Abstract
Context
Phthalates are hypothesized to contribute to diabetes, but longitudinal evidence in humans is limited.
Objective
We examined whether phthalate exposure was associated with a higher incidence of diabetes in a racially/ethnically diverse cohort of midlife women.
Methods
In the Study of Women's Health Across the Nation Multipollutant Study, we followed 1308 women without diabetes in 1999-2000 for 6 years. Eleven phthalate metabolites were measured in spot urine samples in 1999-2000 and 2002-2003. Incident diabetes was ascertained between 1999-2000 and 2005-2006. Cox proportional hazards models with time-varying exposure were used to estimate the hazard ratio (HR) of diabetes associated with each phthalate metabolite, adjusting for demographic, lifestyle, and health-related factors. Effect modification by race/ethnicity was examined with interaction terms.
Results
Sixty-one women developed diabetes over 6 years (cumulative incidence = 4.7%). Among all women, several high-molecular-weight phthalate metabolites were associated with a higher incidence of diabetes, but none were statistically significant. There was effect modification by race/ethnicity. Among White women, each doubling of the concentrations of mono-isobutyl phthalate (MiBP), monobenzyl phthalate, mono-carboxyoctyl phthalate, mono-carboxyisononyl phthalate (MCNP), and mono(3-carboxypropyl) phthalate was associated with a 30% to 63% higher incidence of diabetes (HR = 1.30, 95% CI, 1.03-1.65 for MCNP; HR = 1.63, 95% CI, 1.18-2.25 for MiBP). In contrast, phthalates were not associated with diabetes incidence in Black or Asian women.
Conclusions
Some phthalate metabolites were associated with a higher incidence of diabetes over 6 years, but the associations were inconsistent across racial/ethnic groups. Whether phthalates cause diabetes requires further investigation.
Keywords: phthalates, diabetes, women, endocrine disrupting chemicals
Diabetes is one of the leading causes of death and disability. In 2017-2020, 14.7% of adults in the United States had diabetes (1). Individuals with diabetes are at increased risk of many serious chronic conditions. The disease was estimated to cost the US healthcare system $327 billion in 2017 (2), consuming a significant portion of healthcare expenditures. These enormous costs to individuals and societies have spurred ongoing interest to understand the causes of diabetes to facilitate better prevention and treatment.
The current extraordinary burden of diabetes is the culmination of 6 decades of continuous increases in its prevalence (3). Because this period of increasing diabetes prevalence coincided with the increasing use of synthetic chemicals in industry and commerce, and many synthetic chemicals were found to disrupt energy and glucose metabolism in experimental models, exposure to metabolism-disrupting chemicals has been hypothesized to contribute to diabetes (4, 5). Phthalates, di-esters of 1, 2-benzenedicarboxylic acid, are one of these chemicals. Low-molecular-weight (LMW) phthalates are frequently added to personal care products, such as fragrance, nail polish, and some feminine hygiene products, as solvents, plasticizers, and fixatives (6, 7). High-molecular-weight (HMW) phthalates are frequently added to polyvinyl chloride plastic products, such as plastic food packaging, clothing, and vinyl flooring, as plasticizers (8). Exposure to phthalates is widespread through ingesting food contaminated during processing, packaging, and storage (9-11). Absorption through the skin and potentially the vaginal mucosa is an additional route of exposure particularly relevant for phthalates in personal care products (7, 12).
Because of such widespread exposure, understanding phthalates’ potential diabetogenic effects is important for both risk management and diabetes prevention. In animals, a growing number of studies suggest that exposure to some phthalates adversely affects glucose homeostasis, leading to elevated fasting glucose or worse glucose tolerance (13-15). In humans, epidemiologic studies support an association between phthalate exposure and insulin resistance (16). The association between phthalates and diabetes is less certain. Most studies have been cross-sectional (16). Because diabetes is a chronic disease with a long duration, exposure to phthalates is highly dynamic, and phthalates do not accumulate in the body (12, 17), cross-sectional studies are particularly problematic for causal inference. Phthalate exposure when diabetes is well established may not represent phthalate exposure before disease onset. To our knowledge, only 2 studies have examined phthalates and incident diabetes (18, 19). One study found positive associations between some phthalate metabolites and diabetes in a group of predominantly White nurses in the United States (20). The other study identified mono-isobutyl phthalate (MiBP) as a part of a mixture that improved diabetes incidence prediction over 5 years, although MiBP was not associated with incident diabetes in single-pollutant analysis (19). It is unclear if these findings are generalizable to other populations because the studies were conducted among a predominantly White population in the United States or among older adults in Europe. Further, both studies measured phthalate metabolites at only 1 time point and examined their associations with incident diabetes in the next 5 to 10 years. Because phthalate metabolites in spot urine samples may not accurately reflect habitual exposure (21), the reported associations may be biased toward the null due to substantial exposure measurement error. To address these limitations, we conducted a cohort study on repeatedly-measured phthalates and incident diabetes among a diverse group of midlife women in the United States.
Methods
Study Population
Participants were drawn from the Study of Women's Health Across the Nation (SWAN). SWAN is an ongoing longitudinal study of women's health in midlife with nearly annual follow-up visits. Details about the design of SWAN are available on the study's website (https://www.swanstudy.org/). Briefly, in 1996-1997, women were recruited from 7 study sites: Oakland, California; Los Angeles, California; Chicago, Illinois; the Detroit, Michigan, area; Pittsburgh, Pennsylvania; Boston, Massachusetts; and Newark, New Jersey. Eligibility criteria for SWAN include (1) age between 42 and 52 years in 1996-1997; (2) self-identifying as White, Black, Chinese, Japanese, or Hispanic; (3) having an intact uterus, at least 1 ovary, and at least 1 menstrual period in the past 3 months; and (4) not having used any exogeneous reproductive hormones in the past 3 months. A total of 3302 women met these criteria and participated in SWAN.
The SWAN Multipollutant Study (SWAN-MPS) is an ancillary study that selected SWAN participants for environmental chemical exposure assessments using banked biospecimens from the 1999-2000 and 2002-2003 study visits. Of the 2694 women still active in SWAN in 1999-2000, SWAN-MPS excluded all 646 women from Chicago and Newark because neither site collected urine samples necessary for environmental chemical exposure assessments. An additional 648 women from the other sites were excluded because they lacked sufficient blood or urine samples for environmental chemical exposure assessments. In total, SWAN-MPS included 1400 women, most of whom (N = 1387) had phthalates data at both time points in 1999-2000 and 2002-2003. Because all Hispanic participants of SWAN were recruited from Newark, SWAN-MPS did not include any Hispanic women.
This study aimed to examine the association between time-varying phthalate exposure and incident diabetes between 1999-2000 and 2005-2006. We chose to limit the follow-up time to 6 years because exposure to phthalates is episodic in nature, and phthalates have short half-lives in the body (12, 21). Limiting the follow-up time to 6 years allowed us to have updated information on phthalate exposure in the middle of follow-up. To be eligible for this study, women must be free of diabetes in 1999-2000 and have at least 1 visit with complete data for phthalate metabolites and covariates [urinary creatinine, age in 1999-2000, site, race/ethnicity, education, dietary energy intake, smoking status, physical activity, menopausal status, and body mass index (BMI)] before diabetes onset, loss to follow-up, or end of observation in 2005-2006. Based on these criteria, we excluded 80 women with prevalent diabetes in 1999-2000. We further excluded 12 women with missing covariate data. The analytic sample thus included 1308 women. Of these, 1293 women entered the risk set in 1999-2000 and 15 entered in 2002-2003. The 15 women entered the risk set late because of incomplete covariate data in 1999-2000. The median follow-up time of the entire sample was 6 years.
All SWAN and SWAN-MPS study protocols have been approved by institutional review boards. SWAN participants provided written informed consent to participate in the study.
Phthalate Metabolites
Women provided spot urine samples in polyethylene tubes at in-person visits in 1999-2000 and 2002-2003. The urine samples were stored at −80 °C without thawing until analysis. In 2017-2018, these samples were thawed, and 12 phthalate metabolites were measured using online solid phase extraction coupled to high-performance liquid chromatography-isotope dilution tandem mass spectroscopy (HPLC-MS). Previous studies have shown that phthalate metabolites remain stable in urine samples after long-term storage in freezing conditions (22, 23).
The 12 phthalate metabolites measured included three metabolites of LMW phthalates: mono-ethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), and mono-isobutyl phthalate (MiBP); four metabolites of di(2-ethylhexyl) phthalate (DEHP), a HMW phthalate of particular public health interest: mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP); and five metabolites of other HMW phthalates: monobenzyl phthalate (MBzP), mono-isononyl phthalate (MiNP), mono-carboxyoctyl phthalate (MCOP), mono-carboxy-isononyl phthalate (MCNP), and mono(3-carboxypropyl) phthalate (MCPP). We measured these phthalate metabolites because their parents have been widely used in industry and commerce, and exposure to these phthalates is a national biomonitoring priority (24). The lower limits of detection of each phthalate metabolite and quality control measures of the HPLC-MS assays were previously reported (25). The coefficient of variation of the HPLC-MS assay ranged from an average of 4% for MEHP to 19% for MCOP. We excluded MiNP from all analyses because it was detected in less than 1% of urine samples. For analyses, the concentrations of all other phthalate metabolites, including those below the limits of detection, were used as output by the assay, except for the few negative or zero values. To facilitate log2-transformation, we replaced 7 negative values of MiBP, 5 negative values of MEHP, 1 zero value of MCOP, and 5 negative values of MCPP with each metabolite's median below its limit of detection.
Diabetes
Women's diabetes status was determined longitudinally based on all data from SWAN baseline in 1996-1997 to the most recent follow-up visit in 2016-2017. At SWAN baseline and each follow-up visit, women self-reported doctor's diagnosis of diabetes. In all but 3 visits, women self-reported the use of any antidiabetic medications. In all but 6 visits, women also provided fasting blood samples for the measurement of glucose with a hexokinase assay (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). A woman was classified as ever having diabetes if she (1) reported using antidiabetic medications at any visit, (2) had fasting glucose ≥126 mg/dL for 2 consecutive visits, or (3) self-reported doctor's diagnosis of diabetes at 2 visits and had fasting glucose ≥126 mg/dL at 1 visit. For women who were classified as having diabetes based on medications, the visit of diabetes onset was defined as the first visit with fasting glucose ≥126 mg/dL before the first use of medications; otherwise, the first visit with self-reported diabetes before the first use of medications; otherwise, the first visit at which antidiabetic medication use was reported. For women classified as having diabetes based on the other 2 criteria, the visit of diabetes onset was defined as the first visit with fasting glucose ≥126 mg/dL.
In our analysis, we treated Follow-up Visit 3 in 1999-2000 as the time origin and calculated time to diabetes onset as the time elapsed (in years) between 1999-2000 and the visit of diabetes onset. For women who remained free of diabetes before loss to follow-up or the end of observation at Follow-up Visit 9 in 2005-2006, their time to diabetes onset was right-censored and calculated as the time elapsed (in years) between 1999 and 2000 and the date of their last follow-up visit.
Covariates
Creatinine was measured in urine in 1999-2000 and 2002-2003 with a Cobas Mira analyzer (Horiba ABX, Montpellier, France). Time-fixed confounders included age in 1999-2000, site, race/ethnicity, and education. Time-varying confounders included dietary energy intake, smoking status, physical activity, menopausal status, and BMI.
Age was calculated from visit date in 1999-2000 and date of birth. Site, race/ethnicity, and education were collected in questionnaires at the SWAN study baseline in 1996-1997. Dietary energy intake (kcal/day) was estimated from a modified Block Food Frequency Questionnaire (26). This was administered in 1996-1997 and 2001-2002 only. We used diet data from 1996-1997 and 2001-2002 to approximate diet in 1999-2000 and 2002-2003, respectively. Smoking status (never, past, current), current hormone therapy use (yes, no), self-reported menstrual bleeding frequency, history of gynecologic surgeries, and the frequency of various physical activities were collected via questionnaires in 1999-2000 and 2002-2003. We determined women's menopausal status (pre- or perimenopausal, natural or surgical menopause, and unknown due to hormone therapy use) based on menstrual bleeding frequency, history of gynecologic surgeries, and use of exogeneous hormones. We measured women's physical activity with an index that summarized the frequency and intensity of leisure time physical activity, housework, and active transport (27). BMI was calculated as body weight (kg)/height2 (m2). Body weight was measured in light clothing with a calibrated scale, and height was measured with a stadiometer at each visit. Previous studies showed that the association between phthalates and BMI may be bidirectional. On the one hand, a higher BMI is a marker of lower socioeconomic status and unhealthy behaviors, which are associated with increased phthalate exposure (28). On the other hand, phthalate exposure may lead to more rapid body fat gain (20, 29). Given this potential bidirectionality, we used BMI collected 1 year before phthalate exposure assessments as confounders to strengthen temporality. If BMI 1 year before phthalate exposure assessments was missing (82 observations), we imputed this BMI with BMI obtained up to 3 years before phthalate exposure assessments. Obesity status was defined based on BMI using race/ethnicity-specific cut-points (30): For White and Black women, normal/underweight was defined as BMI < 25 kg/m2, overweight as 25 kg/m2 ≤ BMI < 30 kg/m2, and obese as BMI ≥ 30 kg/m2. For Chinese and Japanese women, normal/underweight was defined as BMI < 23 kg/m2, overweight as 23 kg/m2 ≤ BMI < 27 kg/m2, and obese as BMI ≥ 27 kg/m2.
Statistical Methods
Phthalate metabolite concentrations were adjusted for urine dilution using the covariate-adjusted creatinine standardization method (31). Briefly, each metabolite concentration was divided by the ratio of observed to predicted urinary creatinine. Predictors of creatinine included age, race/ethnicity, BMI, and height (32). The prediction model was developed with data in 1999-2000. We calculated the molar sums of LMW phthalate metabolites, DEHP metabolites, and all HMW phthalate metabolites to assess the impact of aggregate exposure to each group of phthalates.
We obtained descriptive statistics [median (first and third quartiles) for continuous variables; count (%) for categorical variables] of the analytic sample in 1999-2000. To examine the associations between potential confounders and phthalate metabolites in 1999-2000, we obtained the median (first and third quartiles) concentration of each phthalate metabolite by levels of confounders. Differences in median phthalate concentrations across confounder levels were compared with Kruskal-Wallis tests. To examine the associations between incident diabetes status and phthalate metabolites and potential confounders in 1999-2000, we obtained descriptive statistics [median (first and third quartiles) for continuous variables; count (%) for categorical variables] by incident diabetes. Differences in the distribution of phthalate metabolites and confounders by incident diabetes status were compared with Wilcoxon rank-sum tests (continuous variables) and Chi-squared tests (categorical variables).
To examine the association between phthalate exposure and diabetes incidence, for each phthalate metabolite, we fit a series of Cox proportional hazards models with time-varying phthalate metabolites and covariates. Model 1 included the time-varying log2-transformed phthalate metabolite only. Model 2 additionally adjusted for age in 1999-2000; race/ethnicity; site; education; and time-varying physical activity, smoking status, dietary energy intake, and menopausal status. Model 3 additionally adjusted for time-varying BMI. We fit this series of models to examine the impact of confounders on the association between each phthalate metabolite and diabetes incidence. The difference between Models 2 and 3 was of particular interest because BMI was potentially both a confounder and a mediator of the association between phthalates and diabetes. Adjusting for BMI may underestimate the associations between phthalate metabolites and diabetes. From each model, we calculated the hazard ratio (HR) and 95% CI for diabetes per doubling of concentrations for each phthalate metabolite.
We conducted a series of sensitivity analyses to assess the robustness of our findings. First, we fit each phthalate metabolite as tertiles in Cox models to examine potential violation of the linearity assumption. Second, we additionally adjusted for intake frequency of food items associated with phthalate exposure in Cox models to examine potential confounding by these food items. These food items included red meat, poultry, liver, processed meat, dairy, margarine, refined grains, salty snacks, desserts, meat substitutes, pizza, salad dressing, and salsa (10, 11, 32-36). Third, we reanalyzed our data using marginal structural models (MSM) with inverse-probability-of-treatment weights (IPTW) (37). Details about these weights and the MSMs are available in the supplementary materials (38). The IPTW-weighted MSMs allowed us to control for confounding by BMI without adjusting for this variable. Thus, they overcame potential bias induced by BMI adjustment in conventional Cox models. Results from the IPTW-weighted MSMs allowed us to further understand the impact of BMI adjustment on the association between phthalates and diabetes in the main analyses. Fourth, this analysis was based on women who were selected into SWAN-MPS. If selection was related to determinants of phthalate exposure and incident diabetes, HR estimates from our main analyses might be biased. Although we conditioned on many of these determinants in our main analyses (age, race/ethnicity, site, education, smoking status), which should have eliminated bias due to selective participation, we applied inverse-probability-of-selection weights to Cox regression models in sensitivity analyses to further correct for selection bias. Details about these weights are available in the supplementary materials (38). Lastly, because a major difference between this study and existing studies on phthalates and incident diabetes was the racial/ethnic composition of the analytic sample, we included race/ethnicity by phthalate metabolite interaction terms in Cox regression models to investigate potential effect modification by race/ethnicity. In these models, we combined Japanese and Chinese women into 1 group labeled as “Asian” because of the small number of incident diabetes cases among Chinese and Japanese women (4 cases in Chinese; 5 cases in Japanese).
As post hoc analyses, we examined the associations between phthalate metabolites and biomarkers of glucose homeostasis, including fasting glucose and homeostatic model assessment of insulin resistance (HOMA-IR), to see if racial/ethnic differences in the associations between phthalates and diabetes can potentially be explained by racial/ethnic differences in the associations between phthalates and glucose metabolism. We used repeatedly measured fasting glucose and HOMA-IR between 1999-2000 and 2005-2006 as outcomes for these analyses. HOMA-IR was calculated as [fasting insulin (µU/mL) ×fasting glucose (mmol/L)]/22.5 (39). Fasting insulin was measured in serum using a solid phase radioimmunoassay (Coat-A-Count, Diagnostics Product Corp., Los Angeles, CA, USA) (40). Observations obtained while participants were taking antidiabetic medications were excluded. For each phthalate metabolite, percent differences in fasting glucose and HOMA-IR were estimated via mixed effects models. The models included log2-transformed phthalate metabolite, race/ethnicity (White, Black, Asian), their interaction, as well as age, site, education, lagged BMI in 1999-2000, and time-varying smoking status, physical activity, menopausal status, and dietary energy intake as predictors. Random intercepts were included to account for within-woman correlations.
Statistical analyses were conducted in R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) using packages “survival” (version 3.2-13) (41) and “ipw” (version 1.0-11) (42). A 2-sided P-value < 0.05 was considered statistically significant.
Results
Women had a median age of 49.4 (first quartile, third quartile: 47.4, 51.5) in 1999-2000 (Table 1). Approximately half of the participants were White, 20.3% Black, 13% Chinese, and 15.2% Japanese. Approximately half of the participants had a college degree or higher. Most women were never smokers. Most were pre- or perimenopausal. Approximately 29% of the participants were obese.
Table 1.
Participant characteristics in 1999-2000
| Median (Q1, Q3)a | |
|---|---|
| Age (years) | 49.4 (47.4, 51.5) |
| BMI (kg/m2)b | 25.5 (22.3, 30.5) |
| N (%) | |
| Site | |
| Detroit area, MI | 225 (17.4%) |
| Boston, MA | 211 (16.3%) |
| Oakland, CA | 293 (22.7%) |
| Los Angeles, CA | 346 (26.8%) |
| Pittsburgh, PA | 218 (16.9%) |
| Race/ethnicity | |
| White | 667 (51.6%) |
| Black | 262 (20.3%) |
| Chinese | 168 (13.0%) |
| Japanese | 196 (15.2%) |
| Education | |
| High school or less | 222 (17.2%) |
| Some college | 409 (31.6%) |
| College degree | 328 (25.4%) |
| Postgraduate | 334 (25.8%) |
| Smoking | |
| Never | 817 (63.2%) |
| Past | 345 (26.7%) |
| Current | 131 (10.1%) |
| Menopausal status | |
| Pre- or peri menopausal | 913 (70.6%) |
| Natural/surgical menopause | 186 (14.4%) |
| Unknown due to hormone therapy | 194 (15.0%) |
| Obesity statusc | |
| Normal/underweight | 520 (40.2%) |
| Overweight | 395 (30.5%) |
| Obese | 378 (29.2%) |
Abbreviations: BMI, body mass index; Q1, first quartile; Q3, third quartile.
Descriptive data were based on the 1293 women who had complete data in 1999-2000.
BMI data came from the 1998-1999 follow-up visit for 1248 women, the 1997-1998 visit for 36 women, and the 1996-1997 visit for 9 women.
Obesity was defined with BMI using race-specific cut-points. For Black and White, normal/underweight: BMI < 25 kg/m2; overweight: 25 kg/m2 ≤ BMI < 30 kg/m2; obese: BMI ≥ 30 kg/m2. For Chinese and Japanese, normal/underweight: BMI < 23 kg/m2; overweight: 23 kg/m2 ≤ BMI < 27 kg/m2; obese: BMI ≥ 27 kg/m2.
In 1999-2000, the detection frequency of phthalate metabolites ranged from 84.8% for MEHP to 100% for MnBP and MECPP (Table 2). Women who were younger, Black, current smokers, or obese generally had higher concentrations of phthalate metabolites [Supplementary Tables S1-S3 in (38)]. Over 6 years, 61 women developed diabetes (incidence rate = 8.1 per 1000 person-years). Compared to those who did not develop diabetes, women with incident diabetes had significantly higher concentrations of all phthalate metabolites except those of DEHP and MCPP (Table 2).
Table 2.
Phthalate metabolite concentrations in 1999-2000, overall and by incident diabetes status
| Group | Phthalate metabolitea | N (%) detectedb | All (n = 1293) | No diabetes (n = 1232) | Incident diabetes (n = 61) | |
|---|---|---|---|---|---|---|
| Median (Q1, Q3) | Median (Q1, Q3) | Median (Q1, Q3) | P-valuec | |||
| LMW phthalate metabolites | MEP (ng/mL) | 1292 (99.9) | 81.54 (36.64, 212.07) | 80.74 (36.16, 206.44) | 112.82 (47.46, 375.43) | 0.03 |
| MnBP (ng/mL) | 1293 (100.0) | 18.50 (11.63, 33.22) | 18.37 (11.53, 32.01) | 26.21 (14.28, 42.80) | 0.005 | |
| MiBP (ng/mL) | 1266 (97.9) | 2.62 (1.55, 4.51) | 2.60 (1.54, 4.42) | 3.84 (2.04, 5.64) | 0.03 | |
| ∑LMW phthalate metabolites (nmol/mL) | 0.57 (0.28, 1.31) | 0.56 (0.28, 1.30) | 0.70 (0.38, 2.19) | 0.01 | ||
| DEHP metabolites | MEHP (ng/mL) | 1096 (84.8) | 3.06 (1.57, 5.98) | 3.10 (1.59, 6.10) | 2.24 (1.32, 4.95) | 0.11 |
| MEHHP (ng/mL) | 1292 (99.9) | 15.89 (8.24, 30.33) | 15.89 (8.22, 30.19) | 15.78 (9.37, 35.01) | 0.71 | |
| MEOHP (ng/mL) | 1291 (99.8) | 9.54 (5.08, 18.60) | 9.55 (4.99, 18.58) | 9.42 (6.62, 19.10) | 0.64 | |
| MECPP (ng/mL) | 1293 (100.0) | 16.70 (9.74, 31.28) | 16.51 (9.72, 31.32) | 19.00 (11.16, 30.85) | 0.41 | |
| ∑DEHP metabolites (nmol/mL) | 0.15 (0.09, 0.29) | 0.15 (0.09, 0.29) | 0.17 (0.10, 0.28) | 0.64 | ||
| Other HMW phthalate metabolites | MBzP (ng/mL) | 1290 (99.8) | 10.41 (5.81, 18.31) | 10.28 (5.66, 17.94) | 14.14 (7.96, 21.93) | 0.01 |
| MCOP (ng/mL) | 1289 (99.7) | 4.47 (2.63, 7.86) | 4.34 (2.59, 7.65) | 6.72 (3.71, 12.43) | 0.002 | |
| MCNP (ng/mL) | 1289 (99.7) | 2.67 (1.51, 4.94) | 2.63 (1.49, 4.86) | 4.05 (1.77, 5.98) | 0.02 | |
| MCPP (ng/mL) | 1275 (98.6) | 2.69 (1.70, 4.28) | 2.67 (1.69, 4.26) | 3.24 (2.01, 5.03) | 0.09 | |
| ∑HMW phthalate metabolites (nmol/mL) | 0.27 (0.16, 0.46) | 0.27 (0.16, 0.46) | 0.30 (0.19, 0.46) | 0.29 |
Abbreviations: LMW, low molecular weight; HMW, high molecular weight; DEHP, di(2-ethylhexyl) phthalate; MBzP, monobenzyl phthalate; MCNP, mono-carboxy-isononyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MCOP, mono-carboxyoctyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHP, mono(2-ethylhexyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, mono-ethyl phthalate; MiBP, mono-isobutyl phthalate; Q1, first quarter; Q3, third quarter.
∑LMW phthalate metabolites, molar sum of MEP, MnBP, and MiBP; ∑DEHP metabolites, molar sum of MEHP, MEHHP, MEOHP, and MECPP; ∑HMW phthalate metabolites, molar sum of MEHP, MEHHP, MEOHP, MECPP, MBzP, MCOP, MCNP, and MCPP.
All phthalate metabolite concentrations were adjusted for urine dilution using the covariate-adjusted creatinine standardization method. Medians and the Q1 and Q3 are reported.
Descriptive data were based on the 1293 women who had complete data in 1999-2000.
P-values were obtained from Wilcoxon rank-sum tests comparing those who developed diabetes vs those who did not.
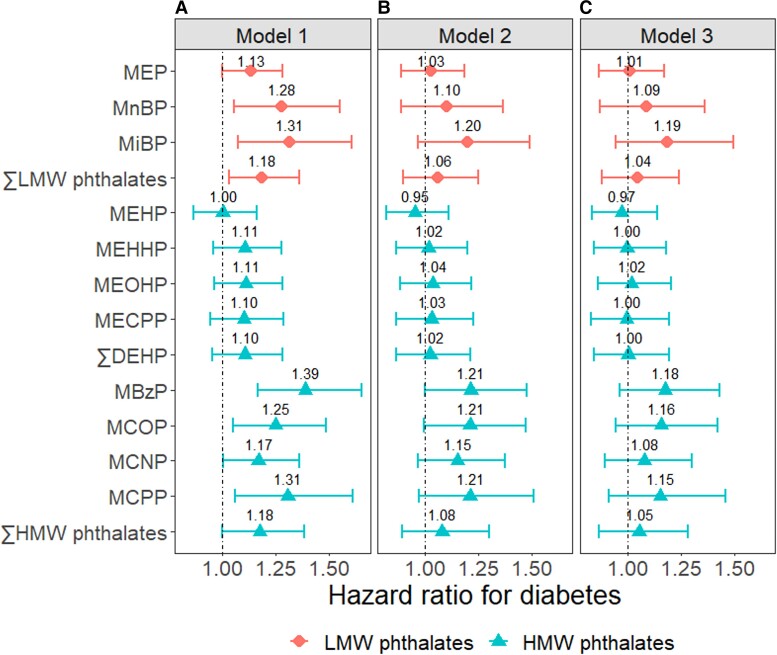
In crude Cox regression models, all phthalate metabolites except DEHP metabolites and the molar sum of HMW phthalates were significantly associated with higher incidence of diabetes [Fig. 1, Panel A; Supplementary Table S5 in (38)]. Adjustment for demographic factors, lifestyle factors, and menopausal status attenuated these associations, so that few associations remained statistically significant [Fig. 1, Panel B; Supplementary Table S5 in (38)]. Further adjustment for BMI led to more attenuations, albeit to a smaller extent [Fig. 1, Panel C; Supplementary Table S5 in (38)]. In fully adjusted Cox regression models, MEP and DEHP metabolites were not associated with the incidence of diabetes (HR = 1). For the other metabolites, each doubling of concentrations was associated with 8% to 19% higher rate of diabetes, but none of the associations were statistically significant.
Figure 1.
Hazard ratios for diabetes per doubling of phthalate metabolite concentrations. Model 1: Crude model. Model 2: Adjusted for age in 1999-2000; race/ethnicity, site, education, and time-varying menopausal status; physical activity; smoking status; and dietary energy intake. Model 3: Model 2 + time-varying BMI. ∑LMW phthalates: molar sum of MEP, MnBP, and MiBP; ∑DEHP: molar sum MEHP, MEHHP, MEOHP, and MECPP; ∑HMW phthalates: molar sum of MEHP, MEHHP, MEOHP, MECPP, MBzP, MCOP, MCNP, and MCPP.
Abbreviations: DEHP, di(2-ethylhexyl) phthalate; HMW, high molecular rate; LMW, low molecular rate; MBzP, monobenzyl phthalate; MCNP, mono-carboxy-isononyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MCOP, mono-carboxyoctyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHP, mono(2-ethylhexyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, mono-ethyl phthalate; MnBP, mono-n-butyl phthalate.
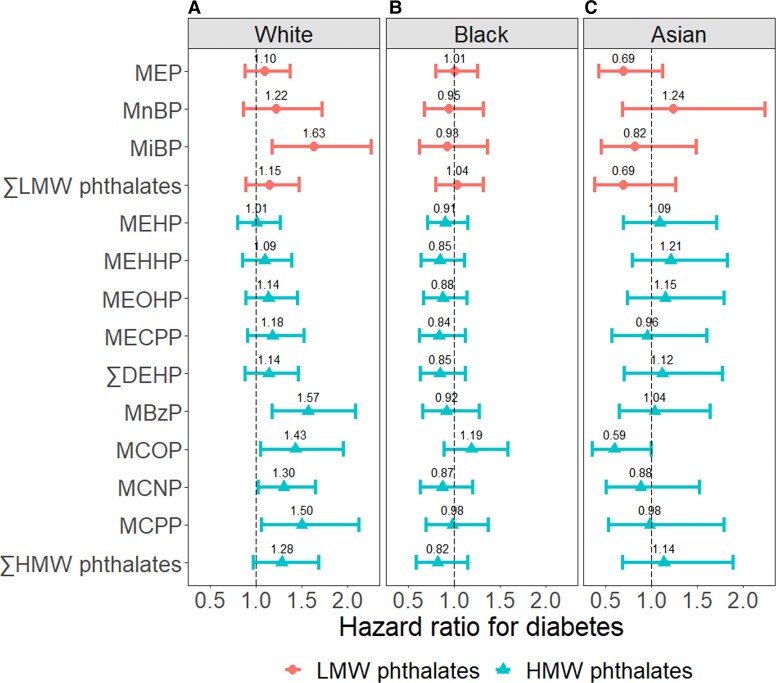
Cox models with phthalate metabolites fitted as tertiles did not reveal notable nonlinear associations [Supplementary Fig. S1 in (38)]. In fact, a statistically significant linear trend was detected for MiBP (P-trend = 0.01). Results did not change with additional adjustment for food items. Results from IPTW-weighted MSMs were nearly identical to those from the fully adjusted Cox models in our main analyses [Supplementary Fig. S2 in (38)]. Applying inverse-probability-of-selection weights did not change our results [Supplementary Fig. S3 in (38)]. Cox models with race/ethnicity by phthalate metabolite interaction terms revealed major differences in the associations between phthalates and diabetes incidence by race/ethnicity. Among White women, MiBP, MBzP, MCOP, MCNP, and MCPP were significantly associated with higher diabetes incidence. Per doubling of concentrations, the HR for diabetes ranged from 1.30 (95% CI, 1.03-1.65) for MCNP to 1.63 (95% CI, 1.18-2.25) for MiBP [Fig. 2, Panel A; Supplementary Table S6 in (38)]. In contrast, among Black and Asian women, none of the phthalate metabolites were associated with increased diabetes incidence [Fig. 2, Panel B and C; Supplementary Table S6 in (38)]. These racial/ethnic differences were inconsistent with the racial/ethnic differences in the associations between phthalate metabolites and glucose homeostasis biomarkers. While the associations between DEHP metabolites and fasting glucose were stronger in White than Black women, the associations between the other phthalate metabolites and fasting glucose did not differ by race/ethnicity or were stronger in Black women [Supplementary Fig. S4 in (38)]. Similarly, the associations between most phthalate metabolites and HOMA-IR did not differ by race/ethnicity or were stronger in Black than White women [Supplementary Fig. S5 in (38)].
Figure 2.
Hazard ratios for diabetes per doubling of phthalate metabolite concentrations by race/ethnicity. The hazard ratios were adjusted for age in 1999-2000; site, education, and time-varying menopausal status; physical activity; smoking status; dietary energy intake; and BMI. ∑LMW phthalates; molar sum of MEP, MnBP, and MiBP; ∑DEHP, molar sum of MEHP, MEHHP, MEOHP, and MECPP; ∑HMW phthalates, molar sum of MEHP, MEHHP, MEOHP, MECPP, MBzP, MCOP, MCNP, and MCPP.
Abbreviations: DEHP, di(2-ethylhexyl) phthalate; HMW, high molecular rate; LMW, low molecular rate; MBzP, monobenzyl phthalate; MCNP, mono-carboxy-isononyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MCOP, mono-carboxyoctyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHP, mono(2-ethylhexyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, mono-ethyl phthalate; MnBP, mono-n-butyl phthalate.
Discussion
In a diverse cohort of midlife women, we found that some phthalate metabolites were associated with a higher incidence of diabetes over 6 years, but these positive associations were apparently limited to White women only. These findings suggest that phthalates may increase the risk of diabetes in some women. However, given inconsistent associations across racial/ethnic groups and phthalate metabolites, a causal relationship between phthalates and diabetes remains uncertain. Additional studies are needed to investigate if phthalate exposure contributes to diabetes.
Phthalates have been shown to disrupt glucose homeostasis in rodents (13-15) and are associated with insulin resistance in diabetes-free adults (16). Whether phthalates increase the risk of diabetes is unclear because few epidemiologic studies have examined the associations between phthalates and incident diabetes. Previously, Sun et al conducted a case-control study among 1941 middle-age or older women from the predominantly White Nurses’ Health Study and Nurses’ Health Study II cohorts. Over approximately 10 years, women at the top quartile of exposure to some phthalates had up to 3 times higher odds of incident diabetes than those at the first quartile (18). Although the strengths of the associations differed by phthalate metabolites and the cohort origin of the participants, findings by Sun et al generally support positive associations between MnBP, MiBP, and DEHP metabolites and incident diabetes in White women. MiBP was also identified as part of a mixture that improved incident diabetes prediction over 5 years in a study among older adults in Sweden, although MiBP was not associated with increased diabetes incidence in single-pollutant analysis (19). Our study confirmed the positive association for MiBP reported by Sun et al. Additionally, we identified 4 more HMW phthalate metabolites associated with significantly increased diabetes risks in White women. Our findings and those by Sun et al and Lind et al are among the few pieces of evidence on phthalates and incident diabetes. While the consistency between some of our findings and those of Sun et al suggests a potential role of phthalates in diabetes development, more studies based on longitudinal data are required to confirm any potential diabetogenic effects of phthalates.
In analyses stratified by race/ethnicity, we found positive associations between some phthalate metabolites and incident diabetes in White but not Black or Asian women. It is unclear what might explain such racial/ethnic differences. It is possible that the effects of phthalates may differ by the route of exposure. For instance, certain menstrual and vaginal products are sources of phthalates (7, 43, 44). Studies suggest that Black women use these products more frequently than White women (7, 45), which may have contributed to not only higher levels of phthalate exposure in Black women but also higher levels of exposure through the vaginal route (7). Unlike the oral route, chemicals absorbed through the vagina enter the circulation without first being metabolized by the liver (45). This may result in differences in the levels and compositions of circulating metabolites for the same parent compound, leading to differences in toxicity.
Alternatively, racial/ethnic differences in the associations between phthalates and incident diabetes may reflect noncausal mechanisms. Our analytic sample included only women who were free of diabetes in 1999-2000. If phthalate exposure increased the risk of diabetes, women who developed diabetes before 1999-2000 due to high levels of past phthalate exposure were excluded from the analytic sample. This process removed highly exposed cases from analysis, creating selection bias and leading to attenuations of the associations between phthalates and incident diabetes. Because Black women are generally exposed to higher levels of phthalates (46) and develop diabetes at a younger age than White women (47), this selection bias may have affected Black women to a greater extent, resulting in greater attenuations in the HRs for incident diabetes. In SWAN-MPS, the urinary concentrations of most phthalate metabolites were higher in Black than White women. In addition, the prevalence of diabetes in 1999-2000 among Black women (11.4%) was nearly 3 times that among White women (4.2%). These patterns seem to support selection bias as a potential explanation for the racial/ethnic differences in the associations between phthalates and incident diabetes. Furthermore, elevated fasting glucose may be a less sensitive criterion to identify incident diabetes among Black and Asian women than White women. Studies have shown that at the same level of whole-body insulin resistance, Black women have lower rates of gluconeogenesis than White women, leading to less frequent fasting hyperglycemia (48). Similarly, the prevalence of impaired fasting glucose is lower in Asian populations than White populations, despite a higher prevalence of impaired glucose tolerance in Asian (49). Using a less sensitive marker of glucose dysregulation in Black and Asian women may have led to greater nondifferential outcome misclassification, which attenuated phthalates’ associations with diabetes in these populations. Unfortunately, without additional data, we are not able to determine which factors may explain the racial/ethnic differences. Our data do indicate that some phthalate metabolites were associated with increased insulin resistance in both White and non-White women. This suggests that all racial/ethnic groups are potentially susceptible to phthalates’ impact on glucose metabolism, and so better-designed studies are needed to quantify the diabetes risks associated with phthalate exposure, particularly in non-White women.
A critical methodological consideration in studies on phthalates and diabetes is the potentially bidirectional relationship between phthalate exposure and adiposity. This bidirectionality means that adjusting for adiposity in conventional regression models to account for confounding may underestimate the associations between phthalates and diabetes. We addressed this concern by reanalyzing our data with IPTW-weighted MSMs, which produced results nearly identical to those from conventional models. The IPTW-weighted MSMs confirmed the validity of BMI adjustment in conventional Cox regression models. In addition, they suggest that, in this study, BMI was likely not a major mediator for phthalates and diabetes. Previously, we found that in SWAN-MPS, phthalate metabolites were associated with more rapid body fat gain primarily in women who were normal/underweight in 1999-2000 (25). In this study, a vast majority (89.9%) of women who developed diabetes over 6 years were overweight or obese in 1999-2000. The weaker associations between phthalates and body fat gain in overweight/obese women may explain why BMI was not a major mediator. Had we had longer follow-up or observed women earlier in the life course before they developed overweight/obesity, we might have found a stronger mediating effect of adiposity and hence greater differences between conventional models and IPTW-weighted MSMs.
Besides increasing body fat, phthalates are thought to increase the risk of diabetes by disrupting glycolysis and gluconeogenesis in liver (50). They may also hinder insulin signaling in liver cells (51, 52), fat cells (51), and skeletal muscle cells (53) through oxidative stress and epigenetic mechanisms, leading to impaired glucose uptake and whole-body insulin resistance. Further, phthalates may increase insulin resistance indirectly by disrupting the synthesis, transportation, or metabolism of hormones important for regulating insulin sensitivity, such as thyroid hormones (54) and sex steroid hormones (55). There is also some evidence that MnBP, MiBP, and MEHP may adversely affect pancreatic β-cell viability and glucose-stimulated insulin secretion (56, 57), but the data all came from in vitro studies and were sometimes conflicting. Intriguingly, many phthalate metabolites activate peroxisome proliferator-activated receptor gamma (PPAR-γ) (58-60). A class of PPAR-γ agonists known as thiazolidinediones is used to treat diabetes because it improves insulin sensitivity through PPAR-γ activation (61). It is unclear if environmental exposure to phthalates at typical levels improves insulin sensitivity similar to thiazolidinediones. If it does, other diabetogenic mechanisms, potentially independent of PPAR-γ, must be present to counter the insulin-sensitizing effects of PPAR-γ activation.
Overall, our study has added some evidence to support the potential diabetogenic effects of phthalates, but it also highlights that much is still unknown about the metabolic effects of these chemicals. The apparent racial/ethnic differences in the associations between phthalates and incident diabetes should be investigated in future studies. Recruiting younger participants and observing them for a longer period of time will also help us understand the effects of phthalates on different stages of the diabetogenic process, including whether body fat gain is an important mediator. Because phthalate exposure is widespread, continued investments in the epidemiology and toxicology research of phthalates are warranted to inform public health policies aimed to manage the risks of these chemicals and reduce the burden of chronic diseases.
This study has several limitations. First, phthalate metabolites were measured in spot urine samples. Because phthalates have short half-lives in the body, and exposure to phthalates is intermittent, phthalate metabolite concentrations in spot urine samples may not accurately reflect habitual exposure. Although our study improved upon previous studies by using phthalate metabolite data from 2, instead of 1, time points (baseline and the middle of follow-up) in analysis, a single spot urine at each time point may not capture exposure variations, and, thus, exposure measurement error is still a concern. Such exposure measurement error generally leads to attenuations of the associations between phthalates and diabetes. Second, we relied on fasting glucose to identify the time of diabetes onset. Diabetes is diagnosed by elevated fasting glucose, impaired glucose tolerance, or elevated hemoglobin A1C, each of which reflects different underlying pathologies and may not identify the same set of patients (62). Using fasting glucose solely to identify time of diabetes onset may have resulted in nondifferential outcome misclassification. Third, we were not able to examine the effects of phthalate metabolite mixtures using state-of-the-art methods such as Bayesian kernel machine regression (63) or quantile-based g-computation (64) because these methods currently do not accommodate the analysis of time-to-event data with time-varying exposures. Fourth, our follow-up time was relatively short and the number of cases was relatively small, which may have limited the study's power. Fifth, due to the small number of incident diabetes cases, we combined Chinese and Japanese women in our analysis of effect modification by race/ethnicity. Chinese and Japanese women may be exposed to phthalates through different sources and at different levels, and they may not have the same metabolic risks (65). The associations between phthalates and diabetes may not be homogeneous among Chinese and Japanese women, but we were not able to examine differences between these 2 ethnic groups. Sixth, as with all observational studies, residual confounding was possible, including confounding by other environmental chemicals (66, 67), although additionally adjusting for methyl paraben, a preservative added to personal care products, did not change our results (data not shown). Lastly, statistical significance should be interpreted cautiously as we did not account for multiple comparisons.
This study also has several strengths. Our cohort design allowed us to ensure temporality between phthalate exposure and diabetes, providing stronger evidence for causal inference. With a diverse population, we also provided data on phthalates and incident diabetes in Black and Asian women. The IPTW-weighted marginal structural models we used in our sensitivity analyses is a novel application of inverse probability weighting in the research on phthalates and diabetes, which not only confirmed the validity of BMI adjustment in our main analyses but also illustrates the utility of inverse probability weighting in the analysis of a time-varying environmental exposure and time-to-event outcome.
Conclusions
In a diverse population of midlife women, exposure to some phthalates was associated with increased incidence of diabetes, but the associations were apparently limited to White women. It is unclear what might explain the racial/ethnic differences, particularly given that some phthalates were associated with increased insulin resistance in Black or Asian women. These findings suggest that phthalate exposure may potentially contribute to diabetes, but more research is needed to further understand phthalates’ impact on glucose homeostasis and diabetes. Given widespread exposure to phthalates and the enormous costs of diabetes to individuals and societies, ongoing investments in the research on phthalates’ metabolic effects are warranted.
Acknowledgments
Clinical Centers: University of Michigan, Ann Arbor—Carrie Karvonen-Gutierrez, PI 2021-present, Siobán Harlow, PI 2011-2021, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999-present; Robert Neer, PI 1994-1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009-present; Lynda Powell, PI 1994-2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011-present, Rachel Wildman, PI 2010-2011; Nanette Santoro, PI 2004-2010; University of Medicine and Dentistry—New Jersey Medical School, Newark-Gerson Weiss, PI 1994-2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
National Institutes of Health Program Office: National Institute on Aging, Bethesda, MD—Rosaly Correa-de-Araujo 2020-present; Chhanda Dutta 2016-2020; Winifred Rossi 2012-2016; Sherry Sherman 1994-2012; Marcia Ory 1994-2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
NIA Biorepository: Rosaly Correa-de-Araujo 2019-Present; SWAN Repository: University of Michigan, Ann Arbor—Siobán Harlow 2013-2018; Dan McConnell 2011-2013; MaryFran Sowers 2000-2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012-present; Kim Sutton-Tyrrell, PI 2001-2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995-2001.
Steering Committee: Susan Johnson, current chair; Chris Gallagher, former chair.
We thank the study staff at each site and all the women who participated in SWAN.
Contributor Information
Mia Q Peng, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, MI, USA.
Carrie A Karvonen-Gutierrez, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, MI, USA.
William H Herman, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, MI, USA; Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, MI, USA.
Bhramar Mukherjee, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, MI, USA; Department of Biostatistics, University of Michigan School of Public Health, Ann Arbor, MI, USA.
Sung Kyun Park, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, MI, USA; Department of Environmental Health Sciences, University of Michigan School of Public Health, Ann Arbor, MI, USA.
Financial Support
The Study of Women’s Health Across the Nation has grant support from the National Institutes of Health, the Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research and the National Institutes of Health Office of Research on Women’s Health (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The study was also supported by the SWAN Repository (U01AG017719). This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131.
This study was also supported by grants from the National Institute of Environmental Health Sciences R01-ES026578, R01-ES026964, and P30-ES017885 and by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health Grant T42-OH008455.
Mia Peng was supported by an Interdisciplinary Research Training on Health and Aging grant (T32 AG-027708) from the National Institute on Aging.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Institute of Nursing Research, Office of Research on Women’s Health, or National Institutes of Health.
Disclosures
The authors declare no competing interests.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Centers for Disease Control and Prevention . National diabetes statistics report 2022. Accessed March 28, 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 2. American Diabetes Association . Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917‐928. doi:10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Long-term trends in diabetes, 2017:6. Accessed December 3, 2021. https://www.cdc.gov/diabetes/statistics/slides/long_term_trends.pdf
- 4. Heindel JJ, Blumberg B, Cave M, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3‐33. doi:10.1016/j.reprotox.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neel BA, Sargis RM. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011;60(7):1838‐1848. doi:10.2337/db11-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo Y, Kannan K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol. 2013;47(24):14442‐14449. doi:10.1021/es4042034 [DOI] [PubMed] [Google Scholar]
- 7. Branch F, Woodruff TJ, Mitro SD, Zota AR. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001-2004. Environ Health. 2015;14(1):57. doi:10.1186/s12940-015-0043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect. 2014;122(3):235‐241. doi:10.1289/ehp.1306681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc B Biol Sci. 2009;364(1526):2097‐2113. doi:10.1098/rstb.2008.0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zota AR, Phillips CA, Mitro SD. Recent fast food consumption and bisphenol A and phthalates exposures among the U.S. population in NHANES, 2003-2010. Environ Health Perspect. 2016;124(10):1521‐1528. doi:10.1289/ehp.1510803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13(1):43. doi:10.1186/1476-069X-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koch HM, Lorber M, Christensen KLY, Pälmke C, Koslitz S, Brüning T. Identifying sources of phthalate exposure with human biomonitoring: results of a 48 h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health. 2013;216(6):672‐681. doi:10.1016/j.ijheh.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 13. Martinelli MI, Mocchiutti NO, Bernal CA. Dietary di(2-ethylhexyl) phthalate-impaired glucose metabolism in experimental animals. Hum Exp Toxicol. 2006;25(9):531‐538. doi:10.1191/0960327106het651oa [DOI] [PubMed] [Google Scholar]
- 14. Klöting N, Hesselbarth N, Gericke M, et al. Di-(2-ethylhexyl)-phthalate (DEHP) causes impaired adipocyte function and alters serum metabolites. PLoS One. 2015;10(12):e0143190. doi:10.1371/journal.pone.0143190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Powell CA, Kay MK, et al. A moderate physiological dose of benzyl butyl phthalate exacerbates the high fat diet-induced diabesity in male mice. Toxicol Res. 2020;9(4):353‐370. doi:10.1093/toxres/tfaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radke EG, Galizia A, Thayer KA, Cooper GS. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environ Int. 2019;132:104768. doi:10.1016/j.envint.2019.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rudel RA, Gray JM, Engel CL, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119(7):914‐920. doi:10.1289/ehp.1003170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun Q, Cornelis MC, Townsend MK, et al. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect. 2014;122(6):616‐623. doi:10.1289/ehp.1307201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lind L, Salihovic S, Lind PM. Mixtures of environmental contaminants and diabetes. Sci Total Environ. 2023;859(Pt 1):159993. doi:10.1016/j.scitotenv.2022.159993 [DOI] [PubMed] [Google Scholar]
- 20. Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes (Lond). 2014;38(12):1532‐1537. doi:10.1038/ijo.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ Int. 2015;85:27‐39. doi:10.1016/j.envint.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samandar E, Silva MJ, Reidy JA, Needham LL, Calafat AM. Temporal stability of eight phthalate metabolites and their glucuronide conjugates in human urine. Environ Res. 2009;109(5):641‐646. doi:10.1016/j.envres.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 23. Baird DD, Saldana TM, Nepomnaschy PA, et al. Within-person variability in urinary phthalate metabolite concentrations: measurements from specimens after long-term frozen storage. J Expo Sci Environ Epidemiol. 2010;20(2):169‐175. doi:10.1038/jes.2009.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention . Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019. Accessed March 26, 2020. https://www.cdc.gov/exposurereport/index.html
- 25. Peng MQ, Karvonen-Gutierrez CA, Herman WH, Mukherjee B, Park SK. Phthalate exposure is associated with more rapid body fat gain in midlife women: the Study of Women’s Health Across the Nation (SWAN) multi-pollutant study. Environ Res. 2023;216(Pt 3):114685. doi:10.1016/j.envres.2022.114685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453‐469. doi:10.1093/oxfordjournals.aje.a114416 [DOI] [PubMed] [Google Scholar]
- 27. Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28(3):313‐323. doi:10.1006/pmed.1998.0470 [DOI] [PubMed] [Google Scholar]
- 28. Reeves KW, Santana MD, Manson JE, et al. Predictors of urinary phthalate biomarker concentrations in postmenopausal women. Environ Res. 2019;169:122‐130. doi:10.1016/j.envres.2018.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Díaz Santana MV, Hankinson SE, Bigelow C, et al. Urinary concentrations of phthalate biomarkers and weight change among postmenopausal women: a prospective cohort study. Environ Health. 2019;18(1):20. doi:10.1186/s12940-019-0458-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joslin Diabetes Center . Asian BMI calculator. Joslin Diabetes Center Asian American Diabetes Initiative 2016. Accessed February 9, 2021. https://www.joslin.org/patient-care/multicultural-programs/asian-american-diabetes-initiative/am-i-risk/aadi-bmi
- 31. O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect. 2016;124(2):220‐227. doi:10.1289/ehp.1509693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ Int. 2019;131:105057. doi:10.1016/j.envint.2019.105057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colacino JA, Harris TR, Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Perspect. 2010;118(7):998‐1003. doi:10.1289/ehp.0901712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trasande L, Sathyanarayana S, Jo Messito M, S. Gross R, Attina TM, Mendelsohn AL. Phthalates and the diets of US children and adolescents. Environ Res. 2013;126:84‐90. doi:10.1016/j.envres.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 35. Bai PY, Wittert GA, Taylor AW, Martin SA, Milne RW, Shi Z. The association of socio-demographic status, lifestyle factors and dietary patterns with total urinary phthalates in Australian men. PLoS One. 2015;10(4):e0122140. doi:10.1371/journal.pone.0122140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varshavsky JR, Morello-Frosch R, Woodruff TJ, Zota AR. Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environ Int. 2018;115:417‐429. doi:10.1016/j.envint.2018.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656‐664. doi:10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peng MQ, Karvonen-Gutierrez CA, Herman WH, Mukherjee B, Park SK. Supplemental-materials-to-Phthalates-and-Diabetes-in-Women-for-peer-review. GitHub. Accessed September 4, 2022. https://github.com/um-mpeg/Supplemental-materials-to-Phthalates-and-Diabetes-in-Women-for-peer-review
- 39. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487‐1495. doi: 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Mukherjee B, Karvonen-Gutierrez CA, et al. Urinary metal mixtures and longitudinal changes in glucose homeostasis: the Study of Women's Health Across the Nation (SWAN). Environ Int. 2020;145:106109. doi:10.1016/j.envint.2020.106109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Therneau T. Package “survival.” Accessed December 28, 2021. https://cran.r-project.org/web/packages/survival/survival.pdf
- 42. van der Wal WM, Geskus RB. Ipw : an R package for inverse probability weighting. J Stat Softw. 2011;43(13):1-23. doi:10.18637/jss.v043.i13 [Google Scholar]
- 43. Gao C-J, Kannan K. Phthalates, bisphenols, parabens, and triclocarban in feminine hygiene products from the United States and their implications for human exposure. Environ Int. 2020;136:105465. doi:10.1016/j.envint.2020.105465 [DOI] [PubMed] [Google Scholar]
- 44. Schildroth S, Wise LA, Wesselink AK, et al. Correlates of non-persistent endocrine disrupting chemical mixtures among reproductive-aged Black women in Detroit, Michigan. Chemosphere. 2022;299:134447. doi:10.1016/j.chemosphere.2022.134447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nicole W. A question for women's health: chemicals in feminine hygiene products and personal lubricants. Environ Health Perspect. 2014;122(3):A70‐A75. doi:10.1289/ehp.122-A70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004;112(3):331‐338. doi:10.1289/ehp.6723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang MC, Shah NS, Carnethon MR, O’Brien MJ, Khan SS. Age at diagnosis of diabetes by race and ethnicity in the United States from 2011 to 2018. JAMA Intern Med. 2021;181(11):1537‐1539. doi:10.1001/jamainternmed.2021.4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chung ST, Courville AB, Onuzuruike AU, et al. Gluconeogenesis and risk for fasting hyperglycemia in Black and White women. JCI Insight. 2018;3(18):e121495. doi:10.1172/jci.insight.121495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yip W, Sequeira I, Plank L, Poppitt S. Prevalence of pre-diabetes across ethnicities: a review of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) for classification of dysglycaemia. Nutrients. 2017;9(11):1273. doi:10.3390/nu9111273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li G, Zhao C-Y, Wu Q, et al. Integrated metabolomics and transcriptomics reveal di(2-ethylhexyl) phthalate-induced mitochondrial dysfunction and glucose metabolism disorder through oxidative stress in rat liver. Ecotoxicol Environ Saf. 2021;228:112988. doi:10.1016/j.ecoenv.2021.112988 [DOI] [PubMed] [Google Scholar]
- 51. Mondal S, Mukherjee S. Long-term dietary administration of diethyl phthalate triggers loss of insulin sensitivity in two key insulin target tissues of mice. Hum Exp Toxicol. 2020;39(7):984‐993. doi:10.1177/0960327120909526 [DOI] [PubMed] [Google Scholar]
- 52. Zhang W, Shen X-Y, Zhang W-W, Chen H, Xu W-P, Wei W. Di-(2-ethylhexyl) phthalate could disrupt the insulin signaling pathway in liver of SD rats and L02 cells via PPARγ. Toxicol Appl Pharmacol. 2017;316:17‐26. doi:10.1016/j.taap.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 53. Wei J, Hao Q, Chen C, et al. Epigenetic repression of miR-17 contributed to di(2-ethylhexyl) phthalate-triggered insulin resistance by targeting Keap1-Nrf2/miR-200a axis in skeletal muscle. Theranostics. 2020;10(20):9230‐9248. doi:10.7150/thno.45253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health Perspect. 2011;119(10):1396‐1402. doi:10.1289/ehp.1103582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou C, Flaws JA. Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol Sci. 2017;156(1):217‐229. doi:10.1093/toxsci/kfw245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weldingh NM, Jørgensen-Kaur L, Becher R, et al. Bisphenol A is more potent than phthalate metabolites in reducing pancreatic β-cell function. Biomed Res Int. 2017;2017:4614379. doi:10.1155/2017/4614379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Karabulut G, Barlas N. The possible effects of mono butyl phthalate (MBP) and mono (2-ethylhexyl) phthalate (MEHP) on INS-1 pancreatic beta cells. Toxicol Res. 2021;10(3):601‐612. doi:10.1093/toxres/tfab045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hurst CH, Waxman DJ. Activation of PPAR and PPAR by environmental phthalate monoesters. Toxicol Sci. 2003;74(2):297‐308. doi:10.1093/toxsci/kfg145 [DOI] [PubMed] [Google Scholar]
- 59. Bility MT, Thompson JT, McKee RH, et al. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci. 2004;82(1):170‐182. doi:10.1093/toxsci/kfh253 [DOI] [PubMed] [Google Scholar]
- 60. Kratochvil I, Hofmann T, Rother S, et al. Mono(2-ethylhexyl) phthalate (MEHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) but not di(2-ethylhexyl) phthalate (DEHP) bind productively to the peroxisome proliferator-activated receptor γ. Rapid Commun Mass Spectrom. 2019;33(Suppl 1):75‐85. doi:10.1002/rcm.8258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012;23(5):205‐215. doi:10.1016/j.tem.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 62. Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130‐1139. doi:10.2337/diacare.2951130 [DOI] [PubMed] [Google Scholar]
- 63. Bobb JF, Claus Henn B, Valeri L, Coull BA. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health. 2018;17(1):67. doi:10.1186/s12940-018-0413-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. 2020;128(4):047004. doi:10.1289/EHP5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ward E, Gold EB, Johnson WO, et al. Patterns of cardiometabolic health as midlife women transition to menopause: a prospective multiethnic study. J Clin Endocrinol Metab. 2019;104(5):1404‐1412. doi:10.1210/jc.2018-00941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Park SK, Wang X, Ding N, et al. Per- and polyfluoroalkyl substances and incident diabetes in midlife women: the Study of Women’s Health Across the Nation (SWAN). Diabetologia. 2022;65(7):1157‐1168. doi:10.1007/s00125-022-05695-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee S, Karvonen-Gutierrez C, Mukherjee B, Herman WH, Harlow SD, Park SK. Urinary concentrations of phenols and parabens and incident diabetes in midlife women: the Study of Women’s Health Across the Nation. Environ Epidemiol. 2021;5(5):e171. doi:10.1097/EE9.0000000000000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.