Abstract
Context
Vasomotor symptoms (VMS) are common, bothersome, and can persist for years before and after menopause.
Objective
We aimed to assess efficacy/safety of fezolinetant for treatment of moderate to severe VMS associated with menopause.
Methods
In this double-blind, placebo-controlled, 12-week phase 3 trial with a 40-week active treatment extension (NCT04003142; SKYLIGHT 2), women aged 40 to 65 years with minimum average 7 moderate to severe VMS/day were randomized to 12 weeks of once-daily placebo, fezolinetant 30 mg, or fezolinetant 45 mg. Completers were rerandomized to fezolinetant 30/45 mg for 40 additional weeks. Coprimary efficacy endpoints were mean daily change from baseline to week 4 (W4) and W12 in VMS frequency and severity. Safety was also assessed.
Results
Both fezolinetant doses statistically significantly reduced VMS frequency/severity at W4 and W12 vs placebo. For VMS frequency, W4 least squares mean (SE) reduction vs placebo: fezolinetant 30 mg, –1.82 (0.46; P < .001); 45 mg, –2.55 (0.46; P < .001); W12: 30 mg, –1.86 (0.55; P < .001); 45 mg, −2.53 (0.55; P < .001). For VMS severity, W4: 30 mg, −0.15 (0.06; P < .05); 45 mg, −0.29 (0.06; P < .001); W12: 30 mg, −0.16 (0.08; P < .05); 45 mg, −0.29 (0.08; P < .001). Improvement in VMS frequency and severity was observed by W1 and maintained through W52. Serious treatment-emergent adverse events were infrequent, reported by 2%, 1%, and 0% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo, respectively.
Conclusion
Daily fezolinetant 30 and 45 mg were efficacious and well tolerated for treating moderate to severe VMS associated with menopause.
Keywords: fezolinetant, vasomotor symptoms, neurokinin 3 receptor antagonist, KNDy, nonhormonal
Vasomotor symptoms (VMS), characterized by hot flashes, affect a large proportion of women during menopausal transition (1-7). Up to 80% of perimenopausal women in the Study of Women's Health Across the Nation reported VMS during the previous 2 weeks when surveyed on an annual basis (7). The International Collaboration for a Life Course Approach to Reproductive Health and Chronic Disease Events study, examining data from 10 countries, found VMS prevalence in women in their late 50s to be 30% to 50% (8). In a cross-sectional study of Australian women aged 65 to 79 years, 33% reported VMS (9). Persisting for a median duration of 7.4 years (10), VMS can significantly affect sleep and quality of life (QoL), lead to fatigue and mood changes, and affect work and relationships (11-15).
Hormone therapy with combined estrogen and progestogen (or estrogen alone) is an effective choice for VMS management. However, it is not appropriate for every woman, depending on underlying medical condition and risk factors, age, time since menopause, or preference (16, 17). Therefore, safe, effective, targeted nonhormonal therapy for relief of VMS associated with menopause is desirable, particularly for women primarily suffering from VMS and unable or unwilling to take hormone therapy.
The thermoregulatory center in the brain hypothalamus is innervated by kisspeptin/neurokinin B/dynorphin (KNDy) neurons. These neurons are stimulated by the neuropeptide neurokinin B, acting at the neurokinin 3 receptors, and inhibited by estrogen. With declining estrogen levels during the menopausal transition, neurokinin 3 receptor-mediated activation is unopposed, leading to hypertrophy of the KNDy neurons, and altered activity on the thermoregulatory center. The thermoregulatory center triggers heat dissipation effectors. Vasodilation in the skin causes heat loss, which is experienced as hot flashes, sweating, and chills (4, 18-20). Fezolinetant, in development for potential treatment of moderate to severe VMS associated with menopause, is a nonhormonal selective neurokinin-3 receptor (NK3R) antagonist that blocks NKB binding on the KNDy neuron, restoring normal sensitivity of the thermoregulatory center (21-23). Its molecular structure and mechanism of action have been described previously (19).
Results from phase 2 trials have demonstrated rapid and substantial reduction in VMS frequency and severity, translating into improvements in health-related QoL (21, 22, 24). The clinical development program for fezolinetant comprises several trials that investigate efficacy and safety of this novel nonhormonal NK3R antagonist. SKYLIGHT 1 (NCT04003155) and SKYLIGHT 2 (NCT04003142) investigate efficacy and safety and are 12-week randomized, placebo-controlled trials of fezolinetant 30 mg/day and 45 mg/day followed by a 40-week active treatment extension period. SKYLIGHT 4 (NCT04003389) focuses on long-term safety and tolerability of fezolinetant 30 mg/day and 45 mg/day in a randomized, placebo-controlled 52-week study. In this manuscript, we focus on the efficacy and safety outcomes from SKYLIGHT 2.
Methods
SKYLIGHT 2 was conducted in accordance with Declaration of Helsinki, Good Clinical Practice, and International Council for Harmonisation guidelines. An independent ethics committee or institutional review board reviewed ethical, scientific, and medical appropriateness of the study at each site before data collection. Written informed consent was obtained from all participants before any study-related procedures.
Study Design
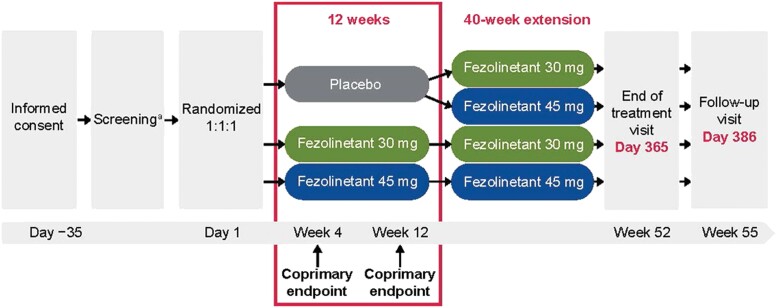
This was a multinational, randomized, double-blind, placebo-controlled, multicenter, phase 3 trial in women aged 40 to 65 years and confirmed as menopausal, with a minimum average of 7 moderate to severe VMS/day, who were seeking treatment or relief for VMS. All women had one of the following: spontaneous amenorrhea for ≥ 12 consecutive months, spontaneous amenorrhea for ≥ 6 months with biochemical criteria of menopause (follicle-stimulating hormone > 40 IU/L), or bilateral oophorectomy ≥ 6 weeks before the screening visit (with or without hysterectomy). Full inclusion and exclusion criteria are presented in Table 1. Demographic data (age, race, sex, height, weight, and smoking status) were collected at screening. The study design is shown in Fig. 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Born female, aged ≥ 40 years and ≤ 65 years at screening | Receiving strong or moderate cytochrome P450 1A2 (CYP1A2) inhibitors, hormone replacement therapy, hormonal contraceptive, or any treatment for VMS (prescription, OTC, or herbal) |
| BMI ≥ 18 kg/m2 and ≤ 38 kg/m2 | Previous/current history of a malignant tumor, except for basal cell carcinoma |
| Seeking treatment/relief for VMS associated with menopause and at the screening visit having: Spontaneous amenorrhea for ≥ 12 consecutive months; Spontaneous amenorrhea for ≥ 6 months with biochemical criteria of menopause (FSH > 40 IU/L); or Had bilateral oophorectomy ≥ 6 weeks prior to the screening visit |
SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg based on an average of 2–3 readings on at least 2 different occasions within the screening period Women who did not meet these criteria may, at the discretion of the investigator, be reassessed after initiation or review of antihypertensive measures Women with a medical history of hypertension could be enrolled at the discretion of the investigator once they are medically clear (stable and compliant) |
| Within 10 days prior to randomization, must have a minimum average of 7–8 moderate to severe VMS/day, or 50–60/week | History within the last 6 months of undiagnosed uterine bleeding |
| Normal/negative or no clinically significant findings on mammogram within the previous 12 months or at screening | A medical condition or chronic disease (including history of neurological, hepatic, renal, CV, GI, pulmonary [eg, moderate asthma], endocrine or gynecological disease) or malignancy that could confound interpretation of the study |
| Normal or not clinically significant Pap test result within the previous 12 months or at screening | Active liver disease, jaundice, or elevated liver aminotransferases (ALT or AST), elevated total or direct bilirubin, elevated INR, or elevated alkaline phosphatase. Participants with mildly elevated ALT or AST up to 1.5 times ULN could be enrolled if total and direct bilirubin were normal. Participants with mildly elevated alkaline phosphatase (up to 1.5 times ULN) could be enrolled if cholestatic liver disease was excluded and no cause other than fatty liver was diagnosed. Participants with Gilbert's syndrome with elevated total bilirubin could be enrolled as long as direct bilirubin, hemoglobin, and reticulocytes were normal |
| Willing to undergo a transvaginal ultrasound to evaluate the uterus and ovaries at screening and at week 52 (EOT), and at early discontinuation for women who withdraw from the study prior to completion | Creatinine >1.5 times ULN; or estimated glomerular filtration rate ≤59 mL/min per 1.73 m2 at screening |
| Willing to undergo an endometrial biopsy at screening and at week 52 (EOT) unless she has had a supracervical or full hysterectomy. The endometrial biopsy obtained at screening must be considered evaluable. In addition, willing to undergo endometrial biopsy in the event of uterine bleeding or early discontinuation of the study or study drug |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CV, cardiovascular; CYP1A2, cytochrome P450 1A2; DBP, diastolic blood pressure; EOT, end of treatment; FSH, follicle-stimulating hormone; GI, gastrointestinal; INR, international normalized ratio; OTC, over the counter; Pap, Papanicolaou; SBP, systolic blood pressure; ULN, upper limit of normal; VMS, vasomotor symptoms.
Figure 1.
Study flow chart. aVasomotor symptoms data collected using an electronic VMS diary. Minimum average of 7 moderate to severe VMS/day for 10 days before randomization.
The study was conducted at 146 sites in 7 countries (United States, Canada, Czechia, Latvia, Poland, Spain, United Kingdom) between July 2019 and April 2021. Participants were randomized 1:1:1 to placebo, fezolinetant 30 mg, or fezolinetant 45 mg for 12 weeks. Randomization was double-blind, and the randomization number was assigned based on information obtained from Interactive Response Technology (Cenduit Ltd, Nottingham, UK), which was used to stratify participants by smoking status (active smoker or nonsmoker [former/never]). The investigators, project team members, clinical staff, and participants were blinded to which treatment was administered. Participants took 2 tablets orally once daily with placebo and active tablets being indistinguishable in appearance and shape (those on fezolinetant 30 mg received one 30-mg tablet and one 15-mg placebo tablet, those on 45 mg received one 15-mg tablet and one 30-mg tablet, those on placebo received 2 placebo tablets [one 30-mg placebo tablet and one 15-mg placebo tablet] to match). After completing 12 weeks of treatment, participants on placebo were rerandomized in a blinded fashion to fezolinetant 30 mg or 45 mg, whereas women initially randomized to either fezolinetant arm continued on their assigned dose for an additional 40 weeks of treatment in an extension period.
Efficacy Assessments
The primary objective was to evaluate the efficacy of fezolinetant vs placebo on the frequency and severity of moderate to severe VMS. Coprimary endpoints were mean change in daily frequency of moderate to severe VMS from baseline to weeks 4 and 12 and mean change in daily severity of moderate to severe VMS from baseline to weeks 4 and 12. Daily VMS data were collected using an electronic VMS diary, completed daily during a 24-hour period by participants from screening through to the follow-up visit. The VMS diary is an interactive, electronic data capture system available for data entry 24 hours/day. Women were provided with a reference guide within the diary, which included definitions: mild: sensation of heat without sweating; moderate: sensation of heat with sweating, able to continue activity; and severe: sensation of heat with sweating, causing cessation of activity (25).
The key secondary endpoint was mean change in the Patient-Reported Outcomes Measurement Information System Sleep Disturbance—Short Form 8b (PROMIS SD SF 8b) total score from baseline to week 12. PROMIS SD SF 8b assesses self-reported sleep disturbance during the prior 7 days and includes perceptions of restless sleep; satisfaction with sleep; refreshing sleep; difficulties sleeping, getting to sleep, or staying asleep; amount of sleep; and sleep quality (26). Responses to the 8 items range from 1 to 5, and the range of possible summed raw scores is 8 to 40. Higher scores on PROMIS SD SF 8b indicate more disturbed sleep. Participants completed PROMIS SD SF 8b electronically via a tablet at each site, without assistance. Other secondary endpoints included mean change in daily frequency and severity of moderate and severe VMS from baseline to each week to week 12. Percentage reductions of at least 50% and 75% in frequency of moderate and severe VMS from baseline were also analyzed each week to week 12.
Exploratory endpoints were Patient Global Impression of Change in Sleep Disturbance (PGI-C SD), mean change from baseline on Patient Global Impression of Severity in Sleep Disturbance (PGI-S SD), and mean change in Menopause-Specific Quality of Life (MENQOL) total score. The PGI-C SD PRO outcomes measure asked women to rate how well they were sleeping at that time compared with the start of the study using a scale ranging from (1) much better to (7) much worse. The PGI-S SD asked women to rate the severity of any current problems while sleeping at night using a scale from (1) no problems to (4) severe problems. The MENQOL is a 29-item patient-reported outcome measure assessing the impact of 4 domains of menopausal symptoms (vasomotor, psychosocial, physical, and sexual) during the prior week. Specific symptoms are rated as present or not present, and if present rated on a scale of (0) not bothersome to (6) extremely bothersome.
Efficacy data (VMS, PROMIS SD SF 8b, PGI-C SD, PGI-S SD, and MENQOL) were collected for up to 52 weeks to assess persistence of effect and were summarized descriptively, with no inferential testing as there was no placebo control.
Safety Assessments
Safety was assessed by frequency of treatment-emergent adverse events (TEAEs) throughout the study. Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA) v23.0 and summarized by System Organ Class and Preferred Term. Clinical laboratory tests were performed at screening and all visits and included hematology and biochemistry, including liver safety assessments (alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, total bilirubin). Endometrial biopsy was performed if there was any uterine bleeding, if the participant discontinued from the study, and at the end of the extension period.
Statistical Analyses
The sample size estimate was 450 women (150 in each treatment arm). A sample size of 450 provided at least 79% power to detect a treatment difference in mean daily frequency of 2 episodes (assuming a SD of 5), to detect a treatment difference in mean severity of 0.4 (assuming a SD of 1), and providing about 95% power to detect a difference of 4.3 from placebo on the key secondary endpoint of the PROMIS SD SF 8b (assuming a SD of 7).
Continuous data were summarized with descriptive statistics (number of participants, mean, SD, minimum, median, maximum). Categorical data were summarized with frequencies and percentages. The efficacy analyses used the full analysis set (FAS) comprising all randomized participants who received ≥ 1 dose of study drug. A sensitivity analysis was also carried out for the coprimary efficacy endpoints based on the per protocol set. The safety analysis set (SAF) also consisted of all randomized participants who received ≥ 1 dose of study drug. Since all participants took the dose they were assigned, the FAS and SAF were identical, comprising all randomized participants who received at least 1 dose of study drug.
All statistical comparisons were conducted using two-sided tests at the α = .05 significance level. For each of the 4 coprimary efficacy endpoints, a mixed model repeated measures (MMRM) analysis of covariance was used with treatment group, week, and smoking status (current vs former or never) as factors, and baseline weight and baseline measurement as covariates, as well as an interaction of treatment by week and of baseline measurement by week. The family-wise type I error rate for comparing the 2 fezolinetant dose groups with placebo for the 4 coprimary efficacy endpoints was controlled using a Hochberg approach. All 4 coprimary endpoints had to be statistically significant for a given dose to be considered successful and the largest P value in each dose group was used because it represented the least significant of the coprimary endpoints. If all coprimary endpoints were statistically significant (fezolinetant at both doses vs placebo), the 5% alpha from the coprimary endpoint analyses passed to testing the key secondary endpoint as part of the family-wise error rate. An unstructured covariance structure shared across treatment groups was used to model the within-patient errors. The Kenward-Roger approximation was used to estimate maximum likelihood-based repeated measures approach. The treatment difference was estimated at all study weeks. The MMRM used all available on-treatment data to inform mean treatment effect estimates without requiring explicit imputation for missing data (ie, discontinued participants). This approach is consistent with the hypothetical strategy used for the estimand (a treatment effect to be estimated as if postrandomization events that may preclude observation of the primary endpoints have not occurred), which is to compare participants as though they had continued the assigned treatment. Generally, the mechanism of missing data was assumed to be missing at random. There was no explicit imputation of missing data for the primary analysis. A sensitivity analysis was conducted to confirm that the data from participants who discontinued the study were missing at random.
Comparisons between the fezolinetant and placebo groups were calculated based on least squares (LS) means. The daily mean frequency and severity per week (eg, week 4 and week 12) were calculated as the average frequency and severity over nonmissing days from 7 days. PROMIS SD SF 8b and MENQOL total score (key secondary endpoints) were analyzed using an MMRM, similar to the primary analysis of the coprimary endpoints, with spatial power as the backup covariance structure. The PGI-C SD and PGI-S SD were analyzed using the Cochran-Mantel-Haenszel test with modified ridit scores.
Results
Study Population
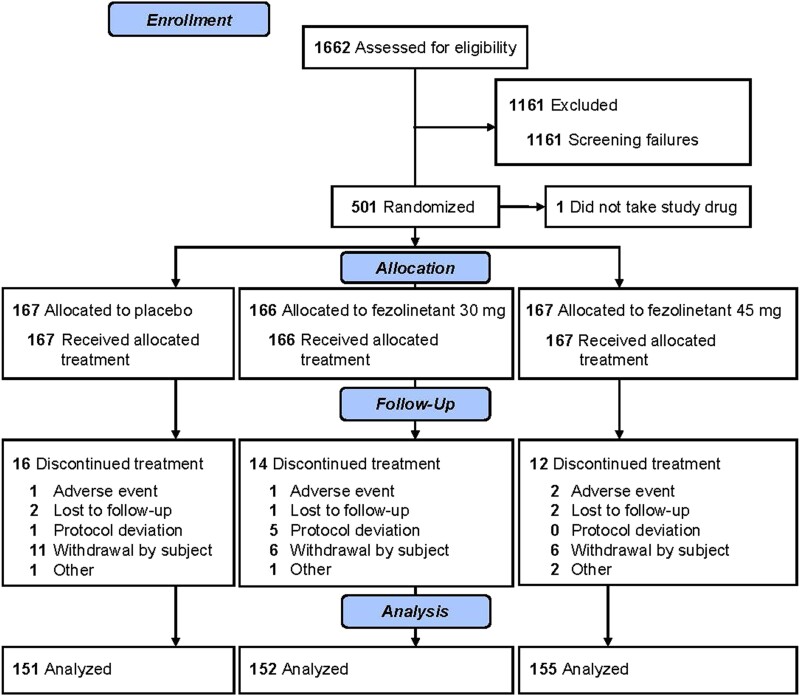
In total, 501 women were randomized and 500 were included in the SAF and FAS, as 1 woman did not take the study drug (placebo, n = 167; fezolinetant 30 mg, n = 166; fezolinetant 45 mg, n = 167; Fig. 2). In both the double-blind and extension parts of the study, all treatment groups were similar with respect to demographics and baseline characteristics (Table 2).
Figure 2.
Flow diagram.
Table 2.
Key participant demographics and baseline characteristics
| 12-Week double-blind period (SAF)a | ||||
|---|---|---|---|---|
| Parameter | Placebo (n = 167) | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) | Total (N = 500) |
| Ethnicity, No. (%) | ||||
| Not Hispanic or Latina | 134 (80.7) | 132 (79.5) | 126 (75.4) | 392 (78.6) |
| Hispanic or Latina | 32 (19.3) | 34 (20.5) | 41 (24.6) | 107 (21.4) |
| Missing | 1 | 0 | 0 | 1 |
| Race, No. (%) | ||||
| American Indian or Alaska Native | 0 | 0 | 1 (0.6) | 1 (0.2) |
| Black or African American | 31 (18.6) | 35 (21.1) | 33 (19.8) | 99 (19.8) |
| Korean | 1 (0.6) | 0 | 0 | 1 (0.2) |
| > 1 race | 1 (0.6) | 0 | 1 (0.6) | 2 (0.4) |
| White | 134 (80.2) | 131 (78.9) | 132 (79.0) | 397 (79.4) |
| Age, mean (SD), y | 54.7 (4.6) | 53.9 (4.9) | 54.3 (5.4) | 54.3 (5.0) |
| Weight, mean (range), kg | 74.57 (46.2–125.0) | 75.33 (48.0–108.4) | 74.62 (45.0–107.4) | 74.84 (45.0–125.0) |
| BMI, mean (range), kg/m2 | 28.16 (18.6–38.0) | 27.94 (18.1–37.6) | 27.91 (18.0–37.5) | 28.00 (18.0–38.0) |
| Current smoker, No. (%) | 35 (21.0) | 34 (20.5) | 34 (20.4) | 103 (20.6) |
| Time since onset of VMS, mean (range), mo | 81.9 (3–364) | 76.2 (3–370) | 81.7 (2–396) | 80.0 (2–396) |
| Amenorrhea, No. (%) | ||||
| No | 8 (4.8) | 3 (1.8) | 5 (3.0) | 16 (3.2) |
| Yes | 159 (95.2) | 163 (98.2) | 162 (97.0) | 484 (96.8) |
| Hysterectomy, No. (%) | ||||
| No | 116 (69.5) | 113 (68.1) | 111 (66.5) | 340 (68.0) |
| Yes | 51 (30.5) | 53 (31.9) | 56 (33.5) | 160 (32.0) |
| Oophorectomy, No. (%) | ||||
| No | 130 (77.8) | 132 (79.5) | 129 (77.2) | 391 (78.2) |
| Yes | 37 (22.2) | 34 (20.5) | 38 (22.8) | 109 (21.8) |
| Start of fezolinetant treatment (SAF-fezolinetant exposure)b | |||||
|---|---|---|---|---|---|
| Parameter | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) | Placebo/Fezolinetant 30 mg (n = 76) | Placebo/Fezolinetant 45 mg (n = 75) | Total (n = 484) |
| Ethnicity, No. (%) | |||||
| Not Hispanic or Latina | 132 (79.5) | 126 (75.4) | 62 (81.6) | 58 (78.4) | 378 (78.3) |
| Hispanic or Latina | 34 (20.5) | 41 (24.6) | 14 (18.4) | 16 (21.6) | 105 (21.7) |
| Missing | 0 | 0 | 0 | 1 | 1 |
| Race, No. (%) | |||||
| American Indian or Alaska Native | 0 | 1 (0.6) | 0 | 0 | 1 (0.2) |
| Black/African American | 35 (21.1) | 33 (19.8) | 11 (14.5) | 18 (24.0) | 97 (20.0) |
| Korean | 0 | 0 | 1 (1.3) | 0 | 1 (0.2) |
| > 1 race | 0 | 1 (0.6) | 1 (1.3) | 0 | 2 (0.4) |
| White | 131 (78.9) | 132 (79.0) | 63 (82.9) | 57 (76.0) | 383 (79.1) |
| Age, mean (SD), y | 53.9 (4.9) | 54.3 (5.4) | 54.3 (4.2) | 55.3 (4.9) | 54.3 (5.0) |
| Weight, mean (range), kg | 75.33 (48.0–108.4) | 74.62 (45.0–107.4) | 75.84 (48.8–112.0) | 74.0 (46.2–125.0) | 74.96 (45.0–125.0) |
| BMI, mean (range), kg/m2 | 27.94 (18.1–37.6) | 27.91 (18.0–37.5) | 28.70 (20.0–38.0) | 27.87 (18.6–37.9) | 28.04 (18.0–38.0) |
| Current smoker, No. (%) | 34 (20.5) | 34 (20.4) | 15 (19.7) | 14 (18.7) | 97 (20.0) |
| Time since onset of VMS, mean (range), mo | 76.2 (3–370) | 81.7 (2–396) | 73.4 (5–308) | 98.2 (3–364) | 81.1 (2–396) |
| Amenorrhea, No. (%) | |||||
| No | 3 (1.8) | 5 (3.0) | 5 (6.6) | 3 (4.0) | 16 (3.3) |
| Yes | 163 (98.2) | 162 (97.0) | 71 (93.4) | 72 (96.0) | 468 (96.7) |
| Hysterectomy, No. (%) | |||||
| No | 113 (68.1) | 111 (66.5) | 51 (67.1) | 52 (69.3) | 327 (67.6) |
| Yes | 53 (31.9) | 56 (33.5) | 25 (32.9) | 23 (30.7) | 157 (32.4) |
| Oophorectomy, No. (%) | |||||
| No | 132 (79.5) | 129 (77.2) | 57 (75.0) | 59 (78.7) | 377 (77.9) |
| Yes | 34 (20.5) | 38 (22.8) | 19 (25.0) | 16 (21.3) | 107 (22.1) |
Data shown in terms of No. (%), unless otherwise stated.
Abbreviations: BMI, body mass index; SAF, safety analysis set; VMS, vasomotor symptoms.
For the double-blind period, data were collected from the first dose of study drug until week 12.
For the extension period, data were collected from the first dose of study drug until week 52 for the fezolinetant groups and from week 13 to week 52 for the placebo/fezolinetant groups.
Efficacy Endpoints
Both fezolinetant doses met statistical significance in reducing VMS frequency and severity/24 hours at weeks 4 and 12 vs placebo with multiplicity adjustment (Table 3). Results were mirrored in the per protocol set (data not shown). For fezolinetant 30 mg, mean (SD) daily VMS frequency was reduced from 11.23 (4.88) at baseline to 5.79 (6.02) at week 4 and 4.80 (5.59) at week 12. For fezolinetant 45 mg, mean (SD) daily VMS was reduced from 11.79 (8.26) at baseline to 5.67 (7.29) at week 4 and 4.49 (5.39) at week 12. In comparison, for placebo, mean (SD) daily VMS frequency was reduced from 11.59 (5.02) at baseline to 8.08 (6.50) at week 4 and 6.73 (7.58) at week 12. This equated to a mean percentage change of −51.60% for fezolinetant 30 mg and −55.16% for fezolinetant 45 mg at week 4, vs −33.60% for placebo. At week 12, mean percentage changes were −58.64% for fezolinetant 30 mg and −64.27% for fezolinetant 45 mg, vs −45.35% for placebo.
Table 3.
Change from baseline to weeks 4 and 12 in daily mean frequency and severity of moderate to severe VMS (FAS)
| Analysis visit | Statistic | Placebo (n = 167) | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) |
|---|---|---|---|---|
| Frequency of daily moderate to severe VMS | ||||
| Baseline | Daily mean (SD) | 11.59 (5.02) | 11.23 (4.88) | 11.79 (8.26) |
| Week 4 | No. | 151 | 155 | 155 |
| Daily mean (SD) | 8.08 (6.50) | 5.79 (6.02) | 5.67 (7.29) | |
| Change from baseline, LS mean (SE) | −3.72 (0.33) | −5.53 (0.33) | −6.26 (0.33) | |
| LS mean (SE) difference vs placebo | — | −1.82 (0.46) | −2.55 (0.46) | |
| 95% CI | — | −2.73, −0.91 | −3.45, −1.64 | |
| Unadjusted P value | — | <.001 | <.001 | |
| Week 12 | No. | 140 | 133 | 145 |
| Daily mean (SD) | 6.73 (7.58) | 4.80 (5.59) | 4.49 (5.39) | |
| Change from baseline, LS mean (SE) | –4.97 (0.39) | –6.83 (0.39) | –7.50 (0.39) | |
| LS mean (SE) difference vs placebo | — | –1.86 (0.55) | –2.53 (0.55) | |
| 95% CI | — | –2.94, –0.78 | –3.60, –1.46 | |
| Unadjusted P value | — | <.001 | <.001 | |
| Severity of daily moderate to severe VMS | ||||
| Baseline | No. | 167 | 166 | 167 |
| Daily mean (SD) | 2.41 (0.32) | 2.44 (0.33) | 2.41 (0.34) | |
| Week 4 | No. | 151 | 155 | 155 |
| Daily mean (SD) | 2.11 (0.56) | 1.97 (0.65) | 1.80 (0.74) | |
| Change from baseline, LS mean (SE) | –0.32 (0.05) | –0.47 (0.05) | –0.61 (0.05) | |
| LS mean (SE) difference vs placebo | — | –0.15 (0.06) | –0.29 (0.06) | |
| 95% CI | — | –0.27, –0.02 | –0.41, –0.16 | |
| Unadjusted P value | — | .021 | <.001 | |
| Week 12 | No. | 140 | 133 | 145 |
| Daily mean (SD) | 1.95 (0.68) | 1.84 (0.79) | 1.66 (0.79) | |
| Change from baseline, LS mean (SE) | –0.48 (0.06) | –0.64 (0.06) | –0.77 (0.06) | |
| LS mean (SE) difference vs placebo | — | –0.16 (0.08) | –0.29 (0.08) | |
| 95% CI | — | –0.33, 0.00 | –0.45, –0.13 | |
| Unadjusted P value | — | <.05 | <.001 | |
Abbreviations: FAS, full analysis set; LS, least squares; VMS, vasomotor symptoms.
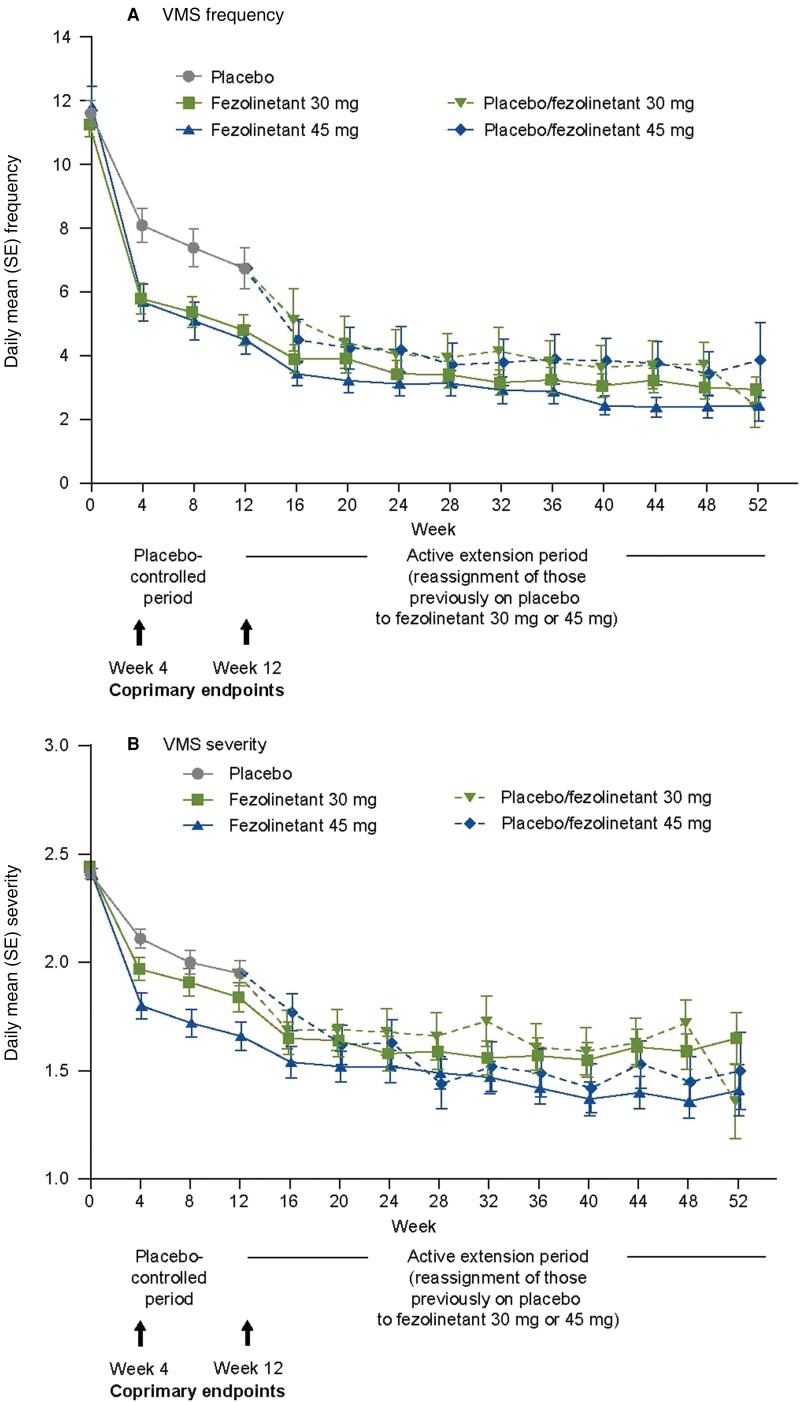
In addition to the differences observed at weeks 4 and 12 (coprimary endpoints), the difference vs placebo was statistically significant for fezolinetant 45 mg at all timepoints between weeks 1 and 12 for both VMS frequency and severity (without multiplicity analysis); fezolinetant 30 mg showed statistically significant differences vs placebo at all weeks for VMS frequency. When women were rerandomized to either fezolinetant 30 mg or 45 mg, a rapid reduction was observed in VMS frequency and severity; this was observed as early as week 1 of treatment and was maintained throughout the 12-week placebo-controlled period (Fig. 3). Persistence of efficacy for all fezolinetant groups was observed during the 40-week active treatment extension period.
Figure 3.
Mean (A) frequency and (B) severity of moderate and severe VMS during the 52-week treatment period (FAS and FAS-fezolinetant exposure). Abbreviations: FAS, full analysis set; VMS, vasomotor symptoms.
Both fezolinetant doses reduced PROMIS SD SF 8b total score vs placebo at week 12 (secondary endpoint) and week 4 (Table 4). Improvement at week 12 was statistically significant for fezolinetant 45 mg (LS mean [SE] difference, −2.0 [0.7]; 95% CI, −3.5 to −0.6; P = .007), but not for fezolinetant 30 mg (LS mean [SE] difference, −0.7 [0.7]; 95% CI, −2.1 to 0.8; P = .381). Improvement in PROMIS SD SF 8b total score was also maintained throughout the extension period. Exploratory analyses of sleep showed that the proportion of participants who reported moderately better and much better PGI-C SD at week 12 was higher in both fezolinetant groups (all P < .05) vs placebo (Fig. 4A). There was also a difference in the proportions of participants reporting sleep disturbance severity problems in the fezolinetant 30 and 45 mg groups vs placebo at week 12 (Fig. 4B).
Table 4.
Change from baseline in PROMIS SD SF 8b total score
| 12-Week double-blind period (FAS)a | ||||
|---|---|---|---|---|
| Analysis visit | Statistics | Placebo (n = 167) | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) |
| Baseline | No. | 166 | 165 | 167 |
| Mean (SD) | 27.4 (7.0) | 27.3 (6.6) | 26.2 (6.6) | |
| Week 4 | No. | 151 | 155b | 158 |
| Mean (SD) | 24.5 (7.6) | 23.4 (7.3) | 21.3 (6.8) | |
| LS mean change from baseline, mean (SE) | −2.6 (0.5) | −3.9 (0.5) | −5.3 (0.5) | |
| LS mean difference vs placebo (SE) | — | –1.30 (0.7) | –2.7 (0.7) | |
| P value vs placeboc | — | .082 | <.001 | |
| Week 12 | No. | 144d | 139 | 145 |
| Mean (SD) | 23.8 (7.0) | 23.0 (7.7) | 21.2 (5.7) | |
| LS mean change from baseline, mean (SE) | −3.4 (0.5) | −4.1 (0.5) | −5.5 (0.5) | |
| LS mean difference vs placebo (SE) | — | −0.7 (0.7) | –2.0 (0.7) | |
| P value vs placeboc | — | .381 | .007 | |
| Start of fezolinetant treatment (SAF-fezolinetant exposure)e | |||||
|---|---|---|---|---|---|
| Analysis visit (duration of fezolinetant exposure) | Statistic | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) | Placebo/Fezolinetant 30 mg (n = 76) | Placebo/fezolinetant 45 mg (n = 75) |
| Baseline | No. | 166 | 167 | 76 | 74 |
| Mean (SD) | 27.4 (6.7) | 26.2 (6.6) | 27.2 (7.4) | 27.6 (6.5) | |
| Week 12 (0 weeks exposure for placebo switchers) | No. | 145 | 149 | f | f |
| Mean (SD) | 23.3 (7.7) | 21.2 (5.7) | |||
| Change from baseline, mean (SD) | –4.4 (8.1) | –4.7 (6.8) | |||
| Week 24 (12 weeks exposure for placebo switchers) | No. | 134 | 138 | 67 | 69 |
| Mean (SD) | 21.9 (7.0) | 21.3 (7.3) | 20.8 (6.7) | 22.5 (7.0) | |
| Change from baseline, mean (SD) | –5.6 (7.3) | –4.7 (7.6) | –6.7 (7.4) | –4.8 (7.9) | |
| Week 52 (40 weeks exposure for placebo switchers) | No. | 107 | 116 | 55 | 54 |
| Mean (SD) | 21.2 (6.9) | 20.2 (7.1) | 20.5 (7.1) | 22.1 (7.1) | |
| Change from baseline, mean (SD) | –6.3 (7.3) | –5.7 (7.9) | –7.6 (8.4) | –4.8 (7.1) | |
Abbreviations: FAS, full analysis set; LS, least squares; PROMIS SD SF 8b, Patient-Reported Outcomes Measurement Information System Sleep Disturbance—Short Form 8b; SAF, safety analysis set.
For the double-blind period, data were collected from the first dose of study drug until week 12.
n = 154 for LS change from baseline.
Two-sided unadjusted P value.
n = 143 for LS change from baseline.
For the extension period, data were collected from the first dose of study drug until week 52 for the fezolinetant groups and from week 13 to week 52 for the placebo/fezolinetant groups.
Exposure to fezolinetant began at week 12.
Figure 4.
(A) Distribution of the Patient Global Impression of Change in Sleep Disturbance at week 12 and (B) the Patient Global Impression of Severity in Sleep Disturbance at week 12 (full analysis set). Abbreviation: NA, not applicable.
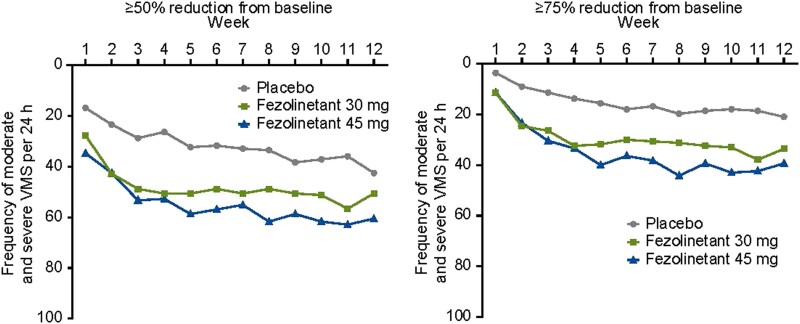
Percentages of participants achieving at least 50% reductions in VMS frequency by week 12 were 50.6% and 60.5% in the fezolinetant 30-mg and 45-mg groups, respectively, vs 42.5% in the placebo group (Fig. 5). Improvements from baseline in MENQOL total score were observed at weeks 4 and 12 in participants treated with fezolinetant 30 and 45 mg vs placebo (P ≤ .002 for fezolinetant 45 mg at weeks 4 and 12 and for fezolinetant 30 mg at week 4; Table 5). Similar results were seen for the other secondary endpoints (data not shown).
Figure 5.
Percentage reduction in frequency of moderate and severe VMS per 24 hours by week (FAS). Abbreviations: FAS, full analysis set; VMS, vasomotor symptoms.
Table 5.
Change from baseline in MENQOL total scorea during the 12-week double-blind period (FAS)
| Analysis visit | Statistics | Placebo (n = 167) | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) |
|---|---|---|---|---|
| Baseline | No. | 165 | 165 | 167 |
| Mean (SD) | 4.40 (1.35) | 4.49 (1.34) | 4.31 (1.31) | |
| Week 4 | No. | 151b | 155c | 158 |
| Mean (SD) | 3.62 (1.39) | 3.30 (1.42) | 3.01 (1.34) | |
| LS mean change from baseline, mean (SE) LS mean difference vs placebo (SE) |
–0.75 (0.10) — |
–1.17 (0.10) –0.42 (0.14) |
–1.34 (0.09) –0.59 (0.14) |
|
| P value vs placebod | — | .002 | <.001 | |
| Week 12 | No. | 144e | 139 | 145 |
| Mean (SD) | 3.43 (1.44) | 3.22 (1.43) | 2.92 (1.33) | |
| LS mean change from baseline, mean (SE) | −0.95 (0.10) | −1.18 (0.10) | −1.43 (0.10) | |
| LS mean difference vs placebo (SE) | — | −0.23 (0.15) | –0.47 (0.15) | |
| P value vs placebod | — | .122 | .001 |
Abbreviations: FAS, full analysis set; LS, least squares; MENQOL, Menopause-Specific Quality of Life.
Comprises all 4 domains and 29 items. A negative change indicates an improvement from baseline.
n = 150 for LS change from baseline.
n = 154 for LS change from baseline.
Mixed model repeated measurements analysis of covariance model with change from baseline as the dependent variable and treatment group, week and smoking status (current vs former/never) as factors, with baseline measurement and baseline weight as covariates, as well as an interaction of treatment by week and an interaction of baseline measurement by week.
n = 142 for LS change from baseline.
Safety
During the 12-week double-blind period, TEAEs were reported by 40% (fezolinetant 30 mg), 36% (fezolinetant 45 mg), and 32% (placebo) of women (Table 6). Headache was the most common TEAE in fezolinetant groups during the double-blind period (3% [fezolinetant 30 mg], 4% [fezolinetant 45 mg], 2% [placebo]). Serious TEAEs were infrequent; these were reported by 2%, 1%, and 0% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo, respectively. There were no serious drug-related TEAEs. TEAEs leading to discontinuation were nonserious and were reported by 1%, 3%, and 1% of those receiving fezolinetant 30 mg, fezolinetant 45 mg, and placebo, respectively. These were fatigue and oropharyngeal pain in 1 participant and alexithymia in 1 participant in the fezolinetant 30 mg group, arthralgia in 1 participant; abdominal pain, hematochezia, nausea, vomiting, and colitis in 1 participant; international normalized ratio increased in 1 participant; nausea in 1 participant; and ALT increased in 1 participant in the fezolinetant 45 mg group; and increased appetite and hot flash in 1 participant in the placebo group.
Table 6.
Overview of adverse events
| 12-Week double-blind period (SAF)a | |||
|---|---|---|---|
| TEAE, No. (%) | Placebo (n = 167) | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) |
| TEAE | 54 (32.3) | 67 (40.4) | 60 (35.9) |
| Drug-related TEAE | 11 (6.6) | 24 (14.5) | 25 (15.0) |
| Serious TEAE | 0 | 3 (1.8)b | 2 (1.2)c |
| Drug-related serious TEAE | 0 | 0 | 0 |
| TEAE leading to permanent discontinuation of study drug | 1 (0.6)d | 2 (1.2)e | 5 (3.0)f |
| Drug-related TEAE leading to permanent discontinuation of study drug | 0 | 1 (0.6) | 5 (3.0) |
| Deaths | 0 | 0 | 0 |
| TEAEs by PT (≥ 2.0% for any group) | |||
| Upper respiratory tract infection | 7 (4.2) | 5 (3.0) | 5 (3.0) |
| Headache | 4 (2.4) | 5 (3.0) | 6 (3.6) |
| Dry mouth | 0 | 4 (2.4) | 4 (2.4) |
| Arthralgia | 1 (0.6) | 5 (3.0) | 1 (0.6) |
| Diarrhea | 4 (2.4) | 1 (0.6) | 2 (1.2) |
| Nasopharyngitis | 4 (2.4) | 3 (1.8) | 0 |
| Nausea | 0 | 3 (1.8) | 4 (2.4) |
| Weight increased | 1 (0.6) | 5 (3.0) | 1 (0.6) |
| TEAEs of special interest | |||
| Depression | 4 (2.4) | 3 (1.8) | 1 (0.6) |
| Liver test elevations | 0 | 2 (1.2) | 3 (1.8) |
| Wakefulness | 1 (0.6) | 3 (1.8) | 1 (0.6) |
| Uterine bleeding | 1 (0.6) | 1 (0.6) | 1 (0.6) |
| Bone fractures | 1 (0.6) | 1 (0.6) | 0 |
| Thrombocytopenia | 0 | 2 (1.2) | 0 |
| Potential abuse liability | 1 (0.6) | 0 | 0 |
| Endometrial hyperplasia/cancer or disordered proliferative endometrium | 0 | 0 | 0 |
| Effect on memory | 0 | 0 | 0 |
| Start of fezolinetant treatment (SAF-fezolinetant exposure)g | ||||
|---|---|---|---|---|
| TEAE, No. (%) | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) | Placebo/Fezolinetant 30 mg (n = 76) | Placebo/Fezolinetant 45 mg (n = 75) |
| TEAE | 107 (64.5) | 106 (63.5) | 43 (56.6) | 45 (60.0) |
| Drug-related TEAE | 33 (19.9) | 30 (18.0) | 8 (10.5) | 8 (10.7) |
| Serious TEAE | 9 (5.4) | 8 (4.8) | 2 (2.6) | 4 (5.3) |
| Drug-related serious TEAE | 0 | 1 (0.6) | 0 | 1 (1.3) |
| TEAE leading to permanent discontinuation of study drug | 4 (2.4) | 7 (4.2) | 2 (2.6) | 3 (4.0) |
| Drug-related TEAE leading to permanent discontinuation of study drug | 1 (0.6) | 6 (3.6) | 1 (1.3) | 2 (2.7) |
| Deaths | 0 | 0 | 0 | 1 (1.3) |
| TEAEs by PT (≥ 4.0% for any group) | ||||
| COVID-19 | 9 (5.4) | 15 (9.0) | 4 (5.3) | 3 (4.0) |
| Headache | 8 (4.8) | 12 (7.2) | 1 (1.3) | 4 (5.3) |
| Arthralgia | 7 (4.2) | 4 (2.4) | 3 (3.9) | 2 (2.7) |
| Back pain | 5 (3.0) | 6 (3.6) | 2 (2.6) | 3 (4.0) |
| Upper respiratory tract infection | 7 (4.2) | 8 (4.8) | 1 (1.3) | 0 |
| Hot flush | 3 (1.8) | 7 (4.2) | 4 (5.3) | 0 |
| Hypertension | 5 (3.0) | 7 (4.2) | 1 (1.3) | 0 |
| Blood creatine phosphokinase increased | 2 (1.2) | 3 (1.8) | 1 (1.3) | 5 (6.7) |
| Weight increased | 8 (4.8) | 2 (1.2) | 1 (1.3) | 0 |
| Pain in extremity | 3 (1.8) | 1 (0.6) | 1 (1.3) | 3 (4.0) |
| Ear infection | 0 | 3 (1.8) | 1 (1.3) | 3 (4.0) |
| Gastroesophageal reflux disease | 2 (1.2) | 2 (1.2) | 0 | 3 (4.0) |
| Anxiety | 1 (0.6) | 0 | 0 | 3 (4.0) |
| TEAEs of special interest | ||||
| COVID-19 | 9 (5.4) | 16 (9.6) | 4 (5.3) | 4 (5.3) |
| Liver test elevations | 4 (2.4) | 9 (5.4) | 1 (1.3) | 1 (1.3) |
| Uterine bleeding | 6 (3.6) | 4 (2.4) | 0 | 0 |
| Depression | 3 (1.8) | 2 (1.2) | 0 | 1 (1.3) |
| Wakefulness | 3 (1.8) | 2 (1.2) | 0 | 0 |
| Bone fractures | 2 (1.2) | 1 (0.6) | 0 | 1 (1.3) |
| Endometrial hyperplasia/cancer or disordered proliferative endometrium | 1 (0.6) | 0 | 1 (1.3) | 1 (1.3) |
| Thrombocytopenia | 2 (1.2) | 0 | 0 | 0 |
| Effect on memory | 0 | 0 | 1 (1.3) | 0 |
| Potential abuse liability | 0 | 0 | 0 | 0 |
Data shown for the SAF (randomized participants who took ≥ 1 dose of study drug). In the double-blind period, 4 participants had confirmed and suspected cases of COVID-19 (1 receiving placebo, 2 receiving fezolinetant 30 mg, and 1 receiving fezolinetant 45 mg). A serious TEAE is a TEAE that, in the view of the investigator or sponsor, results in death, is life-threatening, results in persistent or significant disability/incapacity or substantial disruption of the ability to conduct normal life functions, results in congenital anomaly/birth defect, requires inpatient hospitalization, results in discontinuation due to increases in liver enzymes, results in other medically important events.
Abbreviations: PT, preferred term; SAF, safety analysis set; TEAE, treatment-emergent adverse event.
For the double-blind period, data were collected from the first dose of study drug until week 12.
Atrial fibrillation in 1 participant, tooth infection in 1 participant, and COVID-19 in 1 participant.
Biliary dyskinesia in 1 participant and posterior tibial nerve injury in 1 participant.
Increased appetite and hot flash in 1 participant.
Fatigue and oropharyngeal pain in 1 participant and alexithymia in 1 participant.
Arthralgia in 1 participant; abdominal pain, hematochezia, nausea, vomiting, and colitis in 1 participant; international normalized ratio increased in 1 participant; nausea in 1 participant; and alanine aminotransferase increased in 1 participant.
For the extension period, data were collected from the first dose of study drug until week 52 for the fezolinetant groups and from week 13 to week 52 for the placebo/fezolinetant groups.
Overall, elevations in liver transaminases were asymptomatic and infrequent (Table 7). Of 500 participants receiving study drug, 6 participants had ALT values more than 3 times upper limit of normal (ULN) across treatment groups (2 [fezolinetant 30 mg], 3 [fezolinetant 45 mg], 1 [placebo]). One woman receiving fezolinetant 30 mg had an ALT result more than 5 times ULN during the double-blind period. AST values more than 3 times ULN occurred in 1 fezolinetant 30 mg participant and 1 placebo participant. Increases in ALT or AST were generally asymptomatic, isolated, intermittent or transient, and generally returned to baseline while on treatment or discontinuation. Of the 5 participants on fezolinetant with ALT or AST >3 times ULN during the 12-week placebo-controlled phase, levels returned to within the normal range on treatment in 2 participants, with treatment interruption in 2 participants, and after treatment discontinuation in 1 participant. Importantly, there were no reported cases of Hy's law (ALT or AST > 3 times ULN and bilirubin >2 times ULN with no other reason to explain the combination), an indicator of drug-induced liver injury (27). No new safety signals were observed in the 40-week active treatment extension period that were not evident in the 12-week placebo-controlled period.
Table 7.
Liver safety assessments
| 12-Week double-blind period (SAF)a | |||
|---|---|---|---|
| Category, n/N (%)b | Placebo (n = 167) | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) |
| ALT | |||
| >3 times ULN | 1/161 (0.6) | 2/164 (1.2) | 3/164 (1.8) |
| >5 times ULN | 0/161 | 1/164 (0.6) | 0/164 |
| >8 times ULN | 0/161 | 0/164 | 0/164 |
| AST | |||
| >3 times ULN | 1/161 (0.6) | 1/164 (0.6) | 0/164 |
| >5 times ULN | 0/161 | 0/164 | 0/164 |
| ALT or AST | |||
| ALT or AST > 3 times ULN | 1/161 (0.6) | 2/164 (1.2) | 3/164 (1.8) |
| ALT or AST > 5 times ULN | 0/161 | 1/164 (0.6) | 0/164 |
| ALT or AST > 8 times ULN | 0/161 | 0/164 | 0/164 |
| ALP | |||
| >1.5 times ULN | 4/162 (2.5) | 0/164 | 1/164 (0.6) |
| Total bilirubin >2 times ULN | 0/161 | 0/161 | 0/161 |
| ALT or AST > 3 times ULN and bilirubin > 2 times ULN | 0/161 | 0/161 | 0/161 |
| ALT or AST > 3 times ULN, ALP < 2 times ULN, and bilirubin > 2 times ULN | 0/161 | 0/161 | 0/161 |
| Start of fezolinetant treatment (SAF-fezolinetant exposure)c | ||||
|---|---|---|---|---|
| Category, n/N (%)b | Fezolinetant 30 mg (n = 166) | Fezolinetant 45 mg (n = 167) | Placebo/Fezolinetant 30 mg (n = 76) | Placebo/Fezolinetant 45 mg (n = 75) |
| ALT | ||||
| >3 times ULN | 3/164 (1.8) | 6/164 (3.7) | 0/76 | 2/74 (2.7) |
| >5 times ULN | 1/164 (0.6) | 1/164 (0.6) | 0/76 | 0/74 |
| >8 times ULN | 0/164 | 0/164 | 0/76 | 0/74 |
| AST | ||||
| >3 times ULN | 1/164 (0.6) | 2/164 (1.2) | 0/76 | 0/74 |
| >5 times ULN | 0/164 | 0/164 | 0/76 | 0/74 |
| ALT or AST | ||||
| ALT or AST > 3 times ULN | 3/164 (1.8) | 7/164 (4.3) | 0/76 | 2/74 (2.7) |
| ALT or AST > 5 times ULN | 1/164 (0.6) | 1/164 (0.6) | 0/76 | 0/74 |
| ALT or AST > 8 times ULN | 0/164 | 0/164 | 0/76 | 0/74 |
| ALP | ||||
| >1.5 times ULN | 3/164 (1.8) | 2/164 (1.2) | 3/76 (3.9) | 3/74 (4.1) |
| Total bilirubin >2 times ULN | 0/164 | 0/164 | 0/76 | 0/74 |
| (ALT or AST > 3 times ULN) and bilirubin > 2 times ULN | 0/164 | 0/164 | 0/76 | 0/74 |
| (ALT or AST > 3 times ULN) and ALP < 2 times ULN and bilirubin > 2 times ULN | 0/164 | 0/164 | 0/76 | 0/74 |
Data shown for the safety analysis set (randomized participants who took ≥ 1 dose of study drug; a participant receiving a treatment different from their randomized treatment was assigned to the treatment group received as first dose). A participant could be counted in multiple categories as they were included in all that apply (eg, if a participant had a level > 8 times ULN they were also included in the > 3 times ULN and > 5 times ULN categories). The denominator is the number of participants who had at least one nonmissing value during the 12-week double-blind treatment period.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAF, safety analysis set; ULN, upper limit of normal.
For the double-blind period, data were collected from the first dose of study drug until week 12.
Others were analyzed but are not included due to no events.
For the extension period, data were collected from the first dose of study drug until week 52 for the fezolinetant groups and from week 13 to week 52 for the placebo/fezolinetant groups.
Extension Study Efficacy and Safety
Baseline demographics at the start of the 40-week active treatment extension period are shown in Table 2. A total of 166 women continued to receive fezolinetant 30 mg; 167 continued to receive fezolinetant 45 mg; 76 were rerandomized from placebo to fezolinetant 30 mg; and 75 were rerandomized from placebo to fezolinetant 45 mg. Fezolinetant efficacy persisted throughout the study as shown by the change in VMS frequency and severity over time (Fig. 3) and change in sleep disturbance at weeks 24 and 52 (Table 4).
At least 1 AE was experienced by 56.6% of the participants in the placebo/fezolinetant 30-mg group, 60.0% in the placebo/fezolinetant 45-mg group, 64.5% in the fezolinetant 30-mg group, and 63.5% in the fezolinetant 45-mg group (Table 6). The incidence of AEs by preferred term was balanced across the placebo/fezolinetant 30-mg and 45-mg and fezolinetant 30-mg and 45-mg groups. COVID-19 and headache were the most commonly reported AE; again, there were no cases consistent with Hy's law (Table 7). One participant in the placebo/fezolinetant 45-mg group died due to multiple injuries from a motorcycle passenger accident; this event was considered by the investigator as not related to study intervention.
Discussion
Herein we demonstrate that fezolinetant, a novel nonhormonal treatment for VMS, is effective and safe in reducing this cardinal symptom of menopause by over 50% from baseline. The study successfully met the 4 coprimary efficacy endpoints. Both doses demonstrated statistically significant improvements vs placebo in mean daily VMS frequency and severity at weeks 4 and 12. These results suggest that fezolinetant is efficacious for treatment of moderate to severe VMS at daily doses of 30 and 45 mg. Efficacy of fezolinetant was seen within the first week of treatment and was maintained through week 12, with a daily reduction of 2 to 3 VMS episodes from baseline to week 12 compared with placebo. Efficacy was persistent and reductions in VMS frequency were maintained during the 40-week extension period, at levels consistent with the results of the initial 12 weeks. These results confirm those of phase 2 trials (21, 22), which showed significant reductions in total VMS score (22), and mean frequency of moderate to severe VMS (21), and significant improvements in QoL measures at week 12 vs placebo (21). At week 12, the LS mean reduction in VMS frequency was greater than 50% in both fezolinetant groups, and a 50% reduction is considered clinically significant (28). Additionally, persistence of efficacy was observed during the 52-week treatment period.
The statistically significant reduction in VMS frequency and severity during the 12-week period translated into clinically meaningful improvements in QoL as measured by the MENQOL, a menopause-specific patient-reported outcome tool. Improvement in the MENQOL total score suggests that both fezolinetant doses significantly improved QoL from as early as week 4 of the study. When taken together, the replicate designed SKYLIGHT 1 and SKYLIGHT 2 studies provide data on the efficacy of fezolinetant in more than 1000 women. Data from SKYLIGHT 2 confirm those from SKYLIGHT 1 (29) and provide further evidence of the potential of fezolinetant as a novel nonhormonal therapeutic option for moderate to severe VMS.
Although the study did not require sleep disturbance as an entry requirement, both fezolinetant doses demonstrated numerical improvements in sleep (PROMIS SD SF 8b total score; key secondary endpoint), reaching statistical significance for the 45-mg dose and maintained through the 40-week extension period. This is noteworthy because nearly half of postmenopausal women report sleep impairment, and VMS is associated with poor sleep quality, nighttime awakenings (30), and excessive daytime sleepiness (31). Night sweats commonly result in sleep interruptions and difficulty returning to sleep (32). The magnitude of sleep relief is large compared with paroxetine, which was effective at reducing VMS frequency but had no clinically significant benefit on sleep parameters (33); however, this is limited by being reported in only 2 studies. In contrast, fezolinetant 30 mg did not achieve a statistically significant effect on sleep in the current study. This difference most likely reflects a dose effect. Additionally, reduction in VMS alone may improve sleep, so further investigation is warranted. In the phase 2a trial, fezolinetant improved sleep quality, measured using the Leeds Sleep Evaluation Questionnaire, at all test intervals (22). Patient-reported data in the current study show that a higher proportion of women receiving fezolinetant reported a positive change in PGI-C SD at weeks 4 and 12 and a decrease in the proportion of those with severe sleep problems at weeks 4 and 12 compared with those receiving placebo. Reduction in sleep disturbance may offer a clinical benefit by improved functioning and quality of life, and may potentially reduce the risk of short- and longer-term consequences of sleep deprivation (34).
Through week 12, there was a low incidence of serious AEs, no serious drug-related AEs, and a generally unremarkable safety profile for fezolinetant at both doses. A total of 6 participants across all treatment groups had ALT/AST elevations more than 3 times ULN (2 [fezolinetant 30 mg], 3 [fezolinetant 45 mg], 1 [placebo]). These results support the hepatic safety of fezolinetant, with no cases of Hy's law to suggest drug-induced liver injury. Increases in ALT/AST were generally asymptomatic, isolated, intermittent or transient, and generally returned to baseline while on treatment or after discontinuation. No elevations were associated with evidence of liver function impairment (increased bilirubin or international normalized ratio) or liver-associated clinical symptoms. Although favorable, limited conclusions can be drawn from the 12-week short-term safety data. Data from 52 weeks of study, while not placebo-controlled after 12 weeks, affirm that the safety findings and the overall safety data in SKYLIGHT 2 were similar to those observed in SKYLIGHT 1 (35). Additional data on the long-term safety of fezolinetant are anticipated from SKYLIGHT 4, the 52-week double-blind, placebo-controlled safety study in approximately 1830 women seeking treatment for VMS associated with menopause.
Reductions in VMS frequency and severity in this study were also seen in the placebo group, replicating the well-documented placebo effect in studies investigating potential treatments for VMS (36, 37). Previous studies have reported that treatment of menopausal women with placebo alone reduced hot flash frequency by 33% (38). SKYLIGHT 2 was designed to conform to the U.S. Food and Drug Administration (FDA) Draft Guidance on clinical studies of VMS, with a placebo group and requirement for 4 coprimary endpoints (39). Despite the placebo effect, statistically significant differences were observed for both fezolinetant doses vs placebo at weeks 4 and 12 and continued during the extension period.
A limitation of this study is absence of placebo beyond 12 weeks, although inclusion of placebo for long periods is difficult from a patient perspective. Additionally, other menopause symptoms, such as mood changes and sexual function, were not assessed.
Hormone therapy is considered a standard of care for menopausal symptoms, although it may not be suitable for, or preferred by, all women. Currently, nonhormonal treatments include selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, clonidine, gabapentin, oxybutynin (40), and paroxetine, the only nonhormonal therapy approved by the FDA for VMS (41). NK3R antagonists offer a new selective therapeutic approach and various candidates have been advanced into clinical development (19). Fezolinetant is under development as a nonhormonal treatment option for moderate to severe VMS associated with menopause.
In summary, fezolinetant 30 and 45 mg once daily demonstrated efficacy and were well tolerated for treatment of moderate to severe VMS associated with menopause. There was a rapid onset of effect by week 1, with a full effect by week 4 that was sustained through 52 weeks with a daily reduction of 2 to 3 VMS episodes more than placebo from baseline to week 12 for fezolinetant groups. In addition, fezolinetant 45 mg significantly improved patient-reported sleep. These findings support continued development of fezolinetant as a novel nonhormonal treatment option for VMS associated with menopause.
Acknowledgments
The authors would like to thank the study investigators and staff and all women with moderate to severe VMS who took part in the study. Medical writing support was provided by Sue Cooper CMPP of Envision Scientific Solutions, Horsham, UK, and funded by Astellas Pharma Inc. Written permission has been obtained to include this individual in the acknowledgments. All medications used in this study were prepared by Astellas Pharma Inc. Packaging and labeling was by Fisher Basel.
Abbreviations
- AE
adverse event
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- FAS
full analysis set
- KNDy neurons
kisspeptin/neurokinin B/dynorphin neurons
- LS
least squares
- MENQOL
Menopause-Specific Quality of Life
- MMRM
mixed model repeated measures
- NK3R
neurokinin-3 receptor
- PGI-C SD
Patient Global Impression of Change in Sleep Disturbance
- PGI-S SD
Patient Global Impression of Severity in Sleep Disturbance
- PROMIS SD SF 8b
Patient-Reported Outcomes Measurement Information System Sleep Disturbance—Short Form 8b
- QoL
quality of life
- SAF
safety analysis set
- TEAE
treatment-emergent adverse event
- ULN
upper limit of normal
- VMS
vasomotor symptoms
Contributor Information
Kimball A Johnson, iResearch Atlanta, LLC, Decatur, Georgia 30030, USA.
Nancy Martin, Employee of Astellas Pharma Global Development at the Time of the Study, Northbrook, IL, USA.
Rossella E Nappi, Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, and Research Center for Reproductive Medicine and Gynecological Endocrinology – Menopause Unit, Fondazione Policlinico IRCCS S. Matteo, Pavia 27100, Italy.
Genevieve Neal-Perry, Department of Obstetrics and Gynecology, UNC School of Medicine, Chapel Hill, NC 27599, USA.
Marla Shapiro, Department of Family and Community Medicine, University of Toronto, Toronto, Ontario M5S 1A1, Canada.
Petra Stute, Department of Obstetrics and Gynecology, Inselspital, Bern CH-3010, Switzerland.
Rebecca C Thurston, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Wendy Wolfman, Department of Obstetrics and Gynaecology, University of Toronto, Toronto, Ontario M5G 1E2, Canada.
Marci English, Global Development, Astellas Pharma Global Development, Inc., Northbrook, IL 60062, USA.
Catherine Franklin, Employee of Astellas Pharma Global Development at the Time of the Study, Northbrook, IL, USA.
Misun Lee, Biostatistics, Astellas Pharma Global Development, Inc., Northbrook, IL 60062, USA.
Nanette Santoro, Division of Reproductive Sciences, University of Colorado School of Medicine, Aurora, CO 80045, USA.
Funding
This study was sponsored by Astellas Pharma Inc.
Author Contributions
K.J. was the coordinating investigator for the study and N.S., R.C.T., G.N.-P., P.S., M.S., C.M., and A.C. were Scientific Steering Committee members. N.M., M.L., C.F., and M.E. contributed to the concept and design of the study. M.L. was responsible for the statistical analyses. All authors had access to the study data, were involved in the analysis and interpretation of the data, take responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication. All authors approve the manuscript for submission.
Disclosures
K.J. is a study investigator and consultant for Astellas and a study investigator for iResearch Atlanta, LLC. M.E., and M.L. are employees of Astellas Pharma Inc. N.M. and C.F. were employees of Astellas Pharma Inc at the time of the study. R.E.N. had past financial relationships (lecturer, member of advisory boards, and/or consultant) with Boehringer Ingelheim, Eli Lilly, Endoceutics, Gedeon Richter, HRA Pharma, Merck Sharpe & Dohme, Novo Nordisk, Palatin Technologies, Pfizer, Procter & Gamble, Teva Women's Health, and Zambon; ongoing relationships with Abbott, Astellas, Bayer HealthCare, Exeltis, Fidia, Gedeon Richter, HRA Pharma, Organon, Shionogi, and Theramex; serves as President elect of the International Menopause Society. G.N.-P. is a member of the scientific advisory board for Astellas and Ferring Pharmaceuticals and has received research funding from Merck and NICHD. She is also Vice President of Diversity, Equity and Structural Change for the Society for Reproductive Investigation, is the Programming Committee Chair for the American Society of Reproductive Medicine, holds Committee Membership in the Endocrine Society, and is an Associate Editor for the Journal of Clinical Endocrinology & Metabolism. M.S., C.M. is on the advisory board for and receives consulting fees or honoraria from Aspen, Astellas, Bayer, BioSyent, Duchesnay, GlaxoSmithKline, Merck, Mithra, Pfizer, Searchlight, Sprout, Sunovion, and Therapeutics MD and fulfills a leadership or fiduciary role for the International Menopause Society, the Terry Fox Research Institute, and Research Canada. P.S. is a consultant for Astellas, a board member for DMG and EMAS, and President of the SGEM. R.C.T. is a consultant and advisor for Astellas, a consultant for Bayer HealthCare, Hello Therapeutics, and Happify Health, and a past consultant for Pfizer, Procter and Gamble, and Vira Health. She serves on the Board of Trustees for the North American Menopause Society. W.W. is on the advisory board and consultant/speaker for Astellas, BioSyent, Knight, Merck, Pfizer, and Searchlight. She is the President of the Canadian Menopause Society. N.S. is a study investigator and consultant for Astellas, a member of the scientific advisory board for Amazon (Project Ember), MenoGeniX, and is a consultant for Ansh Labs and QUE Oncology. She is on the Board of Directors for the North American Menopause Society and is Past President of the Society for Reproductive Investigations.
Data Availability
Researchers may request access to anonymized participant-level data, trial-level data and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Previous Presentation
The results from the 12-week part of the SKYLIGHT 2 study were presented at the 32nd North American Menopause Society (NAMS) Annual Meeting; September 22-25, 2021; Washington, DC [late-breaking abstract: Johnson K, Lademacher C, Nappi RE, et al. A phase 3, randomized, placebo-controlled, 12-week, double-blind study, plus a non-controlled extension treatment period, to assess efficacy and safety of fezolinetant, a neurokinin-3 receptor antagonist, in women with moderate to severe vasomotor symptoms associated with menopause. Abstract available at: Menopause. 2021; 28(12):1438-1476]. These data were also presented at the 14th Congress of the European Society of Gynecology; November 10-13, 2021; Venice, Italy [Johnson K, Nappi RE, Neal-Perry G, et al. A phase 3, randomized, placebo-controlled, 12-week, double-blind study, plus a non-controlled extension treatment period, to assess efficacy and safety of fezolinetant, a neurokinin-3 receptor antagonist, in women with moderate to severe vasomotor symptoms associated with menopause. Abstract available at: European Gynecology & Obstetrics. 2021; 3(Supplement 1):75:OP03]. Data from the 52-week study were presented at the 33rd Annual Meeting and Expo of the Endocrine Society (ENDO); June 11-14, 2022; Atlanta, Georgia, USA [Johnson K, Nappi RE, Neal-Perry G, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause: results from a 52-week study (Skylight 2). Abstract available at: Journal of the Endocrine Society. 2022; 6(1): A678-A679].
References
- 1. Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152(12):4894‐4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Padilla SL, Johnson CW, Barker FD, Patterson MA, Palmiter RD. A neural circuit underlying the generation of hot flushes. Cell Rep. 2018;24(2):271‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci U S A. 2012;109(48):19846‐19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010;151(3):1187‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krajewski-Hall SJ, Miranda Dos Santos F, McMullen NT, Blackmore EM, Rance NE. Glutamatergic neurokinin 3 receptor neurons in the median preoptic nucleus modulate heat-defense pathways in female mice. Endocrinology. 2019;160(4):803‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gold EB, Colvin A, Avis N, et al. . Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's Health across the nation. Am J Public Health. 2006;96(7):1226‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mishra GD, Chung H-F, Pandeya N, et al. . The InterLACE study: design, data harmonization and characteristics across 20 studies on women's Health. Maturitas. 2016;92(10):176‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeleke BM, Bell RJ, Billah B, Davis SR. Vasomotor and sexual symptoms in older Australian women: a cross-sectional study. Fertil Steril. 2016;105(1):149‐155.e141. [DOI] [PubMed] [Google Scholar]
- 10. Avis NE, Crawford SL, Greendale G, et al. . Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thurston RC, Joffe H. Vasomotor symptoms and menopause: findings from the study of Women's Health across the nation. Obstet Gynecol Clin North Am. 2011;38(3):489‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whiteley J, DiBonaventura M, Wagner JS, Alvir J, Shah S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J Womens Health (Larchmt). 2013;22(11):983‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009;62(2):153‐159. [DOI] [PubMed] [Google Scholar]
- 14. Worsley R, Bell RJ, Gartoulla P, Robinson PJ, Davis SR. Moderate-severe vasomotor symptoms are associated with moderate-severe depressive symptoms. J Womens Health (Larchmt). 2017;26(7):712‐718. [DOI] [PubMed] [Google Scholar]
- 15. Nappi RE, Kroll R, Siddiqui E, et al. . Global cross-sectional survey of women with vasomotor symptoms associated with menopause: prevalence and quality of life burden. Menopause. 2021;28(8):875‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The NAMS Hormone Therapy Position Statement Advisory Panel . The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2017;24(7):728‐753. [DOI] [PubMed] [Google Scholar]
- 17. de Villiers TJ, Hall JE, Pinkerton JV, et al. . Revised global consensus statement on menopausal hormone therapy. Maturitas. 2016;91(9):153‐155. [DOI] [PubMed] [Google Scholar]
- 18. Archer DF, Sturdee DW, Baber R, et al. . Menopausal hot flushes and night sweats: where are we now? Climacteric. 2011;14(5):515‐528. [DOI] [PubMed] [Google Scholar]
- 19. Depypere H, Lademacher C, Siddiqui E, Fraser GL. Fezolinetant in the treatment of vasomotor symptoms associated with menopause. Expert Opin Investig Drugs. 2021;30(7):681‐694. [DOI] [PubMed] [Google Scholar]
- 20. Krull AA, Larsen SA, Clifton DK, Neal-Perry G, Steiner RA. A comprehensive method to quantify adaptations by male and female mice with hot flashes induced by the neurokinin B receptor agonist senktide. Endocrinology. 2017;158(10):3259‐3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fraser GL, Lederman S, Waldbaum A, et al. . A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause. Menopause. 2020;27(4):382‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Depypere H, Timmerman D, Donders G, et al. . Treatment of menopausal vasomotor symptoms with fezolinetant, a neurokinin 3 receptor antagonist: a phase 2a trial. J Clin Endocrinol Metab. 2019;104(12):5893‐5905. [DOI] [PubMed] [Google Scholar]
- 23. Fraser GL, Hoveyda HR, Clarke IJ, et al. . The NK3 receptor antagonist ESN364 interrupts pulsatile LH secretion and moderates levels of ovarian hormones throughout the menstrual cycle. Endocrinology. 2015;156(11):4214‐4225. [DOI] [PubMed] [Google Scholar]
- 24. Santoro N, Waldbaum A, Lederman S, et al. . Effect of the neurokinin 3 receptor antagonist fezolinetant on patient-reported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause. 2020;27(12):1350‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Center for Drug Evaluation and Research . Draft Guidance. Estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms – recommendations for clinical evaluation. January 2003. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estrogen-and-estrogenprogestin-drug-products-treat-vasomotor-symptoms-and-vulvar-and-vaginal-atrophy. Date of access 13 February 2023.
- 26. Buysse DJ, Yu L, Moul DE, et al. . Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) . Guidance for Industry. Drug-induced liver injury: premarketing clinical evaluation. Vol 20212009. Accessed February 13, 2023. https://www.fda.gov/media/116737/download
- 28. Newton KM, Carpenter JS, Guthrie KA, et al. . Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network. Menopause. 2014;21(1):45‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lederman S, Shapiro M, Stute P, Lee M, Wang X, Neal-Perry G. Phase 3 study of fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause [A132]. Obstetrics & Gynecology. 2022;139(5):395. [Google Scholar]
- 30. Thurston RC, Chang Y, Buysse DJ, Hall MH, Matthews KA. Hot flashes and awakenings among midlife women. Sleep. 2019;42(9):zsz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valiensi SM, Belardo MA, Pilnik S, Izbizky G, Starvaggi AP, Castelo Branco C. Sleep quality and related factors in postmenopausal women. Maturitas. 2019;123(5):73‐77. [DOI] [PubMed] [Google Scholar]
- 32. English M, Stoykova B, Slota C, et al. . Qualitative study: burden of menopause-associated vasomotor symptoms (VMS) and validation of PROMIS sleep disturbance and sleep-related impairment measures for assessment of VMS impact on sleep. J Patient Rep Outcomes. 2021;5(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riemma G, Schiattarella A, La Verde M, et al. . Efficacy of low-dose paroxetine for the treatment of hot flushes in surgical and physiological postmenopausal women: systematic review and meta-analysis of randomized trials. Medicina (Kaunas). 2019;55(9):554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9(5):151‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lederman S, Shapiro CMM, Stute P, Lee M, Wang X, Neal-Perry G. Phase 3 study of fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause. Obstet Gynecol. 2022;139(1):39S. [Google Scholar]
- 36. Goodman NF, Cobin RH, Ginzburg SB, Katz IA, Woode DE. American Association of clinical E. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause: executive summary of recommendations. Endocr Pract. 2011;17(6):949‐954. [DOI] [PubMed] [Google Scholar]
- 37. Miyazaki K, Kaneko M, Narukawa M. Factors associated with high placebo response in clinical studies of hot flashes: a meta-analysis. Menopause. 2021;29(2):239‐246. [DOI] [PubMed] [Google Scholar]
- 38. Freeman EW, Ensrud KE, Larson JC, et al. . Placebo improvement in pharmacologic treatment of menopausal hot flashes: time course, duration, and predictors. Psychosom Med. 2015;77(2):167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. US Food and Drug Administration . Draft guidance for industry: estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvular and vaginal atrophy symptoms - recommendations for clinical evaluation. January 2003 Accessed February 13, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estrogen-and-estrogenprogestin-drug-products-treat-vasomotor-symptoms-and-vulvar-and-vaginal-atrophy
- 40. Simon JA, Gaines T, LaGuardia KD. Extended-release oxybutynin therapy for VMSSG. Extended-release oxybutynin therapy for vasomotor symptoms in women: a randomized clinical trial. Menopause. 2016;23(11):1214‐1221. [DOI] [PubMed] [Google Scholar]
- 41. Simon JA, Portman DJ, Kaunitz AM, et al. . Low-dose paroxetine 7.5mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027‐1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Researchers may request access to anonymized participant-level data, trial-level data and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.