Abstract
Hepatocellular carcinoma (HCC) has a high mortality rate due to the diagnosis of patients at advanced stages and ineffective systemic therapies. Immunotherapy is considered a new treatment option for unresectable HCC alternatives to the limitations of conventional cytotoxic chemotherapy. In this case report, we reported that transarterial radioembolization and immunotherapy such as atezolizumab and bevacizumab can be used together in a manner effectively in the management of HCC treatment.
Keywords: Atezolizumab, fluorodeoxyglucose positron emission tomography-computed tomography, hepatocellular carcinoma, transarterial radioembolization
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies with high mortality rates due to challenging treatment management. Viral hepatitis, alcohol abuse, metabolic liver disease, and obesity cause liver damage. Processing of HCC is a multistep pathway that arises from chronic liver disease and cirrhosis.[1] Hepatic malignancies have been detected in athletes and bodybuilders with a history of using long-term high-dose anabolic androgenic steroids to increase muscle mass. Anabolic androgenic steroids may increase the risk of hepatic tumor development by causing hepatocyte proliferation.[2] Although surgical or locoregional interventions are treatment options in early-stage HCC, the poor prognosis in the patient group with unresectable disease makes treatment challenging.[3] Immunotherapy provides a clinically meaningful improvement in the management of unresectable HCC. In this case, we presented that combining transarterial radioembolization (TARE) and atezolizumab and bevacizumab (Atezo/Bev) therapy can significantly affect the management of unresectable HCC treatment.
Case Report
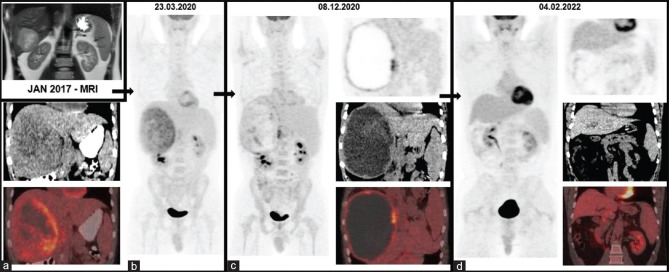
In 2017, a 34-year-old male athlete with a history of using nutritional supplements was admitted to the gastroenterology outpatient clinic suffering from weight loss, nausea, and vomiting. Serum tumor markers (carcinoembryonic antigen, α-fetoprotein, and lactate dehydrogenase) and viral hepatitis screening were negative. Magnetic resonance ımaging (MRI) revealed a well-circumscribed mass measuring 7 cm × 7 cm × 6 cm in liver segment VI [Figure 1a], with rim-shaped contrast enhancement in the arterial phase and a washout pattern in the delayed phase. A hepatic biopsy with a histopathological examination was recommended for a definitive diagnosis; however, the patient refused the interventional procedure and did not follow medical recommendations until 2020. A repeated MRI scan on this date demonstrated the progression of this huge tumor measuring approximately 18 cm × 17 cm × 14 cm. Simultaneous F-18 fluorodeoxyglucose (FDG)-positron emission tomography/computed tomography (PET/CT) images [Figure 1b] also showed increased FDG uptake more prominent in the periphery of the tumor. The consensus of the multidisciplinary liver tumor board was to treat the patient with TARE for HCC, given the radiographic appearance. The patient underwent two sessions of TARE with yttrium-90 (Y-90) at a 2-month interval in April and June. Then, the patient was referred for immunotherapy and received 10 cycles of (Atezo/Bev) between August 2020 and May 2021. This treatment was well tolerated, and no adverse events were observed according to common terminology criteria for adverse events. After these treatments, necrotic and hemorrhagic changes were seen in the mass on a follow-up PET/CT scan [Figure 1c]. At this stage, the multidisciplinary tumor board decided to enucleate the lesion. The postoperative pathological specimens showed no residual tumor histologically and postoperative PET/CT revealed that there was no evidence of malignancy in the rest of the liver parenchyma [Figure 1d].
Figure 1.
MRI revealed a well-circumscribed mass measuring in the liver segment VI (a) in 2017. After 3 years without follow-up in 2020, F-18 FDG PET/CT images (b) also showed increased FDG uptake more prominent in the periphery of the tumor. The consensus of the multidisciplinary liver tumor board was to treat the patient with transarterial radioembolization with yttrium-90 for HCC. Then, the patient was referred for immunotherapy and received 10 cycles of Atezo/Bev. After these treatments, necrotic and hemorrhagic changes were seen in the mass on follow-up F-18 FDG PET/CT scan (c). At this stage, the multidisciplinary tumor board decided to enucleate the lesion and postoperative F-18 PET/CT revealed that there was no evidence of malignancy in the rest of the liver parenchyma (d) MRI: Magnetic resonance ımaging, FDG: Fluorodeoxyglucose, PET/CT: Positron emission tomography/computed tomography, Atezo/Bev: Atezolizumab and bevacizumab treatment, HCC: Hepatocellular carcinoma
Discussion
The prognosis of HCC is precisely related to the stage of cancer at the admission of the patient. Surgical curative treatment options included resection and transplantation in early-stage HCC patients. Patients with unresectable advanced HCC receive systemic treatments, traditionally sorafenib or lenvatinib in the first line and regorafenib, cabozantinib, or ramucirumab in the second line.
Sorafenib is the first multi-tyrosine kinase inhibitor that affects cancer cells by inhibiting angiogenesis and tumor proliferation and has been approved for the treatment of advanced HCC for approximately 10 years.[4] HCC, which develops on the background of chronic inflammation caused by genetic predisposition, alcohol, and cirrhosis, has a heterogeneous tumor immunity.[5] Immunity and the microenvironment of the tumor are essential factors affecting carcinogenesis, invasion and extension of tumor tissue, and metastasis.[6] Immune checkpoint receptors such as programmed death-1 (PD-1) and PD-ligand-1 (PD-L1) are inhibitory pathways that physiologically balance T-cell-mediated immune responses.[7] In patients with HCC with underlying chronic cirrhosis, constant inflammation causes PD 1/PD-L1 overexpression.[8] Immune checkpoint inhibitors (ICIs) (e.g., atezolizumab) precipitate an antitumor effect by blocking the evading mechanism of tumor tissue from the immune system.[9] Bevacizumab is selectively binding circulating vascular endothelial growth factor (VEGF), thereby inhibiting the binding of VEGF to its cell surface receptors.[10] In the management of unresectable HCC, Atezo/Bev treatments provide a clinically meaningful improvement in overall survival/progression-free survival and patients’ quality of life compared to first-line immunotherapies such as sorafenib and lenvatinib.[11-14] ICIs combined with TARE therapy may produce a synergistic effect by exposing hepatocytes to Y-90-labeled microspheres and causing immune cell death. In advanced HCC treatment management, a study presenting the results of the combination of TARE and nivolumab, which is one of the PD-1 targeting agents, has been reported in the literature. However, the results of Atezo/Bev immunotherapy and TARE treatment with Y-90 glass microspheres have not been reported yet. The phase II study of Tai et al. included 40 patients with a received combination of Y-90-resin microspheres with TARE and nivolumab and showed an objective response rate of approximately 30%.[15] Several clinical trials are in progress to evaluate the utility of the combination of Atezo/Bev and TARE with Y-90.[16-18] In this case report, we reported that TARE and immunotherapy can be used together in a manner effectively in the management of HCC treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rinaldi L, Vetrano E, Rinaldi B, Galiero R, Caturano A, Salvatore T, et al. HCC and molecular targeting therapies: Back to the future. Biomedicines. 2021;9:1345. doi: 10.3390/biomedicines9101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solimini R, Rotolo MC, Mastrobattista L, Mortali C, Minutillo A, Pichini S, et al. Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping. Eur Rev Med Pharmacol Sci. 2017;21:7–16. [PubMed] [Google Scholar]
- 3.Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1–61. doi: 10.1016/bs.acr.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fornari F, Giovannini C, Piscaglia F, Gramantieri L. Elucidating the molecular basis of sorafenib resistance in HCC: Current findings and future directions. J Hepatocell Carcinoma. 2021;8:741–57. doi: 10.2147/JHC.S285726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giraud J, Chalopin D, Blanc JF, Saleh M. Hepatocellular carcinoma ımmune landscape and the potential of ımmunotherapies. Front Immunol. 2021;12:655697. doi: 10.3389/fimmu.2021.655697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayant K, Habib N, Huang KW, Warwick J, Arasaradnam R. Recent advances: The ımbalance of ımmune cells and cytokines in the pathogenesis of hepatocellular carcinoma. Diagnostics (Basel) 2020;10:338. doi: 10.3390/diagnostics10050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–51. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valery M, Cervantes B, Samaha R, Gelli M, Smolenschi C, Fuerea A, et al. Immunotherapy and hepatocellular cancer: Where are we now? Cancers (Basel) 2022;14:4523. doi: 10.3390/cancers14184523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: Meta-analysis. BMJ. 2018;362:k3529. doi: 10.1136/bmj.k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung K. Molecular Imaging and Contrast Agent Database (MICAD) 2004–2013. Bethesda (MD): National Center for Biotechnology Information (US); 2008. Alexa Fluor 680-Bevacizumab. [PubMed] [Google Scholar]
- 11.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 12.Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrav|ne150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:991–1001. doi: 10.1016/S1470-2045(21)00151-0. [DOI] [PubMed] [Google Scholar]
- 13.Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:146. doi: 10.1038/s41392-020-00264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura Y, Kobayashi M, Shindoh J, Matsumura M, Okubo S, Muraishi N, et al. Pretreatment positron emission tomography with 18F-fluorodeoxyglucose may be a useful new predictor of early progressive disease following atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma. Oncology. 2022;100:320–30. doi: 10.1159/000523850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai D, Loke K, Gogna A, Kaya NA, Tan SH, Hennedige T, et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): A single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:1025–35. doi: 10.1016/S2468-1253(21)00305-8. [DOI] [PubMed] [Google Scholar]
- 16.Di Federico A, Rizzo A, Carloni R, De Giglio A, Bruno R, Ricci D, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: Preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31:361–9. doi: 10.1080/13543784.2022.2009455. [DOI] [PubMed] [Google Scholar]
- 17.He AR, Kim AY, Toskich BM, Mody K, Kim K, Stein S, et al. Aphase II study of atezolizumab (ATEZO) and bevacizumab (Bev) in combination with Y90 TARE in patients (Pts) with hepatocellular carcinoma (HCC) J Clin Oncol. 2021;39:358. [Google Scholar]
- 18.Park MK, YU SJ. Concurrent transarterial radioembolization and combination atezolizumab/bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: A case report. J Liver Cancer. 2022;22:69–74. doi: 10.17998/jlc.2022.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]