Abstract
CD19-targeted chimeric antigen receptor (CAR) T-cells bearing a crucial costimulatory molecule, which first demonstrated pre-clinical efficacy in 2003,1 have led to impressive response rates and durable remissions in the treatment of relapsed or refractory aggressive non-Hodgkin lymphoma (NHL), including large B cell lymphoma (LBCL).2–18 Following seminal early phase multicenter clinical trials published between 2017 and 2020,2–3,5–8 three CD19-CAR T-cell products received FDA and EMA approval designations in lymphoma in the third-line setting, paving the way for follow-up studies in the second-line.19–22 Meanwhile, investigations into the applications of CAR T-cell therapy have further broadened to treating high-risk patients even prior to completion of first-line conventional chemo-immunotherapy.23 Furthermore, as early trials excluded patients with central nervous system involvement with lymphoma, several studies have recently shown promising efficacy of CD19-CAR T-cells in primary and secondary CNS lymphoma.24–27 Here we provide a detailed overview on clinical data supporting the use of CAR T-cells in patients with LBCL.
CAR T-cell therapy in third and subsequent lines for LBCL
Axicabtagene ciloleucel (axi-cel; KTE-C19) is an autologous CD19-CAR T-cell product bearing a CD28 costimulatory domain. In the pivotal single-arm phase 2, multicenter ZUMA-1 trial, 101 patients with relapsed or refractory (r/r) LBCL after two or more lines of therapy were treated with a single infusion of axi-cel. Patients in the study experienced an objective response rate (ORR) of 82% (95% CI: 73 – 89), including 54% who realized a complete response (CR).2 Responses were durable, with a median duration of response (DOR) of 11.1 months (95% CI: 3.9 - inestimable).2 CAR T-cell-related adverse events were frequent, with 93% of patients experiencing cytokine release syndrome (CRS), including 13% at grade 3 or higher (3+).2 64% of patients experienced immune effector cell-associated neurotoxicity syndrome (ICANS), among which 28% were grade 3+.2 Based on these data, axi-cel received FDA approval for relapsed r/r LBCL after two or more lines of systemic therapy in October 2017, followed by EMA approval 7 months later. On long-term follow-up, the overall 5-year OS rate was 42.6% (95% CI, 32.8–51.9), while complete responders fared better at 64.4% (95% CI: 50.8–75.1).3 As of August 2021, 30% of patients had ongoing CR with a median duration of CR of 62.2 months, highlighting ongoing durability of response and the curative potential of this form of therapy.4 Importantly, results from subsequent real-world analyses were consistent with phase 2 safety and efficacy data.11–12,14–18 For example, a retrospective study of standard of care (SOC) axi-cel from the US lymphoma consortium included 275 patients, 43% of which would not have met ZUMA-1 eligibility criteria due to medical comorbities.12 The ORR was 82% (95% CI: 43.5–62.4) with a CR rate of 64% (95% CI: 43.5–62.4), while the incidence of any CRS and ICANS were 91% and 69%.12
Tisagenlecleucel (tisa-cel; CTL019), an autologous CD19-CAR T-cell bearing a 4–1BB costimulatory domain, was studied in the single-arm, phase 2 JULIET trial, which included 111 patients with r/r LBCL. The best ORR was 52% (95% CI: 41–62) with a CR rate of 40%.6 58% of patients experienced CRS of any grade, including 23% with grade 3+ symptoms, while Grade 3+ ICANS was infrequent with only 11% of patients affected in the study.6 The FDA and EMA subsequently approved tisa-cel as the second CD19-CAR T-cell therapy for LBCL in May and August 2018, respectively. Long-term data at a median follow-up time of 40.3 months showed that an impressive 60.4% (95% CI: 46.1–72.0) of responding patients maintained their response.7 Subsequently published retrospective data from 1159 DLBCL patients treated with tisa-cel was consistent with JULIET efficacy and safety signals, again highlighting the potential for broad application of cellular therapy.18
Lisocabtagene maraleucel (liso-cel; JCAR017) is a CD19 4–1BB CAR T-cell product that varies in manufacturing methodology. CD4 and CD8 T-cells are isolated from the apheresis product, separately transduced, and sequentially infused in two equal target doses of CD4+ and CD8+ CAR+ T-cell subsets. In the pivotal TRANSCEND phase 2 study, 269 patients with r/r LBCL were treated with liso-cel. In contrast to other seminal trials, eligibility criteria for the study were extended to include patients with renal dysfunction (creatinine clearance >30 to <60 mL/min), mild left ventricular dysfunction (ejection fraction ≥40% to <50%), and secondary CNS involvement of lymphoma.8 Notably, the median age was 63 (IQR 18–86) with 41% of patients being 65 or older, compared to 58 and 24% in the ZUMA-1 and 56 and 23% in JULIET.2,6,8 The ORR of the study was 73% (95% CI: 66.8–78.0) and the CR rate was 53% (95% CI: 46.8–59.4).8 CRS of any grade occurred in 42% of patients, including 2% with grade 3+, and iCANS occurred in 30% of patients, including 10% with grade 3+.8 Based on promising safety and efficacy data on interim analysis, the EMA approved liso-cel for relapsed or refractory LBCL in August 2018, followed by the FDA in February 2021. Two-year follow-up data was robust with a median DOR of 23.1 months, a median PFS of 6.8 months, and a median OS of 27.3 months.9
In clinical practice, choosing among the three products is complicated by the lack of studies dedicated to inter-product comparison. While cross-trial comparison should be interpreted with caution due to differences in grading scales, multiple retrospective analyses suggest a higher incidence of toxicity observed in patients treated with axi-cel relative to tisa-cel or liso-cel, though non-relapse mortality is not significantly different.15–17,28 Based on these findings in the context of clinical trial data, axi-cel tends to be reserved for younger and fitter patients, whereas tisa-cel is preferred in patients with significant medical comorbidities, and liso-cel might be appropriate for both groups. One study utilized propensity score matching to compare 418 patients treated in the third-line setting with either axi-cel or tisa-cel and showed improved efficacy outcomes in the former group.16 The one-year PFS and OS for axi-cel and tisa-cel were 46.6% versus 33.2% (HR 0.61; 95% CI: 0.46–0.79; P = 0.0003) and 63.5% versus 48.8% (HR 0.63; 95% CI: 0.45–0.88; P = 0.0072), respectively.16 These findings may need further confirmation. Time between apheresis and infusion was shown to be significantly longer in patients treated with tisa-cel versus axi-cel.15–17 At our center, these data are considered when choosing a CD19-CAR T-cell therapy based on aggressiveness of disease and urgency of next therapy. With the improved toxicity profiles of 4–1BB-containing products, older patients with medical comorbidities previously deemed ineligible are now being considered for CAR T-cell therapy. A sample clinical algorithm is shown based on experience at our center (Figure 1).
Figure 1. Diagnostic and therapeutic algorithm for large B cell lymphoma (LBCL) in first and second or later relapse.
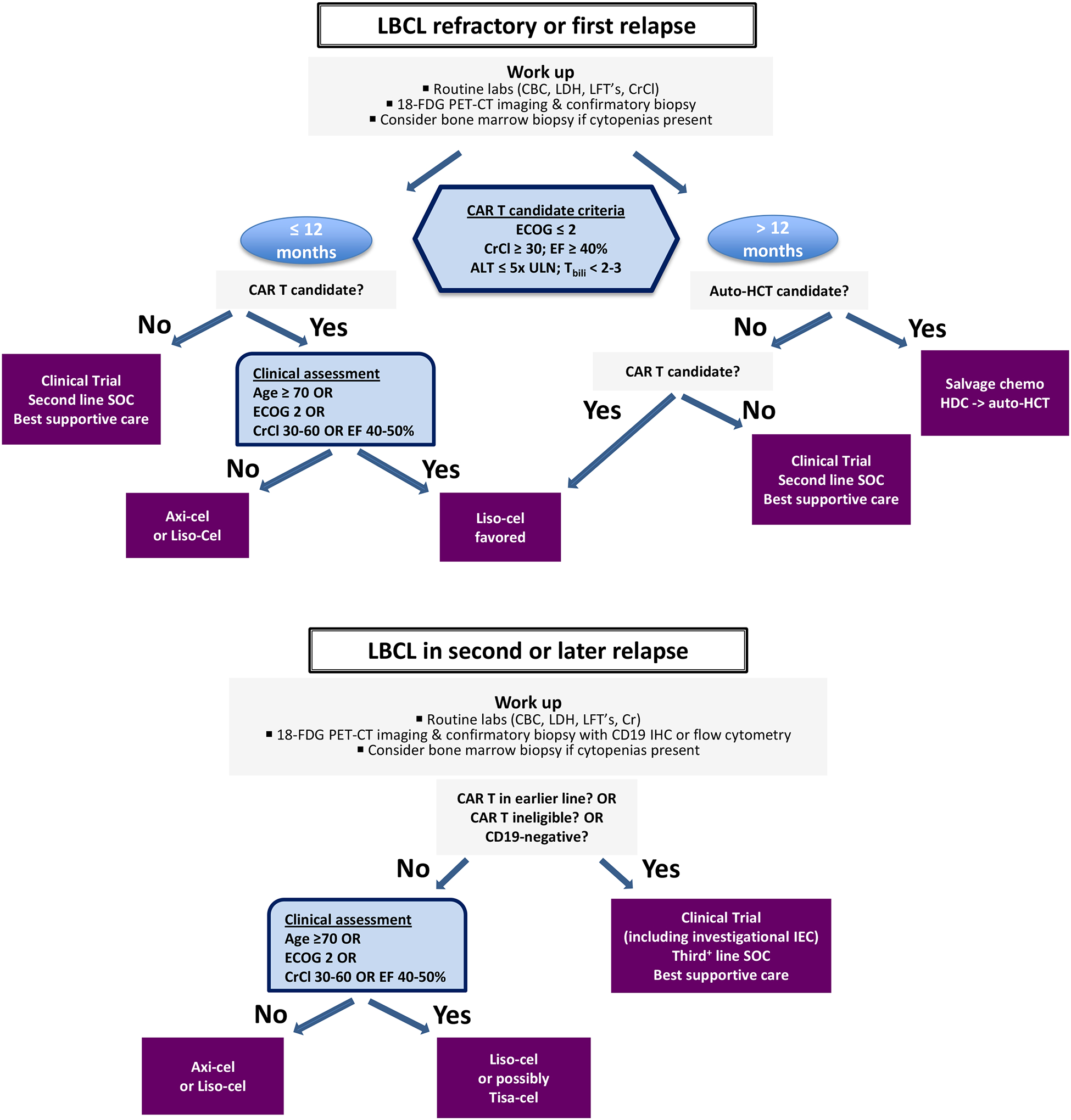
When relapse is suspected clinically, routine laboratory evaluation should include at minimum an assessment of creatinine clearance (CrCl), liver function (LFT’s), blood counts (CBC), and lactate dehydrogenase (LDH) levels. Bone marrow evaluation is typically reserved for patients with cytopenia not explained by other clinical factors. Peripheral blood flow cytometry should be performed if circulating lymphoma cells are suspected. Staging evaluation should be performed with 18F-fluorodeoxyglucose positron-emission tomography and computed tomography (18F-FDG PET-CT). Diagnostic confirmation is based on careful pathological review of biopsy material, preferably from an excisional biopsy. Patients with confirmed first relapse of LBCL at 12 months or less from the time of completion of first-line chemoimmunotherapy should be considered for second-line CAR T-cell therapy. Candidacy is based on Eastern Cooperative Oncology Group (ECOG) performance status less than or equal to 2, CrCl greater than or equal to 30 mL/min, adequate left ventricular function with ejection fraction (EF) greater than or equal to 40%, and adequate liver function with alanine transaminase (ALT) levels less than or equal to 5 times the upper limit of normal (ULN) and total bilirubin (Tbili) less than 2–3 mg/dL. Age might not be a limitation by itself. In the second-line setting, we suggest favoring liso-cel in patients 70 years and older, those with ECOG 2, and those with baseline renal (CrCl 30–60) and/or cardiac (EF 40–50%) dysfunction. Both axi-cel and liso-cel are appropriate for younger and fitter CAR T-cell candidates without organ dysfunction. Transplant-eligible patients with confirmed first relapse greater than 12 months from first-line therapy should undergo platinum-based salvage chemotherapy. Those who respond should proceed to consolidation with high dose chemotherapy and autologous stem cell transplant (auto-HCT). If disease does not respond to salvage therapy, CAR T should be considered. Transplant-ineligible patients should be considered for liso-cel if CAR T-eligible. Patients with suspected second or later relapse of LBCL should undergo similar diagnostic evaluation as above with an additional focus on CD19 immunohistochemistry (IHC) or flow cytometry in those who received CD19-targeted agents. CAR T-cell-naïve patients with CD19+ disease should be considered for axi-cel or liso-cel. We suggest considering liso-cel, or eventually tisa-cel, in patients based on the above criteria regarding age and organ function. Clinical trials incorporating investigational agents should be strongly considered at all phases of therapy, including those evaluating new immune effector cells (IEC).
Significant challenges remain in the use of CAR T-cells in heavily pre-treated patients. Firstly, given the need for rapid change of therapy in aggressive chemo-refractory disease, manufacturing delays impact patient eligibility for autologous cellular therapy. Allogeneic off-the-shelf products have been studied to mitigate treatment delay, though few have induced durable responses. YTB323 (rapcabtagene autoleucel), an autologous CD19-CAR T-cell generated via a proprietary platform which enables a shortened two-day manufacturing process, is being studied in a phase I trial and has shown promising safety and efficacy results (NCT03960840). Another major barrier to improving outcomes in this patient population is intrinsic T-cell dysfunction in the manufacturing product. Early T-cell exhaustion, limiting product expansion and persistence, and “CAR T-regulatory cells” are associated with treatment failure.29–30 Modulation of CD3z immunoreceptor tyrosine-based activation motifs (ITAMs), which enhances CD19-CAR T-cell expansion and potency in mouse models,31 has shown therapeutic promise in a phase 1 study of CD19-Targeted 28z 1XX CAR T Cells (NCT04464200). Lastly, tumor microenvironment suppression, representing an additional obstacle, can be addressed by engineering CAR T cells to modulate inhibitory immune cells.32–34
CAR T-cell therapy in the second-line for LBCL
Three prospective randomized controlled trials: ZUMA-7, BELINDA, and TRANSFORM separately compared CD19-CAR T-cell therapy (axi-cel, tisa-cel, and liso-cel, respectively) to SOC high dose chemotherapy with autologous stem cell transplant (auto-HCT) in LBCL patients with relapsed or refractory disease less than 12 months after completion of first-line chemoimmunotherapy (Table 1).19–21 ZUMA-7 demonstrated improved median event free survival (EFS) in the axi-cel arm relative to SOC (8.3 versus 2.0 months; HR 0.398; P < .0001).19 In the TRANSFORM study, median EFS was superior in the liso-cel arm (10.1 versus 2.3 months; HR: 0.349; P < .0001)), whereas no difference in EFS was shown between the tisa-cel and SOC arms in the BELINDA study (3.0 versus 3.0 months; HR 1.07; P = 0.61).20–21 Between April and June 2022, the FDA and EMA approved both axi-cel and liso-cel for patients with LBCL who experience refractory disease or relapse within 12 months of first-line treatment based on these data.
Table 1.
Summary of results from randomized trials comparing CD19-CAR T versus autologous stem cell transplant in patients with DLBCL who were refractory to R-CHOP or experienced disease progression within one year after the end of R-CHOP.
| Study name and reference | ZUMA-7 (19) | TRANSFORM (21) | BELINDA (20) |
|---|---|---|---|
| CD19-CAR T-cell in experimental arm | Axi-cel | Liso-cel | Tisa-cel |
| Number of patients | 359 | 184 | 322 |
| Patients proceeding to CAR T vs. ASCT (%) | 94% / 36% | 97% / 47% | 96% / 32% |
| Median time from registration to CAR T infusion | 29 days | 34 days | 52 days |
| Bridging therapy allowed (% receiving bridging therapy) | Steroids only | One cycle of salvage chemotherapy (63%) | One cycle or more of salvage chemotherapy (97%, including 54% >1 regimen) |
| Crossover to CAR (% who proceeded) | Not planned (56% received cellular immunotherapy) | Planned per protocol (63%) | Planned per protocol (51%) |
| Median follow-up | 25 months | 17.5 months | 10 months |
| Complete response rate (%) | 65% vs 32% | 74% vs 43% | 28% vs 28% |
| Median event-free survival (months) | 8.3 vs. 2 | Not reached vs. 2.4 | 3 vs 3 |
| Median progression-free survival (months) | 14.7 vs. 3.7 | Not reached vs. 6.2 | na |
| Median overall survival (months) | Not reached vs. 35.1 (0.73) | Not reached vs. 29.9 (0.72) | 16.9 vs. 15.3 |
| Any grade CRS/NE (%) | 92%/60% | 49%/11% | 61%/10% |
| Grade >=3 CRS/NE (%) | 6%/21% | 1%/4% | 5%/2% |
ASCT, high-dose therapy and autologous stem cell support; CAR T, chimeric antigen receptor T-cell therapy; RCHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; CRS, cytokine release syndrome; NE, neurologic event.
While intrinsic differences in product efficacy in refractory LBCL are possible, careful attention to inter-trial differences is warranted while considering the implications of these data on clinical practice. Firstly, the tisa-cel arm of the BELINDA study consisted of a higher proportion of patients with intermediate or higher IPI scores and double-hit lymphoma relative to SOC (65.4 versus 57.5%).20 Importantly, bridging chemotherapy was not allowed in ZUMA-7, thus patients with more aggressive disease behavior at the time of enrollment were potentially excluded.19 Furthermore, patients in TRANSFORM and BELINDA were permitted to receive one or multiple cycles of platinum-based salvage chemotherapy.20–21 ZUMA-7 SOC patients were also only permitted to receive a single line of salvage chemotherapy compared to up to two in the other studies.19 The gap between apheresis and infusion was also longer in BELINDA with a median time of 52 days, relative to 29 and 34 days in ZUMA-7 and TRANSFORM, respectively.19–21 Crossover to CAR T-cell therapy was allowed if a defined event occurred in BELINDA and TRANSFORM whereas patients assigned to SOC in ZUMA-7 could only receive axi-cel off study.19–21 Despite this, crossover rates were comparable (51–56%) across the three studies.19–21 While all three trials used EFS as the primary endpoint, EFS was defined differently offering another potential explanation for discrepant results.19–21
Investigation into the use of CAR T-cells in the second-line also extended to transplant-ineligible patients in the phase 2 PILOT study. 61 patients at a median age of 74 with r/r LBCL who experienced less than a CR to first-line chemoimmunotherapy received a single infusion of liso-cel.22 Ineligibility for transplant was based on age 70 or older, ECOG performance status of 2, and/or end organ dysfunction.22 At a median follow-up time of 12 months, the ORR was 80%, including 54% with CR.22 Patients in CR had a median PFS of 22.6 months while the overall median DOR was about 12 months.22 Importantly, only 49% of patients experienced CRS, ICANS, or both, and the vast majority of symptoms were grade 1–2, consistent with TRANSCEND data.21–22 Based on these results, the FDA expanded the liso-cel approval to include second-line therapy for transplant-ineligible patients in June 2022.
With the advent of expanded use of cellular therapy products, selection of eligible patients is an evolving process. While the HCT-comorbidity index is used to assess transplant eligibility, it has not been shown to predict outcomes or safety of CAR T-cell therapy. On the other hand, several risk scores have been validated in this setting, including the International Prognostic Index (IPI) which was shown to correlate with PFS and neurotoxicity.35 Additionally, as CAR T-cell-related hematopoietic compromise is increasingly being recognized as a frequent and impactful adverse effect after CAR T-cell therapy, the use of the HEMATOTOX model may aid in clinical risk assessment.36 A clinical algorithm for patients with confirmed first relapse of LBCL is shown (Figure 1).
CAR T-cell therapy in the first-line for high-risk LBCL
Patients with high-risk features, including those with MYC, BCL2, and/or BCL6 rearrangement, adverse molecular features,37 and high-intermediate and high-risk IPI scores,38–39 are more likely to experience chemo-refractory disease and shorter overall survival. Furthermore, patients treated in the ZUMA-1 trial who had received fewer prior lines of therapy received CAR T-cells with improved activity profiles, suggesting that less cytotoxic exposure may improve T-cell fitness in the apheresis product.13 The phase 2, multicenter, single-arm ZUMA-12 trial investigated the use of axi-cel as part of first-line therapy in patients with high-risk disease who were PET positive after two cycles of chemoimmunotherapy. High-risk disease was defined as either double- or triple-hit lymphoma or an IPI score of 3 or greater.23 Among 37 treated patients, the ORR was 89% (95% CI, 75–97) and the CR rate was 78% (95% CI, 62–90).23 At a median follow-up time of 16 months, 73% of patients remained in CR, highlighting response durability in the first-line.23 Based on these data, the phase 3 ZUMA-23 study comparing first-line axi-cel to SOC chemo-immunotherapy in patients with high-risk disease is ongoing (NCT05605899).
Though the results in this space are encouraging, feasibility and financial toxicity are major concerns for the nearly 20,000 people in the US who are diagnosed with DLBCL annually. Only about 150 medical centers, mostly in urban areas, offer CAR T-cell therapy, thus severely limiting access.40 Furthermore, as the total estimated cost of a single inpatient treatment is $454,611 (95% CI, $452,466-$458,267), cutting costs is paramount to broadly expand use to the first-line.41 Shifting therapy to the outpatient setting may improve cost-effectiveness. Notably, 25 patients in TRANSCEND received outpatient treatment,8 and one ongoing study is investigating the feasibility of outpatient axi-cel infusion (NCT05108805).
CAR T-cell therapy for LBCL with CNS involvement
Since ICANS was first recognized and characterized, there has been considerable reluctance to administer CAR T-cells to patients with CNS lymphoma both due to efficacy concerns and fear that local inflammatory response will precipitate a fatal neurologic event. In a small case series of two ALL patients, CAR T-cells localized to and persisted for 6 months in the cerebrospinal fluid, consistent with CNS trafficking.42 More recently, intriguing single-cell RNA sequencing data emerged showing CD19 expression in blood-brain barrier-supporting mural cells, suggesting a possible on-target, off-tumor pathogenic mechanism for ICANS.43 As such, patients with secondary CNS involvement by lymphoma were excluded from both ZUMA-1 and JULIET.2,6 TRANSCEND included 7 patients with CNS disease, among which the ORR was 50% and 2 patients experienced grade 3 ICANS,8 yet limited numbers precluded drawing meaningful conclusions.
In the largest single cohort to date, a retrospective analysis of 17 patients with secondary CNS lymphoma (SCNSL) treated with axi-cel showed an ORR of 75%, including 41% of those who remained in a response at 6 months.24 ICANS of any grade and grade 3+ occurred at a similar rate to those in a non-CNS involvement matched cohort.24 Eight SCNSL patients with parenchymal and/or leptomeningeal involvement treated with tisa-cel demonstrated impressive activity with no reports of grade 2 or higher ICANS.25 In primary CNS lymphoma (PCNSL), efficacy and safety signals have also been promising, though similarly limited by small numbers. One ongoing prospective study of 12 patients with PCNSL treated with tisa-cel showed a CR rate of 50%, with only 1 patient experiencing grade 3+ ICANS on interim analysis.26 A recently published meta-analysis combining all published data in CNS lymphoma showed that 30 PCNSL and 98 SCNSL patients treated with CD19 CAR T-cell therapy (70% commercial) had comparable CR rates of 56% and 47%, with 37% and 37% remaining in CR at 6 months, respectively.27 Importantly, the incidence of CRS and ICANS were similar to seminal phase 2 studies.27 Overall, existing data suggest that use of CD19 CAR T-cells in patients with CNS lymphoma is both effective and safe, though larger confirmatory prospective studies are warranted.
Conclusion
CD19-CAR T-cell therapy has revolutionized the way we treat large B cell lymphoma, starting with multiply relapsed disease, and more recently with proven efficacy and comparable safety signal in earlier lines of treatment. Despite these major therapeutic advances, a substantial number of patients experience relapse after CAR T therapy. T-cell intrinsic and extrinsic mechanisms of treatment failure are being addressed by promising engineering strategies that are currently or imminently under investigation in early phase clinical trials. Major ongoing clinical challenges in the field include expansion of patient eligibility, speed of production, and limiting toxicities. Combination with other agents aimed to improve response rate and response duration are being investigated. Notably, off-the-shelf bispecific antibodies are a promising alternative to autologous cellular therapy that have shown impressive efficacy in LBCL, both in first- and subsequent lines of treatment. Rapid production is a significant advantage, yet durability of response and curative potential remain to be determined as the data matures in pivotal phase I/II studies. With imminent approvals of bispecific antibodies in LBCL, well-designed trials will be needed to determine optimal timing and sequencing with CAR T-cell therapy.
Funding
This research was funded in part through the NIH/NCI Cancer Center support grant P30 CA008748 and the 2022 Conquer Cancer ASCO Young Investigator Award (YIA) grant.
Cited References
- 1.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Rivière I, Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003. Mar;9(3):279–86. [DOI] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017. Dec 28;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff P, Flinn IW, Farooq U, Goy A, McSweeney PA, Munoz J, Siddiqi T, Chavez JC, Herrera AF, Bartlett NL, Wiezorek JS, Navale L, Xue A, Jiang Y, Bot A, Rossi JM, Kim JJ, Go WY, Neelapu SS. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019. Jan;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelapu SS, Jacobson CA, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff PJ, Flinn IW, Farooq U, Goy A, McSweeney P, Munoz J, Siddiqi T, Chavez JC, Herrera AF, Bartlett NL, Bot AA, Shen RR, Dong J, Singh K, Miao H, Kim JJ, Zheng Y, Locke FL. 5-Year Follow-Up Supports Curative Potential of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1). Blood. 2023. Feb 23:blood.2022018893. [DOI] [PubMed] [Google Scholar]
- 5.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017. Dec 28;377(26):2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jager U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak O, Salles G, Maziarz RT; JULIET Investigators. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019. Jan 3;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 7.Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, Waller EK, Jaglowski S, Bishop MR, Damon LE, Foley SR, Westin JR, Fleury I, Ho PJ, Mielke S, Teshima T, Janakiram M, Hsu JM, Izutsu K, Kersten MJ, Ghosh M, Wagner-Johnston N, Kato K, Corradini P, Martinez-Prieto M, Han X, Tiwari R, Salles G, Maziarz RT. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021. Oct;22(10):1403–1415. [DOI] [PubMed] [Google Scholar]
- 8.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, Sehgal A, Solomon SR, Ghosh N, Albertson TM, Garcia J, Kostic A, Mallaney M, Ogasawara K, Newhall K, Kim Y, Li D, Siddiqi T. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020. Sep 19;396(10254):839–852. [DOI] [PubMed] [Google Scholar]
- 9.Abramson JS, Palomba LM, Gordon LI, Lunning MA, Wang M, Arnason JE, Purev E, Maloney DG, Andreadis C, Sehgal AR, Solomon SR, Ghosh N, Kostic A, Kim Y, Ogasawara K, Dehner C, Siddiqi T. Two-year follow-up (FU) of transcend NHL 001, a multicenter phase 1 study of lisocabtagene maraleucel (liso-cel) in relapsed or refractory (R/R) large B-cell lymphomas (LBCL). Blood. 2021; 138 (Supplement 1): 2840–2843. [Google Scholar]
- 10.Neelapu SS, Jacobson CA, Oluwole OO, Munoz J, Deol A, Miklos D, Bartlett NL, Braunschweig I, Jiang Y, Kim JJ, Zheng L, Rossi JM, Locke FL. Outcomes of older patients in ZUMA-1, a pivotal study of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood. 2020. Jun 4;135(23):2106–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen PH, Wright K, Lipschitz M, Ritz J, Kamihara Y, Armand P, Nikiforow S, Rogalski M, Maakaron J, Jaglowski S, Maus MV, Chen YB, Abramson JS, Kline J, Budde E, Herrera A, Mei M, Cohen JB, Smith SD, Maloney DG, Gopal AK, Frigault MJ, Acharya UH. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J Clin Oncol. 2020. Sep 20;38(27):3095–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, Dahiya S, Lunning M, Lekakis L, Reagan P, Oluwole O, McGuirk J, Deol A, Sehgal AR, Goy A, Hill BT, Vu K, Andreadis C, Munoz J, Westin J, Chavez JC, Cashen A, Bennani NN, Rapoport AP, Vose JM, Miklos DB, Neelapu SS, Locke FL. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol. 2020. Sep 20;38(27):3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, Oluwole OO, Reagan PM, Lekakis LJ, Lin Y, Sherman M, Better M, Go WY, Wiezorek JS, Xue A, Bot A. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020. Oct 13;4(19):4898–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sermer D, Batlevi C, Palomba ML, Shah G, Lin RJ, Perales MA, Scordo M, Dahi P, Pennisi M, Afuye A, Silverberg ML, Ho C, Flynn J, Devlin S, Caron P, Hamilton A, Hamlin P, Horwitz S, Joffe E, Kumar A, Matasar M, Noy A, Owens C, Moskowitz A, Straus D, von Keudell G, Rodriguez-Rivera I, Falchi L, Zelenetz A, Yahalom J, Younes A, Sauter C. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020. Oct 13;4(19):4669–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon M, Iacoboni G, Reguera JL, Corral LL, Morales RH, Ortiz-Maldonado V, Guerreiro M, Caballero AC, Domínguez MLG, Pina JMS, Mussetti A, Sancho JM, Bastos-Oreiro M, Catala E, Delgado J, Henriquez HL, Sanz J, Calbacho M, Bailén R, Carpio C, Ribera JM, Sureda A, Briones J, Hernandez-Boluda JC, Cebrián NM, Martin JLD, Martín A, Barba P. Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma. Haematologica. 2023. Jan 1;108(1):110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, Beauvais D, Roulin L, Gros FX, Rubio MT, Bories P, Bay JO, Llorente CC, Choquet S, Casasnovas RO, Mohty M, Guidez S, Joris M, Loschi M, Carras S, Abraham J, Chauchet A, Drieu La Rochelle L, Deau-Fischer B, Hermine O, Gastinne T, Tudesq JJ, Gat E, Broussais F, Thieblemont C, Houot R, Morschhauser F. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022. Oct;28(10):2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riedell PA, Hwang WT, Nastoupil LJ, Pennisi M, McGuirk JP, Maziarz RT, Bachanova V, Oluwole OO, Brower J, Flores OA, Ahmed N, Schachter L, Bharucha K, Dholaria BR, Schuster SJ, Perales MA, Bishop MR, Porter DL. Patterns of Use, Outcomes, and Resource Utilization among Recipients of Commercial Axicabtagene Ciloleucel and Tisagenlecleucel for Relapsed/Refractory Aggressive B Cell Lymphomas. Transplant Cell Ther. 2022. Oct;28(10):669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landsburg DJ, Frigault M, Heim M, Foley SR, Hill BT, Ho CM, Jacobson CA, Jaglowski S, Locke FL, Ram R, Riedell PA, Shah GL, Popplewell LL, Tiwari R, Lim S, Majdan M, Masood A, Pasquini MC, Turtle CJ. Real-World Outcomes for Patients with Relapsed or Refractory (R/R) Aggressive B-Cell Non-Hodgkin’s Lymphoma (aBNHL) Treated with Commercial Tisagenlecleucel: Subgroup Analyses from the Center for International Blood and Marrow Transplant Research (CIBMTR) Registry. Blood. 2022; 140 (Supplement 1): 1584–1587. [Google Scholar]
- 19.Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, Ghobadi A, Rapoport AP, McGuirk J, Pagel JM, Muñoz J, Farooq U, van Meerten T, Reagan PM, Sureda A, Flinn IW, Vandenberghe P, Song KW, Dickinson M, Minnema MC, Riedell PA, Leslie LA, Chaganti S, Yang Y, Filosto S, Shah J, Schupp M, To C, Cheng P, Gordon LI, Westin JR; All ZUMA-7 Investigators and Contributing Kite Members. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med. 2022. Feb 17;386(7):640–654. [DOI] [PubMed] [Google Scholar]
- 20.Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, Kato K, Sureda A, Greil R, Thieblemont C, Morschhauser F, Janz M, Flinn I, Rabitsch W, Kwong YL, Kersten MJ, Minnema MC, Holte H, Chan EHL, Martinez-Lopez J, Müller AMS, Maziarz RT, McGuirk JP, Bachy E, Le Gouill S, Dreyling M, Harigae H, Bond D, Andreadis C, McSweeney P, Kharfan-Dabaja M, Newsome S, Degtyarev E, Awasthi R, Del Corral C, Andreola G, Masood A, Schuster SJ, Jäger U, Borchmann P, Westin JR. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N Engl J Med. 2022. Feb 17;386(7):629–639. [DOI] [PubMed] [Google Scholar]
- 21.Abramson JS, Solomon SR, Arnason JE, Johnston PB, Glass B, Bachanova V, Ibrahimi S, Mielke S, Mutsaers PGNJ, Hernandez-Ilizaliturri FJ, Izutsu K, Morschhauser F, Lunning MA, Crotta A, Montheard S, Previtali A, Ogasawara K, Kamdar M. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of phase 3 TRANSFORM study. Blood. 2022. Dec 21:blood.2022018730. [DOI] [PubMed] [Google Scholar]
- 22.Sehgal A, Hoda D, Riedell PA, et al. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): an open-label, phase 2 study. 2022. Aug;23(8):1066–1077. [DOI] [PubMed] [Google Scholar]
- 23.Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, Herrera AF, Ujjani CS, Lin Y, Riedell PA, Kekre N, de Vos S, Lui C, Milletti F, Dong J, Xu H, Chavez JC. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022. Apr;28(4):735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennani NN, Maurer MJ, Nastoupil LJ, et al. Experience with axicabtagene ciloleucel (Axi-cel) in patients with secondary CNS involvement: results from the US lymphoma CAR T consortium. Blood. 2019; 134 (Supplement 1): 763. [Google Scholar]
- 25.Frigault MJ, Dietrich J, Martinez-Lage M, Leick M, Choi BD, DeFilipp Z, Chen YB, Abramson J, Crombie J, Armand P, Nayak L, Panzini C, Riley LS, Gallagher K, Maus MV. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019. Sep 12;134(11):860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frigault MJ, Dietrich J, Gallagher K, Roschewski M, Jordan JT, Forst D, Plotkin SR, Cook D, Casey KS, Lindell KA, Depinho GD, Katsis K, Elder EL, Leick MB, Choi B, Horick N, Preffer F, Saylor M, McAfee S, O’Donnell PV, Spitzer TR, Dey B, DeFilipp Z, El-Jawahri A, Batchelor TT, Maus MV, Chen YB. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood. 2022. Apr 14;139(15):2306–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook MR, Dorris CS, Makambi KH, Luo Y, Munshi PN, Donato M, Rowley S, Saad A, Goy A, Dunleavy K, Ali A. Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: a meta-analysis of 128 patients. Blood Adv. 2023. Jan 10;7(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wudhikarn K, Pennisi M, Garcia-Recio M, Flynn JR, Afuye A, Silverberg ML, Maloy MA, Devlin SM, Batlevi CL, Shah GL, Scordo M, Palomba ML, Dahi PB, Sauter CS, Santomasso BD, Mead E, Perales MA. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Adv. 2020. Jul 14;4(13):3024–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haradhvala NJ, Leick MB, Maurer K, Gohil SH, Larson RC, Yao N, Gallagher KME, Katsis K, Frigault MJ, Southard J, Li S, Kann MC, Silva H, Jan M, Rhrissorrakrai K, Utro F, Levovitz C, Jacobs RA, Slowik K, Danysh BP, Livak KJ, Parida L, Ferry J, Jacobson C, Wu CJ, Getz G, Maus MV. Distinct cellular dynamics associated with response to CAR-T therapy for refractory B cell lymphoma. Nat Med. 2022. Sep;28(9):1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Good Z, Spiegel JY, Sahaf B, Malipatlolla MB, Ehlinger ZJ, Kurra S, Desai MH, Reynolds WD, Wong Lin A, Vandris P, Wu F, Prabhu S, Hamilton MP, Tamaresis JS, Hanson PJ, Patel S, Feldman SA, Frank MJ, Baird JH, Muffly L, Claire GK, Craig J, Kong KA, Wagh D, Coller J, Bendall SC, Tibshirani RJ, Plevritis SK, Miklos DB, Mackall CL. Post-infusion CAR TReg cells identify patients resistant to CD19-CAR therapy. Nat Med. 2022. Sep;28(9):1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feucht J, Sun J, Eyquem J, Ho YJ, Zhao Z, Leibold J, Dobrin A, Cabriolu A, Hamieh M, Sadelain M. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat Med. 2019. Jan;25(1):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain MD, Zhao H, Wang X, Atkins R, Menges M, Reid K, Spitler K, Faramand R, Bachmeier C, Dean EA, Cao B, Chavez JC, Shah B, Lazaryan A, Nishihori T, Hussaini M, Gonzalez RJ, Mullinax JE, Rodriguez PC, Conejo-Garcia JR, Anasetti C, Davila ML, Locke FL. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. 2021. May 13;137(19):2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholler N, Perbost R, Locke FL, Jain MD, Turcan S, Danan C, Chang EC, Neelapu SS, Miklos DB, Jacobson CA, Lekakis LJ, Lin Y, Ghobadi A, Kim JJ, Chou J, Plaks V, Wang Z, Xue A, Mattie M, Rossi JM, Bot A, Galon J. Tumor immune contexture is a determinant of antiCD19 CAR T cell efficacy in large B cell lymphoma. Nat Med. 2022. Sep;28(9):1872–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020. Mar;17(3):147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Recio M, Wudhikarn K, Pennisi M, et al. The International Prognostic Index Is Associated with Outcomes in Diffuse Large B Cell Lymphoma after Chimeric Antigen Receptor T Cell Therapy. Transplant Cell Ther. 2021. Mar;27(3):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rejeski K, Perez A, Sesques P, Hoster E, Berger C, Jentzsch L, Mougiakakos D, Frölich L, Ackermann J, Bücklein V, Blumenberg V, Schmidt C, Jallades L, Fehse B, Faul C, Karschnia P, Weigert O, Dreyling M, Locke FL, von Bergwelt-Baildon M, Mackensen A, Bethge W, Ayuk F, Bachy E, Salles G, Jain MD, Subklewe M. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021. Dec 16;138(24):2499–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, Lawrence MS, Roemer MGM, Li AJ, Ziepert M, Staiger AM, Wala JA, Ducar MD, Leshchiner I, Rheinbay E, Taylor-Weiner A, Coughlin CA, Hess JM, Pedamallu CS, Livitz D, Rosebrock D, Rosenberg M, Tracy AA, Horn H, van Hummelen P, Feldman AL, Link BK, Novak AJ, Cerhan JR, Habermann TM, Siebert R, Rosenwald A, Thorner AR, Meyerson ML, Golub TR, Beroukhim R, Wulf GG, Ott G, Rodig SJ, Monti S, Neuberg DS, Loeffler M, Pfreundschuh M, Trümper L, Getz G, Shipp MA. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018. May;24(5):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993. Sep 30;329(14):987–94. [DOI] [PubMed] [Google Scholar]
- 39.Ruppert AS, Dixon JG, Salles G, Wall A, Cunningham D, Poeschel V, Haioun C, Tilly H, Ghesquieres H, Ziepert M, Flament J, Flowers C, Shi Q, Schmitz N. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood. 2020. Jun 4;135(23):2041–2048. [DOI] [PubMed] [Google Scholar]
- 40.Snyder S, Albertson T, Garcia J, Gitlin M, Jun MP. Travel-Related Economic Burden of Chimeric Antigen Receptor T Cell Therapy Administration by Site of Care. Adv Ther. 2021. Aug;38(8):4541–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyman GH, Nguyen A, Snyder S, Gitlin M, Chung KC. Economic evaluation of chimeric antigen receptor T-cell therapy by site of care among patients with relapsed or refractory large B-cell lymphoma. JAMA Netw Open. 2020. Apr 1;3(4):e202072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013. Apr 18;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker Parker KR, Migliorini D, Perkey E, Yost KE, Bhaduri A, Bagga P, Haris M, Wilson NE, Liu F, Gabunia K, Scholler J, Montine TJ, Bhoj VG, Reddy R, Mohan S, Maillard I, Kriegstein AR, June CH, Chang HY, Posey AD Jr, Satpathy AT. Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell. 2020. Oct 1;183(1):126–142.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]


