth factor-2. Stem Cells. 2016;34(3):768‐780. [DOI] [PubMed] [Google Scholar]
- 42. Luca E, Turcekova K, Hartung A, Mathes S, Rehrauer H, Krützfeldt J. Genetic deletion of microRNA biogenesis in muscle cells reveals a hierarchical non-clustered network that controls focal adhesion signaling during muscle regeneration. Mol Metab. 2020;36:100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fahrner A, Laiferova NA, Ukropcová B, Ukropec B, Ukropec J. Data for activation of PDGF signaling in the adult muscle stem cell niche in patients with type 2 diabetes mellitus. Figshare. Created January 3, 2023 and Published February 9, 2023. 10.6084/m9.figshare.21807111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Skapek SX, Rhee J, Kim PS, Novitch BG, Lassar AB. Cyclin-mediated inhibition of muscle gene expression via a mechanism that is independent of pRB hyperphosphorylation. Mol Cell Biol. 1996;16(12):7043‐7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hietakangas V, Anckar J, Blomster HA, et al. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci U S A. 2006;103(1):45‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bromberg JF, Wrzeszczynska MH, Devgan G Jr., et al. Stat3 as an oncogene. Cell. 1999;98(3):295‐303. [DOI] [PubMed] [Google Scholar]
- 47. Moore JE, Purcaro MJ, Pratt HE, et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020;583(7818):699‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Santos-Rosa H, Schneider R, Bannister AJ, et al. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419(6905):407‐411. [DOI] [PubMed] [Google Scholar]
- 49. Tanaka K, Fisher SE, Craig IW. Characterization of novel promoter and enhancer elements of the mouse homologue of the dent disease gene, CLCN5, implicated in X-linked hereditary nephrolithiasis. Genomics. 1999;58(3):281‐292. [DOI] [PubMed] [Google Scholar]
- 50. Hayama A, Uchida S, Sasaki S, Marumo F. Isolation and characterization of the human CLC-5 chloride channel gene promoter. Gene. 2000;261(2):355‐364. [DOI] [PubMed] [Google Scholar]
- 51. Liu N, Nelson BR, Bezprozvannaya S, et al. Requirement of MEF2A, C, and D for skeletal muscle regeneration. Proc Natl Acad Sci U S A. 2014;111(11):4109‐4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Han J, LaVigne CA, Jones BT, Zhang H, Gillett F, Mendell JT. A ubiquitin ligase mediates target-directed microRNA decay independently of tailing and trimming. Science. 2020;370(6523):eabc9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shi CY, Kingston ER, Kleaveland B, Lin DH, Stubna MW, Bartel DP. The ZSWIM8 ubiquitin ligase mediates target-directed microRNA degradation. Science. 2020;370(6523):eabc9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang L, Hu JJ, Gong F. MG132 Inhibition of proteasome blocks apoptosis induced by severe DNA damage. Cell Cycle. 2011;10(20):3515‐3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brancaccio A, Palacios D. Chromatin signaling in muscle stem cells: interpreting the regenerative microenvironment. Front Aging Neurosci. 2015;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mathes S, Fahrner A, Ghoshdastider U, et al. FGF-2-dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis. Proc Natl Acad Sci U S A. 2021;118(37):e2021013118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barclay RD, Burd NA, Tyler C, Tillin NA, Mackenzie RW. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front Nutr. 2019;6:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kampmann U, Christensen B, Nielsen TS, et al. GLUT4 And UBC9 protein expression is reduced in muscle from type 2 diabetic patients with severe insulin resistance. PLoS One. 2011;6(11):e27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Viswambharan H, Yuldasheva NY, Imrie H, et al. Novel paracrine action of endothelium enhances glucose uptake in muscle and fat. Circ Res. 2021;129(7):720‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suh JM, Jonker JW, Ahmadian M, et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature. 2014;513(7518):436‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mashili FL, Austin RL, Deshmukh AS, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev. 2011;27(3):286‐297. [DOI] [PubMed] [Google Scholar]
- 62. Ying L, Wang L, Guo K, et al. Paracrine FGFs target skeletal muscle to exert potent anti-hyperglycemic effects. Nat Commun. 2021;12(1):7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanchez-Encinales V, Cozar-Castellano I, Garcia-Ocana A, Perdomo G. Targeted delivery of HGF to the skeletal muscle improves glucose homeostasis in diet-induced obese mice. J Physiol Biochem. 2015;71(4):795‐805. [DOI] [PubMed] [Google Scholar]
- 64. Bohm A, Hoffmann C, Irmler M, et al. TGF-beta contributes to impaired exercise response by suppression of mitochondrial key regulators in skeletal muscle. Diabetes. 2016;65(10):2849‐2861. [DOI] [PubMed] [Google Scholar]
- 65. Uezumi A, Fukada S, Yamamoto N, et al. Identification and characterization of PDGFRα+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5(4):e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Theret M, Rossi FMV, Contreras O. Evolving roles of muscle-resident fibro-adipogenic progenitors in health, regeneration, neuromuscular disorders, and aging. Front Physiol. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Farup J, Just J, de Paoli F, et al. Human skeletal muscle CD90 + fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients. Cell Metab. 2021;33(11):2201‐2214.e2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. D'Souza DM, Al-Sajee D, Hawke TJ. Diabetic myopathy: impact of diabetes mellitus on skeletal muscle progenitor cells. Front Physiol. 2013;4:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Teng S, Huang P. The effect of type 2 diabetes mellitus and obesity on muscle progenitor cell function. Stem Cell Res Ther. 2019;10(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kurdiova T, Balaz M, Mayer A, et al. Exercise-mimicking treatment fails to increase Fndc5 mRNA & irisin secretion in primary human myotubes. Peptides. 2014;56:1‐7. [DOI] [PubMed] [Google Scholar]
- 71. Scheele C, Nielsen S, Kelly M, et al. Satellite cells derived from obese humans with type 2 diabetes and differentiated into myocytes in vitro exhibit abnormal response to IL-6. PLoS One. 2012;7(6):e39657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Iovino S, Burkart AM, Warren L, Patti ME, Kahn CR. Myotubes derived from human-induced pluripotent stem cells mirror in vivo insulin resistance. Proc Natl Acad Sci U S A. 2016;113(7):1889‐1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Davegardh C, Sall J, Benrick A, et al. VPS39-deficiency observed in type 2 diabetes impairs muscle stem cell differentiation via altered autophagy and epigenetics. Nat Commun. 2021;12(1):2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Demoulin J-B, Essaghir A. PDGF receptor signaling networks in normal and cancer cells. Cytokine Growth Factor Rev. 2014;25(3):273‐283. [DOI] [PubMed] [Google Scholar]
- 75. Hojman P, Pedersen M, Nielsen AR, et al. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes. 2009;58(12):2797‐2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dhindsa S, Ghanim H, Green K, et al. Acute effects of insulin on skeletal muscle growth and differentiation genes in men with type 2 diabetes. Eur J Endocrinol. 2019;181(6):K55‐K59. [DOI] [PubMed] [Google Scholar]
- 77. Zhou M, Li B, Liu C, et al. M2 macrophage-derived exosomal miR-501 contributes to pubococcygeal muscle regeneration. Int Immunopharmacol. 2021;101(Pt B):108223. [DOI] [PubMed] [Google Scholar]
- 78. Liu X, Zhang F, Chai Y, Wang L, Yu B. The role of bone-derived PDGF-AA in age-related pancreatic β cell proliferation and function. Biochem Biophys Res Commun. 2020;524(1):22‐27. [DOI] [PubMed] [Google Scholar]
- 79. Sakellariou GK, Pearson T, Lightfoot AP, et al. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep. 2016;6(1):33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nilwik R, Snijders T, Leenders M, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48(5):492‐498. [DOI] [PubMed] [Google Scholar]
- 81. Ackland DC, Denton M, Schache AG, Pandy MG, Crossley KM. Hip abductor muscle volumes are smaller in individuals affected by patellofemoral joint osteoarthritis. Osteoarthritis Cartilage. 2019;27(2):266‐272. [DOI] [PubMed] [Google Scholar]
- 82. Hua X, Chen L, Wang J, Li J, Wingender E. Identifying cell-specific microRNA transcriptional start sites. Bioinformatics. 2016;32(16):2403‐2410. [DOI] [PubMed] [Google Scholar]
- 83. Horak M, Novak J, Bienertova-Vasku J. Muscle-specific microRNAs in skeletal muscle development. Dev Biol. 2016;410(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 84. Ermolaeva M, Neri F, Ori A, Rudolph KL. Cellular and epigenetic drivers of stem cell ageing. Nature Reviews Molecular Cell Biology. 2018;19(9):594‐610. [DOI] [PubMed] [Google Scholar]
- 85. Srivastava S, Rathor R, Singh SN, Suryakumar G. Emerging role of MyomiRs as biomarkers and therapeutic targets in skeletal muscle diseases. Am J Physiol Cell Physiol. 2021;321(5):C859‐C875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.